Abstract
Objectives
Transfers between health facilities of people living with HIV attending primary health care (PHC) including hospital to PHC facility, PHC facility to hospital and PHC facility to PHC facility transfers occur frequently, affect health service planning, and are associated with disengagement from care and viraemia. Data on transfer of people living with diabetes attending PHC, particularly transfers between PHC facilities, are few. We assessed the transfer incidence rate of people living with diabetes attending PHC, and the association between transfers between PHC facilities and subsequent HbA1c values.
Methods
We analysed data on HbA1c tests at public sector facilities in the Western Cape Province (2016–March 2020). Individuals with an HbA1c in 2016–2017 were followed-up for 27 months and included in the analysis if ≥18 years at first included HbA1c, ≥2 HbA1cs during follow-up and ≥1 HbA1c at a clinic. A visit interval was the duration between two consecutive HbA1cs. Successive HbA1cs at different facilities of any type indicated any transfer, and HbA1cs at different clinics indicated a clinic-to-clinic transfer. Mixed effects logistic regression adjusted for sex, age, rural/urban facility attended at the start of the visit interval, disengagement (visit interval >14 months) and a hospital visit during follow-up assessed the association between transfers between PHC facilities and HbA1c >8%.
Results
Among 102,813 participants, 22.6% had ≥1 transfers of any type. Including repeat transfers, there were 29,994 transfers (14.4 transfers per 100 person-years, 95% confidence interval [CI] 14.3–14.6). 6,996 (30.1%) of those who transferred had a transfer between PHC facilities. Visit intervals with a transfer between PHC facilities were longer (349 days, IQR 211–503) than those without any transfer (330 days, IQR 182–422). The adjusted relative odds of an HbA1c >8% after a transfer between PHC facilities versus no transfer were 1.20 (95% CI 1.05–1.37).
Conclusion
The volume of transfers involving PHC facilities requires consideration when planning services. Individuals who transfer between PHC facilities require additional monitoring and support.
Keywords: Diabetes Mellitus, Type 2, Primary Health Care, Cohort Studies
Introduction
In low- and middle-income countries (LMIC), the prevalence of chronic non-communicable diseases (NCDs) including diabetes is increasing rapidly, alongside ongoing chronic infectious disease epidemics (1,2). Approximately three-quarters of the 537 million adults with diabetes globally live in LMIC and the prevalence is projected to increase (3,4). In South Africa the prevalence of diabetes increased from 7.1% in 2011 to 10.8% in 2021, with 4.2 million adults estimated to be living with diabetes in 2019 (4,5). Chronic conditions require long-term and continuous care to prevent complications and reduce mortality (6). However, healthcare systems in LMIC have developed to address acute health problems, and provision of care for people with NCDs including for people living with diabetes (PLD) is suboptimal (7–9). In many LMIC including South Africa, levels of retention in care among PLD are low and, among those in care, few are controlled on treatment (10–13). Poor outcomes are largely due to the episodic and unstructured care currently provided in LMIC and strategies to improve the provision of long-term, continuous care in these settings is required. However, most research to inform such changes is from high-income countries and research from LMICs is urgently needed.
Among PLD, reduced continuity of care has been associated with reduced adherence to treatment, impaired glycaemic control, increased diabetes-related complications and increased mortality (14–20). Continuity of care may be disrupted when patients move between health care providers and/or health facilities. Measures of continuity of care include indices assessing continuity to providers and/or facilities, and it is not always clear in these studies whether a transfer between health facilities occurred. Transfers include up-referrals (e.g. transfers from lower to higher levels of care for more complex clinical care), down-referrals (e.g. transfers from hospitals to primary health care [PHC] facilities for ongoing care), and lateral transfers (transfers between facilities at the same level of care, such as between PHC facilities). PHC is integral to providing care for chronic conditions and, among PLH, transfers among PLH attending PHC occur frequently and may affect planning of health care services including resource allocation, drug forecasting, and referral systems. Further, while previous research among PLH has focused on up-referrals (21–23) and down-referrals (24–27), recent analyses have shown that transfers between PHC facilities occur frequently for reasons that include geographic mobility, which occurs frequently in LMIC (28) and that transfers between PHC facilities are associated with viraemia (Odayar).
Among PLD, few studies have assessed the frequency of transfers involving PHC facilities, particularly in LMIC. Studies in high-income countries include one conducted in South Korea: among 457,975 PLD attending hospitals and PHC facilities, 33% transferred between facilities over a one-year period, of whom 53% transferred between PHC facilities (29). In Kenya, people with NCDs including diabetes, asthma and cardiovascular diseases transferred due to medication stockouts (30) and in Uganda, PLD transferred due to due to high costs of transport or treatment or to receive better care (31), indicating that transfers do occur in LMIC. The frequency of transfers in LMIC, however, is unclear. Transfers may affect planning of diabetes health services (32,33). Insufficient monitoring equipment and treatments for diabetes have been described in sub-Saharan Africa (34) and understanding the overall volume of transfers involving PHC facilities is vital to improving the availability of diabetes care at PHC level. Research on outcomes of transfers among PLD have focused on up-referrals (35–37) and down-referrals (38–45). In particular, PLD who are discharged from hospital are at risk of loss to follow-up and readmission, and numerous interventions have been tested to improve outcomes (38–41,43–45). However, data on outcomes of transfers between PHC facilities among PLD are limited.
Levels of geographic mobility are high in LMIC (46), meaning that PLD attending PHC may require transfers between PHC facilities. In addition, decentralization of chronic care services in many LMIC mean that there are increasing numbers of PHC facilities between which people can transfer (47,48). Transfers between PHC facilities among PLD are thus an important area for investigation. To address these gaps, we used routinely collected data from public sector health care facilities across the Western Cape Province to investigate transfers among PLD including the frequency of transfers involving PHC facilities, the frequency of transfers between PHC facilities and the outcomes of transfers between PHC facilities.
Methods
Setting and Data Source
The study was conducted across public sector health care facilities in the Western Cape province. The province is divided into six health districts and 32 sub-districts (49). Of the six sub-districts, one is urban and densely populated (Cape Town Metropole) and five are rural (West Coast, Cape Winelands, Overberg, Garden Route and Central Karoo) (50,51). The Western Cape population was estimated at 6.3 million in 2016, with 64% residing in the Cape Town Metropole (51). Over 80% of the population in the province attend public sector health care facilities (52) which comprise 52 hospitals and 354 PHC facilities (53). Between 2012 and 2019, 64% of public sector health care facility visits by PLD in the province were in the Cape Town Metropole.
