Abstract
Background
Talaromyces marneffei (TM) is the third most prevalent opportunistic infection in HIV-positive patients after tuberculosis and cryptococcosis. However, such infection of non-HIV individuals has rarely been reported.
Case Presentation
We describe a very rare case of a 52-year-old male who presented with a single space-occupying lesion on the right lung and was eventually diagnosed with pulmonary TM infection. The patient was HIV-negative and had liver cirrhosis with portal vein thrombosis. Lung tissue next-generation sequencing (NGS) revealed TM infection. We successfully treated the patient with voriconazole for 8 weeks and observed lesion absorption via subsequent CT. The patient consumed wild bamboo rats two months before admission. Mutations related to congenital immune deficiency were not detected by whole-exome sequencing.
Conclusion
Early and timely diagnosis is critical for improving patient prognosis. NGS plays a vital role in the diagnosis of pulmonary TM infection in patients. To our knowledge, this is the first published case of pulmonary TM infection in an HIV-negative patient with liver cirrhosis.
Keywords: Talaromyces marneffei, pulmonary infection, HIV-negative, PVT
Background
Talaromyces marneffei (TM) is a significant pathogenic thermally dimorphic fungus that causes systemic mycosis in Southeast Asia and South China.1–3 It was originally isolated in 1956 from a bamboo rat, Rhizomys sinensis, by Capponi et al.4 At present, bamboo rats have been widely reported to be reservoirs of TM.
TM is a highly pathogenic fungus that can cause life-threatening fatal systemic mycosis. After TB and cryptococcosis, TM has been the third most common opportunistic illness in HIV-positive people living in endemic regions. However, in recent years, there has been an increase in the number of TM-infected individuals who do not have HIV.5–7 Herein, we report the case of a 52-year-old HIV-negative Chinese man who presented with a pulmonary lesion and was ultimately diagnosed with TM infection via next-generation sequencing (NGS) at our hospital. Upon timely antifungal therapy, his condition improved.
Case Presentation
A 52-year-old Chinese man was admitted to our hospital on 27 February 2023 because of the discovery of portal vein thrombosis for one year. The patient underwent abdominal ultrasound examination at the local hospital, and the results showed “liver cirrhosis, right portal vein thrombosis (PVT), and slow portal vein flow” in March 2022. Therefore, warfarin anticoagulant treatment was given, but the treatment was irregular. In February 2023, the patient underwent another abdominal ultrasound examination, and the results showed that there was no significant blood flow in the main or right portal vein, the flow velocity of the left portal vein branch decreased, and both the splenic vein and superior mesenteric vein dilated. He did not have the following symptoms: fever, cough, phlegm, chest tightness, shortness of breath, bloating, abdominal pain, diarrhea, black stools, etc. After being diagnosed with chronic hepatitis B in 2008, the patient was consistently taking nucleoside drugs. In 2016, the patient experienced gastrointestinal bleeding due to ruptured esophageal and gastric varices and underwent splenectomy. The patient’s condition remained stable after surgery, and there were no decompensated complications of liver cirrhosis. The patient was born and raised in Anhui, China, and had no recent travel history. The patient did not smoke or drink alcohol. During the 2023 Spring Festival, patients consumed bamboo rats. He was transferred to our hospital to further clarify the condition and treatment of PVT on February 27, 2023.
We performed a physical examination of the patient upon admission. The patient’s vital signs were as follows: temperature, 36.5°C; BP, 120/80 mmHg; heart rate, 83 beats/min; and respiratory rate, 18 breaths/min. Upon physical examination, no superficial lymphadenectasis, yellow skin, icteric sclera, untouched right subcostal liver, untouched spleen, liver palm or spider telangiectasias were observed.
During his admission, he underwent routine blood tests, biochemical tests, urine and fecal laboratory tests, etc. Chest CT scans and enhanced CT venography (CTV) of the hepatic portal vein were also performed. The results of the following laboratory routine examinations are shown in Table 1. His blood was HIV-negative, and he had normal CD4 (532.89 cells/μL) counts. Furthermore, the results showed that this patient was infected with HBV without hepatitis A, C, D, or E. Blood tests related to autoimmune liver disease were negative. As shown in Table 1, aspartate transaminase (AST), gamma-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), and total bilirubin (TBIL) were all slightly elevated. Concurrently, albumin (ALB) and platelet count (PLT) decreased slightly. Moreover, all the other indicators were within the normal range, including procalcitonin (PCT), c-reactive protein (CRP), absolute number of neutrophils (NEUH), galactomannan antigen assay (GM), and (1/3)-b-D-glucan assay (G) (Table 1). The patient’s hepatic function suggested a Child Pugh score of 5 points, which was classified as Child-Pugh class A.
