Abstract
Mucoepidermoid carcinoma (MEC) is a common malignancy arising in the parotid gland. The diagnosis of MEC is typically based on its morphological features alone, characteristically containing mucocytes, intermediate cells and epidermoid cells. However, when cystic degeneration is diffuse, it is challenging to distinguish MEC from other benign cystic tumors. This is a case report of a 58-year-old Caucasian man who presented with a parotid mass. H&E sections of the mass reveal multiloculated cysts lined by bland-looking epithelium with only rare papillary architectures. The papillary proliferation contains mucocytes, and epidermoid cells highlighted by the p63 immunohistochemistry study. The diagnosis was confirmed by FISH result of positive MAML2 (11q21) rearrangement. Patient underwent parotidectomy and is disease-free 6 months post-surgery. MEC with cystic degeneration is a common diagnostic pitfall which can mimic many benign lesions in the salivary gland. We present a rare case with MEC with extensive cystic change, its molecular and pathologic findings and review the diagnostic features of MEC, its benign mimickers and useful tools for distinguishing these entities.
Keywords: Salivary gland tumor, low grade mucoepidermoid carcinoma, cystic degeneration, rare tumors, acinic cell carcinoma
Introduction
Mucoepidermoid carcinoma (MEC), the most common type of salivary gland malignancy, usually manifests as a painless swelling mass predominantly located over the parotid glands, for tumor arising from minor salivary glands, the palate, buccal and gingival mucosa, floor of mouth are all possibly sites.1–4 There is a female predominance with an average onset at 49 years and a 5-years overall survival rate of 67% to 90%, depending on the histological grade.1,5 In general, MECs of low to intermediate grade can be adequately treated with surgical excision and have favorable outcomes. However, three major factors contribute to poor outcomes: perineural invasion, extracapsular lymph node extension, and lymph node metastasis. 6 AFIP and Brandwein report that low-grade MEC is often well-circumscribed with cystic components admixed with rich mucous secreting cells.3,7,8 Nuclear atypia, mitosis, or tumor invasion are typically absent.7,8 Based on WHO Classification of Tumors 5th edition, MEC presents histologically with cystic and solid patterns containing mucinous, intermediate, and epidermoid cells. 9 The identification of intermediate and epidermoid cells can be particularly challenging when there is extensive cystic degeneration. Therefore, differentiating MEC from benign cystic lesions can pose a challenge. 10 Herein we describe an interesting case of low-grade MEC with extensive cystic components mimicking benign cystic lesions.
Case presentation
This patient is a 58-year-old Caucasian male with a history of chronic intermittent tinnitus and hearing loss who was referred to otology. MRI IAC was ordered to evaluate possible retrocochlear pathology, and a hypointense right parotid lesion measuring 1.8 × 2.1 cm was incidentally discovered (Figure 1). Following a discussion of the risk, benefit, and alternatives of surgery such as fine needle biopsy (FNA), the patient favored surgery. Total right parotidectomy with facial nerve preservation was performed. Other than transient right-sided facial paresis, no post-operative complications were noted. No adjuvant treatment was applied after tumor board discussion.
Figure 1.
Incidental finding on head MRI with coronal postcontrast fat saturated image of the brain (left) and T2 weighted image of the head (right) demonstrating avidly enhancing bilobed T2 hypointense lesion within the right parotid gland.
Gross examination of the specimen revealed a multiloculated solid-cystic surface with yellow lobulated tissue in the periphery (Figure 2). On H&E section, multiple cysts lined by bland-looking cuboidal to columnar epithelium with lymphoid proliferation in the cystic wall are present (Figure 3(A) and (B)). No mitosis or necrosis are observed. Focal areas were lined by papillary infoldings (Figure 3, (C) and (D)) with scant to abundant mucous cells. Rare groups of intermediate cells with round to oval nuclei and open chromatin are seen (Figure 4 (B)). Lymphocytes and plasma cells are observed in the papillary stroma without well-formed germinal centers. P63 staining was performed to highlight the cyst wall lining and intermediate cell aggregates (Figure 4 (C)). An area of lymphovascular invasion was suspected, which suggested malignant nature (Figure 4 (A)). The resection margins were negative for tumor. FISH study demonstrated MAML2 (11q21) rearrangement, and the diagnosis of low-grade mucoepidermoid carcinoma was rendered. The patient has been regularly followed up for 2 years without further treatment, and no new complaints have been reported.