The prevalence of diabetes in the province was estimated at 11.2% (95% confidence interval [CI] 8.3–15.0) in 2012, which was higher than the national prevalence of 9.5% (95% CI 8.0–11.2) (10). Approximately 18 000 people, of whom 60% are women, start diabetes treatment each year. Most diabetic patients are 40–65 years old (58%) and nearly one-third are >65 years old (54). In the Western Cape in 2010, almost 60% of people previously diagnosed with diabetes were not on treatment and 33% had raised random blood glucose measurements (55). Each sub-district in the province has community-based, PHC and district hospital services (49,56). PHC facilities include community health clinics and community health centres. PLD attending PHC services are managed by nurse practitioners and PHC doctors (51,57). Patients at PHC facilities who require more complex medical care are up-referred to district hospitals and, if necessary, to regional or tertiary hospitals, which are at the provincial level (54). Patients at higher levels of care who are no longer in need of specialized care may be down-referred to a lower level health facility for continued management (58).
National guidelines for the diagnosis of diabetes and HbA1c monitoring at the time of the study are summarized in Supplementary table 1. Diabetes was diagnosed in individuals with either a fasting plasma glucose ≥7.0 mmol/L, a two-hour plasma glucose during an oral glucose tolerance test of ≥11.1mmol/L, an HbA1c ≥6.5%, or symptoms of diabetes and a random plasma glucose ≥11.1 mmol/L(57,59,60). Recommendations for HbA1c testing frequency ranged from three to six monthly if treatment was changed, and from six to 12 monthly if treatment goals were met. Targets for HbA1c varied between guidelines, with some recommending individualized targets. Generally, <7% was considered optimal for most patients, with additional action recommended for an HbA1c >8%.
All public sector health facilities in the province have access to HbA1c testing through the National Health Laboratory Service (NHLS); tests are processed by the NHLS using NGSP certified methods. Data from laboratory test request forms are captured electronically and stored by the NHLS Corporate Data Warehouse. For this analysis, data on all HbA1c tests done at public sector health facilities in the Western Cape, including hospitals and PHC facilities, from 1 January 2016 to 31 December 2021 were obtained from the NHLS. A unique patient identifier is used in the province and multiple tests in the same individual can thus be tracked across health care facilities. Variables obtained included patient sex and age, and the facility, date and result of each HBA1c test.
Inclusion and exclusion criteria:
Individuals <18 years of age at their first included HbA1c test were excluded from the cohort. While data were available up to 31 December 2021, we censored data for this analysis at the end of March 2020 because facility attendance thereafter may have been affected by the national lockdown implemented in response to the COVID-19 pandemic. To allow equal duration of follow-up for all participants, individuals with their first HbA1C after 31 December 2017 were excluded, and those with an HbA1c done between 01 January 2016 to 31 December 2017 were censored 27 months after their first included test. Those with at least two HbA1c tests in the 27-month follow-up period were potentially eligible, and because the focus of the analysis was individuals attending PHC, those without an HbA1c at a PHC facility in this period were excluded. Individuals with one or more HbA1cs conducted at correctional facilities and private or independent health care facilities including care facilitieswere also excluded.
Definitions:
Each HbA1c test represented a health facility visit. A visit interval was defined as the time period between two consecutive visits in one participant (Supplementary figure 1). Community health clinics and community health centres were categorized as PHC facilities. District, regional and tertiary hospitals were categorized as hospitals. A transfer of any type was defined as successive HbA1cs documented at different facilities, regardless of the type of facility, in one individual. Transfers between PHC facilities were defined as an HbA1c at a PHC facility with the subsequent HbA1c at a different PHC facility in the same individual. We were unable to distinguish between self-transfers (a transfer in which the individual did not inform the initial facility) and official transfers (a transfer in which the individual informed the initial facility and obtained a referral letter) using these data, and the above definitions include both types of transfer (61). Attendance at a hospital was determined by record of an HbA1c conducted at a hospital in an individual and was used as an indicator of clinical status. With a maximum recommended duration between HbA1cs of 12 months, we defined a disengagement as >14 months between visits for the primary analysis (57,59,60). As some guidelines recommended a maximum of six months between tests, we conducted a sensitivity analysis with disengagement defined as a visit interval >7 months. Based on South African National Guidelines at the time, a raised HbA1c was defined as ≥8%, with sensitivity analyses defining a raised HbA1c as ≥7%.
Analysis
Data were analysed using STATA/BE version 17.0 (Stata Corporation, College Station, TX, USA). Frequencies and proportions, means with standard deviations or medians with interquartile ranges (IQRs) were calculated to summarise quantitative variables. The proportions of participants with one or more transfers overall, and with at least one transfer between PHC facilities were tabulated. Participant characteristics were described for the whole cohort, for those who did or did not have a transfer of any type, and for those who transferred between PHC facilities.
Transfer rates were calculated for all transfer events from the first HbA1c to the end of the study period, including multiple events per participant. Maximum possible duration of follow-up was 27 months per individual. Participants with at least one HbA1c in the last 14 months of their follow-up (between 13 and 27 months after their first HbA1c) were censored at 27 months after their first HbA1c. Those without an HbA1c in this period were censored seven months after their last HbA1c.
To assess predictors of transfer, generalized estimating equations with an unstructured working correlation with Poisson regression were used to account for repeated measures in participants. Potential confounders were identified a priori. Multivariable models assessing predictors of any transfer were adjusted for age, sex, having at least one HbA1c in a rural district and the value of the first HbA1c test. In addition to these variables, models assessing predictors of transfers between PHC facilities transfers were adjusted for attendance at a hospital during follow-up.
