Abstract
Background: Single-tablet combination therapies (STCTs) combine multiple drugs into one formulation, making drug administration more convenient for patients. STCTs were developed to address concerns with treatment adherence and persistence, but the impact of STCT use is not fully understood across indications.
Objectives: We conducted a systematic literature review (SLR) to examine STCT-associated outcomes across 4 evidence domains: clinical trials, real-world evidence (RWE), health-related quality of life (HRQoL) studies, and economic evaluations.
Methods: Four SLRs were conducted across the aforementioned domains. Included studies compared STCTs as well as fixed-dose combinations ([FDCs] of non-tablet formulations) with the equivalent active compounds and doses in loose-dose combinations (LDCs). Original research articles were included; case reports, case series, and non-English-language sources were excluded. Databases searched included EconLit, Embase, and Ovid MEDLINE® ALL. Two independent reviewers assessed relevant studies and extracted data. Conflicts were resolved with a third reviewer or consensus-based discussion.
Results: In all, 109 studies were identified; 27 studies were identified in more than one SLR. Treatment adherence was significantly higher in patients receiving FDCs vs LDCs in 12 of 13 RWE studies and 3 of 13 clinical trials. All 18 RWE studies reported higher persistence with FDCs. In RWE studies examining clinical outcomes (n = 17), 14 reported positive findings with FDCs, including a reduced need for add-on medication, blood pressure control, and improved hemoglobin A1C. HRQoL studies generally reported numerical improvements with STCTs or similarities between STCTs and LDCs. Economic outcomes favored STCT use. All 6 cost-effectiveness or cost-utility analyses found FDCs were less expensive and more efficacious than LDCs. Four budget impact models found that STCTs were associated with cost savings. Medical costs and healthcare resource use were generally lower with FDCs than with LDCs.
Discussion: Evidence from RWE and economic studies strongly favored STCT use, while clinical trials and HRQoL studies primarily reported similarity between STCTs and LDCs. This may be due to clinical trial procedures aimed at maximizing adherence and HRQoL measures that are not designed to evaluate drug administration.
Conclusions: Our findings highlight the value of STCTs for improving patient adherence, persistence, and clinical outcomes while also offering economic advantages.
Keywords: single-tablet combination therapy, fixed-dose combination, loose-dose combination, cost-effectiveness, real-world outcomes
INTRODUCTION
Chronic diseases result in high healthcare resource utilization (HCRU) and substantial costs for healthcare systems. Patients with chronic diseases frequently have low treatment adherence and persistence rates, which can result in poor clinical outcomes and contribute to higher HCRU and costs.1–6 Poor adherence can be driven by various factors, including a lack of health literacy, comorbidities and polypharmacy, inadequate access to medical care, and the high cost of treatments.7 Mechanisms to improve patient adherence and persistence are increasingly sought after to advance the efficiency of healthcare systems and produce better health outcomes for patients,7–9 particularly patients with chronic diseases where combination therapy is recommended by clinical guidelines.10–12 Single-tablet combination therapies (STCT) that combine multiple drugs into one formulation may address some of the reasons for poor adherence by reducing the pill burden for patients with chronic diseases. Previous work has shown that across multiple indications, STCTs can encourage treatment adherence13 and persistence,14,15 and provide economic evidence supporting the use of STCTs for reducing costs and HCRU.16,17
The number of STCTs that have received regulatory approval in the United States (US) and Europe has increased in recent years. In the US, STCT approvals rose from 12 approvals in the 1980s to 59 approvals in the 2000s,18 while in Europe, 7 STCTs were approved in 2016, compared with just 1 in 2010.19 STCTs are now available for hypertension, HIV, asthma, diabetes, and other chronic diseases. The evaluation of STCT use is relevant to multiple stakeholders, including patients, clinicians, caregivers, and payers. To accurately assess the value provided by STCTs, a comprehensive picture of their impact is needed across indications, countries, and types of evidence. The goal of this systematic literature review (SLR) was to characterize the effects of STCTs and loose-dose combination products (LDC) on treatment adherence, compliance, persistence, clinical outcomes, economic outcomes, and health-related quality of life (HRQoL) across 4 evidence domains: clinical trials, real-world evidence (RWE), HRQoL studies, and economic evaluations.
METHODS
Four SLRs were conducted with unique search strategies to identify the most relevant records under the aforementioned research domains (Supplemental Table 1). Database and registry records published between January 2001 and December 2021 that compared fixed-dose combinations and LDCs were searched according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.20 Only comparisons of fixed-dose combinations and LDCs with the same active compounds and doses were considered. The databases searched included Cochrane, American College of Physicians Journal Club, National Health Service Economic Evaluation Database, EconLit, Embase, and Ovid MEDLINE® ALL. Case reports, case series, and non-English language records were excluded according to the population, intervention, comparator, outcomes and study design criteria (Supplemental Table 2). Two independent reviewers identified relevant studies at the title and abstract level, assessed the full text of included studies, and extracted relevant study data. A third reviewer or consensus-based discussion was used to resolve any conflicts that arose during the screening and data extraction process. Data were extracted to Microsoft Excel® [v.2301]. Figures and tables were created using Microsoft Excel® [v.2301] and PowerPoint® [v.2301].
Real-world and clinical trial evidence are associated with different limitations and advantages for assessing treatment adherence and its effect on clinical outcomes. Clinical trials occur in a controlled setting with protocols to ensure that a direct comparison can be made between study arms. Patient behavior is closely monitored to ensure that patients take medication as prescribed, resulting in higher adherence than would be observed outside of the clinical trial environment. Additionally, patient populations in clinical trials are often more homogenous than in real-world settings.21 In contrast, RWE is based on real-world data, often collected as part of routine healthcare administration and billing. Patients are not randomized and may be assigned to treatments based on physician bias, patient request, or insurance coverage.22 Patient behaviors are typically captured more accurately by RWE. We chose to seek out both clinical trial data and RWE, since information from these sources is often complementary to one another.
The SLRs of RWE and clinical trial data identified treatment adherence, compliance, and persistence outcomes. The terms, while similar, denote different aspects of patient behavior as it relates to clinical recommendations and prescriptions. Adherence is defined as the proportion of prescribed pills taken over a specific interval of time.23 While the adherence threshold can vary across medications, a patient is generally considered adherent if they align with their prescribed dosing schedule 80% of the time.24 Commonly used measures of adherence report the proportion of prescribed medication that a patient acquired at the pharmacy; these include the medication possession ratio (MPR) and the proportion of days covered.25 In contrast to adherence, compliance encompasses a broader definition, referring to the extent to which patients align their medication usage with the recommended dosage, timing, and frequency provided by healthcare professionals in their day-to-day clinical management.26 Treatment persistence refers to the act of continuing to take clinically recommended medication.26 In the literature, some research studies use the terms treatment adherence and treatment compliance interchangeably. No subjective decisions were made to recategorize the data in identified studies; instead, the measures were reported as they were originally described. The number of studies as reported in the results are not mutually exclusive as some studies report multiple outcomes.
When possible, comparable studies that reported the same outcome measures were placed in context with one another for data visualization and reporting, but no data transformations or meta-analyses were conducted. Outcome measures were included when the same metrics were reported across fixed-dose combination and LDC versions of equivalent formulations and doses, either from clinical trials, cohort studies, retrospective database studies, or switching studies, in which patients on LDCs with baseline measurements were switched to treatment with equivalent fixed-dose combinations. Statistically significant and numerical findings were reported as described in each study; no post-hoc statistical tests were performed.
RESULTS
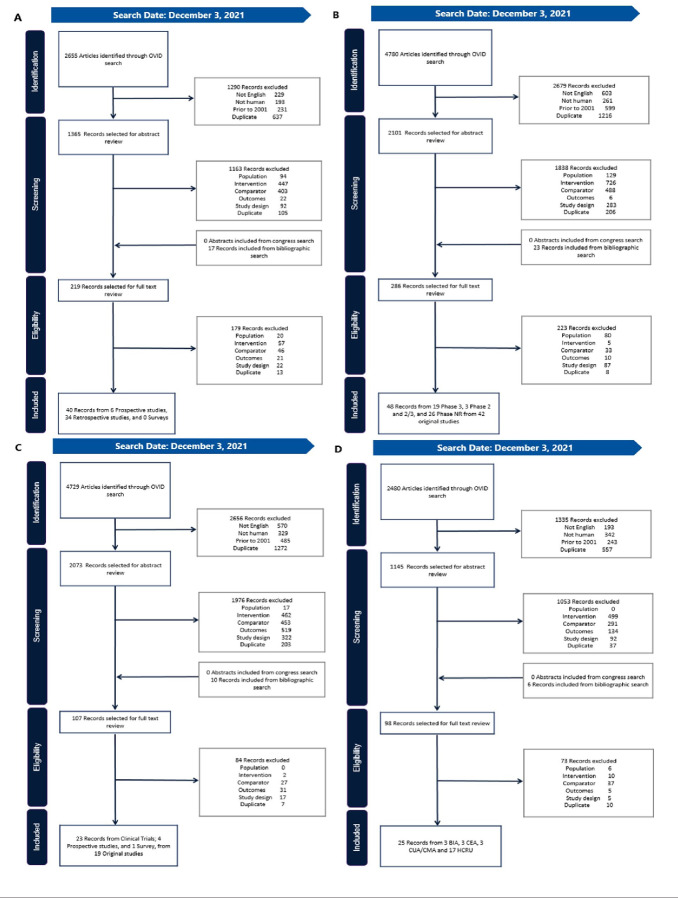
After accounting for duplicate records, 109 original studies were included in our findings (Figure 1).27–134 Twenty-seven of these studies were identified in more than 1 SLR.33,34,37,40,42,44,47,48,51–53,55,65–68,75,87,98,100,106–108,116,119,128,133 A list of the identified studies by SLR, along with study type and the indications included, is presented in Supplemental Table 3. North American, European, and Asian countries were well represented in studies relating to clinical evidence, HRQoL, and economic outcomes, while RWE was reported only from some North American and European countries.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses Diagrams.
Our findings included evidence relating to treatment adherence, treatment persistence, and clinical outcomes in RWE (A, n = 40) and clinical trials (B, n =48), along with HRQoL (C, n = 23) and economic outcomes (D, n = 25).
Abbreviations: BIA, budget impact analysis; CEA, cost-effectiveness analysis; CMA, cost-minimization analysis; CUA, cost-utility analysis; HRQoL, health-related quality of life; HCRU, healthcare resource utilization.
STCTs were the most common fixed-dose formulation (n = 72), followed by inhaled suspensions (n = 18), eye-drop solutions (n = 16), and injected solution and nasal spray (n = 1 each).
