Abstract
Acanthopanax gracilistylus is a deciduous plant in the family Araliaceae, which is commonly used in Chinese herbal medicine, as the root bark has functions of nourishing the liver and kidneys, removing dampness and expelling wind, and strengthening the bones and tendons. Kaurenoic acid (KA) is the main effective substance in the root bark of A. gracilistylus with strong anti-inflammatory effects. To elucidate the KA biosynthesis pathway, second-generation (DNA nanoball) and third-generation (Pacific Biosciences) sequencing were performed to analyze the transcriptomes of the A. gracilistylus leaves, roots, and stems. Among the total 505,880 isoforms, 408,954 were annotated by seven major databases. Sixty isoforms with complete open reading frames encoding 11 key enzymes involved in the KA biosynthesis pathway were identified. Correlation analysis between isoform expression and KA content identified a total of eight key genes. Six key enzyme genes involved in KA biosynthesis were validated by real-time quantitative polymerase chain reaction. Based on the sequence analysis, the spatial structure of ent-kaurene oxidase was modeled, which plays roles in the three continuous oxidations steps of KA biosynthesis. This study greatly enriches the transcriptome data of A. gracilistylus and facilitates further analysis of the function and regulation mechanism of key enzymes in the KA biosynthesis pathway.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-024-01436-7.
Keywords: Acanthopanax gracilistylus, Full-length transcriptome, Kaurenoic acid, Ent-kaurene oxidase, Differentially expressed genes
Introduction
Acanthopanax gracilistylus W. W. Smith is a medicinal plant of the genus Acanthopanax Miq. in the family Araliaceae. The dry root bark of A. gracilistylus, known as Acanthopanacis Cortex is enriched with active ingredients such as volatile oils, terpenes, sterols, and organic acids (Zheng et al. 2015) contributing to various pharmacological activities such as hypoglycemic (Lu et al. 2018), anti-inflammatory (Xu et al. 2018), anti-tumor (Wu et al. 2014), anti-aging (Xie et al. 2003), anti-fatigue (Zhang 2012), and liver protection (Kuang 2015) effects.
Terpenoids are the main components in A. gracilistylus. To date, two sesquiterpenoids, 41 diterpenoids, and 23 triterpenes and their glycosides have been isolated from the root bark, stems, and leaves of A. gracilistylus (Yang et al. 2020). Diterpenoids exhibit significant biological activities in the human body, such as anti-tumor, antibacterial, antiviral, anti-inflammatory, and immunosuppressive effects, along with the ability to lower blood pressure and prevent thrombosis (Hai et al. 2015). Among the diterpenoids, kaurenoic acid (KA) is a potent anti-inflammatory agent, with a content of approximately 1% in the root bark of A. gracilistylus and a high content in other plants such as Eleutherococcus trifoliatus, Aralia continentalis, and Smallanthus sonchifolius; therefore, all of these plants are used in traditional Chinese medicine in the treatment of rheumatoid arthritis (Xie et al. 2013; Sithisarn et al. 2009; Choi et al. 2011; Ragasa et al. 2008). KA has various pharmacological effects such as antibacterial activity; relaxation of the vascular smooth muscle; antispasmodic, antiplatelet aggregation, antitumor, antihypertensive, diuretic, and anti-inflammatory effects; and protection against osteoporosis (Cavalcanti et al. 2006; Fernandes et al. 2013; Park et al. 2010; Lyu et al. 2011; Ndom et al. 2010; Ambrosio et al. 2010; Tirapelli et al. 2004; Yang et al. 2002).
