Abstract
Background and aims
Guidelines recommend that high-risk patients with atherosclerotic cardiovascular disease (ASCVD) be treated with maximally tolerated statins to lower low-density lipoprotein cholesterol (LDL-C) levels and reduce the risk of major adverse cardiovascular events. In patients whose LDL-C remains elevated, non-statin adjunct therapies, including ezetimibe (EZE), bempedoic acid (BA), and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors are recommended.
Methods
The impact of BA and EZE in a fixed-dose combination (FDC) on LDL-C goal attainment was evaluated using a simulation model developed for a United States cohort of high-risk adults with ASCVD. Treatment was simulated for 73,056 patients not at goal (LDL-C >70 mg/dL), comparing BA + EZE (FDC), EZE only, and no oral adjunct therapy (NOAT). The addition of PCSK9 inibitors was assumed after 1 year in patients not at LDL-C goal. Treatment efficacy was estimated from clinical trials. Patient-level outcomes were predicted over a 10-year horizon accounting for treatment discontinuation and general mortality.
Results
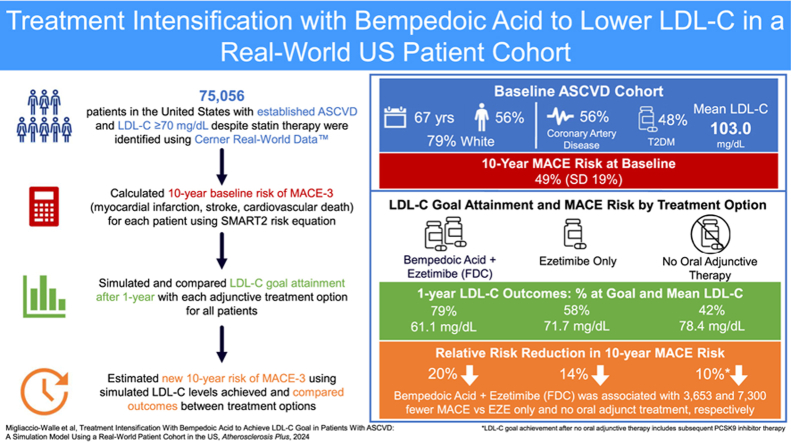
Baseline mean age of the cohort was 67 years, most were White (79%) and male (56%). A majority had established coronary artery disease (75%), 48% had diabetes, and mean LDL-C was 103.0 mg/dL. After 1 year, 79% of patients achieved LDL-C goal (mean, 61.1 mg/dL) with BA + EZE (FDC) compared to 58% and 42% with EZE (71.7 mg/dL) and NOAT (78.4 mg/dL), respectively.
Conclusions
This simulation shows that adding BA + EZE (FDC) to maximally tolerated statins would result in more patients achieving LDL-C goal than adding EZE alone or NOAT.
Graphical abstract
Highlights
-
•
A real-world cohort of ASCVD patients was simulated to estimate the impact of achieving LDL-C goal.
-
•
79% of patients treated with bempadoic acid + ezetimibe [BA + EZE (FDC)] achieved LDL-C goal.
-
•
Adding BA + EZE (FDC) to statins led more patients to LDL-C goal than current standards of care.
-
•
Compared with current standards of care BA + EZE (FDC) reduced the need for adjunctive PCSK9i use.
Atherosclerotic cardiovascular disease (ASCVD) affects approximately 26 million Americans each year [1], resulting in 2 million hospitalizations and 400,000 deaths [2]. People living with ASCVD are at an increased risk for morbidity due to recurrent major adverse cardiovascular events (MACE) including myocardial infarction, stroke, and death. MACE risk is modulated by several factors such as patient history and clinical characteristics, including elevated low-density lipoprotein cholesterol (LDL-C) [3,4]. Treatment guidelines recommend statin therapy as the cornerstone of LDL-C lowering to reduce risk in patients with established ASCVD [[5], [6], [7]]. Guidelines recommend reducing LDL-C to <70 mg/dL in patients at high-risk [5]; more recent recommendations by the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) and the American College of Cardiology (ACC) Expert Consensus Decision Pathway (ECDP) recommend an LDL-C goal of <55 mg/dL in patients at very high-risk [6,7]. Addition of a non-statin lipid-lowering therapy such as ezetimibe (EZE), bempedoic acid (BA), and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors are recommended in patients who do not reach recommended goals to further reduce LDL-C [[5], [6], [7]]. The US Food and Drug Administration approved BA, an adenosine triphosphate citrate lyase inhibitor, in February 2020 for the treatment of adults with established ASCVD or heterozygous familial hypercholesterolemia who require additional LDL-C lowering on maximally tolerated statin therapy alone or in a fixed-dose combination (FDC) with EZE as a once-daily, oral, non-statin, adjunct therapy [8,9]. BA and BA + EZE (FDC) have demonstrated efficacy in lowering LDL-C in patients with ASCVD beyond that achieved with statins alone [10,11], providing an oral treatment alternative which may delay or reduce the need for injectable PCSK9 inhibitors. Recent simulation studies in European populations estimated that use of BA to lower LDL-C resulted in a 25%–30% reduction in the need for PCSK9 inhibitors to achieve LDL-C goals [[12], [13], [14]]. Due to differences in treatment guidelines and patient characteristics, these European studies may not be generalizable to the US population. Thus, this present study aims to conduct a similar analysis in a real-world US population by estimating the impact of adding BA + EZE (FDC) in patients with ASCVD on statin therapy and not at LDL-C goal.
1. Methods
A Monte Carlo simulation model was developed using R and Microsoft® Excel to estimate the impact of adjunctive oral lipid lowering therapy in a real-world cohort of patients diagnosed with ASCVD and above LDL-C goal. The cohort was identified from patient-centric data from the Cerner Real-World Data™ (CRWD), a national (US) de-identified, longitudinal electronic medical records data source [15]. Adults with established ASCVD who had a documented prescription for a statin therapy and LDL-C ≥70 mg/dL were considered for the model population. It was not possible in these data to confirm whether individuals were on a maximally tolerated statin dose, hence it was assumed that all patients with a documented statin prescription were stable at a maximally tolerated dose. The primary outcomes modeled were proportion of patients achieving LDL-C goal, life years spent at goal, number of 3-component MACE (myocardial infarction, stroke, and cardiovascular death), and treatment costs per avoided MACE. A 10-year time-horizon was implemented to align with published risk models [16] and to provide tangible and meaningful estimates for healthcare providers and decision makers. All costs were calculated in 2022 US dollars ($). Costs and health benefits were discounted at an annual rate of 3%.