Table 1.
Laboratory Findings at the First Presentation
| ALT | 50 U/L | G | 30.13 pg/mL |
|---|---|---|---|
| AST | 54 U/L ↑ | GM | <0.1000 ug/L |
| GGT | 181 U/L ↑ | IgG | 21.9 g/L |
| ALP | 146 U/L ↑ | HBsAg | 9.45 IU/mL ↑ |
| ALB | 38.9 g/L ↓ | HBeAb | 0.130 S/CO ↓ |
| TBIL | 21.6 umol/L ↑ | HBcAb | 7.580 S/CO↑ |
| Cr | 61.7 umol/L | HBVDNA | <50 |
| INR | 1.13 | HIV-Ab | (-) |
| PT | 14.5 s | HCV-Ab | (-) |
| PCT | 0.03 ng/mL | HAV-IgM | (-) |
| CRP | <0.499 mg/L | HDV-IgM | (-) |
| WBC | 6.66 10^9/L | HEV-IgM | (-) |
| NEUH | 4 10^9/L | T-spot | (-) |
| LYMPH | 1.91 10^9/L | AMA | (-) |
| PLT | 82 10^9/L ↓ | ANA | (-) |
| CD4 | 532.89 cells/ul | AFP | 3.5 ng/mL |
Notes: ↑: reduction; ↓: elevation.
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; GGT, gamma-glutamyl transpeptidase; ALP, alkaline phosphatase; ALB, albumin; TBIL, total bilirubin; Cr, serum creatinine; INR, international normalized ratio; PT, prothrombin time; PCT, procalcitonin; CRP, c-reactive protein; WBC, white blood cell count; NEUH, absolute number of neutrophils; LYMPH, absolute number of lymphocytes; PLT, platelet count; GM assay, galactomannan antigen assay; G assay, (1/3) -b-D-glucan assay; IgG, immunoglobulin g; HBsAg, hepatitis B surface antigen; T-spot, tuberculosis-sport; AMA, antimitochondrial antibody; ANA, antinuclear antibody; AFP, alphafetoprotein.
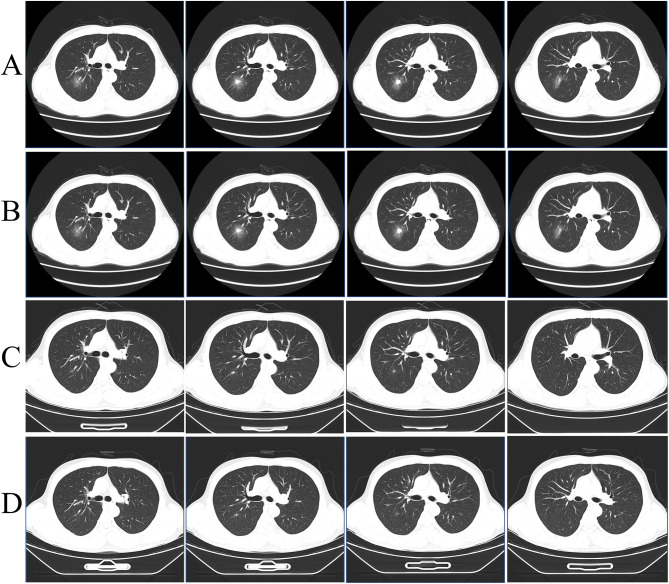
Figure 1 showed the dynamic changes of lung lesions during the patient’s hospitalization, as shown by four chest CT scans. After admission, a chest CT scan revealed a mixed ground glass lesion in the posterior segment of the right upper lobe of the lung, with potential for inflammation or tumor (Figure 1A). He underwent a lung biopsy, and the specimens were sent for pathology review and NGS testing. However, the patient declined bronchoalveolar lavage and lung tissue culture. Biopsy pathology revealed a small focal granuloma with negative periodic-acid-Schiff (PAS) staining and negative Grocott’s methenamine silver (GMS) staining. NGS of the biopsy confirmed lung infection with TM (n = 51 reads) and human gammaherpes virus 4 (n = 17 reads) 2 days later (Figure 2). We further examined the EB virus and found that both the capsid antigen and early antigen IgM were negative. Based on medical history, laboratory tests and radiographic findings, we identified TM lung infection. AMPB was given since March 4 (Figure 3). The dose of AMPB was maintained at 35 mg Qd (0.5 mg/kg/d). After 3 days, the Cr concentration increased to 98 µmol/L. Due to drug-induced renal toxicity, we disabled AMPB and switched to VRC orally (400 mg twice daily, after 24 h, dose-adjusted to 200 mg twice daily) to continue antifungal efficacy. During the next 8 weeks, the trough concentration of voriconazole was monitored (on March 13, 4.72 mg/L; on April 7, 3.16 mg/L), which was within the recommended range (2–5 µg/mL), so the dose was not adjusted. Moreover, 3 chest CT scans, which were performed on March 14, 2023, April 7, 2023, and April 22, 2023, showed that the lung lesion was almost completely absorbed (Figure 1B–D); therefore, VRC was discontinued on April 27, 2023 (Figure 3). Until this article was submitted, there was no recurrence of TM infection. On the other hand, considering that the patient’s HIV test was negative but the total counts of CD4+ did not decrease, we could not rule out congenital immunodeficiency. Blood whole-exome sequencing revealed no mutations related to congenital immune deficiency.
