Abstract
Background and Objectives
Stroke attributable to nonoptimal temperature needs more attention with dramatic climate change. The aim of this study was to estimate the global burden and distribution characteristics of the burden.
Methods
In this ecological study, we collected data from the Climate Research Unit Gridded Time Series, the World Bank databases, and the Global Burden of Diseases study to estimate the distribution of burden. We used the joinpoint model, decomposition analysis, age-period-cohort model, panel data analysis, and health inequality analysis to assess the different types of stroke burden attributable to different climatic conditions.
Results
The burden of stroke attributable to nonoptimal temperature continued to grow, and aging was a key factor in this increase. In 2019, 521,031 (95% uncertainty interval [UI] 402,433–663,996) deaths and 9,423,649 (95% UI 7,207,660–12,055,172) disability-adjusted life years [DALYs] attributable to stroke due to nonoptimal temperature were recorded globally. Globally, men (age-standardized mortality rate [ASMR] 7.70, 95% UI 5.80–9.73; age-standardized DALY rate [ASDR] 139.69, 95% UI 102.96–178.54 in 2019) had a heavier burden than women (ASMR 5.89, 95% UI 4.50–7.60; ASDR 96.02, 95% UI 72.62–123.85 in 2019). Central Asia (ASMR 18.12, 95% UI 13.40–24.53; ASDR 327.35, 95% UI 240.24–440.61 in 2019) had the heaviest burden at the regional level. In the national level, North Macedonia (ASMR 32.97, 95% UI 20.57–47.44 in 2019) and Mongolia (ASDR 568.54, 95% UI 242.03–1,031.14 in 2019) had the highest ASMR/ASDR, respectively. Low temperature currently contributes to the main burden (deaths 474,002, 95% UI 355,077–606,537; DALYs 8,357,198, 95% UI 6,186,217–10,801,911 attributable to low temperature vs deaths 48,030, 95% UI 5,630–104,370; DALYs 1,089,329, 95% UI 112,690–2,375,345 attributable to high temperature in 2019). However, the burden due to high temperature has increased rapidly, especially among people aged older than 10 years, and was disproportionately concentrated in low sociodemographic index (SDI) regions such as Africa. In addition, the rapid increase in the stroke burden due to high temperature in Central Asia also requires special attention.
Discussion
This is the first study to assess the global stroke burden attributed to nonoptimal temperature. The dramatic increase in the burden due to high temperature requires special attention, especially in low-SDI countries.
Introduction
Stroke is a common cardiovascular disease, especially in the elderly, and may be accompanied by severe complications and sequelae. According to the Global Burden of Disease study (GBD), there were 12.2 million incident strokes and 6.55 million deaths due to stroke in 2019.1 Stroke ranked 3rd in 2019 among causes of disability-adjusted life years (DALYs).2
Dramatic temperature changes in recent years have seriously affected human health and have caused widespread concern.3 Among them, the correlation between stroke and nonoptimal temperature has received much attention. Previous studies have noted that extreme temperatures increase stroke burden.4,5 This may be because abnormal temperature interferes with some physiologic activities to increase stroke risk. For instance, the body cools itself by sweating and evaporation at high temperatures. This physiologic response may lead to a hypercoagulable state of the blood, which promotes thrombosis and causes stroke.6 Dehydration can activate the sympathetic nervous system, increasing the metabolic demands of the heart, which may lead to a mismatch between supply and demand, triggering an ischemic event and ultimately a stroke attack.7 The cold environment activates the sympathetic nervous system, which promotes vasoconstriction of the body's blood vessels, leading to increased blood pressure and blood viscosity, which may also increase the risk of stroke.8,9
Although some studies have provided information about the relationship between nonoptimal temperature and stroke, fewer studies have assessed the burden and distributional characteristics of stroke due to nonoptimal temperature across different countries and territories, which is the aim of this study.10,11 Data on the stroke burden attributable to nonoptimal temperature were collected from the GBD 2019. In GBD 2019, the disease burden attributable to nonoptimal temperature is the sum of the burdens due to both low and high temperatures. High-temperature and low-temperature associations are associated with associations above and below the theoretical minimum risk exposure level (TMREL).12
In this study, we first estimated the global, regional, and national burden and trends of stroke attributed to nonoptimal temperature and predicted global trends in the future. Then, we explored the driving factors of disease burden variation and the effects of age, period, and cohort on burden. Next, we revealed the effects of national-level indicators on burden. Previous studies revealed that the distribution of the disease burden attributable to nonoptimal temperature may have great heterogeneity by the socioeconomic level.13 To reveal socioeconomic-related health inequalities in the distribution of stroke burden attributable to nonoptimal temperature, that is, the difference in the distribution of burden between people with low and high socioeconomic levels, we further explored the relationship between the sociodemographic index (SDI) and the burden of disease by health inequality analysis. Finally, we provided a comprehensive landscape of the burden attributed to nonoptimal temperature in 3 subtypes of stroke. In general, our study assesses the burden and distributional characteristics of stroke attributable to nonoptimal temperature, which can assist policy makers in developing targeted policies to reduce the disease burden.