Figure 2.
The specimen was serially sectioned to reveal a multiloculated white to tan solid-cystic mass. The cut surface shows a cystic component (blue arrow) and the white nodular area (black arrow). The mass abuts the inked outer surface.
Figure 3.
Histologic examination of the parotid mass shows multicystic lesions lined with bland epithelium containing pink amorphous material in the lumen (A, 40x; B, 200x) and ample lymphocytic infiltration in the stroma (B, 200x). There are multiple papillary infoldngs along the cyst walls with most devoid of mucous cells (C, 200x). However, several foci of mucinous-rich epithelium arranged in fused papillary structures are noted (D, 200x).
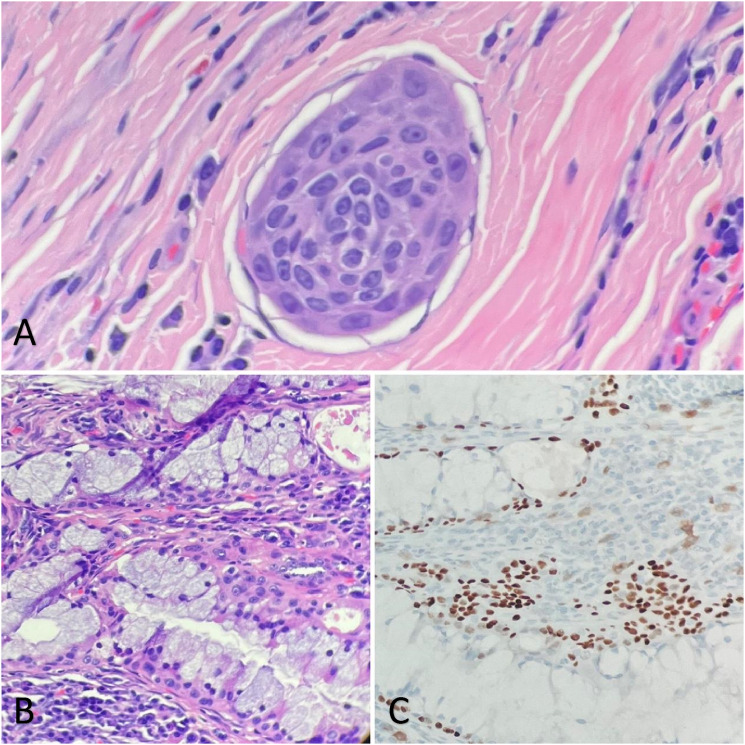
Figure 4.
Tumor cells within an endothelial-lined structure is suspected, raising the possibility for lymphovascular invasion (A, 400x). The papilla with mucous cells also contains epidermoid cells on H&E (B, 200x) that stain positive for p63 (C, 200x) which supports the diagnosis of mucoepidermoid carcinoma.
Discussion
There are different grading systems established for mucoepidermoid carcinoma, according to the extent of aggressiveness.3,7,8 As a result of the subjectivity and heterogeneity of tumor differentiation in large resection specimens, no consensus has been reached. High-grade MEC is characterized by a solid growth pattern, fewer mucous cells, perineural or lymphovascular invasion, and conspicuous nuclear pleomorphism, along with mitotic figures. MECs of intermediate grade fall between the above criteria. Although the AFIP grading system promotes reproducibility, tumor aggressiveness may not directly correlate with grade as some low-grade tumors act more aggressively.7,8
MEC is typically diagnosed by its morphological features alone, and immunohistochemistry (IHC) is not required. The presence of P63 expression with the absence of S100 or SOX10 markers may help differentiate MEC from other salivary tumors. 11 Microscopically, mucous cells appear columnar with abundant intracellular mucin. Epidermoid cells have rich eosinophilic cytoplasm with occasional formation of keratin pearls. Intermediary cells are characterized by smaller nuclei and cytoplasm compared to epidermoid cells. 12 Oncocytic or clear cells may be present in some areas, and it is reported they can constitute the majority of tumor. 1 As a result, MECs are classified as sclerosing, oncocytic, clear-cell, warthin-like, ciliated, spindle-cell, and mucoacinar, based on their highly diverse morphologies.13–18 Translocation at t (11;19) (q21;p13), forming the CRTC1::MAML2 fusion gene is the defining feature of most MECs. 19 There are, however, very few instances of translocation at t (11;15) (q21;q26) with the CRTC3::MAML2 fusion. 20
We presented a case with the initial complaint of hearing loss and subsequent finding of a right painless parotid mass. The presence of a well-circumscribed solid mass with multilocular cysts, positive p63 stain in epidermoid cells, and the FISH finding of MAML2 (11q21) rearrangement support the diagnosis of low-grade MEC. A particular highlight of our study is the low grade mucoepidermoid carcinoma is a great mimicker of benign cystic lesions/neoplasms.
