Abstract
Femoroacetabular impingement (FAI), characterized by a pathological contact between the proximal femur and acetabulum, is a common precursor of hip osteoarthritis. Cam morphology is a bony prominence that causes FAI and frequently forms on the anterosuperior femoral head–neck junction. Despite anatomical consensus regarding the femoral head–neck junction as a boundary area covered by the articular cartilage and joint capsule, it remains unclear whether the joint capsule is continuous with the anterosuperior articular cartilage. For the anatomical consideration of cam morphology formation, this study aimed to investigate the histological characteristics of the capsular attachment on the anterosuperior femoral head–neck junction, particularly focusing on the presence or absence of continuity of the joint capsule to the articular cartilage. A total of 21 anterosuperior regions (seven hips each for the 12:00, 1:30, and 3:00 positions) from seven hips (three males and four females; mean age at death, 68.7 years) were histologically analyzed in this study for quantitative evaluation of the capsular thickness using histological sections stained with Masson's trichrome, as well as qualitative evaluation of the capsular attachment. The present study showed that the joint capsule, which folded proximally to the femoral head–neck junction from the recess, exhibited a blend of the fibrous and synovial regions. Notably, it not only continued with the superficial layer of the articular cartilage, but also attached to the articular cartilage via the fibrocartilage. This continuous region was relatively fibrous with dense connective tissue running in the longitudinal direction. The capsular thickness at the recess point (mean, 1.7 ± 0.9 mm) and those at the distal end of the articular cartilage (0.35 ± 0.16 mm) were significantly greater than the control value for the most superficial layer thickness of the articular cartilage (0.019 ± 0.003 mm) (Dunnett's T3, both p‐value <0.001). Based on the fibrous continuity between the joint capsule and articular cartilage and its thickness, this study suggests the anatomical possibility that some mechanical stress can be transmitted from the joint capsule to the articular cartilage at the frequent sites of cam morphology.
Keywords: articular cartilage, cam morphology, femoral head–neck junction, hip joint capsule, histology
For the anatomical consideration of cam morphology formation, this study aimed to investigate capsular attachment on the anterosuperior femoral head–neck junction. We revealed that the anterosuperior joint capsule (Cap) continued to the superficial layer of the articular cartilage (AC) and attached to the AC via the fibrocartilage (asterisk). Thus, we showed the anatomical possibility that some mechanical stress can be transmitted from the Cap to the AC at the frequent sites of cam morphology.

1. INTRODUCTION
Femoroacetabular impingement (FAI) syndrome is a common painful disorder of the hip, characterized by the pathologic contact between the femur and acetabulum during hip movements, such as repetitive flexion (Ganz et al., 2003; Griffin et al., 2016). Cam morphology is a bony prominence at the femoral head–neck junction that causes the femoral head to become aspheric, which can lead to FAI (Dijkstra et al., 2021; Ganz et al., 2003; Griffin et al., 2016). Thus, individuals with cam morphology are considered to be at a high risk for hip osteoarthritis (Casartelli et al., 2021; Hunter & Bierma‐Zeinstra, 2019; Saberi Hosnijeh et al., 2017). Despite its clinical significance, the cause of cam morphology formation unrelated to a primary disease (e.g., Perthes disease) is not fully understood (Dijkstra et al., 2021; Morris et al., 2018). Cam morphology is reported to be highly prevalent in athletes (Agricola et al., 2012; Siebenrock et al., 2011), and develops primarily during an open growth plate on the femoral head–neck junction (Agricola et al., 2014; Hanke et al., 2021; van Klij et al., 2019). Thus, bony adaptation to stress during skeletal maturation may lead to the formation of cam morphology (Dijkstra et al., 2022). To understand the mechanical stress on the femoral head–neck junction and gain insight into the formation of the cam morphology, precise anatomical knowledge of the femoral head–neck junction is essential.
A common site of occurrence of the cam morphology is the anterosuperior femoral head–neck junction (Dijkstra et al., 2021). Additionally, cartilaginous hypertrophy at the femoral head–neck junction has recently been reported to precede osseous cam morphology (Dijkstra et al., 2021; Palmer et al., 2018). In general, the articular cartilage covers the femoral head and the inner side of the joint capsule covers the femoral neck, folding proximally, and making a recess (Adams, 2021; Pawlina & Ross, 2016; Robben et al., 1999). However, some previous studies have reported that the inner side of the joint capsule ends at the distal end of the articular cartilage (Adams, 2021, Pawlina & Ross, 2016, Robben et al., 1999), while others have reported that the synovial membrane continues to the most superficial layer of the articular cartilage with a thin film (Teshima et al., 1995, 2004). Thus, it remains unclear whether the joint capsule is continuous with the superficial layer of the articular cartilage at the frequent site of occurrence of cam morphology, that is, the anterosuperior region. In addition, the thickness and attachment width of the hip joint capsule have been reported to vary according to location and be interpreted as an adaptation to mechanical stress (Tsutsumi et al., 2019, 2020, 2022). Therefore, the capsular thickness at the distal end of the articular cartilage may be vital in understanding what stresses can be applied structurally to the articular cartilage.
