Abstract
The mammalian sense of smell relies upon a vast array of receptor proteins to detect odorant compounds present in the environment. The proper deployment of these receptor proteins in olfactory sensory neurons is orchestrated by a suite of epigenetic processes that remodel the olfactory genes in differentiating neuronal progenitors. The goal of this review is to elucidate the central role of gene regulatory processes acting in neuronal progenitors of olfactory sensory neurons that lead to a singular expression of an odorant receptor in mature olfactory sensory neurons. We begin by describing the principal features of odorant receptor gene expression in mature olfactory sensory neurons. Next, we delineate our current understanding of how these features emerge from multiple gene regulatory mechanisms acting in neuronal progenitors. Finally, we close by discussing the key gaps in our understanding of how these regulatory mechanisms work and how they interact with each other over the course of differentiation.
Keywords: Olfaction, Olfactory receptors, Heterochromatin, Chromatin, Vomeronasal organ, Gene expression, Chemosensory neurons
1 |. A multitude of odorant chemoreceptors
The sense of smell is both broadly tuned and precise; we can detect and discriminate between a wide range of odorant compounds. The olfactory system maintains this vast receptive field by deploying a multitude of odorant-binding chemoreceptor proteins, which provide the first point of contact with the external environment. The discovery of the first vertebrate odorant receptor genes, by Buck and Axel, in 1991, was a turning point in the long search for the chemosensory proteins that underlie our sense of smell. It was immediately apparent that these odorant receptors comprised a large family of genes, which were subsequently named the olfactory receptor (OR) gene family, and indeed subsequent genome sequencing and annotation efforts have revealed that individual mammalian genomes encode hundreds or thousands of unique OR genes (Barnes et al., 2020; Buck & Axel, 1991). The ORs can be split into two groups on the basis of sequence similarity: the Class I ORs and Class II ORs (Glusman et al., 2000). The Class I ORs family, which is orthologous to OR genes found in aquatic vertebrates, is the smaller of the two groups. In mice, the Class I genes make up only around 10% of the OR gene family, with 116 genes (Bozza et al., 2009; Glusman et al., 2001; Niimura & Nei, 2005; Shi & Zhang, 2007). In contrast, the larger Class II OR genes are massively expanded in terrestrial animals, with 1,315 Class II OR genes in the mouse genome. (Niimura & Nei 2005, Clowney et al., 2011)
Additional classes of chemoreceptor proteins were soon identified, each encoded by a large gene family. Among them were the type I and type II vomeronasal receptors (V1Rs and V2Rs), the Trace Amine-associated receptors (TAARs), and, in rodents, the formyl peptide receptors (FPRs) (Dewan, 2021; Dulac & Axel, 1995; Greer et al., 2016; Herrada & Dulac, 1997; Liberles, 2008; Liberles et al., 2009; Matsunami & Buck, 1997; Rivière et al., 2009; Ryba & Tirindelli, 1997; Tirindelli, 2021). The size of the V1R and V2R gene families varies considerably among mammals, with around 240 V1Rs and 120 V2Rs in mice (Liberles et al., 2009; Tirindelli, 2021). The TAAR gene family contains 15 members in mice, 14 of which are expressed in the olfactory system (Borowsky et al., 2001; Dewan, 2021). The FPR family is smaller, with only 5 genes in mice (Greer et al., 2016; Liberles et al., 2009). All of these receptors are part of the G protein-coupled receptor (GPCR) super-family of seven-pass transmembrane domain receptor proteins. More recently, a non-GPCR family of receptor proteins, the 12 members of the Membrane spanning 4-pass A receptors (MS4As), were also identified as odorant receptors (Greer et al., 2016).
Most mammals have two large olfactory sensory organs known as the main olfactory epithelium (MOE) and the vomeronasal organ, and two smaller structures — the septal organ and the Grueneberg Ganglion. The ORs, TAARs, and MS4As are primarily expressed by olfactory sensory neurons in the MOE, which are specialized for the detection of volatile small molecules. While ORs are broadly receptive to a wide variety of odorants, TAARs are particularly attuned to amine odorants, and MS4As are sensitive to fatty acids and some pheromones (Brann & Datta, 2020; Dewan, 2021; Greer et al., 2016; Kurian et al., 2021; Liberles et al., 2009; Zhang et al., 2004). The V1Rs, V2Rs, and FPRs are primarily expressed by vomeronasal sensory neurons in the vomeronasal organ, which is adapted to detect non-volatile odorants, such as steroid molecules, peptides, and proteins. The septal organ is a smaller olfactory organ, constituting about 1% of the olfactory system (Ma et al., 2003). It resembles the MOE in that it expresses a subset of OR genes and uses the same signal transduction pathway as OR-expressing OSNs from the MOE. The Grueneberg Ganglion is located just inside the nostril, and functions as an independent olfactory subsystem that uses a cGMP signaling pathway to transduce olfactory signals (Fleischer, 2021; Liu et al., 2009).
Individual olfactory sensory neurons (OSNs) in the MOE and vomeronasal sensory neurons (VSN) in the vomeronasal organ do not express all of the odorant chemoreceptor proteins that are available to them. Rather, individual cells “choose” a subset of these receptors for expression. Expression of ORs, TAARs, and V1Rs is monogenic and monoallelic, with each sensory neuron expressing only one allele of one receptor gene (Roppolo et al., 2007; Shykind et al., 2004). V2R expression is combinatorial, with limited coexpression of specific subsets of V2R genes in mature VSNs (Akiyoshi et al., 2018). The exception to this restricted pattern of receptor expression are the non-GPCR MS4A receptors. These receptor genes are coexpressed, with necklace olfactory sensory neurons expressing multiple MS4A genes at the same time (Greer et al., 2016; Zimmerman & Munger, 2021).
The gene regulatory mechanisms that coordinate receptor choice have been the subject of intense interest ever since the OR genes were first discovered. We will review the regulation of chemoreceptor expression in the MOE, with a focus on how class II OR choice is enacted over the process of OSN differentiation. We will also discuss how the concepts that emerge from OR gene regulation may be applicable to the expression of other odorant chemoreceptor gene families.
2 |. Monogenic and monoallelic expression of ORs
From the time of their discovery, it was proposed that individual olfactory sensory neurons (OSNs) were likely to express only a small subset of the large family of receptor genes (Buck & Axel, 1991). Early experiments visualized OSNs that express specific ORs using a variety of methods, including in situ hybridization, genetically tagged OR reporter genes, and OR-specific antibodies (Mombaerts et al., 1996; Ressler et al., 1993; Strotmann et al., 1992, 2004; Vassar et al., 1994). These experiments all consistently observed that individual OR genes were expressed within a particular spatial region, referred to as a zone, of the olfactory epithelium. Within the appropriate zone, ORs are expressed by a seemingly random subset of cells. The axon projection pattern of OSN observed in these experiments further supported monogenic expression. (Mombaerts et al., 1991) OSNs expressing the same labeled OR all project an axon to one of a few specific glomeruli in the olfactory bulb (Ressler et al., 1994; Vassar et al., 1994; Mombaerts et al., 1996). However, because these experiments did not interrogate OR expression by single cells directly, they left open the possibility of transient or coordinated coexpression of OR genes (Mombaerts, 1999).
Experimental approaches that examine OR expression in single cells confirmed monogenic choice as the predominant mode of OR expression. This was first accomplished by combining single-cell RT-PCR with restriction enzyme fingerprinting of OR gene expression, which showed that only a single type of OR transcript can be amplified from individual OSNs (Malnic et al., 1999). Over the past decade, single-cell RNA sequencing (scRNAseq) profiling of cells from the MOE has allowed much higher throughput examination of OR gene expression in large numbers of individual OSNs. These experiments have consistently shown that most mature OSNs (mOSNs) highly express one OR gene at high levels and they rarely detect mOSNs that express two or more OR transcripts (Hanchate et al., 2015; Saraiva et al., 2015; Wu et al., 2018). These experiments also detected TAAR expressing OSNs, which exhibit a similar pattern of monogenic choice (Saraiva et al., 2015). Thus, it is now clear that the vast majority of OSNs express a single OR at high levels.
The single OR gene expressed by each OSN is from only one allele. Monoallelic expression was first demonstrated by cloning OR gene transcripts from mice that were the hybrid progeny of distantly related strains (Chess et al., 1994). When specific ORs were cloned from RNA collected from small numbers of OSNs, either the maternal or paternal allele was detected but not both, reflecting the monoallelic expression of that OR by a single cell in the pool (Chess et al., 1994; Shykind et al., 2004). Monoallelic expression is not observed in bulk RNA preparations, indicating that while monoallelism is observed at the levels of individual OSNs, both alleles are expressed across the population of OSNs in the olfactory epithelium. Subsequent experiments using genetic labeling of OR genes confirmed this observation. Analysis of mice in which two alleles of the same OR gene are differentially labeled reveals that individual OSNs express one or the other allele but not both (Feinstein & Mombaerts, 2004; Shykind et al., 2004). The two alleles are otherwise equivalent; the maternal and paternal alleles are both expressed within the same zone, and OSNs expressing the maternal and paternal allele project to the same glomerulus.
Monogenic OR choice effectively divides OSNs into hundreds of distinct subtypes, with distinct transcriptional profiles. scRNAseq profiling of tens of thousands of OSNs revealed that the set of OSNs expressing the same OR gene have a distinct transcriptome that distinguishes them from other OSN expressing other OR genes (Horgue et al., 2022; Tsukahara et al., 2021a). Accordingly, for each OSN, the expression levels of non-OR genes can be used to predict the identity of the expressed OR with a high degree of accuracy. Critically, the OR-correlated genes include many axon guidance molecules which, together with zonal expression of additional axon guidance molecules (Mori & Sakano, 2011; Takeuchi et al., 2010), accomplishes the targeting of OSN axons to specific glomeruli in the olfactory bulb (Greer et al., 2016; Mombaerts et al., 1996; Nakashima et al., 2019; Shayya et al., 2022; Shykind et al., 2004; I.-H. Wang et al., 2022).
The OR itself has an important role in generating these OR-specific OSN subtypes’ distinct identities. There are several mechanisms that couple OR choice to the transcriptional regulation of non-OR genes These include odorant-invoked neuronal activity, and the intrinsic G protein signaling activity of the OR protein (Connelly et al., 2013; Nakashima et al., 2013, 2019; Serizawa et al., 2003, 2006). However, recent work has demonstrated a direct role for the amino acid sequence of the selected OR in regulating gene expression (Shayya et al., 2022). When a ribosome translates the OR coding sequence into a protein, it leads to the activation of the unfolded protein response (UPR). The degree of UPR activation depends on the primary amino acid sequence of the OR. The UPR, in turn, regulates the expression of hundreds of genes, including guidance molecules that control OSN axon targeting. This UPR-based mechanism allows the OR coding sequence itself to modulate gene expression from the early stages of OR translation, irrespective of odorant stimulation.
3 |. OR choice over OSN differentiation
Monogenic and monoallelic choice of an OR gene is thus central to the organization, structure, and function of the olfactory system, so how is it accomplished? When and how is an OR gene chosen for expression? OR gene choice is coordinated by a dynamic sequence of events that occur in OSN progenitors. These include a cell fate-dependent restriction of the OR genes available for choice, an initial wave of low-level OR gene expression wherein multiple OR genes are coexpressed, and finally, a feedback signal that locks in monoallelic and monogenic choice.
