Abstract
Objectives
Based on enhanced MRI, a prediction model of microvascular invasion (MVI) for hepatocellular carcinoma (HCC) was developed using graph convolutional network (GCN) combined nomogram.
Methods
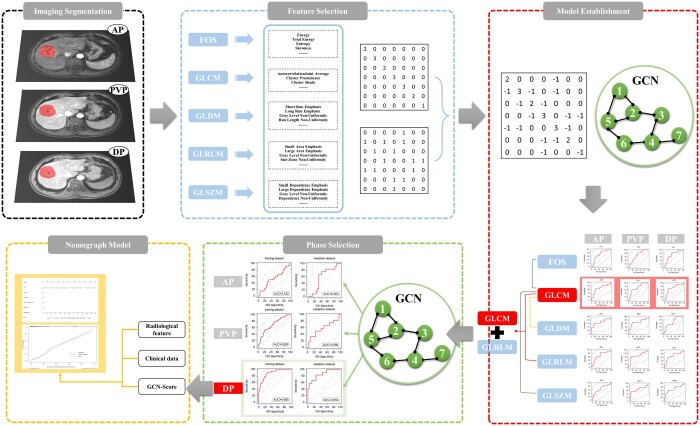
We retrospectively collected 182 HCC patients confirmed histopathologically, all of them performed enhanced MRI before surgery. The patients were randomly divided into training and validation groups. Radiomics features were extracted from the arterial phase (AP), portal venous phase (PVP), and delayed phase (DP), respectively. After removing redundant features, the graph structure by constructing the distance matrix with the feature matrix was built. Screening the superior phases and acquired GCN Score (GS). Finally, combining clinical, radiological and GS established the predicting nomogram.
Results
27.5% (50/182) patients were with MVI positive. In radiological analysis, intratumoural artery (P = 0.007) was an independent predictor of MVI. GCN model with grey-level cooccurrence matrix-grey-level run length matrix features exhibited area under the curves of the training group was 0.532, 0.690, and 0.885 and the validation group was 0.583, 0.580, and 0.854 for AP, PVP, and DP, respectively. DP was selected to develop final model and got GS. Combining GS with diameter, corona enhancement, mosaic architecture, and intratumoural artery constructed a nomogram which showed a C-index of 0.884 (95% CI: 0.829-0.927).
Conclusions
The GCN model based on DP has a high predictive ability. A nomogram combining GS, clinical and radiological characteristics can be a simple and effective guiding tool for selecting HCC treatment options.
Advances in knowledge
GCN based on MRI could predict MVI on HCC.
Keywords: hepatocellular carcinoma, microvascular invasion, deep learning, radiomics, graph convolutional network
Introduction
Hepatocellular carcinoma (HCC) stands as the fourth most common cause of cancer-related death globally.1 Surgical resection and liver transplantation represent two effective methods for treating HCC, with respective 5-year recurrence rates reaching as high as 70% and 25%.2 Previous studies have underscored that microvascular invasion (MVI) serves as a significant risk factor for HCC recurrence post-surgery and liver transplantation. However, the current major clinical challenge is the lack of a diagnostic ability evaluation for MVI.3–6 Therefore, precise preoperative assessment of MVI can help clinicians select a more reasonable treatment plan, thereby reducing the recurrence of HCC patients following surgical treatment and ultimately enhancing patient survival.7,8
Several recent studies have indicated that numerous imaging features are associated with the occurrence of MVI and can serve as predictors of MVI.9–11 However, the reproducibility of these studies, which rely on subjective judgment for assessing imaging features, is controversial, and the predictive value remains to be improved.12,13 Recently, some scholars have utilized texture analysis of radiology images to predict MVI, significantly improving prediction accuracy.14,15 Nevertheless, the features extracted by radiology are complex, and extracting high-latitude features increases the chance of identifying biologically relevant features, albeit with the challenge of potential model overfitting.16 Addressing this issue necessitates including many study samples, entailing substantial manual effort for collecting cases and segmenting regions of interest (ROI). While deep learning has the potential to alleviate the manual segmentation workload, its interpretability has not gained recognition from radiologists.17
Graph convolutional network (GCN) is a deep learning method applied to graph and node classification, serving as a system for learning graph data. GCN can perform end-to-end learning of attributes and structures, yielding interpretable results.18 Specifically, the attribute information contains the inherent properties of the objects in the graph, while the structural information characterizes the association between these objects. GCN extracts fewer features from graph data based on a smaller number of case samples and obtains the embedded representation of the graph through feature classification into nodes, graph classification, and edge prediction. This process enhances the classification and prediction ability.19 Currently, GCN has been successfully applied to tasks for the accurate segmentation of prostate magnetic resonance (MR) images20 and the prediction of brain functional defects and diseases.21 However, its application to related studies on HCC remains unexplored.
Hence, this study aimed to construct a preoperative diagnostic model for MVI using GCN to uncover the correlation between the radiomics signatures of images. The goal is to furnish clinicians with early warning information about MVI in liver cancer in a non-invasive manner.
