Abstract
Background:
The prevalence of neuropathic pain (NP) in burn patients is reported in the literature to be as high as 80%1. Given the complexity of NP in burn patients and the wide range of treatments available, a systematic review of the literature is warranted to summarize our current understanding of management and treatment of NP in this population.
Methods:
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. The following databases were queried to identify relevant articles: PubMed, Cochrane, Embase, Scopus, Ovid, and Web of Science. The main outcome measures were incidence and management of NP. Secondary outcomes included risk factors for NP.
Results:
Included articles presented findings from 11 different countries, capturing outcomes for 4366 patients. Risk factors for neuropathic pain in burn patients were identified, including older age, alcohol and substance abuse, current daily smoking, greater % total body surface area burns (TBSA), and longer hospitalizations. Pharmacologic treatments included gabapentin/pregabalin (n = 7), ascorbic acid (n = 1), and lidocaine (n = 1). Overall, the studies showed varied results regarding the efficacy of pharmacological treatments. While certain studies demonstrated gaba-pentanoids to be effective in reducing neuropathic symptoms, others found conflicting results. With regards to non-pharmacologic treatments, electroconvulsive therapy (n = 1), electropuncture (n = 1), nerve release/reconstruction (n = 2), and somatosensory feedback rehabilitation (n = 1) were used and demonstrated promise in reducing pain intensity and improving functionality.
Conclusions:
Despite NP afflicting the majority of burn patients long after their injury, this systematic review demonstrates insufficient evidence on the pathophysiology, outcomes, and risk factors in NP, as well as the efficacy of various therapies. Future prospective and randomized studies evaluating the etiology of these factors can substantially improve our treatment strategies. This can allow for the development of well-delineated and evidence-based protocols in NP management in hopes of improving quality of life and both psychological and physical function in burn patients.
Keywords: Neuropathic pain, Burns, Itch
1. Introduction
Burn injuries are a significant source of morbidity and mortality worldwide, affecting millions of people each year. While advances in burn care have improved survival rates, many patients continue to experience neuropathic pain (NP) and itch as a result of their injuries [1]. NP is a complex condition arising from damage to the nervous system and is often difficult to manage. This complication of burn injury often leads to reduced quality of life and increased health-care costs [2]. Burn patients can develop NP through a variety of mechanisms, including damage to peripheral nerves, central sensitization, and alterations in the spinal cord and brain [3]. The pain may manifest as tingling, burning, shooting, or electric shock-like sensations. These negative outcomes can be chronic in nature, persisting long after the initial injury has healed [3,4], with rates of NP and itch in the literature to be reported to be as high as 80% [1]. Distinguishing NP from nociceptive pain is crucial, as NP originates from aberrant nervous system signaling rather than tissue damage [5].
Neuropathic itch is another chronic sensation that commonly co-presents with NP, often persisting despite conventional anti-itch measures like scratching. Although both entities arise of an aberration in normal neuronal signaling, evidence suggests they represent distinct mechanisms and clinical manifestations. More specifically, itch arises from activation of pruriceptive pathways that result in tingling or prickling sensations, likely originating from itch-selective primary afferents [6]. Conversely, NP stems from a distinct mechanism involving abnormal signal transduction originating from damaged axons and altered ion channel function, manifesting as heightened responses to noxious stimuli [5,7].
The pathogenesis of post-burn NP emerges from the in-terplay of various molecular and cellular mechanisms. Initially, thermal injury triggers a cascade of inflammatory responses, releasing pro-inflammatory cytokines and activating immune cells, particularly P2×4R+ microglial cells [8–10]. Microglia have been implicated in contributing to the sensitization of the dorsal horn, a division of the grey matter in the spinal cord that plays a major role in the sensory pathway [5]. This inflammatory milieu of proinflammatory cytokines, most predominantly interleukin-1β (IL-1β), contributes to peripheral sensitization, heightening the responsiveness of nociceptive neurons [10]. In addition, axonal damage resulting from burn injury leads to structural alterations in peripheral nerves and central sensitization within the central nervous system. The release of neuro-transmitters such as glutamate and substance P further contribute to pain perception [11].
The timing of NP onset and relief adds additional considerations to its management in burn patients. NP can manifest immediately after burn injuries or exhibit delayed onset, emphasizing the need to understand factors influencing its timing [12]. Relief from NP is also diverse, varying from short-term to persistent, impacting patients long after the initial burn injury [13]. The acute phase immediately following burn injury is marked by intense discomfort, itch and/or pain. If the acute neuropathic symptoms progress to a chronic state, such symptoms can persist, likely with a progressive mild reduction in severity or potential eventual resolution [14,15]. As expected, some preliminary evidence suggests that greater depth and surface area of the primary burn injury were significant risk factors for the progression of acute to chronic NP [15]. The inherently heterogeneous nature of NP presentation and timing further complicate the study of this post-burn sequela.
Defining and assessing neuropathic pain (NP) requires precision in measurement to capture its nuanced aspects. The Visual Analog Scale (VAS) is a commonly used tool where patients rate their pain on a continuous line, providing a quick assessment of pain intensity. Quantitative Sensory Testing (QST) involves a battery of sensory tests to objectively measure responses to stimuli [16]. Numeric Rating Scales (NRS) offer a numerical scale for patients to rate their pain, enhancing quantifiability [17]. Condition-specific scales like the Neuropathic Pain Scale (NPS) delve into the intricacies of NP, considering various dimensions such as sensory symptoms, surface and deep pain, and paroxysmal pain [18]. PROMIS (Patient-Reported Outcomes Measurement Information System) scores, including domains like Pain Interference and Pain Behavior, provide a broader perspective on how pain affects daily life and emotional well-being [19]. Each of these tools contributes uniquely to our understanding of NP, with PROMIS scores offering a more holistic view of the impact of pain on patients’ overall quality of life and functioning. Incorporating these diverse assessment tools into research protocols can enrich the characterization of NP and facilitate cross-study comparisons.
Effective and targeted management of NP in burn patients is essential for improving their quality of life and reducing chronic pain-related disability [20,21]. This demands a multidisciplinary approach that integrates both pharmacological (anticonvulsants, antidepressants, opioids, topical agents) and non-pharmacological (cognitive-behavioral therapy, physical therapy, neuromodulation techniques) interventions,[3,22–24]. However, we find no current standardized guidelines on the implementation of these management strategies (dosage, recommended duration of use, etc.). Given that burn-induced NP is rooted in intricate processes involving peripheral nerve damage and central sensitization, pharmacologic agents used in NP treatment target these mechanisms. Anticonvulsants curb hyperexcitability in sensory neurons [25], while antidepressants balance neuro-chemicals contributing to NP [26]. Opioids bind to central receptors, and topical agents act locally [27]. Understanding how these agents align with NP pathways guides individualized treatment, bridging clinical presentation and molecular mechanisms for improved management.
Given the complexity of NP in burn patients and the wide range of treatment approaches available, a systematic review of the literature is warranted to summarize our current understanding of knowledge gaps in management and treatment strategies for NP. In doing so, we seek to guide future research aimed at improving the management of NP in burn patients.
2. Methods
2.1. Study identification
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [28]. The following databases were queried to identify relevant articles PubMed, Cochrane, Embase, Scopus, Ovid, and Web of Science.
2.2. Search terms and data extraction
Permutations of the following keywords were used in the databases: “neuropathic pain,” “burn injury”, and “burns”. Studies in animals, in-vitro studies, case reports, letters to the editors, and non-English articles independent reviewers were excluded from the review. Three authors (ES, AM, SG) independently screened titles, abstracts, and full texts to determine eligibility for inclusion and exclusion. We found no disagreements between screeners. Included studies were assigned a level of evidence (LOE) after full-text review.
2.3. Outcome measures
The main outcome measures were incidence and management of NP. Secondary outcomes included risk factors.
2.4. Data analysis
Given the heterogeneity of the included articles, quantitative analysis was not performed. Pertinent findings from each study were instead evaluated qualitatively.
3. Results
3.1. Overall
Our initial database search yielded a total of 1770 articles. After title and abstract screening of all total articles and removal of duplicates, 129 articles were left for further review. After full-text review and assessment for final inclusion, 17 studies were left for formal extraction systematic analysis (Fig. 1).
