Abstract
Selective vulnerability of specific brain regions and cell populations is a hallmark of neurodegenerative disorders. Mechanisms of selective vulnerability involve neuronal heterogeneity, functional specializations, and differential sensitivities to stressors and pathogenic factors. We discuss the growing body of literature suggesting that, like neurons, astrocytes are heterogeneous and specialized, respond to and integrate diverse inputs, and induce selective effects on brain function. In disease, astrocytes undergo specific, context-dependent changes that promote different pathogenic trajectories and functional outcomes. We propose that astrocytes contribute to selective vulnerability through maladaptive transitions to context-divergent phenotypes that impair specific brain regions and functions. Further studies on the multifaceted roles of astrocytes in disease may provide new therapeutic approaches to enhance resilience against neurodegenerative disorders.
Keywords: Heterogeneity, glia, dementia, resilience, neuropathology, proteinopathy
Selective vulnerability is a common feature of neurodegenerative disorders
Neurodegenerative disorders are age-related neurological illnesses that cause progressive decline in behavioral, cognitive, social, and emotional processes. Examples of these disorders include Alzheimer’s disease (AD), frontotemporal dementia (FTD), amyotrophic lateral sclerosis (ALS), Huntington’s disease (HD), and Parkinson’s disease (PD). Neurodegenerative disorders are characterized by proteinopathy, brain atrophy, neuronal dysfunction and cell death, glial alterations, neuroimmune responses, and neurovascular changes. The underlying neuropathogenic molecular pathways are complex and include impairments in proteostasis, RNA processing, gene expression, metabolic pathways, and intracellular signaling mechanisms [1].
A central feature of all neurodegenerative disorders is the vulnerability of select neuronal populations and brain regions to progressive dysfunction and pathology, whereas other regions and cell populations are largely spared of pathology or exhibit functional resilience despite the presence of pathology. In AD, impairments typically begin in pyramidal neurons of the entorhinal cortex and hippocampal CA1 region as well as noradrenergic neurons in the locus coeruleus and regions of the neocortex [2]. In FTD, van Economo neurons and fork cells within the frontoinsular cortex and anterior cingulate cortex are more susceptible [3], whereas spinal cord motor neurons are lost early in ALS [4]. In HD, striatal medium spiny GABAergic neurons degenerate [5], whereas PD involves the loss of dopaminergic neurons of the substantia nigra [6]. As these disorders progress, secondary neuronal vulnerability, spread of proteinopathy, and various neuropathological cascades continue to spread selectively across specific brain regions and neuronal populations. In addition to regional differences, there are also differences in impairments and degeneration across affected individuals with similar diagnoses and genetic risk factors. Why particular neural cell populations, brain regions, and individuals are more susceptible to degenerative processes and functional decline while others are resilient remains enigmatic. Identifying the processes underlying selective vulnerability is likely crucial for pinpointing early pathogenic mechanisms and advancing the development of novel prognostic biomarkers, preventative strategies, and therapeutics to halt disease onset and progression.
Although neuronal pathology is a primary hallmark of neurodegenerative diseases, non-neuronal cells of the brain are also affected and play key roles in pathogenesis. Among these cells, astrocytes are a prevalent type of glial cell that enables and regulates neural function and may contribute to pathogenic cascades in disease. Astrocytes are increasingly recognized for their heterogeneity and functional specializations, suggesting that differential astrocytic responses in disease may represent a crucial aspect of selective vulnerability. Given that astrocytes are essential for proper brain function, we posit that exploring astrocytic roles in vulnerability may be paramount to understanding and treating neurodegenerative disorders.
In this review, we discuss recent evidence implicating astrocytes in selective vulnerability associated with neurodegenerative disorders. We first highlight key disease-related mechanisms and the involvement of non-neuronal cells, and then review the fundamental roles of astrocytes as integrators of diverse cues and dynamic modulators of brain function. Focusing on recent studies in the context of neurodegenerative disorders and their mechanisms, we describe converging evidence that astrocytes have context-specific changes and roles in disease that may promote selective vulnerability. These discoveries have important implications for the understanding of astrocyte pathobiology and therapeutic development for neurodegenerative disorders.
Selective vulnerability is multifactorial and involves neuronal, glial, and neurovascular elements
Selective vulnerability of brain regions or neuronal populations is likely a result of multiple converging pathways and variables. In part, it involves cell-autonomous differences in neuronal physiology and pathogenic processes [7,8], including divergence in proteostasis, gene expression, excitability, and cell stress mechanisms. A key signature of neurodegenerative disorders is progressive proteinopathy, which is the accumulation, mislocalization, aggregation, and spread of aberrant proteins, including Aβ, tau, TDP-43, and α-synuclein, in specific neural cell populations and brain regions. Proteostasis, a process that regulates protein expression, folding, trafficking, degradation, and clearance [9,10], is affected in neurodegenerative disorders and its malfunction likely promotes selective neuronal degeneration and related disease cascades [11–14]. Of note, Aβ, tau, and other proteins have the propensity to form different types of pathological aggregates and strains that may result in distinct neuropathological sequelae among individuals [15,16]. Although the extent of proteinopathy in patients does not always correlate with clinical decline, likely due to complex biological reasons, the accumulation and spread of proteinopathy is generally associated with progressive neural impairments and disease severity and has been causally linked to functional impairments in diverse model systems. Additionally, individuals carrying specific genetic variants that promote or prevent proteinopathy often have increased or reduced odds of developing neurodegeneration, respectively [1,17]
Specific brain regions and neuronal circuits may be more vulnerable to disease as a function of their neurotransmitter systems, ion buffering capacity, and other intrinsic factors, which can cause distinct stress-related responses culminating in excitotoxicity, synapse loss, and cell death [18–21]. Neurons also depend on efficient energy generation and recent studies in mouse models and human neural cells point to mitochondrial dysfunction and changes in neuronal metabolism in degeneration of select neuronal populations [22–24]. Notably, aging is a strong risk factor for neurodegenerative disorders and modulates many of the mechanisms underlying neuronal vulnerability to disease [25,26].
In addition to cell-autonomous neuronal mechanisms, recent work emphasizes glial and neurovascular changes as contributors to disease. Concurrent pathological processes, including neuroinflammation, blood-brain barrier and neurovascular dysfunctions, white matter changes, and alterations in brain metabolism are prominent and implicate non-neuronal cell types in neurodegenerative disorders [1,27]. Many genetic risk factors and proteins linked to disease are enriched in non-neuronal cells or affect their functions [28]. Neurovascular impairments are hypothesized to precede functional impairments, and cerebral oxygenation, nutrient transport, and removal of neurotoxic factors are crucial to brain homeostasis [29,30]. In addition, microglial responses and white matter alterations are closely linked to aging and neurodegenerative disease [31–33]. Microglial and oligodendrocyte interactions with neurons and astrocytes impart additional layers of complexity to disease mechanisms. Consequently, a multifactorial and non-linear pathogenic process occurs in neurodegenerative conditions characterized by neuronal and non-neuronal alterations and bidirectional cell-cell interactions among different cell types [34–36] (Figure 1).
Figure 1. Vulnerability to neurodegenerative disorders is multifactorial.
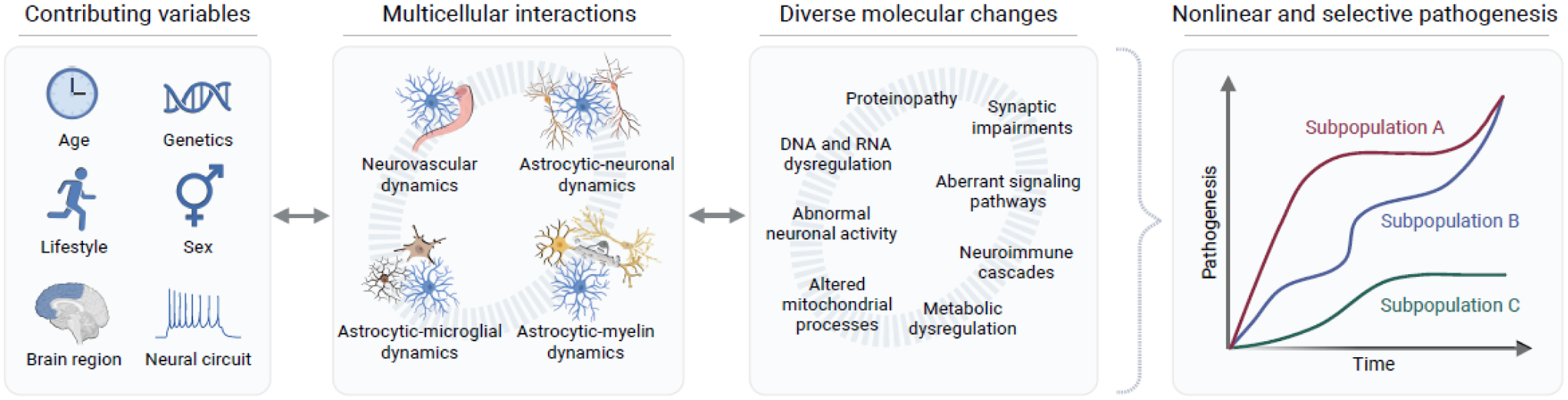
Multiple intrinsic variables affect the onset and progression of neurodegenerative disease, including age and genetics but also environmental and lifestyle factors. These and other variables influence the complex multicellular interactions between different cell types in the brain and their functions. Dynamic molecular changes, detrimental and protective, occur within multiple cell types and affect cell-cell interactions, contributing to disease complexity. Together, these changes result in nonlinear pathogenesis and selective vulnerability within specific subpopulations of cells, brain regions, and individuals. Figure created using BioRender.com.
Many of these disease-associated non-neuronal changes, including astrocyte alterations [37], can precede the onset of apparent clinical symptoms by many years, suggesting that robust compensatory mechanisms are engaged to preserve brain function and promote resilience. These protective mechanisms can also be selective to specific brain regions and cell populations [38] and may further promote variability in disease onset or progression. Therefore, the pathogenesis of neurodegenerative disorders is complex and dynamic, and depends not only on intrinsic neuronal mechanisms but also on glial, neuroimmune, and neurovascular changes and the interplay of pathogenic and protective cascades in these different systems. Moreover, individual lifestyle variables such as diet and physical activity can also affect disease vulnerability [39,40].