KA is the precursor of the synthesis of gibberellin (GA), which is a diterpenoid plant hormone that regulates plant growth and development (Barker et al. 2021). Kasahara et al. demonstrated that GA is mainly synthesized through the methylethritol phosphate (MEP) pathway in Arabidopsis thaliana, based on the detection of 13C-labeled precursors (Kasahara et al. 2002). The contribution of the mevalonate (MVA) pathway to GA synthesis was suggested to depend on the influx of isoprenoid into the plastids from the cytosol (Flügge and Gao 2005). KA is formed from geranylgeranyl diphosphate (GGPP) through a series of enzymatic reactions. The cyclization of GGPP is catalyzed in a two-step reaction by ent-copalyl diphosphate synthase (CPS) and ent-kaurene synthase (KS) to synthesize ent-kaurene. Three continuous oxidation reactions of ent-kaurene further generate ent-kaurenol and ent-kaurenal to finally generate KA (Hedden 2020). Therefore, ent-kaurene oxidase (KO) is considered the key regulatory enzyme involved in the biosynthesis of KA (Morrone et al. 2010; Su et al. 2016; Yamamura et al. 2018).
Third-generation sequencing technology is characterized by single-molecule real-time sequencing, enabling the direct sequencing of transcripts up to 10 kb in length without requiring a reference genome; however, this technology remains limited by a high error rate, high cost per base, and low throughput (Rhoads and Au 2015). Second-generation sequencing results in short read lengths and is often not capable of identifying full-length gene isomers. Nevertheless, second-generation sequencing can be used for the error correction of full-length transcripts. Accordingly, the combination of second- and third-generation sequencing can offer detailed results at the single-nucleotide level through integration with high-throughput and high-precision data, and can further improve the identification of gene isomers and abundance estimation (Magi et al. 2017; Ou et al. 2020; Winand et al. 2019). Sequencing analyses of several Chinese herbal medicinal plants, including Casuarina equisetifolia (Ye et al. 2019), Ipomoea nil (Hoshino et al. 2016), and Chrysanthemum morifolium (Liu et al. 2021), have offered a deeper understanding of the color, properties, growth rate, and stress resistance of the plants. To date, KA has been largely studied in crops such as rice (Sakamoto et al. 2004) and wheat (Huang et al. 2012) with completed transcriptome databases; however, there is no corresponding transcriptome study related to the KA biosynthesis pathway in A. gracilistylus. Therefore, in the present study, we combined the results obtained from second- and third-generation sequencing platforms of the leaves, roots, and stems to construct the first full transcriptome of A. gracilistylus. We further focused on the genes involved in the biosynthesis pathway of the active medicinal ingredient (KA) of A. gracilistylus. These results can lay an experimental foundation for the development of genetic engineering strategies to increase the yield of KA in A. gracilistylus.
Materials and methods
Preparation of experimental materials
A. gracilistylus was collected from the herb garden of Anhui University of Chinese Medicine, which was identified by Professor Qingshan Yang. The fresh leaves, roots, and stems of A. gracilistylus were separated, washed with sterilized distilled water several times, and then dried with filter paper. All tissues were quickly frozen in liquid nitrogen after collection.
Extraction and analysis of KA by high-performance liquid chromatography
The content of KA in the leaves, roots, and stems of A. gracilistylus was determined by high-performance liquid chromatography-diode array detection (HPLC–DAD) on an Agilent 1260 instrument (Agilent Co., Foster City, CA, USA). First, 0.4 g of dried powder of each tissue was weighed, added to 40 mL cyclohexane, and mixed well. The solution was then ultrasonicated (150 W, 40 kHz) at room temperature for 50 min, and 20 mL of the extract was evaporated to dryness. The powder was dissolved again in methanol and the volume was fixed in a 2 mL volumetric flask (Xie et al. 2013). KA (HPLC-grade ≥ 98%, National Institutes for Food and Drug Control) was used as the standard. The samples were run on an Agilent HC-C18 chromatographic column (250 × 4.6 mm, 5 μm) with the column temperature maintained at 30 ℃ and the detection wavelength set to 205 nm. The mobile phase was 1% acetic acid water and methanol (10:90) and the elution procedure was equivalent elution (Lee et al. 2020). The flow rate was 1.0 mL/min and the injection volume was 10 μL. The KA content in each tissue was measured three times.
