Abstract
Frizzled receptors (FZDs) are key contributors intrinsic to the Wnt signaling pathway, activation of FZDs triggering the Wnt signaling cascade is frequently observed in human tumors and intimately associated with an aggressive carcinoma phenotype. It has been shown that the abnormal expression of FZD receptors contributes to the manifestation of malignant characteristics in human tumors such as enhanced cell proliferation, metastasis, chemotherapy resistance as well as the acquisition of cancer stemness. Given the essential roles of FZD receptors in the Wnt signaling in human tumors, this review aims to consolidate the prevailing knowledge on the specific status of FZD receptors (FZD1–10) and elucidate their respective functions in tumor progression. Furthermore, we delineate the structural basis for binding of FZD and its co-receptors to Wnt, and provide a better theoretical foundation for subsequent studies on related mechanisms. Finally, we describe the existing biological classes of small molecule-based FZD inhibitors in detail in the hope that they can provide useful assistance for design and development of novel drug candidates targeted FZDs.
Keywords: Frizzled receptors, Wnt signaling pathway, structural foundation, cancer therapy, FZD inhibitors
Introduction
The human G protein-coupled receptor (GPCR) superfamily comprises ~800 members and contains several classes of receptors. Among the GPCR superfamily, ten frizzled receptors (FZD1–10) and smoothened (SMO) belong to a separate F class [1]. Eleven membrane proteins of the F family share the typical structural features of GPCRs. These features include extracellular N-termini, a transmembrane domain consisting of seven transmembrane α-helical structures (TMD) and an intracellular C-terminus. Additionally, the F family also features a cysteine-rich structural domain (CRD) at their extended N-terminus, which is crucial for binding their endogenous ligands to the receptor [2]. The FZDs are activated by the Wingless/Int-1 (Wnt) family, whereas SMO is indirectly activated by the Hedgehog (Hh) family [3].
SMO plays a crucial role in the Hh signaling pathway. This pathway is essential for regulating tissue regeneration and embryonic development, but can also be associated with birth defects and cancer. Unlike WNT/FZD signaling pathways, SMO is not a direct receptor for Hedgehog. Instead, it is a constitutively active receptor that is kept in an inactive state through the Dodecameric transmembrane receptor Patched (PTCH) [4]. The ligand in this pathway, Hedgehog, is a group of secreted glycol-proteins. There are three Hedgehog homologs present in mammals: Sonic Hedgehog (SHh), Indian Hedgehog (IHh), and Desert Hedgehog (DHh), which encode SHh, IHh, and DHh proteins, respectively. Hedgehog signaling molecule is a protein ligand that is secreted by signaling cells and is locally localized. Its range of action is very small, typically limited to no more than 20 cells. However, Hedgehog plays a crucial role in controlling intercellular signaling and is a significant contributor to mammalian regulation of tissue morphology and directional cell differentiation [5]. Hedgehog signaling also regulates tissue stem cells and wound tissue regeneration. Mutations causing aberrant activation of the Hedgehog pathway can lead to embryonic dysplasia and various diseases, including tumors [6].
The primary molecule that binds to the frizzled receptor FZD1–10 is the Wnt protein family. The Wnt gene was originally discovered in the integrative portion of the mouse mammary adenomavirus and was originally named int1. Later, it was renamed Wnt after it was found to be akin to the Drosophila wingless gene Wingless. The Wnt superfamily is a collection of secreted glycoproteins that are highly conserved, and all have 350–400 amino acids. So far, nearly 100 Wnt genes have been identified in different species, with human genome containing 19 of them [7]. Wnt proteins function by binding to receptors on the cell membrane through autocrine or paracrine means and activating intracellular signaling molecules, which then regulates the expression of target genes. During the development stage, Wnt protein plays different roles in controlling the fate, proliferation, migration, polarity, and death of cells. In adults, Wnt protein is essential for maintaining homeostasis in vivo, and any abnormal activation of the Wnt pathway has been linked to a variety of cancers [8].
FZDs are key contributors intrinsic to the Wnt signaling pathway, and evidence suggests that Wnt signaling pathway transduction is intimately linked to human cancer [9]. FZDs are key contributors intrinsic to the Wnt signaling pathway. The Wnt/FZDs signaling pathway is a highly conserved pathway that includes two types of signaling: classical and non-classical. The classical pathway is the most well-known and is responsible for activating β-catenin proteins, which accumulate and enter the nucleus. The classical pathway plays a crucial role in embryonic development, cell growth, stem cell proliferation, and cancer development [10]. The non-classical pathway, on the other hand, does not involve β-catenin-dependent transcriptional output. Furthermore, it includes two sub-pathways: the Wnt/Ca2+ pathway and the Wnt/PCP pathway. The Wnt/Ca2+ pathway controls the intracellular calcium level through calcium release from the endoplasmic reticulum, while the Wnt/PCP pathway regulates directional cell behaviors, such as migration, division, and morphologic polarization, through the cytoskeleton. In recent years, the importance of both non-classical pathways in tumors has gained increasing public attention [11]. The Wnt and FZD proteins are overexpressed in cancer tissue and homologous cell lines and have an irreplaceable role in tumor cell morphogenesis and differentiation, while they are also closely associated with the tumor immune microenvironment [12]. Blocking the FZDs by antibodies or small molecule inhibitors, the Wnt signaling pathway will be inactivated, ultimately leading to a silent state of downstream related genes [13]. Aberrant expression of FZD receptors is common in tumors. Meanwhile, the profile and frequency of different FZDs alterations vary considerably across cancers, emphasizing the importance of conducting studies on the unique mechanisms associated with different FZD promotion of specific tumor progression. Here, we review the roles of individual FZDs in different cancers, describe the typical Wnt/β-catenin pathway and Wnt/calcium signaling pathway processes, and highlight the potential impact of targeting FZDs for human cancer therapy.
Class frizzled receptors
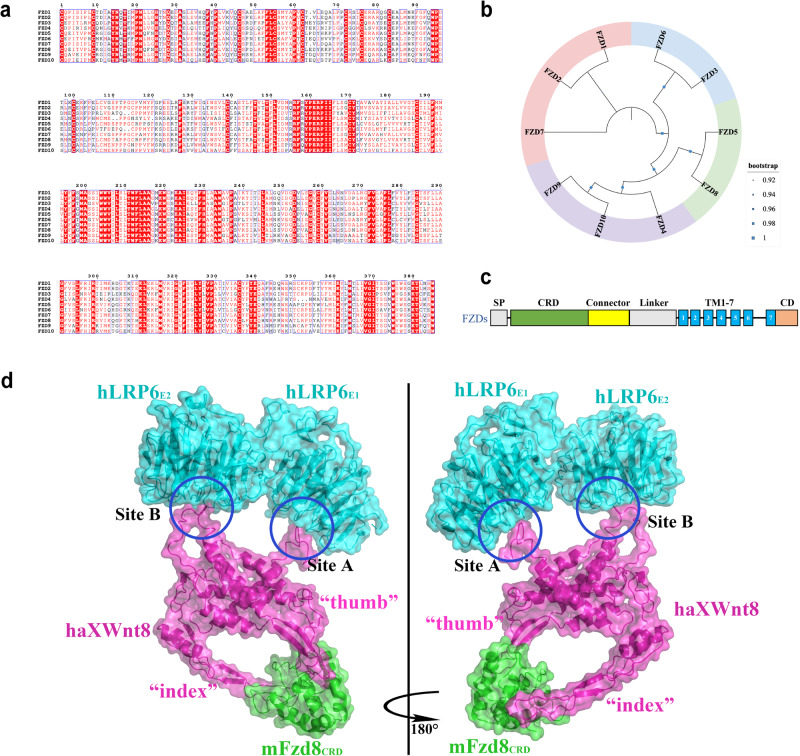
As members of the large GPCR family [14], ten FZDs constitute a highly conserved family of receptors (Fig. 1a). These proteins range in length from 500 to 800 amino acids and can be further divided into four subfamilies on the basis of amino acid sequence identity: FZD1, FZD2, and FZD7 share approximately 78%–80% similarity; FZD3 and FZD6 share 53% similarity; FZD5 and FZD8 share 69% similarity; FZD4, FZD9, and FZD10 share 52%–65% similarity [15] (Fig. 1b).
Fig. 1. Sequence analysis and structure of human FZDs and the binding pattern of haXWnt8–mFzd8CRD–hLRP6E1E2 (PDB code 8CTG).
a Multiple sequence alignment of FZD receptors (after shearing) in https://espript.ibcp.fr/ESPript/ESPript/. Ten FZDs constitute a highly conserved family of receptors sharing highly repetitive sequences. b Phylogenetic tree of human class Frizzled receptors FZD1–10 created with the MEGA software using multiple sequence alignment. The family of FZD1–10 can be further divided into four subfamilies based on the amino acid sequence identity: FZD1, FZD2, and FZD7; FZD3 and FZD6; FZD5 and FZD8; FZD4, FZD9, and FZD10. c Schematic representation of Fz receptors (SP signal peptide; TM transmembrane domain; CD cytoplasmic domain). Extracellular segments of FZDs include a CRD, a Connector, and a Linker between TMs. d hLRP6E1E2 interacts with XWnt8 through two sites of binding (site A and site B) at the top of the structure. XWnt8 interacts with mFzd8CRD through “thumb” and “index” fingers (a special structure similar with a human hand). The ribbon model of Wnt Ternary Complex colored in cyan (hLRP6E1E2), magenta (XWnt8), and green (mFzd8CRD).
The shared structure of ten frizzled receptors consists of an N-terminal signal sequence, a highly conserved CRD in the extracellular region, a seven-pass transmembrane domain (7-TM), and an intracellular C-terminal domain [16]. The CRD of 120 amino acids at the N-terminal end of the FZD protein is the binding site for various ligands. Internally, the CRD of the FZD protein is linked to the first transmembrane helix via a hydrophilic linker containing 70–120 amino acids. Notably, the linkage region in the middle of the CRD and 7-TM may vary and be less conserved in different FZDs [17] (Fig. 1c).
Disheveled (DVL) is a crucial protein that transduces signals for the three Wnt/FZD pathways. It acts as an intracellular hub protein that binds to FZDs inside the cell and forms dynamic signaling complexes called “signalosomes”. These complexes then transmit the signals to downstream effectors. Disheveled has three highly conserved structural domains: N-terminal Disheveled and Axin (DIX) domain, a central Postsynaptic density protein-95, Disk large tumor suppressor, Zonula occludens-1 (PDZ) domain, and a C-terminal Disheveled, Zonula occludens-1, and Zonula occludens-1 domain, which are known as the terminal Disheveled, Egl–10, and Pleckstring (DEP) domains. The DIX structural domain can easily form oligomers, resulting in the creation of dynamic cytoplasmic DVL oligomers. Disheveled also brings Axin (a scaffolding protein for the downstream β-conjugated protein degradation complex) to the plasma membrane through a direct interaction between the DIX structural domain of DVL and the DIX domain of Axin. PDZ structural domains are small modules that recognize PDZ-binding motifs via a peptide-binding groove, usually located at the C-terminus of their binding partners. The function of the PDZ structural domains is currently under debate [18]. Some initial evidence suggests that the PDZ structural domain of DVL directly binds to a highly conserved KTxxxW motif located in the intracellular C-terminal helix8 of FZDs. It is currently believed that the DEP structural domain, rather than the PDZ domain, is responsible for intracellular recognition of FZDs. The DEP domain has been shown to bind to the KTxxxW motif of FZDs, thus facilitating the recruitment of FZDs. The following are the specific dynamics of this process. DVL is recruited to Frizzled by the DEP domain and assembles the Wnt signalosome through the self-association of its DIX and DEP structural regions [19]. The DIX structural domain undergoes dynamic head-to-tail polymerization and produces a stable butterfly cross-link with the DEP structural domain. This cross-link achieves monomer-to-dimer conversion through a domain exchange mechanism [20]. The above events increase the concentration of Disheveled and its affinity for low-affinity effectors such as Axin, enabling it to interact with these effectors even at low cellular concentrations, thus ensuring downstream signaling [21].
The interaction between FZDs and Wnt proteins
As the most crucial ligands of FZDs, members of the Wingless-type (Wnt) family belong to secreted glycoproteins and are generally composed of 350–400 amino acids. Due to the complex association of Wnt proteins with multiple diseases, many studies have focused on Wnt family members in the human genome in recent years. To date, nineteen Wnts have been identified as human Wnt proteins, including Wnt1, Wnt2, Wnt2b, Wnt3, Wnt3a, Wnt4, Wnt5a, Wnt5b, Wnt6, Wnt7a, Wnt7b, Wnt8a, Wnt8b, Wnt10a, Wnt10b, Wnt11, Wnt14, Wnt15, and Wnt16, which share 27%–83% amino acid sequence homology and a conserved sequence of 23 or 24 cysteine residues [22, 23]. The high affinity of Wnt ligands binding to CRD (Kd of 1–10 nM) [24] and the cocrystal structure information of their interaction have been demonstrated (Xenopus Wnt8 in complex with mouse FZD8CRD). As shown in Fig. 1d, the crystal structure of haXWnt8–mFzd8CRD–hLRP6E1E2 was determined by Naotaka Tsutsumi and co-workers through cryo-electron microscopy (cryo-EM) analysis [25], which truly illustrated the binding pattern of soluble Wnt ternary complex. As previously reported, haXWnt8 forms a special structure similar to a human hand with a central “palm” stretching a “thumb” plus an “index finger” that clamps the mFzd8CRD globular structure. Both contact sites involve highly chemically conserved residues, indicating that most Wnts interact with the corresponding FZD receptor by specifically interacting with amino acids in the two areas. Meanwhile, the β-propellers of hLRP6E1 and hLRP6E2 interact with XWnt8 through two sites of binding (sites A and B) at the top of the XWnt8-mFzd8CRD complex, respectively. The N-terminal loop of XWnt8 forms site A, while site B is formed by the linker between N- and C-terminal domains (NC-linker) of XWnt8. It could indicate that binding with hLRP6 is beneficial for stabilizing the N-terminal loop and NC-linker structures in XWnt8–mFzd8CRD complex [25] (Fig. 1d).