Treatment Adherence, Compliance, Persistence, and Clinical Outcomes in RWE
Forty RWE studies (n = 34 retrospective observational31,33,36,38,40,42,47,54–56,58,59,74,76,85–88,94,96,97,99,100,110–115,121,122,126,127,129 and n = 6 prospective observational)49,63,65,71,103,104 reported information on adherence rates, persistence rates, compliance, or clinical outcomes. The included clinical indications were frequently those treated with multiple drugs, and/ or with common comorbidities that added to the pill burden, such as cardiovascular disease (n = 23, including 19 hypertension),40,42,49,54,59,63,71,74,76,86–88,94,96,97,99,100,110–115 type 2 diabetes mellitus (n = 10),31,36,38,47,55,58,103,104,126,127 HIV (n = 3),33,85,129 asthma (n = 2),121,122 and 1 study each for chronic obstructive pulmonary disease (COPD)65 and Parkinson’s disease.56
Thirteen studies reported treatment adherence in patients taking STCTs vs LDCs, using MPR (n = 11), proportion of days covered (n = 7), or mean adherence (n = 3). In 12 of those studies, treatment adherence was significantly higher in patients who received fixed-dose combinations compared with LDCs36,40,42,55,56,76,85–87,96,97,122; in the remaining study, patients taking STCTs had a numerically higher adherence.49 An additional 8 studies described treatment adherence in patients who switched from LDCs or monotherapy to fixed-dose combinations,31,38,47,54,58,71,126,127 with reported follow-up times ranging from 3 months63 to 36 months.38 Patients who switched to STCTs had higher treatment adherence in all studies except for one, in which patients switched from sulfonylureas (including glyburide) plus metformin to glyburide/ metformin for type 2 diabetes.58 Even in this study, the STCT had better clinical outcomes (improvement of glycemic control, A1C reduction of 0.6% (p = .002), and the difference in adherence was not statistically significant (92.4% vs 90.9% after the switch to STCT). Of the studies that reported improved adherence with STCTs, 8 were of patients with hypertension40,42,49,54,86,87,96,97 and 6 were of patients with type 2 diabetes mellitus.31,36,38,47,55,126
Four studies reported treatment compliance with STCTs or fixed-dose inhalers compared with LDCs.65,103,104,110 Treatment compliance was significantly lower in patients taking LDCs in 2 of the 4 studies.103,104 In the remaining 2 studies, compliance was numerically high with both fixed-dose combinations and LDCs (70.0%-80.0%),110 and similar in 1 study using a fixed-dose inhaler (compliance defined as residual doses in the inhalation device returned by the patient 97.1% in the fixed dose inhaler group vs 98.4% in the 2-inhaler group).65
Of the 18 studies that examined treatment persistence, all studies reported higher persistence in patients taking STCTs or fixed-dose inhalers compared with LDCs. Among these 18 studies, 16 reported significantly higher persistence in patients taking STCTs or fixed-dose inhalers compared with LDCs in 1 or more treatment arms40,42,56,59,74,87,96,97,100,110,112–115,121,122; while the remaining 2 studies did not report statistical significance, only a numerically higher treatment persistence with STCTs.88,111 Thirteen studies reported mean 1-year persistence in STCTs vs LDCs (Table 1)40,42,59,74,87,88,96,110–115; of these, 9 studies reported ≥20% higher persistence in patients taking STCT.42,59,74,87,96,111–113,115 When summarized across studies, mean 1-year persistence was 51.9% for STCTs and 31.5% for LDCs. In a large retrospective study that included 81 958 patients with hypertension, treatment persistence ranged from 16% to 42% greater in patients on STCTs compared with LDCs.40
Table 1. Summary of RWE Findings for Persistence, Compliance, and Adherence, Where Comparable.
| Source | Indication | Population | Intervention | STCT | LDC | ΔSTCT–LDC |
| Mean 1-year persistence (%) | ||||||
| Bramlage et al, 201840 | HTN | Patients with HTN | Ramipril/amlodipine | 65.7 | 48.6 | 17.10 |
| Candesartan/amlodipine | 55.5 | 43.1 | 12.40 | |||
| Brixner et al, 200842 | HTN | Adults with HTN | Valsartan/ hydrochlorothiazide |
44.0 | 16.0 | 28.00 |
| Ehlken et al, 201159 | HTN | Patients with HTN | Olmesartan/ hydrochlorothiazide |
44.6 | 25.0 | 19.60 |
| Olmesartan/amlodipine | 47.3 | 27.4 | 19.90 | |||
| Valsartan/ hydrochlorothiazide |
39.6 | 13.7 | 25.90 | |||
| Valsartan/amlodipine | 44.6 | 25.2 | 19.40 | |||
| Jackson et al, 200674 | HTN | Adults with HTN | Valsartan/ hydrochlorothiazide |
44.0 | 16.0 | 28.00 |
| Machnicki et al, 201587 | HTN | Adults with HTN | Amlodipine/valsartan/ hydrochlorothiazide |
46.8 | 23.6 | 23.20 |
| Maggioni et al, 201988 | ACS | Patients with ACS | Aspirin/clopidogrel | 81.5 | 72.9 | 8.60 |
| Ong et al, 201496 | HTN | Patients with HTN | Amlodipine/valsartan/ hydrochlorothiazide (Exforge) HCT) |
47.2 | 23.6 | 23.60 |
| Sandberg et al, 2011110 | HTN | Patients with HTN | Olmesartan/ hydrochlorothiazide |
44.6 | 25.0 | -19.6 |
| Olmesartan/amlodipine | 47.3 | 27.4 | 19.9 | |||
| Simons et al, 2011112 | HTN | Patients with HTN | Amlodipine/atorvastatin | 67.0 | 41.0 | 26.00 |
| Simons et al, 2017111 | HTN | Patients with HTN | Amlodipine/atorvastatin | 66.0 | 43.0 | 23.00 |
| Simonyi et al, 2015113 | HTN | Patients with HTN | Ramipril/amlodipine | 54.0 | 34.0 | 20.00 |
| Simonyi et al, 2016114 | HTN | Patients with HTN | Perindopril/amlodipine | 40.0 | 27.0 | 13.00 |
| Simonyi et al, 2017115 | HTN | Patients with HTN | Ramipril/amlodipine | 54.0 | 34.0 | 20.00 |
| Mean compliance (%) | ||||||
| Hagedorn et al, 201365 | COPD | Adults with stage 3 or 4 COPD |
Fluticasone/salmeterol | 97.1 | 98.4 | -1.30a |
| Rombopoulos et al, 2012104 | T2DM | Adults with T2DM | Vildagliptin/metformin | 68.0 | 56.0 | 12.00 |
| Rombopoulos et al, 2015103 | T2DM | Adults with T2DM | Vildagliptin/metformin | 98.9 | 84.6 | 14.30 |
| Mean adherence (%) | ||||||
| Degli et al, 201854 | HTN | Adult with HTN | Perindopril + amlodipine to perindopril/amlodipine |
79.8 | 70.9 | 8.90 |
| Duckworth, 200358 | T2DM | Adults with T2DM who had been treated with glipizide or glyburide plus metformin 6 mo prior to switching to glyburide/ metformin |
Glyburide + metformin or glipizide + metformin to glyburide/metformin |
90.9 | 92.4 | -1.50b |
| Legorreta et al, 200585 | HIV | Adults with HIV who initiated therapy on or after September 1997 |
Lamivudine/zidovudine | 85.0 | 75.0 | 10.00 |
aStatistical significance not measured.
bNo statistically significant difference.
Abbreviations: ACS, acute coronary syndrome; COPD, chronic obstructive pulmonary disease; HTN, hypertension; LDC, loose-dose combination; STCT, single-tablet combination therapy; T2DM, type 2 diabetes mellitus.
Clinical outcomes were reported by 17 studies, across indications of asthma, COPD, hypertension, and type 2 diabetes mellitus (Table 2).36,40,42,49,58,63,65,71,94,99,100,103,104,111,121,122,126 Positive findings with STCTs or fixed-dose inhalers were reported in all but 3 studies that described similar outcomes with STCTs/fixed-dose inhalers and LDCs.65,104,121 Of the studies that reported improved clinical outcomes with STCTs, 3 contained caveats that may weaken the finding, such as the likelihood of residual confounders,36,94,111 and 2 reported improvements in subgroups of adherent or persistent patients only.42,94 Significantly improved clinical outcomes included hemoglobin A1C (n = 3),36,58,126 the need for add-on medication (n = 3),40,42,122 all-cause hospitalization (n = 2),99,100 blood pressure control (n = 1),63 and all-cause mortality (n = 1).99 Six studies reported statistical significance in both clinical outcome improvement and adherence, compliance, or persistence improvement.40,42,100,103,122,126
Table 2. Reported Clinical Outcomes With STCTs.
| Source | Intervention | Clinical Outcome Compared With LDC | Adherence, Compliance, and/or Persistence Compared With LDC |
| Asthma studies | |||
| Stempel et al, 2005121 | Ramipril/amlodipine | No difference in the need for add-on SABA medication | Persistence improvementa |
| Stoloff et al, 2004122 | Fluticasone/salmeterol | Reduced need for add-on SABA medicationa |
|
| Cardiovascular disease studies | |||
| Ofili et al, 201794 | Isosorbide dinitrate/ hydralazine hydrochloridec |
Among adherent Black American patients, improved 1-year overall survivala | Worsened adherence |
| Predel et al, 2020100 | Aspirin/ramipril/ atorvastatin |
Reduced incidence rate ratios for:
|
Persistence improvementa |
| Predel et al, 202199 | Ramipril/amlodipine |
Reduced incidence rate ratios for:
|
Not measured |
| COPD | |||
| Hagerdorn et al, 201365 | Fluticasone/salmeterol |
No difference in:
|
No difference in compliance |
| Hypertension studies | |||
| Bramlage et al, 201840 | Ramipril/amlodipine |
|
|
| Candesartan/ amlodipine |
|
|
|
| Brixner et al, 200842 | Valsartan/ hydrochlorothiazide |
|
|
| Czarnecka et al, 201549 | Bisoprolol/amlodipine |
|
Adherence improvement |
| Gaciong et al, 201763 | Bisoprolol/ acetylsalicylic acid |
|
Not measured |
| Hostalek et al, 201571 | Bisoprolol/amlodipine |
|
Not measured |
| Simons 2017111 | Amlodipine/ perindopril |
|
Persistence improvement |
| T2DM studies | |||
| Blonde et al, 200336 | Glyburide/metformin | A1C improvementa | Adherence improvementb |
| Duckworth 200358 | Glyburide/metformin | A1C improvementa | Worsened persistence |
| Rombopoulos et al, 2012104 | Vildagliptin/ metformin |
No difference in A1C | Compliance improvementa |
| Rombopoulos et al, 2015103 | Vildagliptin/ metformin |
A1C improvement | Compliance improvementa |
| Thayer et al, 2010126 | Rosiglitazone/ sulfonylurea |
A1C improvementa | Adherence improvementa |
aStatistically significant result.
bLinear regression did not find a relationship between adherence and improved A1C.
cSTCT may not be bioequivalent to its LDC comparator.14
dStudy authors attributed result to residual confounders.
Abbreviations: A1C, hemoglobin A1C; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; FEV1, forced expiratory volume in 1 second; IVC, inspiratory vital capacity; LDC, loose-dose combination therapy; SABA, short- acting β-agonist; SBP, systolic blood pressure; STCT, single-tablet combination therapy; T2DM, type 2 diabetes mellitus.
Treatment Adherence, Compliance, Persistence, and Clinical Outcomes in Clinical Trials
The SLR for clinical trial evidence identified 48 records from 42 studies across the indications of glaucoma/ocular hypertension (n = 10),30,35,57,61,66–69,81,134 hypertension (n = 6),41,60,83,92,124,131 asthma (n = 7),37,72,75,98,106,107,133 tuberculosis (n = 6),28,32,79,91,123,125,132 HIV (n = 3),48,108,128 and others. Twenty-six studies did not identify the study phase,28,29,32,34,44,50,57,60,61,75,80,81,83,92,93,98,106–108,123–125,128,131–133 14 were Phase 3 studies,27,30,35,37,39,41,48,51–53,66–69,72,82,89,116,134 1 was Phase 2,45 and 1 was Phase 2/3.79,91 Clinical trials were geographically diverse, with more studies including participants from Africa and Asia compared with the other study types. Thirteen studies reported adherence outcomes with fixed-dose combinations and LDCs: 9 reported similar outcomes with fixed-dose combinations and LDCs,28,48,75,80,89,92,98,108,133 3 reported a statistically significant improvement in adherence with STCTs,45,83,124 and 1 reported a numerical improvement with fixed-dose eye drops.30 Among the studies that reported a significant improvement in adherence with STCTs, 2 also reported improvements in blood pressure control.83,124 All 4 studies that described compliance outcomes reported similar findings with STCTs/fixed-dose inhalers and LDCs.27,28,37,123
Health-Related Quality of Life Findings
HRQoL findings were reported in 23 records of 19 studies,34,37,44,48,51–53,64–68,73,75,95,98,106–108,116,119,128,133 which included 14 clinical trials34,37,44,48,51–53,66–68,75,98,106–108,116,128,133 and 5 observational studies64,65,73,95,119 primarily from North American and European countries. The studies included indications consisting of asthma (n = 7),37,75,98,106,107,119,133 HIV (n = 3),48,108,128 COPD (n = 2),51–53,65 and glaucoma (n = 2),66–68,73 among others. The study findings were largely noncomparable due to the heterogeneous methods used to assess HRQoL, which included generic and disease-specific scales, patient-reported outcome measures, and symptom reporting. Findings from the studies were mixed, with 5 of 8 treatment arms in the observational studies reporting a numerical (n = 4)64,65,73,95 or significant (n = 1)64 improvement in HRQoL with STCTs, and the remaining 3 treatment arms describing similar outcomes with fixed-dose combinations and LDCs.65,73,119 Results were similar between fixed-dose combinations and LDCs in the majority (n = 9)34,44,48,53,68,98,107,128,133 of the interventional HRQoL studies, but numerical improvements with fixed-dose combinations (n = 3)37,108,116 and LDCs (n = 1)75 were also reported.