Fig. 1 –
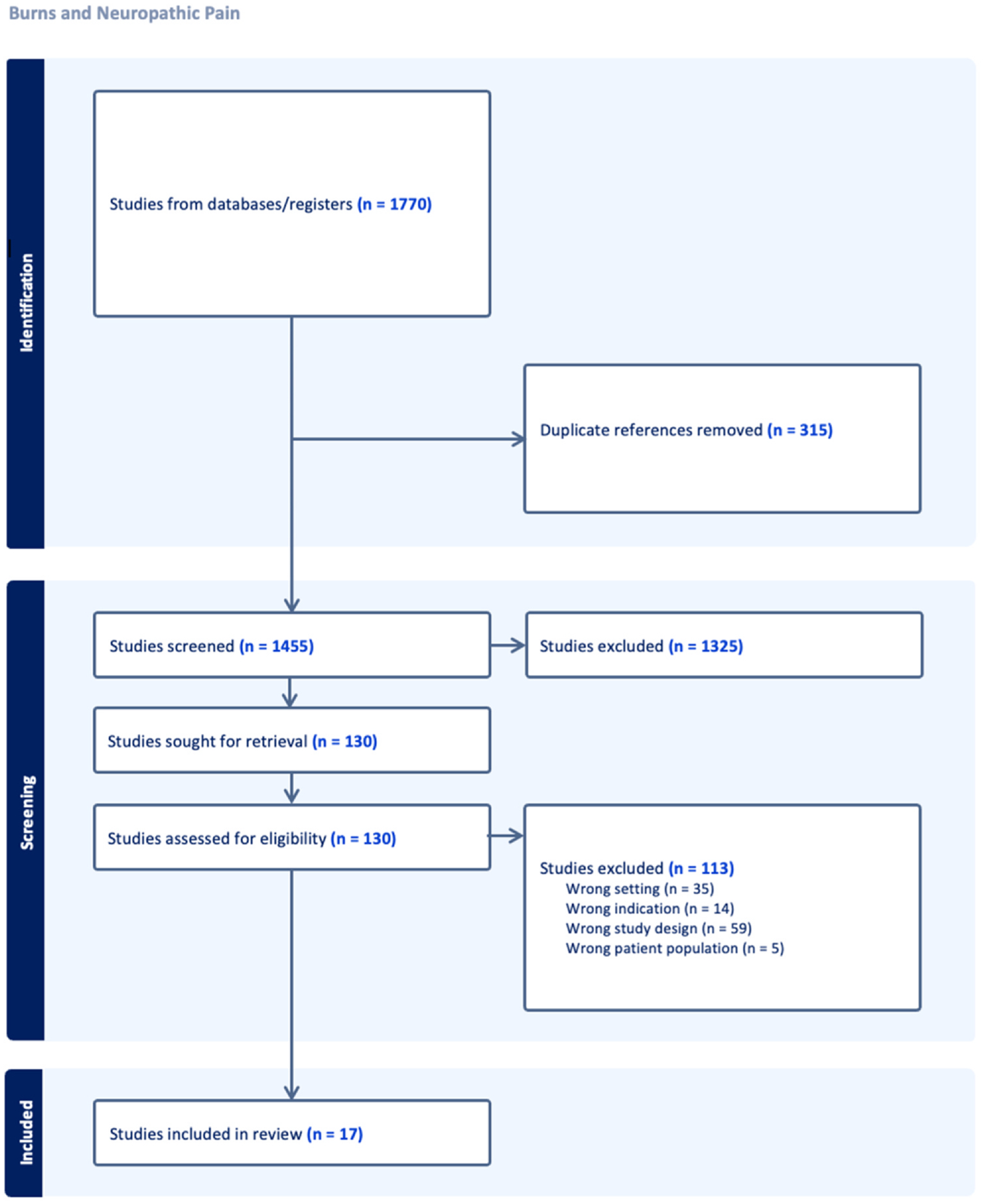
Preferred Reporting Items for Systematic Reviews and Meta-Analysis-P (PRISMA-P) Literature Search.
Included articles presented findings from 11 different countries, overall capturing outcomes for 4366 patients. The study design most implemented was retrospective cohort (n = 8), followed by randomized/non-randomized controlled trial (n = 6), prospective cohort (n = 2), and case series (n = 1). Outcome measures assessed by the articles included pain, itch, sensory thresholds, nerve conduction, response to various treatment modalities, length of stay, and risk factors for NP. Pharmacologic treatments included gabapentin/pregabalin (n = 7), ascorbic acid (n = 1), and lidocaine (n = 1). In regard to non-pharmacologic treatments, electroconvulsive therapy (n = 1), electropuncture (n = 1), nerve release/reconstruction (n = 2), and somatosensory feedback rehabilitation (n = 1) were used. Extracted data can be found in Table 1.
Table 1 –
Included Articles and Extracted Data.
| Title | Author (Year) | Type of Study | Location of study (institution, city, country) | Level of Evidence | Population | Cohort size | Aim of study | Methods | Mean age (%female) | Inclusion Criteria | Exclusion Criteria | Mechanism of burn injury | Graft (Y/N) | If yes graft, what type | Avg size of burn injury (%TBSA) | Pain score/assessment tool used | Avg. Pain score | Treatment used (i.e. medication, laser, etc.) | Mean Follow-Up | Outcomes Measured | Result | Comments | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| The effects of electroacupuncture on analgsia and peripheral sensory thresholds in patients with burn scar pain | Cugnet O, Pirlot A, Ortiz S, Rose T (2015) | Non-randomized prospective | Burn Center, Milatary Hospital, Rue Bruyn, Belgian | Level 3 | Belgian military post-burn patients | 32 patients | N/A | Investigate the efficacy of electroacupuncture in addressing burn-related pain and its effects on sensory thresold | 3 weekly 30-min sessions of standardized EA, then pain level assessed | Age (46 years) and 31.3% female | Patients clinical signs of post-burn scarring and neuropathic pain despite treatmnet | Patietns with pacemakers, epilepsy, pre-existing neurological problem (stroke, head injury), peripheral neuropathy or conditions that may lead to it, history of previous burns, 3rd degree burns, TBSA > 25%, or a SIRS during hispitalizatiom | Unspecified | Unspecified | Unspecified | 6% TBSA | Visual Analog Scale (VAS) and Quantitaive Sensory Testing (QST) | VAS: 6.8 pre-treatment and 4.5 post-treatment | Electropuncture | 1 week before and after treatment | Pain on the VAS scale and QST measurment | There was a education of VAS pain score (statistically significant but not clinically significant) 6.8 pre treatment to 4.5 post-treatment. QST score increased, indicating increase in sensory threshold (60 to 91 in the burned region and 63 to 106 in the unaffected region). | N/A |
| Pregabalin in severe burn injury pain: a double-blind, randomised placeblo-controlled trial | Gray P, et al. (2011) | Randomized, double-blind, placebo-controlled | The university of Queensland, School of Medicine, Brisbane, Australia | Level 1 | Hospitalized adults with a history of major burns | 90 patients (46 treatment and 44 placebo) | 44 patients | Investigate the efficacy of pregabalin in reducing post-burn pain | Patients randomly assigned to pregabalin or placebo group for 28 days starting at 75 mg BID to 300 mg BID, pain level were assessed daily , and then at 6 months follow up | Age (35.7 years) and 9% female | 18–65 years old with an affected BSA of 5%+ and with a neuropathic pain scale score 4+ | Patients using an anti-epileptic drug, pre-burn injury neuropathic pain, intravenous lidocaine or oral mexiletine after the burn injury, allergy to pregabalin or gabapentin, and significant pyschiatric hostory | Unspecified | Yes; 76% of the patients had grafts | Unspecified | Unspecified | Neuropathic Pain Scale (NPS) | Unspecified | Pregabalin | Every 6 months | Pain levels as an NPS score 0–10), procedural pain score (0–10 on a Likert scale) associated with drug administration, and side effects scale (0–4) for nausea, vomitting, drowsiness, giddiness. | Hot and sharp pain were reduced in the experimental group receiving pregabalin relative to control. Also, itch, surface pain, and procedural pain were reduced in the pregabalin group too. No difference in length of hospitalization or presence of pain after 6 months. | Better response in pts who did not require grafting |
| Neuropathic pain in post-burn hypertrophic scare: a psychophysical and neurophysiological study | Gianluca Isoardo 1, et al. (2012) | Non-randomized control | Department of Plastic Surgery, Burn Center, Hospital CTO–Maria Adelaide, Torino, Italy | Level 3 | Post-burn plastic with hypertrophic scarring | 102 patients (13 treatment and 89 placebo) | 89 patients (9 patients with non-painful PPS, 52 healthy subjects, and 28 patients with carpal tunnel syndrome) w/o burn history | Investigate the nature and cause of neuropathic pain associated with post-burn scarring | Both groups nderwent testing with the DN4 questionnaire; nerve conduction studies (NCS) of median, ulnar, and radial nerves; QST for cold- (CDT) and heat-induced pain threshold evaluation; and cutaneous silent period (CSP) testing of the abductor pollicis brevis | Age (48.6 years old) and 23% female | Patients with post-burn scars that involved both the dorsum and the palmar surface of at least one hand and reported pain site of scar | Patients with history of depression; medicated with neuroleptics, anti-epileptics, benzodiazepines, or antidepressants; history of diabets and/or other known cause of autoimmune, metabolic, or toxic peripheral neuropathies | Unspecified | Unspecified | Unspecified | Unspecified | Quantitative Sensory Testing (QST), Visual Analog Scale (VAS), Douleur Neuropathique en 4 Questions (DN4) | 5.6 (VAS scale) and 7 (DN4 scale) | N/A | N/A | DN4 pain score, nerve conduction evaluation of ulnar, radial, and median nerves, pain threshold evaluation by QST | Nerve conduction studies indicated no difference between ulnar and radial nerves, but increased incidence of carpal tunnel syndrome (CTS) in the post-burn hypertrophics scarring group. Cutaneous silent period (CSP) testing showed significantly shorter duration in post-burn patients. QST showed that cold pain threshold (CDT) was lower in the index finger in hands with painful PPS than in control; hot pain threshold (HDT) was not different. | N/A |
| Use of gabapentin and pregabalin for pruritus and neuropathic pain associated with major burn injury: A retrospective chart review | Kaul I, et al. (2017) | Retrospective chart review | University of Texas and Shriners Hospitals for Children, TX, USA | Level 4 | Pediatric burn survivors up to 20 years of age | 136 patients (112 gabapentin-receiving only and 24 gabapentin + pregabalin-receiving) | N/A | Investigate if gabapentin and/or pregabalin are effective in treating post-burn pruritis and neuropathic pain in the pediatric population | Conduct retrospective chart review to analyze does, age and weight of patients, presence of neuropathic pain and pruritus, reported response to medication, and side effects of pregabalin and gabapentin in pediatric burn survivors | Unspecified | Patients 0–20 yrs old who have history of burn and subsequent complaint or suspected neuropathic pain and pruritis and received pregabalin and gabapentin at Shriners Hospitals for Children | Patients who did not receive gabapentin and/or pregabalin, those without complaints of pruritus and pain and those older than age 20 | Unspecified | Unspecified | Unspecified | Unspecified | Clinician-based evaluation | Unspecified | Pregabalin and gapapentin | Variable | Improvement in pain and pruritis post-burn, average effective dosages, and side effects reported | The average effective doses of gabapentin was 23.9mg/kg/day for children 5years, 27.0mg/kg/day for children 6–13 years and 34.1mg/kg/day for children >12years. The average effective dose of pregabalin was 6.5mg/kg/day for children 6–12 years and 4.7mg/kg/day for children >12years. In the gabapentin only group, 91.4% had a significant response for pruritus, 100% for neuropathic pain, and 43.3% for both. 100% of individuals treated with both gabapentin and pregabalin had a significant response for pruritus and 88.2% had an adequate response for both pruritus and pain. | Side effects reported were: hyperactivity/s edation, nausea/vomitting/headaches, all of which were associated w/gabapentin |
