Abstract
Introduction:
Mammarenaviruses are negative-sense bisegmented enveloped RNA viruses that are endemic in Africa, the Americas, and Europe. Several are highly virulent, causing acute human diseases associated with high case fatality rates, and are considered to be significant with respect to public health impact or bioterrorism threat.
Areas covered:
This review summarizes the status quo of treatment development, starting with drugs that are in advanced stages of evaluation in early clinical trials, followed by promising candidate medical countermeasures emerging from bench analyses and investigational animal research.
Expert opinion:
Specific therapeutic treatments for diseases caused by mammarenaviruses remain limited to the off-label use of ribavirin and transfusion of convalescent sera. Progress to identify novel candidate medical countermeasures against mammarenavirus infection has been slow in part because of the biosafety and biosecurity requirements. However, novel methodologies and tools have enabled increasingly efficient high-throughput molecular screens of regulatory-agency-approved small-molecule drugs and led to identification of several compounds that could be repurposed for treatment of infection with several mammarenaviruses. Unfortunately, most of them have not yet been evaluated in vivo. The most promising treatment under development is a monoclonal antibody cocktail that is protective against multiple lineages of Lassa virus in nonhuman primate disease models.
Keywords: Arenaviridae, arenavirus, drug, high-throughput, Lassa, mammarenavirus, medical countermeasure, repurposing, small molecule, treatment
1. Introduction
Arenaviridae, a family of order Bunyavirales, comprises a wide variety of segmented ambisense animal RNA viruses that produce enveloped spherical to pleomorphic (monopartite) particles [1]. Arenavirids are classified into five genera: Antennavirus, Hartmanivirus, Innmovirus, Mammarenavirus, and Reptarenavirus [2]. Antennaviruses have been discovered via metagenomic sequencing in actinopterygian fish [3–5]; hartmaniviruses and reptarenaviruses were discovered and isolated from captive and wild healthy and diseased boid and pythonid snakes [6–11]; and inmoviruses were discovered in river sediment samples [12] and are likely of actinopterygian and chondrichthyan fish origin [5, 13, 14]. Mammarenaviruses subclinically infect mammals, in particular phyllostomid bats [15–17] and possibly some of their ixodid ectoparasites [18], eulipotyphlans [19], lagomorphs [20, 21], and dipodid and muroid rodents [22–24]. Additional unclassified mammal viruses populate a sixth genus-rank clade that awaits taxonomic placement [15].
Nine arenavirids are known to be pathogenic for humans (Table 1). All human-infecting arenavirids are muroid-borne mammarenaviruses that are maintained in cricetid rodents (in South America) or in murid rodents (in Africa) in areas of disease endemicity. Zoonotic transmission of these viruses primarily occurs through contact with contaminated rodent excreta or secreta (e.g., inhaled aerosolized dried urine and feces particles produced by sweeping or consumption of food contaminated with rodent excrements) and infected rodents or their tissues (e.g., emptying of traps, aerosolization of whole rodents in harvest machinery, and preparation of rodents as food) [25, 26]. Thus, the natural geographic distribution, migration patterns, and population size of mammarenavirus-specific host rodents determine the endemicity of mammarenavirus diseases [25–28]. Person-to-person transmission is rare but may occur through contact with bodily secretions of infected individual or fomites, in particular in health care settings (e.g., aerosolization of bodily secretions during cardiopulmonary resuscitation, intubation, or sample centrifugation) [29, 30]. Sexual transmission of mammarenaviruses has not yet been proven but is not unlikely at least as an occasional occurrence [29, 31–33].
Table 1.
Mammarenaviruses pathogenic for humans (updated and modified from [208])
| Virus (abbreviation) [2] | Endemicity [25, 26, 30, 209] | Natural reservoir host(s) [25, 26, 30, 209] | Human disease name (abbreviation; ICD-11 code [210]) | Significance [48, 211–215] |
|---|---|---|---|---|
| South American-clade mammarenaviruses (“Tacaribe serocomplex”) | ||||
| Chapare virus (CHAPV) | Bolivia | Unidentified cricetids | Other specified arenavirus disease (1D61.Y), informally also “Chapare hemorrhagic fever (CHHF)” | Biosafety:
|
| Flexal virus (FLEV) | Brazil | Unidentified cricetids | Other specified arenavirus disease (1D61.Y) | Biosafety:
|
| Guanarito virus (GTOV) | Venezuela | Predominantly cricetid short-tailed zygodonts (Zygodontomys brevicauda J.A. Allen & Chapman, 1893) but also cricetid Alston’s cotton rats (Sigmodon alstoni Thomas, 1881) | Venezuelan hemorrhagic fever (VeHF; 1D61.3) | Biosafety:
|
| Junín virus (JUNV) | Argentina | Predominantly cricetid drylands lauchas (Calomys musculinus (Thomas, 1913)) but also cricetid Azara’s akodonts (Akodon azarae J. Fischer, 1829) little lauchas (Calomys laucha Fischer, 1814) | Argentinian hemorrhagic fever (AHF; 1D61.0) | Biosafety:
|
| Machupo virus (MACV) | Bolivia | Cricetid big lauchas (Calomys callosus Rengger, 1830) | Bolivian hemorrhagic fever (BHF; 1D61.1) | Biosafety:
|
| Sabiá virus (SBAV) | Brazil | Unidentified cricetids | Other specified arenavirus disease (1D61.Y), informally also “Brazilian hemorrhagic fever (BzHF)” | Biosafety:
|
| African-clade mammarenaviruses (“Lassa–lymphocytic choriomeningitis serocomplex”) | ||||
| Lassa virus (LASV) | Western Africa (Benin, Burkina Faso, Côte d’Ivoire, Guinea, Ghana, Liberia, Mali, Nigeria, Sierra Leone, Togo) | Predominanly murid Natal mastomys (Mastomys natalensis Smith, 1834) but also reddish-white mastomys (Mastomys erythroleucus (Temminck, 1853)), African hylomuscus (Hylomyscus pamfi Nicolas, Olayemi, Wendelen & Colyn, 2010), and Baoule’s mice (Mus baoulei (Vermeiren & Verheyen, 1980)) | Lassa fever (LF; 1D61.2) | Biosafety:
|
| Lujo virus (LUJV) | Zambia | unknown | Other specified arenavirus disease (1D61.Y), informally also “Lujo hemorrhagic fever” | Biosafety:
|
| lymphocytic choriomeningitis virus (LCMV) | worldwide | Predominantly murid house mice (Mus musculus Linnaeus, 1758), but also murid long-tailed field mice (Apodemus sylvaticus (Linnaeus, 1758)), soft-furred mice (Praomys spp.), and golden hamsters (Mesocricetus auratus Waterhouse, 1839) | Lymphocytic choriomeningitis (LCM; 1C8F), informally also “aseptic meningitis” | Biosafety:
|
BSL-4, biosafety level 4; CDC, Centers for Disease Control and Prevention; ICD-11, International Classification of Diseases 11th Revision; NIAID, National Institute of Allergy and Infectious Diseases; R&D, research and development; WHO, World Health Organization
As Table 1 clarifies, two of the human-pathogenic mammarenaviruses, lymphocytic choriomeningitis virus (LCMV) and Flexal virus (FLEV), are neither considered Select Agents nor Priority Pathogens and hence will not be discussed here. Of the remaining seven viruses, some are relatively rarely encountered. Chapare virus (CHAPV) [29, 34], Guanarito virus (GTOV) [35, 36], Lujo virus (LUJV) [30], and Sabiá virus (SBAV) [37–39] have caused very limited outbreaks, with one individual to dozens of cases. However, Junín virus (JUNV) [40, 41] (several hundred thousand infections over the last 60 years) and Machupo virus (MACV) [42–45] (>1,000 cases) have caused more extensive focal outbreaks with extremely high case fatality rates (sometimes >50%). Based on the low number of recorded cases but high virulence, these six viruses are primarily of bioterrorism/biowarfare concern. However, more clearly transmissible Lassa virus (LASV) causes hundreds of thousands of often subclinical infections annually over broad geographic areas, including a significant burden of disease associated with high case fatality, and is therefore primarily of public health concern [46–48].
1.1. Mammarenaviruses other than Lassa virus
The clinical presentation of human CHAPV [29, 34, 49], GTOV [35, 50, 51], JUNV [52–59], LUJV [60], MACV [42, 44, 61], and SBAV [38, 62] infections is similar: After an incubation period of approximately 1–2 weeks, patients develop influenza-like symptoms/signs, such as fever, headaches, myalgia and general malaise, and anorexia. A few days later, increasingly severe clinical manifestations involving multiple organ systems appear; in particular, early symptoms/signs are associated with the gastrointestinal (nausea with vomiting, constipation, or mild diarrhea), central nervous (photophobia with retro-orbital pain, disorientation), or coagulation (conjunctival injection, petechiae, or flushing of the head and upper torso) systems. In the second week after system onset, 20‒30% of patients develop severe neurologic signs (convulsions, seizures, tremors, delirium, coma) and hemorrhagic signs (mucosal hemorrhages ecchymoses) that are associated with fatal outcomes. Survivors convalesce over several weeks, often reporting sequelae (such as, alopecia, dizziness, fatigue, and hearing loss) that are generally poorly characterized [63–65].
1.2. Lassa virus
In contrast to CHAPV, GTOV, JUNV, LUJV, MACV, and SBAV infections, LASV causes predominantly asymptomatic infection or mild febrile disease. However, approximately 20% of patients develop acute disease that is generally reminiscent of the diseases caused by the other pathogenic mammarenaviruses; after a 1‒3-week incubation period, patients develop sudden and progressive fever and general malaise and then, within a few days, a wide array of clinical manifestations, including arthralgia/myalgia, headache (sometimes with dizziness or tinnitus), and gastrointestinal involvement (abdominal pain, nausea, diarrhea, and/or constipation). However, the clinical presentation also variably includes cardiac (chest pain, tachycardia), hepatic (hepatomegaly with jaundice), lymphatic (lymphadenopathy, splenomegaly), ocular (conjunctivitis), respiratory (cough, pharyngitis, rales, rhonchi, wheezing), and other manifestations. Patients either recover or develop progressive multi-system dysfunction, including acute kidney injury, pulmonary (pneumonia, pleural effusion, acute lung injury), cardiac (pericardial effusion, myocarditis), or hematologic dysfunction, hemorrhagic signs (ecchymoses, epistaxis, hematemesis, hematuria, hemoptysis, melena, petechia), and/or neurologic manifestations (confusion, convulsions, encephalopathy, tremors, coma) that often precede shock and death. LASV survivors may recover, sometimes over long periods of time, without sequelae, but approximately 13–30% of them suffer from permanent unilateral or bilateral sensorineural deafness, alopecia, and/or pericarditis; in general, post-acute clinical sequelae are poorly characterized. [66–74].
2. Mammarenavirus biology
2.1. Virions and genomes
Mammarenaviruses produce enveloped pleomorphic (50–200) virions containing evolutionary negative-sense (functionally ambisense) bisegmented RNA genomes, totaling about 10.5 kb. The virion surface contains club-shaped peplomers that are 8–10 nm in length and consist of clearly distinguishable head (GP1) and stalk (GP2) domains. The two internal ribonucleoprotein (RNP) complexes consist of the small (S) and large (L) genomic RNA segments that are independently encapsidated by nucleoproteins (NPs; 60–68 kDa) [75–77].
Both segments (which may be present in virions in multiple copies) each encode two structural proteins from open reading frames (ORFs) arranged in ambisense orientation, separated by highly structured intergenic regions (IGRs), and flanked by termini that include inverted complementary sequences containing highly conserved transcription and replication initiation signals. The complementary sequences force the segments into not covalently closed panhandle-like structures [78–80].