3.1 |. Stages of OSN differentiation
For the purposes of this review, we will be classifying the different stages of OSN development as described in Fletcher et al., 2017. (Figure 1) - Horizontal Basal Cells (HBCs), Globose Basal Cells (GBCs), Immediate Neuronal Progenitors (INPs), Immature olfactory sensory neurons (iOSNs) and finally mature OSNs (mOSNs). HBCs constitute a quiescent pool of multipotent stem cells that are maintained throughout life, and that can be activated to proliferate and regenerate the MOE following an injury (Leung et al., 2007). In contrast, GBCs constitute the proliferative stem cell population that continuously replaces epithelial cells lost over time. GBCs can give rise to all of the cell types of the olfactory epithelium, including OSNs and cell types outside the OSN lineage, such as microvillus cells, sustentacular cells, and cells of the Bowman’s gland (Caggiano, 1994, Chen et al 2014). Differentiating cells are fate-restricted to become OSNs after the GBC stage when they become Immediate neuronal precursors (INP). INPs can be subdivided into three sequential stages (INP1, INP2, INP3) based on gene expression patterns (Fletcher et al., 2017). Each differentiating GBC can give rise to multiple OSNs as progenitor cells remain mitotically active in the INP1 stage.
Figure 1: Fate Restriction of Olfactory Sensory Neurons leading to stochastically chosen Olfactory Receptor gene.
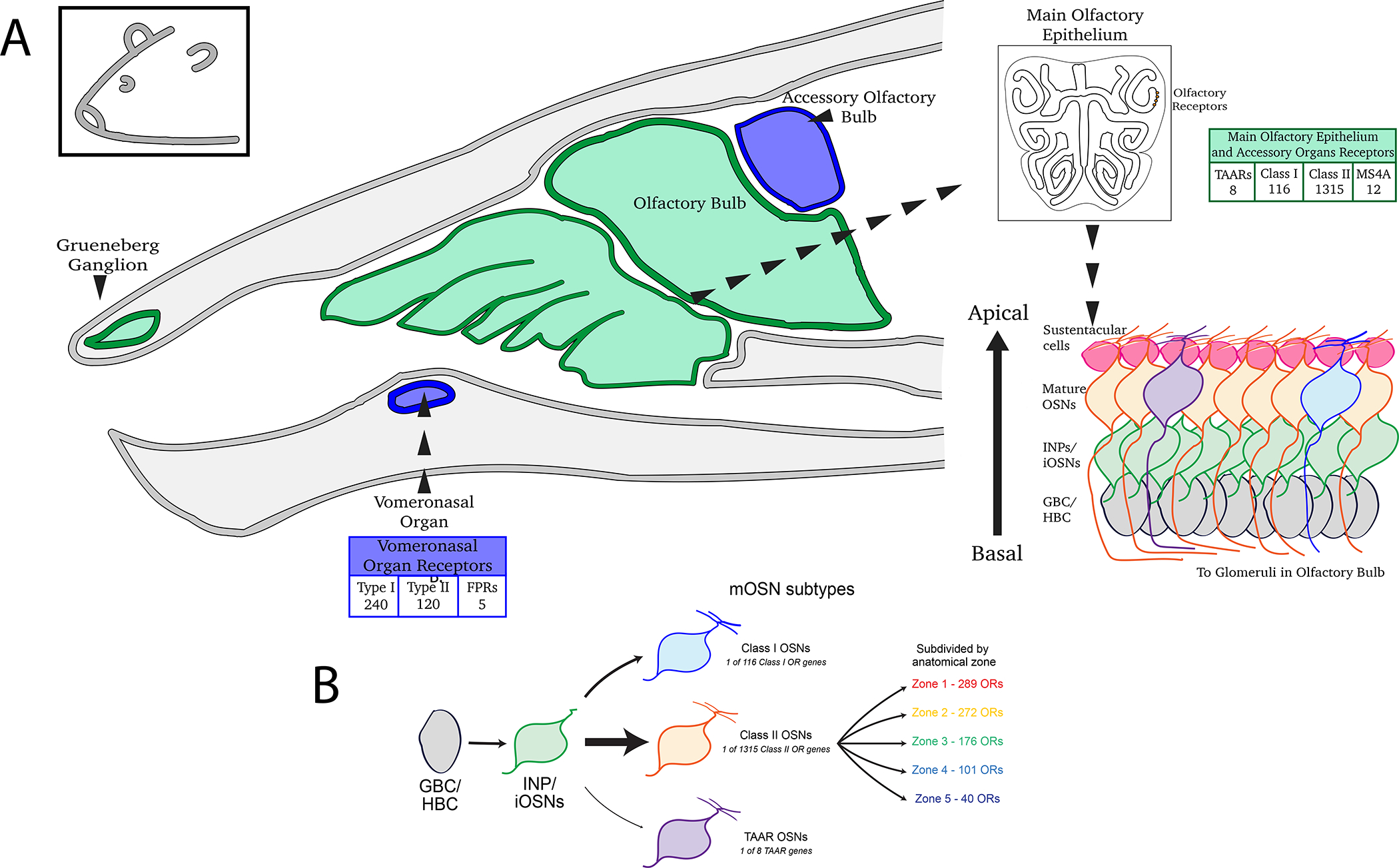
Olfactory sensory neurons go through several stages of differentiation to reach a mature olfactory sensory neuron state. A. Anatomy and classification of the different types of odorant receptors based on where they are present, including total numbers of each receptor type. Type I, Type II and Formyl Peptide Receptors (FPRs) are present in the Vomeronasal Organ. Trace Amine-Associated Receptor, Class I, Class II and MS4A receptors are present in the main olfactory epithelium and accessory organs like septum and Gruenberg ganglion. B. During differentiation, two levels of cell-fate determination occur. Global Basal Cells (GBCs) or Horizontal Basal Cells (HBCs) are stem cells that can give rise to intermediate neuronal progentiors (INP) and then immature OSN (iOSN) stage. They are restricted into either a class I or class II or TAAR type of OSN, followed by stochastic expression of chosen olfactory receptor (OR). Class II OSNs are further estricted into zonal identities (Zone 1–5) that correspond to specific anatomical regions of the tissue.
3.2 |. Restriction of OR gene choice
A given OSN can only choose from a restricted subset of the more than 2000 OR gene alleles encoded in the mouse genome. There are at least two levels of cell-fate determination that restrict OR choice to tens or hundreds of OR genes. At the highest level, cells commit to a fate tied to a specific class of receptors (Figure 1). Within the main olfactory epithelium, OSN progenitors are restricted to express either class I or class II OR genes based upon expression of a transcriptional repressor - Bcl11b or Ctip2 (Enomoto et al., 2019). Class I OR choice is the default fate of OSNs. Expression of Bcl11b suppresses the class I OR enhancer, J element, and induces Class II OR choice. TAAR deletion allele expression studies have shown that OSNs that express TAARs are restricted to their fate separately from class I or II ORs; and the activity of enhancer elements that activate TAAR genes is restricted to this set of cells (Fei et al., 2021; Pacifico et al., 2012; Shah et al., 2021).
For progenitors fated to select class II ORs, there is further restriction of the available OR pool which reflects a commitment to a specific zonal identity, related to the spatial position of the OSN within the olfactory epithelium (Figure 1). As described above, ORs tend to be expressed within specific bands of cells, termed “zones” (Vassar et al 1994, Ressler et al., 1994, Miyamichi et al., 2005), that are located at specific positions along the dorsal-medial axis of the tissue. This phenomenon was first observed by RNA in situ hybridization, with probes targeting specific OR genes. For individual ORs, their specific expression in a specific zone depends, at least in part, on unidentified cis-regulatory elements located near the OR gene (Qasba & Reed, 1998; F. Wang et al., 1998). As discussed below, these elements might control zonal expression by recruiting heterochromatin to OR genes.
How many zonal identities are there, and are these identities smoothly distributed or granular and distinct? (Tan et al., 2018) produced the first comprehensive anatomical map of OR expression patterns by sequencing microdissected fragments of olfactory epithelium tissue. They were then able to assign zonal positions to nearly the full set of OR genes based upon their co-occurrence in these fragments with ORs with known zonal expression patterns. These zonal assignments have largely been confirmed by 3D transcriptomic maps assembled from the sequencing of tissue sections (Ruiz Tejada Segura et al., 2022). Spatial gradients of OR expression are also evident from scRNAseq analysis of OSNs; each OR subtype expresses characteristic levels of a set of genes that vary with anatomical position within the tissue (Tsukahara et al., 2021). However, zonal restriction may be more granular than the spatial zones identified through these bulk spatial strategies. In particular, high-resolution mapping of multiple combinations of ORs suggests that there are distinct subpopulations of OSNs that reside within, and sometimes across, zones, with each subpopulation capable of expressing a specific pool of OR genes (Zapiec & Mombaerts, 2020). OSNs within each zonal subpopulation stochastically choose one OR gene out of a couple hundred possibilities, rather than choosing from the full repertoire of over 1000. It will be exciting to see whether new, higher-resolution spatial transcriptomics approaches are able to precisely and comprehensively define the full set of zonal OSN subpopulations.
3.3 |. Transient expression of multiple OR genes
When are OR genes first transcriptionally activated during OSN differentiation, and what are the properties of this initial OR expression? In addition to allowing direct observation of monogenic OR expression in OSNs, the application of scRNA-seq to the olfactory epithelium also revealed that OSN progenitors transiently express multiple OR genes. This observation emerged from the first scRNAseq study of the MOE by (Hanchate et al., 2015). This study used a microfluidic strategy, which allowed deep sequencing of a limited number of cells, to generate transcriptome data for 85 cells extracted from adult mouse olfactory epithelium. These cells were assigned to developmental stages using the expression of known marker genes. OR gene expression is first detectable in early INP stages. Remarkably, this early expression is characterized by the expression of multiple OR genes at low levels. This observation was subsequently confirmed by multiple groups (Tan et al., 2015; Saraiva et al., 2015; Scholz et al., 2016). The ORs that are coexpressed by individual INP cells usually come from multiple chromosomes, reaching as many as 9 in some cells. However, the coexpression of OR genes from the same chromosome is also observed in some cells (Bashkirova et al., 2023). Taken together, these observations suggest that there is a broad initial wave of OR transcription in INP cells, involving the transcriptional activation of OR genes across multiple loci on multiple chromosomes, but that this initial coexpression is at low transcript levels.
It remains unclear how early coexpression relates to subsequent OR choice. Intriguingly, there is an asymmetric relationship between early coexpression and zonality (Bashkirova et al., 2023). OSN progenitors extracted from dorsal zones of the olfactory epithelium coexpress the dorsally expressed ORs. However, OSN progenitors extracted from ventral zones of the olfactory epithelium do not exhibit this zonal restriction. Rather, they coexpress ORs from all zones. This difference may reflect the mechanisms that control zonal restriction, as discussed below.
3.4 |. A feedback signal stabilizes singular choice
Once a single receptor gene is stochastically selected from the available pool, this choice kicks off a feedback signal that triggers OSN maturation and prevents the activation of additional OR genes. The effects of this feedback signal was first observed in experiments that used transgenes to introduce labeled OR genes into the mouse genome. In these experiments cells that expressed a labeled transgene would not express any other OR genes, not even additional copies of the same OR gene with identical regulatory sequences (Fleischmann et al., 2008; Serizawa et al., 2003). This effect, termed allelic exclusion, requires the OR coding sequence. Replacement or mutation of the OR coding sequence eliminates allelic exclusion, allowing coexpression with other OR genes (Feinstein & Mombaerts, 2004; Lewcock & Reed, 2004; Serizawa et al., 2003). This requirement for an intact coding sequence prevents the OR pseudogenes, which number in the hundreds, from triggering the feedback mechanisms and getting stably chosen.