| Prevalence and associated predictors for patients developing chronic neuropathic pain following burns | Klifto K, Dellon A, Hultman C (2020) | Retrospective chart review | The John Hopkins Burn Center, Baltimore, MD, USA | Level 4 | Burn patients older than 15 years admitted to John Hopkins Burn Center | 1880 patients (113 with chronic neuropathic pain) | N/A | Determine the prevalance and associated predictors for chronic neuropathic pain (CNP) in burn patients | Conduct retrospective chart review of patients admitted to burn center and analyze prevalance of CNP and associated predictors in the group of patients that developed chronic neuropathic pain | Age no-pain group (46 years), age in CNP group (54 years) and % female 34.1% | Patients older than 15 years that sustained a burn imjury; patients were evaluated and diagnosed by at least two heathcare providers during follow up visit and had CNP that lasted greater than 6 months | Patients younger than 15 years old and those with pain due an underlying illness or medication | Unspecified | Unspecified | Unspecified | No-pain group = 3.5% and CNP group = 6.66% | Clinician-based evaluation | Unspecified | N/A | Unspecified | Prevalance of CNP patients treated at burn center over 5-year period | 113 of the 1880 burn patients developed CNP as a direct result of burn injury over 5 years with a prevalence of 6.01%. Patients who developed CNP were a significantly older median age, abused alcohol, abused substance, were current daily smoker, sufferd more full-thickness burns, greater %TBSA burns (6 vs. 3.5, p > 0.001), were more often intubated on mechanical ventilation, greater median number of surgeries and longer hospital lenght of stay, compared to those who did not develop CNP. | Disproportionate comparative sizes of groups between CNP group (n=113) and no-pain group (n=1767). |
| Chronic Neuropathic Pain Following Hand Burns: Etiology, Treatment, and Long-Term Outcomes | Klifto K, et al. (2020) | Retrospective chart review | The Johns Hopkins Burn Center, Baltimore, MD, USA | Level 4 | Burn patients older than 15 years admitted to John Hopkins Burn Center with hand/upper extremity burns | 914 patients (55 developed chronic neuropathic pain) | N/A | Analyze patients with pain after hand/upper extremity burns to help guide risk stratifiaction and treatment strategies | Conducted retrospective chart review of patients admitted to burn center for hand/upper extremity burns and analyze risk factor risk factor for developing CNP and refractory pain among hand/upper extremity burn patients | Age of CNP patients (50.6 years), age of no CNP patients (46.1 years) and %female of CNP patients 22%, %female of no CNP patients 31.7% | Older than 15 years, sustained burn injury to hand and/or upper extremity, admitted to the burn center | Patients with preexisting neuropathic pain due to underlying medical illness or medication use, patients with less than 6 months of follow-up data, patients under 15 years of age | Burn mechanisms in CNP group unclude flame, scald, contact electrical, cold | Yes; 44/55 patients in CNP group underwent skin grafting | Unspecified | CNP group=16.5% and non-CNOP group = 6.9% | Visual analog scale (VAS) | Avg pain in CNP group w/o refractory pain = 4, avg pain in CNP group with refractory pain = 5 (recorded at time of discharge) | Gabapentin/pregbalin, SNRIs, TCAs, NSAIDs, acetaminophen, ascorbic acid, opioid, neuroma excison, nerve release, nerve repair (as per chart review) | CNP patients w/o refractory pain = 23 months, CNP patients w.refractory pain = 25 months | Development of CNP after hand/upper extremity burn injury and refractory pain among CNP patient | 55/914 (6%) patients developed CNP after hand/upper extremity burns. 29/55 (53%) of CNP patients had refractory pain. Significant risk factor for developing CNP included hx of substance/tobacco use, Among CNP patients, significant risk factor for developing refractory pain include sx of burning sensations. Gabapentin and ascorbic acid were associated w/ significant decreases in pain scores on f/u. | N/A |
| The Effects of Early Neuropathic Pain Control With Gabapentin on Long-Term Chronic Pain and Itech in Burn Patients | Kneib, et al. (2019) | Retrospective cohort | University Washington Scattle, WA, USA | Level 2 | Patients with burn-related chronic pain and pruritis | 147 receiving gabapentin within 72hrs after injury and and 64 receieving gabapentin after 72 hours after injury | 192 patients | Investigate the effect of a neuropathic pain control protocol, as well as early gabapentin initiation (>72 hours from injury) on total inpatient opioid use, chronic pain, and itch | Demographics, injury characteristic, long-term patient-reported outcomes on pain, itch and general health status were collected using the Burn Model System National Database | Age (44.7 years) and 24.8% female | Patients >14yo admitted to the burn unit with burn injury and adequate chart data | Patients with inadquate chart data | Unspecified | Yes | Unspecified | 18.40% | Short From-12 physical component summary (PCS) and Short From-12 mental component summary (MCS) | Pain and itch severity odds ratios (OR) did not significantly change at any time point with any use of gabapentin, early or late (P > .05) | Gabapentin (none, early, late) Neuropathic pain protocol: Patients admitted after February 2015 with complaints of itch or scratching/rubbing of wounds were initiated on the neuropathic pain protocol starting with cetirizine 10 mg followed by a titration of gabapentin strarting at 30 mg |
24 months | Mental heath score, pain score, pain, itch | Pain and itch severity odds ratios (OR) with any use of gabapentin, early or late (P > .05, Table 3). SF-12 physical and mental composite summaries with early gabapentin intiation were not significantly changed at any time point except for a significant decrease in MCS at discharge with late gabapentin initiation(−3.82, p = .04). | N/A |
| Scrambler Therapy for Chronic Pain after Burns and Its Effect on the Cerebral Pain Network: A Prospective, Double-Blineded, Randomized Controlled Trial | Lee, et al. (2022) | Prospective. double-blind, randomized control | Soonchunhyang University, Bouchen, South Korea | Level 4 | Patients with chronic burn-related pain | 20 patients | 23 patients | Use magnetic resonance imaging (MRI) to evalute the pain network-related mechanisms that underlie the clinical effect of scramble therapy (ST) in patients with chronic burn-related pain | Use of MRI in randomly-assigned patients who experienced chronic neuropathic pain after unilateral burn injuries to assess scrambler therapy | Age (48 years) and 7% female | Patients with unilateral burn injurys with VAS scores >= 5 despite tx with gabapentin or other physical modalities; >18y old | Patients with a cardiac history, neurological disease, pain resulting from other causes (e.g., neuromuscular diseases) as confirmed via imaging, phychiatric disorder, abnormal renal function, contraindication for MRI, pregnancy | Unspecified | Unspecified | Unspecified | ST group = 11% and control group = 20% | Visual analogue scale (VAS) and Hamilton Depression Rating Scale (HDRS) | Unspecified | Pain Scrambler Therapy device (Electrocutaneous nerve stimulation) | 10 weeks | VAS score, HDRS score, MRI cerebral blood volume (CBV) changes | ECT group had a significant reduction in pain relative to the control group, which correlated to post-ST MRI evaluations that demonstrated decreased CBV in the in the orbito-frontal gyrus, middle frontal gyrus, superior frontal gyrus, and gyrus rectus and increased CBV in the precentral gyrus and postcentral gyrus of the burned group compared with the control group. | High drop-out rate, short follow-up |