2.2. Proteins
The S genomic fragment encodes NP and the glycoprotein complex (GP) precursor (GPC; 70–80 kDa, whereas the L segment encodes a bunyaviral-typical L protein (250–450 kDa), including an RNA-directed RNA polymerase (RdRp) domain and a zinc-binding protein (Z; 10–14 kDa). NP, the most abundant mammarenavirus protein in infected cells, oligomerizes and encapsidates both genomic and antigenomic segments, functions as an exoribonuclease, and is an interferon antagonist [75, 81–83]. GP is heterotrimeric and consists of a stable signal peptide (SSP), a receptor-binding subunit (GP1), and a class I fusion membrane fusion subunit (GP2)—all generated via proteolytic cleavage of GPC. GP mediates the adsorption of the virion to the host cell and subsequent virion entry and, via the selectivity of GP for particular cellular molecules, thereby determines virus cell and tissue tropism [84–87]. The L protein mediates both transcription and replication via its RdRp domain as well as cellular mRNA cap-snatching for virus subgenomic RNA capping [88, 89]. Like NP, Z is an interferon antagonist that functions as a matrix protein for polymerization at membranes and thereby facilitates virion assembly and budding [89–92].
2.3. Lifecycle
Figure 1 depicts a simplified lifecycle of mammarenaviruses. Mammarenaviruses enter cells via adsorption to cell-surface attachment factors, which vary among viruses [93]. At the host–cell surface, LASV attaches to matriglycan, a unique disaccharide modification of dystroglycan 1 (DAG1; previously often called α-dystroglycan and abbreviated α-DG) [94, 95]. LUJV attaches to neuropilin 2 (NRP2) [96], whereas CHAPV, GTOV, JUNV, MACV, and SBAV engage transferrin receptor 1 (TFRC; previously often abbreviated TfR1) [97–99]. Attachment is followed by endocytosis. The increasingly acidic environment in the endosome triggers pH-dependent fusion, resulting in the release of the S and L RNPs into the cytoplasm, where viral RNA genome replication and gene transcription take place. In the case of LASV and LUJV, engagement of intracellular/endosomal proteins, lysosomal-associated membrane protein 1 (LAMP1) and the CD63 molecule (CD63), respectively, is necessary to progress to fusion [96, 100]. Transcription of NP- and L-encoding subgenomic RNAs, which lack poly(A) tails, is initiated by the RNP complexes at promoters located in the 3’ termini of the genomic RNA segments and terminated by structural motifs within the IGRs. The RNPs switch from transcription to replication, generating full-length antigenome RNAs that then serve as templates for transcription of the GPC- and Z-encoding ORFs. In addition, progeny genomes are amplified from the antigenomic RNA segment templates [101, 102]. Virion assembly occurs at the cell surface, near membranes enriched with GP and mediated by Z, which contains canonical late-budding motifs to recruit components of the endosomal sorting complex required for transport (ESCRT) pathway [90, 103, 104].
Figure 1. Mammarenavirus lifecycle.
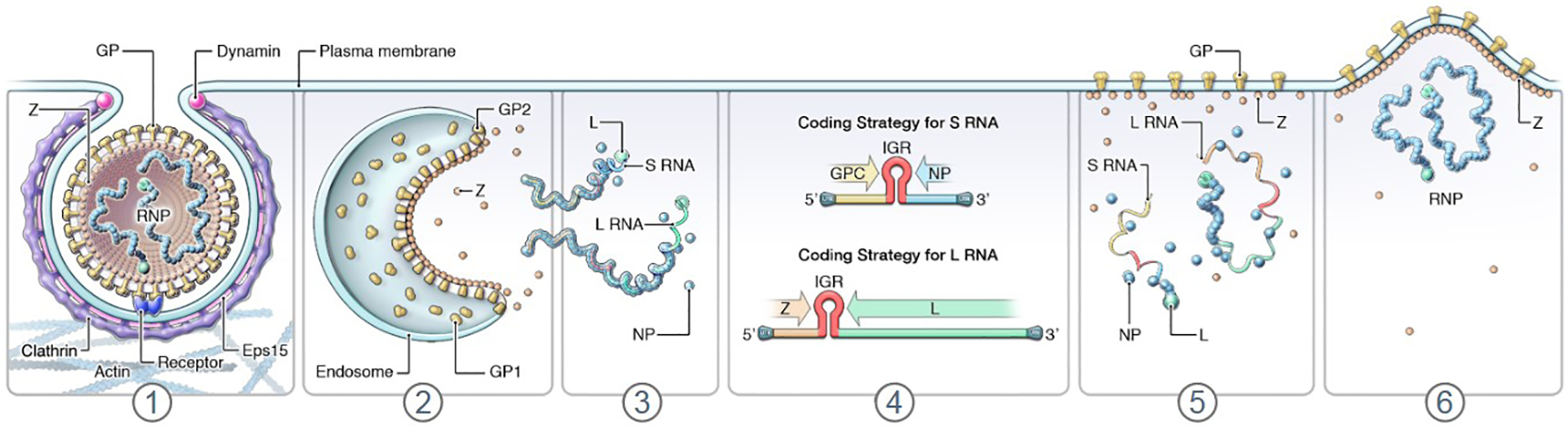
(1) Mammarenavirions adsorb to cell-surface factors, followed by endocytic uptake and engagement of intracellular receptors; (2, 3) membrane fusion, uncoating, and release of S and L genomic RNP complexes into the cytosol; (4) transcription of viral subgenomic RNAs, translations of viral proteins NP, GPC, L, and Z, and genome replication via antigenomic intermediates (antigenomes); (5, 6) Z-mediated virion morphogenesis and budding. GP, glycoprotein complex; GP1, glycoprotein 1 subunit; GP2, glycoprotein 2 subunit; GPC, glycoprotein precursor; IGR, intergenic region; L, large; NP, nucleoprotein; RNP, ribonucleoprotein complex; S, small; Z, zinc-binding protein. Modified and updated from [2] as well as from [148] with permission of Taylor & Francis.
3. Progress in treatment of mammarenavirus infections
3.1. Clinical development
Identification, development, and evaluation (safety and efficacy) of candidate medical countermeasures (MCMs) against human mammarenaviruses are a priority because no prophylactic or therapeutic treatments are approved by the U.S. Food and Drug Administration (FDA) or other major agencies, leaving solely limited strategies to prevent (via minimizing rodent exposure and isolating patients) and treat (to supportive care or the non-standard use of virus-specific MCMs). Only four small molecules (ribavirin, favipiravir, LHF-535, and ARN-75039), all targeting LASV (Table 2), have been or are being evaluated in clinical trials. Despite limited evidence and no regulatory approval, ribavirin has been standardly used off-label in endemic areas for many years. Other candidates are only in advanced preclinical development or in early phases of human clinical trials.
Table 2.
Clinical trials to evaluate mammarenavirus antivirals [216].
| https://clinicaltrials.gov/ Identifier | Title | Type/Phase | Status |
|---|---|---|---|
| NCT02483260 | Intravenous Ribavirin Protocol to Treat Individuals With Viral Hemorrhagic Fever | Interventional/NA | Recruiting |
| NCT03889106 | Cardiovascular Function and Ribavirin Pharmacokinetics and Pharmacodynamics in Patients With Lassa Fever | Observational/NA | Terminated |
| NCT03993704 | Multiple Ascending Oral Dose 14-Day Trial of LHF-535 in Healthy Participants | Interventional/Phase 1 | Completed |
| NCT04285034 | Cardiovascular Function and Ribavirin PK/PD in Lassa Fever in Lassa Fever | Observational/NA | Completed |
| NCT04907682 | Pharmacokinetics, Tolerability and Safety of Favipiravir Compared to Ribavirin for the Treatment of Lassa Fever (SAFARI) | Interventional/Phase 2 | Completed |
| NCT05735249 | A Study to Assess the Safety, Tolerability, and Pharmacokinetics of Oral ARN-75039 in Healthy Adult Subjects | Interventional/Phase 1 | Recruiting |
3.1.1. Ribavirin
Ribavirin is a guanosine prodrug that is phosphorylated after administration to an active form that interferes with diverse cellular pathways. Ribavirin’s antiviral activity is hypothesized to be related to decreased cellular availability of guanosine triphosphate (GTP) and deoxyguanosine GTP (dGTP) and subsequent prevention of viral subgenomic RNA capping, leading to viral RNA degradation; interference with RdRp-mediated transcription and replication, leading to lethal mutagenesis in newly synthesized viral RNAs; and/or inhibition of macrophage activation and lymphocyte proliferation [105–112]. Off-label intravenous administration of ribavirin is the most-used specific treatment option for mammarenavirus infections. Rationale for this use is derived from a clinical study of Lassa fever patients in Sierra Leone in the late 1970s and early 1980s [113]. In this non-blinded study, early initiation of ribavirin for ten days in patients with high viral load appeared to reduce the case fatality rate compared to a control group [113]. However, this and other studies have come under scrutiny for methodologic and analytic flaws to truly determine efficacy; furthermore, reanalysis of the data indicates that ribavirin may actually have been harmful to some patients [114–116]. Ribavirin has also been used clinically to treat patients infected with JUNV, MACV, SBAV, or LUJV [60, 62, 117, 118], but therapeutic benefit in all of these cases continues to be hotly debated. Indeed, the adverse side effects of ribavirin, which include infusion-related rigors, severe anemia, thrombocytosis, and congenital disorders [119, 120], pose a conundrum for physicians weighing the potentially limited antiviral effects versus risks posed by the drug [110, 114]. That said, ribavirin has repeatedly been shown to have preclinical prophylactic and therapeutic value against a range of mammarenaviruses both in vitro and in a range of animal models, including nonhuman primates [121–126]; in the absence of and until identification of other effective therapeutics, these data suggest that ribavirin should not yet be dismissed. Indeed, efforts are underway to create and evaluate ribavirin analogues that may be equally effective while causing fewer side effects [127].
3.1.2. Favipiravir
The pyrazine derivative favipiravir (T-705) is a broad-spectrum purine nucleoside mimic that interferes with mammarenavirus transcription and replication [128–130], possibly through multiple mechanisms (termination of transcription via binding to RdRp and/or incorporation into nascent viral RNAs leading to error catastrophe) [131–134]. In domesticated guinea pigs exposed to the LASV Josiah isolate, treatment with favipiravir (300 mg/kg/day dosed subcutaneously) beginning at 2, 5, or even 7 days after virus exposure resulted in uniform survival with a 2–3 log infectious titer reduction compared to the control group [135]. Similarly, in domesticated guinea pigs exposed to JUNV, initiation (1 or 2 days after exposure) of favipiravir (300 mg/kg/day dosed orally or intraperitoneally twice daily for two weeks) led to 20% and 33–40% survival in the oral and intraperitoneally dosed groups, respectively [136]. In crab-eating macaques (Macaca fascicularis Raffles, 1821) exposed to LASV, initiation (4 days after exposure) of favipiravir treatment (300 mg/kg/day dosed intravenously once and subcutaneously thereafter for 13 days) led to uniform survival although all animal developed clinical signs [137]. Notably, in all published animal study comparisons, favipiravir outperformed ribavirin. Favipiravir escape mutants have been discovered in treated JUNV-infected cells [134], although these in vitro experiments used an attenuated JUNV (Candid#1 vaccine) strain rather than wild-type virus; consideration of combination treatments in the clinic to avoid emergence of therapeutic-resistant variants may be warranted. Combination treatment with favipiravir and ribavirin for two Lassa fever patients reduced viremia in both [31], but whether the combination led to the patients’ survival is unclear from these anecdotal descriptions. In an immunocompromised laboratory mouse model of Lassa fever, combination treatment with favipiravir and ribavirin led to a decline in viremia and protected against lethality; however, cessation of treatment led to a reoccurrence of viremia in all mice [138]. Ongoing phase 2 clinical studies are comparing the pharmacokinetics, safety, and tolerability of favipiravir compared to ribavirin in patients with Lassa fever in Nigeria.