Identification of the ASCVD cohort was based on International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) diagnosis codes and defined as acute coronary syndrome, history of myocardial infarction, stable or unstable angina, coronary or other arterial revascularization, stroke, transient ischemic attack, and/or peripheral arterial disease including aortic aneurysm consistent with recent guidelines [5]. Mean age and gender distributions were obtained from the CRWD population along with additional clinical characteristics, such as high-density lipoprotein cholesterol, total cholesterol, and systolic blood pressure, and risk factors including current smoking status, diagnosis of diabetes mellitus, and current anticoagulant therapy use. Patients with no record of LDL-C measurement (n = 141,667) and/or no record of statin use (n = 76,925) were excluded from the analysis, as were those missing records of high-density lipoprotein cholesterol (n = 136,022) and/or total cholesterol (n = 127,612). In addition, patients with documented EZE or PCSK9 inhibitor use at baseline and/or a diagnosis of dialysis-dependent chronic kidney disease were excluded. In the remaining cohort of 73,056 patients, for those missing any record of smoking status (n = 58,615), the patient was assumed not to smoke. If creatinine (n = 71,294) or systolic blood pressure (n = 1990) was missing, the patient was assigned the population median value (median creatinine, 1.2 mg/dL; median systolic blood pressure, 132 mmHg). A full list of characteristics and risk factors is presented in Table 1. As all data were fully deidentified, this study was approved as exempt by the University of Texas Southwestern Institutional Review Board.
Table 1.
Baseline demographic and clinical characteristics.
| Parameter | Patient cohort (N = 73,056) |
|---|---|
| Male, n (%) | 40,853 (56) |
| Mean age, years (SD) | 67.4 (10.8) |
| Clinical characteristics, mean (SD) | |
| LDL-C, mg/dL | 103.0 (30.6) |
| HDL-C, mg/dL | 48.4 (15.0) |
| Total cholesterol, mg/dL | 179.0 (37.5) |
| Systolic blood pressure, mmHg | 134.7 (21.0) |
| Creatinine, mg/dL | 1.4 (2.2) |
| High-sensitivity C-reactive protein, mg/dL | 3.1 (3.1) |
| Years since ASCVD diagnosis | 4.2 (9.0) |
| Risk factors and history, n (%)a | |
| Current smoking | 7752 (11) |
| Diabetes mellitus | 35,369 (48) |
| Coronary artery disease | 54,567 (75) |
| Cerebrovascular disease | 23,617 (32) |
| Peripheral artery disease | 17,766 (24) |
| Aortic aneurysm | 2108 (3) |
| Current anticoagulant therapy | 9900 (14) |
ASCVD = atherosclerotic cardiovascular disease; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; SD = standard deviation.
Patients may be represented in more than 1 diagnostic category.
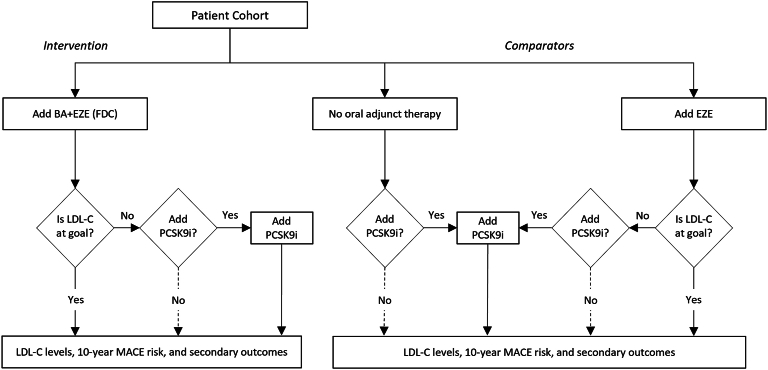
Treatment pathways and LDL-C goal achievement were simulated for the cohort of patients not at goal (LDL-C ≥70 mg/dL) despite statin therapy at baseline, as shown in Fig. 1. LDL-C goal achievement (<70 mg/dL and <55 mg/dL) in this cohort was estimated for BA + EZE (FDC) as the intervention and compared to (1) EZE alone, to reflect current standard-of-care recommendations [7], and (2) no additional oral adjunct therapy (i.e., direct to PCSK9 inhibitor), to approximate real-world prescribing practices [17]. Consistent with guidelines and clinical recommendations, patients in the cohort who did not reach LDL-C goal with the addition of a non-statin lipid-lowering therapy (i.e., BA + EZE [FDC], EZE only) were eligible to add a PCSK9 inhibitor [7]. It was assumed that half (50%) of eligible patients would, in practice, add a PCSK9 inhibitor. Patients escalating to adding a PCSK9 inhibitor were not modeled to switch therapies.
Fig. 1.
Model structure.
LDL-C goal was <70 mg/dL in base case; <55 mg/dL explored in scenario analysis. Dashed lines were 50% in base case, and 0% or 100% in scenarios. BA = bempedoic acid; EZE = ezetimibe; FDC = fixed-dose combination; LDL-C = low density lipoprotein cholesterol; MACE = major adverse cardiovascular event; PCSK9i = proprotein convertase subtilisin/kexin type 9 inhibitor.
The impact of treatment on LDL-C reduction and treatment discontinuation rates were obtained from published clinical trials [10,11,[18], [19], [20], [21]] (Table 2) and applied to patients in the CRWD population to predict individual LDL-C goal achievement. PCSK9 inhibitor efficacy was based on a published meta-analysis of 8 clinical trials of evolocumab, alirocumab, and inclisiran [19]. In the no oral adjunct comparison, it was assumed that 100% of patients remain above LDL-C goal. Treatment discontinuation was applied over the first year following initiation of each treatment, after which treatment was assumed to continue until death or the end of the model time-horizon, whichever happened first.
Table 2.
Clinical inputs.
| Treatment | LDL-C reduction (95% CI), % | Annual treatment discontinuation, % |
|---|---|---|
| BA + EZE (FDC) | 38.0 (29.6–46.5) [10] | 8.2 [10] |
| EZE | 16.8 (16.0–17.5) [18] | 10.0 [18] |
| PCSK9 inhibitors (pooled) [40],a | 51.2 (41.2–61.2) [19] | 5.8 [20] |
BA = bempedoic acid; EZE = ezetimibe; FDC = fixed-dose combination; PCSK9 = proprotein convertase subtilisin/kexin type 9.