| 5% Lidocaine Medicated Plaster Use in Children with Neuropathic Pain from Burn Sequelae | Silva (2013) | Prospective, uncontrolled | Corporation of Aid to Burned Children, Chile | Level 3 | Pediatric patients with burn sequelae neuropathic pain | 14 patients | N/A | Evaluate the effectiveness and safety of 5 % lidocaine medicated plaster to treat neuropathic pain in children with burn sequelae | Plasma lidocaine levels were measured before applications of the 5% lidocaine medicated plaster, and 12, 36, and 60 hours after application, using chromatography with detection by mass spectrometry, and assessment of pain level and functionality over 3 months | Age (11 years) and 75% female | Males and female aged >6 years of age; bodyweight >20 kg; scar or graft with no open wounds, completely re-epthelialized; localized neuropathic pain in postburn scar or graft; and signed informed consent approved by the ethics comittee | Patient with a history of psychiatric disorders, or if peripheral nerve injury was present | Unspecified | Yes | Unspecified | Unspecified | FACES scale | FACES (pre-6.8 and post-0) | 5% lidocaine plaster | 3 months | Demographics, burn and pain evolution (type, intensity using FACES score), DN4 score, patient functionality. Plasma lidocaine levels were measured at 0, 12, 36, and 60 hours after treatment commencement | All patients had improved functionality and improved pain intensity scores. No adverse reactions. No qualitative analysis performed. | Qualitative study; small sample size |
| Somatosensory Rehabilitation for Neuropathic Pain in Burn Survivors: A Case Series | Nedelec, et al. (2016) | Restrospective case series | McGill University, Quebec, Canada | Level 2 | Burn survivors with reported pain | 17 patients | N/A | To evaluate the outcomes of burn survivors with neuropathic pain following somatosensory rehabilitation | Pain score were collected pre- and post-somatosensory treatment retrospectively | Age (47.2 years) and 29% female | Patients with neuropathic chronic pain defined as abnormal if lasted >3m on the McGill Pain Questionnaire; patients were defined as having neuropathic pain if they used neuropathic-like pain descriptors including pins and needles, stabbing, burning cold, electric shock, or shooting pain to describe their pain | N/A | Fire/flame, chemical, electrical, friction | Unspecified | Unspecified | 21% | Rainbow Pain Scale (mechanical allodynia surface area assessment), McGill Pain Questionnaire | Reported in % improvement: SWM scores improved by 27 ± 21% (n = 14) and 29 ± 26% (n = 12) at 2 and 3 months posttreatment, respectively. QDSA score improved by 9 ± 14% (n = 8) and 23 ± 23% (n = 6) at 2 and 3 months posttreatment, respectively | Somatosensery rehabilitation (touch discrimination, vibratory stimulation, touch perception/tactile exercises) | 3 months | Rainbow Pain Scale % change, McGill Pain Questionnaire % change | Rainbow Pain Scale/Semmes Weinstein Monofilaments score improved by 27 ± 21% (n = 14) and 29 ± 26% (n = 12) at 2 and 3 months post-treatment, respectively by 9 ± 14% (n = 8) and 23 ± 23% (n = 6) at 2 and 3 months posttreatment, respectively. Overall, the majority of patients (13/17 or 76%) showed substantial improvements after somatosensory rehabilitation. | N/A |
| Under Pressure Applying Practice-Based Learning and Improvement to the Treatment of Chronic Neuropathic Pain in Patients with Burns | Rapolti, et al. (2017) | Retrospective chart | University of North Carolina, Chapel Hill, NC, USA | Level 2 | Patients needing burn reconstruction who underwent elective peripheral nerve surgery at UNC by a single surgeon, from 200 – 2015 | 223 patients | N/A | Evaluate the effectiveness of the practice based learning and improvement (PBLI) model in affecting outcomes for patients undergoing burn surgeries | Master database was created to identify patients with procedure history of decompression, neurolysis, transposition, excision and/or implantation of peripheral nerves by the surgeon, and then split into two cohorts: patients admitted from 2000–2015 (non-PBLI cohort) and 2011–2015 cohort (PBLI cohort). | Age of 2000–2010 (non-PBLI) cohort (38.2 years) and age of 2011–2015 (37.6 years) and % female unspecified | Patients that underwent burn reconstructive nerve surgery from 2000–2015 at UNC by a single surgeon | Asymptomatic patients who underwent nerve decompression, as part of contracture release or exposure of deeper structures | Flame, scald, electrical, chemical, contact, abrasion, cold, radiation | Unspecified | Unspecified | Non-PBLI cohort = 18.7% and PBLI cohort = 12.5% | Unspecified | 86.7% of non-PBLI patient cohort reported definitive to moderate improvement in neuropathic sx, compared to 95.8% of patients in PBLI cohort (P=0.015) | Wide-awake local anesthesia no tourniquet (WALANT) intro practice, favoring in-situ nreve decompression rathee than transposition, more aggressive with neuroma exploration/resection, optimizing pharmacologic regimen preop, using laser resurfacing, laser-assisted steroid delivery, and fat grafting | 13.7 months across entire study group, 16.5 months for non-PBLI group, 11.05 months for PBLI group | Improvement in neuropathic pain and motor funvtion and reduction in pharmacologic regimen | Ovwrall complication rates between the two cohorts remained unchanged (19.0% in non-PBLI and 16.9% in PBLI). However, complications per anatomic site did decreased significant (12.7% > 7.3%, P=.039). Primary driver for this statistically sifnificant decrease seems to be lower rate of dehiscence (15.2% > 6.8%) in PBLI group. Furthermore, 86.7% of non-PBLI patient cohort reported definitive to moderate improvement in neuropathic symptoms, compared to 95.8% of patients in PBLI cohort (p=0.015) | Several interventions were introduced as part of PBLI cohort. Many variable factors in this study can lead to confusing/confounding results. |
| Analysis of the predictors of hypertrophic scarring pain and neuropathic pain after burn | Shu (2021) | Retrospective single cohort | The First Affliated Hospital of Naval Medical University, Shanghai, China | Level 2 | Patients with postburn hypertophic scarring pain between 2017–2020 in a burn center in Shanghai | 123 patients | N/A | Explore risk factors for hypertophics scarring pain and further identify whether it is neuropathic pain in postburn patients | Asssess burn scar characteristics and pain scores were collected through Changhai Hospital Medical Information system, pt questionnaire, and physician assessment | Age (41.5 years) and %female 43.1% | Hypertrophic scarring pts with localized between July 2017-Sept 2020. Patients aged 16y or older with a definitve clinical dignosis of hypertrophic scars caused by flame, hot liquid, lecricity, or chemicals were recruited | Patients with pre-burn pain, Patients with keloids and atrophics scars. Patients with hypertrophic scare that were a result of trauma, surgery, acne or other skin injures | Fire, hot liquid, other | Unspecified | Unspecified | 44.40% | Modified Vancouver Scar Scale (mVSS), Visual Analog Scale (VAS), Brief Pain Inventory (BPI) | BPI (total) = 36.0, pain severity = 12.0, pain interference = 24.1 | N/A | Unspecified | Patient demographics, cause of burn, median age of pts, TBSA, wound healing time of target scar, hyperplasia time, scar location | Sex, age, target scar location, and pliability were linked with severe VAS score. Burn depth or target scar, hyperplasia time, vascularity, and pliability were associated with higher BPI score. Adjusting for confounder, hyperplasia time was found to be the only independent risk factor for hyperplasia time was found to be the only independent risk factor for hypertrophics scarring neuropathic pain | N/A |
