Abstract
A new atom-economical synthesis of β-alkenyl-substituted BODIPYs via indium(III)-catalyzed intermolecular alkyne hydroarylation with meso-substituted BODIPYs is described. While catalysis with InI3 allows the double β-functionalization of BODIPY, resulting in regioselectively branched β,β′-disubstituted alkenyl BODIPYs, catalytic InCl3 enables the formation of linear β-substituted alkenyl BODIPYs. Subsequent In(III)-catalyzed intermolecular alkyne hydroarylation allows the synthesis of unsymmetrical push–pull BODIPY derivatives. Therefore, indium catalysis offers complementary regioselectivity in good chemical yields and functional group tolerance. The resulting BODIPY dyes displayed bathochromically shifted absorption and emission according to the electron-nature of the substituents in the alkenyl moiety with high molar extinction coefficients (ε up to 88,200 M–1 cm–1) and quantum yields (0.14–0.96).
Introduction
4,4-Difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) and derivatives are a class of valuable small-molecule fluorophores of interest in diverse research areas like bioimaging,1 fluorescent probes,2 photodynamic therapy,3 photocatalysis,4 optoelectronic devices,5 and so forth.6 The growing success of these rigid π-conjugated complexes lies to their excellent features such as narrow absorption and emission bands, high fluorescent quantum yield, good photostability, and solubility.7 Hitherto, a plethora of efforts have been made to fine-tuning their properties by introduction of suitable substituents at the different positions of the BODIPY scaffold and in particular, conjugated substituents at C2 and C6 (β) positions through alkenyl extension usually led to red-shifted emission bands with application as fluorescence probes to biothiols,8 DNA,9 or as photosensitizer.10
Among the different synthetic approaches so far employed to obtain β-alkenyl BODIPYs, the metal-catalyzed cross-coupling reactions from halogenated or borylated derivatives represent a straightforward methodology (Scheme 1a).11,12 Late-stage functionalization of the BODIPY core has also been exploited via C–H functionalization using palladium-mediated oxidative olefination (Scheme 1b)13 To the best of our knowledge, the synthesis of branched and linear β-alkenyl BODIPYs through intermolecular metal-catalyzed alkyne hydroarylation with BODIPY is unknown, despites its great potential in terms of economy. By taking advantage of the reactivity of C2(6) positions of the BODIPY framework,7 we envisioned the synthesis of alkenyl-substituted BODIPYs through intermolecular In(III)-catalyzed alkyne hydroarylation (Scheme 1c).
Scheme 1. Synthetic Approaches to Obtain β-Alkenyl BODIPYs.
Since Shirakawa and Kawakami reported the first examples of Friedel–Crafts alkenylation of arenes using alkynes with indium(III) triflate as the catalyst,14 this transformation has received much attention, because this approach offers an attractive alternative to Heck and cross-coupling reactions, as can be catalyzed by different transition metals such as palladium, platinum, gold, and iron,15 that was later expanded to aromatic heterocycles.16 Indium(III) has also proven to be an efficient π-Lewis acid in inter- and intramolecular hydroarylation transformations which offers advantages in terms of cost and low toxicity.17 In this research area, we have developed In(III)-catalyzed intramolecular hydroarylation reaction of arenes and hydroalkoxylation and hydroamination reactions,17a including tandem cycloisomerization reactions.18 Herein, we report the indium(III)-catalyzed intermolecular alkyne hydroarylation with BODIPY dyes to access to β-alkenyl BODIPYs and the study of their photophysical properties (Scheme 1c).
Results and Discussion
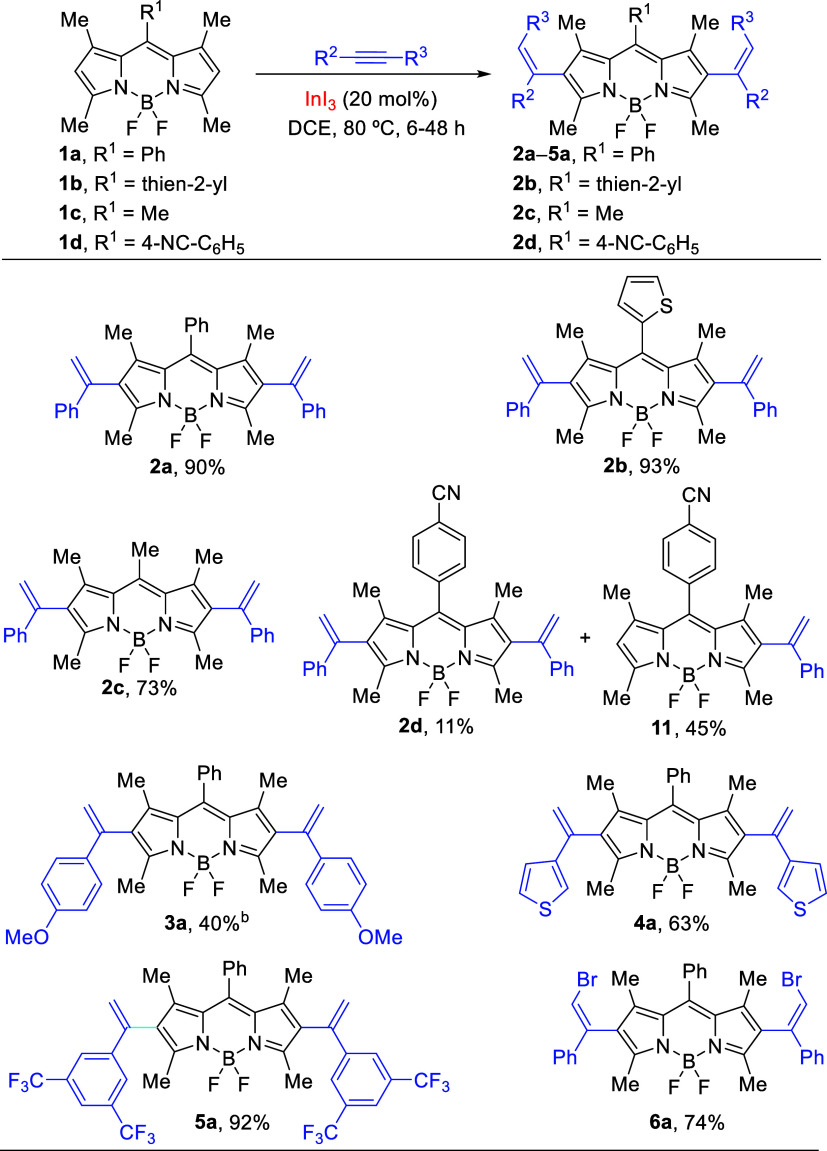
At the outset of this investigation, a variety of meso-substituted BODIPYs 1a–d were synthesized through our recently reported efficient microwave-assisted one-pot pathway.19 Initially, based on our previous experience, commercially available InI3 was chosen as the catalyst. Treatment of BODIPY 1a with phenylacetylene (molar ratio of 1:5) in the presence of InI3 (10 mol %) in DCE (∼0.1 M) at 80 °C afforded branched β-alkenyl BODIPY 2 in 11% yield, as a result of intermolecular hydroarylation with Markovnikov regioselectivity, along with a large amount of remaining unreacted 1a (entry 1, Table 1). No other regioisomer was detected in the reaction mixture. Likewise, when the molar ratio was increased from 1:5 to 1:10 and to 1:14, compound 2 was obtained in 21% and 38% yields, respectively, accompanied by the formation of 2,6-dialkenyl BODIPY 2a (entries 2–3, respectively). Other solvents such as toluene and CH3CN were less effective (entries 4–5). Moreover, the use of 10 mol % of Au(III), Au(I), or Pt(II) salts was ineffective catalysts recovering in all cases the starting compound 1a (entries 6–8). However, using 20 mol % of InI3, 2a was isolated in 90% yield after 8 h (entry 9). It was found that both InBr3 and InCl3 catalysts allowed a mixture of 2 and 2a in moderate yields (entries 10–11, respectively). On the other hand, the use of In(OTf)3 (20 mol %) in DCE at 80 °C or toluene at 100 °C did not afford the target product 2a (entries 12–13).
Table 1. Intermolecular In(III)-Catalyzed Double Hydroarylation with Phenylacetylene with BODIPY 1a.
| entry | catalyst (mol %) | solventa | time (h) | yield (%)b |
|
|---|---|---|---|---|---|
| 2 | 2a | ||||
| 1c | InI3 (10) | DCE | 48 | 11 | - |
| 2d | InI3 (10) | DCE | 24 | 21 | 4 |
| 3 | InI3 (10) | DCE | 24 | 38 | 14 |
| 4 | InI3 (10) | toluene | 24 | 25 | 1 |
| 5 | InI3 (10) | CH3CN | 24 | nr | nr |
| 6 | AuCl3 (10) | DCE | 48 | nr | nr |
| 7 | PtCl2 (10) | DCE | 48 | nr | nr |
| 8e | Au(PPh3)Cl (10) | DCE | 48 | nr | nr |
| 9 | InI3 (20) | DCE | 8 h | - | 90f |
| 10 | InBr3 (20) | DCE | 48 | 36 | 29 |
| 11 | InCl3 (20) | DCE | 48 | 39 | 10 |
| 12 | In(OTf)3 (20) | DCE | 72 | 12 | - |
| 13g | In(OTf)3 (20) | toluene | 48 | nr | nr |
Reactions were carried out using 1a (1 mmol, ∼ 0.1 M) and 14 equiv of phenylacetylene.
Yields estimated by 1H NMR using CH2Br2 as an internal standard.
Reaction using 5 equiv of phenylacetylene.
