Abstract
Reference conditions are difficult to find in the Anthropocene but essential for effective biodiversity conservation. Aquatic ecosystems in the Andes‐Amazon transition zone of Colombia are now at high risk due to expanded human activities after peace agreements in 2016 ended armed conflict because lands formerly controlled by FARC and other armed groups are now prone to agricultural and urban expansion. Particularly, expanding human land use may reduce fish diversity across the altitudinal gradient, especially in the premontane streams (i.e., <500 m a.s.l.) because lands are more amenable to human use than at greater altitudes. We evaluated fish α‐diversity (measured as species richness, total abundance, and effective species number) and β‐diversity (spatial and temporal) in 12 sites over 8 years bracketing the end of armed conflict. All α‐diversity and β‐diversity analyses were evaluated relative to categorical altitude (< or >500 m) and continuous altitude. Strong differences in fish community structure among sites occurred as a function of altitude. Fish communities exhibit altitudinal biodiversity gradients that are consistent in space and time, and that need to be accounted for conservation and management considerations. Our results provide a reference to identify short‐ and long‐term changes due to impending human land use at a critical moment for the conservation of tropical fish diversity. Similar studies in other areas of the upper Amazon Basin are needed to evaluate effects of subsequent human activities on diversity patterns and our study area to compare to reference conditions reported here.
Keywords: abundance, altitudinal gradient, Amazon piedmont, beta diversity, effective diversity, land use, multivariate analysis, species richness
Aquatic ecosystems in the Andes‐Amazon transition zone of Colombia are now at high risk due to expanded human activities after peace agreements in 2016 ended armed conflict. Fish communities exhibit altitudinal biodiversity gradients that are consistent in space and time, and that need to be accounted for conservation and management considerations. Our results provide a baseline to identify short‐term and long‐term changes due to impending human land use at a critical moment for the conservation of tropical fish diversity.
1. INTRODUCTION
Increased human activities have already transformed and degraded many ecosystems worldwide due to urbanization, agriculture, and extraction of natural resources (Achiso, 2020; Feng et al., 2022; IPBES, 2019). As a result, diversity is often reduced, measured as a decrease in both species richness and relative abundance (Newbold et al., 2015), and it is difficult to understand reference conditions before human impacts occurred. However, some areas are more affected by humans than others, where differences may be related to human access and landscape suitability for human uses (e.g., agriculture, urban expansion). Among regions undergoing anthropogenic land use change, the Amazon is known for its remarkable biodiversity and endemics (Mittermeier et al., 2003). Amazonian lowlands contain the largest biodiversity in the world (Gentry, 1988; Wilson, 1992), including both terrestrial and aquatic faunas (Myers et al., 2000; Reis et al., 2003). Amazonia hosts about 17% and 10% of all known vascular plants and vertebrate species, respectively (Lundberg et al., 2000; Myers et al., 2000). Freshwater aquatic ecosystems of the Amazon Basin are megadiverse (Myers et al., 2000) and host the most diverse ichthyofauna in the world (Lundberg, 2001; Reis et al., 2003). In highly diverse systems such as the Amazon Basin, many species are relatively rare and occupy specific niches accordingly to their morphological and physiological traits (Hercos et al., 2013). In addition, most species are not evenly distributed, whether measured in presence/absence or in abundance (Bell, 2005; Magurran, 2004; Magurran & Henderson, 2003).
The high freshwater diversity of the Amazon provides crucial ecosystem services, especially fish as food that often represents the sole source of animal protein and financial income for the human population in the region (Agudelo et al., 2006; Agudelo Córdoba et al., 2011). Within Colombia, Amazonian aquatic ecosystems are now at high risk due to expanded human activities, especially expanding agriculture after the establishment of the peace agreements to end armed conflict in 2016 (Tellez, 2019). This scenario sets up a critical moment for the establishment of management and conservation policies (Agudelo Hz et al., 2023; Clerici et al., 2019) to avoid negative conservation outcomes (Feng et al., 2022).
Much of the Amazonian basin is lowland, but not everywhere. The Colombian Amazon includes the Andean‐Amazon transition, where natural ecosystems of basimontane altitudes (i.e., 500–1700 m a.s.l.) are less affected by human activities than those in the premontane zone (i.e., <500 m a.s.l.) because the steep basimontane landscape complicates human activities whereas flat and smooth premontane terrain enables most human activities, despite infertile soils (Galvis et al., 2007).
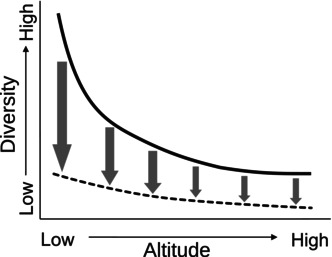
Altitudinal gradients in diversity are well‐recognized worldwide (Keller et al., 2013; Lomolino et al., 2010), where diversity is generally expected to decrease with altitude because more stringent environmental conditions occur at greater altitude (Figure 1; Heegaard & Vandvik, 2009; De La Barra et al., 2016). However, a hump‐shaped diversity ~ altitude pattern is common (Fischer et al., 2011). Environmental conditions along altitudinal gradients are particular to specific locations (Körner, 2007) and the combined effects of these conditions produce diversity patterns (Dyer et al., 2007; Schemske et al., 2009). Different selective conditions across the altitudinal gradient provide different selective conditions, leading to different diversities. For example, basimontane fishes more often have morphological adaptions for attachment to surfaces and behaviors and morphologies to reduce flow forces acting on the fish body (Maldonado‐Ocampo et al., 2005). In comparison, lowland species are more often adapted for less flow forces and instead develop physiological adaptations for warmer temperatures, lower dissolved oxygen, and different pH and conductivity (Bogotá‐Gregory et al., 2020; Saint‐Paul et al., 2000). Therefore, diversity patterns are defined via selection for associated physiological and morphological traits, where the natural conditions act as an environmental filter (Chase & Leibold, 2003). On the other hand, for some taxa, altitudinal patterns might be the result of random mechanisms, which can be described by the mid‐domain effect (Colwell & Hurtt, 1994; Colwell & Lees, 2000).
FIGURE 1.