The OR-induced feedback signal passes through the unfolded protein response (UPR) (Read & Schröder, 2021). When transcripts encoding an intact OR coding sequence are translated by OSN progenitors, it leads, through an unknown mechanism, to the activation of the UPR (Dalton et al., 2013). Activation of the UPR leads to the translation of a transcription factor, Atf5, which upregulates genes required for OSN maturation. These include key signaling proteins, such as Adenylyl cyclase 3, and chaperones, such as RTP1 and RTP2, that help traffic OR proteins (Sharma et al., 2017). Critically, subsequent signaling by Adenylyl cyclase 3 results in downregulation of factors that are required to activate new OR genes, including a histone demethylase, Lsd1 (Dalton et al., 2013). Accordingly, mutations affecting the UPR pathway block OSN maturation, leaving OSN progenitors arrested as iOSNs, and result in chronically switching between expression of different OR genes. Thus translation of an OR with a functional coding sequence is a key checkpoint, which leads to OSN maturation and blocks the transcriptional activation of additional OR genes.
Based on these observations, a major outstanding question pertains to the timing of this feedback signal and how it relates to OR coexpression. Exogenous transcriptional activation of a functional OR gene is sufficient to induce this feedback signal (Dalton et al., 2013) and exogenous transcriptional activation of an OR gene early in differentiation can skew OR choice toward the activated OR gene. For example, the insertion of a Tet-operator binding site into the promoter of OR gene allows transcriptional activation of that OR by the expression of a tet-transactivator (tTA) protein (Fleischmann et al., 2008, 2013; Nguyen et al., 2007). If tTA expression is expressed in OSN progenitors, then the TetO-driven OR is chosen at high frequency across all zones, and this choice is stably maintained even if tTA expression later stops (Bashkirova et al., 2023; Fleischmann et al., 2013). Strikingly, OR activation by tTA can be blocked by expression of an endogenous OR, or by ubiquitous expression of an OR transgene, presumably because the feedback signal had already occurred in these cells (Fleischmann et al., 2013; Nguyen et al., 2007). These results suggest that there may be a threshold level of expression that is sufficient to activate choice and that breaching this threshold marks the transition from early co-expression to singular choice. Indeed, transgenic OR expression systems suggest that there is a threshold level of OR expression above which OR expression suppresses the expression of other OR genes (Abdus-Saboor et al., 2016). What then is the purpose, if any, of OR coexpression, and what leads to the upregulation of a single OR gene that leads to choice?
4 |. Regulation of OR gene expression
What are the key regulatory elements and factors that control the expression of OR genes? Given the huge size of the OR gene family, there are many genes that need to be regulated in a coordinated fashion. Examination of the regulatory elements that govern OR expression has revealed shared features that likely serve to coordinate their expression. These features include the clustering of OR genes in the genome, a large family of regulatory enhancer elements, and shared sequence motifs.
4.1 |. Genomic organization of OR genes and regulatory elements
Most OR genes are located in multi-gene clusters (Monahan et al., 2017). These clusters vary considerably in size, and, at the high end, can encompass hundreds of receptor genes and several megabases of DNA (Zhang & Firestein, 2002). In mice, all of the class I OR genes are located in a single large gene cluster located on chromosome 7. The Class II ORs, in contrast, are widely spread, with dozens of clusters that are spread across almost every mouse chromosome, including their regulatory enhancer elements (Figure 2, Table S1). These clusters have common properties, such as high AT content and enrichment for LINE retrotransposon elements, that could possibly distinguish them from surrounding non-OR genomic regions (Brovkina et al., 2023; Glusman et al., 2001; Ormundo et al., 2020). Moreover, OR clusters also behave as a unit in regards to how they are packaged with nuclear proteins to form chromatin. As discussed below, OR genes are marked with histone modifications associated with heterochromatin over the course of OSN differentiation (Magklara et al., 2011). When these repressive marks are deposited, they are added cluster-wide.
Figure 2: Location of class I and class II OR and TAAR genes across mouse genome.
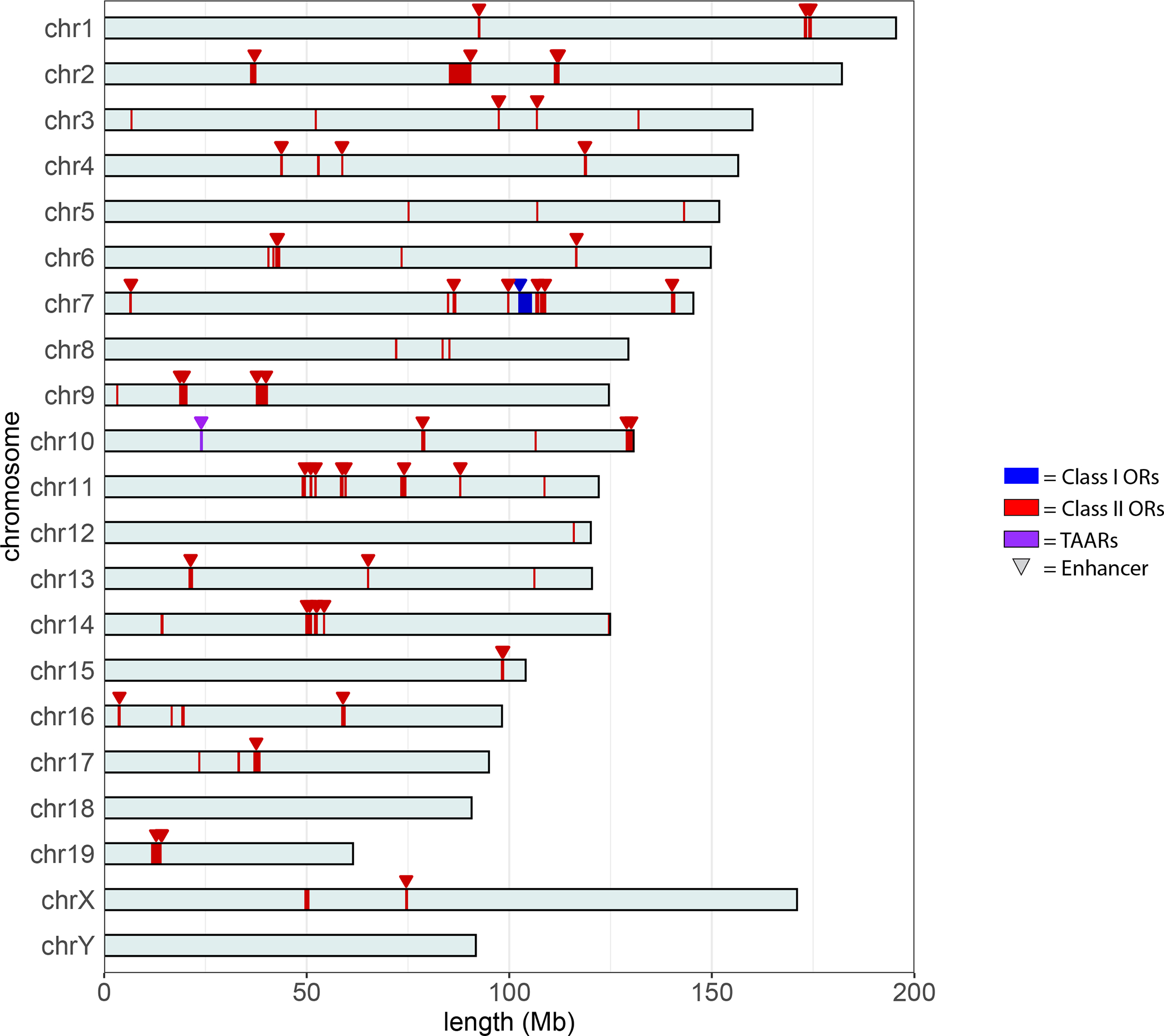
Class I OR genes (blue) are located in one cluster in chromosome 7 and TAAR gene cluster (purple) is in chromosome 10. Meanwhile, class II OR gene clusters (red) are spread across multiple chromosomes.
Within OR gene clusters, the OR genes themselves each have their own promoter element. OR gene promoters are unusual; they are very AT-rich relative to most gene promoters and they generally lack CpG islands (Clowney et al., 2011). In addition to OR promoters, OR clusters also contain regulatory DNA elements, called enhancers, that can activate the expression of the surrounding OR genes (Pourmorady & Lomvardas, 2022). The first OR enhancers, H and P, were identified in class II OR clusters on the basis of sequence similarity and enhancer activity in transgene reporters (Bozza et al., 2009; Serizawa et al., 2003). Many more enhancers have subsequently been identified in class II clusters on the basis of chromatin state and the binding of specific transcription factors in OSNs (Markenscoff-Papadimitriou et al., 2014; Monahan et al., 2017). Intriguingly, recent work using single-cell chromatin accessibility assays have shown that OR enhancers are most accessible in OR progenitor cells, and suggest that additional enhancer elements remain to be identified (Poumorady et al 2024, Xie, Wu et al, 2023). These elements, many of which are named after Greek Islands, are capable of driving the expression of a reporter transgene in the olfactory epithelium (Markenscoff-Papadimitriou et al., 2014), and targeted deletions of individual OR enhancer elements have shown that they are required for normal OR gene expression of nearby OR genes (Fuss et al., 2007; Khan et al., 2011; Markenscoff-Papadimitriou et al., 2014; Nishizumi et al., 2007).
4.2 |. Shared transcription factors
A common feature of both OR promoters and enhancers is the presence of specific sequence motifs. The most frequently shared motifs across OR promoters are an O/E motif that is recognized by members of the Ebf family and a homeodomain motif that can bind homeodomain proteins, including Lhx2 and Emx2 (Michaloski et al., 2006; Clowney et al., 2011; Plessy et al., 2012). Nearly all of the OR enhancers contain these same motifs (Monahan et al., 2017). Chromatin immunoprecipitation experiments have shown that the enhancer sites are bound by Lhx2 and members of the Ebf-family in mOSNs, but that these factors are absent from OR promoters. This difference may be because these factors only bind when the promoter is active, which would make them undetectable in heterogeneous populations of bulk OSNs. Nonetheless, these binding sites are functional in the context of OR promoters; Ebf and homeodomain sites are required for the full activity of an OR-promoter transgene and of an endogenous OR promoter (Rothman et al., 2005; Vassalli et al., 2002). In addition, multimerization of an extended version of the homeodomain motif can drive strong transgene expression in OSNs throughout the olfactory epithelium (D’Hulst et al., 2016; Vassalli et al., 2011). Intriguingly, OR enhancers are also enriched for a distinct composite motif in which Ebf and Lhx2 sites are located immediately adjacent to each other (Monahan et al., 2017). This close juxtaposition of Ebf and Lhx2 sites in OR enhancers may allow cooperative binding of these factors. This model is supported by the observation that conditional deletion of Lhx2 in OSNs also results in loss of Ebf binding to these sites. Thus Ebf proteins and Lhx2 act as common regulatory factors that are widely distributed in OR gene regulatory sequences, and OR enhancers, in particular, are characterized by cooperative binding of these factors to closely spaced sites. Future studies should determine whether the close spacing of Ebf and Lhx2 binding sites in OR enhancers creates a distinctive protein complex that could account for the ability of these elements to interact with each other to form interchromosomal enhancer hubs, as is discussed below.
Notably, the class I ORs and TAAR clusters are also regulated by enhancers that contain Ebf and homeodomain sites, but these elements also contain binding sites for additional factors that likely restrict their activity to the appropriate population of cells. A single conserved enhancer, the J element, is located in the type I OR cluster and is required for the expression of most class I OR genes (Iwata et al., 2017). The J enhancer contains conserved Ebf and homeodomain motifs but also contains a highly conserved sequence that is not found in class II OR enhancers. It will be interesting to determine whether this additional motif is responsible for the suppression of the J enhancer by Bcl11b expression in class II-fated OSNs (Enomoto et al., 2019). The TAAR gene cluster contains a pair of conserved enhancers that cooperatively activate TAAR genes (Fei et al., 2021; Shah et al., 2021). The TAAR enhancers share a long, conserved ~30bp sequence motif that could coordinate TAAR-fated OSN-specific activation of these genes (Shah et al., 2021). Intriguingly, the TAAR enhancers also contain multiple homeodomain motifs, Ebf motifs, and composite motifs resembling those observed in OR enhancers (Fei et al., 2021). The J element and the TAAR enhancers are not strongly bound by Ebf or Lhx2 in bulk populations of OSNs, but it remains possible these factors bind to these elements specifically in class I OR-fated or TAAR-fated OSNs.