Methods
Data Sources
In this ecological study, the number and rate of stroke deaths and DALYs attributable to nonoptimal temperature were extracted from GBD 2019 for different SDI quintiles, regions, countries, and territories; age groups; and sexes from 1990 to 2019.14 Details about GBD 2019 were published elsewhere and in the supplemental methods (eMethods).2 We obtained land temperature information for a 0.5° latitude by 0.5° longitude grid from 1990 to 2019 from the Climate Research Unit Gridded Time Series.15 We then calculated the following metrics for winter (December-January in the Northern Hemisphere and June-August in the Southern Hemisphere) and summer (June-August in the Northern Hemisphere and December-January in the Southern Hemisphere) for the different countries and territories: the monthly mean daily mean temperature (TMP), the monthly mean daily minimum temperature (TMN), and the monthly mean daily maximum temperature (TMX). Data on other national indicators among different countries and territories from 1990 to 2019 were extracted from the World Bank databases. Detailed information on these indicators can be found in the eMethods and the World Bank databases.16
Estimation of the Burden of Nonoptimal Temperature-Related Stroke
A 3-step process was used to estimate the burden of stroke attributable to nonoptimal temperature. (1) Data collection: GBD collaborators obtained temperature estimates for each place from the European Centre for Medium-Range Weather Forecasts and then calculated daily averages of temperatures for use in burden estimation. Individual patient death information was collected from vital registration data sources in the GBD cause of death database. Stroke is a tertiary cause of death associated with nonoptimal temperature. (2) Exposure-response modelling: TMRELs of temperature were estimated as the temperatures with the lowest mortality risk for each location and year. High-temperature exposure refers to exposure to temperatures above the TMREL, and low-temperature exposure refers to exposure to temperatures below the TMREL. Temperature zones are defined by mean annual temperature. The robust meta-regression framework implemented through the MR-Bayesian, Regularized, Trimmed tool was used to estimate cause-specific mortality according to the temperature zone and average daily temperature. (3) Calculation of disease burden indicators: stroke-specific death and DALY data were estimated according to internationally recognized standard methods. Detailed methods are described in previous studies.2,12
Statistical Analysis
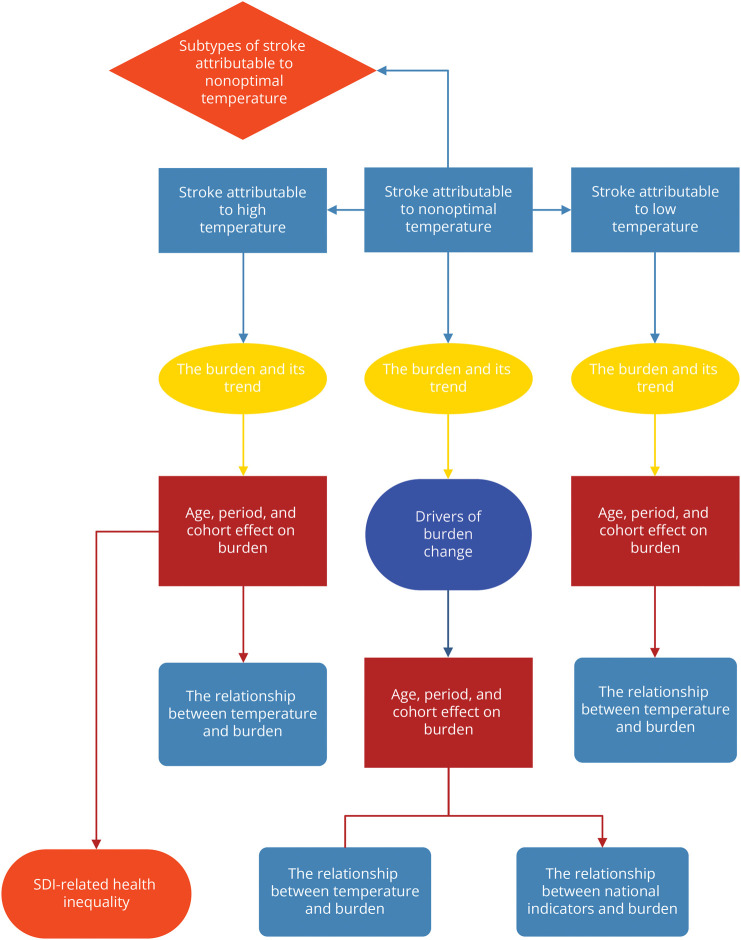
Identifying changes in disease trends can help to analyze stroke mortality and DALY data attributable to nonoptimal temperature. Time trends of disease burden were estimated using joinpoint regression models, a set of statistical linear models. The Bayesian Information Criterion is calculated in the joinpoint regression model to identify change points in trends in the data set. The annual percentage change of each identified trend was then calculated by fitting the regression line to the natural logarithm of the rates using calendar year as the regression variable.17,18 To further predict future trends in the Global Burden of Disease, we applied a Bayesian age-period-cohort (a-p-c) model, which calculates hypothetical probability distributions based on 3 factors—age, period, and cohort—by applying the Bayesian formula and combines prior information with sample data to derive posterior information.19 Then, we used an a-p-c model framework to analyze the underlying trends in burden by age, period, and birth cohort.20,21 The a-p-c model estimated mean percent change per year in age-specific rates, longitudinal age-specific rates (i.e., age effects), and period/cohort effects, expressed as the relative risk of rates by period/cohort. A decomposition analysis with aging, epidemiologic change, and population growth was used to explore the driving factors of stroke burden change from 1990 to 1991–2019. To explore potential national-level indicators (including temperature, demographic indicators, and economic indicators) that may be associated with the burden of stroke attributable to nonoptimal temperature, we conducted a fixed-effect panel data analysis. In addition, we explored SDI-related health inequalities in disease burden. The slope index was used to characterize absolute inequalities. The concentration index was used to characterize relative inequalities. The flowchart of the analysis process is illustrated in Figure 1. All analyses mentioned above were conducted in R (version 4.1.3) and joinpoint software (version 4.9.1). p < 0.05 was considered significant.