PCSK9 inhibitor pooled costs are a weighted average of recent market shares.
The baseline 10-year cumulative risk of MACE before applying the treatment effect on LDL-C was estimated for all individuals within the ASCVD cohort using the updated Secondary Manifestations of ARTerial disease (SMART2) risk equation and was assumed to be constant over time [16]. The SMART2 equation utilizes patient age, age squared, male gender, current smoker, systolic blood pressure, diabetes diagnosis, coronary artery disease, cerebrovascular disease, peripheral artery disease, aortic aneurysm, years since ASCVD diagnosis, years since ASCVD diagnosis squared, log of non–high-density lipoprotein cholesterol, estimated glomerular filtration rate, estimated glomerular filtration rate squared, log of C-reactive protein, and current use of anticoagulants as predictors of 10-year MACE risk [22]. Following the guidance of the SMART2 authors, glomerular filtration rate was estimated using the gender-dependent Chronic Kidney Disease Epidemiology Collaboration formulas [23]. Each MACE predicted was associated with a 5.5% chance that the event was fatal [16]. In addition to MACE outcomes, general population mortality was taken into account using 2019 US life tables, excluding the rate of CVD mortality to avoid double-counting fatal MACE [24].
The impact of treatment-related LDL-C reduction on subsequent MACE risk was estimated using the cardiovascular risk reduction observed in a meta-analysis of over 300,000 patients from 52 randomized controlled studies of LDL-C lowering therapies, where each 1 mmol/L (38.67 mg/dL) reduction in LDL-C was associated with a 19% decrease in the 10-year risk of MACE [21]. For patients escalated to a PCSK9 inhibitor, a 20% reduction in MACE risk, in line with published cardiovascular outcomes data [19], was applied over the remaining time on treatment to avoid overestimating the real-world risk reduction for patients on PCSK9 inhibitor therapy. Thus, the 10-year risk of a MACE event after treatment was calculated as:
-
•
.
-
•
Where is the LDL-C level at time without accounting for the LDL-C reduction due to PCSK9 inhibitors, and is a binary variable that indicates if a patient is receiving a PCSK9 inhibitor or not.
Drug acquisition costs were calculated based on dosages published in the US Prescribing Information for each product and the corresponding wholesale acquisition costs from RedBook and reported in 2022 US dollars [8,9,[25], [26], [27], [28], [29]]. Treatment administration costs were applied only for inclisiran [28,30] (Table 3). No cost offsets were considered for the potential costs associated with LDL-C reduction and related sequelae, resulting in a conservative estimate of costs (i.e., treatment costs only).
Table 3.
Drug acquisition and administration costs.
| Treatment | Acquisition cost per dose [29] | Administration cost per dose | Doses per 4-week cycle | Cost per 4-week cycle | Annual cost |
|---|---|---|---|---|---|
| BA + EZE (FDC) | $12.81 | $0.00 | 28 | $358.80 | $4677.23 |
| EZE | $0.18 | $0.00 | 28 | $5.04 | $65.66 |
| PCSK9 inhibitor (pooled)a | – | – | – | $503.83 | $6549.79 |
| Evolocumab | $259.91 | $0.00 | 2 | $519.82 | $6757.66 |
| Alirocumab | $231.75 | $0.00 | 2 | $463.50 | $6025.50 |
| Inclisiran | $3250.00 | $15.88 [30] | 0.15 | $502.44 | $6531.76 |
BA = bempedoic acid; EZE = ezetimibe; FDC = fixed-dose combination; PCSK9 = proprotein convertase subtilisin/kexin type 9.
PCSK9 inhibitor pooled costs are a weighted average of recent market shares [40].
Analyses were conducted to estimate the 1-year and long-term clinical and economic outcomes with BA + EZE (FDC) and each comparator option. To estimate the range of uncertainty, the simulation results were repeatedly sampled and summarized to generate bootstrapped confidence intervals. One percent of the patients’ histories were sampled with replacement 1000 times to generate these uncertainty estimates. Because PCSK9 inhibitor use is highly variable between clinical guideline recommendations and real-world clinical practice, and the costs can be substantial, scenario analyses were conducted to assess outcomes by varying the percent of patients receiving PCSK9 inhbitors and the rate of PCSK9 inhibitor discontinuation. An additional scenario analysis was conducted by assuming an LDL-C treatment goal of <55 mg/dL to more closely align to the 2019 ESC/EAS guidelines [6] and the recommendation for very high-risk patients [5]. (Table 4).
Table 4.
Key scenario parameters for PCSK9 inhibitors.
| Parameter | Base case | Real-world PCSK9 inhibitor discontinuation | Unrestricted access to PCSK9 inhibitor | No PCSK9 inhibitor | LDL-C goal <55 mg/dL |
|---|---|---|---|---|---|
| Baseline MACE risk [22] | SMART2 | SMART2 | SMART2 | SMART2 | SMART2 |
| LDL-C goal [5] | <70 mg/dL | <70 mg/dL | <70 mg/dL | <70 mg/dL | <55 mg/dL |
| Eligible patients receiving PCSK9 inhibitor, % | 50 [41,42] | 50 [41,42] | 100a | 0 | 50 [41,42] |
| Annual discontinuation rates, % | |||||
| BA + EZE (FDC) [10] | 8.2 | 8.2 | 8.2 | 8.2 | 8.2 |
| EZE [18] | 9.95 | 9.95 | 9.95 | 9.95 | 9.95 |
| PCSK9 inhibitors | 5.8b | 20.0 [43] | 5.8 [17]b | n/a | 5.8 [17]b |
BA = bempedoic acid; EZE = ezetimibe; FDC = fixed-dose combination; LDL-C = low-density lipoprotein cholesterol; MACE = major adverse cardiovascular event; PCSK9 = proprotein convertase subtilisin/kexin type 9.
Assumption.
The modeled discontinuation rate was prorated from a published 12.2% discontinuation rate over 26 months.