5 |. Epigenetic programs for OR choice
As OSN progenitors differentiate, they enact an epigenetic program that remodels how OR genes are packaged and organized within the nucleus (Figure 3). This program results in widespread, coordinated changes in the chromatin state and the spatial positioning of OR gene clusters and enhancers. The outcome of this process is the sequestration of the non-chosen OR genes into nuclear structures where they are transcriptionally silenced, and the assembly of an activating nuclear structure, called an enhancer hub, that drives the expression of the single chosen OR allele.
Figure 3: Model of Olfactory Receptor gene arrangement.
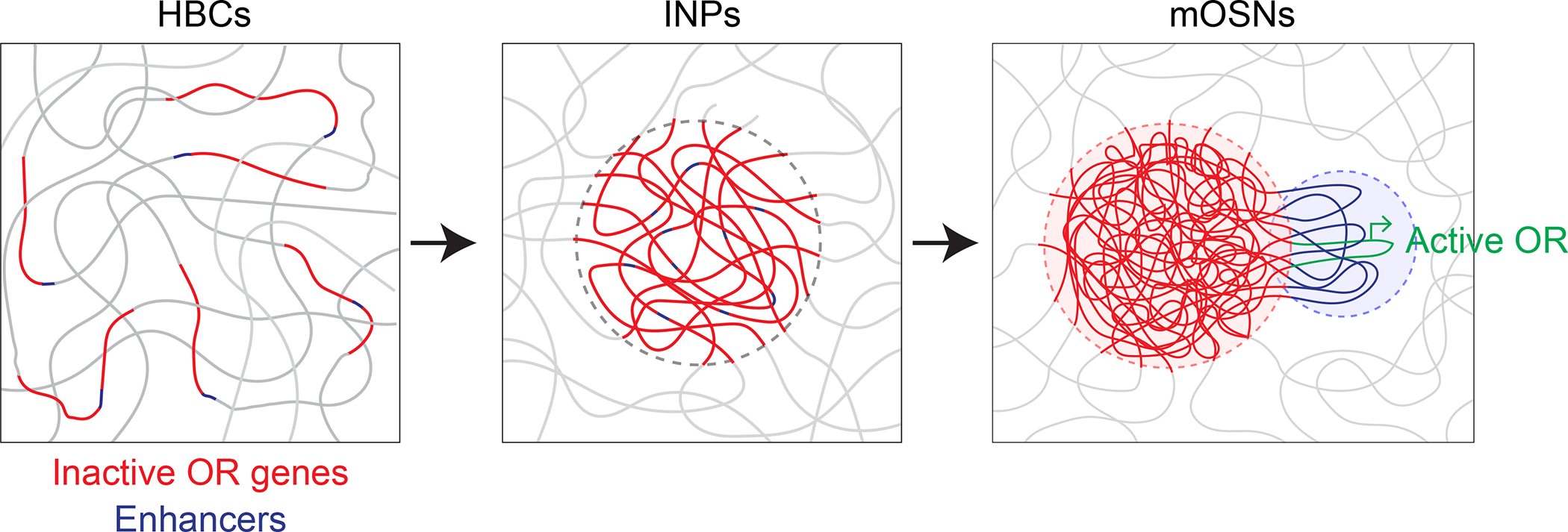
Inactive Olfactory Receptor (OR) genes rearrange themselves in 3D space during the course of differentiation from Horizontal Basal Cells (HBC) to Immediate Neuronal Progenitors (INPs) to Mature Olfactory Sensory Neurons (mOSNs). The active OR is looped out of the constitutively silenced heterochromatin compartment and associates with the enhancer hub so that the single chosen OR is ready for transcription in mOSNs.
5.1 |. OR choice is encoded in chromatin structure in OSNs
In eukaryotic cells, genomic DNA is packaged together with proteins to form chromatin, and the local organization of chromatin both reflects and influences gene expression. In particular, DNA is wrapped around histone proteins to form nucleosomes. Histones can be modified through either acetylation, methylation or phosphorylation, which influence the recruitment of regulatory proteins that modify chromatin structure and/or alter gene expression (Bannister & Kouzarides, 2011).
In OSNs, Class II OR gene clusters bear histone modifications typically observed on constitutive heterochromatin, which typically keeps genes tightly repressed and transcriptionally silent. In particular, chromatin immunoprecipitation experiments performed on primary, purified OSNs show that class II OR gene clusters are broadly enriched for histone H3, lysine 9 trimethylation (H3K9me3), and histone H4, lysine 20 trimethylation (H4K20me3) (Magklara et al., 2011). In contrast, these marks are absent from the TAAR gene cluster and much less enriched on the class I OR genes, although it remains possible that these marks are present in the specific subpopulations of OSNs that express these genes (Bashkirova et al., 2023; Fei et al., 2021). The enrichment of heterochromatin marks on OR genes can also be detected by imaging of OSNs in tissue sections; combined immunohistochemical and DNA-FISH experiments reveal that probes that label OR gene loci overlap with heterochromatin marks (Clowney et al., 2012; Armelin-Correa et al., 2014). Direct interrogation of chromatin compaction using sucrose gradient sedimentation reveals that OR genes chromatin are densely compacted in mature OSNs (Magklara et al., 2011), which suggests that these marks do indeed seem to reflect heterochromatinization of OR genes in OSNs, rather than some alternative cell-type specific function.
The heterochromatin marks observed on OR genes in mOSNs reflect the chromatin state of the non-chosen OR genes; in contrast, the chromatin around the active OR gene is open and accessible. Since only one allele is active per cell, marks associated with silencing predominate on all OR genes in bulk populations of OSNs. However, imaging cells that express a specific OR reveals that the active OR allele is looped out of the heterochromatin foci that contain most OR genes, and instead localized to euchromatic regions of the nucleus (Clowney et al., 2012; Markenscoff-Papadimitriou et al., 2014). Combined immunohistochemical and in situ hybridization experiments showed that the active OR alleles have minimal overlap with heterochromatic markers like H3K9me3, H4K20me3, and heterochromatin protein 1B (HP1B). Instead the active OR allele resides in euchromatic portions of the nucleus. Accordingly, chromatin immunoprecipitation analysis of OSNs purified on the basis of their expression of a specific OR, Olfr1507, reveals that the active Olfr1507 allele lacks heterochromatin marks (Magklara et al., 2011). Moreover, ATAC-seq analysis of three OSN subtypes, purified using GFP labeled endogenous OR genes, reveals that in each case the chosen OR gene is open and accessible (Monahan et al., 2017).
5.2 |. OR gene chromatin is remodeled over OSN differentiation
What processes generate the different chromatin states at the non-chosen and chosen ORs? Heterochromatic histone modifications are deposited on OR gene clusters in OSN progenitors (Bashkirova et al., 2023; Magklara et al., 2011). ChIP-seq analysis of FACS purified GBCs, INPs, iOSNs, and mOSNs reveals that H3K9me3 marks and H3K79me3 marks, which also label OR clusters in OSNs, are first detectable in INP cells and then increase substantially by the iOSN stage, at which point they resemble the levels seen in mOSNs. It remains unknown how these marks are targeted to OR gene clusters. However genetic evidence suggests that heterochromatin deposition has an important role in influencing OR gene choice. In particular, conditional knockout of a pair of histone methyltransferases, G9a (KMT1C) and GLP (KMT1D), that catalyze mono- or di-methylation of H3K9, which may then be further converted to the tri-methyl state, results in reduced intensity of H3K9me3 staining and less compact organization of OR genes in OSNs (Lyons et al., 2014). Strikingly, OR expression in these mice is skewed toward a handful of OR genes, and these overexpressed OR genes are frequently coexpressed. OR choice also requires a histone demethylase, Lsd1, which, among other targets, is able to demethylate H3K9me2 (Lyons et al., 2013). Conditional deletion of Lsd1 prior to OSN differentiation results in widespread loss of OR expression. Notably, Lsd1 is one of the genes that is downregulated by the OR-induced feedback signal that blocks the activation of additional OR genes, and overexpression of Lsd1 leads to unstable OR expression.
Taken together, these observations suggest that OR gene choice is preceded by a cycle of heterochromatin deposition, involving G9a/Glp, and then heterochromatin removal by Lsd1. This cycle could possibly serve to balance the probability of OR choice by initially silencing all OR genes in a way that prevents intrinsic differences in promoter strength or access to enhancers from skewing choice (Lyons et al., 2014). Alternatively, heterochromatin deposition could also serve as a regulatory checkpoint delimiting the pool of OR genes available for choice. It is unclear whether this cycle involves H3K9me3 or an intermediate mark such as H3K9me1/2. All of the enzymes implicated by genetic evidence either deposit or remove mono- or di-methyl H3K9 marks. It is possible that OR genes available for choice receive an H3K9me1/2, and then the non-chosen OR genes are subsequently converted to H3K9me3 for permanent silencing.
5.3 |. The 3D positioning of OR genes and enhancers coordinates singular choice
Despite being dispersed across many chromosomes, many class II OR genes from multiple chromosomes are colocalized in the nucleus of mOSNs. The first indications of spatial localization of OR gene clusters from different chromosomes came from circular chromosome conformation capture (4C) experiments, which detect 3D spatial proximity between pairs of loci through the sequencing of proximity-dependent ligation products (Lomvardas et al., 2006). These experiments showed that the H enhancer interacts with many OR genes from multiple chromosomes specifically in OSNs. Subsequently, in situ Hi-C, which uses the same proximity-ligation approach as 4C but on a genome-wide scale (Belton et al., 2012; Pal et al., 2019), was used to map spatial interactions between every locus in the genome in mouse mOSNs. Hi-C detects a vast network of strong interactions between class II OR gene clusters in mature OSNs (Monahan et al., 2019). These include interactions between OR clusters on the same chromosome (cis intrachromosomal contacts) and between clusters located on different chromosomes (trans interchromosomal contacts). The high resolution of Hi-C also revealed that class II OR enhancers specifically interact with each other, forming a network of interactions that is distinct from, stronger, and more specific than the interactions between OR clusters. By sorting cells expressing specific labeled OR genes it was shown that the chosen OR gene associates with this network of enhancer interactions. Importantly, a pair of OR enhancer-binding proteins, Lhx2, and its cofactor Ldb1, are required for the enhancer interactions; conditional deletion of either Lhx2 or Ldb1 in OSNs strongly reduces interaction between the OR enhancers and also significantly reduces the expression of nearly all class II OR genes. These findings collectively suggest a model where the non-chosen OR genes aggregate together into large heterochromatic foci or “hubs”, while the chosen OR allele associates instead with a network, or hub, of OR enhancers.
