Abstract
In environments characterized by extended multi-stress conditions, pathogens develop a variety of immune escape mechanisms to enhance their ability to infect the host. The capsules, polymers that bacteria secrete near their cell wall, participates in numerous bacterial life processes and plays a crucial role in resisting host immune attacks and adapting to their niche. Here, we discuss the relationship between capsules and bacterial virulence, summarizing the molecular mechanisms of capsular regulation and pathogenesis to provide new insights into the research on the pathogenesis of pathogenic bacteria.
Subject terms: Biofilms, Bacteriology, Pathogens
Introduction
In bacteria, polymers known as capsules are generated at the periphery of the cell wall, enveloping the entire cell. Capsules connect to the peptidoglycan in Gram-negative bacteria or the plasma membrane in Gram-positive bacteria via covalent attachments to either phospholipid or lipid-A molecules. Capsules may also establish direct connections with surface proteins on the bacterial membrane1. For most bacteria, capsules primarily consist of polysaccharides, exemplified by Streptococcus suis (S. suis)2. Some capsules primarily consist of polypeptides, as in Bacillus anthracis3, while others, like Bacillus megaterium4, contain both polysaccharides and polypeptides. Capsular polysaccharides (CPS) were initially described as “halo” by Pasteur in 1881. CPS was isolated and discovered by Avery and Dochez in 19175. It was not until 1925 that Avery elucidated the carbohydrate nature of the substance in the microbial capsule6. Capsules are prevalent in natural pathogens and participate in various bacterial cellular processes. Capsules regulate the size and dispersion of bacterial biofilm, contributing to sustained infections within hosts7, reducing the efficacy of antimicrobial peptides and complement8, suppressing phagocytosis by innate immune cells, promoting intracellular survival7,9, and aiding in defence against antimicrobial agents10. In this review, we summarize the role of capsules in bacterial virulence and analyze their regulation mechanisms.
Capsules biosynthesis
Capsules primarily consist of high molecular weight polysaccharides, which are essentially oligosaccharide repeating units. However, some bacterial capsules are atypical: the capsule of Yersinia pestis is a protein polymer composed of 17-kDa subunits11,12, and the capsule of Bacillus anthracis consists of D-glutamic acid13. Additionally, the O-antigen capsule and the capsule-like complex (CLC) are identified as capsules of Francisella tularensis (F. tularensis)14. The O-antigen capsule is composed of mannose, rhamnose, and dideoxy sugars, whereas the CLC comprises proteins and carbohydrates15. However, the precise role these capsules play in the virulence of F. tularensis remain to be fully elucidated. Further research is required to clarify the structure and contributions of each capsule to the pathogenesis and virulence of F. tularensis.
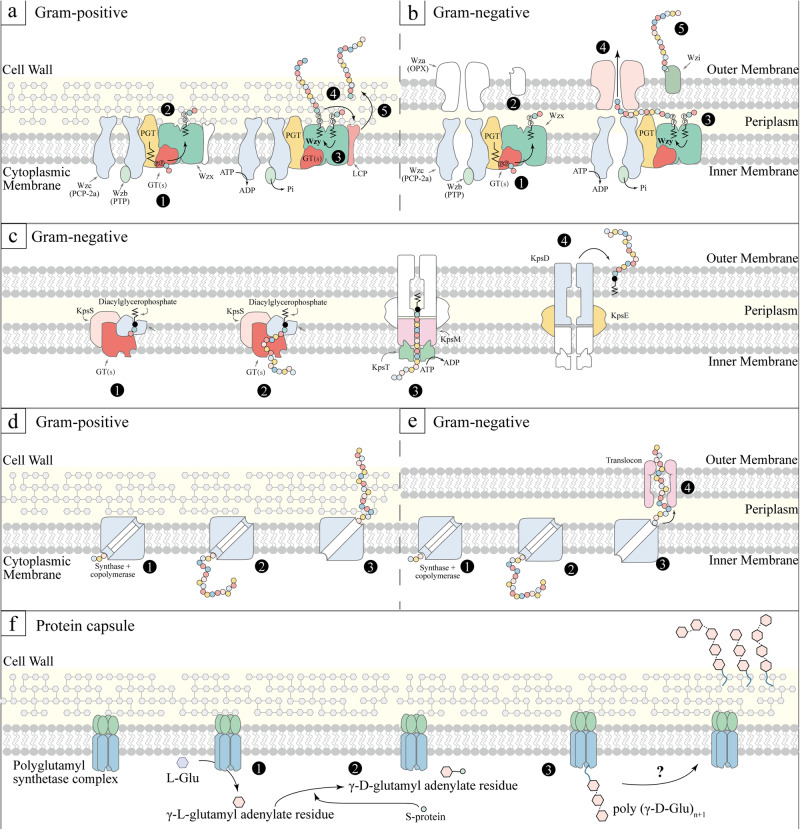
Currently, three primary capsule synthesis pathways are recognized: the Wzx/Wzy-dependent mechanism, the ATP-binding cassette (ABC) transporter-dependent mechanism, and the synthase-dependent mechanism16,17. Over 90% of S. pneumoniae serotypes synthesize capsules via the Wzx/Wzy-dependent mechanism, characterized by the formation of repeat units and nonprocessive polymerization18. This mechanism is also fundamental to the synthesis of group I and group IV capsules in Gram-negative bacteria19. The initial step involves transferring a 1-phosphate to the lipid carrier (undecyl isoprene phosphate) on the cell membrane’s cytoplasmic surface. Following this, the complete repeat unit is turned outward by the Wzx flip enzyme, and the Wzy polymerase attaches the growing polymer chain to the newly formed repeat unit (Fig. 1, a, b)19. In S. pneumoniae serotypes, both with and without glucose (Glc), this step is accomplished by liposome and Glc-1-phosphotransferase (Glc-1-P) CpsE/WchA and CPS site transferase from Wcil, WcjG or WcjH homologous groups, respectively20. Group II and group III capsules are called ABC-dependent capsule. In this pathway, new polysaccharide chains undergo polymerization in the cytoplasm and are associated with phospholipid receptors. These chains are then transported across the intima by ABC transporters (Fig. 1c). Even with these variations, both Wzx/Wzy and ABC-dependent mechanisms employ similar outer membrane proteins belonging to the polysaccharide export family for the transportation of capsules across the bacteria’s outer membrane16. For group IV capsules, CPS synthesis relies on Wzy polymerase and does not involve the Wzx flip enzyme. Additionally, CPS synthesis can occur via the synthase-dependent mechanism, wherein specific enzymes are responsible for initiating, polymerizing, and translocating the capsules (Fig. 1, d, e)21. For instance, S. pneumoniae serotype 3 and 37 utilize a single enzyme mechanism, which initiates capsule synthesis by transferring sugar to lipid receptors and subsequently adding additional sugars for extension22. However, it remains a puzzle why the Wzx/Wzy-dependent mechanism predominates in most Gram-positive bacteria, while the synthase-dependent mechanism is less common23.
Fig. 1. Capsule synthesis mechanisms.
Wzy-Dependent mechanism. The first three steps are universal: ①, phosphoglycosyltransferase (PGT) and serotype-specific glycosyltransferases (GT(s)) synthesize undecaprenyl diphosphate-linked oligosaccharide repeat units; ②, Wzx facilitates the translocation of these units across the membrane, followed by Wzy-mediated polymerization; ③, The phosphorylation cycle of Wzc, catalyzed by Wzb, is a crucial step. In Gram-positive bacteria (a): ④, The newly formed polymer is transferred onto a peptidoglycan assembly intermediate; ⑤, It gets anchored to the cell wall through LCP activity. In Gram-negative bacteria (b): ④, The polymer translocates across the outer membrane via Wza; ⑤, In some prototype and other species, Wzi assists in organizing the translocated polymer into surface-associated capsule structures. c ABC Transporter-Dependent mechanism. For Gram-negative bacteria, ①-② involve the assembly of sugar chains; ③, the ABC transporter (KpsMT) plays a pivotal role in exporting these chains; ④, KpsE and KpsD are essential for translocating the assembled chains across the periplasm and outer membrane. The specifics of the ABC-dependent pathway in Gram-positive bacteria remain unclear. Synthase-Dependent mechanism: ①, Initial synthesis of the short sugar chains; ②, Extension of these chains by glycosyltransferases. In Gram-positive bacteria (d): ③, Transfer of the polymer to the outer membrane. In Gram-negative bacteria (e): ③, Polymer translocation and anchoring to the cell wall via a translocation anchor. (f): Protein capsule synthesis pathway. The synthesis of the poly (y-D-glutamyl) capsule in Bacillus licheniformis involves a series of membrane-associated enzymatic reactions. Here, a polyglutamyl synthetase complex catalyzes the activation, racemization, and polymerization of L-glutamic acid, resulting in a high molecular weight polymer of y-D-glutamic acid. ① L-Glu + ATP ⇌ γ-L-Glu-AMP + PPi; ② γ-L-Glu-AMP + S-protein⇌ γ-L-Glu-S-protein + AMP; γ-L-Glu-S-protein ⇌ γ-D-Glu-S-protein; ③, γ-D-Glu-S-protein + poly (γ-D-Glu)n → poly (γ-D-Glu)n+1 + S-protein. GT glycosyltransferase, LCP LytR-CpsA-Psr, OPX outer membrane polysaccharide, PCP polysaccharide copolymerase; PGT, phosphoglycosyltransferase, PHP polymerase and histidinol phosphatase, SA polysaccharide A, PTP phosphotyrosine phosphatase, Glu glutamate, S-protein a protein-bound thioester, as a second intermediate for the growing polymeric chains.
The initial identification of protein capsules occurred in Bacillus anthracis24,25, and subsequent elucidation of their biosynthetic pathways was achieved in Bacillus licheniformis26. In the absence of hydroxylamine, γ-L-glutamyl adenylate residue forms, which is then converted to γ-D-glutamyl adenylate residue upon activation. A protein-bound thioester (S-protein) may serve as a second intermediate in this process27. The final step involves transferring the activated glutamate to an endogenous membrane-bound poly (γ-D-glutamyl) acceptor. Consequently, polyglutamyl chain extension occurs by adding new glutamyl units to the terminal amino group of the receptor-bound glutamyl residue (Fig, 1f). In summary, gaps remain in understanding protein capsule synthesis and assembly, particularly regarding specific molecular mechanisms, such as the completed polypeptide chain outside the membrane.
Serotypes and pathogenicity
Capsule types are influenced by various factors, including the number and sequence of monosaccharide components, the position of glycosidic linkages, the configuration (L or D) of components, and the degree of chemical modification, such as O-acetylation. Consequently, capsular structures are diverse, including hyaluronic acid (HA)28, heparosan29, chondroitin30, polysialic acid (PSA)31, and colanic acid32,33. The composition of the capsules facilitates further differentiation of bacteria into distinct groups (serotypes, serovars). Escherichia coli (E. coli) produces approximately 80 distinct capsule types, which were further categorized into four major groups: Group I, Group II, Group III, and Group IV, including the PSA-containing K1 capsule, chondroitin-containing K4 capsule, and heparosan-containing K5 capsule (Fig. 2A)34. Colanic acid is a capsule whose structure is similar to the group I capsule. Its biosynthesis occurs via the Wzx/Wzy-dependent pathway as in the the group I capsule35. A notable distinction is that E. coli cultured in laboratory conditions at 37 °C does not produce colanic acid36. In S. pneumoniae, 93 capsular serotypes have been identified, most of which are capable of causing infection18,37–39. S. pneumoniae primarily synthesized its CPS in trisaccharide units, though variations exist among serotypes. For serotype 12 F, CPS synthesis is based on hexasaccharides40. In serotype 4, CPS comprises a tetrasaccharide repeating unit with pyruvate modifications (Fig. 2B)41. Serotype 6 A’ CPS, based on tetrasaccharides, includes a rhamnose modification42. Streptococcus suis (S. suis) strains were initially classified into 35 serotypes based on CPS antigenicity43–45, later revised to 33 serotypes46.
Fig. 2. Bacterial capsule structure.