Economic Outcomes
Economic outcomes were evaluated in 25 studies from 11 countries in North and South America, Europe, and Asia, and reported on indications of hypertension (n = 10),40,42,46,62,70,77,78,87,102,120 asthma (n = 4),43,90,105,119 diabetes (n = 3),47,55,109 cardiovascular disease (n = 3),100,117,118 COPD (n = 2),65,101 glaucoma/ocular hypertension (n = 2),84,130 and HIV (n = 1).33 Six cost-effectiveness or cost-utility analyses compared STCT or fixed-dose inhalers with LDCs (n = 6, Table 3),43,62,77,101,102,120 including 3 branded STCTs62,77,101 and 1 branded fixed-dose inhaler.43 All 6 analyses found that fixed-dose combinations were both less expensive and more efficacious than LDCs (in economic terminology, fixed-dose products dominated LDCs). Several budget impact analyses evaluated the use of STCTs and LDCs for patients with cardiovascular diseases, including hypertension and hyperlipidemia (n = 4, Table 4).62,78,117,118 The scope of the analyses varied, with some studies estimating the cost savings from public payer62,78 and patient perspectives78 and others calculating the costs of medication117 or treatment.118 In all 4 studies, STCTs were associated with savings compared with LDCs.
Table 3. Findings from Cost-Effectiveness Models and Cost-Utility Analyses.
| Reference | Study Design | Country | Population | Intervention (STCT Brand) | Main Message |
|---|---|---|---|---|---|
| Kawalec et al, 201577 | CEA + CUA | Poland | Patients with arterial hypertension |
Indapamide + amlodipine (Tertens-AM) |
STCT was less expensive than treatment with LDCs |
| Subgroup: STCT patients with higher adherence |
STCT dominates LDCs, in both NHF and patient’s perspective |
||||
| Ren et al, 2020102 | CEA + CUA | China | Hypertensive adults | Olmesartan + amlodipine (Sevikar) |
STCT dominates LDCs |
| Fujii et al, 201562 | CEA, BIA | Brazil | Systemic arterial hypertension |
Bisoprolol + amlodipine (Concor AM) |
STCT dominates LDCs |
| Stawowczyk et al, 2014120 | CUA | Poland | Hypertensive patients | Indapamide + amlodipine (Natrixam) |
Public payer perspective: STCT dominates LDCs Patient’s perspective: Net monetary benefit was higher with STCT |
| Price et al, 2014101 | CMA | Sweden | Moderate-to-severe COPD patients |
Indacaterol + glycopyrronium (Utibron) |
STCT dominates LDCs in all horizons, and in PSA iterations |
| Brüggenjürgen et al, 201043 | CMA | Germany | Asthma | Beclomethasone dipropionate + formoterol fumarate (CHF 1535) |
STCT dominates LDCs |
Note: The result of the CEA from Fujii et al (2015) is shown here; findings from the BIA from Fujii et al (2015) is reported in Table 4.
Abbreviations: BIA, budget impact analysis; CEA, cost-effectiveness analysis; CMA, cost-minimization analysis; COPD, chronic obstructive pulmonary disease; CUA, cost-utility analysis; LDC, loose-dose combination; NHF, National Health Fund; PSA, probabilistic sensitivity analysis; STCT, single-tablet combination therapy.
Table 4. Findings from Budget Impact Analyses.
| Short Reference | Country; Currency | Time Horizon | Population |
Intervention (STCT
Brand) |
Savings | Budget Impact | Main Message |
|---|---|---|---|---|---|---|---|
| Fujii et al, 201562 | Brazil; Brazilian real (BRL) | 10 y | Systemic arterial hypertension | Bisoprolol + amlodipine (Concor AM) | NR | -300 321 412 | There were financial resource savings with Concor AM, in 2014- 2025 |
| Kawalec et al, 201478 | Poland; Polish zloty (PLN) | 3 y | Hypertensive patients | Indapamide + amlodipine (Natrixam) | Cost savings at year 3: NHF perspective: -725 965 Patient perspective: -8 328 480 |
NR | Treatment with indapamide/amlodipine STCTs vs LDCs generates significant savings both from the public payer and patient perspectives |
| Stafylas et al, 2018117 | Greece; euros (€) | 5 y | Essential hypertension and/or stable coronary artery disease in association with primary hypercholesterolemia or mixed hyperlipidemia | Atorvastatin + perindopril + amlodipine | Year 5: -1 903 639 |
Cumulative over 5 y: -6 107 965 |
Introduction of STCTs at a price lower than that of the LDCs of the same agents will reduce medication costs |
| Stafylas et al, 2019118 | Greece; euros (€) | 5 y | Hyperlipidemia patients | Rosuvastatin + ezetimibe |
Year 5: -395 447 |
Cumulative over 5 y: -2 351 952 |
The introduction of STCTs of ezetimibe/ rosuvastatin is expected to reduce treatment costs |
Note: The result of the BIA from Fujii et al (2015) is shown here; findings from the CEA from Fujii et al (2015) is reported in Table 3.
Abbreviations: BIA, budget impact analysis; CEA, cost-effectiveness analysis; COPD, chronic obstructive pulmonary disease; CUA, cost-utility analysis; LDC, loose-dose combination; NHF, National Health Fund; NR, not reported; PSA, probabilistic sensitivity analysis; STCT, single-tablet combination therapy
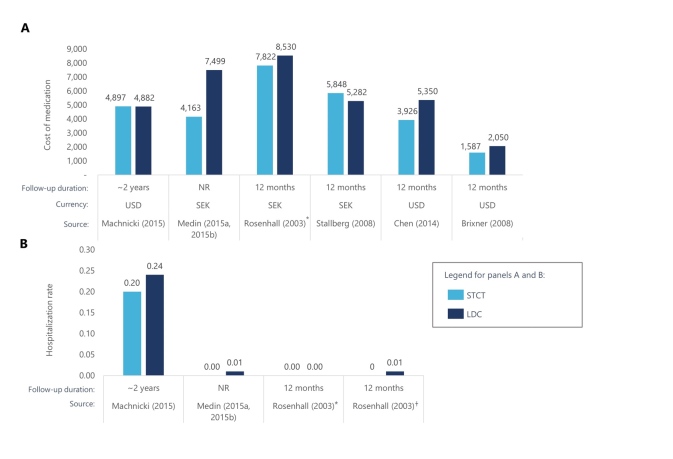
Among the 16 studies that examined medical costs and HCRU, findings were largely favorable toward fixed-dose combinations.33,40,42,46,47,55,65,70,84,87,90,100,105,109,119,130 Medication costs over time were generally lower for fixed-dose combinations compared with LDCs (n = 6,42,46,87,90,105,119 Figure 2A). Several studies reported lower overall medical costs with STCTs,42,46,87 including a retrospective cohort study that included 8711 US patients with hypertension.42 Patients received valsartan plus hydrochlorothiazide as an STCT or LDC and HCRU costs were compared after 12 months.42 The average annual medical costs for patients receiving an STCT were $474 lower than for patients who received an LDC during the study period of 1996 to 2004. When patients were both persistent and adherent to medication, medical costs were $608 lower. There was also evidence of STCTs and fixed-dose inhalers reducing HCRU, with 3 studies reporting lower hospitalization rates over time (n = 3).87,90,105 STCTs were also associated with reductions in emergency department costs87 and utilization rates.46,87,100
Figure 2. Medication Costs for STCTs and LDCs.
Several of the identified economic studies reported the cost of medication over time for STCTs vs LDCs. Of these, 3 studies also reported hospitalization rates for STCTs vs LDCs.
*Patients in this comparison received budesonide + formoterol in a single inhaler or 2 inhalers
†Patients in this comparison received budesonide + formoterol + terbutaline PRN in 2 or 3 inhalers Abbreviations: LDC, loose-dose combination product; NR, not reported; SEK, Swedish krona; STCT, single-tablet combination therapy; USD, US dollar.
Sources: References 42, 46, 87, 90, 105, 119.
DISCUSSION
The results of this study reflect the growing body of evidence that link STCTs to increased treatment adherence and persistence, along with positive clinical and economic outcomes. The strongest evidence supporting STCT use came from the RWE and economic studies. Improved adherence and persistence with STCTs were seen consistently across RWE studies, including studies where patients switched from an LDC to an STCT. The majority of the studies that reported clinical outcomes with STCTs described improvements, particularly in glycemic control and the reduced need for add-on medication. Several studies linked improved adherence or persistence outcomes with improved clinical efficacy. Studies that did not find a link between adherence/persistence and clinical outcomes included patients with complex, chronic diseases, such as asthma, COPD, and cardiovascular disease. It is possible that unmeasured confounders, such as disease severity, time since diagnosis, or symptom presentation affected the measurement of clinical outcomes, and more work is needed to better describe the impact of STCTs for patients with these indications. Only 4 studies were identified that described compliance outcomes, perhaps due to evolving terminology that increasingly favors “adherence” over “compliance,” and retrospective studies that often include adherence and persistence measures based on patient records. The economic findings in favor of STCTs were robust and suggested that the improved clinical outcomes associated with STCTs can lead to reduced medication costs, hospitalizations, and overall medical costs over time. All the identified cost-effectiveness, cost-utility, and budget impact analyses demonstrated savings with STCTs compared with LDCs.
While some clinical and HRQoL studies reported improvement in adherence and clinical outcomes with STCTs, the results were more heterogeneous. This may be a result of data collection methodology that is not optimized to compare outcomes with STCTs and LDCs. The controlled environment of clinical trials is designed to facilitate high treatment adherence, which may increase the adherence and persistence outcomes for LDCs and affect LDC-STCT comparisons in ways unrelated to pill burden. In studies that reported HRQoL outcomes, there was some evidence of improved outcomes among patients who received STCTs compared with those who received LDCs. However, most studies focused primarily on patient symptoms and general measures of HRQoL, rather than pill burden or drug administration processes. There is a need for future studies to compare STCTs and LDCs with HRQoL measures that more closely reflect the aspects of drug administration. Since there is evidence linking poor treatment satisfaction to poor adherence in patients with chronic diseases,135,136 validated tools to measure treatment satisfaction, such as the Treatment Satisfaction Questionnaire for Medication137 and the Diabetes Medication System Rating Questionnaire,138 may be useful in understanding how STCTs can affect HRQoL.
Our results, which included studies from countries on 6 continents, indicated that STCTs can boost adherence, clinical outcomes, and economic savings in most countries, but some considerations may be especially important in local markets. Much of the identified RWE and economic studies were based in the US and Europe. As a general recommendation, future research should include patients in other regions to shed light on global variation and common themes across STCT use and outcomes.
While this article may be especially useful for payers, the benefits of STCT use are relevant to multiple stakeholders, including patients, clinicians, and caregivers. For patients, improved treatment adherence with STCTs can result in positive clinical outcomes, reduced medical costs, and improved treatment satisfaction. STCT use helps to align patient actions with the recommendations of clinicians, contributing to positive clinical outcomes. Caregivers benefit from STCTs through reduced medication costs, co-pay costs, and drug administration burden. Finally, payers benefit from the reduced economic burden and HCRU that is accompanied by improved clinical outcomes.
Strengths
The substantial amount of literature reporting outcomes of STCTs vs LDCs, spanning the last 2 decades and various countries, greatly contributed to the value of this study. The use of common measures in RWE studies and cost- effectiveness and budget impact analyses resulted in comparability across many studies. Furthermore, in contrast to previously published SLRs that primarily focused on the effects of STCTs in single indications including hypertension,139,140 diabetes,16 or HIV,141 our searches were not limited by indication or intervention, which allowed for broad commonalities to emerge in the comparison of STCTs and LDCs. Not only do the results of our SLR support the observations that use of STCTs may improve adherence and clinical effectiveness while also lowering costs as presented in previously published SLRs,16,139–142 our study further provides analysis among patients with Parkinson’s,143 asthma,43 and more general cardiovascular disease,94,99,100 which have previously not been reported.
Limitations
Comparability in this study was limited by the variation in the outcomes that were measured and reported by the included studies. Additionally, in some cases, studies that did not report favorable results for the use of STCTs by one metric reported broadly favorable results for STCT use when considering other metrics. One example is a study that reported the results of switching from LDCs to STCTs in patients with type 2 diabetes mellitus.58 Although treatment adherence was high before and after switching, adherence slightly declined after patients switched to STCTs (92.4% to 90.9%).58 Despite this, hemoglobin A1C values were significantly improved with STCTs.58 The context of the original study, though largely favorable to STCT use, is lost when reporting aggregated adherence results.