3.1.3. LHF-535
Benzimidazole derivative LHF-535, related to previously promising antiviral ST-193 [139, 140], is a small-molecule viral-entry inhibitor that targets the mammarenavirus GP. Through interaction with the SSP–GP2 prefusion structure, LHF-535 is thought to prevent the conformational changes necessary within the complex to mediate membrane fusion [141]. The molecule was first shown to be inhibit cell transduction by noninfectious lentiviral particles pseudotyped with GPs of LASV lineages II, III, and IV (50% inhibitory concentration [IC50] = 0.1–0.3 nM) but was equally active against JUNV (IC50 = 0.1 nM) and MACV (IC50 = 0.1 nM) pseudotypes, whereas much less activity was measured against CHAPV, SBAV, and LUJV pseudotypes [141]. In the established lethal domesticated guinea pig (Cavia porcellus (Linnaeus, 1758)) model of Lassa fever [142], intraperitoneal injection of 50 mg/kg/day of LHF-535 starting at day 1 or day 3 after exposure to a typically lethal dose of guinea pig-adapted LASV (lineage IV Josiah isolate) protected all animals from fatal outcome and led to reduced viremia and less severe disease compared to control animals [143]. In two phase 1 clinical trials in healthy volunteers, the safety (well-tolerated) and pharmacokinetics (rapid absorption and long half-life) of oral LHF-535 were favorable for further development [144]. LHF-535 pharmacokinetics at the protective dose in domesticated guinea pigs revealed plasma concentrations within the range observed in the phase 1 trial volunteers [143, 144], supporting the continued development of LHF-535 as a LASV-specific therapeutic.
3.1.4. ARN-75039
ARN-75039 is a potentially orally bioavailable imidazopyridine fusion inhibitor that is highly active at low doses against noninfectious vesicular stomatitis Indiana virus particles pseudotyped with JUNV, MACV, or LASV GPs (half-maximal response [EC50] ≤ 1 nM) and wild-type JUNV and LASV in vitro (EC50 <3.3 nM) [145]. In the domesticated guinea pig model of Argentinian hemorrhagic fever [146], oral administration of ARN-75039 led to uniform survival of JUNV-exposed animals when administered on day 2 after virus exposure and to partial survival when administered on day 4 or even day 6 [147]. Production company news indicate that the compound may be equally active against LASV in domesticated guinea pigs. Phase I studies of the tolerability, safety, and pharmacokinetics of oral ARN-75039 in healthy volunteers are ongoing.
4. Preclinical development
4.1. Small-molecule inhibitors
The identification of potentially promising novel small molecules that could be developed as future candidate MCMs is increasingly performed using standardized high-throughput screens. These screens are often established around mammarenavirus surrogates (e.g., reporter protein-encoding non-infectious retrovirion- or vesciculovirion-like particles pseudotyped with mammarenavirus GPs for identification of virion entry inhibitors or non-infectious minigenomes and derived systems consisting of transcription- and replication-regulatory sequences surrounding a reporter protein-encoding gene to identify L protein inhibitors) to enable screening at biosafety level 2. Alternatively, these screens are adapted for use of replicative viruses at biosafety level 4. Variations of screening include the modification of cell lines to encode reporter proteins that are induced (or abrogated) upon pseudotype transduction of virus infection and the use of recombinant mammarenaviruses that encode reporter proteins. Alternatively, screening platforms are developed specifically for identification of mammarenavirus protein-specific inhibitors [148–157].
“Repurposing” of regulatory agency-approved drugs, i.e., their use for a different indication (in this case treatment of mammarenavirus infections) could shorten the time and reduce costs and risks in preclinical development of novel drugs. Consequently, libraries of approved drugs are increasingly evaluated against mammarenaviruses (or surrogate systems) using in vitro high-throughput platforms [158–160]. Such approaches have led to the identification of, for instance:
afatinib, a pan-ErbB tyrosine kinase inhibitor that potently inhibits LASV RNP activity and authentic JUNV replication [161];
AM-251, AM-281, and rimonabant (SR141716), known biarylpyrazole cannabinoid receptor 1 (CB1) antagonists, that robustly inhibit fusion affecting LUJV pseudotype transduction entry in the high nanomolar range [162];
isavuconazonium, a triazole antifungal that inhibits CHAPV, GTOV, LASV (lineages I–IV), JUNV, MACV, and SBAV pseudotype transduction via inhibition of SSP-GP2-mediated membrane fusion [163];
lacidipine, a known calcium channel blocker, as GTOV and LASV and d-phenothrin (sumithrin), a pyrethroid, as LASV pseudotype transduction inhibitors that act at the low-pH-induced membrane fusion step [164];
losmapimod (GW856553X), a selective p38α/β mitogen-activated protein kinase (MAPK) inhibitor, as a LASV pseudotype transduction inhibitor that interferes with SSP-GP2-mediated membrane fusion [165];
teriflunomide as a potent inhibitor of RNA synthesis and thereby JUNV replication [166];
manidipine, lercanidipine, and trametinib as LUJV pseudotype transduction inhibitors in the micromolar range. Manidipine and lercanidipine were identified as dihydropyridine calcium channel blockers, whereas trametinib, which also inhibited CHAPV, GTOV, JUNV, LASV, MACV, and SBAV pseudotype transduction and is a known MEK inhibitor, was identified as a mammarenavirion fusion inhibitor [167]; and
umifenovir, niclosamide, and sertraline as disruptors of LASV replication; and combinatorial umifenovir plus aripiprazole or sertraline as synergistic inhibitors of JUNV and LASV pseudotypes [168, 169].
Although numerous drugs have been identified as in vitro inhibitors via this approach, most have not progressed to in vivo preclinical evaluation. An exception is stampidine, the nucleoside analog derived from stavudine (d4T) and a known retroviral reverse transcriptase inhibitor, which increased survival of laboratory mice exposed to LASV as compared to controls [170].
Alternatively, high-throughput screens are performed with large libraries of chemical compounds that are not yet approved for any treatment. Such approaches have led to the identification of, for instance:
16G8, 17C8 as inhibitors of LASV, JUNV, MACV GTOV replication via entry interference (IC50 ≈200–350 nM) [171];
bergamottin and casticin as entry inhibitors of CHAPV, GTOV, JUNV, LASV, LUJV, and MACV transduction in the micromolar range [172];
BEZ-235, a dual phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin (Rpm) (mTOR) inhibitor and LY294002, a PI3K inhibitor, as inhibitors of LASV particle production [173];
BIBX 1382 and OSU-03012, two kinase inhibitors, as inhibitors of LASV replication [174];
cap-dependent endonuclease inhibitors that inhibit JUNV and LASV replication at micromolar concentrations [175];
CP100356, a specific P-glycoprotein inhibitor, as an inhibitor of LASV replication (IC50 = 0.062 μM) and JUNV, LASV, MACV, and SBAV pseudotype transduction [176];
F1204 and F1781, benzotriazole derivates that target the LASV endonuclease activity in a minigenome system [157];
F1920 and F1965, which inhibit LASV pseudotype transduction [152];
PF-429242, an inhibitor of cellular site 1 protease (S1P)-mediated processing of LASV GPV, as a disruptor of LASV maturation in the low micromolar range [177];
quercetin, a flavonol that interrupts the PI3K/Akt pathway, as an inhibitor of JUNV replication via entry interference [178];
BEZ-235 and LY294002, which together target the PI3K/Akt pathway inhibit viral budding of LASV and replication [173];
ST-193, ST-294, ST-336, and TRAM-34, which are clotrimazole-derivatives that inhibit GTOV, JUNV, and MACV replication by interference with GP2 [179, 180], and ST-193 as a survival-increasing inhibitor in a LASV domesticated guinea pig experiment [140]; and
tangeretin, a flavone, as a fusion inhibitor that interrupts replicative LASV entry in the submicromolar range and CHAPV, GTOV, JUNV, LASV, LUJV, MACV, and SBAV pseudotype transduction [181].
4.2. Antibodies
4.2.1. Passive antibody therapy
Transfusion of convalescent sera (or plasma) from survivors of mammarenavirus infections is a plausible and seemingly straightforward therapeutic intervention. For instance, administration of such sera reduces the case fatality rate of Argentinian hemorrhagic fever to <1–2% when administered within the first eight days of disease, and hence is the current treatment of choice for patients with acute disease. However, receipt of convalescent plasma has been associated with late neurologic sequelae (headache, cerebellar tremor, acranial nerve palsies) in 10% of treated patients [182–186]. Data supporting convalescent serum treatment of patients with Lassa fever are less clear; though repeatedly administered to individuals during outbreaks and after accidental laboratory infections [187–189] , these descriptive and observational data, often not obtained under well-controlled conditions, do not enable clear determination of safety and efficacy. Investigational animal studies indicated the potential usefulness of convalescent sera for treatment of LASV infections but only if repeated treatment begins almost immediately after virus exposure and contains high titers of virus-neutralizing antibodies [190, 191]. Likewise, very limited investigational animal studies indicated that GTOV and MACV infections could be treated with convalescent sera as well [192, 193].
4.2.2. Monoclonal antibodies
Even if efficacious, convalescent sera (or plasma) is typically available in limited quantity (in particular, due to a low number of cases and/or in the absence of a program for standardized collection, processing, and administration), is difficult to standardize, and poses a potential risk of transfusion-associated transmission of pathogens, such as HIV-1, hepatitis viruses, or plasmodia. Thus, a major focus of MCM development for mammarenavirids has focused on the identification and characterization of protective monoclonal antibodies (mAbs) from human survivors (or from animals after experimental mammarenavirus infections) which could then be manufactured as a standardized biological product. Indeed, a proof-of-concept study demonstrated that identified neutralizing murine anti-JUNV mAbs expressed as mouse-human chimeric antibodies provided complete protection to JUNV-exposed domesticated guinea pigs when administered on day 2 post-exposure. The mAb J199 provided complete protection when administered on day 6 and offered 92% protection when administered on day 7 [194]. Similarly, human Lassa fever survivor anti-GP mAbs 8.9F, 12.1F, 37.2D, and 37.7H [195] completely protected LASV-exposed crab-eating macaques when administered intravenously at 15 mg/kg at days 0, 4, and 8 post-exposure [196, 197]. Administration of a thoroughly characterized cocktail containing 15 mg/kg each of mAbs 8.9F, 12.1F, and 37.2D (now known as arevirumab-3) protected all macaques when treatment was initiated as late as 8 days after exposure during an advanced stage of disease [196–198]. Importantly, arevirumab-3 completely protected macaques against disease caused by lineage II, III, and IV LASV on day 8 and against lineage VII LASV when treatment was initiated on day 7 [199, 200].
5. Conclusion
Increased understanding of the molecular biology of arenavirids in general, and that of mammarenaviruses in particular, combined with the use of ever more sophisticated high-throughput small molecule screening platforms, resulted in the identification of a plethora of small molecules with anti-mammarenaviral activity. However, most of these molecules were only evaluated using mammarenavirus surrogate systems (usually viral pseudotypes or in minigenome systems) and, even in those settings, required micromolar concentrations to exert effects. Few molecules have been tested in vivo against authentic mammarenaviruses and rarely have they been evaluated against multiple mammarenaviruses side-by-side to identify broad-spectrum options that could, ideally, also protect against newly emerging viruses. Only a handful of promising molecules have resulted in considerable therapeutic benefit in investigational animal models of mammarenavirus disease and only three candidates (arevirumab-3, favipiravir, and ribavirin) were active in nonhuman primate models of, primarily, Lassa fever.
6. Expert opinion
At least two mammarenaviruses, JUNV and LASV, are significant public health concerns because they cause tens to hundreds of thousands of human infections and numerous deaths annually in endemic areas and result in case exportations to other continents (Argentina and Western Africa, respectively) [1, 71]. It is unsurprising, therefore, that the majority of research and development (R&D), including the limited data for the most advanced treatment options, is focused on these two viruses; treatments include off-label use of ribavirin for humans infected with any mammarenaviruses [62, 113, 117, 118], transfusion of convalescent sera to treat human JUNV infections [182–186], and arevirumab-3 as the currently most promising therapeutic under development against LASV infections [196–200]. However, real risk-benefit concerns regarding the safety and efficacy of ribavirin treatment remain unanswered by limited data[110, 114–116, 119, 120], and widespread use of convalescent sera is impractical, possibly unsafe, and poorly evidenced; absent data from well-designed clinical trials of either therapeutic, field clinicians are heavily reliant on supportive care strategies to improve patient outcomes.