Reaction using 10 equiv of phenylacetylene.
AgSbF6 (10 mol %) was also used.
Isolated yield.
Reaction carried out at 100 °C.
After the optimal reaction conditions were determined, we examined the scope of the reaction by using meso-substituted BODIPYs 1b–d (Scheme 2). The reaction of 1b, with a thien-2-yl group at the meso position, using 20 mol % of InI3 in DCE at 80 °C gave the double hydroarylation product 2b in 93% yield. In a similar way, meso-methyl BODIPY 1c also produced the double hydroarylated product 2c in 73% yield. The role of electronic effects was also explored using BODIPY 1d bearing a 4-cyanophenyl substituent at the meso position. Under the previous reaction conditions, the reaction of 1d with phenylacetylene provided only 2,6-dialkenyl BODIPY 2d in 11% along with the monosubstituted BODIPY 11 in 45% yield. This result could be attributed to the basic nitrogen of cyano group or to the low reactivity of BODIPY, resulting in the electron-withdrawing effect of the cyano group.
Scheme 2. Synthesis of Branched 2,6-Dialkenyl BODIPY Dyes through π-acid Catalysis.
Reactions were carried out using 1a–1d (1 mmol, ∼ 0.1 M) and 14 equiv of arylacetylene.
Monohydroarylated product was also obtained in 32% yield.
To further extend the scope of this transformation, the electronic effect of the substituents with respect to the alkyne component was explored. In this endeavor, the reaction of 1a with 4-methoxyphenylacetylene provided 3a in moderate yield (40%) accompanied by monosubstituted BODIPY (32% yield). In addition, treatment of 1a with 3-ethynylthiophene afforded 4a in 63% yield. The indium(III)-catalyzed procedure with BODIPY 1a also proved efficient using 1-ethenyl-3,5-bis(trifluoromethyl)benzene as the alkyne partner, arising selectively the dihydroarylated product 5a in 92% yield. Interestingly, the polyfluorinated fluorophores based on the BODIPY framework are of particular interest for the preparation of fluorescent fluorocarbon nanoemulsions, which can be used for simultaneous fluorescent and 19F MRI imaging.20
During our research, we also examined (bromoethynyl)benzene21 as an alkyne partner in the InI3-catalyzed reaction with BODIPY 1a. The participation of haloalkynes in the metal-catalyzed nucleophilic addition22 to generate alkenyl-halides constitutes a transformation with significant synthetic value.23 Alkenyl halides are also a pivotal class of compounds for chemical transformations in natural product total synthesis24 and also have found applications in photodynamic therapy when are linked to a BODIPY scaffold.25 In the event, the reaction of 1a with (bromoethynyl)benzene under the stablished reaction conditions (20 mol % of InI3, DCE, 80 °C) gave selectively (Z)-2,6-dialkenyl BODIPY 6a in 74% yield (Scheme 2). The Markovnikov regioselectivity was determined in 6a by the NOE experiment (see the Supporting Information).
Remarkably, indium(III) demonstrated a good ability to promote intermolecular alkyne hydroarylation with meso-substituted BODIPY dyes, yielding branched 2,6-alkenyl BODIPYs with Markovnikov regioselectivity independent of the electronic nature of the alkyne and BODIPY moieties.
As the next step, we studied the hydroarylation reaction of BODIPY with electron deficient alkynes under indium(III) catalysis. In this endeavor, we found that 1a reacts regioselectively with ethyl propiolate under the optimal reaction conditions (20 mol % of InI3, DCE, 80 °C), affording the linear β-addition product 7a in 20% yield (Scheme 3). The stereochemistry of (E)-2-alkenyl BODIPY 7a was assigned by 1H NMR spectroscopy on the basis of the coupling constants of alkenyl hydrogens (J = 16.2 Hz, see the Supporting Information). Interestingly, we found that using InCl3 (20 mol %) as the catalyst in DCE at 80 °C, the yield increased up to 40%. This result could be attributed to the oxophilic σ-Lewis acid character of the InCl3,26 and therefore, we hypothesized that the coordination mode of ethyl propiolate to InCl3 implies a σ-catalysis instead of π-catalysis. Furthermore, the reaction of 1b with ethyl propiolate afforded the expected β-addition, yielding (E)-2-alkenyl BODIPY 7b (56% yield). In addition, the reactions of 1a with 3-substituted propiolates, under the same reaction conditions, provided the trisubstituted alkenyl BODIPYs 8a and 9a in 42% and 36% yields, respectively, with (Z)-stereochemistry (Scheme 3). The assignment of stereochemistry of both compounds was confirmed by NOESY spectra (see the Supporting Information). Interestingly, the products obtained containing ester moieties are synthetically useful and might provide opportunities to development of BODIPY conjugates.27
Scheme 3. Synthesis of Linear 2-Alkenyl BODIPY Dyes through σ-Catalysis.
Reactions were carried out using 1a–1b (1 mmol, ∼ 0.1 M) and 14 equiv of alkyne.
InI3 (20 mol %) was used.
In parentheses, yield of the 2,6-dialkenyl products.
These experimental findings revealed that the branched 2,6-dialkenyl BODIPY dyes can be obtained regioselectively as a result of an initial activation of alkyne by π-coordination to InI3, followed by the anti-addition of the BODIPY through the β-position, further aromatization and protodemetalation, although a mechanism involving an alkenyl cation intermediate could not be discarded.17b,17d On the other hand, linear (E)-β-alkenyl BODIPYs could be formed through an initial σ-coordination of InCl3 with the carbonyl moiety followed by the β-conjugated addition of the BODIPY to form a zwitterionic allenyl enolate. The formation of (Z)-trisubstituted alkenes in the reaction of substituted propiolates can be attributed to the high Lewis acidity of InCl3, which would induce an alkene isomerization equilibrium.17d
At this point, we explored the synthesis of unsymmetrical 2,6-dialkenyl BODIPYs by sequential hydroarylation reactions. In the event, the reaction of β-alkenyl BODIPY 7b with phenylacetylene in the presence of InI3 (20 mol %) in DCE at 80 °C allowed the synthesis of unsymmetrical 2,6-dialkenyl BODIPY 8b in 80% yield (Scheme 4). Analogously, we carried out the InI3-catalyzed intermolecular hydroarylation with BODIPY 9a with 3-ethynylthiophene to give compound 10a in 73% yield. It is remarkably to note that the electron-withdrawing effect of the alkenyl group at C2 does not affect the reactivity in the hydroarylation reaction at C6 in contrast with substitution at the meso position.
Scheme 4. Synthesis of push–pull 2,6-Dialkenyl BODIPY Dyes.
The optical properties of synthesized fluorophores containing substituted alkenyl moieties at C2(6) positions of the BODIPYs 1a–d were measured in CHCl3 (Table 2). In general, BODIPY dyes are characterized by a narrow absorption band assigned to the strong S1 ← S0 transition. Indeed, the absorption maxima of the linear β-alkenyl and branched β,β′-dialkenyl BODIPY dyes 2a–10a, 2b–d, and 7b–8b induced a bathochromic shift (12–31 nm) by the electron-donating or electron-withdrawing substituents (Figure 1a,c). Besides, most compounds present high extinction coefficients (ε) such as the meso-phenyl-BODIPYs 3a and 4a (up to 88,200 M–1 cm–1) as the electron-donating ability at C2 and C6 positions increases, a required property in practical applications such as photodynamic therapy and photocatalysis.6b,28
Table 2. UV–Vis and PL Data of Compounds 2a–10a, 2b–d, and 7b–8b in CHCl3.
| comp.a | λAbsmax (nm) [ε × 103(M–1 cm–1)] | λPLmax (nm) | Stokes shift (nm) | ΦFb |
|---|---|---|---|---|
| 1a | 503 (74.4) | 512 | 9 | nd |
| 1b | 515 (66.5) | 522 | 7 | nd |
| 1c | 499 (54.4) | 508 | 9 | nd |
| 1d | 507 (47.4) | 519 | 12 | nd |
| 2a | 526 (64.9) | 552 | 26 | 0.72 |
| 2b | 540 (77.7) | 565 | 25 | 0.14 |
| 2c | 520 (38.4) | 554 | 34 | 0.96 |
| 2d | 532 (64.8) | 562 | 30 | 0.49 |
| 3a | 528 (83.3) | 558 | 30 | 0.82 |
| 4a | 521 (88.2) | 547 | 26 | 0.72 |
| 5a | 523 (79.2) | 543 | 20 | 0.79 |
| 6a | 523 (55.0) | 538 | 15 | 0.38 |
| 7a | 528 (56.1) | 549 | 21 | 0.79 |
| 7b | 541(43.2) | 566 | 25 | 0.18 |
| 8a | 515 (40.3) | 528 | 13 | 0.69 |
| 8b | 546 (48.5) | 575 | 29 | 0.16 |
| 9a | 514 (75.3) | 526 | 12 | 0.61 |
| 10a | 524 (51.6) | 544 | 20 | 0.77 |
All spectra were recorded in CHCl3 solutions at room temperature at 7.5 × 10–7 M for UV–vis and PL spectra, excited at the respective under λmax.
Fluorescence quantum yields determined relative to rhodamine 6G in as standard (ΦF = 0.94 in EtOH).32
Figure 1.
Normalized (a) absorbance and (b) emission spectra of BODIPY dyes 1a–8a, 8b, and 10a in CHCl3 (7.5 × 10–7 M, excited at the respective under λmax). (c) Absorbance and (d) emission spectra of BODIPY dyes 2a–d in CHCl3 (7.5 × 10–7 M, excited at the respective under λmax). (e) Photograph of the BODIPYs 2a–10a and 7b–8b under UV irradiation (λ = 365 nm).