| Gabapentin is ineffective as an analgesic adjunct in the immediate postburn period | Wibbenmeyer, et al. (2014) | Rrandomized, double-blind, placebo-controlled | University of Iowa Hospitals and Clinics, Iowa City, IA, USA | Level 2 | Patients with acute burn injury history at University of Iowa Hospitals and Clinics Burn Unit | 53 total patients (27 treatment and 26 placebo) | 26 patients | Investigate the efficacy of gabapentin for pain in post-burn patients soon after acute burn injury | For the trearment group, 8 patients received a dosage of 900mg daily, 24 received 1800mg, 14 received 2400mg, and 7 received 3600mg. For the control group, 4 patients received a dosage of 900mg daily, 10 received 1800mg, 8 received 2400mg, and 4 received 3600mg | Age (41.8 years) and 11.3% female | Patients 18 years or older, recent 5%+ BSA burn injury and an expected length of stay of 48 hours, complaint of pain post-burn | Patients who were pregnant, lactating, prisoners, or who had renal insufficiency/disease | Unspecified | Graft/surgical intervention was not significant between control and experimental groups (22/26 placebo and 21/27 treatment patients received graft surgery) | N/A | 15.85% | Neurophic Pain Scale (NPS) and Hospital Anxiety and Depression Scale (HADS) | NPS scale 0–100: placebo 41.02 and tretment 38.51; HADS scale 0–10: placebo 5.04 and treatment 4.23 | Gabapentin | Daily | NPS score to assess the persence and severity of neuropathic pain in the immediate post-burn period | There was no significant improvement in pain (NPS score change of +0.22 from treatment effect, p=0.96) and anxiety (change of −0.83 from tratment effect, p=0.34). Author conclude gabapentin is not an effective therapy for pain in the immediate post-burn period. | N/A |
| Acute Neuropathic Pain Assessment in Burn Injured Patients | Taverner T, Prience J (2016) | Retrospective chart review | University British Columbia, Vancouver, British Columbia, Canada | Level 3 | patients with second- and third-degree burn injuries | 76 patients | N/A | Acess the prevalence and nature of acute neuropathic pain in patients with burn injuries | Retrospective chart review of patients with acute pain post-burn to assess nature of pain | Age (51 years and 29% female) | Patients with complete charts and those with acute burn injury history who were twice-daily assessed for neuropathic pain | Patients without twice-daily neuropathic pain assessments recorded n charts, incomlete charts, death following burn injury | Fire/flame, electrical, and scalding | Unspecified | N/A | 11% | Douleur Neuropathique instrument score (DN4) | Unspecified | N/A | twice-daily neuropathic pain assessments | Prevelance of pain and mean TBSA associated with post-burn pain | 42% of patients with acute burn injury met criteria of neuropathic pain, Most signs of pain onset within the first week of burn injury. The patients most at risk of developing pain were younger and had more than 10% BSA burn injury | N/A |
| Distinct behavioral response of primary motor cortex stimulation in itch and pain after burn injury | Aurore Tibaut, et al. (2019) | Randomized control | Harvard Medical School Boston, MA, USA | Level 1 | Patients with acute burn injury at least 3 weeks before study and patients reporting pain and/or itch as moderate or severe (≥4 on the visual Analogue Scale) | 31 patients (16 treatment and 15 placebo) | 15 patients | Investigate the efficacy of transcrabial direct current stimulation (tDCS) in modulating pain and itch response in post-burn injury | Experimental group received tDCS vs. control not receiving therapy and subsequent assessment of pain and itch with the BPI and VAS scale | Unspecified | Patients 18 y/o or older who had acute burn injury at least 3 weeks before study and reported complaint of pain and itch post-burn | Patients w itch/pain rated as mild according to scoring systems, patients taking Carbamazepine (Tegretol), Oxcarbaepine (Trileptil), or Phenytoin (Dilantin) or prior neurological history | Unspecified | Unspecified | N/A | Unspecified | Brief Pain Inventory (BPI) and Visual Analogue Scale (VAS). | Unspecified | Transcranial direct current stimulation (tDCS) | At baseline after the stimulation periods, at 2, 4 and 8-week post-stimulation | Reduction in itch and pain according to the VAS and BPI scales at baseline vs. after tDCS therapy | tDCS was not deemed an effective therapy for itch or pain, as pain and itch levels were not affected at any follow up period. | N/A |
| Sensory alteration patterns in burned patients | Tirado-Esteban, et al. (2019) | Retrospective observational | University Hospital of Vall d’Hebron, Spain | Level 2 | Patients referred to the Neurophysiology Department whose discomfort had become painful/started to affect quality | 26 patients | N/A | Determine patterns of heat, heat-pain, cold, cold-pain, sympathetic skin response and touch following severe burns | Retrospectively analyze the patterns of type of pain and response in post-burn injury | Age (55 years and 9 months) and gender unspecified | Women and men over 18 years, with either superficial second degree burns, deep second degree burns, or third degree burns on an upper or lower limb | Patients under 18 years, less than 5cm squared TBSA, and no corresponding contralateral healthy area | Unspecified | Unspecified | N/A | Unspecified | Quantitative Sensory Test (QST), electrical SSR and the Von Frey filaments | Unspecified | N/A | Unspecified | area vs contralateral healthy area regarding heat detectio, heat pain detection, cold detection, cold pain detection, sympathetic cutaneous response, and perecption of touch | burn areas perceive heat while lower temperature are required to preceive cold. There were no significant diffrence in result of heat-pain and cold-pain variable. They also found post-burn and healthy skin detected cold pain at 0 degrees C, suggesting lower temps are needed in both areas to detect cold-pain. No significant difference in sympathetic cutaneous responses. Post-burn skin areas required more pressure to be able to detect touch sensation. | N/A |
| Role of burn severity and posttraumatic stress symptoms in the cooccurrence of itch and neuropathic pain after burns: A longitudinal study. | Van Loey, et al. (2022) | Longitudina l prospective | Multiple burn centers, the Netherlands and Belgium | Level 2 | Adult patients admitted for >24h between April 2010 and December 2012 from five burn centers in Netherlands and Belgium | 192 patients | N/A | Document neuropathic pain prevalence as well as potential symptom profiles of itch and neuropathic pain that may present after burns, and to explore potential predictors | Prospective cohort study that assesses neuropathic pain and itch profiles with self reported itch and neuropathic pain at 2 weeks post-discharge, 3, 6, 12, and 18 months post-discharge | Age (41.56 years) and % female = 33% | Adult patients admitted for >24h between april 2010 and dec 2012 to five burn centers in netherlands/belgium | Patients with poor Dutch proficiency, acute/chronic cognitive problems, or when injury was deliberate | Unspecified | 53.1% of participants required one or more skin graft surgeries | N/A | Transient itch/pain group = 7.6%, chronic itch group = 11.78%, chronic itch and pain group = 12.16% | Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) Pain Scale, Likert scale, Impact of Event Scale-Revised (IES-R) | Unspecified | N/A | Unspecified | Demographics, neuropathic pain, itch, and posttraumatic stress symptoms | Neuropathic pain symptoms were reported by 54% of participants at 2 weeks post-discharge, which declined to 24% at 18 mo post-burn. For itch, prevalence rates were 78% at 2 weeks post discharge and 43% at 18 mo postburn. Itch intensity was most severe in the chronic itch and pain group. Chronic itch group and chronic itch and pain group were associated w/more severe burns. Chronic itch and pain group were associated with more PTS symptoms. Findings suggest that biological and psycho-dermatological processes underlie both CNP and itch processes in burn scars. | Final sample size 192, and several patients were lost to follow up |
The following scales were implemented across the different studies to assess NP-related outcomes: Visual Analog Scale (VAS), Quantitative Sensory Testing (QST), Short Form-12 Physical Component Summary (PCS), Short Form-12 (SF-12) Mental Component Summary (MCS), Numeric Rating Scales (NRS), Hamilton Depression Rating Scale, Rainbow Pain Scale, McGill Pain Questionnaire, Modified Vancouver Scar Scale (mVSS), Brief Pain Inventory (BPI), Neuropathic Pain Scale (NPS), Douleur Neuropathique Instrument Score, Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) Pain Scale. Across the studies, mean follow-up periods varied from one week to 27 months.