Hypothetical effects of potential land use on fish α‐diversity across elevation gradients. The solid curve represents a decay of diversity with altitude. The lower dashed curve represents potential diversity after natural lands are converted to agriculture and other human uses, where greatest diversity loss (arrows) occurs at lower altitude.
The Andes‐Amazon transition zone provides a double opportunity to evaluate natural diversity patterns across a strong altitudinal gradient and to evaluate reference conditions for differential and impending anthropogenic effects. The natural altitudinal diversity gradient should interact with spatially biased anthropogenic effects of land use to further modify diversity patterns (Figure 1), where diversity reduction may not be uniform across altitudes (Penjor et al., 2022). Anthropogenic effects may be local and point source (e.g., mining; Rehmana et al., 2024), but more generally extensive, nonpoint source anthropogenic effects occur, related to land use (e.g., agriculture, urban systems). Land use change is often greatest and earliest in lowlands due to simple economics of access and labor (Shively, 2001). Well‐known effects include habitat loss and fragmentation on lands (Adhikari & Hansen, 2018; Plieninger, 2006) and nonpoint source pollution (e.g., sediment loading, nutrient runoff) in streams and rivers (Ikeda et al., 2009; Martinelli et al., 1989). This bias of human activities among terrains seems general, given it is repeated in other landscapes (e.g., Bürgi et al., 2017; Mclain et al., 2013).
Expanding human land use in the Colombian Andes‐Amazon transition zone may reduce diversity but is unlikely to cause biological homogenization between altitudinal levels because the altitudinal gradient is selective (e.g., fishes adapted to low‐slope streams may not move into high‐slope conditions). Instead, premontane streams should become more similar to each other in species richness and abundance, though with different suites of species in lowlands than in uplands (Figure 1). For practical reasons, premontane land use may also be expected to precede that in basimontane regions, with matching timing for diversity effects.
Here we evaluate fish α‐diversity (local species richness, abundance, and effective species number) and β‐diversity (spatial and temporal) patterns in basimontane and premontane streams of the Caquetá River basin in the Colombian Andes‐Amazon transition zone, using data from samples collected between 2013 and 2022 in 12 localities. We expected two general outcomes. First, a greater fish α‐diversity and β‐diversity in premontane streams than in basimontane streams, corresponding to an altitudinal gradient. Second, no great change pre‐ and post‐2016 in patterns because land use had not yet accelerated, because this represents a “transition” period.
Our data represent barely impacted conditions soon after the conflict ended and before the development of formerly avoided lands due to strongholds of FARC and other armed groups (Agudelo Hz et al., 2023; Calle‐Rendón et al., 2018), as well as the COVID‐19 pandemic. Results are especially relevant considering the importance of Andean‐Amazonian connectivity (Anderson et al., 2018; Clerici et al., 2019; Melack & Fosberg, 2001) and the high rates of endemism that characterize the area (Tognelli et al., 2016). Our study sets a baseline for evaluating future changes in Andean‐Amazonian biodiversity while accounting for biodiversity patterns that are fundamental for the elaboration of conservation and management planning. This is particularly relevant in light of environmental degradation, especially under the climate change context.
2. METHODS
2.1. Study area
The Caquetá River basin is a western Amazonian affluent of Andean origin, formed at 3850 m above sea level (a.s.l.) by the confluence of three different minor tributaries in the Peñas Blancas Páramo, located in the East Mountain Chain in the Southeast Region in Colombia (IGAC, 1996). It runs over 1200 km in a southeast direction before it merges to the main channel of the Amazon River, crossing the Caquetá, Putumayo, and Amazonas departments in Colombia, until it is named the Rio Japurá at the Colombian‐Brazilian border (IGAC, 1996, 1999).
The upper section of the Caquetá River drains most of the western uplift of the Guyana Shield, a formation from the Miocene characterized by a crystalline basement. Shields are very evolved soils with low nutrient and organic contents. Above 500 m a.s.l. (Figure 2a), the aquatic systems of the Caquetá́ basin are typically Andean ecosystems with dominant rocky substrates, abrupt slopes, and high flow (Figure 2b). Below the 500 m a.s.l., denoted as Amazonian Piedmont, aquatic ecosystems are characterized by basement alluvial fans (Figure 2b) of volcanic origin with elements from the Andes Mountain chain (Galvis et al., 2007; Hoorn, 1994).
FIGURE 2.

(a) Geographical location of the sample sites. (b) Examples of the aquatic ecosystems within the altitudinal levels, which are the ones recognized by van der Hammen and dos Santos (1995).
2.2. Data collection in situ
The sample sites are located in the Caquetá Department in the municipalities of Belén de los Andaquíes, Florencia, and Morelia, between 200 and 1500 m a.s.l. (Figure 2, Table 1). Sample sites were selected based on location within the basimontane (i.e., >500 m a.s.l.) and premontane levels (i.e., <500 m a.s.l.) and access permission. Sampling occurred during a “transition period” when dialog toward peace agreements was occurring, enabling sites to be accessible. Twelve sites (four sites at the basimontane level and eight at the premontane level) were sampled during the falling water season in each of eight years (2013, 2015–2019, 2021, and 2022). However, three sites were not sampled in 2019 due to access restrictions because of weather conditions. In total, data represent 91 sample events.
TABLE 1.
Sample localities, geographical coordinates, and altitude (m a.s.l.)
| Sites | Locality | Latitude | Longitude | Altitude |
|---|---|---|---|---|
| S1 | La Portada Stream | 1.8139 | −75.6591 | 1253 |
| S2 | Sucre River | 1.7953 | −75.6465 | 1013 |
| S3 | Paraíso River | 1.7472 | −75.6283 | 676 |
| S4 | Las Doradas Stream | 1.7289 | −75.6464 | 530 |
| S5 | La Sardina Stream | 1.6797 | −75.6225 | 386 |
| S6 | La Carbona Stream | 1.7064 | −75.6253 | 439 |
| S7 | La Yuca Stream | 1.6078 | −75.6383 | 277 |
| S8 | La Mochilero Stream | 1.5508 | −75.6756 | 285 |
| S9 | Aguas Calientes Stream | 1.4758 | −75.7694 | 286 |
| S10 | La Chocho Stream | 1.4472 | −75.8117 | 316 |
| S11 | La Arenosa Stream | 1.4644 | −75.8633 | 360 |
| S12 | Bodoquerito River | 1.4971 | −75.8741 | 382 |
Fish were sampled using SAMUS725M electrofishing equipment, making multiples passes along a 100 m stretch in each of the sample sites. This sampling methodology follows the standardized sampling technique of Maldonado‐Ocampo et al. (2005) for subsequent data comparisons. Fish were euthanized with clove oil and fixed in a 10% formaldehyde solution. Prior to species taxonomic identifications, specimen vouchers were transferred for preservation in a 75% ethanol solution. All the fish specimens were deposited in the Ichthyological Collection of the Colombian Amazon‐CIACOL at the SINCHI Amazonian Scientific Research Institute in Leticia, Amazonas, Colombia (https://sinchi.org.co/ciacol).