The RWE findings clearly showed that STCT use is associated with improved outcomes but do not provide a mechanistic explanation for this within the context of drug administration. Since STCTs affect several distinct administration processes by reducing the number of medications to be prescribed, filled at the pharmacy, and consumed, studies that examine these processes directly could differentiate between benefits provided by STCTs and other administration options, like bundling prescriptions to be filled simultaneously at the pharmacy.144
Lastly, some heterogeneity in our results may be due to the inherent nature of different diseases that vary in patient population demographics, drug administration protocols, symptom severity, and perceived mortality risk—factors that can influence patient behavior and treatment adherence.
CONCLUSIONS
The use of STCTs resulted in positive outcomes compared with LDCs across clinical studies, RWE, HRQoL studies, and economic evaluations. In RWE studies, STCTs were associated with consistent improvements in treatment adherence, persistence, and compliance, with evidence for greater adherence and persistence resulting in positive clinical outcomes. STCTs consistently dominated LDCs in cost-effectiveness and cost-utility analyses, and all identified budget impact analyses found cost savings with STCTs. This study provides a solid foundation for understanding the benefits of STCTs across clinical indications and the need for these to be thoughtfully considered in formulary decision-making. The findings highlight opportunities for future research to contribute by reporting STCT outcomes in additional countries and measuring drug administration–related HRQoL.
Author Contributions
C.P. contributed to the planning, critical review of the manuscript, and approved the final manuscript; J.L. contributed to the planning, critical review of the manuscript, and approved the final manuscript; J.W. contributed to the planning, critical review of the manuscript, and approved the final manuscript; K.G. performed the systematic literature review, data extraction, and approved the final manuscript; D.T. performed the systematic literature review and data extraction, and approved the final manuscript; and S.G. performed the systematic literature review, data extraction, and approved the final manuscript.
Disclosures
C.P. is an employee of Janssen Pharmaceutical Companies of Johnson & Johnson and J.L. is an employee of Janssen-Cliag of Johnson & Johnson, both with stock or stock options at Johnson & Johnson. J.W. was an employee of Janssen Pharmaceutical Companies of Johnson & Johnson at the time of the study. D.T. and S.G. are employees of Cytel, Inc. K.G. was an employee of Cytel, Inc. at the time of the study.
Supplementary Material
Acknowledgments
Acknowledgments
Writing support was provided by Allison Brackley, PhD, and Elizabeth Hubscher, PhD of Cytel, Inc.
Funding Statement
This study and its reporting were funded by Janssen Pharmaceuticals.
References
- Cutler Rachelle Louise, Fernandez-Llimos Fernando, Frommer Michael, Benrimoj Charlie, Garcia-Cardenas Victoria. BMJ Open. 1. Vol. 8. BMJ; Economic impact of medication non-adherence by disease groups: a systematic review; p. e016982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adherence to treatment and related factors among patients with chronic conditions in primary care: a cross-sectional study. Fernandez-Lazaro Cesar I., García-González Juan M., Adams David P., Fernandez-Lazaro Diego, Mielgo-Ayuso Juan, Caballero-Garcia Alberto, Moreno Racionero Francisca, Córdova Alfredo, Miron-Canelo Jose A. Sep 14;2019 BMC Family Practice. 20(1):132. doi: 10.1186/s12875-019-1019-3. doi: 10.1186/s12875-019-1019-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medication adherence, hospitalization, and healthcare resource utilization and costs in patients with pulmonary arterial hypertension treated with endothelin receptor antagonists or phosphodiesterase type-5 inhibitors. Frantz Robert P., Hill Jerrold W., Lickert Cassandra A., Wade Rolin L., Cole Michele R., Tsang Yuen, Drake William III. Jan;2020 Pulmonary Circulation. 10(1):2045894019880086. doi: 10.1177/2045894019880086. doi: 10.1177/2045894019880086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adherence to disease-specific drug treatment among patients with pulmonary arterial hypertension or chronic thromboembolic pulmonary hypertension. Kjellström Barbro, Sandqvist Anna, Hjalmarsson Clara, Nisell Magnus, Näsman Per, Ivarsson Bodil. Oct;2020 ERJ Open Research. 6(4) doi: 10.1183/23120541.00299-2020. doi: 10.1183/23120541.00299-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primary nonadherence to chronic disease medications: a meta-analysis. Lemstra Mark, Nwankwo Chijioke, Bird Yelena, Moraros John. May;2018 Patient Preference and Adherence. 12:721–731. doi: 10.2147/ppa.s161151. doi: 10.2147/ppa.s161151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treatment patterns, healthcare resource utilization, and healthcare costs among patients with pulmonary arterial hypertension in a real-world US database. Studer Sean, Hull Michael, Pruett Janis, Koep Eleena, Tsang Yuen, Drake William, III. 2019Pulmonary Circulation. 9(1):1–11. doi: 10.1177/2045894018816294. doi: 10.1177/2045894018816294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrador Marta, Cabral Ana Cristina, Castel-Branco Margarida, Figueiredo Isabel Vitoria, Fernandez-Llimos Fernando. Aging. Vol. 2023. Elsevier; Polypharmacy and medication adherence; pp. 435–453. [DOI] [Google Scholar]
- Interventions to improve medication adherence: a review. Kini Vinay, Ho P. Michael. Dec 18;2018 JAMA. 320(23):2461–2473. doi: 10.1001/jama.2018.19271. doi: 10.1001/jama.2018.19271. [DOI] [PubMed] [Google Scholar]
- Medication adherence interventions and outcomes: an overview of systematic reviews. Wilhelmsen Nina C, Eriksson Tommy. 2019European Journal of Hospital Pharmacy. 26(4):187–192. doi: 10.1136/ejhpharm-2018-001725. doi: 10.1136/ejhpharm-2018-001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes—2023. ElSayed Nuha A., Aleppo Grazia, Aroda Vanita R., Bannuru Raveendhara R., Brown Florence M., Bruemmer Dennis, Collins Billy S., Hilliard Marisa E., Isaacs Diana, Johnson Eric L., Kahan Scott, Khunti Kamlesh, Leon Jose, Lyons Sarah K., Perry Mary Lou, Prahalad Priya, Pratley Richard E., Seley Jane Jeffrie, Stanton Robert C., Gabbay Robert A. Dec 12;2022 Diabetes Care. 46(suppl 1):S140–S157. doi: 10.2337/dc23-s009. doi: 10.2337/dc23-s009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Initiative for Asthma (GINA) Global Strategy for Asthma Management and Prevention, 2023 Update. https://ginasthma.org/wp-content/uploads/2023/05/GINA-2023-Full-Report-2023-WMS.pdf
- 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and European Renal Association (ERA) Mancia G., Kreutz R., Brunström M.., et al. 2023J Hypertens. 41(12):1874–2071. doi: 10.1097/HJH.0000000000003480. [DOI] [PubMed] [Google Scholar]
- Effects of a fixed-dose combination strategy on adherence and risk factors in patients with or at high risk of CVD: the UMPIRE randomized clinical trial. Thom Simon, Poulter N, Field J, et al. Sep 4;2013 JAMA. 310(9):918–929. doi: 10.1001/jama.2013.277064. doi: 10.1001/jama.2013.277064. [DOI] [PubMed] [Google Scholar]
- The impact of fixed-dose combination versus free-equivalent combination therapies on adherence for hypertension: a meta-analysis. Du Li-Ping, Cheng Zhong-Wei, Zhang Yu-Xuan, Li Ying, Mei Dan. Apr 27;2018 The Journal of Clinical Hypertension. 20(5):902–907. doi: 10.1111/jch.13272. doi: 10.1111/jch.13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effectiveness of fixed-dose combination therapy in hypertension: systematic review and meta-analysis. Kawalec Paweł, Holko Przemysław, Gawin Małgorzata, Pilc Andrzej. 2018Archives of Medical Science. 14(5):1125–1136. doi: 10.5114/aoms.2018.77561. doi: 10.5114/aoms.2018.77561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A systematic review of adherence, treatment satisfaction and costs, in fixed-dose combination regimens in type 2 diabetes. Hutchins Valerie, Zhang Bin, Fleurence Rachael L., Krishnarajah Girishanthy, Graham John. Apr 5;2011 Current Medical Research and Opinion. 27(6):1157–1168. doi: 10.1185/03007995.2011.570745. doi: 10.1185/03007995.2011.570745. [DOI] [PubMed] [Google Scholar]
- Fixed-dose and fixed-ratio combination therapies in type 2 diabetes. Schlosser Robert. Aug;2019 Canadian Journal of Diabetes. 43(6):440–444. doi: 10.1016/j.jcjd.2019.05.005. doi: 10.1016/j.jcjd.2019.05.005. [DOI] [PubMed] [Google Scholar]
- Fixed-dose combination drug approvals, patents and market exclusivities compared to single active ingredient pharmaceuticals. Hao Jing, Rodriguez-Monguio Rosa, Seoane-Vazquez Enrique. Oct 15;2015 PLoS One. 10(10):e0140708. doi: 10.1371/journal.pone.0140708. doi: 10.1371/journal.pone.0140708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Body of evidence and approaches applied in the clinical development programme of fixed-dose combinations in the European Union from 2010 to 2016. Nøhr-Nielsen Asbjørn, De Bruin Marie Louise, Thomsen Mikael, Pipper Christian Bressen, Lange Theis, Bjerrum Ole Jannik, Lund Trine Meldgaard. Jun 17;2019 British Journal of Clinical Pharmacology. 85(8):1829–1840. doi: 10.1111/bcp.13986. doi: 10.1111/bcp.13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Page Matthew J, McKenzie Joanne E, Bossuyt Patrick M, Boutron Isabelle, Hoffmann Tammy C, Mulrow Cynthia D, Shamseer Larissa, Tetzlaff Jennifer M, Akl Elie A, Brennan Sue E, Chou Roger, Glanville Julie, Grimshaw Jeremy M, Hróbjartsson Asbjørn, Lalu Manoj M, Li Tianjing, Loder Elizabeth W, Mayo-Wilson Evan, McDonald Steve, McGuinness Luke A, Stewart Lesley A, Thomas James, Tricco Andrea C, Welch Vivian A, Whiting Penny, Moher David. Mar 29;2021 BMJ. 372:n71. doi: 10.1136/bmj.n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Eudocia, Wen Patrick. ESMO Open. suppl 4. Vol. 5. Elsevier BV; Gender and sex disparity in cancer trials; p. e000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Examining the use of real-world evidence in the regulatory process. Beaulieu-Jones Brett K., Finlayson Samuel G., Yuan William, Altman Russ B., Kohane Isaac S., Prasad Vinay, Yu Kun-Hsing. 2020Clinical Pharmacology & Therapeutics. 107(4):843–852. doi: 10.1002/cpt.1658. doi: 10.1002/cpt.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho P. Michael, Bryson Chris L., Rumsfeld John S. Circulation. 23. Vol. 119. Ovid Technologies (Wolters Kluwer Health); Medication adherence; pp. 3028–3035. [DOI] [Google Scholar]
