Abstract
Objective
This study aims to analyze a severe adverse reaction of pulmonary fibrosis induced by dronedarone hydrochloride tablets, and to provide a reference for clinical rational medication through drug precautions.
Methods
A case of pulmonary fibrosis induced by dronedarone hydrochloride tablets, along with related literature was retrospectively analyzed.
Results
Patients over 65 years old with a history of exposure to amiodarone may increase the incidence of pulmonary toxicity induced by dronedarone, and dronedarone should not be selected as a substitute treatment drug for patients with amiodarone-induced pulmonary toxicity.
Conclusions
It is recommended that clinicians monitor the diffusion capacity of carbon monoxide and lung ventilation function of patients before and after using dronedarone for treatment. For patients with a history of amiodarone exposure, intermittent monitoring of chest X-rays and lung function is necessary. If lung function decreases, dronedarone should be immediately discontinued.
Key words: Adverse reactions, Amiodarone hydrochloride tablets, Dronedarone hydrochloride tablets, Drug precaution, Pulmonary fibrosis
Introduction
Amiodarone is a classic class III antiarrhythmic drug commonly used to treat arrhythmias. However, it has a long half-life, which can lead to cumulative poisoning, coupled with various adverse reactions. Pulmonary toxicity is one of its severe adverse reactions.1 Dronedarone hydrochloride is a deiodinated benzofuran derivative structurally similar to amiodarone (Figure 1). Its mechanism of action in antiarrhythmic therapy is similar to that of amiodarone but with significantly reduced adverse reactions. This makes it a good choice for patients with toxic reactions caused by amiodarone during treatment.2 Currently, there are few reports on dronedarone causing pulmonary fibrosis, although there have been cases reported of interstitial lung disease.3 Here, we report a severe adverse reaction of pulmonary fibrosis induced by dronedarone hydrochloride tablets in our hospital to provide a reference for the clinical use of this drug. Meanwhile, we also analyze and discuss the rationality of replacing amiodarone with dronedarone after amiodarone-induced pulmonary toxicity to promote clinically rational medication.
Figure 1.
Comparison of the structures of amiodarone and dronedarone.
Case Description
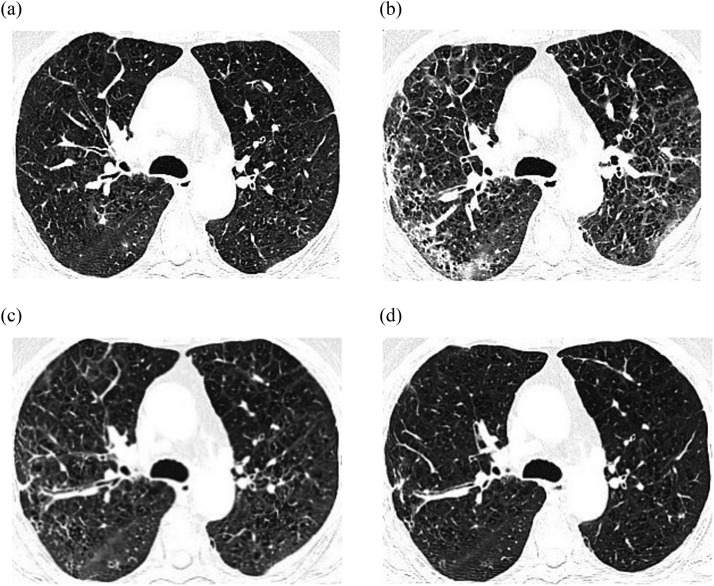
A 66-year-old male patient was admitted on April 22, 2022, with “paroxysmal atrial fibrillation” and underwent radiofrequency ablation. During the surgery, the patient was infused with 300 mg of amiodarone hydrochloride injection, followed by oral administration of amiodarone hydrochloride tablets at a gradually decreasing dosage: 200 mg q8h within 5 days after the surgery, 200 mg q12h from day 6 to 12, and 200 mg qd from day 12 onwards. On February 1, 2023, the patient had chest tightness and shortness of breath, which worsened after physical activity and lasted for several minutes. These symptoms recurred and progressed over time. On February 5, arterial blood gas analysis showed a PO2 of 58.1 mmHg, and a chest CT scan showed interstitial proliferation and scattered small nodules in both lungs (Figure 2A). On February 7, due to amiodarone-induced hypothyroidism, the patient was administered dronedarone hydrochloride tablets 400 mg orally twice a day as a replacement therapy. On day 17, the patient had chest tightness and shortness of breath, accompanied by intermittent coughing and white sputum, who also felt weak and had a sore throat. On day 22, the arterial blood gas analysis showed a PO2 of 41.1 mmHg. On day 23, lung function tests showed FEV1/FVC at 50%, FEV1% predicted at 30%, and the lung CO diffusion capacity is 31% predicted value, which suggested severe mixed ventilation dysfunction and severe diffusion dysfunction, moreover, chest CT scan showed pulmonary fibrosis in both lungs (Figure 2B). The patient was suspected to have drug-related interstitial lung disease and type I respiratory failure, so dronedarone hydrochloride tablets were discontinued, and he was administered methylprednisolone sodium succinate 40 mg intravenously once a day for 6 days. His respiratory symptoms improved, and the chest CT scan showed an improvement in pulmonary fibrosis (Figure 2C). On day 30, the patient's dosage was changed to prednisone 40 mg orally once a day. CT re-examination on day 56 (Figure 2D) showed that there was no significant increase in interstitial proliferation in both lungs, and the bilateral pulmonary fibrosis was improved compared to day 30. Changes in the course of the patient's illness are shown in Figure 3.