Methods
Patients
This study received approval from the Institutional Review Board (KY2021-05). Given its retrospective nature, informed consent was waived. Contrast-enhanced magnetic resonance imaging (MRI) was performed on 282 patients suspected of having HCC between June 2017 and August 2020. The recruitment process and inclusion and exclusion criteria for patients are illustrated in Figure 1. Ultimately, 182 patients (144 men and 38 women, mean age ± standard deviation (SD), 57.9 ± 9.0 years) were included in this retrospective study. Patients were randomly assigned to training (n = 146) and validation (n = 36) groups using an 8:2 randomization ratio.
Figure 1.
The patient recruitment pathway and the inclusion and exclusion criteria.
Note: HCC = hepatocellular carcinoma; ICC = intrahepatic cholangiocarcinoma; MVI = microvascular invasion.
Inclusion criteria were as follows: (1) patients with histologically confirmed HCC post-surgical resection; (2) patients with HCC who underwent contrast-enhanced MR imaging within two weeks before surgery; (3) patients without preoperative cancer-related treatments (radiotherapy, arterial chemoembolization, radiofrequency ablation, and preoperative neoadjuvant chemotherapy).
Exclusion criteria included the following: (1) diffuse HCC or HCC combined with hepatobiliary cell carcinoma; (2) HCC with intravascular tumour thrombosis; (3) HCC pathological examination without MVI assessment; (4) poor quality MR images.
Clinical data
Clinical baseline data, including age, gender, Child-Pugh classification of liver function, hypertension, number of lesions, serum alpha-fetoprotein (AFP) level, alanine aminotransferase (ALT) level, aspartate aminotransferase (AST) level, total serum bilirubin (TB), and Barcelona Clinic Liver Cancer (BCLC), were collected.
Image acquisition
All patients underwent contrast-enhanced MRI utilizing a 3.0 T MR system (Ingenia, Philips Healthcare, Best, The Netherlands) equipped with a 32-channel phased-array coil. Initially, an axial T2-weighted single-shot turbo spin-echo sequence with fat-suppressed respiratory triggering was performed for routine liver MRI, employing the following parameters: repetition time (TR)/echo time (TE) = 1000/70 milliseconds, slice thickness/gap = 7/1 mm, field of view (FOV) = 400 × 350 mm, and matrix size = 208 × 207. Subsequently, an axial dual-echo T1-weighted fast field-echo with breath-hold was also conducted with TR/TE1/TE2 = 8.7/1.15/2.3 milliseconds, slice thickness/gap = 7/1 mm, FOV = 400 × 350 mm, and matrix size =252 × 211. Finally, a fat-suppressed dynamic 3D T1-weighted sequence (TR/TE = 3.7/1.32 milliseconds, slice thickness/gap = 4/−2 mm, FOV = 400 × 350 mm, matrix size = 252 × 207) with volumetric interpolation and breath-hold was performed before and after the bolus injection of 0.1 mmol/kg gadoteric acid (Hengrui Pharmaceutical Co., Ltd, Jiangsu, China) at a flow rate of 2 mL/s through the cubital vein, followed by a 20 mL saline chaser administered at the same rate. The dynamic enhanced sequence included several phases: the pre-enhanced phase, AP (25-30 seconds), PVP (55 seconds), and DP (180 seconds).
Image analysis
Digital Imaging and Communications in Medicine (DICOM) files of MR scans were retrieved and evaluated by two abdominal radiologists, each possessing 3 and 5 years of experience in liver MRI diagnosis. The imaging characteristics of each selected observed lesion included the following: (1) nonrim AP hyperenhancement, (2) nonperipheral washout, (3) enhancing capsule, (4) mosaic architecture, (5) fat in mass, (6) corona enhancement, (7) intratumoural artery, (8) restricted diffusion, and (9) mild-to-moderate T2WI hyperintensity. The maximum long diameter at the lesion’s maximum level along the axis was measured in the PVP. In cases of multiple lesions, the largest lesion was selected for evaluation (Figure 2).
Figure 2.
(A) The HCC lesion (blue arrow) in the right lobe of the liver is shown on contrast-enhanced MR image, presenting a “mosaic architecture” on T2WI. (B) HCC lesion (blue arrow) in the left lobe of the liver on contrast-enhanced MR image and “intratumoral artery” inside the lesion (red arrow) on AP image. (C) HCC lesion (blue arrow) in the right lobe of the liver is shown on contrast-enhanced MR image. The lesion appears as nonrim AP hyperenhancement on the AP image, and “corona enhancement” is shown around the lesion (white arrow). (D) HCC lesion (blue arrow) in the right lobe of the liver is demonstrated on contrast-enhanced MR image, the regression of lesion enhancement on PVP image is shown as “nonperipheral washout,” and the edge of the visible lesion shows an “enhancing capsule.”
Histopathological evaluation
The postoperative pathological specimens of all HCC patients were examined by two pathologists, each with 5 and 15 years of experience in pathological diagnosis. These pathologies were kept unaware of the clinical and imaging information related to the patients. MVI was defined as tumour invasion confined solely to a microscopically visible portal vein or hepatic vein within the surrounding liver tissue contiguous with the tumour.