A Gram-negative bacteria. B Gram-positive bacteria. Glc, glucose; GlcA (GlcUA), glucuronic acid; Gal galactose, Fuc fucose; Fru fructose; Man mannose; Rha, rhamnose; GlcNAc N-acetylglucosamine; ManNAc, N-acetylmannosamine; GalNAc, N-acetylgalactosamine; ManNAcA, N-acetylmannosaminuronic acid; FucNAc, N-acetylfucosamine; NeuNAc (Neu5Ac), N‑Acetylneuraminic Acid (sialic acid); Qui4NMal, 4-(2-carboxyacetamido)-4,6-dideoxyglucose; p, phosphate; Ac, acetate; The structures have been published elsewhere: Escherichia coli (K1, K4, K5, K10, K30, colanic acid)19; Pasteurella multocida (A, B)207; Neisseria meningitidis (B, W)132; Klebsiella pneumoniae (K1, K2)208,209; Streptococcus pneumoniae (3, 4, 12F)17; Streptococcus agalactiae (Ia)209; Staphylococcus aureus (5, 8)210; Streptococcus suis (2)211.
Significant variation in capsule structure exists between different bacteria and among serotypes within the same species, contributing to diverse virulence (Table 1). Specific S. pneumoniae serotypes (1, 4, 5, 8, 12F, 18C and 19A) are highly invasive, while others (6A, 6B, 11A, and 23F) are reported to be less aggressive47–50. In S. aureus, serotypes 5 and 8, out of 11 known serotypes, are predominantly responsible for human infections51. The polysaccharides of serotypes 5 and 8 differ only in sugar linkages and O-acetylation sites of the mannosaminuronic acid residues (Fig. 2B)52,53. In Haemophilus influenzae (H influenzae), the capsules composed of polyribose ribitol phosphate connected by phosphodiester bonds render serotype b (Hib) the most virulent, followed by serotype a (Hia) and other capsular types54. Streptococcus pyogenes (GAS) groups A-C causing pharyngitis are typically not associated with skin infections (group D), and the opposite is also true, although certain serotypes show no particular tissue preference (group E)55,56. Considering these observations, researchers speculate that capsule virulence may be correlated with the frequency of monomer repetition or the specific type of monomer. In a mouse infection model, highly virulent serotypes of Klebsiella pneumoniae (K. pneumoniae), notably K2, which is associated with bacteremia, are devoid of the mannose-α-2/3-mannose structure that present in less virulent strains57,58.
Table 1.
Capsular-related bacterial diseases
| Species | Capsule type | Diseases | Function | Refs |
|---|---|---|---|---|
| Gram-negative bacteria | ||||
| Escherichia coli | K1, 4 | Sepsis | Immune evasion | 85,177,178 |
| K1 | Meningitis | Invasion | 103 | |
| K1 | Spontaneous bacterial peritonitis | Translocation | 179 | |
| K2, K5 | Urinary tract infections; Ulcerative colitis | Adhesion | 180,181 | |
| Pseudomonas aeruginosa | Sepsis; Septic shock | Immune evasion | 182 | |
| Klebsiella pneumoniae | K1, K1, K2, K16, K28, K57, K63 | Bacteremia; liver abscess | Immune evasion | 183,184 |
| K2, K1, K57, K5, K20, K54 | Meningitis | Serum resistance; Biofilm formation | 185,186 | |
| K20 | Burn infection | Unknown | 58 | |
| K1 | Urinary tract infections | Biofilm formation; Invasion | 187 | |
| Gram-positive bacteria | ||||
| Streptococcus pneumoniae | 6B | Meningitis | Immune evasion | 188 |
| 1, 2, 4 and 9V | Pneumonia | Immune evasion | 189,190 | |
| Endocarditis | Adhesion | 191 | ||
| 2, 4, 6B, 7F | Meningitis | Immune evasion; Translocation | 190,192–194 | |
| 3, 6, 9, 15, 19, 23 | Acute conjunctivitis; Endophthalmitis | Immune evasion | 195,196 | |
| 4, 3, 19A | Respiratory tract infection; Acute otitis media | Adhesion | 78,197 | |
| Staphylococcus aureus | 5, 5 | Bacteriemia; Atopic dermatitis | Adhesion; Immune evasion | 198,199 |
| Streptococcus pyogenes | M18 | Pharyngitis | Immune evasion | 200 |
| Toxic shock syndrome | Immune evasion | 201 | ||
| Streptococcus suis | 2, 14 | Meningitis | Anti phagocytosis; Adhesion | 202–204 |
| 1 | Polyarthritis | Immune evasion | 205 | |
| Bacillus anthracis | Poly-γ-D-glutamic acid capsule | Septicemia | Immune evasion | 3,206 |
Capsular switching is common in bacterial populations, exemplified by S. pneumoniae serotypes 11A and 11E. Serotype 11E, while rare in nasopharyngeal (NP) isolates, is frequently found in invasive pneumococcal disease (IPD) isolates59. Serotype 11E is identical to 11A, except that wcjE is inactivated, resulting in a lack of binding to ficolin-260,61. In addition, most serotype 11E isolates possess wcjE mutations, including missense and nonsense mutations, single-base insertions and deletions, as well as transposon insertions61. This indicates that serotype 11A evolves into serotype 11E within populations, facilitating escape from ficolin-2-mediated phagocytosis during invasive S. pneumoniae infections. Similarly, the distinction between serotypes 9V and 9A lies solely in wcjE mutations, with the inactivation supporting the evolution from 9V to 9A62,63. Frequent variations through homologous recombination and horizontal gene transfer result in numerous uncharacterized components within capsular systems, including genetic components, proteins, or other molecular structures64. As a result, only a limited number of studies have reported on the frequency and diversity of capsules. To address this, Rendueles et al. developed protein profile models for identifying key components of various capsule biosynthetic pathways65. Wick et al. introduced Kaptive Web, an online tool for rapidly typing Klebsiella’s K and O loci66,67. This method was also applied to identify Acinetobacter baumannii (A. baumannii)’s KL and OCL loci68. These developments offer significant technical support for identifying prokaryotic capsules.
Biological functions and pathogenicity of capsules
Adherence
Capsules facilitate bacterial adhesion to surfaces and other bacteria, enhancing colonization in diverse niches and fostering biofilm formation. The bacterial biofilm matrix consists of polysaccharides and is enriched with extracellular proteins and various small molecules, including extracellular DNA (eDNA)69. Eliminating the frwC gene, encoding a hypothetical fructose-specific enzyme II C, can stimulate magA (also known as wzy, encoding a polymerase essential for capsule synthesis) transcription, thus enhancing CPS production and in vitro biofilm formation in K. pneumoniae70. Self-phosphorylating Wza proteins facilitate CPS assembly. The absence of wza impairs CPS transport to the outer membrane and its fixation, markedly diminishing cell adhesion in A. baumannii and leading to in vitro biofilm defects71. There is also a mechanism different from the “classic” static biofilm formation. During periods of high CPS expression, floating S. pyogenes aggregates connect via CPS to facilitate in vitro biofilm formation (Fig. 3A, a). However, the “classic” mechanism can overshadow this biofilm formation mechanism72. Biofilm resulting from bacterial attachment can have far-reaching effects. For example, the biofilm formation of retention tubes in inpatients will cause more severe nosocomial infections73. The Pseudomonas aeruginosa biofilm in patients with cystic fibrosis forms a permeable barrier that can resist the function of antibiotics74.
Fig. 3. The functions of capsule in the process of pathogenic bacterial infection.
A The influence of capsules on bacterial adherence. (a) Capsules promote or inhibits the formation of bacterial biofilm. (b) The addition of exogenous CPS changes the physical properties of the surface of non-biological materials, make them carry high negative charge and inhibit the adhesion of bacteria. B Interfering with host immune responses. (a) S. pneumoniae masks body surface antigens through capsule to avoid phagocytosis by macrophages. (b) F.tularensis inhibits the metabolic transformation of phagocytes through the synthesis of capsules, and finally inhibits the secretion of cytokines. C Assisting bacterial BBB penetration. Bacteria break through the blood-brain barrier by stimulating actin cytoskeleton rearrangement and various signal transduction pathways, including phosphatidylinositol-3-kinase (PI3K) and cytoplasmic phospholipase A2 (cPLA2). But at present, the effect on capsules is only phenotypic. D The impact of capsules on antibiotic resistance. (a) A. baumanii regulates the expression of K locus gene through the BfmRS TCS, which further promotes the synthesis of CPS and resists the killing of antibiotics, but the mechanism of CPS is still unknown. (b) Exogenous CPS adsorbs antimicrobial peptides (AMPs) and polymyxin, thus promoting the survival of bacteria. (c) The CPS located on the surface of bacteria adhere to polymyxin, which promotes the cleavage of polymyxin to the bacterial cell wall and finally promotes the death of bacteria. Positive regulation is indicated by red arrow, and negative regulation by blue arrow.
Nevertheless, the impact of capsules on bacterial adhesion remains a subject of debate. An alternative perspective posits that capsules formation primarily occurs during the mature stage of bacterial biofilm, where CPS secretion facilitates biofilm dissociation (Fig. 3A, a), enabling bacterial dispersion and subsequent biofilm development7. This effect could be due to the masking of adhesive proteins on the bacterial surface or the capsule’s physical and chemical properties. Antigen 43 (Ag43), a surface protein facilitating cell-to-cell aggregation75, is hindered in its function when a K1 or K5 capsule is expressed, as the extended polysaccharides sterically block adhesion76. Likewise, CPS production can obscure ClfA (Clumping Factor A) on the cell wall, thereby reducing S. aureus’s capacity to adhere to platelets77. Additionally, certain studies indicate that capsules may negatively regulate initial in vitro biofilm formation. The negative charge carried by CPS is thought to hinder early bacterial accumulation, consequently inhibiting in vitro biofilm formation78. This phenomenon, far from being accidental, has garnered attention since the early 21st century, with the capsule’s biological properties considered promising for medical surface coatings. Group II capsules extract of E. coli can induce a significant charge reversal at latex particles’ interface ζ (zeta) potential, resulting in a highly anionic nature79. Besides electrostatic modifications, active supernatants can also remodel colloid surface properties, potentially involving surface hydration and steric repulsion. Consequently, Group II capsules significantly reduce biofilm formation on glass surfaces by E. coli and various Gram-positive and Gram-negative pathogens, primarily by weakening cell-surface contacts and reducing cell-cell interactions (Fig. 3A, b).
The influence of capsule serotypes on in vitro biofilm varies. In Bacteroides thetaiotaomicron biofilm grown in a chemostat for 8 days, CPS 8 expression was upregulated, whereas capsules 1, 3, 4, and 6 showed downregulated80. However, there is no evidence that increased in vitro adhesion correlates with improved colonization in axenic mice, indicating that enhanced in vitro biofilm formation may not predict in vivo colonization capacity81. Capsules-mediated bacterial adhesion is also linked to the bacterial growth cycle. S. aureus exhibits stronger adhesion to human endothelial cells (EC) during the exponential growth phase compared to the stationary phase82. Ashbaugh et al. conducted comparative experiments with primate models and revealed that CPS-free mutants were eliminated from the baboon pharynx more rapidly than wild-type strains in short-term colonization studies83. In long-term carrier models, it was observed that carriage isolates developed mutations leading to reduced or absent hyaluronic acid production, suggesting that CPS enhances transmission and initial colonization. Additionally, the late downregulation or loss of CPS synthesis may aid in the long-term survival of the strain in vivo84.
Resistance to host immunity
In invasive bacterial infections, the capsule’s interaction with the host immune system is pivotal in determining infection outcomes85. Capsules confer resistance to nonspecific host defense mechanisms, particularly without specific antibodies. Such mechanisms involve the activation of the complement cascade and C3b-mediated neutrophil phagocytosis via alternative pathways. Activation of the alternative pathway occurs via the nonspecific attachment of the serum protein C3b to the bacterial surface. Upon attachment, C3b engages with factor B, forming C3 convertase (C3bBb). This leads to enhanced C3 attachment and the development of a membrane attack complex (MAC) on the bacterial surface, culminating in lysis and the demise of the bacteria86. CPS impedes the binding of immunoglobulin G (IgG) to bacteria by masking antigen-binding sites on the bacterial surface and diminishes the effect of C3b/iC3b by obstructing the conversion of C3b to iC3b on the bacterial surface (Fig. 3B, a)87. CPS that contains N-acetylneuraminic acid do not initiate the alternative pathway. The inhibition of this pathway can be ascribed to the direct interaction of N-acetylneuraminic acid in polysaccharides with factor H. The bound factor H serves as a cofactor, initiating the combination of factor I and C3b to form iC3b, thus preventing MAC formation88. The capsule collaborates with cell surface structures like O antigen to resist complement-mediated killing. Consequently, this specific combination exhibited by the bacteria confers significant resistance to complement-mediated killing89. Additionally, CPS can counteract complement-mediated opsonophagocytosis. The capsules mask C3b on the inner cell surface, thereby preventing its binding to the C3b receptor (e.g., CR1) on the phagocyte surface90,91. Hurst et al. noted in their immunohistochemical study of a mouse infection model that neutrophils infiltrated the core of GAS infections, irrespective of HA capsule expression. Within 24 h post-infection, there was an upsurge in the neutrophil population (GR1+), and strains with capsular defects were rapidly eliminated by 48 h. Moreover, this does not lead to nasopharyngeal restructuring conducive to favorable inflammation. Furthermore, capsules may play a role in pathogenesis by preventing the entrapment of S. pneumoniae in neutrophil extracellular traps92.