Figure 2.
Comparison of chest CT changes in the patient.
Figure 3.
Changes in the course of the patient's illness.
Discussion
Amiodarone has a wide range of antiarrhythmic effects and generally does not induce new arrhythmia, making it a commonly used antiarrhythmic drug in the clinic. However, in the case of the patient mentioned above, after using amiodarone hydrochloride tablets for 9 months, he developed chest tightness, shortness of breath, decreased oxygen partial pressure, and changes in pulmonary function and imaging (Figure 2A), as well as hypothyroidism. Therefore, dronedarone hydrochloride tablets were administered. However, about 20 days later, the patient's respiratory symptoms worsened, accompanied by a progressive decrease in arterial blood oxygen partial pressure and further changes in pulmonary imaging. We posit that there exists a temporal correlation between the occurrence of this event and the use of dronedarone. Studies have reported that the incidence of amiodarone-induced pulmonary fibrosis is between 1% and 5%, which usually occurs 12 to 60 months after medication. The risk factors include high total cumulative doses (treatment time exceeding 2 months) and high maintenance doses (>400 mg/day).4 In this case, after the patient was administered dronedarone, his chest tightness, shortness of breath, pulmonary function, and pulmonary imaging did not improve, instead, a progressive exacerbation of these symptoms was observed (Figure 2B). Therefore, the possibility of amiodarone playing a synergistic role in adverse reactions was considered, but the patient was given steroid treatment after stopping dronedarone hydrochloride tablets, resulting in a significant improvement in respiratory symptoms and a significant reduction in pulmonary fibrosis (Figure 2C, D). The patient did not have a history of underlying lung disease, rendering the influence of previous diseases unlikely. Furthermore, the patient had long-term exposure to amlodipine and levothyroxine, which were reported to lead to dyspnea. However, there have been no reports of adverse reactions associated with interstitial pneumonia or pulmonary fibrosis caused by these medications. It was noteworthy that the patient continued to use the aforementioned drugs after developing pulmonary interstitial fibrosis, and their clinical symptoms and pulmonary imaging did not show significant worsening. Thus, there is a high possibility that dronedarone hydrochloride tablets have caused pulmonary fibrosis.
Dronedarone hydrochloride tablets (Multaq, Sanofi company) were approved by the US Food and Drug Administration in July 2009 for the treatment of nonpermanent atrial fibrillation or flutter.5 The introduction of a methylsulfonyl group in its structure reduces the half-life, lipophilicity, and tissue accumulation of the drug, thereby reducing its amiodarone-like toxicity, particularly the adverse effects on the thyroid and lungs.2 Early animal experimentation studies have shown that dronedarone can cause damage to the alveoli, but controlled trials in humans have yet to establish a relationship between dronedarone and alveolar damage. Currently, adverse reactions of pulmonary fibrosis caused by dronedarone have been reported with increasing clinical use (Table).2,3,6,7 Hernández Voth et al3 reported on a 73-year-old man who developed dyspnea, cough, and high-resolution chest CT showed thickening of the interstitium and diffuse opacity of the parenchyma after using dronedarone, suggesting the possibility of pulmonary toxicity caused by dronedarone, which must be used with caution. In addition, Siu et al7 reported 2 cases of pulmonary fibrosis caused by dronedarone, which suggests that patients receiving dronedarone therapy, especially those with a history of amiodarone exposure, need to be monitored intermittently with chest X-rays to detect adverse drug reactions timely.
Table.