Image processing
Image segmentation
All data were stored in the DICOM format, and the workflow of this study is illustrated in Figure 3. A radiologist possessing 5 years of experience in abdominal imaging diagnosis read the images using MATLAB software (MathWorks, Natick, MA, USA). The radiologist selected the maximum cross-section of the tumour in the DP, manually delineated the ROI along the edge of the lesion, and mapped the ROI onto the corresponding slices of AP and PVP. Segmentation results were reviewed and corrected by another radiologist with 15 years of experience in liver cancer diagnosis.
Figure 3.
Flowchart of the model development process in this study.
Feature extraction
Radiomics features were extracted using PyRadiomics v3.0.1, an open-source Python package.22 A total of 81 radiomics features, comprising 11 first-order statistical parameters and 70 texture parameters, were extracted from the MR image. The first-order statistical (FOS) features, consisting of 11 parameters, provided information on the distribution of voxel intensities within the MRI through commonly used and basic metrics. Textural features were computed using various matrices, including the GLCM (24 parameters), grey-level dependency matrix (GLDM, 14 parameters), GLRLM (16 parameters), and grey-level size region matrix (GLSZM, 16 parameters). In total, 243 radiomics features were extracted in three phases (AP, PVP, DP) for each patient.
Radiomics features selection
Redundant radiomics features are eliminated based on the following mathematical model applied to each image, as described in equation (2-1):
| #(2-1) |
Here, represents the mean of positive samples, is the negative sample mean, is the positive sample variance, and is the negative sample variance. This mathematical model serves as the evaluation function for a genetic algorithm, enabling the selection of the optimal combination of radiomics features for each image at each time phase.
Establishment of GCN prediction model
The graph structure was formed by creating the distance matrix with the feature matrix. Initially, the feature vector , for each node (patient) was calculated based on the selected feature . Here = , where m represents the node (patient), n represents the feature group (FOS, GLCM, GLDM, GLRLM, GLSZM, and x signifies the number of features after selection). Subsequently, the eigenvector matrix, comprising all nodes, was obtained. The Euclidian distance X(i, j) was calculated using the following equation (2-2):
| #(2-2) |
Here, i and j represent two different patients. The Euclidean distance was chosen to establish the graph structure, and an iterative threshold was employed to determine whether a connection is formed between two nodes (patients).
The constructed graph structure was fed into the GCN for learning, and subsequently, the GCN prediction model of MVI was developed. The models were validated using the 5-fold crossover method, and the diagnostic performance of each model was compared. The model with the highest area under the receiver operating characteristic curve (AUC) was selected as the optimal GCN prediction model, providing the GCN score (GS) (Figure 4).
Figure 4.

The AUC of each GCN combination model is based on the AP, PVP, and DP images of the training and validation groups, respectively.
Construction of nomogram
Variables with P < 0.1 were selected through univariate analysis of clinical data, radiological characteristics, and the GS to construct a nomogram model for predicting MVI. Calibration curves were adopted to analyse the diagnostic performance of the nomogram. Decision curve analysis (DCA) was employed to evaluate the clinical utility of nomograms by calculating the net benefit at different threshold probabilities (Figure 5).
Figure 5.
(A) Nomogram predicting the probability of MVI in HCC patients. Total points were calculated by adding up the points of each variable on the point scale and indicating the probability of MVI presence according to the bottom scales. GS, GCN-Score. (B) DCA is for the combined nomogram. The grey or black line hypothesizes that all patients were MVI positive or negative, respectively. The red line represents the net benefit of the nomogram at different threshold probabilities. (C) The calibration curve for predicting the presence of MVI. The combined nomogram-predicted MVI presence is plotted on the X-axis, and the actual MVI presence is plotted on the Y-axis. A plot along the 45 ° line would indicate a perfect calibration model.
Statistical analysis
The univariate analysis employed the chi-square test, Fisher's test, or Kruskal-Wallis test to compare categorical variables, and the t-test or Mann-Whitney U-test to compare continuous variables. The Cohen kappa statistic assessed interobserver agreement, with a kappa greater than 0.80 considered excellent, 0.61-0.80 considered good, 0.41-0.60 considered moderate, and less than or equal to 0.40 considered poor. Multivariate analysis was performed for variables with P < 0.1 in univariate analysis. SPSS software (version 25.0, IBM) was utilized for all statistical analyses, with a value of P < 0.05 considered statistically significant.
Results
Clinical and radiological characteristics
The patients were divided into the MVI-negative group (n = 132) and the MVI-positive group (n = 50) based on the results of pathological examination. Agreement between the two observers was good to excellent, with kappa values ranging from 0.759 to 0.954 for all MRI characteristics. Univariate analysis revealed a significant difference in diameter between the MVI-positive and MVI-negative groups (P = 0.001) (Table 1). Radiographic characteristics, including mosaic architecture (P = 0.001), corona enhancement (P = 0.047), and intratumoural artery (P < 0.001), exhibited significant differences between MVI-positive and MVI-negative groups (Table 2). Multivariate analysis indicated that only the presence of an intratumoural artery was significantly different between the MVI-positive and MVI-negative groups (P < 0.05) (Table 3).
Table 1.