Examination of the spatial co-localization of OR genes and enhancers in single cells revealed that the nuclei of mOSNs contain multiple hubs of OR genes and OR enhancers. When olfactory epithelium tissue sections are stained with a complex “pan-OR” probe that fluorescently labels all OR genes, the OR genes appear in a small number of large foci in OSN nuclei, rather than dispersed throughout the nuclear volume as in other cell types (Armelin-Correa et al., 2014; Clowney et al., 2012; Markenscoff-Papadimitriou et al., 2014). Multi-color DNA fluorescent in situ hybridization also revealed that multiple OR enhancers associate with each other and with the active OR allele in OSN nuclei (Clowney et al., 2012; Markenscoff-Papadimitriou et al., 2014). Experiments using a single-cell version of the Hi-C assay, called DIP-C, allowed the visualization of OR gene and enhancer interactions in individual OSNs (Tan et al., 2019). DIP-C revealed that most OSNs contain multiple OR gene hubs, which aligns the multiple OR gene foci detected by in situ hybridization with pan-OR probe (Armelin-Correa et al., 2014; Clowney et al., 2012). Interestingly, DIP-C also reveals multiple similarly sized OR enhancer hubs per cell (Tan et al., 2019). The identity of the active OR is not known in these experiments, and the spatial resolution of DIP-C is not sufficient to resolve the presence of a single OR gene associated with one of these hubs. Nonetheless, these observations suggest that if enhancer hubs do indeed coordinate singular expression, there must be some mechanism to selectively activate one of several enhancer hubs present in each OSN (Pourmorady & Lomvardas, 2022).
It is possible that the changing patterns of OR gene expression over OSN differentiation reflect the dynamic assembly of OR gene hubs and enhancer hubs. (Figure 3) Hi-C analysis of OSN progenitors shows that the interactions between OR gene clusters and the interactions between OR enhancers form as OSN progenitors differentiate. The quiescent olfactory stem cells, HBCs, do not have interchromosomal interactions between OR gene clusters or between OR enhancers (Monahan et al., 2019). However, by the INP/iOSN stage, interchromosomal interactions between OR clusters are present but are not yet as strong as those observed in mOSNs. Interestingly, there is not a distinct network of OR enhancers in iOSNs, instead OR enhancers make abundant contacts with the surrounding OR gene clusters in these cells. One possibility is that this dispersed enhancer state reflects the stage of low-level OR coexpression, with many dispersed OR enhancers weakly activating transcription of nearby OR genes. Then the transition from low-level coexpression to singular choice could correspond to the transition from dispersed OR enhancers to multi-enhancer hubs. The temporal dynamics of this transition may result in one enhancer hub to become active first, allowing OR-induced feedback to stabilize expression of an OR from this hub and block the activation of other hubs (Pourmorady & Lomvardas, 2022). Application of DIP-C and Linking mRNA to Chromatin Architecture (LiMCA) methods to defined populations of OSN progenitors is allowing this model to be tested and refined. Indeed, recent reports show that multiple enhancer hubs form in OR progenitors (Poumorady et al, 2024, Xie, Wu, et al, 2023). Moreover, the process of OR transcription may form the basis of competition between hubs. Transcription of the OR gene results in the recruitment of additional enhancers, stabilizing the local hub, while the OR RNA molecules that are produced inhibit other enhancer hubs (Poumorady et al, 2024, Xie, Wu, et al, 2023). OR enhancers become less accessible as OSNs develop where only the chosen OR maintains it’s interactions with the enhancer hub that remains (Xie, Wu et al 2023). This provides a plausible mechanism that would allow multiple potentially active hubs to resolve to a single active enhancer hub in mOSNs.
5.4 |. Chromatin structure and 3D folding together coordinate zonal OR choice
How do heterochromatin deposition and the formation of chromatin hubs relate to each other? And how do they influence OR gene choice? Analysis of the zonal restriction of OR choice suggests that OR choice emerges from a dynamic interplay between these two processes, and shows that transcription factors can influence choice by controlling these processes. There is a striking correlation between OR zonal expression, heterochromatin marks, and localization of class II OR genes to OR gene hubs (Bashkirova et al., 2023). In bulk preparations of OSNs from whole olfactory epithelium tissue, the class II OR genes expressed in most dorsal part of the olfactory epithelium have the highest level of heterochromatin and the strongest interchromosomal Hi-C contacts, class II OR genes expressed in the middle of the olfactory epithelium have intermediate levels, and the most ventral OR genes have the lowest levels. (Bashkirova et al., 2023) provided a crucial insight into the nuances of this relationship by microdissecting different zones of the olfactory epithelium and then purifying cells at different stages of OSNs differentiation from this dissected tissue. This revealed that the deposition of heterochromatin marks and the strength of Hi-C contacts actually vary in a graded manner within the tissue. Dorsal OSNs only have intermediate levels of heterochromatin marks and Hi-C contacts on dorsal ORs, and none on ventral ORs. In contrast, ventral OSNs have high levels of heterochromatin marks on all OR genes except for the medial ORs, which have intermediate levels. Across the tissue, the set of OR genes available for choice is consistently observed to have an intermediate level of heterochromatin marks and Hi-C contacts (Bashkirova et al., 2023) go on to show that the zonal variation in the deposition of heterochromatin marks and subsequent zonal OR choice is coordinated by transcription factors from the Nfi-family, establishing a relationship between transcription factors that coordinate zonal restriction of OR choice and deposition of heterochromatin marks. These observations, taken together with the zonal skew in early OR gene expression observed by single-cell RNA-seq, also reveal a correlation between OR transcription during the coexpression stage and heterochromatin deposition. Future studies should explore whether nascent OR transcripts recruit the heterochromatin deposition machinery in OSN progenitors.
6 |. Future directions
The olfactory system deploys a huge number of odorant receptors to detect the volatile odorant compounds present everywhere in the world around us. The monogenic and monoallelic choice of a single olfactory receptor by olfactory sensory neurons is central to the organization of this sensory system. OR choice relies upon a core set of transcription factors that are able to broadly regulate OR genes and is accompanied by a dramatic remodeling of the chromatin structure and 3D organization of OR genes and enhancers. We are just beginning to understand the molecular mechanisms that coordinate these changes (Figure 4), but enough has been learned to sketch the outline of a model. It seems likely that heterochromatin deposition on OR gene clusters, perhaps recruited by OR transcription, is an important step. Intermediate levels of heterochromatin seem to label the subset of OR genes that are available for stochasctic choice and to correlate with the recruitment of these OR genes to OR gene hubs in the nucleus of OSNs. As OR gene hubs assemble, they will bring OR enhancers into spatial proximity, which may be important for nucleating the formation of enhancer hubs. Once an enhancer hub has reached some critical threshold it is able to drive sufficient expression of an OR gene to trigger feedback. Feedback could then, through mechanisms that remain unknown, stabilize expression from the existing enhancer hub and block the activation of additional, nascent enhancer hubs.
Figure 4: Summary of epigenetic events during olfactory sensory neuron differentiation.
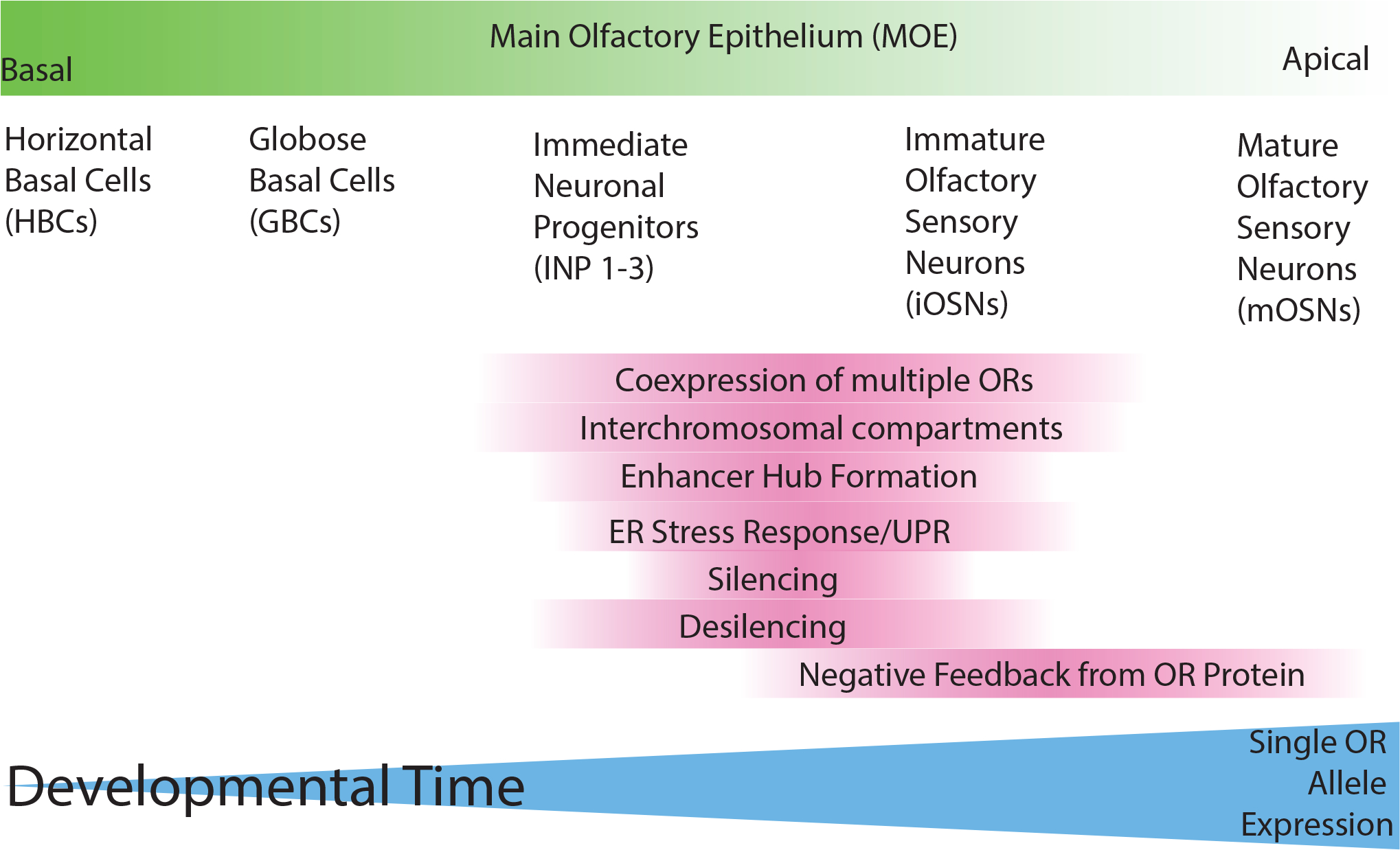
Multiple key developmental processes occur in the immediate neuronal progenitors (INP) and immature olfactory sensory neuron (iOSN) stages that lead to single OR allele expression in mature OSNs (mOSNs).
Single-cell RNA-seq has massively improved our ability to monitor the changes in gene expression that accompany OSN differentiation. This will allow a much finer-grained understanding of the dynamic changes in the expression of transcription factors and regulatory proteins that control OR choice, and the identification of gene expression programs that these factors control. Recent analyses have mapped the temporal dynamics of known regulatory factors, for example, revealing that several factors that regulate OR gene expression are dramatically upregulated as OSNs differentiate, and showing that the timing of this upregulation coincides with the onset of OR coexpression (Hussainy et al., 2022). These analyses have also combined gene expression trajectories and motif analysis to identify new candidate factors that could be involved in the regulation of OR choice. Future studies should bring in additional modalities, including integrating single-cell chromatin accessibility together with single-cell RNA-seq to reveal how the timing and location of transcription factor binding regulates OR coexpression, the deposition of heterochromatin on OR genes, and the formation of OR chromatin hubs.
One key point that bears further investigation is the relationship between OSN neuronal activity and OR gene choice. The olfactory epithelium may adapt to the olfactory environment by increasing or decreasing the number of OSNs expressing a given OR depending on the prevalence of ligands for that OR in the environment. This adaptation could occur through activity-dependent changes in OSN longevity; if OSNs that are activated more often tend to live longer, this will gradually make them more abundant in the MOE (Santoro & Dulac, 2012). However, there is also evidence that OSN neuronal activity can influence choice directly (C. van der Linden et al., 2018; C. J. van der Linden et al., 2020). It will be fascinating to explore how information related to OR-specific neuronal activity can be communicated to progenitor cells in a way that can influence OR choice.