This study aimed to investigate the capsular attachment on the anterosuperior femoral head–neck junction using combined micro‐computed tomography (micro‐CT) and histological analysis. We hypothesized that the joint capsule continued with the superficial layer of the articular cartilage on the anterosuperior femoral head, and the capsular thickness at the distal end of the anterosuperior articular cartilage was significantly greater than that of the most superficial layer of the anterosuperior articular cartilage on the femoral head.
2. MATERIALS AND METHODS
2.1. Specimen preparations
Ten hips (four left and six right) from 10 cadavers of Japanese body donors (five males and five females; mean age at death, 73.4 years; age range, 49–95 years) donated to the Department of Anatomy at Tokyo Medical and Dental University were used in this study. The appropriate sample size was determined based on the average sample size of previous similar studies (Robben et al., 1999; Teshima et al., 1995, 2004). Before death, all donors voluntarily declared that their remains be donated for educational and research purposes. This study complied with the Japanese law entitled “Act on Body Donation for Medical and Dental Education,” and the study design was approved by the Medical Research Ethics Committee of Tokyo Medical and Dental University (approval no. #M2018‐006). All procedures were performed in accordance with the Japanese “Ethical Guidelines for Medical and Health Research Involving Human Subjects.”
Anatomical specimens that had no joint contracture in the lower limbs and had not undergone hip surgery during their lifetime were included in this study. The exclusion criteria were the presence of obvious synovial hyperplasia, labral tear, or any osteoarthritic changes in the histological analysis described below. All the anatomical specimens were fixed in 8% formalin and preserved in 30% ethanol. Hip specimens were obtained from the anatomical specimens by cutting the anterior superior iliac spine as the upper end and the lesser trochanter of the femur as the lower end using a diamond saw (EXAKT 312; EXAKT Advanced Technologies, Norderstedt, Germany; blade thickness, 300 μm). After removal of the skin and subcutaneous tissues, analyses were performed in the following order: (1) micro‐CT, and (2) histological analyses.
2.2. Micro‐CT analysis
Micro‐CT (inspeXio SMX‐100CT; Shimadzu, Kyoto, Japan) with a 200‐μm resolution was performed on all hip specimens. To verify the presence of the cam morphology, the morphological features of the femoral head–neck junction were analyzed from the micro‐CT image series by measuring the alpha angles on predefined radial sections (Nötzli et al., 2002; Siebenrock et al., 2011). To obtain predefined radial sections, the scanned sequential images were resliced perpendicular to the femoral neck axis using the ImageJ software version 1.54 (National Institutes of Health, Bethesda, MD, USA). The axis of the femoral neck was defined as a line passing through the center of the circle on the femoral head and parallel to a line connecting the centers of two circles set on the femoral neck (Bouma et al., 2014). Then, the 12 o'clock plane was defined as the plane parallel to the axis of the proximal femur diaphysis and radial planes were rotated clockwise in 45° intervals around the femoral neck axis using the “Radial slice” plugin of the ImageJ software (Figures 1 and 2, 3, 4). Finally, the alpha angles were measured at the anterosuperior positions, namely the radial sections, at 12:00, 1:30, and 3:00.
FIGURE 1.
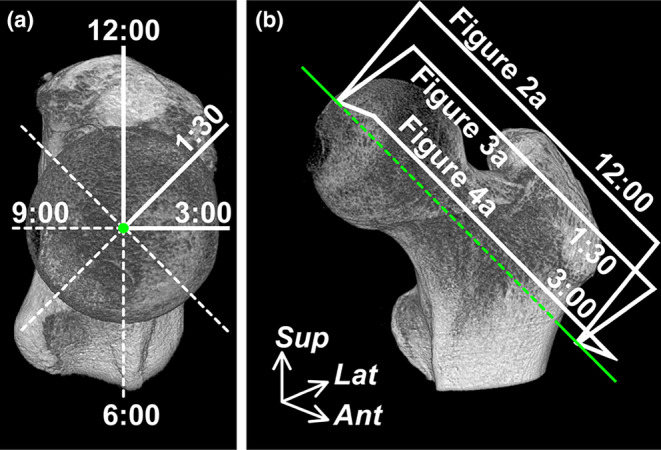
Radial plane locations on the micro‐CT images of the proximal femur. (a) Proximal aspect perpendicular to the femoral neck axis. The radial planes rotate clockwise in 45° intervals around the femoral neck axis (green dot). The 12 o'clock plane is parallel to the axis of the proximal femur diaphysis. (b) Anteromedial aspect of the proximal femur. The radial boxed regions are analyzed, as shown in Figure 2a (12:00), 3a (1:30) and 4a (3:00). Ant, Anterior; CT, computed tomography; Green line, femoral neck axis; Lat, lateral; Sup, superior.
FIGURE 2.