2-Alkenyl and 2,6-dialkenyl BODIPYs also show narrow emission bandwidths covering the spectral range from 526 to 575 nm, exhibiting a more pronounced bathochromic effect (14–53 nm), related to the electron richness of the substituent on the alkenyl moiety (Figure 1b,d and Table 2). Interestingly, branched alkenyl BODIPY 2d equipped with a 4-cyanophenyl group at the meso position also showed a bathochromic shift, that could be attributed to a stabilization of the LUMO level due to the electron-withdrawing effect of the cyano group.29
Besides, 2-alkenyl BODIPY 7b, with extending conjugation containing an acrylate moiety, is bathochromically shifted (44 nm) related to 1b due to its D-π-A character. Moreover, when a second alkenyl substituent is placed at the C6 position, the Stokes shift was increased to 53 nm, which confirmed the results from a strong internal charge transfer (ICT) effect in unsymmetrical BODIPY 8b induced by greater push–pull electron movement.
Remarkably, the BODIPY dyes showed in general good-to-excellent quantum yields (up to ΦF = 0.96), suggesting a very rigid structures (Table 2). The quenched fluorescence observed for 2b, 7b, and 8b is basically due to the greater freedom of rotation of thien-2-yl group at the meso position, increasing the energy lost to nonradiative decay.30 In the case of 2d, the electron-withdrawing effect of the cyano group could lead to a deactivation process through photoinduced electron-transfer (PET) from the electron-rich BODIPY core to the electron poor cyano moiety, reducing the quantum yield.7,31 Meanwhile, the low ΦF of BODIPY 6a possessing heavy atoms (bromine) could be attributed that might arise an intersystem crossing from an excited singlet state to an excited triplet state, induced by the halogens linked to the BODIPY core through the alkenyl moiety.6
In general, the BODIPYs equipped with substituted alkenyl groups showed a remarkable effect on the absorption and fluorescence properties. Indeed, these compounds exhibit greater photophysical properties compared to the reported BODIPY dyes containing a p-dimethylamino substituent at the α-position of the alkene moiety.11d The molar absorption coefficients and quantum yield values tend to be excellent in some dyes. Therefore, compounds exhibit brightness when irradiated, as shown in Figure 1e, and show properties of great interest in fluorescence bioimaging.
Conclusions
A new method for the synthesis of β-alkenyl-substituted BODIPYs by the indium(III)-catalyzed intermolecular alkyne hydroarylation reaction of meso-substituted BODIPYs has been developed. Depending on the different nature of the interaction of the alkyne to the indium(III) salt resulted in two different types of products. The double intermolecular hydroarylation reaction of phenylacetylene and analogues with meso-substituted-BODIPYs takes place through the electrophilic π-activation mode using indium triiodide as the catalyst, to provide branched 2,6-dialkenyl BODIPYs with Markovnikov regioselectivity. Conversely, when ethyl propiolate was used, the hydroarylation occurs through the σ-activation mode in the presence of indium trichloride as the catalyst, to give rise linear (E)-2-alkenyl BODIPYs coming from a β-addition. This atom-economical dual catalysis allowed the synthesis of push–pull dialkenyl BODIPY dyes with tuneable spectral properties. The majority of compounds synthesized have brilliant fluorescence with emissions that span from 526 to 575 nm with high quantum yields (0.14–0.96). Further research will be focused on exploring further functionalization, photophysical properties, and applications in bioimaging.
Experimental Section
General Methods
All reactions were carried out in dried glassware, under the argon atmosphere, using standard gastight syringes and septa. Dry toluene, DCE, CH3CN, and other commercially available reagents were used as received. Reaction temperatures refer to external bath temperatures. All indium(III) salts were used as received under argon in a glovebox system. The BODIPY substrates (1a–d) were prepared according to the literature.19 Reactions were monitored by thin-layer chromatography, in precoated silica gel foils, using UV light as the visualizing agent and ethanolic phosphomolybdic acid as the developing agent. Flash column chromatography was performed using 230–400 mesh silica gel. 1H NMR, 19F{H} NMR, and 13C{H} NMR spectra were recorded at room temperature in CDCl3 using a 300 or 500 MHz spectrometer and calibrated to the solvent peak. DEPT data were used to assign carbon types. Chemical shifts are reported in ppm (δ) relative to the solvent CDCl3 (δH 7.26 ppm and δC 77.1 ppm). 19F NMR are internally referenced with respect to CFCl3 (δF 0.0 ppm). Structural assignments were made with additional information from NOESY, COSY, HSQC, and HMBC experiments. Mass spectra were recorded on a Magnetic Sector EI spectrometer or a QSTAR ESI mass spectrometer, operating in the positive ionization mode. The IR spectra were recorded with attenuated total reflectance (ATR). Ultraviolet/visible (UV/vis) absorption and fluorescence spectra were recorded with standard 1 cm quartz cells. Emission spectra were recorded using a spectrofluorometer equipped with a pulsed xenon flash-lamp as a light source. Compounds were excited at their excitation maxima (band of lowest energy) to record the emission spectra. The concentration of the compound solutions (in CHCl3) was adjusted to 7.5 × 10–7 M. Fluorescence quantum yield (ΦF) values were determined by comparison with rhodamine 6G in ethanol as a reference (ΦF = 0.94)32 using the equation below, where ΦST is the quantum yield of the reference, m is the slope of the line obtained from the best linear fit of the integrated fluorescence intensity versus absorbance data, and η is the refractive index of the solvent.
General Procedure for the Indium(III)-Catalyzed Intermolecular Alkyne Hydroarylation Reaction with BODIPYs
A Schlenk tube equipped with a magnetic stir bar was charged with the indium salt (0.04 mmol, 0.2 equiv) inside a glovebox. Then, the corresponding BODIPY (0.2 mmol, 1 equiv), DCE (2 mL), and the corresponding alkyne (2.8 mmol, 14 equiv) were added. The reaction mixture was heated in an oil bath at 80 °C and monitored by TLC until the starting material was consumed. The reaction mixture was cooled to rt, and the solvent was removed under reduced pressure. The resulting crude was purified by flash chromatography on silica gel (EtOAc/hexanes) to afford after concentration, and high vacuum drying, the corresponding 2-alkenyl or 2,6-dialkyne BODIPYs.
5,5-Difluoro-1,3,7,9-tetramethyl-10-phenyl-2,8-bis(1-phenylvinyl)-5H-4λ4,5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine (2a)
Following the general procedure, the reaction of 1a (49.9 mg, 0.154 mmol) with InI3 (15.3 mg, 0.081 mmol) and phenylacetylene (0.24 mL, 2.16 mmol) in DCE (1.5 mL) was heated at 80 °C for 8 h. After purification by chromatography (2–4% EtOAc/hexanes), compound 2a (73.2 mg, 90%) was obtained as a pink solid: mp = 106–107 °C; λabsmax (CHCl3) = 526 nm (ε = 64,944 M–1 cm–1); λemmax (CHCl3) = 552 nm; ΦF (CHCl3) = 0.72; IR (ATR) νmax = 2957, 2924, 2853, 1529, 1176 cm–1; 1H NMR (300 MHz, CDCl3) δ 7.43–7.49 (m, 3H), 7.34–7.36 (m, 2H), 7.26–7.31 (m, 10H), 5.85 (d, J = 1.5 Hz, 2H), 5.11 (d, J = 1.5 Hz, 2H), 2.40 (s, 6H), 1.23 (s, 6H); 13C{1H} NMR (75 MHz, CDCl3) δ 154.9 (2 × C), 141.9 (2 × C), 141.0 (C), 140.5 (2 × C), 140.2 (2 × C), 135.3 (2 × C), 133.2 (2 × C), 131.3 (C), 129.2 (2 × CH), 129.0 (CH), 128.5 (4 × CH), 128.1 (2 × CH), 127.8 (2 × CH), 126.3 (4 × CH), 117.3 (2 × CH2), 13.3 (2 × CH3), 12.8 (2 × CH3); 19F{1H} NMR (282 MHz, CDCl3) δ −145.80 (q, JB–F = 32.5 Hz, 2F); HRMS (ESI) m/z: [M + Na]+ calculated for C35H31BF2N2Na, 551.2446, found, 551.2441.
5,5-Difluoro-1,3,7,9-tetramethyl-2,8-bis(1-phenylvinyl)-10-(thiophen-2-yl)-5H-4λ4,5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine (2b)
Following the general procedure, the reaction of 1b (40.1 mg, 0.121 mmol) with InI3 (12.0 mg, 0.024 mmol) and phenylacetylene (0.19 mL, 1.69 mmol) in DCE (1.5 mL) was heated at 80 °C for 8 h. After purification by chromatography (2% EtOAc/hexanes), compound 2b (59.8 mg, 93%) was obtained as a pink solid: mp = 112–113 °C. λabsmax (CHCl3) = 540 nm (ε = 77,698 M–1 cm–1); λemmax (CHCl3) = 565 nm; ΦF (CHCl3) = 0.14; IR (ATR) νmax = 2962, 2918, 2851, 1259, 1023 cm–1; 1H NMR (300 MHz, CDCl3) δ 7.47 (dd, J = 1.3, 5.1 Hz, 1H), 7.26–7.32 (m, 10H), 7.10 (dd, J = 3.4, 5.1 Hz, 1H), 7.04 (dd, J = 1.2, 3.4 Hz, 1H), 5.87 (d, J = 1.5 Hz, 2H), 5.14 (d, J = 1.5 Hz, 2H), 2.40 (s, 6H), 1.44 (s, 6H); 13C{1H} NMR (75 MHz, CDCl3) δ 155.6 (2 × C), 140.8 (2 × C), 140.7 (2 × C), 140.1 (2 × C), 135.0 (2 × C), 134.1 (C), 133.5 (2 × C), 132.3 (C), 128.5 (4 × CH), 128.0 (CH), 127.9 (2 × CH), 127.6 (CH), 127.5 (CH), 126.3 (4 × CH), 117,4 (2 × CH2), 13.4 (2 × CH3), 12.0 (2 × CH3); 19F{1H} NMR (282 MHz, CDCl3) δ −145.74 (q, JB–F = 32.6 Hz, 2F); HRMS (ESI) m/z: [M + Na]+ calculated for C33H29BF2N2SNa, 557.2010, found, 557.2001.