Figure 1. Flowchart of Analysis.
SDI = sociodemographic index.
Details of the method described above and the interpretation of other variables appearing in the manuscript are given in the eMethods.
Standard Protocol Approvals, Registrations, and Patient Consents
All data used in this study did not contain identifiable personal or medical information. For GBD 2019, a waiver of informed consent was reviewed and approved by the Institutional Review Board at the University of Washington. All information on ethical standards is available through the official website.22 Therefore, no additional ethical approval was required for this study. Consent to participate was not required for this study.
Data Availability
The data for this study are available on request from the corresponding author.
Results
Stroke Burden Attributed to Nonoptimal Temperature
Burden and Its Trend
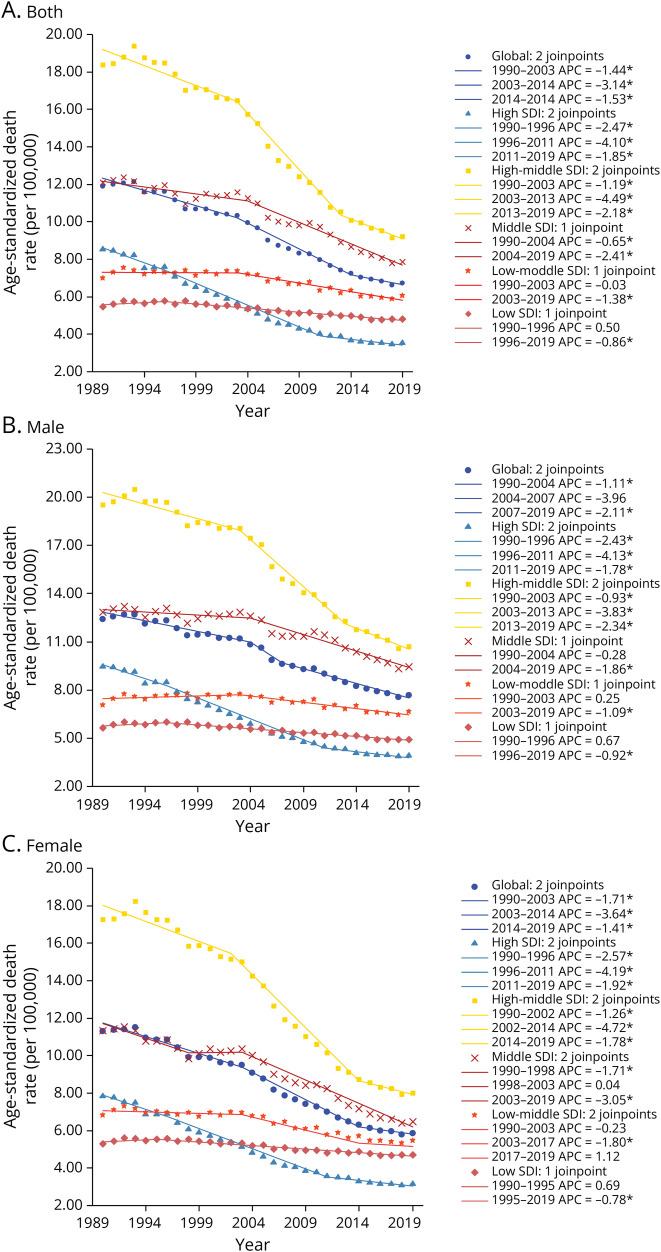
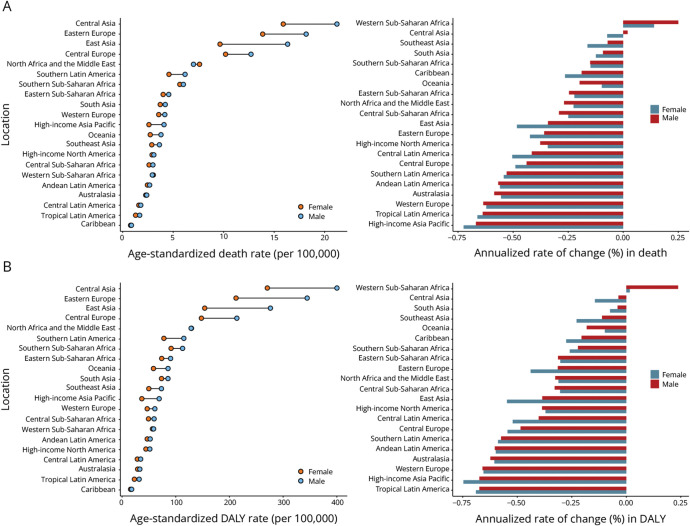
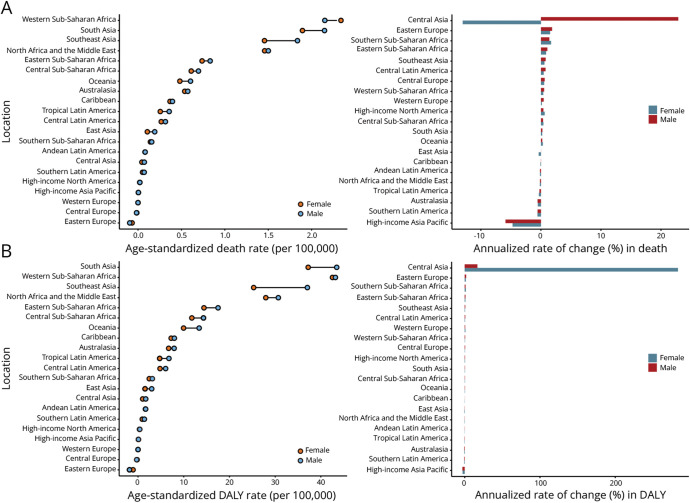
Globally, 521,031 (95% uncertainty interval [UI] 402,433–663,996) deaths and 9,423,649 (95% UI 7,207,660–12,055,172) DALYs attributable to stroke due to nonoptimal temperature were recorded in 2019 (eTable 1). The age-standardized mortality rate (ASMR) and age-standardized DALY rate (ASDR) of stroke attributable to nonoptimal temperature decreased continuously globally (annualized rate of change [ARC] in ASMR −0.43% per year, 95% UI −0.49% to −0.36%; ARC in ASDR −0.45% per year, 95% UI −0.51% to −0.36%) (Figure 2 and eFigure 1). Men (ASMR 7.70, 95% UI 5.80–9.73; ASDR 139.69, 95% UI 102.96–178.54 in 2019) had a heavier burden than women (ASMR 5.89, 95% UI 4.50–7.60; ASDR 96.02, 95% UI 72.62–123.85 in 2019) globally. Similar patterns are shown in our prediction until 2030 (eFigure 2, A and B). Moreover, most disease burdens in places with different SDIs displayed a declining trend. High-middle–SDI countries consistently had the highest burden, with high-SDI countries having the lowest burden in recent years. In 2019, the top 3 stroke burdens in 21 regions occurred in Central Asia (ASMR 18.12, 95% UI 13.4–24.53; ASDR 327.35, 95% UI 240.24–440.61), Eastern Europe (ASMR 15.71, 95% UI 9.34–25.13; ASDR 268.96, 95% UI 159.42–430.71), and East Asia (ASMR 12.48, 95% UI 9.48–15.95; ASDR 210.56, 95% UI 158.19–273.44). From 1990 to 2019, the stroke burden in most regions decreased (Figure 3 and eTable 2). At the national level, the top 3 stroke ASMRs were noted in North Macedonia (32.97, 95% UI 20.57–47.44), Mongolia (27.85, 95% UI 11.82–50.58), and Montenegro (27.85, 95% UI 11.82–50.58), while Mongolia (25.1, 95% UI 17.37–34.97), North Macedonia (475.61, 95% UI 293.57–691.66), and Tajikistan (441.44, 95% UI 235.67–711.17) had the top 3 stroke ASDRs (eFigure 3 and eTable 3).
Figure 2. Age-Standardized Death Rate of Stroke Attributable to Nonoptimal Temperature by SDI Quintiles for Both Sexes (A), Male Population (B), and Female Population (C), 1990–2019.
APC = annual percentage change; SDI = sociodemographic index.
Figure 3. Age-Standardized Death Rate and Its Annualized Rate of Change (A), Age-Standardized DALY Rate and Its Annualized Rate of Change (B) in Stroke Attributable to Nonoptimal Temperature by Region.
DALY = disability-adjusted life year.
Drivers of Burden Change: Aging, Epidemiologic Change, and Population Growth
Both global stroke deaths and DALYs attributable to nonoptimal temperature were larger in 2019 than in 1990. To explore the extent to which the forces of aging, population growth, and epidemiologic change influenced changes in stroke burden attributable to nonoptimal temperature over the 30-year period, we conducted a decomposition analysis of burden change by population, age structure, and age and population standardized rates referred to here as epidemiologic change. The analysis showed that aging and population growth promoted an increase in burden and that epidemiologic change promoted a reduction in burden (eFigures 4A and 5A). Across different SDI quintiles, stroke deaths and DALYs have increased sharply since 1990, except in high-SDI and high-middle–SDI countries. The contribution of aging and population growth to burden changes generally increased over the study period (eFigures 4, B–F and 5, B–F).