Statistics of cases of fibrosis caused by dronedarone.
| Serial number | Author | Time | Journal | Title | Patient's age | Gender | Medication time | History of amiodarone exposure |
|---|---|---|---|---|---|---|---|---|
| 1 | Stack S2 | 2015 | Chest. | Diffuse alveolar damage in a patient receiving dronedarone. | 68 | Female | 6 months | None |
| 2 | Hernández Voth AR3 | 2012 | Am J Respir Crit Care Med | A 73-year-old man with interstitial lung disease due to dronedarone. | 73 | Male | 70 days | 2 years |
| 3 | Thornton D6 | 2015 | J R Coll Physicians Edinb | Organising pneumonia due to dronedarone. | 67 | Male | 2 weeks | None |
| 4 | Siu CW7 | 2012 | Arch Intern Med | Fatal lung toxic effects related to dronedarone use. | 72 | Female | 9 months | 14 months |
| 83 | Male | 12 days | 10 days |
Previous literature research has demonstrated that the safety of dronedarone is better than that of amiodarone, and it is generally recommended as a substitute treatment for amiodarone. Immordino et al8 recommend using dronedarone as a substitute for amiodarone based on the patient's condition within 48 hours. This study retrospectively analyzed the effectiveness and safety of dronedarone in patients with atrial fibrillation who stopped amiodarone within 2 days (Group A, n = 98) and those who had not been treated with amiodarone within 2 months (Group B, n = 680) in the EURIDIS and ADONIS databases. The results showed a similar incidence of slow arrhythmia between the 2 groups, but the discontinuation of medication due to this event was significantly higher in Group A than in Group B. Additionally, Group A experienced more severe heart failure events and heart failure hospitalizations, while the incidence of severe adverse events related to the gastrointestinal tract and events leading to hospitalization or death was similar between the 2 groups. Furthermore, a study by Naccarelli et al9 showed that switching immediately from amiodarone to dronedarone in atrial fibrillation patients after amiodarone loading doses or long-term treatment did not increase the recurrence of arrhythmias or major adverse events, including QTc interval prolongation or drug-induced bradycardia. However, the study excluded patients who changed their amiodarone treatment plan due to amiodarone-related toxicity, such as interstitial lung disease or thyroid disease. Previous studies have demonstrated that dronedarone does not affect thyroid function and can be used as an alternative treatment for patients with amiodarone-induced hyperthyroidism.10 Therefore, can dronedarone be selected as a substitute treatment for patients with amiodarone-induced pulmonary toxicity?
Currently, cases of dronedarone-induced pulmonary fibrosis have been reported to occur from 12 days to 9 months after medication and related risk factors are still unclear. Associated reports have shown that the incidence of adverse reactions in individuals aged 65 and above is twice that of young people.11 The dronedarone product label indicates that the exposure in patients aged 65 and above is 23% higher than that in patients under 65. As shown in Table, all cases of dronedarone-induced pulmonary toxicity occurred in patients over the age of 65, suggesting age may be a relevant risk factor. Furthermore, while there is research supporting the safety of dronedarone as a substitute for amiodarone, the related research did not include analysis of patients with amiodarone-induced pulmonary toxicity. Table also shows that patients with dronedarone-induced pulmonary toxicity had a history of amiodarone exposure. In this case, the patient experienced interstitial proliferation changes after using amiodarone, which progressed to interstitial fibrosis after switching to dronedarone. Therefore, amiodarone has a long half-life of 55 to 60 days and tends to accumulate in adipose tissue and lungs, leading to the possibility of progression of pulmonary toxicity even after discontinuation of the drug for weeks or months. At the same time, patients experienced pulmonary toxicity from amiodarone, who are not recommended to reintroduce amiodarone or other drugs that have been shown to have pulmonary toxicity due to the high risk of recurrence of pulmonary toxicity.12 In conclusion, it is recommended to closely monitor patients with a prior history of amiodarone exposure for any alterations in clinical symptoms, pulmonary function, and lung imaging. Additionally, timely evaluation of amiodarone-associated pulmonary toxicity is strongly recommended. Meanwhile, they should avoid the use of dronedarone as an alternative therapeutic agent for patients with amiodarone-induced pulmonary toxicity and could choose beta-blockers, propafenone, and other drugs with low pulmonary toxicity, while systemic hormone therapy may also be recommended to patients with progressive disease.
