Abstract
Background
Immuno‐oncology has been focused on T cell‐centric approaches until the field recently started appreciating the importance of tumor‐reactive antibody production by tumor‐infiltrating plasma B cells, and the necessity of developing novel therapeutic antibodies for the treatment of different cancers.
Recent Findings
B lymphocytes often infiltrate solid tumors and the extent of B cell infiltration normally correlates with stronger T cell responses while generating humoral responses against malignant progression by producing tumor antigens‐reactive antibodies that bind and coat the tumor cells and promote cytotoxic effector mechanisms, reiterating the fact that the adaptive immune system works by coordinated humoral and cellular immune responses. Isotypes, magnitude, and the effector functions of antibodies produced by the B cells within the tumor environment differ among cancer types. Interestingly, apart from binding with specific tumor antigens, antibodies produced by tumor‐infiltrating B cells could bind to some non‐specific receptors, peculiarly expressed by cancer cells. Antibody‐based immunotherapies have revolutionized the modalities of cancer treatment across the world but are still limited against hematological malignancies and a few types of solid tumor cancers with a restricted number of targets, which necessitates the expansion of the field to have newer effective targeted antibody therapeutics.
Conclusion
Here, we discuss about recent understanding of the protective spontaneous antitumor humoral responses in human cancers, with an emphasis on the advancement and future perspectives of antibody‐based immunotherapies in cancer.
Keywords: antibody‐based immunotherapies, immunoglobulin class‐switching, tumor microenvironment, tumor‐infiltrating B lymphocytes
1. INTRODUCTION
It is well accepted in the field of infection immunology that the two arms of the adaptive immune system in humans, formally known as humoral and cell‐mediated immune responses, work in coordination to elicit a sustained, robust protective immune response. Surprisingly, humoral response driven by antibodies produced by tumor‐infiltrating B lymphocytes (TIL‐B) was correlated with disease progression in fast‐progressing murine models 1 , 2 that do not recapitulate the complex immune response network in humans. Conversely, all recent evidence correlating the extent of B cell infiltration in human solid tumors and survival of patients in multiple cancer types indicated that increased TIL‐B accumulation in the tumor microenvironment and associated humoral responses predict superior patient survival. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 The effector functions of antibodies produced by the TIL‐B cells depend on the magnitude of different isotypes of antibodies produced. 17
Studies with major solid tumor cancer types have shown that class‐switched IgG and IgA antibodies dominate the milieu of antibodies in tumor beds. 13 , 14 , 18 , 19 , 20 , 21 Conditions that promote one or the other type of class‐switched antibody production are still elusive. Recent reports have shown that the main antibody‐producing machinery in solid tumors is known as tertiary lymphoid structures (TLS), 22 , 23 , 24 which are specialized germinal center‐like structures formed by orchestration of conglomerates of B cells and surrounding T cells. 22 , 25 Intratumoral TGF‐β silences Satb1, a known genomic organizer, which warrants T follicular helper cell (TFH) differentiation, and adoptive transfer of TFH cells induces intratumoral TLS formation and decreases tumor growth in vivo. 25 One area of utmost importance is the effectiveness of these auto‐antibodies to govern disease progression. Strikingly, despite extensive spontaneous production of IgG antibodies at tumor beds, the magnitude of the response is not dramatic. In human serous ovarian cancer and endometrial cancer, although IgA is the dominant class of antibody, IgG is also extensively produced. 13 , 14 So far, virtually all approved therapeutic antibody preparations for clinical practice including for cancer treatment are in IgG backbone, 26 while recently the importance of implementing dimeric IgA‐based therapeutic antibodies has been underscored. 27 , 28
In this review, we have summarized a comprehensive, up‐to‐date understanding of how the antibody‐producing B cells function within the tumor microenvironment, how the class‐switched antibody production is governed and its outcome, and the crosstalk of B lymphocytes with other immune cells in the tumor microenvironment. We have also discussed about major therapeutic antibodies in clinical practice and future prospects of targeting intracellular targets by specialized dimeric IgA antibodies or engineered IgG antibodies.
2. FUNCTION OF B CELLS AND PLASMA CELLS IN THE TUMOR MICROENVIRONMENT
Apart from a few instances where research groups have focused on IL‐10‐producing regulatory B lymphocytes in cancers 29 , 30 and correlated with immunosuppression, 31 , 32 growing converging evidence confirms that TIL‐B and their antibodyproducing plasma cell subsets predict improved outcomes of patients in multiple cancer types. 7 , 8 , 9 , 13 , 14 , 33 The biological function of B lymphocytes includes antigen presentation to CD4 helper T cells as antigen‐presenting cells 34 , 35 along with their principal function of antibody production when stimulated by cytokines, produced by activated CD4 T cells. 36 Besides some virus‐induced cancer models, 37 the comprehensive understanding of the antigen presentation function of B cells in solid tumor malignancies is still lacking. In epithelial ovarian cancer, increasing immunogenic advancement of the disease positively correlates with the amount of B cells in the tumor and the extent of antibody production by plasma cells. 38 In human endometrial cancer 14 and high‐grade serous ovarian cancer, 13 increased infiltration of B lymphocytes has been associated with superior patient survival. Correspondingly, in other epithelial carcinomas, such as in human breast, 10 , 21 lung, 18 hepatocellular, 5 and colorectal cancers, 3 , 4 B cell infiltration has been associated with improved response to therapies and better survival. Equally important, B cell infiltration in non‐epithelial cancers such as sarcoma 9 and melanoma 7 is also a predictor of increased survival and therapy responses in patients. Helmink B. and colleagues demonstrated among melanoma patients that, individuals with higher mRNA expression for B cell‐ and antibody‐related genes such as marginal zone b and b1 cell‐specific protein, immunoglobulin lambda like polypeptide, or J‐chain, in the cancer tissues, positively correlate with response to immune checkpoint blockade therapies and survive more. 7 Petitprez F. et al showed that B cells drive immunogenicity in soft tissue sarcoma, and they also demonstrated that B cell signatures in the tumor are associated with better responses to immunotherapies and survival outcomes in patients. 9
The main antibody‐producing machinery in solid tumors is lymph node‐like structures formally known as TLS. Virtually in all instances, researchers have found over the years that a higher number of TIL‐B cells positively correlates with the size and density of TLS germinal centers, in different human cancer types. 39 , 40 , 41 However, the types and extent of different immunoglobulin isotypes produced by TIL‐B cells are not consistent across the cancer types, 42 and largely determine the effector functions of spontaneous humoral responses in cancer. Though others have found that plasma cells in the tumor are localized both inside and outside of the TLS, 43 , 44 and both in tumor islets and stroma, 45 , 46 Mazor and colleagues interestingly have demonstrated that tumor‐reactive IgG antibody‐producing plasma cells localize adjacent to the CD20+ B cell cluster in the TLS34. While the cornerstone for TLS formation in epithelial cancers is TFH cells, 25 , 47 both B lymphocytes and TFH cells get accumulated in the tumor microenvironment by a common chemokine, CXCL13 48 because both cell types express CXCR5, 49 the only known receptor of CXCL13. 50 , 51 , 52 , 53 The extent of antibody‐dependent cytotoxic or phagocytic killing of tumor cells and complement fixation depend on the ratio of the different antibody isotypes produced by the TLS‐orchestrated TIL‐B cells. Therapeutic interventions that enhance the formation and maturation of TLS could boost spontaneous, tumor‐destroying humoral responses.
