Abstract
The anaerobic cultivation of fecal microbiota is a promising approach to investigating how gut microbial communities respond to specific intestinal conditions and perturbations. Here, we describe a flexible protocol using 96-deepwell plates to cultivate stool-derived gut microbiota. Our protocol aims to address gaps in high-throughput culturing in an anaerobic chamber. We characterized the influence of the gas phase on the medium chemistry and microbial physiology and introduced a modular medium preparation process to enable the testing of several conditions simultaneously. Furthermore, we identified a medium formulation that maximized the compositional similarity of ex vivo cultures and donor microbiota while limiting the bloom of Enterobacteriaceae. Lastly, we validated the protocol by demonstrating that cultivated fecal microbiota responded similarly to dietary fibers (resistant dextrin, soluble starch) and drugs (ciprofloxacin, 5-fluorouracil) as reported in vivo. This high-throughput cultivation protocol has the potential to facilitate culture-dependent studies, accelerate the discovery of gut microbiota-diet-drug-host interactions, and pave the way to personalized microbiota-centered interventions.
Keywords: ex vivo, in vitro, microbial ecology, xenobiotics, fibers, personalized microbiota response, anaerobic chamber, 16S rRNA gene sequencing, short-chain fatty acids, fecal microbiota
Introduction
The human gut is inhabited by a diverse and dynamic assembly of microbes unique to each individual [1]. This microbial community contributes to key health functions such as nutrient metabolism, pathogen colonization resistance, gut barrier integrity maintenance, and host immune system education [2]. Despite advances in understanding the human gut microbiota’s diversity and functionality, the mechanisms governing ecological and physiological dynamics in response to specific intestinal conditions and perturbations remain poorly understood. Numerous studies have documented changes in the gut microbial community in response to dietary interventions [3–5], antibiotic/non-antibiotic medications [6–8], or alterations of the host physiology (e.g. intestinal pH, oxidative stress, osmotic pressure, antimicrobial peptides) [9–11]. However, high inter- and intra-individual variability of the gut microbiota, combined with the complex interactions between diet-, drug-, and host-related factors, has limited our capacity to generalize microbial responses to a broader population [12].
Ex vivo fecal cultivation holds promise for investigating the direct impact of relevant intestinal conditions on individuals’ gut microbiota in a controlled manner. Different setups have been employed for cultivating intestinal communities, ranging from simple batch incubation using anaerobic tube-based techniques [13–15] or multiwell plates [16–20] to a fully controlled, continuous multistage model [21–23]. Although continuous models more closely mimic in vivo intestinal conditions and enable more fundamental studies, they are labor-intensive, time-consuming, and limited by the number of donor microbiota and conditions that can be evaluated simultaneously. Further, many responses and activities are specific to donors [24–27], strains [28–31], and the physicochemical environment of the gut [9–11]. Cultivation with a higher throughput is thus crucial to advance our understanding of gut microbiota-diet-drug–host interactions and facilitate the development of personalized microbiota-centered interventions [4, 14]. Different high-throughput cultivation methods have been developed for multiwell plates in the anaerobic chamber [16–20, 32], with important efforts directed toward evaluating and optimizing medium formulation [17, 18, 20, 33–36], streamlining the workflow from feces to sample analysis [19, 37], and providing detailed technical procedures for cultivation [38].
Here, we aimed to contribute to the field by addressing remaining gaps in anaerobic high-throughput cultivation, including the need for (i) characterizing the influence of the gas phase on pH control and fecal microbiota growth and metabolism, (ii) designing a highly customizable medium preparation process to enable the testing of a wide range of factors and their combinations, and (iii) establishing conditions capable of maintaining the main characteristics of fecal inoculum from healthy donors while mitigating the bloom of Enterobacteriaceae, i.e. a taxonomic group associated with dysbiosis (e.g. irritable bowel syndrome, inflammatory bowel disease, obesity, colorectal cancer) [39] and prone to overgrowth during ex vivo cultivation [16, 18, 20, 21, 36, 40]. At the core of our approach is the use of a standardized basal medium that can be flexibly modified to test fecal microbiota responses to factors related to diet, drugs, and the host, individually or in combination. We highlighted the abiotic and biotic effects of the gas phase, buffer composition, and the cultivation process in a 96-deepwell plate within an anaerobic chamber (open gas-exchange system) compared to the well-established Hungate technique (closed tube-based system). Importantly, we evaluated the capacity of distinct mixtures of fibers and mucin to maintain the composition and activity of the gut microbiota from healthy adult donors while preventing Enterobacteriaceae overgrowth. Finally, we validated our protocol by showing similar ex vivo responses to known dietary fibers (resistant dextrin, soluble starch) and drugs (ciprofloxacin, 5-fluorouracil [5-FU]) as previously reported in vivo. A comprehensive protocol detailing the necessary steps and procedures for material preparation, high-throughput cultivation, and sample handling for analyses is provided in the Supplementary File 1.
Materials and methods
Growth media and anaerobic procedures
To cultivate fecal microbiota and pure cultures, we modified the yeast casitone fatty acid (YCFA) medium [41]. Specifically, we reduced nitrogen (N)-sources 10-fold, resulting in a basal minimal media, referred to as basal YCFA (bYCFA). bYCFA consisted of (L−1): 1 g amicase (Sigma-Aldrich Chemie GmbH, Buchs, Switzerland), 1.25 g yeast extract (Lesaffre, Marcq-en-Barœul, France), 0.5 g meat extract (Sigma-Aldrich), 150 ml mineral solution I (0.52 g K2HPO4), 150 ml mineral solution II (0.52 g KH2PO4, 0.9 g NaCl, 0.9 g (NH4)2SO4, 90 mg MgSO4, 90 mg CaCl2), 1 g L-cysteine HCl, and 4 g NaHCO3, 0.1 ml vitamin solution (10 μg biotin, 10 μg cobalamin, 30 μg 4-aminobenzoic acid, 50 μg folic acid and 150 μg pyridoxamine, Sigma-Aldrich), and 6.2 ml of volatile fatty acids solution (2.11 ml acetic acid (Sigma-Aldrich), 0.74 ml propionic acid (Sigma-Aldrich), 0.12 ml valeric acid (VWR International AG, Dietikon, Switzerland), 0.12 ml isovaleric acid (Sigma-Aldrich), and 0.12 ml isobutyric acid (Sigma-Aldrich) in 3.1 ml 5 M NaOH). The basal medium was prepared 1.33-fold concentrated since it was then complemented with supplements within the chamber (Fig. 1A; Table S1). Thus, all compounds except L-cysteine and NaHCO3 were dissolved in 75% of the final volume. The basal medium was adjusted to pH 7, boiled (10 min), and the remaining compounds were added after cooling to ~60 °C while constantly flushing with CO2 (or N2). After another 10 min, the medium was transferred into DURAN® Pressure flasks for plate-based cultivation (DWK Life Sciences GmbH, Wertheim, Germany) or Hungate tubes for tube-based cultivation (Belleco Glass, Vineland, NJ) and autoclaved.
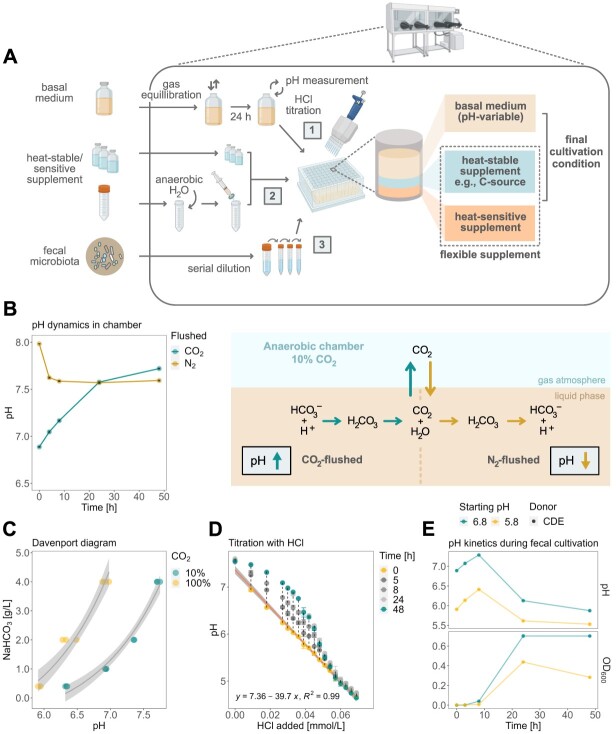
Figure 1.
Overview of the high-throughput cultivation protocol and effect of the anaerobic chamber atmosphere on the medium’s chemistry and microbial physiology; (A) overview of the step-by-step cultivation protocol adapted for the anaerobic chamber setup; in a 96-deepwell plate, heat-stable/sensitive supplements (1) are mixed with HCl-titrated bYCFA (2) and inoculated with diluted feces (3); (B) characterization of pH dynamics of CO2 and N2-flushed bYCFA (uninoculated) in a 96-deepwell plate in an anaerobic chamber; (C) relationship between pH, aqueous NaHCO3 concentration, and gaseous CO2 levels (~10% in the anaerobic chamber, ~100% in gas-tight tube) in bYCFA after 48-h equilibration; (D) change of pH over time upon titration of bYCFA with HCl, with subsequent pH increase over 48 h; the resulting titration curve (at time 0 h) can be used to adjust the pH to different values; (E) pH and growth (OD600) during 48-h batch fecal cultivation in bYCFA (initial pH 6.8 and 5.8; containing 4 g/l NaHCO3 and 3 g/l resistant dextrin) titrated with HCl immediately before inoculation (donor CDE; 1% inoculation of 10−4 fecal dilution); data are presented as the mean and standard deviation of three technical replicates; all pH dynamic analyses were conducted in the 60 central experimental wells to circumvent the edge effect (Supplementary File 1, Step 4).
