Methods
In a double-blinded cross-over design, 30 adults (mean age = 25.57, SD = 3.74; all male) were administered racemic ketamine and compared against saline infusion as a control. Both task-driven (auditory oddball paradigm) and resting-state EEG were recorded. HOI were computed using advanced multivariate information theory tools, allowing us to quantify nonlinear statistical dependencies between all possible electrode combinations. Results: Ketamine increased redundancy in brain dynamics, most significantly in the alpha frequency band. Redundancy was more evident during the resting state, associated with a shift in conscious states towards more dissociative tendencies. Furthermore, in the task-driven context (auditory oddball), the impact of ketamine on redundancy was more significant for predictable (standard stimuli) compared to deviant ones. Finally, associations were observed between ketamine’s HOI and experiences of derealization. Conclusions: Ketamine appears to increase redundancy and genuine HOI across metrics, suggesting these effects correlate with consciousness alterations towards dissociation. HOI represents an innovative method to combine all signal spatial interactions obtained from low-density dry EEG in drug interventions, as it is the only approach that exploits all possible combinations from different electrodes. This research emphasizes the potential of complexity measures coupled with portable EEG devices in monitoring shifts in consciousness, especially when paired with low-density configurations, paving the way for better understanding and monitoring of pharmacological-induced changes.
1. Introduction
Ketamine, a non-competitive N-Methyl-d-aspartate (NMDA) receptor antagonist considered a non-serotonergic psychedelic compound, has garnered attention for its capacity to induce alterations in the global dynamics of conscious states1,2. It holds promising implications for pharmacological interventions, especially in the treatment of depression and other mood-related disorders3,4. Specifically, it has been linked to experiences of derealization (i.e., feeling detached from surroundings), depersonalization (i.e., feeling detached from self), and altered perception of the body, environment, and time1,2. Various studies employing behavioral analyses and neuroimaging have illustrated shifts in neural patterns during ketamine administration (see reviews:5,6). Although ketamine does not directly target serotonergic receptors like classical psychedelics, evidence from neuroimaging and electrophysiological studies suggest common signatures and potential mechanisms of altered states of consciousness7–9. Both ketamine and serotonergic psychedelics have been linked to modulations of the Default Mode Network (DMN)10 as well as increases of brain entropy7,11. Many of the brain pattern shifts observed under ketamine and psychedelics entail a decrease in top-down brain organization paired with an enhanced emphasis on the lower hierarchies of sensory information12–15. These observations are consistent with the Relaxed Beliefs Under Psychedelics (REBUS) model proposed by Carhart-Harris and Friston, which suggests that psychedelics alter consciousness by reducing the weight of prior beliefs when processing bottom-up sensory information emerging from the periphery15. The reduced top-down control from higher brain hierarchies results in a richer conscious experience and sometimes prediction errors manifesting as perceptual illusions or experiences of dissociation12,15. During the ketamine experience, this dissociation may be further evidenced through complexity measures of brain activity, as reflected in increased entropy15–18.
Emergent innovations in clinical trials emphasize the adoption of portable dry electrodes, addressing a significant gap in the demand for accessible, reliable, and economical biomarkers to monitor drug effects19,20. However, studies exploring the effects of ketamine with low-density electrodes are scarce. An innovative and robust approach to probe into these effects in low-density setups encompasses brain high-order interactions (HOI)21. Three salient features made HOI critically relevant22–26. First, as opposed to standard event-related potentials (ERP), oscillations, and connectivity metrics, HOI can compute all possible interactions between signals (here, electrodes). Despite the advantages of having high-density arrays (as source localization, increased spatial resolution, and interpolation of bad electrodes), the HOI approach becomes computationally challenging in these arrays, inducing a selection bias imposed by dimensionality reduction techniques. In contrast, despite their reduced spatial resolution, low-density arrays allow us to feasibly compute all potential interactions, thereby maximizing information conveyance—a feature unmatched by any other technique. Thus, leveraging HOI provides a unique and novel improvement to understanding brain dynamics using low-density arrays, which usually reduce data’s granularity. Second, HOI effectively captures the global dynamics of brain organization22–26, a critical component of different consciousness states (disorder of consciousness, anesthesia, transitions, and conscious access27–31), making them ideal for elucidating the effects of pharmacology. Furthermore, the evidence underscores HOI’s superior robustness when compared to traditional connectivity metrics across various modalities, including fMRI and EEG, and across different brain conditions23–26. In brief, when combined with entropy measures and low-density EEG, HOI may constitute a crucial approach for assessing global dynamic changes induced by ketamine and other drugs used in clinical trials settings.
