Nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs) are evolutionarily conserved intracellular pattern recognition receptors (PRRs) that sense microbial and danger signals, trigger immediate host defenses, and prime the adaptive immune response for long-lasting protection [1]. In humans, 22 known NLRs exist, and mutations in NLR family genes are associated with a wide range of inflammatory and autoimmune conditions, including hereditary periodic fever syndromes, Crohn’s disease, Blau’s syndrome, infantile enterocolitis, multiple sclerosis, and asthma [2, 3]. Collectively, these disorders can be categorized as NLR-associated autoinflammatory diseases.
The hallmark feature of NLRs is their central NOD (or NACHT) domain—an ATPase domain—while the N-terminal and C-terminal domains exhibit variability. Most NLRs have a C-terminal leucine-rich repeat (LRR). The N-terminal varies among NLRs and is responsible for homotypic protein-protein interactions [4]. The C-terminal LRR functions as an inhibitory domain in NLRs, and the NACHT domain hydrolyzes ATP upon activation. After ligand binding, the C-terminal LRR domain of most NLRs undergoes a conformational change, exposing the N-terminal domain. This conformational shift allows for the formation of oligomeric scaffolds, which subsequently interact with downstream signaling adaptors or effectors. Notably, ATPase activity within NLRs is essential for complex formation [4]. Despite the diversity of inflammatory pathways activated by different NLR family members, recent research has revealed a common checkpoint of ATPase activation that could govern the activation of all NLRs [5].
Protein pelota homolog (PELO) is a conserved component of the ribosome-associated quality control machinery. It functions as a surveillance factor in translational quality control and ribosome rescue [6–10]. Recently, we reported that PELO is essential for the activation of all the cytosolic NLR family proteins we tested. The underlying mechanism involves PELO recruitment by NLR proteins upon activation, catalyzing the activation of the ATPase within the NACHT domain of NLRs [5]. Because ATPase activity is essential for NLR activation, the effect(s) of pathogenic and nonpathogenic alterations on NLR ATPase activation mediated by PELO deserves investigation.
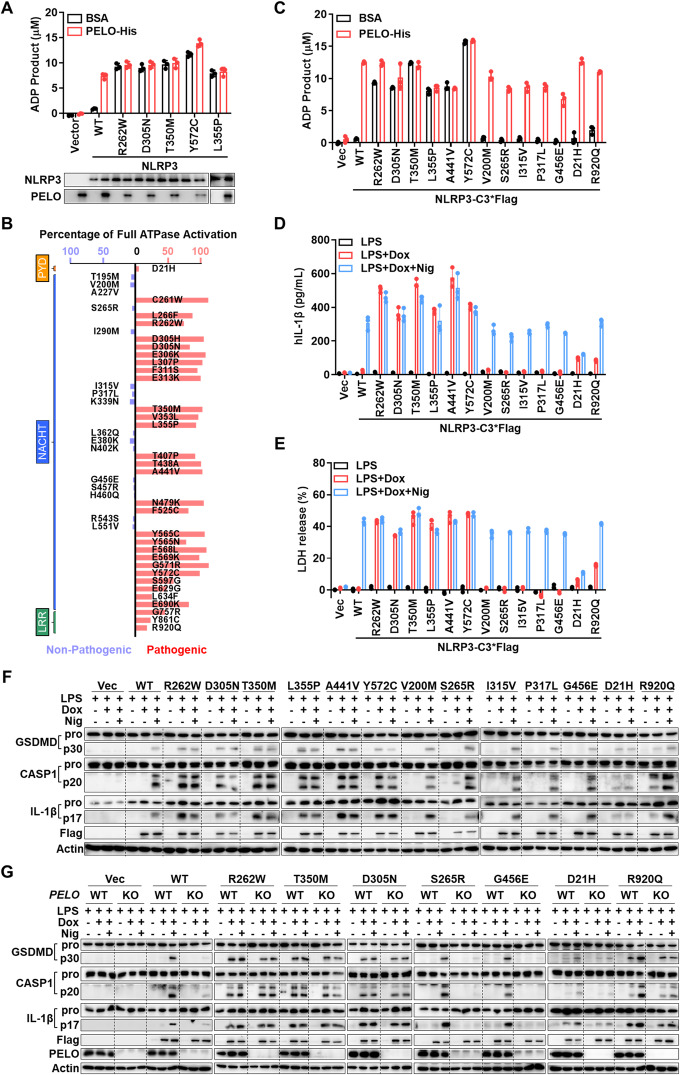
In our analysis, we focused on NLRP3, one of the most extensively studied NLR family members [11]. NLRP3 forms an inflammasome—an intracellular multimeric protein complex—in response to various exogenous microbial infections and endogenous danger signals [12]. Numerous mutations in NLRP3 have been reported, and these mutations are associated with autoinflammatory diseases such as cryopyrin-associated periodic syndrome (CAPS). Interestingly, CAPS can occur not only as a result of an inherited genetic mutation but also sporadically due to de novo or somatic mosaic NLRP3 mutations [13]. In our investigation, we initially analyzed the five most frequent gain-of-function germline mutants of NLRP3 to assess their impact on ATPase activation by PELO [13]. We expressed and purified NLRP3 and its mutants from PELO knockout HEK293T cells (Supplementary Fig. S1A) and measured their ATPase activity. Consistent with previous reports [5], the addition of purified recombinant PELO protein substantially increased the ATPase activity of NLRP3 (Fig. 1A). Interestingly, these five NLRP3 mutants exhibited high ATPase activity, and the addition of PELO had minimal or no effect on further enhancing their ATPase activity (Fig. 1A). Consequently, these disease-causing NLRP3 mutants can bypass the PELO-mediated activation step because they are capable of self-activation.