Clinicopathologic characteristics of the MVI analysis population.
| Variable | MVI absence | MVI presence | P value | |
|---|---|---|---|---|
| (n = 132) | (n = 50) | |||
| Age | ||||
| ≤50 years | 28 (21.2) | 11 (22.0) | ||
| >50 years | 104 (78.8) | 39 (78.0) | 0.908 | |
| Sex | ||||
| male | 103 (78.0) | 41 (82.0) | ||
| female | 29 (22.0) | 9 (18.0) | 0.556 | |
| AST | ||||
| ≤40 U/L | 88 (66.7) | 33 (66.0) | ||
| >40 U/L | 44 (33.3) | 17 (34.0) | 0.932 | |
| ALT | ||||
| ≤50 U/L | 107 (81.1) | 42 (84.0) | ||
| >50 U/L | 25 (18.9) | 8 (16.0) | 0.646 | |
| AFP | ||||
| ≤400 ng/ml | 101 (82.8) | 32 (71.1) | ||
| >400 ng/ml | 21 (17.2) | 13 (28.9) | 0.096 | |
| TB | ||||
| ≤19 µmol/L | 113 (85.6) | 41 (82.0) | ||
| >19 µmol/L | 19 (14.4) | 9 (18.0) | 0.547 | |
| Portal hypertension | ||||
| absent | 114 (86.4) | 42 (84.0) | ||
| present | 18 (13.6) | 8 (16.0) | 0.684 | |
| Number | ||||
| single | 105 (79.5) | 35 (70.0) | ||
| multiple | 27 (20.5) | 15 (30.0) | 0.172 | |
| Diameter | ||||
| ≤3 cm | 40 (30.3) | 3 (6.0) | ||
| >3 cm | 92 (69.7) | 47 (94.0) | 0.001* | |
| Child-pugh | ||||
| A | 131 (99.2) | 50 (100.0) | ||
| B | 1 (0.8) | 0 (0.0) | 1.000 | |
| BCLC | ||||
| 0 | 3 (2.3) | 0 (0.0) | ||
| A1 | 52 (39.4) | 9 (18.0) | ||
| A2 | 11 (8.3) | 5 (10.0) | ||
| A3 | 6 (4.5) | 3 (6.0) | ||
| A4 | 18 (19.6) | 9 (18.0) | ||
| B | 42 (31.8) | 24 (48.0) | 0.073 | |
Note: Data are presented as number (%) of patients unless otherwise noted. Categorical variables were compared using the chi-square test or Fisher’s exact test.
Abbreviations: AFP = alpha-fetoprotein; ALT = alanine aminotransferase; AST = aspartate aminotransferase; BCLC = Barcelona Clinic Liver Cancer; TB = total serum bilirubin.
P < 0.05, significant difference.
Table 2.
Radiological characteristics of the MVI analysis population.
| Variable | MVI negative | MVI positive | P value |
|---|---|---|---|
| n = 132 | n = 50 | ||
| Nonrim AP hyperenhancement | |||
| absent | 8 (6.1) | 3 (6.0) | |
| present | 124 (93.9) | 47 (94.0) | 1.000 |
| Nonperipheral washout | |||
| absent | 18 (13.6) | 5 (10.0) | |
| present | 114 (86.4) | 45 (90.0) | 0.510 |
| Enhancing capsule | |||
| absent | 5 (3.8) | 2 (4.0) | |
| present | 127 (95.6) | 48 (96.0) | 1.000 |
| Mosaic architecture | |||
| absent | 84 (63.6) | 18 (36.0) | |
| present | 48 (36.4) | 32 (64.0) | 0.001* |
| Fat in mass | |||
| absent | 111 (84.1) | 44 (88.0) | |
| present | 21 (15.9) | 6 (12.0) | 0.508 |
| Corona enhancement | |||
| absent | 92 (69.7) | 27 (54.0) | |
| present | 40 (30.3) | 23 (46.0) | 0.047* |
| Intratumoural artery | |||
| absent | 121 (91.7) | 33 (66.0) | |
| present | 11 (8.3) | 17 (34.0) | <0.001* |
| Restricted diffusion | |||
| absent | |||
| present | 132 (100.0) | 50 (100.0) | |
| Mild-to-moderate T2 hyperintensity | |||
| absent | 3 (2.3) | 1 (2.0) | |
| present | 129 (97.7) | 49 (98.0) | 1.000 |
Note: Data are presented as number (%) of patients unless otherwise noted. Categorical variables were compared using the chi-square test or Fisher’s exact test.
P < 0.05, significant difference.
Table 3.
Univariate and multivariate logistic analysis for MVI.
| Variable | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| B | OR (95% CI) | P value | B | OR (95% CI) | P value | |
| AFP | 0.67 | 1.95(0.88-4.34) | 0.1 | 0.521 | 1.68(0.68-4.17) | 0.261 |
| Diameter | 1.919 | 6.81(2.00-23.18) | 0.002* | 1.148 | 3.15(0.84-11.84) | 0.089 |
| BCLC | 0.281 | 1.32(1.09-1.61) | 0.005* | 0.065 | 1.07(0.83-1.37) | 0.608 |
| Mosaic architecture | 1.135 | 3.11(1.58-6.13) | 0.001* | −0.543 | 0.58(0.25-1.36) | 0.211 |
| Corona enhancement | 0.673 | 1.96(1.00-3.82) | 0.049* | 0.504 | 1.66(0.74-3.67) | 0.218 |
| Intratumoural artery | 1.735 | 5.67(2.42-12.27) | <0.001* | 1.43 | 4.17(1.48-11.80) | 0.007* |
Note: Variables from univariate analysis were applied to multivariate analysis.