Wnt/FZD signaling paradigms
The Wnt/FZD signaling pathways are fundamental in maintaining embryonic tissue development and homeostasis of adult tissue. Once out of control, they promote cancer development through multiple channels and mechanisms. The ligand-protein Wnt binds to individual FZDs and their coreceptors to activate intracellular signaling and trigger modifications in cell morphology and motility [26, 27]. The intracellular signaling system consists of three principal streams: one canonical Wnt/β-catenin pathway and two noncanonical Wnt/β-catenin pathways. Specifically, the two non-canonical Wnt/β-catenin pathways mainly encompass the Wnt/planar cell polarity (PCP) pathway and the Wnt/calcium signaling pathway [28].
The canonical Wnt/FZD signaling pathway
The canonical Wnt/β-catenin signaling pathway, which is highly conserved in biological evolution from lower Drosophila to higher mammals, regulates the stability of β-catenin and depends on β-catenin gene expression. Due to the inhibition of the degradation complex, β-catenin is no longer degraded and continuously accumulates in the cytoplasm. It is then transferred to the nucleus and regulates the activation of transcription of relevant target genes together with LEF/TCF transcription factors [29].
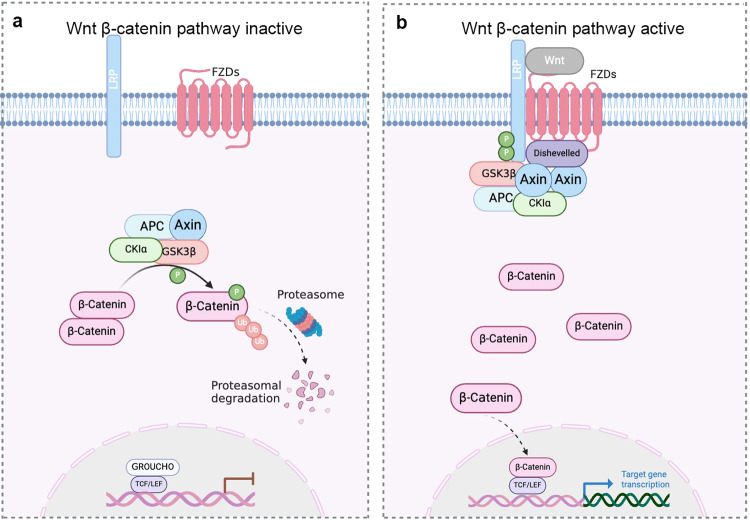
In the absence of the extracellular Wnt ligands, the signaling switch is not activated, the canonical pathway is disabled, and the central effector β-catenin is continuously phosphorylated and degraded, thus preventing the abnormal cellular responses triggered by the canonical pathway. Casein kinase 1α, axis inhibition protein (AXIN), adenomatous polyposis coli (APC), and the serine/threonine kinase glycogen synthase kinase3β (GSK3β) together form the β-catenin degradation complex. GSK3β phosphorylates the serine and threonine residues at the N-terminal end of β-catenin with the assistance of the other three proteins. Phosphorylation of β-catenin will further trigger its degradation (Fig. 2a).
Fig. 2. Canonical Wnt/FZD signal transduction.
(Created with BioRender.com). a In the absence of the extracellular Wnt ligands, the signaling switch is not activated. Then β-catenin is continuously phosphorylated and degraded, Casein kinase 1α (CK1α), axis inhibition protein (AXIN), adenomatous polyposis coli (APC), and the serine/threonine kinase glycogen synthase kinase3β (GSK3β) together form the β-catenin degradation complex. Phosphorylation of β-catenin by the degradation complex will further trigger its degradation, thus preventing the abnormal cellular responses triggered by the canonical pathway. b In the presence of Wnt, it binds to two coreceptors on the cell membrane and the degradation complex is inhibited. Free β-catenin is no longer phosphorylated and accumulates in the cytoplasm, then they are translocated to the nucleus to promote transcription of downstream target genes.
When Wnt protein is present and functional, the Wnt ligand binds to two coreceptors on the cell membrane: an FZD family receptor and a member of the low-density lipoprotein receptor-related protein (LRP) family. Subsequently, LRP undergoes phosphorylation, and DVL is recruited to the corresponding sites and undergoes oligomerization. The degradation complex then binds to the receptor and DVL, and the activated DVL protein enhances GSK3β phosphorylation, thereby inhibiting GSK3β. Free β-catenin is no longer phosphorylated and accumulates in the cytoplasm before being translocated to the nucleus. As a category of transcription factors with bidirectional regulatory functions, intranuclear T-cell factor (TCF)/lymphatic enhancer binding factor (LEF) binding to Groucho represses gene transcription, while its binding to β-catenin promotes transcription of downstream target genes. In a Wnt-deficent state in which the FZD-related signaling is cut off, TCF/LEF binds to specific sequences in the promoter/enhancer regions of target genes and exerts a repressive effect on gene expression together with Groucho transcriptional repressors. While Wnt is present, β-catenin degradation complexes lose its phosphorylation activity, then β-catenin levels in the nucleus are elevated, and β-catenin binds to TCF/LEF to promote altered transcriptional machinery and activation of target genes, ultimately regulating cellular behaviors such as cell cycle progression and stem cell self-renewal (Fig. 2b).
The canonical Wnt/β-catenin signaling pathway plays a dual role in human development and disease progression. While its native job is to promote normal body development and to participate in the regulation of cell proliferation and differentiation as well as in the maintenance of stem cells, its abnormal activation can lead to imbalances in the body and even become an accomplice in the development of cancer [30].
Non-canonical Wnt/FZD signaling
The non-classical Wnt pathway consists of Wnt ligands mainly Wnt4, Wnt5a, Wnt5b, Wnt6, Wnt7a, and Wnt11. When these ligands bind to cell surface FZD receptors and co-receptors, the DVL-dependent effector proteins or Ca2+-dependent signaling cascade is activated, which then triggers intracellular signal transduction. The co-receptors specifically refer to the tyrosine kinase-like receptor alone 1/2 (ROR1/2)/receptor-associated tyrosinase (RYK) coreceptor. The noncanonical Wnt signaling pathway consists of two major branches: the planar cell polarity (PCP) pathway and the Wnt/Ca2+ pathway.
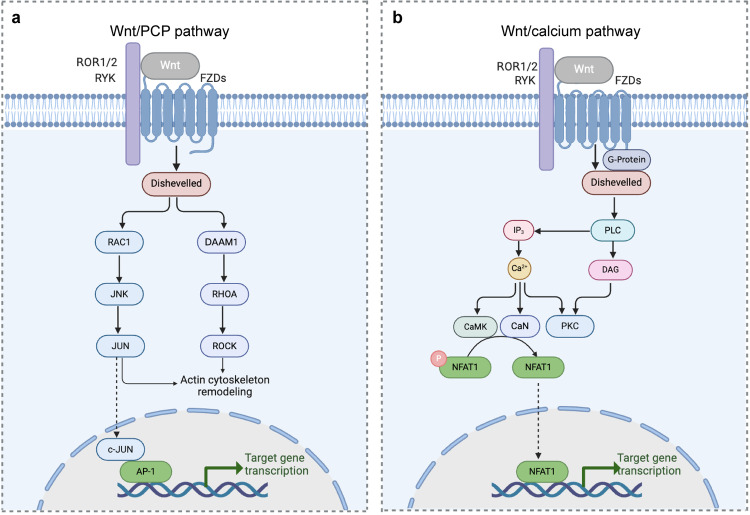
The PCP pathway involves DVL-associated morphogenetic activator 1 (DAMM1), RHOA-ROCK and RAC-JNK and is involved in the regulation of actin cytoskeletal structure and cell motility. In the Wnt/PCP pathway, the binding of Wnt to FZD and the coreceptor RYK causes the activation of DVL recruitment and binding to DAMM1. DAMM1 activates Rho GTPase via guanine exchange factors and subsequently activates Rho-associated protein kinase and myosin, causing actin skeleton alterations and rearrangements, ultimately altering cell polarity and migration. Alternatively, the PCP pathway can initiate the JNK signaling cascade by triggering RAC. RAC activation is not dependent on DAMM1, and activated c-Jun further promotes activator protein 1-dependent gene transcription, which ultimately regulates cytoskeletal remodeling and cell adhesion [31] (Fig. 3a).
Fig. 3. Non-canonical WNT pathways.
(Created with BioRender.com). a In the Wnt/PCP pathway, the binding of Wnt to FZD and the coreceptor tyrosine kinase-like receptor alone 1/2 (ROR1/2)/receptor-associated tyrosinase (RYK) coreceptor causes the activation of DVL recruitment. The binding of DVL to DAMM1 activates Rho-associated protein kinase and ultimately cause actin skeleton alterations and rearrangements. The activation of DVL can also initiate the JNK signaling cascade by triggering RAC. Then the activation of c-Jun further promotes activator protein 1 (AP-1)-dependent gene transcription, which ultimately regulates cytoskeletal remodeling and cell adhesion. b The Wnt/Ca2+ cascade activates phospholipase C (PLC) via heterotrimeric G proteins to generate diacylglycerol and inositol 1,4,5-trisphosphate-3 (IP3), followed by an increase in cytoplasmic Ca2+ concentration. The accumulation of Ca2+ activates Ca2+-sensitive enzymes such as calcium-regulated protein kinase II (CaMKII) and protein kinase C (PKC), and calcium activation of CaMKII and CaN dephosphorylates nuclear factor of transcription factor-activated T cells (NFAT), leading to their nuclear import and subsequent NFAT-mediated gene expression.
The Wnt/Ca2+ cascade activates phospholipase C via heterotrimeric G proteins to generate diacylglycerol and inositol 1,4,5-trisphosphate-3, followed by an increase in cytoplasmic Ca2+ concentration. The release and intracellular accumulation of Ca2+ activates Ca2+-sensitive enzymes such as calcium-regulated protein kinase II (CaMKII) and protein kinase C (PKC), and calcium activation of CaMKII and CaN dephosphorylates nuclear factor of transcription factor-activated T cells (NFAT), leading to their nuclear import and subsequent NFAT-mediated gene expression. In conclusion, the Wnt/Ca2+ pathway is centered on elevated intracellular Ca2+ and activates signaling factors such as PKC, CaMKII, CaN, NFAT, and transcription factors to exert cell adhesion and gene expression [32] (Fig. 3b).
Noncanonical Wnt signaling can contribute to developing diseases such as inflammation and cancer in the human body by affecting cell motility, polarity, and migration. In recent years, more studies have focused on the effects of non-canonical Wnt signaling on the formation and maintenance of tumor stem cells and on tumor immunity [33].
Frizzled receptors in human cancers
While mechanistic overlaps exist, each FZD receptor plays distinct roles in physiological and pathological processes. Numerous studies have shown that the abnormal upregulation of FZDs is significantly associated with the development of human cancers, in which most FZDs play oncogenic roles (Table 1). In contrast, individual frizzled receptors may have tumor-suppressive effects on specific types of cancer. The frequent association of FZDs with certain oncogenic functions contributes to cancer development, such as chemoresistance, EMT, metastasis, and angiogenesis. The biological functions and mechanisms of different FZDs in cancer development will be described in detail here.
Table 1.
Oncogenic roles of FZD proteins in human cancers.
| FZDs | Cancers | Main functions | Ref. |
|---|---|---|---|
| FZD1 | NB, NSCLC, PADC, CCRCC, AML, Uterine sarcoma, Ovarian cancer | Chemo-resistance | [34–41] |
| Breast cancer, GC | EMT and metastasis | [42, 43] | |
| GBM | Poor prognosis | [44] | |
| FZD2 | Breast cancer, TSCC, ESCC, Ovarian cancer, HCC, Prostate cancer | EMT and metastasis | [46–53] |
| HCC, Breast cancer, Prostate cancer | Chemo-resistance | [54, 55] | |
| FZD3 | Cervical cancer, GC | Metastasis | [59, 60] |
| HCC, ESCC | Chemo-resistance | [61, 62] | |
| CLL | Proliferation | [63] | |
| HCC | Cancer stemness and attenuated antitumor immunity | [64] | |
| FZD4 | Osteosarcoma, NSCLC, Intrahepatic cholangiocarcinoma | Progression | [68, 69] |
| GBM | Cancer stemness | [70] | |
| HNSCC, Glioma, HCC, colon | Angiogenesis | [73–76] | |
| FZD5 | TNBC, Bladder urothelial carcinoma | EMT, stemness, chemo-resistance | [77, 78] |
| RNF-43-mutant pancreatic tumor | Growth | [79] | |
| Breast cancer | Angiogenesis | [80] | |
| FZD6 | Cervical cancer, Glioblastoma | Proliferation | [85, 86] |
| Cervical cancer, Melanoma | Invasion and EMT | [86, 87] | |
| Bladder cancer, OSCC, Colorectal cancer, Liver | Metastasis | [88–90] | |
| Breast cancer, ESCC, AML | Poor prognosis | [91–93] | |
| FZD7 | Pancreatic cancer, Ovarian cancer, Breast cancer, TSCC, HCC | Stemness, EMT, chemo-resistance | [99–104] |
| FZD8 | Breast | Chemo-resistance | [105] |
| NSCLC | ECM expression and myofibroblast differentiation | [108] | |
| HNSCC | Stemness, recurrence and metastasis | [109] | |
| GC, Prostate cancer, Thyroid cancer | Poor prognosis and metastasis | [110–112] | |
| FZD9 | Astrocytomas | Proliferation and angiogenesis | [116] |
| Osteosarcoma | Proliferation, migration and invasion | [117] | |
| Breast cancer | Poor prognosis | [118] | |
| FZD10 | Synovial sarcomas | Cell growth and destruction of cytoskeleton structure | [123] |
| Ovarian cancer, | Chemo-resistance | [124, 125] | |
| HCC | Stemness and drug resistance | [126] | |
| NPC | Recurrence, radio-resistance, poor prognosis | [127, 128] | |
| Breast cancer | Metastasis | [129] | |
| Colorectal and GC | Proliferation and poor prognosis | [130] |
FZD1
FZD1 is one of the proteins expressed in the central nervous system and is extremely closely related to the development and evolution of the human nervous system. It can exert a neuroprotective effect [34]. The specific influence of FZD1 on chemoresistance has been investigated. FZD1 mediates chemoresistance in neuroblastoma (NB) by increasing MDR1 (multidrug resistance gene) transcription [35], non-small cell lung cancer (NSCLC) [36], pancreatic ductal adenocarcinoma (PDAC) [37], clear cell renal cell carcinoma [38], acute myeloid leukemia (AML) [39], human uterine sarcoma cells [40], and ovarian cancer cells [41]. FZD1 has also been revealed to facilitate EMT and metastasis in both breast [42] and gastric cancer [43]. In addition, FZD1 has been reported to play an influential role in the pathological development and tumor formation of glioblastoma (GBM) [44]. In addition, FZD1 may be a TME-directed efficacious treatment target for clinical colon cancer, given its role in regulating the pathological process of colon cancer through the complex environment of the TME [45]. Based on the above, it can be concluded that FZD1 plays an essential contributory role in cancer development by altering the responsiveness and sensitivity of various human cancers to different chemotherapeutic agents.