- A systematic review of medication adherence thresholds dependent of clinical outcomes. Baumgartner Pascal C., Haynes R. Brian, Hersberger Kurt E., Arnet Isabelle. Nov 20;2018 Frontiers in Pharmacology. 9:1290. doi: 10.3389/fphar.2018.01290. doi: 10.3389/fphar.2018.01290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proportion of days covered as a measure of medication adherence. Loucks Jennifer, Zuckerman Autumn D, Berni Angelica, Saulles Adam, Thomas Gosia, Alonzo Amy. 2022American Journal of Health-System Pharmacy. 79(6):492–496. doi: 10.1093/ajhp/zxab392. doi: 10.1093/ajhp/zxab392. [DOI] [PubMed] [Google Scholar]
- Medication compliance and persistence: terminology and definitions. Cramer Joyce A., Roy Anuja, Burrell Anita, Fairchild Carol J., Fuldeore Mahesh J., Ollendorf Daniel A., Wong Peter K. Jan;2008 Value in Health. 11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- Open-label randomized non-inferiority trial of a fixed-dose combination of glimepiride and atorvastatin for the treatment of people whose Type 2 diabetes is uncontrolled on metformin. Ambery P., Stylianou A., Atkinson G., Dott C., Baylor Curtis L., Haque N., LaCroix K., Min K. W., on behalf of the Glimepiride/Atorvastatin Investigational Team 2016Diabetic Medicine. 33(8):1084–1093. doi: 10.1111/dme.13003. doi: 10.1111/dme.13003. [DOI] [PubMed] [Google Scholar]
- Efficacy and safety of ‘fixed dose’ versus ‘loose’ drug regimens for treatment of pulmonary tuberculosis in two high TB-burden African countries: a randomized controlled trial. Aseffa Abraham, Chukwu Joseph N., Vahedi Mahnaz, Aguwa Emmanuel N., Bedru Ahmed, Mebrahtu Tesfamariam, Ezechi Oliver C., Yimer Getnet, Yamuah Lawrence K., Medhin Girmay, Connolly Cathy, Rida Wasima, Aderaye Getachew, Zumla Alimuddin I., Onyebujoh Philip C., 4FDC Study Group Jun 20;2016 PloS One. 11(6):e0157434. doi: 10.1371/journal.pone.0157434. doi: 10.1371/journal.pone.0157434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An open label randomized comparison of mefloquine–artesunate as separate tablets vs. a new co-formulated combination for the treatment of uncomplicated multidrug-resistant falciparum malaria in Thailand. Ashley Elizabeth A., Lwin Khin Maung, McGready Rose, Simon Win Htay, Phaiphun Lucy, Proux Stephane, Wangseang Nantawan, Taylor Walter, Stepniewska Kasia, Nawamaneerat Wimon, Thwai Kyaw Lay, Barends Marion, Leowattana Wattana, Olliaro Piero, Singhasivanon Pratap, White Nicholas J., Nosten François. Oct 5;2006 Tropical Medicine & International Health. 11(11):1653–1660. doi: 10.1111/j.1365-3156.2006.01724.x. doi: 10.1111/j.1365-3156.2006.01724.x. [DOI] [PubMed] [Google Scholar]
- Adherence to fixed-combination versus unfixed travoprost 0.004%/timolol 0.5% for glaucoma or ocular hypertension: a randomized trial. Barnebey Howard S., Robin Alan L. Apr;2017 American Journal of Ophthalmology. 176:61–69. doi: 10.1016/j.ajo.2016.12.002. doi: 10.1016/j.ajo.2016.12.002. [DOI] [PubMed] [Google Scholar]
- Adherence to oral antidiabetic agents with pioglitazone and metformin: comparison of fixed-dose combination therapy with monotherapy and loose-dose combination therapy. Barner Jamie C. Sep;2011 Clinical Therapeutics. 33(9):1281–1288. doi: 10.1016/j.clinthera.2011.07.016. doi: 10.1016/j.clinthera.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Comparison of a four-drug fixed-dose combination regimen with a single tablet regimen in smear-positive pulmonary tuberculosis. Bartacek A., Schütt D., Panosch B., Borek M., Rimstar® FDCSG 2009Int J Tuberc Lung Dis. 13(6):760–766. [PubMed] [Google Scholar]
- Lower healthcare costs associated with the use of a single-pill ARV regimen in the UK, 2004–2008. Beck Eduard J., Mandalia Sundhiya, Sangha Roshni, Youle Mike, Brettle Ray, Gompels Mark, Johnson Margaret, Pozniak Anton, Schwenk Achim, Taylor Stephen, Walsh John, Wilkins Ed, Williams Ian, Gazzard Brian, for the NPMS-HHC Steering Group Oct 30;2012 PLoS One. 7(10):e47376. doi: 10.1371/journal.pone.0047376. doi: 10.1371/journal.pone.0047376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safety and efficacy of moxifloxacin-dexamethasone eyedrops as treatment for bacterial ocular infection associated with bacterial blepharitis. Belfort Rubens, Gabriel Luis, Martins Bispo Paulo José, Muccioli Cristina, Zacharias Serapicos Patricia Cabral, Clark Linda, Bell Belinda, Bartell John, Stroman David W., Höfling-Lima Ana Luisa. May;2012 Advances in Therapy. 29(5):416–426. doi: 10.1007/s12325-012-0018-8. doi: 10.1007/s12325-012-0018-8. [DOI] [PubMed] [Google Scholar]
- Efficacy and safety of benzalkonium chloride-free fixed-dose combination of latanoprost and timolol in patients with open-angle glaucoma or ocular hypertension. Bhagat P, Sodimalla K, Paul C, et al. Jun;2014 Clinical Ophthalmology. 8:1241–1252. doi: 10.2147/opth.s64584. doi: 10.2147/opth.s64584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greater reductions in A1C in type 2 diabetic patients new to therapy with glyburide/metformin tablets as compared to glyburide co-administered with metformin. Blonde L., Wogen J., Kreilick C., Seymour A. A. Nov;2003 Diabetes, Obesity and Metabolism. 5(6):424–431. doi: 10.1046/j.1463-1326.2003.00297.x. doi: 10.1046/j.1463-1326.2003.00297.x. [DOI] [PubMed] [Google Scholar]
- Efficacy and safety of fluticasone and formoterol in a single pressurized metered dose inhaler. Bodzenta-Lukaszyk Anna, Pulka Grażyna, Dymek Andrzej, Bumbacea Dragos, McIver Tammy, Schwab Birgit, Mansikka Heikki. May;2011 Respiratory Medicine. 105(5):674–682. doi: 10.1016/j.rmed.2010.11.011. doi: 10.1016/j.rmed.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Regimen simplification and medication adherence: Fixed-dose versus loose-dose combination therapy for type 2 diabetes. Böhm Anna-Katharina, Schneider Udo, Aberle Jens, Stargardt Tom. May 4;2021 PLoS One. 16(5):e0250993. doi: 10.1371/journal.pone.0250993. doi: 10.1371/journal.pone.0250993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The effect of combined calcium and cholecalciferol supplementation on bone mineral density in elderly women with moderate chronic kidney disease. Bosworth Cortney, de Boer Ian H., Targher Giovanni, Kendrick Jessica, Smits Gerard, Chonchol Michel. May 1;2012 Clinical Nephrology. 77(5):358–365. doi: 10.5414/cn107180. doi: 10.5414/cn107180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fixed-dose vs free-dose combinations for the management of hypertension—an analysis of 81 958 patients. Bramlage Peter, Schmidt Stefanie, Sims Helen. Feb 19;2018 The Journal of Clinical Hypertension. 20(4):705–715. doi: 10.1111/jch.13240. doi: 10.1111/jch.13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safety and efficacy of indapamide sustained release/amlodipine fixed-dose combination in essential hypertension. Bricout-Hennel S., Zelveian P., Nedogoda S. Jun;2018 Journal of Hypertension. 36(suppl 1):e45. doi: 10.1097/01.hjh.0000539081.92227.69. doi: 10.1097/01.hjh.0000539081.92227.69. [DOI] [Google Scholar]
- Assessment of adherence, persistence, and costs among valsartan and hydrochlorothiazide retrospective cohorts in free-and fixed-dose combinations. Brixner Diana I., Jackson Kenneth C., II, Sheng Xiaoming, Nelson Richard E., Keskinaslan Abdulkadir. Aug 6;2008 Current Medical Research and Opinion. 24(9):2597–2607. doi: 10.1185/03007990802319364. doi: 10.1185/03007990802319364. [DOI] [PubMed] [Google Scholar]
- Brüggenjürgen B., Ezzat N., Kardos P., Buhl R. Allergy. 9. Vol. 65. Wiley; Economic evaluation of BDP/formoterol fixed vs two single inhalers in asthma treatment; pp. 1108–1115. [DOI] [PubMed] [Google Scholar]
- Efficacy and tolerability of a combined gatifloxacin plus prednisolone formulation for topical prophylaxis after LASIK. Campos M, Muccioli C, Malta J B N S, Gerade R A, la Salame A, Belfort R., Jr. Feb;2011 Clinical Ophthalmology. 5(1):209–214. doi: 10.2147/opth.s17059. doi: 10.2147/opth.s17059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A polypill strategy to improve adherence: results from the FOCUS project. Castellano José M., Sanz Ginés, Peñalvo José L., Bansilal Sameer, Fernández-Ortiz Antonio, Alvarez Luz, Guzmán Luis, Linares Juan Carlos, García Fernando, D’Aniello Fabiana, Arnáiz Joan Albert, Varea Sara, Martínez Felipe, Lorenzatti Alberto, Imaz Iñaki, Sánchez-Gómez Luis M., Roncaglioni Maria Carla, Baviera Marta, Smith Sidney C. Jr., Taubert Kathryn, Pocock Stuart, Brotons Carlos, Farkouh Michael E., Fuster Valentin. Nov;2014 Journal of the American College of Cardiology. 64(20):2071–2082. doi: 10.1016/j.jacc.2014.08.021. doi: 10.1016/j.jacc.2014.08.021. [DOI] [PubMed] [Google Scholar]
- Health care utilization and cost comparison between adherent hypertension patients treated by single exforge HCT and amlodipine/valsartan/hydrochlorothiazide free combination. Chen W, Wei Z, Ong S H, Machnicki G, Kristijan K. Nov;2014 Value in Health. 17(7):A723. doi: 10.1016/j.jval.2014.08.036. doi: 10.1016/j.jval.2014.08.036. [DOI] [PubMed] [Google Scholar]
- Patient adherence and reimbursement amount for antidiabetic fixed-dose combination products compared with dual therapy among Texas Medicaid recipients. Cheong Chelim, Barner Jamie C., Lawson Kenneth A., Johnsrud Michael T. Oct;2008 Clinical Therapeutics. 30(10):1893–1907. doi: 10.1016/j.clinthera.2008.10.003. doi: 10.1016/j.clinthera.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Cohen Calvin J., Kubota Marshall, Brachman Philip S., Harley William B., Schneider Stefan, Williams Vanessa C., Sutherland-Phillips Denise H., Lim Michael L., Balu Rukmini B., Shaefer Mark S. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 3. Vol. 28. Wiley; Short-term safety and tolerability of a once-daily fixed-dose abacavir-lamivudine combination versus twice-daily dosing of abacavir and lamivudine as separate components: findings from the ALOHA study; pp. 314–322. [DOI] [PubMed] [Google Scholar]
- Benefits of a fixed-dose combination of bisoprolol and amlodipine in the treatment of hypertension in daily practice: results of more than 4000 patients. Czarnecka D., Koch E.M.W., Gottwald-Hostalek U. Apr 8;2015 Current Medical Research and Opinion. 31(5):875–881. doi: 10.1185/03007995.2015.1027676. doi: 10.1185/03007995.2015.1027676. [DOI] [PubMed] [Google Scholar]
- da Cunha Patrícia Abreu Ferreira, Shinzato Flavio Araujo, Tecchio Geraldine Trevisan, La Porta Weber Sarah, Brasil Alexandre, Avakian Amaryllis. Clinics. 6. Vol. 68. Elsevier BV; Efficacy and tolerability of a gatifloxacin/prednisolone acetate fixed combination for topical prophylaxis and control of inflammation in phacoemulsification: a 20-day-double-blind comparison to its individual components; pp. 834–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Once-daily QVA149 provides the same efficacy as the free combination of its monocomponents indacaterol and glycopyrronium: The BEACON study. Dahl Ronald, Jadayel Dalal, Alagappan Vijay, Chen Hungta, Banerji D. 2013Eur Resp J. 42 doi: 10.2147/COPD.S49615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efficacy and safety of once-daily QVA149 compared with the free combination of its monocomponents: The Beacon Study. Dahl Ronald, Jadayel Dalal, Alagappan Vijay, Chen Hungta, Banerji Donald. Mar;2014 Chest. 145(3 MEETING ABSTRACT):407A. doi: 10.1378/chest.1824459. doi: 10.1378/chest.1824459. [DOI] [Google Scholar]