Capsular attachment on femoral head–neck junction at the 12:00 position. (a) Micro‐CT images of the boxed region (12:00) in Figure 1b. (b) Macroscopic section of the white boxed region in Figure 2a. (c) Histological section (Masson's trichrome staining) of the dotted boxed region in Figure 2b. Femoral capsular attachment makes the recess (T1, capsular thickness at the recess point) and partly extends to the femoral head–neck junction. Black arrow heads indicate the fibrous region and red open arrow heads indicate the synovial region. (d) Magnified images of the black boxed region in Figure 2c. Joint capsule (Cap) not only continues to the superficial layer of the articular cartilage (AC) but also attaches to the AC via the fibrocartilage (asterisk). T2 indicates the capsular thickness at the distal end of the AC. (e) and (f) Correspond Safranin O staining sections with (c) and (d), respectively. CT, computed tomography; GMa, gluteus maximus; GMe, gluteus medius; GMi, Gluteus minimus; GT, Greater trochanter; HF, head of femur; Labrum, acetabular labrum; NF, neck of femur; OI, Obturator internus; RFr, reflected head of the rectus femoris.
FIGURE 3.

Capsular attachment on femoral head–neck junction at the 1:30 position. (a) Micro‐CT images of the boxed region (1:30) in Figure 1b. (b) Macroscopic section of the white boxed region in Figure 3a. (c) Histological section (Masson's trichrome staining) of the dotted boxed region in Figure 3b. T1 indicates capsular thickness at the recess point. Black arrow heads indicate the fibrous region and red open arrow heads indicate the synovial region. (d) Magnified images of the black boxed region in Figure 3c. Joint capsule (Cap) partly attaches to the articular cartilage (AC) via the fibrocartilage (asterisk). T2 indicates the capsular thickness at the distal end of the AC. AIIS, anterior inferior iliac spine; CT, computed tomography; GMe, gluteus medius; GMi, Gluteus minimus; GT, Greater trochanter; HF, head of femur; Ip, iliopsoas; Labrum, acetabular labrum; NF, neck of femur; RF, rectus femoris; Sa, sartorius; TFL, tensor fascia latae.
FIGURE 4.

Capsular attachment on femoral head–neck junction at the 3:00 position. (a) Micro‐CT images of the boxed region (3:00) in Figure 1b. (b) Macroscopic section of the white boxed region in Figure 4a. (c) Histological section (Masson's trichrome staining) of the dotted boxed region in Figure 4b. T1 indicates capsular thickness at the recess point. Black arrow heads indicate the fibrous region and red open arrow heads indicate the synovial region. (d) Magnified images of the black boxed region in Figure 4c. Joint capsule (Cap) partly attaches to the articular cartilage (AC) via the fibrocartilage (asterisk). T2 indicates the capsular thickness at the distal end of the AC. CT, computed tomography; GMi, gluteus minimus; GT, Greater trochanter; HF, head of femur; Ip, iliopsoas; Labrum, acetabular labrum; NF, neck of femur; RF, rectus femoris; Sa, sartorius; TFL, tensor fasciae latae; VL, vastus lateralis.
2.3. Histological analysis
After micro‐CT analysis, the bony elements of the posterior part of the acetabulum, posterior joint capsule, and hip muscles (gluteus maximum, adductor magnus, quadratus femoris, obturator externus, and complex of the gemellus superior, gemellus inferior, and obturator internus) were partly removed as little as necessary to expose the femoral head, neck, and its axis at approximately 9:00. Using a diamond saw, based on the exposed femoral neck axis and three‐dimensional reconstruction images obtained using ImageJ, the hip specimens were cut approximately in the radial plane at 9:00 and 3:00. Based on this cutting plane, all specimens were sectioned into 5‐mm thick segments at 12:00, 1:30, and 3:00 positions using a band saw (WN‐25‐3; Nakajima Seisakusho, Osaka, Japan; blade thickness, 0.5 mm) after embedding in a 3% agar solution and were frozen at −80°C.
After removing the excess agar from the sectioned specimens, we harvested a block from each radial section, including the capsular attachment of the femur, head–neck junction, and acetabular labrum, and decalcified the blocks for 3 weeks with Plank–Rychlo solution (AlCl3:6H2O [70.0 [g/L]], HCl [85.0 [mL/L]], and HCOOH [50.0 [mL/L]]) (Plank & Rychlo, 1952). After decalcification, each block was dehydrated and embedded in paraffin. The paraffin‐embedded tissue was sliced into 5‐μm sections from the side consistent with each clock position. The first slice and its sequential slices, in which all anatomical structures in the block could be observed, were selected as the analysis slices. The remaining blocks were also sliced into 5‐μm sections with a 1‐mm interval; the sections were stained using the Masson trichrome and partly Safranin O staining protocol. All histological sections were digitized using a whole‐slide scanner (NanoZoomer‐SQ; Hamamatsu Photonics, Hamamatsu, Japan) with a 20‐fold objective lens (numerical aperture, 0.75; scan resolution, 0.46 μm/pixel). Based on histological analysis, three hip specimens were excluded based on the aforementioned exclusion criteria. Therefore, 21 selected clock positions (7 specimens each for the 12:00, 1:30, and 3:00 positions) from seven hips (three males and four females; mean age at death, 68.7 years; age range, 49–82 years) were analyzed in this study.