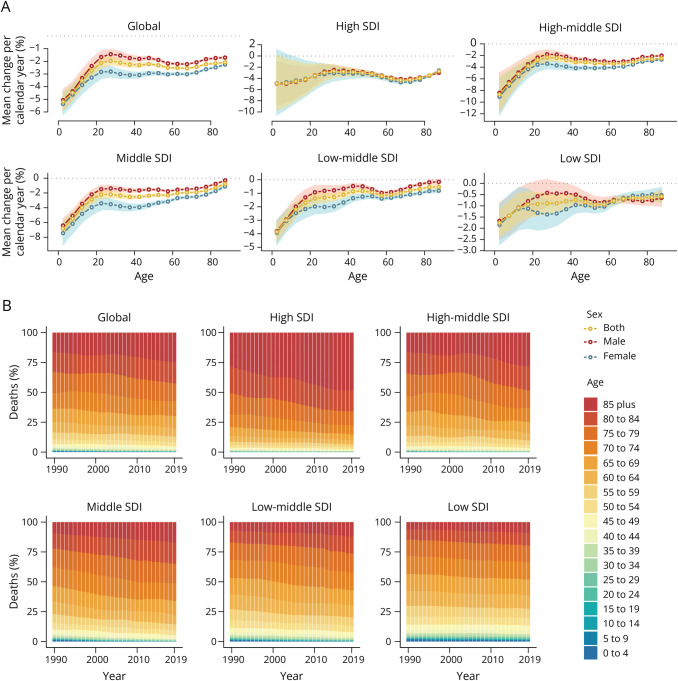
Time Trends for Different Age Groups
For each 5-year age group, the mean percent change per year (MPC) in stroke death and DALY rates are shown in Figure 4A and eFigure 6A. Globally, stroke death and DALY rates attributed to nonoptimal temperature decreased across all age groups (MPCs for all groups <0% per year), and the largest decline occurred in the group with the youngest population (MPC for 0–4 years group: death −5.19% per year, 95% CI −5.87% to −4.51%; DALY −5.22% per year, 95% CI −5.70% to −4.74%). The absolute values of MPC tended to be smaller in the older group than in the younger group, implying that the downward trend in the burden over 30 years was smaller in the older group than in the younger group. The degree of burden decrease was often lower in male than in female population. In addition, the decline in burden across age groups was generally greatest in the high-middle–SDI areas and least in the low-SDI areas. There was an increase in the proportion of stroke deaths and DALYs in the elderly population, especially in high-SDI countries and territories (Figure 4B and eFigure 6B).
Figure 4. Local Drifts of Mortality (A) and Age Distribution of Deaths (B) for Stroke Attributable to Nonoptimal Temperature by SDI Quintiles From 1990 to 2019.
SDI = sociodemographic index.
Individual Age, Period, and Cohort Effect of Stroke Burden
eFigures 7 and 8 present the effects of individual age, cohort, and period on stroke death and DALY rates by a-p-c analysis. Generally, age was a risk factor for disease burden. The burden tended to be greater for men than for women, and the difference in burden between the higher and lower age groups was also generally greater for men than for women, especially in high-SDI and high-middle–SDI areas. For period and cohort effects, declining trends were observed over the study period.
Associations Between the National Level Indicators and Burden
Based on the analysis of the panel data model, for every 1-unit increase in carbon dioxide emission and PM2.5 air pollution, ASMR would increase by 0.15 (95% CI 0.12–0.19, p < 0.001) and 0.16 (95% CI 0.13–0.19, p < 0.001), respectively. A lower ASMR was observed in countries with lower population density (β = −1.32E-03, 95% CI −1.76E-03 to −8.77E-04, p < 0.001), GDP (β = −1.09E-04, 95% CI −1.15E-04 to −1.03E-04, p < 0.001), urban population (β = −0.12, 95% CI −0.14 to −0.11, p < 0.001), current health expenditure per capita (β = −1.09E-03, 95% CI −2.01E-03 to −1.79E-03, p < 0.001), physicians per 1,000 people (β = −0.48, 95% CI −0.62 to −0.34, p < 0.001), and forest area (β = −0.09, 95% CI −0.12 to −0.07, p < 0.001) (eTable 4). A similar relationship between these indicators and ASDR was also observed (eTable 4).
Trends in Stroke Burden Attributed to Low and High Temperatures
Burden and Its Trends
According to the classification of nonoptimal temperature, we further analyzed the stroke burden situation attributable to high and low temperatures. Globally, low temperature (deaths 474,002, 95% UI 355,077–606,537; DALYs 8,357,198, 95% UI 6,186,217–10,801,911) contributed more to stroke deaths and DALYs than high temperature (deaths 48,030, 95% UI 5,630–104,370; DALYs 1,089,329, 95% UI 112,690–2,375,345) in 2019 (eTables 5 and 6).
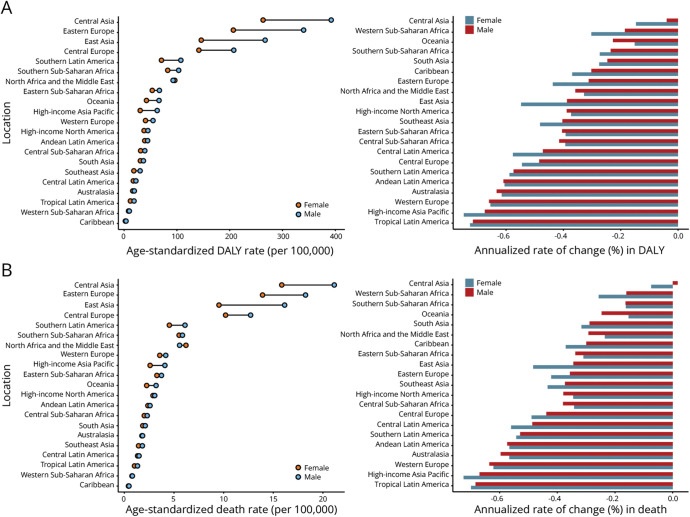
Since 1990, the ASMR and ASDR of stroke attributable to high temperature have increased continuously globally. Across different SDIs, it also generally displayed an increasing trend except in high-middle–SDI countries and territories. Lower SDIs were often accompanied by a higher burden of disease. A greater disease burden occurred in men (eFigures 9 and 10). Notably, in the future, the stroke burden attributable to high temperature may increase sharply (eFigure 2, C and D). In 21 regions, South Asia had the highest stroke burden in 2019 (ASMR 2.02, 95% UI 0.37–3.6; ASDR 40.32, 95% UI 8.06–71.2). From 1990 to 2019, the stroke burden in most regions increased (Figure 5 and eTable 7). Burden in Central Asian showed dramatic increases over 30 years and required special attention. At the national level, Mali had the top stroke ASMRs (7.31, 95% UI 2.22–16.28), while Niger had the top stroke ASDRs (141.34, 95% UI 43.19–303.14) in 2019 (eFigure 11 and eTable 8).
Figure 5. Age-Standardized Death Rate and Its Annualized Rate of Change (A), Age-Standardized DALY Rate and Its Annualized Rate of Change (B) in Stroke Attributable to High Temperature by Region.
DALY = disability-adjusted life year.
The ASMR and ASDR of stroke attributable to low temperature decreased globally. Burden has also continued to decline in all SDI places, except for low-SDI countries. Men had a higher burden of disease than women (eFigures 12 and 13). Moreover, the stroke burden attributed to low temperature may continue to decrease in the future (eFigure 2, E and F). Among the 21 regions, Central Asia stood out as having the highest stroke burden in 2019 (ASMR 18.07, 95% UI 13.08–24.58; ASDR 326.14, 95% UI 235.53–440.11). Since 1990, the burden has declined sharply in almost all regions, with the exception of Central Asia, where it has tended to increase among men (Figure 6 and eTable 9). At the national level, North Macedonia had the top stroke ASMR (833.66, 95% UI 513.58–1,212.00), while Mongolia had the top stroke ASDR (14,275.23, 95% UI 6,087.67–26,191.04) in 2019 (eFigure 14 and eTable 10).
Figure 6. Age-Standardized Death Rate and Its Annualized Rate of Change (A), Age-Standardized DALY Rate and Its Annualized Rate of Change (B) in Stroke Attributable to Low Temperature by Region.