3. ANTIBODY ISOTYPES, CLASS‐SWITCHING, AND THEIR FUNCTION IN SOLID TUMOR CANCERS
Antibodies, in nature, can be one of the five different classes. 54 Naïve B cells produce IgM and IgD 55 while antigen experiencing of B cells drives class‐switching through V‐(D)‐J recombination 56 and production of the other three isotypes, IgG, IgA and IgE. 56 Immunoglobulin isotype switching from constant‐μ (IgM) or constant‐δ (IgD) to downstream isotypes constant‐α (IgA) or constant‐γ (IgG) or constant‐ε (IgE) occurs by an event known as class switch recombination (CSR), which is essentially an intra‐chromosomal deletional recombination whereas the change from IgM to IgD happens through a process known as alternative splicing of the constant‐μ to constant‐δ genes. 57 , 58 Each isotype, except IgD, has a unique genomic region, known as the switch (S) region, upstream of each of the constant heavy regions known as the S regions. S regions (~1–12 kb long) are composed of 20‐80 bp long, G‐rich tandem repeats. 59 , 60 The process of CSR, occurs by end‐joining recombination, rather than by homologous recombination, 61 , 62 involves recombination between the S regions, and results in a change from IgM/IgD on naive B cells to one of the downstream isotypes (IgA/IgG or IgE). 59 , 60
Among the five antibody isotypes, IgG, IgD, and IgE only form monomers while secreted IgA and IgM form dimers and pentamers, respectively. 54 The basic structure of all antibodies includes a pair of heavy chain and light chain. 54 Though the antigen‐specificity of all isotypes of immunoglobulin is determined by the variable region sequences in the heavy and light chains, more precisely, by the complementarity determining regions (CDRs), 54 their function is determined by their Fc regions which also define their isotypes. 54 The major effector functions of antibodies are antibody‐dependent cell‐mediated cytotoxicity and phagocytosis (ADCC and ADCP) through the accumulation of cytotoxic NK cells, neutrophils, or macrophages, promoting opsonization and through activation of the complement cascade. 54 Different antibody isotypes have different levels of abilities to drive ADCC/ADCP, opsonization or complement fixation. 54
The two major isotypes present in human solid tumor cancers are IgG and IgA. 13 , 14 , 21 , 27 , 63 Among the four subclasses of IgG antibodies (IgG1, IgG2, IgG3, and IgG4), IgG1 is the most efficient one for promoting ADCC/ADCP. 64 All IgG antibodies fix complement except IgG4, 65 while IgG2 does not function as an opsonin like other IgG subtypes. 65 IgA is the most abundant antibody produced in humans, 66 and it is the second most abundant in blood, 66 while more than ninety percent of total IgA belongs to the IgA1 subclass. For polymeric IgA and IgM, the Fc chains of individual monomers are brought together by another small protein known as the J chain. 54 A receptor expressed on the basolateral surfaces of mucosal epithelium, known as polymeric immunoglobulin receptor (PIGR) binds with the J chain of polymeric IgA/IgM 54 and governs transcytosis of the antibody 67 where the antibody gets complexed with a fragment of PIGR, known as a secretory component, and released from the apical surface of the mucosal epithelium. 67 By this mechanism, polymeric IgA antibodies clear viruses from virally infected cells. 68 , 69 On the other hand, fetal neonatal receptor (FCRN), expressed by placental epithelium helps in transcytosis of IgG antibodies from the mother to the growing fetus. 70 , 71 , 72 Recent studies suggest that PIGR (and perhaps FCRN) is expressed in several cancer tissues. 73 While dimeric IgA binds PIGR expressed on the mucosal epithelium or cancer epithelial cells, 67 monomeric IgA specifically binds to FcαRI (CD89) 74 and Fcα/μR (CD351) 75 while IgG antibodies bind to Fc gamma receptors (FcγRs). 54 The significance of aberrant expression of some of these receptors within the tumor microenvironment is still elusive. Theoretically, some of these receptors could sequester tumor‐reactive antibodies, produced by TIL‐B cells, by re‐routing their binding with specific tumor antigens. However, binding of dimeric IgA antibodies with PIGR, which is quasi‐universally expressed by virtually all epithelial cancers, elicits transcytosis of dimeric IgA that upregulates DUSP phosphatases, which, in turn, dephosphorylates ERK1/2 and thereby dampens RAS pathway, and also sensitizes the cancer cells for MHC‐independent killing by cytotoxic T cells. 13 , 76 IgA‐PIGR interaction also thwarts DNA repair pathways and ER stress response proteins such as CHOP. 14 Reportedly, CD47–SIRPα checkpoint blockade enhances tumor cell killing by neutrophils, induced by IgA. 77 Very recently it has been demonstrated that utilizing the tumor‐penetrating abilities of dimeric IgA, intracellular mutated oncodrivers‐specific dimeric IgA antibodies could target and expel the oncoproteins out of PIGR+ cancer cells. 27 The consequence of FCRN expression in cancer is yet to be clearly understood. FCRN is expressed by breast cancer cells which is retained in metastatic cells found in draining lymph nodes, 78 and downregulation of FCRN is associated with aggressive disease progression. 79 Similarly, in hepatocellular carcinoma 80 and non‐small cell lung cancer, 81 decreased FCRN expression is associated with worse outcome in patients. Interestingly, though NK cells do not express FCRN, fcgrt −/− mice show impaired NK cell development, maturation, and interferon secretion with increased melanoma metastasis into the lung. 82 , 83 Presumably, NK cell development and maturation need neighboring FCRN+ immune cells.
4. CROSSTALK OF B LYMPHOCYTES WITH OTHER IMMUNE CELLS WITHIN THE TUMOR MICROENVIRONMENT
B cells in the tumor microenvironment do not work alone, rather they work closely with T cells. B lymphocytes and T cells orchestrate to form specialized germinal center‐like structures, formally known as TLS. Maturation of TLS into an antibody‐producing germinal center is characterized by the presence of high endothelial venules 84 , 85 and requires expression of BCL6, 86 GL7 25 and cytokine LIGHT. 25 In classical TLS, B cells are normally found in the center while T cells are on the periphery (Figure 1A). However, in murine ovarian cancer model, it has been demonstrated that in early developmental stages, B cells rather make the periphery surrounding the central T cell zone. 25 The intimate crosstalk between B cells and T lymphocytes in the TLS determines the extent of humoral response within the tumor. The formation of TLS is governed mainly by the T follicular helper (TFH) cells 25 , 47 and chemokine CXCL13 48 (Figure 1A). While TIL‐B cells' activity is positively correlated with effective T cell responses, B cell exhaustion often correlates with the frequency of FOXP3+ regulatory T cells (Tregs). 87 Apart from interaction with T cell subsets, much is not known yet about the interactions of TIL‐B cells with other immune cells in the tumor microenvironment. Among other cells in the tumor microenvironment, NK cells could promote B cell activation and antibody class‐switching by CD40‐CD40L interaction and IFN‐γ production 88 , 89 (Figure 1B). A major proportion of human γδ cells, (Vγ9Vδ2 cells) express CXCR5 and therefore could co‐infiltrate the tumor microenvironment with CXCR5+ B cells 90 (Figure 1A). Similar to NK cells, γδ cells also promote class‐switched antibody production 90 , 91 (Figure 1B). Nonetheless, tumor‐associated macrophages (TAMs) secrete B cells‐activating factor (BAFF) and a proliferation‐inducing ligand (APRIL) and govern B cell proliferation, 92 and also support B cell development by secreting IL‐6 93 (Figure 1B). Chen Z et al found that TAMs drive IgG‐producing plasma cells in hepatocellular carcinoma. 63 In a very intriguing study, Shaul M et al have demonstrated that the differentiation of tumor‐infiltrating CD45+B220+CD138− B cells into IgG‐producing B220+CD138+ plasma cells requires physical contact with tumor‐associated neutrophils, which they found to be independent of the involvement of tumor‐infiltrating T cells. 94 Notably, they have also found that tumor‐associated neutrophils drive B cell recruitment to the tumor microenvironment by secreting TNF‐α. 94 In a mouse model of lung cancer, Wang Y et al demonstrated that with an increasing number of myeloid‐derived suppressor cells (MDSCs)‐infiltration into the tumor mass they observed impaired B cell functions, indicated by decreased serum IgG levels. 95 MDSCs 96 in the tumor microenvironment force B cell differentiation into IL‐10, IL‐35, and TGF‐β‐producing 97 immunosuppressive regulatory B cells (Bregs), 98 , 99 however, Bregs do not have any unique identification marker like FOXP3 for Tregs, and therefore the classification of Bregs among total B cells is elusive.