Extraction of RNA from A. gracilistylus
After sterilizing and autoclaving the required utensils, the A. gracilistylus tissues were ground in a mortar with the addition of liquid nitrogen. The powder was mixed and placed in centrifuge tubes and the supernatant was collected after centrifugation. Total RNA was extracted from each tissue type with an RNA kit (Omega Bio-Tek, Norcross, GA, USA) according to the manufacturer’s protocol. For mRNA enrichment, the total RNA was mixed with magnetic beads containing OligodT with a polyA tail. A DNA probe was used to hybridize rRNA and then RNaseH was used to digest the DNA/RNA hybrid chain. Finally, DNaseI was used to digest the DNA probe and obtain the required RNA sample after purification.
Library preparation and database annotation
The DNB-seq platform was used for three repeated sequencing of leaves, roots, and stems of A. gracilistylus, and the PacBio iso-seq platform was used to sequence the full-length transcriptome of the mixture of leaves, roots and stems. The raw reads obtained by transcriptome sequencing on the DNB-seq platform were filtered with SOAPnuke (v1.5.2) software (Cock et al. 2010). Reads containing adapters, an unknown base (N) content > 10%, and of low quality were removed to obtain clean reads. After detection and analysis of full-length transcripts from the PacBio iso-seq platform, full-length non-chimeric sequences were clustered. For each cluster composed of highly similar sequences, we applied the Arrow algorithm of PacBio to correct errors and integrate them into a consistent sequence. We filtered the data according to the sequence quality value after error correction and retained only the consistent sequence with high reliability. The library preparation and sequencing were carried out at Beijing Genomics Institute (Shenzhen, China). Finally, the high-quality sequences obtained after clustering and error correction were combined to remove redundancies enabling isoform identification and annotation to seven major databases (Supplementary Table S1).
Isoform expression and differential expression analysis
Bowtie 2 (v.2.2.5) (Langmead and Salzberg 2012) and RSEM (v1.2.8) (Li and Dewey 2011) software were used to calculate the isoform expression of the three tissues of A. gracilistylus, expressed as fragments per kilobase of exon model per million mapped fragments (FPKM). Based on the Poisson distribution principle, the differentially expressed genes (DEGs) of the tissues were analyzed and subject to functional annotation. DEGs were identified according to a false discovery rate (FDR) ≤ 0.001 and more than two fold difference in expression level between groups (tissue types).
Transcription factor (TF) analysis
The getorf (EMBOSS: 6.5.7.0) tool was used to determine the open reading frame (ORF) of each isoform in A. gracilistylus. The ORFs were then compared to the TF protein domain by hmmsearch (v3.0). Finally, the probability of each isoform being a TF was identified according to TF family characteristics described in PlantTFDB.
Structural analysis of KO in A. gracilistylus
ExPASy (https://web.expasy.org/translate/) was used to determine the ORF sequence of KO in A. gracilistylus. Using MEGA 5.0 (Tamura et al. 2011) and CLUSTALX 1.83 (Jeanmougin et al. 1998) software, the amino acid sequences of KO in A. gracilistylus were compared with KO sequences in other plants recorded in the National Center for Biotechnology Information (NCBI) international database. The conserved active site was searched and the secondary structure of KO in A. gracilistylus was displayed using ESPript 3.0 (https://espript.ibcp.fr/ESPript/cgi-bin/ESPript.cgi). Finally, the tertiary structure of KO in A. gracilistylus was simulated by Swiss-Model (https://swissmodel.expasy.org/) and visualized using Pymol (v2.3.2.0) software (Shan et al. 2020).
Validation of isoform expression of key enzymes
The key enzyme isoforms related to the biosynthesis of KA in A. gracilistylus identified by transcriptome analysis were selected for experimental validation by real-time quantitative polymerase chain reaction (RT-qPCR). The reaction mixture was placed in a 0.2 mL PCR tube comprising 7.5 μL 2 × qPCR Mix, 1.5 μL of 2.5 μM each forward and reverse primer (Supplementary Table S2), 2.0 μL of the reverse transcription product (cDNA), and 4.0 μL nuclease-free water. PCR amplification comprised a pre-denaturation step at 95℃ for 30 s, followed by 40 cycles of denaturation at 95℃ for 15 s and annealing and extension at 60℃ for 30 s. The relative expression of each independent isoform was normalized to the poly-ubiquitinase isoform (isoform_52190), and the relative isoform expression was calculated by the 2−ΔΔCT method (Livak and Schmittgen 2001).