The current study (Fig. 1) investigated changes in HOI during a drug-induced altered state of consciousness, adopting a double-blinded cross-over design, enrolling 30 adults (but only 29 were used in this analysis), and administering racemic ketamine via a continuous infusion protocol (Fig. 1A). We juxtapose the effects of ketamine against a saline infusion as a control, leveraging both task-driven (gamified auditory oddball paradigm) and resting-state EEG sampling methodologies32,33. Using a low-density, wireless, dry electrode EEG system34, participants’ responses to ketamine were captured. Alongside EEG data, the subjective effects of ketamine administration were measured through self-reported and clinician-administered questionnaires. Our analyses focused on HOI assessed with entropy-based measures35. We predicted that HOI would yield consistent results across designs (resting state, task) and conditions (ketamine vs saline and deviant vs. standard stimuli in the task). We expected that effects would mainly be observed in an alpha band which is systematically reported to be altered in ketamine studies32,33. Such effects are anticipated to be more pronounced during resting state than in task36, given ketamine’s hypothesized role in diminishing top-down control, manifesting as redundancy of bottom-up signals in brain dynamics15. To assess this specific hypothesis of increased redundancy associated with the reduction of top-down hierarchical control, we include other measures of global dynamics (see below). Furthermore, during task-driven conditions, particularly a passive and gamified auditory oddball paradigm, the more predictable stimuli (i.e., standard) are expected to yield similar increased effects in contrast to the deviant stimuli, as prediction may be more impacted by the decreased top-down influences. Finally, we assessed whether HOI-associated changes during ketamine administration correlate with subjective consciousness-altered states in terms of dissociative states (i.e., derealization scores)37–39. In brief, this study aims to establish the ability of a portable, low-density dry EEG device to capture the overarching global brain dynamics of ketamine-induced shifts, both in resting and task-engaged states, serving as potential brain signatures of altered states of consciousness.
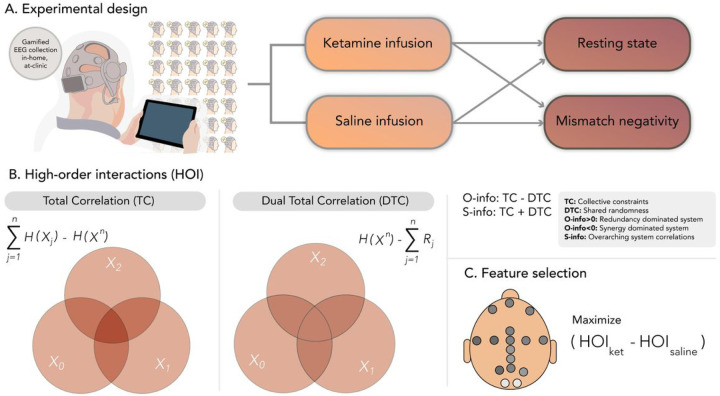
Figure 1. Overview of experimental design and data analysis.
A). Subjects participated in a double-blind crossover design using portable EEG, capturing both resting states and task-based recordings (namely, a gamified oddball paradigm inducing a typical mismatch negativity). In randomized sessions, participants received both ketamine and saline infusions. B). Analysis of high-order interactions (HOI) entailed measurements of total correlation (TC) and dual total correlation (DTC), O-information and S-information (see Methods). C). Feature selection across the two designs (rest and task) was employed to pinpoint the primary differences between the ketamine and saline conditions.
2. Methods
EEG data (16 electrodes, Cumulus Neuroscience dry-sensor 16 electrode headset) were recorded during a resting state and a mismatch negativity task under saline or ketamine injection (Fig. 1A). A pipeline based on multivariate information theory was applied to investigate whether saline could be discriminated against ketamine under both rest and task. The complete set of possible combinations between electrodes at all orders of interactions (from 2 to 16) was assessed (Fig. 1B–C). The EEG used here has been presented previously on reference40, where a detailed description can be found.
2.1. Participants
Study participants (N = 30 males, 25.57 +/− 3.74y) were carefully selected to ensure a consistent and controlled environment for the research. To qualify, individuals had to be males aged between 18 and 55. Women were excluded to avoid undetected pregnancy risk and to reduce sample variability. Participants’ health was critically assessed through a physical examination, medical history, vital signs, a 12-lead Electrocardiogram (ECG), and clinical laboratory tests. Participants were excluded if they had a current or past history of psychiatric disorders as per the ICD-10, especially those with a history of drug or alcohol dependence/abuse in the last 6 months. Furthermore, any serious unstable illnesses, including but not limited to hepatic, renal, respiratory, cardiovascular, endocrine, and neurological disorders, led to exclusion. This also encompassed subjects with unresolved seizure causes, conditions that likely alter brain morphology or physiology such as uncontrolled hypertension or diabetes, significant acute illnesses a week before the drug administration, notable history of drug or food allergies, and the use of specific medications including antidepressants, anti-psychotics, anxiolytics, and others. Additionally, color-blind individuals were also not considered for this study. Each participant’s commitment to the study’s guidelines and restrictions was crucial, and they had to demonstrate their understanding and willingness to participate. This study was approved by the Institutional Review Board of the Otto-von-Guericke University Magdeburg, Germany, and informed consent was obtained from all participants (Approbation code number: 123/18).
2.2. Study Design
The study was a saline-controlled, double-blind, randomized, crossover study with healthy participants in their homes, designed to investigate the acute and delayed effects of ketamine on EEG and behavioral measures. Participants were invited to the laboratory to complete repeated measurements: at enrollment, on the days of infusion of ketamine or saline administration (randomized across two visits), and on the days after infusion (Fig. 1). The two infusion days occurred four weeks apart following the same study protocol. All EEG recordings were performed with the portable dry EEG system developed by Cumulus Neuroscience (www.cumulusneuro.com).