Beyond passive responses to the host immune system, bacterial capsules actively modulate host immune responses by directly influencing cytokine release and disrupting the coordination of host cell-mediated immune responses93. For instance, the capsule of Francisella tularensis (F. tularensis) can impede the R848-induced increase in lactic acid secretion. This inhibition subsequently impedes phagocyte metabolic transition from oxidative phosphorylation to glycolysis, ultimately suppressing cytokine secretion94 (Fig. 3B, b). However, the mechanism of action of the capsules is still unclear, and the capsules’ direct mediation of immune cell responses remains to be explored.
Niche adaptation
Capsules frequently constitute the outermost layer of cells and facilitate direct interaction between bacteria and their environment, thus influencing bacterial adaptation to new niches. Species encoding capsule, particularly environmental bacteria and facultative pathogens with multiple capsule genes, exhibit higher levels of genetic diversification than their counterparts, contributing to broader environmental adaptability95.
During the invasion of a host by pathogenic bacteria, these organisms encounter significant environmental changes, including low pH, elevated temperature, reduced oxygen pressure, and altered osmotic pressure. When pathogenic bacteria invade the epithelial barrier and enter the bloodstream, they are exposed to a 0.15 M sodium chloride osmotic pressure, triggering prioritized CPS synthesis96. Upon entering the bloodstream and invading deeper tissues and organs, bacteria encounter the challenge of low oxygen pressure in these environments. In S. pneumoniae, CPS synthesis is reduced under hyperoxic conditions compared to hypoxic growth, a phenomenon linked to CpsB phosphatase activity, not CpsD phosphorylation levels97. Considering S. pneumoniae’s growth environment, its benign colonization in high-oxygen nasopharyngeal areas leads to decreased capsule synthesis. Upon transfer to host defense sites with lower oxygen levels, an increase in capsule synthesis is observed18. A similar phenomenon is observed in S. aureus, where capsule synthesis in three serotypes is inhibited in environments supplemented with 1%-5% CO298. Further research indicated that CO2 impedes the transcription of the cap gene99. However, it is important to note that higher oxygen concentrations are not more beneficial. In S. pneumoniae, high oxygen concentrations regulate CpsB phosphatase activity, inhibiting CpsD phosphorylation. Impaired CPS regulation due to tyrosine phosphorylation in CpsD affects S. pneumoniae’s capacity to transition from the lungs to the bloodstream100. Furthermore, factors such as iron-limited culture101, acidic conditions102,103, and the nutrient richness104 of the environment also play crucial roles in capsule synthesis.
The influence of capsules presence on bacterial adaptation and its role in strain evolution were not empirically confirmed until Nucci et al. conducted the first relevant evolutionary study105. In an evolutionary experiment spanning 675 generations (102 days) with three phylogenetically distant strains of K. pneumoniae, Nucci et al. discovered that both capsulated and non-capsulated populations possessed a competitive edge over their progenitor strains, with average fitness increases of 58% and 36%, respectively. This finding suggests that capsules play a significant role in enhancing the average fitness of populations105. The presence of capsules in adapting populations influences phenotypic changes significantly. Evolved capsulated bacteria exhibit an increase in or the emergence of a hypermucoidy phenotype (HMP), while non-capsulated populations adapt through higher population yields, enhanced surface polysaccharides, and biofilm formation105. The competitive advantage of the HMP has been demonstrated in bacteria and fungi, with HMP preventing predation by amoeba or bacteria on Klebsiella106,107. Cryptococcus neoformans develops resistance to amoeba by increasing its capsules size108. Furthermore, E. coli exhibits increased mucoidy when interacting with macrophages or predatory bacteria, suggesting that this phenotype is advantageous outside the host109,110. The research by Nucci et al. suggests that capsulated and non-capsulated populations adapt to niches via distinct pathways. Capsulated strains frequently demonstrate genetic mutations that directly impact capsule synthesis105. So, how do non-capsulated cells adapt to the new environment? Firstly, non-capsulated strains adapt through increased production of alternative extracellular polysaccharides on the cell surface, facilitated by Wzi (a functional lectin-binding protein), thereby mimicking capsule functionality111,112. Secondly, most non-capsulated clones accumulate mutations in the capsule’s regulatory elements, reducing in the expression cost of other genes within the operon113. This reduction in capsule expression may confer an advantage when capsules are regained through horizontal gene transfer. Furthermore, this can result in capsule swapping, expressing a novel serotype with a different biochemical composition among strains with similar chemical compositions113,114. Ultimately, the co-adaptation of bacterial populations, both encapsulated and non-encapsulated, leads to a more complex population structure and an increase in cellular interactions115,116.
Other functions
Capsules are known for their contribution to bacterial adhesion and anti-phagocytosis, so these two properties have been most widely studied. With the increasingly in-depth study of capsules, other virulence potentials have gained attention.
Presently, the mortality and morbidity rates linked to bacterial meningitis remain alarmingly high. Earlier studies have reported capsules’ role in enabling bacteria to breach the blood-brain barrier (BBB). E. coli with the K1 CPS is particularly prominent among isolates that cause neonatal meningitis. Investigations have revealed that microbial elements, including the K1 capsule, are crucial for the invasiveness of E. coli. The K1-cps locus is present in a quarter of bloodstream infection isolates and has independently emerged in at least four ExPEC phylogroups over the last 500 years34. Furthermore, the K1 capsule aids in the invasion of brain microvascular endothelial cells (BMEC) by E. coli117. Unlike other meningitis-causing bacteria like Group B Streptococci (GBS)118, E. coli K1’s invasion does not compromise the integrity of the cell monolayer structure. E. coli K1 invades BMEC via a zipper-like mechanism and travels through enclosed vacuoles119. Employing a reverse-oriented Transwell filter system with porcine choroid plexus epithelial cells (PCPEC), Tenenbaum et al. explored the process of bacterial invasion and movement from the basolateral (blood) aspect to the apical (cerebrospinal fluid) aspect, a novel approach in this field. Their findings suggest that S. suis translocation through PCPEC could be regulated by capsule-derived signals dependent on the lipid kinase phosphatidylinositol 3-kinase pathway (Fig. 3C)120. However, there exist conflicting research findings indicating that capsular presence may in fact attenuate bacterial virulence. According to Gendrin et al., GBS strains enhance their intracellular survival and propagation by abandoning their capsule. This mechanism does not involve concealment within macrophages (also called the “Trojan horse” mechanism121,122). Concurrently, there is an observed increase in the permeability of the BBB associated with GBS123.
The contribution of capsules to bacterial drug resistance is well-documented in scientific literature. In A. baumannii, antibiotics induce stress, leading to increased transcription of the K locus genes (responsible for capsule biosynthesis) via the BfmRS two-component regulatory system124. This sequence of events triggers capsule synthesis, which confers resistance to chloramphenicol and erythromycin (Fig. 3D, a). In High-alcohol-producing K. pneumoniae (HiAlc Kpn), glucose inhibits the expression of crp and enhances CPS production, thereby increasing the strain’s drug resistance125. Furthermore, several studies have demonstrated that externally added capsules can bind antimicrobial peptides (AMPs), potentially blocking their entry into the cell and diminishing the efficacy of polymyxin (Fig. 3D, b)126,127. When the capsule functions naturally, attached to the outer membrane, it increases antimicrobials’ concentration near the cell (Fig. 3D, c)128. This elevated concentration of antimicrobial peptides benefits agents like colistin, which disrupt the outer cell membrane and induce cell lysis by binding to lipopolysaccharides and phospholipids129. Gendrin et al. noted that reducing capsule density could enhance antibiotic evasion in GBS123. Given the limited research, the contribution of the capsule to bacterial antibiotic resistance persists as a debated topic, and the investigation into whether this role is specific to particular antibiotic species remains imperative.
Regulation mechanisms
Two-component systems regulate capsules
Two-component systems (TCS) represent a predominant mechanism in bacterial signal transduction130. In bacteria, TCS plays a pivotal role in gene regulation, responding to environmental changes. These protein families are implicated in adapting to diverse stress conditions and in essential cellular pathways131.
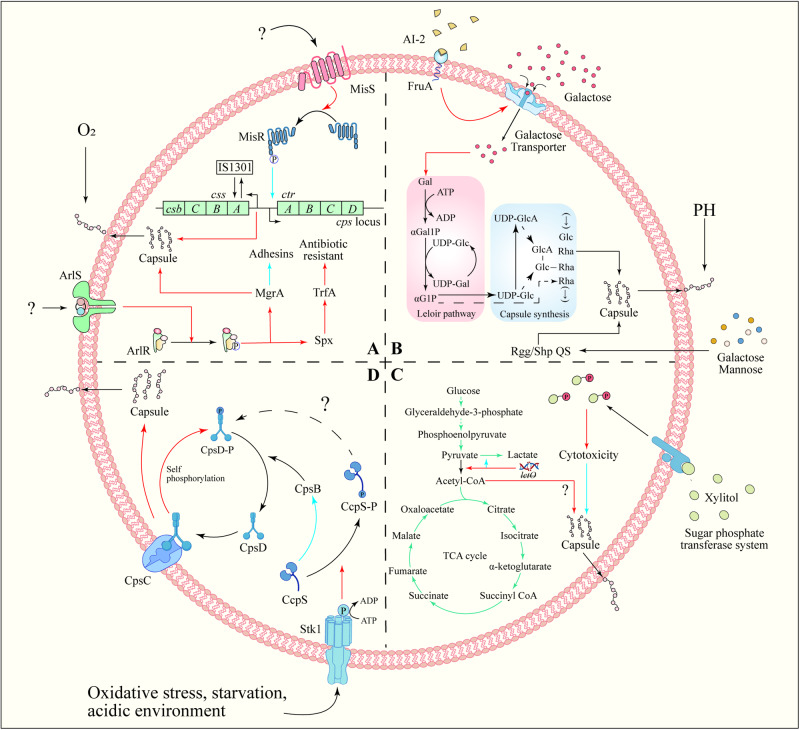
The cps locus promoter in Neisseria meningitidis is situated in the intergenic region between biosynthesis and the conserved envelope transport operon. The MisR/MisS TCS negatively regulates CPS production by directly binding to the promoter region (Fig. 4A)132. In GAS, the CsrRS TCS as a regulator of capsule production. This system comprises the loci csrR and csrS (also known as covR and covS)133. While the inactivation of csrR did not alter M protein expression or hemolytic activity, it resulted in a sixfold increase in capsule production, whereas subsequent studies showed that the system influences the expression of additional virulence factors, including streptokinase (ska), mitogenic factor (speMF) and cysteine protease (speB)28. The RstAB TCS positively regulates CPS synthesis, aiding Photobacterium damselae in evading fish host cells’ defense134. ArlR directly activates the expression of global transcription factors MgrA and Spx in S. aureus135 (Fig. 4A), influencing capsule synthesis genes, wall-anchored adhesins (ebh, sdrD), cell wall remodeling genes (lytN, ddh) and anaerobic metabolism genes (adhE, pflA, nrdDG). This activation promotes capsule synthesis and impacts the TrfA protein, a component of the Clp proteasome complex, which plays a role in resisting cell wall-targeting antibiotics, thereby enhancing antibiotic resistance136,137.
Fig. 4. Capsule regulation mechanisms.