FIGURE 1.
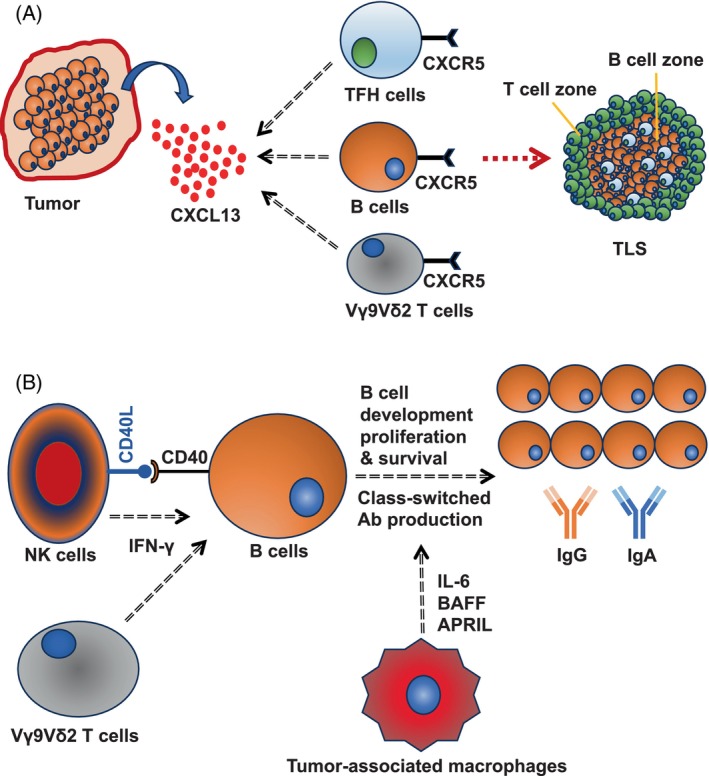
Tumor microenvironment and B lymphocytes. (A) CXCL13, secreted in the tumor microenvironment, attracts CXCR5+ B cells, CXCR5+ TFH cells, and CXCR5+ Vγ9Vδ2 γδ T cells that together promote formation of tertiary lymphoid structures. (B) NK cells promote B cell activation by engaging with CD40 on B cell surface through CD40L. NK cells and γδ T cells promote B cell class‐switching by secreting IFN‐γ. Tumor‐associated macrophages promote B cell development, proliferation, and survival by secreting B cells‐activating factor (BAFF) and a proliferation‐inducing ligand (APRIL).
5. THERAPEUTIC ANTIBODIES IN THE CLINICS AND FUTURE PERSPECTIVES
Despite the huge early success of two monoclonal antibody‐based immunotherapies for cancer, rituximab 100 , 101 and trastuzumab 102 , 103 against CD20‐positive B cell lymphoma and Her2positive estrogen receptor (ER)/progesterone receptor (PR)negative breast cancer, respectively, the field of immunotherapy research gradually became more focused on cellular therapies such as adoptive TIL therapy, 104 , 105 CAR T cells therapy, 106 , 107 CAR NK therapy 108 , 109 etc. after their success in solid tumors. The importance and effectiveness of these cellular therapies are unquestionable, however, the cost of such treatments is very expensive in their current states 110 while antibody‐based therapies are significantly cost‐effective and therefore affordable to a larger proportion of eligible patients. Primarily the effector functions of immunotherapeutic antibodies against cancer lie in their ability to induce ADCC/ADCP or complement fixation. Rituximab, a monoclonal IgG1 antibody, binds with CD20 on malignant B cells and induces NK cells‐ and macrophage‐mediated killing or complement‐mediated lysis of the cancer cells. 111 Similarly, Daratumumab, a monoclonal IgG1 antibody, binds with CD38 on myeloma cells and is extensively used for treating multiple myeloma patients. 112 Resistance to rituximab in relapsed refractory lymphoma patients is common 113 and therefore alternative approaches have been introduced later in the clinical practice, for example targeting CD79b by Polatuzumab 114 or using bi‐specific antibodies, such as Glofitamab which is a CD20‐directed, CD3 T cells engager. 115 , 116 Trastuzumab, which is also an IgG1 monoclonal antibody, works in a similar fashion to Rituximab or Daratumumab by binding with surface protein Her2 on breast cancer cells and is a life‐saving drug for ER/PRnegative Her2positive breast cancer patients. 117 Apart from killing target cancer cells, rescuing antitumor cytotoxic functions of TILs resulted in significant increase in antitumor immunities and corresponding reduction in cancer progression by targeting CTLA‐4 118 , 119 and PD‐1 120 , 121 by monoclonal antibodies were demonstrated in parallel by James P. Allison and colleagues; and Tasuku Honjo and associates, respectively, popularly known as immune checkpoint blockade (ICB) therapies, which produced the Joint‐Nobel prize in physiology or medicine in the year 2018. 122 The anti‐PD‐1 antibodies (Nivolumab or Pembrolizumab) are made in IgG4 backbone, 123 , 124 to annihilate the elimination of antibody‐bound PD‐1 positive T cells by ADCC/ADCP or complement, though the anti‐CTLA‐4 antibody, Ipilimumab, was developed in human IgG1 backbone. 125
The field started considering antibody‐mediated immunotherapies more seriously for solid tumor cancer treatments after the success of checkpoint inhibitor monoclonal antibodies in the clinics against PD‐1, CTLA‐4, and PD‐L1 (ligand of PD‐1). 126 , 127 , 128 , 129 Approaches for targeting intracellular oncoproteins and mutated oncodrivers have been restricted to the development of novel small molecule inhibitors. 130 Some of them are like different EGFR inhibitors. 131 recently FDA‐approved Sotorasib 132 targeting G12C‐mutated KRAS or MRTX1133 133 targeting KRASG12D which is awaiting FDA‐approval. Unfortunately, small‐molecule inhibitors have a smaller half‐life than antibodies 134 and the development of resistance is very common. 135 , 136 Dimeric IgA antibodies, which bind naturally expressed PIGR 137 on the basolateral surface of mucosal epithelium, through their J‐chain, 54 and undergo transcytosis by taking a portion of PIGR (known as secretory component), and released by the apical surface, 67 and through this mechanism they clear viruses from the gut. 68 , 69 Notably, PIGR is heavily expressed in a majority of the epithelial cancer types and it has been exhibited that dimeric IgA can also undergo PIGR‐driven transcytosis through cancer epithelial cells, 13 , 14 which instigated their use for targeting intracellular oncoproteins. Recent findings demonstrated that specific dimeric IgA antibodies, but not the same antibodies in IgG backbone, against G12D mutation of KRAS and R132H mutation of IDH1, could target KRASG12D and IDH1R132H, respectively, inside the cancer cells harboring these mutations and significantly abrogate tumor progression in vivo 27 , 138 (Figure 2). This opens a novel opportunity to develop dimeric IgA‐based (or perhaps engineered IgG with the ability to bind and internalize with PIGR) therapeutic antibodies against virtually any intracellular oncoproteins. In future, strategies such as developing antibodies permeable through the cell membrane and reachable to the subcellular locations like the nucleus (for example against mutant p53) could shift the paradigm of antibody‐bases immunotherapies against cancer and other diseases.