There are many different etiologies that can present as a parotid mass with prominent cystic architecture, ranging from benign to malignant processes. There are three main categories:: cysts without malignancy, benign cystic tumors, and macrocystic tumors with malignant transformation. 21 The list includes parotid duct cyst, mucinous cystadenoma, mucocele, infarcted pleomorphic adenoma, sialometaplasia, Warthin tumor, lymphoepithelial cyst, mucoepidermoid carcinoma and very rarely squamous cell carcinoma. Parotid duct cysts can be congenital or secondary to trauma or chronic inflammation. 22 The majority of congenital parotid gland cysts involve the superficial lobe of the parotid gland, which is sometimes referred to as a branchial cleft cyst.23,24 In patients with a history of salivary duct obstruction, acquired parotid duct cysts are often suspected. 25 Parotid duct cysts are usually lined with stratified squamous epithelium and subepithelial lymphoid tissue. Typically, complex papillary architecture is not observed along the cystic lining. 23 There may be sparse to moderate lymphocytic infiltration of the cyst wall. In some cases, ductal obstruction leads to oncocytic metaplasia. 25 Cystadenomas are well-circumscribed, benign tumors, half of which arise in the parotid gland, malignant transformation of benign cystadenomas can be seen in exceedingly rare occasions.26, 27 The tumor presents histologically as a multicystic lesion lined by proliferative epithelium with varying proportions of papillary architecture and oncocytic change. 28 Fibrous stroma can sometimes contain foci of lymphocytic cell aggregates. 29 In addition, several subtypes are described according to their unique diagnostic features. Mucinous cystadenoma is defined by a predominant mucinous columnar-lined cyst wall without papillary architecture. 30 The cystic cavity is commonly filled with amorphous mucinous material. Mucocytes are always present, but mucinous cystadenomas typically do not harbor multiple cell populations alone the cystic lining.30,31 Cystadenomas can also be of the papillary oncocytic subtype, where the epithelium is lined with oncocytoid cells with prominent papillary architecture that can manifest as multicystic lesions. 32 CK7, CK5/6, and mammaglobin may be positive, p63 highlights the myoepiethlial layer. 32
Warthin tumors are benign tumors consisting of oncocytic epithelium arranged in cystic or papillary patterns overlying rich lymphoid stroma, seen most commonly in males ages 60 to 70. 33 On gross examination, it can appear as a solid area within a multicystic lesion with admixed papillary projections. 34 The cystic line is composed of the bilayered epithelium with a luminal layer of tall, columnar or oncocytic cells and basal triangular cells. A great mimicker is MEC, Warthin-like subtype. Unlike the aforementioned tumors, pleomorphic adenomas are rarely characterized by cystic changes at their initial stage although post-biopsy/procedure infarctions or necrosis can result in secondary degenerative cystic changes. 35 Moreover, none of the three main components for diagnosis, ductal cells, myoepithelial cells, and chondromyxoid stroma, were identified in this case. Another differential diagnosis is acinic cell carcinoma, especially when presents with papillary cystic (macro-cystic) architectures. Microscopically, it shows papillary projection of tumors or a hobnailing morphology, where secretions can be observed in the cystic lumen. 36 Acinar cells are large and polyhedral, with basophilic granular cytoplasm. The granules can be highlighted be diastase resistant positive periodic acid-Schiff (PAS) reaction. Positive DOG1 stain and negative for p63 are useful tools to make the discretion between other malignant salivary gland tumors. 37
Lastly, lymphoepithelial cysts are another epithelial-lined cystic lesion affecting the salivary glands, with stratified squamous epithelium reported in most cases. 38 Bilateral lymphoepithelial cysts are commonly seen in individuals who have contracted the human immunodeficiency virus (HIV).39 Fewer cases of mixed mucous and cuboidal-lined epithelium have been reported. It is characterized by lymphoid tissue with germinal centers along the stroma as well as the absence of cytological atypia. 38 Squamous cell carcinoma, which is often metastatic from other sites (such as skin or mucosal origin), shows very prominent cytological atypia and lacks intermediate cells and mucocytes. It usually shows well-developed keratinization.