FZD2
An early study found that FZD2 is elevated in various metastatic cancer cell lines and is strongly associated with EMT, which is a hallmark of cancer metastasis. FZD2 can drive EMT through a noncanonical pathway involving Fyn and STAT3 [46]. Further studies have confirmed that the abnormal expression of FZD2 plays oncogenic roles and regulates tumor metastasis via STAT3 signaling in breast cancer [47], tongue cancer [48], esophageal squamous cancer carcinoma (ESCC) [49], serous ovarian cancer [50], hepatocellular carcinoma (HCC) [51] and prostate cancer [52]. Furthermore, FZD2 can also regulate EMT by activating typical Wnt signaling and thus facilitate metastasis of endometrial cancer [53]. The biological function of drug resistance of FZD2 via noncanonical pathways is indicated in hepatocellular carcinoma [54], breast cancer [47], and APC [55]. Interestingly, a study showed that FZD2 could exert tumor inhibitory functions by curbing cell growth and migration in cystic carcinomas of the salivary gland [56]. In summary, FZD2 primarily exerts carcinogenic functions in various cancers by driving EMT to regulate tumor metastasis despite its anti-carcinogenic effect in salivary adenoid cystic carcinomas.
FZD3
FZD3 is also predominantly expressed in the nervous system, and its persistence in the adult sensory nervous system is critical for transferring information between the extremities and the brain. The fact that it can alter pain sensitivity in a specific manner can also be used for in-depth studies of cancer-related pain [57]. A study has shown that FZD3 determines the aggressive phenotype of human melanoma by affecting the MAPK pathway [58]. More studies have defined FZD3 as an essential protein for the critical stage of metastasis in cervical cancer (CC) [59] and gastric cancer [60]. Regarding drug resistance, FZD3 knockdown could increase sensitivity to chemotherapy in HCC [61] and ESCC [62]. In addition, FZD3 is involved in the malignant development of chronic lymphocytic leukemia [63] and tumor stem cell properties, as well as attenuated antitumor immunity in HCC [64]. However, a report suggested that FZD3 exerts an antitumor effect in renal cell carcinoma (RCC), which could be related to the inhibitory effect of FZD3 on glycolysis in RCC cells [65]. FZD3 can be labeled as a key receptor during malignant transformation in various human cancers, especially in human melanoma.
FZD4
FZD4 is indispensable for the normal growth and development of vascular endothelial cells [66]. FZD4 deficiency affects endothelial cell proliferation and, thus, the formation of small blood vessels in tissues and organs, providing a potential target for the treatment of retinal and other vascular diseases [67]. As a critical Wnt/β-catenin signaling component, FZD4 is a critical pathogenesis function in many human malignancies, such as osteosarcoma [68] and intrahepatic cholangiocarcinoma [69]. FZD4 is suggested to promote the induction of stem cell-like cells with highly invasive potential in GBM [70]. Angiogenesis is one of the key hallmarks of tumors and an important condition for tumor development, infiltration, and metastasis, making targeting pro-angiogenic genes a research hotspot for tumor treatment and prevention. More importantly, in the frizzled family, FZD4 is the only receptor that can promote angiogenesis by binding to the protein Norrin, a secreted retinal growth factor with angiogenic and neuroprotective properties [71]. Norrin/FZD4 is necessary to preserve the completeness of the blood-retinal barrier [72]. Initiation of the Norrin/FZD4 signaling axis is a prerequisite for the regulation of tumor angiogenesis in colon cancer [73], HNSCC [74], glioma [75], and HCC [76]. Identified as a pro-angiogenic signature, FZD4 can be targeted to influence the sensitivity of tumors to antiangiogenic therapy. Investigating the mode of action of FZD4 in human tumors can provide a more solid theoretical basis for antitumor angiogenesis and alteration of the state of the tumor vascular microenvironment to improve the therapeutic effect.
FZD5
FZD5 is required to initiate primary invagination of the archenteron in sea urchin and is needed in the Wnt-mediated presynaptic formation in the hippocampus. In terms of disease progression, it has been validated that FZD5 contributes to EMT, stemness, and chemoresistance in triple-negative breast cancer [77] and bladder urothelial carcinoma [78]. A study employing genome-wide gene editing techniques also confirmed that FZD5 is a suppressible target affecting the prognosis of specific subtypes of pancreatic tumors [79]. Furthermore, inhibition of endothelial FZD5 resulted in loss of normal endothelial cell function and further affected the expression levels of essential factors related to angiogenesis [80]. Further studies revealed a pro-angiogenic function of FZD5 through NF-κB/ERK signaling [81] or calcium/NFAT signaling [82] in human cancer cell development and progression. These studies demonstrate the potential of FZD5 in tumor angiogenesis and provide a new effective target for vascular microenvironment-directed tumor therapy. Targeted inhibition of FZD5 in the afore-mentioned tumors could lead to the inhibition of the tumor cells themselves and to the further eradication of the tumors by cutting off their nutritional sources (inhibiting tumor angiogenesis). Surprisingly, in gastric cancer, FZD5 slows down the development of EMT and promotes patient survival, and it is likely to be a specific suppressor of gastric cancer [83]. The double-sided phenomenon of FZD5 raises some warnings for its targeted therapy, as FZD5 should be treated with caution and should never be used generically in all tumor types to prevent the unexpected deterioration of individual tumors, including gastric cancer.
FZD6
FZD6 is the protein closely related to the non-canonical PCP signaling pathway among the ten receptors. As a key molecule in the PCP signaling pathway, FZD6 is not only instructive in the process of cell motility necessary to maintain organismal homeostasis but also contributes to tumor formation [84]. In addition, the molecular involvement of FZD6 in the biological complexity of human cancers has gained an in-depth understanding in the past decade. On the one hand, the association of FZD6 with aggressive behavior of cancer has been validated in glioblastoma [85], cervical cancer [86], melanoma [87], bladder cancer [88], OSCC [89], colorectal cancer [90], breast cancer [91], AML [92], and ESCC [93]. In liver tumorigenesis, FZD6 is the only frizzled gene associated with tumor recurrence and metastasis [94].
Interestingly, FZD6 is also one of the key brakes of the Wnt signaling cascade and plays a role in eliminating malignant tumor cells in a small subset of cancers. In prostate cancer, FZD6 significantly attenuates the stemness and self-renewal ability of PC and is most likely defined as a tumor suppressor in prostate cancer [95]. FZD6 represses cell proliferation and motility in gastric cancer by revitalizing the non-canonical Wnt signaling pathway [96]. In GBM, high levels of FZD6 attenuate Wnt signaling by activating CaMKII-TAK1-NLK [97]. The profile of FZD6 in the pathogenesis of cancer is not definitive: it can serve as an oncogene promoting cancer development in most human cancers, whereas now it could also be identified as a tumor suppressor in PC, GC, and GBM.
FZD7
FZD7 is involved in the normal development and pathological progression of human embryonic stem cells (hESCs) and adult intestinal stem cells [98]. As a key receptor of Wnt proteins, FZD7 is upregulated in multiple cancers and drives tumor phenotype progression at various stages. The specific aspect of carcinogenesis regulated by FZD7 must be mentioned is cancer stemness, which is increased in cancer cells undergoing EMT. High expression of FZD7 could strengthen stemness and EMT and is responsible for drug resistance in pancreatic cancer [99], ovarian cancer [100], breast cancer [101], gastric cancer [102], tongue cancer [103], and hepatocellular carcinoma [104] through both canonical and non-canonical Wnt signaling pathways. FZD7 is significantly upregulated in a variety of malignancies and is significantly associated with tumor chemotherapy tolerance and low patient survival. Targeting FZD7 has shown promising antitumor effects in vitro and in vivo. Thus, highly selective inhibitors of FZD7 will break new bright prospects for the treatment and prognosis improvement of malignant tumors.
FZD8
In the human FZD family, FZD8 shares a very high similarity with FZD5. FZD8 mediates chemoresistance in triple-negative breast cancer, making targeted inhibition of FZD8 one of the optimal strategies to attenuate breast cancer chemoresistance and improve patient well-being [105]. As a functional gene candidate that can worsen the prognosis of human lung cancer, in addition to the pro-inflammatory role of fibroblasts in regulating airway inflammation [106] and TGF-β1-induced ECM expression and myofibroblast differentiation [107], FZD8 also increases the aggressive characteristics of NSCLC [108]. In HNSCC, FZD8 is highly expressed in cancer stem cells (CSCs) and is a crucial mediator of c-Met-maintained HN-CSCs, which are responsible for the recurrence and metastasis of HNSCC [109]. In addition, FZD8 promotes focal metastasis in gastric cancer and predicts a worse quality of life and shorter survival [110], prostate cancer [111], and thyroid cancer [112] by activating canonical Wnt signaling. Research on FZD8 in human tumors is relatively limited, but FZD8 is still a potential target for certain tumors, and there are already antibodies targeting the FZD5/8 subfamily.
FZD9
The FZD9 gene is mainly expressed in the brain, muscle, testis, eye, skeletal muscle, and kidney tissues and plays a vital role in maintaining stable growth and differentiation of stem cell populations in blood, skin, and intestine [113]. FZD9 ensures normal bone formation through non-canonical Wnt signaling [114]. On the one hand, FZD9 promotes the formation and malignant progression of some human tumors, including pancreatic islet cancer [115], astrocytomas [116], osteosarcoma [117], and breast cancer [118]. On the other hand, FZD9 serves as a brake on the malignant progression of tumors in acute myeloid leukemia [119] and lung cancer [120]. In the lung, binding of FZD9 to Wnt7a gives activation instructions to peroxisome proliferator-activated receptor γ (PPARγ), leading to normal lung epithelial maintenance. In contrast, the deletion of FZD9 creates a lung microenvironment conducive to malignant disease and tumorigenesis, making FZD9 a candidate protein for effective lung cancer treatment. Regarding angiogenesis, FZD9 could be upregulated after angiogenic induction by differentiating stem cells towards endothelial cells and might be associated with angiogenesis in astrocytomas [120]. In conclusion, more in-depth studies are needed to clarify the specific inhibitory or promotive mechanisms of FZD9 in different tumors, and the targeting of FZD9 needs to be treated with caution.
FZD10
Since FZD10 is absent in almost all typically developing sound tissues, the high tumor specificity of FZD10 makes it a potentially highly sensitive tumor marker [121]. Increased expression of FZD10 will potentially be an essential indicator for human tumor diagnosis and malignancy determination [122]. FZD10 upregulation can be detected in most synovial sarcomas, making FZD10 a putative therapeutic target for synovial sarcoma. Above all, the anti-FZD10 antibody (OTSA101) has achieved complete remission and has been identified as a new and efficient pharmacological treatment option for synovial sarcoma [123]. N6-methyladenosine (m6A) modification of FZD10 mRNA dramatically contributes to its effect on tumors. In epithelial ovarian cancer, m6A-mediated stabilization of FZD10 enhances poly (ADP-ribose) polymerase inhibitors resistance by activating typical Wnt signaling [124]. In highly malignant plasma ovarian cancer, FZD10 silencing causes significant sensitization toward cisplatin treatment [125]. In HCC, m6A-mediated FZD10 overexpression augments hepatic tumor stem cell properties and lenvatinib resistance via canonical Wnt and Hippo signaling pathways, suggesting that FZD10 is a marker protein for hepatic tumor stem cells and may be a potential target for hepatocellular carcinoma prevention and treatment [126]. In addition, FZD10 is involved in the progression of nasopharyngeal carcinoma [127, 128], breast cancer [129], colorectal cancer, and gastric cancer [130]. As a member of the FZD4/9/10 subfamily, FZD10 is a hotspot for targeted antibody research, an antibody specifically targeting FZD10 is in the phase of clinical research [123]. Identified as a relatively novel target in tumor research, FZD10 is incredibly hopeful to become an independent predictive biomarker for cancer therapy and may improve the survival outcomes of patients suffering from tumors.
The significance of metastatic properties and drug resistance for malignant tumor progression cannot be overstated and is particularly evident in advanced tumors. These properties are intricately linked to the complex microenvironment of high-grade tumors. Although individual FZDs have been identified as tumor suppressors in certain cancers (Table 2), the frequent association of FZDs with certain oncogenic functions contributing to cancer development, such as chemoresistance, EMT, metastasis, and angiogenesis, suggests that targeting FZDs can prevent the acquisition of malignant phenotypes from multiple stages and directions of tumor development.
Table 2.