FIGURE 2.
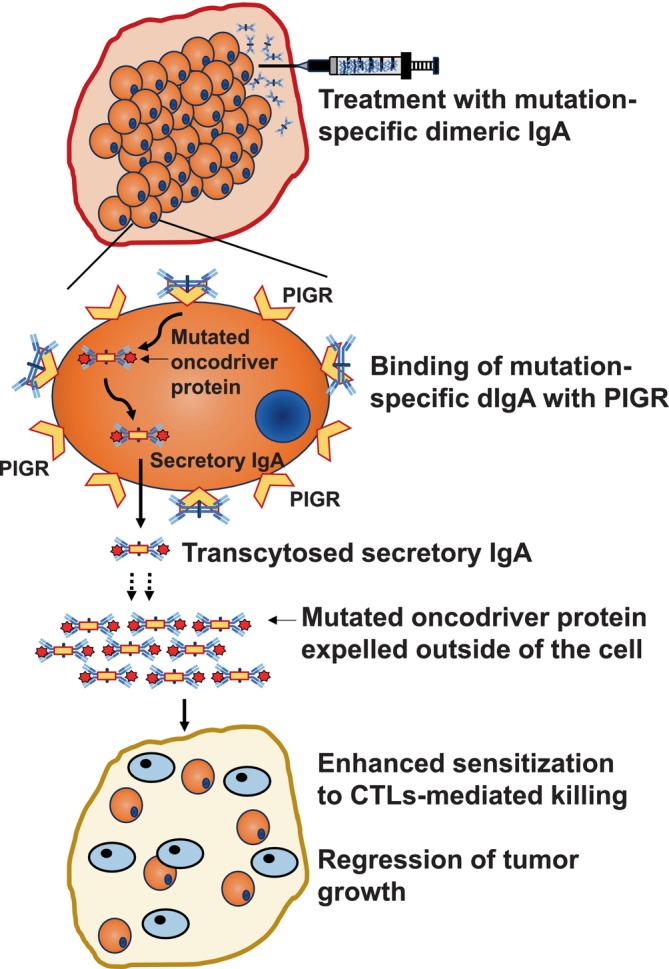
Intracellular targeting of mutated oncoproteins by dimeric IgA antibodies. Mutation‐specific dimeric IgA antibodies can bind with PIGR expressed on the epithelial cancer cells of major cancer types and get internalized with a fragment of PIGR known as the secretory component. Internalized dimeric IgA antibodies encounter target antigens in the cytoplasm and could haul them outside of the cells through transcytosis whereby the mutated oncodrivers could be expelled into the extracellular space by secretory IgA. Treatment of PIGR+ tumor cells sensitizes them for CD8+ cytotoxic T cell‐mediated killing.
6. CONCLUDING REMARKS
Though the role of T cells in antitumor immunity is indisputable, it is time to appreciate the importance of coordinated humoral and cellular immune response in human malignancies and look for strategies that induce sustained, robust antitumor immune responses by driving simultaneously both the arms of immune system. Apart from the suitability of targeting intracellular targets by dimeric IgA antibodies, their suitability to synergize with existing immunotherapies and chemotherapies should also be evaluated. Previously it has been shown that non‐antigen specific dimeric IgA could enhance T cell‐mediated killing of PIGR‐expressing cancer cells in an MHC‐I independent manner, therefore, there is a high chance that therapeutic use of dimeric IgA antibodies could also promote cytotoxic killing by CD8 T cells, in addition to ADCC/ADCP. The development of penetrable IgG antibodies is also important, pertinent to the fact that IgG antibodies have a much higher half‐life, and therefore industrial production of monomeric IgG is cost‐effective than dimeric IgA antibodies.
AUTHOR CONTRIBUTIONS
Gunjan Mandal: Conceptualization; funding acquisition; writing – original draft; supervision; visualization; writing – review and editing; methodology. Suchismita Pradhan: Writing – original draft; writing – review and editing.
CONFLICT OF INTEREST STATEMENT
The authors do not have any conflict of interest.
ACKNOWLEDGMENTS
We sincerely acknowledge Institute of Life Sciences and the Department of Biotechnology, Govt. of India for providing core funding support.
Mandal G, Pradhan S. B cell responses and antibody‐based therapeutic perspectives in human cancers. Cancer Reports. 2024;7(3):e2056. doi: 10.1002/cnr2.2056
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Andreu P, Johansson M, Affara NI, et al. FcRgamma activation regulates inflammation‐associated squamous carcinogenesis. Cancer Cell. 2010;17:121‐134. doi: 10.1016/j.ccr.2009.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Visser KE, Korets LV, de Coussens LM. Novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411‐423. doi: 10.1016/j.ccr.2005.04.014 [DOI] [PubMed] [Google Scholar]
- 3. Meshcheryakova A, Tamandl D, Bajna E, et al. B cells and ectopic follicular structures: novel players in anti‐tumor programming with prognostic power for patients with metastatic colorectal cancer. PLoS One. 2014;9:e99008. doi: 10.1371/journal.pone.0099008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Richards CH, Flegg KM, SD Roxburgh C, et al. The relationships between cellular components of the peritumoural inflammatory response, clinicopathological characteristics and survival in patients with primary operable colorectal cancer. Br J Cancer. 2012;106:2010‐2015. doi: 10.1038/bjc.2012.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang Z, Ma L, Goswami S, et al. Landscape of infiltrating B cells and their clinical significance in human hepatocellular carcinoma. Onco Targets Ther. 2019;8:e1571388. doi: 10.1080/2162402X.2019.1571388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ladanyi A. Prognostic and predictive significance of immune cells infiltrating cutaneous melanoma. Pigment Cell Melanoma Res. 2015;28:490‐500. doi: 10.1111/pcmr.12371 [DOI] [PubMed] [Google Scholar]
- 7. Helmink BA, Reddy SM, Gao J, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577:549‐555. doi: 10.1038/s41586-019-1922-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cabrita R, Lauss M, Sanna A, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577:561‐565. doi: 10.1038/s41586-019-1914-8 [DOI] [PubMed] [Google Scholar]
- 9. Petitprez F, de Reyniès A, Keung EZ, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020;577:556‐560. doi: 10.1038/s41586-019-1906-8 [DOI] [PubMed] [Google Scholar]
- 10. Zhang Z, Zhu Y, Wang Z, Zhang T, Wu P, Huang J. Yin‐yang effect of tumor infiltrating B cells in breast cancer: from mechanism to immunotherapy. Cancer Lett. 2017;393:1‐7. doi: 10.1016/j.canlet.2017.02.008 [DOI] [PubMed] [Google Scholar]
- 11. Nielsen JS, Sahota RA, Milne K, et al. CD20+ tumor‐infiltrating lymphocytes have an atypical CD27‐ memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clinic Cancer Res. 2012;18:3281‐3292. doi: 10.1158/1078-0432.CCR-12-0234 [DOI] [PubMed] [Google Scholar]
- 12. Cillo AR, Kürten CHL, Tabib T, et al. Immune landscape of viral‐ and carcinogen‐driven head and neck cancer. Immunity. 2020;52:183‐199. doi: 10.1016/j.immuni.2019.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Biswas S, Mandal G, Payne KK, et al. IgA transcytosis and antigen recognition govern ovarian cancer immunity. Nature. 2021;591:464‐470. doi: 10.1038/s41586-020-03144-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mandal G, Biswas S, Anadon CM, et al. IgA‐dominated humoral immune responses govern Patients' outcome in endometrial cancer. Cancer Res. 2022;82:859‐871. doi: 10.1158/0008-5472.Can-21-2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Handley KF, Mehta S, Martin AL, et al. Actionable spontaneous antibody responses antagonize malignant progression in ovarian carcinoma. Gynecol Oncol. 2023;173:114‐121. doi: 10.1016/j.ygyno.2023.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Laumont CM, Banville AC, Gilardi M, Hollern DP, Nelson BH. Tumour‐infiltrating B cells: immunological mechanisms, clinical impact and therapeutic opportunities. Nat Rev Cancer. 2022;22:414‐430. doi: 10.1038/s41568-022-00466-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kretschmer A, Schwanbeck R, Valerius T, Rösner T. Antibody isotypes for tumor immunotherapy. Transfus Med Hemother. 2017;44:320‐326. doi: 10.1159/000479240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Germain C, Gnjatic S, Tamzalit F, et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med. 2014;189:832‐844. doi: 10.1164/rccm.201309-1611OC [DOI] [PubMed] [Google Scholar]