Future studies should also explore the regulation of V1R and V2R gene choice in the vomeronasal organ. Recent single-cell and developmental studies have begun to reveal the key regulatory mechanisms that control vomeronasal sensory neuron development, including the mechanism that decides V1R versus V2R cell fate (Katreddi et al., 2022; Lin et al., 2022; Tirindelli, 2021). However, the gene regulatory mechanism that controls the choice of a single V1R gene or coordinated selection of specific combinations of V2R genes remains unknown. The V1Rs and V2Rs genes have a similar organization, to the class II ORs, with multiple clusters on multiple chromosomes. Future studies should investigate whether specialized enhancers, chromatin remodeling, and nuclear organization have a similar role in regulating V1R and V2R gene expression. Notably, the rodent-specific conversion of FPRs from their ancestral function as immune sensors to their new role as VNO chemoreceptors may provide insights into these mechanisms. Expression of FPR genes in the VNO in rodents coincides with the location of FPR genes in close proximity to both V1R and V2R genes. This proximity likely allowed them to hijack V1R and V2R regulatory elements (Dietschi et al., 2017). Future experiments should investigate whether this type of hijacking is limited to the VNO or if they are employed by other olfactory receptors as well.
The development of single-cell genomic assays, in combination with the continuing development of mouse genetics strategies for labeling and manipulating cells in vivo, is opening a window into the process of OSN neuronal differentiation. By tracking and manipulating this process with much greater resolution and precision, future studies will uncover the complex series of epigenetic events that coordinate OR choice, thus making our sense of smell possible.
Supplementary Material
Table S1: List of OR Enhancers This table lists the enhancers that are labeled along with the OR clusters in Figure 2 with genome coordinates (including references).
Acknowledgements:
We thank Nader Boutros-Ghali with help in editing this manuscript. This manuscript was supported by NIH grants 1R35GM146901 (KM) and F31DC021641 (NY) and a Rita Allen Scholar Award (KM). The authors have no conflicting interests.
References:
- Abdus-Saboor I, Al Nufal MJ, Agha MV, Ruinart de Brimont M, Fleischmann A, & Shykind BM (2016). An Expression Refinement Process Ensures Singular Odorant Receptor Gene Choice. Current Biology: CB, 26(8), 1083–1090. 10.1016/j.cub.2016.02.039 [DOI] [PubMed] [Google Scholar]
- Akiyoshi S, Ishii T, Bai Z, & Mombaerts P (2018). Subpopulations of vomeronasal sensory neurons with coordinated coexpression of type 2 vomeronasal receptor genes are differentially dependent on Vmn2r1. The European Journal of Neuroscience, 47(7), 887–900. 10.1111/ejn.13875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armelin-Correa LM, Gutiyama LM, Brandt DYC, & Malnic B (2014). Nuclear compartmentalization of odorant receptor genes. Proceedings of the National Academy of Sciences of the United States of America, 111(7), 2782–2787. 10.1073/pnas.1317036111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister AJ, & Kouzarides T (2011). Regulation of chromatin by histone modifications. Cell Research, 21(3), 381–395. 10.1038/cr.2011.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes IHA, Ibarra-Soria X, Fitzgerald S, Gonzalez JM, Davidson C, Hardy MP, Manthravadi D, Van Gerven L, Jorissen M, Zeng Z, Khan M, Mombaerts P, Harrow J, Logan DW, & Frankish A (2020). Expert curation of the human and mouse olfactory receptor gene repertoires identifies conserved coding regions split across two exons. BMC Genomics, 21(1), 196. 10.1186/s12864-020-6583-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashkirova EV, Klimpert N, Pourmorady A, Monahan K, Campbell CE, Osinski JM, Tan L, Schieren I, Stecky B, Barnea G, Sunney Xie X, Abdus-Saboor I, Shykind B, Marlin BJ, Gronostajski RM, Fleischmann A, & Lomvardas S (2023). Opposing, spatially-determined epigenetic forces impose restrictions on stochastic olfactory receptor choice. eLife, 12. 10.7554/eLife.87445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belton J-M, McCord RP, Gibcus JH, Naumova N, Zhan Y, & Dekker J (2012). Hi-C: a comprehensive technique to capture the conformation of genomes. Methods, 58(3), 268–276. 10.1016/j.ymeth.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Lakhlani PP, Bonini JA, Pathirana S, Boyle N, Pu X, Kouranova E, Lichtblau H, Ochoa FY, Branchek TA, & Gerald C (2001). Trace amines: identification of a family of mammalian G protein-coupled receptors. Proceedings of the National Academy of Sciences of the United States of America, 98(16), 8966–8971. 10.1073/pnas.151105198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza T, Vassalli A, Fuss S, Zhang J-J, Weiland B, Pacifico R, Feinstein P, & Mombaerts P (2009). Mapping of class I and class II odorant receptors to glomerular domains by two distinct types of olfactory sensory neurons in the mouse. Neuron, 61(2), 220–233. 10.1016/j.neuron.2008.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann DH, & Datta SR (2020). Finding the Brain in the Nose. Annual Review of Neuroscience, 43, 277–295. 10.1146/annurev-neuro-102119-103452 [DOI] [PubMed] [Google Scholar]
- Brovkina MV, Chapman MA, Holding ML, & Clowney EJ (2023). Emergence and influence of sequence bias in evolutionarily malleable, mammalian tandem arrays. BMC Biology, 21(1), 179. 10.1186/s12915-023-01673-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck L, & Axel R (1991). A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell, 65(1), 175–187. 10.1016/0092-8674(91)90418-x [DOI] [PubMed] [Google Scholar]
- Caggiano M, Kauer JS, Hun (1994). Globose basal cells are neuronal progenitors in the olfactory epithelium: A lineage analysis using a replication-incompetent retrovirus. Neuron, 13(2), 339–352. 10.1016/0896-6273(94)90351-4 [DOI] [PubMed] [Google Scholar]
- Chen M, Tian S, Yang X, Lane AP, Reed RR, Liu H (2014) Wnt-Responsive Lgr5+ Globose Basal Cells Function as Multipotent Olfactory Epithelium Progenitor Cells. Journal of Neuroscince. 34 (24) 8268–8276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess A, Simon I, Cedar H, & Axel R (1994). Allelic inactivation regulates olfactory receptor gene expression. Cell, 78(5), 823–834. 10.1016/s0092-8674(94)90562-2 [DOI] [PubMed] [Google Scholar]
- Clowney EJ, LeGros MA, Mosley CP, Clowney FG, Markenskoff-Papadimitriou EC, Myllys M, Barnea G, Larabell CA, & Lomvardas S (2012). Nuclear aggregation of olfactory receptor genes governs their monogenic expression. Cell, 151(4), 724–737. 10.1016/j.cell.2012.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowney EJ, Magklara A, Colquitt BM, Pathak N, Lane RP, & Lomvardas S (2011). High-throughput mapping of the promoters of the mouse olfactory receptor genes reveals a new type of mammalian promoter and provides insight into olfactory receptor gene regulation. Genome Research, 21(8), 1249–1259. 10.1101/gr.120162.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly T, Savigner A, & Ma M (2013). Spontaneous and sensory-evoked activity in mouse olfactory sensory neurons with defined odorant receptors. Journal of Neurophysiology, 110(1), 55–62. 10.1152/jn.00910.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton RP, Lyons DB, & Lomvardas S (2013). Co-opting the unfolded protein response to elicit olfactory receptor feedback. Cell, 155(2), 321–332. 10.1016/j.cell.2013.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewan A (2021). Olfactory signaling via trace amine-associated receptors. Cell and Tissue Research, 383(1), 395–407. 10.1007/s00441-020-03331-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hulst C, Mina RB, Gershon Z, Jamet S, Cerullo A, Tomoiaga D, Bai L, Belluscio L, Rogers ME, Sirotin Y, & Feinstein P (2016). MouSensor: A Versatile Genetic Platform to Create Super Sniffer Mice for Studying Human Odor Coding. Cell Reports, 16(4), 1115–1125. 10.1016/j.celrep.2016.06.047 [DOI] [PubMed] [Google Scholar]
- Dietschi Q, Tuberosa J, Rösingh L, Loichot G, Ruedi M, Carleton A, & Rodriguez I (2017). Evolution of immune chemoreceptors into sensors of the outside world. Proceedings of the National Academy of Sciences of the United States of America, 114(28), 7397–7402. 10.1073/pnas.1704009114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac C, & Axel R (1995). A novel family of genes encoding putative pheromone receptors in mammals. Cell, 83(2), 195–206. 10.1016/0092-8674(95)90161-2 [DOI] [PubMed] [Google Scholar]
- Enomoto T, Nishida H, Iwata T, Fujita A, Nakayama K, Kashiwagi T, Hatanaka Y, Kondo H, Kajitani R, Itoh T, Ohmoto M, Matsumoto I, & Hirota J (2019). Bcl11b controls odorant receptor class choice in mice. Communications Biology, 2, 296. 10.1038/s42003-019-0536-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei A, Wu W, Tan L, Tang C, Xu Z, Huo X, Bao H, Kong Y, Johnson M, Hartmann G, Talay M, Yang C, Riegler C, Herrera KJ, Engert F, Xie XS, Barnea G, Liberles SD, Yang H, & Li Q (2021). Coordination of two enhancers drives expression of olfactory trace amine-associated receptors. Nature Communications, 12(1), 3798. 10.1038/s41467-021-23823-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein P, & Mombaerts P (2004). A contextual model for axonal sorting into glomeruli in the mouse olfactory system. Cell, 117(6), 817–831. 10.1016/j.cell.2004.05.011 [DOI] [PubMed] [Google Scholar]
- Fleischer J (2021). The Grueneberg ganglion: signal transduction and coding in an olfactory and thermosensory organ involved in the detection of alarm pheromones and predator-secreted kairomones. Cell and Tissue Research, 383(1), 535–548. 10.1007/s00441-020-03380-w [DOI] [PubMed] [Google Scholar]
- Fleischmann A, Abdus-Saboor I, Sayed A, & Shykind B (2013). Functional interrogation of an odorant receptor locus reveals multiple axes of transcriptional regulation. PLoS Biology, 11(5), e1001568. 10.1371/journal.pbio.1001568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann A, Shykind BM, Sosulski DL, Franks KM, Glinka ME, Mei DF, Sun Y, Kirkland J, Mendelsohn M, Albers MW, & Axel R (2008). Mice with a “monoclonal nose”: perturbations in an olfactory map impair odor discrimination. Neuron, 60(6), 1068–1081. 10.1016/j.neuron.2008.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher RB, Das D, Gadye L, Street KN, Baudhuin A, Wagner A, Cole MB, Flores Q, Choi YG, Yosef N, Purdom E, Dudoit S, Risso D, & Ngai J (2017). Deconstructing Olfactory Stem Cell Trajectories at Single-Cell Resolution. Cell Stem Cell, 20(6), 817–830.e8. 10.1016/j.stem.2017.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss SH, Omura M, & Mombaerts P (2007). Local and cis effects of the H element on expression of odorant receptor genes in mouse. Cell, 130(2), 373–384. 10.1016/j.cell.2007.06.023 [DOI] [PubMed] [Google Scholar]