DALY = disability-adjusted life year.
Time Trends of Burden Across Different Age Groups
Globally, the young group (<10 years old) revealed a downward trend in stroke death and DALY rates attributed to high temperature over 30 years, with the opposite being true in the group over approximately 10 years old. MPC exhibited an increasing trend with age before approximately 40 years old, followed by a reduction until approximately 60 years of age. Moreover, across almost all age groups in low-SDI countries and territories, there was an increasing trend of death and DALY rates (eFigures 15A and 16A). Notably, the proportion of stroke deaths and DALYs increased in the young population from high-SDI countries and territories (eFigures 15B and 16B).
Under low temperature, MPC in stroke death and DALY rates for each age group are shown in eFigures 17A and 18A. Globally, stroke death and DALY rates attributed to low temperature decreased across all groups. Notably, there was an increase in the proportion of stroke deaths and DALYs in the elderly population, especially in high-SDI countries and territories (eFigures 17B and 18B).
Individual Age, Period, and Cohort Effect of Stroke Burden
Individual age, cohort, and period effects on stroke death and DALY rates attributable to high temperature are presented in eFigures 19 and 20. Generally, the high-risk paralleled age growth. The period effect indicated an increasing trend except in high-middle–SDI countries and territories. The cohort effect first increased and then fell after approximately 1990. This tendency was more obvious with increasing SDI.
eFigures 21 and 22 show individual age, cohort, and period effects on stroke death and DALY rates attributed to low temperature. In general, older people tend to have a greater risk of stroke. This effect was more severe in men. Period and cohort had declining effects on stroke burden over the study period.
Correlation Between Temperature and Burden
As shown in eFigures 23 and 24, regardless of whether TMN, TMX, or TMP was considered, the lower the temperature was, the higher the burden attributed to low temperature. Higher temperatures paralleled the heavier burden attributed to high temperatures.
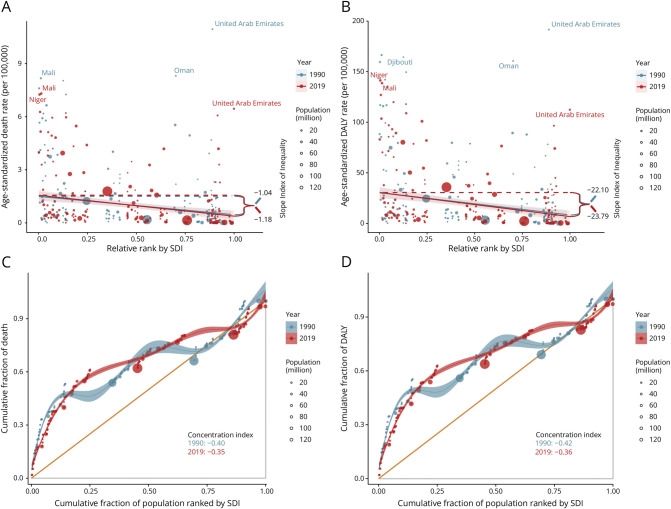
SDI-Related Health Inequality in Stroke Burden Attributed to High Temperature
Although no significant correlation was observed between SDI and stroke burden attributable to nonoptimal or low temperatures (Figure 2 and eFigures 1, 12, 13), a significant negative correlation was found between SDI and burden attributable to high temperature (eFigures 9 and 10). Therefore, a health inequality analysis was conducted to further explore this relationship. As shown in Figure 7, SDI-related absolute health inequalities of the burden attributable to high temperature, as measured by the slope index, were significant and increasingly evident (slope index for 1990 ASMR −1.04, 95% CI −1.35 to −0.78; 1990 ASDR −22.10, 95% CI −27.61 to −18.10; 2019 ASMR −1.18, 95% CI −1.42 to −0.97, and 2019 ASDR −23.79, 95% CI −28.77 to −18.86), showing that countries with higher SDIs bear a higher burden of disease than those with lower SDIs and that this difference has been increasing over time. The concentration index also confirmed the existence of significant relative inequalities, with the burden disproportionately concentrated in low-SDI countries (concentration index for 1990 ASMR −0.40, 95% CI −0.52 to −0.31; 1990 ASDR −0.42, 95% CI −0.55 to −0.35; 2019 ASMR −0.35, 95% CI −0.44 to −0.27; and 2019 ASDR −0.36, 95% CI −0.45 to −0.29).
Figure 7. SDI-Related Health Inequality Regression Lines (A, B) and Concentration Curves (C, D) for the Age-Standardized Death and DALY Rates Due to Stroke Attributable to High Temperature, 1990 and 2019.
DALY = disability-adjusted life year; SDI = sociodemographic index.
Trends of Subtypes of Stroke Burden Attributed to Nonoptimal Temperature
Based on categories in the GBD database, we roughly depicted the proportion of ASMR and ASDR of 3 stroke subtypes, that is, subarachnoid hemorrhage (SAH), ischemic stroke (IS), and intracerebral hemorrhage (IA). IA and IS attributed to nonoptimal temperature accounted for the main stroke deaths and DALYs (2019 death: 40.73% with IA and 53.67% with IS, 2019 DALY: 48.31% with IA and 44.1% with IS). Notably, IA occupied the main part of stroke burden attributed to high temperature (2019 death: 51.26% with IA, 2019 DALY: 58.25% with IA). Compared with 1990, the proportion of IS burden in each temperature subtypes increased obviously (eFigure 25).
Discussion
In this study, we assessed the global, regional, and national burden and distributional characteristics of stroke due to nonoptimal temperature. Although the ASMR and ASDR for stroke burden have declined since 1990, their numbers have continued to rise, and this upward trend is particularly evident in low-SDI countries. Spatially, the burden is mainly concentrated in Eastern Europe, Central Asia, and East Asia. The male and elderly populations have a greater stroke burden. In temperature subtypes, while low temperature currently contributes to the main burden, the burden attributable to high temperature has been rising rapidly, especially among people over 10 years, and will do so in the coming decades. The dramatic increase in ASDR among women in Central Asia requires special attention. In addition, the stroke burden due to high temperature was disproportionately concentrated in low-SDI countries.
The current burden of stroke due to nonoptimal temperature is enormous. The mechanism for the association between nonoptimal temperature and increased risk of stroke may be as follows: low temperature stimulates the sympathetic nervous system, resulting in high pressure.23,24 Cold exposure is also related to thermogenesis and inflammation.25 In addition, high temperature may cause dehydration, elevated blood viscosity, and dyslipidemia.26,27
In recent years, the ASMR and ASDR of stroke burden attributed to nonoptimal temperature decreased globally, which may benefit from advanced medical management and acute therapies.28,29 However, the increasing number of stroke deaths and DALYs attributable to nonoptimal temperature cannot be neglected, especially in low-SDI countries, which may place a heavy burden on the public health system in the future.