- Efficacy and safety of QVA149 compared to the concurrent administration of its monocomponents indacaterol and glycopyrronium: the BEACON study [Erratum in: Int J Chron Obstruct Pulmon Dis. 2014;9:85 Note: MEDLINE/PubMed abstract corrected; Dosage error in article text.] Dahl Ronald, Jadayel Dalal, Alagappan Vijay, Chen Hungta, Banerji D. Oct;2013 International Journal of Chronic Obstructive Pulmonary Disease. 8:501–518. doi: 10.2147/copd.s49615. doi: 10.2147/copd.s49615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modifications in drug adherence after switch to fixed-dose combination of perindopril/amlodipine in clinical practice. Results of a large-scale Italian experience. The amlodipine-perindopril in real settings (AMPERES) study. Degli Esposti Luca, Perrone Valentina, Veronesi Chiara, Gambera Marco, Nati Giulio, Perone Francesco, Tagliabue Paola Fausta, Buda Stefano, Borghi Claudio. Feb 22;2018 Current Medical Research and Opinion. 34(9):1571–1577. doi: 10.1080/03007995.2018.1433648. doi: 10.1080/03007995.2018.1433648. [DOI] [PubMed] [Google Scholar]
- Add-on Therapy with rosiglitazone (RSG)/metformin (MET) as a fixed-dose combination (FDC) vs RSG plus MET or RSG plus sulfonylurea (SU) as separate pills (SP): retrospective study of outcomes and costs. Delea T.E., Arondekar B., Kartashov A. 2007Diabetes. 56(suppl 1):2200–P0. [Google Scholar]
- Adherence with levodopa/carbidopa/entacapone versus levodopa/carbidopa and entacapone as separate tablets in patients with Parkinson’s disease. Delea Thomas E., Thomas Simu K., Hagiwara May, Mancione L. Apr 30;2010 Current Medical Research and Opinion. 26(7):1543–1552. doi: 10.1185/03007991003780628. doi: 10.1185/03007991003780628. [DOI] [PubMed] [Google Scholar]
- A 12-week, randomized, double-masked, multicenter study of the fixed combination of latanoprost and timolol in the evening versus the individual components. Diestelhorst Michael, Larsson Lill-Inger, European-Canadian Latanoprost Fixed Combination Study Group Jan;2006 Ophthalmology. 113(1):70–76. doi: 10.1016/j.ophtha.2005.06.027. doi: 10.1016/j.ophtha.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Improvements in glycemic control in type 2 diabetes patients switched from sulfonylurea coadministered with metformin to glyburide-metformin tablets. Duckworth Wiliam. May;2003 Journal of Managed Care Pharmacy. 9(3):256–262. doi: 10.18553/jmcp.2003.9.3.256. doi: 10.18553/jmcp.2003.9.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PCV104 persistence in hypertension treatment with olmesartan medoxomil versus valsartan - analysis of real-life prescription data in Germany. Ehlken B., Kostev K., Breitscheidel L., Sandberg A., Holz B., Oberdiek A.M. Nov;2011 Value in Health. 14(7):A383. doi: 10.1016/j.jval.2011.08.824. doi: 10.1016/j.jval.2011.08.824. [DOI] [Google Scholar]
- Calcium channel blocker and angiotensin-converting enzyme (ACE) inhibitor antihypertensive regimen: comparing fixed vs. free combination therapy. Finelli R, Pascale A V, Battimelli A, et al. Oct 9;2014 High Blood Pressure & Cardiovascular Prevention. 21(4):333–334. doi: 10.1007/s40292-014-0066-z. doi: 10.1007/s40292-014-0066-z. [DOI] [Google Scholar]
- Comparing the fixed combination dorzolamide-timolol (Cosopt) to concomitant administration of 2% dorzolamide (Trusopt) and 0.5% timolol -- a randomized controlled trial and a replacement study. Francis B. A., Du L. T., Berke S., Ehrenhaus M., Minckler D. S., Cosopt Study G Aug;2004 Journal of Clinical Pharmacy and Therapeutics. 29(4):375–380. doi: 10.1111/j.1365-2710.2004.00574.x. doi: 10.1111/j.1365-2710.2004.00574.x. [DOI] [PubMed] [Google Scholar]
- Cost-effectiveness analysis and budget impact of concor® am versus bisoprolol plus amlodipine in systemic arterial hypertension treatment, from the perspective of the Brazilian public health system. Fujii R.K., Restrepo M., Fernandes R.A., Haas L., Pepe C., Junqueira M. May;2015 Value in Health. 18(3):A138. doi: 10.1016/j.jval.2015.03.801. doi: 10.1016/j.jval.2015.03.801. [DOI] [Google Scholar]
- Compliance and acceptance of fixed-dose combination of bisoprolol and aspirin. Open-label multicenter study. Gaciong Z., Hostalek U., Kurzeja A. Sep;2017 Journal of Hypertension. 35([LB.03.16]):e340–e341. doi: 10.1097/01.hjh.0000524012.97940.f0. doi: 10.1097/01.hjh.0000524012.97940.f0. [DOI] [Google Scholar]
- Approaches to increase efficacy of antihypertensive treatment: results of the Russian observational program forsage. Glezer M. 2016Journal of Hypertension. 34(suppl 2):e297–e298. doi: 10.1097/01.hjh.0000523870.41484.74. doi: 10.1097/01.hjh.0000523870.41484.74. [DOI] [Google Scholar]
- Influence of salmeterol/fluticasone via single versus separate inhalers on exacerbations in severe/very severe COPD. Hagedorn Cordula, Kässner Frank, Banik Norbert, Ntampakas Paris, Fielder Karin. Apr;2013 Respiratory Medicine. 107(4):542–549. doi: 10.1016/j.rmed.2012.12.020. doi: 10.1016/j.rmed.2012.12.020. [DOI] [PubMed] [Google Scholar]
- Preservative-free tafluprost 00015%/timolol 05% fixed dose combination: a 6-month double-masked, randomized multicenter P-III comparison to concomitant use of the individual preservative-free components in patients with glaucoma or ocular hypertension. Hollo G., Hommer A., Anton A., Ropo A. 20142013 Annual Meeting of the Association for Research in Vision and Ophthalmology. 54(15) [Google Scholar]
- Efficacy, safety, and tolerability of preservative-free fixed combination of tafluprost 0.0015%/timolol 0.5% versus concomitant use of the ingredients. Holló Gábor, Hommer Anton, Antón López Alfonso, Ropo Auli. Aug;2014 Journal of Ocular Pharmacology and Therapeutics. 30(6):468–475. doi: 10.1089/jop.2013.0229. doi: 10.1089/jop.2013.0229. [DOI] [PubMed] [Google Scholar]
- Intraocular pressure decrease with preservative-free fixed and unfixed combination of tafluprost and timolol in pseudoexfoliative glaucoma. Holló Gábor, Ropo Auli. 2015Current Medical Research and Opinion. 31(1):13–16. doi: 10.1185/03007995.2014.972500. doi: 10.1185/03007995.2014.972500. [DOI] [PubMed] [Google Scholar]
- A double-masked, randomized, parallel comparison of a fixed combination of bimatoprost 0.03%/timolol 0.5% with non-fixed combination use in patients with glaucoma or ocular hypertension. Hommer A., Ganfort Investigators Group Jan;2007 European Journal of Ophthalmology. 17(1):53–62. doi: 10.1177/112067210701700108. doi: 10.1177/112067210701700108. [DOI] [PubMed] [Google Scholar]
- Dynamic view on affordability of fixed-dose combination antihypertensive drug therapy. Hong S. H., Wang J., Tang J. Mar 18;2013 American Journal of Hypertension. 26(7):879–887. doi: 10.1093/ajh/hpt035. doi: 10.1093/ajh/hpt035. [DOI] [PubMed] [Google Scholar]
- Hostalek Ulrike, Czarnecka Danuta, Koch Ernst M. W. Cardiology and Therapy. 2. Vol. 4. Springer Science and Business Media LLC; Treatment of hypertensive patients with a fixed-dose combination of bisoprolol and amlodipine: results of a cohort study with more than 10,000 patients; pp. 179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung function and asthma control with beclomethasone and formoterol in a single inhaler [Comment in: Respir Med. 2009;103(12):1969-70; author reply 1971-1972] Huchon G., Magnussen H., Chuchalin A., Dymek L., Gonod F. Bonnet, Bousquet J. Jan;2009 Respiratory Medicine. 103(1):41–49. doi: 10.1016/j.rmed.2008.09.002. doi: 10.1016/j.rmed.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Inoue Kenji, Shiokawa Minako, Sugahara Michitaka, Wakakura Masato, Soeda Shoichi, Tomita Goji. Japanese Journal of Ophthalmology. 6. Vol. 56. Springer Science and Business Media LLC; Three-month evaluation of dorzolamide hydrochloride/timolol maleate fixed-combination eye drops versus the separate use of both drugs; pp. 559–563. [DOI] [PubMed] [Google Scholar]
- Compliance and persistence of fixed dose versus free dose combination therapy with valsartan and HCTZ for patients with hypertension. Jackson KC, Brixner D, Oderda GM, Oberg B, Sheng X, Keskinaslan A. Nov;2006 Value in Health. 9(6):A363. doi: 10.1016/s1098-3015(10)63700-x. doi: 10.1016/s1098-3015(10)63700-x. [DOI] [Google Scholar]
- Jenkins Christine, Kolarikova Renata, Kuna Piotr, CAILLAUD Denis, SANCHIS Joaquin, POPP Wolfgang, PETTERSSON Eva. Respirology. 3. Vol. 11. Wiley; Efficacy and safety of high-dose budesonide/formoterol (Symbicort®) compared with budesonide administered either concomitantly with formoterol or alone in patients with persistent symptomatic asthma; pp. 276–286. [DOI] [PubMed] [Google Scholar]
- Adherence to single-pill combination versus multiple-pill combination lipid-modifying therapy among patients with mixed dyslipidemia in a managed care population. Kamat Siddhesh A., Bullano Michael F., Chang Chun-Lan, Gandhi Sanjay K., Cziraky Mark J. Mar 7;2011 Current Medical Research and Opinion. 27(5):961–968. doi: 10.1185/03007995.2011.562494. doi: 10.1185/03007995.2011.562494. [DOI] [PubMed] [Google Scholar]
- Kawalec Paweł, Holko Przemysław, Stawowczyk Ewa, Borowiec Łukasz, Filipiak Krzysztof J. Kardiologia Polska. 9. Vol. 73. Polskie Towarzystwo Kardiologiczne; Economic evaluation of single-pill combination of indapamide and amlodipine in the treatment of arterial hypertension in the Polish setting; pp. 768–780. [DOI] [PubMed] [Google Scholar]
- Budget impact analysis of hypertensive treatment with indapamide and amlodipine single-pill combination in the Polish setting. Kawalec P., Stawowczyk E., Holko P., Borowiec L., Filipiak K.J. Nov;2014 Value in Health. 17(7):A479. doi: 10.1016/j.jval.2014.08.1381. doi: 10.1016/j.jval.2014.08.1381. [DOI] [PubMed] [Google Scholar]
- Fallah Saeid, Khodakarim Soheila, Bazmi Elham, Lak ShakerSalari, Raeisi Vahideh, Behnoush Behnam. The Egyptian Journal of Chest Diseases and Tuberculosis. 3. Vol. 69. Medknow; Comparison of sputum conversion time in tuberculosis treatment with fix-dose combination drugs and separate drug regimens; p. 468. [DOI] [Google Scholar]
- Pharmacodynamic effects of a new fixed-dose clopidogrel–aspirin combination compared with separate administration of clopidogrel and aspirin in patients treated with coronary stents: the ACCEL-COMBO trial. Koh Jin-Sin, Park Yongwhi, Tantry Udaya S., Ahn Jong-Hwa, Kang Min Gyu, Kim Kyehwan, Jang Jeong Yoon, Park Hyun Woong, Park Jeong Rang, Hwang Seok-Jae, Kwak Choong Hwan, Hwang Jin-Yong, Gurbel Paul A., Jeong Young-Hoon. 2017Platelets. 28(2):187–193. doi: 10.1080/09537104.2016.1206197. doi: 10.1080/09537104.2016.1206197. [DOI] [PubMed] [Google Scholar]
- Twenty-four-hour efficacy of the brimonidine/timolol fixed combination versus therapy with the unfixed components. Konstas A G P, Katsimpris I E, Kaltsos K, Georgiadou I, Kordelou A, Nelson L A, Stewart W C. 2008Eye. 22(11):1391–1397. doi: 10.1038/sj.eye.6702906. doi: 10.1038/sj.eye.6702906. [DOI] [PubMed] [Google Scholar]