- Gadye L, Das D, Sanchez MA, Street K, Baudhuin A, Wagner A, Cole MB, Choi YG, Yosef N, Purdom E, Dudoit S, Risso D, Ngai J, & Fletcher RB (2017). Injury Activates Transient Olfactory Stem Cell States with Diverse Lineage Capacities. Cell Stem Cell, 21(6), 775–790.e9. 10.1016/j.stem.2017.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glusman G, Bahar A, Sharon D, Pilpel Y, White J, & Lancet D (2000). The olfactory receptor gene superfamily: data mining, classification, and nomenclature. Mammalian Genome: Official Journal of the International Mammalian Genome Society, 11(11), 1016–1023. 10.1007/s003350010196 [DOI] [PubMed] [Google Scholar]
- Glusman G, Yanai I, Rubin I, & Lancet D (2001). The complete human olfactory subgenome. Genome Research, 11(5), 685–702. 10.1101/gr.171001 [DOI] [PubMed] [Google Scholar]
- Greer PL, Bear DM, Lassance J-M, Bloom ML, Tsukahara T, Pashkovski SL, Masuda FK, Nowlan AC, Kirchner R, Hoekstra HE, & Datta SR (2016). A Family of non-GPCR Chemosensors Defines an Alternative Logic for Mammalian Olfaction. Cell, 165(7), 1734–1748. 10.1016/j.cell.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanchate NK, Kondoh K, Lu Z, Kuang D, Ye X, Qiu X, Pachter L, Trapnell C, & Buck LB (2015). Single-cell transcriptomics reveals receptor transformations during olfactory neurogenesis. Science, 350(6265), 1251–1255. 10.1126/science.aad2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrada G, & Dulac C (1997). A novel family of putative pheromone receptors in mammals with a topographically organized and sexually dimorphic distribution. Cell, 90(4), 763–773. 10.1016/s0092-8674(00)80536-x [DOI] [PubMed] [Google Scholar]
- Horgue LF, Assens A, Fodoulian L, Marconi L, Tuberosa J, Haider A, Boillat M, Carleton A, & Rodriguez I (2022). Transcriptional adaptation of olfactory sensory neurons to GPCR identity and activity. Nature Communications, 13(1), 2929. 10.1038/s41467-022-30511-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussainy M, Korsching SI, & Tresch A (2022). Pseudotime analysis reveals novel regulatory factors for multigenic onset and monogenic transition of odorant receptor expression. Scientific Reports, 12(1), 16183. 10.1038/s41598-022-20106-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata T, Niimura Y, Kobayashi C, Shirakawa D, Suzuki H, Enomoto T, Touhara K, Yoshihara Y, & Hirota J (2017). A long-range cis-regulatory element for class I odorant receptor genes. Nature Communications, 8(1), 885. 10.1038/s41467-017-00870-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katreddi RR, Taroc EZM, Hicks SM, Lin JM, Liu S, Xiang M, & Forni PE (2022). Notch signaling determines cell-fate specification of the two main types of vomeronasal neurons of rodents. Development, 149(13). 10.1242/dev.200448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Vaes E, & Mombaerts P (2011). Regulation of the probability of mouse odorant receptor gene choice. Cell, 147(4), 907–921. 10.1016/j.cell.2011.09.049 [DOI] [PubMed] [Google Scholar]
- Kurian SM, Naressi RG, Manoel D, Barwich A-S, Malnic B, & Saraiva LR (2021). Odor coding in the mammalian olfactory epithelium. Cell and Tissue Research, 383(1), 445–456. 10.1007/s00441-020-03327-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CT, Coulombe PA, Reed RR (2007). Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nature Neuroscience, 10(6)l 720–6. doi: 10.1038/nm1882. [DOI] [PubMed] [Google Scholar]
- Lewcock JW, & Reed RR (2004). A feedback mechanism regulates monoallelic odorant receptor expression. Proceedings of the National Academy of Sciences of the United States of America, 101(4), 1069–1074. 10.1073/pnas.0307986100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberles SD (2008). A Novel Family of Sensory Receptors in the Nose. Chemical Senses. [Google Scholar]
- Liberles SD, Horowitz LF, Kuang D, Contos JJ, Wilson KL, Siltberg-Liberles J, Liberles DA, & Buck LB (2009). Formyl peptide receptors are candidate chemosensory receptors in the vomeronasal organ. Proceedings of the National Academy of Sciences of the United States of America, 106(24), 9842–9847. 10.1073/pnas.0904464106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JM, Mitchell TA, Rothstein M, Pehl A, Taroc EZM, Katreddi RR, Parra KE, Zuloaga DG, Simoes-Costa M, & Forni PE (2022). Sociosexual behavior requires both activating and repressive roles of Tfap2e/AP-2ε in vomeronasal sensory neurons. eLife, 11. 10.7554/eLife.77259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CY, Fraser SE, & Koos DS (2009). Grueneberg ganglion olfactory subsystem employs a cGMP signaling pathway. The Journal of Comparative Neurology, 516(1), 36–48. 10.1002/cne.22096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomvardas S, Barnea G, Pisapia DJ, Mendelsohn M, Kirkland J, & Axel R (2006). Interchromosomal interactions and olfactory receptor choice. Cell, 126(2), 403–413. 10.1016/j.cell.2006.06.035 [DOI] [PubMed] [Google Scholar]
- Lyons DB, Allen WE, Goh T, Tsai L, Barnea G, & Lomvardas S (2013). An epigenetic trap stabilizes singular olfactory receptor expression. Cell, 154(2), 325–336. 10.1016/j.cell.2013.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons DB, Magklara A, Goh T, Sampath SC, Schaefer A, Schotta G, & Lomvardas S (2014). Heterochromatin-mediated gene silencing facilitates the diversification of olfactory neurons. Cell Reports, 9(3), 884–892. 10.1016/j.celrep.2014.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magklara A, Yen A, Colquitt BM, Clowney EJ, Allen W, Markenscoff-Papadimitriou E, Evans ZA, Kheradpour P, Mountoufaris G, Carey C, Barnea G, Kellis M, & Lomvardas S (2011). An epigenetic signature for monoallelic olfactory receptor expression. Cell, 145(4), 555–570. 10.1016/j.cell.2011.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnic B, Hirono J, Sato T, & Buck LB (1999). Combinatorial receptor codes for odors. Cell, 96(5), 713–723. 10.1016/s0092-8674(00)80581-4 [DOI] [PubMed] [Google Scholar]
- Ma M, Grosmaitre X, Iwema CL, Baker H, Greer CA, & Shepherd GM (2003). Olfactory Signal Transduction in the Mouse Septal Organ. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23(1), 317–324. 10.1523/JNEUROSCI.23-01-00317.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markenscoff-Papadimitriou E, Allen WE, Colquitt BM, Goh T, Murphy KK, Monahan K, Mosley CP, Ahituv N, & Lomvardas S (2014). Enhancer interaction networks as a means for singular olfactory receptor expression. Cell, 159(3), 543–557. 10.1016/j.cell.2014.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunami H, & Buck LB (1997). A multigene family encoding a diverse array of putative pheromone receptors in mammals. Cell, 90(4), 775–784. 10.1016/s0092-8674(00)80537-1 [DOI] [PubMed] [Google Scholar]
- Michaloski JS, Galante PAF, Malnic B (2006) Identification of potential regulatory motifs in odorant receptor genes by analysis of promoter sequences. Genome Research. (9):1091–8. doi: 10.1101/gr.5185406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamichi K, Serizawa S, Kimura HM, & Sakano H (2005). Continuous and overlapping expression domains of odorant receptor genes in the olfactory epithelium determine the dorsal/ventral positioning of glomeruli in the olfactory bulb. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 25(14), 3586–3592. 10.1523/JNEUROSCI.0324-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P (1999). Molecular biology of odorant receptors in vertebrates. Annual Review of Neuroscience, 22, 487–509. 10.1146/annurev.neuro.22.1.487 [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, & Axel R (1996). Visualizing an olfactory sensory map. Cell, 87(4), 675–686. 10.1016/s0092-8674(00)81387-2 [DOI] [PubMed] [Google Scholar]
- Monahan K, Horta A, & Lomvardas S (2019). LHX2- and LDB1-mediated trans interactions regulate olfactory receptor choice. Nature, 565(7740), 448–453. 10.1038/s41586-018-0845-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan K, Schieren I, Cheung J, Mumbey-Wafula A, Monuki ES, & Lomvardas S (2017). Cooperative interactions enable singular olfactory receptor expression in mouse olfactory neurons. eLife, 6. 10.7554/eLife.28620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, & Sakano H (2011). How is the olfactory map formed and interpreted in the mammalian brain? Annual Review of Neuroscience, 34, 467–499. 10.1146/annurev-neuro-112210-112917 [DOI] [PubMed] [Google Scholar]
- Nakashima A, Ihara N, Shigeta M, Kiyonari H, Ikegaya Y, & Takeuchi H (2019). Structured spike series specify gene expression patterns for olfactory circuit formation. Science, 365(6448). 10.1126/science.aaw5030 [DOI] [PubMed] [Google Scholar]
- Nakashima A, Takeuchi H, Imai T, Saito H, Kiyonari H, Abe T, Chen M, Weinstein LS, Yu CR, Storm DR, Nishizumi H, & Sakano H (2013). Agonist-independent GPCR activity regulates anterior-posterior targeting of olfactory sensory neurons. Cell, 154(6), 1314–1325. 10.1016/j.cell.2013.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MQ, Zhou Z, Marks CA, Ryba NJP, & Belluscio L (2007). Prominent Roles for Odorant Receptor Coding Sequences in Allelic Exclusion. Cell, 131(5), 1009–1017. 10.1016/j.cell.2007.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimura Y, & Nei M (2005). Evolutionary dynamics of olfactory receptor genes in fishes and tetrapods. Proceedings of the National Academy of Sciences of the United States of America, 102(17), 6039–6044. 10.1073/pnas.0501922102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizumi H, Kumasaka K, Inoue N, Nakashima A, & Sakano H (2007). Deletion of the core-H region in mice abolishes the expression of three proximal odorant receptor genes in cis. Proceedings of the National Academy of Sciences of the United States of America, 104(50), 20067–20072. 10.1073/pnas.0706544105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormundo LF, Machado CF, Sakamoto ED, Simões V, & Armelin-Correa L (2020). LINE-1 specific nuclear organization in mice olfactory sensory neurons. Molecular and Cellular Neurosciences, 105, 103494. 10.1016/j.mcn.2020.103494 [DOI] [PubMed] [Google Scholar]
- Pacifico R, Dewan A, Cawley D, Guo C, & Bozza T (2012). An olfactory subsystem that mediates high-sensitivity detection of volatile amines. Cell Reports, 2(1), 76–88. 10.1016/j.celrep.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal K, Forcato M, & Ferrari F (2019). Hi-C analysis: from data generation to integration. Biophysical Reviews, 11(1), 67–78. 10.1007/s12551-018-0489-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessy C, Pascarella G, Bertin N, Akalin A, Carrieri C, Vassalli A, Lazarevic D, Severin J, Vlachouli C, Simone R, Faulkner GJ, Kawai J, Daub CO, Zucchelli S, Hayashizaki Y, Mombaerts P, Lenhard B, Gustincich S, & Carninci P (2012). Promoter architecture of mouse olfactory receptor genes. Genome Research, 22(3), 486–497. 10.1101/gr.126201.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourmorady A, & Lomvardas S (2022). Olfactory receptor choice: a case study for gene regulation in a multi-enhancer system. Current Opinion in Genetics & Development, 72, 101–109. 10.1016/j.gde.2021.11.003 [DOI] [PubMed] [Google Scholar]