Based on these anterosuperior positions slices, the thickness of the joint capsule, and the most superficial layer of the articular cartilage were measured using the viewer software of the digitized histological sections (NDP.view2; Hamamatsu Photonics, Hamamatsu, Japan). The thickness of the joint capsule was measured at two locations: the most distal part of the recess, formed by the joint capsule folding proximally at the femoral attachment, and the distal end of the articular cartilage. For the measurement site of the most superficial layer of the articular cartilage, three locations were randomly selected on the femoral head proximal to the areas covered by the acetabulum or acetabular labrum, and the average of the three locations was recorded. All measurements were repeated twice in 21 sections randomly on different days by a single observer using the same method and the mean values were used for statistical analysis. Intraclass correlation coefficients (ICCs) were calculated to determine the intra‐rater reliability of each measured value.
2.4. Statistical analyses
Statistical comparisons of the thicknesses of three locations (the joint capsule at the recess point, those at the distal end of the articular cartilage, and the most superficial layer of the articular cartilage) in 21 sections were performed using SPSS software (version 27.0; IBM Corp., Armonk, NY, USA). Data are expressed as mean and standard deviation (SD). Levene's test revealed that data showed heterogeneous variance. Dunnett's T3 test was applied to consider whether the joint capsule at the recess point and those at the distal end of the articular cartilage were statistically thicker with the most superficial layer of the articular cartilage as the control group, and each mean differences and its 95% confidence interval (CI) was calculated. Additionally, to perform the subgroup analyses based on sex, the Welch's t‐test was applied to compare the capsular thickness between the males and females at the three locations, and Dunnett's T3 test was also applied to consider whether the joint capsule at the recess point and those at the distal end of the articular cartilage were thicker than the most superficial layer thickness of the articular cartilage for males and females, respectively. The significance level of comparisons was set at 0.05, and the effect size (Cohen's d) was calculated.
ICCs were determined for the intra‐rater reliability of each measured value. A score of >0.75 was considered an excellent agreement (Cicchetti, 1994). The ICCs of the capsular thickness at the recess point, those at the distal end of the articular cartilage, and the most superficial layer thickness of the articular cartilage were 1.00 [95% CI 0.99–1.00], 0.995 [95% CI 0.987–0.998] and 0.917 [95% CI 0.799–0.966], respectively. All ICCs were ≥0.799 (range 0.799–1.000), indicating excellent agreement.
3. RESULTS
In the seven hips, the mean alpha angle was 39.7° (standard deviation [SD], 4.5°; range, 32.8–45.5) at the 12:00 position, 55.2° (SD, 8.3°; range, 42.0–65.4) at the 1:30 position, and 46.6° (SD, 8.3°; range, 31.8–57.3) at the 3:00 position.
Based on the macroscopic sections, the structures superficial to the joint capsule were mainly the gluteus minimus at the 12:00 and 1:30 positions, and the iliopsoas and adipose tissue, which was lateral to the iliopsoas and deep to the rectus femoris, at the 3:00 position (Figures 2, 3, 4). In all anterosuperior regions (12:00, 1:30, and 3:00 positions), the histological analysis confirmed that the joint capsule folded proximally at the femoral attachment while making the recess and blended with the periosteum of the femoral neck. The joint capsule on the femoral neck after folding proximally did not have a distinct two‐layered structure, consisting of synovial and fibrous layers. Instead, it blended with the fibrous region with dense connective tissue running in the longitudinal direction (Figures 2, 3, 4, black arrow heads) and a synovial region with loose connective tissue and adipose cells (Figures 2, 3, 4, red open arrow heads and Figure S1). After extending proximally to the femoral head–neck junction, the joint capsule not only continued with the superficial layer of the articular cartilage but also attached to the articular cartilage via the fibrocartilage (Figures 2c–f, 3c,d and 4c,d). This continuous region was relatively fibrous with dense connective tissue running in the longitudinal direction.
Capsular thickness at the recess point (T1, mean 1.7 [SD 0.9] mm; Figures 2, 3, 4), distal end of the articular cartilage (T2, 0.35 [0.16] mm; Figures 2, 3, 4), and most superficial layer thickness of the articular cartilage (Tms, 0.019 [0.003] mm; Figure S2) are summarized in Table 1. The results of subgroup analyses based on sex are also shown in Table 1. A significant difference in terms of sex was observed only for T2 (male vs female, 0.45 [0.11] mm vs 0.28 [0.16] mm, p = 0.010). With regard to the comparisons among the three locations (T1, T2, and Tms), capsular thicknesses at T1 and T2 were significantly greater than those at the most superficial layer of the articular cartilage (T1 vs Tms, p < 0.001; T2 vs Tms, p < 0.001; Table 2). The subgroup analyses based on sex revealed significant differences in both males and females.
TABLE 1.