2. Results
2.1. Study population
The final cohort from the CRWD was 73,056 ASCVD patients with a documented prescription for a statin therapy and LDL-C ≥70 mg/dL. At baseline, the mean age of the cohort was 67.4 years (standard deviation [SD], 10.8), a majority were male (56%), and most patients were White (79%). Mean LDL-C at baseline was 103 mg/dL (SD, 30.6), with over half of patients (58%) having baseline LDL-C between 70 and 99 mg/dL and 2% having LDL-C over 190 mg/dL. Just over half of patients obtained health coverage through a private network (57%), and approximately one-third through Medicare (33%; Table S1).
2.2. LDL-C goal attainment at 1 year
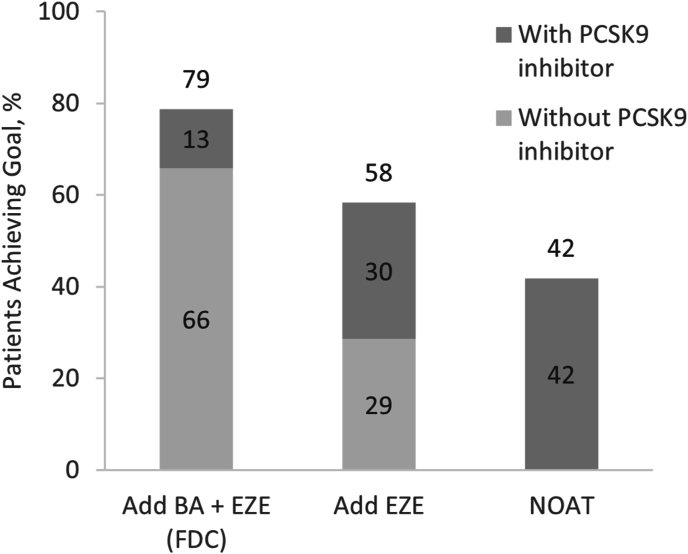
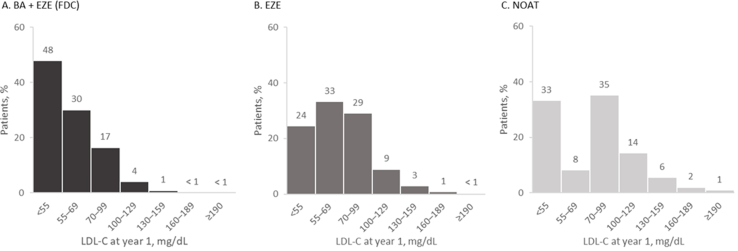
BA + EZE (FDC) treatment was associated with a mean LDL-C of 61.1 mg/dL (SD, 20.7) at year 1, corresponding to an absolute reduction of 41.9 mg/dL. Of the 71,653 (98%) of patients alive at year 1, 47,345 (66%) were predicted to achieve LDL-C goal with BA + EZE (FDC) alone (mean LDL-C, 67.5 mg/dL [SD, 23.7]) without requiring escalation to a PCSK9 inhibitor (Fig. 2). The addition of a PCSK9 inhibitor in half of eligible patients who were not at goal after BA + EZE (FDC) resulted in an additional 13% achieving goal at year 1. In addition, almost half (48%) of all BA + EZE (FDC) patients were predicted to achieve LDL-C <55 mg/dL at year 1 (Fig. 3).
Fig. 2.
Proportion of patients in the overall population predicted to reach LDL-C goal at year 1 with and without PCSK9 inhibitor.
Values may not add up to the total values due to rounding.
BA = bempedoic acid; EZE = ezetimibe; FDC = fixed-dose combination; LDL-C, low-density lipoprotein cholesterol; NOAT = no oral adjunct therapy; PCSK9 = proprotein convertase subtilisin/kexin type 9.
Fig. 3.
Predicted LDL-C levels at year 1, by treatment pathway.
LDL-C levels at year 1 for patients recieving (A) BA + EZE (FDC) (n = 71,645), (B) EZE (n = 71,737), and (C) NOAT (n = 71,587).
Percentages may not add up to 100% due to rounding. The total number of patients included in each analysis represents the number of patients simulated to be alive at year 1.
BA = bempedoic acid; EZE = ezetimibe; FDC = fixed-dose combination; LDL-C = low density lipoprotein cholesterol; NOAT = no oral adjunct therapy.
In comparison, treatment with EZE alone would result in a mean LDL-C of 71.7 mg/dL (SD, 27.7) at year 1, corresponding to a 31.4 mg/dL absolute reduction from baseline, with 58% of patients alive at year 1 achieving LDL-C goal. Half of patients reaching goal, or approximately one-third (29%, n = 20,495) of the total cohort, did so without requiring escalation to a PCSK9 inhibitor (mean LDL-C, 87.7 mg/dL [SD, 26.7]). In addition, 24% were estimated to reach LDL-C <55 mg/dL at year 1. Less than half (42%) of the cohort reached goal when no oral adjunct therapy was simulated, resulting in a mean LDL-C of 78.4 mg/dL (SD, 36.1) at year 1 (24.6 mg/dL change from baseline). All LDL-C reduction and goal attainment in this cohort was attributable to adding a PCSK9 inhibitor. Overall, BA + EZE (FDC) reduced the need for adjunctive PCSK9 inhibitor treatment by 57% compared with EZE alone and 69% compared with no oral adjunct therapy.
2.3. Cardiovascular event reduction
The baseline risk of MACE in the CRWD ASCVD cohort was estimated to be 49% (SD, 19) over 10 years. Adding BA + EZE (FDC) was associated with a 20% relative risk reduction in 10-year MACE risk from baseline compared with 14% with the addition of EZE only. This translated to 3653 fewer MACE predicted over 10 years with BA + EZE (FDC) versus adding EZE alone. The option of no oral adjunct therapy was associated with a relative risk reduction of 10% from baseline, which was a result of downstream PCSK9 inhibitor use. Adjunct BA + EZE (FDC) was estimated to result in 7300 fewer MACE compared with no oral adjunct treatment in the CRWD ASCVD cohort over 10 years.
Patients with ASCVD were estimated to spend more life years at LDL-C goal after treatment with BA + EZE (FDC) and EZE only (521 and 387 per 100 patients, respectively), and have a smaller number of MACE (49 and 54 per 100 patients, respectively; Table 5). Those with no oral adjunct therapy were estimated to have 231 fewer life years per 100 patients and 10 additional MACE per 100 patients compared with the BA + EZE (FDC) group.
Table 5.