A TCS regulation of capsule synthesis. TCS often regulates capsule biosynthesis by directly binding to the promoters of capsule synthesis genes, thereby responding to environmental changes. The ArlRS TCS of S. aureus can directly activate the global transcription factors MgrA and Spx expression, thereby regulating capsule synthesis and antibiotic resistance. N. meningitidis MisR/MisS TCS can negatively regulate the production of CPS by directly binding to the promoter region. B QS regulation of capsule synthesis. AI-2 regulates the phosphotransferase system on the membrane surface to limit the intake of galactose, ultimately affecting the synthesis of CPS. C Metabolic regulation of capsule synthesis. The synthesis of bacterial capsule is related to the fate of pyruvate. Pyruvate is converted into acetyl-CoA and enters the TCA cycle, which is beneficial to capsule synthesis. Therefore, inhibiting the conversion of pyruvate to lactic acid can promote the synthesis of bacterial capsule. In addition, excessive xylitol intake will stimulate the phosphotransferase system on the cell membrane, and phosphorylated xylitol impairs capsule synthesis and ultimately affects bacterial adhesion. D External environmental conditions promote Stk1/Stp1 system phosphorylate CcpS, thus relieving the inhibition of CcpS on CpsB. CpsB assists the dephosphorylation of phosphorylated CpsD (CpsD-P). Finally, CpsD binds to CpsC again and promotes CPS synthesis. Positive regulation is indicated by red arrow, and negative regulation by blue arrow.
In summary, most TCS are recognized as key molecular mechanisms regulating bacterial capsule synthesis, and the understanding of their interrelationships is becoming increasingly comprehensive.
Quorum sensing regulates capsules
Quorum sensing (QS) represents a form of inter-bacterial ‘communication’73. In this process, bacteria synthesize and detect extracellular signaling molecules, termed auto-inducers (AI), leading to either the activation of regulatory proteins or the suppression of specific gene expression. This intricate system plays a crucial role in bacterial coordination and collective behavior. This process allows for the control of the physiological characteristics of microbial populations, including traits like motility, biofilm formation, immunosuppression, and nutrient utilization138,139. Studies have indicated that QS-regulated genes constitute 10% and 20% of the entire genome140,141. As research on QS deepens, more attention is being directed toward understanding the relationship between QS and capsules.
FruA, a fructose-specific phosphotransferase system component in S. pneumoniae, can sense AI-2. This sensing mechanism leads to the up-regulation of the galactose ABC transporter and the Leloir pathway (Fig. 4B). Subsequently, it increases CPS synthesis, resulting in a high virulence phenotype142. Rgg/Shp144 and Rgg/Shp939 were proved to be another QS of S. pneumoniae by Zhi et al. The Rgg/Shp QS includes Rgg proteins (alternatively termed Gad or Mut), which are part of a conserved group of independent transcriptional regulators, and short hydrophobic peptides (Shp)143,144. Rgg/Shp1517 QS is necessary for S. pneumoniae to use galactose and mannose. However it is a negative regulator of capsular expression, which may involve binding Rgg to the capsular locus promoter (Fig. 4B)145. Additionally, the regulation of CPS by Rgg/Shp QS varies depending on the response to specific sugars. Rgg144 and Rgg939 were most significantly induced by mannose, followed by galactose, whereas Rgg1518 was primarily stimulated by galactose146. Furthermore, other quorum sensing systems have also been reported to be associated with capsule synthesis. For example, GtaR/I QS is involved in regulating Rhodobacter capsulatus (RcGTA) growth and capsules’ formation, and the capsules also play a role in RcGTA adhesion as a receptor147. Agr (accessory gene regulator) QS positively regulates the production of type-5 capsular polysaccharide (CP5) in S. aureus, enhancing adhesion to inner epidermal cells (EC)82.
Metabolic activity regulates capsules
Capsules are crucial in bacterial invasive infections, acting as an energy-consuming virulence factor. Bacteria frequently utilize carbohydrates via glycolysis and other central carbon metabolism (CCM) to support the energy demands of capsule synthesis104. CCM encompasses enzymatic reactions converting carbon into energy, including glycolysis, the tricarboxylic acid (TCA) cycle, the gluconeogenesis, the pentose phosphate pathway (PPP), the glyoxylate shunt, the γ-aminobutyric acid (GABA) shunt, and the methylcitrate cycle148. As a central component of material metabolism, the TCA cycle plays an irreplaceable role in the life process of organisms. Pyruvate from glycolysis is converted to acetyl-CoA by pyruvate dehydrogenase, initiating the TCA cycle and providing energy for cells. Following the simultaneous deletion of spxB (pyruvate oxidase) and lctO (lactate dehydrogenase), acetyl-CoA and capsule production were restored in S. pneumoniae type 4, though the underlying mechanism remains elusive149. The prevailing hypothesis posits that the absence of lactate dehydrogenase leads to increased lactic acid levels, inhibiting lactate dehydrogenase activity. This inhibition facilitates bacterial capture of pyruvate, thus enhancing the conversion of pyruvate to acetyl-CoA (Fig. 4C). Although S. aureus possesses pyruvate dehydrogenase, it directly converts pyruvate to acetic acid150. However, the impact of this conversion on acetyl-CoA and capsule synthesis remains unknown. Excessive sugar intake is deleterious, given that the glucose phosphotransferase system swiftly transports xylitol into cells and phosphorylates it, while the accumulation of excessive xylitol phosphate can exert toxic efforts on S. pneumoniae, impairing CPS production and diminishing adhesion to nasopharyngeal cells (Fig. 4C)151. CPS in S. aureus serotype 5 was eliminated in the presence of glucose, but this did not affect CPS synthesis in the ccpA (coding catabolite control protein A) deletion strain MST14 serotype 5. Seidl et al. found that cap operon expression in S. aureus serotype 5 was significantly lower compared to MST14, yet no prominent catabolite-responsive elements (CREs) were identified in the cap operon’s genomic region, suggesting an indirect effect of CcpA on cap transcription152.
Additionally, a complex relationship exists between capsule types and metabolic cost. For instance, capsule exchange in S. pneumoniae may result in diminished fitness or viability, a consequence modulated by the carbon content and CPS charge of each polysaccharide153. Hathaway et al. compared growth phenotypes of S. pneumoniae across different capsular serotypes, finding that strains with a lower metabolic burden exhibited growth advantages104. Schipper et al. examined the impact of meningococcal CPS structure on the lethality of zebrafish embryos and neutrophil consumption post-infection. They observed a close relationship between the CPS structure and the carbon number in each polysaccharide repeat unit. Consequently, the variation in virulence among different capsule types may stem from metabolic cost differences rather than molecular interactions with host immune components154.
Other regulation mechanisms
Tyrosine phosphorylation, initially viewed as crucial in eukaryotic regulation155, is now recognized as a critical factor in bacterial physiology156. Although phosphorylation is a longstanding recognized posttranslational regulatory mechanism in bacteria, the significance of tyrosine phosphorylation was highlighted with the discovery of a tyrosine-phosphorylated protein in Acinetobacter johnsonii157. The cocci bacteria encode the first four genes, cpsABCD, in the cps locus, a sequence broadly conserved across species18. The cpsA gene encodes LytR-Cps2A-Psr (LCP) protein, which is believed to conjugate CPS to peptidoglycan (PG)158. The cpsB, cpsC, and cpsD genes form a tyrosine phosphoregulatory system controlling CPS assembly machinery159,160. CpsC is essential for CpsD’s tyrosine phosphorylation. When CpsD self-phosphorylates (utilizing bound ATP), the resulting tyrosine phosphorylated CpsD (CpsD-p) dissociates from CpsC, reducing CPS production. CpsB assists in CpsD-p dephosphorylation, facilitating its interaction with CpsC, leading to an accelerated rate of CPS biosynthesis/polymerization161,162. The CpsBCD bacterial tyrosine kinase system responds to environmental changes (e.g., oxygen content18), with the mechanism clarified by Tang et al.’s study in S. suis, enhancing understanding of the relationship between CpsBCD and signal transduction. CcpS, a protein regulating phosphatase CpsB’s activity, links the Stk1/Stp1 system (a serine/threonine kinase system controlling bacterial phosphosignaling) with the Wzx-Wzy pathway in bacteria. Stk1/Stp1 specially mediates Thr-phosphorylation of the CcpS protein. Non-phosphorylated CcpS can inhibit CpsB-catalyzed dephosphorylation of CpsD-P in vivo, leading to abnormal CPS synthesis in S. suis163 (Fig. 4D).
The second messenger cyclic AMP (cAMP), small RNA, and iron-acquisition systems are also found to be associated with capsule synthesis. Cyclic-3’,5’-adenosine monophosphate (cAMP) is a ubiquitous second messenger, orchestrating essential processes in bacteria and eukaryotes164. K. pneumoniae regulates CPS production through cAMP-dependent carbon catabolite repression (CCR), enhancing protection from serum killing and phagocytosis and modifying oxidative stress resistance, improving phagosome survival165. In Vibrio parahaemolyticus, AI-2 QS controls the capsular synthesis and bacterial aggregation through self-inducing signals (S signals) affecting c-di-GMP levels166. Small RNAs (sRNAs), such as rss04 and rss03, are critical regulators of bacterial virulence, inhibiting CPS production after S. suis enters the brain, exacerbating the inflammatory response, and promoting meningitis167. The iron uptake system, a crucial regulatory mechanism in bacteria, has become a focal point of research. Bacteria stringently regulate iron transport and storage via Fur (ferric uptake regulator) to maintain iron homeostasis. Under iron-replete conditions, dimeric Fur complexed with Fe(II) binds to a 19 bp consensus DNA sequence in the promoters of iron uptake genes, inhibiting their transcription168. In K. pneumoniae, Fur suppresses CPS biosynthesis by inhibiting RmpA and RcsA. Interestingly, sRNA also plays a role in these regulatory activities169. sRNA RyhB activates the transcription of orf1 and orf16, components of the cps gene cluster open reading frames (ORFs)), independently of RmpA and RcsA170. However, since K. pneumoniae requires CPS for survival in the host, other positive regulatory systems responding to external iron influence CPS biosynthesis. IscR, a protein harboring a [2Fe-2S] cluster and encoded by the first gene of the iscRSUA operon171, orchestrates the regulation of genes engaged in diverse cellular processes that respond to environmental stimuli such as oxidative stress and iron172. With the [2Fe-2S] clusters, IscR’s DNA binding specificity is broadened, enabling holo-IscR to interact with both type 1 and type 2 IscR box173, positively influencing CPS biosynthesis174.
Outlook
Given the substantial immunomodulatory characteristics of CPS, they have garnered considerable attention in vaccine development. Extensive research over the years has established the efficacy of vaccines based on polyvalent pneumococcal polysaccharides. This focus aligns with CPS’s ability to modulate immune responses, underscoring its potential in preventive healthcare strategies. Mutagenesis-induced removal of CPS reveals antigenic cell wall proteins usually obscured by the dense capsular shell. The nonencapsulated mutant is anticipated to elicit a more potent immune response. Although the capsule’s structural diversity, biosynthesis, and immunogenicity have been extensively studied, further research is required on their role in pathogen adhesion and regulatory mechanisms. Secondly, research on immune evasion predominantly centers on how capsules mask surface antigens of pathogenic bacteria. Studies on the capsule’s active mediation of host immunity (e.g., F. tularensis94) remain limited. Conducting further research will undoubtedly deepen our understanding of the capsule’s biological functions and aid in developing treatment strategies. Additionally, bacteria can express multiple capsule types concurrently65,175. This capability of co-expression broadens the bacteria’s range of environmental adaptabilities, including enhanced adhesion and virulence, thereby facilitating their ecological transition towards host colonization and pathogenesis176. Therefore, in the future, it is necessary to study in detail the mechanisms that lead to the acquisition of multiple capsules.
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (32172852), Excellent Youth Foundation of Henan Scientific Committee (222300420005), He’nan Provincial Science and Technology Research Project (232102110095), Program for Innovative Research Team (in Science and Technology) in University of Henan Province (24IRTSTHN033) and Key Scientific Research Projects of Higher Education Institutions of Henan Province (24A230013).
Author contributions
S.G., W.J., and Y.W.: Writing—original draft preparation; Y.W.: Writing—review & editing; Y.Q. and Y.L.: References collection; S.Y. and Y.S.: Tables and figures organization. L.Y., and Y.W.: Acquisition of funds; Y.W.: Visualization, investigation. All authors have agreed to its publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shuji Gao, Wenjie Jin.
Contributor Information
Yuxin Wang, Email: wangyuxin_1991@163.com.
Yang Wang, Email: wangyocean@163.com.