2.3. Data analysis
We evaluated the efficiency of our overall sampling effort for basimontane and premontane levels using rarefaction curves and the Chao1 species estimator for data pooled across sample years (Chao, 1984). Extrapolating a species accumulation curve to its asymptote and estimating species richness via rarefaction and the Chao 1, respectively, both provide an estimate of the performance of the proposed sampling method (Chao et al., 2009; Magurran, 2004).
We evaluated diversity components using both univariate and multivariate analyses. We used generalized linear mixed effects models to evaluate the effect of altitude on species richness, total abundance, effective diversity (i.e., e H′, where H′ is the Shannon diversity index; Jost, 2006) and temporal β‐diversity (Jaccard index). Sampling year was a random effect because we used a repeated measures sampling design. Sample sites were also included as a random effect to address unmeasured differences in sites. As a result of this analytical model, results are general for altitudinal effects on fish diversity in the Caquetá River through years, including pre‐ and postconflict time‐periods, and among sites. Alternative models were compared using the corrected Akaike's Information Criterion (AICc) with the bbmle package (Bolker et al., 2022), where we emphasized AICc weights to identify the most plausible model after discounting for model complexity (Burnham & Anderson, 2002). Different alternative residual distributions (e.g., Gaussian, Gamma, negative binomial) in generalized linear models were iteratively evaluated and compared to log‐transforms of response variables to best meet model assumptions (using check_model in the performance package; Lüdecke, 2023). Given a chosen distribution, alternative models were then compared by AICc, where compared models included a null, random effects only, potential fixed effects (i.e., a dummy variable representing pre‐ and postcease fire conditions and altitude effects), and combinations of fixed and random effects. Finally, we used nonmetric multidimensional scaling (NMDS; Bray‐Curtis based) with PERMANOVA to evaluate differences in community structure (β‐diversity). between basimontane and premontane sites.
Data management and statistical analyses were performed in R (R Core Team, 2021) using functions from the glmmTMB (Brooks et al., 2020), vegan (Oksanen et al., 2020), devtools (Hadley et al., 2022), iNEXT (Hsieh et al., 2016), ggplot2 (Kassambara, 2018), MASS (Ripley et al., 2019), multcompView (Graves & Piepho, 2022), and performance (Lüdecke, 2023) packages.
3. RESULTS
Samples included 4216 fish, belonging to 100 species, 58 genera, 24 families, and six orders (Appendix S1). Rarefaction curves reached asymptotes for both basimontane and premontane levels (Figure 3a). Premontane sites had >3 times more fish and >5 times more species than those recorded in basimontane sites (Figure 3b). In addition, basimontane sites had a different taxonomic composition than premontane sites. Basimontane sites had similar numbers of Characiformes and Siluriformes and some (<10%) of the Cyprinodontiformes (Figure 3c). In contrast, the premontane level was clearly dominated (>75%) by the Characiformes fishes which comprised a higher diversity at the order level (Figure 3c). Fish community structure disparities between altitudinal levels are confirmed by the PERMANOVA (F = 3.04, R = .23, p < .05), and the NMDS in which two clear groups, corresponding to the basimontane and premontane sites, are depicted in the multidimensional space representing the analysis (Figure 3d).
FIGURE 3.

Comparisons of premontane and basimontane fish communities. (a) Rarefaction curves approach asymptotes, indicating representative sampling. Premontane sites were inhabited by more species, as supported by evidence in (b) for total abundance, richness, and effective diversity. (c) Taxonomic composition differed between premontane and basimontane sites, where Siluriformes were more prominent in basimontane sites than in premontane sites. Characiformes were dominant in both site types, but in different proportions. (d) NMDS of fish communities from the sampled sites. Dots represent pooled temporal data (2013–2022) for sites. Polygons represent fish communities based on altitudinal levels recognized by van der Hammen and dos Santos (1995).
Species richness was most efficiently modeled as a power law (i.e., log–log) function of altitude and the random effects of year and site (Table S1; Table 2). Although altitude alone has a good predictive power (R 2 = .42) predictions improve with the random effects (R 2 = .62), as confirmed by the model performance procedure (Figure S1). The model output suggests a significant mean decrease by 0.98 log(species richness) per unit of log‐altitude (Table 2; see Figure 4a for regression line).
TABLE 2.
Details for the most efficient models to predict α‐diversity species richness, total abundance, and effective diversity (1D = exp(Shannon entropy)) of fishes in the Caquetá River, Colombia, based on 91 samples among 12 sites collected in 8 years during the 2013–2022 interval.
| Model distribution | Fixed effects R 2 | Fixed + random R 2 | Random effects | Std. deviation | Fixed effects | Coefficient | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| Species richness | Gaussian | .42 | .62 | Year | 0.1950 | Intercept | 7.724 | 2.229 |
| Sites | 0.2612 | log(Altitude) | −0.979 | 0.363 | ||||
| Total abundance | Gamma (log link) | .13 | .48 | Year | 0.359 | Intercept | 7.987 | 3.657 |
| Sites | 0.437 | log(Altitude) | −0.716 | 0.594 | ||||
| Effective diversity | Gamma (log link) | .43 | .57 | Year | 0.146 | Intercept | 6.526 | 1.622 |
| Sites | 0.170 | log(Altitude) | −0.834 | 1.164 |
FIGURE 4.

Regression lines for species richness (a), total abundance (b), and effective diversity (c) ~ altitude, where regression lines represent GLMM predicted values and error bars. Note log axes; regressions represent power law functions.