Capsular thickness at the femoral head–neck junction.
| T1 | T2 | Tms | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Total | 1.7 | 0.9 | 0.35 | 0.16 | 0.019 | 0.003 |
| Sex | ||||||
| Male | 2.0 | 1.1 | 0.45 | 0.11 | 0.020 | 0.002 |
| Female | 1.6 | 0.8 | 0.28 | 0.16 | 0.019 | 0.004 |
| Clock positions | ||||||
| 12:00 | 2.8 | 0.7 | 0.40 | 0.18 | 0.018 | 0.003 |
| 1:30 | 1.2 | 0.5 | 0.39 | 0.19 | 0.021 | 0.003 |
| 3:00 | 1.2 | 0.5 | 0.27 | 0.10 | 0.019 | 0.002 |
Abbreviations: SD, standard deviation; T1, thickness at the recess point; T2, thickness at the distal end of the articular cartilage; Tms, thickness of the most superficial layer of the articular cartilage.
TABLE 2.
Comparison of the capsular thickness at the femoral head–neck junction.
| Comparisons | Mean difference | p value | Cohen' d |
|---|---|---|---|
| (95% CI) | (95% CI) | ||
| T1 versus Tms | |||
| Total | 1.7 (1.2 to 2.2) | <0.001 | 2.59 (1.75 to 3.41) |
| Male | 2.0 (0.9 to 3.0) | 0.0015 | 2.61 (1.79 to 3.46) |
| Female | 1.5 (0.9 to 2.2) | <0.001 | 2.57 (1.74 to 3.40) |
| T2 versus Tms | |||
| Total | 0.33 (0.24 to 0.43) | <0.001 | 2.93 (2.05 to 3.81) |
| Male | 0.43 (0.32 to 0.54) | <0.001 | 5.51 (4.15 to 6.86) |
| Female | 0.26 (0.14 to 0.39) | <0.001 | 2.35 (1.55 to 3.14) |
Note: Statistical comparisons were performed using Dunnett's T3 test, and the significance level was set at p < 0.05. Unit of mean difference = mm.
Abbreviations: CI, confidence interval; T1, thickness at the recess point; T2, thickness at the distal end of the articular cartilage; Tms, thickness of the most superficial layer of the articular cartilage.
4. DISCUSSION
The present study revealed that the anterosuperior region of the joint capsule not only continued to the superficial layer of the femoral head articular cartilage but also attached to the articular cartilage via the fibrocartilage (Figure 5). The capsular thickness at the recess point (1.7 [0.9] mm) and distal end of the articular cartilage (0.35 [0.16] mm) was significantly greater than the control value for the most superficial layer thickness of the articular cartilage (0.019 [0.003] mm) (both p values, <0.001).
FIGURE 5.
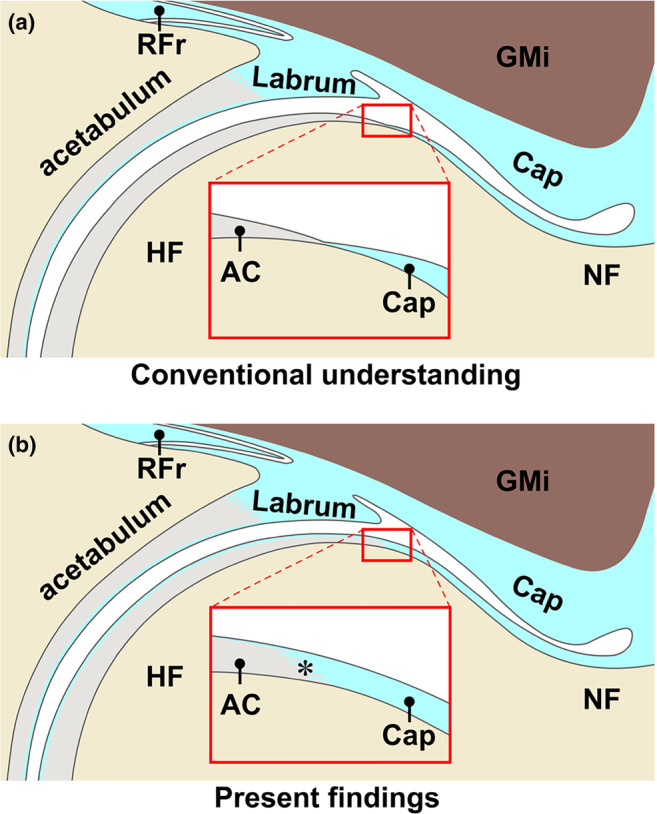
Schematic diagram of the capsular attachment on femoral head–neck junction. Comparison of the conventional understanding (a) and present findings (b) at the 12 o'clock position. The joint capsule (Cap) has generally been reported to end at the distal margin of the articular cartilage (AC) on the femoral head (HF); however, the present study revealed that the anterosuperior cap continued to the superficial layer of the AC and attached to the AC via the fibrocartilage (asterisk). GMi, gluteus minimus; Labrum, acetabular labrum; NF, neck of femur; RFr, reflected head of rectus femoris.