To complement the basal medium, a range of heat-stable and heat-sensitive supplement solutions were prepared (Table S2). Heat-stable compounds were dissolved in dH2O, adjusted to pH 7, boiled for 10 min, cooled under N2, and autoclaved separately soon after. Heat-sensitive compounds were dissolved in anaerobic dH2O (except for omeprazole, which was dissolved in dimethyl sulfoxide (DMSO); 0.2% final concentration) in the anaerobic chamber, adjusted to pH 7, and filter sterilized.
The day before the experiment, the autoclaved basal medium was equilibrated with the gas phase of the anaerobic chamber (10% CO2, 5% H2, and 85% N2, Coy Laboratory Products Inc., Grass Lake, MI) under constant stirring, without the lid but covered with Breathe-Easier Sealing film (Diversified Biotech, Dedham, MA). On the day of the experiment, the pH was adjusted to 6.8 (if not indicated otherwise) by adding HCl 3 M based on a predetermined titration curve (Fig. 1E) and was mixed with the corresponding heat-stable/sensitive supplements (Tables S1 and S2).
A complete step-by-step description of the preparation of the bYCFA medium and heat-stable/sensitive supplement solutions for the high-throughput cultivation in 96-deepwell plates (Ritter GmbH, Schwabenmünchen, Germany) is presented in the Supplementary File 1.
Fecal sample collection and ex vivo cultivation
Fourteen healthy donors (7 females and 7 males, ages ranging between 26 and 36) consented to provide one or more fresh fecal samples. Donors were excluded if they had taken antibiotics in the past 6 months. The sample collection was processed in an anonymized (using randomly generated three-letter codes) and non-interventional manner, and the ethics committee of ETH Zurich exempted this study from review.
Self-collected samples were provided in a plastic container containing an Oxoid™ AnaeroGen™ pouch (Thermo Fisher Diagnostics AG, Pratteln, Switzerland) to generate anaerobiosis during transport. Within 3 h, samples were relocated into the anaerobic chamber. Approximately 1 g of fecal matter was transferred using a plastic spoon into a 50 ml Falcon tube and homogenized with 9 ml of anaerobic phosphate buffer (pH 7.0). The resulting 10−1 dilution was then further diluted to 10−4 and inoculated (1%, v/v, in triplicates) into a plate (2 ml) or tube (8 ml) containing previously pH-adjusted basal medium complemented with respective supplements as listed in Table S1. After incubation for 48 h at 37 °C, cultures were transferred out of the chamber and centrifuged (5′500 rpm, 20 min and 4 °C). Cell pellets and supernatants were recovered and stored separately (−80 °C) until further processing (Supplementary File 1, Step 8).
Technical replicates exhibited small compositional differences (median Bray-Curtis distance 0.11) compared with the between-sample differences (median Bray–Curtis distance 0.72; Fig. S1). Therefore, when indicated, replicates were pooled before centrifugation by transferring 0.5 ml from each culture into a fresh plate (Supplementary File 1, Step 8) to reduce sample analysis time, cost, and storage space.
Cultivation of pure intestinal strains
A panel of 10 human intestinal strains, representing diverse fiber and intermediate metabolite utilizers, were assessed for their growth and metabolic capabilities using the plate- and tube-based technique. The strains were acquired from the German Collection of Microorganisms and Cell Culture GmbH (DSMZ, Braunschweig, Germany) and PharmaBiome AG (Zürich, Switzerland; Table S3). They were stored at −80 °C in anaerobic glycerol stocks (25%, v/v) and reactivated by inoculating (1.25%, v/v) Hungate tubes containing bYCFA (pH 6.8) supplemented with the respective carbon sources (final concentrations of 3 g/l for fibers, 30 mM for glucose and intermediate metabolites; Table S3). Precultures were incubated for 24 h at 37 °C before experiments. Then, plates and tubes were filled with bYCFA, complemented with the corresponding C-sources, and inoculated (1% v/v). After incubation (48 h, 37 °C), 200 μl of cultures were transferred into a 96-well plate for OD600 measurement. Supernatants were recovered by centrifugation (4700 rpm, 20 min and 4 °C) for organic acids quantification.
pH dynamics and bicarbonate buffering system in the anaerobic chamber
We characterized the relationship between pH, NaHCO3 concentration, and CO2 levels (0% or 100% in gas-tight tubes, compared to 10% in the anaerobic chamber) by measuring the pH of the basal medium containing different NaHCO3 concentrations (0.4, 1, 2, and 4 g/l). When required, pH dynamics were monitored over time. Measurements were performed in a 96-deepwell plate filled with 1.5 ml basal medium using a pH microelectrode (diameter 6 mm; Metrohm AG, Herisau, Switzerland) and a pH meter (type 913; Metrohm AG). A titration of bYCFA medium with HCl 3 M was performed and served as a calibration to support the pH adjustment of bYCFA in the chamber (Supplementary File 1, Step 6).
Organic acids quantification
Short-chain fatty acids (SCFAs; acetate, propionate and butyrate) and intermediate metabolites (formate, lactate and succinate) were quantified by high-performance liquid chromatography equipped with a refractive index detector (HPLC-RI) as reported before [14]. All listed compound concentrations were corrected with blank values of the basal medium to estimate the net production of SCFA and intermediates. To quantify total metabolites, blank-corrected concentrations were summed, and metabolite fractions were calculated by dividing concentrations of respective metabolites by total metabolites.
DNA extraction and 16S rRNA gene sequencing
Cell pellets were preprocessed using the Maxwell® HT 96 gDNA Blood Isolation Kit (Promega AG, Dübendorf, Switzerland) combined with a FastPrep homogenizer (FastPrep-24TM; MP Biomedicals, Illkirch-Graffenstaden, France), and followed by DNA purification using KingFisher Flex instrument (Thermo Fisher Scientific, Waltham, MA), according to the manufacturer’s instructions. The total DNA concentration after extraction was quantified using the Qubit® dsDNA HS Assay kit. Amplification and barcoding of the V4 hypervariable region of the 16S rRNA gene were performed using a polymerase chain reaction (PCR) with the barcoded primer 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) [42, 43]. The pooled library was then sequenced using the amplifying primers (515F and 806R) and the Illumina MiSeq platform [44] at the Genetic Diversity Centre (ETH Zurich, Switzerland).
Sequencing reads were processed using the metabaRpipe R package (v0.9) [45]. In brief, reads were inferred with the DADA2 R package (v1.14.1) [46] with read filtering set to c(260, 250) and maximal error rates set to c(3, 4). Bimeras were removed, and taxonomy was assigned to amplicon sequence variants (ASVs) using the SILVA database (v138.1) [47, 48]. Reads were filtered with a per-sample abundance threshold of 0.1% [49] and then analyzed using the phyloseq package (v1.40.0) of the R software (v4.2.0) [50]. Alpha and beta-diversity analyses were done on rarefied samples (with an average rarefication depth of 2374 reads). Data meeting the criteria of homoscedasticity were evaluated with a permutational multivariate analysis using the adonis2 function (999 permutations) of the vegan package (v2.6–4). Differential abundance analysis was performed on non-rarified data using ALDEx2 [51]. Compositional taxa bar plots were generated using comp_barplot from the microViz package (v0.10.8) [52]. Using the aldex.clr function from the mia package (v1.5.17), the data were centered log(2)-ratio (clr) transformed using 1000 Dirichlet Monte-Carlo instances [53]. Significantly altered taxa were identified based on the P-value of Welch’s t-test, corrected according to the Benjamini–Hochberg Method.
Data analysis and visualization
Data were analyzed using R software (v4.2.0) and visualized using the ggplot package (v3.3.6). Pearson correlation lines were plotted using ggpmisc (v0.5.1). Data were evaluated for normality using the Shapiro–Wilk Normality Test and when P > .05, the comparison of means was performed using a t-test. Otherwise, a Wilcoxon test using the rstatix package (v0.7.0) was used. P-values were corrected for multiple testing using the Holm–Bonferroni method. All codes used for data analysis in this study have been made publicly available and can be accessed at https://github.com/janinazuend/HTP.
Results
A modular system for medium preparation and pH control for high-throughput cultivation in the anaerobic chamber
To formulate a standardized high-throughput cultivation protocol, we opted for 96-deepwell plates, gas-permeable sealing membranes, and a standard anaerobic chamber. This setup enables simultaneous testing of multiple conditions, with 60 wells/plate allocated for experiments surrounded by water-filled wells to minimize evaporation and edge effects [54] (Supplementary File 1, Step 4), while wells at the edges of the plate can be used to allocate contamination controls. The working volume of 2 ml/well provides enough samples to conduct a range of analyses on the whole culture (e.g. OD600, pH), the cell-free supernatant (e.g. metabolites), and the cell pellet (e.g. DNA-based analyses) (Supplementary File 1, Step 8).
To provide flexibility for testing diverse culture conditions, we designed a modular procedure in which an anaerobic basal medium solution (containing N-sources, fatty acids, salts, buffers, trace elements, and heat-stable vitamins) is complemented within the chamber with a carbon (C)-source solution, and any other solution with specific components (heat stable/sensitive) to assess (Fig. 1A). As the basal medium, we selected bYCFA, which is a modified version of Yeast extract Casitone and Fatty Acids (YCFA) medium (C-depleted, 10-fold reduction N-sources), which has a long history of use in cultivating gut microbes and microbiota [41, 55]. The minimal nutrient availability of bYCFA enables the identification of supplement-specific responses.