Similar to results for species richness, a power law model for total abundance using altitude as a fixed effect and the random effects was most predictive compared to other models (Table S2; Table 2). Altitude alone represented 42% of variation in species richness, but model predictions improve with random effects (R 2 = .62; Figure S2). The model output suggests a 0.72 significant decrease in log(fish abundance) per unit of log‐altitude (Table 2; see Figure 4b for regression line).
Effective diversity was also most plausibly modeled as a power law (Table S3; Table 2). Altitude alone has substantial predictive power (R‐squared = .433), but adding year as a random effect slightly improved fit (R‐squared = .456; Figure S3). The model output suggests a significant 0.834 mean significant decrease in log(effective diversity) per unit of log‐altitude (Table 2; see Figure 4c for regression line).
Premontane sites (<500 m) maintained greater temporal β‐diversity (Jaccard index) through time than basimontane sites (>500 m; Figure 5). As a reminder, temporal β‐diversity was calculated through time (i.e., t + 1 – t) for each site. Thus, lower elevation stream sites that are more vulnerable to human land use effects had more taxonomic heterogeneity than did the higher elevation sites.
FIGURE 5.

Temporal β‐diversity (Jaccard dissimilarity) of premontane (dark green) and basimontane (light green) sites. Temporal β‐diversity compares time t to time t + 1 for each site. Linear regressions are shown with 95% confidence intervals. Data represent Jaccard index for each site between consecutive samples, and adjusted R 2 values are low because trends are essentially flat through time, as indicated by nonsignificant slope coefficients for Temporal Interval (premontane p = .96, basimontane p = 17).
Overall, for all of the diversity components evaluated herein, using altitudinal level as fixed effect and site and year as mixed effects was rather predictive, and no pre‐ and postconflict difference was yet apparent in results (i.e., results here represent conditions before further human land use effects). Fish diversity predictably decreased with altitude as a power law function, and fish communities were different in pre‐ and basimontane streams.
4. DISCUSSION
Our study is the first of its kind to evaluate fish community structure in the Colombian Andes‐Amazon transition zone. Here we had the opportunity to evaluate altitudinal gradients using altitude from two perspectives, as a continuous and a categorial variable. Results here provide a baseline for conservation of regional streams across premontane and basimontane levels because data represent the period when armed conflict ended but before potential development of formerly avoided lands (Calle‐Rendón et al., 2018).
Our results confirmed that altitudinal gradients in fish community assembly are important (van der Hammen & dos Santos, 1995) and that fish diversity is greater at lower altitudes, no matter how we estimated diversity (e.g., Jaramillo‐Villa et al., 2010; Lomolino et al., 2010; Lujan et al., 2013). Most of the times it is difficult to understand process causing patterns due to the many mechanisms that might be involved (Ricklefs, 2004). We provide evidence that deterministic processes are more important for freshwater fish diversity patterns along an altitudinal gradient; otherwise, patterns would not be consistent in space and time. Furthermore, fish diversity patterns are strongly associated with altitude in our study system, whether altitude is viewed as a categorical (basimontane and premontane) or continuous predictor. The strong altitude gradient and changes in associated stream conditions appear to be responsible for deterministic processes at local scales (Dyer et al., 2007; Schemske et al., 2009) that are stronger than regional scale mechanisms related to dispersal that may blur the strong gradient (Qian & Ricklefs, 2007; Ricklefs, 1987). Considering that effective mitigation of anthropogenic pressures on streams and fish assemblages should account for site‐specific conditions and at different scales (Newbold et al., 2015; Poiani et al., 2000), altitudinal gradients in the study area may be predictive for conservation and management. We note that the basimontane streams are important to conserve despite their relatively low diversity because fishes there are notably different from those downstream.
Changes in community composition through time are typically complex and depend on spatial and land use contexts (Allen et al., 2019; Hill et al., 2021). We expect that fish diversity might be most heavily affected at the premontane level over time due to human activities that will stronger and earlier at lower altitudes. Conservation practices (e.g., riparian buffer zones, runoff settling ponds) to maintain stream conditions can be implemented early in regional land changes to best conserve diversity.
Given species extinction rates due to habitat degeneration in ecosystems similar to those sampled here (Manjarrés‐hernández et al., 2021), this study is valuable as a baseline of barely impacted conditions as the conflict ended in Colombia and before encroaching development of formerly avoided lands (Calle‐Rendón et al., 2018). We expect future sampling will compare results to those reported here to document changes in fish assemblages due to land use changes. We also expect land use to be most changed at lower altitudes, where soils and slopes are more amenable to agriculture, roads, and housing. If so, then it is possible that fish assemblages will be degraded by increased land use to become simpler and later affected by upstream effects (Figure 1). If ongoing deforestation and encroaching anthropogenic land use in the hyper‐diverse Amazon basin is to be managed to minimize species losses, then some lands and streams must be preserved (Granado‐Lorencio et al., 2007; Mckinney & Lockwood, 1999; Rull, 2007). Results here suggest that land surrounding premontane streams should take priority to conserve the most species in areas that are most vulnerable. Upstream catchments in basimontane areas can also be preserved to maintain water quality flowing into premontane streams and conserve unique fishes in those upstream reaches. Future work in the study area should evaluate relative effects of changing land use in upper and lower catchments on fish diversity in the lower reaches.
So far, most emphasis on ecological studies on understanding diversity has been on spatial patterns of biological diversity rather than analyzing temporal patterns. Understanding temporal changes is essential to predict possible scenarios of the most diverse world's biota in natural ecosystems of the Amazon. Fish assemblages are affected by processes occurring at multiple scales, including those covered in our analyses (Jackson et al., 2001; Livingstone et al., 1982; Oberdoff et al., 2011; Tedesco et al., 2005). Future surveys could integrate data at scales obtained here with even greater spatial and temporal extents. We regard results here as a potential reference for future work in the same drainages, where future work may reveal effects of coming anthropogenic land use in the Amazon basin. Our data integrated spatial and temporal analysis to elucidate the consistent altitudinal gradient under relatively unimpacted conditions. By implication, similar conditions exist to be conserved in other headwaters that host the most diverse fish fauna in the world.