Generalized mixed effects logistic models assessed the association between the occurrence of any transfer during a visit interval and an HbA1c ≥8% at the end of the interval adjusting for sex, age at the start of the interval, location of the visit at the start of the visit interval (rural vs urban) and occurrence of a disengagement during the visit interval (visit interval >14 months). In addition to these variables, models assessing the association between transfers between PHC facilities and an HbA1c ≥8% at the subsequent visit were adjusted for the occurrence of a hospital visit during follow-up. Further models assessed the association between transfers between PHC facilities during a visit interval and HbA1c value at the end of the visit interval as a continuous variable. HbA1c percentage was not normally distributed and was log-transformed for linear analyses. Sensitivity analyses were done to assess the association between transfers between PHC facilities and an HbA1c ≥8% when including only visit intervals in which the HbA1c at the start of the interval was <8%, and to assess alternate definitions of the outcome (HbA1c ≥7%). Disengagement was defined as a visit interval >14 months for all analyses except for one sensitivity analysis assessing the association between transfers between PHC facilities and an HbA1c ≥8% at the subsequent visit using an alternate definition of disengagement (visit interval >7 months). Lastly, we conducted stratified analyses to assess effect modification of the relationship between transfers between PHC facilities and HbA1c by the occurrence of disengagement (visit interval >14 months) in the visit interval.
Ethical approval was obtained from the University of Cape Town Human Research Ethics Committee (R026/2022, current approval date 31 May 2023).
Results
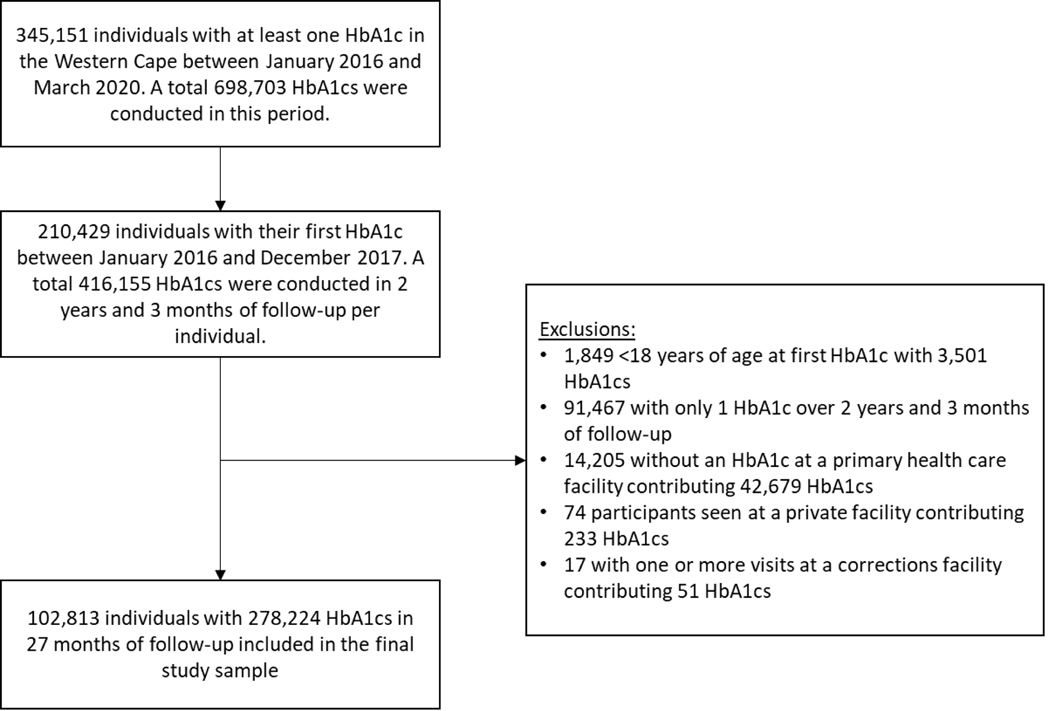
A total of 345,151 individuals had at least one HbA1c test in the Western Cape between January 2016 and March 2020 (Figure 1). Among these, 134,722 had their first HbA1c after December 2017 and were excluded. Of the 210,429 who had their first HbA1c between January 2016 and December 2017, 1,849 were <18 years of age at the point of their first included HbA1c and were excluded. Including 2 years and 3 months of follow-up per individual, 91,467 had only one HbA1c during follow-up, 14,205 did not have an HbA1c at a PHC facility, 78 attended a private or independently run facility and 17 had an HbA1c at a correctional facility and were excluded from the study cohort.
FIGURE 1.
Flowchart of participant inclusion.
The remaining 102,813 individuals included in the analysis contributed 278,224 HbA1cs performed at 383 facilities over 208,030 person-years of follow-up. Median age at first HbA1c test was 56 years (IQR 48–64), approximately two-thirds of participants were female (n = 68,090; 66.2%) and approximately one-third (n = 35,443; 34.5%) had at least one HbA1c in a rural district (Table 1). Median number of visits was 2 (2–3) and median duration between visits was 321 days (IQR 175–424). Across all visits, 68,151 (66.3%) participants had at least one HbA1c ≥8%.
Table 1:
Patient characteristics overall and by transfer status (n=102,813)
| All participants | ≥1 transfer | Between PHC facilities | No transfer | |
|---|---|---|---|---|
| Number of participants, n (%) | 102,813 | 23,277 | 6,996 | 79,536 |
| Age at first HbA1c (years), median (IQR) | 56 (48–64) | 56 (46–65) | 56 (47–65) | 56 (48–64) |
| Female, n (%) | 68,090 (66.2) | 15,075 (64.8) | 4,587 (65.6) | 53,015 (66.7) |
| Number of visits, median (IQR) | 2 (2–3) | 3 (2–4) | 3 (2–3) | 2 (2–3) |
| Duration of follow up (days), median (IQR) | 821.8 (821.8–821.8) | 821.8 (821.8–821.8) | 821.8 (821.8–821.8) | 821.8 (821.8–821.8) |
| First visit in a rural region | 34,730 (33.8) | 6,370 (27.4) | 2,230 (31.9) | 28,360 (35.7) |
| At least one HbA1c conducted in a rural region | 35,443 (34.5) | 7,083 (30.4) | 2,563 (36.6) | 28,360 (35.7) |
| First HbA1c value (%), median (IQR) | 8.4 (6.7–10.6) | 8.7 (6.8–11.1) | 8.6 (6.9–10.8) | 8.3 (6.7–10.5) |
| At least one HbA1c ≥6.5% | 88,126 (85.7) | 20,341 (87.4) | 6,206 (88.7) | 67,785 (85.2) |
| At least one HbA1c ≥7.0% | 80,934 (78.7) | 18,858 (81.0) | 5,736 (82.0) | 62,076 (78.1) |
| At least one HbA1c ≥7.5% | 74,326 (72.3) | 17,571 (75.5) | 5,344 (76.4) | 56,755 (71.4) |
| At least one HbA1c ≥8.0% | 68,151 (66.3) | 16,345 (70.2) | 4,932 (70.5) | 51,806 (65.1) |
| At least one hospital visit | 16,793 (16.3) | 16,793 (72.1) | 512 (7.3) | 0 |
Overall, 23,277/102,813 (22.6%) participants transferred at least once (including all transfer types) during follow-up. Among the 23,227 participants who transferred one or more times, 5,542 (23.8%) had evidence of multiple transfers. Including repeat transfers per individual, a total of 29,994 episodes of transfer were documented for a transfer rate of 14.4 (95% confidence interval [CI] 14.3–14.6) transfers per 100 person-years. Of the 29,994 total transfers, 7,884 (26.3%) were between PHC facilities and of the 23,277 participants who had at least one transfer of any type, 6,996 (30.1%) had at least one transfer between PHC facilities.