Cultivation in anaerobic chambers poses diverse challenges, including maintaining strict anaerobiosis to prevent the proliferation of facultative anaerobes, such as Enterobacteriaceae. Our protocol highlights key practical steps for mitigating O2 contamination (Supplementary File 1, Step 2), with a particular focus on sustaining proper hydrogen levels (>2.5%) to achieve efficient O2 elimination and reproducible anaerobic microbial growth, as demonstrated in Fig. S2. Another challenge is pH control, which is influenced by the equilibrium between gaseous and dissolved CO2, with the latter forming carbonic acid (H2CO3) in an aqueous solution [56]. During the preparation of the basal medium, 100% CO2 or N2 is flushed to remove traces of O2 (Supplementary File 1, Step 1), resulting in dissolved CO2 levels that are out of equilibrium with the chamber atmosphere (here, 10% CO2). As a result, introducing CO2-flushed medium in the chamber led to a significant abiotic increase of pH (+0.16 ± 0.05 after 4 h and + 0.83 ± 0.03 after 48 h in bYCFA). In contrast, a rapid drop of pH was observed when introducing N2-flushed medium in the chamber (−0.36 ± 0.02 after 4 h in bYCFA, Fig. 1B). These changes in pH reflect the release of CO2 into the gas phase (CO2-flushed) or the solubilization of CO2 into the medium (N2-flushed) to equilibrate with the levels in the chamber’s atmosphere (Fig. 1B). The pH at equilibrium depended on the CO2 level in the atmosphere and on the concentration of NaHCO3 in the medium (Davenport diagram, Fig. 1C), which was set at 4 g/l in YCFA [41]. To establish a standard basal medium that can be flexibly adjusted to a range of pH values, we kept the original NaHCO3 concentration of 4 g/l and adjusted the pH of the medium (equilibrated in the chamber for 24 h) via HCl titration (Supplementary File 1, Step 6). This approach allowed rapid adjustment of the initial pH but also led to a slow increase in pH over time (Fig. 1D) as HCl titration shifts the bicarbonate buffer system toward CO2, which is gradually released again, resulting in alkalinization. The pH should thus be adjusted right before inoculation to maintain the initial pH in the desired range. To validate this approach, we titrated bYCFA with HCl (to pH 6.8 and pH 5.8) immediately before inoculation with fecal microbiota and incubated the cultures at 37 °C for 48 h (Fig. 1E). After an initial pH increase of +0.48 ± 0.07 (pH 6.8) and + 0.45 ± 0.02 (pH 5.8) after 8 h, the pH decreased and reached final values of 5.90 ± 0.04 (pH 6.8) and 5.51 ± 0.04 (pH 5.8) after 48 h (Fig. 1E). The degree of alkalinization resulting from the disrupted bicarbonate buffer was small compared to the acidification caused by microbial activity (i.e. organic acid production), and the pH remained within a range permitting the growth of common gut anaerobes [57]. Therefore, HCl-titration within the chamber was integrated into the protocol to rapidly set the initial pH to the desired value, thereby facilitating the testing of diverse pH. This step requires a pH meter in the chamber and a predetermined titration curve (Fig. 1D; Supplementary File 1, Step 6).
Effect of the cultivation techniques on the microbial community and metabolism of fecal cultures
Having established a modular procedure for preparing the medium and adjusting culture conditions, we next evaluated whether the plate-based cultivation system is equally suited to assess condition-specific microbial responses as the well-established and strict anaerobic Hungate technique. Indeed, differences might arise as a 96-deepwell plate can be regarded an open system, here exposed to an atmosphere of 10% CO2, 5% H2, and 85% N2. In turn, a Hungate tube functions as a closed system that is better protected from O2 contaminations, contains 100% CO2,and retains gases produced by microorganisms (e.g. CO2, H2, and H2S; Fig. 2A). Using both techniques, we cultured two fecal microbiota (48 h, 37 °C) in bYCFA containing two different C-sources (resistant dextrin, soluble starch, or H2O as control; Fig. 2A) that have previously shown to evoke strong microbial responses when using the Hungate technique [14]. As an indicator of microbial biomass [58], we observed similar or higher DNA concentrations in plates compared to tubes (Fig. S3A). The richness of fecal cultures (observed ASVs) strongly correlated between plates and tubes (R = 0.92, Fig. 2B); only cultures from donor BCY supplemented with dextrin exhibited lower richness in plates. Permutational multivariate analysis (Adonis2) of Bray–Curtis distances revealed that the cultivation technique significantly affected the community, albeit with a low effect size (F = 4.5, P ≤ .01; Fig. 2C), compared to the donor microbiota (F = 76.9, P < .001) and the supplemented C-source (F = 26.5, P ≤ .001). Despite slightly differing community structures, the taxa enriched in the two tested C-source conditions correlated between plates and tubes (R = 0.78, Fig. 2D). Notably, though not significant across all conditions and donors, plate cultivation is more prone to the proliferation of Enterobacteriaceae compared to tubes (Fig. S3B).
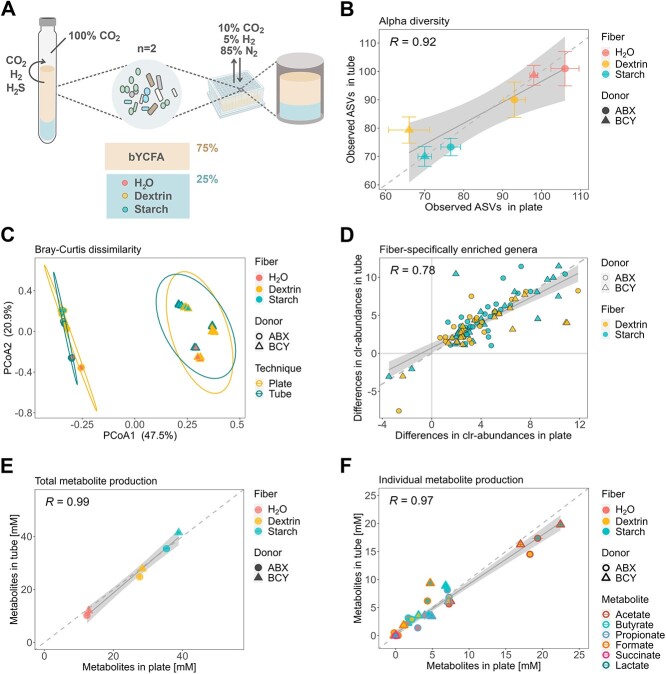
Figure 2.
Impact of the cultivation technique (96-deepwell plates vs. gas-tight tubes) on the metabolism and composition of ex vivo cultures (donor ABX and BCY) cultivated in bYCFA for 48 h, 37 °C, in the presence of resistant dextrin (3 g/l), soluble starch (3 g/l), or H2O as control (technical triplicates); (A) experimental setup for comparing the plate- and tube-based techniques; all analyses were performed after 48h cultivation; (B) correlation of observed ASVs; points represent means, and bars represent standard deviations; (C) PCoA plot of Bray–Curtis distances with 95% confidence ellipses (calculated separately for each donor) for the two cultivation techniques; (D) taxa that are significantly different between each fiber (dextrin, starch) and H2O (P < .05); each data point corresponds to the median difference of the clr-abundance (genus-level) between plate and tube; (E) correlation of the total metabolite production; total metabolites were calculated by summing the medium-corrected concentrations of organic acids (SCFA and intermediates); (F) correlation of individual metabolites; points represent means of blank corrected concentrations and bars represent standard deviations.
The concentrations of total metabolites (R = 0.99, Fig. 2E) and individual metabolites (R = 0.97, Fig. 2F) were similar between the two cultivation techniques. The main difference was a lower accumulation of the intermediate metabolite formate (P < .001, −3.5 ± 1.6) in plates as compared to tubes when starch was added (Fig. 2F). To identify whether the difference in metabolites produced might be attributed to changes in community composition or to variations in the metabolic outputs of individual strains exposed to the distinct gas atmosphere, we cultured a panel of pure gut bacteria using both techniques (Fig. S4A). The selected bacteria covered a range of nutritional preferences (e.g. primary degraders of complex fibers, utilizers of intermediate metabolites) and metabolic capabilities (e.g. acetate-, propionate-, and butyrate-producers). All bacteria grew well using both techniques, though six out of eight strains reached lower OD600 values after 48 h in plates compared to tubes (Fig. S4B). The cultivation technique influenced the carbohydrate metabolism of the tested strains, with all cultures exhibiting higher levels of end-metabolites (the SCFAs acetate, propionate and butyrate) in plates as compared to tubes (Fig. S4C). The two butyrate-producing species Agathobacter rectalis and Eubacterium ramulus yielded significantly lower levels of formate in plate compared to tubes (9.5 ± 2 vs. 12.5 ± 1 mM, P ≤ .05, and 1.1 ± 0.03 vs. 3.5 ± 0.1 mM, P ≤ .001, respectively; Fig. S4C). When grown in the chamber, the propionate-producing species Phocaeicola dorei showed higher conversion of succinate (1.8 ± 0.03 vs. 3.0 ± 0.1 mM, P ≤ .001) to propionate (5.7 ± 0.3 vs. 3.9 ± 0.1 mM, P ≤ .05; Fig. S4C) compared to tubes.