Figure 1.
Chest CT image. (A) A mixed ground glass (4.2*3.7 cm) in the posterior segment of the upper lobe of the right lung on admission (2023-2-28). (B) After effective antifungal therapy, the mixed ground glass opacity (2.5*1.4 cm) in the posterior segment of the upper lobe of the right lung was reduced on March 14, 2023. (C) After effective antifungal therapy, the nodule (11*7 mm) in the posterior segment of the upper lobe of the right lung continued to reduce on April 7, 2023. (D) The nodule (approximately 8 mm) in the posterior segment of the upper lobe of the right lung continued to reduce on April 22, 2023.
Figure 2.
Confirmation of TM specific amplification from lung tissue by NGS. (A) The reads mapped to TM derived from NGS data. A total of 51 reads were mapped to TM in the reference database, which contains approximately 2764 pathogen genomes and has a total coverage of 0.0127%. (B) The distribution of microbe sequences (N = 2764 reads) identified in patient plasma, including TM (N = 51; 1.85%), human gammaherpesvirus 4, Pseudomonas sp.R9.37 and others.
Figure 3.
Timeline of the patient with relevant data on the episodes and interventions.
Abbreviations: PVT portal vein thrombosis, LMWH low molecular weight heparin, TMF amitinofovir, NGS next generation sequencing, TM Talaromyces marneffei, Cr creatinine, AMPB amphotericin B liposomes, VRC voriconazole.
After admission, the CTV of the hepatic portal vein demonstrated liver cirrhosis and partial thrombosis of the right portal vein. An upper endoscopy revealed severe gastric and esophageal varices and portal hypertensive gastropathy (Figure 4). Partial thrombosis of the right portal vein and portal hypertension were ultimately diagnosed. This patient was given carvedilol to decrease portal pressure and low molecular weight heparin (LMWH) for thrombolysis. Telephone follow-up is continuing to clarify the presence or absence of recurrence of TM infection and the thrombolytic efficacy of LMWH.
Figure 4.
Gastroscopy: gastroesophageal varices (severe), portal hypertensive gastropathy.
Systematic Review
Twenty-seven cases of HIV-negative adults with TM infection published from 2016 to 2024 have been reported in the English literature, and case reports of HIV-negative children were excluded. Their characteristics are detailed in Table 2. All patients were HIV negative, with a mean age of approximately 46 years. Eighteen patients were male, and 9 were female. Twenty-five patients were from China, and 2 patients were from southern California, USA. Four patients had a clear travel history to an area of the TM. One patient had a history of pet rat bites. Seven patients had no underlying illnesses, while the remaining patients had underlying diseases.
Table 2.