Previous studies have provided evidence of the continuity between the joint capsule and superficial layer of the articular cartilage on the femoral head. This evidence was obtained using polarizing microscopy, scanning electron microscopy, and immunohistochemical collagen analysis (Teshima et al., 1995, 2004). However, these microscopic analyses were unable to elucidate the localization. The present study, using combined micro‐CT and histological analyses, not only confirmed the continuity at the anterosuperior region of the femoral head–neck junction but also revealed that the continuity between the joint capsule and articular cartilage transitioned through the fibrocartilage.
Furthermore, capsular thickness was significantly greater at the distal end of the articular cartilage than at the most superficial layer of the articular cartilage. At the distal end of the articular cartilage, the joint capsule was relatively fibrous with dense connective tissue and maintained its continuity with the articular cartilage. In general, the joint capsule folding from capsular recess is depicted to consist of the synovial layer of the joint capsule (Adams, 2021). However, when the fibrous layer is defined to include dense fibrous tissue and the synovial layer is characterized by more cellular and loose fibrous layers with adipose cells (Fawcett & Bloom, 1986), considering the joint capsule at the continuous region to the articular cartilage as a synovial layer is not appropriate. In addition, most studies have focused on the capsular thickness at the acetabular or trochanter sides attachments or substance regions (Philippon et al., 2014; Telleria et al., 2014; Tsutsumi et al., 2019, 2020; Wagner et al., 2012); however, capsular thickness proximal to the femoral head–neck junction has been rarely analyzed due to the lack of attention given to its presence. At the acetabular side, width of the capsular attachment has been suggested to be related to the adaptation to mechanical stress (Tsutsumi et al., 2019). Therefore, the thickness of the fibrous continuity between the joint capsule and the articular cartilage may be interpreted as the morphology that allows the joint capsule to transmit mechanical stress to the articular cartilage of the femoral head.
Regarding clinical relevance, the present study provides hypothetical insights into the formation of cam morphology. This study identified the continuity between the joint capsule and articular cartilage via both the superficial layer of the articular cartilage and fibrocartilage at frequent sites of cam morphology. Most prospective studies to follow the developmental processes of cam morphology have focused only on the cartilage or bony structures (including the growth plate) and not on the soft tissues, including the joint capsule (Agricola et al., 2014; Hanke et al., 2021; van Klij et al., 2019). One cross‐sectional study suggested that osseous cam morphology is preceded by cartilaginous hypertrophy at the femoral head–neck junction (Palmer et al., 2018). Based on the continuity between the joint capsule and articular cartilage, some mechanical stress, whether due to traction force or impact, can be transmitted from the joint capsule to the articular cartilage at the anterosuperior femoral head–neck junction, a frequent site of cam morphology.
4.1. Limitations
First, this study was purely anatomical; therefore, the formation of the cam morphology could not be proved, and our explanations remain speculative. Future investigations are required to examine the fiber orientation related to the application of mechanical stress across various cross‐sections (e.g., perpendicular to the femoral neck). Additionally, higher magnifications analyses, including those using electron microscopy, will be crucial for a more detailed understanding. Second, the sample size was not determined based on the prior‐power analysis; therefore, this study was suitable to be considered a pilot study. However, a post‐hoc power analysis using G*Power software version 3.1.9.6 based on the capsular thickness values showed that the power was >0.99. Therefore, a larger sample size may not have been necessary for this study. Based on the cam morphology classification (van Klij et al., 2020), the threshold of an alpha angle is ≥60° and two specimens showed cam morphology in this study. Further analysis with a suitable sample size should be performed to compare the specimens with and without cam morphology and to evaluate whether specimens with cam morphology show similar characteristics as observed in the present study. Third, the lack of radiographic evaluation meant that hips with osteoarthritis could not be entirely excluded. Based on the OARSI assessment of the histological section (Pritzker et al., 2006), two hips were OARSI Grade 0, and five hips were Grade 1. Thus, a future study completely excluding the specimens with osteoarthritis changes is warranted. Finally, the individuals from whom the anatomical specimens were obtained were relatively older. A wide range of age specimens should be analyzed to examine the relationship between joint capsule anatomy and the development of cam morphology with age. In addition, cam morphology is reported to develop until growth plate closure around 18 years of age (Agricola et al., 2014; Hanke et al., 2021; van Klij et al., 2019), although analyzing anatomical donors of such age is not practical. With advances in imaging technology, it is anticipated that in vivo imaging of microscale analysis at the same or higher level as that used in this study will be feasible in the future.
5. CONCLUSION
The anterosuperior joint capsule not only continued with the superficial layer of the articular cartilage on the femoral head but also attached to the articular cartilage via the fibrocartilage. The thickness of the fibrous continuity between the joint capsule and articular cartilage was significantly greater than that of the most superficial layer of the articular cartilage. This study identified the anatomical possibility that some mechanical stress can be transmitted from the joint capsule to the articular cartilage at the common sites of cam morphology.
AUTHOR CONTRIBUTIONS
All authors contributed to the conception and design of this study. M.T. and A.N. prepared the materials and performed data collection and analysis. M.T. wrote the first draft of the manuscript, and all authors commented on previous versions of the manuscript. All the authors have read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
None.