In this broader view of disease pathogenesis, astrocytes occupy a crucial niche at the interface of neurons, other glial cells, the vasculature, and infiltrating immune cells and peripheral factors [41,42]. Historically, astrocytes have been viewed as largely homogeneous support cells with similar functions and responses to disease. However, emerging evidence is revealing a rich diversity of astrocytic features and functions within and across brain regions (Figure 2), which is likely due to their developmental lineage, positional identity, and sensitivity to various local inputs and cues in their microenvironment [43]. Considering their multifunctional roles in neurovascular dynamics, brain metabolism, neuronal activities, neural stress responses, and neuroimmune cascades, astrocytes are well-positioned to affect many of the mechanisms linked to selective vulnerability or resilience in disease. In the following section, we briefly summarize recent evidence indicating that astrocytes have extensive functional heterogeneity and respond to stimuli in a versatile and context-dependent manner. Thereafter, we discuss the potential involvement of astrocytes in selective pathogenesis linked to neurodegenerative disorders.
Figure 2. Molecular, structural, and functional diversity in astrocytes.
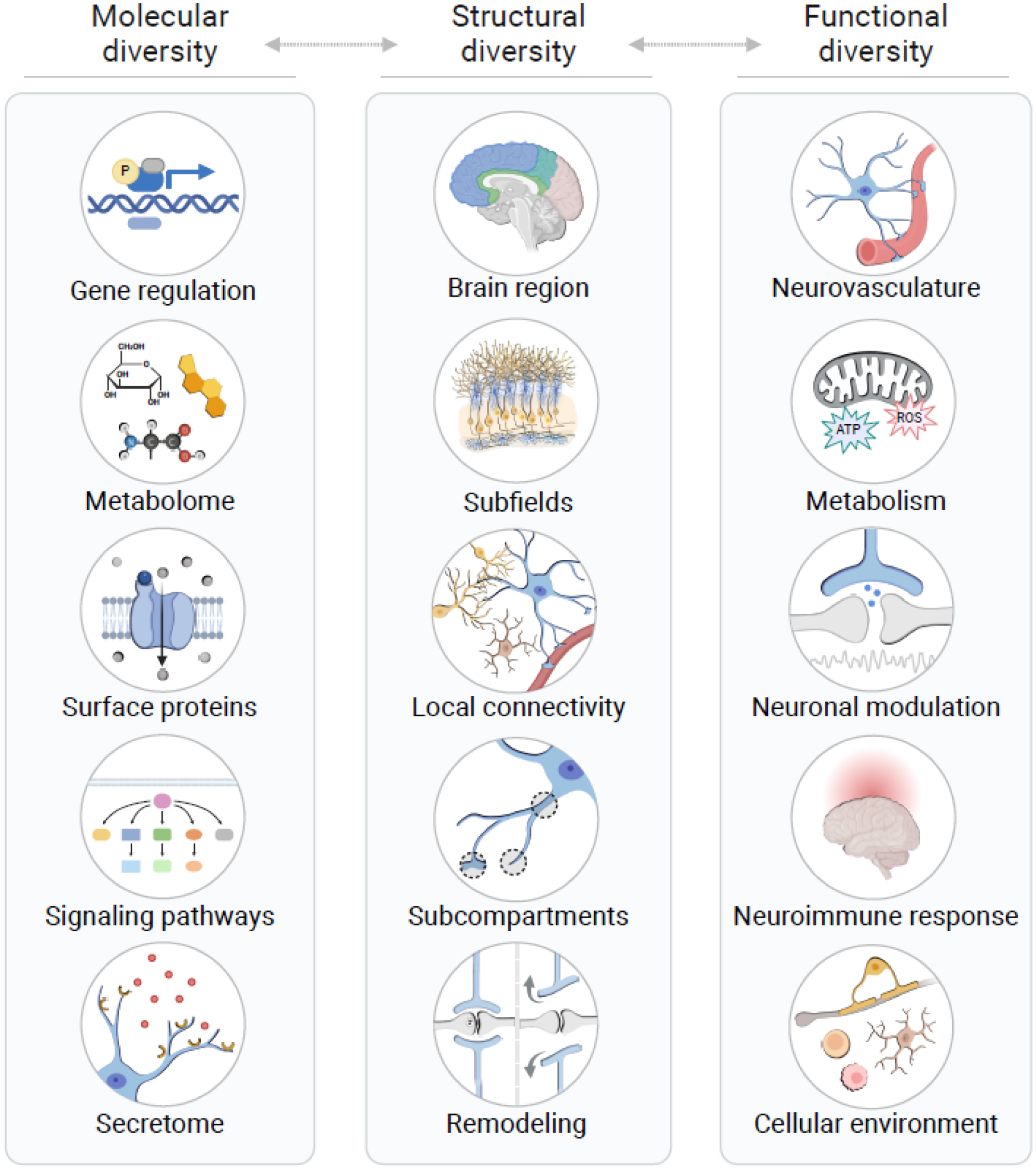
Astrocyte diversity involves cell-autonomous molecular features, structural and positional identity of astrocytes throughout the brain, and the integration of astrocyte populations into their functional contexts. All three aspects of heterogeneity are interconnected, and facilitate precise, dynamic, and multifaceted responses by astrocytes to various physiological and pathological signals. Ultimately, astrocytic responses have direct and indirect effects on neuronal function and survival and thereby have major roles in the pathogenesis of neurodegenerative diseases. Abbreviations: ATP, adenosine triphosphate; ROS, reactive oxygen species. Figure created using BioRender.com.
Astrocytes integrate numerous cues and have diverse features and functions
Astrocytes are prevalent glial cells that tile the brain and are estimated to contact hundreds of thousands of synapses and interact with microglia, oligodendrocytes, and neurovascular elements [44]. Astrocytes are involved in a wide range of normal and pathogenic processes [45] through diverse arrays of receptors, channels, adhesion molecules, and other surface factors that sense neurotransmitters, cytokines, growth factors, and other types of molecules produced by surrounding cells, afferent synaptic inputs, and the periphery [46,47]. In response to these various cues, astrocytes regulate synaptic and neural circuit functions, other glial cells, vascular elements, and homeostatic processes. Historically, astrocytes were frequently studied in isolation or in the context of injury or disease, which can alter their phenotype and function. In addition, long-enduring dogmas asserting that astrocytes are non-excitable and slow to respond have perpetuated a notion that they are unable to effectively detect, store, and transmit behaviorally relevant neural information. These challenges, together with the slow development of tools to precisely target and manipulate astrocytes in vivo, have delayed our understanding of astrocyte biology. However, recent studies using more advanced methods suggest that astrocytes are crucial integrators and conductors of brain function, including behavior, in part due to their intimate associations with neurons and other cell types and their precise effects on neural function.
Astrocytes, like neurons, are heterogeneous and have region-specific morphological and molecular signatures, as well as region- and context-specific calcium dynamics and neurotransmitter release properties [48–50]. Astrocytes regulate diverse neuronal functions, such as basal synaptic strength in hippocampal CA1 neurons, inhibition in cortical circuits related to goal-directed behaviors, and modulation of sensory transmission in arousal and sleep [51–53], as established primarily in rodent models. Astrocytes exhibit distinct functional properties involving fast and slow signaling mechanisms [53,54], and modulate neural network excitation and synchronization required for sensory information processing, storage, and behavioral output [55,56]. Indeed, in mouse models, astrocytic signaling has been shown to regulate cognitive function and behavior [57,58], including learning and memory-linked processes [59–61]. Aberrant changes in the activation of astrocytic G-protein coupled signaling can lead to neural hyperactivity and specific behavioral deficits [59,62–64]. Studies are also revealing astrocytic contributions to social and emotion-related behaviors and neuropsychiatric conditions, as recently reviewed [65].
Evidence in rodent and ferret models suggests that astrocyte engagement in neural circuit activities is selective, dynamic, and dependent on context. Astrocytes in the striatum can discriminate and differentially regulate neuronal activities in specific circuits despite being physically intermingled with multiple circuits [66], and these astrocytes lack evoked calcium-based responses to neocortical inputs, but gain aberrant evoked responsiveness in the context of Huntington’s disease (HD)-related pathology [67]. Somewhat similarly, astrocytes in the neocortex discriminate between cholinergic inputs from neuronal populations in the nucleus basalis activated by visual stimuli [68], tune their responses to distinct features of visual stimuli [69], and shift their responsiveness and calcium signaling patterns based on the sleep-wake cycle and levels of norepinephrine [49,53,70,71]. In addition, astrocytes in the CA1 region of the hippocampus can selectively modulate CA1 neurons projecting to the anterior cingulate cortex but not those projecting to the nucleus accumbens, thereby modulating remote memory [62]. Moreover, CA1 astrocytes participate in frequency-specific dendritic integration [72]. Thus, in contrast to previous views that astrocytes are functionally homogeneous, emerging studies reveal a high degree of context-dependent functional precision and adaptation in astrocytic-neuronal interactions across a range of circuits and domains [73].
Developmental patterning affects astrocyte maturation and contributes to early heterogeneity in astrocytes across different brain regions. Local neuronal cues also play important roles in defining astrocytic phenotypes, including their gene expression and functional features, such as specific metabolic fluxes and transporter currents [74–76]. Notably, neuronal cues may continually refine astrocytic properties and promote distinct transcriptional, morphological, and functional features [77,78]. Astrocytic properties are also heterogeneous within brain regions, namely within neocortical layers [79,80] and hippocampal subregions, including distinct areas of the dentate gyrus [50,81,82]. Astrocyte heterogeneity is postulated to subserve specific neural processes and requirements in the local neural environment. There are also structural and molecular differences within subcellular compartments of individual astrocytes, which have distinct and potentially specialized proteomic signatures [83], intracellular calcium dynamics [84], and mitochondrial distributions [85], revealing a high degree of organizational sophistication and complexity at the subcellular level (Figure 2).