- 19. Shalapour S, Lin XJ, Bastian IN, et al. Inflammation‐induced IgA+ cells dismantle anti‐liver cancer immunity. Nature. 2017;551:340‐345. doi: 10.1038/nature24302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Montfort A, Pearce O, Maniati E, et al. A strong B‐cell response is part of the immune landscape in human high‐grade serous ovarian metastases. Clinic Cancer Res. 2017;23:250‐262. doi: 10.1158/1078-0432.Ccr-16-0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garaud S, Zayakin P, Buisseret L, et al. Antigen specificity and clinical significance of IgG and IgA autoantibodies produced in situ by tumor‐infiltrating B cells in breast cancer. Front Immunol. 2018;9:2660. doi: 10.3389/fimmu.2018.02660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Domblides C, Rochefort J, Riffard C, et al. Tumor‐associated tertiary lymphoid structures: from basic and clinical knowledge to therapeutic manipulation. Front Immunol. 2021;12:698604. doi: 10.3389/fimmu.2021.698604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Teillaud JL, Dieu‐Nosjean MC. Tertiary lymphoid structures: an anti‐tumor School for Adaptive Immune Cells and an antibody factory to fight cancer? Front Immunol. 2017;8:830. doi: 10.3389/fimmu.2017.00830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pimenta EM, Barnes BJ. Role of tertiary lymphoid structures (TLS) in anti‐tumor immunity: potential tumor‐induced cytokines/chemokines that regulate TLS formation in epithelial‐derived cancers. Cancers. 2014;6:969‐997. doi: 10.3390/cancers6020969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chaurio RA, Anadon CM, Lee Costich T, et al. TGF‐β‐mediated silencing of genomic organizer SATB1 promotes Tfh cell differentiation and formation of intra‐tumoral tertiary lymphoid structures. Immunity. 2022;55:115‐128.e119. doi: 10.1016/j.immuni.2021.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu J, Song Y, Tian W. How to select IgG subclasses in developing anti‐tumor therapeutic antibodies. J Hematol Oncol. 2020;13:45. doi: 10.1186/s13045-020-00876-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Biswas S, Mandal G, Anadon CM, et al. Targeting intracellular oncoproteins with dimeric IgA promotes expulsion from the cytoplasm and immune‐mediated control of epithelial cancers. Immunity. 2023;56:2570‐2583.e2576. doi: 10.1016/j.immuni.2023.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sterlin D, Gorochov G. When therapeutic IgA antibodies might come of age. Pharmacology. 2021;106:9‐19. doi: 10.1159/000510251 [DOI] [PubMed] [Google Scholar]
- 29. Sarvaria A, Madrigal JA, Saudemont A. B cell regulation in cancer and anti‐tumor immunity. Cell Mol Immunol. 2017;14:662‐674. doi: 10.1038/cmi.2017.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Piersiala K, Hjalmarsson E, da Silva PFN, et al. Regulatory B cells producing IL‐10 are increased in human tumor draining lymph nodes. Int J Cancer. 2023;153:854‐866. doi: 10.1002/ijc.34555 [DOI] [PubMed] [Google Scholar]
- 31. Fremd C, Schuetz F, Sohn C, Beckhove P, Domschke C. B cell‐regulated immune responses in tumor models and cancer patients. Onco Targets Ther. 2013;2:e25443. doi: 10.4161/onci.25443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schioppa T, Moore R, Thompson RG, et al. B regulatory cells and the tumor‐promoting actions of TNF‐α during squamous carcinogenesis. Proc Natl Acad Sci U S A. 2011;108:10662‐10667. doi: 10.1073/pnas.1100994108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mazor RD, Nathan N, Gilboa A, et al. Tumor‐reactive antibodies evolve from non‐binding and autoreactive precursors. Cell. 2022;185:1208‐1222.e1221. doi: 10.1016/j.cell.2022.02.012 [DOI] [PubMed] [Google Scholar]
- 34. Hong S, Zhang Z, Liu H, et al. B cells are the dominant antigen‐presenting cells that activate naive CD4(+) T cells upon immunization with a virus‐derived nanoparticle antigen. Immunity. 2018;49:695‐708.e694. doi: 10.1016/j.immuni.2018.08.012 [DOI] [PubMed] [Google Scholar]
- 35. Hua Z, Hou B. The role of B cell antigen presentation in the initiation of CD4+ T cell response. Immunol Rev. 2020;296:24‐35. doi: 10.1111/imr.12859 [DOI] [PubMed] [Google Scholar]
- 36. Hoffman W, Lakkis FG, Chalasani G. B cells, antibodies, and more. Clin J Am Soc Nephrol. 2016;11:137‐154. doi: 10.2215/cjn.09430915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schultz KR, Klarnet JP, Gieni RS, HayGlass KT, Greenberg PD. The role of B cells for in vivo T cell responses to a friend virus‐induced leukemia. Science. 1990;249:921‐923. doi: 10.1126/science.2118273 [DOI] [PubMed] [Google Scholar]
- 38. Lundgren S, Berntsson J, Nodin B, Micke P, Jirström K. Prognostic impact of tumour‐associated B cells and plasma cells in epithelial ovarian cancer. J Ovarian Res. 2016;9:21. doi: 10.1186/s13048-016-0232-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Garaud S, Buisseret L, Solinas C, et al. Tumor infiltrating B‐cells signal functional humoral immune responses in breast cancer. JCI Insight. 2019;5:e129641. doi: 10.1172/jci.insight.129641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qin M, Hamanishi J, Ukita M, et al. Tertiary lymphoid structures are associated with favorable survival outcomes in patients with endometrial cancer. Cancer Immunol Immunother. 2022;71:1431‐1442. doi: 10.1007/s00262-021-03093-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Germain C, Devi‐Marulkar P, Knockaert S, et al. Tertiary lymphoid structure‐B cells narrow regulatory T cells impact in lung cancer patients. Front Immunol. 2021;12:626776. doi: 10.3389/fimmu.2021.626776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Playoust E, Remark R, Vivier E, Milpied P. Germinal center‐dependent and ‐independent immune responses of tumor‐infiltrating B cells in human cancers. Cell Mol Immunol. 2023;20:1040‐1050. doi: 10.1038/s41423-023-01060-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Meylan M, Petitprez F, Becht E, et al. Tertiary lymphoid structures generate and propagate anti‐tumor antibody‐producing plasma cells in renal cell cancer. Immunity. 2022;55:527‐541.e525. doi: 10.1016/j.immuni.2022.02.001 [DOI] [PubMed] [Google Scholar]
- 44. Fridman WH, Meylan M, Petitprez F, Sun CM, Italiano A, Sautès‐Fridman C. B cells and tertiary lymphoid structures as determinants of tumour immune contexture and clinical outcome. Nat Rev Clin Oncol. 2022;19:441‐457. doi: 10.1038/s41571-022-00619-z [DOI] [PubMed] [Google Scholar]
- 45. Kroeger DR, Milne K, Nelson BH. Tumor‐infiltrating plasma cells are associated with tertiary lymphoid structures, Cytolytic T‐cell responses, and superior prognosis in ovarian cancer. Clinic Cancer Res. 2016;22:3005‐3015. doi: 10.1158/1078-0432.CCR-15-2762 [DOI] [PubMed] [Google Scholar]