Abbreviations: AFP = alpha-fetoprotein; B = β Coefficient; BCLC = Barcelona Clinic Liver Cancer; CI = confidence interval; OR = odds ratio.
P < 0.05, significant difference.
GCN model development and optimal phase selection
The GCN determined the AUCs for different radiomics feature groups related to images in each phase (Table 4). The GLCM feature group exhibited higher AUCs in the three-phase images (0.70 for AP-GLCM, 0.72 for PVP-GLCM, and 0.72 for DP-GLCM). GLCM on images in each phase (AP, PVP, and DP) was modelled in combination with other radiomics feature groups to improve the diagnostic ability further. The results revealed that combining GLCM with GLRLM in the DP achieved the highest AUC in the training and validation groups, precisely 0.874 and 0.854, respectively (Figure 4).
Table 4.
AUC for the prediction of MVI by the radiomics feature groups about the validation group.
| Radiomics feature group | AP (95% CI) | PVP | DP |
|---|---|---|---|
| FOS | 0.69(0.512-0.831) | 0.71(0.534-0.848) | 0.70(0.529-0.844) |
| GLCM | 0.70(0.529-0.844) | 0.72(0.547-0.857) | 0.72(0.548-0.858) |
| GLDM | 0.70(0.524-0.840) | 0.68(0.501-0.823) | 0.70(0.529-0.844) |
| GLRLM | 0.71(0.536-0.849) | 0.69(0.518-0.836) | 0.71(0.534-0,848) |
| GLSZM | 0.68(0.501-0.823) | 0.68(0.506-0.827) | 0.71(0.536-0.849) |
| GLCM-FOS | 0.58(0.401-0.739) | 0.67(0.490-0.814) | 0.74(0.570-0.874) |
| GLCM-GLDM | 0.76(0.586-0.884) | 0.61(0.431-0.766) | 0.75(0.578-0.879) |
| GLCM-GLRLM | 0.58(0.408-0.745) | 0.58(0.404-0.742) | 0.85(0.697-0.949) |
| GLCM-GLSZM | 0.71(0.530-0.845) | 0.56(0.388-0.727) | 0.84(0.684-0.943) |
Abbreviations: AP = arterial phase; CI = confidence interval; DP = delayed phase; FOS = first-order statistics; GLCM = grey-level cooccurrence matrix; GLDM = grey-level dependency matrix; GLRLM = grey-level run-length matrix; GLSZM = grey-level size region matrix; PVP = portal venous phase.
Nomogram for MVI
A nomogram predicting MVI was developed based on the results of univariate analysis, incorporating the diameter, corona enhancement, mosaic architecture, intratumoural artery, and GS from DP imaging (P < 0.05). The nomogram exhibited a C-index of 0.884 (95% CI: 0.829-0.927). The nomogram and the corresponding calibration curve are presented in Figure 5. The calibration curve demonstrated satisfactory consistency between the nomogram’s prediction of MVI and pathological findings.
Discussion
The non-invasive assessment of MVI holds significant guiding implications for treatment option selection. In this study, we developed and validated a GCN model for the preoperative prediction of MVI. Additionally, we constructed a nomogram for MVI diagnosis based on clinical data, radiological characteristics, and the GS. Our results indicated that features from the DP exhibited the highest predictive power in developing the GCN model. Furthermore, the nomogram of GCN-clinical-radiological demonstrated high diagnostic accuracy for predicting MVI, with an AUC of 0.884 and specificity and sensitivity rates of 87.9% and 78.0%, respectively. Our study highlights that GCN can serve as an interpretable deep learning method to explore the correlation between the radiomics signature of images and MVI, holding great potential for the preoperative identification of MVI.
Our study represents the first application of the GCN modelling approach for predicting MVI in HCC patients. The results revealed that the combination of GLCM-GLRLM demonstrated the best predictive ability for MVI among the various combinations of radiomics feature groups. A convolutional neural network (CNN) is one of the most popular methods for predicting MVI. Zhang et al23 established 3D-CNN models based on T2-weighted images, T2 fat-compression images, and PVP images, respectively. Their study found that the combined model fused with the three MR sequences exhibited the highest predictive ability, with an AUC of 0.72 at validation. Zhang’s study represents a reasonable attempt at using CNN to predict MVI. However, this also suggests that the diagnostic ability of CNN models based on image analysis alone needs improvement. Jiang et al24 developed a 3D-CNN model that combined clinical data, computed tomography (CT) radiological characteristics, and radiomics signature, achieving an AUC of 0.906 at validation. While clinical data and radiological characteristics are essential for predicting MVI, CNN demands substantial data, limiting its universal applicability in clinical medical research. Our study established an efficient GCN model with fewer radiomics features, yielding high diagnostic power (AUC = 0.854). The diagnostic ability was further enhanced after combining clinical data and radiological characteristics (AUC = 0.884), and the model exhibited good stability with higher results in both the training and validation groups. As an interpretable deep learning method, we considered that GCN could precisely predict diseases, holding excellent development prospects and potential application value in imaging diagnosis.