One-year and 10-year patient outcomes, and treatment costs (N = 73,056).
| BA + EZE (FDC) | EZE | Difference between BA + EZE (FDC) and EZE | No oral adjunct therapy | Difference between BA + EZE (FDC) and no oral adjunct therapy | |
|---|---|---|---|---|---|
| Alive at year 1, n (%) | 71,654 (98) | 71,737 (98) | −83 (0) | 71,587 (98) | 67 (0) |
| Patients reaching LDL-C goal before PCSK9 inhibitor, n (%) | 47,224 (66) | 20,504 (29) | 26,810 (37) | 0 (0) | 47,224 (66) |
| Patients reaching LDL-C goal at year 1, n (%) | 56,416 (79) | 41,862 (58) | 14,364 (20) | 29,991 (42) | 26,425 (37) |
| Mean (SD) LDL-C achieved at year 1, mg/dL | 61.1 (20.7) | 71.7 (27.7) | −10.6 (34.6) | 78.4 (36.1) | −17.3 (41.6) |
| Mean LDL-C reduction at year 1, mg/dL | 41.9 | 31.4 | 10.6 | 24.6 | 17.3 |
| Overall proportion simulated to receive PCSK9 inhibitor treatment at year 1, n (%)a | 10,445 (14) | 24,911 (34) | −14,466 | 36,406 (50) | −25,961 |
| Overall 10-year treatment cost per patient (2022), US$ | 34,731 | 15,182 | 19,549 | 21,367 | 13,364 |
| Non-PCSK9 inhibitor costs | 28,441 | 380 | 28,061 | 0 | 28,441 |
| PCSK9 inhibitor costs | 6290 | 14,802 | −8512 | 21,367 | −15,077 |
| Mean (SD) 10-year MACE risk overall, %b | 39 (16) | 42 (17) | −3 (23) | 44 (18) | −5 (24) |
| MACE, 10-year totalc | 49 | 54 | −5 | 58 | −10 |
| MACE, 10-year non-fatalc | 46 | 51 | −4 | 55 | −9 |
| MACE, 10-year fatalc | 3 | 3 | 0 | 3 | 0 |
| Life Years at LDL-C goalc | 521 | 387 | 133 | 290 | 231 |
BA = bempedoic acid; EZE = ezetimibe; FDC = fixed dose combination; LDL-C = low density lipoprotein cholesterol; MACE = major adverse cardiovascular event; PCSK9 = proprotein convertase subtilisin/kexin type 9; SD = standard deviation; US = United States.
In the base case, each eligible patient has a 50% chance of getting a PCSK9 inhibitor.
10-year MACE risk at baseline, mean (SD) was 49% (19%).
Events reported per 100 patients. MACE values may not sum to total due to rounding.
2.4. Treatment costs
The clinical benefits associated with adding BA + EZE (FDC) to reduce LDL-C to goal in patients uncontrolled on statins would come at an increased treatment cost (Table 5). Adjunct BA + EZE (FDC) would cost an additional $19,549 per patient per year compared with adjunctive EZE alone (when considering drug costs only), and an additional $13,364 compared with using no oral adjunct therapy. When considering the source of the costs, the addition of PCSK9 inhibitors accounts for 18% of the overall treatment cost. In comparison, the estimated costs associated with EZE only and no oral adjunct therapy in patients achieving goal without PCSK9 inhibitors (29% and 42%, respectively) were $380 and $0 with a majority (97.5% and 100%) of the overall costs ($15,182 and $21,367, respectively) associated with PCSK9 inhibitor use. As such, the use of BA + EZE (FDC) would correspond to a $8512 decrease (58%) in the cost of treatment with PCSK9 inhibitors compared with use of EZE alone, and a $15,077 decrease (71%) in the cost of treatment with a PCSK9 inhibitor compared with no oral adjunct therapy.
2.5. Scenario analyses
Because PCSK9 inhibitor use is highly variable between clinical guideline recommendations and real-world clinical practice, and their costs can be substantial, 3 scenarios were evaluated to examine the impact of PCSK9 inhibitor utilization assumptions on treatment outcomes (Table 4, Table S2). The first scenario assumed 50% of eligible patients received PCSK9 inhibitors, but treatment discontinuation was increased. Real-world discontinuation rates (20%) were applied to closely resemble the clinical practice and real-world use of PCSK9 inhibitors [17]. Overall goal achievement was similar to, but lower than, the base case across all treatment pathways.
The second scenario examined the potential impact of unrestricted access to PCSK9 inhibitors for all eligible patients (ie, all eligible patients received PCSK9 inhibitor therapy). Under this assumption, more patients achieved goal compared with restricted access (50% of eligible patients in the base case), and the overall proportion of patients achieving LDL-C goal at 1 year was similar between BA + EZE (FDC) and EZE only (91% and 88%, respectively). However, even if all eligible patients received a PCSK9 inhibitor in the no oral adjunct therapy group, fewer would achieve goal at 1 year (84%). Mean LDL-C values and reduction from baseline for both BA + EZE (FDC) and EZE only groups were similar. Total costs would also be highest for the no oral adjunct therapy group in this scenario, driven by the high PCSK9 inhibitor use to get patients to goal.
The third scenario examined the impact of no access to PCSK9 inhibitors. Fewer patients overall achieved goal in a scenario excluding PCSK9 inhibitor utilization compared with the base case. As expected, the results for the no oral adjunct therapy group in this scenario closely resembled the baseline statistics.
The final scenario examined the impact of a lower LDL-C target goal (<55 mg/dL). As per the 2022 ACC treatment guidelines [7], and alignment with European Society of Cardiology 2019 guidelines [6] and simulation studies [[12], [13], [14]], the proportion of patients in each modeled treatment differed from those in the base case. In the BA + EZE (FDC) group, more than half of patients (52% vs 66% under the base case LDL-C goal of <70 mg/dL) were predicted to reach the LDL-C goal on BA + EZE (FDC) alone, and an additional 19% (vs 13%) were predicted to reach goal with the addition of PCSK9 inhibitor, even with the stricter LDL-C level goals (<55 mg/dL; Table S2). Across the therapeutic options, 37%–71% patients would achieve LDL-C goal compared with the base case of 42%–79% (Table S2).