AUTHOR CONTRIBUTIONS
David G. Jenkins: Conceptualization (lead); formal analysis (lead); methodology (lead); writing – review and editing (lead). Astrid Acosta‐Santos: Data curation (equal). Edwin Agudelo Córdoba: Resources (supporting). Juan David Bogota‐Gregory: Conceptualization (lead); data curation (lead); formal analysis (lead); investigation (lead); methodology (lead); project administration (lead); resources (lead); writing – original draft (lead).
CONFLICT OF INTEREST STATEMENT
All authors declare that there are no competing interests.
Supporting information
Figure S1
Appendix S1
Table S1
ACKNOWLEDGEMENTS
This study was funded by the projects “Investigación Conservación y Aprovechamiento Sostenible de la Diversidad Biológica Socioeconómica y Cultural de la Amazonia Colombiana‐Amazonas, Caquetá, Putumayo, Guaviare, Vaupés y Guainía” and “Expedición Colombia‐Bio a la Biodiversidad en la Transición Andino‐Amazónica del Departamento del Caquetá”. Grupo de Investigación en Ecosistemas Acuáticos Amazónicos of the SINCHI Institute. Marcela Núñez and Iván González for their support during the fieldwork. Members of the headquarters of the SINCHI Institute at Leticia (Amazonas) and Florencia (Caquetá).
Bogota‐Gregory, J. D. , Jenkins, D. G. , Acosta‐Santos, A. , & Agudelo Córdoba, E. (2024). Fish diversity of Colombian Andes‐Amazon streams at the end of conflict is a reference for conservation before increased land use. Ecology and Evolution, 14, e11046. 10.1002/ece3.11046
DATA AVAILABILITY STATEMENT
Supporting data are available at https://doi.org/10.5281/zenodo.10424407.
REFERENCES
- Achiso, Z. (2020). Biodiversity and human livelihoods in protected areas: World‐ wide perspective—A review. SSR Institute of International Journal of Life Sciences, 6(3), 2565–2578. [Google Scholar]
- Adhikari, A. , & Hansen, A. J. (2018). Landscape and urban planning land use change and habitat fragmentation of wildland ecosystems of the North Central United States. Landscape and Urban Planning, 177, 196–216. 10.1016/j.landurbplan.2018.04.014 [DOI] [Google Scholar]
- Agudelo Córdoba, E. , Sánchez, C. , Rodríguez, C. , Bonilla‐Castillo, C. , & Gómez, G. (2011). Diagnóstico de la pesquería en la cuenca Amazonas. In Lasso C. A., Morales‐Betancourt M. A., González‐Cañón G., Ajiaco‐Martínez R. E., Valderrama Barco M., Hernández Barrero S., Ortega Lara A., Rodríguez Fernández C. A., Álvarez León R., Agudelo Córdoba E., Bonilla Castillo C. A., Gómez Hurtado G. A., Barreto‐Reyes C., & Sánchez Páez C. L. (Eds.), II. Pesquerías continentales de Colombia: cuencas del Magda‐ lena‐Cauca, Sinú, Canalete, Atrato, Orinoco, Amazonas y vertiente del Pacífico. Serie Editorial Recursos Hidrobiológicos y Pesqueros Continentales de Colombia, 13(2152), 1–14. (pp. 143–166). Instituto de Investigación de los Recursos Biológicos Alexander von Humboldt. [Google Scholar]
- Agudelo, E. , Alonso, J. C. , & Moya, L. (2006). Perspectivas para el ordenamiento de la pesca y la acuicultura en el área de integración fronteriza Colombo‐Peruana del río Putumayo .
- Agudelo Hz, W. J. , Castillo‐Barrera, N. C. C. , & Murcia‐García, U. (2023). Scenarios of land use and land cover change in the Colombian Amazon to evaluate alternative post ‐ conflict pathways. Scientific Reports, 1–14. 10.1038/s41598-023-29243-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, D. C. , Bateman, H. L. , Warren, P. S. , De Albuquerque, F. S. , Arnett‐Romero, S. , & Harding, B. (2019). Long‐term effects of land‐use change on bird communities depend on spatial scale and land‐use type. Ecosphere, 10(11), 1–18. 10.1002/ecs2.2952 [DOI] [Google Scholar]
- Anderson, E. , Jenkins, C. N. , Heilpern, S. , Maldonado‐Ocampo, J. A. , Carvajal‐Vallejos, F. M. , Encalada, A. C. , Rivadeneira, J. F. , Hidalgo, M. , Cañas, C. M. , Ortega, H. , Salcedo, N. , Maldonado, M. , & Tedesco, P. A. (2018). Fragmentation of Andes‐to‐Amazon connectivity by hydropower dams. Science Advances, 4(1), 1–8. 10.1126/sciadv.aao1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, G. (2005). The co‐distribution of species in relation to the neutral theory of community ecology. Ecology, 86(7), 1757–1770. 10.1890/04-1028 [DOI] [Google Scholar]
- Bogotá‐Gregory, J. D. , Lima, F. C. T. , Correa, S. B. , Silva‐Oliveira, C. , Jenkins, D. G. , Ribeiro, F. R. , Lovejoy, N. R. , Reis, R. E. , & Crampton, W. G. R. (2020). Biogeochemical water type influences community composition, species richness, and biomass in megadiverse Amazonian fish assemblages. Scientific Reports, 10(1), 15349. 10.1038/s41598-020-72349-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolker, B. M. , Team, R. D. C. , & Giné‐Vázquez, I. (2022). bbmle: Tools for general maximum likelihood estimation (pp. 1–32). R package version 1.0.15. 2012.
- Brooks, M. , Bolker, B. , Kristensen, K. , Maechler, M. , Magnusson, A. , McGillycuddy, M. , Skaug, H. , Nielsen, A. , Berg, C. , van Bentham, K. , Sadat, N. , Lüdecke, D. , Lenth, R. , O’Brien, J. , Geyer, C. J. , Jagan, M. , Wiernik, B. , & Stouffer, D. B. (2020). Package glmmTMB . R package version 0.2.0. Generalized Linear Mixed Models using Template Model Builder. R topics documented.