3.2. Risk factors for neuropathic pain
Among burn patients, one study identified that individuals who developed chronic pain were significantly older, abused alcohol and substances, were current daily smokers, suffered more full-thickness burns, had a greater median %TBSA burns, were more often intubated on mechanical ventilation, had a greater median number of surgeries, and had a longer median hospital length of stay compared to those who did not develop chronic pain [29].
3.3. Pharmacologic treatments
In one study, pregabalin was found to be effective in reducing hot and sharp pain, as well as itch, surface pain, and procedural pain [30]. However, no difference in the length of hospitalization or the presence of pain was found after six months. Kaul et al. found gabapentin to be effective in reducing pruritus and NP, with a higher effective dose for children > 12 years [31]. Gabapentin and ascorbic acid were associated with significant decreases in pain scores in all chronic NP patients. Gabapentin use did not significantly affect SF-12 physical and mental composite summaries except for a decrease in MCS at discharge with late initiation. Another study demonstrated gabapentin to be ineffective in the immediate post-burn period [32]. Similarly, Kneib et al. found that early gabapentin use for NP failed to improve long-term pain, itch, or PCS or PCS scores post-burn injury [33]. One study assessed the use of 5% lidocaine plaster and found all patients had improved functionality and improved pain intensity scores with no adverse reactions [34]. We found no studies on other drugs such as opioids, NSAIDs, or acet-aminophen.
3.4. Non-pharmacological treatments
One study implemented electroconvulsive therapy (ECT) and found patients receiving ECT had significant reductions in pain relative to the control group. This correlated to post-therapy MRI evaluations that demonstrated decreased cerebral blood volume (CBV) in the orbito-frontal gyrus, middle frontal gyrus, superior frontal gyrus, and gyrus rectus and increased CBV in the precentral gyrus and postcentral gyrus of the burned group compared with the control group [35] Another study investigated rehabilitation for sensory dys-function. The study protocol involved first avoiding any tactile stimulation in the allodynic zone while simultaneously providing proximal sensory and vibratory counter stimulation to the surrounding areas. Once the mechanical allodynia subsided, the underlying hypoesthesia was addressed through the re-establishment of tactile function via targeted training in touch discrimination, texture perception, and vibratory stimulation. This intervention led to improvement in Rainbow Pain Scale/Semmes Weinstein Monofilaments scores by 27 ± 21% at two months post-treatment and 29 ± 26% at three months post-treatment. Overall, the majority of patients (76%) showed substantial improvements after somatosensory rehabilitation [36].
Tibaut et al. implemented a transcranial Doppler simulator and found that it was not effective in reducing itch or pain, although a mild placebo effect was observed in the control group for itch at the 2-week follow-up [37]. Finally, one study evaluated the effects of pressure bandage-like immobilization (PBLI) following nerve decompression surgery for NP and showed that patients who had PBLI had a significantly greater improvement in NP symptoms compared with those who did not receive pressure bandages (PBLI: 95.8% symptom improvement vs. non-PBLI: 86.7% symptom improvement, p = 0.015) [38].
4. Discussion
The present study examines available literature regarding the management and outcomes of chronic NP in burn patients. Studies involved 4366 patients spanning 11 countries for a variety of perspectives with different patient populations in our search query. Despite the challenges in integrating the data to perform a formal quantitative analysis, our findings provide insight into the current perspectives, outcomes, and approaches to the treatment and management of NP. Unfortunately, it also highlights that while NP is a common and frequently debilitating problem, large knowledge gaps are present with critical areas for improvement.
This systematic review identified certain risk factors to be significantly associated with the development of NP in burn patients, including older age, alcohol, and substance abuse, current daily smoking, greater %TBSA burns, and a longer hospital length of stay. These findings emphasize the importance of risk stratification in assessing the likelihood for developing chronic NP. More informed data on preventable risk factors (e.g., nicotine use) will help increase health literacy in the prevention of developing debilitating long-term pain.
Overall, the studies showed mixed results regarding the efficacy of pharmacological treatments. The most notable class of medication for this treatment has been the gabapentinoids with mixed results. Some studies suggested that medications such as pregabalin and gabapentin were successful in reducing NP while others showed no statistical differences. We found no guidelines on quantity or duration of treatment with this class of drugs. Therefore, further investigation regarding the efficacy of gabapentinoids, more specifically regarding the optimal duration, timing, and application of these pharmacological treatments is recommended. We could not find other studies on other pharmacological classes of medications in the treatment of NP. As the treatment of NP with medications proves to be inconclusive, providers have turned to non-pharmacological treatments, such as ECT and somatosensory feedback rehabilitation, to reduce pain intensity and improve functionality.
Currently, no standards of care exist for managing NP in burn patients, despite it having significant impacts on both functional and psychological aspects of an individual’s life [3]. The manifestations of NP can be systemic as well. For example, depression is a well-documented comorbidity in this patient population, with a prevalence rate reported in the literature of 34% [39]. The relationship between pain and depression is reciprocal and dynamic, leading to a vicious cycle of symptom exacerbation [40,41]. NP can lead to feelings of helplessness and reduced quality of life, which can contribute to the development of depression. Depression, in turn, can amplify pain perception and lead to increased pain sensitivity and reduced pain threshold [42].
From the above, multidisciplinary, multimodal interventions addressing both physical and psychological aspects of pain management will improve function and quality of life in these patients. However, one of the most significant results of our study show that current data on the efficacy of psychosocial interventions for NP is sparse [43]. Patients with NP after traumatic spinal cord injury were found to quickly adopt pain-related coping mechanisms which may require further multidisciplinary support [44]. Further studies to better understand the impact of psychological treatments in conjunction with those geared toward physical symptoms (i.e., pain, numbness, and tingling) can help elucidate this complex physical-psychological relationship.
Despite improvements in modern burn care, rates of NP remain high and pose significant morbidity to patients following traumatic injuries [3]. As such, while standard pharmacologic modalities such as gabapentin/pregabalin remain to be the mainstay of treatment, more nuanced and personalized treatment strategies are on the horizon. Our results demonstrating mixed efficacies regarding both acute and long-term benefits of gabapentin in this patient population show personalized treatment regimens are necessary. In addition to the more classically-studied NP drain gabapentin, future studies should encompass a more diverse array of pharmacologic agents, including Ketamine, Clonidine, Capsaicin, Lidocaine, Namenda, and Amantadine. These agents, with their distinct mechanisms of action, represent promising avenues for a nuanced and multi-modal approach to NP management, though were not captured by the current systematic review and further studies should be conducted to better understand their role in NP management.