3. Discussion
The CLEAR outcomes trial demonstrated significantly lower CV risk with BA use in actual practice among statin-intolerant patients over a 3.5-year period compared with no oral adjunct therapy [17]. In the absence of a comparable outcomes trial examining BA + EZE (FDC) in the broader ASCVD population, the current model simulated a US patient cohort with established ASCVD and LDL-C above 70 mg/dL despite having a documented statin prescription. Following simulations of the INTERCATH [12], IQVIA™ German [13], and SANTORINI [14] cohorts in Europe, we sought to assess the clinical and economic outcomes of utilizing adjunct BA + EZE (FDC) after statins and before PCSK9 inhibitors in a real-world US patient population.
Our simulation compared 3 treatment pathways for patients needing additional LDL-C lowering after statins: adjunct therapy with BA + EZE (FDC), EZE alone, or no oral adjunct therapy. Clinical guidelines unanimously recommend the addition of EZE alone for patients unable to reach LDL-C goals on statins alone, yet real-world studies have consistently shown dishearteningly low use. Observed utilization of EZE in the CRWD dataset among patients on statins who were not at LDL-C goal was extremely limited (∼4%) [[31], [32], [33], [34]]. As such, the no oral adjunct therapy pathway was included to closely resemble routine clinical practice. Results of our simulation estimated that a larger proportion of patients treated with BA + EZE (FDC), compared with EZE alone and no oral adjunct therapy, would potentially achieve AHA/ACC LDL-C treatment goal (<70 mg/dL) [5,7] without the use of PCSK9 inhibitors. The addition of BA + EZE (FDC) would potentially provide additional long-term clinical benefit for those reaching LDL-C goal (<70 mg/dL) within the first year of therapy by reducing the occurrence of future MACE. The predicted difference in simulated MACE outcomes prevented was greatest between adjunct BA + EZE (FDC) and no oral and adjunct therapy (9 MACE per 100 people).
Though the cost of BA + EZE (FDC) therapy was expected to be higher than the alternatives of adding EZE only and using no oral adjunct therapy, this may underestimate the overall value of BA + EZE (FDC) because our simulation factored in only treatment costs, and did not consider medical costs asssociated with managing ASCVD or downstream MACE. As such, the incremental clinical benefits of BA + EZE (FDC) (ie, LDL-C goal attainment and MACE avoidance) were predicted to come at a higher treatment cost that was modestly offset by delaying or reducing the need to escalate to PCSK9 inhibitor therapy. In context, a recent study noted that the overall healthcare costs for patients with ASCVD in the US are extensive and exceed approximately $20,000 annually, with average annual out-of-pocket spending exceeding $2000 [35]. If the costs of acute events and post-acute management had been included, the cost-offsets simulated with BA + EZE (FDC) would likely have been greater through reduction in the costs associated with MACE [36,37].
Importantly, this study assumed that all patients with documented statin therapy at baseline were at the maximum tolerated dose and eligible to add adjunctive therapy. The result is that some patients in our cohort were modeled on adjunctive therapy when in actuality they may have been able to optimize their statin therapy to reach goal without additional treatment. The no oral adjunct therapy option provided a comparison to the potential outcomes in patients who may not have reached maximally tolerated statin utilization.
Several factors related to patient cohort selection are also of note. We took a pragmatic approach to records with missing data from CRWD in an effort to preserve the sample size. This included interpolating key missing data elements required for use of the SMART2 risk equation, such as smoking status (assumed patient did not smoke if missing) and creatinine and systolic blood pressure measures (assumed the population mean when missing). The population mean for creatinine was slightly lower than the overall cohort mean (1.2 mg/dL vs 1.4 mg/dL) and systolic blood pressure was similar (132 mmHg vs 134 mmHg). As these assumptions were applied equally to cohorts across all treatment options, it was expected that only the absolute number of predicted outcomes would differ if all data were available or alternative assumptions were made. It was the relative effects of BA + EZE (FDC) compared with EZE alone and no oral adjunct therapy that were at the center of this analysis and would not be expected to meaningfully change since only the impact on, and of, LDL-C was simulated. Estimates of MACE were projections based on adjustment of 1 clinical factor (LDL-C) and age out of 16 factors known to impact event risk. Thus, the incremental benefits on MACE outcomes at 10 years were less pronounced, likely due to the impact of other SMART2 risk factors that were assumed to remain constant from baseline (e.g., systolic blood pressure and diabetes mellitus). In addition, the SMART2 does not factor in the inflammatory biomarker, high sensitivity C-reactive protein (hsCRP) when calculating MACE risk, and as such, our analysis only considered the LDL-C lowering effects of BA + EZE (FDC) on cardiovsacular risk reduction. An analysis of the CLEAR Outcomes trial of 13,970 patients with or at high-risk for ASCVD found that hsCRP was as strong or stronger of a predictor for cardiovascular events and mortality as LDL-C [38]. As BA reduced hsCRP by 21.6% in CLEAR Outcomes, our simulation may underestimate the actual cardiovascular risk reduction that could occur over the long-term [38].
Long-term MACE outcomes were estimated by first calculating the composite risk of MACE and then determining the specific event type (myocardial infarction, stroke, cardiovascular death) by applying a distribution to assign unique counts for individual events. This approach was a simplification of the complexities of real-world clinical experience and prognosis influenced by many factors including the occurrence of the events themselves. In addition, the assumption that this underlying distribution itself remained constant over time (10 years) was also a simplification. As with our patient cohort selection, this approach and assumption was applied equally to all treatment comparators and was not expected to systematically impact the relative findings or interpretation.
In this simulation of a US patient cohort with established ASCVD, a larger proportion of patients on treatment with BA + EZE (FDC) were predicted to achieve LDL-C treatment goal (<70 mg/dL) without the need for escalating to PCSK9 inhibitors, compared with those on EZE alone. This resulted in more patients who would achieve LDL-C goals without needing to escalate to PCSK9 inhibitor therapy, thus delaying and reducing the need for PCSK9 inhibitor initiation and associated costs. These findings were consistent with those observed in previous European simulation studies [[12], [13], [14]] and similar to those in a simulated US cohort [39].
Implementation of BA + EZE (FDC) in the treatment pathway to lower LDLC levels demonstrated the anticipated benefit of providing an oral adjunct treatment option for patients who need further LDL-C lowering to achieve recommended treatment goals. The full context of individual patient factors, comorbidities, and history, along with relevant cost considerations, were shown to be important factors to take into account when making treatment decisions and recommendations. In the future, real-world outcomes studies should be conducted to confirm the validity of these simulation findings.