Fig. 1.
Pathogenic NLRP3 mutations lead to the bypass of PELO-mediated ATPase activation of NLRP3 via an increase in ATPase activity, and each gain-of-function NLRP3 mutation spontaneously elicits inflammasome activation. A Purified Flag-WT or one of the five mutated NLRP3 proteins (100 ng) as indicated were incubated with BSA or recombinant PELO protein (50 ng) in the presence of 20 μM ATP, and the amount of the ADP product was measured using the ADP-Glo assay. B The experiments were performed as in A with 47 NLRP3 mutants. Schematics showing the basal ATPase activity of each NLRP3 mutant relative to the full activation induced by incubation with PELO. Pathogenic mutations are shown in red, and nonpathogenic mutations are shown in blue. The positions of the bars of each mutant in the schematic diagram correspond to the domain location of each mutation site. C ATPase activity of the 13 selected NLRP3 mutants. The data are derived from Supplementary Fig. S1B. D–F NLRP3 KO THP-1 cells with stable integration of a Dox-inducible expression vector containing Flag-NLRP3 WT or one of the selected NLRP3 mutants were first primed with 1 mg/ml LPS for 4 hours. Following priming, the medium was replaced with medium supplemented with or without 1 μg/ml doxycycline (Dox). Six hours later, the cells were stimulated with or without 5 μM nigericin (Nig) for another hour. The supernatants were analyzed for IL-1β (D) and LDH (E); the pooled cell extracts and supernatants were analyzed by immunoblotting with anti-GSDMD, anti-caspase-1 (CASP1), anti-IL-1β and anti-actin (F) antibodies. G NLRP3 KO THP-1 cells with and without additional PELO KO were stably transfected with a Dox-inducible expression vector of Flag-NLRP3 WT or one of the designated mutants and were treated with the stimuli described in F. The levels of processed GSDMD, caspase-1 (CASP1) and IL-1β in the pooled cell extracts and supernatants were analyzed by immunoblotting. Equal loading of the proteins in the ATPase activity assay was monitored by Western blotting (WB). The data are presented as the mean ± SD of triplicate samples (A, C, D and E). The rearranged noncontiguous WB lanes from the parallel processed gels are separated by a thin frame. All results are representative of at least two independent experiments
To investigate the linkage between PELO-mediated ATPase activation and disease-causing NLRP3 mutants, we expanded our analysis to include 47 naturally occurring NLRP3 mutations. This comprehensive set encompasses germline and somatic mosaic mutants, as well as pathogenic and nonpathogenic variants [13]. The ATPase activities of these strains with and without PELO activation are shown in Supplementary Fig. S1B. To show the relevant information collectively, we calculated the basal activity levels of each mutant relative to the full activation achieved through incubation with PELO based on the data in Supplementary Fig. S1B, which should represent the degree by which these mutants bypass PELO-mediated activation; we included the domain locations of each mutant and their disease-causing potential and showed all of this information in Fig. 1B. Our findings revealed a consistent pattern; pathogenic mutations within the NACHT domain exhibited high basal ATPase activity, whereas