Beyond these drugs, recent studies have investigated the important role of microglia and NDMA in both instigating and maintaining NP through the release of cytokines and other inflammatory molecules [40,45]. These molecular signaling pathways ultimately lead to synaptic remodeling and maladaptive connectivity that can lead a burn patient to experience a chronic state of pain hypersensitivity [46]. Microglia activation has been implicated in the maintenance and amplification of neuropathic pain. Upon nerve injury, microglia undergo morphological changes and release proinflammatory factors, contributing to the sensitization of adjacent neurons. This process, known as microglial activation, is a key component of neuroinflammation in NP [47]. NMDA receptors, particularly in the spinal cord, also play a crucial role in the development and maintenance of NP [45]. The activation of NMDA receptors leads to increased calcium influx and excitatory neurotransmission, contributing to synaptic plasticity and central sensitization [48]. Antagonizing NMDA receptors has been a target for NP management, though little study has been done in burn patients [49]. While preliminary efforts are underway to target microglia molecules and NDMA and their associated signaling pathways in preclinical studies [50,51], future in-vivo clinical studies will provide a more specific treatment for patients with NP.
Fractional carbon dioxide lasers have also demonstrated promise in reducing burn scar morbidity and greater patient post-burn satisfaction. Lasers have been found to provide significant improvements in sensation, pain, itch, and appearance with limited adverse outcomes and relatively low cost [55]. These lasers, utilizing a carbon dioxide medium, deliver precise and controlled energy to targeted tissues, facilitating a range of therapeutic effects. In burn injury cases, CO2 lasers have demonstrated effectiveness in reducing scar morbidity and enhancing patient satisfaction. Studies exploring the application of CO2 lasers in burn scars have reported significant improvements in sensory outcomes, pain reduction, itch alleviation, and overall scar appearance [56]. Beyond the physical aspects, CO2 laser therapy holds potential to address NP associated with burn injuries. While the precise mechanisms are still under investigation, the ability of laser therapy to modulate scar tissue and influence sensory outcomes suggests a multifaceted impact. Further research is warranted to elucidate the specific pathways through which CO2 lasers exert their therapeutic effects in neuropathic pain, paving the way for optimized treatment strategies and improved quality of life for individuals affected by burn-related NP.
While our systematic review focused on elucidating the broad landscape of neuropathic pain in burn patients, the relevance of these specific interventions and their relation to NP was not found to be discussed in our included studies in depth. These modalities represent nuanced treatments of NP, and their inclusion in future research and clinical considerations holds promise for advancing our understanding and management of burn patients.
Pain conditions that overlap with NP such as allodynia, fibromyalgia, and complex regional pain syndrome (CRPS) represent significant facets of NP signaling and merit further consideration in the context of burn injuries. Allodynia, characterized by pain in response to normally non-painful stimuli, underscores the complexity of sensory processing alterations in neuropathic pain [52]. Fibromyalgia, a condition involving widespread musculoskeletal pain, fatigue, and sleep disturbances, shares overlapping features with neuropathic pain, contributing to the intricate spectrum of pain experiences [53]. CRPS, marked by disproportionate pain in response to injury may also be an important area of study in these patients [54]. Literature discussing these conditions was not captured in the search query for the current systemic review. However, as the aforementioned pain manifestations are closely related to NP, additional study of these in burn patients may offer valuable insights into the regional manifestations of neuropathic pain following burns.
Several limitations of this systematic review exist. Firstly, many of the treatment modalities mentioned in the discussion were not captured by our search query with little literature on these modalities specifically for the treatment of NP in burn patients. Another limitation is the heterogeneity of the definition of NP utilized in these studies. The definition of NP varies by clinician and patient which makes it challenging to address on a large systematic scale. The included studies have significant variability in study design, sample size, outcome measures, follow-up periods, and overall quality. Moreover, some studies presented conflicting results or lacked appropriate control groups, which limits the reliability of the findings. Many studies lacked a randomized controlled trial design, precluding an establishment of causality. Additionally, the heterogeneity of assessing pain scales, medications, and dosages in included studies reduced the power of conclusions drawn in this systematic review. Further studies should seek to prospectively describe and classify the different NP reported by burn patients. Finally, although our study utilized several different databases for our literature search, our study’s database selection may contribute to biases in study selection.
While up to 80% of burn patients experience NP following their injury [1], our systematic analysis of the literature highlights a knowledge gap in our ability to solve this common and disabling problem. Treatment strategies will need to employ a multidisciplinary and multimodal approach, consisting of social and psychological support, occupational and physical therapy, medications, and laser therapy. Given the high incidence of this chronic and pervasive morbidity, additional prospective studies are warranted to better characterize the combinatorial effects of these interventions in NP modulation.
5. Conclusions
This systematic review demonstrates insufficient evidence on the pathophysiology, outcomes, and risk factors in NP, as well as the efficacy of various therapies. Future prospective and randomized studies evaluating the etiology of these factors can substantially improve our treatment strategies. This can allow for the development of well-delineated and evidence-based protocols in NP management in hopes of improving patient quality of life and both psychological and physical function in burn patients.
External funding
The contents of this manuscript were developed under a grant from the National Institute on Disability, Independent Living, and rehabilitation Research (NIDILRR grant number 90DPBU0007). NIDILRR is a Center within the Administration for Community Living (ACL), Department of health and Human Services (HHS). The contents of this manuscript do not necessarily represent the policy of NIDILRR, ACL, or HHS, and you should not assume endorsement by the Federal Government.
Footnotes
Conflicts of Interest and Disclosures
The authors report no conflicts of interest or financial disclosures related to this manuscript.
CRediT authorship contribution statement
A detailed description of each author’s contribution is as follows: Eloise Stanton contributed to the majority of manuscript writing, data collection and analysis, study protocol design, and table/figure creation. Paul Won contributed to data collection and manuscript writing and editing. Artur Manasyan and Sandeep Gurram contributed to data collection and manuscript writing and editing. Gillenwater and Haig Yenikomshian, contributed to project conception, manuscript editing, and final approval of the version to be published.
REFERENCES
- [1].Van Loey NEE, de Jong AEE, Hofland HWC, van Laarhoven AIM. Role of burn severity and posttraumatic stress symptoms in the co-occurrence of itch and neuropathic pain after burns: a longitudinal study. Front Med 2022;9:997183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Shu F, Liu H, Lou X, et al. Analysis of the predictors of hypertrophic scarring pain and neuropathic pain after burn. Burns 2022;48(6):1425–34. [DOI] [PubMed] [Google Scholar]
- [3].Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Prim 2017;3:17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gray P, Williams B, Cramond T. Successful use of gabapentin in acute pain management following burn injury: a case series. Pain Med 2008;9(3):371–6. [DOI] [PubMed] [Google Scholar]
- [5].Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron 2006;52(1):77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hachisuka J, Chiang MC, Ross SE. Itch and neuropathic itch. Pain 2018;159(3):603–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Klein MC, Oaklander AL. Ion channels and neuropathic pain. Elife 2018;7. 10.7554/eLife.42849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Beggs S, Trang T, Salter MW. P2×4R+ microglia drive neuropathic pain. Nat Neurosci 2012;15(8):1068–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Inoue K Purinergic signaling in microglia in the pathogenesis of neuropathic pain. Proc Jpn Acad Ser B Phys Biol Sci 2017;93(4):174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett 2004;361(1–3):184–7. [DOI] [PubMed] [Google Scholar]