Scale-Up Experiment for 2b
A Schlenk tube equipped with a magnetic stir bar was charged with InI3 (120 mg, 0.242 mmol) inside a glovebox. Then, a solution of 1b (400 mg, 1.21 mmol) in DCE (5.6 mL) and phenylacetylene (1.86 mL, 16.9 mmol) were added. The resulting mixture was heated in an oil bath at 80 °C for 8 h. The reaction mixture was cooled to rt, and the solvent was removed under reduced pressure. The residue was purified by flash chromatography on silica gel (2 to 8% EtOAc/hexanes) to afford after concentration, and high vacuum drying, 2b (517.4 mg, 80%) as a pink solid.
5,5-Difluoro-1,3,7,9,10-pentamethyl-2,8-bis(1-phenylvinyl)-5H-4λ4,5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine (2c)
Following the general procedure, the reaction of 1c (40.1 mg, 0.153 mmol) with InI3 (15.1 mg, 0.031 mmol) and phenylacetylene (0.24 mL, 2.14 mmol) in DCE (1.5 mL) was heated at 80 °C for 24 h. After purification by chromatography (2% EtOAc/hexanes), compound 2c (51.6 mg, 73%) was obtained as a pink solid: mp = 168–169 °C. λabsmax (CHCl3) = 520 nm (ε = 38,421 M–1 cm–1); λemmax (CHCl3) = 554 nm; ΦF (CHCl3) = 0.96; IR (ATR) νmax = 2956, 2920, 2851, 1544, 1193 cm–1; 1H NMR (300 MHz, CDCl3) δ 7.29–7.36 (m, 10H), 5.95 (d, J = 1.5 Hz, 2H), 5.18 (d, J = 1.5 Hz, 2H), 2.67 (s, 3H), 2.37 (s, 6H), 2.27 (s, 6H); 13C{1H} NMR (75 MHz, CDCl3) δ 153.1 (2 × C), 141.5 (C), 141.0 (2 × C), 140.3 (2 × C), 138.3 (2 × C), 133.0 (2 × C), 132.2 (2 × C), 128.5 (4 × CH), 127.9 (2 × CH), 126.3 (4 × CH), 117.4 (2 × CH2), 17.1 (2 × CH3), 15.5 (2 × CH3), 13.2 (CH3); 19F{1H} NMR (282 MHz, CDCl3) δ −146.21 (q, JB–F = 32.8 Hz, 2F); HRMS (IE) m/z: [M]+ calculated for C30H29BF2N2, 466.2392, found, 466.2376.
4-(5,5-Difluoro-1,3,7,9-tetramethyl-2,8-bis(1-phenylvinyl)-5H-4λ4,5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinin-10-yl)benzonitrile (2d)
Following the general procedure, the reaction of 1d (80.4 mg, 0.229 mmol) with InI3 (22.7 mg, 0.046 mmol) and phenylacetylene (0.35 mL, 3.21 mmol) in DCE (2 mL) was heated at 80 °C for 48 h. After purification by chromatography (4% EtOAc/hexanes), compound 2d (13.7 mg, 11%) and 2-alkenyl BODIPY 11 (46.4 mg, 45%) were obtained as pink and orange solids, respectively.
Data for 2d
mp = 92–93 °C; λabsmax (CHCl3) = 532 nm (ε = 64,780 M–1 cm–1); λemmax (CHCl3) = 562 nm; ΦF (CHCl3) = 0.49; IR (ATR) νmax = 2957, 2922, 2851, 2325, 1587, 1180 cm–1; 1H NMR (300 MHz, CDCl3) δ 7.79 (d, J = 8.0 Hz, 2H), 7.54 (d, J = 8.0 Hz, 2H), 7.28 (s, 10H), 5.86 (s, 2H), 5.11 (s, 2H), 2.40 (s, 6H), 1.20 (s, 6H); 13C{1H} NMR (75 MHz, CDCl3) δ 156.2 (C), 140.7 (2 × C), 140.4 (2 × C), 140.0 (2 × C), 139.8 (2 × C), 138.8 (C), 133.8 (C), 133.0 (2 × CH), 130.6 (C), 129.6 (2 × CH), 128.6 (4 × CH), 128.1 (2 × CH), 126.3 (4 × CH), 118.0 (2 × C), 117.6 (2 × CH2), 113.4 (2 × C), 13.5 (2 × CH3), 13.1 (2 × CH3); 19F{1H} NMR (282 MHz, CDCl3) δ −145.78 (q, JB–F = 33.2 Hz, 2F); HRMS (ESI) m/z: [M + Na]+ calculated for C36H30BF2N3Na, 576.2399, found, 576.2390.
Data for 11
mp = 77–78 °C; IR (ATR) νmax = 2954, 2921, 2851,2338, 1462, 1377 cm–1; 1H NMR (300 MHz, CDCl3) δ 7.80 (d, J = 8.1 Hz, 2H), 7.50 (d, J = 8.0 Hz, 2H), 7.28 (s, 5H), 6.03 (s, 1H) 5.85 (s, 1H), 5.09 (s, 1H), 2.57 (s, 3H), 2.38 (s, 3H), 1.37 (s, 3H), 1.19 (s, 3H); 13C{1H} NMR (75 MHz, CDCl3) δ 156.7 (C), 156.0 (C), 142.6 (C), 140.7 (C), 140.2 (2 × C), 139.9 (2 × C), 139.7 (C), 138.7 (C), 132.9 (2 × CH), 129.5 (2 × CH), 129.4 (C), 128.5 (2 × CH), 128.0 (CH), 126.3 (2 × CH), 121.9 (CH), 118.1 (C), 117.6 (CH2), 113.3 (C), 14.7 (2 × CH3), 13.4 (CH3), 13.0 (CH3); 19F{1H} NMR (282 MHz, CDCl3) δ −145.96 (q, JB–F = 32.3 Hz, 2F); HRMS (ESI) m/z: [M + Na]+ calculated for C28H24BF2N3Na, 474.1929, found, 474.1918.
5,5-Difluoro-2,8-bis(1-(4-methoxyphenyl)vinyl)-1,3,7,9-tetramethyl-10-phenyl-5H-4λ4,5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine (3a)
Following the general procedure, the reaction of 1a (79.9 mg, 0.247 mmol) with InI3 (24.5 mg, 0.05 mmol) and 1-ethynyl-4-methoxybenzene (0.45 mL, 3.46 mmol) in DCE (2 mL) was heated at 80 °C for 24 h. After purification by chromatography (1–3% EtOAc/hexanes), compound 3a (57.9 mg, 40%) was obtained as a pink solid: mp = 107–108 °C; λabsmax (CHCl3) = 528 nm (ε = 83,256 M–1 cm–1); λemmax (CHCl3) = 558 nm; ΦF (CHCl3) = 0.82; IR (ATR) νmax = 2956, 2925, 2837, 1533, 1172 cm–1; 1H NMR (300 MHz, CDCl3) δ 7.36–7.39 (m, 3H), 7.26–7.28 (m, H), 7.14–7.18 (m, 4H), 6.73 (d, J = 8.6 Hz, 4H), 5.67 (s, 2H), 4.92 (s, 2H), 3.72 (s, 6H), 2.32 (s, 6H), 1.15 (s, 6H); 13C{1H} NMR (75 MHz, CDCl3) δ 160.0 (2 × C), 155.4 (C), 142.3 (C), 140.9 (C), 140.8 (4 × C), 135.9 (C), 134.0 (C), 133.3 (4 × C), 131.8 (C), 129.7 (2 × CH), 129.5 (CH), 128.6 (2 × CH), 128.0 (4 × CH), 115.8 (2 × CH2), 114.3 (4 × CH), 55.8 (2 × CH3), 13.9 (2 × CH3), 13.3 (2 × CH3); 19F{1H} NMR (282 MHz, CDCl3) δ −145.83 (q, JB–F = 32.9 Hz, 2F); HRMS (ESI) m/z: [M + Na]+ calculated for C37H35BF2N2O2Na, 611.2657, found, 611.2650.