Conclusion
Our study describes a diagnostic dilemma of low grade mucoepidermoid carcinoma with prominent cystic appearance mimicking benign cystic lesions. Careful examination of advanced papillary structures containing mucous cells is critical. However, due to the challenge in identifying epidermoid cells and intermediate cells across the extensive cystic background, p63 immunohistochemistry and molecular confirmation of MAML2 rearrangement are useful tools for establishing the correct diagnosis.
Footnotes
Author contributions: Wangpan Shi: Drafting the main manuscript including pathology pictures and discussion. Timothy Law: Edition the manuscript. Kevin Brumund: Provision surgery procedure and edition of manuscript. Jennifer Chang: Provision MRI study results. Charmi Patel: edition of the manuscript. Grace Lin: edition of the manuscript. Jingjing Hu: Provision design of the manuscript and edition of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical statment
Informed consent
Written patient informed consent is obtained.
ORCID iD
Jingjing Hu https://orcid.org/0000-0003-1957-2706
References
- 1.Peraza A, Gómez R, Beltran J, et al. Mucoepidermoid carcinoma. An update and review of the literature. J Stomatol Oral Maxillofac Surg 2020; 121: 713–720. DOI: 10.1016/j.jormas.2020.06.003 [DOI] [PubMed] [Google Scholar]
- 2.Stewart FW, Foote FW, Becker WF. Muco-epidermoid tumors of salivary glands. Ann Surg 1945; 122: 820–844. DOI: 10.1097/00000658-194511000-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandwein MS, Ivanov K, Wallace DI, et al. Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol 2001; 25: 835–845. DOI: 10.1097/00000478-200107000-00001 [DOI] [PubMed] [Google Scholar]
- 4.Brookstone MS, Huvos AG. Central salivary gland tumors of the maxilla and mandible: a clinicopathologic study of 11 cases with an analysis of the literature. J Oral Maxillofac Surg 1992; 50: 229–236. DOI: 10.1016/0278-2391(92)90317-S [DOI] [PubMed] [Google Scholar]
- 5.Boahene DK, Olsen KD, Lewis JE, et al. Mucoepidermoid carcinoma of the parotid gland: the Mayo clinic experience. Arch Otolaryngol Head Neck Surg 2004; 130: 849–856. DOI: 10.1001/archotol.130.7.849 [DOI] [PubMed] [Google Scholar]
- 6.Dahan LS, Giorgi R, Vergez S, et al. Mucoepidermoid carcinoma of salivary glands: a French Network of Rare Head and Neck Tumors (REFCOR) prospective study of 292 cases. Eur J Surg Oncol 2021; 47: 1376–1383. DOI: 10.1016/j.ejso.2020.11.123 [DOI] [PubMed] [Google Scholar]
- 7.Auclair PL, Goode RK, Ellis GL. Mucoepidermoid carcinoma of intraoral salivary glands. Evaluation and application of grading criteria in 143 cases. Cancer 1992; 69: 2021-2030. DOI: [DOI] [PubMed] [Google Scholar]
- 8.Goode RK, Auclair PL, Ellis GL. Mucoepidermoid carcinoma of the major salivary glands: clinical and histopathologic analysis of 234 cases with evaluation of grading criteria. Cancer 1998; 82: 1217–1224. DOI: [DOI] [PubMed] [Google Scholar]
- 9.WcoTE B. WHO classification of tumours series. Lyon, France: International Agency for Research on Cancer, 2022. [Google Scholar]
- 10.McHugh CH, Roberts DB, El-Naggar AK, et al. Prognostic factors in mucoepidermoid carcinoma of the salivary glands. Cancer 2012; 118: 3928–3936. DOI: 10.1002/cncr.26697 [DOI] [PubMed] [Google Scholar]