At least historically, R&D strategies around mammarenavirus MCMs have unfortunately (but perhaps necessarily) been driven by and targeted more urgent needs in response to outbreak disease burden (and the resultant virus- and disease-specific questions and opportunities inherent to those “felt” needs); however, a narrow focus in the short term has not translated well into broader longer-term vision and strategy. First, the overall focus on JUNV and LASV neglects the possibility that the other highly virulent mammarenaviruses (CHAPV, GTOV, LUJV, MACV, SBAV), currently considered exotic pathogens causing only a handful to a few hundred of cases over decades, are potential pandemic pathogens [1]. Because all mammarenaviruses are mammal-borne, disturbances in, for instance, rodent habitats and concomitant rodent migrations leading to increased human–rodent contact makes this scenario a distinct possibility. Pathogenic mammarenaviruses are also the minority of known members of genus Mammarenavirus, which currently includes 43 species for 50 classified viruses and is associated with a list of numerous unclassified viruses that grows steadily [2]. Consequently, R&D efforts need to anticipate the emergence of novel pathogenic mammarenaviruses. Second, most R&D efforts rely on the use of surrogate systems rather than authentic viruses due to the risk to the laboratory worker that is associated with these pathogens (and hence the need for maximum containment laboratories, of which there a few worldwide) and/or the lack of access to the viruses. This reliance skews research towards identification of entry inhibitors, because pseudotyped viruses are the only truly amenable, available, and easily established for high-throughput drug screens. However, entry dynamics, mechanics, and kinetics of pseudotypes differ considerable from those of authentic viruses and EC50 values and other important drug indices obtained with surrogate systems are rarely extrapolatable to or predictive for authentic virus infections. Third, when human clinical trials are either not logistically feasible or ethically unjustifiable, investigational animal model data become crucial for drug development, possible under the FDA Animal Rule [201]. However, only a few animal models of mammarenavirus diseases have been rigorously established and, once again, are biased toward JUNV and LASV infection models that require maximum containment facilities [142, 146, 202]. Fourth, there is likely considerable intra-species diversity among all pathogenic mammarenavirus. LASV is the only virus for which ample genomic data are available, and these data clearly delineate at least seven genomically diverse lineages that differ in geographic distribution, likely host spectrum [71], and also in response to MCMs [200]. However, until recently, the vast majority of MCM discovery and evaluation efforts have only targeted LASV lineage IV isolates (or, in case of pseudotype surrogate systems, GPs thereof). Together, these observations indicate a need for a more concerted, community-wide effort to create and make available standardized reagents that include well-characterized viruses (and genes and proteins thereof) that represent all major clades of mammarenaviruses and also all major intra-specific lineages so that drug candidates can be tested systematically to predict their value as broad-spectrum antivirals. In addition, MCM R&D needs to consider different field realities for their applications. Ideally, rigorous clinical trials show an MCM to be safe and efficacious as well as practicable in relevant settings (i.e., effective in the real world). Because most pathogenic mammarenaviruses are endemic in underdeveloped and often rural areas, affordability, and availability to local populations, including farm workers or hospital staff, are barriers to improving clinical outcomes. Studies cited in this manuscript rarely mention real-world applicability in connection with a given “promising” antiviral. To the contrary, the most products under development are based on monoclonal antibodies [196–200], which are expensive to produce and purify and require technological and manufacturing capabilities rarely available in areas most in need. Private–public partnerships could possibly mitigate these challenges in the long term by building local capabilities that are not pathogen-specific [203]. In the meantime, “repurposing” of regulatory-agency-approved drugs may be pursued to help mitigate the health impacts of local mammarenavirus disease outbreaks; drugs already approved for other indications a) may already be available in quantities necessary for distribution, b) may not be expensive especially if intellectual property protections have already expired, and c) may be quickly approved due to their known human safety profile. Repurposing screens ought to focus on drugs that are administered orally, stable at room temperature, have a long shelf life, and can be combined with additional out-of-class MCMs to prevent the evolution of drug-resistance traits in the targeted viruses.
It should be noted that recent pandemic preparedness efforts have underscored the need to more broadly characterize high-risk viral families (rather than just focus only their notorious members); however, the selection of “prototype pathogen” viruses that might serve as proxy for mammarenaviral family members is particularly challenging given known (and unknown) within-virus and within-family genetic variations. The degree of “representativeness” in the face of this diversity needs to be carefully considered, especially for prioritization of preclinical development efforts [204].
Even in the diseases that have received the most attention, there is a need for robust clinical trials data to inform clinical decision-making, most obvious in the common non-standard use of ribavirin in Lassa fever patients based on inadequate data from many decades ago. That non-standard (but now routine) use of ribavirin complicates the design of current and future randomized clinical trials, namely whether ribavirin’s use over many years obligates its inclusion either alone or in combination in clinical trials. The recent establishment of well-characterized Lassa fever clinical cohorts in Western Africa, as well as preparation and pre-positioning for clinical trials of advanced MCMs in these clinical networks, shows promise for clearing up some long-held therapeutic ambiguity. For example, results from LASCOPE (NCT03655561), a prospective observational cohort study of Lassa fever aiming to more fully characterizing the natural history of disease, identify risk factors, and standardize case management, also defined a reference mortality rate to inform future clinical trial endpoints and suggested that the need for dialysis should also be considered an important clinical outcome in any evaluation of therapeutics [205]. Increasingly, clinical trial evaluation also must consider the specific needs of vulnerable populations (e.g., those with HIV/AIDS or diabetes, pregnant women) that are at risk of acquiring mammarenavirus infections. Furthermore, as with many human viral infections, it is likely that combination therapeutics that include highly effective mAb and small-molecule antivirals (with potentially advantages in tissue distribution) may be needed to optimize acute and convalescent outcomes after mammarenavirus infections. Central nervous system manifestations (encephalopathy, coma, seizures) have been associated with poor outcomes in these cohorts [74] and LASV has been detected in the cerebrospinal fluid [206]; the relative penetration of advanced therapeutic candidates into the central nervous system is unknown but is plausibly less likely for mAb-based strategies [207]. The pathogenesis of hearing loss during acute Lassa fever and in survivors is uncertain; notably, ribavirin use during acute Lassa fever does not appear to prevent the development of Lassa-fever-associated sensorineural deafness [68]. Although related transmission events have not been documented, recent descriptions of LASV persistence in the semen of male survivors at least argue that therapeutic penetration into immune-privileged tissues merits consideration for public health reasons. Indeed, future clinical trials might include secondary endpoints targeting the post-acute clinical sequelae or viral persistence endpoints. Finally, though outside the scope of this review, mammarenavirus-specific therapeutic approaches cannot be considered in isolation; safe and effective antiviral strategies must be coupled and bundled with appropriate supportive or critical care to improve patient outcomes in an optimal future.
Article highlights.
Several mammarenaviruses can cause severe human diseases with high case fatality rates and hence are considered public health and bioterrorism threats.
Therapeutic options for mammarenavirus infections and diseases remain highly limited and controversial.
High-throughput screening of regulatory-agency-approved drugs for the treatment of a variety of diseases identified numerous molecules that could be “repurposed” as medical countermeasures (MCMs) against mammarenaviruses.
However, due to their classification as Risk Group 4 agents, further development of identified candidate MCMs has been slow and antiviral activity evaluation of identified candidates in animal models has rarely occurred.
The most promising treatment under development is a cocktail consisting of three monoclonal antibodies that protects macaques against multiple lineages of Lassa virus even in advanced stages of disease.
This box summarizes key points contained in the article.
Acknowledgments
We would like to thank Fabian de Kok-Mercado and Jiro Wada (Integrated Research Facility at Fort Detrick) for creating/modifying Figure 1.
Funding
This work was supported in part through Laulima Government Solutions, LLC, prime contract with the National Institutes of Health National Institute of Allergy and Infectious Diseases under Contract No. HHSN272201800013C. IA Nunez, A Crane, and G Worwa performed this work as employees of Laulima Government Solutions, LLC. JH Kuhn performed this work as an employee of Tunnell Government Services, a subcontractor of Laulima Government Solutions, LLC, under Contract No. HHSN272201800013C. This work was also supported in part with federal funds from the NIH National Cancer Institute (NCI), under Contract No. 75N91019D00024 with Leidos Biomedical Research, Inc. I Crozier performed this work as an employee of Leidos Biomedical Research, Inc., as supported by the Clinical Monitoring Research Program Directorate, Frederick National Lab for Cancer Research, sponsored by NCI.
List of abbreviations used in the manuscript
- CB1
cannabinoid receptor 1
- CD63
CD63 molecule
- CHAPV
Chapare virus
- DAG1
dystroglycan 1
- dGTP
deoxyguanosine GTP
- EC50
half-maximal response
- ESCRT
endosomal sorting complex required for transport
- FDA
U.S. Food and Drug Administration
- FLEV
Flexal virus
- GP
glycoprotein complex
- GP1
glycoprotein subunit 1
- GP2
glycoprotein subunit 2
- GPC
glycoprotein precursor
- GTOV
Guanarito virus
- GTP
guanosine triphosphate
- IC50
50% inhibitory concentration
- IGR
intragenic region
- JUNV
Junín virus
- L
large
- LAMP1
lysosomal-associated membrane protein 1
- LASV
Lassa virus
- LCMV
lymphocytic choriomeningitis virus
- LUJV
Lujo virus
- MACV
Machupo virus
- MAPK
mitogen-activated protein kinase
- MCM
medical countermeasure
- NP
nucleoprotein
- NRP2
neuropilin 2
- ORF
open reading frame
- R&D
research and development
- RdRp
RNA-directed RNA polymerase
- RNA
ribonucleic acid
- RNP
ribonucleoprotein
- S
small
- S1P
site 1 protease
- SBAV
Sabiá virus
- SSP
stable signal peptide
- TFRC
transferrin receptor 1
- Z
zinc-binding protein
Footnotes
Declaration of Interest:
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures:
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Annotated bibliography
Papers of special note have been highlighted as either of interest (●) or of considerable interest
(●●) to readers.