2.3. Ketamine infusion
During the infusion, participants were seated in a comfortable chair, and their legs were elevated on a footrest. Overall mobility was restricted as a cannula was placed in each arm; one for the delivery of ketamine or saline and the other to draw blood samples. Participants were administered a single IV infusion (of a total volume of 50 ml) of 0.5 mg/kg of racemic ketamine hydrochloride over 40 mins or IV saline solution (0.9%) over 40 mins. During the infusions, the tablet was mounted on a tablet holder and operated by the researchers as participants could not bend their arms at that time.
2.4. EEG recordings
EEG data was collected using the wireless 16-channel dry sensor EEG system developed by Cumulus Neuroscience (Cumulus, www.cumulusneuro.com), suitable for use in various supervised and unsupervised settings34. The analog front-end is based on the ADS1299 chip-set from Texas Instruments, incorporating a high input impedance of 1GΩ, a configurable driven bias function for common-mode rejection, built-in impedance checking, and configurable gain and sampling rates. The left mastoid is used for reference and the right mastoid for driven-bias, with single-use, snap-on electrodes attached to wires extending from the headset. An onboard processor and Bluetooth module transmit 250Hz EEG data to another device (an Android tablet in this case), transferring it to a cloud server for storage and processing. Flexible Ag/AgCl coated polymer sensors of a comb-design (ANT-Neuro/eemagine GmbH) are used to achieve a stable and dermatologically safe contact to the scalp through the hair. The electronics and sensors are mounted on a flexible neoprene net for comfort and ease of montage. Incorporating natural landmarks in the headset form factor and the stretchable structure enable consistent placement by non-experts in line with the 10–20 sensor system.
2.5. Resting-state session
Eyes-closed resting state EEG recordings were collected during the first 20 mins after either saline or ketamine infusion. Participants were instructed to rest with closed eyes and remain still during the recording. For the resting state, the first 10 minutes post-injection were not considered in the analysis to discard transients associated with injection, yielding 10 minutes of stable post-injection resting state performed at home.
2.6. Passive-listening auditory oddball paradigm
During the last 15 minutes of each infusion, we used an app-based version of the passive auditory oddball task developed by Cumulus – Sonic Scenes – eliciting the Mismatch Negativity (MMN) of infrequent ‘deviant’ stimuli in a train of ‘standard’ stimuli. The task was performed passively – the subject merely needed to remain still while listening to repetitive auditory stimuli. The participant was prompted to put on headphones and adjust the volume until he could clearly hear some sample tones. When ready, the participant asked to press play on a silent movie, which lasted 15 mins. Tones played throughout while the participant watched the film. Eight short films were used in an arbitrary cycled order, each consisting of silent clip montages compiled from stock footage. There were no narratives or subtitles. Each movie session incorporated 1000 ‘standard’ tones (100ms; 1000Hz) and 200 pitch deviants (100ms; 1200Hz).
2.7. Subjective scores of dissociative states
We assessed the Clinician-Administered Dissociative States Scale (CADSS), which measures dissociative states41, and the 5D-ABZ, which assesses self-reported altered states of consciousness42. CADSS has 3 subscales (“Amnesia”, “Derealization”, “Depersonalization”), while 5D-ASC entails five dimensions (“Oceanic Boundlessness”, “Dread of Ego Dissolution (DED)”, “Visionary Restructuralization”, “Auditory Alterations”, “Vigilance Reduction“). Both questionnaires were administered in the laboratory 1h before and 1.5hr after the infusion. The scores’ difference between before and after infusion were used to rate the subjective changes for both saline and ketamine infusion. All questionnaires used validated German-language versions, except for the CADSS questionnaire translated by the research team as no validated translation was available when the study was conducted.
2.8. EEG data preprocessing
Using Cumulus’ proprietary algorithms, EEG data were pre-processed to correct the integrity of timing information and eye-movement artifacts. For the oddball paradigm, baseline-adjusted EEG data were band-filtered between 0.25–40 Hz and analyzed in 30-s epochs. Corrective procedures are applied for epochs with missing and anomalous data, including eye and movement artifacts. To preserve the same number of channels (16) during the whole analysis, time points from all channels were removed if at least one channel had a flat signal in those points, which was required to explore all the possible high-order interactions. One subject was removed from further resting state analysis because there was < 1s of valid data in the ketamine condition. No differences in the number of selected points were found after the artifact removal (Supplementary Tables 1 and 2) across conditions (ketamine/saline) and recordings (rest/task). Resting-state data were bandpass filtered in the canonical EEG frequency bands: δ: 0.5–4 Hz, θ: 4–8 Hz, α: 8–12 Hz, β: 12–30 Hz, γ: 30–40 Hz). Given that our goal was not to compute ERPs, we pooled all the valid trials (with at least 125ms valid signal) corresponding to the deviant and standard tones separately to perform the analysis. In the following analysis, the 5 bands plus broadband (0.5–40 Hz) data was used for the resting state, while for the tasks only broadband data were used to focus only on the evoked rather than the induced response. EEG data obtained appeared reliable as the standard effects on ketamine vs. saline solution were noticeable in the EEG spectrum (across the alpha peak and typical frequency/power decay, see Figure S1).
2.9. Pairwise and high-order interactions
To assess the hypothesis of ketamine-induced specific effects of redundancy in brain dynamics, different measures of entropy characterizing different properties of brain dynamics were computed. We used tools from multivariate information theory i) to quantify the nonlinear statistical dependencies between all the possible combinations between electrodes and ii) to distinguish the nature of these dependencies in terms of collective constraints (total correlation, TC), shared randomness (dual total correlation, DTC), synergy (O-info < 0), redundancy (O-info > 0), and overarching correlations (S-info) (see reference35 for a detailed explanation of these measures).