Astrocytic alterations and contributions to pathogenic processes are selective and context-dependent
Astrocytes have been implicated in diverse neurological and neuropsychiatric conditions, including most neurodegenerative disorders. Various perturbations in the brain can induce reactive astrocyte phenotypes, which are broadly characterized as changes in astrocytic morphological, molecular, and functional features in response to pathological or stress-associated conditions. Although general markers of astrocyte reactivity, such as increased glial fibrillary acidic protein (GFAP) expression, have historically served as indicators of astrocytic alterations, the use of binary characterization (e.g., normal or reactive) is now recognized as insufficient and potentially misleading due to the diversity of astrocytic changes and their influence across different contexts [86,87]. For example, in mouse models, astrocytes in the spinal cord and striatum adopt diverse and dynamic reactive states across different pathological contexts through changes in the activities of multiple transcriptional regulators, as assessed by chromatin accessibility measures, RNA sequencing, and gene perturbation [88,89].
Aging and neuroinflammation are major risk factors for neurodegenerative disorders and facilitate disease onset and progression [25,90]. The effects of aging and immune responses on glial cell phenotypes are brain region-specific and appear to be pronounced in the white matter [32,91]. Aging induces divergent transcriptional profiles in astrocytes within different brain regions and is associated with diminished astrocyte-mediated synaptic plasticity in mice and humans [92–94]. It has not been firmly established, however, whether certain aging-induced astrocytic changes in specific brain areas promote regional vulnerability or resilience to dementia-linked pathology, and if so – how. In animal models, astrocytes also have regional and time-dependent responses in lipopolysaccharide (LPS)-induced neuroinflammation, which induces distinct subpopulations of astrocytes with differing transcriptional profiles [95,96]. Therefore, spatiotemporal differences in astrocytic phenotypes consequent to aging and inflammation may contribute to differential brain vulnerability to disease, possibly synergizing with specific neuronal alterations in susceptible regions and giving rise to selective and localized neurodegenerative cascades. Below, we highlight further evidence that astrocytes are differentially affected by dementia-associated factors and pathological processes and in turn influence neuronal functions and pathogenic trajectories in a selective and context-dependent manner (Figure 3).
Figure 3. Astrocytes may contribute to selective vulnerability in disease through divergent, context-dependent phenotypes that affect specific brain regions and functions.
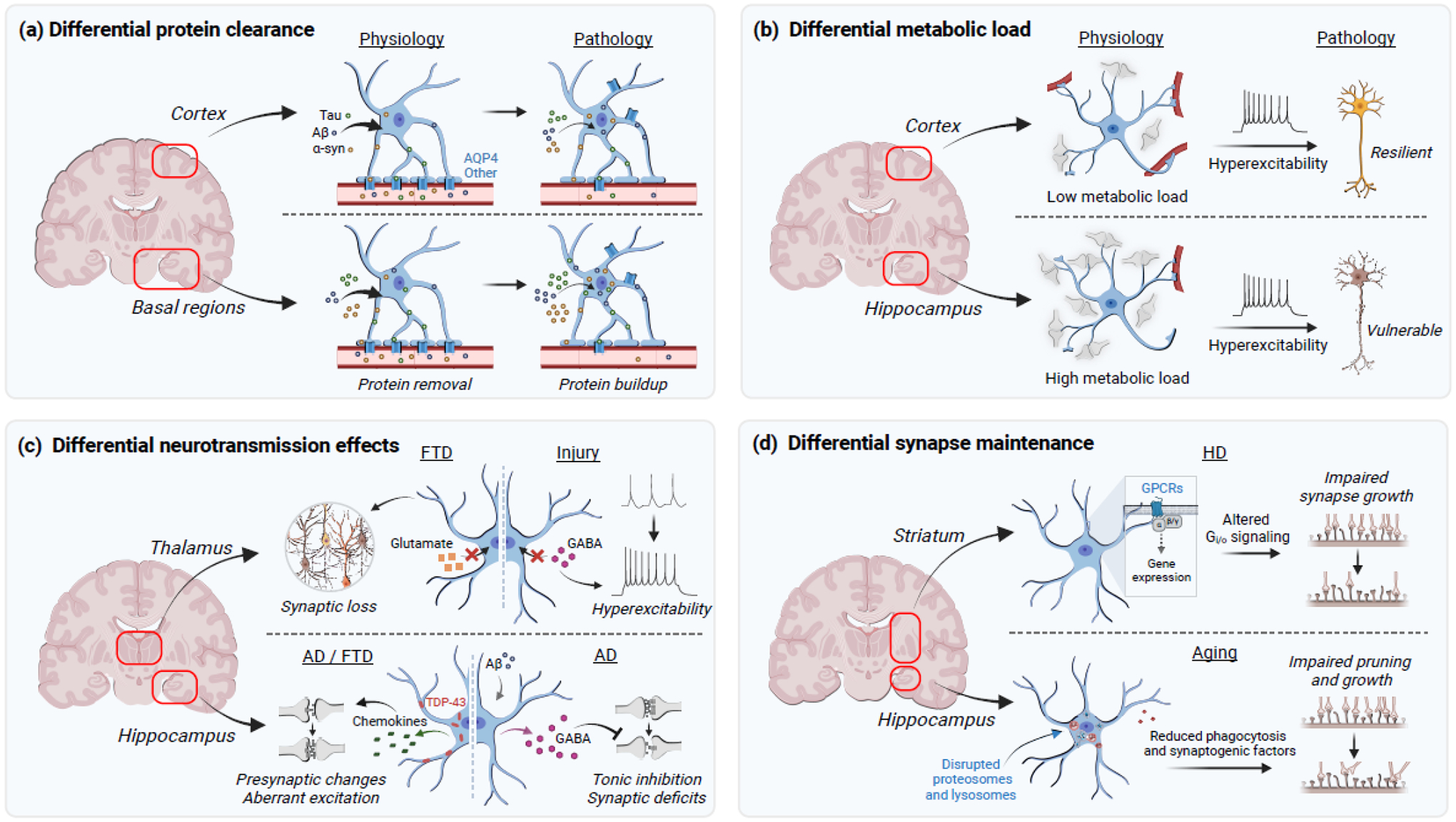
(a) Aberrant proteins implicated in disease are cleared from the brain through various mechanisms, including AQP4-dependent protein removal. Astrocytes in basal brain regions may have a higher clearance burden and, in conjunction with changes in AQP4 localization and expression, might promote selective vulnerability in the basal regions. (b) Cortical astrocytes contact more blood vessels and fewer synapses per cell as compared to hippocampal astrocytes. The resulting higher metabolic load carried by hippocampal astrocytes may predispose hippocampal synapses and other neural elements to metabolic vulnerability and the detrimental effects of neural hyperexcitability. (c) Astrocytes regulate neurotransmission in a state-dependent and region-specific manner. In FTD, thalamic astrocytes may remove less glutamate via surface transporters and promote synaptic loss and, in brain injury, remove less GABA and induce hyperexcitability. Aberrant TDP-43 accumulation in hippocampal astrocytes promotes region-specific expression of chemokines that induce presynaptic changes and aberrant excitatory transmission. Hippocampal astrocytes in AD overproduce GABA and impair neuronal function through increases in tonic inhibition. (d) Astrocytes can differentially modulate synaptic formation and loss. Striatal astrocytes in the context of HD-related pathology have altered Gi/o-coupled signaling leading to gene expression changes that impair synaptogenesis. In aging, subpopulations of hippocampal astrocytes have disrupted proteosomes and lysosomes, which impairs synaptic pruning and secretion of synaptogenic factors. Abbreviations: α-syn, alpha-synuclein; Aβ, amyloid-beta; AD, Alzheimer’s disease; AQP4, aquaporin-4; FTD, frontotemporal dementia; GABA, γ-aminobutyric acid; Gi/o, Gi/o-coupled receptor signaling; GPCRs, G protein-coupled receptors; TDP-43, transactive response DNA-binding protein of 43 kDa. Figure created using BioRender.com.
Proteinopathy
Astrocytes can differentially modulate proteinopathy and its effects on brain function. The uptake and clearance of protein aggregates by astrocytes can become progressively impaired in pathological conditions [97]. Astrocytes take up protein aggregates to either degrade them intracellularly or deliver them to waste removal systems, though alternative pathways might also exist. Brain clearance of protein aggregates depends in part on bulk flow through perivascular spaces [98], and water movement through astrocytic aquaporin 4 (AQP4) channels is hypothesized to facilitate Aβ removal [99–101]. Notably, AQP4 levels are reduced in astrocytic endfeet in mouse models of AD pathology and in individuals with AD or cerebral amyloid angiopathy [102], which is characterized by aberrant Aβ accumulation in perivascular spaces. Reductions in clearance are associated with increased Aβ load in humans [103], implicating changes in astrocytic clearance mechanisms in AD pathogenesis. AQP4 levels differ among astrocyte subpopulations and subcompartments, with AQP4 enrichment in astrocytic endfeet [104,105]. Differences in AQP4 function may promote local variations in Aβ clearance and affect the capacity of brain regions to cope with protein accumulation, thereby correlating with pathogenesis and neurocognitive changes. In contrast to AQP4 reductions in AD, AQP4 levels are enhanced in FTD linked to progranulin mutations [107], highlighting potential disease-specific changes in clearance mechanisms.
In addition to potential differences in blood-brain barrier (BBB)-dependent clearance, astrocyte subpopulations in human organoids and mouse models have differential expression of phagocytosis and autophagy factors with aging and disease [108–110]. Reduced autophagy in human and mouse astrocytes may be a driver of astrocytic impairments and consequent neuronal damage, especially in those with genetic risk factors for neurodegeneration [111]. Considering that astrocytes serve as gatekeepers positioned between brain parenchyma and glymphatic waste removal, reduced phagocytic and AQP4-dependent clearance mechanisms may synergize to hasten the accumulation and aggregation of aberrant proteins. Most CSF is hypothesized to drain at the base of the brain via lymphatic vessels running in parallel to cranial nerves [112]. Accordingly, despite generally similar levels of astrocytic AQP4 throughout the brain, there is likely a high local burden on astrocytic clearance mechanisms in basal brain regions. Interestingly, these regions are also among the earliest to display signs of Aβ, tau, and α-synuclein accumulation in humans, including basal brain regions and midbrain, suggesting that astrocytes in those regions could be affected by aging and other risk factors and promote regional vulnerability to altered proteostasis and onset of pathogenic cascades that subsequently spread to other brain areas.