Collectively, the data indicate that cultivation in 96-deepwell plates results in similar community characteristics (alpha- and beta-diversity, DNA concentration) as the well-established Hungate technique. Fiber-specific taxonomic changes were highly comparable between the two protocols despite the higher susceptibility of plate cultures to Enterobacteriaceae proliferation. Furthermore, the open nature of the plate system and the composition of the gas used improved fermentability, favoring the conversion of C-sources to end-metabolites. Specific procedures for cultivating fecal microbiota (Supplementary File 1, Step 5.1) and pure cultures (Supplementary File 1, Step 5.2) are detailed in the high-throughput protocol.
Formulation of a growth medium to mimic the microbial composition of healthy adult feces during ex vivo cultivation
Next, we sought to formulate an optimal mix of C-sources to complement bYCFA basal medium and maximize the compositional similarity between ex vivo cultures and feces from human adults. Addressing a previously identified challenge in plate-based systems, we also assessed whether we could minimize the commonly observed ex vivo overgrowth of Enterobacteriaceae, a family associated with dysbiosis [39]. By maintaining the main characteristics of the donor’s community in culture, we aimed to enhance the relevance of ex vivo testing in assessing the effect mediated by diet-, drug-, and host-related factors. We cultivated fecal samples from eight healthy adults and examined whether the bacterial composition of feces could be better maintained using complex mixtures of carbohydrates (3C and 6C; 3 g/l total) and mucin (±Muc), as compared to glucose and H2O (C-depleted control; Fig. 3A). The composition of 3C was designed to mirror the C-sources present in the M2GSC medium (33% starch, 33% cellobiose, and 33% glucose), which supports the growth of numerous gut microbes [55]. In comparison, 6C contained the carbohydrates found in the Macfarlane medium (15% starch, 15% pectin, 15% xylan, 8% arabinogalactan, 8% guar gum, and 38% inulin) [59], a nutrient-rich medium that closely mimics the gut chyme of healthy adults [59], and is commonly used in continuous intestinal in vitro models [60]. Finally, we incorporated two conditions designed to replicate the nutritional composition of media previously used for gut microbiota culturing, namely Brain Heart Infusion (BHI) medium [20, 61] and Gut Microbiota Medium (GMM) [20, 35, 61]. Since we showed that the bicarbonate concentration affects the pH within the chamber, and given that BHI and GMM inherently lack bicarbonate, we formulated BHI-like and GMM-like media to improve the comparability of results without introducing confounding factors related to pH. Specifically, the BHI-like and GMM-like media are composed of bYCFA, supplemented with the necessary C- and N-sources to match the composition of BHI and GMM (Table S2).
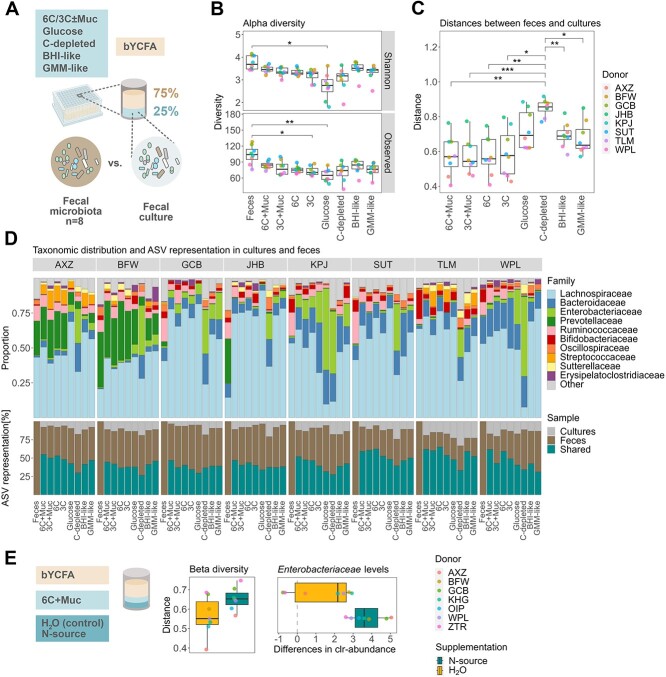
Figure 3.
Formulation of a growth medium maintaining the microbial composition of healthy adult feces in ex vivo cultures during high-throughput cultivation (48 h, 37 °C) in bYCFA medium; (A) experimental setup for determining the optimal C-source mix to maintain the composition of the original feces in cultures; all analyses were performed after 48-h cultivation; (B) number of observed ASVs and Shannon-Index in feces and cultures with different C-sources; (C) Bray–Curtis distances between the feces and the respective culture; (D) visual representation of the community composition (top) and the shared ASVs between feces and cultures (bottom); taxonomic relative abundance values are provided in Supplementary File 2; (E) evaluation of the impact of additional N-source supplementation (amicase and yeast extract) on Bray-Curtis distances and on the difference in Enterobacteriaceae clr-abundance as compared to the feces; all analyses were performed on pooled samples from three independent replicates for each donor microbiota; a total of 11 distinct donors were used, i.e. AXZ, BFW, GCB, JHB, KPJ, SUT, TLM, WPL, KHG, OIP, and ZTR; multiple comparisons across all conditions were performed using a t-test and P-values were corrected using the Holm–Bonferroni method; statistically significant results are marked by stars, with * indicating P ≤ .05, **P ≤ .01, and ***P ≤ .001.
The fecal richness (observed ASVs with relative abundance >0.1%; median of 104 among donors) was best maintained in culture conditions containing both mucin and complex C-sources, with the 6C + Muc condition exhibiting the highest median of observed ASVs (84.5) together with BHI-like (84.5), followed by GMM-like (76.5), 3C + Muc (76.5), 6C (75.0), 3C (70.5), and glucose (65.0; Fig. 3B). A similar trend was observed when including microbial evenness (Shannon-Index; Fig. 3B). Though 74 ASVs were detected in the C-depleted controls, a significant portion of detected taxa potentially originated from resting or dead cells from the fecal inoculum, as limited growth (OD600) was observed after 48 h (Fig. S5A). When comparing the taxonomic composition of the fecal cultures to the original feces using Bray–Curtis distances (ranging from 0: all ASVs shared, to 1: no ASVs shared), the conditions containing complex C-sources demonstrated significantly higher similarity to the feces (6C + Muc: 0.57; 3C + Muc: 0.54; 6C: 0.56; and 3C: 0.58) than the C-depleted conditions (H2O: 0.86) and, although not significantly, than those with glucose (0.70), BHI- (0.69) and GMM-like supplements (0.64; Fig. 3C). In line, shared ASVs between feces and cultures were highest in 6C + Muc (53 ± 7%), followed by 6C (51 ± 11%), 3C + Muc (48 ± 9%), 3C (44 ± 7%), BHI-like (43 ± 7%), GMM-like (43 ± 7%), and lowest with glucose (39 ± 7%) or in the absence of a C-source (33 ± 5%; Fig. 3D). At the family level, the main fecal taxa were represented in cultures containing complex C-sources (Fig. 3D), except for donor JHB, for which a relative loss of Prevotellaceae abundance was observed ex vivo (0.5 ± 0.4% among all complex C-source cultures vs. 31.8% in feces; Fig. 3D). Notably, adding mucin and complex C-sources limited the relative growth of Enterobacteriaceae, with the smallest increase in clr-abundance between cultures and feces observed with 6C + Muc (21.2-fold increase) and the largest in the presence of glucose (23.2-fold increase) and H2O (24.3-fold increase; Fig. S5B). Overall, among all cultures containing 6C + Muc, the maximum relative abundance of Enterobacteriaceae detected after 48 h was 9% (Donor KPJ; Fig. 3D).
When evaluating the metabolic outputs of fecal cultures, a significant increase in metabolite production was observed in the presence of complex C-sources (Fig. S5C). However, substantial amounts of formate accumulated (Fig. S5D), indicating incomplete fermentation. Conversely, BHI-like conditions resulted in more metabolites production compared to the 6C + Muc condition, with lower relative concentrations of formate (1.3 ± 1.5% vs. 8.5 ± 3%; Fig. S5D). Thus, we assessed whether the addition of N-source in the 6C + Muc condition could promote end-metabolite production [62]. We cultivated fecal samples from seven healthy adults in bYCFA with 6C + Muc and supplemented an additional 7.2 g/l amicase and 1 g/l yeast extract (Fig. 3E). With additional N-sources, a reduction of formate levels after 48 h (from 14.4 ± 3.8% to 1.3 ± 2.6%) and a representative SCFA ratio [63] was observed, with 61.5 ± 5.1% acetate, 18.9 ± 1.9% butyrate, and 16.4 ± 5.4% propionate among all donors (Fig. S6A). However, N-source addition did not help maintain the composition of the original feces and resulted in a slight reduction of richness (74 observed ASVs with extra N-sources and 81 ASVs in control; Fig. S6B) and increased Bray–Curtis distances between cultures and feces (extra N-source: 0.65; control: 0.55; Fig. 3E). Notably, the clr-abundance of Enterobacteriaceae was enhanced by additional N-sources (Fig. 3E). Taken together, we recommend complementing bYCFA with 6C + Muc without additional N-sources to better retain the compositional characteristics from adult feces and limit Enterobacteriaceae bloom during cultivation (Supplementary File 1).