- 1.Radoshitzky SR, Buchmeier MJ, de la Torre JC. Arenaviridae: the viruses and their replication. In: Howley PM, Knipe DM, Whelan SPJ, eds. Fields Virology. 7th ed. Philadelphia, Pennsylvania, USA: Wolters Kluwer/Lippincott Williams & Wilkins; 2020:784–809. [Google Scholar]
- 2.Radoshitzky SR, Buchmeier MJ, Charrel RN, et al. ICTV virus taxonomy profile: Arenaviridae 2023. J Gen Virol 2023. Sep;104(9):001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mordecai GJ, Miller KM, Di Cicco E, et al. Endangered wild salmon infected by newly discovered viruses. Elife 2019. Sep 3;8:e47615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi M, Lin X-D, Chen X, et al. The evolutionary history of vertebrate RNA viruses. Nature 2018. Apr;556(7700):197–202. [DOI] [PubMed] [Google Scholar]
- 5.Grimwood RM, Holmes EC, Geoghegan JL. A novel rubi-like virus in the Pacific electric ray (Tetronarce californica) reveals the complex evolutionary history of the Matonaviridae. Viruses 2021. Mar 31;13(4):585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hepojoki J, Hepojoki S, Smura T, et al. Characterization of Haartman Institute snake virus 1 (HISV-1) and HISV-like viruses—the representatives of genus Hartmanivirus, family Arenaviridae. PLoS Pathog 2018. Nov;14(11):e1007415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hepojoki J, Salmenperä P, Sironen T, et al. Arenavirus coinfections are common in snakes with boid inclusion body disease. J Virol 2015. Aug;89(16):8657–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alfaro-Alarcón A, Hetzel U, Smura T, et al. Boid inclusion body disease is also a disease of wild boa constrictors. Microbiol Spectr 2022. Oct 26;10(5):e0170522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hetzel U, Sironen T, Laurinmäki P, et al. Isolation, identification, and characterization of novel arenaviruses, the etiological agents of boid inclusion body disease. J Virol 2013. Oct;87(20):10918–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stenglein MD, Sanders C, Kistler AL, et al. Identification, characterization, and in vitro culture of highly divergent arenaviruses from boa constrictors and annulated tree boas: candidate etiological agents for snake inclusion body disease. mBio 2012;3(4):e00180–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abba Y, Hassim H, Hamzah H, et al. In vitro isolation and molecular identification of reptarenavirus in Malaysia. Virus Genes 2016. Oct;52(5):640–50. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y-M, Sadiq S, Tian J-H, et al. RNA virome composition is shaped by sampling ecotype. SSRN Electronic Journal 2021:https://ssrn.com/abstract=3934022. [Google Scholar]
- 13.Costa VA, Mifsud JCO, Gilligan D, et al. Metagenomic sequencing reveals a lack of virus exchange between native and invasive freshwater fish across the Murray-Darling Basin, Australia. Virus Evol 2021. Jan;7(1):veab034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geoghegan JL, Di Giallonardo F, Wille M, et al. Virome composition in marine fish revealed by meta-transcriptomics. Virus Evol 2021. Jan;7(1):veab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y-M, Hu S-J, Lin X-D, et al. Host traits shape virome composition and virus transmission in wild small mammals. Cell 2023. Oct 12;186(21):4662–75 e12. [DOI] [PubMed] [Google Scholar]
- 16.Downs WG, Anderson CR, Spence L, et al. Tacaribe virus, a new agent isolated from Artibeus bats and mosquitoes in Trinidad, West Indies. Am J Trop Med Hyg 1963. Jul;12:640–6. [DOI] [PubMed] [Google Scholar]
- 17.Bentim Góes LG, Fischer C, Almeida Campos AC, et al. Highly diverse arenaviruses in neotropical bats, Brazil. Emerg Infect Dis 2022. Dec;28(12):2528–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sayler KA, Barbet AF, Chamberlain C, et al. Isolation of Tacaribe virus, a Caribbean arenavirus, from host-seeking Amblyomma americanum ticks in Florida. PLoS One 2014;9(12):e115769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reuter G, Boros Á, Takáts K, et al. A novel mammarenavirus (family Arenaviridae) in hedgehogs (Erinaceus roumanicus) in Europe. Arch Virol 2023. Jun 8;168(7):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo X-L, Lu S, Qin C, et al. Emergence of an ancient and pathogenic mammarenavirus. Emerg Microbes Infect 2023. Dec;12(1):e2192816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui X, Fan K, Liang X, et al. Virus diversity, wildlife-domestic animal circulation and potential zoonotic viruses of small mammals, pangolins and zoo animals. Nat Commun 2023. Apr 29;14(1):2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowen MD, Peters CJ, Nichol ST. Phylogenetic analysis of the Arenaviridae: patterns of virus evolution and evidence for cospeciation between arenaviruses and their rodent hosts. Mol Phylogenet Evol 1997. Dec;8(3):301–16. [DOI] [PubMed] [Google Scholar]
- 23.Hugot JP, Gonzalez JP, Denys C. Evolution of the Old World Arenaviridae and their rodent hosts: generalized host-transfer or association by descent? Infect Genet Evol 2001. Jul;1(1):13–20. [DOI] [PubMed] [Google Scholar]
- 24.Wu Z, Du J, Lu L, et al. Detection of hantaviruses and arenaviruzses [sic] in three-toed jerboas from the Inner Mongolia Autonomous Region, China. Emerg Microbes Infect 2018. Mar 21;7(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smither AR, Bell-Kareem AR. Ecology of Lassa virus. Curr Top Microbiol Immunol 2023. Feb 11;440:67–86. [DOI] [PubMed] [Google Scholar]
- 26.Tapia-Ramírez G, Lorenzo C, Navarrete D, et al. A review of mammarenaviruses and rodent reservoirs in the Americas. Ecohealth 2022. Mar;19(1):22–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redding DW, Gibb R, Dan-Nwafor CC, et al. Geographical drivers and climate-linked dynamics of Lassa fever in Nigeria. Nat Commun 2021. Oct 1;12(1):5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redding DW, Moses LM, Cunningham AA, et al. Environmental-mechanistic modelling of the impact of global change on human zoonotic disease emergence: a case study of Lassa fever. Methods Ecol Evol 2016. Jun;7(6):646–55. [Google Scholar]
- 29.Loayza Mafayle R, Morales-Betoulle ME, Romero C, et al. Chapare hemorrhagic fever and virus detection in rodents in Bolivia in 2019. N Engl J Med 2022. Jun 16;386(24):2283–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Briese T, Paweska JT, McMullan LK, et al. Genetic detection and characterization of Lujo virus, a new hemorrhagic fever-associated arenavirus from southern Africa. PLoS Pathog 2009. May;5(5):e1000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raabe VN, Kann G, Ribner BS, et al. Favipiravir and ribavirin treatment of epidemiologically linked cases of Lassa fever. Clin Infect Dis 2017. Sep 1;65(5):855–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salu OB, Amoo OS, Shaibu JO, et al. Monitoring of Lassa virus infection in suspected and confirmed cases in Ondo State, Nigeria. Pan Afr Med J 2020;36(1):253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thielebein A, Ighodalo Y, Taju A, et al. Virus persistence after recovery from acute Lassa fever in Nigeria: a 2-year interim analysis of a prospective longitudinal cohort study. Lancet Microbe 2022. Jan;3(1):e32–e40. [DOI] [PubMed] [Google Scholar]; ● (Describes Lassa virus RNA shedding and persistence of infectious virus in seminal fluid of survivors).
- 34.Delgado S, Erickson BR, Agudo R, et al. Chapare virus, a newly discovered arenavirus isolated from a fatal hemorrhagic fever case in Bolivia. PLoS Pathog 2008. Apr 18;4(4):e1000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salas R, Pacheco ME, Ramos B, et al. Venezuelan haemorrhagic fever. Lancet 1991. Oct 26;338(8774):1033–6. [DOI] [PubMed] [Google Scholar]
- 36.Silva-Ramos CR, Montoya-Ruíz C, Faccini-Martínez ÁA, Rodas JD. An updated review and current challenges of Guanarito virus infection, Venezuelan hemorrhagic fever. Arch Virol 2022. Sep;167(9):1727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryder RW, Gandsman EJ. Laboratory-acquired Sabiá virus infection. N Engl J Med 1995. Dec 21;333(25):1716. [DOI] [PubMed] [Google Scholar]
- 38.de Mello Malta F, Amgarten D, Nastri A, et al. Sabiá virus-like mammarenavirus in patient with fatal hemorrhagic fever, Brazil, 2020. Emerg Infect Dis 2020. Jun;26(6):1332–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nastri AC, Duarte-Neto AN, Casadio LVB, et al. Understanding Sabiá virus infections (Brazilian mammarenavirus). Travel Med Infect Dis 2022. Jul-Aug;48:102351. [DOI] [PubMed] [Google Scholar]
- 40.Maiztegui J, Feuillade M, Briggiler A. Progressive extension of the endemic area and changing incidence of Argentine hemorrhagic fever. Med Microbiol Immunol 1986;175(2–3):149–52. [DOI] [PubMed] [Google Scholar]
- 41.García Gili MI, Zampetti A, Asencio MD, et al. Fiebre hemorrágica argentina: comunicación de dos casos en zona no endémica. Medicina (B Aires) 2023;83(1):129–32. [PubMed] [Google Scholar]
- 42.Mackenzie RB. Epidemiology of Machupo virus infection. I. Pattern of human infection, San Joaquín, Bolivia, 1962–1964. Am J Trop Med Hyg 1965. Sep;14(5):808–13. [DOI] [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention. Bolivian hemorrhagic fever - El Beni Department, Bolivia, 1994. MMWR Morb Mortal Wkly Rep 1994. Dec 23;43(50):943–6. [PubMed] [Google Scholar]
- 44.Aguilar PV, Camargo W, Vargas J, et al. Reemergence of Bolivian hemorrhagic fever, 2007–2008. Emerg Infect Dis 2009. Sep;15(9):1526–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silva-Ramos CR, Faccini-Martinez AA, Calixto OJ, Hidalgo M. Bolivian hemorrhagic fever: A narrative review. Travel Med Infect Dis 2021. Mar-Apr;40:102001. [DOI] [PubMed] [Google Scholar]
- 46.Simons D Lassa fever cases suffer from severe underreporting based on reported fatalities. Int Health 2023. Sep 1;15(5):608–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richmond JK, Baglole DJ. Lassa fever: epidemiology, clinical features, and social consequences. BMJ 2003. Nov 29;327(7426):1271–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.World Health Organization. R&D Blueprint. https://www.who.int/teams/blueprint/. 2023.; ●● (Identifies LASV MCM R&D as an utmost priority).
- 49.Escalera-Antezana JP, Rodriguez-Villena OJ, Arancibia-Alba AW, et al. Clinical features of fatal cases of Chapare virus hemorrhagic fever originating from rural La Paz, Bolivia, 2019: a cluster analysis. Travel Med Infect Dis 2020. Jul-Aug;36:101589. [DOI] [PubMed] [Google Scholar]
- 50.Coimbra TLM, Nassar ES, Burattini MN, et al. New arenavirus isolated in Brazil. Lancet 1994. Feb 12;343(8894):391–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Manzione N, Salas RA, Paredes H, et al. Venezuelan hemorrhagic fever: clinical and epidemiological studies of 165 cases. Clin Infect Dis 1998. Feb;26(2):308–13. [DOI] [PubMed] [Google Scholar]
- 52.Arribalzaga RA. Una nueva enfermedad epidémica a germen desconocido: hipertermia nefrotóxica, leucopénica y enantemática. Día Méd 1955. Jun 16;27(40):1204–10. [PubMed] [Google Scholar]
- 53.Harrison LH, Halsey NA, McKee KT Jr., et al. Clinical case definitions for Argentine hemorrhagic fever. Clin Infect Dis 1999. May;28(5):1091–4. [DOI] [PubMed] [Google Scholar]
- 54.Molteni HD, Guarinos HC, Petrillo CO, Jaschek F. Estudio clínico estadístico sobre 338 pacientes afectados por la fiebre hemorrágica epidémica del noroeste de la provincia de Buenos Aires. Sem Méd 1961. Apr 24;118:839–55. [PubMed] [Google Scholar]
- 55.Pirosky I, Zuccarini J, Molinelli EA, et al. Virosis hemorrágica del noroeste bonaerense (endemo-epidémica, febril, enantémática, y leucopenica). Ministerio de Asistencia Social y Salud Pública. Buenos Aires, Argentina: Buenos Aires Instituto Nacional de Microbiología; 1959. [Google Scholar]
- 56.Ruggiero HR, Parodi AS, Ruggiero HG, et al. Fiebre hemorrágica argentina. Periodo de incubación e invasión. Rev Asoc Med Argent 1964. May;78:221–6. [PubMed] [Google Scholar]
- 57.Rugiero HR, Cintora FA, Magnoni C, et al. Fiebre hemorrágica argentina. IV. Formas clínicas. Rev Asoc Med Argent 1964. Sep;78:500–10. [PubMed] [Google Scholar]
- 58.Rugiero HR, Ruggiero H, González Cambaceres C, et al. Fiebre hemorrágica argentina. II. Estudio clínico descriptivo. Rev Asoc Med Argent 1964. Jun;78:281–94. [PubMed] [Google Scholar]
- 59.Schwarz ER, Mando OG, Maiztegui JI, Vilches AM. Síntomas y signos iniciales de mayor valor diagnóstico en la fiebre hemorrágica argentina. Medicina (B Aires) 1970. Sep;30 Suppl 1:8–14. [PubMed] [Google Scholar]
- 60.Sewlall NH, Richards G, Duse A, et al. Clinical features and patient management of Lujo hemorrhagic fever. PLoS Negl Trop Dis 2014;8(11):e3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stinebaugh BJ, Schloeder FX, Johnson KM, et al. Bolivian hemorrhagic fever. A report of four cases. Am J Med 1966. Feb;40(2):217–30. [DOI] [PubMed] [Google Scholar]
- 62.Barry M, Russi M, Armstrong L, et al. Treatment of a laboratory-acquired Sabiá virus infection. N Engl J Med 1995. Aug 3;333(5):294–6. [DOI] [PubMed] [Google Scholar]
- 63.Frank MG, Beitscher A, Webb CM, Raabe V. South American hemorrhagic fevers: a summary for clinicians. Int J Infect Dis 2021. Apr;105:505–15. [DOI] [PubMed] [Google Scholar]
- 64.Peters CJ. Human infection with arenaviruses in the Americas. Curr Top Microbiol Immunol 2002;262:65–74. [DOI] [PubMed] [Google Scholar]
- 65.Vainrub B, Salas R. Latin American hemorrhagic fever. Infect Dis Clin North Am 1994. Mar;8(1):47–59. [PubMed] [Google Scholar]
- 66.Bausch DG, Demby AH, Coulibaly M, et al. Lassa fever in Guinea: I. Epidemiology of human disease and clinical observations. Vector Borne Zoonotic Dis 2001. Winter;1(4):269–81. [DOI] [PubMed] [Google Scholar]
- 67.Cummins D, Bennett D, Fisher-Hoch SP, et al. Lassa fever encephalopathy: clinical and laboratory findings. J Trop Med Hyg 1992. Jun;95(3):197–201. [PubMed] [Google Scholar]
- 68.Cummins D, McCormick JB, Bennett D, et al. Acute sensorineural deafness in Lassa fever. J Am Med Assoc 1990. Oct 24–31;264(16):2093–6. [PubMed] [Google Scholar]
- 69.Frame JD. Clinical features of Lassa fever in Liberia. Rev Infect Dis 1989. May-Jun;11 Suppl 4:S783–9. [DOI] [PubMed] [Google Scholar]
- 70.Frame JD, Baldwin JM Jr., Gocke DJ, Troup JM. Lassa fever, a new virus disease of man from West Africa. I. Clinical description and pathological findings. Am J Trop Med Hyg 1970. Jul;19(4):670–6. [DOI] [PubMed] [Google Scholar]
- 71.Garry RF. Lassa fever - the road ahead. Nat Rev Microbiol 2023. Feb;21(2):87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]; ●● (Highly informative and in-depth expert review of Lassa fever).