Summary of HIV-Negative Adults with TM Infection
| Year | References | NO.cases | Age(Years) /Sex | Residence | Underlying Disease | Animal Exposure | Travel history in Epidemic Areas | Infection Site | Antifungal Therapy (Duration) | Outcomes | Immunodeficiency |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2016 | Qiu Y8 | 2 | 64/F | China | LCH | No | No | Lung, skin, bones, lymph node | AMPB-VRC-ITRA (1 year) | Death | NA |
| 57/M | China | No | No | Yes | Lymphadenopathy, skin, lymph node | VRC-AMPB-ITRA (5 months) | Recovered | NA | |||
| 2017 | Zhang J9 | 1 | 45/F | China | Mycobacteria kansasii with papillary thyroid cancer | NA | NA | Lung, skin, thyroid, bones, lymph node | AMPB-ITRA (17months) | Recovered | NA |
| 2018 | Yu X10 | 1 | 41/M | Zhejiang, China | Pulmonary sarcoidosis | No | No | Lung, skin | VRC (12 months) | Recovered | NA |
| 2018 | Zhu YM11 | 1 | 22/M | China | No | NA | NA | Lung, bone marrow, central nervous system, skin | AMPB-ITRA (>1month) | Improved | NA |
| 2019 | Lin F12 | 1 | 50/F | China | LELC | No | No | Lung, lymph node | VRC-ITRA (9.5 months) | Recovered | NA |
| 2020 | Chen Y13 | 1 | 55/M | Zhejiang, China | No | No | No | Lung, skin, bones | AMPB-VRC (6 months) | Recovered | NA |
| 2020 | Zhang W14 | 1 | 34/F | Hangzhou, China | Chronic hepatitis B | No | No | Lung | ITRA (3 months) | Improved | STAT3 mutation |
| 2020 | Chen D15 | 1 | 24/M | China | No | NA | NA | Lymph node, intestinal wall | AMPB-VRC (2 weeks) | Death | NA |
| 2021 | Lin F16 | 2 | 63/M | China | Lung adenocarcinoma | No | No | Lung, skin | VRC-AMPB-ITRA (7 months) | Death | AIGAs (+) |
| 57/M | Guangxi, China | Lung adenocarcinoma | NA | Yes | Lung, lymph node | VRC-AMPB-ITRA (9 months) | Death | AIGAs (+) | |||
| 2021 | Shen Q17 | 1 | 24/M | Zhejiang, China | Bronchopulmonary dysplasia | No | No | Lung | VRC (7 months) | Recovered | TSC2 mutation |
| 2021 | Huang H18 | 1 | 28/F | China | No | Yes | No | Lymph node, lung, skin, bones | AMPB-VRC (28 months) | Improved | NA |
| 2021 | Yang Z19 | 1 | 51/M | Southern China | Diabetes, NSHL | No | NA | Blood, lungs, skin, and subcutaneous tissue | AMPB-VRC-ITRA (7 months) | Death | NA |
| 2021 | Liu W20 | 1 | 59/F | China | Advanced lung adenocarcinoma | No | No | Lung | AMPB-ITRA (4 months) | Recovered | EGFR mutation |
| 2022 | Zhan Y21 | 4 | 21/M | China | No | NA | NA | Lung, lymph nodes, bone | AMPB-VRC | Improved | AIGAs (+) |
| 41/M | China | Nephrotic syndrome and hypertension | NA | NA | Lung | AMPB-VRC | Recovered | NA | |||
| 24/M | China | Hyper IgE syndrome and bronchiectasis | NA | NA | Lung | AMPB-ITRA | Recurrence | STAT3 deficiency | |||
| 62/M | China | Chronic renal failure | NA | NA | Lung, skin | AMPB-VRC | Improved | NA | |||
| 2022 | Chen L22 | 1 | 32/F | Guangdong, China | Chronic hepatitis B | No | Yes | Mediastinal mass | AMPB-ITRA (3.5 months) | Improved | NA |
| 2022 | Qiu Y23 | 1 | 56/M | China | Coronary atherosclerotic heart disease | NA | NA | Lung, lymph node, bones | AMPB+VRC (3 days) | Death | AIGAs (+) |
| 2022 | Cai DH24 | 1 | 51/M | Jiangxi, China | Post renal transplantation | NA | Yes | Lung | VRC (3 Months) | Recovered | NA |
| 2023 | Du R25 | 1 | 30/F | China | No | No | No | Lung, spine | AMPB-ITRA (9 months) | Recovered | AIGAs (+) |
| 2023 | Singh MK26 | 2 | 76/M | Southern California, USA | Squamous cell lung carcinoma | No | No | Lung | AMPB-VRC-ITRA | Symptom improved (progression of pulmonary malignancy) | NA |
| 63/M | Southern California, USA | Multiple sites of poorly differentiated adenocarcinoma | No | No | Lung | ITRA | Symptom improved (died due to complications of his cancer) | NA | |||
| 2023 | Chen Z27 | 1 | 51/F | China | Myasthenia gravis | No | No | Lung | VRC | Recovered | NA |
| 2024 | Yang H28 | 1 | 66/M | Northern China | Hypertension | No | No | Liver, spleen, lymph node, bones | AMPB | Death | NA |
TM Talaromyces marneffei, F female, M male, NA, not available, VRC voriconazole, ITRA, itraconazole, AMPB amphotericin B, LCH Langerhans cell histiocytosis, LELC primary pulmonary lymphoepithelioma-like carcinoma, STAT3 transcription 3, AIGAs anti-interferon-gamma autoantibodies, TSC2 tuberous sclerosis complex subunit 2, NSHL nodular sclerosing Hodgkin lymphoma, EGFR epidermal growth factor receptor, HIES hyper IgE syndrome.