The median duration of visit intervals was shorter when any transfer occurred (275 days, IQR 143–436) in the interval compared to when a transfer did not occur (330 days, IQR 182–422; Supplementary table 2). However, visit intervals in which a transfer between PHC facilities occurred were of longer duration (349 days, IQR 211–503) compared to those in which no transfer occurred. The median value of the first HbA1c result was higher in participants with any transfer (HbA1c 8.7%, IQR 6.8–11.1) and in those who transferred between PHC facilities (HbA1c 8.6%, 95% CI 6.9–10.8) compared to those who did not transfer (8.3%, IQR 6.7–10.5). A slightly higher proportion of individuals who transferred between PHC facilities had at least one HbA1c in a rural region (n=2,563, 36.6%) compared to those who did not transfer (n = 28,360, 35.7%). The majority of transfers between PHC facilities occurred within districts (n = 7,043, 89.3%) and within subdistricts (n = 4,985, 63.2%).
In a multivariable GEE Poisson regression model modelling the occurrence of any transfer, male sex (incidence rate ratio [IRR] 1.04, 95% CI 1.01–1.06) and a first HbA1c ≥8% (IRR 1.21, 95% CI 1.18–1.24) were associated with an increased rate of transfer, while having at least one HbA1c in a rural district was associated with a decreased rate of transfer (IRR 0.84, 95% CI 0.82–0.86; Table 2). When assessing factors associated with a transfer between PHC facilities, a first HbA1c ≥8% was similarly associated with an increased transfer rate (IRR 1.17, 95% CI 1.11–1.22). However, in contrast to any transfer, having at least one HbA1c in a rural district was associated with an increased rate of transfers between PHC facilities (IRR 1.14, 95% CI 1.09–1.19). People with at least one hospital visit were 61% less likely to transfer between PHC facilities (aOR 0.39, 95% CI 0.36–0.42).
Table 2:
GEE Poisson regression model assessing predictors any transfer in primary care (n=102,813 individuals)
| Any transfer within primary care | Transfer between PHC facilities | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Univariable | Multivariable | Univariable | Multivariable | |||||||||
|
| ||||||||||||
| Unadjusted IRR | 95% CI | p | Adjusted IRR | 95% CI | p | Unadjusted IRR | 95% CI | p | Adjusted IRR | 95% CI | p | |
| Male | 1.03 | 1.01–1.06 | 0.007 | 1.04 | 1.01–1.06 | 0.003 | 1.00 | 0.95–1.05 | 0.975 | 1.01 | 0.96–1.06 | 0.656 |
| Age at first HbA1c (years) | 0.993 | 0.992–0.994 | <0.001 | 0.994 | 0.993–0.994 | <0.001 | 0.998 | 0.996–1.000 | 0.019 | 0.998 | 0.996–1.000 | 0.028 |
| First HbA1c ≥8.0% | 1.22 | 1.20–1.25 | <0.001 | 1.21 | 1.18–1.24 | <0.001 | 1.17 | 1.11–1.22 | <0.001 | 1.17 | 1.11–1.22 | <0.001 |
| At least one HbA1c in a rural district | 0.86 | 0.84–0.88 | <0.001 | 0.84 | 0.82–0.86 | <0.001 | 1.19 | 1.14–1.25 | <0.001 | 1.14 | 1.09–1.19 | <0.001 |
| At least one hospital visit | - | - | - | - | 0.39 | 0.36–0.42 | <0.001 | 0.39 | 0.36–0.42 | <0.01 | ||
Abbreviations: CI, confidence interval; GEE, generalized estimating equations; IRR, incidence rate ratio.
In a mixed effects logistic model, the adjusted relative odds of an HbA1c ≥8% when any transfer occurred in a visit interval versus no transfer were 1.02 (95% CI 0.95–1.10; Supplementary table 3). The adjusted relative odds of an HbA1c ≥8% at the end of a visit interval in which a transfer between PHC facilities occurred compared to when no transfer occurred were 1.20 (IQR 1.05–1.37; Table 3). Using an alternate definition of disengagement (>7 months between HbA1cs) did not substantially alter the odds of HbA1c ≥8% after a transfer between PHC facilities versus no transfer (aOR 1.20, 95% CI 1.05–1.36; Supplementary table 4). In addition, the adjusted relative odds of an HbA1c ≥7% (aOR 1.16, 95% CI 1.02–1.32) after a transfer between PHC facilities compared to no transfer were similar to those for an HbA1c ≥8%. A mixed effects linear regression model modelling log HbA1c values showed similar results, with transfers between PHC facilities associated with a statistically significant increase in log HbA1c (coefficient 0.0112, 95% CI 0.0065 to 0.0160) versus no transfer (Table 4). Further sensitivity analyses restricted to visit intervals in which the HbA1c at the start of the interval was <8% produced consistent findings regarding the association between transfers between PHC facilities and increased HbA1c percentage (aOR 1.21, 95% CI 1.08–1.35; Supplementary table 5). In adjusted models stratified to include only visit intervals in which a disengagement occurred, the increased relative odds of an HbA1c ≥8% at the end of visit intervals in which a transfer between PHC facilities occurred versus intervals in which a transfer between PHC facilities did not occur persisted (aOR 1.12, 95% CI 1.03–1.21; Supplementary table 6). Including only visit intervals in which a disengagement did not occur, the effect estimate remained above one but was reduced and was not statistically significant (aOR 1.09, 95% CI 0.94–1.27).