Carcinostasis effect of certain FZD proteins in human cancers.
| FZDs | Cancers | Roles in tumorigenesis | Mechanism | Ref. |
|---|---|---|---|---|
| FZD2 | Salivary carcinomas | Inhibiting cell growth and migration | FZD2/PAI-1 axis | [56] |
| FZD3 | Renal cell carcinoma | Inhibiting cell proliferation, migration, invasion and glycolysis | MiR-340/FZD3 axis | [65] |
| FZD5 | Gastric cancer | Preventing EMT and associating with longer survival | WNT7b/FZD5/ELF3 axis | [83] |
| FZD6 | Prostate cancer | Suppressing tumor stemness | Luteolin/FZD6/β-catenin | [95] |
| Gastric cancer | Repressing cell proliferation and migration | MiR21/FZD6/PCP signaling and FZD6/β-catenin axis | [96] | |
| Glioblastoma | Preventing proliferation and associating with longer survival | MiR-93/FZD6 axis | [97] | |
| FZD9 | Acute myeloid leukemia | Inhibiting neoplastic transformation and indicating better clinical outcomes | Aberrant DNA Methylation of FZD9 | [119] |
| Lung cancer | Preventing EMT | FZD9/PPARγ axis | [120] |
Frizzled receptors as pharmacological targets
The Wnt/FZD signaling pathway has a dual role in embryonic development and carcinogenesis, and it is dysregulated at almost all stages of tumorigenesis, including malignant metastasis, proliferation, and drug resistance. This pathway also interferes with the immune surveillance of cancer and promotes immune escape and resistance to immunotherapy. Therefore, research on the Wnt/FZD signaling pathway inhibitors has been an enduring hot topic. However, targeting other Wnt/FZD signaling proteins usually comes at a higher cost, triggering more severe toxic effects due to their broad spectrum of adaptations. This leaves the key cell membrane receptor protein FZD as the best target for inhibition. A multitude of inhibitors targeting FZD with clinical translation potential are already available, including small molecule inhibitors and biomolecules (Table 3), such as blocking antibodies, peptide inhibitors, and small molecule inhibitors. Therapeutic strategies based on antibodies and small molecule inhibitors selectively targeting the FZD receptor can treat cancer without affecting normal tissue homeostasis. Therefore, we describe here the available inhibitors targeting the FZD receptor.
Table 3.
Antibodies and engineered proteins targeting FZDs.
| Name | Categories | Targets | Cancer types | Ref |
|---|---|---|---|---|
| OMP-18R5 | Antibody | FZD1/2/5/7/8 | Breast cancer, pancreatic cancer, HNSCC | [121, 122] |
| IgG-2919 and IgG-2919 | Antibody | FZD5 | Pancreatic cancer | [68] |
| mAb92-13 | Antibody | FZD10 | Synovial sarcoma | [111] |
| sFZD7 | Peptide | FZD7 | Hepatocellular carcinoma | [134] |
| RHPDs | Peptide | FZD7 | Hepatocellular carcinoma | [135] |
| dFZD7-21 | Peptide | FZD7 | Intestinal organoids | [136] |
| OMP-54F28 | Engineered antibody | FZD8 | Pancreatic cancer; ovarian cancer; hepatocellular cancer HNSCC | [125–127] |
| F2.A | Engineered antibody | FZD1/2/4/5/7/8 | Pancreatic cancer | [128] |
| FZD7-scFv | Engineered antibody | FZD7 | Breast cancer | [131, 132] |
| TT641 | Engineered antibody | FZD10 | Synovial sarcomas | [110] |
| FZD7-NS | Engineered antibody | FZD7 | Breast cancer | [133] |
| DRPBs | Engineered protein | FZD1/2/4/5/7/8 | Intestinal crypt stem cell | [129] |
| FZD7-Fab | Engineered protein | FZD7 | Human embryonic stem cells | [87] |
Antibodies and engineered proteins against FZDs
Vantictumab (OMP-18R5) is a human monoclonal antibody that inhibits canonical Wnt signaling by binding to the extracellular regions of FZD1, 2, 5, 7, and 8 and preventing phosphorylation of the coreceptor LRP [131]. In an open phase 1b dose-climbing clinical trial investigating the safety, tolerability, and pharmacokinetics of the combination of OMP-18R5 and paclitaxel in patients with locally recurrent and metastatic breast cancer already treated with chemotherapy, this monoclonal antibody showed clinically relevant and favorable results. However, clinical trials adverse effects (high fracture rate) limit the Vantictumab future study [132, 133].
Ipafricept (IPA; OMP-54F28) is a recombinant protein developed to target FZD8 using the extracellular Wnt binding region of the human FZD8 receptor [134]. As a broad and potent inhibitor of the Wnt pathway, this fusion protein can bind competitively to all Wnts, thereby inhibiting this signaling pathway [135]. OMP-54F28 can inhibit the growth of many types of tumors, and phase 1b clinical trials of this inhibitor in combination with nab-paclitaxel and gemcitabine for pancreatic cancer [136], with carboplatin and paclitaxel in ovarian cancer [137], and with sorafenib in hepatocellular cancer have yielded favorable therapeutic benefits. In an animal model of HNSCC patient-derived xenografts, OMP-18R5 and OMP-54F28 showed promising inhibition by significantly inhibiting Wnt signaling in tumors [138]. Unfortunately, since OMP-54F28 cross-reacts with multiple receptor members, its lack of target protein specificity may lead to off-target effects and compromise the efficacy of the antibodies. Second, OMP-54F28 may trigger dose-related fragility fractures, and these drawbacks greatly limit the general applicability of this antibody. A new antibody F2.A with higher specificity compared to OMP-18R5, was developed through phage display technology to target FZD4 in addition to FZD1/2/7/5/8, and the potential inhibition of tumor vasculature obtained by targeting FZD4 provides additional therapeutic benefit to this treatment strategy. This variant antibody showed a more optimized efficacy than OMP-18R5 in inhibiting the proliferation of RNF43 mutant PDAC cells [139]. Antibody drugs are more specific and targeted, are effective in activating the body’s immune function and usually show better antitumor effects. The above experimental results provide much worthwhile information for the safety and efficacy of FZD-targeted antibody inhibitors and provide directions for optimizing subsequent antibody inhibitors.
Using a phage display fragment antigen binding (Fab) library, two Fabs with high affinity for human FZD5-CRD were screened, IgG-2919 and IgG-2921. These two full-length human recombinant antibodies exhibited superior binding properties and efficacy in RNF43-mutated pancreatic cancer cells, significantly inhibiting the growth of the corresponding cell lines and transplanted tumors [79]. Another study used Fab, an antigen-binding fragment molecule, targeting FZD7 to block the messaging mediated by the binding of Wnt3a protein and FZD7, resulting in hESCs no longer being able to maintain a stemness state. This specific FZD receptor binding protein (FZDs-Fab) provides a novel targeted therapeutic candidate for the clinical treatment of tumors [98]. Precise targeting of individual proteins of a highly closely related family of proteins is a frequent challenge in drug discovery. The desire to reduce cross-reactivity and achieve desired therapeutic effects with minimal off-target side effects requires more cutting-edge drug screening and synthesis technologies. The design of high-affinity anchor protein conjugates using inverse screening techniques has shown potential for excellent therapeutic agents. Repeat protein binders (DRPBs) targeting specific receptor subtypes of the FZD family exhibit high specificity and affinity. DRPBs that specifically bind the CRD region of FZD5, FZD8, FZD4, and FZD7 have been developed [140].
Identifying the functional position of receptors is one of the critical challenges in developing efficient receptor inhibitors. Rapid advances in contemporary bioinformatics have greatly ameliorated this dilemma. Single-chain fragile variable (scFv) antibodies result from combining bioinformatics and recombinant antibodies, which combine heavy and light-chain variable regions through short peptide junctions [141]. The human attributes, less space-occupying position, and high affinity confer significant effectiveness of this antibody in clinical trials. Anti-FZD7 scFv has been developed with surprising inhibitory effects against triple-negative breast cancer [142] and Wilms tumors [143]. Compared with antibodies delivered freely to the body, antibodies delivered by nanoparticles have greater affinity and better therapeutic benefits than those delivered freely to the body, significantly reducing production costs and therapeutic doses, and are a boon to oncology treatment. A study evaluated the inhibitory effect of antibody-functionalized nanoparticles on triple-negative breast cancer. This antibody-nanoshell conjugate against FZD7 (FZD7-NS) exhibited a striking Wnt signaling pathway disruption effect and tumor treatment benefit [144]. As an extracellular peptide of FZD7, soluble recombinant FZD7 protein (sFZD7) was developed to bind Wnt3 ligand competitively with FZD7, thereby inhibiting the transcriptional activity of the canonical Wnt signaling pathway in human hepatoma cells. In vitro and in vivo experiments showed that sFZD7 effectively inhibited hepatocellular carcinoma cell death growth without affecting normal hepatocytes. As an effective therapeutic agent with specificity, sFZD7 can be used with other chemotherapeutic agents to improve the disease management of hepatocellular carcinoma [145]. Small interfering peptide (RHPD) is another novel competitive receptor antagonist developed in recent years that can generate high affinity between this antagonist and the PDZ structural domain of DVL, thus effectively inhibiting the proliferative ability of hepatocellular carcinoma cells and promoting apoptosis in vitro and in vivo. This recombinant protein provides a safe and effective option for managing hepatocellular carcinoma [146]. A potent peptide ligand (Fz7-21) was screened from a peptide phage library that binds to the proximal end of the FZD7 lipid-binding groove and alters its structure, with the CRD metastable to the active conformation. The above alterations disrupt FZD-dependent stem cell signaling in the intestine. This peptide shows that lipid-binding groove targeting promises to be a way to achieve heteromer-selective FZD receptor inhibition, providing a powerful tool for the study of the biological function of specific FZD receptors and a pharmacological means to explore further the function of FZD7 in stem cells and oncology [147].
A polyclonal antibody (TT641 pAb) against human-derived FZD10 has been used to treat synovial sarcomas and other tumors that overexpress FZD10 and has shown potent antitumor potential both in vivo and in vitro [121]. Compared to TT641 pAb, murine-derived mAb92-13 has higher specificity as a monoclonal antibody and exhibits significant negative regulation of synovial sarcoma in mice by potent binding to FZD10. mAb92-13 provides a high value option for clinical treatment and prognostic improvement of synovial sarcoma and other FZD10 high-expressing tumors [122]. Given that FZD10 is an established therapeutic target for synovial sarcoma progression, targeted inhibition of FZD10 in synovial sarcoma has also undergone several human trials. The biodistribution, safety, and recommended dose of the 90Y-labeled anti-FZD10 antibody OTSA101 have been scientifically validated in synovial sarcoma patients [148]. Later, a study using 225Ac-labeled OTSA101 and a mouse model for safety and efficacy assessment found a higher bioeffective dose than 90Y-labeled OTSA101, and a 60% complete response could be achieved [123].
Using antibodies and peptides to target FZD is a relatively promising clinical translation approach. Antibodies have more vital specificity and targeting and will have better antitumor effects, and recombinant proteins or peptides also provide safe and effective options for the clinical treatment of a variety of human tumors.
Small molecule inhibitors
While macromolecules such as antibodies and peptides targeting FZD CRDs possess significant efficacy and high specificity, small molecules also have clear advantages: being more suitable for oral administration, avoiding serious immune-related adverse events by modulating half-life, easy transport and storage, and high stability and membrane permeability. The available small molecule inhibitors against FZD are more limited (Table 4).
Table 4.
Small molecule inhibitors targeting FZDs.
A complex set of quality control mechanisms in the organism is used to help proteins fold correctly and repair or degrade misfolded proteins. Molecular chaperones are essential for quality control in the correct folding of proteins. Ligand proteins can also be chaperoned to achieve stable binding by chaperoning their receptors. Organic molecules can bind to specific receptors in the form of protein-folding chaperones, thereby achieving conformational changes and abnormal activity on the target receptor [149]. FZM1, an organic molecule, can act as a pharmacological chaperone to elicit conformational changes in FZD4-WT (wild type), ultimately enabling it to inhibit downstream factor activity. FZM1 achieved a significant inhibitory effect on U87MG glioblasts and Caco-2 cells by chaperoning FZD4 at 10 µM, demonstrating FZD4 heterodimeric chaperone molecules should be given more attention as antitumor agents [150].
As the importance of the drug development field, the roles of GPCRs have been studied in kinds of cancers. It is well-accepted that targeting the TMD of GPCRs is a clinically validated strategy for the development of different drug candidates. It has been established the FZDs share some common structural characterizations with the GPCRs: specific structures in the transmembrane structural domain (TMD) of FZDs, including the conventional 7TM core, the intracellular C-terminal helix-8, conserved cysteine residues in the extracellular loop, and other common residues in the TMD, are highly consistent with GPCRs [151]. Therefore, targeting FZD-TMD could be an attractive therapeutic strategy to improve the cancer prognosis by modulating WNT/FZD signaling. A small molecule inhibitor (SRI37892) that acts on the TMD of FZD7 was screened by structure-based virtual screening. As a FZD7 inhibitor, the antitumor effects of this small molecule were initially evaluated in a breast cancer cell model. This small molecule compound significantly inhibited breast cancer cell proliferation by inhibiting the Wnt/FZD7 signaling cascade with an IC50 value of ~2 µM. This experiment was the first to use FZD7-TMD as a specific inhibitory region and demonstrated the medicinal value of this region [152]. The use of FZD-TMD as a target of the drug has a broad scope for drug development and significant value.
A study performed homology modeling and ab initio modeling of FZD7 and virtual screening for ligands using the ZINC database, followed by docking analysis to assess the binding free energy between FZD7-CRD and the selected ligands. Molecular docking results identified 30 ligands that bind to the CRD of FZD7, among which Zinc05972969 has a binding free energy of -8.1 kcal/mol, significantly better than the control molecule. Zinc05972969 has two hydrogen bonds to Lys61 and Gln55 and universal hydrophobic contact with FZD7-CRD. This is one of the first lead small molecules targeting the structural domain of FZD7-CRD, and more biological experiments are needed to verify its value [153].