- [11].Basbaum AI. Distinct neurochemical features of acute and persistent pain. Proc Natl Acad Sci USA 1999;96(14):7739–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Strong AL, Agarwal S, Cederna PS, Levi B. Peripheral neuropathy and nerve compression syndromes in burns. Clin Plast Surg 2017;44(4):793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Schneider JC, Harris NL, El Shami A, et al. A descriptive review of neuropathic-like pain after burn injury. J Burn Care Res 2006;27(4):524–8. [DOI] [PubMed] [Google Scholar]
- [14].Fiore NT, Debs SR, Hayes JP, Duffy SS, Moalem-Taylor G. Pain-resolving immune mechanisms in neuropathic pain. Nat Rev Neurol 2023;19(4):199–220. [DOI] [PubMed] [Google Scholar]
- [15].Klifto KM, Dellon AL, Hultman CS. Risk factors associated with the progression from acute to chronic neuropathic pain after burn-related injuries. Ann Plast Surg 2020;84(6S Suppl 5):S382–5. [DOI] [PubMed] [Google Scholar]
- [16].Krumova EK, Geber C, Westermann A, Maier C. Neuropathic pain: is quantitative sensory testing helpful? Curr Diab Rep 2012;12(4):393–402. [DOI] [PubMed] [Google Scholar]
- [17].Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res 2011;63(Suppl 11):S240–52. [DOI] [PubMed] [Google Scholar]
- [18].Garg A, Pathak H, Churyukanov MV, Uppin RB, Slobodin TM. Low back pain: critical assessment of various scales. Eur Spine J 2020;29(3):503–18. [DOI] [PubMed] [Google Scholar]
- [19].Askew RL, Cook KF, Keefe FJ, et al. A PROMIS measure of neuropathic pain quality. Value Health 2016;19(5):623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Longson D Neuropathic pain–pharmacological management: the pharmacological management of neuropathic pain in adults in non-specialist settings. NICE clinical guideline. [Google Scholar]
- [21].Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol 2010;17(9):1113–88. [DOI] [PubMed] [Google Scholar]
- [22].Emery MA, Eitan S. Drug-specific differences in the ability of opioids to manage burn pain. Burns 2020;46(3):503–13. [DOI] [PubMed] [Google Scholar]
- [23].Yenikomshian HA, Curtis EE, Carrougher GJ, Qiu Q, Gibran NS, Mandell SP. Outpatient opioid use of burn patients: a retrospective review. Burns 2019;45(8):1737–42. [DOI] [PubMed] [Google Scholar]
- [24].Akyuz G, Kenis O. Physical therapy modalities and rehabilitation techniques in the management of neuropathic pain. Am J Phys Med Rehabil 2014;93(3):253–9. [DOI] [PubMed] [Google Scholar]
- [25].Raja SN, Ringkamp M, Guan Y, Campbell JN, Bonica John J. Award lecture: peripheral neuronal hyperexcitability: the “low-hanging” target for safe therapeutic strategies in neuropathic pain. Pain 2020;161(Suppl 1):S14–26. Suppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Obata H Analgesic mechanisms of antidepressants for neuropathic pain. Int J Mol Sci 2017;18(11). 10.3390/ijms18112483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fornasari D Pharmacotherapy for neuropathic pain: a review. Pain Ther 2017;6(Suppl 1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Klifto KM, Yesantharao PS, Dellon AL, Hultman CS, Lifchez SD. Chronic neuropathic pain following hand burns: etiology, treatment, and long-term outcomes. J Hand Surg Am 2021;46(1):e1–67. e9. [DOI] [PubMed] [Google Scholar]
- [30].Gray P, Kirby J, Smith MT, et al. Pregabalin in severe burn injury pain: a double-blind, randomised placebo-controlled trial. Pain 2011;152(6):1279–88. [DOI] [PubMed] [Google Scholar]
- [31].Kaul I, Amin A, Rosenberg M, Rosenberg L, Meyer 3rd WJ. Use of gabapentin and pregabalin for pruritus and neuropathic pain associated with major burn injury: A retrospective chart review. Burns 2018;44(2):414–22. [DOI] [PubMed] [Google Scholar]
- [32].Wibbenmeyer L, Eid A, Liao J, et al. Gabapentin is ineffective as an analgesic adjunct in the immediate postburn period. J Burn Care Res 2014;35(2):136–42. [DOI] [PubMed] [Google Scholar]
- [33].Kneib CJ, Sibbett SH, Carrougher GJ, Muffley LA, Gibran NS, Mandell SP. The effects of early neuropathic pain control with gabapentin on long-term chronic pain and itch in burn patients. J Burn Care Res 2019;40(4):457–63. [DOI] [PubMed] [Google Scholar]
- [34].Orellana Silva M, Yañez V, Hidalgo G, Valenzuela F, Saavedra R. 5% lidocaine medicated plaster use in children with neuropathic pain from burn sequelae. Pain Med 2013;14(3):422–9. [DOI] [PubMed] [Google Scholar]
- [35].Lee SY, Park CH, Cho YS, et al. Scrambler therapy for chronic pain after burns and its effect on the cerebral pain network: a prospective, double-blinded, randomized controlled trial. J Clin Med Res 2022;11(15). 10.3390/jcm11154255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nedelec B, Calva V, Chouinard A, et al. Somatosensory rehabilitation for neuropathic pain in burn survivors: a case series. J Burn Care Res 2016;37(1):e37–46. [DOI] [PubMed] [Google Scholar]
- [37].Thibaut A, Schiff N, Giacino J, Laureys S, Gosseries O. Therapeutic interventions in patients with prolonged disorders of consciousness. Lancet Neurol 2019;18(6):600–14. [DOI] [PubMed] [Google Scholar]
- [38].Rapolti M, Wu C, Schuth OA, Hultman CS. Under pressure: applying practice-based learning and improvement to the treatment of chronic neuropathic pain in patients with burns. Clin Plast Surg 2017;44(4):925–34. [DOI] [PubMed] [Google Scholar]
- [39].Gustorff B, Dorner T, Likar R, et al. Prevalence of self-reported neuropathic pain and impact on quality of life: a prospective representative survey. Acta Anaesthesiol Scand 2008;52(1):132–6. [DOI] [PubMed] [Google Scholar]
- [40].Donnelly CR, Andriessen AS, Chen G, et al. Central nervous system targets: glial cell mechanisms in chronic pain. Neurotherapeutics 2020;17(3):846–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rahman S, Alzarea S. Glial mechanisms underlying major depressive disorder: potential therapeutic opportunities. Prog Mol Biol Transl Sci 2019;167:159–78. [DOI] [PubMed] [Google Scholar]
- [42].Hermesdorf M, Berger K, Baune BT, Wellmann J, Ruscheweyh R, Wersching H. Pain sensitivity in patients with major depression: differential effect of pain sensitivity measures, somatic cofactors, and disease characteristics. J Pain 2016;17(5):606–16. [DOI] [PubMed] [Google Scholar]
- [43].Eccleston C, Hearn L, Williams AC de C. Psychological therapies for the management of chronic neuropathic pain in adults. Cochrane Database Syst Rev 2015;2015(10):CD011259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Taylor J, Huelbes S, Albu S, Gómez-Soriano J, Peñacoba C, Poole HM. Neuropathic pain intensity, unpleasantness, coping strategies, and psychosocial factors after spinal cord injury: an exploratory longitudinal study during the first year. Pain Med 2012;13(11):1457–68. [DOI] [PubMed] [Google Scholar]
- [45].Finnerup NB, Kuner R, Jensen TS. Neuropathic pain: from mechanisms to treatment. Physiol Rev 2021;101(1):259–301. [DOI] [PubMed] [Google Scholar]
- [46].Inoue K, Tsuda M. Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci 2018;19(3):138–52. [DOI] [PubMed] [Google Scholar]
- [47].Kohno K, Shirasaka R, Yoshihara K, et al. A spinal microglia population involved in remitting and relapsing neuropathic pain. Science 2022;376(6588):86–90. [DOI] [PubMed] [Google Scholar]
- [48].Li XH, Miao HH, Zhuo M. NMDA receptor dependent long-term potentiation in chronic pain. Neurochem Res 2019;44(3):531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kreutzwiser D, Tawfic QA. Expanding role of NMDA receptor antagonists in the management of pain. CNS Drugs 2019;33(4):347–74. [DOI] [PubMed] [Google Scholar]
- [50].Matsumura Y, Yamashita T, Sasaki A, et al. A novel P2×4 receptor-selective antagonist produces anti-allodynic effect in a mouse model of herpetic pain. Sci Rep 2016;6:32461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bhattacharya A, Biber K. The microglial ATP-gated ion channel P2×7 as a CNS drug target. Glia 2016;64(10):1772–87. [DOI] [PubMed] [Google Scholar]
- [52].Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol 2014;13(9):924–35. [DOI] [PubMed] [Google Scholar]
- [53].Martínez-Lavín M Fibromyalgia in women: somatisation or stress-evoked, sex-dimorphic neuropathic pain? Clin Exp Rheuma 2021;39(2):422–5. [PubMed] [Google Scholar]
- [54].Taylor SS, Noor N, Urits I, et al. Complex regional pain syndrome: a comprehensive review. Pain Ther 2021;10(2):875–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Choi KJ, Williams EA, Pham CH, et al. Fractional CO2 laser treatment for burn scar improvement: a systematic review and meta-analysis. Burns 2021;47(2):259–69. [DOI] [PubMed] [Google Scholar]
- [56].Levi B, Ibrahim A, Mathews K, et al. The Use of CO2 fractional photothermolysis for the treatment of burn scars. J Burn Care Res 2016;37(2):106–14. [DOI] [PubMed] [Google Scholar]