nonpathogenic mutants exhibited no spontaneous activation (Fig. 1A, B and Supplementary Fig. S1B). Interestingly, mutations occurring outside the NACHT domain—such as those in the LRR domain—can also increase basal ATPase activity, which is further enhanced by PELO (Fig. 1B and Supplementary Fig. S1B). Remarkably, even though these LRR mutations only exhibit modest basal ATPase activity, they are pathogenic. Notably, the gain-of-function effects of the pathogenic NLRP3 mutants on PELO-mediated ATPase activation are not linked to any changes in their ability to interact with PELO (Supplementary Fig. S1C). Thus, the ability to bypass the PELO checkpoint and directly self-activate ATPase has emerged as a common feature among pathogenic NLRP3 mutants.
To investigate whether gain-of-function mutations in ATPase activate NLRP3, we analyzed inflammasome activation using an NLRP3-knockout human monocyte cell line (THP-1). The key features of inflammasome activation include caspase-1 activation, which initiates the maturation of the proinflammatory cytokines interleukin-1β (IL-1β) and/or IL-18, and cleavage of gasdermin D (GSDMD) to generate the N-terminal fragment, which induces pore formation, cytokine release, and pyroptosis [11]. Although NLRP3 and pro-IL-1β expression is typically induced by lipopolysaccharide (LPS) in standard cell models, our study employed NLRP3 knockout THP-1 cells in which NLRP3 or its mutants were induced via a doxycycline (Dox)-inducible system. Thirteen mutants harboring both pathogenic and nonpathogenic mutations across distinct domains of NLRP3 were selected for evaluation. As already described in Supplementary Fig. S1B, the pathogenic mutants in these 13 mutants had spontaneous activity, while the nonpathogenic mutants required PELO for ATPase activation (Fig. 1C). In the standard experimental procedure, nigericin (Nig) was used to induce caspase-1 activation, IL-1β maturation and secretion, and pyroptosis in LPS-primed THP-1 cells. The experimental procedure was as follows: following LPS priming, the cells were stimulated with either nothing, Dox alone, or Dox in combination with Nig. IL-1β secretion was quantified by assessing IL-1β levels in the cell culture medium (Fig. 1D), while pyroptosis was evaluated through lactate dehydrogenase (LDH) release into the medium (Fig. 1E). Additionally, IL-1β maturation, caspase-1 cleavage, and GSDMD cleavage were determined by Western blotting (Fig. 1F). The results revealed an unresponsive phenotype in NLRP3 knockout THP-1 cells to LPS, LPS+Dox, and LPS+Dox+Nig. Induction of NLRP3 expression by Dox restored the response to LPS+Nig to a level comparable to that in wild-type THP-1 cells. The induced expression of NLRP3 mutants with spontaneous ATPase activation directly triggers caspase-1 activation, GSDMD cleavage, and IL-1β maturation and secretion in the absence of secondary stimulation (nigericin). In contrast, mutants that do not exhibit gain-of-function ATPase activity do not directly activate the NLRP3 inflammasome; instead, they behave similarly to wild-type NLRP3 and respond to nigericin. Notably, the D21H mutant exhibits distinct behavior from other pathogenic mutants, likely due to its location in the PYD domain, which interacts with the downstream adaptor protein ASC [14]. Collectively, these data support the notion that ATPase activation by PELO serves as a checkpoint for NLRP3 inflammasome activation and that gain-of-function ATPase mutations bypass this checkpoint.