5,5-Difluoro-1,3,7,9-tetramethyl-10-phenyl-2,8-bis(1-(thiophen-3-yl)vinyl)-5H-4λ4,5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine (4a)
Following the general procedure, the reaction of 1a (79.8 mg, 0.247 mmol) with InI3 (24.5 mg, 0.05 mmol) and 3-ethynylthiophene (0.34 mL, 3.46 mmol) in DCE (2 mL) was heated at 80 °C for 23 h. After purification by chromatography (1–3% EtOAc/hexanes), compound 4a (83.8 mg, 63%) was obtained as a pink solid: mp = 98–100 °C; λabsmax (CHCl3) = 521 nm (ε = 88,163 M–1 cm–1); λemmax (CHCl3) = 547 nm; ΦF (CHCl3) = 0.72; IR (ATR) νmax = 2956, 2929, 2853, 1536, 1178 cm–1; 1H NMR (300 MHz, CDCl3) δ 7.35–7.42 (m, 3H), 7.23–7.27 (m, 2H), 7.16–7.19 (m, 2H), 7.11–7.13 (m, 2H), 6.83–6.85 (m, 2H), 5.71 (s, 2H), 4.95 (s, 2H), 2.37 (s, 6H), 1.17 (s, 6H); 13C{1H} NMR (75 MHz, CDCl3) δ 155.2 (2 × C), 142.7 (C), 142.5 (C), 140.7 (2 × C), 136.3 (2 × C), 135.7 (2 × C), 133.9 (C), 131.7 (C), 129.7 (2 × CH), 129.7 (C), 129.6 (CH), 128.6 (2 × CH), 128.5 (C), 126.6 (2 × CH), 125.9 (2 × CH), 123.2 (2 × CH), 116.5 (2 × CH2), 13.8 (2 × CH3), 13.3 (2 × CH3); 19F{1H} NMR (282 MHz, CDCl3) δ −145.88 (q, JB–F = 32.7 Hz, 2F); HRMS (ESI) m/z: [M + Na]+ calculated for C31H27BF2N2S2Na, 563.1574, found, 563.1568.
2,8-Bis(1-(3,5-bis(trifluoromethyl)phenyl)vinyl)-5,5-difluoro-1,3,7,9-tetramethyl-10-phenyl-5H-4λ4,5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine (5a)
Following the general procedure, the reaction of 1a (44.8 mg, 0.139 mmol) with InI3 (13.8 mg, 0.028 mmol) and 1-ethynyl-3,5-bis(trifluoromethyl)benzene (0.34 mL, 1.94 mmol) in DCE (2 mL) was heated at 80 °C for 6 h. After purification by chromatography (2% EtOAc/hexanes), compound 5a (102.1 mg, 92%) was obtained as a pink solid: mp = 106–107 °C; λabsmax (CHCl3) = 523 nm (ε = 79,174 M–1 cm–1); λemmax (CHCl3) = 543 nm; ΦF (CHCl3) = 0.79; IR (ATR) νmax = 2926,2854, 1535, 1279, 1131, 1136 cm–1; 1H NMR (300 MHz, CDCl3) δ 7.78 (s, 2H), 7.72 (s, 4H), 7.46–7.54 (m, 3H), 7.35–7.38 (m, 2H), 6.00 (s, 2H), 5.35 (s, 2H), 2.44 (s, 6H), 1.21 (s, 6H); 13C{1H} NMR (75 MHz, CDCl3) δ 154.8 (2 × C), 143.0 (C), 142.4 (2 × C), 141.0 (2 × C), 138.7 (2 × C), 134.8 (2 × C), 132.0 (q, 2JCF = 33.4 Hz, 4 × C), 131.6 (C), 131.4 (C), 129.4 (2 × CH), 129.3 (CH), 128.0 (C), 127.8 (2 × CH), 126.2 (4 × CH), 123.2 (q, 1JCF = 272.3 Hz, 4 × CF3), 121.6 (2 × CH), 120.9 (2 × CH2), 13.4 (CH3), 13.0 (3 × CH3); 19F{1H} NMR (282 MHz, CDCl3) δ −62.86 (s, 4CF3), −145.73 (q, JB–F = 33.1 Hz, 2F); HRMS (ESI) m/z: [M + Na]+ calculated for C39H27BF14N2Na, 823.1941, found, 823.1941.
2,8-Bis((Z)-2-bromo-1-phenylvinyl)-5,5-difluoro-1,3,7,9-tetramethyl-10-phenyl-5H-4λ4,5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinine (6a)
Following the general procedure, the reaction of 1a (20.1 mg, 0.062 mmol) with InI3 (6.1 mg, 0.012 mmol) and (bromoethynyl)benzene (156.4 mg, 0.86 mmol) in DCE (0.7 mL) was heated at 80 °C for 3 h. After purification by chromatography (3% EtOAc/hexanes), compound 6a (31.3 mg, 74%) was obtained as a pink solid: mp = 105–106 °C; λabsmax (CHCl3) = 523 nm (ε = 54,973 M–1 cm–1); λemmax (CHCl3) = 538 nm; ΦF (CHCl3) = 0.38; IR (ATR) νmax = 2955, 2923, 2851, 1461, 1377 cm–1; 1H NMR (300 MHz, CDCl3) δ 7.38–7.49 (m, 5H), 7.27–7.31 (m, 6H), 7.20–7.24 (m, 4H), 6.95 (s, 2H), 2.41 (s, 6H), 1.23 (s, 6H); 13C{1H} NMR (75 MHz, CDCl3) δ 154.6 (2 × C), 142.2 (C), 141.0 (C), 139.6 (C), 139.6 (C), 139.0 (2 × C), 138.9 (2 × C), 135.0 (2 × C), 131.5 (C), 130.8 (C), 129.4 (CH), 129.2 (CH), 129.1 (CH), 128.8 (CH), 128.37 (CH), 128.19 (CH), 128.1 (CH), 128.0 (4 × CH), 126.4 (4 × CH), 109.4 (CH), 109.4 (CH), 13.4 (2 × CH3), 12.9 (2 × CH3); 19F{1H} NMR (282 MHz, CDCl3) δ −145.67 (q, JB–F = 33.1 Hz, 2F); HRMS (ESI) m/z: [M + Na]+ calculated for C35H29BF2N2Br2Na, 707.0656, found, 707.0653.
Ethyl (E)-3-(5,5-difluoro-1,3,7,9-tetramethyl-10-phenyl-5H-4λ4,5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinin-2-yl)acrylate (7a)
Following the general procedure, the reaction of 1a (44.8 mg, 0.138 mmol) with InCl3 (6.1 mg, 0.028 mmol) and ethyl propiolate (0.20 mL, 1.93 mmol) in DCE (2 mL) was heated at 80 °C for 48 h. After purification by chromatography (5–20% EtOAc/hexanes), compound 7a (23.4 mg, 40%) was obtained as a pink solid: mp = 155–156 °C; λabsmax (CHCl3) = 528 nm (ε = 56,128 M–1 cm–1); λemmax (CHCl3) = 549 nm; ΦF (CHCl3) = 0.79; IR (ATR) νmax = 2955, 2919, 2851, 1711, 1540, 1166 cm–1; 1H NMR (300 MHz, CDCl3) δ 7.55 (d, J = 16.5 Hz, 1H), 7.45–7.47 (m, 3H), 7.21–7.26 (m, 2H), 6.01 (s, 1H), 5.98 (d, J = 16.5 Hz, 1H), 4.17 (q, J = 7.1 Hz, 2H), 2.65 (s, 3H), 2.53 (s, 3H), 1.40 (s, 3H), 1.34 (s, 3H), 1.25 (t, J = 7.2 Hz, 3H); 13C{1H} NMR (75 MHz, CDCl3) δ 167.6 (C), 158.5 (C), 154.45 (C), 145.4 (C), 142.4 (C), 140.5 (C), 135.8 (CH), 134.7 (C), 132.7 (C), 130.6 (C), 129.3 (2 × CH), 129.3 (CH), 128.0 (2 × CH), 125.0 (C), 122.7 (CH), 117.5 (CH), 60.4 (CH2), 14.8 (CH3), 14.7 (CH3), 14.4 (CH3), 14.0 (CH3), 12.7 (CH3); 19F{1H} NMR (282 MHz, CDCl3) δ −144.94 (q, JB–F = 32.9 Hz, 2F); HRMS (ESI) m/z: [M + Na]+ calculated for C24H25BF2N2O2Na, 445.1875, found, 445.1869.
Ethyl (E)-3-(5,5-difluoro-1,3,7,9-tetramethyl-10-(thiophen-2-yl)-5H-4λ4,5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinin-2-yl)acrylate (7b)
Following the general procedure, the reaction of 1b (40.1 mg, 0.121 mmol) with InCl3 (5.4 mg, 0.024 mmol) and ethyl propiolate (0.17 mL, 1.69 mmol) in DCE (2 mL) was heated at 80 °C for 48 h. After purification by chromatography (3–15% EtOAc/hexanes), compound 7b (29.2 mg, 56%) was obtained as a pink solid: mp = 155–156 °C; λabsmax (CHCl3) = 541 nm (ε = 43,246 M–1 cm–1); λemmax (CHCl3) = 566 nm; ΦF (CHCl3) = 0.18; IR (ATR) νmax = 2956, 2924, 2852, 1708, 1539, 1284, 1164 cm–1; 1H NMR (300 MHz, CDCl3) δ 7.61 (d, J = 16.3 Hz, 1H), 7.53 (d, J = 4.8 Hz, 1H), 7.16 (t, J = 4.1 Hz, 1H), 7.01 (d, J = 3.2 Hz, 1H), 6.08 (s, 1H), 6.03 (d, J = 16.3 Hz, 1H), 4.23 (q, J = 7.1 Hz, 2H), 2.69 (s, 3H), 2.58 (s, 3H), 1.65 (s, 3H), 1.61 (s, 3H), 1.31 (t, J = 7.2 Hz, 3H); 13C{1H} NMR (75 MHz, CDCl3) δ 167.5 (C), 159.2 (C), 155.0 (C), 145.7 (C), 140.7 (C), 135.7 (CH), 134.8 (C), 134.3 (2 × C), 131.4 (C), 128.2 (CH), 127.9 (CH), 127.8 (CH), 125.3 (C), 123.0 (CH), 117.8 (CH), 60.4 (CH2), 14.9 (CH3), 14.4 (CH3), 14.1 (CH3), 13.9 (CH3), 12.0 (CH3); 19F{1H} NMR (319 MHz, CDCl3) δ −144.85 (q, JB–F = 32.4 Hz, 2F); HRMS (ESI) m/z: [M + Na]+ calculated for C22H23BF2N2O2SNa, 451.1439, found, 451.1431.