Multiphase dynamic contrast-enhanced scanning plays a vital role in diagnosing liver diseases, and exploring a superior phase for radiomics-related studies is beneficial for clinical practice. In a study by Ma et al,15 the radiomics signature was employed to predict MVI in HCC patients preoperatively. The results demonstrated that the radiomics features of enhanced CT images in PVP were superior to those of AP and DP. Similarly, Huang et al25 obtained a comparable result, indicating that AP images exhibited a relatively poor predictive ability when dealing with dual-phenotypic HCC. They concluded that AP MR images might cause artefacts that affect the extraction of texture parameters. Contrastingly, our findings revealed that the predictive ability of DP MR images (AUC = 0.854) is superior to that of other phases (AUC = 0.583, 0.580). We speculate that DP images are more stable and eliminate human factors associated with selecting personal AP and PVP. Therefore, we recommend the application of DP images in various radiomics fields.
In the multivariate analysis of radiological characteristics, the intratumoural artery emerged as the only independent predictor of MVI. However, contrary to previous literature, in our study, we utilized diameter, corona enhancement, mosaic architecture, and intratumoural artery as combined indicators in univariate analysis (P < 0.05) to construct the nomogram, achieving an excellent predictive effect on MVI. Most previous studies have indicated that larger tumour size is one of the preoperative correlates of MVI.15,26,27 Reports by Chen et al28 and Wei et al29 suggest that mosaic architecture is an essential radiological characteristic for predicting MVI, defined as the presence of randomly distributed internal nodules or components of different densities, intensities, enhancement, and fibre separation on images.30 Some scholars argue that tumour diameter and mosaic architecture are crucial characteristics of signal heterogeneity of tumours on images, clearly distinguishable by the naked eye, indicating a more aggressive nature of tumours.31,32 Peritumoural enhancement in the AP, also known as corona enhancement,33 has been reported several times in recent years and is considered a vital characteristic indicating the presence of MVI. The appearance of motion sickness may be attributed to MVI causing the obstruction of the microportal vein and increased compensation of hepatic artery supply.34 Lin et al’s study35 found the intratumoural artery to be an independent predictor of MVI positivity, aligning with our findings. It has been demonstrated that intratumoural arteries are associated with poor differentiation, angiogenesis, cell proliferation, and stromal invasion.36 These results can elucidate the relationship between intratumoural arteries and the aggressiveness of tumours at the pathological level and biological behaviour. Therefore, utilizing these predictors closely related to tumour aggressiveness is beneficial for improving diagnostic ability.
Diagnostic institutes specializing in radiology have acknowledged nomograms as a visually predictive model widely accepted by clinicians. In a study focused on establishing a model for predicting MVI, Lei et al26 utilized a nomogram constructed by combining tumour diameter, number, capsule, AFP, platelet level, hepatitis B virus (HBV)-DNA load, and enhanced MRI radiological characteristics to predict the MVI of HBV-related HCC within the Milan criteria, achieving a validated AUC of 0.80. Lin et al35 developed a nomogram to predict MVI by combining independent predictors such as diameter, intratumoural artery, tumour type, and AFP, obtaining a validated AUC of 0.814. Ma et al15 created a nomogram for predicting MVI by combining four clinical factors (age, diameter, AFP, and hepatitis B antigen) with seven PVP radiological characteristics, achieving a validation C index of 0.820. In this study, the radiographic characteristics of the patients were combined with the GS, and the established nomogram for predicting MVI yielded an AUC of 0.884 in validation. Compared to other studies, the GCN model demonstrated a further improvement in diagnostic ability.
This study has limitations: First, it is a single-centre retrospective study without multicentre validation. Second, for feature extraction, the study selected the maximum cross-sectional image of the lesion instead of measuring the entire volume. Third, the multiphase dynamic enhancement employed fixed-time scanning due to technical limitations, and variations in patient individualization may introduce discrepancies in the images of each phase.
Conclusion
We present a novel model for predicting MVI using the GCN method, which utilizes fewer radiomics features. This study introduces a new approach for interpretable deep learning in radiological image analysis. Furthermore, nomograms are constructed after combining clinical data and radiological characteristics, potentially enhancing the preoperative predictive capability for MVI. This model aims to contribute to the refinement of precise preoperative staging for liver cancer, playing an essential role in selecting appropriate treatment options for HCC patients.
Acknowledgements
The authors thank J.L., PhD (Philips Healthcare, China), for technical support.
Contributor Information
Yang Liu, Department of Radiology, Harbin Medical University Cancer Hospital, Harbin 150010, Heilongjiang, China.
Ziqian Zhang, Department of Radiology, Harbin Medical University Cancer Hospital, Harbin 150010, Heilongjiang, China.
Hongxia Zhang, Department of Radiology, Harbin Medical University Cancer Hospital, Harbin 150010, Heilongjiang, China.
Xinxin Wang, Department of Radiology, Harbin Medical University Cancer Hospital, Harbin 150010, Heilongjiang, China.