- 72.Knobloch J, McCormick JB, Webb PA, et al. Clinical observations in 42 patients with Lassa fever. Tropenmed Parasitol 1980. Dec;31(4):389–98. [PubMed] [Google Scholar]
- 73.Monson MH, Cole AK, Frame JD, et al. Pediatric Lassa fever: a review of 33 Liberian cases. Am J Trop Med Hyg 1987. Mar;36(2):408–15. [DOI] [PubMed] [Google Scholar]
- 74.Okokhere P, Colubri A, Azubike C, et al. Clinical and laboratory predictors of Lassa fever outcome in a dedicated treatment facility in Nigeria: a retrospective, observational cohort study. Lancet Infect Dis 2018. Jun;18(6):684–95. [DOI] [PMC free article] [PubMed] [Google Scholar]; ● (Observational study describing acute kidney injury in acute Lassa fever patients).
- 75.Buchmeier MJ. Arenaviruses: protein structure and function. Curr Top Microbiol Immunol 2002;262:159–73. [DOI] [PubMed] [Google Scholar]
- 76.Li S, Sun Z, Pryce R, et al. Acidic pH-induced conformations and LAMP1 binding of the Lassa virus glycoprotein spike. PLoS Pathog 2016. Feb;12(2):e1005418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Neuman BW, Adair BD, Burns JW, et al. Complementarity in the supramolecular design of arenaviruses and retroviruses revealed by electron cryomicroscopy and image analysis. J Virol 2005. Mar;79(6):3822–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hass M, Westerkofsky M, Müller S, et al. Mutational analysis of the Lassa virus promoter. J Virol 2006. Dec;80(24):12414–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Auperin DD, Compans RW, Bishop DHL. Nucleotide sequence conservation at the 3’ termini of the virion RNA species of New World and Old World arenaviruses. Virology 1982. Aug;121(1):200–3. [DOI] [PubMed] [Google Scholar]
- 80.Harnish DG. Arenavirus replication In: Salvato M, ed. The Arenaviridae. New York: Plenum Press; 1993:157–74. [Google Scholar]
- 81.Hastie KM, Kimberlin CR, Zandonatti MA, et al. Structure of the Lassa virus nucleoprotein reveals a dsRNA-specific 3’ to 5’ exonuclease activity essential for immune suppression. Proc Natl Acad Sci U S A 2011. Feb 8;108(6):2396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hastie KM, Liu T, Li S, et al. Crystal structure of the Lassa virus nucleoprotein-RNA complex reveals a gating mechanism for RNA binding. Proc Natl Acad Sci U S A 2011. Nov 29;108(48):19365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martínez-Sobrido L, Emonet S, Giannakas P, et al. Identification of amino acid residues critical for the anti-interferon activity of the nucleoprotein of the prototypic arenavirus lymphocytic choriomeningitis virus. J Virol 2009. Nov;83(21):11330–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bederka LH, Bonhomme CJ, Ling EL, Buchmeier MJ. Arenavirus stable signal peptide is the keystone subunit for glycoprotein complex organization. mBio 2014. Oct 28;5(6):e02063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kunz S, Edelmann KH, de la Torre J-C, et al. Mechanisms for lymphocytic choriomeningitis virus glycoprotein cleavage, transport, and incorporation into virions. Virology 2003. Sep 15;314(1):168–78. [DOI] [PubMed] [Google Scholar]
- 86.Pennington HN, Lee J. Lassa virus glycoprotein complex review: insights into its unique fusion machinery. Biosci Rep 2022. Feb 25;42(2):BSR20211930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Burri DJ, da Palma JR, Kunz S, Pasquato A. Envelope glycoprotein of arenaviruses. Viruses 2012. Oct 17;4(10):2162–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kranzusch PJ, Schenk AD, Rahmeh AA, et al. Assembly of a functional Machupo virus polymerase complex. Proc Natl Acad Sci U S A 2010. Nov 16;107(46):20069–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu X, Peng R, Peng Q, et al. Cryo-EM structures of Lassa and Machupo virus polymerases complexed with cognate regulatory Z proteins identify targets for antivirals. Nat Microbiol 2021. Jul;6(7):921–31. [DOI] [PubMed] [Google Scholar]; ●● (Provides in-depth understanding of the mammarenavirus polymerase complex to enable targeted antiviral development).
- 90.Perez M, Craven RC, de la Torre JC. The small RING finger protein Z drives arenavirus budding: implications for antiviral strategies. Proc Natl Acad Sci U S A 2003. Oct 28;100(22):12978–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fehling SK, Lennartz F, Strecker T. Multifunctional nature of the arenavirus RING finger protein Z. Viruses 2012. Nov 9;4(11):2973–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hastie KM, Zandonatti M, Liu T, et al. Crystal structure of the oligomeric form of Lassa virus matrix protein Z. J Virol 2016. May;90(9):4556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vela EM, Zhang L, Colpitts TM, et al. Arenavirus entry occurs through a cholesterol-dependent, non-caveolar, clathrin-mediated endocytic mechanism. Virology 2007. Dec 5;369(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cao W, Henry MD, Borrow P, et al. Identification of α-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science 1998. Dec 11;282(5396):2079–81. [DOI] [PubMed] [Google Scholar]
- 95.Sheikh MO, Capicciotti CJ, Liu L, et al. Cell surface glycan engineering reveals that matriglycan alone can recapitulate dystroglycan binding and function. Nat Commun 2022. Jun 24;13(1):3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Raaben M, Jae LT, Herbert AS, et al. NRP2 and CD63 are host factors for Lujo virus cell entry. Cell Host Microbe 2017. Nov 8;22(5):688–96 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Radoshitzky SR, Abraham J, Spiropoulou CF, et al. Transferrin receptor 1 is a cellular receptor for New World haemorrhagic fever arenaviruses. Nature 2007. Mar 1;446(7131):92–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Flanagan ML, Oldenburg J, Reignier T, et al. New World clade B arenaviruses can use transferrin receptor 1 (TfR1)-dependent and -independent entry pathways, and glycoproteins from human pathogenic strains are associated with the use of TfR1. J Virol 2008. Jan;82(2):938–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Radoshitzky SR, Kuhn JH, Spiropoulou CF, et al. Receptor determinants of zoonotic transmission of New World hemorrhagic fever arenaviruses. Proc Natl Acad Sci U S A 2008. Feb 19;105(7):2664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jae LT, Raaben M, Herbert AS, et al. Lassa virus entry requires a trigger-induced receptor switch. Science 2014. Jun 27;344(6191):1506–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Meyer BJ, de la Torre JC, Southern PJ. Arenaviruses: genomic RNAs, transcription, and replication. Curr Top Microbiol Immunol 2002;262:139–57. [DOI] [PubMed] [Google Scholar]
- 102.Meyer BJ, Southern PJ. Concurrent sequence analysis of 5’ and 3’ RNA termini by intramolecular circularization reveals 5’ nontemplated bases and 3’ terminal heterogeneity for lymphocytic choriomeningitis virus mRNAs. J Virol 1993. May;67(5):2621–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eichler R, Strecker T, Kolesnikova L, et al. Characterization of the Lassa virus matrix protein Z: electron microscopic study of virus-like particles and interaction with the nucleoprotein (NP). Virus Res 2004. Mar 15;100(2):249–55. [DOI] [PubMed] [Google Scholar]
- 104.Strecker T, Eichler R, ter Meulen J, et al. Lassa virus Z protein is a matrix protein and sufficient for the release of virus-like particles J Virol 2003. Oct;77(19):10700–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Canonico PG. Efficacy, toxicology and clinical applications of ribavirin against virulent RNA viral infections. Antiviral Res 1985. 1985/01/01/;5 Suppl 1:75–81. [DOI] [PubMed] [Google Scholar]
- 106.Graci JD, Cameron CE. Therapeutically targeting RNA viruses via lethal mutagenesis. Future Virol 2008. Nov;3(6):553–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Parker WB. Metabolism and antiviral activity of ribavirin. Virus Res 2005. Feb;107(2):165–71. [DOI] [PubMed] [Google Scholar]
- 108.Moreno H, Grande-Pérez A, Domingo E, Martín V. Arenaviruses and lethal mutagenesis. Prospects for new ribavirin-based interventions. Viruses 2012. Nov 6;4(11):2786–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ölschläger S, Neyts J, Günther S. Depletion of GTP pool is not the predominant mechanism by which ribavirin exerts its antiviral effect on Lassa virus. Antiviral Res 2011. Aug;91(2):89–93. [DOI] [PubMed] [Google Scholar]
- 110.Snell NJ. Ribavirin - current status of a broad spectrum antiviral agent. Expert Opin Pharmacother 2001. Aug;2(8):1317–24. [DOI] [PubMed] [Google Scholar]
- 111.Crotty S, Maag D, Arnold JJ, et al. The broad-spectrum antiviral ribonucleoside ribavirin is an RNA virus mutagen. Nat Med 2000. Dec;6(12):1375–9. [DOI] [PubMed] [Google Scholar]
- 112.Carrillo-Bustamante P, Nguyen THT, Oestereich L, et al. Determining ribavirin’s mechanism of action against Lassa virus infection. Sci Rep 2017. Sep 15;7(1):11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McCormick JB, King IJ, Webb PA, et al. Lassa fever. Effective therapy with ribavirin. N Engl J Med 1986. Jan 2;314(1):20–6. [DOI] [PubMed] [Google Scholar]
- 114.Salam AP, Cheng V, Edwards T, et al. Time to reconsider the role of ribavirin in Lassa fever. PLoS Negl Trop Dis 2021. Jul;15(7):e0009522. [DOI] [PMC free article] [PubMed] [Google Scholar]; ●● (Crucial critical assessment of ribavirin as a treatment of Lassa fever).