Methyl (Z)-3-(5,5-difluoro-1,3,7,9-tetramethyl-10-phenyl-5H-4λ4,5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinin-2-yl)-3-phenylacrylate (8a)
Following the general procedure, the reaction of 1a (60.1 mg, 0.185 mmol) with InCl3 (8.2 mg, 0.037 mmol) and ethyl 3-phenylpropiolate (0.38 mL, 2.59 mmol) in DCE (2 mL) was heated at 80 °C for 24 h. Then, the temperature was raised to 100 °C, and the reaction was left for 2 more days. After purification by chromatography (5–20% EtOAc/hexanes), compound 8a (37.2 mg, 42%) was obtained as a pink solid: mp = 101–102 °C; λabsmax (CHCl3) = 515 nm (ε = 40,299 M–1 cm–1); λemmax (CHCl3) = 528 nm; ΦF (CHCl3) = 0.69; IR (ATR) νmax = 3059, 2951, 1722, 1615, 1157 cm–1; 1H NMR (300 MHz, CDCl3) δ 7.43–7.47 (m, 3H), 7.29–7.37 (m, 7H), 6.49 (s, 1H), 6.01 (s, 1H), 3.68 (s, 3H), 2.58 (s, 3H), 2.34 (s, 3H), 1.39 (s, 3H), 1.11 (s, 3H); 13C{1H} NMR (75 MHz, CDCl3) δ 165.7 (C), 155.9 (C), 154.4 (C), 148.7 (2 × C), 143.5 (C), 141.9 (C), 140.4 (C), 139.6 (2 × C), 135.0 (2 × C), 129.7 (CH), 129.2 (CH), 129.1 (CH), 129.0 (CH), 128.8 (2 × CH), 128.2 (CH), 128.1 (CH), 128.0 (CH), 127.4 (2 × CH), 119.7 (CH), 49.9 (CH3), 14.7 (CH3), 14.5 (CH3), 13.3 (CH3), 12.8 (CH3); 19F{1H} NMR (282 MHz, CDCl3) δ −146.00 (dq, JF–F = 253.9 Hz, JB–F = 33.0 Hz, 2F); HRMS (ESI) m/z: [M + Na]+ calculated for C29H27BF2N2O2Na, 507.2031, found, 507.2028.
Methyl (Z)-3-(5,5-difluoro-1,3,7,9-tetramethyl-10-phenyl-5H-4λ4,5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinin-2-yl)but-2-enoate (9a)
Following the general procedure, the reaction of 1a (80.1 mg, 0.247 mmol) with InCl3 (10.9 mg, 0.049 mmol) and methyl but-2-ynoate (0.35 mL, 3.46 mmol) in DCE (2.5 mL) was heated at 80 °C for 24 h. Then, the temperature was raised to 100 °C, and the reaction was left for 2 more days. After purification by chromatography (3–10% EtOAc/hexanes), compound 9a (37.5 mg, 36%) was obtained as a pink solid: mp = 134–135 °C; λabsmax (CHCl3) = 514 nm (ε = 75,310 M–1 cm–1); λemmax (CHCl3) = 526 nm; ΦF (CHCl3) = 0.61; IR (ATR) νmax = 2954, 2921, 2851, 1783, 1542, 1462 cm–1; 1H NMR (300 MHz, CDCl3) δ 7.37–7.42 (m, 3H), 7.25–7.29 (m, 1H), 7.18–7.22 (m, 1H), 5.96 (q, J = 1.4 Hz, 1H), 5.89 (s, 1H), 3.50 (s, 3H), 2.47 (s, 3H), 2.36 (s, 3H), 1.90 (t, J = 1.3 Hz, 3H), 1.29 (s, 3H), 1.15 (s, 3H); 13C{1H} NMR (75 MHz, CDCl3) δ 165.3 (C), 155.3 (C), 152.8 (C), 148.5 (2 × C), 143.0 (C), 141.6 (C), 138.2 (C), 135.1 (2 × C), 145.1 (C), 129.1 (2 × CH), 129.1 (CH), 128.9 (CH), 128.2 (CH), 128.0 (CH), 121.2 (CH), 51.2 (CH3), 26.3 (CH3), 14.6 (CH3), 14.3 (CH3), 13.1 (CH3), 12.5 (CH3); 19F{1H} NMR (282 MHz, CDCl3) δ −146.12 (dq, JF–F = 308.9, JB–F = 33.2 Hz, 2F); HRMS (ESI) m/z: [M + Na]+ calculated for C24H25BF2N2O2Na, 445.1875, found, 445.1876.
Ethyl (E)-3-(5,5-difluoro-1,3,7,9-tetramethyl-8-(1-phenylvinyl)-10-(thiophen-2-yl)-5H-4λ4,5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinin-2-yl)acrylate (8b)
Following the general procedure, the reaction of 7b (24.2 mg, 0.058 mmol) with InI3 (5.6 mg, 0.012 mmol) and phenylacetylene (0.090 mL, 0.82 mmol) in DCE (1 mL) was heated at 80 °C for 48 h. After purification by chromatography (2–5% EtOAc/hexanes), compound 8b (23.9 mg, 80%) was obtained as a pink solid: mp = 96–97 °C; λabsmax (CHCl3) = 546 nm (ε = 48,515 M–1 cm–1); λemmax (CHCl3) = 575 nm; ΦF (CHCl3) = 0.16; IR (ATR) νmax = 2955, 2923, 2852, 1733, 1532, 1461 cm–1; 1H NMR (300 MHz, CDCl3) δ 7.63 (d, J = 16.3 Hz, 1H), 7.53 (dd, J = 1.1, 5.1 Hz, 1H), 7.27–7.33 (m, 5H), 7.13–7.16 (m, 1H), 7.04 (dd, J = 1.2, 3.5 Hz, 1H), 6.08 (d, J = 16.3 Hz, 1H), 5.89 (d, J = 1.3 Hz, 1H), 5.14 (d, J = 1.25 Hz, 1H), 4.25 (q, J = 7.1 Hz, 2H), 2.71 (s, 3H), 2.40 (s, 3H), 1.67 (s, 3H), 1.46 (s, 3H), 1.32 (t, J = Hz, 3H);13C{1H} NMR (75 MHz, CDCl3) δ 167.5 (C), 158.8 (C), 155.0 (C), 142.5 (C), 140.7 (C), 140.3 (2 × C), 139.6 (2 × C), 135.7 (CH), 134.8 (C), 134.5 (C), 128.6 (2 × CH), 128.5 (C), 128.2 (CH), 128.1 (CH), 127.9 (CH), 127.8 (CH), 126.9 (C), 126.3 (CH), 126.2 (2 × CH), 117.8 (CH2), 60.4 (CH2), 14.4 (CH3), 14.1 (CH3), 13.7 (CH3), 12.2 (CH3), 12.1 (CH3); 19F{1H} NMR (282 MHz, CDCl3) δ −144.59 (q, JB–F = 31.7 Hz, 2F); HRMS (ESI) m/z: [M + Na]+ calculated for C30H29BF2N2O2SNa, 553.1909, found, 553.1890.
Methyl (Z)-3-(5,5-difluoro-1,3,7,9-tetramethyl-10-phenyl-8-(1-(thiophen-3-yl)vinyl)-5H-4λ4,5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinin-2-yl)but-2-enoate (10a)
Following the general procedure, the reaction of 9a (29.0 mg, 0.069 mmol) with InI3 (6.8 mg, 0.014 mmol) and 3-ethynylthiophene (0.10 mL, 0.96 mmol) in DCE (1 mL) was heated at 80 °C for 23 h. After purification by chromatography (3–10% EtOAc/hexanes), compound 10a (26.3 mg, 73%) was obtained as a pink solid: mp = 102–104 °C; λabsmax (CHCl3) = 524 nm (ε = 51,560 M–1 cm–1); λemmax (CHCl3) = 544 nm; ΦF (CHCl3) = 0.77; IR (ATR) νmax = 2955, 2922, 2851, 1536, 1463 cm–1; 1H NMR (300 MHz, CDCl3) δ 7.36–7.41 (m, 3H), 7.27–7.32 (m, 1H), 7.22–7.26 (m, 1H), 7.17–7.19 (m, 1H), 7.10–7.12 (m, 1H), 6.84 (dd, J = 1.3, 3.0 Hz, 1H), 5.97 (q, J = 1.5 Hz, 1H), 5.71 (d, J = 1.5 Hz, 1H), 4.93 (d, J = 1.5 Hz, 1H), 3.52 (s, 3H), 2.38 (s, 3H), 2.36 (s, 3H), 1.92 (s, 3H), 1.16 (s, 3H), 1.15 (s, 3H); 13C{1H} NMR (75 MHz, CDCl3) δ 165.4 (C), 154.3 (C), 153.1 (C), 148.5 (C), 142.2 (C), 141.8 (C), 140.0 (C), 138.3 (C), 135.9 (C), 135.2 (2 × C), 133.2 (C), 132.3 (C), 131.0 (C), 129.1 (CH), 129.1 (CH), 129.0 (CH), 128.2 (CH), 128.1 (CH), 126.0 (CH), 125.4 (CH), 122.6 (CH), 121.3 (CH), 115.9 (CH2), 51.2 (CH3), 26.2 (2 × CH3), 13.1 (CH3), 12.7 (CH3), 12.6 (CH3); 19F{1H} NMR (282 MHz, CDCl3) δ −145.97 (dq, JF–F = 262.9, JB–F = 33.2 Hz, 2F); HRMS (ESI) m/z: [M + Na]+ calculated for C30H29BF2N2O2SNa, 553.1909, found, 553.1909.