Carbamazepine is commonly used to treat epilepsy. However, a recent study observed carbamazepine binding to a novel pocket of FZD8-CRD during a small molecule screen by using surface plasmon resonance and determined the crystal structure of the complex with a resolution of 1.7 Å. Furthermore, the unique residue Tyr52 of FZD8 becomes a carbamazepine-binding specific recognition site, distinguishing FZD8 from other FZD receptors that are highly similar or even FZD5. Notably, no drug specifically recognizes either FZD8 or FZD5 alone. This experiment revealed a significant concentration-dependent response of FZD8-CRD to carbamazepine (KD of 17 µM and important inhibitory concentration of 8 µM) through a series of cell function experiments. The high-resolution crystal structure of the target protein is the gold standard for structure-guided drug design. This is the first time that structural information on the binding of small molecules to FZD receptors has been obtained, enabling the identification of binding sites that can be used to distinguish different FZD receptors with high homology and providing new ideas and candidates for the development of highly specific FZD inhibitors [154].
There is no doubt that this knowledge-driven approach has become an essential part of the drug design process, leveraging existing knowledge and theory of receptor-ligand interactions to guide drug discovery and optimize routes. Despite the initial success in the mining of FZD inhibitors, there is a need to integrate existing technologies that organically combine the fields of chemistry, structural biology, bioinformatics, and artificial intelligence to exploit the full potential of FZD targets as much as possible and apply them fully in efficient cancer chemoprevention strategies.
Conclusion
The Wnt pathway is a multilinked, multisite pathway co-opted by human tumors, and the FZD protein, as a specific key protein in the Wnt signaling cascade, is also a hot research topic of interest. Dysregulation of different members of the FZD family has been detected in different clinical cancer tissues. Aberrant expression of these members is significantly and positively correlated with tumor conventional chemotherapy tolerance, disease recurrence, poor prognosis, and low survival rates. A growing number of biological experiments have demonstrated the powerful effects of these receptors in the malignant progression of human tumors, including the promotion of cell proliferation and metastatic capacity, chemoresistance, and tumor stemness. These results suggest that members of our FZD family can serve as potentially effective therapeutic targets for a variety of malignancies. Several inhibitors have been developed to antagonize FZDs, including blocking antibodies, peptide inhibitors, small molecule inhibitors, and non-coding RNAs. The inhibitory effects of these inhibitors on cultured tumor cells, tumor cell xenografts, mouse tumor models, and patient-derived xenografts have been demonstrated on a basic level, and the success of FZD-targeted therapies in combination with chemotherapy in preclinical settings, including clinical phase 1b, demonstrates the clinical translational promise of this combination therapy.
Structure-based antibodies and optimized lead molecule design provide a high-level platform for developing and optimizing FZD receptor inhibitors. The specificity and affinity of FZD inhibitors can be continuously improved while reducing their toxic side effects. The structure of human FZD4-7TM alone has been reported, accompanied by some structure-based molecular dynamics simulation data [155]. The cryoelectronic microscopic structure of human FZD5, previously observed at 3.7 Å resolution, is a significant development in the structural exploration of FZDs [17]. CRD, as a key protein region for binding FZDs to their ligands, is currently the main target of pharmacological research and drug development. The crystal structures of the Wnt/FZD-CRD complexes corresponding to different FZD receptors need to be explored further. Moreover, a more precise understanding of the FZD-7TMD region, which is highly homologous to GPCRs, will lead to promising new ideas for drug development. Based on the pharmacological structural characteristics of different FZDs, more highly specific inhibitors for the treatment of cancer should be developed.
In addition to the specific FZD crystal structure studies that need to be intensified, challenges for FZDs include the unique identification and binding of individual Wnts and FZDs. The specific regulation of individual Wnt/FZD cascades for different human tumors is a prerequisite for cancer therapy. Current studies on frizzled receptors affecting tumors mostly focus on the effects of frizzled receptors themselves on tumors, and studying Wnt/frizzled as a unit will have far-reaching implications for guidance. Determining how individual Wnt and frizzled are coupled and the overall role such couplings play in tumors has important guiding and therapeutic implications. Kinetic analysis of Wnt/FZD signaling allows us to describe receptor signaling and pharmacology in greater detail. To fully understand Wnt/FZD binding, receptor specificity, and possible signaling, we need access to all pure Wnts. Since the initial purification of Wnt-3A, it has become possible to acutely stimulate cells with relatively well-defined concentrations of agonists, but the highly hydrophobic nature of Wnt due to the presence of fatty acyl groups makes it difficult to express, solubilize, and purify, and the lack of purified, active, and labeled Wnt remains a major obstacle. Certain formulations, such as liposome packaging, have yielded a more stable and biologically active Wnt3A, which is in the early stages of clinical trials [156]. However, such formulations require purification of individual WNTs, which has so far only been achieved in a few individual Wnt proteins. In addition, WNT lacks selectivity for FZDs due to conserved interaction sites between WNT and the extracellular cysteine-rich structural domains of FZDs, and Wnt is also highly promiscuous in its interactions with ten FZDs (FZDs 1–10) and two LRPs (LRP5 and LRP6), each of which can activate multiple FZD and LRP pairs. Ultimately, the tool molecules described to date do not target all the FZDs that activate Wnt/β-catenin signaling, and establishing a method to monitor and quantify Wnt/FZD interactions will improve our understanding of receptor function, ligand interactions, and endogenous Wnt inhibitors, as well as aid in the screening of small-molecule drugs that target FZDs.
The exploitation of the potential of FZD cannot be limited to cell survival, motility, and drug resistance; FZD proteins are essential for the maintenance and differentiation of various normal adult stem cells. The Wnt signaling pathway is a major driver of adult stem cell self-renewal and cell fate differentiation in the gut, liver, skin, and many other organs, while sustained activation of the Wnt/β-catenin pathway confers self-renewal growth properties to cancer cells and is also associated with therapeutic resistance. The impact of Wnt/β-catenin signaling depends not only on a simple “on/off” principle but also on the coordination of different “modes” in a temporally and spatially controlled manner. In healthy adult stem cells, Wnt pathway activity is carefully controlled by core pathway tumor suppressors and negative feedback regulators. To prevent excessive signaling levels, Wnt-induced responses are often balanced by critical negative feedback regulators. For example, the membrane-bound E3 ligases ring finger protein 43 and zinc and ring finger protein 39 play an important role in the maintenance of stem cell homeostasis by mediating the ubiquitination of Wnt receptors and reducing the cell’s sensitivity to Wnt proteins [157]. At the same time, Wnt signaling can also be engaged in tumor formation through stem cells, and when key proteins of the Wnt pathway are abnormally activated or regulated, the normal behavior of stem cells can be disturbed and cancer can be triggered. It has been demonstrated that sustained expression of Wnt target gene-survivin during human embryonic stem cell differentiation in vivo will promote teratoma formation [158]. It has also been shown that the delicate balance between the coactivator proteins CREB-binding protein and E1A binding protein p300 is an important determinant for stem cells to maintain proliferation or initiate differentiation [159]. The investigation of these proteins will provide critical and useful information on the mechanisms by which the Wnt pathway is aberrantly regulated to promote cancer progression. More to the point, drug studies targeting the Wnt/frizzled signaling pathway for the regulation of stem cell proliferation and differentiation will provide insights into regenerative medicine and malignancy treatment.
In addition, more focus should be placed on the effect of FZD on tumor immune escape and the tumor microenvironment. It was found that Wnts in the tumor microenvironment can bind to the DC cell-expressed coreceptor LRP5/6 (expressed by DC cells) and suppress the activity of effector T cells while activating signaling pathways, contributing to the tumor microenvironment [160]. Therefore, in addition to examining the inhibitory effect of FZD inhibitors on tumor cells, their effect on various immune cells needs to be considered. Bioinformatics analysis revealed that FZD2 in this family correlates significantly with macrophages and CD4+ T cells in the tumor microenvironment as well as with immune checkpoints [161]. Given that immunotherapy may be a useful adjuvant therapy for tumors overexpressing one or more FZD receptors, the specific effects and connections between FZD and the tumor immune microenvironment need to be explored biologically in more depth in vitro and in vivo to provide a more theoretical basis and broad ideas for FZD-related tumor-targeted therapy.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant number 82172686), and Beijing Natural Science Foundation (Grant numbers 7202088, 7212152).
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Hui-yu Liu, Xiao-jiao Sun
Contributor Information
Zhen-ming Liu, Email: zmliu@bjmu.edu.cn.
Xiao-cong Pang, Email: pangxiaocong1227@163.com.
Tian-cheng Li, Email: litiancheng@bjmu.edu.cn.
References
- 1.Schulte G, Wright SC. Frizzleds as GPCRs—more conventional than we thought! Trends Pharmacol Sci. 2018;39:828–42. 10.1016/j.tips.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 2.Schulte G, Kozielewicz P. Structural insight into class F receptors—what have we learnt regarding agonist-induced activation? Basic Clin Pharmacol Toxicol. 2020;126:17–24. 10.1111/bcpt.13235 [DOI] [PubMed] [Google Scholar]
- 3.Schulte G. International union of basic and clinical pharmacology. LXXX. The class frizzled receptors. Pharmacol Rev. 2010;62:632–67. 10.1124/pr.110.002931 [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Liu ZL, Jia JH. Mechanisms of smoothened regulation in hedgehog signaling. Cells. 2021;10:2138. 10.3390/cells10082138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang YX, Beachy PA. Cellular and molecular mechanisms of hedgehog signalling. Nat Rev Mol Cell Biol. 2023;24:668–87. 10.1038/s41580-023-00591-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang J. Hedgehog signaling mechanism and role in cancer. Semin Cancer Biol. 2022;85:107–22. 10.1016/j.semcancer.2021.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirai H, Matoba K, Mihara E, Arimori T, Takagi J. Crystal structure of a mammalian wnt-frizzled complex. Nat Struct Mol Biol. 2019;26:372–9. 10.1038/s41594-019-0216-z [DOI] [PubMed] [Google Scholar]
- 8.Parsons MJ, Tammela T, Dow LE. Wnt as a sriver and dependency in cancer. Cancer Discov. 2021;11:2413–29. 10.1158/2159-8290.CD-21-0190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw HV, Koval A, Katanaev VL. A high-throughput assay pipeline for specific targeting of frizzled GPCRs in cancer. Methods Cell Biol. 2019;149:57–75. 10.1016/bs.mcb.2018.08.006 [DOI] [PubMed] [Google Scholar]
- 10.Albrecht LV, Tejeda-Muñoz N, De Robertis EM. Cell biology of canonical wnt signaling. Annu Rev Cell Dev Biol. 2021;37:369–89. 10.1146/annurev-cellbio-120319-023657 [DOI] [PubMed] [Google Scholar]
- 11.Rim EY, Clevers H, Nusse R. The wnt pathway: from signaling mechanisms to synthetic modulators. Annu Rev Biochem. 2022;91:571–98. 10.1146/annurev-biochem-040320-103615 [DOI] [PubMed] [Google Scholar]
- 12.Rhee CS, Sen M, Lu D, Wu C, Leoni L, Rubin J, et al. Wnt and frizzled receptors as potential targets for immunotherapy in head and neck squamous cell carcinomas. Oncogene. 2002;21:6598–605. 10.1038/sj.onc.1205920 [DOI] [PubMed] [Google Scholar]