Author contributions
Kristen Migliaccio-Walle: Conceptualization, Methodology, Investigation, Writing – Original Draft, Writing – Review & Editing, Visualization, Supervision, Project Administration. David Elsea: Methodology, Validation, Formal analysis, Writing – Review & Editing. Anand Gupta: Software, Validation, Formal analysis, Investigation, Resources, Data Curation, Writing – Review & Editing. Evelyn Sarnes: Conceptualization, Methodology, Writing – Review & Editing, Supervision, Project administration, Funding acquisition. Kristel Griffith: Methodology, Validation, Formal analysis, Writing – Review & Editing, Visualization. Rajshree Pandey: Conceptualization, Investigation, Writing – Original Draft, Writing – Review & Editing. Kristin Gillard: Conceptualization, Methodology, Writing – Review & Editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Kristen Migliaccio-Walle reports financial support was provided by Esperion Therapeutics Inc. Kristen Migliaccio-Walle reports writing assistance was provided by Spark Therapeutics Inc. David Elsea, Kristel Griffith, Rajshree Pandey reports financial support was provided by Esperion Therapeutics Inc. Anand Gupta reports financial support was provided by Esperion Therapeutics Inc. Evelyn Sarnes, Kristin Gillard reports writing assistance was provided by Spark Therapeutics Inc. Evelyn Sarnes, Kristin Gillard reports a relationship with Esperion Therapeutics Inc that includes: employment and equity or stocks. co-author previously employed by Genetech Inc - RP.
Acknowledgements
Editorial support, funded by Esperion Therapeutics, Inc. was provided by Dave Buist, PhD, and Isobel Markham, MSc, of Spark Medica Inc. who assisted with copyediting and formatting of the manuscript for submission.
Footnotes
Study funding provided by Esperion Therapeutics, Inc., Ann Arbor, Michigan.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.athplu.2024.01.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article::
References
- 1.Virani S.S., Alonso A., Aparicio H.J., Benjamin E.J., Bittencourt M.S., et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 2.Ritchey M.D., Wall H.K., Owens P.L., Wright J.S. Vital signs: state-level variation in nonfatal and fatal cardiovascular events targeted for prevention by million hearts 2022. MMWR Morb Mortal Wkly Rep. 2018;67:974–982. doi: 10.15585/mmwr.mm6735a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhindsa D.S., Sandesara P.B., Shapiro M.D., Wong N.D. The evolving understanding and approach to residual cardiovascular risk management. Review. Front Cardiovasc Med. 2020;7:88. doi: 10.3389/fcvm.2020.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Angelantonio E., Gao P., Pennells L., Kaptoge S., Caslake M., et al. Lipid-related markers and cardiovascular disease prediction. JAMA. 2012;307:2499–2506. doi: 10.1001/jama.2012.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grundy S.M., Stone N.J., Bailey A.L., Beam C., Birtcher K.K., et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;73:3168–3209. doi: 10.1016/j.jacc.2018.11.002. 2019. [DOI] [PubMed] [Google Scholar]
- 6.Mach F., Baigent C., Catapano A.L., Koskinas K.C., Casula M., et al. ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2019;41:111–188. doi: 10.1093/eurheartj/ehz455. 2020. [DOI] [PubMed] [Google Scholar]
- 7.Lloyd-Jones D.M., Morris P.B., Ballantyne C.M., Birtcher K.K., Covington A.M., et al. ACC Expert Consensus Decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology solution set oversight committee. J Am Coll Cardiol. 2022;80(14):1366–1418. doi: 10.1016/j.jacc.2022.07.006. 2022. [DOI] [PubMed] [Google Scholar]
- 8.Esperion Therapeutics Inc NEXLETOL® (bempedoic acid) [package insert] 2023. https://pi.esperion.com/nexletol/nexletol-pi.pdf
- 9.Esperion Therapeutics Inc NEXLIZET® (bempedoic acid and ezetimibe) [package insert] 2023. https://pi.esperion.com/nexlizet/nexlizet-pi.pdf
- 10.Ballantyne C.M., Laufs U., Ray K.K., Leiter L.A., Bays H.E., et al. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur J Prev Cardiol. 2020;26:593–603. doi: 10.1177/2047487319864671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banach M., Duell P.B., Gotto A.M., Jr., Laufs U., Leiter L.A., et al. Association of bempedoic acid administration with atherogenic lipid levels in phase 3 randomized clinical trials of patients with hypercholesterolemia. JAMA Cardiol. 2020;5:1124–1135. doi: 10.1001/jamacardio.2020.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blaum C., Brunner F.J., Goßling A., Kröger F., Bay B., et al. Target populations and treatment cost for bempedoic acid and PCSK9 inhibitors: a simulation study in a contemporary CAD cohort. Clin Therapeut. 2021;43:1583–1600. doi: 10.1016/j.clinthera.2021.07.019. [DOI] [PubMed] [Google Scholar]
- 13.Katzmann J.L., Becker C., Bilitou A., Laufs U. Simulation study on LDL cholesterol target attainment, treatment costs, and ASCVD events with bempedoic acid in patients at high and very-high cardiovascular risk. PLoS One. 2022;17 doi: 10.1371/journal.pone.0276898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toplak H., Bilitou A., Alber H., Auer J., Clodi M., et al. Simulation of bempedoic acid and ezetimibe in the lipid-lowering treatment pathway in Austria using the contemporary SANTORINI cohort of high and very high risk patients. Wien Klin Wochenschr. 2023;135:364–374. doi: 10.1007/s00508-023-02221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corporation Cerner. HealtheDataLab in AWS; real_world_data_2021_q2: 2021. Cerner real-world data. [Google Scholar]
- 16.van't Klooster C.C., Bhatt D.L., Steg P.G., Massaro J.M., Dorresteijn J.A.N., et al. Predicting 10-year risk of recurrent cardiovascular events and cardiovascular interventions in patients with established cardiovascular disease: results from UCC-SMART and REACH. Int J Cardiol. 2021;325:140–148. doi: 10.1016/j.ijcard.2020.09.053. [DOI] [PubMed] [Google Scholar]
- 17.Nissen S.E., Lincoff A.M., Brennan D., Ray K.K., Mason D., et al. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med. 2023;388:1353–1364. doi: 10.1056/NEJMoa2215024. [DOI] [PubMed] [Google Scholar]