Consider a system of n random variables denoted by Xn = (X1, … ,Xn). The TC, DTC, O-information (O), and S-information (S) are generalizations of the mutual information (MI)35, which can be respectively expressed as:
| 1 |
| 2 |
| 3 |
| 4 |
where H(X1, …, Xn) is the joint Shannon’s entropy of the n variables, H(Xi) the entropy of the i-th region H (Xi|Xn−i) is the entropy of the i-th region conditioned by the activity of the whole system without it – which is known as “residual entropy,” and is denoted as Ri. Above, X−in represents the vector of all variables except Xi, i.e., (X1, …, Xi−1, Xi+1, …, Xn). Estimations were performed using the Gaussian copula approximation25,43. As for n = 2 TC = DTC = mutual information, only the TC was computed for each possible pair of electoreds. For the high-order interactions (from 3 to 16) all metrics were computed for each possible combination of electrodes at each order of interaction (Fig. 1B and C).
Broadband (0.5–40 Hz) and filtered EEG signals (δ: 0.5–4 Hz, θ: 4–8 Hz, α: 8–12 Hz, β: 12–30 Hz, γ: 30– 40 Hz) were analyzed considering all the possible combinations of electrodes at each order of interaction, here denoted by k (120 interactions for k = 2, 560 for k = 3, 1.820 for k = 4, 4.368 for k = 5, 8.008 for k = 6, 11.440 for k = 7, 12.870 for k = 8, 11.440 for k = 9, 8.008 for k = 10, 4.368 for k = 11, 1.820 for k = 12, 560 for k = 13, 120 for k = 14, 16 for k = 15 and 1 for k = 16). An n-plet represents a particular combination of n electrodes, and the effect of ketamine on it was assessed via the effect sizes.
2.10. Effect sizes
To characterize the size of the effect associated with each measurement, we used Cohen’s d effect size for paired samples44:
| (5), |
where μket and μsal are the average measure of the ketamine and saline condition, respectively, and s is the standard deviation of the difference of means (i.e μket – μsal). This metric measures a standardized mean difference between paired samples, and its sign indicates the direction of the effect, i.e. if d > 0 means that ketamine increases the measure.
2.11. Statistical analyses
For the selected features, a non-parametric Wilcoxon sign rank test for paired samples with the False Discovery Rate (FDR) correction for multiple comparisons was performed. As in previous work on HIOs22–26, the statistical correction was not directly assessed for each HOI given the non-selective data approach, including all interactions45. Conversely, we used Cohen’s D to report the effect size of HOI22,24,25 as p-values can be artificially inflated. To compute the association between HOI and subjective scores, the change (ketamine - saline) in HOI was (Pearson) correlated with the change in subjective scores, yielding one R2 per n-plet and subjective score. We evaluated the significance of associations by a permutation test followed by post-hoc FDR correction for multiple comparisons. First, we generated a null distribution of R2 values by randomly permuting the correspondence between HOI and subjective scores 1000 times. Only correlations values above the 99.9-th percentile of their respective null distribution were submitted to FDR correction (p < 0.005).
3. Results
3.1. Ketamine increases redundancy in the alpha band during resting state
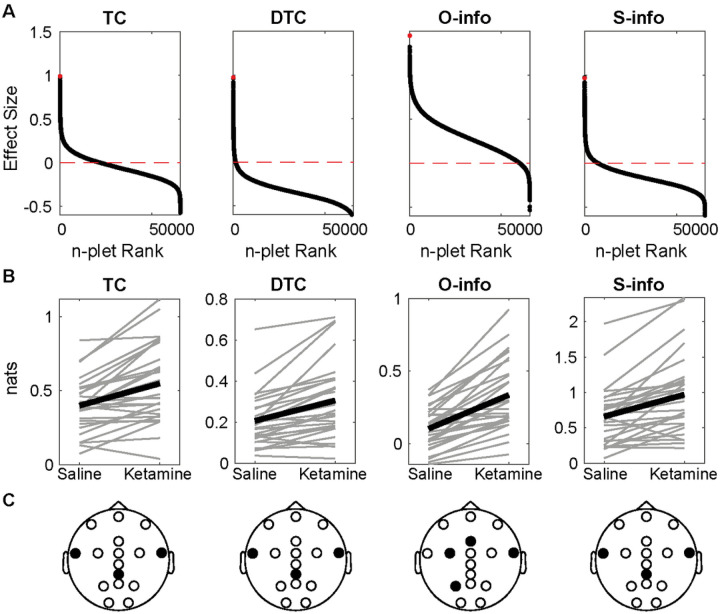
Results evidenced positive and negative effect sizes for all the combinations between filtering bands and metrics. The alpha band showed the largest (metric increase with ketamine) and smallest effect sizes (metric decrease with ketamine) for all the metrics (Fig. 2A and S1). Indeed, only O-info and S-info significantly increased in the alpha band (Fig. 1B, Wilcoxon sign rank test, p < 0.001 after FDR correction). Among the 4 metrics in the alpha band, the largest increase was found for the O-info, which included the P3, FCC3, Fz, and FT8 electrodes (Fig. 2C). The S-info was related to the CPz, FT7, and FT8 electrodes. Despite the other filtering bands also exhibiting a marked tendency both for increases and decreases, no significant effect was found (Figure S2). These results indicate that the effect of ketamine can be better explained as an increase in the overarching correlations –Specifically redundancy– between temporal and parietal recordings in the alpha band during the resting state.