To confirm that PELO controls this checkpoint, we conducted the aforementioned experiments in cells with and without additional PELO deletion. As expected, PELO deficiency affected only wild-type NLRP3 and the mutants that did not exhibit basal ATPase activity (Fig. 1G). Conclusively, each gain-of-function ATPase mutation in NLRP3 bypassed PELO for ATPase activation, resulting in spontaneous inflammasome activation.
Next, we investigated whether the conclusions drawn from the study of NLRP3 could be extended to other NLR family members. NLRC4, another well-studied NLR, is associated with autoinflammatory diseases such as autoinflammation and infantile enterocolitis (AIFEC). Similar to our NLRP3 study, we selected specific NLRC4 mutants and assessed their ATPase activation by PELO in vitro (Supplementary Fig. S2A, B). The results mirrored those obtained for NLRP3: all mutants (highlighted in red) with pathogenic mutations in the NACHT domain bypassed the PELO checkpoint and gained basal ATPase activity. Conversely, PELO remained necessary for ATPase activation in mutants with nonpathogenic mutations (highlighted in blue) in the NACHT domain. In line with the NLRP3 findings, pathogenic mutations in the CARD domain showed no functional relationship with the PELO checkpoint for ATPase activation, while LRR domain mutations partially bypassed this checkpoint. Although mutations have been identified in most, if not all, NLR family members, disease-associated studies have primarily focused on NLRP3 and NLRC4 with some studies on NOD1, NOD2, NLRP7, and NLRP12. To further explore the impact of the PELO checkpoint on these additional NLRs, we examined the effect of PELO on the ATPase activity of these NLRs with pathogenic mutations in the NACHT domain. As depicted in Supplementary Fig. S2C–F, all of these mutations led to the bypass of PELO-mediated ATPase activation. Our data collectively demonstrate that bypassing the PELO checkpoint through gain-of-function ATPase mutations represents a common pathogenic mechanism in NLR-associated autoinflammatory diseases.
As the identification of sequence variations (including germline and somatic mutations) in NLRs continues to grow, assessing whether the PELO checkpoint for ATPase activation has been bypassed can serve as a valuable tool for classifying pathogenesis in patients with NLR mutations. Computational modeling of NLRP3 has been instrumental in evaluating the potential relationship between clinical severity and structural disruptions caused by mutations [15], but its feasibility is poor. By quantitatively determining both the extent of the bypass of the ATPase activation checkpoint by a mutation and whether PELO can further enhance ATPase activity in a given NLR mutant, we can explore the clinical implications. This includes assessing vulnerability to sterile or infectious inflammation and the potential for excessive immune reactions.
Supplementary information
Acknowledgements
We thank Lu Zhou for help with proofreading the manuscript. This work was supported by the National Natural Science Foundation of China (82388201 to JH, 32170751 to Z-HY), the Fundamental Research Funds for the Central Universities (226-2024-00015 to XW), the National Key R&D Program of China (2020YFA0803500 to JH), the CAMS Innovation Fund for Medical Science (2019-I2M-5-062 to JH), Fujian Province Central to Local Science and Technology Development Special Program (No. 2022L3079 to JH) and the Fu-Xia-Quan Zi-Chuang District Cooperation Program (No. 3502ZCQXT2022003 to JH).