- 11.Sams RN, Gnepp DR. P63 expression can be used in differential diagnosis of salivary gland acinic cell and mucoepidermoid carcinomas. Head Neck Pathol 2013; 7: 64–68. DOI: 10.1007/s12105-012-0403-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katabi N, Ghossein R, Ali S, et al. Prognostic features in mucoepidermoid carcinoma of major salivary glands with emphasis on tumour histologic grading. Histopathology 2014; 65: 793–804. DOI: 10.1111/his.12488 [DOI] [PubMed] [Google Scholar]
- 13.Yabuki K, Matsuyama A, Shiba E, et al. Sclerosing mucoepidermoid carcinoma in the parotid gland with CRTC1-MAML2 fusion: a case report. Int J Surg Pathol 2018; 26: 250–255. DOI: 10.1177/1066896917742721 [DOI] [PubMed] [Google Scholar]
- 14.Tajima S, Namiki I, Koda K. A clear cell variant of mucoepidermoid carcinoma harboring CRTC1-MAML2 fusion gene found in buccal mucosa: report of a case showing a large clear cell component and lacking typical epidermoid cells and intermediate cells. Med Mol Morphol 2017; 50: 117–121. DOI: 10.1007/s00795-015-0120-5 [DOI] [PubMed] [Google Scholar]
- 15.Ishibashi K, Ito Y, Masaki A, et al. Warthin-like mucoepidermoid carcinoma: a combined study of fluorescence in situ hybridization and whole-slide imaging. Am J Surg Pathol 2015; 39: 1479–1487. DOI: 10.1097/pas.0000000000000507 [DOI] [PubMed] [Google Scholar]
- 16.Bishop JA, Cowan ML, Shum CH, et al. MAML2 rearrangements in variant forms of mucoepidermoid carcinoma: ancillary diagnostic testing for the ciliated and warthin-like variants. Am J Surg Pathol 2018; 42: 130–136. DOI: 10.1097/pas.0000000000000932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goh GH, Lim CM, Vanacek T, et al. Spindle cell mucoepidermoid carcinoma of the palatine tonsil with CRTC1-MAML2 fusion transcript: report of a rare case in a 17-year-old boy and a review of the literature. Int J Surg Pathol 2017; 25: 705–710. DOI: 10.1177/1066896917714890 [DOI] [PubMed] [Google Scholar]
- 18.Bundele M, Weinreb I, Xu B, et al. Mucoacinar carcinoma: a rare variant of mucoepidermoid carcinoma. Am J Surg Pathol 2021; 45: 1028–1037. DOI: 10.1097/pas.0000000000001752 [DOI] [PubMed] [Google Scholar]
- 19.Seethala RR, Dacic S, Cieply K, et al. A reappraisal of the MECT1/MAML2 translocation in salivary mucoepidermoid carcinomas. Am J Surg Pathol 2010; 34: 1106–1121. DOI: 10.1097/PAS.0b013e3181de3021 [DOI] [PubMed] [Google Scholar]
- 20.Nakayama T, Miyabe S, Okabe M, et al. Clinicopathological significance of the CRTC3-MAML2 fusion transcript in mucoepidermoid carcinoma. Mod Pathol 2009; 22: 1575–1581. DOI: 10.1038/modpathol.2009.126 [DOI] [PubMed] [Google Scholar]
- 21.Takita H, Takeshita T, Shimono T, et al. Cystic lesions of the parotid gland: radiologic-pathologic correlation according to the latest world health organization 2017 classification of head and neck tumours. Jpn J Radiol 2017; 35: 629–647. DOI: 10.1007/s11604-017-0678-z [DOI] [PubMed] [Google Scholar]
- 22.Work WP, Hecht DW. Non-neoplastic lesions of the parotid gland. Ann Otol Rhinol Laryngol 1968; 77: 462–467. DOI: 10.1177/000348946807700308 [DOI] [PubMed] [Google Scholar]
- 23.Cohen MN, Rao U, Shedd DP. Benign cysts of the parotid gland. J Surg Oncol 1984; 27: 85–88. DOI: 10.1002/jso.2930270206 [DOI] [PubMed] [Google Scholar]