The lesions in 9 patients were only in the lungs, while the remaining 18 patients had multiple organ involvement. Nine patients had clear immune deficiencies, while the remaining 18 patients had not reported any immune issues.
Overall, the mortality rate is approximately 31%. In patients with only lung lesions, there was one recurrence of TM infection, and two other patients had poor prognoses due to their own tumor, while the remaining patients had good prognoses. These patients have a variety of congenital immune deficiency conditions. TM infection was observed in five patients with AIGAs, three of whom eventually died. AIGAs have been shown to underlie severe TM infection in HIV-negative patients. AIGAs may thus be considered a new form of late-onset immunodeficiency conferring a predisposition not only to severe mycobacterial infections but also to some bacterial and fungal infections.29 One patient was diagnosed with a STAT3 mutation, and the other patient had STAT3 hyper-IgE syndrome. STAT3-HIES is most likely a susceptibility factor for TM infections among HIV-negative patients and has a high case fatality rate.30 Either hyperactivation or inactivation of STAT3 results in human disease.31 STAT3 hyper-IgE syndrome comprises a heterogeneous group of immune-sharing manifestations, including increased infection susceptibility.32 Two other genetic defects were detected, including mutations in TSC2 and EGFR. Patients with TSC2 mutations that induce bronchopulmonary dysplasia may be potential hosts for TM.17 Genetically impaired lung cancer might increase the likelihood of developing malignancies, and lung cancer will impair the immune system, but whether this is an important cause of TM remains to be confirmed by future studies.20
Seventeen patients with favorable prognoses received prompt diagnosis and antifungal therapy. The antifungal therapy lasted anywhere from three months to twenty-eight months at its longest. Individuals with a poor outcome typically have many lesions, diffuse TM infection, or an uncertain or delayed diagnosis. These non-HIV-infected patients were frequently misdiagnosed with other illnesses or were not properly identified because of coinfections. Adverse responses to antifungal medications prevented several patients from receiving effective antiviral therapy.
Discussion
In the 1990s, TM infections were documented in an increasing number of HIV-negative patients with poor cell-mediated immunity. TM is a dimorphic fungus with mycelial and yeast forms at 25 and 37°C, respectively.33 The bamboo rat can serve as a significant natural reservoir for TM since inhalation of airborne conidia is believed to be the major infection route. In our study, this patient consumed bamboo rats. TM has the ability to infiltrate several organs, including the lungs, skin, and lymph nodes in HIV-negative people. A 10-year retrospective cohort study showed that HIV-negative patients with TM infection have greater neutrophil counts, more organ damage and greater mortality than HIV-positive patients.7 This patient had only one lung lesion that did not spread to other organs. The infection is more prevalent in Guangxi Province and Guangdong Province in mainland China, and respiratory signs, fever, lymphadenopathy, skin lesions, anemia, and hepatosplenomegaly are the primary clinical manifestations of TM infections in HIV-negative adults.34 In our case, the patient presented no fever, cough, phlegm, chest tightness, or shortness of breath, which suggested that the symptoms were occult.