References
- 1.Rajagopal M, Walker S. Envelope structures of gram-positive bacteria. Curr. Top. Microbiol. Immunol. 2017;404:1–44. doi: 10.1007/82_2015_5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng Y, et al. Attenuation of Streptococcus suis virulence by the alteration of bacterial surface architecture. Sci. Rep. 2012;2:710. doi: 10.1038/srep00710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moayeri M, et al. Anthrax pathogenesis. Annu Rev. Microbiol. 2015;69:185–208. doi: 10.1146/annurev-micro-091014-104523. [DOI] [PubMed] [Google Scholar]
- 4.Tomcsik J, Baumann-Grace JB. Polysaccharide capsule of Bacillus megaterium. Proc. Soc. Exp. Biol. Med. Soc. Exp. Biol. Med. 1959;101:570–571. doi: 10.3181/00379727-101-25019. [DOI] [PubMed] [Google Scholar]
- 5.Dochez AR, Avery OT. The elaboration of specific soluble substance by pneumococcus during growth. J. Exp. Med. 1917;26:477–493. doi: 10.1084/jem.26.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avery OT, Morgan HJ. Immunological reactions of the isolated carbohydrate and protein of Pneumococcus. J. Exp. Med. 1925;42:347–353. doi: 10.1084/jem.42.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee KJ, et al. Role of capsular polysaccharide (CPS) in biofilm formation and regulation of CPS production by quorum-sensing in Vibrio vulnificus. Mol. Microbiol. 2013;90:841–857. doi: 10.1111/mmi.12401. [DOI] [PubMed] [Google Scholar]
- 8.Cole JN, et al. M protein and hyaluronic acid capsule are essential for in vivo selection of covRS mutations characteristic of invasive serotype M1T1 group A Streptococcus. mBio. 2010;1:e00191–10. doi: 10.1128/mBio.00191-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tram G, Jennings MP, Blackall PJ, Atack JM. Streptococcus suis pathogenesis-A diverse array of virulence factors for a zoonotic lifestyle. Adv. Micro. Physiol. 2021;78:217–257. doi: 10.1016/bs.ampbs.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Campos MA, et al. Capsule polysaccharide mediates bacterial resistance to antimicrobial peptides. Infect. Immun. 2004;72:7107–7114. doi: 10.1128/IAI.72.12.7107-7114.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrews GP, et al. Fraction 1 capsular antigen (F1) purification from Yersinia pestis CO92 and from an Escherichia coli recombinant strain and efficacy against lethal plague challenge. Infect. Immun. 1996;64:2180–2187. doi: 10.1128/iai.64.6.2180-2187.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Runco LM, Myrczek S, Bliska JB, Thanassi DG. Biogenesis of the fraction 1 capsule and analysis of the ultrastructure of Yersinia pestis. J. Bacteriol. 2008;190:3381–3385. doi: 10.1128/JB.01840-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ezzell JW, Welkos SL. The capsule of bacillus anthracis, a review. J. Appl Microbiol. 1999;87:250. doi: 10.1046/j.1365-2672.1999.00881.x. [DOI] [PubMed] [Google Scholar]
- 14.Freudenberger Catanzaro KC, Inzana TJ. The Francisella tularensis Polysaccharides: What is the real capsule? Microbiol Mol. Biol. Rev. 2020;84:e00065–19. doi: 10.1128/MMBR.00065-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bandara AB, et al. Isolation and mutagenesis of a capsule-like complex (CLC) from Francisella tularensis, and contribution of the CLC to F. tularensis virulence in mice. PLoS One. 2011;6:e19003. doi: 10.1371/journal.pone.0019003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuthbertson L, Kos V, Whitfield C. ABC transporters involved in export of cell surface glycoconjugates. Microbiol Mol. Biol. Rev. 2010;74:341–362. doi: 10.1128/MMBR.00009-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geno KA, et al. Pneumococcal capsules and their types: past, present, and future. Clin. Microbiol Rev. 2015;28:871–899. doi: 10.1128/CMR.00024-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yother J. Capsules of Streptococcus pneumoniae and other bacteria: paradigms for polysaccharide biosynthesis and regulation. Annu Rev. Microbiol. 2011;65:563–581. doi: 10.1146/annurev.micro.62.081307.162944. [DOI] [PubMed] [Google Scholar]
- 19.Whitfield C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu Rev. Biochem. 2006;75:39–68. doi: 10.1146/annurev.biochem.75.103004.142545. [DOI] [PubMed] [Google Scholar]
- 20.Bentley SD, et al. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2006;2:e31. doi: 10.1371/journal.pgen.0020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shikina EV, Kovalevsky RA, Shirkovskaya AI, Toukach PV. Prospective bacterial and fungal sources of hyaluronic acid: A review. Comput Struct. Biotechnol. J. 2022;20:6214–6236. doi: 10.1016/j.csbj.2022.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luck JN, Tettelin H, Orihuela CJ. Sugar-coated killer: Serotype 3 Pneumococcal disease. Front Cell Infect. Microbiol. 2020;10:613287. doi: 10.3389/fcimb.2020.613287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitfield C, Wear SS, Sande C. Assembly of bacterial capsular polysaccharides and exopolysaccharides. Annu Rev. Microbiol. 2020;74:521–543. doi: 10.1146/annurev-micro-011420-075607. [DOI] [PubMed] [Google Scholar]
- 24.Avakyan AA, Katz LN, Levina KN, Pavlova IB. Structure and composition of the Bacillus anthracis capsule. J. Bacteriol. 1965;90:1082–1095. doi: 10.1128/jb.90.4.1082-1095.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nordberg BK, Thorsell W. The effect of certain enzyme systems on the capsule of Bacillus anthracis. J. Bacteriol. 1955;69:367–371. doi: 10.1128/jb.69.4.367-371.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Troy FA. Chemistry and biosynthesis of the poly(-D-glutamyl) capsule in Bacillus licheniformis. I. Properties of the membrane-mediated biosynthetic reaction. J. Biol. Chem. 1973;248:305–315. doi: 10.1016/S0021-9258(19)44475-X. [DOI] [PubMed] [Google Scholar]
- 27.Fouet A, Mesnage S. Bacillus anthracis cell envelope components. Curr. Top. Microbiol. Immunol. 2002;271:87–113. doi: 10.1007/978-3-662-05767-4_5. [DOI] [PubMed] [Google Scholar]
- 28.Wessels, M. R. Capsular Polysaccharide of Group A Streptococcus. Microbiol Spectr. 7, (2019). [DOI] [PMC free article] [PubMed]
- 29.Yan L, et al. A revised structure for the glycolipid terminus of Escherichia coli K5 Heparosan capsular polysaccharide. Biomolecules. 2020;10:1516. doi: 10.3390/biom10111516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Ambrosio S, et al. Production and purification of higher molecular weight chondroitin by metabolically engineered Escherichia coli K4 strains. Sci. Rep. 2020;10:13200. doi: 10.1038/s41598-020-70027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lizak C, et al. X-ray crystallographic structure of a bacterial polysialyltransferase provides insight into the biosynthesis of capsular polysialic acid. Sci. Rep. 2017;7:5842. doi: 10.1038/s41598-017-05627-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez-Torres AJ, Stout V. Role of colanic acid polysaccharide in serum resistance in vivo and in adherence. Curr. Microbiol. 1996;33:383–389. doi: 10.1007/s002849900132. [DOI] [PubMed] [Google Scholar]
- 33.Pando JM, et al. The Rcs-regulated colanic acid capsule maintains membrane potential in Salmonella enterica serovar Typhimurium. mBio. 2017;8:e00808–e00817. doi: 10.1128/mBio.00808-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arredondo-Alonso S, et al. Evolutionary and functional history of the Escherichia coli K1 capsule. Nat. Commun. 2023;14:3294. doi: 10.1038/s41467-023-39052-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reid AN, Whitfield C. functional analysis of conserved gene products involved in assembly of Escherichia coli capsules and exopolysaccharides: evidence for molecular recognition between Wza and Wzc for colanic acid biosynthesis. J. Bacteriol. 2005;187:5470–5481. doi: 10.1128/JB.187.15.5470-5481.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Majdalani N, Gottesman S. The Rcs phosphorelay: a complex signal transduction system. Annu. Rev. Microbiol. 2005;59:379–405. doi: 10.1146/annurev.micro.59.050405.101230. [DOI] [PubMed] [Google Scholar]
- 37.Bratcher PE, et al. Identification of natural pneumococcal isolates expressing serotype 6D by genetic, biochemical and serological characterization. Microbiology. 2010;156:555–560. doi: 10.1099/mic.0.034116-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu J, Carvalho M, Beall B, Nahm MH. A rapid pneumococcal serotyping system based on monoclonal antibodies and PCR. J. Med Microbiol. 2008;57:171–178. doi: 10.1099/jmm.0.47549-0. [DOI] [PubMed] [Google Scholar]
- 39.Park IH, et al. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J. Clin. Microbiol. 2007;45:1225–1233. doi: 10.1128/JCM.02199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seeberger PH, Pereira CL, Govindan S. Total synthesis of a Streptococcus pneumoniae serotype 12F CPS repeating unit hexasaccharide. Beilstein J. Org. Chem. 2017;13:164–173. doi: 10.3762/bjoc.13.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pereira CL, Geissner A, Anish C, Seeberger PH. Chemical synthesis elucidates the immunological importance of a pyruvate modification in the capsular polysaccharide of Streptococcus pneumoniae Serotype 4. Angew. Chem. (Int. ed. Engl.) 2015;54:10016–10019. doi: 10.1002/anie.201504847. [DOI] [PubMed] [Google Scholar]
- 42.Chaudhury A, Mukherjee MM, Ghosh R. Synthetic avenues towards a tetrasaccharide related to Streptococcus pneumonia of serotype 6A. Beilstein J. Org. Chem. 2018;14:1095–1102. doi: 10.3762/bjoc.14.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perch B, Pedersen KB, Henrichsen J. Serology of capsulated streptococci pathogenic for pigs: six new serotypes of Streptococcus suis. J. Clin. Microbiol. 1983;17:993–996. doi: 10.1128/jcm.17.6.993-996.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Z, et al. Development of multiplex PCR assays for the identification of the 33 serotypes of Streptococcus suis. PLoS One. 2013;8:e72070. doi: 10.1371/journal.pone.0072070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Athey TB, et al. Determining Streptococcus suis serotype from short-read whole-genome sequencing data. BMC Microbiol. 2016;16:162. doi: 10.1186/s12866-016-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hill JE, et al. Biochemical analysis, cpn60 and 16S rDNA sequence data indicate that Streptococcus suis serotypes 32 and 34, isolated from pigs, are Streptococcus orisratti. Vet. Microbiol. 2005;107:63–69. doi: 10.1016/j.vetmic.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Sá-Leão R, et al. Analysis of invasiveness of pneumococcal serotypes and clones circulating in Portugal before widespread use of conjugate vaccines reveals heterogeneous behavior of clones expressing the same serotype. J. Clin. Microbiol. 2011;49:1369–1375. doi: 10.1128/JCM.01763-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rivera-Olivero IA, et al. Carriage and invasive isolates of Streptococcus pneumoniae in Caracas, Venezuela: the relative invasiveness of serotypes and vaccine coverage. Eur. J. Clin. Microbiol. Infect. Dis. 2011;30:1489–1495. doi: 10.1007/s10096-011-1247-5. [DOI] [PubMed] [Google Scholar]
- 49.Yildirim I, et al. Serotype specific invasive capacity and persistent reduction in invasive pneumococcal disease. Vaccine. 2010;29:283–288. doi: 10.1016/j.vaccine.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song JY, Nahm MH, Moseley MA. Clinical implications of pneumococcal serotypes: invasive disease potential, clinical presentations, and antibiotic resistance. J. Korean Med. Sci. 2013;28:4–15. doi: 10.3346/jkms.2013.28.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Riordan K, Lee JC. Staphylococcus aureus capsular polysaccharides. Clin. Microbiol Rev. 2004;17:218–234. doi: 10.1128/CMR.17.1.218-234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu TC, Park JT. Chemical characterization of a new surface antigenic polysaccharide from a mutant of Staphylococcus aureus. J. Bacteriol. 1971;108:874–884. doi: 10.1128/jb.108.2.874-884.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fournier JM, Vann WF, Karakawa WW. Purification and characterization of Staphylococcus aureus type 8 capsular polysaccharide. Infect. Immun. 1984;45:87–93. doi: 10.1128/iai.45.1.87-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ulanova M, Tsang RSW. Haemophilus influenzae serotype a as a cause of serious invasive infections. Lancet Infect. Dis. 2014;14:70–82. doi: 10.1016/S1473-3099(13)70170-1. [DOI] [PubMed] [Google Scholar]