- 46. Yeong J, Lim JCT, Lee B, et al. High densities of tumor‐associated plasma cells predict improved prognosis in triple negative breast cancer. Front Immunol. 2018;9:1209. doi: 10.3389/fimmu.2018.01209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Garaud S, Dieu‐Nosjean MC, Willard‐Gallo K. T follicular helper and B cell crosstalk in tertiary lymphoid structures and cancer immunotherapy. Nat Commun. 2022;13:2259. doi: 10.1038/s41467-022-29753-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ukita M, Hamanishi J, Yoshitomi H, et al. CXCL13‐producing CD4+ T cells accumulate in the early phase of tertiary lymphoid structures in ovarian cancer. JCI Insight. 2022;7:1‐15. doi: 10.1172/jci.insight.157215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moser B, Schaerli P, Loetscher P. CXCR5(+) T cells: follicular homing takes center stage in T‐helper‐cell responses. Trends Immunol. 2002;23:250‐254. doi: 10.1016/s1471-4906(02)02218-4 [DOI] [PubMed] [Google Scholar]
- 50. Biswas S, Sengupta S, Roy Chowdhury S, et al. CXCL13‐CXCR5 co‐expression regulates epithelial to mesenchymal transition of breast cancer cells during lymph node metastasis. Breast Cancer Res Treat. 2014;143:265‐276. doi: 10.1007/s10549-013-2811-8 [DOI] [PubMed] [Google Scholar]
- 51. Mitkin NA, Hook CD, Schwartz AM, et al. p53‐dependent expression of CXCR5 chemokine receptor in MCF‐7 breast cancer cells. Sci Rep. 2015;5:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Biswas S, Chowdhury SR, Mandal G, et al. RelA driven co‐expression of CXCL13 and CXCR5 is governed by a multifaceted transcriptional program regulating breast cancer progression. Biochim Biophys Acta Mol Basis Dis. 2019;1865:502‐511. [DOI] [PubMed] [Google Scholar]
- 53. Legler DF, Loetscher M, Roos RS, Clark‐Lewis I, Baggiolini M, Moser B. B cell‐attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J Exp Med. 1998;187:655‐660. doi: 10.1084/jem.187.4.655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schroeder HW Jr, Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010;125:S41‐S52. doi: 10.1016/j.jaci.2009.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Noviski M, Mueller JL, Satterthwaite A, Garrett‐Sinha LA, Brombacher F, Zikherman J. IgM and IgD B cell receptors differentially respond to endogenous antigens and control B cell fate. Elife. 2018;7:1‐29. doi: 10.7554/eLife.35074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chi X, Li Y, Qiu X. V(D)J recombination, somatic hypermutation and class switch recombination of immunoglobulins: mechanism and regulation. Immunology. 2020;160:233‐247. doi: 10.1111/imm.13176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annu Rev Immunol. 2008;26:261‐292. doi: 10.1146/annurev.immunol.26.021607.090248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stavnezer J, Schrader CE. IgH chain class switch recombination: mechanism and regulation. J Immunol. 2014;193:5370‐5378. doi: 10.4049/jimmunol.1401849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Min IM, Rothlein LR, Schrader CE, Stavnezer J, Selsing E. Shifts in targeting of class switch recombination sites in mice that lack mu switch region tandem repeats or Msh2. J Exp Med. 2005;201:1885‐1890. doi: 10.1084/jem.20042491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dunnick W, Hertz GZ, Scappino L, Gritzmacher C. DNA sequences at immunoglobulin switch region recombination sites. Nucleic Acids Res. 1993;21:365‐372. doi: 10.1093/nar/21.3.365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Manis JP, Tian M, Alt FW. Mechanism and control of class‐switch recombination. Trends Immunol. 2002;23:31‐39. doi: 10.1016/s1471-4906(01)02111-1 [DOI] [PubMed] [Google Scholar]
- 62. Stavnezer J. Antibody class switching. Adv Immunol. 1996;61:79‐146. doi: 10.1016/s0065-2776(08)60866-4 [DOI] [PubMed] [Google Scholar]
- 63. Chen Z, Zhang G, Ren X, et al. Cross‐talk between myeloid and B cells shapes the distinct microenvironments of primary and secondary liver cancer. Cancer Res. 2023;83:3544‐3561. doi: 10.1158/0008-5472.Can-23-0193 [DOI] [PubMed] [Google Scholar]
- 64. Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520. doi: 10.3389/fimmu.2014.00520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dalakas MC. IgG4‐mediated neurologic autoimmunities: understanding the pathogenicity of IgG4, ineffectiveness of IVIg, and Long‐lasting benefits of anti‐B cell therapies. Neurol Neuroimmunol Neuroinflamm. 2022;9:1‐7. doi: 10.1212/nxi.0000000000001116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kerr MA. The structure and function of human IgA. Biochem J. 1990;271:285‐296. doi: 10.1042/bj2710285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wei H, Wang JY. Role of polymeric immunoglobulin receptor in IgA and IgM Transcytosis. Int J Mol Sci. 2021;22:1‐19. doi: 10.3390/ijms22052284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Turula H, Wobus CE. The role of the polymeric immunoglobulin receptor and secretory immunoglobulins during mucosal infection and immunity. Viruses. 2018;10:1‐15. doi: 10.3390/v10050237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mazanec MB, Kaetzel CS, Lamm ME, Fletcher D, Nedrud JG. Intracellular neutralization of virus by immunoglobulin a antibodies. Proc Natl Acad Sci U S A. 1992;89:6901‐6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rodewald R, Kraehenbuhl JP. Receptor‐mediated transport of IgG. J Cell Biol. 1984;99:159s‐164s. doi: 10.1083/jcb.99.1.159s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Story CM, Mikulska JE, Simister NE. A major histocompatibility complex class I‐like fc receptor cloned from human placenta: possible role in transfer of immunoglobulin G from mother to fetus. J Exp Med. 1994;180:2377‐2381. doi: 10.1084/jem.180.6.2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Borghi S, Bournazos S, Thulin NK, et al. FcRn, but not FcγRs, drives maternal‐fetal transplacental transport of human IgG antibodies. Proc Natl Acad Sci U S A. 2020;117:12943‐12951. doi: 10.1073/pnas.2004325117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chae J, Choi J, Chung J. Polymeric immunoglobulin receptor (pIgR) in cancer. J Cancer Res Clin Oncol. 2023;149:17683‐17690. doi: 10.1007/s00432-023-05335-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Monteiro RC, Van De Winkel JG. IgA fc receptors. Annu Rev Immunol. 2003;21:177‐204. doi: 10.1146/annurev.immunol.21.120601.141011 [DOI] [PubMed] [Google Scholar]
- 75. Sakamoto N, Shibuya K, Shimizu Y, et al. A novel fc receptor for IgA and IgM is expressed on both hematopoietic and non‐hematopoietic tissues. Eur J Immunol. 2001;31:1310‐1316. doi: [DOI] [PubMed] [Google Scholar]
- 76. Harjes U. IgA strikes twice in ovarian cancer. Nat Rev Cancer. 2021;21:215. doi: 10.1038/s41568-021-00342-4 [DOI] [PubMed] [Google Scholar]
- 77. Treffers LW, Ten Broeke T, Rösner T, et al. IgA‐mediated killing of tumor cells by neutrophils is enhanced by CD47‐SIRPα checkpoint inhibition. Cancer Immunol Res. 2020;8:120‐130. doi: 10.1158/2326-6066.Cir-19-0144 [DOI] [PubMed] [Google Scholar]