Kun Wang, College of Intelligent Systems Science and Engineering, Harbin Engineering University, Harbin 150001, China.
Rui Yang, Department of Medical Oncology, Harbin Medical University Cancer Hospital, No.150 Haping Road, Nangang District, Harbin 150081, Heilongjiang Province, China.
Peng Han, Department of Surgical Oncology, Harbin Medical University Cancer Hospital, No.150 Haping Road, Nangang District, Harbin 150081, Heilongjiang Province, China.
Kuan Luan, College of Intelligent Systems Science and Engineering, Harbin Engineering University, Harbin 150001, China.
Yang Zhou, Department of Radiology, Harbin Medical University Cancer Hospital, Harbin 150010, Heilongjiang, China.
Author contributions
Study concepts: Yang Liu, Ziqian Zhang, Yang Zhou. Study design: Yang Liu, Ziqian Zhang, Yang Zhou, Xinxin Wang, Kun Wang. Data acquisition: Xinxin Wang, Ziqian Zhang, Rui Yang. Quality control of data: Hongxia Zhang, Yang Liu, Kuan Luan, Peng Han. Data analysis and interpretation: Ziqian Zhang, Kun Wang, Kuan Luan. Statistical analysis: Yang Liu, Ziqian Zhang, Xinxin Wang, Rui Yang. Manuscript preparation: Yang Liu, Ziqian Zhang, Hongxia Zhang, Yang Zhou. Manuscript editing and reviewing: all authors. All authors read and approved the final manuscript. Yang Liu and Ziqian Zhang contributed equally to this work and should be considered co-first authors.
Funding
This study was funded by Natural Science Foundation of Heilongjiang Provincial Government (LH2022H067); Funding for postdoctoral research of Heilongjiang Provincial Government (LBH-Z15170) and (LBH-Q20144).
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Ethical approval was obtained by the local institutional review board (Ethical Committee of Harbin Medical University Cancer Hospital).
Consent for publication
Not applicable.
Data availability
The datasets used and/or analysed during this study are available from the corresponding author on reasonable request.
References
- 1. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR.. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bruix J, Gores GJ, Mazzaferro V.. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63(5):844-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jin YJ, Lee JW, Lee OH, et al. Transarterial chemoembolization versus surgery/radiofrequency ablation for recurrent hepatocellular carcinoma with or without microvascular invasion. J Gastroenterol Hepatol. 2014;29(5):1056-1064. [DOI] [PubMed] [Google Scholar]
- 4. Kluger MD, Salceda JA, Laurent A, et al. Liver resection for hepatocellular carcinoma in 313 Western patients: tumor biology and underlying liver rather than tumor size drive prognosis. J Hepatol. 2015;62(5):1131-1140. [DOI] [PubMed] [Google Scholar]
- 5. Lim KC, Chow PK, Allen JC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg. 2011;254(1):108-113. [DOI] [PubMed] [Google Scholar]
- 6. Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10(1):35-43. [DOI] [PubMed] [Google Scholar]
- 7. Hirokawa F, Hayashi M, Miyamoto Y, et al. Outcomes and predictors of microvascular invasion of solitary hepatocellular carcinoma. Hepatol Res. 2014;44(8):846-853. [DOI] [PubMed] [Google Scholar]
- 8. Zhao H, Chen C, Gu S, et al. Anatomical versus non-anatomical resection for solitary hepatocellular carcinoma without macroscopic vascular invasion: A propensity score matching analysis. J Gastroenterol Hepatol. 2017;32(4):870-878. [DOI] [PubMed] [Google Scholar]
- 9. Lee S, Kim SH, Lee JE, Sinn DH, Park CK.. Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J Hepatol. 2017;67(3):526-534. [DOI] [PubMed] [Google Scholar]
- 10. Zhao H, Hua Y, Dai T, et al. Development and validation of a novel predictive scoring model for microvascular invasion in patients with hepatocellular carcinoma. Eur J Radiol. 2017;88:32-40. [DOI] [PubMed] [Google Scholar]
- 11. Zhu F, Yang F, Li J, Chen W, Yang W.. Incomplete tumor capsule on preoperative imaging reveals microvascular invasion in hepatocellular carcinoma: a systematic review and meta-analysis. Abdom Radiol (NY). 2019;44(9):3049-3057. [DOI] [PubMed] [Google Scholar]
- 12. Wei Y, Huang Z, Tang H, et al. IVIM improves preoperative assessment of microvascular invasion in HCC. Eur Radiol. 2019;29(10):5403-5414. [DOI] [PubMed] [Google Scholar]
- 13. Zhang J, Liu X, Zhang H, et al. Texture analysis based on preoperative magnetic resonance imaging (MRI) and conventional MRI features for predicting the early recurrence of single hepatocellular carcinoma after hepatectomy. Acad Radiol. 2019;26(9):1164-1173. [DOI] [PubMed] [Google Scholar]
- 14. Liu Q, Li J, Liu F, et al. A radiomics nomogram for the prediction of overall survival in patients with hepatocellular carcinoma after hepatectomy. Cancer Imaging. 2020;20(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ma X, Wei J, Gu D, et al. Preoperative radiomics nomogram for microvascular invasion prediction in hepatocellular carcinoma using contrast-enhanced CT. Eur Radiol. 2019;29(7):3595-3605. [DOI] [PubMed] [Google Scholar]