Table 3:
Results of generalized mixed effects logistic regression modeling the association between transfers between PHC facilities and HbA1c category post-transfer (102,813 participants with 175,411 visit intervals).
| HbA1c ≥8.0% | HbA1c ≥7.0% | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Univariable | Multivariable | Univariable | Multivariable | |||||
|
|
||||||||
| Unadjusted OR (95% CI) | p | Adjusted OR (95% CI) | p | Unadjusted OR (95% CI) | p | Adjusted OR (95% CI) | p | |
| Transfer between PHC facilities during the visit interval | 1.16 (1.04–1.30) | 0.008 | 1.20 (1.05–1.37) | 0.008 | 1.17 (1.03–1.34) | 0.017 | 1.16 (1.02–1.32) | 0.025 |
| One or more hospital visits during follow-up | 0.87 (0.80–0.95) | 0.002 | 0.88 (0.78 – 1.00) | 0.056 | 0.73 (0.66 –0.81) | <0.001 | 0.68 (0.62–0.75) | <0.001 |
| Male sex | 0.67 (0.62–0.72) | <0.001 | 0.42 (0.37–0.47) | <0.001 | 0.66 (0.61–0.71) | <0.001 | 0.65 (0.60–0.70) | <0.001 |
| HbA1c at start of visit interval done at a facility in a rural district | 2.07 (1.93–2.22) | <0.001 | 2.29 (2.05–2.56) | <0.001 | 2.70 (2.51–2.91) | <0.001 | 2.66 (2.46–2.86) | <0.001 |
| Age at start of the visit interval (years) | 0.881 (0.879–0.884) | <0.001 | 0.890 (0.887–0.893) | <0.001 | 0.954 (0.951–0.957) | <0.001 | 0.954 (0.951–0.957) | <0.001 |
| Disengagement in the visit interval (>14 months between HbA1c tests) | 1.04 (0.98–1.10) | 0.193 | 1.10 (1.02–1.18) | 0.012 | 1.00 (0.93–1.06) | 0.805 | 0.95 (0.89 – 1.01) | 0.095 |
Table 4:
Results of generalized mixed effects linear regression modeling the association between transfers between PHC facilities and log HbA1c values (102,813 participants with 175,411 visit intervals)
| Univariable | Multivariable | |||
|---|---|---|---|---|
|
|
||||
| Unadjusted Coefficient (95% CI) | p | Adjusted coefficient 95% CI) | p | |
| Transfer between PHC facilities during the visit interval | 0.0111 (0.0063 to 0.0158) | <0.001 | 0.0113 (0.0066 to 0.0161) | <0.001 |
| One or more hospital visits during follow-up | 0.0023 (−0.0020 to 0.0067) | 0.299 | 0.0045 (0.0002 to 0.0088) | 0.04 |
| Male sex | −0.0219 (−0.0253 to −0.0184) | <0.001 | −0.0222 (−0.0256 to −0.0188) | <0.001 |
| HbA1c at start of visit interval done at a facility in a rural district | 0.0470 (0.0436 to 0.0503) | <0.001 | 0.0464 (0.0431 to 0.0497) | <0.001 |
| Age at first visit (years) | −0.0031 (−0.0033 to −0.0030) | <0.001 | −0.0031 (−0.0032 to – 0.0030) | <0.001 |
| Disengagement in the visit interval (>14 months between HbA1c tests) | 0.0103 (0.0079 to 0.0127) | <0.001 | 0.0120 (0.0096 to 0.0144) | <0.001 |
Discussion
This analysis demonstrated high numbers of transfers of PLD attending PHC facilities, including transfers between PHC facilities. Approximately 23% of participants transferred once or more over the study period, of whom 30% transferred between PHC facilities. Risk factors for a PHC facility to PHC facility transfer included an HbA1c ≥8% at the first included visit and, compared to no transfer, transfers between PHC facilities were associated with a 19% increased relative odds of an HbA1c ≥8%.
The finding that almost one-quarter of individuals transferred at least once over the study period was slightly less than found in South Korea where 33% of individuals transferred one or more times (29). In India, 42% of individuals with diabetes living in an urban slum transferred between health facilities but the study included only 60 people (62). The overall transfer incidence rate over the study period of 14.4 (95% CI 14.3–14.6) per 100 person-years is similar to the incidence rate of 12.7 per 100 person-years (95% CI 12.6–12.8) found among PLH attending PHC facilities in the Western Cape between 2011 and 2018 (29,63). The rate of transfers is thus similar in these two distinct diseases with differing disease profiles, suggesting that transfer should be investigated for other chronic diseases, including NCDs. Further, large proportions of PLD are not in care and numerous health system inadequacies have been identified in sub-Saharan Africa including insufficient availability of monitoring equipment and treatments (34). Transfers have implications for resource allocation and health system planning, and any efforts to improve access to diabetes care and availability of treatments and equipment at PHC level should thus consider the volume of transfers (32,33). Additional details on facility types and locations involved in transfers are required to facilitate planning.
The occurrence of any transfer in individuals attending PHC was not associated with a change in HbA1c percentage. However, this included up- and down referrals; patient characteristics, reasons for transfer and transfer processes likely differ for up-referrals, down-referrals and lateral transfers, and outcomes of these types of transfers may thus also differ. When considering transfers between PHC facilities specifically, transfer was associated with an increased HbA1c percentage and this was consistent across numerous sensitivity analyses. Worse outcomes among those who transferred between PHC facilities compared to those who did not transfer may be related to reduced continuity of care. Transfers may impair relational continuity which refers to an ongoing relationship between a patient and provider and has been associated with better quality of care for reasons that include better knowledge of the patient’s history and better communication (64). Transfers may also affect informational continuity, which involves the use of information on past events to make care decisions, and managerial continuity, which refers to a consistent and coherent approach to patient management (64). Continuity is also associated with improved patient satisfaction, which may lead to improved adherence to medical recommendations. These results also indicate that the occurrence of disengagement among those transferring between PHC facilities may affect outcomes. In stratified analyses, the association between transfers between PHC facilities and an HbA1c ≥8% was maintained when a disengagement occurred, with a 12% increase in the odds of an HbA1c ≥8% when a transfer between PHC facilities occurred compared to no transfer. This association was attenuated when a disengagement did not occur but was still increased, with a 9% in the odds an HbA1c ≥8% when a transfer between PHC facilities occurred compared to no transfer. However, this was not statistically significant. These results suggest that the effect of transfer on HbA1c is modified by the presence of disengagement. In this analysis, the duration between visits was longer when a transfer between PHC facilities occurred compared to no transfer. Improving access to care and developing strategies to prevent disengagement in people who transfer between PHC facilities may thus help improve outcomes. Follow-up of patients who officially transfer-out and of patients who are lost-to follow-up and may require access to care at a different facility should be considered to identify those in need of support.