- 78. Cianga P, Cianga C, Cozma L, Ward ES, Carasevici E. The MHC class I related fc receptor, FcRn, is expressed in the epithelial cells of the human mammary gland. Hum Immunol. 2003;64:1152‐1159. doi: 10.1016/j.humimm.2003.08.025 [DOI] [PubMed] [Google Scholar]
- 79. Jansen MP. Molecular classification of tamoxifen‐resistant breast carcinomas by gene expression profiling. J Clin Oncol. 2005;23:732‐740. doi: 10.1200/jco.2005.05.145 [DOI] [PubMed] [Google Scholar]
- 80. Shi L, Zhang W, Zou F, Mei L, Wu G, Teng Y. KLHL21, a novel gene that contributes to the progression of hepatocellular carcinoma. BMC Cancer. 2016;16:815. doi: 10.1186/s12885-016-2851-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dalloneau E, Baroukh N, Mavridis K, et al. Downregulation of the neonatal fc receptor expression in non‐small cell lung cancer tissue is associated with a poor prognosis. Oncotarget. 2016;7:54415‐54429. doi: 10.18632/oncotarget.10074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Castaneda DC, Dhommée C, Baranek T, et al. Lack of FcRn impairs natural killer cell development and functions in the tumor microenvironment. Front Immunol. 2018;9:2259. doi: 10.3389/fimmu.2018.02259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pasero C, Gravis G, Granjeaud S, et al. Highly effective NK cells are associated with good prognosis in patients with metastatic prostate cancer. Oncotarget. 2015;6:14360‐14373. doi: 10.18632/oncotarget.3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jones E, Gallimore A, Ager A. Defining high endothelial Venules and tertiary lymphoid structures in cancer. Methods in Molecular Biology. 2018;1845:99‐118. doi: 10.1007/978-1-4939-8709-2_7 [DOI] [PubMed] [Google Scholar]
- 85. Li Q, Liu X, Wang D, et al. Prognostic value of tertiary lymphoid structure and tumour infiltrating lymphocytes in oral squamous cell carcinoma. Int J Oral Sci. 2020;12:24. doi: 10.1038/s41368-020-00092-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Delvecchio FR, Fincham REA, Spear S, et al. Pancreatic cancer chemotherapy is potentiated by induction of tertiary lymphoid structures in mice. Cell Mol Gastroenterol Hepatol. 2021;12:1543‐1565. doi: 10.1016/j.jcmgh.2021.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bruno TC, Ebner PJ, Moore BL, et al. Antigen‐presenting Intratumoral B cells affect CD4(+) TIL phenotypes in non‐Small cell lung cancer patients. Cancer Immunol Res. 2017;5:898‐907. doi: 10.1158/2326-6066.Cir-17-0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Blanca IR, Bere EW, Young HA, Ortaldo JR. Human B cell activation by autologous NK cells is regulated by CD40‐CD40 ligand interaction: role of memory B cells and CD5+ B cells. J Immunol. 2001;167:6132‐6139. doi: 10.4049/jimmunol.167.11.6132 [DOI] [PubMed] [Google Scholar]
- 89. Yuan D, Koh CY, Wilder JA. Interactions between B lymphocytes and NK cells. FASEB J. 1994;8:1012‐1018. doi: 10.1096/fasebj.8.13.7926365 [DOI] [PubMed] [Google Scholar]
- 90. Caccamo N. CXCR5 identifies a subset of Vgamma9Vdelta2 T cells which secrete IL‐4 and IL‐10 and help B cells for antibody production. J Immunol. 2006;177:5290‐5295. doi: 10.4049/jimmunol.177.8.5290 [DOI] [PubMed] [Google Scholar]
- 91. Schönefeldt S, Wais T, Herling M, et al. The diverse roles of γδ T cells in cancer: from rapid immunity to aggressive lymphoma. Cancer. 2021;13:1‐31. doi: 10.3390/cancers13246212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Craxton A, Magaletti D, Ryan EJ, Clark EA. Macrophage‐ and dendritic cell—dependent regulation of human B‐cell proliferation requires the TNF family ligand BAFF. Blood. 2003;101:4464‐4471. doi: 10.1182/blood-2002-10-3123 [DOI] [PubMed] [Google Scholar]
- 93. Thies FG, Laurindo MFL, Perez EC, Novaes e Brito RR, Mariano M, Popi AF. Cross talk between peritoneal macrophages and B‐1 cells in vitro. PLoS One. 2013;8:e62805. doi: 10.1371/journal.pone.0062805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Shaul ME, Zlotnik A, Tidhar E, et al. Tumor‐associated neutrophils drive B‐cell recruitment and their differentiation to plasma cells. Cancer Immunol Res. 2021;9:811‐824. doi: 10.1158/2326-6066.Cir-20-0839 [DOI] [PubMed] [Google Scholar]
- 95. Wang Y. Myeloid‐derived suppressor cells impair B cell responses in lung cancer through IL‐7 and STAT5. J Immunol. 2018;201:278‐295. doi: 10.4049/jimmunol.1701069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lee‐Chang C, Rashidi A, Miska J, et al. Myeloid‐derived suppressive cells promote B cell‐mediated immunosuppression via transfer of PD‐L1 in glioblastoma. Cancer Immunol Res. 2019;7:1928‐1943. doi: 10.1158/2326-6066.Cir-19-0240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Flores‐Borja F, Blair P. Mechanisms of induction of regulatory B cells in the tumour microenvironment and their contribution to immunosuppression and pro‐tumour responses. Clin Exp Immunol. 2022;209:33‐45. doi: 10.1093/cei/uxac029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Shang J, Zha H, Sun Y. Phenotypes, functions, and clinical relevance of regulatory B cells in cancer. Front Immunol. 2020;11:582657. doi: 10.3389/fimmu.2020.582657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Nelson MA, Ngamcherdtrakul W, Luoh SW, Yantasee W. Prognostic and therapeutic role of tumor‐infiltrating lymphocyte subtypes in breast cancer. Cancer Metastasis Rev. 2021;40:519‐536. doi: 10.1007/s10555-021-09968-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Feugier P. A review of rituximab, the first anti‐CD20 monoclonal antibody used in the treatment of B non‐Hodgkin's lymphomas. Future Oncol. 2015;11:1327‐1342. doi: 10.2217/fon.15.57 [DOI] [PubMed] [Google Scholar]
- 101. Salles G, Barrett M, Foà R, et al. Rituximab in B‐cell hematologic malignancies: a review of 20 years of clinical experience. Adv Ther. 2017;34:2232‐2273. doi: 10.1007/s12325-017-0612-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Boekhout AH, Beijnen JH, Schellens JH. Trastuzumab. Oncologist. 2011;16:800‐810. doi: 10.1634/theoncologist.2010-0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2‐overexpressing breast cancer. Ann Oncol. 2007;18:977‐984. doi: 10.1093/annonc/mdl475 [DOI] [PubMed] [Google Scholar]