Supporting information
Figure S1.
Figure S2.
ACKNOWLEDGMENTS
The authors acknowledge and thank the anonymous individuals who generously donated their bodies for this study, as well as their families. This study was supported by the JSPS KAKENHI (grant number JP 22k17645). Micro‐CT analysis was performed at the Research Core of Tokyo Medical and Dental University (TMDU). The authors also thank Editage (www.editage.com) for the English language editing.
Tsutsumi, M. , Nimura, A. , Utsunomiya, H. , Kudo, S. & Akita, K. (2024) Capsular attachment on the anterosuperior femoral head–neck junction: A hypothesis about femoroacetabular impingement. Journal of Anatomy, 245, 231–239. Available from: 10.1111/joa.14046
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Adams, M.A. (2021) Functional anatomy of the musculoskeltal system. In: Standring, S. (Ed.) Gray's anatomy: the anatomical basis of clinical practice. New York: Elsevier Health Sciences, pp. 85–126. [Google Scholar]
- Agricola, R. , Bessems, J.H. , Ginai, A.Z. , Heijboer, M.P. , van der Heijden, R. , Verhaar, J.A. et al. (2012) The development of cam‐type deformity in adolescent and young male soccer players. American Journal of Sports Medicine, 40(5), 1099–1106. [DOI] [PubMed] [Google Scholar]
- Agricola, R. , Heijboer, M.P. , Ginai, A.Z. , Roels, P. , Zadpoor, A.A. , Verhaar, J.A.N. et al. (2014) A cam deformity is gradually acquired during skeletal maturation in adolescent and young male soccer players: a prospective study with minimum 2‐year follow‐up. American Journal of Sports Medicine, 42(4), 798–806. [DOI] [PubMed] [Google Scholar]
- Bouma, H. , Slot, N.J. , Toogood, P. , Pollard, T. , van Kampen, P. & Hogervorst, T. (2014) Where is the neck? Alpha angle measurement revisited. Acta Orthopaedica, 85(2), 147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casartelli, N.C. , Maffiuletti, N.A. , Valenzuela, P.L. , Grassi, A. , Ferrari, E. , van Buuren, M.M.A. et al. (2021) Is hip morphology a risk factor for developing hip osteoarthritis? A systematic review with meta‐analysis. Osteoarthritis and Cartilage, 29(9), 1252–1264. [DOI] [PubMed] [Google Scholar]
- Cicchetti, D.V. (1994) Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychological Assessment, 6(4), 284–290. [Google Scholar]
- Dijkstra, H.P. , Ardern, C.L. , Serner, A. , Mosler, A.B. , Weir, A. , Roberts, N.W. et al. (2021) Primary cam morphology; bump, burden or bog‐standard? A concept analysis. British Journal of Sports Medicine, 55(21), 1212–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra, H.P. , Mc Auliffe, S. , Ardern, C.L. , Kemp, J.L. , Mosler, A.B. , Price, A. et al. (2022) Oxford consensus on primary cam morphology and femoroacetabular impingement syndrome: part 1‐definitions, terminology, taxonomy and imaging outcomes. British Journal of Sports Medicine, 57(6), 325–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett, D.W. & Bloom, W. (1986) A textbook of histology. Philadelphia: Saunders. [Google Scholar]
- Ganz, R. , Parvizi, J. , Beck, M. , Leunig, M. , Nötzli, H. & Siebenrock, K.A. (2003) Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clinical Orthopaedics and Related Research, 417, 112–120. [DOI] [PubMed] [Google Scholar]
- Griffin, D.R. , Dickenson, E.J. , O'Donnell, J. , Agricola, R. , Awan, T. , Beck, M. et al. (2016) The Warwick agreement on femoroacetabular impingement syndrome (FAI syndrome): an international consensus statement. British Journal of Sports Medicine, 50(19), 1169–1176. [DOI] [PubMed] [Google Scholar]
- Hanke, M.S. , Schmaranzer, F. , Steppacher, S.D. , Reichenbach, S. , Werlen, S.F. & Siebenrock, K.A. (2021) A cam morphology develops in the early phase of the final growth spurt in adolescent ice hockey players: results of a prospective MRI‐based study. Clinical Orthopaedics and Related Research, 479(5), 906–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter, D.J. & Bierma‐Zeinstra, S. (2019) Osteoarthritis. Lancet, 393(10182), 1745–1759. [DOI] [PubMed] [Google Scholar]
- Morris, W.Z. , Li, R.T. , Liu, R.W. , Salata, M.J. & Voos, J.E. (2018) Origin of cam morphology in femoroacetabular impingement. American Journal of Sports Medicine, 46(2), 478–486. [DOI] [PubMed] [Google Scholar]
- Nötzli, H.P. , Wyss, T.F. , Stoecklin, C.H. , Schmid, M.R. , Treiber, K. & Hodler, J. (2002) The contour of the femoral head‐neck junction as a predictor for the risk of anterior impingement. Journal of Bone and Joint Surgery, 84(4), 556–560. [DOI] [PubMed] [Google Scholar]