- The effect of combined calcium and vitamin D3 supplementation on serum intact parathyroid hormone in moderate CKD. Kooienga Laura, Fried Linda, Scragg Robert, Kendrick Jessica, Smits Gerard, Chonchol Michel. Mar;2009 American Journal of Kidney Diseases. 53(3):408–416. doi: 10.1053/j.ajkd.2008.09.020. doi: 10.1053/j.ajkd.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Koval Sergiy M., Snihurska Iryna O., Starchenko Tetyana G., Penkova Marina Yu., Mysnychenko Olga V., Yushko Kostyantin O., Lytvynova Olga M., Vysotska Olena, Berezin Alexander E. Biomedical Research and Therapy. 11. Vol. 6. Biomedical Research and Therapy; Efficacy of fixed dose of triple combination of perindopril-indapamide-amlodipine in obese patients with moderate-to-severe arterial hypertension: an open-label 6-month study; pp. 3501–3512. [DOI] [Google Scholar]
- Topical glaucoma therapy cost in Mexico. Lazcano-Gomez Gabriel, Hernandez-Oteyza Alejandra, Iriarte-Barbosa María José, Hernandez-Garciadiego Carlos. 2014International Ophthalmology. 34(2):241–249. doi: 10.1007/s10792-013-9823-6. doi: 10.1007/s10792-013-9823-6. [DOI] [PubMed] [Google Scholar]
- Legorreta A., Yu A., Chernicoff H., Gilmore A., Jordan J., Rosenzweig J. C. AIDS Care. 8. Vol. 17. Informa UK Limited; Adherence to combined Lamivudine+Zidovudine versus individual components: a community-based retrospective medicaid claims analysis; pp. 938–948. [DOI] [PubMed] [Google Scholar]
- Patient adherence to olmesartan/amlodipine combinations: fixed versus extemporaneous combinations. Levi Miriam, Pasqua Alessandro, Cricelli Iacopo, Cricelli Claudio, Piccinni Carlo, Parretti Damiano, Lapi Francesco. Mar;2016 Journal of Managed Care & Specialty Pharmacy. 22(3):255–262. doi: 10.18553/jmcp.2016.22.3.255. doi: 10.18553/jmcp.2016.22.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comparison of amlodipine/valsartan/hydrochlorothiazide single pill combination and free combination: adherence, persistence, healthcare utilization and costs. Machnicki G., Ong S.H., Chen W., Wei Z.J., Kahler K.H. Nov 11;2015 Current Medical Research and Opinion. 31(12):2287–2296. doi: 10.1185/03007995.2015.1098598. doi: 10.1185/03007995.2015.1098598. [DOI] [PubMed] [Google Scholar]
- The use of antiplatelet agents after an acute coronary syndrome in a large community Italian setting of more than 12 million subjects. Maggioni Aldo P, Dondi Letizia, Pedrini Antonella, Ronconi Giulia, Calabria Silvia, Cimminiello Claudio, Martini Nello. 2019European Heart Journal: Acute Cardiovascular Care. 8(6):527–535. doi: 10.1177/2048872618801252. doi: 10.1177/2048872618801252. [DOI] [PubMed] [Google Scholar]
- Multicap to improve adherence after acute coronary syndromes: results of a randomized controlled clinical trial. Mariani Javier, Rosende Andrés, De Abreu Maximiliano, Gonzalez Villa Monte Gabriel, D’Imperio Heraldo, Antonietti Laura, Lemonnier Gabriela, de Bonis Alejandra, Tajer Carlos. Jan;2020 Therapeutic Advances in Cardiovascular Disease. 14 doi: 10.1177/1753944720912071. doi: 10.1177/1753944720912071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updated medication costs from a real-life cost-effectiveness evaluation of budesonide/formoterol maintenance and reliever therapy in asthma maintenance and reliever therapy in asthma [abstract] Medin E, Safioti G, Lindqvist F, Torvinen S. Nov;2015 Value in Health. 18(7):A500. doi: 10.1016/j.jval.2015.09.1413. doi: 10.1016/j.jval.2015.09.1413. [DOI] [Google Scholar]
- Treatment outcome in smear-positive pulmonary tuberculosis patients treated with a fixed-dose drug combination regimen in comparison with a separate regimen: a randomized clinical trial. Fallah Saeid, Nazari Seyed SaeedHashemi, Raeisi Vahideh. 2021The Egyptian Journal of Chest Diseases and Tuberculosis. 70(1):26–30. doi: 10.4103/ejcdt.ejcdt_110_19. doi: 10.4103/ejcdt.ejcdt_110_19. [DOI] [Google Scholar]
- Nedogoda Sergey V., Stojanov Vesna J. Cardiology and Therapy. 1. Vol. 6. Springer Science and Business Media LLC; Single-pill combination of perindopril/indapamide/amlodipine in patients with uncontrolled hypertension: a randomized controlled trial; pp. 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The efficacy of the fixed combination of mometasone furoate and azelastine hydrochloride as a nasal spray in adult patients with perennial rhinitis. Nenasheva N, Nosulya E, Kim I, Ryazantsev S, Ovchinnikov A, Berdnikova N. Aug;2019 Allergy. 74(suppl 106):400–401. doi: 10.1111/all.13961. doi: 10.1111/all.13961. [DOI] [Google Scholar]
- Ofili Elizabeth, Anand Inder, Williams Richard Allen, Akinboboye Ola, Xu Liou, Puckrein Gary. Advances in Therapy. 8. Vol. 34. Springer Science and Business Media LLC; Fixed-dose versus off-label combination of isosorbide dinitrate plus hydralazine hydrochloride: retrospective propensity-matched analysis in black Medicare patients with heart failure; pp. 1976–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramipril/amlodipine single pill – effectiveness, tolerance and patient satisfaction with antihypertensive therapy in relation to nutritional status. Olszanecka-Glinianowicz Magdalena, Smertka Mike, Almgren-Rachtan Agnieszka, Chudek Jerzy. Dec;2014 Pharmacological Reports. 66(6):1043–1049. doi: 10.1016/j.pharep.2014.06.020. doi: 10.1016/j.pharep.2014.06.020. [DOI] [PubMed] [Google Scholar]
- Persistence and adherence with exforge HCT single pill combination versus amlodipine/valsartan/hydrochlorothiazide free combination: a comparison controlling for demographic and clinical factors. Ong S, Machnicki G, Chen W, Wei Z J, Kahler K H. Sep 2;2014 European Heart Journal. 35(suppl_1):1–172. doi: 10.1093/eurheartj/ehu322. doi: 10.1093/eurheartj/ehu322. [DOI] [Google Scholar]
- Adherence with single-pill amlodipine/atorvastatin vs a two-pill regimen. Patel B., Leslie R., Thiebaud P.., et al. 2008Vasc Health Risk Manag. 4(3):673–681. [PMC free article] [PubMed] [Google Scholar]
- Randomized controlled trial of adherence with single or combination inhaled corticosteroid/long-acting β-agonist inhaler therapy in asthma. Perrin Kyle, Williams Mathew, Wijesinghe Meme, James Kate, Weatherall Mark, Beasley Richard. Sep;2010 Journal of Allergy and Clinical Immunology. 126(3):505–510. doi: 10.1016/j.jaci.2010.06.033. doi: 10.1016/j.jaci.2010.06.033. [DOI] [PubMed] [Google Scholar]
- The single pill concept leads to improved persistence of medication, clinical outomes and reduced all-cause mortaility in hypertensive patients - results from the START project. Predel Hans-Georg, Weisser Burkhard, Wassmann Sven, Gillessen Anton, Blettenberg Jörg, Fournier Valérie, Noetel Andrea, Randerath Olaf, Böhm Michael, Schmieder Roland E. Apr;2021 Journal of Hypertension. 39(suppl 1):e378. doi: 10.1097/01.hjh.0000748928.45804.cf. doi: 10.1097/01.hjh.0000748928.45804.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persistence and cardiovascular outcomes with ramipril, atorvastatin, ASA as a single pill compared to the multi pill combination. A subanalysis of the START study, a claims data analysis. Predel H.G, Weisser B, Wassmann S, Schmieder R.E, Blettenberg J, Gillessen A, Fournier V, Noetel A, Randerath O, Boehm M. Nov 1;2020 European Heart Journal. 41(suppl_2):ehaa946.2964. doi: 10.1093/ehjci/ehaa946.2964. doi: 10.1093/ehjci/ehaa946.2964. [DOI] [Google Scholar]
- Cost-effectiveness of the LABA/LAMA dual bronchodilator indacaterol/glycopyrronium in a Swedish healthcare setting. Price D., Keininger D., Costa-Scharplatz M.., et al. 2014Resp Med. 108(12):1786–1793. doi: 10.1016/j.rmed.2014.09.015. doi: 10.1016/j.rmed.2014.09.015. [DOI] [PubMed] [Google Scholar]
- Economic evaluation of olmesartan/amlodipine fixed-dose combination for hypertension treatment in China. Ren Maodong, Xuan Dennis, Lu Yongji, Fu YuYan, Xuan Jianwei. Jan 25;2020 Journal of Medical Economics. 23(4):394–400. doi: 10.1080/13696998.2019.1699799. doi: 10.1080/13696998.2019.1699799. [DOI] [PubMed] [Google Scholar]
- Treatment compliance with fixed-dose combination of vildagliptin/metformin in patients with Type 2 diabetes mellitus inadequately controlled with metformin monotherapy: a 24-week observational study. Rombopoulos Grigorios, Hatzikou Magdalini, Athanasiadis Athanasios, Elisaf Moyses. 2015International Journal of Endocrinology. 2015(251485):1–5. doi: 10.1155/2015/251485. doi: 10.1155/2015/251485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preliminary results of a multicenter observational study of treatment compliance with free-combination versus fixed combination treatment in Type II diabetes mellitus patients in Greece (Less Study) Rombopoulos G., Hatzikou M., Kossiva E., Athanasiadis A., Elisaf M. Nov;2012 Value in Health. 15(7)(PDB53):A503. doi: 10.1016/j.jval.2012.08.1696. doi: 10.1016/j.jval.2012.08.1696. [DOI] [Google Scholar]
- Budesonide/formoterol in a single inhaler (Symbicort) reduces healthcare costs compared with separate inhalers in the treatment of asthma over 12 months. Rosenhall L, Borg S, Andersson F, Ericsson K. Oct;2003 International Journal of Clinical Practice. 57(8):662–667. doi: 10.1111/j.1742-1241.2003.tb10584.x. doi: 10.1111/j.1742-1241.2003.tb10584.x. [DOI] [PubMed] [Google Scholar]
- One-year safety and efficacy of budesonide/formoterol in a single inhaler (Symbicort® Turbuhaler®) for the treatment of asthma. Rosenhall L., Elvstrand A., Tilling B., VINGE I., JEMSBY P., STAHL E., JERRE F., BERGQVIST P.B.F. Jun;2003 Respiratory Medicine. 97(6):702–708. doi: 10.1053/rmed.2003.1504. doi: 10.1053/rmed.2003.1504. [DOI] [PubMed] [Google Scholar]
- Budesonide/formoterol (Symbicort) is well tolerated and effective in patients with moderate persistent asthma. Rosenhall L, Heinig JH, Lindqvist A, Leegaard J, Ståhl E, Bergqvist PBF. Jul;2002 International Journal of Clinical Practice. 56(6):427–433. doi: 10.1111/j.1742-1241.2002.tb11292.x. doi: 10.1111/j.1742-1241.2002.tb11292.x. [DOI] [PubMed] [Google Scholar]
- Patient-reported outcomes and low-level residual HIV-RNA in adolescents perinatally infected with HIV-1 after switching to one-pill fixed-dose regimen. Rosso Raffaella, Di Biagio Antonio, Maggiolo Franco, Nulvesu Loredana, Callegaro Anna Paola, Taramasso Lucia, Bruzzone Bianca, Viscoli Claudio. 2012AIDS Care. 24(1):54–58. doi: 10.1080/09540121.2011.596511. doi: 10.1080/09540121.2011.596511. [DOI] [PubMed] [Google Scholar]
- Pharmacoeconomic evaluation: cost effectiveness analysis of oral antidiabetic therapy in a tertiary care hospital. Saju Sereena, Varghese Fedrin, R S Deena, N R Nivetha, Chetty Samhitha, Mahesh K. Uma. Jan 15;2021 International Journal of Pharmaceutical Sciences Review and Research. 66(1):31–37. doi: 10.47583/ijpsrr.2021.v66i01.008. doi: 10.47583/ijpsrr.2021.v66i01.008. [DOI] [Google Scholar]
- PCV62 Persistence and compliance in hypertension treatment with olmesartan medoxomil analysis of real-life prescription data. Sandberg A., Kostev K., Ehlken B., Holz B., Oberdiek A. May;2011 Value in Health. 14(3):A43. doi: 10.1016/j.jval.2011.02.249. doi: 10.1016/j.jval.2011.02.249. [DOI] [Google Scholar]