- 55.McMillan DJ, et al. Updated model of group A Streptococcus M proteins based on a comprehensive worldwide study. Clin. Microbiol. Infect. 2013;19:E222–E229. doi: 10.1111/1469-0691.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanderson-Smith M, et al. A systematic and functional classification of Streptococcus pyogenes that serves as a new tool for molecular typing and vaccine development. J. Infect. Dis. 2014;210:1325–1338. doi: 10.1093/infdis/jiu260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu VL, et al. Virulence characteristics of Klebsiella and clinical manifestations of K. pneumoniae bloodstream infections. Emerg. Infect. Dis. 2007;13:986–993. doi: 10.3201/eid1307.070187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hasani A, et al. Serotyping of Klebsiella pneumoniae and its relation with capsule-associated virulence genes, antimicrobial resistance pattern, and clinical infections: a descriptive study in medical practice. Infect. Drug Resist. 2020;13:1971–1980. doi: 10.2147/IDR.S243984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Calix JJ, et al. Differential occurrence of Streptococcus pneumoniae serotype 11E between asymptomatic carriage and invasive pneumococcal disease isolates reflects a unique model of pathogen microevolution. Clin. Infect. Dis. 2012;54:794–799. doi: 10.1093/cid/cir953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brady AM, et al. Low invasiveness of pneumococcal serotype 11A is linked to ficolin-2 recognition of O-acetylated capsule epitopes and lectin complement pathway activation. J. Infect. Dis. 2014;210:1155–1165. doi: 10.1093/infdis/jiu195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Calix JJ, Nahm MH. A new pneumococcal serotype, 11E, has a variably inactivated wcjE gene. J. Infect. Dis. 2010;202:29–38. doi: 10.1086/653123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Calix JJ, et al. Streptococcus pneumoniae serotype 9A isolates contain diverse mutations to wcjE that result in variable expression of serotype 9V-specific epitope. J. Infect. Dis. 2011;204:1585–1595. doi: 10.1093/infdis/jir593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Calix JJ, Saad JS, Brady AM, Nahm MH. Structural characterization of Streptococcus pneumoniae serotype 9A capsule polysaccharide reveals role of glycosyl 6-O-acetyltransferase wcjE in serotype 9V capsule biosynthesis and immunogenicity. J. Biol. Chem. 2012;287:13996–14003. doi: 10.1074/jbc.M112.346924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wyres KL, et al. Pneumococcal capsular switching: a historical perspective. J. Infect. Dis. 2013;207:439–449. doi: 10.1093/infdis/jis703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rendueles O, et al. Abundance and co-occurrence of extracellular capsules increase environmental breadth: Implications for the emergence of pathogens. PLoS Pathog. 2017;13:e1006525. doi: 10.1371/journal.ppat.1006525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lam MMC, et al. Kaptive 2.0: updated capsule and lipopolysaccharide locus typing for the Klebsiella pneumoniae species complex. Microb. Genom. 2022;8:000800. doi: 10.1099/mgen.0.000800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wick RR, Heinz E, Holt KE, Wyres KL. Kaptive Web: User-friendly capsule and lipopolysaccharide serotype prediction for Klebsiella Genomes. J. Clin. Microbiol. 2018;56:e00197–18. doi: 10.1128/JCM.00197-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wyres KL, et al. Identification of Acinetobacter baumannii loci for capsular polysaccharide (KL) and lipooligosaccharide outer core (OCL) synthesis in genome assemblies using curated reference databases compatible with Kaptive. Microb. Genom. 2020;6:e000339. doi: 10.1099/mgen.0.000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li JP, et al. Paeoniflorin reduce luxS/AI-2 system-controlled biofilm formation and virulence in Streptococcus suis. Virulence. 2021;12:3062–3073. doi: 10.1080/21505594.2021.2010398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin D, et al. The fructose-specific Phosphotransferase system of Klebsiella pneumoniae is regulated by global regulator CRP and linked to virulence and growth. Infect. Immun. 2018;86:e00340–00318. doi: 10.1128/IAI.00340-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Niu T, et al. Wza gene knockout decreases Acinetobacter baumannii virulence and affects Wzy-dependent capsular polysaccharide synthesis. Virulence. 2020;11:1–13. doi: 10.1080/21505594.2019.1700659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matysik A, Kline KA. Streptococcus pyogenes capsule promotes microcolony-independent biofilm formation. J. Bacteriol. 2019;201:e00052–19. doi: 10.1128/JB.00052-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fan Q, et al. Contribution of quorum sensing to virulence and antibiotic resistance in zoonotic bacteria. Biotechnol. Adv. 2022;59:107965. doi: 10.1016/j.biotechadv.2022.107965. [DOI] [PubMed] [Google Scholar]
- 74.Pang Z, et al. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019;37:177–192. doi: 10.1016/j.biotechadv.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 75.Hasman H, Chakraborty T, Klemm P. Antigen-43-mediated autoaggregation of Escherichia coli is blocked by fimbriation. J. Bacteriol. 1999;181:4834–4841. doi: 10.1128/JB.181.16.4834-4841.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schembri MA, Dalsgaard D, Klemm P. Capsule shields the function of short bacterial adhesins. J. Bacteriol. 2004;186:1249–1257. doi: 10.1128/JB.186.5.1249-1257.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Misawa Y, et al. Staphylococcus aureus colonization of the mouse gastrointestinal tract is modulated by wall teichoic acid, capsule, and surface proteins. PLoS Pathog. 2015;11:e1005061. doi: 10.1371/journal.ppat.1005061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zafar MA, et al. Capsule type and amount affect shedding and transmission of Streptococcus pneumoniae. mBio. 2017;8:e00989–17. doi: 10.1128/mBio.00989-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Valle J, et al. Broad-spectrum biofilm inhibition by a secreted bacterial polysaccharide. Proc. Natl Acad. Sci. USA. 2006;103:12558–12563. doi: 10.1073/pnas.0605399103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.TerAvest MA, et al. Regulated expression of polysaccharide utilization and capsular biosynthesis loci in biofilm and planktonic Bacteroides thetaiotaomicron during growth in chemostats. Biotechnol. Bioeng. 2014;111:165–173. doi: 10.1002/bit.24994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Béchon N, et al. Capsular polysaccharide cross-regulation modulates Bacteroides thetaiotaomicron Biofilm Formation. mBio. 2020;11:e00729–20. doi: 10.1128/mBio.00729-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pöhlmann-Dietze P, et al. Adherence of Staphylococcus aureus to endothelial cells: influence of capsular polysaccharide, global regulator agr, and bacterial growth phase. Infect. Immun. 2000;68:4865–4871. doi: 10.1128/IAI.68.9.4865-4871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ashbaugh CD, et al. Bacterial determinants of persistent throat colonization and the associated immune response in a primate model of human group A streptococcal pharyngeal infection. Cell Microbiol. 2000;2:283–292. doi: 10.1046/j.1462-5822.2000.00050.x. [DOI] [PubMed] [Google Scholar]
- 84.Flores AR, et al. Asymptomatic carriage of group A streptococcus is associated with elimination of capsule production. Infect. Immun. 2014;82:3958–3967. doi: 10.1128/IAI.01788-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.An H, et al. Functional vulnerability of liver macrophages to capsules defines virulence of blood-borne bacteria. J. Exp. Med. 2022;219:e20212032. doi: 10.1084/jem.20212032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Heesterbeek DAC, et al. Complement-dependent outer membrane perturbation sensitizes Gram-negative bacteria to Gram-positive specific antibiotics. Sci. Rep. 2019;9:3074. doi: 10.1038/s41598-019-38577-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hyams C, et al. Streptococcus pneumoniae capsular serotype invasiveness correlates with the degree of factor H binding and opsonization with C3b/iC3b. Infect. Immun. 2013;81:354–363. doi: 10.1128/IAI.00862-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Frank MM, Joiner K, Hammer C. The function of antibody and complement in the lysis of bacteria. Rev. Infect. Dis. 1987;9:S537–S545. doi: 10.1093/clinids/9.Supplement_5.S537. [DOI] [PubMed] [Google Scholar]
- 89.Cross AS. The biologic significance of bacterial encapsulation. Curr. Top. Microbiol. Immunol. 1990;150:87–95. doi: 10.1007/978-3-642-74694-9_5. [DOI] [PubMed] [Google Scholar]
- 90.Agrahari G, et al. Streptococcus pyogenes employs strain-dependent mechanisms of C3b inactivation to inhibit phagocytosis and killing of bacteria. J. Biol. Chem. 2016;291:9181–9189. doi: 10.1074/jbc.M115.704221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moses AE, et al. Relative contributions of hyaluronic acid capsule and M protein to virulence in a mucoid strain of the group A Streptococcus. Infect. Immun. 1997;65:64–71. doi: 10.1128/iai.65.1.64-71.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moorthy AN, et al. Capsules of virulent pneumococcal serotypes enhance formation of neutrophil extracellular traps during in vivo pathogenesis of pneumonia. Oncotarget. 2016;7:19327–19340. doi: 10.18632/oncotarget.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cress BF, et al. Masquerading microbial pathogens: capsular polysaccharides mimic host-tissue molecules. FEMS Microbiol Rev. 2014;38:660–697. doi: 10.1111/1574-6976.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wyatt EV, et al. Metabolic reprogramming of host cells by virulent Francisella tularensis for optimal replication and modulation of inflammation. J. Immunol. 2016;196:4227–4236. doi: 10.4049/jimmunol.1502456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rendueles O, et al. Genetic exchanges are more frequent in bacteria encoding capsules. PLoS Genet. 2018;14:e1007862. doi: 10.1371/journal.pgen.1007862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hu X, et al. Vi capsular polysaccharide: Synthesis, virulence, and application. Crit. Rev. Microbiol. 2017;43:440–452. doi: 10.1080/1040841X.2016.1249335. [DOI] [PubMed] [Google Scholar]
- 97.Geno KA, Hauser JR, Gupta K, Yother J. Streptococcus pneumoniae phosphotyrosine phosphatase CpsB and alterations in capsule production resulting from changes in oxygen availability. J. Bacteriol. 2014;196:1992–2003. doi: 10.1128/JB.01545-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Herbert S, et al. Regulation of Staphylococcus aureus capsular polysaccharide type 5: CO2 inhibition in vitro and in vivo. J. Infect. Dis. 1997;176:431–438. doi: 10.1086/514061. [DOI] [PubMed] [Google Scholar]
- 99.Herbert S, et al. Regulation of Staphylococcus aureus type 5 and type 8 capsular polysaccharides by CO(2) J. Bacteriol. 2001;183:4609–4613. doi: 10.1128/JB.183.15.4609-4613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morona JK, Miller DC, Morona R, Paton JC. The effect that mutations in the conserved capsular polysaccharide biosynthesis genes cpsA, cpsB, and cpsD have on virulence of Streptococcus pneumoniae. J. Infect. Dis. 2004;189:1905–1913. doi: 10.1086/383352. [DOI] [PubMed] [Google Scholar]