Lastly, we evaluated the stability of ex vivo culture compositions in bYCFA with 6C + Muc over multiple passages to provide a potential setup for studying microbial community recovery patterns after perturbations [16]. Two fecal cultures were re-inoculated (1% v/v) into fresh medium over three successive passages (P1, P2, P3) at two different intervals (24 or 48 h; Fig. S7A). Repeated inoculation maintained the cultures’ richness (Fig. S7B) but increased the dissimilarity between the cultured communities and the original fecal samples (Bray–Curtis distances; Fig. S7B). These findings indicate that although re-inoculation shifts the community structure away from the original fecal composition, this simple approach could serve for studying the recovery dynamics of gut microbes after perturbations (e.g. applied in P1, with recovery evaluated in P2 onwards), provided that appropriate controls are implemented to account for community shifts.
Treating ex vivo cultures with dietary fibers or drugs results in physiologically relevant microbial responses
Finally, we aimed to validate our ex vivo high-throughput cultivation protocol by testing whether supplementation with specific dietary fibers (resistant dextrin and soluble starch; Fig. 4A) or drugs (omeprazole, ciprofloxacin, and 5-FU; Fig. 4D) could induce microbial changes that are consistent with previous in vivo findings.
Figure 4.
Treatment with dietary fibers (donor n = 8) or drugs (donor n = 7) elicits physiologically relevant changes in the ex vivo cultures’ metabolism and community composition (bYCFA, 48 h, 37 °C); (A) experimental setup to test the fiber-specific response of fecal cultures. All analyses were performed after 48-h cultivation; (B) SCFA production by fecal cultures when bYCFA was supplemented with resistant dextrin (3 g/l), soluble starch (3 g/l), or no C-source (H2O as control); (C) median clr-differences of taxa significantly differed between each fiber (dextrin, starch) and H2O; genera significantly different (P ≤ .05) are labeled and located above the dotted red line; (D) experimental setup to test the effect of drugs (i.e. omeprazole, ciprofloxacin and 5-FU) as compared to the vehicle control (DMSO or H2O) in bYCFA supplemented with 6C + Muc; all analyses were performed after 48h cultivation; (E) number of observed ASVs as compared to the vehicle control; (F) Bray–Curtis distances between controls and the drug-treated cultures; (G) Clr-differences of the 15 most abundant families; significantly altered families, as compared to the control, are marked by stars; all the analyses were performed on pooled samples from incubations for each donor microbiota; multiple comparisons across all conditions were performed using a t-test, and P-values were corrected using the Holm–Bonferroni method; statistically significant results are marked by stars, with * indicating P ≤ .05, **P ≤ .01, ***P ≤ .001, and ns, non-significant.
We chose resistant dextrin (3 g/l) and soluble starch (3 g/l) as dietary fibers due to their well-known ability to modulate the gut microbiota and mediate propiogenic [64] and butyrogenic [65–67] effects, respectively. Using feces from eight healthy donors, we performed enrichment experiments in bYCFA containing each fiber as the sole C-source. Among all donors, we found significantly higher levels of propionate after 48 h in the presence of dextrin (10.9 ± 2.9 mM) compared to starch (6.0 ± 2.4 mM; P ≤ .05) or the control without C-sources (3.9 ± 1.2 mM; P ≤ .001). Conversely, starch supplementation led to significantly higher levels of butyrate (10.1 ± 2.1 mM) compared to dextrin (4.5 ± 1.1 mM; P ≤ .001) or the control (2.4 ± 1.5 mM; P ≤ .01; Fig. 4B). At the taxonomic level, resistant dextrin increased the relative abundance of Fusicatenibacter (P ≤ .05) and Blautia (P > .05) among all donors (Fig. 4C), consistent with previous ex vivo and in vivo studies [14, 68, 69]. Human trials also identified a dextrin-specific increase of Parabacteroides [14, 70], which was increased in our ex vivo cultures (23.5-fold increase), though the effect was not significant. Starch supplementation most prominently enriched Faecalibacterium (P ≤ .01) and Agathobacter (P ≤ .05; formerly Eubacterium, Fig. 4C), which were also promoted in previous human trials [25, 66, 67, 71]. Relative abundances of Flavonifractor (P < .05) and Bilophila were reduced with starch (P < .05; Fig. 4C).
Many commonly used medications have been shown to disrupt gut microbial communities [6, 72]. Conversely, microbial activities can alter drug efficacy and toxicity [73–75]. To test the direct interaction between the gut microbiota and drugs, we exposed the feces from seven individuals (in bYCFA with 6C + Muc) to a single dose of 11 μM omeprazole (proton-pump inhibitor), 50 μM ciprofloxacin (antibiotic), or 50 μM 5-FU (cytostatic), representing the respective expected intestinal concentrations [16, 76, 77]. Compared to the vehicle control cultures, treatment with ciprofloxacin and 5-FU negatively impacted the microbial communities’ diversity (Fig. 4E) and structure (Fig. 4F). Ciprofloxacin led to a relative increase in Lachnospiraceae (P ≤ .05) (Fig. 4G). The reduction in community diversity and persistence of Lachnospiraceae agrees with previous findings in mice [16] and ciprofloxacin-treated patients [78]. Treatment with 5-FU inhibited Sutterellaceae (P ≤ .05) and Erysipelatoclostridiaceae (P ≤ .01) and promoted the relative abundance of Akkermansiaceae (P ≤ .05) (Fig. 4G). Several mice studies have demonstrated the reduction in diversity and the drastic bloom of Akkermansiaceae in response to 5-FU [79–81]. In contrast, we observed no significant effect of omeprazole on fecal cultures at the tested concentration (Fig. 4E and F). This common proton pump inhibitor has been shown to decrease the richness and enrich the abundance of oral bacteria (e.g. Streptococcaceae) in the gut [82]. However, it was proposed that the effect of omeprazole on the gut microbiota might not be directly caused by the molecule but rather by an elevation of gastric pH, disrupting the barrier between the upper and lower gastrointestinal tract [82]. This hypothesis aligns with our ex vivo findings. It is worth noting that omeprazole was dissolved in DMSO at a final concentration of 0.2% DMSO in fecal culture. At this concentration, DMSO did not affect the communities’ diversity and structure (Fig. S8), indicating its suitability as a solvent for testing compounds with low solubility in an aqueous phase.
Discussion
A well-controlled protocol for cultivating stool-derived microbial communities in the anaerobic chamber has the potential to advance our understanding of the causal interactions between gut microbes, diet, drugs, and host physiology [83]. Such a protocol should possess a high-throughput capacity to effectively capture the complex and individualistic nature of the gut microbiota, with heterogenous compositions among individuals [24–27], variations in activities at the strain level [28–31], and the influence of environmental factors on ecological and physiological dynamics [6, 10]. An increasing number of studies have reported the development of anaerobic high-throughput systems for cultivating stool-derived gut microbiota to generate personalized culture collections of gut microbes [84, 85], map the ability of the human gut microbiota to metabolize small molecule drugs [18, 75], or evaluate the response of fecal communities to specific stimuli [16, 17]. These studies have highlighted the promise of high-throughput ex vivo modeling by demonstrating a strong correlation between ex vivo and in vivo gut microbiota responses [16, 17]. Here, we addressed some of the practical, technical, and biological gaps in fecal cultivation that we consider essential to create a widely applicable, reproducible and accessible protocol, and advance the field of gut microbiota research.
First, we conducted an in-depth characterization of potential confounding factors associated with anaerobic chamber cultivation by comparing plate-based cultivation with the well-established Hungate technique. Plate cultures are more susceptible to O2 contamination than Hungate tube cultures, with a higher risk of disrupting the growth of commensals (e.g. Faecalibacterium prausnitzii, Figs S2) and promoting the commonly observed bloom of facultative anaerobes (e.g. Enterobacteriaceae, Fig. S3B). To minimize the risk of O2 contamination, we strongly recommend adhering to the manufacturer’s recommendations for proper chamber preparation by maintaining high H2 levels and regenerating the palladium catalysts (Supplementary File 1, Step 2). Although it is theoretically possible to generate anaerobiosis by prereducing a sterile aerobic medium inside the chamber for several days before the experiment [16–20], we recommend an additional step of medium boiling and flushing (CO2 or N2) prior to sterilization (Supplementary File 1, Step 1), as practiced in the Hungate technique [13]. Despite the increased relative Enterobacteriaceae abundances in plates compared to tubes, both protocols resulted in strong and comparable substrate-specific responses, indicating that both methods are equally suited for assessing supplement-specific responses.
Second, we highlighted that the equilibrium between gaseous and dissolved CO2 and the concentration of NaHCO3 in the solution influenced the pH of the medium in the chamber (Fig. 1B–E). The bicarbonate buffer is more challenging to control in open systems (e.g. 96-deepwell plates) where CO2 can escape. Still, the bicarbonate buffer optimally maintains pH in the range of the human adult colon from 5.7 (proximal) to 6.7 (distal) [86] when potential pH shifts are accounted for, e.g. by performing pH adjustment with HCl immediately before inoculation (Supplementary File 1, Step 6). Note that bYCFA basal medium also contains a phosphate buffer to maintain the pH between 6.4 and 7.4.
Third, we identified a few metabolic differences between cultivation in plates and tubes. The typically lower CO2 concentration in the chamber (~10%) compared to the concentration in tubes (~100%) mediated an increase of succinate to propionate conversion in pure cultures of P. dorei (Fig. S4C) [87, 88]. Moreover, the plate-based system allows microbially-produced gases to be released. H2, for instance, is a common growth-limiting factor that accumulates in tube cultures during fermentation [89] and promotes the accumulation of intermediate metabolites [90]. Our data support this finding, as tube cultures exhibited a higher accumulation of intermediate products (i.e. formate) than plate cultivation (Fig. 2C and Fig. S4C). Overall, the typical gas composition in the anaerobic chamber (i.e. 5% H2, 10% CO2, and 85% N2) is close to the physiological conditions reported in vivo for H2 and CO2, with concentrations of 2.9 ± 0.7% and 9.9 ± 1.6%, respectively [91].