- Palmer, A. , Fernquest, S. , Gimpel, M. , Birchall, R. , Judge, A. , Broomfield, J. et al. (2018) Physical activity during adolescence and the development of cam morphology: a cross‐sectional cohort study of 210 individuals. British Journal of Sports Medicine, 52(9), 601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlina, W. & Ross, M.H. (2016) Histology: a text and atlas: with correlated cell and molecular biology. Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
- Philippon, M.J. , Michalski, M.P. , Campbell, K.J. , Goldsmith, M.T. , Devitt, B.M. , Wijdicks, C.A. et al. (2014) An anatomical study of the acetabulum with clinical applications to hip arthroscopy. The Journal of Bone and Joint Surgery, 96(20), 1673–1682. [DOI] [PubMed] [Google Scholar]
- Plank, J. & Rychlo, A. (1952) A method for quick decalcification. Zentralblatt Fur Allgemeine Pathologie u. Pathologische Anatomie, 89(8), 252–254. [PubMed] [Google Scholar]
- Pritzker, K.P. , Gay, S. , Jimenez, S.A. , Ostergaard, K. , Pelletier, J.P. , Revell, P.A. et al. (2006) Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis and Cartilage, 14(1), 13–29. [DOI] [PubMed] [Google Scholar]
- Robben, S.G. , Lequin, M.H. , Diepstraten, A.F. , den Hollander, J.C. , Entius, C.A. & Meradji, M. (1999) Anterior joint capsule of the normal hip and in children with transient synovitis: US study with anatomic and histologic correlation. Radiology, 210(2), 499–507. [DOI] [PubMed] [Google Scholar]
- Saberi Hosnijeh, F. , Zuiderwijk, M.E. , Versteeg, M. , Smeele, H.T.W. , Hofman, A. , Uitterlinden, A.G. et al. (2017) Cam deformity and acetabular dysplasia as risk factors for hip osteoarthritis. Arthritis and Rheumatology, 69(1), 86–93. [DOI] [PubMed] [Google Scholar]
- Siebenrock, K.A. , Ferner, F. , Noble, P.C. , Santore, R.F. , Werlen, S. & Mamisch, T.C. (2011) The cam‐type deformity of the proximal femur arises in childhood in response to vigorous sporting activity. Clinical Orthopaedics and Related Research, 469(11), 3229–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telleria, J.J. , Lindsey, D.P. , Giori, N.J. & Safran, M.R. (2014) A quantitative assessment of the insertional footprints of the hip joint capsular ligaments and their spanning fibers for reconstruction. Clinical Anatomy (New York, N.Y.), 27(3), 489–497. [DOI] [PubMed] [Google Scholar]
- Teshima, R. , Ono, M. , Yamashita, Y. , Hirakawa, H. , Nawata, K. & Morio, Y. (2004) Immunohistochemical collagen analysis of the most superficial layer in adult articular cartilage. Journal of Orthopaedic Science, 9(3), 270–273. [DOI] [PubMed] [Google Scholar]
- Teshima, R. , Otsuka, T. , Takasu, N. , Yamagata, N. & Yamamoto, K. (1995) Structure of the most superficial layer of articular cartilage. Journal of Bone and Joint Surgery, 77(3), 460–464. [PubMed] [Google Scholar]
- Tsutsumi, M. , Nimura, A. & Akita, K. (2020) New insight into the iliofemoral ligament based on the anatomical study of the hip joint capsule. Journal of Anatomy, 236(5), 946–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi, M. , Nimura, A. & Akita, K. (2022) Clinical anatomy of the musculoskeletal system in the hip region. Anatomical Science International, 97(2), 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi, M. , Nimura, A. , Honda, E. , Utsunomiya, H. , Uchida, S. & Akita, K. (2019) An anatomical study of the anterosuperior capsular attachment site on the acetabulum. Journal of Bone and Joint Surgery, 101(17), 1554–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Klij, P. , Heijboer, M.P. , Ginai, A.Z. , Verhaar, J.A.N. , Waarsing, J.H. & Agricola, R. (2019) Cam morphology in young male football players mostly develops before proximal femoral growth plate closure: a prospective study with 5‐yearfollow‐up. British Journal of Sports Medicine, 53(9), 532–538. [DOI] [PubMed] [Google Scholar]
- van Klij, P. , Reiman, M.P. , Waarsing, J.H. , Reijman, M. , Bramer, W.M. , Verhaar, J.A.N. et al. (2020) Classifying cam morphology by the alpha angle: a systematic review on threshold values. Orthopaedic Journal of Sports Medicine, 8(8), 2325967120938312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, F.V. , Negrão, J.R. , Campos, J. , Ward, S.R. , Haghighi, P. , Trudell, D.J. et al. (2012) Capsular ligaments of the hip: anatomic, histologic, and positional study in cadaveric specimens with MR arthrography. Radiology, 263(1), 189–198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Figure S2.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