Further research into reasons for transfers between PHC facilities, transfer processes, and possible reasons for increased HbA1c values in PLD who transfer between PHC facilities is required to develop strategies to improve outcomes. The relationship between transfers and disengagement requires elucidation. In addition, monitoring of overall transfer numbers and outcomes is relevant to programme evaluation. Both HIV and TB programmes use well-established cohort monitoring systems to monitor individual and programme level outcomes; however, neither system reports outcomes in people who transfer (9,65). The importance of cohort analyses to improve the PHC response to the diabetes epidemic is well recognised and these results underscore the importance of monitoring transfer and transfer outcomes as part of chronic care programmes (9,66,67).
Strengths of this study include access to data from health facilities throughout the Western Cape. This, together with the use of a unique patient identifier in the province, allowed tracking of patient movement across facilities. HbA1c testing is an objective measure of disease control and is currently the standard of care monitoring test for PLD; we were thus able to monitor changes in disease control using an objective marker. Limitations of this analysis include that the data included only records of visits at which HbA1cs were taken. HbA1cs are not done at all visits, and the number of visits and the number of transfers will thus be underestimated. In addition, some patients may have had HbA1cs processed at private laboratories and records of these tests would not be included in NHLS records, but this number is expected to be small. We were unable to differentiate between silent and official transfers, and did not have data on a number of potential confounders including duration since diagnosis, comorbidities, treatment and complications of diabetes. HbA1c can be used as a diagnostic test and it is possible that some individuals in the analysis did not have diabetes. However, this number is expected to be small because at least two HbA1cs were required for inclusion in the cohort. In addition, 86% of participants had an HbA1c ≥6.5% which is diagnostic of diabetes. Lastly, these data are from one province of South Africa and research on transfer is required in other settings; however, we believe that these results may have relevance to other LMIC with high levels of mobility.
In conclusion, almost 23% of individuals attending PHC facilities transferred between health facilities one or more times during follow-up. Transfers between PHC facilities were associated with an increase in HbA1c percentage. Additional research is required to understand the reasons for transfers between PHC facilities among PLD, and how to improve outcomes in patients who transfer between PHC facilities. Tracking of patient transfer should be considered as part of patient and diabetes programme monitoring.
Supplementary Material
Funding:
Jasantha Odayar received training in research that was supported by the Fogarty International Centre of the National Institutes of Health under Award Numbers D43 TW010559 and D43 TW00934D.
Data availability statement:
Data are available upon reasonable request.
References
- 1.Nyirenda MJ. Non-communicable diseases in sub-Saharan Africa: understanding the drivers of the epidemic to inform intervention strategies. Int Health. 2016;8(3):157–8. [DOI] [PubMed] [Google Scholar]
- 2.Oni T, Youngblood E, Boulle A, McGrath N, Wilkinson RJ, Levitt NS. Patterns of HIV, TB, and non-communicable disease multi-morbidity in peri-urban South Africa- a cross sectional study. BMC Infect Dis. 2015;15:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunachie S, Chamnan P. The double burden of diabetes and global infection in low and middle- income countries. Trans R Soc Trop Med Hyg. 2019;113:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Diabetes Federation. IDF Diabetes Atlas, 10th edition. Brussels, Belgium: International Diabetes Federation; 2021. [Google Scholar]
- 5.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Innovative care for chronic conditions: Building blocks for action. Geneva: Noncommunicable Diseases and Mental Health, World Health Organization; 2002. [Google Scholar]
- 7.Beaglehole R, Epping-Jordan J, Patel V, Chopra M, Ebrahim S, Kidd M, et al. Improving the prevention and management of chronic disease in low-income and middle-income countries: a priority for primary health care. Lancet. 2008;372:940–9. [DOI] [PubMed] [Google Scholar]
- 8.Gouda HN, Charlson F, Sorsdahl K, Ahmadzada S, Ferrari AJ, Erskine H, et al. Burden of non-communicable diseases in sub-Saharan Africa, 1990–2017: results from the Global Burden of Disease Study 2017. Lancet Glob Health. 2019;7(10):e1375–87. [DOI] [PubMed] [Google Scholar]
- 9.Kapur A, Harries AD. Cohort monitoring - As a tool to improve diabetes care services. Diabetes Res Clin Pract. 2013;102(3):260–4. [DOI] [PubMed] [Google Scholar]
- 10.Stokes A, Berry KM, Mchiza Z, Parker W ah, Labadarios D, Chola L, et al. Prevalence and unmet need for diabetes care across the care continuum in a national sample of South African adults: Evidence from the SANHANES-1, 2011–2012. PLoS One. 2017;12(10):e0184264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garrib A, Njim T, Adeyemi O, Moyo F, Halloran N, Luo H, et al. Retention in care for type 2 diabetes management in Sub-Saharan Africa: A systematic review. Trop Med Int Health. 2023;28(4):248–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adeniyi OV, Yogeswaran P, Longo-Mbenza B, Goon D Ter, Ajayi AI, Adeniyi V. Cross-sectional study of patients with type 2 diabetes in OR Tambo district, South Africa. BMJ Open. 2016;6(7):e010875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manne-goehler J, Geldsetzer P, Agoudavi K, Andall-Brereton G, Aryal KK, Bicaba BW, et al. Health system performance for people with diabetes in 28 low- and middle-income countries: A cross-sectional study of nationally representative surveys. PLoS Med. 2019;16(3):e1002751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weir DL, Mcalister FA, Majumdar SR, Eurich DT. The Interplay Between Continuity of Care, Multimorbidity, and Adverse Events in Patients With Diabetes. 2016;54(4):386–93. [DOI] [PubMed] [Google Scholar]
- 15.Lustman A, Comaneshter D, Vinker S. Interpersonal continuity of care and type two diabetes. Prim Care Diabetes. 2016;10(3):165–70. [DOI] [PubMed] [Google Scholar]