Accurate and early diagnosis of TM infection is important but challenging. Previously, TM was identified by separating the fungus from clinical samples. The protracted incubation period makes prompt diagnosis challenging. Using NGS, the physician was able to immediately establish the presence of TM within 48 hours after the pathologist was unable to identify the pathogen solely based on microscopic morphology. NGS played a critical role in the diagnosis of TM infection in 10 patients, as shown in Table 2. This case report details how unbiased NGS, in which sequence reads corresponding to TMs are found in tissues, was used to diagnose TM infection in an HIV-negative patient. In this case, the results of routine pathogen detection were negative; there was only one lung lesion, the patient exhibited no symptoms such as fever or cough, and only a small number of NGS sequences were available. Based on the above, we speculate that the disease could still be in the very early stages of infection. However, there were no encouraging findings in the pathological results of the lung tissue. Unfortunately, tissue culture and bronchial lavage were not performed.
HIV-negative patients with TM infection are generally severely immunosuppressed, especially those with solid-organ transplants, bone marrow recipients, or long-term steroid use. As shown in Table 2, HIV-negative individuals with anti-IFN-γ autoantibodies or STAT3 hyper-IgE syndrome typically have relapsing, refractory, and fatal infections, such as TM. Congenital immune deficiency was ruled out for the patient in this case, and he denied long-term use of immunosuppressants. However, the patient had liver cirrhosis. Liver injury and persistent inflammation lead to the development of liver cirrhosis. Long-term inflammation causes the liver to accumulate fibrous tissue and regenerate nodules, which distorts the hepatic vascular system and impairs liver function. Cirrhosis impairs the liver’s capacity to maintain homeostasis and hepatic immunosurveillance, impairing the body’s immune system. Liver cirrhosis is characterized by immune dysfunction, which includes immunodeficiency and systemic inflammation. Cirrhosis-associated immune dysfunction contributes to the high rate of infection in patients with decompensated cirrhosis.35 Patients with cirrhosis are at risk of developing a number of potentially fatal consequences, including infections, regardless of the etiology. Numerous clinical studies have demonstrated that innate and adaptive immune responses, including monocyte/macrophage, dendritic cell, natural killer (NK) cell, and T cell responses, become dysfunctional as a result of chronic HBV infection.36 A complicated interaction between the immune system and the liver is responsible for the frequency and severity of infections that worsen cirrhotic portal hypertension, which leads to a range of systemic immunological disorders affecting both the innate and humoral systems.37 The patient’s immune system was impaired by liver cirrhosis, which may have been a significant factor in the patient’s infection with this fungus even though genetic testing revealed no hereditary immune weakness and no decline in CD4+ T-cell count.
The first-line treatment for TM infection is amphotericin B, while voriconazole and itraconazole are similarly effective. However, the patient developed kidney damage after treatment with amphotericin, and oral voriconazole was administered successively after 8 weeks of cumulative treatment. The final outcomes met our expectations. The blood level of voriconazole must be carefully monitored during the treatment period.
Conclusions
Clinicians should be aware that they may encounter TM infection in HIV-negative patients with liver cirrhosis. However, diagnosis is challenging, and missed diagnosis of TM infection may result in fatal spread. The application of NGS significantly aids in the quick and accurate diagnosis of TM infection.
Acknowledgments
We would like to acknowledge the reviewers for their helpful comments on this paper.
Funding Statement
This work was supported by the internal research fund of Shanghai Public Health Clinical Center (NO. KY- GW-2024-11) and the Clinical Research Plan of SHDC (No. SHDC2020CR1037B China).
Data and Material Availability
All data included in this study are available upon request by contact with the corresponding author.