Fourth, we convey our findings in a simple and comprehensive step-by-step protocol (Supplementary File 1), which includes a reproducible and modular medium preparation process. The basal medium bYCFA is heat-stable (sterilization via autoclave) and contains a limited amount of undefined ingredients to increase batch-to-batch reproducibility. Furthermore, it can be flexibly complemented with C-sources or any other molecules to test, and the pH can be easily adjusted, allowing for testing of a wide range of culture conditions with minimal medium preparation effort. Here, we showcased two examples of experiments by using the presented protocol. We first complemented bYCFA with defined fibers to identify taxa associated with functional niches (Fig. 4A–C). The results corroborated with a previous tube-based enrichment study, with the validity of this approach being further supported by a parallel nutritional intervention study [14]. We then tested the microbial responses to antibiotic and non-antibiotic drugs in fecal cultures (Fig. 4D–G), using an optimized mix of C-sources to maximize compositional similarity to feces (6C + Muc; Fig. 3B–D). This setup could be extended to study the effects of other xenobiotics (e.g. pollutants), dietary compounds (e.g. N-sources, vitamins), host-derived factors (e.g. pH, redox potential, oxidative stress, bile salts, antimicrobial peptides), or their combinations. Importantly, the medium composition containing 6C + Muc surpassed other commonly used media for fecal microbiota ex vivo cultivation, such as BHI-like and GMM-like, especially in maintaining the gut microbiota community structure (Fig. 3C and D). By complementing bYCFA with a mix of mucin and complex carbohydrates (6C + Muc), we were able to mitigate the blooming of Enterobacteriaceae (Fig. S5B). A previous study suggested that the ex vivo overgrowth of Enterobacteriaceae in the starch-containing medium, modified Gifu Anaerobic Medium, could be prevented by mixing it with the Bryant and Burkey medium [18, 20]. The latter contains acetate, a known inhibitor of Enterobacteriaceae taxa, such as Escherichia coli [92]. Another study highlighted the importance of mucin as a growth factor of Firmicutes [35]. Based on these previous findings and our data, it is tempting to speculate that combining mucin and complex carbohydrates in the 6C + Muc solution may foster the proliferation of commensals and, at the same time, organic acids in bYCFA may serve as growth inhibitors for Enterobacteriaceae.
Altogether, this protocol encompasses key features like flexibility (e.g. testing of various molecules and conditions alone or in combination; complex communities or pure cultures), reproducibility (e.g. reduced media complexity; preparation of stable stock solutions), and simplicity (e.g. minimized hands-on time; easy pH adjustment; reduced evaporation rate). It is important to recognize that common limitations of batch cultivation setups (e.g. limited cultivation time, nutrient depletion, acidification, accumulation of inhibitory metabolites) are inherent to the system presented [21]. Therefore, the applicability of the protocol must be carefully evaluated based on the research question and results have to be interpreted accordingly. Although batch cultivation models are valuable screening tools for growth capacities, metabolic potentials, and inter-individual variations, more complex in vitro models (i.e. continuous culture) are undoubtedly superior for in-depth studies of ecological and evolutionary dynamics, as they provide accurate readouts with stable conditions and continuous sampling. In addition, continuous fermentations are optimal for validating mechanism hypotheses generated with batch cultivation setups.
In conclusion, our high-throughput cultivation protocol is a valuable tool for fundamental and applied gut microbiota research. It enables rapid and reproducible characterization of diet-, host-, or drug-specific effects, microbe–microbe interactions [93], strain-specific [28, 31], and donor-specific [27] responses and activities. The protocol can also be applied to cost-efficient screening of drug metabolism by the gut microbiota [75, 76], preselection of treatments or dosage prior to costly animal or human trials [94], development of personalized microbiota-targeted nutritional interventions [95], identification of responders and non-responders to drugs and prebiotics [27], and for discovery and investigation of novel live biotherapeutics [96].
Supplementary Material
Acknowledgements
We thank Alfonso Die (ETH Zürich) for their assistance with HPLC-RI analyses. We thank Florentin Constancias for his support with statistical analysis. We thank Anna Greppi for her valuable feedback during the preparation of the manuscript. We thank Roquette for providing NUTRIOSE® (resistant dextrin). Data were generated in collaboration with the Genetic Diversity Centre (GDC), ETH Zurich. Graphic elements BioRender.com have been used to create Figs 1–3.
Contributor Information
Janina N Zünd, Laboratory of Food Biotechnology, Institute of Food, Nutrition and Health, Department of Health Sciences and Technology, ETH Zürich, 8092 Zürich, Switzerland.
Serafina Plüss, Laboratory of Food Biotechnology, Institute of Food, Nutrition and Health, Department of Health Sciences and Technology, ETH Zürich, 8092 Zürich, Switzerland.
Denisa Mujezinovic, Laboratory of Food Biotechnology, Institute of Food, Nutrition and Health, Department of Health Sciences and Technology, ETH Zürich, 8092 Zürich, Switzerland.
Carmen Menzi, Laboratory of Food Biotechnology, Institute of Food, Nutrition and Health, Department of Health Sciences and Technology, ETH Zürich, 8092 Zürich, Switzerland; PharmaBiome AG, 8952 Schlieren, Switzerland.
Philipp R von Bieberstein, Laboratory of Food Biotechnology, Institute of Food, Nutrition and Health, Department of Health Sciences and Technology, ETH Zürich, 8092 Zürich, Switzerland; PharmaBiome AG, 8952 Schlieren, Switzerland.
Tomas de Wouters, PharmaBiome AG, 8952 Schlieren, Switzerland.
Christophe Lacroix, Laboratory of Food Biotechnology, Institute of Food, Nutrition and Health, Department of Health Sciences and Technology, ETH Zürich, 8092 Zürich, Switzerland.
Gabriel E Leventhal, PharmaBiome AG, 8952 Schlieren, Switzerland.
Benoit Pugin, Laboratory of Food Biotechnology, Institute of Food, Nutrition and Health, Department of Health Sciences and Technology, ETH Zürich, 8092 Zürich, Switzerland.
Author contributions
Benoit Pugin, Christophe Lacroix, Tomas de Wouters, and Gabriel E. Leventhal conceptualized the project and provided financial support. Janina N. Zünd, Benoit Pugin, and Gabriel E. Leventhal planned the experiments. Janina N. Zünd, Serafina Plüss, Denisa Mujezinovic, Carmen Menzi performed wet-lab experiments and analyses. Janina N. Zünd, Philipp R. von Bieberstein, Gabriel E. Leventhal curated the data. Janina N. Zünd and Philipp R. von Bieberstein performed data analysis. Janina N. Zünd, Benoit Pugin, Gabriel E. Leventhal , Christophe Lacroix interpreted the data. Janina N. Zünd, Serafina Plüss, and Benoit Pugin wrote the step-by-step protocol. Janina N. Zünd and Benoit Pugin wrote the original draft of the manuscript. All authors critically reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Conflicts of interest
C.M., P.B., T.W., and G.L. are or were employees of PharmaBiome AG. T.W. and C.L. are founders of PharmaBiome AG. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by a grant from the Swiss Innovation Agency (51128.1 IP-LS) in collaboration with PharmaBiome AG. This work was also supported by an ETH Research Grant ETH-38 20-1.
Data availability
All data reported in this paper are deposited in the ENA repository, with the accession number PRJEB68331, https://www.ebi.ac.uk/ena/browser/view/PRJEB68331.