In addition to extracellular clearance defects, intracellular protein dysregulation in astrocytes is sufficient to cause progressive neuronal impairments and behavioral decline. Pioneering studies in the context of familial ALS suggest that astrocytic protein aggregates in transgenic SOD1 mouse models promote neurodegeneration by disturbing glutamate homeostasis, and that genetic deletion of mutant SOD1 selectively in mouse astrocytes slows disease progression [113]. Moreover, in mouse models involving targeted viral vector manipulations, tau accumulation in hilar astrocytes of the hippocampus was sufficient to cause bioenergetic failure, reductions in neurogenesis, and selective impairments in inhibitory neurons [114]. Similarly, TDP-43 accumulation induced in hippocampal astrocytes of adult mice causes progressive memory loss through alterations in astrocytic antiviral pathways and selective impairments in presynaptic function [115]. Of note, learning, motor function, and innate behaviors in mice were not affected by astrocytic TDP-43 mislocalization, even when occurring throughout the brain [115], suggesting that TDP-43 pathology in astrocytes has region-selective effects on brain function. Mutant huntingtin and tau also disturb astrocytic cholesterogenesis, which is essential for neuronal function [116–118]. Therefore, heterogeneous proteinopathy in astrocytes, along with local variations in BBB-linked clearance mechanisms, could result in selective vulnerabilities in protein handling and specific types of neuronal and neurocognitive impairments. In support, correlational analyses of Aβ, tau, and astrocyte pathology, as assessed by plasma GFAP levels, suggest that astrocytic alterations in humans may reflect an early pathogenic transition in preclinical AD, predicting tau pathology and thereby linking Aβ accumulation to clinical symptoms [119].
Metabolic pathways
Neurodegenerative disorders also involve changes in astrocytic metabolic pathways [120–123]. Astrocytes supply essential metabolites to neurons and other cells, including lactate for energy, cholesterol for membrane homeostasis, glutamine as a precursor for neurotransmitters, and factors to maintain redox homeostasis [124,125]. Astrocytes also regulate cerebral blood flow to sustain neuronal energy and potentially integrate peripheral metabolic cues [47,126]. Moreover, astrocyte subpopulations have been identified with varying expression of genes involved in energy-producing processes, including glycolysis and oxidative phosphorylation, functions that might be compromised in aging, according to transcriptomic studies in mice [32,93], and these differential bioenergetic states could promote impairments in select circuits or brain regions.
Alterations to astrocyte metabolism have multifactorial and context-dependent effects. In mouse models, changes in astrocytic urea metabolism protect from Aβ toxicity but also result in GABA overproduction and cognitive deficits [127]. Astrocytes also buffer neuronal fatty acid (FA) lipotoxicity upon stress or increased neuronal firing [128,129]. However, in the context of HD-linked pathology in mice, a metabolic shift toward FA oxidation in striatal astrocytes increases the levels of reactive oxygen species that promote disease [130]. In contrast, loss of the ability to catabolize FAs can contribute to astrocyte reactivity in certain subpopulations and cause neurotoxic FA release by mouse astrocytes [123,131,132]. ApoE expression, a key regulator of lipid transport, is enriched in astrocytes as compared to other neural cell types, and ApoE4 is a strong genetic risk factor for AD. ApoE4 diminishes astrocytic clearance of neuronal lipids and degradation of FAs, thereby possibly contributing to lipid dysregulation and neuronal metabolic stress [121,133]. In human induced pluripotent stem cell (iPSC)-derived astrocytes, ApoE4 also increases astrocytic cholesterol release that can exacerbate neuronal amyloid precursor protein (APP) processing and Aβ production [134]. Thus, ApoE and other genetic variants linked to disease risk can shift metabolic functions of astrocytes and promote specific vulnerabilities. A better understanding is needed of the metabolic repertoire of different astrocyte populations and states, and their respective capacity to respond to alterations in metabolic supply and demand associated with disease. Of note, studies in mice increasingly link astrocyte metabolism to cognitive function [135–137], suggesting that therapeutic targeting of these mechanisms might reduce pathology as well as restore or enhance cognitive processes.
Regional variations in cytoarchitecture and astrocytic-neurovascular contacts may also contribute to localized or variable metabolic vulnerabilities. Astrocytic endfeet line capillaries throughout the brain, enable neurovascular coupling, and modulate the flux of essential metabolites, waste products, trophic factors, and other molecules across the BBB [138]. However, there are differences in the degree of vessel coverage by astrocytes across brain regions [78]. Particularly, mouse cortical astrocytes contact more vessels than hippocampal astrocytes, and a subset of astrocytes without vessel contact have been detected in the hippocampus but not other examined regions [139]. Hippocampal astrocytes are estimated to contact roughly 100,000 excitatory synapses, whereas striatal astrocytes contact about half as many synapses [77]. Together, these findings suggest that hippocampal astrocytes bear a larger load of excitatory synapses but contact fewer blood vessels, which might cause insufficient energy supply and altered metabolic states upon increases in neuronal activity and neural network dysregulation in disease [140]. Of note, astrocytes in AD have enhanced connexin-43 expression, a gap junction channel that can connect adjacent astrocytes [141], which may reflect a compensatory response to distribute the increased metabolic load across a broader astrocyte network. Dynamic changes in astrocytic morphology could also affect their physical interactions with the vasculature and synaptic elements. In mouse models, disrupted metabolic supply can recruit astrocytic metabolic stores from nearby unstressed brain areas, potentially leaving those areas more susceptible to stressors [142]. Hence, variations in physical contacts and redistribution of metabolites across astrocyte networks could contribute to differential and progressive susceptibility of healthy regions and the spread of pathology across brain areas.
Neurotransmission and synaptic function
Astrocytes constitute an essential element of synaptic transmission and plasticity, and their engagement in these processes is crucial for brain function [143]. In particular, astrocytes regulate neurotransmitter synthesis and cycling, and alterations in these mechanisms have profound effects on neuronal function and vulnerability to disease [125,144]. In mouse and human iPSC-based models of FTD with progranulin mutations, reduced glutamate uptake by astrocytes promoted synaptic degeneration in thalamocortical circuits [107]. Additionally, reduced astrocytic GABA uptake upon injury induces neuronal hyperexcitability in the thalamus [145], and astrocytic GABA overproduction in transgenic mice with AD-related amyloid pathology impairs spike probability in the hippocampus [144]. These astrocytic functions can vary across brain regions. For instance, astrocytic glutamate uptake is faster in the mouse frontal cortex as compared to the somatosensory cortex [146], and vesicular glutamate is released by a specific subset of hippocampal and cortical astrocytes that express genes required for calcium-regulated glutamate exocytosis, as detected in mouse and human brains [50]. These specialized astrocytic functions might differentially influence local changes in neurotransmission and synaptic plasticity in disease.
Astrocytes are also involved in maintaining synaptic structure and density through the release of synaptogenic factors and active elimination of synapses. These functions can vary across regions and types of synapses. In aging mice, a specific population of hippocampal astrocytes has impaired autophagy and synaptogenic mechanisms for regulating synapses [110]. In a mouse model of HD, dysfunctional Gi/o-coupled receptor signaling in striatal astrocytes can selectively dysregulate synaptogenesis and cause impairments in attention-associated behaviors [88]. In mouse models and human cases of ALS, a selective loss of tripartite synapses may occur, pointing to greater vulnerability of synapses containing astrocytic processes as compared to bipartite synapses [147]. Human and mouse astrocytes are also implicated in increased AD pathology-associated synapse elimination [148]. Of note, astrocytic synapse elimination in a mouse model of tauopathy preferentially targets excitatory synapses as compared to inhibitory synapses, which may be engulfed more by microglia [149]. Astrocytes can also modulate microglia-mediated synapse elimination [150] and could influence microglial roles in brain resilience to tauopathy [31], but these potential astrocytic-microglial interactions require further investigation. In addition to enhanced synapse removal, there is also evidence that astrocytes in transgenic mice with amyloid pathology are impaired in the clearance of dystrophic synapses [151]. In summary, astrocytes are known to influence synaptic structure and function, and recent studies suggest that these effects are likely region-specific and selective, mediated by distinct astrocytic subpopulations, differentially affected by pathogenic cascades, and potentially intertwined with microglial activities.
Astrocytic context-dependent roles in disease may inform therapeutic approaches
As discussed above, astrocytes are essential for proper CNS function, and their molecular and morphological features and functional properties vary across brain regions, circuits, and synapses and can strongly depend on the exact biological context and disease process. These diverse and dynamic properties are likely due in large part to astrocytic sensitivity to numerous incoming signals that provide information regarding animal maturity and age [93,110,152], biological sex [153,154], genetic factors [122,155], metabolism [47,156,157], sensory inputs and arousal [158], and various other brain activities and systemic factors. The integrated processing of local and long-range signals likely generates a variety of astrocytic phenotypes and functional states that are finetuned and integrated into their specific neural context. We speculate that context integration enables astrocytes to properly support and modulate many different types of neural circuits, synapses, and functions in diverse and dynamic biological conditions (Figure 4). As described in the last section, astrocytes also have heterogeneous responses and selective roles in neurodegenerative disease-associated pathogenic processes. Particularly, aberrant cues and factors associated with aging, proteinopathy, inflammation, and other pathological processes induce specific types of changes in subsets and subcompartments of astrocytes, affecting their gene expression, protein handling, and immunometabolic pathways, and their regulation of specific types of neuronal circuits, synapses, and other surrounding cells. These changes result in dysfunctional engagement (or disengagement) of astrocytes in their defined microenvironment, which we call context divergence. Shifting from context-integrated to context-divergent states could set the stage for selective pathogenesis by preferentially disrupting (or failing to maintain) specific astrocyte-regulated neural circuits, signaling mechanisms, and local brain activities (Figure 4).