Figure 2. Ketamine increases redundancy in the resting state alpha band.
A)Each panel shows the effect sizes of each n-plet (i.e. each possible combination of electrodes from 2 to 16) sorted in decreasing order, with a red horizontal line showing the 0. The red dot denotes the maximum effect size. B) The n-plet with the largest effect under saline and ketamine for each subject (gray) and for the average (black). Only O and S-info yielded FDR-corrected Wilcoxon sign rank p-values<0.001. C) EEG layout with the electrodes composing the n-plet in black.
3.2. Ketamine increases redundancy for predictable stimuli
We found both positive and negative effect sizes for all the combinations between metrics and stimuli type (STD, standard; DEV, deviant) for the auditory oddball task (Fig. 3A, S4). We compared the STD and DEV response under saline and under ketamine, without comparing the STD to the DEV tone. The deviant tone showed slightly larger absolute effect sizes. Significant differences were found only for the O-info in the standard tone (Fig. 3B, FDR-corrected Wilcoxon sign rank test, p < 0.005), which included CPz, Cz and FCz electrodes. The results for the rest of the metrics and the deviant tones are shown in Figure S4. The same set of electrodes was found for both conditions in the tasks. These results indicate that ketamine significantly increases the evoked responses for predictable but not deviant stimuli.
Figure 3. Redundancy increases in the standard tone for mismatch negativity task.
A) Effect sizes of each n-plet for the oddball paradigm, sorted in decreasing order, with a red horizontal line showing the 0. The red dot denotes the maximum effect size B) The n-plet with the largest effect under saline and ketamine for each subject (gray) and for the average (black). C) The electrodes involved in the best feature. Wilcoxon sign rank FDR-corrected p-value<0.005.
3.3. Derealization correlates with changes in resting state theta-band high-order interactions
Finally, we investigated the association between the change in resting state HOI and the change in subjective scores, as measured via the CADSS and the 5D-ASC (see Methods). We found the largest number of significant correlations for the O-info in the theta band and CADSS Derealization score, followed by the alpha band in the ‘Dread of ego dissolution’ item (Fig. 4B). Indeed, the theta band showed the largest association values for all the subjective scores, followed by the alpha band (Figure S5). The presence of the alpha band is consistent with results of the previous section, where the largest effect sizes were found in the alpha-band.
Figure 4. Association between subjective scores and HOI.
A) Average R2 only for the significant (FDR, p<0.005) associations between the change in each dimension of subjective score (see Methods) and change in HOI. B) Same as A, but showing the number of significant HOI in log10 scale to improve visualization.
This finding suggests that changes in the alpha band are not only indicative of the change in the global state of consciousness, but also of more subtler aspects of the experience. In turn, changes in the theta band HOI may be indicative of the level of derealization experienced by the subjects following ketamine infusion, as compared to saline.
4. Discussion
This study aimed to investigate the neuropharmacological effects of ketamine on consciousness, employing a novel and robust approach using HOI, with portable EEG in a double-blinded cross-over design. This exploration centered around low-density arrays, addressing a current gap in research methodology. Key findings indicate that ketamine administration induced a significant increase in the correlations and redundancy of brain dynamics, particularly evident in the alpha frequency band, consistent with observations across classical EEG studies of ketamine. Results open a new avenue for future studies using portable, low-density recordings captured during pharmacological interventions that maximize the combined information across electrodes.
Our findings bolster the application of HOI for low-density arrays. The shifts in brain dynamics, Specifically an increase in redundancy, are most pronounced in alpha (echoing previous reports focused on this band, tasks and rest46–48). Such alterations, particularly in the resting state, support the potential of ketamine to decrease top-down control and increase the sensitization to bottom-up signals1,2,12–15. This phenomenon is manifested by an upswing in redundancy within brain dynamics, possibly reflecting a reduced top-down inhibition and an amplification of lower hierarchies of sensory information12–15. The increase in redundancy following ketamine administration was larger during resting states than tasks, suggesting the spontaneous shift of the conscious state towards less controlled states during resting state, as dissociation. Also, the app-based auditory oddball paradigm revealed that the effects on redundancy are more conspicuous for predictable (standard) stimuli than for deviant stimuli, accentuating ketamine’s influence on spontaneous, less controlled cognitive processes12,15, as low-level stimuli prediction. Furthermore, a significant correlation emerged between the effects of ketamine on resting-state HOI in the alpha and theta band and the subjective experiences of DED and derealization, respectively, both core facets of ketamine-induced dissociative states. DED refers to a change in the perception of selfhood and subject-object boundaries, while derealization is the feeling of disconnection from the surroundings12,15,16. These results confirm reported associations between the alpha-band and ´ego-integrity’ 49and derealization measures and theta band50,51, particularly observed with ketamine12. Our findings support the idea that subjective alterations in consciousness are anchored in changes in brain dynamics and functional organization and thus can be properly tracked with measures of neural complexity52.