- 24.Upile T, Jerjes W, Al-Khawalde M, et al. Branchial cysts within the parotid salivary gland. Head Neck Oncol 2012; 4: 24. DOI: 10.1186/1758-3284-4-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hecht DW, Work WP. Surgery for nonneoplastic parotid disease. Arch Otolaryngol 1970; 92: 463–467. DOI: 10.1001/archotol.1970.04310050045007 [DOI] [PubMed] [Google Scholar]
- 26.Gontarz M, Orłowska-Heitzman J, Gąsiorowski K, et al. Myoepithelial carcinoma arising in a salivary duct cyst of the parotid gland: case presentation. Medicina 2023; 59: 2023. DOI: 10.3390/medicina59020184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seifert G. Mucoepidermoid carcinoma in a salivary duct cyst of the parotid gland. Contribution to the development of tumours in salivary gland cysts. Pathol Res Pract 1996; 192: 1211–1217. DOI: 10.1016/s0344-0338(96)80153-1 [DOI] [PubMed] [Google Scholar]
- 28.Hellquist H, Paiva-Correia A, Vander Poorten V, et al. Analysis of the clinical relevance of histological classification of benign epithelial salivary gland tumours. Adv Ther 2019; 36: 1950–1974. DOI: 10.1007/s12325-019-01007-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tjioe KC, de Lima HG, Thompson LD, et al. Papillary cystadenoma of minor salivary glands: report of 11 cases and review of the English literature. Head Neck Pathol 2015; 9: 354–359. DOI: 10.1007/s12105-014-0602-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rai S, Rana AS, Gupta V, et al. Mucinous cystadenoma: a rare entity. Dent Res J 2013; 10: 685–688. [PMC free article] [PubMed] [Google Scholar]
- 31.Girotra C, Padhye MN, Mahajan P, et al. A rare case report of mucinous cystadenoma with immunohistochemical analysis and review of literature. J Maxillofac Oral Surg 2015; 14: 426–434. DOI: 10.1007/s12663-014-0656-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsurumi Kunio, Kamiya Hiroaki, Yokoi Motoo, Kameyama Yoichiro, et al. Papillary oncocytic cystadenoma of palatal minor salivary gland: A case report. Journal of Oral and Maxillofacial Surgery. 2003; 61(5): 631–633. [DOI] [PubMed] [Google Scholar]
- 33.Pinkston JA, Cole P. Cigarette smoking and Warthin’s tumor. Am J Epidemiol 1996; 144: 183–187. DOI: 10.1093/oxfordjournals.aje.a008906 [DOI] [PubMed] [Google Scholar]
- 34.Nagao TGD, Simpson RHW, Vielh P. World Health Organization classification of head and neck tumors. 4 ed. Lyon, France: International Agency for Research on Cancer, 2017. [Google Scholar]
- 35.Kaveri H, Gopalkrishnana K, Venkatesh A. Cystic and florid squamous metaplasia in pleomorphic adenoma of palate – a diagnostic dilemma. Asian J Med Sci 2014; 5: 108–110. DOI: 10.3126/ajms.v5i4.10434 [DOI] [Google Scholar]
- 36.Liew C, Witherow H, Ketheeswaranathan V, et al. Papillary cystic variant of the acinic cell adenocarcinoma. Oral Oncol Extra 2005; 41: 146–149. DOI: 10.1016/j.ooe.2005.03.005 [DOI] [Google Scholar]
- 37.Chênevert J, Duvvuri U, Chiosea S, et al. DOG1: a novel marker of salivary acinar and intercalated duct differentiation. Mod Pathol 2012; 25: 919–929. DOI: 10.1038/modpathol.2012.57 [DOI] [PubMed] [Google Scholar]
- 38.Skouteris CA, Patterson GT, Sotereanos GC. Benign cervical lymphoepithelial cyst: report of cases. J Oral Maxillofac Surg 1989; 47: 1106–1112. DOI: 10.1016/0278-2391(89)90194-8 [DOI] [PubMed] [Google Scholar]