- 13.Zeng CM, Chen Z, Fu L. Frizzled receptors as potential therapeutic targets in human cancers. Int J Mol Sci. 2018;19:1543. 10.3390/ijms19051543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nichols AS, Floyd DH, Bruinsma SP, Narzinski K, Baranski TJ. Frizzled receptors signal through G proteins. Cell Signal. 2013;25:1468–75. 10.1016/j.cellsig.2013.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Umar SA, Dong B, Nihal M, Chang H. Frizzled receptors in melanomagenesis: from molecular interactions to target identification. Front Oncol. 2022;12:1096134. 10.3389/fonc.2022.1096134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schenkelaars Q, Fierro-Constrain L, Renard E, Hill AL, Borchiellini C. Insights into frizzled evolution and new perspectives. Evol Dev. 2015;17:160–9. 10.1111/ede.12115 [DOI] [PubMed] [Google Scholar]
- 17.Tsutsumi N, Mukherjee S, Waghray D, Janda CY, Jude KM, Miao Y, et al. Structure of human frizzled5 by fiducial-assisted cryo-EM supports a heterodimeric mechanism of canonical Wnt signaling. Elife. 2020;9:e58464. 10.7554/eLife.58464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mieszczanek J, Strutt H, Rutherford TJ, Strutt D, Bienz M, Gammons MV. Selective function of the PDZ domain of dishevelled in noncanonical wnt signalling. J Cell Sci. 2022;135:259547. 10.1242/jcs.259547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beitia GJ, Rutherford TJ, Freund SMV, Pelham HR, Bienz M, Gammons MV. Regulation of dishevelled DEP domain swapping by conserved phosphorylation sites. Proc Natl Acad Sci USA. 2021;118:e2103258118. 10.1073/pnas.2103258118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babcock RL, Pruitt K. Letting go: Dishevelled phase separation recruits Axin to stabilize β-catenin. J Cell Biol. 2022;221:e202211001. 10.1083/jcb.202211001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsieh JC, Rattner A, Smallwood PM, Nathans J. Biochemical characterization of wnt-frizzled interactions using a soluble, biologically active vertebrate wnt protein. Proc Natl Acad Sci USA. 1999;96:3546–51. 10.1073/pnas.96.7.3546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacDonald BT, He X. Frizzled and LRP5/6 Receptors for wnt/β-catenin signaling. Cold Spring Harb Perspect Biol. 2012;4:a007880. 10.1101/cshperspect.a007880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.González-Sancho JM, Brennan KR, Castelo-Soccio LA, Brown AMC. Wnt proteins induce dishevelled phosphorylation via an LRP5/6-independent mechanism, irrespective of their ability to stabilize β-catenin. Mol Cell Biol. 2004;24:4757–68. 10.1128/MCB.24.11.4757-4768.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu CH, Nusse R. Ligand receptor interactions in the wnt signaling pathway in Drosophila. J Biol Chem. 2005;280:31340. 10.1016/S0021-9258(20)79413-5 [DOI] [PubMed] [Google Scholar]
- 25.Tsutsumi N, Hwang S, Waghray D, Hansen S, Jude KM, Wang N, et al. Structure of the wnt-frizzled-LRP6 initiation complex reveals the basis for coreceptor discrimination. Proc Natl Acad Sci USA. 2023;120:e2218238120. 10.1073/pnas.2218238120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Logan CY, Nusse R. The wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:81–810. 10.1146/annurev.cellbio.20.010403.113126 [DOI] [PubMed] [Google Scholar]
- 27.Galluzzi L, Spranger S, Fuchs E, López-Soto A. Wnt signaling in cancer immunosurveillance. Trends Cell Biol. 2019;29:44–65. 10.1016/j.tcb.2018.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang YS, Chang H, Rattner A, Nathans J. Frizzled receptors in development and disease. Essays Dev Biol. 2016;117:113–39. 10.1016/bs.ctdb.2015.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–99. 10.1016/j.cell.2017.05.016 [DOI] [PubMed] [Google Scholar]
- 30.Peifer M, Polakis P. Cancer-wnt signaling in oncogenesis and embryogenesis-a look outside the nucleus. Science. 2000;287:1606–9. 10.1126/science.287.5458.1606 [DOI] [PubMed] [Google Scholar]
- 31.Butler MT, Wallingford JB. Planar cell polarity in development and disease. Nat Rev Mol Cell Biol. 2017;18:375–88. 10.1038/nrm.2017.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thrasivoulou C, Millar M, Ahmed A. Activation of intracellular calcium by multiple wnt ligands and translocation of β-catenin into the nucleu. J Biol Chem. 2013;288:35651–9. 10.1074/jbc.M112.437913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao Q, Chen ZX, Jin XZ, Mao RY, Chen ZQ. The many postures of noncanonical wnt signaling in development and diseases. Biomed Pharmacother. 2017;93:359–69. 10.1016/j.biopha.2017.06.061 [DOI] [PubMed] [Google Scholar]
- 34.González P, González-Fernández C, Campos-Martín Y, Mollejo M, Carballosa-Gautam M, Marcillo A, et al. Frizzled 1 and wnt1 as new potential therapeutic targets in the traumatically injured spinal cord. Cell Mol Life Sci. 2020;7:4631–62. 10.1007/s00018-019-03427-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flahaut M, Meier R, Coulon A, Nardou KA, Niggli FK, Martinet D, et al. The wnt receptor FZD1 mediates chemoresistance in neuroblastoma through activation of the wnt/β-catenin pathway. Oncogene. 2009;28:2245–56. 10.1038/onc.2009.80 [DOI] [PubMed] [Google Scholar]
- 36.Su WM, Mo YL, Wu FP, Guo KG, Li JM, Luo YP, et al. MiR-135b reverses chemoresistance of non-small cell lung cancer cells by downregulation of FZD1. Biomed Pharmacother. 2016;84:123–9. 10.1016/j.biopha.2016.09.027 [DOI] [PubMed] [Google Scholar]
- 37.Yang LL, Yang ZL, Li DQ, Li ZR, Zou Q, Yuan Y, et al. Overexpression of FZD1 and CAIX are associated with invasion, metastasis, and poor-prognosis of the pancreatic ductal adenocarcinoma. Pathol Oncol Res. 2018;24:899–906. 10.1007/s12253-017-0284-5 [DOI] [PubMed] [Google Scholar]
- 38.Peng Q, Wang L, Zhao DF, Lv YL, Wang HZ, Chen G, et al. Overexpression of FZD1 is associated with a good prognosis and resistance of sunitinib in clear cell renal cell carcinoma. J Cancer. 2019;10:1237–51. 10.7150/jca.28662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang YH, Lmai Y, Shiseki M, Tanaka J, Motoji T. Knockdown of the wnt receptor frizzled-1 (FZD1) reduces MDR1/P-glycoprotein expression in multidrug resistant leukemic cells and inhibits leukemic cell proliferation. Leuk Res. 2018;67:99–108. 10.1016/j.leukres.2018.01.020 [DOI] [PubMed] [Google Scholar]
- 40.Hung TH, Chen CM, Tseng CP, Shen CJ, Wang HL, Choo KB, et al. FZD1 activates protein kinase C delta-mediated drug resistance in multidrug-resistant MES-SA/Dx5 cancer cells. Int J Biochem Cell Biol. 2014;53:55–65. 10.1016/j.biocel.2014.04.011 [DOI] [PubMed] [Google Scholar]
- 41.Zhang H, Jing XX, Wu XJ, Hu J, Zhang XF, Wang X, et al. Suppression of multidrug resistance by rosiglitazone treatment in human ovarian cancer cells through downregulation of FZD1 and MDR1 genes. Anti-Cancer Drugs. 2015;26:706–15. 10.1097/CAD.0000000000000236 [DOI] [PubMed] [Google Scholar]
- 42.Liu ZJ, Sun LC, Cai YP, Shen SQ, Zhang T, Wang NN, et al. Hypoxia-induced suppression of alternative splicing of MBD2 promotes breast cancer metastasis via activation of FZD1. Cancer Res. 2021;81:1265–78. 10.1158/0008-5472.CAN-20-2876 [DOI] [PubMed] [Google Scholar]
- 43.Sun JG, Li XB, Yin RH, Li XF. lncRNA VIM-AS1 promotes cell proliferation, metastasis and epithelial-mesenchymal transition by activating the wnt/β-catenin pathway in gastric cancer. Mol Med Rep. 2020;22:4567–78. 10.3892/mmr.2020.11577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jia F, Zhang LX, Jiang ZY, Tan GW, Wang ZX. FZD1/KLF10-hsa-miR-4762-5p/miR-224-3p-circular RNAs axis as prognostic biomarkers and therapeutic targets for glioblastoma: a comprehensive report. BMC Med Genomics. 2023;16:21. 10.1186/s12920-023-01450-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ludwig K, Tse ES, Wang JYJ. Colon cancer cells adopt an invasive phenotype without mesenchymal transition in 3-D but not 2-D culture upon combined stimulation with EGF and crypt growth factors. BMC Cancer. 2013;13:221. 10.1186/1471-2407-13-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gujral TS, Chan M, Peshkin L, Sorger PK, Kirschner MW, Macbeath G. A noncanonical frizzled2 pathway regulates epithelial-mesenchymal transition and metastasis. Mol Biol Cell. 2014;159:844–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yin P, Wang W, Gao J, Bai Y, Wang Z, Na L, et al. Fzd2 contributes to breast cancer cell mesenchymal-like stemness and drug resistance. Oncol Res. 2020;28:273–84. 10.3727/096504020X15783052025051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang EJ, Li ZN, Xu ZF, Duan WY, Sun CF, Lu L. Frizzled2 mediates the migration and invasion of human oral squamous cell carcinoma cells through the regulation of the signal transducer and activator of transcription-3 signaling pathway. Oncol Rep. 2015;34:3061–7. 10.3892/or.2015.4285 [DOI] [PubMed] [Google Scholar]
- 49.Fu YF, Zheng Q, Mao YY, Jiang XY, Chen X, Liu P, et al. Wnt2-Mediated FZD2 stabilization regulates esophageal cancer metastasis via STAT3 signaling. Front Oncol. 2020;10:1168. 10.3389/fonc.2020.01168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo M, Zhou L, Zhan SJ, Cheng LJ, Li RN, Wang H, et al. ALPL regulates the aggressive potential of high grade serous ovarian cancer cells via a non-canonical wnt pathway. Biochem Biophys Res Commun. 2019;513:528–33. 10.1016/j.bbrc.2019.04.016 [DOI] [PubMed] [Google Scholar]
- 51.Asano T, Yamada S, Fuchs BC, Takami H, Hayashi M, Sugimoto H, et al. Clinical implication of frizzled 2 expression and its association with epithelial-to-mesenchymal transition in hepatocellular carcinoma. Int J Oncol. 2017;50:1647–54. 10.3892/ijo.2017.3937 [DOI] [PubMed] [Google Scholar]
- 52.Nath D, Li X, Mondragon C. Post Dawn, Chen M, White JR, et al. Abi1 loss drives prostate tumorigenesis through activation of EMT and non-canonical wnt signaling. Cell Commun Signal. 2019;17:120. 10.1186/s12964-019-0410-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bian YD, Chang XW, Liao Y, Wang JY, Li YR, Wang K, et al. Promotion of epithelial-mesenchymal transition by frizzled2 is involved in the metastasis of endometrial cancer. Oncol Rep. 2016;36:803–10. 10.3892/or.2016.4885 [DOI] [PubMed] [Google Scholar]
- 54.Golkowski M, Lau HT, Chan M, Kenerson H, Vidadala VN, Shoemaker A, et al. Pharmacoproteomics identifies kinase pathways that drive the epithelial-mesenchymal transition and drug resistance in hepatocellular carcinoma. Cell Syst. 2020;11:196–207. 10.1016/j.cels.2020.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ning S, Liu CF, Lou W, Yang JC, Lombard AP, D’Abronzo LS, et al. Bioengineered BERA-Wnt5a siRNA targeting Wnt5a/FZD2 signaling suppresses advanced prostate cancer tumor growth and enhances enzalutamide treatment. Mol Cancer Ther. 2022;21:1594–607. 10.1158/1535-7163.MCT-22-0216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding LC, Huang XY, Zheng FF, Xie J, She L, Feng Y, et al. FZD2 inhibits the cell growth and migration of salivary adenoid cystic carcinomas. Oncol Rep. 2016;35:1006–12. 10.3892/or.2015.3811 [DOI] [PubMed] [Google Scholar]
- 57.Hua ZL, Jeon S, Caterina MJ, Nathans J. Frizzeld3 is required for the development of multiple axon tracts in the mouse central nervous system. Proc Natl Acad Sci USA. 2014;111:E3005–3014. 10.1073/pnas.1406399111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li C, Nguyen V, Clark KN, Zahed T, Sharkas S, Filipp FV, et al. Down-regulation of FZD3 receptor suppresses growth and metastasis of human melanoma independently of canonical wnt signaling. Proc Natl Acad Sci USA. 2019;116:4548–57. 10.1073/pnas.1813802116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang A, Wang Y, Zhan J, Chen J. MicroRNA-186-5p inhibits the metastasis of cervical cancer by targeting FZD3. J Buon. 2021;26:677–83. [PubMed] [Google Scholar]
- 60.Feng L, Zheng H, Zhang HP, Cao MX, Zhou H. LncRNA FGD5-AS1 drives the malignant development of gastric cancer by negatively interacting with FZD3. Pol J Pathol. 2022;73:72–9. 10.5114/pjp.2022.117179 [DOI] [PubMed] [Google Scholar]
- 61.Meng ZF, Liu Q, Liu YF, Yang YM, Shao CF, Zhang SQ. Frizzled-3 suppression overcomes multidrug chemoresistance by wnt/β-catenin signaling pathway inhibition in hepatocellular carcinoma cells. J Chemother. 2023;35:653–61. 10.1080/1120009X.2023.2182573 [DOI] [PubMed] [Google Scholar]
- 62.Yao WJ, Wang JJ, Meng FR, Zhu ZB, Jia XB, Lei X, et al. Circular RNA circPVT1 inhibits 5-fluorouracil chemosensitivity by regulating ferroptosis through miR-30a-5p/FZD3 axis in esophageal cancer cells. Front Oncol. 2021;11:780938. 10.3389/fonc.2021.780938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu D, Zhao YD, Tawatao R, Cottam HB, Sen M, Leoni LM, et al. Activation of the Wnt signaling pathway in chronic lymphocytic leukemia. Proc Natl Acad Sci USA. 2004;101:3118–23. 10.1073/pnas.0308648100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu GQ, Wang Y, Wang B, Liu WR, Dong SS, Chen EB, et al. Targeting HNRNPM inhibits cancer stemness and enhances antitumor immunity in wnt-activated hepatocellular carcinoma. Cell Mol Gastroenterol Hepatol. 2022;13:1413–47. 10.1016/j.jcmgh.2022.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xiang M, Huang YQ, Dai CJ, Zou GD. MiR-340 regulates the growth and metabolism of renal cell carcinoma cells by targeting frizzled class receptor 3. Arch Pharm Res. 2021;44:219–29. 10.1007/s12272-021-01310-0 [DOI] [PubMed] [Google Scholar]