- 16.Kim JH, Park EC. Can diabetes patients seeking a second hospital get better care? Results from nested case–control study. PLoS One. 2019;14(1):e0210809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao PJ, Lin ZY, Huang JC, Hsu KH. The relationship between type 2 diabetic patients’ early medical care-seeking consistency to the same clinician and health care system and their clinical outcomes. Medicine. 2015;94(7):e554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mainous AG, Koopman RJ, Gill JM, Baker R, Pearson WS. Relationship between Continuity of Care and Diabetes Control: Evidence from the Third National Health and Nutrition Examination Survey. Am J Public Health. 2004;94(1):66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan KS, Wan EYF, Chin WY, Cheng WHG, Ho MK, Yu EYT, et al. Effects of continuity of care on health outcomes among patients with diabetes mellitus and/or hypertension: a systematic review. BMC Fam Pract. 2021;22(145). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen CC, Tseng CH, Cheng SH. Continuity of Care, Medication Adherence, and Health Care Outcomes Among Patients With Newly Diagnosed Type 2 Diabetes A Longitudinal Analysis. Med Care. 2012;51(3):231–7. [DOI] [PubMed] [Google Scholar]
- 21.Sheik S, Willems B. Estimating qualification and factors associated with third-line antiretroviral therapy referral in the Western Cape. S Afr J HIV Med. 2021; 22(1):a1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muiruri C, Corneli A, Cooper L, Dombeck C, Gray S, Longenecker CT, et al. Perspectives of HIV specialists and cardiologists on the specialty referral process for people living with HIV: a qualitative descriptive study. BMC Health Serv Res. 2022;22:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGettrick P, Ghavami-Kia B, Tinago W, Macken A, O’Halloran J, Lambert JS, et al. The HIV Care Cascade and sub-analysis of those linked to but not retained in care: the experience from a tertiary HIV referral service in Dublin Ireland. HIV Clin Trials. 2017;18(3):93–9. [DOI] [PubMed] [Google Scholar]
- 24.Eaton AD, Chan Carusone S, Craig SL, Telegdi E, Mccullagh JW, Mcclure D, et al. The ART of conversation: Feasibility and acceptability of a pilot peer intervention to help transition complex HIV-positive people from hospital to community. BMJ Open. 2019;9:e026674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bassett IV, Huang M, Cloete C, Candy S, Giddy J, Frank SC, et al. Using national laboratory data to assess cumulative frequency of linkage after transfer to community-based HIV clinics in South Africa. 2016;22:e25326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Decroo T, Panunzi I, das Dores C, Maldonado F, Biot M, Ford N, et al. Lessons learned during down referral of antiretroviral treatment in Tete, Mozambique. J Int AIDS Soc. 2009;12(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moen M, Doede M, Johantgen M, Taber D, Adesanya I, Werthman E, et al. Nurse-led hospital-to-community care, clinical outcomes for people living with HIV and health-related social needs. J Adv Nurs. 2023;79(5):1949–58. [DOI] [PubMed] [Google Scholar]
- 28.Espinosa Dice AL, Bengtson AM, Mwenda KM, Colvin CJ, Lurie MN. Quantifying clinic transfers among people living with HIV in the Western Cape, South Africa: A retrospective spatial analysis. BMJ Open. 2021;11(12):e055712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin JY, Choi NK, Jung SY, Kim YJ, Seong JM, Park BJ. Overlapping medication associated with healthcare switching among Korean elderly diabetic patients. J Korean Med Sci. 2011;26(11):1461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng G, Raskin E, Wirtz VJ, Banks KP, Laing RO, Kiragu ZW, et al. Coping with access barriers to non-communicable disease medicines: qualitative patient interviews in eight counties in Kenya. BMC Health Serv Res. 2021;21:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rutebemberwa E, Bagonza J, Tweheyo R. Pathways to diabetic care at hospitals in rural Eastern Uganda: A cross sectional study. BMC Health Serv Res. 2019;19:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alegana VA, Wright JA, Pentrina U, Noor AM, Snow RW, Atkinson PM. Spatial modelling of healthcare utilisation for treatment of fever in Namibia. Int J Health Geogr. 2012;11:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bae KH, Jones M, Evans G, Antimisiaris D. Simulation modelling of patient flow and capacity planning for regional long-term care needs: a case study. Health Systems. 2019. Jan 2;8(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atun R, Davies JI, Gale EAM, Bärnighausen T, Beran D, Kengne AP, et al. Diabetes in sub-Saharan Africa: from clinical care to health policy. Lancet Diabetes Endocrinol. 2017;5(8):622–67. [DOI] [PubMed] [Google Scholar]
- 35.Mtshali S, Mahomed O. Prevalence, patient predictors, and referral patterns for diabetes-related complications treated at a central hospital in Kwazulu Natal. Diabetes Metab Syndr Obes. 2021;14:4181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh MY, Kim SS, Kim IJ, Lee IK, Baek HS, Lee HW, et al. Clinical characteristics of diabetic patients transferred to Korean referral hospitals. Diabetes Metab J. 2014;38(5):388–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bresnick G, Cuadros JA, Khan M, Fleischmann S, Wolff G, Limon A, et al. Adherence to ophthalmology referral, treatment and follow-up after diabetic retinopathy screening in the primary care setting. BMJ Open Diabetes Res Care. 2020;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ostling S, Wyckoff J, Ciarkowski SL, Pai CW, Choe HM, Bahl V, et al. The relationship between diabetes mellitus and 30-day readmission rates. Clin Diabetes Endocrinol. 2017;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Enomoto LM, Shrestha DP, Rosenthal MB, Hollenbeak CS, Gabbay RA. Risk factors associated with 30-day readmission and length of stay in patients with type 2 diabetes. J Diabetes Complications. 2017;31(1):122–7. [DOI] [PubMed] [Google Scholar]
- 40.Gregory NS, Seley JJ, Dargar SK, Galla N, Gerber LM, Lee JI. Strategies to Prevent Readmission in High-Risk Patients with Diabetes: the Importance of an Interdisciplinary Approach. Curr Diab Rep. 2018;(8):54. [DOI] [PubMed] [Google Scholar]
- 41.Wheeler K, Crawford R, McAdams D, Benel S. Inpatient to Outpatient Transfer of Care in Urban Patients With Diabetes. Arch Intern Med. 2004;164(4):447–53. [DOI] [PubMed] [Google Scholar]