Conclusions
In recent years, the clinical use of dronedarone hydrochloride tablets has gradually increased, but reports on dronedarone-induced pulmonary fibrosis are relatively rare. Therefore, clinicians should pay attention to the adverse reactions of dronedarone. The above case analysis and literature reviews suggest that patients over the age of 65 may be at risk for dronedarone-induced lung toxicity. Therefore, it is recommended that clinicians should monitor lung function and arterial blood gas levels during medication. For patients with a history of amiodarone exposure, whose chest X-rays and pulmonary function should be intermittently monitored, and if lung function declines, dronedarone should be discontinued immediately.2,7 Additionally, for patients who experience pulmonary toxicity during amiodarone use, dronedarone is not recommended as a substitute treatment. Currently, there are limited data on dronedarone-induced pulmonary fibrosis, and the conclusions of this study may have some limitations.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Declaration of Generative AI and AI-Assisted Technologies in the Writing Process
This work does not use generative AI and AI-assisted technologies in the writing process.
Acknowledgments
Funding: None.
Author Contributions
All authors contributed to the research involved in the manuscript and article preparation. Yuyan Chen, Zhendong Fu, Peng Wang, Jin Zhang, Jun Ren, and Rong Wang contributed to study conceptualization. Yuyan Chen, Xue Wen, Mingxia Zhang, Qiong Min, and Wenbin Li contributed to study methodology and review of results. Yuyan Chen and Zhendong Fu contributed to manuscript preparation and finalization.
Contributor Information
Jun Ren, Email: 819149226@qq.com.
Wenbin Li, Email: yfcs2002@163.com.
Rong Wang, Email: wangrong-69@163.com.
References
- 1.Budin CE, Cocuz IG, Sabău AH, et al. Pulmonary fibrosis related to amiodarone—is it a standard pathophysiological pattern? A case-based literature review. Diagnostics (Basel) 2022;12(12):3217. doi: 10.3390/diagnostics12123217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stack S, Nguyen DV, Casto A, Ahuja N. Diffuse alveolar damage in a patient receiving dronedarone. Chest. 2015;147(4):e131–e133. doi: 10.1378/chest.14-1849. [DOI] [PubMed] [Google Scholar]
- 3.Hernández Voth AR, Catalán JS, Benavides Mañas PD, et al. A 73-year-old man with interstitial lung disease due to dronedarone. Am J Respir Crit Care Med. 2012;186(2):201–202. doi: 10.1164/ajrccm.186.2.201. [DOI] [PubMed] [Google Scholar]
- 4.Money DB, Lee DH, Hadar A, et al. Amiodarone for the treatment of arrhythmias in COVID-19 patients does not increase the risk of pulmonary fibrosis: a retrospective cohort study. Cureus. 2023;15(1):e34109. doi: 10.7759/cureus.34109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prescribing information for dronedarone. http://products.sanofi.us/multaq/multaq.html. Accessed September 18, 2013.
- 6.Thornton D, Avery S, Edey AJ, Medford AR. Organising pneumonia due to dronedarone. J R Coll Physicians Edinb. 2015;45(3):213–214. doi: 10.4997/JRCPE.2015.308. [DOI] [PubMed] [Google Scholar]
- 7.Siu CW, Wong MP, Ho CM, et al. Fatal lung toxic effects related to dronedarone use. Arch Intern Med. 2012;172(6):516–517. doi: 10.1001/archinternmed.2011.1681. [DOI] [PubMed] [Google Scholar]
- 8.Immordino L, Connolly S, Crijns H, et al. Effects of dronedarone started rapidly after amiodarone discontinuation. Clin Cardiol. 2013;36(2):88–95. doi: 10.1002/clc.22090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naccarelli GV, Bhatt DL, Camm AJ, et al. Evaluation of the switch from amiodarone to dronedarone in patients with atrial fibrillation: results of the ARTEMIS AF studies. J Cardiovasc Pharmacol Ther. 2020;25(5):425–437. doi: 10.1177/1074248420926874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perrone MA, Babu Dasari J, Intorcia A, et al. Efficacy and safety of dronedarone in patients with amiodarone-induced hyperthyroidism: a clinical study. Eur Rev Med Pharmacol Sci. 2018;22(23):8502–8508. doi: 10.26355/eurrev_201812_16551. [DOI] [PubMed] [Google Scholar]
- 11.Zazzara MB, Palmer K, Vetrano DL, et al. Adverse drug reactions in older adults: a narrative review of the literature. Eur Geriatr Med. 2021;12(3):463–473. doi: 10.1007/s41999-021-00481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spagnolo P, Bonniaud P, Rossi G, et al. Drug-induced interstitial lung disease. Eur Respir J. 2022;60(4) doi: 10.1183/13993003.02776-2021. [DOI] [PubMed] [Google Scholar]