References
- 1. Arumugam M, Raes J, Pelletier E et al. Enterotypes of the human gut microbiome. Nature 2011;473:174–80. 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Valdes AM, Walter J, Segal E et al. Role of the gut microbiota in nutrition and health. BMJ 2018;361:k2179–44. 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Poeker SA, Geirnaert A, Berchtold L et al. Understanding the prebiotic potential of different dietary fibers using an in vitro continuous adult fermentation model (PolyFermS). Sci Rep 2018;8:1–12. 10.1038/s41598-018-22438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wastyk HC, Fragiadakis GK, Perelman D et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021;184:4137–4153.e14. 10.1016/j.cell.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Djekic D, Shi L, Brolin H et al. Effects of a vegetarian diet on cardiometabolic risk factors, gut microbiota, and plasma metabolome in subjects with ischemic heart disease: a randomized, crossover study. J Am Hear Assoc Cardiovasc Cerebrovasc Dis 2020;9:e016518. 10.1161/JAHA.120.016518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jackson MA, Verdi S, Maxan ME et al. Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat Commun 2018;9:2655. 10.1038/s41467-018-05184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palleja A, Mikkelsen KH, Forslund SK et al. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat Microbiol 2018;3:1255–65. 10.1038/s41564-018-0257-9. [DOI] [PubMed] [Google Scholar]
- 8. Klünemann M, Andrejev S, Blasche S et al. Bioaccumulation of therapeutic drugs by human gut bacteria. Nature 2021;597:533–8. 10.1038/s41586-021-03891-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hashimoto T, Perlot T, Rehman A et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 2012;487:477–81. 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tropini C, Moss EL, Merrill BD et al. Transient osmotic perturbation causes long-term alteration to the gut microbiota. Cell 2018;173:1742–1754.e17. 10.1016/j.cell.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roager HM, Hansen LBS, Bahl MI et al. Colonic transit time is related to bacterial metabolism and mucosal turnover in the gut. Nat Microbiol 2016;1:1–9. 10.1038/nmicrobiol.2016.93. [DOI] [PubMed] [Google Scholar]
- 12. Murga-Garrido SM, Hong Q, Cross TWL et al. Gut microbiome variation modulates the effects of dietary fiber on host metabolism. Microbiome 2021;9:117. 10.1186/s40168-021-01061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hungate RE, Chapter IV. A roll tube method for cultivation of strict anaerobes. Methods Microbiol 1969;3:117–32. [Google Scholar]
- 14. Anthamatten L, von Bieberstein PR, Thabuis C. et al. Mapping gut bacteria into functional niches reveals the ecological structure of human gut microbiomes. bioRxiv 2023;2023.07.04.547750. 10.1101/2023.07.04.547750 [DOI]
- 15. Pérez-Burillo S, Molino S, Navajas-Porras B et al. An in vitro batch fermentation protocol for studying the contribution of food to gut microbiota composition and functionality. Nat Protoc 2021;16:3186–209. 10.1038/s41596-021-00537-x. [DOI] [PubMed] [Google Scholar]
- 16. Aranda-Díaz A, Ng KM, Thomsen T et al. Establishment and characterization of stable, diverse, fecal-derived in vitro microbial communities that model the intestinal microbiota. Cell Host Microbe 2022;30:260–272.e5. 10.1016/j.chom.2021.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li L, Abou-Samra E, Ning Z et al. An in vitro model maintaining taxon-specific functional activities of the gut microbiome. Nat Commun 2019;10:4146. 10.1038/s41467-019-12087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Javdan B, Lopez JG, Chankhamjon P et al. Personalized mapping of drug metabolism by the human gut microbiome. Cell 2020;181:1661–1679.e22. 10.1016/j.cell.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li L, Ning Z, Zhang X et al. RapidAIM: a culture- and metaproteomics-based rapid assay of individual microbiome responses to drugs. Microbiome 2020;8:1–16. 10.1186/s40168-020-00806-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tao X, Huang W, Pan L et al. Optimizing ex vivo culture conditions to study human gut microbiome. ISME Commun 2023;3:1–10. 10.1038/s43705-023-00245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Isenring J, Bircher L, Geirnaert A et al. In vitro human gut microbiota fermentation models: opportunities, challenges, and pitfalls. Microbiome Res Reports 2023;2:2. 10.20517/mrr.2022.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zihler Berner A, Fuentes S, Dostal A et al. Novel polyfermentor intestinal model (PolyFermS) for controlled ecological studies: validation and effect of pH. PLoS One 2013;8:e77772. 10.1371/journal.pone.0077772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Molly K, Vande Woestyne M, Verstraete W. Development of a 5-step multi-chamber reactor as a simulation of the human intestinal microbial ecosystem. Appl Microbiol Biotechnol 1993;39:254–8. 10.1007/BF00228615. [DOI] [PubMed] [Google Scholar]
- 24. Sandberg J, Kovatcheva-Datchary P, Björck I et al. Abundance of gut Prevotella at baseline and metabolic response to barley prebiotics. Eur J Nutr 2019;58:2365–76. 10.1007/s00394-018-1788-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walker AW, Ince J, Duncan SH et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J 2011;5:220–30. 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cantu-Jungles TM, Bulut N, Chambry E et al. Dietary fiber hierarchical specificity: the missing link for predictable and strong shifts in gut bacterial communities. mBio 2021;12:e01028–1. 10.1128/mBio.01028-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hjorth MF, Christensen L, Larsen TM et al. Pretreatment Prevotella-to-Bacteroides ratio and salivary amylase gene copy number as prognostic markers for dietary weight loss. Am J Clin Nutr 2020;111:1079–86. 10.1093/ajcn/nqaa007. [DOI] [PubMed] [Google Scholar]
- 28. Hall AB, Yassour M, Sauk J et al. A novel Ruminococcus gnavus clade enriched in inflammatory bowel disease patients. Genome Med 2017;9:1–12. 10.1186/s13073-017-0490-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Karcher N, Pasolli E, Asnicar F et al. Analysis of 1321 Eubacterium rectale genomes from metagenomes uncovers complex phylogeographic population structure and subspecies functional adaptations. Genome Biol 2020;21:138. 10.1186/s13059-020-02042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sorbara MT, Littmann ER, Fontana E et al. Functional and genomic variation between human-derived isolates of Lachnospiraceae reveals inter- and intra-species diversity. Cell Host Microbe 2020;28:134–146.e4. 10.1016/j.chom.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Otaru N, Ye K, Mujezinovic D et al. GABA production by human intestinal Bacteroides spp.: prevalence, regulation, and role in acid stress tolerance. Front Microbiol 2021;12:656895. 10.3389/fmicb.2021.656895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Müller P, de la Cuesta-Zuluaga J, Kuhn M et al. High-throughput anaerobic screening for identifying compounds acting against gut bacteria in monocultures or communities. Nat Protoc 2023;19:668–99. 10.1038/s41596-023-00926-4. [DOI] [PubMed] [Google Scholar]
- 33. Średnicka P, Roszko MŁ, Popowski D et al. Effect of in vitro cultivation on human gut microbiota composition using 16S rDNA amplicon sequencing and metabolomics approach. Sci Rep 2023;13:3026. 10.1038/s41598-023-29637-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alessandri G, Fontana F, Mancabelli L et al. Exploring species-level infant gut bacterial biodiversity by meta-analysis and formulation of an optimized cultivation medium. NPJ Biofilms Microbiomes 2022;8:1–12. 10.1038/s41522-022-00349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li L, Zhang X, Ning Z et al. Evaluating in vitro culture medium of gut microbiome with orthogonal experimental design and a metaproteomics approach. J Proteome Res 2018;17:154–63. 10.1021/acs.jproteome.7b00461. [DOI] [PubMed] [Google Scholar]
- 36. Barrack KE, Hampton TH, Valls RA. et al. An in vitro medium for modeling gut dysbiosis associated with cystic fibrosis. Journal of Bacteriology 2024;206:e00286-23. 10.1128/jb.00286-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang X, Walker K, Mayne J et al. Evaluating live microbiota biobanking using an ex vivo microbiome assay and metaproteomics. Gut Microbes 2022;14:2035658. 10.1080/19490976.2022.2035658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li L, Mayne J, Beltran A. et al. RapidAIM 2.0: a high-throughput assay to study functional response of human gut microbiome to xenobiotics. bioRxiv 2022;2022.08.03.502618. 10.1101/2022.08.03.502618 [DOI] [PMC free article] [PubMed]
- 39. Zeng MY, Inohara N, Nuñez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol 2017;10:18–26. 10.1038/mi.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Celis AI, Relman DA, Huang KC. The impact of iron and heme availability on the healthy human gut microbiome in vivo and in vitro. Cell Chem Biol 2023;30:110–126.e3. 10.1016/j.chembiol.2022.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Duncan SH, Hold GL, Harmsen HJM et al. Growth requirements and fermentation products of Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. nov. Int J Syst Evol Microbiol 2002;52:2141–6. [DOI] [PubMed] [Google Scholar]
- 42. Caporaso JG, Lauber CL, Walters WA et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 2012;6:1621–4. 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Caporaso JG, Lauber CL, Walters WA et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci USA 2011;108:4516–22. 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Walters W, Hyde ER, Berg-Lyons D et al. Improved bacterial 16S rRNA gene (V4 and V4-5) and fungal internal transcribed spacer marker gene primers for microbial community surveys. mSystems 2015;1:10–1128. 10.1128/mSystems.00009-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Constancias F, Mahé F. fconstancias/metabaRpipe: v0.9 (v0.9) Zenodo, 2022.
- 46. Callahan BJ, McMurdie PJ, Rosen MJ et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 2016;13:581–3. 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Quast C, Pruesse E, Yilmaz P et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 2013;41:D590–6. 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yilmaz P, Parfrey LW, Yarza P et al. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res 2014;42:D643–8. 10.1093/nar/gkt1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reitmeier S, Hitch TCA, Treichel N et al. Handling of spurious sequences affects the outcome of high-throughput 16S rRNA gene amplicon profiling. ISME Commun 2021;1:1–12. 10.1038/s43705-021-00033-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McMurdie PJ, Holmes S. Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 2013;8:e61217. 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fernandes AD, Reid JNS, Macklaim JM et al. Unifying the analysis of high-throughput sequencing datasets: characterizing RNA-seq, 16S rRNA gene sequencing and selective growth experiments by compositional data analysis. Microbiome. 2014;2:1–13. 10.1186/2049-2618-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Barnett DJM, Arts ICW, Penders J. microViz: an R package for microbiome data visualization and statistics. J Open Source Softw 2021;6:3201. 10.21105/joss.03201. [DOI] [Google Scholar]
- 53. Ernst FGM, Shetty SA, Borman T, Lahti L. mia: Microbiome Analysis. R package version 1.11.5. 2024. https://github.com/microbiome/mia.
- 54. Douglas S, Auld PD, Peter A. et al. Microplate selection and recommended practices in high-throughput screening and quantitative biology. In: Assay Guidance Manual [Internet] 2020.