Ethics Approval and Patient Consent
Written informed consent was obtained from the patient for the publication of the case details. The study was approved by the medical ethics committee of Shanghai Public Health Clinical Center.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, execution, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the final version to be published; and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Vanittanakom N, Cooper CR, Fisher MC, Sirisanthana T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev. 2006;19(1):95–110. doi: 10.1128/CMR.19.1.95-110.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu Y, Zhang J, Li X, et al. Penicillium marneffei infection: an emerging disease in mainland China. Mycopathologia. 2013;175(1–2):57–67. doi: 10.1007/s11046-012-9577-0 [DOI] [PubMed] [Google Scholar]
- 3.Yilmaz N, Visagie CM, Houbraken J, Frisvad JC, Samson RA. Polyphasic taxonomy of the genus Talaromyces. Stud Mycol. 2014;78:175–341. doi: 10.1016/j.simyco.2014.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capponi M, Segretain G, Sureau P. Pénicillose de Rhizomys sinensis [Penicillosis from Rhizomys sinensis]. Bull Soc Pathol Exot Filiales. 1956;49(3):418–421. French. [PubMed] [Google Scholar]
- 5.Wang Y, Mo X, Zhang J, et al. Clinical features of Talaromyces marneffei infection in HIV-positive and HIV-negative individuals: a retrospective study in southern China. Med Mycol. 2023;61(8). doi: 10.1093/mmy/myad083 [DOI] [PubMed] [Google Scholar]
- 6.Qiu Y, Liu AL, Huang J, et al. Comparison of the clinical features of HIV-positive and HIV-negative hosts infected with Talaromyces marneffei: a multicenter, retrospective study. Int J Infect Dis. 2023;132:93–98. doi: 10.1016/j.ijid.2023.04.398 [DOI] [PubMed] [Google Scholar]
- 7.Yu Q, Wei M, Xiao R, et al. Clinical characteristics, course, and long-term outcomes in patients with talaromyces marneffei infection: a 10-year retrospective cohort study. Infect Dis Ther. 2023;12(5):1283–1297. doi: 10.1007/s40121-023-00801-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiu Y, Pan M, Zhang J, et al. Two unusual cases of human immunodeficiency virus-negative patients with talaromyces marneffei infection. Am J Trop Med Hyg. 2016;95(2):426–430. doi: 10.4269/ajtmh.15-0789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J, Huang X, Zhang X, et al. Coinfection of disseminated Talaromyces marneffei and Mycobacteria kansasii in a patient with papillary thyroid cancer: a case report. Medicine. 2017;96(52):e9072. doi: 10.1097/MD.0000000000009072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu X, Miao K, Zhou C, et al. T. marneffei infection complications in an HIV-negative patient with pre-existing pulmonary sarcoidosis: a rare case report. BMC Infect Dis. 2018;18(1):390. doi: 10.1186/s12879-018-3290-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu YM, Ai JW, Xu B, et al. Rapid and precise diagnosis of disseminated T. Marneffei infection assisted by high-throughput sequencing of multifarious specimens in a HIV-negative patient: a case report. BMC Infect Dis. 2018;18(1):379. doi: 10.1186/s12879-018-3276-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin F, Qiu Y, Zeng W, Liang Y, Zhang J. Talaromyces marneffei infection in a lung cancer patient: a rare case report. BMC Infect Dis. 2019;19(1):336. doi: 10.1186/s12879-019-3968-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y. A Talaromyces marneffei infection with osteolytic lesions in an HIV-negative patient at non-endemic areas: a case report. SAGE Open Med Case Rep. 2020;8:2050313x20938242. doi: 10.1177/2050313X20938242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang W, Ye J, Qiu C, et al. Rapid and precise diagnosis of T. marneffei pulmonary infection in a HIV-negative patient with autosomal-dominant STAT3 mutation: a case report. Ther Adv Respir Dis. 2020;14:1753466620929225. doi: 10.1177/1753466620929225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen D, Chang C, Chen M, et al. Unusual disseminated Talaromyces marneffei infection mimicking lymphoma in a non-immunosuppressed patient in East China: a case report and review of the literature. BMC Infect Dis. 2020;20(1):800. doi: 10.1186/s12879-020-05526-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin F, Yang Z, Qiu Y, Zeng W, Liu G, Zhang J. Talaromyces marneffei infection in lung cancer patients with positive AIGAs: a rare case report. Infect Drug Resist. 2021;14:5005–5013. doi: 10.2147/IDR.S340694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen Q, Sheng L, Zhou J. HIV-negative case of Talaromyces marneffei pulmonary infection with a TSC2 mutation. J Int Med Res. 2021;49(5):3000605211016761. doi: 10.1177/03000605211016761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang H, Deng J, Qin C, Zhou J, Duan M. Disseminated Coinfection by Mycobacterium fortuitum and Talaromyces marneffei in a Non-HIV Case. Infect Drug Resist. 