Acknowledgments
We thank the Ministerio de Ciencia e Innovación–Agencia Estatal de Investigación (Spain, PID2021-122335NB-I00, MCIN/AEI/10.13039/501100011033/FEDER, UE) and Xunta de Galicia (GRC2022/039) for financial support. We also thank Prof. E. Pazos research group (Universidade da Coruña) for sharing their spectrofluorometer equipment. A.D.L. thanks the Xunta de Galicia for a predoctoral fellowship (EDA 481A-2020/017). We also thank the funding for open access: Universidade da Coruña/CISUG.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.3c02951.
Copies of 1H NMR, 13C NMR, and 19F NMR spectra of all compounds; NOE and 2D NMR spectra for compounds 6a, 7a, 8a, and 9a; and UV–vis and emission spectra for all compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Ni Y.; Wu J. Far-Red and near Infrared BODIPY Dyes: Synthesis and Applications for Fluorescent pH Probes and Bio-Imaging. Org. Biomol. Chem. 2014, 12, 3774–3791. 10.1039/c3ob42554a. [DOI] [PubMed] [Google Scholar]; b Samanta S.; Lai K.; Wu F.; Liu Y.; Cai S.; Yang X.; Qu J.; Yang Z. Xanthene, Cyanine, Oxazine and BODIPY: The Four Pillars of the Fluorophore Empire for Super-Resolution Bioimaging. Chem. Soc. Rev. 2023, 52, 7197–7261. 10.1039/D2CS00905F. [DOI] [PubMed] [Google Scholar]
- Kowada T.; Maeda H.; Kikuchi K. BODIPY-Based Probes for the Fluorescence Imaging of Biomolecules in Living Cells. Chem. Soc. Rev. 2015, 44, 4953–4972. 10.1039/C5CS00030K. [DOI] [PubMed] [Google Scholar]
- a Awuah S. G.; You Y. Boron Dipyrromethene (BODIPY)-Based Photosensitizers for Photodynamic Therapy. RSC Adv. 2012, 2, 11169–11183. 10.1039/c2ra21404k. [DOI] [Google Scholar]; b Kamkaew A.; Lim S. H.; Lee H. B.; Kiew L. V.; Chung L. Y.; Burgess K. BODIPY Dyes in Photodynamic Therapy. Chem. Soc. Rev. 2013, 42, 77–88. 10.1039/C2CS35216H. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Bassan E.; Gualandi A.; Cozzi P. G.; Ceroni P. Design of BODIPY Dyes as Triplet Photosensitizers: Electronic Properties Tailored for Solar Energy Conversion, Photoredox Catalysis and Photodynamic Therapy. Chem. Sci. 2021, 12, 6607–6628. 10.1039/D1SC00732G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana P.; Singh N.; Majumdar P.; Singh S. P. Evolution of BODIPY/Aza-BODIPY Dyes for Organic Photoredox/Energy Transfer Catalysis. Coord. Chem. Rev. 2022, 470, 214698. 10.1016/j.ccr.2022.214698. [DOI] [Google Scholar]
- Singh S. P.; Gayathri T. Evolution of BODIPY Dyes as Potential Sensitizers for Dye-Sensitized Solar Cells. Eur. J. Org Chem. 2014, 2014, 4689–4707. 10.1002/ejoc.201400093. [DOI] [Google Scholar]
- a Bertrand B.; Passador K.; Goze C.; Denat F.; Bodio E.; Salmain M. Metal-Based BODIPY Derivatives as Multimodal Tools for Life Sciences. Coord. Chem. Rev. 2018, 358, 108–124. 10.1016/j.ccr.2017.12.007. [DOI] [Google Scholar]; b Li F.-Z.; Yin J.-F.; Kuang G.-C. BODIPY-Based Supramolecules: Construction, Properties and Functions. Coord. Chem. Rev. 2021, 448, 214157. 10.1016/j.ccr.2021.214157. [DOI] [Google Scholar]; c Mao Z.; Kim J. H.; Lee J.; Xiong H.; Zhang F.; Kim J. S. Engineering of BODIPY-Based Theranostics for Cancer Therapy. Coord. Chem. Rev. 2023, 476, 214908. 10.1016/j.ccr.2022.214908. [DOI] [Google Scholar]
- Loudet A.; Burgess K. BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. 10.1021/cr078381n. [DOI] [PubMed] [Google Scholar]
- Isik M.; Ozdemir T.; Turan I. S.; Kolemen S.; Akkaya E. U. Chromogenic and Fluorogenic Sensing of Biological Thiols in Aqueous Solutions Using BODIPY-Based Reagents. Org. Lett. 2013, 15 (1), 216–219. 10.1021/ol303306s. [DOI] [PubMed] [Google Scholar]
- Prusty D. K.; Herrmann A. A Fluorogenic Reaction Based on Heavy-Atom Removal for Ultrasensitive DNA Detection. J. Am. Chem. Soc. 2010, 132, 12197–12199. 10.1021/ja105181v. [DOI] [PubMed] [Google Scholar]
- Ye S.; Rao J.; Qiu S.; Zhao J.; He H.; Yan Z.; Yang T.; Deng Y.; Ke H.; Yang H.; Zhao Y.; Guo Z.; Chen H. Rational Design of Conjugated Photosensitizers with Controllable Photoconversion for Dually Cooperative Phototherapy. Adv. Mater. 2018, 30, 1801216. 10.1002/adma.201801216. [DOI] [PubMed] [Google Scholar]
- Revisions and selected references:; a Clarke R. G.; Hall M. J. Recent Developments in the Synthesis of the BODIPY Dyes. Adv. Heterocycl. Chem. 2019, 128, 181–261. 10.1016/bs.aihch.2018.12.001. [DOI] [Google Scholar]; b Boens N.; Verbelen B.; Ortiz M. J.; Jiao L.; Dehaen W. Synthesis of BODIPY Dyes through Postfunctionalization of the Boron Dipyrromethene Core. Coord. Chem. Rev. 2019, 399, 213024. 10.1016/j.ccr.2019.213024. [DOI] [Google Scholar]; c Ahrens J.; Haberlag B.; Scheja A.; Tamm M.; Bröring M. Conjugated BODIPY DYEmers by Metathesis Reactions. Chem. - Eur. J. 2014, 20, 2901–2912. 10.1002/chem.201303468. [DOI] [PubMed] [Google Scholar]; d Gai L.; Mack J.; Lu H.; Yamada H.; Kuzuhara D.; Lai G.; Li Z.; Shen Z. New 2,6-Distyryl-Substituted BODIPY Isomers: Synthesis, Photophysical Properties, and Theoretical Calculations. Chem. - Eur. J. 2014, 20, 1091–1102. 10.1002/chem.201303291. [DOI] [PubMed] [Google Scholar]
- Chen J.; Mizumura M.; Shinokubo H.; Osuka A. Functionalization of Boron Dipyrrin (BODIPY) Dyes through Iridium and Rhodium Catalysis: A Complementary Approach to a- and β-Substituted BODIPYs. Chem. - Eur. J. 2009, 15, 5942–5949. 10.1002/chem.200802541. [DOI] [PubMed] [Google Scholar]
- a Thivierge C.; Bandichhor R.; Burgess K. Spectral Dispersion and Water Solubilization of BODIPY Dyes via Palladium-Catalyzed C–H Functionalization. Org. Lett. 2007, 9, 2135–2138. 10.1021/ol0706197. [DOI] [PubMed] [Google Scholar]; b Luo L.; Wu D.; Li W.; Zhang S.; Ma Y.; Yan S.; You J. Regioselective Decarboxylative Direct C–H Arylation of Boron Dipyrromethenes (BODIPYs) at 2,6-Positions: A Facile Access to a Diversity-Oriented BODIPY Library. Org. Lett. 2014, 16, 6080–6083. 10.1021/ol502883x. [DOI] [PubMed] [Google Scholar]; c Wang J.; Li Y.; Gong Q.; Wang H.; Hao E.; Lo P.-C.; Jiao L. β-AlkenylBODIPY Dyes: Regioselective Synthesis via Oxidative C–H Olefination, Photophysical Properties, and Bioimaging Studies. J. Org. Chem. 2019, 84, 5078–5090. 10.1021/acs.joc.9b00020. [DOI] [PubMed] [Google Scholar]; d Wang J.; Wu Q.; Gong Q.; Cheng K.; Liu Q.; Yu C.; Hao E.; Jiao L. Direct β-Selective Styrylation of BODIPY Dyes via Palladium(II)-Catalyzed C–H Functionalization. Adv. Synth. Catal. 2019, 361, 769–777. 10.1002/adsc.201801338. [DOI] [Google Scholar]; e Shimada T.; Mori S.; Ishida M.; Furuta H. Regioselectively α- and β-alkynylated BODIPY dyes via gold(I)-catalyzed direct C–H functionalization and their photophysical properties. Beilstein J. Org. Chem. 2020, 16, 587–595. 10.3762/bjoc.16.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchimoto T.; Maeda T.; Shirakawa E.; Kawakami Y. Friedel-Crafts Alkenylation of Arenes Using Alkynes Catalysed by Metal Trifluoromethanesulfonates. Chem. Commun. 2000, 1573–1574. 10.1039/b003702h. [DOI] [Google Scholar]