Statistical analysis
The experimental data are expressed as mean ± standard deviation. GraphPad Prism 8 software was used for plotting and statistical analysis. Single-factor analysis of variance was used to compare the mean values between groups. The OmicShare Tools (https://www.omicshare.com/tools/) was used to analyze the correlation between the expression level of isoforms and KA content.
Results
Determination of KA content in A. gracilistylus
HPLC–DAD demonstrated that KA was present in all three tissues of A. gracilistylus, with a significantly higher content in the roots, followed by the stems, and the leaves had the lowest content overall (Fig. 1, Supplementary Table S3, Supplementary Fig. S1).
Fig. 1.
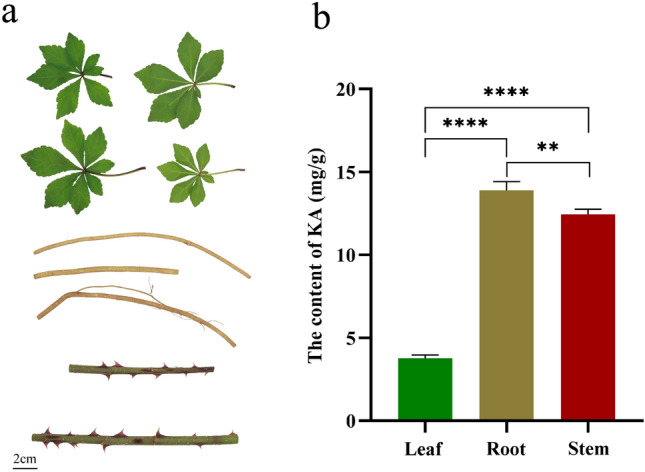
Determination of KA content in A. gracilistylus. a Morphological characteristics of A. gracilistylus. b Results of KA determination in three tissues of A. gracilistylus (error bars indicate standard error from three biological replicates). **p < 0.01, ****p < 0.0001
Transcriptome sequencing and data assembly
The DNB-seq and PacBio iso-seq platforms were used for transcriptome sequencing of A. gracilistylus. The RNA-sequencing datasets from the three A. gracilistylus tissues were deposited in the NCBI Sequence Read Archive (SRA) database (accession: PRJNA945217). The DNB-seq platform was used to sequence the leaves, roots, and stems of A. gracilistylus separately, with a total of 358,103 genes detected and 229,522 genes co-expressed in the three tissues (Supplementary Fig. S2). The average output of each sample was 69.48 MB (Table 1). The PacBio iso-seq platform was used to detect the mixed samples of the leaves, roots, and stems of A. gracilistylus, resulting in 63.59 GB of polymerase read sequences and 62.70 GB of subreads. After insert processing of the reads, we obtained 715,020 circular consensus sequences (CCS). The average read length of the CCS was 5830 bp and the average read quality was 0.97. After assembling the full-length non-chimeric reads into high-quality sequences and removing redundancies 505,880 transcripts were finally generated. The N50 value was 1552 bp and the N90 value was 586 bp. The minimum length was 197 bp, the maximum length was 28,796 bp, and the GC content was 40.71% (Table 2, Supplementary Fig. S3).
Table 1.
Comparison of clean reads and reference gene sequence obtained in second-generation sequencing
| Sample | Total clean reads (M) | Total mapping (%) | Uniquely mapping (%) |
|---|---|---|---|
| Ag_L_1 | 70.19 | 86.05 | 3.85 |
| Ag_L_2 | 69.45 | 84.81 | 3.93 |
| Ag_L_3 | 69.85 | 85.55 | 4.04 |
| Ag_R_1 | 68.75 | 84.26 | 5.10 |
| Ag_R_2 | 69.75 | 82.91 | 5.20 |
| Ag_R_3 | 70.04 | 82.60 | 5.10 |
| Ag_S_1 | 70.18 | 85.05 | 3.84 |
| Ag_S_2 | 68.20 | 84.20 | 4.00 |
| Ag_S_3 | 68.89 | 82.34 | 4.93 |
Table 2.