Figure 4. Astrocytes promote context-dependent effects in health and disease.
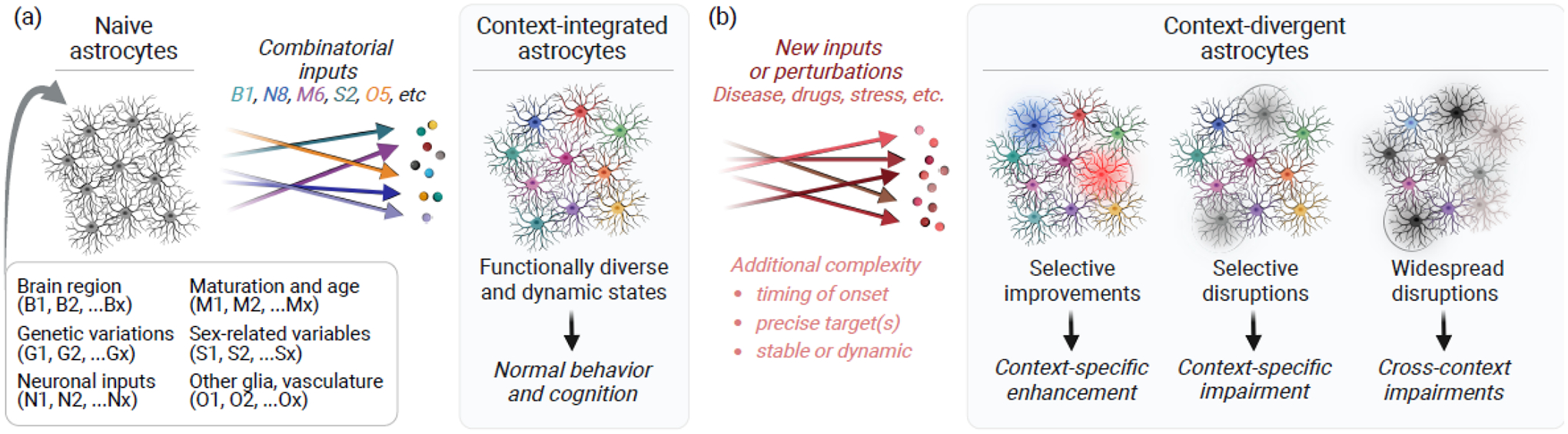
(a) Astrocytes are sensitive to various cues in their microenvironment and have diverse molecular, structural, and functional states as a result of the specific combination of cues received by individual astrocytes. These combinatorial inputs instruct astrocytes to assume various context-integrated states that enable normal brain function. (b) In disease and other conditions, aberrant inputs and perturbations affect individual astrocytes differently and may shift subsets or subcompartments of astrocytes into context-divergent states that selectively enhance or disrupt specific aspects of brain function. Figure created using BioRender.com.
Further investigation of the exact molecular and cellular basis for selective vulnerability holds great potential for elucidating disease pathogenesis and developing more effective treatment options, with the possibility for earlier and more precise interventions. With regards to the contributions of astrocytes to disease progression, recent studies argue against the notion that disease-related impairments are triggered largely by dysfunction in neuronal or other glial populations with secondary or ancillary responses in astrocytes. Instead, studies are converging on a theory that dysfunctions in astrocytes are operating in concert with neuronal predispositions and potential abnormalities in other cells to trigger selective neurodegenerative cascades and progressive behavioral alterations.
Considering this emerging view, effective therapeutic strategies may require targeting of astrocytic functions that are linked to specific neuronal circuits most susceptible to impairment in a particular type of disorder and patient population. Whether targeting a discrete disease-associated astrocyte subcluster or a broader astrocyte network within a given brain region is required for therapeutic efficacy remains an important open question. Further investigations are required to unravel exactly how different astrocytic subpopulations, compartments, and functional states are organized and dynamically tuned to different brain activities, and how these astrocytic states are selectively compromised and could be therapeutically targeted in neurodegenerative conditions.
Concluding remarks and future perspectives
Mounting evidence of astrocytic heterogeneity and functional selectivity suggests that astrocytes could promote selective vulnerability in disease. Significant progress has been made in broadly profiling molecular changes and uncovering novel mechanisms that mediate astrocytic-neuronal crosstalk and effects on pathogenic processes. However, an integrated understanding of astrocytic and neuronal functions and their interconnected changes in disease is still lacking. Additional studies are needed that address how astrocytes differentially regulate and contribute to brain function and pathology in distinct biological and environmental contexts (see Outstanding questions). In elucidating these roles, it is important to be mindful of the limitations of current models of neurodegenerative disorders and explore neuropathological evidence and multiomic datasets from human samples and use human iPSC-derived systems when possible. Human astrocytes can differ from mouse astrocytes in certain morphological features, metabolic changes, and inflammatory pathways, emphasizing the importance of cross-species validation [159,160]. Future studies should investigate the roles of circuit-specific and molecularly defined subpopulations of astrocytes, take advantage of advanced genetically encoded indicators and actuators, and rigorously compare astrocytic features and functions across different biological and pathogenic contexts.
Outstanding Questions.
The mechanisms by which astrocytes regulate various types of disease processes and neural impairments are incompletely understood. When and how do astrocytes engage in different pathological cascades, synaptic alterations, and neural circuit dysfunctions?
It remains unclear whether astrocytes are a diverse population with developmentally defined and constant states, whether they exhibit dynamic shifts in function that generates apparent heterogeneity, or whether both scenarios are occurring in vivo. What are the exact triggers and regulatory mechanisms that promote regional astrocyte heterogeneity in health and disease?
What are the effects of disease-linked astrocytic changes on specific neuronal subpopulations that are known to be most vulnerable to disease? What is the nature of astrocyte contributions to cognitive and behavioral deficits across disorders and what are their specific pathophysiological mechanisms?
Individual astrocytes have structural and functional subcompartments, including endfeet and leaflets. What are the disease-linked changes in these subcellular compartments, and how do these changes affect presynaptic and postsynaptic elements, neurovascular cells, and other glia?
Astrocytes are the primary cell type involved in the synthesis of brain lipoproteins and lipids, including ApoE and cholesterol, which are essential for brain function and implicated in neurodegenerative disorders. How is astrocytic lipid metabolism and lipid-based crosstalk with other cell types regulated by neuronal and neurovascular activities and altered by pathological processes?
Sex differences affect behavioral and cognitive processes and are prevalent in aging and neurodegenerative disorders. In astrocytes, sex-specific features are evident in the hypothalamus and may occur in other regions, but most studies on astrocytes in behavior and disease have not addressed potential differences among sexes. Are astrocytic effects on cognition and disease pathogenesis sex-dependent?
Highlights.
Selective vulnerability in neurodegenerative disorders affects specific neuronal populations and may involve non-neuronal cells like astrocytes, which have multifaceted roles in brain function.
Astrocytes are phenotypically and functionally heterogeneous and responsive to diverse signals as a function of age, brain region, environmental conditions, and other variables.
Astrocytic responses and contributions to neural impairments and pathogenic processes in neurodegenerative conditions are emerging as selective and context-dependent.
Astrocytes may promote selective vulnerability in neurodegenerative disorders by inducing distinct effects across different contexts.
Acknowledgements
This work was supported by the National Institutes of Health: R01AG068091 (A.G.O) and RF1NS118569 (A.G.O.), BrightFocus Foundation: BFA2023008F (T.S.Z.), and the Alzheimer’s Association: 23AARF-1029892 (T.S.Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare no competing interests in relation to this work.