- Bürgi, M. , Östlund, L. , & Mladenoff, D. J. (2017). Legacy effects of human land use : Ecosystems as time‐lagged systems. Ecosystems, 20, 94–103. 10.1007/s10021-016-0051-6 [DOI] [Google Scholar]
- Burnham, K. P. , & Anderson, D. R. (2002). Model selection and multimodel inference: A practical information‐theoretic approach (2nd ed.). Springer. [Google Scholar]
- Calle‐Rendón, B. R. , Moreno, F. , & Hilário, R. R. (2018). Vulnerability of mammals to land‐use changes in Colombia's post‐conflict era. Nature Conservation, 92, 79–92. 10.3897/natureconservation.29.28943 [DOI] [Google Scholar]
- Chao, A. (1984). Nonparametric estimation of the number of classes in a population. Scandinavian Journal of Statistics, 11(4), 265–270. 10.2307/4615964 [DOI] [Google Scholar]
- Chao, A. , Colwell, R. K. , Lin, C. W. , & Gotelli, N. J. (2009). Sufficient sampling for asymptotic minimum species richness estimators. Ecology, 90(4), 1125–1133. 10.1890/07-2147.1 [DOI] [PubMed] [Google Scholar]
- Chase, J. M. , & Leibold, M. A. (2003). Ecological niches: Linking classic and contemporary approaches. University of Chicago Press. [Google Scholar]
- Clerici, N. , Salazar, C. , Pardo‐Díaz, C. , Jiggins, C. D. , Richardson, J. E. , & Linares, M. (2019). Peace in Colombia is a critical moment for Neotropical connectivity and conservation: Save the northern Andes – Amazon biodiversity bridge. Conservation Letters, 12, e12594. 10.1111/conl.12594 [DOI] [Google Scholar]
- Colwell, R. K. , & Hurtt, G. C. (1994). Nonbiological gradients in species richness and a spurious Rapoport effect. American Naturalist, 144, 570–595. [Google Scholar]
- Colwell, R. K. , & Lees, D. C. (2000). The mid‐domain effect: Geometric constraints on the geography of species richness. Trends in Ecology & Evolution, 15, 70–76. [DOI] [PubMed] [Google Scholar]
- De La Barra, E. , Zubieta, J. , Aguilera, G. , Maldonado, M. , Pouilly, M. , & Oberdorff, T. (2016). ¿Qué factores determinan la distribución altitudinal de los peces de ríos tropicales andinos? Biología Tropical, 64(1), 173–192. [PubMed] [Google Scholar]
- Dyer, L. A. , Singer, M. S. , Lill, J. T. , Stireman, J. O. , Gentry, G. L. , Marquis, R. J. , Ricklefs, R. E. , Greeney, H. F. , Wagner, D. L. , Morais, H. C. , Diniz, I. R. , Kursar, T. A. , & Coley, P. D. (2007). Host specificity of Lepidoptera in tropical and temperate forests. Nature, 448, 686–699. [DOI] [PubMed] [Google Scholar]
- Feng, C. , Cao, M. , Liu, F. Z. , Zhou, Y. , du, J. H. , Zhang, L. B. , Huang, W. J. , Luo, J. W. , Li, J. S. , & Wang, W. (2022). Improving protected area effectiveness through consideration of different human‐pressure baselines. Conservation Biology, 36, 1–10. 10.1111/cobi.13887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, A. , Blaschke, M. , & Bässler, C. (2011). Altitudinal gradients in biodiversity research: The state of the art and future perspectives under climate change aspects. Biodiv ersitäts‐Forschung, 11, 35–47. [Google Scholar]
- Galvis, G. , Sánchez‐Duarte, P. , Mesa‐Salazar, L. M. , López‐Pinto, Y. , Gutiérrez‐e, M. A. , Gutiérrez‐Cortés, Á. , Castaño, M. L. , & Castillo, C. C. (2007). Peces de la Amazonía Colombiana con énfasis en especies de interés ornamental. Incoder, Universidad Nacional, SINCHI. [Google Scholar]
- Gentry, A. H. (1988). Tree species richness of upper Amazonian forests. Proceedings of the National Academy of Sciences of the United States of America, 85, 156–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granado‐Lorencio, C. , Cerviá, J. L. , & Lima, C. R. M. A. (2007). Floodplain lake fish assemblages in the Amazon River: Directions in conservation biology. Biodiversity and Conservation, 16(3), 679–692. 10.1007/s10531-005-3742-4 [DOI] [Google Scholar]
- Graves, S. , & Piepho, H. (2022). Package “multcompView” .
- Hadley, A. , Hester, J. , & Chang, W. (2022). Package “devtools” R topics documented .
- Heegaard, E. , & Vandvik, V. (2009). Climate change affects the outcome of competitive interactions – An application of principal response curves. Oecologia, 139(3), 459–466. 10.1007/s00442-004- [DOI] [PubMed] [Google Scholar]
- Hercos, A. P. , Sobansky, M. , Queiroz, H. L. , & Magurran, A. E. (2013). Local and regional rarity in a diverse tropical fish assemblage. Proceedings of the Royal Society B, 280(1751), 20122076. 10.1098/rspb.2012.2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, M. J. , White, J. C. , Biggs, J. , Briers, R. A. , Gledhill, D. , Ledger, M. E. , Thornhill, I. , Wood, P. J. , & Hassall, C. (2021). Local contributions to beta diversity in urban pond networks: Implications for biodiversity conservation and management. Diversity and Distributions, 27, 887–900. 10.1111/ddi.13239 [DOI] [Google Scholar]
- Hoorn, C. (1994). An environmental reconstruction of the palaeo‐Amazon river system (Middle‐Late Miocene, NW Amazonia). Palaeogeography, Palaeoclimatology, Palaeoecology, 112, 187–238. [Google Scholar]
- Hsieh, T. C. , Ma, K. H. , & Chao, A. (2016). iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods in Ecology and Evolution, 7, 1451–1456. 10.1111/2041-210X.12613 [DOI] [Google Scholar]
- IGAC . (1996). Diccionario Geográfico de Colombia. Instituto Geográfico Agustín Codazzi, Ministerio de Hacienda y Crédito Público. [Google Scholar]
- IGAC . (1999). Paisajes fisiográficos de Orinoquia‐Amazonia (ORAM) Colombia. Análisis Geográficos, Ministerio de Hacienda y Crédito Público, Instituto Geográfico Agustín Codazzi. [Google Scholar]
- Ikeda, S. , Osawa, K. , & Akamatsu, Y. (2009). Review sediment and nutrients transport in watershed and their impact on coastal environment. Proceedings of the Japan Academy Series, 85, 2–12. 10.2183/pjab.85.374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPBES . (2019). Summary for policymakers of the global assessment report on biodiversityand ecosystem services .