- 101.Lee JC, Takeda S, Livolsi PJ, Paoletti LC. Effects of in vitro and in vivo growth conditions on expression of type 8 capsular polysaccharide by Staphylococcus aureus. Infect. Immun. 1993;61:1853–1858. doi: 10.1128/iai.61.5.1853-1858.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tahoun A, et al. Capsular polysaccharide inhibits adhesion of Bifidobacterium longum 105-A to enterocyte-like Caco-2 cells and phagocytosis by macrophages. Gut Pathog. 2017;9:27. doi: 10.1186/s13099-017-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fan Y, et al. YbdO promotes the pathogenicity of Escherichia coli K1 by regulating capsule synthesis. Int J. Mol. Sci. 2022;23:5543. doi: 10.3390/ijms23105543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hathaway LJ, et al. Capsule type of Streptococcus pneumoniae determines growth phenotype. PLoS Pathog. 2012;8:e1002574. doi: 10.1371/journal.ppat.1002574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nucci A, Rocha EPC, Rendueles O. Adaptation to novel spatially-structured environments is driven by the capsule and alters virulence-associated traits. Nat. Commun. 2022;13:4751. doi: 10.1038/s41467-022-32504-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nasser W, et al. Bacterial discrimination by dictyostelid amoebae reveals the complexity of ancient interspecies interactions. Curr. Biol. 2013;23:862–872. doi: 10.1016/j.cub.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baker M, et al. Measuring and modelling the response of Klebsiella pneumoniae KPC prey to Bdellovibrio bacteriovorus predation, in human serum and defined buffer. Sci. Rep. 2017;7:8329. doi: 10.1038/s41598-017-08060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fu MS, et al. Amoeba predation of Cryptococcus neoformans results in Pleiotropic changes to traits associated with virulence. mBio. 2021;12:e00567–21. doi: 10.1128/mBio.00567-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Miskinyte M, et al. The genetic basis of Escherichia coli pathoadaptation to macrophages. PLoS Pathog. 2013;9:e1003802. doi: 10.1371/journal.ppat.1003802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nair RR, et al. Bacterial predator-prey coevolution accelerates genome evolution and selects on virulence-associated prey defences. Nat. Commun. 2019;10:4301. doi: 10.1038/s41467-019-12140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mike LA, et al. A systematic analysis of hypermucoviscosity and capsule reveals distinct and overlapping genes that impact Klebsiella pneumoniae fitness. PLoS Pathog. 2021;17:e1009376. doi: 10.1371/journal.ppat.1009376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rendueles O. Deciphering the role of the capsule of Klebsiella pneumoniae during pathogenesis: A cautionary tale. Mol. Microbiol. 2020;113:883–888. doi: 10.1111/mmi.14474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Haudiquet M, Buffet A, Rendueles O, Rocha EPC. Interplay between the cell envelope and mobile genetic elements shapes gene flow in populations of the nosocomial pathogen Klebsiella pneumoniae. PLoS Biol. 2021;19:e3001276. doi: 10.1371/journal.pbio.3001276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Holt KE, et al. Diversity and evolution of surface polysaccharide synthesis loci in Enterobacteriales. ISME J. 2020;14:1713–1730. doi: 10.1038/s41396-020-0628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Queller DC. A general model for kin selection. Int. J. Org. Evol. 1992;46:376–380. doi: 10.2307/2409858. [DOI] [PubMed] [Google Scholar]
- 116.Saxer G, Doebeli M, Travisano M. Spatial structure leads to ecological breakdown and loss of diversity. Proc. Biol. Sci. 2009;276:2065–2070. doi: 10.1098/rspb.2008.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim, K. S. Human Meningitis-associated Escherichia coli. EcoSal Plus7, 10.1128/ecosalplus.ESP-0015-2015 (2016). [DOI] [PMC free article] [PubMed]
- 118.Doran KS, et al. Host-pathogen interactions in bacterial meningitis. Acta Neuropathol. 2016;131:185–209. doi: 10.1007/s00401-015-1531-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Das A, et al. Differential role of cytosolic phospholipase A2 in the invasion of brain microvascular endothelial cells by Escherichia coli and Listeria monocytogenes. J. Infect. Dis. 2001;184:732–737. doi: 10.1086/322986. [DOI] [PubMed] [Google Scholar]
- 120.Tenenbaum T, et al. Polar bacterial invasion and translocation of Streptococcus suis across the blood-cerebrospinal fluid barrier in vitro. Cell Microbiol. 2009;11:323–336. doi: 10.1111/j.1462-5822.2008.01255.x. [DOI] [PubMed] [Google Scholar]
- 121.Gutiérrez-Jiménez C, et al. Neutrophils as Trojan horse vehicles for Brucella abortus macrophage infection. Front Immunol. 2019;10:1012. doi: 10.3389/fimmu.2019.01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hsueh PT, et al. Burkholderia pseudomallei-loaded cells act as a Trojan horse to invade the brain during endotoxemia. Sci. Rep. 2018;8:13632. doi: 10.1038/s41598-018-31778-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gendrin C, et al. Diminished capsule exacerbates virulence, blood-brain barrier penetration, intracellular persistence, and antibiotic evasion of Hyperhemolytic Group B Streptococci. J. Infect. Dis. 2018;217:1128–1138. doi: 10.1093/infdis/jix684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Geisinger E, Isberg RR. Antibiotic modulation of capsular exopolysaccharide and virulence in Acinetobacter baumannii. PLoS Pathog. 2015;11:e1004691. doi: 10.1371/journal.ppat.1004691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fan Z, et al. Glucose induces resistance to polymyxins in high-alcohol-producing Klebsiella pneumoniae via increasing capsular polysaccharide and maintaining intracellular ATP. Microbiol Spectr. 2023;11:e0003123. doi: 10.1128/spectrum.00031-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sabnis A, Ledger EVK, Pader V, Edwards AM. Antibiotic interceptors: Creating safe spaces for bacteria. PLoS Pathog. 2018;14:e1006924. doi: 10.1371/journal.ppat.1006924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Llobet E, Tomás JM, Bengoechea JA. Capsule polysaccharide is a bacterial decoy for antimicrobial peptides. Microbiology. 2008;154:3877–3886. doi: 10.1099/mic.0.2008/022301-0. [DOI] [PubMed] [Google Scholar]
- 128.D’Angelo F, Rocha EPC, Rendueles O. The capsule increases susceptibility to last-resort polymyxins, but not to other antibiotics, in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2023;67:e0012723. doi: 10.1128/aac.00127-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen CH, Lu TK. Development and challenges of antimicrobial peptides for therapeutic applications. Antibiotics. 2020;9:24. doi: 10.3390/antibiotics9010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Groisman EA. Feedback control of two-component regulatory systems. Annu. Rev. Microbiol. 2016;70:103–124. doi: 10.1146/annurev-micro-102215-095331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jacob-Dubuisson F, Mechaly A, Betton JM, Antoine R. Structural insights into the signalling mechanisms of two-component systems. Nat. Rev. Microbiol. 2018;16:585–593. doi: 10.1038/s41579-018-0055-7. [DOI] [PubMed] [Google Scholar]
- 132.Tzeng YL, Thomas J, Stephens DS. Regulation of capsule in Neisseria meningitidis. Crit. Rev. Microbiol. 2016;42:759–772. doi: 10.3109/1040841X.2015.1022507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Levin JC, Wessels MR. Identification of csrR/csrS, a genetic locus that regulates hyaluronic acid capsule synthesis in group A Streptococcus. Mol. Microbiol. 1998;30:209–219. doi: 10.1046/j.1365-2958.1998.01057.x. [DOI] [PubMed] [Google Scholar]
- 134.Matanza XM, Lopez-Suarez L, do Vale A, Osorio CR. The two-component system RstAB regulates production of a polysaccharide capsule with a role in virulence in the marine pathogen Photobacterium damselae subsp. damselae. Environ. Microbiol. 2021;23:4859–4880. doi: 10.1111/1462-2920.15731. [DOI] [PubMed] [Google Scholar]
- 135.Crosby HA, et al. The Staphylococcus aureus ArlRS two-component system regulates virulence factor expression through MgrA. Mol. Microbiol. 2020;113:103–122. doi: 10.1111/mmi.14404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bai J, et al. The role of ArlRS in regulating oxacillin susceptibility in methicillin-resistant Staphylococcus aureus indicates it is a potential target for antimicrobial resistance breakers. Emerg. microbes Infect. 2019;8:503–515. doi: 10.1080/22221751.2019.1595984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jousselin A, et al. The Staphylococcus aureus thiol/oxidative stress global regulator Spx controls trfA, a gene implicated in cell wall antibiotic resistance. Antimicrob. Agents Chemother. 2013;57:3283–3292. doi: 10.1128/AAC.00220-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Azimi S, Klementiev AD, Whiteley M, Diggle SP. Bacterial Quorum sensing during infection. Annu Rev. Microbiol. 2020;74:201–219. doi: 10.1146/annurev-micro-032020-093845. [DOI] [PubMed] [Google Scholar]
- 139.Yi L, et al. Establishment of Streptococcus suis biofilm infection model in vivo and comparative analysis of gene expression profiles between in vivo and in vitro biofilms. Microbiol Spectr. 2023;11:e0268622. doi: 10.1128/spectrum.02686-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ng CK, How KY, Tee KK, Chan KG. Characterization and Transcriptome studies of autoinducer synthase gene from multidrug resistant acinetobacter baumannii Strain 863. Genes (Basel) 2019;10:282. doi: 10.3390/genes10040282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shah N, et al. Investigation of the quorum-sensing regulon of the biocontrol bacterium Pseudomonas chlororaphis strain PA23. PLoS One. 2020;15:e0226232. doi: 10.1371/journal.pone.0226232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Trappetti C, et al. Autoinducer 2 signaling via the Phosphotransferase FruA drives Galactose utilization by Streptococcus pneumoniae, resulting in Hypervirulence. mBio. 2017;8:e02269–02216. doi: 10.1128/mBio.02269-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fleuchot B, et al. Rgg proteins associated with internalized small hydrophobic peptides: a new quorum-sensing mechanism in streptococci. Mol. Microbiol. 2011;80:1102–1119. doi: 10.1111/j.1365-2958.2011.07633.x. [DOI] [PubMed] [Google Scholar]
- 144.Cook LC, Federle MJ. Peptide pheromone signaling in Streptococcus and Enterococcus. FEMS Microbiol. Rev. 2014;38:473–492. doi: 10.1111/1574-6976.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shlla B, et al. The Rgg1518 transcriptional regulator is a necessary facet of sugar metabolism and virulence in Streptococcus pneumoniae. Mol. Microbiol. 2021;116:996–1008. doi: 10.1111/mmi.14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Junges R, et al. A Quorum-sensing system that regulates Streptococcus pneumoniae biofilm formation and surface polysaccharide production. mSphere. 2017;2:e00324–17. doi: 10.1128/mSphere.00324-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Brimacombe CA, et al. Quorum-sensing regulation of a capsular polysaccharide receptor for the Rhodobacter capsulatus gene transfer agent (RcGTA) Mol. Microbiol. 2013;87:802–817. doi: 10.1111/mmi.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cumming BM, Steyn AJ. Metabolic plasticity of central carbon metabolism protects mycobacteria. Proc. Natl Acad. Sci. USA. 2015;112:13135–13136. doi: 10.1073/pnas.1518171112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Echlin H, et al. Pyruvate oxidase as a critical link between metabolism and capsule biosynthesis in streptococcus pneumoniae. PLoS Pathog. 2016;12:e1005951. doi: 10.1371/journal.ppat.1005951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang X, Bayles KW, Luca S. Staphylococcus aureus CidC Is a Pyruvate:Menaquinone Oxidoreductase. Biochemistry. 2017;56:4819–4829. doi: 10.1021/acs.biochem.7b00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tapiainen T, et al. Ultrastructure of Streptococcus pneumoniae after exposure to xylitol. J. Antimicrob. Chemother. 2004;54:225–228. doi: 10.1093/jac/dkh302. [DOI] [PubMed] [Google Scholar]
- 152.Seidl K, et al. Staphylococcus aureus CcpA affects virulence determinant production and antibiotic resistance. Antimicrob. Agents Chemother. 2006;50:1183–1194. doi: 10.1128/AAC.50.4.1183-1194.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Trzciński K, et al. Effect of Serotype on Pneumococcal competition in a mouse colonization model. mBio. 2015;6:e00902–e00915. doi: 10.1128/mBio.00902-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schipper K, et al. Meningococcal virulence in zebrafish embryos depends on capsule polysaccharide structure. Front Cell Infect. Microbiol. 2022;12:1020201. doi: 10.3389/fcimb.2022.1020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Heneberg P. Finding the smoking gun: protein tyrosine phosphatases as tools and targets of unicellular microorganisms and viruses. Curr. Med. Chem. 2012;19:1530–1566. doi: 10.2174/092986712799828274. [DOI] [PubMed] [Google Scholar]
- 156.Whitmore SE, Lamont RJ. Tyrosine phosphorylation and bacterial virulence. Int. J. oral. Sci. 2012;4:1–6. doi: 10.1038/ijos.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Duclos B, et al. Autophosphorylation of a bacterial protein at tyrosine. J. Mol. Biol. 1996;259:891–895. doi: 10.1006/jmbi.1996.0366. [DOI] [PubMed] [Google Scholar]
- 158.Kawai Y, et al. A widespread family of bacterial cell wall assembly proteins. EMBO J. 2011;30:4931–4941. doi: 10.1038/emboj.2011.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Su T, et al. Decoding capsule synthesis in Streptococcus pneumoniae. FEMS Microbiol. Rev. 2021;45:fuaa067. doi: 10.1093/femsre/fuaa067. [DOI] [PubMed] [Google Scholar]