References
- 1.Wilson DM 3rd et al. (2023) Hallmarks of neurodegenerative diseases. Cell 186, 693–714. 10.1016/j.cell.2022.12.032 [DOI] [PubMed] [Google Scholar]
- 2.Serrano-Pozo A et al. (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1, a006189. 10.1101/cshperspect.a006189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santillo AF et al. (2013) von Economo neurones are selectively targeted in frontotemporal dementia. Neuropathol Appl Neurobiol 39, 572–579. 10.1111/nan.12021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cleveland DW and Rothstein JD (2001) From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci 2, 806–819. 10.1038/35097565 [DOI] [PubMed] [Google Scholar]
- 5.Ross CA et al. (2014) Huntington disease: natural history, biomarkers and prospects for therapeutics. Nat Rev Neurol 10, 204–216. 10.1038/nrneurol.2014.24 [DOI] [PubMed] [Google Scholar]
- 6.Dickson DW (2012) Parkinson’s disease and parkinsonism: neuropathology. Cold Spring Harb Perspect Med 2. 10.1101/cshperspect.a009258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu H et al. (2018) Selective vulnerability in neurodegenerative diseases. Nat Neurosci 21, 1350–1358. 10.1038/s41593-018-0221-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roussarie JP et al. (2020) Selective Neuronal Vulnerability in Alzheimer’s Disease: A Network-Based Analysis. Neuron 107, 821–835 e812. 10.1016/j.neuron.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva MC et al. (2020) Prolonged tau clearance and stress vulnerability rescue by pharmacological activation of autophagy in tauopathy neurons. Nat Commun 11, 3258. 10.1038/s41467-020-16984-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourdenx M et al. (2021) Chaperone-mediated autophagy prevents collapse of the neuronal metastable proteome. Cell 184, 2696–2714 e2625. 10.1016/j.cell.2021.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nana AL et al. (2019) Neurons selectively targeted in frontotemporal dementia reveal early stage TDP-43 pathobiology. Acta Neuropathol 137, 27–46. 10.1007/s00401-018-1942-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin LC et al. (2019) Preferential tau aggregation in von Economo neurons and fork cells in frontotemporal lobar degeneration with specific MAPT variants. Acta Neuropathol Commun 7, 159. 10.1186/s40478-019-0809-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau A et al. (2020) alpha-Synuclein strains target distinct brain regions and cell types. Nat Neurosci 23, 21–31. 10.1038/s41593-019-0541-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogel JW et al. (2023) Connectome-based modelling of neurodegenerative diseases: towards precision medicine and mechanistic insight. Nat Rev Neurosci. 10.1038/s41583-023-00731-8 [DOI] [PubMed] [Google Scholar]
- 15.Vaquer-Alicea J and Diamond MI (2019) Propagation of Protein Aggregation in Neurodegenerative Diseases. Annu Rev Biochem 88, 785–810. 10.1146/annurev-biochem-061516-045049 [DOI] [PubMed] [Google Scholar]
- 16.Uemura N et al. (2020) Cell-to-Cell Transmission of Tau and alpha-Synuclein. Trends Mol Med 26, 936–952. 10.1016/j.molmed.2020.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arboleda-Velasquez JF et al. (2019) Resistance to autosomal dominant Alzheimer’s disease in an APOE3 Christchurch homozygote: a case report. Nat Med 25, 1680–1683. 10.1038/s41591-019-0611-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selvaraj BT et al. (2018) C9ORF72 repeat expansion causes vulnerability of motor neurons to Ca(2+)-permeable AMPA receptor-mediated excitotoxicity. Nat Commun 9, 347. 10.1038/s41467-017-02729-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang F et al. (2020) beta-amyloid redirects norepinephrine signaling to activate the pathogenic GSK3beta/tau cascade. Sci Transl Med 12. 10.1126/scitranslmed.aay6931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leng K et al. (2021) Molecular characterization of selectively vulnerable neurons in Alzheimer’s disease. Nat Neurosci 24, 276–287. 10.1038/s41593-020-00764-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao R et al. (2022) Activity disruption causes degeneration of entorhinal neurons in a mouse model of Alzheimer’s circuit dysfunction. Elife 11. 10.7554/eLife.83813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Traxler L et al. (2022) Warburg-like metabolic transformation underlies neuronal degeneration in sporadic Alzheimer’s disease. Cell Metab 34, 1248–1263 e1246. 10.1016/j.cmet.2022.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moya MV et al. (2022) Unique molecular features and cellular responses differentiate two populations of motor cortical layer 5b neurons in a preclinical model of ALS. Cell Rep 38, 110556. 10.1016/j.celrep.2022.110556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez-Rodriguez P et al. (2021) Disruption of mitochondrial complex I induces progressive parkinsonism. Nature 599, 650–656. 10.1038/s41586-021-04059-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou Y et al. (2019) Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol 15, 565–581. 10.1038/s41582-019-0244-7 [DOI] [PubMed] [Google Scholar]
- 26.Wegmann S et al. (2019) Experimental evidence for the age dependence of tau protein spread in the brain. Sci Adv 5, eaaw6404. 10.1126/sciadv.aaw6404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun N et al. (2023) Single-nucleus multiregion transcriptomic analysis of brain vasculature in Alzheimer’s disease. Nat Neurosci 26, 970–982. 10.1038/s41593-023-01334-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gleichman AJ and Carmichael ST (2020) Glia in neurodegeneration: Drivers of disease or along for the ride? Neurobiol Dis 142, 104957. 10.1016/j.nbd.2020.104957 [DOI] [PubMed] [Google Scholar]
- 29.Sweeney MD et al. (2018) The role of brain vasculature in neurodegenerative disorders. Nat Neurosci 21, 1318–1331. 10.1038/s41593-018-0234-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park L et al. (2020) Tau induces PSD95-neuronal NOS uncoupling and neurovascular dysfunction independent of neurodegeneration. Nat Neurosci 23, 1079–1089. 10.1038/s41593-020-0686-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Udeochu JC et al. (2023) Tau activation of microglial cGAS-IFN reduces MEF2Cmediated cognitive resilience. Nat Neurosci 26, 737–750. 10.1038/s41593-023-01315-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hahn O et al. (2023) Atlas of the aging mouse brain reveals white matter as vulnerable foci. Cell 186, 4117–4133 e4122. 10.1016/j.cell.2023.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blanchard JW et al. (2022) APOE4 impairs myelination via cholesterol dysregulation in oligodendrocytes. Nature 611, 769–779. 10.1038/s41586-022-05439-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goutman SA et al. (2022) Emerging insights into the complex genetics and pathophysiology of amyotrophic lateral sclerosis. Lancet Neurol 21, 465–479. 10.1016/S1474-4422(21)00414-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simons M et al. (2023) Tipping points in neurodegeneration. Neuron. 10.1016/j.neuron.2023.05.031 [DOI] [PubMed] [Google Scholar]
- 36.Murdock MH and Tsai LH (2023) Insights into Alzheimer’s disease from single-cell genomic approaches. Nat Neurosci 26, 181–195. 10.1038/s41593-022-01222-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chatterjee P et al. (2021) Plasma glial fibrillary acidic protein is elevated in cognitively normal older adults at risk of Alzheimer’s disease. Transl Psychiatry 11, 27. 10.1038/s41398-020-01137-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barker SJ et al. (2021) MEF2 is a key regulator of cognitive potential and confers resilience to neurodegeneration. Sci Transl Med 13, eabd7695. 10.1126/scitranslmed.abd7695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faraco G et al. (2019) Dietary salt promotes cognitive impairment through tau phosphorylation. Nature 574, 686–690. 10.1038/s41586-019-1688-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi SH et al. (2018) Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 361. 10.1126/science.aan8821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanmarco LM et al. (2021) Functional immune cell-astrocyte interactions. J Exp Med 218. 10.1084/jem.20202715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brandebura AN et al. (2023) Astrocyte contribution to dysfunction, risk and progression in neurodegenerative disorders. Nat Rev Neurosci 24, 23–39. 10.1038/s41583-022-00641-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khakh BS and Deneen B (2019) The Emerging Nature of Astrocyte Diversity. Annu Rev Neurosci 42, 187–207. 10.1146/annurev-neuro-070918-050443 [DOI] [PubMed] [Google Scholar]
- 44.Torres-Ceja B and Olsen ML (2022) A closer look at astrocyte morphology: Development, heterogeneity, and plasticity at astrocyte leaflets. Curr Opin Neurobiol 74, 102550. 10.1016/j.conb.2022.102550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blanco-Suarez E et al. (2017) Role of astrocyte-synapse interactions in CNS disorders. J Physiol 595, 1903–1916. 10.1113/JP270988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sofroniew MV (2020) Astrocyte Reactivity: Subtypes, States, and Functions in CNS Innate Immunity. Trends Immunol 41, 758–770. 10.1016/j.it.2020.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nampoothiri S et al. (2022) Glial cells as integrators of peripheral and central signals in the regulation of energy homeostasis. Nat Metab 4, 813–825. 10.1038/s42255-022-00610-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lanjakornsiripan D et al. (2018) Layer-specific morphological and molecular differences in neocortical astrocytes and their dependence on neuronal layers. Nat Commun 9, 1623. 10.1038/s41467-018-03940-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsunematsu T et al. (2021) Region-Specific and State-Dependent Astrocyte Ca(2+) Dynamics during the Sleep-Wake Cycle in Mice. J Neurosci 41, 5440–5452. 10.1523/JNEUROSCI.2912-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Ceglia R et al. (2023) Specialized astrocytes mediate glutamatergic gliotransmission in the CNS. Nature 622, 120–129. 10.1038/s41586-023-06502-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chipman PH et al. (2021) Astrocyte GluN2C NMDA receptors control basal synaptic strengths of hippocampal CA1 pyramidal neurons in the stratum radiatum. Elife 10. 10.7554/eLife.70818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mederos S et al. (2021) GABAergic signaling to astrocytes in the prefrontal cortex sustains goal-directed behaviors. Nat Neurosci 24, 82–92. 10.1038/s41593-020-00752-x [DOI] [PubMed] [Google Scholar]
- 53.Wang F et al. (2023) Distinct astrocytic modulatory roles in sensory transmission during sleep, wakefulness, and arousal states in freely moving mice. Nat Commun 14, 2186. 10.1038/s41467-023-37974-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stobart JL et al. (2018) Cortical Circuit Activity Evokes Rapid Astrocyte Calcium Signals on a Similar Timescale to Neurons. Neuron 98, 726–735 e724. 10.1016/j.neuron.2018.03.050 [DOI] [PubMed] [Google Scholar]