Author contributions
XW and Z-HY performed most of the experiments; YZ and JW participated in the experiments; and XW, Z-HY, and JH analyzed the data and wrote the manuscript. XW and JH conceived the project and supervised the study.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Xiurong Wu, Zhang-Hua Yang.
Contributor Information
Xiurong Wu, Email: xiurongwu@zju.edu.cn.
Jiahuai Han, Email: jhan@xmu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41423-024-01162-w.
References
- 1.Chen G, Shaw MH, Kim YG, Nunez G. NOD-like receptors: role in innate immunity and inflammatory disease. Annu Rev Pathol. 2009;4:365–98. doi: 10.1146/annurev.pathol.4.110807.092239. [DOI] [PubMed] [Google Scholar]
- 2.Geddes K, Magalhaes JG, Girardin SE. Unleashing the therapeutic potential of NOD-like receptors. Nat Rev Drug Discov. 2009;8:465–79. doi: 10.1038/nrd2783. [DOI] [PubMed] [Google Scholar]
- 3.Zhong Y, Kinio A, Saleh M. Functions of NOD-like receptors in human diseases. Front Immunol. 2013;4:333. doi: 10.3389/fimmu.2013.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meunier E, Broz P. Evolutionary convergence and divergence in NLR function and structure. Trends Immunol. 2017;38:744–57. doi: 10.1016/j.it.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Wu X, Yang ZH, Wu J, Han J. Ribosome-rescuer PELO catalyzes the oligomeric assembly of NOD-like receptor family proteins via activating their ATPase enzymatic activity. Immunity. 2023;56:926–43.e927. doi: 10.1016/j.immuni.2023.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Doma MK, Parker R. Endonucleolytic cleavage of eukaryotic mRNAs with stalls in translation elongation. Nature. 2006;440:561–4. doi: 10.1038/nature04530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shoemaker CJ, Eyler DE, Green R. Dom34:Hbs1 promotes subunit dissociation and peptidyl-tRNA drop-off to initiate no-go decay. Science. 2010;330:369–72. doi: 10.1126/science.1192430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pisareva VP, Skabkin MA, Hellen CU, Pestova TV, Pisarev AV. Dissociation by Pelota, Hbs1 and ABCE1 of mammalian vacant 80S ribosomes and stalled elongation complexes. EMBO J. 2011;30:1804–17. doi: 10.1038/emboj.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuboi T, Kuroha K, Kudo K, Makino S, Inoue E, Kashima I, et al. Dom34:hbs1 plays a general role in quality-control systems by dissociation of a stalled ribosome at the 3’ end of aberrant mRNA. Mol Cell. 2012;46:518–29. doi: 10.1016/j.molcel.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Guydosh NR, Green R. Dom34 rescues ribosomes in 3’ untranslated regions. Cell. 2014;156:950–62. doi: 10.1016/j.cell.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407–20. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- 12.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–87. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louvrier C, Assrawi E, El Khouri E, Melki I, Copin B, Bourrat E, et al. NLRP3-associated autoinflammatory diseases: Phenotypic and molecular characteristics of germline versus somatic mutations. J Allergy Clin Immunol. 2020;145:1254–61. doi: 10.1016/j.jaci.2019.11.035. [DOI] [PubMed] [Google Scholar]
- 14.Hochheiser IV, Behrmann H, Hagelueken G, Rodríguez-Alcázar JF, Kopp A, Latz E, et al. Directionality of PYD filament growth determined by the transition of NLRP3 nucleation seeds to ASC elongation. Sci Adv. 2022;8:eabn7583. doi: 10.1126/sciadv.abn7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samson JM, Ravindran Menon D, Vaddi PK, Kalani Williams N, Domenico J, Zhai Z, et al. Computational modeling of NLRP3 identifies enhanced ATP binding and multimerization in Cryopyrin-associated periodic syndromes. Front Immunol. 2020;11:584364. doi: 10.3389/fimmu.2020.584364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.