- 115.Eberhardt KA, Mischlinger J, Jordan S, et al. Ribavirin for the treatment of Lassa fever: A systematic review and meta-analysis. Int J Infect Dis 2019. Oct;87:15–20. [DOI] [PubMed] [Google Scholar]
- 116.Cheng H-Y, French CE, Salam AP, et al. Lack of evidence for ribavirin treatment of Lassa fever in systematic review of published and unpublished studies. Emerg Infect Dis 2022. Aug;28(8):1559–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Enria DA, Maiztegui JI. Antiviral treatment of Argentine hemorrhagic fever. Antiviral Res 1994. Jan;23(1):23–31. [DOI] [PubMed] [Google Scholar]
- 118.Kilgore PE, Ksiazek TG, Rollin PE, et al. Treatment of Bolivian hemorrhagic fever with intravenous ribavirin. Clin Infect Dis 1997. Apr;24(4):718–22. [DOI] [PubMed] [Google Scholar]
- 119.McKee KT Jr., Huggins JW, Trahan CJ, Mahlandt BG. Ribavirin prophylaxis and therapy for experimental Argentine hemorrhagic fever. Antimicrob Agents Chemother 1988. Sep;32(9):1304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Enria DA, Briggiler AM, Levis S, et al. Tolerance and antiviral effect of ribavirin in patients with Argentine hemorrhagic fever. Antiviral Res 1987. Jul;7(6):353–9. [DOI] [PubMed] [Google Scholar]
- 121.Stephen EL, Jones DE, Peters CJ, et al. Ribavirin treatment of toga-, arena-, bunyavirus infection in subhuman primates and other laboratory animal species. In: Smith RA, Kirkpatrick W, eds. Ribavirin: A Broad Spectrum Antiviral Agent. New York, New York, USA: Academic Press; 1980:169–83. [Google Scholar]
- 122.Kenyon RH, Canonico PG, Green DE, Peters CJ. Effect of ribavirin and tributylribavirin on Argentine hemorrhagic fever (Junin virus) in guinea pigs. Antimicrob Agents Chemother 1986. Mar;29(3):521–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jahrling PB, Hesse RA, Eddy GA, et al. Lassa virus infection of rhesus monkeys: pathogenesis and treatment with ribavirin. J Infect Dis 1980. May;141(5):580–9. [DOI] [PubMed] [Google Scholar]
- 124.Weissenbacher MC, Calello MA, Merani MS, et al. Therapeutic effect of the antiviral agent ribavirin in Junín virus infection of primates. J Med Virol 1986. Nov;20(3):261–7. [DOI] [PubMed] [Google Scholar]
- 125.Jahrling PB, Peters CJ, Stephen EL. Enhanced treatment of Lassa fever by immune plasma combined with ribavirin in cynomolgus monkeys. J Infect Dis 1984. Mar;149(3):420–7. [DOI] [PubMed] [Google Scholar]
- 126.Lingas G, Rosenke K, Safronetz D, Guedj J. Lassa viral dynamics in non-human primates treated with favipiravir or ribavirin. PLoS Comput Biol 2021. Jan;17(1):e1008535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Contin M, Sepúlveda C, Fascio M, et al. Modified ribavirin analogues as antiviral agents against Junín virus. Bioorg Med Chem Lett 2019. Feb 15;29(4):556–59. [DOI] [PubMed] [Google Scholar]
- 128.Gowen BB, Wong M-H, Jung K-H, et al. In vitro and in vivo activities of T-705 against arenavirus and bunyavirus infections. Antimicrob Agents Chemother 2007. Sep;51(9):3168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mendenhall M, Russell A, Juelich T, et al. T-705 (favipiravir) inhibition of arenavirus replication in cell culture. Antimicrob Agents Chemother 2011. Feb;55(2):782–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gowen BB, Smee DF, Wong M-H, et al. Treatment of late stage disease in a model of arenaviral hemorrhagic fever: T-705 efficacy and reduced toxicity suggests an alternative to ribavirin. PLoS One 2008;3(11):e3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci 2017;93(7):449–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Goldhill DH, Langat P, Xie H, et al. Determining the mutation bias of favipiravir in influenza virus using next-generation sequencing. J Virol 2019. Jan 15;93(2):e01217–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Furuta Y, Gowen BB, Takahashi K, et al. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res 2013. Nov;100(2):446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zadeh VR, Afowowe TO, Abe H, et al. Potential and action mechanism of favipiravir as an antiviral against Junin virus. PLoS Pathog 2022. Jul;18(7):e1010689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Safronetz D, Rosenke K, Westover JB, et al. The broad-spectrum antiviral favipiravir protects guinea pigs from lethal Lassa virus infection post-disease onset. Sci Rep 2015. Oct 12;5:14775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gowen BB, Juelich TL, Sefing EJ, et al. Favipiravir (T-705) inhibits Junín virus infection and reduces mortality in a guinea pig model of Argentine hemorrhagic fever. PLoS Negl Trop Dis 2013;7(12):e2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rosenke K, Feldmann H, Westover JB, et al. Use of favipiravir to treat Lassa virus infection in macaques. Emerg Infect Dis 2018. Sep;24(9):1696–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Oestereich L, Rieger T, Lüdtke A, et al. Efficacy of favipiravir alone and in combination with ribavirin in a lethal, immunocompetent mouse model of Lassa fever. J Infect Dis 2016. Mar 15;213(6):934–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Larson RA, Dai D, Hosack VT, et al. Identification of a broad-spectrum arenavirus entry inhibitor. J Virol 2008. Nov;82(21):10768–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cashman KA, Smith MA, Twenhafel NA, et al. Evaluation of Lassa antiviral compound ST-193 in a guinea pig model. Antiviral Res 2011. Apr;90(1):70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Madu IG, Files M, Gharaibeh DN, et al. A potent Lassa virus antiviral targets an arenavirus virulence determinant. PLoS Pathog 2018. Dec;14(12):e1007439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sattler RA, Paessler S, Ly H, Huang C. Animal models of Lassa fever. Pathogens 2020. Mar 6;9(3):197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cashman KA, Wilkinson ER, Posakony J, et al. Lassa antiviral LHF-535 protects guinea pigs from lethal challenge. Sci Rep 2022. Nov 19;12(1):19911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Amberg SM, Snyder B, Vliet-Gregg PA, et al. Safety and pharmacokinetics of LHF-535, a potential treatment for Lassa fever, in healthy adults. Antimicrob Agents Chemother 2022. Nov 15;66(11):e0095122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Plewe MB, Gantla VR, Sokolova NV, et al. Discovery of a novel highly potent broad-spectrum heterocyclic chemical series of arenavirus cell entry inhibitors. Bioorg Med Chem Lett 2021. Jun 1;41:127983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Golden JW, Hammerbeck CD, Mucker EM, Brocato RL. Animal models for the study of rodent-borne hemorrhagic fever viruses: arenaviruses and hantaviruses. Biomed Res Int 2015;2015:793257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Westover JB, Naik S, Bailey KW, et al. Severe mammarenaviral disease in guinea pigs effectively treated by an orally bioavailable fusion inhibitor, alone or in combination with favipiravir. Antiviral Res 2022. Dec;208:105444. [DOI] [PMC free article] [PubMed] [Google Scholar]; ● (Shows synergistic activity of ARN-75039 in combination with favipiravir and improved survival outcomes in guinea pigs exposed to JUNV).
- 148.Radoshitzky SR, Kuhn JH, de Kok-Mercado F, et al. Drug discovery technologies and strategies for Machupo virus and other New World arenaviruses. Expert Opin Drug Discov 2012. Jul;7(7):613–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Welch SR, Spengler JR, Genzer SC, et al. Screening and identification of Lujo virus inhibitors using a recombinant reporter virus platform. Viruses 2021. Jun 28;13(7):1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mizutani T, Ohba Y, Mizuta S, et al. An antiviral drug screening platform with a FRET biosensor for measurement of arenavirus Z assembly. Cell Struct Funct 2020. Dec 25;45(2):155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Löw K, Möller R, Stegmann C, et al. Luminescent reporter cells enable the identification of broad-spectrum antivirals against emerging viruses. J Med Virol 2023. Nov;95(11):e29211. [DOI] [PubMed] [Google Scholar]
- 152.Hou Y, Liu Y, Jia X, et al. Screening and identification of Lassa virus entry inhibitors from a fragment-based drug discovery library. Viruses 2022. Nov 27;14(12):2649. [DOI] [PMC free article] [PubMed] [Google Scholar]; ● (Implementation of a novel high-throughput screening technique to fragment-based drug librarys to identify LASV cell entry inhibitors).
- 153.Oestereich L, Wurr S, Becker-Ziaja B, et al. Establishment of recombinant trisegmented Mopeia virus expressing two reporter genes for screening of mammarenavirus inhibitors. Viruses 2022. Aug 25;14(9):1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cubitt B, Ortiz-Riano E, Cheng BYH, et al. A cell-based, infectious-free, platform to identify inhibitors of Lassa virus ribonucleoprotein (vRNP) activity. Antiviral Res 2020. Jan;173:104667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Caì Y, Iwasaki M, Beitzel BF, et al. Recombinant Lassa virus expressing green fluorescent protein as a tool for high-throughput drug screens and neutralizing antibody assays. Viruses 2018. Nov 20;10(11):655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Welch SR, Guerrero LW, Chakrabarti AK, et al. Lassa and Ebola virus inhibitors identified using minigenome and recombinant virus reporter systems. Antiviral Res 2016. Dec;136:9–18. [DOI] [PubMed] [Google Scholar]
- 157.Lan X, Zhang Y, Jia X, et al. Screening and identification of Lassa virus endonuclease-targeting inhibitors from a fragment-based drug discovery library. Antiviral Res 2022. Jan;197:105230. [DOI] [PubMed] [Google Scholar]
- 158.Mercorelli B, Palu G, Loregian A. Drug repurposing for viral infectious diseases: how far are we? Trends Microbiol 2018. Oct;26(10):865–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Strittmatter SM. Overcoming drug development bottlenecks with repurposing: old drugs learn new tricks. Nat Med 2014. Jun;20(6):590–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kim YJ, Cubitt B, Chen E, et al. The ReFRAME library as a comprehensive drug repurposing library to identify mammarenavirus inhibitors. Antiviral Res 2019. Sep;169:104558. [DOI] [PMC free article] [PubMed] [Google Scholar]; ● (Highlights drug repurposing as a strategy to identify antiviral drug candidates).
- 161.Mizuma K, Takashima A, Cubitt B, et al. The Pan-ErbB tyrosine kinase inhibitor afatinib inhibits multiple steps of the mammarenavirus life cycle. Virology 2022. Nov;576:83–95. [DOI] [PubMed] [Google Scholar]
- 162.Kimura M, Matsuoka R, Taniguchi S, et al. Inhibitors of cannabinoid receptor 1 suppress the cellular entry of Lujo virus. Virology 2023. Oct;587:109867. [DOI] [PubMed] [Google Scholar]
- 163.Zhang X, Tang K, Guo Y. The antifungal isavuconazole inhibits the entry of Lassa virus by targeting the stable signal peptide-GP2 subunit interface of Lassa virus glycoprotein. Antiviral Res 2020. Feb;174:104701. [DOI] [PubMed] [Google Scholar]
- 164.Wang P, Liu Y, Zhang G, et al. Screening and identification of Lassa virus entry inhibitors from an FDA-Approved drug library. J Virol 2018. Aug 15;92(16):e00954–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang X, Yan F, Tang K, et al. Identification of a clinical compound losmapimod that blocks Lassa virus entry. Antiviral Res 2019. Jul;167:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sepúlveda CS, García CC, Damonte EB. Antiviral activity of A771726, the active metabolite of leflunomide, against Junín virus. J Med Virol 2018. May;90(5):819–27. [DOI] [PubMed] [Google Scholar]
- 167.Cao J, Dong S, Liu Y, et al. Screening and identification of Lujo virus entry inhibitors from an Food and Drug Administration-approved drugs library. Front Microbiol 2021;12:793519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hulseberg CE, Fénéant L, Szymańska-de Wijs KM, et al. Arbidol and other low-molecular-weight drugs that inhibit Lassa and Ebola viruses. J Virol 2019. Apr 15;93(8):e02185–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Herring S, Oda JM, Wagoner J, et al. Inhibition of arenaviruses by combinations of orally available approved drugs. Antimicrob Agents Chemother 2021. Mar 18;65(4):e01146–20. [DOI] [PMC free article] [PubMed] [Google Scholar]; ● (Orally available and FDA-approved drugs that suppress mammarenavirus cell entry in vitro offer a new strategy for controling mammaarenaviral infection in endemic areas).