This study has limitations that open different avenues for further research. Compared to high-density electrodes, low-density dry electrodes offer limited information and have a lower signal-to-noise ratio. However, we attempted to mitigate these shortcomings with several internal controls in our design, including (a) no significant difference in the number of artifacts across conditions; (b) using a robust double-blinded cross-over design, which minimized individual heterogeneity, variance, and the distribution of random effects from any confounding variables across conditions; (c) verification of the expected effects of ketamine on the power spectrum (supplementary data). As a result, we found a systematic directional consistency of the effects of HOI across various measures. Moreover, since the primary HOI differentiating the ketamine vs. saline conditions did not involve frontal areas, the results reduce the possibility that differences in eye movement across conditions could account for these effects. Despite these measures, our findings require further validation using high-density electrode arrays, which might entail reducing the number of HOI. Exploring other brain measures like fMRI, where HOI can be assessed, would also be beneficial. While our sample size was modest, it remains comparable to, or even larger than, similar studies in the field (see reviews:5,6,12,13). In any case, future studies must replicate the findings in larger samples. This study only included male participants, so the effects of gender and the potential particular changes observed in women require further investigation. Lastly, our study does not explore brain dynamics in correlation with plasma measurements of ketamine and potential whole-body effects, such as cardio-dynamics and sensorimotor activity. These aspects offer promising avenues for future research.
5. Conclusions
Our results suggest that HOI provides a novel approach to maximizing the information obtained from low-density EEG in pharmacological interventions. More Specifically, ketamine fosters an increase in redundancy and HOI across measures suggesting changes in how the brain processes information, leading towards dissociation. Further research is needed to evaluate the potential of HOI to track the potential therapeutic effect of ketamine for psychiatric diseases as depression. These effects offer a deeper understanding of the neuropharmacological actions of ketamine and underscore the potential of portable EEG devices in charting alterations in consciousness, especially when combined with the HOI in low-density setups. This could lay the foundation for future endeavors aimed at better capturing the subject’s preparedness and the subsequent pharmacological-induced changes in therapeutic settings across neuropsychiatric conditions.
Funding
This study was funded by Cumulus Neuroscience Ltd. Cumulus Neuroscience employees took an active role in study design, data collection and analysis. IA is partially supported by grants from ANID/FONDECYT Regular (1210195 and 1210176 and 1220995); ANID/FONDAP/15150012; ANID/PIA/ANILLOS ACT210096; ANID/FONDAP 15150012; and the MULTI-PARTNER CONSORTIUM TO EXPAND DEMENTIA RESEARCH IN LATIN AMERICA [ReDLat, supported by Fogarty International Center (FIC) and National Institutes of Health, National Institutes of Aging (R01 AG057234, R01 AG075775, R01 AG21051, CARDS-NIH), Alzheimer’s Association (SG-20-725707), Rainwater Charitable foundation - Tau Consortium, the Bluefield Project to Cure Frontotemporal Dementia, and Global Brain Health Institute)]. MW is funded by the German Center for Mental Health (DZPG) and the BMBF (01EW2010A -NeuroMarKet) in the frame of ERA-Net NEURON. The contents of this publication are solely the authors’ responsibility and do not represent the official views of these institutions. The funders had no role in study design, data collection and analysis, decision to publish, or manuscript preparation.
Competing interests
FB, MI, HN, BM and LR-D are employees and shareholders of Cumulus Neuroscience Ltd. MW is a member of the following advisory boards and has given presentations to the following companies: Bayer AG, Germany; Boehringer Ingelheim, Germany; and Biologische Heilmittel Heel GmbH, Germany. MW has further conducted studies with institutional research support from HEEL and Janssen Pharmaceutical Research for a clinical trial (IIT) on ketamine in patients with MDD, unrelated to this investigation. MW did not receive any financial compensation from the companies mentioned above. DLB and PO were employees and shareholders of Takeda Pharmaceuticals at the time of the original study.
The other authors declare that they have no competing interests.
Funding Statement
This study was funded by Cumulus Neuroscience Ltd. Cumulus Neuroscience employees took an active role in study design, data collection and analysis. IA is partially supported by grants from ANID/FONDECYT Regular (1210195 and 1210176 and 1220995); ANID/FONDAP/15150012; ANID/PIA/ANILLOS ACT210096; ANID/FONDAP 15150012; and the MULTI-PARTNER CONSORTIUM TO EXPAND DEMENTIA RESEARCH IN LATIN AMERICA [ReDLat, supported by Fogarty International Center (FIC) and National Institutes of Health, National Institutes of Aging (R01 AG057234, R01 AG075775, R01 AG21051, CARDS-NIH), Alzheimer’s Association (SG-20-725707), Rainwater Charitable foundation - Tau Consortium, the Bluefield Project to Cure Frontotemporal Dementia, and Global Brain Health Institute)]. MW is funded by the German Center for Mental Health (DZPG) and the BMBF (01EW2010A -NeuroMarKet) in the frame of ERA-Net NEURON. The contents of this publication are solely the authors’ responsibility and do not represent the official views of these institutions. The funders had no role in study design, data collection and analysis, decision to publish, or manuscript preparation.
Footnotes
Supplementary Files
Contributor Information
Agustin Ibanez, Trinity College Dublin.
Nooshin Javaheripour, University Hospital Jena.