Third-generation sequencing results and data quality control
| Item | Number | Mean length (bp) |
|---|---|---|
| Polymerase reads | 820,253 | 77,529.66 |
| Subreads | 12,794,646 | 4900.68 |
| Circular consensus sequences (CCS) | 715,020 | 5830 |
| Full-length non-chimeric reads (FLNC) | 681,426 | 948 |
Functional annotation and expression analysis of isoforms
To predict the potential functions of the isoforms identified in A. gracilistylus, the 505,880 isoforms were annotated by seven functional databases, including the Non-redundant (NR; 385,189 isoforms), Nucleotide (NT; 331,924 isoforms), Swissprot (291,516 isoforms), Kyoto Encyclopedia of Genes and Genomes (KEGG; 296,226 isoforms), Eukaryotic Orthologous Groups of proteins (KOG; 294,088 isoforms), Pfam (227,250 isoforms), and Gene Ontology (GO; 224,994 isoforms) databases. Among them, 103,418 isoforms were jointly annotated by the seven databases, accounting for 20.44% of the total, and 408,954 isoforms were annotated by any one of the seven major databases, accounting for 80.84% of all isoforms (Table 3).
Table 3.
Annotation results of the transcripts with seven functional databases
| Database | Annotated number | Proportion (%) |
|---|---|---|
| NR | 385,189 | 76.14 |
| NT | 331,924 | 65.61 |
| Swissprot | 291,516 | 57.63 |
| KEGG | 296,226 | 58.56 |
| KOG | 294,088 | 58.13 |
| Pfam | 227,250 | 44.92 |
| GO | 224,994 | 44.48 |
| Overall | 408,954 | 80.84 |
The expression levels of 358,103 isoforms in the nine samples of the leaves, roots, and stems of A. gracilistylus are shown in Fig. 2a, with higher expression detected in the stems than in the leaf and root tissues (Fig. 2b).
Fig. 2.
Expression of isoforms in the three tissues of A. gracilistylus. a Distribution of the number of isoforms with different expression levels in the three tissues. b Boxplots of isoforms expressed in three tissues. L, leaf; R, root; S, stem
Annotations and functional classification of isoforms
The 385,189 A. gracilistylus isoforms annotated in the NR database were further analyzed, demonstrating the highest similarity with Daucus carota var. sativa. (48.21%), followed by Vitis vinifera, Sesamum indicum, and Helianthus annuus (Supplementary Fig. S4). This comparison indicated that D. carota has the closest homologous relationship with A. gracilistylus among the plants in the known gene database. The KOG database annotated 395,108 isoforms, which were divided into 25 categories. These were mainly annotated to 62,042 entries in “general function prediction only,” 47,848 entries in “signal transduction mechanisms,” and 37,403 entries in “posttranslational modification, protein turnover, chaperones” (Fig. 3a).
Fig. 3.
Annotations and functional classification of isoforms in A. gracilistylus. a Gene functional annotation in the KOG database. b Gene functional annotation in the GO database
The isoforms annotated in the NR database were further compared to the GO database, resulting in the annotation of 224,994 isoforms, which were divided into three GO categories: biological process, cellular component, and molecular function. The isoforms were mainly clustered in the biological processes “cellular process” (89,896 isoforms), “metabolic process” (85,703 isoforms), and “biological regulation” (28,321 isoforms). The isoforms mainly aggregated in the cellular components “cell” (90,525 isoforms), “cell part” (88,823 isoforms), and “membrane” (73,223 isoforms). The isoforms were mainly concentrated in the molecular functions “binding” (111,422 isoforms), “catalytic activity” (102,904 isoforms), and “transporter activity” (10,917 isoforms) (Fig. 3b).