- 66.Robitaille J, MacDonald MLE, Kaykas A, Sheldahl LC. Zeisler Jutta, Dubé MP, et al. Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nat Genet. 2002;32:326–30. 10.1038/ng957 [DOI] [PubMed] [Google Scholar]
- 67.Descamps B, Sewduth R, Tojais NF, Jaspard B, Reynaud A, Sohet F, et al. Frizzled 4 regulates arterial network organization through noncanonical wnt/planar cell polarity signaling. Circ Res. 2012;110:47–58. 10.1161/CIRCRESAHA.111.250936 [DOI] [PubMed] [Google Scholar]
- 68.Feng ZH, Zheng L, Yao T, Tao SY, Wei XA, Zheng ZY, et al. EIF4A3-induced circular RNA PRKAR1B promotes osteosarcoma progression by miR-361-3p-mediated induction of FZD4 expression. Cell Death Dis. 2021;12:1025. 10.1038/s41419-021-04339-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xiang DM, Gu MY, Liu JY, Dong W, Yang ZS, Wang K, et al. m6A RNA methylation-mediated upregulation of HLF promotes intrahepatic cholangiocarcinoma progression by regulating the FZD4/β-catenin signaling pathway. Cancer Lett. 2023;560:216144. 10.1016/j.canlet.2023.216144 [DOI] [PubMed] [Google Scholar]
- 70.Jin X, Jeon HY, Joo KM, Kim JK, Jin JY, Kim SH, et al. Frizzled 4 regulates stemness and invasiveness of migrating glioma cells established by serial intracranial transplantation. Cancer Res. 2011;71:3066–75. 10.1158/0008-5472.CAN-10-1495 [DOI] [PubMed] [Google Scholar]
- 71.Ohlmann A, Tamm ER. Norrin: molecular and functional properties of an angiogenic and neuroprotective growth factor. Prog Retinal Eye Res. 2012;1:243–57. 10.1016/j.preteyeres.2012.02.002 [DOI] [PubMed] [Google Scholar]
- 72.Paes KT, Wang E, Henze K, Vogel P, Read R, Suwanichkul A, et al. Frizzled 4 is required for retinal angiogenesis and maintenance of the blood-retina barrier. Invest Ophthalmol Vis Sci. 2011;52:6452–61. 10.1167/iovs.10-7146 [DOI] [PubMed] [Google Scholar]
- 73.Planutis K, Planutiene M, Holcombe RF. A novel signaling pathway regulates colon cancer angiogenesis through Norrin. Sci Rep. 2014;4:5630. 10.1038/srep05630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gyanchandani R, Alves MVO, Myers JN, Kim S. A proangiogenic signature is revealed in FGF-mediated bevacizumab-resistant head and neck squamous cell carcinoma. Mol Cancer Res. 2013;11:1585–96. 10.1158/1541-7786.MCR-13-0358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen TL, Zhang FB, Liu J, Huang ZL, Zheng YF, Deng SK, et al. Dual role of wnt5a in promoting endothelial differentiation of glioma stem cells and angiogenesis of glioma derived endothelial cells. Oncogene. 2021;40:5081–94. 10.1038/s41388-021-01922-2 [DOI] [PubMed] [Google Scholar]
- 76.Li X, Lv JX, Hou LY, Guo XF. Circ0001955 acts as a miR-646 sponge to promote the proliferation, metastasis and angiogenesis of hepatocellular carcinoma. Digest Dis Sci. 2022;67:2257–68. 10.1007/s10620-021-07053-8 [DOI] [PubMed] [Google Scholar]
- 77.Sun Y, Wang Z, Na L, Dong D, Wang W, Zhao CH. FZD5 contributes to TNBC proliferation, DNA damage repair and stemness. Cell Death Dis. 2020;11:1060. 10.1038/s41419-020-03282-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Na L, Wang Z, Bai Y, Sun Y, Dong D, Wang W, et al. WNT7B represses epithelial-mesenchymal transition and stem-like properties in bladder urothelial carcinoma. Biochim Biophys Acta-Mol Basis Dis. 2022;1868:166271. 10.1016/j.bbadis.2021.166271 [DOI] [PubMed] [Google Scholar]
- 79.Steinhart Z, Pavlovic Z, Chandrashekhar M, Hart T, Wang X, Zhang X, et al. Genome-wide CRISPR screens reveal a Wnt-FZD5 signaling circuit as a druggable vulnerability of RNF43-mutant pancreatic tumors. Nat Med. 2017;23:60–8. 10.1038/nm.4219 [DOI] [PubMed] [Google Scholar]
- 80.Brandt MM, Dijk CGMV, Chrifi I, et al. L, et al. Endothelial loss of Fzd5 stimulates PKC/Ets1-mediated transcription of Angpt2 and Flt1. Angiogenesis. 2018;21:805–21. 10.1007/s10456-018-9625-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wan X, Guan SD, Hou YX, Qin YL, Zeng H, Yang LP, et al. FOSL2 promotes VEGF-independent angiogenesis by transcriptionnally activating Wnt5a in breast cancer-associated fibroblasts. Theranostics. 2022;12:6157–8. 10.7150/thno.77019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peterson YK, Nasarre P. Bonilla IV, Hilliard E, Samples J, Morinelli TA, et al. Frizzled-5: a high-affinity receptor for secreted frizzled-related protein-2 activation of nuclear factor of activated T-cells c3 signaling to promote angiogenesis. Angiogenesis. 2017;20:615–28. 10.1007/s10456-017-9574-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dong D, Na L, Zhou K, Wang Z, Sun Y, Zheng Q, et al. FZD5 prevents epithelial-mesenchymal transition in gastric cancer. Cell Commun Signal. 2021;19:21. 10.1186/s12964-021-00708-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Corda G, Sala A. Non-canonical WNT/PCP signalling in cancer: Fzd6 takes centre stage. Oncogenesis. 2017;6:e364. 10.1038/oncsis.2017.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang DN, Ma SC, Zhang CB, Li PL, Mao BB, Guan XD, et al. MicroRNA-935 directly targets FZD6 to inhibit the proliferation of human glioblastoma and correlate to glioma malignancy and prognosis. Front Oncol. 2021;11:566492. 10.3389/fonc.2021.566492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bowin CF, Kozielewicz P, Grätz L, Kowalski-Jahn M, Schihada H, Schulte G. WNT stimulation induces dynamic conformational changes in the Frizzled-Dishevelled interaction. Sci Signal. 2023;16:e4974. 10.1126/scisignal.abo4974 [DOI] [PubMed] [Google Scholar]
- 87.Dong B. FZD6 promotes melanoma cell invasion but not proliferation by regulating canonical wnt signaling and epithelial‒mesenchymal transition. J Invest Dermatol. 2023;143:621–9. 10.1016/j.jid.2022.09.658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhan YH, Zhang LH, Yu SB, Wen JG, Liu YC, Zhang XP. Long non-coding RNA CASC9 promotes tumor growth and metastasis via modulating FZD6/Wnt/β-catenin signaling pathway in bladder cancer. J Exp Clin Cancer Res. 2020;39:136. 10.1186/s13046-020-01624-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sun S, Wang J, Liu J, Yin F, Xin C, Zeng X, et al. MiR-302b suppresses tumor metastasis by targeting frizzled 6 in OSCC. J Dent Res. 2021;100:739–45. 10.1177/0022034520986551 [DOI] [PubMed] [Google Scholar]
- 90.Kim BK, Yoo HI, Kim IJ, Park JK, Yoon SK. FZD6 expression is negatively regulated by miR-199a-5p in human colorectal cancer. BMB Rep. 2015;48:360–6. 10.5483/BMBRep.2015.48.6.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Corda G, Sala G, Lattanzio R, Iezzi M, Sallese M, Fragassi G, et al. Functional and prognostic significance of the genomic amplification of frizzled 6 (FZD6) in breast cancer. J Pathol. 2017;241:350–61. 10.1002/path.4841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yuan Y, Wang Q, Ma SL, Xu LQ, Liu MY, Han B, et al. lncRNA PCAT-1 interacting with FZD6 contributes to the malignancy of acute myeloid leukemia cells through activating Wnt/β-catenin signaling pathway. Am J Transl Res. 2019;11:7104–14. [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang J, Wang JL, Zhang CY, Ma YF, Zhao R, Wang YY. The prognostic role of FZD6 in esophageal squamous cell carcinoma patients. Clin Transl Oncol. 2020;22:1172–9. 10.1007/s12094-019-02243-3 [DOI] [PubMed] [Google Scholar]
- 94.Chen ZZ, Gao YF, Yao LT, Liu YT, Huang L, Yan ZY, et al. LncFZD6 initiates Wnt/β-catenin and liver TIC self-renewal through BRG1-mediated FZD6 transcriptional activation. Oncogene. 2018;37:3098–112. 10.1038/s41388-018-0203-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Han K, Lang T, Zhang Z, Zhang Y, Sun Y, Shen Z, et al. Luteolin attenuates wnt signaling via upregulation of FZD6 to suppress prostate cancer stemness revealed by comparative proteomics. Sci Rep. 2018;8:8537. 10.1038/s41598-018-26761-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yan J, Liu TY, Zhou XY, Dang YN, Yin CQ, Zhang GFZD6. targeted by miR-21, represses gastric cancer cell proliferation and migration via activating non-canonical wnt pathway. J Gastroenterol Hepatol. 2016;31:88. [PMC free article] [PubMed] [Google Scholar]
- 97.Huang TZ, Alvarez AA, Pangeni RP, Horbinski CM, Lu SJ, Kim SH, et al. A regulatory circuit of miR-125b/miR-20b and Wnt signalling controls glioblastoma phenotypes through FZD6-modulated pathways. Nat Commun. 2016;7:12885. 10.1038/ncomms12885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fernandez A, Huggins IJ, Perna L, Brafman D, Lu D, Yao SY, et al. The WNT receptor FZD7 is required for maintenance of the pluripotent state in human embryonic stem cells. Proc Natl Acad Sci USA. 2014;111:1409–14. 10.1073/pnas.1323697111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang ZB, Xu YH. FZD7 accelerates hepatic metastases in pancreatic cancer by strengthening EMT and stemness associated with TGF-β/SMAD3 signaling. Mol Med. 2022;28:82. 10.1186/s10020-022-00509-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang YN, Zhao GY, Condello S, Huang H, Cardenas H, Tanner EJ, et al. Frizzled-7 identifies platinum-tolerant ovarian cancer cells susceptible to ferroptosis. Cancer Res. 2021;81:384–99. 10.1158/0008-5472.CAN-20-1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yin P, Bai Y, Wang Z, Sun Y, Gao J, Na L, et al. Non-canonical Fzd7 signaling contributes to breast cancer mesenchymal-like stemness involving col6a1. Cell Commun Signal. 2020;18:143. 10.1186/s12964-020-00646-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheng YZ, Li L, Pan SR, Jiang HL, Jin HY. Targeting Frizzled-7 decreases stemness and chemotherapeutic resistance in gastric cancer cells by suppressing myc expression. Med Sci Monit. 2019;25:8637–44. 10.12659/MSM.918504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xie SL, Fan S, Zhang SY, Chen WX, Li QX, Pan GK, et al. SOX8 regulates cancer stem-like properties and cisplatin-induced EMT in tongue squamous cell carcinoma by acting on the Wnt/beta-catenin pathway. Int J Cancer. 2018;142:1252–65. 10.1002/ijc.31134 [DOI] [PubMed] [Google Scholar]
- 104.Lo RC, Leung CO, Chan KK, Ho DW, Wong CM, Lee TK, et al. Cripto-1 contributes to stemness in hepatocellular carcinoma by stabilizing Dishevelled-3 and activating Wnt/beta-catenin pathway. Cell Death Differ. 2018;25:1426–41. 10.1038/s41418-018-0059-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yin SP, Xu LP, Bonfil RD, Banerjee S, Sarkar FH, Sethi S, et al. Tumor-initiating cells and FZD8 play a major role in drug resistance in triple-negative breast cancer. Mol Cancer Ther. 2013;12:491–8. 10.1158/1535-7163.MCT-12-1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Spanjer AIR, Menzen MH, Dijkstra AE, Berge MVD, Boezen HM, Nickle DC, et al. A pro-inflammatory role for the Frizzled-8 receptor in chronic bronchitis. Thorax. 2016;71:312–22. 10.1136/thoraxjnl-2015-206958 [DOI] [PubMed] [Google Scholar]
- 107.Spanjer AIR, Baarsma HA, Oostenbrink LM, Jansen SR, Kuipers CC, Lindner M, et al. TGF-β-induced profibrotic signaling is regulated in part by the WNT receptor Frizzled-8. FASEB J. 2016;30:1823–35. 10.1096/fj.201500129 [DOI] [PubMed] [Google Scholar]
- 108.Liu R, Chen YJ, Shou T, Hu J, Qing C. MiRNA-99b-5p targets FZD8 to inhibit non-small cell lung cancer proliferation, migration and invasion. Oncotargets Ther. 2019;12:2615–21. 10.2147/OTT.S199196 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 109.Sun SY, Liu SL, Duan SZ, Zhang L, Zhou HH, Hu YJ, et al. Targeting the c-Met/FZD8 signaling axis eliminates patient-derived cancer stem-like cells in head and neck squamous carcinomas. Cancer Res. 2014;74:7546–59. 10.1158/0008-5472.CAN-14-0826 [DOI] [PubMed] [Google Scholar]
- 110.Chen W, Liu ZW, Mai WL, Xiao Y, You XL, Qin L. FZD8 indicates a poor prognosis and promotes gastric cancer invasion and metastasis via β-catenin signaling pathway. Ann Clin Lab Sci. 2020;50:13–23. [PubMed] [Google Scholar]
- 111.Li QJ, Ye LP, Zhang X, Wang M, Lin CY, Huang S, et al. FZD8, a target of p53, promotes bone metastasis in prostate cancer by activating canonical Wnt/β-catenin signaling. Cancer Lett. 2017;402:166–76. 10.1016/j.canlet.2017.05.029 [DOI] [PubMed] [Google Scholar]
- 112.Ma P, Han JL. Overexpression of miR-100-5p inhibits papillary thyroid cancer progression via targeting FZD8. Open Med. 2022;17:1172–82. 10.1515/med-2022-0490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang YK, Spörle R, Paperna T, Schughart K, Francke U. Characterization and expression pattern of the frizzled gene Fzd9, the mouse homolog of FZD9 which is deleted in Williams-Beuren syndrome. Genomics. 1999;57:235–48. 10.1006/geno.1999.5773 [DOI] [PubMed] [Google Scholar]