Decomposition analyses of changes in the burden showed that aging was an important driver of the increased burden. The age effect exhibited by the a-p-c model also suggests that older people tend to have greater death and DALY rates than younger people. At the same time, while stroke death and DALY rates attributable to nonoptimal temperature have trended downward since 1990 for all age groups, the downward trend has been slightest in the older age groups. A study supported that temperature extremes cause increased mortality risk in the elderly population compared with the young population.30 Physiologic activity changes such as endothelial cell dysfunction and abnormal immune responses attributable to various geriatric diseases result in deterioration of microvascular repair and infiltration of immune cells to increase poor prognosis and death of stroke in elderly patients.31,32 Downward trends in the period effect may be related to hygiene improvement and social development since 1990. Regarding the cohort effect, advanced diagnosis, prevention, and management contribute to the improvement in the disease burden of young cohorts.33-35
Regarding sex, a heavier stroke burden attributed to nonoptimal temperature occurs in men. A Chinese study showed higher stroke mortality in men than in women aged younger than 80 years.36 Another study directly supported that abnormal temperature causes a greater stroke burden in men than in women.37 This phenomenon is associated with different physiologic activities in different sexes. For instance, estrogen-dependent protection was observed in female astrocytes in a stroke model in mice.38 In addition, men sweat more and are at higher risk of electrolyte imbalance, which may lead to a greater risk of stroke in men than in women when temperatures rise.39
The panel data analysis confirms that increased air pollution is closely associated with a rising burden of stroke. PM2.5 triggers oxidative damage in vessels and abnormal blood pressure responses, which may increase the risk of death from stroke.40 Carbon dioxide emissions often mean more industrialization and more emissions of other air pollution, which increases the stroke burden.41 Of interest, a study pointed out that an urban population is a risk factor for stroke.42 Another study emphasized urban characteristics as risk factors for temperature-related mortality associated with the urban heat island effect.43 However, better medical resources and stroke prevention in urban areas decreased stroke burden explained by a previous study.44 Enough forest area can relieve the negative health effects of heat exposure.45,46 Its protective effect on stroke patients was also reported in a previous study.47 High coverage of greenness will decrease the influence of air pollution and levels of sympathetic activation and oxidative stress, which benefits patients with stroke.48
In temperature subtypes, low temperature currently causes the main stroke burden, but the rate gradually attenuated over the study period, similar to the results of a previous study.49 Noticeably, the stroke burden attributed to high temperature dramatically increased and will do so in the future. The process of global warming may be the driver. At the regional levels, we found a sharp upward trend in the ASDR of female population due to high temperature in Central Asia. We hypothesize that this situation may be related to unbalanced medical resources. Compared with men, local women may not receive enough medical treatment, which causes more DALYs.
No significant correlation was observed between the burden of stroke due to low or overall nonoptimal temperatures and SDI. However, the burden of stroke attributable to high temperature was significantly concentrated in areas with a lower SDI, and previous studies have reached similar conclusions.50 This may be related to lower medical levels, less air conditioning, less awareness of health care, and higher poverty rates in lower SDI places. In addition, most low-SDI places are located at low latitudes, increasing heat exposure.51,52
Our study had several limitations. First, due to the features of ecological studies, our study can only illustrate the association between burden and related risk factors. We cannot explore causality at the individual level. Meanwhile, limited by data from the GBD database without relevant risk factors, we cannot explore some classical cofounders affecting our results, such as hypertension and high cholesterol levels. Third, limited by the data source, we were only able to follow the GBD stroke classification (IA, IS, and SAH) to explore stroke subtype burden and were unable to study stroke subtype burden according to other classification criteria, such as Trial of ORG 10172 in Acute Stroke Treatment (TOAST). We next plan to collect epidemiologic data from other sources to assess the burden of various TOAST subtypes of stroke and the relationship between classical stroke risk factors and temperature. Fourth, the data from low-SDI countries may not exactly reflect the disease burden of stroke due to undeveloped public health systems and volatile national situations.53,54
Our study has several advantages. First, we comprehensively assessed the global burden and distribution of stroke attributable to nonoptimal temperature. Second, we not only depicted the effect of total nonoptimal temperature but also compared the burden from temperature subtypes and stroke subtypes, which reminds us that countries with different climates should pay special attention to different stroke subtypes. Third, we confirm the presence of health inequalities in the burden and emphasize that increased attention should be given to underdeveloped countries.
Further exploration of the effect of nonoptimal temperature on stroke in older people and responses to it is essential in the context of increasing aging trends. The burden is high in Central Asia and Eastern Europe, but less research has been performed for these regions, and more attention is needed. More research is also needed for low-SDI countries to determine the precise disease burden and to target solutions to address health inequalities. It is more important that future research should aim to reduce the threat of abnormal temperatures to human health, such as searching for suitable and effective health policies.
Our ecological study provides various pieces of information for clinical practice and public health work on reducing stroke burden attributed to nonoptimal temperature. The burden associated with high temperature has been gradually increasing and will grow sharply in the future. Higher SDI regions such as Central Asia and Eastern Europe need to increase research on strokes attributed to low temperatures, while low-SDI countries urgently need to focus on stroke attributed to high temperature. Increasing vegetation coverage and reducing pollution are important measures that can be concluded in making policy. Special prevention management and treatment guidelines need to be considered for special populations, such as male and elderly individuals.
Acknowledgment
The authors thank GBD collaborators for their work. The authors also thank Dr. Siqi Li (Joint Shantou International Eye Center of Shantou University and the Chinese University of Hong Kong) and Dr. Qingwei Zhang (Renji Hospital) for their help in improving the readability of the text. The authors are grateful to the High Performance Computing Center of Central South University for partial support of this work.
Glossary
- a-p-c
age-period-cohort
- ARC
annualized rate of change
- ASDR
age-standardized DALY rate
- ASMR
age-standardized mortality rate
- DALY
disability-adjusted life year
- GBD
Global Burden of Disease study
- IA
intracerebral hemorrhage
- IS
ischemic stroke
- MPC
mean percent change per year
- SAH
subarachnoid hemorrhage
- SDI
sociodemographic index
- TMN
the monthly mean daily minimum temperature
- TMP
the monthly mean daily mean temperature
- TMX
the monthly mean daily maximum temperature
- TMREL
theoretical minimum risk exposure level
- TOAST
Trial of ORG 10172 in Acute Stroke Treatment
- UI
uncertainty interval
Appendix. Authors
| Name | Location | Contribution |
| Chunrun Qu, BM | Department of Neurosurgery, National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, and XiangYa School of Medicine, Central South University, Changsha, Hunan, China | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data; additional contributions: software, investigation |
| Yu Chen, BM | Department of Neurosurgery, National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, and XiangYa School of Medicine, Central South University, Changsha, Hunan, China | Drafting/revision of the manuscript for content, including medical writing for content; additional contributions: software, investigation; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Chen Liu, BM | XiangYa School of Medicine, Central South University, Changsha, Hunan, China | Drafting/revision of the manuscript for content, including medical writing for content; additional contributions: validation, visualization |
| Zhiwen Hu, BM | XiangYa School of Medicine, Central South University, Changsha, Hunan, China | Drafting/revision of the manuscript for content, including medical writing for content; additional contributions: validation, visualization |
| Jingwei Zhang, MD | Department of Neurosurgery, and National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, Hunan, China | Drafting/revision of the manuscript for content, including medical writing for content; additional contributions: visualization |
| Luzhe Yan, BM | XiangYa School of Medicine, Central South University, Changsha, Hunan, China | Drafting/revision of the manuscript for content, including medical writing for content; additional contributions: visualization, investigation |
| Hao Zhang, MD | Department of Neurosurgery, The Second Affiliated Hospital, Chongqing Medical University, China | Drafting/revision of the manuscript for content, including medical writing for content; additional contributions: visualization, investigation |
| Yifan Liu, BM | XiangYa School of Medicine, Central South University, Changsha, Hunan, China | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design |
| Wanyao Liu | XiangYa School of Medicine, Central South University, Changsha, Hunan, China | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design |
| Quan Cheng, MD | Department of Neurosurgery, and National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, Hunan, China | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; additional contributions: supervision |
| Peng Luo, MD | Department of Oncology, Zhujiang Hospital, Southern Medical University, Guangzhou, Guangdong, China | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; additional contributions: supervision |
| Zhixiong Liu, MD | Department of Neurosurgery, and National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, Hunan, China | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; additional contributions: supervision |
Study Funding
Hunan Youth Science and Technology Talent Project (No. 2023RC3074).