- 16. Zhao B. Understanding sources of variation to improve the reproducibility of radiomics. Front Oncol. 2021;11:633176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dong H, Suarez-Paniagua V, Whiteley W, Wu H.. Explainable automated coding of clinical notes using hierarchical label-wise attention networks and label embedding initialisation. J Biomed Inform. 2021;116:103728. [DOI] [PubMed] [Google Scholar]
- 18. Kojima R, Ishida S, Ohta M, Iwata H, Honma T, Okuno Y.. kGCN: a graph-based deep learning framework for chemical structures. J Cheminform. 2020;12(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Song X, Zhou F, Frangi AF, et al. Graph convolution network with similarity awareness and adaptive calibration for disease-induced deterioration prediction. Med Image Anal. 2021;69:101947. [DOI] [PubMed] [Google Scholar]
- 20. Tian Z, Li X, Zheng Y, et al. Graph-convolutional-network-based interactive prostate segmentation in MR images. Med Phys. 2020;47(9):4164-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jiang H, Cao P, Xu M, Yang J, Zaiane O.. Hi-GCN: a hierarchical graph convolution network for graph embedding learning of brain network and brain disorders prediction. Comput Biol Med. 2020;127:104096. [DOI] [PubMed] [Google Scholar]
- 22. van Griethuysen J, Fedorov A, Parmar C, et al. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017;77(21):e104-e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang Y, Lv X, Qiu J, et al. Deep learning with 3D convolutional neural network for noninvasive prediction of microvascular invasion in hepatocellular carcinoma. J Magn Reson Imaging. 2021;54(1):134-143. [DOI] [PubMed] [Google Scholar]
- 24. Jiang YQ, Cao SE, Cao S, et al. Preoperative identification of microvascular invasion in hepatocellular carcinoma by XGBoost and deep learning. J Cancer Res Clin Oncol. 2021;147(3):821-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang X, Long L, Wei J, et al. Radiomics for diagnosis of dual-phenotype hepatocellular carcinoma using Gd-EOB-DTPA-enhanced MRI and patient prognosis. J Cancer Res Clin Oncol. 2019;145(12):2995-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lei Z, Li J, Wu D, et al. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma within the Milan criteria. JAMA Surg. 2016;151(4):356-363. [DOI] [PubMed] [Google Scholar]
- 27. Xu X, Zhang HL, Liu QP, et al. Radiomic analysis of contrast-enhanced CT predicts microvascular invasion and outcome in hepatocellular carcinoma. J Hepatol. 2019;70(6):1133-1144. [DOI] [PubMed] [Google Scholar]
- 28. Chen J, Zhou J, Kuang S, et al. Liver imaging reporting and data system category 5: MRI predictors of microvascular invasion and recurrence after hepatectomy for hepatocellular carcinoma. AJR Am J Roentgenol. 2019;213(4):821-830. [DOI] [PubMed] [Google Scholar]
- 29. Wei Y, Pei W, Qin Y, Su D, Liao H.. Preoperative MR imaging for predicting early recurrence of solitary hepatocellular carcinoma without microvascular invasion. Eur J Radiol. 2021;138:109663. [DOI] [PubMed] [Google Scholar]
- 30. Cerny M, Chernyak V, Olivie D, et al. LI-RADS version 2018 ancillary features at MRI. Radiographics. 2018;38(7):1973-2001. [DOI] [PubMed] [Google Scholar]
- 31. Khatri G, Merrick L, Miller FH.. MR imaging of hepatocellular carcinoma. Magn Reson Imaging Clin N Am. 2010;18(3):421-450, x. [DOI] [PubMed] [Google Scholar]
- 32. McHugh PP, Gilbert J, Vera S, Koch A, Ranjan D, Gedaly R.. Alpha-fetoprotein and tumour size are associated with microvascular invasion in explanted livers of patients undergoing transplantation with hepatocellular carcinoma. HPB (Oxford). 2010;12(1):56-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shinmura R, Matsui O, Kobayashi S, et al. Cirrhotic nodules: association between MR imaging signal intensity and intranodular blood supply. Radiology. 2005;237(2):512-519. [DOI] [PubMed] [Google Scholar]
- 34. Matsui O, Kobayashi S, Sanada J, et al. Hepatocelluar nodules in liver cirrhosis: hemodynamic evaluation (angiography-assisted CT) with special reference to multi-step hepatocarcinogenesis. Abdom Imaging. 2011;36(3):264-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lin S, Ye F, Rong W, et al. Nomogram to assist in surgical plan for hepatocellular carcinoma: a prediction model for microvascular invasion. J Gastrointest Surg. 2019;23(12):2372-2382. [DOI] [PubMed] [Google Scholar]
- 36. Lee JH, Lee JM, Kim SJ, et al. Enhancement patterns of hepatocellular carcinomas on multiphasicmultidetector row CT: comparison with pathological differentiation. Br J Radiol. 2012;85(1017):e573-e583. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during this study are available from the corresponding author on reasonable request.