2021;14:3619–3625. doi: 10.2147/IDR.S316881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Z, Zeng W, Qiu Y, Liu G, Zhang J. Nodular sclerosing Hodgkin lymphoma combined with disseminated talaromyces marneffei infection: a case report. Infect Drug Resist. 2021;14:5671–5678. doi: 10.2147/IDR.S340192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu W, Xu J, Lin B, et al. Pneumonia caused by Talaromyces marneffei in an epidermal growth factor receptor (EGFR) mutation-positive advanced lung adenocarcinoma patient: a case report. Ann Palliat Med. 2021;10(1):759–766. doi: 10.21037/apm-20-2137 [DOI] [PubMed] [Google Scholar]
- 21.Zhan Y, Lu C, Li S, et al. Successful management of mixed mycosis in HIV-negative patients with different immune status: a case series report. Front Cell Infect Microbiol. 2022;12:851891. doi: 10.3389/fcimb.2022.851891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L, Zhang M, Guo W, et al. Case report: acute Talaromyces marneffei mediastinitis in an HIV-negative patient. Front Microbiol. 2022;13:1045660. doi: 10.3389/fmicb.2022.1045660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu Y, Pan M, Yang Z, et al. Talaromyces marneffei and Mycobacterium tuberculosis co-infection in a patient with high titer anti-interferon-γ autoantibodies: a case report. BMC Infect Dis. 2022;22(1):98. doi: 10.1186/s12879-021-07015-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai DH, Wang J, Fang XL. Successful treatment of Talaromyces marneffei pneumonia in a HIV-negative renal transplantation recipient: a case report. Medicin. 2022;101(40):e30958. doi: 10.1097/MD.0000000000030958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du R, Feng Y, Mao H. Case report: diagnosis of Talaromyces marneffei infection in an HIV-negative patient with septic shock and high-titer anti-interferon gamma autoantibodies by metagenomic next-generation sequencing. Front Cell Infect Microbiol. 2023;13:1163846. doi: 10.3389/fcimb.2023.1163846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh MK, Borson S, Lei V, Molloy R, Weng B, Sutjita M. Rare cases of Talaromyces pneumonia in individuals with underlying cancer and no travel to endemic areas. IDCases. 2023; 33:e01831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Z, Luo XL, Li WQ, Deng ZP. Talaromyces marneffei infection in an HIV-negative patient in a non-endemic area: a case report and literature review. Clin Lab. 2023;69(9). doi: 10.7754/Clin.Lab.2023.230334 [DOI] [PubMed] [Google Scholar]
- 28.Yang H, Liu M, Xu N, et al. Disseminated Talaromyces marneffei infection associated with haemophagocytic syndrome in an HIV-negative patient in northern China: a case report. BMC Infect Dis. 2024;24(1):63. doi: 10.1186/s12879-023-08953-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shih HP, Ding JY, Yeh CF, Chi CY, Ku CL. Anti-interferon-γ autoantibody-associated immunodeficiency. Curr Opin Immunol. 2021;72:206–214. doi: 10.1016/j.coi.2021.05.007 [DOI] [PubMed] [Google Scholar]
- 30.Li Z, Yang J, Qiu Y, et al. Disseminated talaromyces marneffei infection With STAT3-Hyper-IgE syndrome: a case series and literature review. Open Forum Infect Dis. 2023;10(4):ofac614. doi: 10.1093/ofid/ofac614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hillmer EJ, Zhang H, Li HS, Watowich SS. STAT3 signaling in immunity. Cytokine Growth Factor Rev. 2016;31:1–15. doi: 10.1016/j.cytogfr.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsilifis C, Freeman AF, Gennery AR. STAT3 Hyper-IgE syndrome-an update and unanswered questions. J Clin Immunol. 2021;41(5):864–880. doi: 10.1007/s10875-021-01051-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andrianopoulos A. Laboratory maintenance and growth of talaromyces marneffei. Curr Protoc Microbiol. 2020;56(1):e97. doi: 10.1002/cpmc.97 [DOI] [PubMed] [Google Scholar]
- 34.He L, Mei X, Lu S, et al. Talaromyces marneffei infection in non-HIV-infected patients in mainland China. Mycoses. 2021;64(10):1170–1176. doi: 10.1111/myc.13295 [DOI] [PubMed] [Google Scholar]
- 35.Irvine KM, Ratnasekera I, Powell EE, Hume DA. Causes and consequences of innate immune dysfunction in cirrhosis. Front Immunol. 2019;10:293. doi: 10.3389/fimmu.2019.00293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li TY, Yang Y, Zhou G, Tu ZK. Immune suppression in chronic hepatitis B infection associated liver disease: a review. World J Gastroenterol. 2019;25(27):3527–3537. doi: 10.3748/wjg.v25.i27.3527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tranah TH, Edwards LA, Schnabl B, Shawcross DL. Targeting the gut-liver-immune axis to treat cirrhosis. Gut. 2021;70(5):982–994. doi: 10.1136/gutjnl-2020-320786 [DOI] [PubMed] [Google Scholar]