- 170.Uckun FM, Petkevich AS, Vassilev AO, et al. Stampidine prevents mortality in an experimental mouse model of viral hemorrhagic fever caused by Lassa virus. BMC Infect Dis 2004. Jan 13;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lee AM, Rojek JM, Spiropoulou CF, et al. Unique small molecule entry inhibitors of hemorrhagic fever arenaviruses. J Biol Chem 2008. Jul 4;283(27):18734–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Liu Y, Guo J, Cao J, et al. Screening of botanical drugs against Lassa virus entry. J Virol 2021. Mar 25;95(8):e02429–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Urata S, Ngo N, de la Torre JC. The PI3K/Akt pathway contributes to arenavirus budding. J Virol 2012. Apr;86(8):4578–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mohr EL, McMullan LK, Lo MK, et al. Inhibitors of cellular kinases with broad-spectrum antiviral activity for hemorrhagic fever viruses. Antiviral Res 2015. Aug;120:40–7. [DOI] [PubMed] [Google Scholar]
- 175.Toba S, Sato A, Kawai M, et al. Identification of cap-dependent endonuclease inhibitors with broad-spectrum activity against bunyaviruses. Proc Natl Acad Sci U S A 2022. Sep 6;119(36):e2206104119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Takenaga T, Zhang Z, Muramoto Y, et al. CP100356 hydrochloride, a P-glycoprotein inhibitor, inhibits Lassa virus entry: implication of a candidate pan-mammarenavirus entry inhibitor. Viruses 2021. Sep 3;13(9):1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Urata S, Yun N, Pasquato A, et al. Antiviral activity of a small-molecule inhibitor of arenavirus glycoprotein processing by the cellular site 1 protease. J Virol 2011. Jan;85(2):795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Alvarez De Lauro AE, Pelaez MA, Marquez AB, et al. Effects of the natural flavonoid quercetin on arenavirus Junín infection. Viruses 2023. Aug 15;15(8):1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bolken TC, Laquerre S, Zhang Y, et al. Identification and characterization of potent small molecule inhibitor of hemorrhagic fever New World arenaviruses. Antiviral Res 2006. Feb;69(2):86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Torriani G, Trofimenko E, Mayor J, et al. Identification of clotrimazole derivatives as specific inhibitors of arenavirus fusion. J Virol 2019. Mar 15;93(6):e01744–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tang K, He S, Zhang X, et al. Tangeretin, an extract from Citrus peels, blocks cellular entry of arenaviruses that cause viral hemorrhagic fever. Antiviral Res 2018. Dec;160:87–93. [DOI] [PubMed] [Google Scholar]
- 182.Maiztegui JI, Fernandez NJ, de Damilano AJ. Efficacy of immune plasma in treatment of Argentine haemorrhagic fever and association between treatment and a late neurological syndrome. Lancet 1979. Dec 8;314/ii(8154):1216–7. [DOI] [PubMed] [Google Scholar]; ● (Clinical trial that linked treatment with immune plasma to the development of neurological signs).
- 183.Enria DA, Briggiler AM, Fernandez NJ, et al. Importance of dose of neutralising antibodies in treatment of Argentine haemorrhagic fever with immune plasma. Lancet 1984. Aug 4;324/ii(8397):255–6. [DOI] [PubMed] [Google Scholar]
- 184.Enria DA, de Damilano AJ, Briggiler AM, et al. Síndrome neurológico tardío en enfermos de fiebre hemorrágica argentina tratados con plasma inmune. Medicina (B Aires) 1985;45(6):615–20. [PubMed] [Google Scholar]
- 185.Enria DA, Briggiler AM, Sánchez Z. Treatment of Argentine hemorrhagic fever. Antiviral Res 2008. Apr;78(1):132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ruggiero HA, Pérez Isquierdo F, Milani HA, et al. Traitement de la fièvre hémorragique argentine par le plasma de convalescent. 4,433 cas. Presse Méd 1986. Dec 20;15(45):2239–42. [PubMed] [Google Scholar]
- 187.Leifer E, Gocke DJ, Bourne H. Lassa fever, a new virus disease of man from West Africa. II. Report of a laboratory-acquired infection treated with plasma from a person recently recovered from the disease. Am J Trop Med Hyg 1970. Jul;19(4):677–9. [DOI] [PubMed] [Google Scholar]
- 188.White HA. Lassa fever. A study of 23 hospital cases. Trans R Soc Trop Med Hyg 1972;66(3):390–401. [DOI] [PubMed] [Google Scholar]
- 189.Frame JD, Verbrugge GP, Gill RG, Pinneo L. The use of Lassa fever convalescent plasma in Nigeria. Trans R Soc Trop Med Hyg 1984;78(3):319–24. [DOI] [PubMed] [Google Scholar]
- 190.Jahrling PB. Protection of Lassa virus-infected guinea pigs with Lassa-immune plasma of guinea pig, primate, and human origin. J Med Virol 1983;12(2):93–102. [DOI] [PubMed] [Google Scholar]
- 191.Jahrling PB, Peters CJ. Passive antibody therapy of Lassa fever in cynomolgus monkeys: importance of neutralizing antibody and Lassa virus strain. Infect Immun 1984. May;44(2):528–33. [DOI] [PMC free article] [PubMed] [Google Scholar]; ●● (Demonstrates the importance of neutralizing antibody titers in the defense against typically lethal exposure of NHPs to LASV).
- 192.Eddy GA, Wagner FS, Scott SK, Mahlandt BJ. Protection of monkeys against Machupo virus by the passive administration of Bolivian haemorrhagic fever immunoglobulin (human origin). Bull World Health Organ 1975;52(4–6):723–7. [PMC free article] [PubMed] [Google Scholar]
- 193.Kenyon RH, Green DE, Eddy GA, Peters CJ. Treatment of Junin virus-infected guinea pigs with immune serum: development of late neurological disease. J Med Virol 1986. Nov;20(3):207–18. [DOI] [PubMed] [Google Scholar]
- 194.Zeitlin L, Geisbert JB, Deer DJ, et al. Monoclonal antibody therapy for Junin virus infection. Proc Natl Acad Sci U S A 2016. Apr 19;113(16):4458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cross RW, Mire CE, Branco LM, et al. Treatment of Lassa virus infection in outbred guinea pigs with first-in-class human monoclonal antibodies. Antiviral Res 2016. Sep;133:218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mire CE, Cross RW, Geisbert JB, et al. Human-monoclonal-antibody therapy protects nonhuman primates against advanced Lassa fever. Nat Med 2017. Oct;23(10):1146–49. [DOI] [PMC free article] [PubMed] [Google Scholar]; ● (Important proof-of-concept study in NHPs to advance monoclonal antibody therapies against LASV infection).
- 197.Cross RW, Hastie KM, Mire CE, et al. Antibody therapy for Lassa fever. Curr Opin Virol 2019. Aug;37:97–104. [DOI] [PubMed] [Google Scholar]
- 198.Li H, Buck T, Zandonatti M, et al. A cocktail of protective antibodies subverts the dense glycan shield of Lassa virus. Sci Transl Med 2022. Oct 26;14(668):eabq0991. [DOI] [PMC free article] [PubMed] [Google Scholar]; ● (Further development of monoclonal antibody-based therapeutic opportunities to control LASV replication).
- 199.Cross RW, Heinrich ML, Fenton KA, et al. A human monoclonal antibody combination rescues nonhuman primates from advanced disease caused by the major lineages of Lassa virus. Proc Natl Acad Sci U S A 2023. Aug 22;120(34):e2304876120. [DOI] [PMC free article] [PubMed] [Google Scholar]; ●● (Promising cocktail of LASV monoclonal antibodies provides protection against LASV of two lineages of LASV in NHPs).
- 200.Woolsey C, Cross RW, Prasad AN, et al. Monoclonal antibody therapy demonstrates increased virulence of a lineage VII strain of Lassa virus in nonhuman primates. Emerg Microbes Infect 2024. Jan 2;13:2301061. [DOI] [PMC free article] [PubMed] [Google Scholar]; ● (Highlights the importance of time-sensitive therapeutic delivery of a monoclonal antibody cocktail to achieve protection from LASV in NHPs).
- 201.Allio T The FDA Animal Rule and its role in protecting human safety. Expert Opin Drug Saf 2018. Oct;17(10):971–73. [DOI] [PubMed] [Google Scholar]
- 202.Vela E Animal models, prophylaxis, and therapeutics for arenavirus infections. Viruses 2012. Sep;4(9):1802–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Stevens H, Huys I. Innovative approaches to increase access to medicines in developing countries. Front Med (Lausanne) 2017;4:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Cassetti MC, Pierson TC, Patterson LJ, et al. Prototype pathogen approach for vaccine and monoclonal antibody development: a critical component of the NIAID plan for pandemic preparedness. J Infect Dis 2023. Jun 15;227(12):1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Duvignaud A, Jaspard M, Etafo IC, et al. Lassa fever outcomes and prognostic factors in Nigeria (LASCOPE): a prospective cohort study. Lancet Glob Health 2021. Apr;9(4):e469–e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Okokhere PO, Erameh CO, Alikah F, et al. Acute Lassa virus encephalitis with Lassa virus in the cerebrospinal fluid but absent in the blood: a case report with a positive outcome. Case Rep Neurol 2018. May-Aug;10(2):150–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Shah DK, Betts AM. Antibody biodistribution coefficients: inferring tissue concentrations of monoclonal antibodies based on the plasma concentrations in several preclinical species and human. MAbs 2013. Mar-Apr;5(2):297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kuhn JH, Radoshitzky SR, Jahrling PB. Pathogens causing viral hemorrhagic fevers. In: Katz R, Zilinskas RA, eds. Encyclopedia of Bioterrorism Defense. 2nd ed. Hoboken, New Jersey, USA: Wiley-Blackwell; 2011:489–98. [Google Scholar]
- 209.Vilibic-Cavlek T, Savic V, Ferenc T, et al. Lymphocytic choriomeningitis—emerging trends of a neglected virus: a narrative review. Trop Med Infect Dis 2021. May 25;6(2):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.World Health Organization. International Classification of Diseases 11th Revision. https://icd.who.int/browse11/l-m/en. 2023.
- 211.U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, Health NIo. Biosafety in Microbiological and Biomedical Laboratories (BMBL) 6th Edition. https://www.cdc.gov/labs/BMBL.html. 2020. [Google Scholar]
- 212.The Australia Group. List of human and animal pathogens and toxins for export control. https://www.dfat.gov.au/publications/minisite/theaustraliagroupnet/site/en/human_animal_pathogens.html. 2022.
- 213.U.S. Centers for Disease Control and Prevention. Bioterrrorism Agents/Diseases. https://emergency.cdc.gov/agent/agentlist-category.asp. 2018.
- 214.U.S. Department of Health and Human Services, U.S. Department of Agriculture. HHS and USDA Select Agents and Toxins. 7CFR Part 331, 9 CFR Part 121, and 42 CFR Part 73 https://www.selectagents.gov/sat/list.htm. 2023.
- 215.U.S. National Institute of Allergy and Infectious Diseases. NIAID Emerging Infectious Diseases/Pathogens. https://www.niaid.nih.gov/research/emerging-infectious-diseases-pathogens. 2018.
- 216.U.S. National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/. 2023. [DOI] [PubMed]; ●● (Lists clinical trials for evaluation of antivirals).