- 18.Cannon C.P., Blazing M.A., Giugliano R.P., McCagg A., White J.A., et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 19.Talasaz A.H., Ho A.J., Bhatty F., Koenig R.A., Dixon D.L., et al. Meta-analysis of clinical outcomes of PCSK9 modulators in patients with established ASCVD. Pharmacotherapy. 2021;41:1009–1023. doi: 10.1002/phar.2635. [DOI] [PubMed] [Google Scholar]
- 20.Sabatine M.S., Giugliano R.P., Keech A.C., Honarpour N., Wiviott S.D., et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 21.Wang N., Fulcher J., Abeysuriya N., Park L., Kumar S., et al. Intensive LDL cholesterol-lowering treatment beyond current recommendations for the prevention of major vascular events: a systematic review and meta-analysis of randomised trials including 327 037 participants. Lancet Diabetes Endocrinol. 2020;8:36–49. doi: 10.1016/S2213-8587(19)30388-2. [DOI] [PubMed] [Google Scholar]
- 22.Hageman S.H.J., McKay A.J., Ueda P., Gunn L.H., Jernberg T., et al. Estimation of recurrent atherosclerotic cardiovascular event risk in patients with established cardiovascular disease: the updated SMART2 algorithm. Eur Heart J. 2022;43:1715–1727. doi: 10.1093/eurheartj/ehac056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y.L., Castro A.F., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miniño A.M., Klein R.J. Health E-Stats National Center for Health Statistics; 2010. Health mortality from major cardiovascular diseases: United States, 2007. [Google Scholar]
- 25.Organon L.L.C. ZETIA® (ezetimibe) [package insert] 2023. https://www.organon.com/product/usa/pi_circulars/z/zetia/zetia_pi.pdf
- 26.Amgen Inc. REPATHA® (evolocumab) [package insert] 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125522orig2s000lbl.pdf
- 27.Regeneron Pharmaceuticals Inc PRALUENT® (alirocumab) [package insert] 2021. https://www.regeneron.com/downloads/praluent_pi.pdf
- 28.Novartis Pharmaceuticals Corporation LEQVIO® (inclisiran) [package insert] 2023. https://www.novartis.com/us-en/sites/novartis_us/files/leqvio.pdf
- 29.Micromedex Merative. RED BOOK® database. 2022. https://www.micromedexsolutions.com/
- 30.Centers for Medicare & Medicaid Services . List of CPT/HCPCS Codes; 2022. HCPCS code CPT 96372. [Google Scholar]
- 31.Chen G., Farris M.S., Cowling T., Pinto L., Rogoza R.M., et al. Prevalence of atherosclerotic cardiovascular disease and subsequent major adverse cardiovascular events in Alberta, Canada: a real-world evidence study. Clin Cardiol. 2021;44:1613–1620. doi: 10.1002/clc.23732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradley C.K., Kolkailah A.A., Shah N.P., Page C.B., Peterson E.D., et al. Uptake of non-statin lipid-lowering therapies for secondary prevention in community practice. J Clin Lipidology. 2023;17:412–414. doi: 10.1016/j.jacl.2023.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Navar A.M., Kolkailah A.A., Gupta A., Gillard K.K., Israel M.K., et al. Gaps in guideline-based lipid-lowering therapy for secondary prevention in the United States: a retrospective cohort study of 322 153 patients. Circ Cardiovasc Qual Outcomes. 2023;16:533–543. doi: 10.1161/CIRCOUTCOMES.122.009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah N.P., Peterson E.D., Page C., Blanco R., Navar A.M. Generalizability of an EHR-network dataset to the United States for cardiovascular disease conditions: comparison of Cerner real world data with the national inpatient sample. Am Heart J. 2023;263:64–72. doi: 10.1016/j.ahj.2023.05.009. [DOI] [PubMed] [Google Scholar]
- 35.Khera R., Valero-Elizondo J., Okunrintemi V., Saxena A., Das S.R., et al. Association of out-of-pocket annual health expenditures with financial hardship in low-income adults with atherosclerotic cardiovascular disease in the United States. JAMA Cardiol. 2018;3:729–738. doi: 10.1001/jamacardio.2018.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao Y., Zabriski S., Bertram C. Associations between statin adherence level, health care costs, and utilization. J Manag Care Spec Pharm. 2014;20:703–713. doi: 10.18553/jmcp.2014.20.7.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korsnes J.S., Davis K.L., Ariely R., Bell C.F., Mitra D. Health care resource utilization and costs associated with nonfatal major adverse cardiovascular events. J Manag Care Spec Pharm. 2015;21:443–450. doi: 10.18553/jmcp.2015.21.6.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ridker P.M., Lei L., Louie M.J., Haddad T., Nicholls S.J., et al. Inflammation and cholesterol as predictors of cardiovascular events among 13 970 contemporary high-risk patients with statin intolerance. Circulation. 2024;149:28–35. doi: 10.1161/CIRCULATIONAHA.123.066213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cannon C.P., Khan I., Klimchak A.C., Sanchez R.J., Sasiela W.J., et al. Simulation of impact on cardiovascular events due to lipid-lowering therapy intensification in a population with atherosclerotic cardiovascular disease. Am Heart J. 2019;216:30–41. doi: 10.1016/j.ahj.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Data on File . 2022. Esperion market research 2022: PCSK9 inhibitors market shares. [Google Scholar]
- 41.Hess G.P., Natarajan P., Faridi K.F., Fievitz A., Valsdottir L., et al. Proprotein convertase subtilisin/kexin type 9 inhibitor therapy: payer approvals and rejections, and patient characteristics for successful prescribing. Circulation. 2017;136:2210–2219. doi: 10.1161/CIRCULATIONAHA.117.028430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Navar A.M., Mulder H.M., Wojdyla D.M., Peterson E.D. Have the major cardiovascular outcomes trials impacted payer approval rates for PCSK9 inhibitors? Circ Cardiovasc Qual Outcomes. 2020;13 doi: 10.1161/CIRCOUTCOMES.119.006019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donald D.R., Reynolds V.W., Hall N., DeClercq J., Choi L. Exploring rates of PCSK9 inhibitor persistence and reasons for treatment non-persistence in an integrated specialty pharmacy model. J Clin Lipidol. 2022;16:315–324. doi: 10.1016/j.jacl.2022.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.