- 55.Lines J et al. (2020) Astrocytes modulate sensory-evoked neuronal network activity. Nat Commun 11, 3689. 10.1038/s41467-020-17536-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reitman ME et al. (2023) Norepinephrine links astrocytic activity to regulation of cortical state. Nat Neurosci 26, 579–593. 10.1038/s41593-023-01284-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Santello M et al. (2019) Astrocyte function from information processing to cognition and cognitive impairment. Nat Neurosci 22, 154–166. 10.1038/s41593-018-0325-8 [DOI] [PubMed] [Google Scholar]
- 58.Akther S and Hirase H (2022) Assessment of astrocytes as a mediator of memory and learning in rodents. Glia 70, 1484–1505. 10.1002/glia.24099 [DOI] [PubMed] [Google Scholar]
- 59.Orr AG et al. (2015) Astrocytic adenosine receptor A2A and Gs-coupled signaling regulate memory. Nat Neurosci 18, 423–434. 10.1038/nn.3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robin LM et al. (2018) Astroglial CB(1) Receptors Determine Synaptic D-Serine Availability to Enable Recognition Memory. Neuron 98, 935–944 e935. 10.1016/j.neuron.2018.04.034 [DOI] [PubMed] [Google Scholar]
- 61.Doron A et al. (2022) Hippocampal astrocytes encode reward location. Nature 609, 772–778. 10.1038/s41586-022-05146-6 [DOI] [PubMed] [Google Scholar]
- 62.Kol A et al. (2020) Astrocytes contribute to remote memory formation by modulating hippocampal-cortical communication during learning. Nat Neurosci 23, 1229–1239. 10.1038/s41593-020-0679-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nagai J et al. (2021) Specific and behaviorally consequential astrocyte G(q) GPCR signaling attenuation in vivo with ibetaARK. Neuron 109, 2256–2274 e2259. 10.1016/j.neuron.2021.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Delepine C et al. (2023) Differential Effects of Astrocyte Manipulations on Learned Motor Behavior and Neuronal Ensembles in the Motor Cortex. J Neurosci 43, 2696–2713. 10.1523/JNEUROSCI.1982-22.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shigetomi E and Koizumi S (2023) The role of astrocytes in behaviors related to emotion and motivation. Neurosci Res 187, 21–39. 10.1016/j.neures.2022.09.015 [DOI] [PubMed] [Google Scholar]
- 66.Martin R et al. (2015) Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science 349, 730–734. 10.1126/science.aaa7945 [DOI] [PubMed] [Google Scholar]
- 67.Jiang R et al. (2016) Dysfunctional Calcium and Glutamate Signaling in Striatal Astrocytes from Huntington’s Disease Model Mice. J Neurosci 36, 3453–3470. 10.1523/JNEUROSCI.3693-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen N et al. (2012) Nucleus basalis-enabled stimulus-specific plasticity in the visual cortex is mediated by astrocytes. Proc Natl Acad Sci U S A 109, E2832–2841. 10.1073/pnas.1206557109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schummers J et al. (2008) Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science 320, 1638–1643. 10.1126/science.1156120 [DOI] [PubMed] [Google Scholar]
- 70.Paukert M et al. (2014) Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron 82, 1263–1270. 10.1016/j.neuron.2014.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bojarskaite L et al. (2020) Astrocytic Ca(2+) signaling is reduced during sleep and is involved in the regulation of slow wave sleep. Nat Commun 11, 3240. 10.1038/s41467-020-17062-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bohmbach K et al. (2022) An astrocytic signaling loop for frequency-dependent control of dendritic integration and spatial learning. Nat Commun 13, 7932. 10.1038/s41467-022-35620-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Murphy-Royal C et al. (2023) A conceptual framework for astrocyte function. Nat Neurosci 26, 1848–1856. 10.1038/s41593-023-01448-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Farmer WT et al. (2016) Neurons diversify astrocytes in the adult brain through sonic hedgehog signaling. Science 351, 849–854. 10.1126/science.aab3103 [DOI] [PubMed] [Google Scholar]
- 75.Hasel P et al. (2017) Neurons and neuronal activity control gene expression in astrocytes to regulate their development and metabolism. Nat Commun 8, 15132. 10.1038/ncomms15132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Farhy-Tselnicker I et al. (2021) Activity-dependent modulation of synapse-regulating genes in astrocytes. Elife 10. 10.7554/eLife.70514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chai H et al. (2017) Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron 95, 531–549 e539. 10.1016/j.neuron.2017.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Endo F et al. (2022) Molecular basis of astrocyte diversity and morphology across the CNS in health and disease. Science 378, eadc9020. 10.1126/science.adc9020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bayraktar OA et al. (2020) Astrocyte layers in the mammalian cerebral cortex revealed by a single-cell in situ transcriptomic map. Nat Neurosci 23, 500–509. 10.1038/s41593-020-0602-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tan CX et al. (2023) delta-Catenin controls astrocyte morphogenesis via layer-specific astrocyte-neuron cadherin interactions. J Cell Biol 222. 10.1083/jcb.202303138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karpf J et al. (2022) Dentate gyrus astrocytes exhibit layer-specific molecular, morphological and physiological features. Nat Neurosci 25, 1626–1638. 10.1038/s41593-022-01192-5 [DOI] [PubMed] [Google Scholar]
- 82.Viana JF et al. (2023) Astrocyte structural heterogeneity in the mouse hippocampus. Glia 71, 1667–1682. 10.1002/glia.24362 [DOI] [PubMed] [Google Scholar]
- 83.Soto JS et al. (2023) Astrocyte-neuron subproteomes and obsessive-compulsive disorder mechanisms. Nature 616, 764–773. 10.1038/s41586-023-05927-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bindocci E et al. (2017) Three-dimensional Ca(2+) imaging advances understanding of astrocyte biology. Science 356. 10.1126/science.aai8185 [DOI] [PubMed] [Google Scholar]
- 85.Salmon CK et al. (2023) Organizing principles of astrocytic nanoarchitecture in the mouse cerebral cortex. Curr Biol 33, 957–972 e955. 10.1016/j.cub.2023.01.043 [DOI] [PubMed] [Google Scholar]
- 86.Escartin C et al. (2021) Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci 24, 312–325. 10.1038/s41593-020-00783-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Patani R et al. (2023) Functional roles of reactive astrocytes in neuroinflammation and neurodegeneration. Nat Rev Neurol 19, 395–409. 10.1038/s41582-023-00822-1 [DOI] [PubMed] [Google Scholar]
- 88.Yu X et al. (2020) Context-Specific Striatal Astrocyte Molecular Responses Are Phenotypically Exploitable. Neuron 108, 1146–1162 e1110. 10.1016/j.neuron.2020.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Burda JE et al. (2022) Divergent transcriptional regulation of astrocyte reactivity across disorders. Nature 606, 557–564. 10.1038/s41586-022-04739-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen X and Holtzman DM (2022) Emerging roles of innate and adaptive immunity in Alzheimer’s disease. Immunity 55, 2236–2254. 10.1016/j.immuni.2022.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Allen WE et al. (2023) Molecular and spatial signatures of mouse brain aging at single-cell resolution. Cell 186, 194–208 e118. 10.1016/j.cell.2022.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Soreq L et al. (2017) Major Shifts in Glial Regional Identity Are a Transcriptional Hallmark of Human Brain Aging. Cell Rep 18, 557–570. 10.1016/j.celrep.2016.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boisvert MM et al. (2018) The Aging Astrocyte Transcriptome from Multiple Regions of the Mouse Brain. Cell Rep 22, 269–285. 10.1016/j.celrep.2017.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Csemer A et al. (2023) Astrocyte- and NMDA receptor-dependent slow inward currents differently contribute to synaptic plasticity in an age-dependent manner in mouse and human neocortex. Aging Cell 22, e13939. 10.1111/acel.13939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Diaz-Castro B et al. (2021) Molecular and functional properties of cortical astrocytes during peripherally induced neuroinflammation. Cell Rep 36, 109508. 10.1016/j.celrep.2021.109508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hasel P et al. (2021) Neuroinflammatory astrocyte subtypes in the mouse brain. Nat Neurosci 24, 1475–1487. 10.1038/s41593-021-00905-6 [DOI] [PubMed] [Google Scholar]
- 97.Smethurst P et al. (2022) The role of astrocytes in prion-like mechanisms of neurodegeneration. Brain 145, 17–26. 10.1093/brain/awab366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rasmussen MK et al. (2022) Fluid transport in the brain. Physiol Rev 102, 1025–1151. 10.1152/physrev.00031.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sapkota D et al. (2022) Aqp4 stop codon readthrough facilitates amyloid-beta clearance from the brain. Brain 145, 2982–2990. 10.1093/brain/awac199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Simon M et al. (2022) Loss of perivascular aquaporin-4 localization impairs glymphatic exchange and promotes amyloid beta plaque formation in mice. Alzheimers Res Ther 14, 59. 10.1186/s13195-022-00999-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gomolka RS et al. (2023) Loss of aquaporin-4 results in glymphatic system dysfunction via brain-wide interstitial fluid stagnation. Elife 12. 10.7554/eLife.82232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Greenberg SM et al. (2020) Cerebral amyloid angiopathy and Alzheimer disease - one peptide, two pathways. Nat Rev Neurol 16, 30–42. 10.1038/s41582-019-0281-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.de Leon MJ et al. (2017) Cerebrospinal Fluid Clearance in Alzheimer Disease Measured with Dynamic PET. J Nucl Med 58, 1471–1476. 10.2967/jnumed.116.187211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boulay AC et al. (2017) Translation in astrocyte distal processes sets molecular heterogeneity at the gliovascular interface. Cell Discov 3, 17005. 10.1038/celldisc.2017.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Batiuk MY et al. (2020) Identification of region-specific astrocyte subtypes at single cell resolution. Nat Commun 11, 1220. 10.1038/s41467-019-14198-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zeppenfeld DM et al. (2017) Association of Perivascular Localization of Aquaporin-4 With Cognition and Alzheimer Disease in Aging Brains. JAMA Neurol 74, 91–99. 10.1001/jamaneurol.2016.4370 [DOI] [PubMed] [Google Scholar]
- 107.Marsan E et al. (2023) Astroglial toxicity promotes synaptic degeneration in the thalamocortical circuit in frontotemporal dementia with GRN mutations. J Clin Invest 133. 10.1172/JCI164919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Szebenyi K et al. (2021) Human ALS/FTD brain organoid slice cultures display distinct early astrocyte and targetable neuronal pathology. Nat Neurosci 24, 1542–1554. 10.1038/s41593-021-00923-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jiwaji Z et al. (2022) Reactive astrocytes acquire neuroprotective as well as deleterious signatures in response to Tau and Abeta pathology. Nat Commun 13, 135. 10.1038/s41467-021-27702-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee E et al. (2022) A distinct astrocyte subtype in the aging mouse brain characterized by impaired protein homeostasis. Nat Aging 2, 726–741. 10.1038/s43587-022-00257-1 [DOI] [PubMed] [Google Scholar]