Identification of genes involved in KA biosynthesis by KEGG analysis
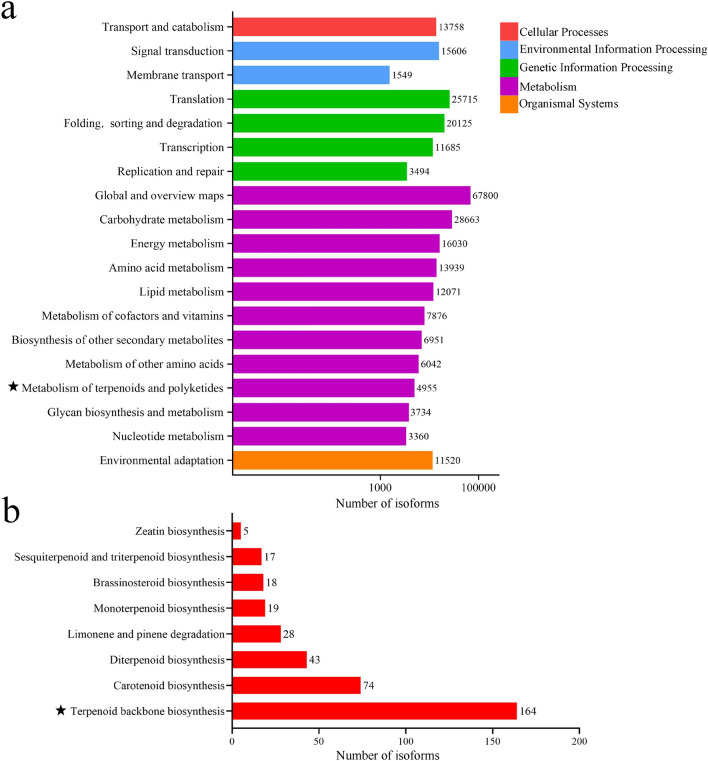
A total of 296,226 isoforms in the transcriptome of A. gracilistylus were annotated in the KEGG database, including five categories (Fig. 4a): cellular processes (13,758 isoforms), environmental information processing (17,155 isoforms), genetic information processing (58,019 isoforms), metabolism (171,421 isoforms), and organic systems (11,520 isoforms), involving a total of 132 metabolic pathways.
Fig. 4.
Annotation of A. gracilistylus isoforms in the KEGG database. a Five categories of biosynthesis pathways and the number of related isoforms. b The biosynthesis pathway of terpenoids and polyketones and the number of related isoforms
A total of 1728 isoforms were annotated to the terpenoid backbone biosynthesis (ko00900, 1142 isoforms) and diterpenoid biosynthesis (ko00904, 586 isoforms) pathways, which are two metabolic pathways of KA synthesis (Fig. 4b). Based on the screening conditions of Q value < 0.05 and FPKM > 1 of isoforms, eight, one, and two isoforms were associated with the 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMGR), mevalonate kinase (MK), and isopentenyl diphosphate isomerase (IDI) enzymes in the MVA pathway, respectively. In the MEP pathway, there were nine, six, and four isoforms encoding the enzymes 1-deoxy-d-xylulose-5-phosphate synthase (DXS), 1-deoxy-d-xylulose 5-phosphate reductoisomerase (DXR), and 2C-methyl-d-erythritol 2,4-cyclodiphosphate synthase (MCS), respectively. In addition, there were four, forteen, three, one, and eight isoforms encoding geranyl diphosphate synthase (GPPS), GGPP synthase (GGPPS), CPS, KS, and KO enzymes, respectively, in the pathway involved in the conversion of isopentenyl diphosphate (IPP) to KA (Supplementary Table S4). The relative expression levels of the isoforms of these key enzymes in each tissue are displayed in the form of a heat map in Fig. 5a. Most of these isoforms were highly expressed in the leaves and roots, whereas the isoforms related to KO were highly expressed in the roots of A. gracilistylus and had lower expression in the leaves and stems.
Fig. 5.