- Long-term persistence with single-pill, fixed-dose combination therapy versus two pills of amlodipine and perindopril for hypertension: Australian experience. Simons Leon A., Chung Eric, Ortiz Michael. Aug 24;2017 Current Medical Research and Opinion. 33(10):1783–1787. doi: 10.1080/03007995.2017.1367275. doi: 10.1080/03007995.2017.1367275. [DOI] [PubMed] [Google Scholar]
- Persistence with a single pill versus two pills of amlodipine and atorvastatin: the Australian experience, 2006–2010. Simons Leon A, Ortiz Michael, Calcino Gordon. Aug;2011 Medical Journal of Australia. 195(3):134–137. doi: 10.5694/j.1326-5377.2011.tb03240.x. doi: 10.5694/j.1326-5377.2011.tb03240.x. [DOI] [PubMed] [Google Scholar]
- Persistence of fixed and free combination of ramipril and amlodipine in hypertension. Simonyi G., Ferenci T. 2015Eur Heart J. 36:326–327. [Google Scholar]
- Persistence of fixed and free combination of perindopril and amlodipine in hypertension. Simonyi G, Ferenci T, Medvegy M. Aug 1;2016 European Heart Journal. 37(suppl_1):191–598. doi: 10.1093/eurheartj/ehw432. doi: 10.1093/eurheartj/ehw432. [DOI] [Google Scholar]
- Which is the best choice? One year persistence of ramipril, ramipril/amlodipin free and fixed dose combination therapy in hypertension. Simonyi G., Ferenczi T., Medvegy M., Finta E. Aug 1;2017 European Heart Journal. 38(suppl_1)(P1654):ehx502. doi: 10.1093/eurheartj/ehx502.p1654. doi: 10.1093/eurheartj/ehx502.p1654. [DOI] [Google Scholar]
- Efficacy and safety of a fixed combination of intramuscular diclofenac 75 mg + thiocolchicoside 4 mg in the treatment of acute low back pain: a phase III, randomized, double blind, controlled trial. Sproviero Egidio, Albamonte Emilio, Costantino Cosimo, Giossi Attilio, Mancuso Maurizio, Rigamonti Alberto, Tornari Paolo, Caggiano Giuseppe. Sep;2018 European Journal of Physical and Rehabilitation Medicine. 54(5):654. doi: 10.23736/s1973-9087.17.04923-1. doi: 10.23736/s1973-9087.17.04923-1. [DOI] [PubMed] [Google Scholar]
- Budget impact analysis of the introduction of a single-pill combination of atorvastatin, perindopril and amlodipine in the Greek setting. Stafylas P., Karaiskou M., Zouka M. Oct;2018 Value in Health. 21(PCV43):S99–S100. doi: 10.1016/j.jval.2018.09.590. doi: 10.1016/j.jval.2018.09.590. [DOI] [Google Scholar]
- Cost analysis of the introduction of a single-pill combination of rosuvastatin and ezetimibe in the Greek setting. Stafylas P., Stamuli E., Karaiskou M., Panteris E., Chotzagiannoglou V., Beletsi A. Nov;2019 Value in Health. 22(PCV44):S548. doi: 10.1016/j.jval.2019.09.769. doi: 10.1016/j.jval.2019.09.769. [DOI] [Google Scholar]
- A real-life cost-effectiveness evaluation of budesonide/formoterol maintenance and reliever therapy in asthma. Ställberg B., Ekström T., Neij F., Olsson P., Skoogh B.-E., Wennergren G., Löfdahl C.-G. Oct;2008 Respiratory Medicine. 102(10):1360–1370. doi: 10.1016/j.rmed.2008.06.017. doi: 10.1016/j.rmed.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Cost-utility analysis of hypertensive treatment with indapamide and amlodipine single-pill combination in the Polish setting. Stawowczyk E., Holko P., Kawalec P., Borowiec L., Filipiak K.J. Nov;2014 Value in Health. 17(7):A491. doi: 10.1016/j.jval.2014.08.1450. doi: 10.1016/j.jval.2014.08.1450. [DOI] [PubMed] [Google Scholar]
- Adherence to asthma controller medication regimens. Stempel D.A., Stoloff S.W., Carranza Rosenzweig J.R., Stanford R.H., Ryskina K.L., Legorreta A.P. Oct;2005 Respiratory Medicine. 99(10):1263–1267. doi: 10.1016/j.rmed.2005.03.002. doi: 10.1016/j.rmed.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Improved refill persistence with fluticasone propionate and salmeterol in a single inhaler compared with other controller therapies. Stoloff Stuart W, Stempel David A, Meyer Jay, Stanford Richard H, Carranza Rosenzweig Jacqueline R. Feb;2004 Journal of Allergy and Clinical Immunology. 113(2):245–251. doi: 10.1016/j.jaci.2003.10.011. doi: 10.1016/j.jaci.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Fixed-dose combination chemotherapy (Rifater/Rifinah) for active pulmonary tuberculosis in Taiwan: a two-year follow-up. Su W.J., Perng R.P. 2002Int J Tuberc Lung Dis. 6(11):1029–1032. [PubMed] [Google Scholar]
- Adherence to triple-component antihypertensive regimens is higher with single-pill than equivalent two-pill regimens: a randomized controlled trial. Sung Jidong, Ahn Kye Taek, Cho Byung-Ryul, Lee Sung Yun, Kim Byung Jin, Kim Dae Kyeong, Park Joong-il, Lee Wang-Soo. Feb 13;2021 Clinical and Translational Science. 14(3):1185–1192. doi: 10.1111/cts.12979. doi: 10.1111/cts.12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Is there an increased risk of TB relapse in patients treated with fixed-dose combination drugs in Indonesia? Suryanto A.A., van denBroek J., Hatta M., de Soldenhoff R., van der Werf M.J. 2008Int J Tuberc Lung Dis. 12(2):174–179. [PubMed] [Google Scholar]
- Thayer Sarah, Arondekar Bhakti, Harley Carolyn, Darkow Theodore E. Annals of Pharmacotherapy. 5. Vol. 44. SAGE Publications; Adherence to a fixed-dose combination of rosiglitazone/glimepiride in subjects switching from monotherapy or dual therapy with a thiazolidinedione and/or a sulfonylurea; pp. 791–799. [DOI] [PubMed] [Google Scholar]
- Adherence to a fixed-dose combination of rosiglitazone maleate/metformin hydrochloride in subjects with type 2 diabetes mellitus: a retrospective database analysis. Vanderpoel Daniel R., Hussein Mohamed A., Watson-Heidari Teresa, Perry Andrew. Dec;2004 Clinical Therapeutics. 26(12):2066–2075. doi: 10.1016/j.clinthera.2004.12.018. doi: 10.1016/j.clinthera.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Simplification of HAART therapy on ambulatory HIV patients in Malaysia: a randomized controlled trial. Velvanathan Tineshwaran, Islahudin Farida, Sim Benedict L., Taha Nur A. Dec 31;2016 Pharmacy Practice. 14(4):830. doi: 10.18549/pharmpract.2016.04.830. doi: 10.18549/pharmpract.2016.04.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benefits of ART simplification on adherence, clinical and economic outcomes. Vera J, Aragão F, Guimaraes M, Vaz Pinto I. Nov;2012 Journal of the International AIDS Society. 15(S4):18064. doi: 10.7448/ias.15.6.18064. doi: 10.7448/ias.15.6.18064. [DOI] [Google Scholar]
- Comparison of the cost of topical therapy for glaucoma between generic and brand medicines in Mexico. Vidaurre Mora E.V., Moreno U, Lazcano G, Jiménez Román J. 2019Invest Ophthalmol Visual Sci. 60(9):5472–5472. [Google Scholar]
- Larger blood pressure reduction by fixed-dose compared to free dose combination therapy of ACE inhibitor and calcium antagonist in hypertensive patients. Visco V., Finelli R., Pascale A.V.., et al. 2017Transl Med UniSa. 16:17–23. [PMC free article] [PubMed] [Google Scholar]
- Clinical evaluation and monitoring of adverse effects for fixed multidose combination against single drug therapy in pulmonary tuberculosis patients. Zaka-Ur-Rehman Z., Jamshaid M., Chaudhry A. 2008Pak J Pharm Sci. 21(2):185–194. [PubMed] [Google Scholar]
- Improved asthma control with budesonide/formoterol in a single inhaler, compared with budesonide alone. Zetterström O., Buhl R., Mellem H., Perpiñá M., Hedman J., O'Neill S., Ekström T. Aug;2001 European Respiratory Journal. 18(2):262–268. doi: 10.1183/09031936.01.00065801. doi: 10.1183/09031936.01.00065801. [DOI] [PubMed] [Google Scholar]
- Comparative efficacy and safety of the fixed versus unfixed combination of latanoprost and timolol in Chinese patients with open-angle glaucoma or ocular hypertension. Zhao Jia-Liang, Ge Jian, Li Xiao-Xin, Li Yu-Min, Sheng Yao-Hua, Sun Nai-Xue, Sun Xing-Huai, Yao Ke, Zhong Zheng. Aug 19;2011 BMC Ophthalmology. 11(1):23. doi: 10.1186/1471-2415-11-23. doi: 10.1186/1471-2415-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medication adherence in inflammatory bowel disease. Chan Webber, Chen Andy, Tiao Darren, Selinger Christian, Leong Rupert. 2017Intestinal Research. 15(4):434–445. doi: 10.5217/ir.2017.15.4.434. doi: 10.5217/ir.2017.15.4.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Factors affecting adherence to disease-modifying therapies in multiple sclerosis: systematic review. Washington F.A.-O., Langdon D. 2021J Neurol. 269:1432–1459. doi: 10.1007/s00415-021-10850-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Atkinson Mark J, Sinha Anusha, Hass Steven L, Colman Shoshana S, Kumar Ritesh N, Brod Meryl, Rowland Clayton R. 2004Health and Quality of Life Outcomes. 2(1):12. doi: 10.1186/1477-7525-2-12. doi: 10.1186/1477-7525-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Validation of a tool to assess medication treatment satisfaction in patients with Type 2 diabetes: the Diabetes Medication System Rating Questionnaire (DMSRQ) Peyrot M, Harshaw Q Fau - Shillington A C, Xu Y, Rubin R R. doi: 10.1111/j.1464-5491.2011.03538.x. [DOI] [PubMed]
- Impact of single-pill combination therapy on adherence, blood pressure control, and clinical outcomes: a rapid evidence assessment of recent literature. Tsioufis Konstantinos, Kreutz Reinhold, Sykara Georgia, van Vugt Joris, Hassan Tarek. Jun;2020 Journal of Hypertension. 38(6):1016–1028. doi: 10.1097/hjh.0000000000002381. doi: 10.1097/hjh.0000000000002381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The impact of fixed-dose combination versus free-equivalent combination therapies on adherence for hypertension: a meta-analysis. Du Li-Ping, Cheng Zhong-Wei, Zhang Yu-Xuan, Li Ying, Mei Dan. Apr 27;2018 The Journal of Clinical Hypertension. 20(5):902–907. doi: 10.1111/jch.13272. doi: 10.1111/jch.13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay P.G., Nag S., Graham C.M., Narayanan S. Medicine. 42. Vol. 94. Ovid Technologies (Wolters Kluwer Health); Meta-analysis of studies comparing single and multi-tablet fixed dose combination HIV treatment regimens; p. e1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Single-pill vs free-equivalent combination therapies for hypertension: a meta-analysis of health care costs and adherence. Sherrill Beth, Halpern Michael, Khan Shahnaz, Zhang Jie, Panjabi Sumeet. Nov 7;2011 The Journal of Clinical Hypertension. 13(12):898–909. doi: 10.1111/j.1751-7176.2011.00550.x. doi: 10.1111/j.1751-7176.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adherence with levodopa/carbidopa/entacapone versus levodopa/carbidopa and entacapone as separate tablets in patients with Parkinson’s disease. Delea Thomas E., Thomas Simu K., Hagiwara May, Mancione L. Apr 30;2010 Current Medical Research and Opinion. 26(7):1543–1552. doi: 10.1185/03007991003780628. doi: 10.1185/03007991003780628. [DOI] [PubMed] [Google Scholar]
- Help patients safely handle medications to improve adherence. Maxik K., Kimble C., Coustasse A. 2021Pharmacy Times.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.