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795-820. doi: 10.1016/S1474-4422(21)00252-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204-1222. doi: 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebi KL, Vanos J, Baldwin JW, et al. Extreme weather and climate change: population health and health system implications. Annu Rev Public Health. 2021;42:293-315. doi: 10.1146/annurev-publhealth-012420-105026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan R, Okada A, Yamana H, et al. Association between ambient temperature and cause-specific cardiovascular disease admissions in Japan: a nationwide study. Environ Res. 2023;225:115610. doi: 10.1016/j.envres.2023.115610 [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Cao Y, Hong D, et al. Ambient temperature and stroke occurrence: a systematic review and meta-analysis. Int J Environ Res Public Health. 2016;13(7):698. doi: 10.3390/ijerph13070698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keatinge WR, Coleshaw SR, Easton JC, Cotter F, Mattock MB, Chelliah R. Increased platelet and red cell counts, blood viscosity, and plasma cholesterol levels during heat stress, and mortality from coronary and cerebral thrombosis. Am J Med. 1986;81(5):795-800. doi: 10.1016/0002-9343(86)90348-7 [DOI] [PubMed] [Google Scholar]
- 7.Peters A, Schneider A. Cardiovascular risks of climate change. Nat Rev Cardiol. 2021;18:1-2. doi: 10.1038/s41569-020-00473-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keatinge WR, Coleshaw SR, Cotter F, Mattock M, Murphy M, Chelliah R. Increases in platelet and red cell counts, blood viscosity, and arterial pressure during mild surface cooling: factors in mortality from coronary and cerebral thrombosis in winter. Br Med J (Clin Res Ed). 1984;289(6456):1405-1408. doi: 10.1136/bmj.289.6456.1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryti NR, Guo Y, Jaakkola JJ. Global association of cold spells and adverse health effects: a systematic review and meta-analysis. Environ Health Perspect. 2016;124(1):12-22. doi: 10.1289/ehp.1408104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi J, Tian F, Ai S, et al. Association between ambient temperature and years of life lost from stroke: 30 PLADs, China, 2013-2016. China CDC Wkly. 2021;3(23):485-489. doi: 10.46234/ccdcw2021.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravljen M, Bajrovic F, Vavpotic D. A time series analysis of the relationship between ambient temperature and ischaemic stroke in the Ljubljana area: immediate, delayed and cumulative effects. BMC Neurol. 2021;21(1):23. doi: 10.1186/s12883-021-02044-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1223-1249. doi: 10.1016/S0140-6736(20)30752-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song J, Pan R, Yi W, et al. Ambient high temperature exposure and global disease burden during 1990-2019: an analysis of the Global Burden of Disease Study 2019. Sci Total Environ. 2021;787:147540. doi: 10.1016/j.scitotenv.2021.147540 [DOI] [PubMed] [Google Scholar]
- 14.Institute for Health Metrics and Evaluation Global Health Data Exchange. GBD Results. Accessed May 7, 2023. ghdx.healthdata.org/gbd-results-tool.
- 15.Science and Technology Facilities Council. The CEDA Archive. Accessed May 3, 2023. archive.ceda.ac.uk/.
- 16.The World Bank. DataBank. Accessed May 10, 2023. databank.Worldbank.org/home.aspx.
- 17.Kim HJ, Chen HS, Midthune D, et al. Data-driven choice of a model selection method in joinpoint regression. J Appl Stat. 2023;50(9):1992-2013. doi: 10.1080/02664763.2022.2063265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Ma B, Han X, Ding S, Li Y. Global, regional, and national burdens of HIV and other sexually transmitted infections in adolescents and young adults aged 10-24 years from 1990 to 2019: a trend analysis based on the Global Burden of Disease Study 2019. Lancet Child Adolesc Health. 2022;6(11):763-776. doi: 10.1016/S2352-4642(22)00219-X [DOI] [PubMed] [Google Scholar]
- 19.Hu W, Fang L, Zhang H, Ni R, Pan G. Global disease burden of COPD from 1990 to 2019 and prediction of future disease burden trend in China. Public Health. 2022;208:89-97. doi: 10.1016/j.puhe.2022.04.015 [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg PS, Anderson WF. Age-period-cohort models in cancer surveillance research: ready for prime time? Cancer Epidemiol Biomarkers Prev. 2011;20(7):1263-1268. doi: 10.1158/1055-9965.EPI-11-0421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberg PS, Check DP, Anderson WF. A web tool for age-period-cohort analysis of cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2296-2302. doi: 10.1158/1055-9965.EPI-14-0300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.healthdata.org/gbd/2019.