- Jackson, D. A. , Peres‐Neto, P. R. , & Olden, J. D. (2001). What controls who is where in freshwater fish communities – the roles of biotic, abiotic, and spatial factors. Canadian Journal of Fisheries and Aquatic Sciences, 58(1), 157–170. 10.1139/cjfas-58-1-157 [DOI] [Google Scholar]
- Jaramillo‐Villa, U. , Maldonado‐Ocampo, J. A. , & Escobar, F. (2010). Altitudinal variation in fish assemblage diversity in streams of the central Andes of Colombia. Journal of Fish Biology, 76(10), 2401–2417. 10.1111/j.1095-8649.2010.02629.x [DOI] [PubMed] [Google Scholar]
- Jost, L. (2006). Entropy and diversity. OIKOS, 113(2), 363–375. [Google Scholar]
- Kassambara, A. (2018). ggpubr: “ggplot2” based publication ready plots . R package version 0.2. https://CRAN.R‐project.org/package=ggpubr; https://CRAN.R‐project.org/package=ggpubr
- Keller, I. , Alexander, J. M. , Holderegger, R. , & Edwards, O. J. (2013). Widespread phenotypic and genetic divergence along altitudinal gradients in animals. Journal of Evolutionary Biology, 26(12), 2527–2543. 10.1111/jeb.12255 [DOI] [PubMed] [Google Scholar]
- Körner, C. (2007). The use of “altitude” in ecological research. Trends in Ecology and Evolution, 22, 569–574. [DOI] [PubMed] [Google Scholar]
- Livingstone, D. A. , Rowland, M. , & Bailey, P. E. (1982). On the size of African riverine fish faunas. American Zoologist, 22(2), 361–369. [Google Scholar]
- Lomolino, M. V. , Riddle, B. R. , Whittaker, R. J. , & Brown, J. H. (2010). Biogeography (4th ed.). Sinauer Associates, Inc. [Google Scholar]
- Lüdecke, D. (2023). Package “performance” (p. 97). 10.1098/rsif.2017.0213 [DOI]
- Lujan, N. K. , Roach, K. A. , Jacobsen, D. , Winemiller, K. O. , Vargas, V. M. , Ching, V. R. , & Maestre, J. A. (2013). Aquatic community structure across an Andes‐to‐Amazon fluvial gradient. Journal of Biogeography, 40(9), 1715–1728. 10.1111/jbi.12131 [DOI] [Google Scholar]
- Lundberg, J. (2001). Freshwater richness of the Amazon. Natural History, 110(7), 36–43. [Google Scholar]
- Lundberg, J. G. , Kottelat, M. , Smith, G. R. , Stiassny, M. L. J. , & Gill, A. C. (2000). So many fishes, so little time: An overview of recent ichthyological discovery in continental waters. Annals of the Missouri Botanical Garden, 87, 26–62. [Google Scholar]
- Magurran, A. E. (2004). Measuring biological biodiversity. Blackwell Science Ltd. [Google Scholar]
- Magurran, A. E. , & Henderson, P. A. (2003). Explaining the excess of rare species in natural species abundance distributions. Nature, 422(6933), 714–716. 10.1038/nature01547 [DOI] [PubMed] [Google Scholar]
- Maldonado‐Ocampo, J. A., Oretega‐Lara, A., Usama Oviedo, J. S., Gálvis Vergara, G., Villa‐Navarro, F. A., Vásquez Gamboa, L., Prada‐Pedreros, S., & Ardila Rodríguez, C. (2005). Peces de los Andes de Colombia. Instituto de Investigación de Recursos Biológicos Alexander von Humboldt. [Google Scholar]
- Manjarrés‐hernández, A. , Guisande, C. , García‐Roselló, E. , Heine, J. , Pelayo‐Villamil, P. , Pérez‐Costas, E. , González‐Vilas, L. , González‐Dacosta, J. , Duque, S. R. , Granado‐Lorencio, C. , & Lobo, J. M. (2021). Predicting the effects of climate change on future freshwater fish diversity at global scale. Nature Conservation, 24, 1–24. 10.3897/natureconservation.43.58997 [DOI] [Google Scholar]
- Martinelli, L. A. , Victoria, R. L. , Devol, A. H. , Richey, J. E. , & Forsberg, B. R. (1989). Suspended sediment load in the Amazon Basin: An overview. GeoJournal, 19(4), 381–389. [Google Scholar]
- Mckinney, M. L. , & Lockwood, J. L. (1999). Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends in Ecology & Evolution, 14(11), 450–453. 10.1016/S0169-5347(99)01679-1 [DOI] [PubMed] [Google Scholar]
- Mclain, R. J., Cerveny, L., Besser, D., Banis, D., & Todd, A. (2013). Mapping human‐environment connections on the Olympic Peninsula: An Atlas of landscape values. Occasional Papers in Geography No. 7.