- 104. Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299‐308. doi: 10.1038/nrc2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Morotti M, Albukhari A, Alsaadi A, et al. Promises and challenges of adoptive T‐cell therapies for solid tumours. Br J Cancer. 2021;124:1759‐1776. doi: 10.1038/s41416-021-01353-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Cappell KM, Kochenderfer JN. Long‐term outcomes following CAR T cell therapy: what we know so far. Nat Rev Clin Oncol. 2023;20:359‐371. doi: 10.1038/s41571-023-00754-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sterner RC, Sterner RM. CAR‐T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11:69. doi: 10.1038/s41408-021-00459-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhang L, Meng Y, Feng X, Han Z. CAR‐NK cells for cancer immunotherapy: from bench to bedside. Biomark Res. 2022;10:12. doi: 10.1186/s40364-022-00364-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J. CAR‐NK cells: a promising cellular immunotherapy for cancer. EBioMedicine. 2020;59:102975. doi: 10.1016/j.ebiom.2020.102975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Geethakumari PR, Ramasamy DP, Dholaria B, Berdeja J, Kansagra A. Balancing quality, cost, and access during delivery of newer cellular and immunotherapy treatments. Curr Hematol Malig Rep. 2021;16:345‐356. doi: 10.1007/s11899-021-00635-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Boross P, Leusen JH. Mechanisms of action of CD20 antibodies. Am J Cancer Res. 2012;2:676‐690. [PMC free article] [PubMed] [Google Scholar]
- 112. Sanchez L, Wang Y, Siegel DS, Wang ML. Daratumumab: a first‐in‐class CD38 monoclonal antibody for the treatment of multiple myeloma. J Hematol Oncol. 2016;9:51. doi: 10.1186/s13045-016-0283-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Rezvani AR, Maloney DG. Rituximab resistance. Best Pract Res Clin Haematol. 2011;24:203‐216. doi: 10.1016/j.beha.2011.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab Vedotin in relapsed or refractory diffuse large B‐cell lymphoma. J Clin Oncol. 2020;38:155‐165. doi: 10.1200/jco.19.00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Birtas Atesoglu E, Gulbas Z, Uzay A, et al. Glofitamab in relapsed/refractory diffuse large B‐cell lymphoma: real‐world data. Hematol Oncol. 2023;41:663‐673. doi: 10.1002/hon.3174 [DOI] [PubMed] [Google Scholar]
- 116. Dickinson MJ, Carlo‐Stella C, Morschhauser F, et al. Glofitamab for relapsed or refractory diffuse large B‐cell lymphoma. N Engl J Med. 2022;387:2220‐2231. doi: 10.1056/NEJMoa2206913 [DOI] [PubMed] [Google Scholar]
- 117. Nahta R, Esteva FJ. Herceptin: mechanisms of action and resistance. Cancer Lett. 2006;232:123‐138. doi: 10.1016/j.canlet.2005.01.041 [DOI] [PubMed] [Google Scholar]
- 118. Leach DR, Krummel MF, Allison JP. Enhancement of Antitumor Immunity by CTLA‐4 Blockade. Science. 1996;271:1734‐1736. doi: 10.1126/science.271.5256.1734 [DOI] [PubMed] [Google Scholar]
- 119. Small EJ, Tchekmedyian NS, Rini BI, Fong L, Lowy I, Allison JP. A pilot trial of CTLA‐4 blockade with human anti‐CTLA‐4 in patients with hormone‐refractory prostate cancer. Clinic Cancer Res. 2007;13:1810‐1815. doi: 10.1158/1078-0432.Ccr-06-2318 [DOI] [PubMed] [Google Scholar]
- 120. Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD‐1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027‐1034. doi: 10.1084/jem.192.7.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Iwai Y, Terawaki S, Honjo T. PD‐1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int Immunol. 2005;17:133‐144. doi: 10.1093/intimm/dxh194 [DOI] [PubMed] [Google Scholar]
- 122. Huang PW, Chang JW. Immune checkpoint inhibitors win the 2018 Nobel prize. Biom J. 2019;42:299‐306. doi: 10.1016/j.bj.2019.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Brahmer JR, Hammers H, Lipson EJ. Nivolumab: targeting PD‐1 to bolster antitumor immunity. Future Oncol. 2015;11:1307‐1326. doi: 10.2217/fon.15.52 [DOI] [PubMed] [Google Scholar]
- 124. Kwok G, Yau TC, Chiu JW, Tse E, Kwong YL. Pembrolizumab (Keytruda). Hum Vaccin Immunother. 2016;12:2777‐2789. doi: 10.1080/21645515.2016.1199310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Tarhini A, Lo E, Minor DR. Releasing the brake on the immune system: ipilimumab in melanoma and other tumors. Cancer Biother Radiopharm. 2010;25:601‐613. doi: 10.1089/cbr.2010.0865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Tan S, Zhang CW, Gao GF. Seeing is believing: anti‐PD‐1/PD‐L1 monoclonal antibodies in action for checkpoint blockade tumor immunotherapy. Signal Transduct Target Ther. 2016;1:16029. doi: 10.1038/sigtrans.2016.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Sato H, Okonogi N, Nakano T. Rationale of combination of anti‐PD‐1/PD‐L1 antibody therapy and radiotherapy for cancer treatment. Int J Clin Oncol. 2020;25:801‐809. doi: 10.1007/s10147-020-01666-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Hou K, Xu X, Ge X, Jiang J, Ouyang F. Blockade of PD‐1 and CTLA‐4: a potent immunotherapeutic approach for hepatocellular carcinoma. Biofactors. 2023:1‐16. doi: 10.1002/biof.2012 [DOI] [PubMed] [Google Scholar]
- 129. Willsmore ZN, Coumbe BGT, Crescioli S, et al. Combined anti‐PD‐1 and anti‐CTLA‐4 checkpoint blockade: treatment of melanoma and immune mechanisms of action. Eur J Immunol. 2021;51:544‐556. doi: 10.1002/eji.202048747 [DOI] [PubMed] [Google Scholar]
- 130. Zhong L, Li Y, Xiong L, et al. Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct Target Ther. 2021;6:201. doi: 10.1038/s41392-021-00572-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zubair T, Bandyopadhyay D. Small molecule EGFR inhibitors as anti‐cancer agents: discovery, mechanisms of action, and opportunities. Int J Mol Sci. 2023;24:1‐19. doi: 10.3390/ijms24032651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Hong DS, Fakih MG, Strickler JH, et al. KRAS(G12C) inhibition with Sotorasib in advanced solid tumors. N Engl J Med. 2020;383:1207‐1217. doi: 10.1056/NEJMoa1917239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wang X, Allen S, Blake JF, et al. Identification of MRTX1133, a noncovalent, potent, and selective KRAS(G12D) inhibitor. J Med Chem. 2022;65:3123‐3133. doi: 10.1021/acs.jmedchem.1c01688 [DOI] [PubMed] [Google Scholar]
- 134. Huck BR, Kötzner L, Urbahns K. Small molecules drive big improvements in Immuno‐oncology therapies. Angewandte Chemie. 2018;57:4412‐4428. doi: 10.1002/anie.201707816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Koga T, Suda K, Fujino T, et al. KRAS secondary mutations that confer acquired resistance to KRAS G12C inhibitors, Sotorasib and Adagrasib, and overcoming strategies: insights from in vitro experiments. J Thoracic Oncol. 2021;16:1321‐1332. doi: 10.1016/j.jtho.2021.04.015 [DOI] [PubMed] [Google Scholar]
- 136. Awad MM, Liu S, Rybkin II, et al. Acquired resistance to KRAS(G12C) inhibition in cancer. N Engl J Med. 2021;384:2382‐2393. doi: 10.1056/NEJMoa2105281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Strugnell RA. When secretion turns into excretion – the different roles of IgA. Front Immunol. 2022;13:1076312. doi: 10.3389/fimmu.2022.1076312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Prince S, Hollmén M. Dimeric IgA specifically disables intracellular mutated oncodrivers. Immunity. 2023;56:2461‐2463. doi: 10.1016/j.immuni.2023.10.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