Heatmap of isoforms related to KA biosynthesis in A. gracilistylus. a The expression level of key enzyme isoforms in various tissues of the KA biosynthesis pathway. b Correlation analysis between the content of KA and the expression of isoforms involved in the KA biosynthesis pathway. L, leaf; R, root; S, stem. *0.01 < p < 0.05, **0.001 < p < 0.01, ***p ≤ 0.001
The correlation analysis showed that the levels of two isoforms of HMGR (isoform_13399, isoform_292476), one isoform of MK (isoform_33932), one isoform of IDI (isoform_58522), two isoforms of CPS (isoform_144483, isoform_305763), and two isoforms of KO (isoform_18099, isoform_445625) were significantly correlated with the content of KA (Fig. 5b). The correlation coefficients between the expression levels of these isoforms and the content of KA were all greater than or equal to 0.8. These results demonstrated a strong correlation between KA content and gene expression levels, indicating that these isoforms may be the key contributors to the biosynthesis of KA.
Analysis of DEGs
In the comparison of the leaves and roots, 101,473 DEGs were identified, 47,363 of which were up-regulated and 54,110 of which were down-regulated in the roots (Supplementary Table S5). In the comparison of the stems and leaves, 69,335 DEGs were identified, 29,476 of which were up-regulated and 39,859 of which were down-regulated in the leaves (Supplementary Table S6). In the comparison of the roots and stems, 51,195 DEGs were identified, 31,986 of which were up-regulated and 19,209 of which were down-regulated in the stems (Fig. 6a, Supplementary Table S7). We identified 12,048 common DEGs among the three comparisons (Fig. 6b).
Fig. 6.
Quantity and enrichment analysis of DEGs. a Up-regulated and down-regulated DEGs in different tissues. b Venn diagram of DEGs in different comparison groups. c Enrichment of KEGG pathways for DEGs in the leaves compared to the roots. d Enrichment of KEGG pathways for DEGs in the stems compared to the leaves. e Enrichment of KEGG pathways for DEGs in the roots compared to the stems
The KEGG database was used to annotate the biological functions of DEGs. The 40,542 DEGs identified between the leaves and roots were annotated to 135 metabolic pathways, mainly enriched in carbon metabolism, plant hormone signal transduction, and plant–pathogen interaction (Fig. 6c). The 27,957 DEGs identified between the stems and leaves were annotated to 135 metabolic pathways, mainly enriched in carbon metabolism, biosynthesis of amino acids, and plant hormone signal transduction (Fig. 6d). The 21,880 DEGs identified between the roots and stems were also annotated to 135 metabolic pathways, mainly enriched in carbon metabolism, ribosome, and plant hormone signal transduction (Fig. 6e).
A total of 652 DEGs in the leaf-to-root comparison were annotated to the KA biosynthesis pathway, with 358 of the DEGs specifically up-regulated in the roots. A total of 386 DEGs in the stem-to-leaf comparison were involved in the KA biosynthesis pathway, with 160 DEGs specifically up-regulated in the leaves. A total of 373 DEGs in the root-to-stem comparison were involved in the KA biosynthesis pathway, with 180 DEG specifically up-regulated in the stems (Table 4).
Table 4.