- 114.David R. Developement: strong bones: got FZD9? Nat Rev Mol Cell Biol. 2011;12:280. [DOI] [PubMed] [Google Scholar]
- 115.Zacarías-Fluck MF, Jauset T, Martínez-Martín S, Kaur J, Casacuberta-Serra S, Massó-Vallés D, et al. The wnt signaling receptor Fzd9 is essential for myc-driven tumorigenesis in pancreatic islets. Life Sci Alliance. 2021;4:e201900490. 10.26508/lsa.201900490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang Z, Schittenhelm J, Guo K, Bühring HJ, Trautmann K, Meyermann R, et al. Upregulation of frizzled 9 in astrocytomas. Neuropathol Appl Neurobiol. 2006;32:615–24. 10.1111/j.1365-2990.2006.00770.x [DOI] [PubMed] [Google Scholar]
- 117.Wang QZ, Liu HC, Wang Q, Zhou FH, Xiu YX, Zhang YW, et al. Involvement of c-Fos in cell proliferation, migration, and invasion in osteosarcoma cells accompanied by altered expression of Wnt2 and Fzd9. PLoS One. 2017;12:e0180558. 10.1371/journal.pone.0180558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.de Bastos DR, Conceição MPF, Michelli APP. Leite JMRS, da Silva RA, Cintra RC, et al. An analysis identified FZD9 as a potential prognostic biomarker in triple-negative breast cancer patients. Eur J Breast Health. 2021;17:42–52. 10.4274/ejbh.2020.5804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang YJ, Jiang Q, Kong XL, Yang LL, Hu WZ, Lv CF, et al. Methylation status of the promoter region of the human frizzled 9 gene in acute myeloid leukemia. Mol Med Rep. 2016;14:1339–44. 10.3892/mmr.2016.5387 [DOI] [PubMed] [Google Scholar]
- 120.Sompel K, Dwyer-Nield LD, Smith AJ, Elango A, Backos DS, Zhang BC, et al. Iloprost requires the Frizzled-9 receptor to prevent lung cancer. Iscience. 2022;25:e104442. 10.1016/j.isci.2022.104442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nagayama S, Fukukawa C, Katagiri T, Okamoto T, Aoyama T, Oyaizu N, et al. Therapeutic potential of antibodies against FZD10, a cell-surface protein, for synovial sarcomas. Oncogene. 2005;24:6201–12. 10.1038/sj.onc.1208780 [DOI] [PubMed] [Google Scholar]
- 122.Fukukawa C, Hanaoka H, Nagayama S, Tsunoda T, Toguchida J, Endo K, et al. Radioimmunotherapy of human synovial sarcoma using a monoclonal antibody against FZD10. Cancer Sci. 2008;99:432–40. 10.1111/j.1349-7006.2007.00701.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sudo H, Tsuji AB, Sugyo A, Harada Y, Nagayama S, Katagiri T, et al. FZD10-targeted alpha-radioimmunotherapy with (225) Ac-labeled OTSA101 achieves complete remission in a synovial sarcoma model. Cancer Sci. 2022;113:721–32. 10.1111/cas.15235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fukumoto T, Zhu HR, Nacarelli T, Karakashev S, Fatkhutdinov N, Wu S, et al. The N6-methylation of adenosine (M6a) in Fzd10 mRNA contributes to resistance to parp Inhibitor. Clin Cancer Res. 2019;25:204–204. 10.1158/1557-3265.OVCASYMP18-NT-093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tomar T, Alkema NG, Schreuder L, Meersma GJ, Meyer TD, Criekinge WV, et al. Methylome analysis of extreme chemoresponsive patients identifies novel markers of platinum sensitivity in high-grade serous ovarian cancer. BMC Med. 2017;15:116. 10.1186/s12916-017-0870-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang J, Yu HM, Dong W, Zhang C, Hu MT, Ma WC, et al. N6-methyladenosine-mediated up-regulation of FZD10 regulates liver cancer stem cells’ properties and lenvatinib resistance through wnt/β-catenin and hippo signaling pathways. Gastroenterology. 2023;164:990–1005. 10.1053/j.gastro.2023.01.041 [DOI] [PubMed] [Google Scholar]
- 127.Tulalamba W, Ngernsombat C, Larbcharoensub N, Janvilisri T. Transcriptomic profiling revealed FZD10 as a novel biomarker for nasopharyngeal carcinoma recurrence. Front Oncol. 2023;12:1084713. 10.3389/fonc.2022.1084713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen JY, Zhang F, Ren XS, Wang YH, Huang WH, Zhang JT, et al. Targeting fatty acid synthase sensitizes human nasopharyngeal carcinoma cells to radiation via downregulating frizzled class receptor 10. Cancer Biol Med. 2020;17:740–52. 10.20892/j.issn.2095-3941.2020.0219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gong C, Qu SH, Lv XB, Liu BD, Tan WG, Nie Y, et al. BRMS1L suppresses breast cancer metastasis by inducing epigenetic silence of FZD10. Nat Commun. 2014;5:5406. 10.1038/ncomms6406 [DOI] [PubMed] [Google Scholar]
- 130.Scavo MP, Rizzi F, Depalo N, Armentano R, Coletta S, Serino G, et al. Exosome released FZD10 increases Ki-67 expression via phospho-ERK1/2 in colorectal and gastric cancer. Front Oncol. 2021;11:730093. 10.3389/fonc.2021.730093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L, et al. Wnt pathway inhibition via the targeting of frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci USA. 2012;109:11717–22. 10.1073/pnas.1120068109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Diamond JR, Becerra C, Richards D, Mita A, Osborne C, O’Shaughnessy J, et al. Phase Ib clinical trial of the anti-frizzled antibody vantictumab (OMP-18R5) plus paclitaxel in patients with locally advanced or metastatic HER2-negative breast cancer. Breast Cancer Res Treat. 2020;184:53–62. 10.1007/s10549-020-05817-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Davis SL, Cardin DB, Shahda S, Lenz H, Dotan E, O’Neil BH, et al. A phase 1b dose escalation study of wnt pathway inhibitor vantictumab in combination with nab-paclitaxel and gemcitabine in patients with previously untreated metastatic pancreatic cancer. Invest New Drugs. 2020;38:821–30. 10.1007/s10637-019-00824-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Le PN, McDermott JD, Jimeno A. Targeting the wnt pathway in human cancers: therapeutic targeting with a focus on OMP-54F28. Pharmacol Ther. 2015;146:1–11. 10.1016/j.pharmthera.2014.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jimeno A, Gordon M, Chugh R, Messersmith W, Mendelson D, Dupont J, et al. A first-in-human phase 1 study of anticancer stem cell agent OMP-54F28 (FZDS-Fe), decoy receptor for wnt ligands, in patients with advanced solid tumors. J Clin Oncol. 2017;23:7490–7. [DOI] [PubMed] [Google Scholar]
- 136.Dotan E, Cardin DB, Lenz H, Messersmith W, O’Neil B, Cohen SJ, et al. Phase Ib study of wnt inhibitor ipafricept with gemcitabine and nab-paclitaxel in patients with previously untreated stage IV pancreatic cancer. Clin Cancer Res. 2020;26:5348–57. 10.1158/1078-0432.CCR-20-0489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Moore KN, Gunderson CC, Sabbatini P, McMeekin DS, Mantia-Smaldone G, Burger RA, et al. A phase 1b dose escalation study of ipafricept (OMP-54F28) in combination with paclitaxel and carboplatin in patients with recurrent platinum-sensitive ovarian cancer. Gynecol Oncol. 2019;154:294–301. 10.1016/j.ygyno.2019.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Le PN, Keysar SB, Miller B, Eagles JR, Chimed T, Reisinger J, et al. Wnt signaling dynamics in head and neck squamous cell cancer tumor-stroma interactions. Mol Carcinog. 2019;58:398–410. 10.1002/mc.22937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pavlovic Z, Adams JJ, Blazer LL, Gakhal AK, Jarvik N, Steinhart Z, et al. A synthetic anti-frizzled antibody engineered for broadened specificity exhibits enhanced anti-tumor properties. Mabs. 2018;10:1157–67. 10.1080/19420862.2018.1515565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dang LT, Miao Y, Ha A, Yuki K, Park K, Janda CY, et al. Receptor subtype discrimination using extensive shape complementary designed interfaces. Nat Struct Mol Biol. 2019;26:407–14. 10.1038/s41594-019-0224-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mohammadi M, Nejatollahi F, Sakhteman A, Zarei N. Insilico analysis of three different tag polypeptides with dual roles in scFv antibodies. J Theor Biol. 2016;402:100–6. 10.1016/j.jtbi.2016.04.016 [DOI] [PubMed] [Google Scholar]
- 142.Zarei N, Fazeli M, Mohammadi M, Nejatollahi F. Cell growth inhibition and apoptosis in breast cancer cells induced by anti-FZD7 scFvs: involvement of bioinformatics-based design of novel epitopes. Breast Cancer Res Treat. 2018;169:427–36. 10.1007/s10549-017-4641-6 [DOI] [PubMed] [Google Scholar]
- 143.Pode-Shakked N, Harari-Steinberg O, Haberman-Ziv Y, Rom-Gross E, Bahar S, Omer D, et al. Resistance or sensitivity of Wilms’ tumor to anti-FZD7 antibody highlights the wnt pathway as a possible therapeutic target. Oncogene. 2011;30:1664–80. 10.1038/onc.2010.549 [DOI] [PubMed] [Google Scholar]
- 144.Riley RS, Day ES. Frizzled7 antibody-functionalized nanoshells enable multivalent binding for wnt signaling inhibition in triple-negative breast cancer cells. Small. 2017;13:544. 10.1002/smll.201700544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wei W, Chua M, Grepper S, So SK. Soluble frizzled-7 receptor inhibits wnt signaling and sensitizes hepatocellular carcinoma cells towards doxorubicin. Mol Cancer. 2011;10:16. 10.1186/1476-4598-10-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nambotin SB, Lefrancois L, Sainsily X, Berthillon P, Kim M, Wands JR. Pharmacological inhibition of frizzled-7 displays anti-tumor properties in hepatocellular carcinoma. J Hepatol. 2011;54:288–99. 10.1016/j.jhep.2010.06.033 [DOI] [PubMed] [Google Scholar]
- 147.Nile AH, de Sousa E, Melo F, Mukund S, Piskol R, Hansen S, et al. A selective peptide inhibitor of Frizzled 7 receptors disrupts intestinal stem cells. Nat Chem Biol. 2018;14:582–90. Corrected and republished from: Nat Chem Biol. 2018;14: 902. 10.1038/s41589-018-0035-2 [DOI] [PubMed] [Google Scholar]
- 148.Giraudet AL, Cassier PA, Iwao-Fukukawa C, Garin G, Badel J, Kryza D, et al. A first-in-human study investigating biodistribution, safety and recommended dose of a new radiolabeled MAb targeting FZD10 in metastatic synovial sarcoma patients. BMC Cancer. 2018;18:646. 10.1186/s12885-018-4544-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ulloa-Aguirre A, Conn PM. Pharmacoperones: a new therapeutic approach for diseases caused by misfolded G protein-coupled receptors. Recent Pat Endocr Metab Immune Drug Discov. 2011;5:13–24. 10.2174/187221411794351851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Generoso SF, Giustiniano M, Regina GL, Bottone S, Passacantilli S, Maro SD, et al. Pharmacological folding chaperones act as allosteric ligands of frizzled4. Nat Chem Biol. 2015;11:280–6. 10.1038/nchembio.1770 [DOI] [PubMed] [Google Scholar]
- 151.Lagerström MC, Schiöth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7:542. 10.1038/nrd2592 [DOI] [PubMed] [Google Scholar]
- 152.Zhang W, Lu WY, Ananthan S, Suto MJ, Li YH. Discovery of novel frizzled-7 inhibitors by targeting the receptor’s transmembrane domain. Oncotarget. 2017;8:91459–70. 10.18632/oncotarget.20665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pinto IA, Da Silveira NJF. In Silico identification of potential inhibitors of the wnt signaling pathway in human breast cancer. J Comput Biol. 2020;27:999–1010. 10.1089/cmb.2019.0311 [DOI] [PubMed] [Google Scholar]
- 154.Zhao YG, Ren JS, Hillier J, Lu WX, Jones EY. Antiepileptic drug Carbamazepine binds to a novel pocket on the wnt receptor frizzled-8. J Med Chem. 2020;63:3252–60. 10.1021/acs.jmedchem.9b02020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yang SF, Wu YR, Xu TH, de Waal PW, He YZ, Pu MC, et al. Crystal structure of the frizzled 4 receptor in a ligand-free state. Nature. 2018;560:666–70. 10.1038/s41586-018-0447-x [DOI] [PubMed] [Google Scholar]
- 156.Zhong Q, Zhao YY, Ye FF, Xiao ZY, Huang GXY, Xu M, et al. Cryo-EM structure of human wntless in complex with wnt3a. Nat Commun. 2021;12:4541. 10.1038/s41467-021-24731-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bugter JM, Fenderico N, Maurice MM. Mutations and mechanisms of wnt pathway tumour suppressors in cancer. Nat Rev Cancer. 2021;21:64. 10.1038/s41568-020-00316-y [DOI] [PubMed] [Google Scholar]
- 158.Blum B, Bar-Nur O, Golan-Lev T, Benvenisty N. The anti-apoptotic gene survivin contributes to teratoma formation by human embryonic stem cells. Nat Biotechnol. 2009;27:281–7. 10.1038/nbt.1527 [DOI] [PubMed] [Google Scholar]
- 159.Lukaszewicz AI, Nguyen C, Melendez E, Lin DP, Teo JL, Lai KKY, et al. The mode of stem cell division is dependent on the differential interaction of β-catenin with the Kat3 coactivators CBP or p300. Cancers. 2019;11:962. 10.3390/cancers11070962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Suryawanshi A, Manoharan I, Hong Y, Swafford D, Majumdar T, Taketo MM, et al. Canonical wnt signaling in dendritic cells regulates Th1/Th17 Responses and suppresses autoimmune neuroinflammation. J Immunol. 2015;194:3295–304. 10.4049/jimmunol.1402691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhou MM, Sun XZ, Zhu YH. Analysis of the role of frizzled 2 in different cancer types. FEBS Open Bio. 2021;11:1195–208. 10.1002/2211-5463.13111 [DOI] [PMC free article] [PubMed] [Google Scholar]