- 111.Chandrasekaran A et al. (2021) Astrocytic reactivity triggered by defective autophagy and metabolic failure causes neurotoxicity in frontotemporal dementia type 3. Stem Cell Reports 16, 2736–2751. 10.1016/j.stemcr.2021.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ma Q et al. (2017) Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat Commun 8, 1434. 10.1038/s41467-017-01484-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yamanaka K et al. (2008) Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci 11, 251–253. 10.1038/nn2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Richetin K et al. (2020) Tau accumulation in astrocytes of the dentate gyrus induces neuronal dysfunction and memory deficits in Alzheimer’s disease. Nat Neurosci 23, 1567–1579. 10.1038/s41593-020-00728-x [DOI] [PubMed] [Google Scholar]
- 115.Licht-Murava A et al. (2023) Astrocytic TDP-43 dysregulation impairs memory by modulating antiviral pathways and interferon-inducible chemokines. Sci Adv 9, eade1282. 10.1126/sciadv.ade1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Benraiss A et al. (2021) Cell-intrinsic glial pathology is conserved across human and murine models of Huntington’s disease. Cell Rep 36, 109308. 10.1016/j.celrep.2021.109308 [DOI] [PubMed] [Google Scholar]
- 117.Glasauer SMK et al. (2022) Human tau mutations in cerebral organoids induce a progressive dyshomeostasis of cholesterol. Stem Cell Reports 17, 2127–2140. 10.1016/j.stemcr.2022.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gangwani MR et al. (2023) Neuronal and astrocytic contributions to Huntington’s disease dissected with zinc finger protein transcriptional repressors. Cell Rep 42, 111953. 10.1016/j.celrep.2022.111953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bellaver B et al. (2023) Astrocyte reactivity influences amyloid-beta effects on tau pathology in preclinical Alzheimer’s disease. Nat Med 29, 1775–1781. 10.1038/s41591-023-02380-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Le Douce J et al. (2020) Impairment of Glycolysis-Derived l-Serine Production in Astrocytes Contributes to Cognitive Deficits in Alzheimer’s Disease. Cell Metab 31, 503–517 e508. 10.1016/j.cmet.2020.02.004 [DOI] [PubMed] [Google Scholar]
- 121.Qi G et al. (2021) ApoE4 Impairs Neuron-Astrocyte Coupling of Fatty Acid Metabolism. Cell Rep 34, 108572. 10.1016/j.celrep.2020.108572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tcw J et al. (2022) Cholesterol and matrisome pathways dysregulated in astrocytes and microglia. Cell 185, 2213–2233 e2225. 10.1016/j.cell.2022.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mi Y et al. (2023) Loss of fatty acid degradation by astrocytic mitochondria triggers neuroinflammation and neurodegeneration. Nat Metab 5, 445–465. 10.1038/s42255-023-00756-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bonvento G and Bolanos JP (2021) Astrocyte-neuron metabolic cooperation shapes brain activity. Cell Metab 33, 1546–1564. 10.1016/j.cmet.2021.07.006 [DOI] [PubMed] [Google Scholar]
- 125.Andersen JV et al. (2022) Astrocyte energy and neurotransmitter metabolism in Alzheimer’s disease: Integration of the glutamate/GABA-glutamine cycle. Prog Neurobiol 217, 102331. 10.1016/j.pneurobio.2022.102331 [DOI] [PubMed] [Google Scholar]
- 126.Institoris A et al. (2022) Astrocytes amplify neurovascular coupling to sustained activation of neocortex in awake mice. Nat Commun 13, 7872. 10.1038/s41467-022-35383-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ju YH et al. (2022) Astrocytic urea cycle detoxifies Abeta-derived ammonia while impairing memory in Alzheimer’s disease. Cell Metab 34, 1104–1120 e1108. 10.1016/j.cmet.2022.05.011 [DOI] [PubMed] [Google Scholar]
- 128.Fecher C et al. (2019) Cell-type-specific profiling of brain mitochondria reveals functional and molecular diversity. Nat Neurosci 22, 1731–1742. 10.1038/s41593-019-0479-z [DOI] [PubMed] [Google Scholar]
- 129.Ioannou MS et al. (2019) Neuron-Astrocyte Metabolic Coupling Protects against Activity-Induced Fatty Acid Toxicity. Cell 177, 1522–1535 e1514. 10.1016/j.cell.2019.04.001 [DOI] [PubMed] [Google Scholar]
- 130.Polyzos AA et al. (2019) Metabolic Reprogramming in Astrocytes Distinguishes Region-Specific Neuronal Susceptibility in Huntington Mice. Cell Metab 29, 1258–1273 e1211. 10.1016/j.cmet.2019.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Guttenplan KA et al. (2021) Neurotoxic reactive astrocytes induce cell death via saturated lipids. Nature 599, 102–107. 10.1038/s41586-021-03960-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen ZP et al. (2023) Lipid-accumulated reactive astrocytes promote disease progression in epilepsy. Nat Neurosci 26, 542–554. 10.1038/s41593-023-01288-6 [DOI] [PubMed] [Google Scholar]
- 133.Fleeman RM et al. (2023) Apolipoprotein E epsilon4 modulates astrocyte neuronal support functions in the presence of amyloid-beta. J Neurochem 165, 536–549. 10.1111/jnc.15781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee SI et al. (2021) APOE4-carrying human astrocytes oversupply cholesterol to promote neuronal lipid raft expansion and Abeta generation. Stem Cell Reports 16, 2128–2137. 10.1016/j.stemcr.2021.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Descalzi G et al. (2019) Lactate from astrocytes fuels learning-induced mRNA translation in excitatory and inhibitory neurons. Commun Biol 2, 247. 10.1038/s42003-019-0495-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen MB et al. (2020) Persistent transcriptional programmes are associated with remote memory. Nature 587, 437–442. 10.1038/s41586-020-2905-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Morant-Ferrando B et al. (2023) Fatty acid oxidation organizes mitochondrial supercomplexes to sustain astrocytic ROS and cognition. Nat Metab 5, 1290–1302. 10.1038/s42255-023-00835-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kaplan L et al. (2020) Neuronal regulation of the blood-brain barrier and neurovascular coupling. Nat Rev Neurosci 21, 416–432. 10.1038/s41583-020-0322-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hosli L et al. (2022) Direct vascular contact is a hallmark of cerebral astrocytes. Cell Rep 39, 110599. 10.1016/j.celrep.2022.110599 [DOI] [PubMed] [Google Scholar]
- 140.Palop JJ and Mucke L (2016) Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat Rev Neurosci 17, 777–792. 10.1038/nrn.2016.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mathys H et al. (2019) Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 570, 332–337. 10.1038/s41586-019-1195-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cooper ML et al. (2020) Redistribution of metabolic resources through astrocyte networks mitigates neurodegenerative stress. Proc Natl Acad Sci U S A 117, 18810–18821. 10.1073/pnas.2009425117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Semyanov A and Verkhratsky A (2021) Astrocytic processes: from tripartite synapses to the active milieu. Trends Neurosci 44, 781–792. 10.1016/j.tins.2021.07.006 [DOI] [PubMed] [Google Scholar]
- 144.Koh W et al. (2023) GABA tone regulation and its cognitive functions in the brain. Nat Rev Neurosci 24, 523–539. 10.1038/s41583-023-00724-7 [DOI] [PubMed] [Google Scholar]
- 145.Cho FS et al. (2022) Enhancing GAT-3 in thalamic astrocytes promotes resilience to brain injury in rodents. Sci Transl Med 14, eabj4310. 10.1126/scitranslmed.abj4310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Romanos J et al. (2019) Differences in glutamate uptake between cortical regions impact neuronal NMDA receptor activation. Commun Biol 2, 127. 10.1038/s42003-019-0367-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Broadhead MJ et al. (2022) Selective vulnerability of tripartite synapses in amyotrophic lateral sclerosis. Acta Neuropathol 143, 471–486. 10.1007/s00401-022-02412-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tzioras M et al. (2023) Human astrocytes and microglia show augmented ingestion of synapses in Alzheimer’s disease via MFG-E8. Cell Rep Med 4, 101175. 10.1016/j.xcrm.2023.101175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dejanovic B et al. (2022) Complement C1q-dependent excitatory and inhibitory synapse elimination by astrocytes and microglia in Alzheimer’s disease mouse models. Nat Aging 2, 837–850. 10.1038/s43587-022-00281-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vainchtein ID et al. (2018) Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science 359, 1269–1273. 10.1126/science.aal3589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sanchez-Mico MV et al. (2021) Amyloid-beta impairs the phagocytosis of dystrophic synapses by astrocytes in Alzheimer’s disease. Glia 69, 997–1011. 10.1002/glia.23943 [DOI] [PubMed] [Google Scholar]
- 152.Lattke M et al. (2021) Extensive transcriptional and chromatin changes underlie astrocyte maturation in vivo and in culture. Nat Commun 12, 4335. 10.1038/s41467-021-24624-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Baier MP et al. (2022) Selective ablation of Sod2 in astrocytes induces sex-specific effects on cognitive function, D-serine availability, and astrogliosis. J Neurosci 42, 5992–6006. 10.1523/JNEUROSCI.2543-21.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Luengo-Mateos M et al. (2023) Hypothalamic astrocytic-BMAL1 regulates energy homeostasis in a sex-dependent manner. Cell Rep 42, 112949. 10.1016/j.celrep.2023.112949 [DOI] [PubMed] [Google Scholar]
- 155.Arnaud L et al. (2022) APOE4 drives inflammation in human astrocytes via TAGLN3 repression and NF-kappaB activation. Cell Rep 40, 111200. 10.1016/j.celrep.2022.111200 [DOI] [PubMed] [Google Scholar]
- 156.Jimenez-Blasco D et al. (2020) Glucose metabolism links astroglial mitochondria to cannabinoid effects. Nature 583, 603–608. 10.1038/s41586-020-2470-y [DOI] [PubMed] [Google Scholar]
- 157.Morant-Ferrando B et al. (2023) Fatty acid oxidation organizes mitochondrial supercomplexes to sustain astrocytic ROS and cognition. Nat Metab. 10.1038/s42255-02300835-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rasmussen RN et al. (2023) Astrocytes: integrators of arousal state and sensory context. Trends Neurosci 46, 418–425. 10.1016/j.tins.2023.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li J et al. (2021) Conservation and divergence of vulnerability and responses to stressors between human and mouse astrocytes. Nat Commun 12, 3958. 10.1038/s41467-021-24232-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Preman P et al. (2021) Human iPSC-derived astrocytes transplanted into the mouse brain undergo morphological changes in response to amyloid-beta plaques. Mol Neurodegener 16, 68. 10.1186/s13024-021-00487-8 [DOI] [PMC free article] [PubMed] [Google Scholar]