- 160.Paton, J. C. & Trappetti, C. Streptococcus pneumoniae Capsular Polysaccharide. Microbiol Spectr. 7, 10.1128/microbiolspec.GPP3-0019-2018 (2019). [DOI] [PMC free article] [PubMed]
- 161.Morona JK, Paton JC, Miller DC, Morona R. Tyrosine phosphorylation of CpsD negatively regulates capsular polysaccharide biosynthesis in streptococcus pneumoniae. Mol. Microbiol. 2000;35:1431–1442. doi: 10.1046/j.1365-2958.2000.01808.x. [DOI] [PubMed] [Google Scholar]
- 162.Nourikyan J, et al. Autophosphorylation of the bacterial Tyrosine-Kinase CpsD connects capsule synthesis with the cell cycle in Streptococcus pneumoniae. PLoS Genet. 2015;11:e1005518. doi: 10.1371/journal.pgen.1005518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tang J, et al. A link between STK signalling and capsular polysaccharide synthesis in Streptococcus suis. Nat. Commun. 2023;14:2480. doi: 10.1038/s41467-023-38210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Matange N. Revisiting bacterial cyclic nucleotide phosphodiesterases: cyclic AMP hydrolysis and beyond. FEMS Microbiol. Lett. 2015;362:fnv183. doi: 10.1093/femsle/fnv183. [DOI] [PubMed] [Google Scholar]
- 165.Lin CT, et al. Role of the cAMP-dependent carbon catabolite repression in capsular polysaccharide biosynthesis in Klebsiella pneumoniae. PLoS One. 2013;8:e54430. doi: 10.1371/journal.pone.0054430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Trimble MJ, McCarter LL. Bis-(3′-5′)-cyclic dimeric GMP-linked quorum sensing controls swarming in Vibrio parahaemolyticus. Proc. Natl Acad. Sci. 2011;108:18079–18084. doi: 10.1073/pnas.1113790108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wu Z, et al. The Streptococcus suis transcriptional landscape reveals adaptation mechanisms in pig blood and cerebrospinal fluid. RNA. 2014;20:882–898. doi: 10.1261/rna.041822.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Griggs DW, Konisky J. Mechanism for iron-regulated transcription of the Escherichia coli cir gene: metal-dependent binding of fur protein to the promoters. J. Bacteriol. 1989;171:1048–1054. doi: 10.1128/jb.171.2.1048-1054.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lin CT, et al. Fur regulation of the capsular polysaccharide biosynthesis and iron-acquisition systems in Klebsiella pneumoniae CG43. Microbiology. 2011;157:419–429. doi: 10.1099/mic.0.044065-0. [DOI] [PubMed] [Google Scholar]
- 170.Huang SH, et al. Role of the small RNA RyhB in the Fur regulon in mediating the capsular polysaccharide biosynthesis and iron acquisition systems in Klebsiella pneumoniae. BMC Microbiol. 2012;12:148. doi: 10.1186/1471-2180-12-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Giel, J. L. et al. Regulation of iron-sulphur cluster homeostasis through transcriptional control of the Isc pathway by [2Fe-2S]-IscR in Escherichia coli. Mol Microbiol.87 478–492 (2013). [DOI] [PMC free article] [PubMed]
- 172.Yeo WS, Lee JH, Lee KC, Roe JH. IscR acts as an activator in response to oxidative stress for the suf operon encoding Fe-S assembly proteins. Mol. Microbiol. 2006;61:206–218. doi: 10.1111/j.1365-2958.2006.05220.x. [DOI] [PubMed] [Google Scholar]
- 173.Rajagopalan S, et al. Studies of IscR reveal a unique mechanism for metal-dependent regulation of DNA binding specificity. Nat. Struct. Mol. Biol. 2013;20:740–747. doi: 10.1038/nsmb.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wu CC, et al. IscR regulation of capsular polysaccharide biosynthesis and iron-acquisition systems in Klebsiella pneumoniae CG43. PLoS One. 2014;9:e107812. doi: 10.1371/journal.pone.0107812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hsieh SA, Donermeyer DL, Horvath SC, Allen PM. Phase-variable bacteria simultaneously express multiple capsules. Microbiology. 2021;167:001066. doi: 10.1099/mic.0.001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Brézillon C, et al. Capsules, toxins and AtxA as virulence factors of emerging Bacillus cereus biovar anthracis. PLoS Neglect. Tropic. Dis. 2015;9:e0003455. doi: 10.1371/journal.pntd.0003455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Biran D, Rosenshine I, Ron EZ. Escherichia coli O-antigen capsule (group 4) is essential for serum resistance. Res. Microbiol. 2020;171:99–101. doi: 10.1016/j.resmic.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 178.Biran D, et al. Surviving Serum: the Escherichia coli iss gene of Extraintestinal Pathogenic E. coli is required for the synthesis of Group 4 Capsule. Infect. Immun. 2021;89:e0031621. doi: 10.1128/IAI.00316-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang MC, et al. Role of K1 capsule antigen in cirrhotic patients with Escherichia coli spontaneous bacterial peritonitis in southern Taiwan. Eur. J. Clin. Microbiol. Infect. Dis. 2013;32:407–412. doi: 10.1007/s10096-012-1757-9. [DOI] [PubMed] [Google Scholar]
- 180.Buckles EL, et al. Role of the K2 capsule in Escherichia coli urinary tract infection and serum resistance. J. Infect. Dis. 2009;199:1689–1697. doi: 10.1086/598524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Heimesaat MM, et al. Disruption of Escherichia coli Nissle 1917 K5 Capsule Biosynthesis, through loss of distinct kfi genes, Modulates interaction with intestinal epithelial cells and impact on cell health. Plos One. 2015;10:e0120430. doi: 10.1371/journal.pone.0120430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Alamu J, Kakithakara Vajravelu L, Venkatesan B, Thulukanam J. Correlation of phenotypic and genotypic virulence markers, antimicrobial susceptibility pattern, and outcome of Pseudomonas aeruginosa sepsis infection. Micro. Pathog. 2022;170:105716. doi: 10.1016/j.micpath.2022.105716. [DOI] [PubMed] [Google Scholar]
- 183.Short FL, et al. Genomic profiling reveals distinct routes to complement resistance in Klebsiella pneumoniae. Infect. Immun. 2020;88:e00043–20. doi: 10.1128/IAI.00043-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Huang X, et al. Capsule type defines the capability of Klebsiella pneumoniae in evading Kupffer cell capture in the liver. PLoS Pathog. 2022;18:e1010693. doi: 10.1371/journal.ppat.1010693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ku YH, et al. Klebsiella pneumoniae Isolates from Meningitis: Epidemiology, virulence and antibiotic resistance. Sci. Rep. 2017;7:6634. doi: 10.1038/s41598-017-06878-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tan YH, et al. Cell envelope defects of different capsule-null mutants in K1 hypervirulent Klebsiella pneumoniae can affect bacterial pathogenesis. Mol. Microbiol. 2020;113:889–905. doi: 10.1111/mmi.14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ernst CM, et al. Adaptive evolution of virulence and persistence in carbapenem-resistant Klebsiella pneumoniae. Nat. Med. 2020;26:705–711. doi: 10.1038/s41591-020-0825-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hupp S, et al. Pneumolysin and the bacterial capsule of Streptococcus pneumoniae cooperatively inhibit taxis and motility of microglia. J. Neuroinflamm. 2019;16:105. doi: 10.1186/s12974-019-1491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wartha F, et al. Capsule and D-alanylated lipoteichoic acids protect Streptococcus pneumoniae against neutrophil extracellular traps. Cell Microbiol. 2007;9:1162–1171. doi: 10.1111/j.1462-5822.2006.00857.x. [DOI] [PubMed] [Google Scholar]
- 190.Shainheit MG, Mulé M, Camilli A. The core promoter of the capsule operon of Streptococcus pneumoniae is necessary for colonization and invasive disease. Infect. Immun. 2014;82:694–705. doi: 10.1128/IAI.01289-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Henriet SSV, et al. Endocarditis caused by Nontypeable Streptococcus pneumoniae. Clin. Infect. Dis. 2022;75:719–722. doi: 10.1093/cid/ciac079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hyams C, et al. The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect. Immun. 2010;78:704–715. doi: 10.1128/IAI.00881-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Brissac T, et al. Capsule promotes intracellular survival and vascular endothelial cell translocation during invasive pneumococcal disease. mBio. 2021;12:e0251621. doi: 10.1128/mBio.02516-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hathaway LJ, et al. Streptococcus pneumoniae capsule determines disease severity in experimental pneumococcal meningitis. Open Biol. 2016;6:150269. doi: 10.1098/rsob.150269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Porat N, et al. The important role of nontypable Streptococcus pneumoniae international clones in acute conjunctivitis. J. Infect. Dis. 2006;194:689–696. doi: 10.1086/506453. [DOI] [PubMed] [Google Scholar]
- 196.Sanders ME, et al. The Streptococcus pneumoniae capsule is required for full virulence in pneumococcal endophthalmitis. Investig. Ophthalmol. Vis. Sci. 2011;52:865–872. doi: 10.1167/iovs.10-5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rodrigues F, et al. Multiple Streptococcus pneumoniae serotypes in aural discharge samples from children with acute otitis media with spontaneous otorrhea. J. Clin. Microbiol. 2013;51:3409–3411. doi: 10.1128/JCM.01303-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Suligoy CM, et al. Acapsular Staphylococcus aureus with a non-functional agr regains capsule expression after passage through the bloodstream in a bacteremia mouse model. Sci. Rep. 2020;10:14108. doi: 10.1038/s41598-020-70671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Key FM, et al. On-person adaptive evolution of Staphylococcus aureus during treatment for atopic dermatitis. Cell Host Microbe. 2023;31:593–603.e597. doi: 10.1016/j.chom.2023.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hurst JR, et al. The Streptococcus pyogenes hyaluronic acid capsule promotes experimental nasal and skin infection by preventing neutrophil-mediated clearance. PLoS Pathog. 2022;18:e1011013. doi: 10.1371/journal.ppat.1011013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Iida K, et al. Capsule of Streptococcus pyogenes is essential for delayed death of mice in a model of streptococcal toxic shock syndrome. Microbiol Immunol. 2006;50:127–130. doi: 10.1111/j.1348-0421.2006.tb03777.x. [DOI] [PubMed] [Google Scholar]
- 202.Seitz M, et al. Role of capsule and suilysin in mucosal infection of complement-deficient mice with Streptococcus suis. Infect. Immun. 2014;82:2460–2471. doi: 10.1128/IAI.00080-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Segura M, Fittipaldi N, Calzas C, Gottschalk M. Critical streptococcus suis virulence factors: are they all really critical? Trends Microbiol. 2017;25:585–599. doi: 10.1016/j.tim.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 204.Roy D, et al. Role of the capsular polysaccharide as a virulence factor for Streptococcus suis serotype 14. Can. J. Vet. Res. 2015;79:141–146. [PMC free article] [PubMed] [Google Scholar]
- 205.Mayer L, et al. Survival patterns of Streptococcus suis serotypes 1 and 14 in porcine blood indicate cross-reactive bactericidal antibodies in naturally infected pigs. Vet. Microbiol. 2021;260:109183. doi: 10.1016/j.vetmic.2021.109183. [DOI] [PubMed] [Google Scholar]
- 206.Goossens PL, Tournier JN. Crossing of the epithelial barriers by Bacillus anthracis: the Known and the Unknown. Front. Microbiol. 2015;6:1122. doi: 10.3389/fmicb.2015.01122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Guan L, Xue Y, Ding W, Zhao Z. Biosynthesis and regulation mechanisms of the Pasteurella multocida capsule. Res Vet. Sci. 2019;127:82–90. doi: 10.1016/j.rvsc.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 208.Corsaro MM, et al. 1H and 13C NMR characterization and secondary structure of the K2 polysaccharide of Klebsiella pneumoniae strain 52145. Carbohydr. Res. 2005;340:2212–2217. doi: 10.1016/j.carres.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 209.Ovodov YS. Bacterial capsular antigens. Structural patterns of capsular antigens. Biochem. Biokhimiia. 2006;71:937–954. doi: 10.1134/S000629790609001X. [DOI] [PubMed] [Google Scholar]
- 210.Jones C. Revised structures for the capsular polysaccharides from Staphylococcus aureus Types 5 and 8, components of novel glycoconjugate vaccines. Carbohydr. Res. 2005;340:1097–1106. doi: 10.1016/j.carres.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 211.Van Calsteren MR, et al. Structure determination of Streptococcus suis serotype 2 capsular polysaccharide. Biochem Cell Biol. 2010;88:513–525. doi: 10.1139/O09-170. [DOI] [PubMed] [Google Scholar]