- Melack, J. M. , & Fosberg, B. R. (2001). Biogeochemistry of Amazon floodplain lakes and associated wetlands. In McClain M. E., Victoria R. L., & Richey J. E. (Eds.), The biogeochemistry of the Amazon basin (pp. 235–274). Oxford University Press. [Google Scholar]
- Mittermeier, R. A. , Mittermeier, C. G. , Brooks, T. M. , Pilgrim, J. D. , Konstant, W. R. , da Fonseca, G. A. B. , & Kormos, C. (2003). Wilderness and biodiversity conservation. Proceedings of the National Academy of Sciences of the United States of America, 100(18), 10309–10313. 10.1073/pnas.1732458100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers, N. , Mittermeier, R. A. , Mittermeier, C. G. , da Fonseca, G. A. B. , & Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature, 403, 853–858. [DOI] [PubMed] [Google Scholar]
- Newbold, T. , Hudson, L. N. , Hill, S. L. L. , Contu, S. , Lysenko, I. , Senior, R. A. , Börger, L. , Bennett, D. J. , Choimes, A. , Collen, B. , Day, J. , De Palma, A. , Díaz, S. , Echeverria‐Londoño, S. , Edgar, M. J. , Feldman, A. , Garon, M. , Harrison, M. L. K. , Alhusseini, T. , … Purvis, A. (2015). Global effects of land use on local terrestrial biodiversity. Nature, 520, 45–50. 10.1038/nature14324 [DOI] [PubMed] [Google Scholar]
- Oberdoff, T. , Tedesco, P. A. , Hugueny, B. , Leprieur, F. , Beauchard, O. , Brosse, S. , & Dürr, H. H. (2011). Global and regional patterns in riverine fish species richness: A review. International Journal of Ecology, 2011, 1–12. [Google Scholar]
- Oksanen, J., Simpson, G. L., Guillaume Blanchet, F., Kindt, R., Legendre, P., Minchin, P. R., Solymos, P., Stevens, M. H. H., Szoecs, E., Wagner, H., Barbour, M., Bedward, M., Bolker, B., Borcard, D., Carvalho, G., Chirico, M., De Caceres, M., Durand, S., … Weedon, J. (2020). vegan: Community ecology package . R package version 2.5‐7. https://cran.r‐project.org/package=vegan
- Penjor, U. , Jamtsho, R. , & Sherub, S. (2022). Anthropogenic land‐use change shapes bird diversity along the eastern Himalayan altitudinal gradient. Journal of Applied Ecology, 59, 847–859. [Google Scholar]
- Plieninger, T. (2006). Habitat loss, fragmentation, and alteration – Quantifying the impact of land‐use changes on a Spanish dehesa landscape by use of aerial photography and GIS (pp. 91–105). 10.1007/s10980-005-8294-1 [DOI]
- Poiani, K. A. , Richter, B. D. , Anderson, M. G. , & Richter, H. E. (2000). Biodiversity conservation at multiple scales: Functional sites, landscapes, and networks. BioScience, 50(2), 133–146. [Google Scholar]
- Qian, H. , & Ricklefs, R. E. (2007). A latitudinal gradient in large‐scalebeta diversity for vascular plants in North America. Ecology Letters, 10(8), 737–744. 10.1111/j.1461-0248.2007.01066.x [DOI] [PubMed] [Google Scholar]
- R Core Team . (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing. [Google Scholar]
- Rehmana, G. , Khattak, I. , Hamayun, M. , Rahman, A. , Haseeb, M. , Umar, M. , Ali, S. , Iftikhar, M. , Shams, W. A. , & Pervaiz, R. (2024). Impacts of mining on local fauna of wildlife in District Mardan & District Mohmand Khyber Pakhtunkhwa Pakistan. Brazilian Journal of Biology, 84, 1–11. [DOI] [PubMed] [Google Scholar]
- Reis, R. E. , Kullander, S. O. , & Ferraris, C. J. (2003). Checklist of the freshwater fishes of south and Central America. Edipucrs. [Google Scholar]
- Ricklefs, R. E. (1987). Community Diversity: Relative Roles of Local and Regional Processes. Science, 235(4785), 167–171. 10.1126/science.2354785.167 [DOI] [PubMed] [Google Scholar]
- Ricklefs, R. E. (2004). A comprehensive framework for global patterns in biodiversity. Ecology Letters, 7(1), 1–15. 10.1046/j.1461-0248.2003.0054.x [DOI] [Google Scholar]
- Ripley, B., Venables, B., Bates, D. M., Hornik, K., Gebhardt, A., Firth, D. (2019). Package “MASS” (Version 7.3‐51.4) . Cran‐R Project. http://www.stats.ox.ac.uk/pub/MASS4/Contact
- Rull, V. (2007). The Guayana Highlands: A promised (but threatened) land for ecological and evolutionary science. Biotropica, 39(1), 31–34. 10.1111/j.1744-7429.2006.00215.x [DOI] [Google Scholar]
- Saint‐Paul, U. , Zuanon, J. , Correa, M. A. V. , García, M. , Fabré, N. N. , Berger, U. , & Junk, W. J. (2000). Fish communities in central Amazonian white‐ and blackwater floodplains. Environmental Biology of Fishes, 57(3), 235–250. 10.1023/a:1007699130333 [DOI] [Google Scholar]
- Schemske, D. W. , Mittelbach, G. G. , Cornell, H. V. , Sobel, J. M. , & Roy, K. (2009). Is there a latitudinal gradient in the importance of biotic interactions? Annual Review of Ecology, Evolution, and Systematics, 40, 245–269. 10.1146/annurev.ecolsys.39.110707.173430 [DOI] [Google Scholar]
- Shively, G. E. (2001). Agricultural change, rural labor markets, and forest clearing: An illustrative case from The Philippines. Land Economics, 77(2), 17. [Google Scholar]
- Tedesco, P. A. , Oberdorff, T. , Lasso, C. A. , Zapata, M. , & Hugueny, B. (2005). Evidence of history in explaining diversity patterns in tropical riverine fish. Journal of Biogeography, 32(11), 1899–1907. 10.1111/j.1365-2699.2005.01345.x [DOI] [Google Scholar]
- Tellez, J. F. (2019). Peace agreement design and public support for peace: Evidence from Colombia. Journal of Peace Research, 56(6), 827–844. [Google Scholar]
- Tognelli, M. F. , Lasso, C. A. , Bota‐Sierra, C. A. , & Jiménez‐Segura, L. F. (2016). Estado de Conservación y Distribución de la Biodiversidad de Agua Dulce en los Andes Tropicales. UICN. [Google Scholar]
- van der Hammen, T. , & dos Santos, A. (Eds.). (1995). La Cordillera central Colombiana transecto parque los Nevados (Tercera parte). In Studies on tropical Andean ecosystems. Volumen 4. J. Cramer. der Gebrüder Borntraeger Verlagsbuchhandlung. [Google Scholar]
- Wilson, E. O. (1992). The diversity of life. W. W. Norton & Company. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Appendix S1
Table S1
Data Availability Statement
Supporting data are available at https://doi.org/10.5281/zenodo.10424407.