- 55. Miyazaki K, Martin JC, Marinsek-Logar R et al. Degradation and utilization of xylans by the rumen anaerobe Prevotella bryantii (formerlyP. ruminicolasubsp.brevis) B14. Anaerobe 1997;3:373–81. 10.1006/anae.1997.0125. [DOI] [PubMed] [Google Scholar]
- 56. Kohn RA, Dunlap TF. Calculation of the buffering capacity of bicarbonate in the rumen and in vitro. J Anim Sci 1998;76:1702–9. 10.2527/1998.7661702x. [DOI] [PubMed] [Google Scholar]
- 57. Duncan SH, Louis P, Thomson JM et al. The role of pH in determining the species composition of the human colonic microbiota. Environ Microbiol 2009;11:2112–22. 10.1111/j.1462-2920.2009.01931.x. [DOI] [PubMed] [Google Scholar]
- 58. Contijoch EJ, Britton GJ, Yang C et al. Gut microbiota density influences host physiology and is shaped by host and microbial factors. Elife 2019;8:e40553. 10.7554/eLife.40553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Macfarlane GT, Macfarlane S, Gibson GR. Validation of a three-stage compound continuous culture system for investigating the effect of retention time on the ecology and metabolism of bacteria in the human colon. Microb Ecol 1998;35:180–7. 10.1007/s002489900072. [DOI] [PubMed] [Google Scholar]
- 60. Isenring J, Stevens MJA, Jans C et al. Identification of valerate as carrying capacity modulator by analyzing Lactiplantibacillus plantarum colonization of colonic microbiota in vitro. Front Microbiol 2022;13:1855. 10.3389/fmicb.2022.910609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yousi F, Kainan C, Junnan Z et al. Evaluation of the effects of four media on human intestinal microbiota culture in vitro. AMB Exp 2019;9:1–10. 10.1186/s13568-019-0790-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Walker AW, Duncan SH, Carol McWilliam Leitch E et al. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl Environ Microbiol 2005;71:3692–700. 10.1128/AEM.71.7.3692-3700.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cummings JH, Pomare EW, Branch HWJ et al. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987;28:1221–7. 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Guerin-Deremaux L, Ringard F, Desailly F et al. Effects of a soluble dietary fibre NUTRIOSE® on colonic fermentation and excretion rates in rats. Nutr Res Pract 2010;4:470–6. 10.4162/nrp.2010.4.6.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Louis P, Scott KP, Duncan SH et al. Understanding the effects of diet on bacterial metabolism in the large intestine. J Appl Microbiol 2007;102:1197–208. 10.1111/j.1365-2672.2007.03322.x. [DOI] [PubMed] [Google Scholar]
- 66. Venkataraman A, Sieber JR, Schmidt AW et al. Variable responses of human microbiomes to dietary supplementation with resistant starch. Microbiome. 2016;4:1–9. 10.1186/s40168-016-0178-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Baxter NT, Schmidt AW, Venkataraman A et al. Dynamics of human gut microbiota and short-chain fatty acids in response to dietary interventions with three fermentable fibers. mBio 2019;10:e02566–18. 10.1128/mBio.02566-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Barber C, Sabater C, Ávila-Gálvez MÁ et al. Effect of resistant dextrin on intestinal gas homeostasis and microbiota. Nutrients 2022;14:4611. 10.3390/nu14214611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Perreau C, Thabuis C, Verstrepen L et al. Ex vivo colonic fermentation of NUTRIOSE® exerts immuno-modulatory properties and strong anti-inflammatory effects. Nutrients 2023;15:4229. 10.3390/nu15194229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Thirion F, Da Silva K, Plaza Oñate F et al. Diet supplementation with NUTRIOSE, a resistant dextrin, increases the abundance of Parabacteroides distasonis in the human gut. Mol Nutr Food Res 2022;66:e2101091. 10.1002/mnfr.202101091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vital M, Howe A, Bergeron N et al. Metagenomic insights into the degradation of resistant starch by human gut microbiota. Appl Environ Microbiol 2018;84:e01562–18. 10.1128/AEM.01562-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vich Vila A, Collij V, Sanna S et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat Commun 2020;11:362. 10.1038/s41467-019-14177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. van Kessel SP, Frye AK, El-Gendy AO et al. Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson’s disease. Nat Commun 2019;10:310. 10.1038/s41467-019-08294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yuan L, Zhang S, Li H et al. The influence of gut microbiota dysbiosis to the efficacy of 5-Fluorouracil treatment on colorectal cancer. Biomed Pharmacother 2018;108:184–93. 10.1016/j.biopha.2018.08.165. [DOI] [PubMed] [Google Scholar]
- 75. Zimmermann M, Zimmermann-Kogadeeva M, Wegmann R et al. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 2019;570:462–7. 10.1038/s41586-019-1291-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Maier L, Pruteanu M, Kuhn M et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018;555:623–8. 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. LaCourse KD, Zepeda-Rivera M, Kempchinsky AG et al. The cancer chemotherapeutic 5-fluorouracil is a potent Fusobacterium nucleatum inhibitor and its activity is modified by intratumoral microbiota. Cell Rep 2022;41:111625. 10.1016/j.celrep.2022.111625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Stewardson AJ, Gaïa N, François P et al. Collateral damage from oral ciprofloxacin versus nitrofurantoin in outpatients with urinary tract infections: a culture-free analysis of gut microbiota. Clin Microbiol Infect 2015;21:344.e1–11. 10.1016/j.cmi.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 79. Li HL, Lu L, Wang XS et al. Alteration of gut microbiota and inflammatory cytokine/chemokine profiles in 5-fluorouracil induced intestinal mucositis. Front Cell Infect Microbiol 2017;7:455. 10.3389/fcimb.2017.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hamouda N, Sano T, Oikawa Y et al. Apoptosis, dysbiosis and expression of inflammatory cytokines are sequential events in the development of 5-fluorouracil-induced intestinal mucositis in mice. Basic Clin Pharmacol Toxicol 2017;121:159–68. 10.1111/bcpt.12793. [DOI] [PubMed] [Google Scholar]
- 81. Carvalho R, Vaz A, Pereira FL et al. Gut microbiome modulation during treatment of mucositis with the dairy bacterium Lactococcus lactis and recombinant strain secreting human antimicrobial PAP. Sci Rep 2018;8:15072. 10.1038/s41598-018-33469-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jackson MA, Goodrich JK, Maxan ME et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut 2016;65:749–56. 10.1136/gutjnl-2015-310861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Clavel T, Horz HP, Segata N et al. Next steps after 15 stimulating years of human gut microbiome research. Microb Biotechnol 2022;15:164–75. 10.1111/1751-7915.13970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hitch TCA, Afrizal A, Riedel T et al. Recent advances in culture-based gut microbiome research. Int J Med Microbiol 2021;311:151485. 10.1016/j.ijmm.2021.151485. [DOI] [PubMed] [Google Scholar]
- 85. Goodman AL, Kallstrom G, Faith JJ et al. Extensive personal human gut microbiota culture collections characterized and manipulated in gnotobiotic mice. Proc Natl Acad Sci USA 2011;108:6252–7. 10.1073/pnas.1102938108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Fallingborg J. Intraluminal pH of the human gastrointestinal tract. Dan Med Bull 1999;46:183–96. [PubMed] [Google Scholar]
- 87. Fischbach MA, Sonnenburg JL. Eating for two: how metabolism establishes interspecies interactions in the gut. Cell Host Microbe 2011;10:336–47. 10.1016/j.chom.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Macy JM, Ljungdahl LG, Gottschalk G. Pathway of succinate and propionate formation in Bacteroides fragilis. J Bacteriol 1978;134:84–91. 10.1128/jb.134.1.84-91.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Smith NW, Shorten PR, Altermann EH et al. Hydrogen cross-feeders of the human gastrointestinal tract. Gut Microbes 2019;10:270–88. 10.1080/19490976.2018.1546522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Van Lingen HJ, Plugge CM, Fadel JG et al. Thermodynamic driving force of hydrogen on rumen microbial metabolism: a theoretical investigation. PLoS One 2016;11:e0161362. 10.1371/journal.pone.0161362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Modesto A, Cameron NR, Varghese C et al. Meta-analysis of the composition of human intestinal gases. Dig Dis Sci 2022;67:3842–59. 10.1007/s10620-021-07254-1. [DOI] [PubMed] [Google Scholar]
- 92. Pinhal S, Ropers D, Geiselmann J et al. Acetate metabolism and the inhibition of bacterial growth by acetate. J Bacteriol 2019;201:147–66. 10.1128/JB.00147-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Shetty SA, Kuipers B, Atashgahi S et al. Inter-species metabolic interactions in an in-vitro minimal human gut microbiome of core bacteria. NPJ Biofilms Microbiomes 2022;8:21. 10.1038/s41522-022-00275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Liu Y, Gibson GR, Walton GE. An in vitro approach to study effects of prebiotics and probiotics on the faecal microbiota and selected immune parameters relevant to the elderly. PLoS One 2016;11:e0162604. 10.1371/journal.pone.0162604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gibbons SM, Gurry T, Lampe JW et al. Perspective: leveraging the gut microbiota to predict personalized responses to dietary, prebiotic, and probiotic interventions. Adv Nutr 2022;13:1450–61. 10.1093/advances/nmac075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Relizani K, Le Corf K, Kropp C et al. Selection of a novel strain of Christensenella minuta as a future biotherapy for Crohn’s disease. Sci Rep 2022;12:6017. 10.1038/s41598-022-10015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper are deposited in the ENA repository, with the accession number PRJEB68331, https://www.ebi.ac.uk/ena/browser/view/PRJEB68331.