- Pourmorady A, Bashkirova EV, Chiariello AM, Belagzhal H, Kodra A, Duffle R, Kahiapo J, Monahan K, Pulupa J, Schieren I, Osterhoudt A, Dekker J, Nicodermi M, Lomvardas S (2024) RNA-mediated symmetry breaking enables singular olfactory receptor choice. Nature. 625, 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qasba P, & Reed RR (1998). Tissue and zonal-specific expression of an olfactory receptor transgene. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 18(1), 227–236. 10.1523/JNEUROSCI.18-01-00227.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read A, & Schröder M (2021). The Unfolded Protein Response: An Overview. Biology, 10(5). 10.3390/biology10050384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, & Buck LB (1993). A zonal organization of odorant receptor gene expression in the olfactory epithelium. Cell, 73(3), 597–609. 10.1016/0092-8674(93)90145-g [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, & Buck LB (1994). Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb. Cell, 79(7), 1245–1255. 10.1016/0092-8674(94)90015-9 [DOI] [PubMed] [Google Scholar]
- Rivière S, Challet L, Fluegge D, Spehr M, & Rodriguez I (2009). Formyl peptide receptor-like proteins are a novel family of vomeronasal chemosensors. Nature, 459(7246), 574–577. 10.1038/nature08029 [DOI] [PubMed] [Google Scholar]
- Roppolo D, Vollery S, Kan C-D, Lüscher C, Broillet M-C, & Rodriguez I (2007). Gene cluster lock after pheromone receptor gene choice. The EMBO Journal, 26(14), 3423–3430. 10.1038/sj.emboj.7601782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman A, Feinstein P, Hirota J, & Mombaerts P (2005). The promoter of the mouse odorant receptor gene M71. Molecular and Cellular Neurosciences, 28(3), 535–546. 10.1016/j.mcn.2004.11.006 [DOI] [PubMed] [Google Scholar]
- Ruiz Tejada Segura ML, Abou Moussa E, Garabello E, Nakahara TS, Makhlouf M, Mathew LS, Wang L, Valle F, Huang SSY, Mainland JD, Caselle M, Osella M, Lorenz S, Reisert J, Logan DW, Malnic B, Scialdone A, & Saraiva LR (2022). A 3D transcriptomics atlas of the mouse nose sheds light on the anatomical logic of smell. Cell Reports, 38(12), 110547. 10.1016/j.celrep.2022.110547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryba NJ, & Tirindelli R (1997). A new multigene family of putative pheromone receptors. Neuron, 19(2), 371–379. 10.1016/s0896-6273(00)80946-0 [DOI] [PubMed] [Google Scholar]
- Santoro SW, & Dulac C (2012). The activity-dependent histone variant H2BE modulates the life span of olfactory neurons. eLife, 1. 10.7554/eLife.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva LR, Ibarra-Soria X, Khan M, Omura M, Scialdone A, Mombaerts P, Marioni JC, & Logan DW (2015). Hierarchical deconstruction of mouse olfactory sensory neurons: from whole mucosa to single-cell RNA-seq. Scientific Reports, 5, 18178. 10.1038/srep18178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz P, Kalbe B, Jansen F, Altmueller J, Becker C, Mohrhardt J, Schreiner B, Gisselmann G, Hatt H, & Osterloh S (2016). Transcriptome Analysis of Murine Olfactory Sensory Neurons during Development Using Single Cell RNA-Seq. Chemical Senses, 41(4), 313–323. 10.1093/chemse/bjw003 [DOI] [PubMed] [Google Scholar]
- Serizawa S, Miyamichi K, Nakatani H, Suzuki M, Saito M, Yoshihara Y, & Sakano H (2003). Negative feedback regulation ensures the one receptor-one olfactory neuron rule in mouse. Science, 302(5653), 2088–2094. 10.1126/science.1089122 [DOI] [PubMed] [Google Scholar]
- Serizawa S, Miyamichi K, Takeuchi H, Yamagishi Y, Suzuki M, & Sakano H (2006). A neuronal identity code for the odorant receptor-specific and activity-dependent axon sorting. Cell, 127(5), 1057–1069. 10.1016/j.cell.2006.10.031 [DOI] [PubMed] [Google Scholar]
- Shah A, Ratkowski M, Rosa A, Feinstein P, & Bozza T (2021). Olfactory expression of trace amine-associated receptors requires cooperative cis-acting enhancers. Nature Communications, 12(1), 3797. 10.1038/s41467-021-23824-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Ishimaru Y, Davison I, Ikegami K, Chien M-S, You H, Chi Q, Kubota M, Yohda M, Ehlers M, & Matsunami H (2017). Olfactory receptor accessory proteins play crucial roles in receptor function and gene choice. eLife, 6. 10.7554/eLife.21895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayya HJ, Kahiapo JK, Duffié R, Lehmann KS, Bashkirova L, Monahan K, Dalton RP, Gao J, Jiao S, Schieren I, Belluscio L, & Lomvardas S (2022). ER stress transforms random olfactory receptor choice into axon targeting precision. Cell, 185(21), 3896–3912.e22. 10.1016/j.cell.2022.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P, & Zhang J (2007). Comparative genomic analysis identifies an evolutionary shift of vomeronasal receptor gene repertoires in the vertebrate transition from water to land. Genome Research, 17(2), 166–174. 10.1101/gr.6040007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shykind BM, Rohani SC, O’Donnell S, Nemes A, Mendelsohn M, Sun Y, Axel R, & Barnea G (2004). Gene switching and the stability of odorant receptor gene choice. Cell, 117(6), 801–815. 10.1016/j.cell.2004.05.015 [DOI] [PubMed] [Google Scholar]
- Strotmann J, Levai O, Fleischer J, Schwarzenbacher K, & Breer H (2004). Olfactory receptor proteins in axonal processes of chemosensory neurons. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 24(35), 7754–7761. 10.1523/JNEUROSCI.2588-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strotmann J, Wanner I, Krieger J, Raming K, & Breer H (1992). Expression of odorant receptors in spatially restricted subsets of chemosensory neurones. Neuroreport, 3(12), 1053–1056. 10.1097/00001756-199212000-00005 [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Inokuchi K, Aoki M, Suto F, Tsuboi A, Matsuda I, Suzuki M, Aiba A, Serizawa S, Yoshihara Y, Fujisawa H, & Sakano H (2010). Sequential arrival and graded secretion of Sema3F by olfactory neuron axons specify map topography at the bulb. Cell, 141(6), 1056–1067. 10.1016/j.cell.2010.04.041 [DOI] [PubMed] [Google Scholar]
- Tang JCY, Rudolph S, Dhande OS, Abraira VE, Choi S, Lapan SW, Drew IR, Drokhlyansky E, Huberman AD, Regehr WG, & Cepko CL (2015). Cell type-specific manipulation with GFP-dependent Cre recombinase. Nature Neuroscience, 18(9), 1334–1341. 10.1038/nn.4081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, Li Q, Xie XS (2015) Olfactory sensory neurons transiently express multiple oflactory receptors during development. Molecular Systems Biology. 8;11(12):844. doi: 10.15252/msb.20156639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, Xie XS (2018). A Near-Complete Spatial Map of Olfactory Receptors in the Mouse Main Olfactory Epithelium. Chemical Senses. 5:43(6):427–432. doi: 10.1093/chemse/bjy030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, Xing D, Daley N, & Xie XS (2019). Three-dimensional genome structures of single sensory neurons in mouse visual and olfactory systems. Nature Structural & Molecular Biology, 26(4), 297–307. 10.1038/s41594-019-0205-2 [DOI] [PubMed] [Google Scholar]
- Tirindelli R (2021). Coding of pheromones by vomeronasal receptors. Cell and Tissue Research, 383(1), 367–386. 10.1007/s00441-020-03376-6 [DOI] [PubMed] [Google Scholar]
- Tsukahara T, Brann DH, Pashkovski SL, Guitchounts G, Bozza T, & Datta SR (2021). A transcriptional rheostat couples past activity to future sensory responses. Cell, 184(26), 6326–6343.e32. 10.1016/j.cell.2021.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linden C, Jakob S, Gupta P, Dulac C, & Santoro SW (2018). Sex separation induces differences in the olfactory sensory receptor repertoires of male and female mice. Nature Communications, 9(1), 5081. 10.1038/s41467-018-07120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linden CJ, Gupta P, Bhuiya AI, Riddick KR, Hossain K, & Santoro SW (2020). Olfactory Stimulation Regulates the Birth of Neurons That Express Specific Odorant Receptors. Cell Reports, 33(1), 108210. 10.1016/j.celrep.2020.108210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli A, Feinstein P, & Mombaerts P (2011). Homeodomain binding motifs modulate the probability of odorant receptor gene choice in transgenic mice. Molecular and Cellular Neurosciences, 46(2), 381–396. 10.1016/j.mcn.2010.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli A, Rothman A, Feinstein P, Zapotocky M, & Mombaerts P (2002). Minigenes impart odorant receptor-specific axon guidance in the olfactory bulb. Neuron, 35(4), 681–696. 10.1016/s0896-6273(02)00793-6 [DOI] [PubMed] [Google Scholar]
- Vassar R, Chao SK, Sitcheran R, Nuñez JM, Vosshall LB, & Axel R (1994). Topographic organization of sensory projections to the olfactory bulb. Cell, 79(6), 981–991. 10.1016/0092-8674(94)90029-9 [DOI] [PubMed] [Google Scholar]
- Wang F, Nemes A, Mendelsohn M, & Axel R (1998). Odorant receptors govern the formation of a precise topographic map. Cell, 93(1), 47–60. 10.1016/s0092-8674(00)81145-9 [DOI] [PubMed] [Google Scholar]
- Wang I-H, Murray E, Andrews G, Jiang H-C, Park SJ, Donnard E, Durán-Laforet V, Bear DM, Faust TE, Garber M, Baer CE, Schafer DP, Weng Z, Chen F, Macosko EZ, & Greer PL (2022). Spatial transcriptomic reconstruction of the mouse olfactory glomerular map suggests principles of odor processing. Nature Neuroscience, 25(4), 484–492. 10.1038/s41593-022-01030-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Ma L, Duyck K, Long CC, Moran A, Scheerer H, Blanck J, Peak A, Box A, Perera A, & Yu CR (2018). A Population of Navigator Neurons Is Essential for Olfactory Map Formation during the Critical Period. Neuron, 100(5), 1066–1082.e6. 10.1016/j.neuron.2018.09.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Wu H, Zhang J, Jian F, Chen J, Zheng Y, Tan L (2023). Simultaneous single-cell three-dimensional genome and gene expression profiling uncovers dynamic enhancer connectivity underlying olfactory receptor choice. Preprint https://www.researchsquare.com/article/rs-3210240/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapiec B, & Mombaerts P (2020). The Zonal Organization of Odorant Receptor Gene Choice in the Main Olfactory Epithelium of the Mouse. Cell Reports, 30(12), 4220–4234.e5. 10.1016/j.celrep.2020.02.110 [DOI] [PubMed] [Google Scholar]
- Zhang X, & Firestein S (2002). The olfactory receptor gene superfamily of the mouse. Nature Neuroscience, 5(2), 124–133. 10.1038/nn800 [DOI] [PubMed] [Google Scholar]
- Zhang X, Rogers M, Tian H, Zhang X, Zou D-J, Liu J, Ma M, Shepherd GM, & Firestein SJ (2004). High-throughput microarray detection of olfactory receptor gene expression in the mouse. Proceedings of the National Academy of Sciences of the United States of America, 101(39), 14168–14173. 10.1073/pnas.0405350101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman AD, & Munger SD (2021). Olfactory subsystems associated with the necklace glomeruli in rodents. Cell and Tissue Research, 383(1), 549–557. 10.1007/s00441-020-03388-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: List of OR Enhancers This table lists the enhancers that are labeled along with the OR clusters in Figure 2 with genome coordinates (including references).