- 23.Park S, Kario K, Chia YC, et al. The influence of the ambient temperature on blood pressure and how it will affect the epidemiology of hypertension in Asia. J Clin Hypertens (Greenwich). 2020;22(3):438-444. doi: 10.1111/jch.13762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hintsala H, Kentta TV, Tulppo M, et al. Cardiac repolarization and autonomic regulation during short-term cold exposure in hypertensive men: an experimental study. PLoS One. 2014;9(7):e99973. doi: 10.1371/journal.pone.0099973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai J, Meng X, Wang C, et al. The cold effects on circulatory inflammation, thrombosis and vasoconstriction in type 2 diabetic patients. Sci Total Environ. 2016;568:271-277. doi: 10.1016/j.scitotenv.2016.06.030 [DOI] [PubMed] [Google Scholar]
- 26.Nayha S. Environmental temperature and mortality. Int J Circumpolar Health. 2005;64(5):451-458. doi: 10.3402/ijch.v64i5.18026 [DOI] [PubMed] [Google Scholar]
- 27.Halonen JI, Zanobetti A, Sparrow D, Vokonas PS, Schwartz J. Outdoor temperature is associated with serum HDL and LDL. Environ Res. 2011;111(2):281-287. doi: 10.1016/j.envres.2010.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wardlaw JM, Murray V, Berge E, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet. 2012;379(9834):2364-2372. doi: 10.1016/S0140-6736(12)60738-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell BCV, Mitchell PJ, Churilov L, et al. Endovascular thrombectomy for ischemic stroke increases disability-free survival, quality of life, and life expectancy and reduces cost. Front Neurol. 2017;8:657. doi: 10.3389/fneur.2017.00657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scovronick N, Sera F, Acquaotta F, et al. The association between ambient temperature and mortality in South Africa: a time-series analysis. Environ Res. 2018;161:229-235. doi: 10.1016/j.envres.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pacinella G, Ciaccio AM, Tuttolomondo A. Endothelial dysfunction and chronic inflammation: the cornerstones of vascular alterations in age-related diseases. Int J Mol Sci. 2022;23(24):15722. doi: 10.3390/ijms232415722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuohy MC, Hillman EMC, Marshall R, Agalliu D. The age-dependent immune response to ischemic stroke. Curr Opin Neurobiol. 2023;78:102670. doi: 10.1016/j.conb.2022.102670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boodt N, Compagne KCJ, Dutra BG, et al. Stroke etiology and thrombus computed tomography characteristics in patients with acute ischemic stroke: a MR CLEAN registry substudy. Stroke. 2020;51(6):1727-1735. doi: 10.1161/STROKEAHA.119.027749 [DOI] [PubMed] [Google Scholar]
- 34.Bosch J, Lonn EM, Dagenais GR, et al. Antihypertensives and statin therapy for primary stroke prevention: a secondary analysis of the HOPE-3 trial. Stroke. 2021;52(8):2494-2501. doi: 10.1161/STROKEAHA.120.030790 [DOI] [PubMed] [Google Scholar]
- 35.van der Steen W, van de Graaf RA, Chalos V, et al. Safety and efficacy of aspirin, unfractionated heparin, both, or neither during endovascular stroke treatment (MR CLEAN-MED): an open-label, multicentre, randomised controlled trial. Lancet. 2022;399(10329):1059-1069. doi: 10.1016/S0140-6736(22)00014-9 [DOI] [PubMed] [Google Scholar]
- 36.Wang W, Jiang B, Sun H, et al. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults. Circulation. 2017;135(8):759-771. doi: 10.1161/CIRCULATIONAHA.116.025250 [DOI] [PubMed] [Google Scholar]
- 37.Polcaro-Pichet S, Kosatsky T, Potter BJ, Bilodeau-Bertrand M, Auger N. Effects of cold temperature and snowfall on stroke mortality: a case-crossover analysis. Environ Int. 2019;126:89-95. doi: 10.1016/j.envint.2019.02.031 [DOI] [PubMed] [Google Scholar]
- 38.Roy-O'Reilly M, McCullough LD. Age and sex are critical factors in ischemic stroke pathology. Endocrinology. 2018;159(8):3120-3131. doi: 10.1210/en.2018-00465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vodonos A, Novack V, Horev A, Abu Salameh I, Lotan Y, Ifergane G. Do gender and season modify the triggering effect of ambient temperature on ischemic stroke? Womens Health Issues. 2017;27(2):245-251. doi: 10.1016/j.whi.2016.11.002 [DOI] [PubMed] [Google Scholar]
- 40.Munzel T, Gori T, Al-Kindi S, et al. Effects of gaseous and solid constituents of air pollution on endothelial function. Eur Heart J. 2018;39(38):3543-3550. doi: 10.1093/eurheartj/ehy481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tran PM, Warren JL, Leifheit EC, Goldstein LB, Lichtman JH. Associations between long-term air pollutant exposure and 30-day all-cause hospital readmissions in US patients with stroke. Stroke. 2023;54(4):e126-e129. doi: 10.1161/STROKEAHA.122.042265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X, Dai J, Li W, Yang Y. High-risk population and factors of stroke has changed among middle-aged and elderly Chinese-Evidence from 1989 to 2015. Front Public Health. 2023;11:1090298. doi: 10.3389/fpubh.2023.1090298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sera F, Armstrong B, Tobias A, et al. How urban characteristics affect vulnerability to heat and cold: a multi-country analysis. Int J Epidemiol. 2019;48(4):1101-1112. doi: 10.1093/ije/dyz008 [DOI] [PubMed] [Google Scholar]
- 44.Wang Z, Liu W, Ren Y, et al. Loss of life expectancy due to stroke and its subtypes in urban and rural areas in China, 2005-2020. Stroke Vasc Neurol. 2023;8(5):349-357. doi: 10.1136/svn-2022-001968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kenney WL, Craighead DH, Alexander LM. Heat waves, aging, and human cardiovascular health. Med Sci Sports Exerc. 2014;46(10):1891-1899. doi: 10.1249/MSS.0000000000000325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hasan S, Choi W, Kang S. Associations of urban and green land covers and heat waves in 49 U.S. cities between 1992 and 2019. Int J Environ Res Public Health. 2022;19(13):7688. doi: 10.3390/ijerph19137688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He F, Wei J, Dong Y, et al. Associations of ambient temperature with mortality for ischemic and hemorrhagic stroke and the modification effects of greenness in Shandong Province, China. Sci Total Environ. 2022;851(pt 1):158046. doi: 10.1016/j.scitotenv.2022.158046 [DOI] [PubMed] [Google Scholar]
- 48.Yeager R, Riggs DW, DeJarnett N, et al. Association between residential greenness and cardiovascular disease risk. J Am Heart Assoc. 2018;7(24):e009117. doi: 10.1161/JAHA.118.009117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao Q, Guo Y, Ye T, et al. Global, regional, and national burden of mortality associated with non-optimal ambient temperatures from 2000 to 2019: a three-stage modelling study. Lancet Planet Health. 2021;5(7):e415-e425. doi: 10.1016/S2542-5196(21)00081-4 [DOI] [PubMed] [Google Scholar]
- 50.Bo Y, Zhu Y, Lu R, et al. Burden of stroke attributable to high ambient temperature from 1990 to 2019: a global analysis. Int J Stroke. 2023;18(9):1121-1131. doi: 10.1177/17474930231183858 [DOI] [PubMed] [Google Scholar]
- 51.Ramin BM, McMichael AJ. Climate change and health in sub-Saharan Africa: a case-based perspective. Ecohealth. 2009;6(1):52-57. doi: 10.1007/s10393-009-0222-4 [DOI] [PubMed] [Google Scholar]
- 52.Watts N, Amann M, Arnell N, et al. The 2020 report of The Lancet Countdown on health and climate change: responding to converging crises. Lancet. 2021;397(10269):129-170. doi: 10.1016/S0140-6736(20)32290-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Global Burden of Disease Health Financing Collaborator Network. Future and potential spending on health 2015-40: development assistance for health, and government, prepaid private, and out-of-pocket health spending in 184 countries. Lancet. 2017;389(10083):2005-2030. doi: 10.1016/S0140-6736(17)30873-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Global Burden of Disease Health Financing Collaborator Network. Past, present, and future of global health financing: a review of development assistance, government, out-of-pocket, and other private spending on health for 195 countries, 1995-2050. Lancet. 2019;393(10187):2233-2260. doi: 10.1016/S0140-6736(19)30841-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data for this study are available on request from the corresponding author.