- 42.Azzi M, Constantino M, Pont L, Mcgill M, Twigg S, Krass I. Medication safety: An audit of medication discrepancies in transferring type 2 diabetes mellitus (T2DM) patients from Australian primary care to tertiary ambulatory care. Int J Qual Health Care. 2014;26(4):397–403. [DOI] [PubMed] [Google Scholar]
- 43.Mukherjee T, Robbins T, Lim Choi Keung SN, Sankar S, Randeva H, Arvanitis TN. A systematic review considering risk factors for mortality of patients discharged from hospital with a diagnosis of diabetes. J Diabetes Complications. 2020;34(11):107705. [DOI] [PubMed] [Google Scholar]
- 44.Magny-Normilus C, Nolido NV., Borges JC, Brady M, Labonville S, Williams D, et al. Effects of an Intensive Discharge Intervention on Medication Adherence, Glycemic Control, and Readmission Rates in Patients With Type 2 Diabetes. J Patient Saf. 2021;17(2):73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wexler DJ, Beauharnais CC, Regan S, Nathan DM, Cagliero E, Larkin ME. Impact of inpatient diabetes management, education, and improved discharge transition on glycemic control 12 months after discharge. Diabetes Res Clin Pract. 2012;98(2):249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ginsburg C, Collinson MA, Gómez-Olivé FX, Gross M, Harawa S, Lurie MN, et al. Internal migration and health in South Africa: determinants of healthcare utilisation in a young adult cohort. BMC Public Health. 2021;21:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nglazi MD, Kaplan R, Orrell C, Myer L, Wood R, Bekker LG, et al. Increasing Transfers-Out from an Antiretroviral Treatment Service in South Africa: Patient Characteristics and Rates of Virological Non-Suppression. PLoS One. 2013;8(3):e57907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilkinson LS, Skordis-Worrall J, Ajose O, Ford N. Self-transfer and mortality amongst adults lost to follow-up in ART programmes in low- and middle-income countries: Systematic review and meta-analysis. Trop Med Int Health. 2015;20(3):365–79. [DOI] [PubMed] [Google Scholar]
- 49.Schneider H, Schaay N, Dudley L, Goliath C, Qukula T. The challenges of reshaping disease specific and care oriented community based services towards comprehensive goals: A situation appraisal in the Western Cape Province, South Africa. BMC Health Serv Res. 2015;15:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Statistics South Africa. Census 2011 Provincial Profile: Western Cape. Pretoria, South Africa: Statistics South Africa; 2014. [Google Scholar]
- 51.Boake M, Mash R. Diabetes in the Western Cape, South Africa: A secondary analysis of the diabetes cascade database 2015 – 2020. Prim Care Diabetes. 2022;16(4):555–61. [DOI] [PubMed] [Google Scholar]
- 52.Kula N, Fryatt RJ. Public-private interactions on health in South Africa: Opportunities for scaling up. Health Policy Plan. 2014;29(5):560–9. [DOI] [PubMed] [Google Scholar]
- 53.Boulle A, Heekes A, Tiffin N, Smith M, Mutemaringa T, Zinyakatira N, et al. Data centre profile: The Provincial Health Data Centre of the Western Cape Province, South Africa. Int J Popul Data Sci. 2019;4(2):1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coetzee D, Zweigenthal V, Chiwire P, Mahomed H, Pienaar D, London L, et al. Western Cape Burden of Disease. [Google Scholar]
- 55.Bailey S Lou, Ayles H, Beyers N, Godfrey-Faussett P, Muyoyeta M, du Toit E, et al. Diabetes mellitus in Zambia and the Western Cape province of South Africa: Prevalence, risk factors, diagnosis and management. Diabetes Res Clin Pract. 2016;118:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Western Cape Government: Health. Annual Report 2018–2019. Cape Town: Western Cape Government: Health; 2018. [Google Scholar]
- 57.South African National Department of Health. Management of type 2 diabetes in adults at primary care level. Pretoria; South African National Department of Health; 2014. [Google Scholar]
- 58.South African National Department of Health. Referral Policy for South African Health Services and Referral Implementation Guidelines. Pretoria: South African National Department of Health; 2020. [Google Scholar]
- 59.South African National Department of Health. Standard Treatment Guidelines and Essential Medicines List for South Africa, Primary Healthcare Level. Pretoria: South African National Department of Health; 2020. [Google Scholar]
- 60.South African National Department of Health. Adult primary care, Symptom-based integrated approach to the adult in primary care. Pretoria: South African National Department of Health; 2019. [Google Scholar]
- 61.Geng EH, Glidden DV., Bwana MB, Musinguzi N, Emenyonu N, Muyindike W, et al. Retention in care and connection to care among HIV-infected patients on antiretroviral therapy in Africa: Estimation via a Sampling-Based approach. PLoS One. 2011;6(7):e21797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nimesh V, Halder A, Mitra A, Kumar S, Joshi A, Joshi R, et al. Patterns of healthcare seeking behavior among persons with diabetes in Central India: A mixed method study. J Family Med Prim Care. 2019;8(2):677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Odayar J, Chi BH, Phillips TK, Mukonda E, Hsiao NY, Lesosky M, et al. Transfer of Patients on Antiretroviral Therapy Attending Primary Health Care Services in South Africa. J Acquir Immune Defic Syndr. 2022;90(3):309–15. [DOI] [PubMed] [Google Scholar]
- 64.Schwarz D, Hirschhorn LR, Kim JH, Ratcliffe HL, Bitton A. Continuity in primary care: A critical but neglected component for achieving high-quality universal health coverage. BMJ Global Health. 2019;4(3):e001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Odayar J, Myer L. Transfer of primary care patients receiving chronic care: The next step in the continuum of care. Int Health. 2019;11(6):432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maher D. The power of health information - the use of cohort monitoring in managing patients with chronic non-communicable diseases. Trop Med Int Health. 2012;17(12):1567–8. [DOI] [PubMed] [Google Scholar]
- 67.Allain TJ, van Oosterhout JJ, Douglas GP, Joukes S, Gadabu OJ, Darts C, et al. Applying lessons learnt from the “DOTS” Tuberculosis Model to monitoring and evaluating persons with diabetes mellitus in Blantyre, Malawi. Tropical Medicine and International Health. 2011;16(9):1077–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.