- a Jia C.; Piao D.; Oyamada J.; Lu W.; Kitamura T.; Fujiwara Y. Efficient Activation of Aromatic C-H Bonds for Addition to C-C Multiple Bonds. Science 2000, 287, 1992–1995. 10.1126/science.287.5460.1992. [DOI] [PubMed] [Google Scholar]; b Jia C.; Lu W.; Oyamada J.; Kitamura T.; Matsuda K.; Irie M.; Fujiwara Y. Novel Pd(II)- and Pt(II)-Catalyzed Regio- and Stereoselective trans-Hydroarylation of Alkynes by Simple Arenes. J. Am. Chem. Soc. 2000, 122, 7252–7263. 10.1021/ja0005845. [DOI] [Google Scholar]; c Reetz M. T.; Sommer K. Gold-Catalyzed Hydroarylation of Alkynes. Eur. J. Org Chem. 2003, 2003 (18), 3485–3496. 10.1002/ejoc.200300260. [DOI] [Google Scholar]; d Shi Z.; He C. Efficient Functionalization of Aromatic C–H Bonds Catalyzed by Gold(III) under Mild and Solvent-Free Conditions. J. Org. Chem. 2004, 69, 3669–3671. 10.1021/jo0497353. [DOI] [PubMed] [Google Scholar]; e Li R.; Wang S. R.; Lu W. FeCl3-Catalyzed Alkenylation of Simple Arenes with Aryl-Substituted Alkynes. Org. Lett. 2007, 9, 2219–2222. 10.1021/ol070737u. [DOI] [PubMed] [Google Scholar]
- a Lu W.; Jia C.; Kitamura T.; Fujiwara Y. Pd-Catalyzed Selective Addition of Heteroaromatic C–H Bonds to C–C Triple Bonds under Mild Conditions. Org. Lett. 2000, 2, 2927–2930. 10.1021/ol006156l. [DOI] [PubMed] [Google Scholar]; b Murakami M.; Hori S. Ruthenium-Mediated Regio- and Stereoselective Alkenylation of Pyridine. J. Am. Chem. Soc. 2003, 125, 4720–4721. 10.1021/ja029829z. [DOI] [PubMed] [Google Scholar]; c McLean E. B.; Cutolo F. M.; Cassidy O. J.; Burns D. J.; Lee A.-L. Selectivity Control in Gold-Catalyzed Hydroarylation of Alkynes with Indoles: Application to Unsymmetrical Bis(Indolyl)Methanes. Org. Lett. 2020, 22, 6977–6981. 10.1021/acs.orglett.0c02526. [DOI] [PubMed] [Google Scholar]
- Revision and selected references:; a Pérez Sestelo J.; Sarandeses L. A.; Martínez M. M.; Alonso-Marañón L. Indium(III) as π-Acid Catalyst for the Electrophilic Activation of Carbon–Carbon Unsaturated Systems. Org. Biomol. Chem. 2018, 16, 5733–5747. 10.1039/C8OB01426D. [DOI] [PubMed] [Google Scholar]; b Song C.; Jung D.; Choung S.; Roh E.; Lee S. Dramatic Enhancement of Catalytic Activity in an Ionic Liquid: Highly Practical Friedel–Crafts Alkenylation of Arenes with Alkynes Catalyzed by Metal Triflates. Angew. Chem., Int. Ed. 2004, 43, 6183–6185. 10.1002/anie.200460292. [DOI] [PubMed] [Google Scholar]; c Yoon M. Y.; Kim J. H.; Choi D. S.; Shin U. S.; Lee J. Y.; Song C. E. Metal Triflate-Catalyzed Regio- and Stereoselective Friedel–Crafts Alkenylation of Arenes with Alkynes in an Ionic Liquid: Scope and Mechanism. Adv. Synth. Catal. 2007, 349, 1725–1737. 10.1002/adsc.200700039. [DOI] [Google Scholar]; d Bhaskar G.; Saikumar C.; Perumal P. T. Indium(III) Bromide-Catalyzed Hydroarylation of Alkynes with Indoles. Tetrahedron Lett. 2010, 51, 3141–3145. 10.1016/j.tetlet.2010.04.036. [DOI] [Google Scholar]
- a Pérez-Guevara R.; Sarandeses L. A.; Martínez M. M.; Pérez Sestelo J. Indium-Catalyzed Synthesis of Benzannulated Spiroketals by Intramolecular Double Hydroalkoxylation of ortho-(Hydroxyalkynyl)Benzyl Alcohols. Org. Chem. Front. 2022, 9, 6894–6901. 10.1039/D2QO01600A. [DOI] [Google Scholar]; b Seoane-Carabel F.; Alonso-Marañón L.; Sarandeses L. A.; Pérez Sestelo J. Synthesis of 1H-Isochromenes and 1,2-Dihydroisoquinolines by Indium(III)-Catalyzed Cycloisomerization of ortho-(Alkynyl)Benzyl Derivatives. Synthesis 2023, 55, 1714–1723. 10.1055/s-0042-1751383. [DOI] [Google Scholar]
- Da Lama A.; Pérez Sestelo J.; Sarandeses L. A.; Martínez M. M. Microwave-Assisted Direct Synthesis of BODIPY Dyes and Derivatives. Org. Biomol. Chem. 2022, 20, 9132–9137. 10.1039/D2OB01349E. [DOI] [PubMed] [Google Scholar]
- Janjic J. M.; Srinivas M.; Kadayakkara D. K. K.; Ahrens E. T. Self-Delivering Nanoemulsions for Dual Fluorine-19 MRI and Fluorescence Detection. J. Am. Chem. Soc. 2008, 130, 2832–2841. 10.1021/ja077388j. [DOI] [PubMed] [Google Scholar]
- Zeng L.; Lin Y.; Li J.; Sajiki H.; Xie H.; Cui S. Skeletal Reorganization Divergence of N-Sulfonyl Ynamides. Nat. Commun. 2020, 11, 5639. 10.1038/s41467-020-19467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadierno V. Metal-Catalyzed Hydrofunctionalization Reactions of Haloalkynes. Eur. J. Inorg. Chem. 2020, 2020, 886–898. 10.1002/ejic.201901016. [DOI] [Google Scholar]
- Liu C.; Xue Y.; Ding L.; Zhang H.; Yang F. Au-Catalyzed Addition of Nucleophiles to Chloroalkynes: A Regio- and Stereoselective Synthesis of (Z)-Alkenyl Chlorides. Eur. J. Org Chem. 2018, 2018, 6537–6540. 10.1002/ejoc.201801222. [DOI] [Google Scholar]
- a Dounay A. B.; Overman L. E. The Asymmetric Intramolecular Heck Reaction in Natural Product Total Synthesis. Chem. Rev. 2003, 103, 2945–2964. 10.1021/cr020039h. [DOI] [PubMed] [Google Scholar]; b Nicolaou K. C.; Bulger P. G.; Sarlah D. Palladium-Catalyzed Cross-Coupling Reactions in Total Synthesis. Angew. Chem., Int. Ed. 2005, 44, 4442–4489. 10.1002/anie.200500368. [DOI] [PubMed] [Google Scholar]
- Dartar S.; Ucuncu M.; Karakus E.; Hou Y.; Zhao J.; Emrullahoglu M. BODIPY–Vinyl Dibromides as Triplet Sensitisers for Photodynamic Therapy and Triplet–Triplet Annihilation Upconversion. Chem. Commun. 2021, 57, 6039–6042. 10.1039/D1CC01881G. [DOI] [PubMed] [Google Scholar]
- Yata T.; Kita Y.; Nishimoto Y.; Yasuda M. Regioselective Synthesis of 5-Metalated 2-Pyrones by Intramolecular Oxymetalation of Carbonyl-Ene-Yne Compounds Using Indium Trihalide. J. Org. Chem. 2019, 84, 14330–14341. 10.1021/acs.joc.9b02186. [DOI] [PubMed] [Google Scholar]
- Antina E.; Bumagina N.; Marfin Y.; Guseva G.; Nikitina L.; Sbytov D.; Telegin F. BODIPY Conjugates as Functional Compounds for Medical Diagnostics and Treatment. Molecules 2022, 27, 1396. 10.3390/molecules27041396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J.; Xu K.; Yang W.; Wang Z.; Zhong F. The Triplet Excited State of Bodipy: Formation, Modulation and Application. Chem. Soc. Rev. 2015, 44, 8904–8939. 10.1039/C5CS00364D. [DOI] [PubMed] [Google Scholar]
- Boens N.; Leen V.; Dehaen W. Fluorescent Indicators Based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. 10.1039/C1CS15132K. [DOI] [PubMed] [Google Scholar]
- Gibbs J. H.; Robins L. T.; Zhou Z.; Bobadova-Parvanova P.; Cottam M.; McCandless G. T.; Fronczek F. R.; Vicente M. G. H. Spectroscopic, Computational Modeling and Cytotoxicity of a Series of Meso-Phenyl and Meso-Thienyl-BODIPYs. Bioorg. Med. Chem. 2013, 21, 5770–5781. 10.1016/j.bmc.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu H.; Liu J.; O’Connor H. M.; Gunnlaugsson T.; James T. D.; Zhang H. Photoinduced Electron Transfer (PeT) Based Fluorescent Probes for Cellular Imaging and Disease Therapy. Chem. Soc. Rev. 2023, 52, 2322–2357. 10.1039/D1CS01097B. [DOI] [PubMed] [Google Scholar]
- Kubin R. F.; Fletcher A. N. Fluorescence quantum yields of some rhodamine dyes. J. Lumin. 1982, 27, 455–462. 10.1016/0022-2313(82)90045-x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.