Zümrüt Sen, Jena University Hospital.
Martin Walter, Jena University Hospital.
Patricio Odonnell, Sage Therapeutics.
References
- 1.Ballard E. D. & Zarate C. A. Jr. The role of dissociation in ketamine’s antidepressant effects. Nat. Commun. 11, 6431 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krystal J. H. et al. Subanesthetic Effects of the Noncompetitive NMDA Antagonist, Ketamine, in Humans: Psychotomimetic, Perceptual, Cognitive, and Neuroendocrine Responses. Arch. Gen. Psychiatry 51, 199–214 (1994). [DOI] [PubMed] [Google Scholar]
- 3.Murrough J. W., Abdallah C. G. & Mathew S. J. Targeting glutamate signalling in depression: progress and prospects. Nat. Rev. Drug Discov. 16, 472–486 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Caddy C., Giaroli G., White T. P., Shergill S. S. & Tracy D. K. Ketamine as the prototype glutamatergic antidepressant: pharmacodynamic actions, and a systematic review and meta-analysis of efficacy. Ther Adv Psychopharmacol 4, 75–99 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwertner A., Zortea M., Torres F. V. & Caumo W. Effects of Subanesthetic Ketamine Administration on Visual and Auditory Event-Related Potentials (ERP) in Humans: A Systematic Review. Front. Behav. Neurosci. 12, 70 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zavaliangos-Petropulu A. et al. Neuroimaging-Derived Biomarkers of the Antidepressant Effects of Ketamine. Biol Psychiatry Cogn Neurosci Neuroimaging 8, 361–386 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schartner M. M., Carhart-Harris R. L., Barrett A. B., Seth A. K. & Muthukumaraswamy S. D. Increased spontaneous MEG signal diversity for psychoactive doses of ketamine, LSD and psilocybin. Sci. Rep. 7, 46421 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vlisides P. E. et al. Subanaesthetic ketamine and altered states of consciousness in humans. Br. J. Anaesth. 121, 249–259 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonhomme V. et al. Resting-state Network-specific Breakdown of Functional Connectivity during Ketamine Alteration of Consciousness in Volunteers. Anesthesiology 125, 873–888 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Zacharias N. et al. Ketamine effects on default mode network activity and vigilance: A randomized, placebo-controlled crossover simultaneous fMRI/EEG study. Hum. Brain Mapp. 41, 107–119 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carhart-Harris R. L. et al. The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front. Hum. Neurosci. 8, 20 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de la Salle S. et al. Effects of Ketamine on Resting-State EEG Activity and Their Relationship to Perceptual/Dissociative Symptoms in Healthy Humans. Front. Pharmacol. 7, 348 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nugent A. C. et al. Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects. Mol. Psychiatry 24, 1040–1052 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang S., Seo H., Wang M. & Arnsten A. F. T. NMDAR Neurotransmission Needed for Persistent Neuronal Firing: Potential Roles in Mental Disorders. Front. Psychiatry 12, 654322 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carhart-Harris R. L. & Friston K. J. REBUS and the Anarchic Brain: Toward a Unified Model of the Brain Action of Psychedelics. Pharmacol. Rev. 71, 316–344 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajpal H. et al. Psychedelics and schizophrenia: Distinct alterations to Bayesian inference. Neuroimage 263, 119624 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanz Perl Y. et al. Nonequilibrium brain dynamics as a signature of consciousness. Phys Rev E 104, 014411 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Dasilva M. et al. Modulation of cortical slow oscillations and complexity across anesthesia levels. Neuroimage 224, 117415 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Byrom B., McCarthy M., Schueler P. & Muehlhausen W. Brain Monitoring Devices in Neuroscience Clinical Research: The Potential of Remote Monitoring Using Sensors, Wearables, and Mobile Devices. Clin. Pharmacol. Ther. 104, 59–71 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whelan R., Barbey F. M., Cominetti M. R., Gillan C. M. & Rosická A. M. Developments in scalable strategies for detecting early markers of cognitive decline. Transl. Psychiatry 12, 473 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herzog R., Haghayegh S., Ibáñez A. & Hu K. 0052 A Novel Biomarker of Alzheimer’s Disease Based on High-order Interactions of Low-density Electroencephalography. Sleep 46, A25–A25 (2023). [Google Scholar]
- 22.Battiston F. et al. The physics of higher-order interactions in complex systems. Nat. Phys. 17, 1093–1098 (2021). [Google Scholar]
- 23.Zhu H., Wang J., Zhao Y.-P., Lu M. & Shi J. Contrastive Multi-View Composite Graph Convolutional Networks Based on Contribution Learning for Autism Spectrum Disorder Classification. IEEE Trans. Biomed. Eng. 70, 1943–1954 (2023). [DOI] [PubMed] [Google Scholar]
- 24.Plis S. M. et al. High-order interactions observed in multi-task intrinsic networks are dominant indicators of aberrant brain function in schizophrenia. Neuroimage 102 Pt 1, 35–48 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herzog R. et al. Genuine high-order interactions in brain networks and neurodegeneration. Neurobiol. Dis. 175, 105918 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varley T. F., Pope M., Maria Grazia, Joshua & Sporns, O. Partial entropy decomposition reveals higher-order information structures in human brain activity. Proc. Natl. Acad. Sci. U. S. A. 120, e2300888120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frohlich J. et al. Neural complexity is a common denominator of human consciousness across diverse regimes of cortical dynamics. Commun Biol 5, 1374 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mediano P. A. M., Rosas F. E., Bor D., Seth A. K. & Barrett A. B. The strength of weak integrated information theory. Trends Cogn. Sci. 26, 646–655 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Atasoy S., Deco G., Kringelbach M. L. & Pearson J. Harmonic Brain Modes: A Unifying Framework for Linking Space and Time in Brain Dynamics. Neuroscientist 24, 277–293 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Mashour G. A. & Hudetz A. G. Neural Correlates of Unconsciousness in Large-Scale Brain Networks. Trends Neurosci. 41, 150–160 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cofré R. et al. Whole-Brain Models to Explore Altered States of Consciousness from the Bottom Up. Brain Sci 10, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muthukumaraswamy S. D. et al. Evidence that Subanesthetic Doses of Ketamine Cause Sustained Disruptions of NMDA and AMPA-Mediated Frontoparietal Connectivity in Humans. J. Neurosci. 35, 11694–11706 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thiebes S. et al. Alterations in interhemispheric gamma-band connectivity are related to the emergence of auditory verbal hallucinations in healthy subjects during NMDA-receptor blockade. Neuropsychopharmacology 43, 1608–1615 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McWilliams E. C. et al. Feasibility of Repeated Assessment of Cognitive Function in Older Adults Using a Wireless, Mobile, Dry-EEG Headset and Tablet-Based Games. Front. Psychiatry 12, 574482 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosas F. E., Mediano P. A. M., Gastpar M. & Jensen H. J. Quantifying high-order interdependencies via multivariate extensions of the mutual information. Physical Review E 100, 32305 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Farnes N., Juel B. E., Nilsen A. S., Romundstad L. G. & Storm J. F. Increased signal diversity/complexity of spontaneous EEG, but not evoked EEG responses, in ketamine-induced psychedelic state in humans. PLoS One 15, e0242056 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bredlau A. L., Thakur R., Korones D. N. & Dworkin R. H. Ketamine for pain in adults and children with cancer: a systematic review and synthesis of the literature. Pain Med. 14, 1505–1517 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Lineham A., Avila-Quintero V. J., Bloch M. H. & Dwyer J. The Relationship Between Acute Dissociative Effects Induced by Ketamine and Treatment Response in Adolescent Patients with Treatment-Resistant Depression. J. Child Adolesc. Psychopharmacol. 33, 20–26 (2023). [DOI] [PubMed] [Google Scholar]
- 39.Acevedo-Diaz E. E. et al. Can ‘floating’ predict treatment response to ketamine? Data from three randomized trials of individuals with treatment-resistant depression. J. Psychiatr. Res. 130, 280–285 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barbey F. M. et al. Neuroscience from the comfort of your home: Repeated, self-administered wireless dry EEG measures brain function with high fidelity. Front Digit Health 4, 944753 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bremner J. D. et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J. Trauma. Stress 11, 125–136 (1998). [DOI] [PubMed] [Google Scholar]
- 42.Studerus E., Gamma A. & Vollenweider F. X. Psychometric evaluation of the altered states of consciousness rating scale (OAV). PLoS One 5, e12412 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ince R. A. A. et al. A statistical framework for neuroimaging data analysis based on mutual information estimated via a gaussian copula. Human Brain Mapping 38, 1541–1573 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sawilowsky S. S. New Effect Size Rules of Thumb. J. Mod. Appl. Stat. Methods 8, 26 (2009). [Google Scholar]
- 45.Kriegeskorte N., Simmons W. K., Bellgowan P. S. F. & Baker C. I. Circular analysis in systems neuroscience: the dangers of double dipping. Nat. Neurosci. 12, 535–540 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anijärv T. E. et al. Spectral Changes of EEG Following a 6-Week Low-Dose Oral Ketamine Treatment in Adults With Major Depressive Disorder and Chronic Suicidality. Int. J. Neuropsychopharmacol. 26, 259–267 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liley D. T. J. & Muthukumaraswamy S. D. Evidence that alpha blocking is due to increases in system-level oscillatory damping not neuronal population desynchronisation. Neuroimage 208, 116408 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Keavy D. et al. The qEEG Signature of Selective NMDA NR2B Negative Allosteric Modulators; A Potential Translational Biomarker for Drug Development. PLoS One 11, e0152729 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stoliker D., Egan G. F., Friston K. J. & Razi A. Neural Mechanisms and Psychology of Psychedelic Ego Dissolution. Pharmacol. Rev. 74, 876–917 (2022). [DOI] [PubMed] [Google Scholar]
- 50.Soffer-Dudek N., Todder D., Shelef L., Deutsch I. & Gordon S. A neural correlate for common trait dissociation: Decreased EEG connectivity is related to dissociative absorption. J. Pers. 87, 295–309 (2019). [DOI] [PubMed] [Google Scholar]
- 51.Krüger C., Bartel P. & Fletcher L. Dissociative mental states are canonically associated with decreased temporal theta activity on spectral analysis of EEG. J. Trauma Dissociation 14, 473–491 (2013). [DOI] [PubMed] [Google Scholar]
- 52.Sarasso S. et al. Consciousness and complexity: a consilience of evidence. Neurosci Conscious niab023 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]