Number of specific up-regulated isoforms between each tissue
| Metabolic pathway name | Pathway number | Number of up-regulated isoforms | ||
|---|---|---|---|---|
| Leaf vs Root | Stem vs Leaf | Root vs Stem | ||
| Terpenoid skeleton biosynthesis | ko00900 | 166 | 105 | 129 |
| Diterpenoid biosynthesis | ko00904 | 192 | 55 | 51 |
Structural characterization of KO from A. gracilistylus
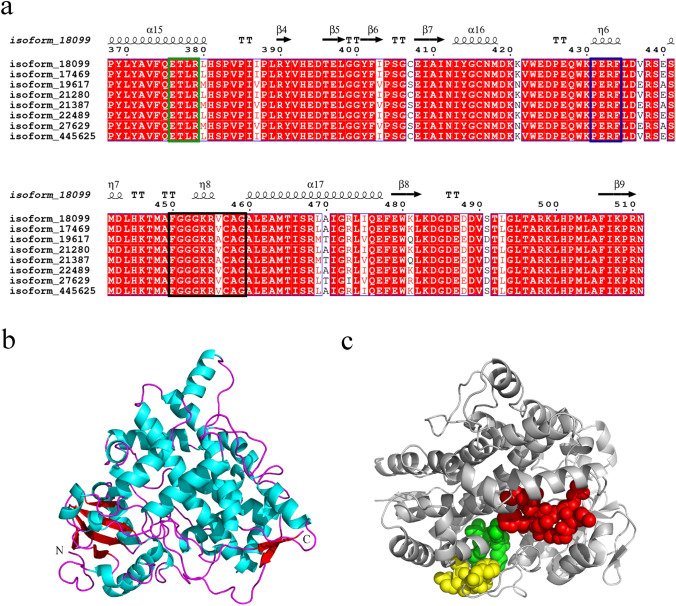
KO is the key enzyme in the KA synthesis pathway, which catalyzes the three consecutive oxidation reactions of ent-kaurene to finally generate KA. The ORF length of isoform_18099 in the transcriptome of A. gracilistylus was 1536 bp, encoding KO with 511 amino acids. Sequence alignment analysis of KO in A. gracilistylus showed 74.76% sequence homology with KO in Daucus carota var. sativa (DcKO, XP_017253618.1), 73.19% sequence homology with KO in Trachyspermum ammi (TaKO, AUZ98413.1), 69.25% sequence homology with KO in Camellia sinensis (CsKO, AUD40399.1), and 68.22% sequence homology with KO in Nicotiana tomentosiformis (NtKO, XP_009592896.1). Therefore, the KO gene in A. gracilistylus shows high consistency with that of other plants. The secondary structure analysis of KO in A. gracilistylus showed that the protein mainly consists of 17 α-helices and nine β-sheets (Fig. 7a, Supplementary Fig. S5).
Fig. 7.
Sequence alignment and protein structure model of KO in A. gracilistylus. a Sequence alignment and secondary structure of KO in A. gracilistylus. The substrate-binding domain ETLR, redox site PERF, and heme-binding domain FGGGKRVCAG are labeled with green, blue, and black boxes, respectively. b Cartoon model of the structure of KO in A. gracilistylus; α-helices and β-sheets are represented in cyan and red, respectively. c Active site of KO in A. gracilistylus. The redox sites, substrate-binding domains, and heme-binding domains are indicated as yellow, green, and red spheres, respectively
The three-dimensional structure of KO in A. gracilistylus was predicted using CYP76AH1 (SMTL ID: 5ylw.1.A) from Salvia miltiorrhiza as a model, which showed 33% (L60-N529) sequence similarity to the KO sequence of A. gracilistylus (Fig. 7b). KO in A. gracilistylus possesses the redox site PERF of the eukaryotic CYP450 enzyme, the substrate-binding domain ETLR, and the heme-binding domain FGGGKRVCAG (Fig. 7c).
TFs involved in KA biosynthesis
Based on the A. gracilistylus transcriptome data, 11,528 TFs were identified belonging to 58 TF families (Fig. 8a). The most abundant TF families were MYB (1252 isoforms), AP2-EREBP (1170 isoforms), and WRKY (799 isoforms). According to the functional classification of these TFs by the KEGG database, 64 TFs were found to participate in the metabolism of terpenes and polyketones, all of which belong to the forkhead-associated domain protein (FHA) family. The 64 TFs were screened using FPKM > 1 as the screening condition, and seven highly expressed TFs were obtained. Protein–protein interaction (PPI) network analysis was performed between the seven TFs with FPKM > 1 and key enzymes in the KA biosynthesis pathway. The PPI network contained 13 nodes and 61 edges, showing that DXR and DXS have high interactions with FHA (Fig. 8b).
Fig. 8.
Identification of TFs and the interaction network with key enzymes in the KA biosynthesis pathway of A. gracilistylus. a Classification of TF families to which isoforms belong. b Network interactions between key enzymes in the KA biosynthesis pathway and the FHA family. The size of the circle and the thickness of the line represent the strength of the interaction between proteins
Verification of key enzyme isoform expression levels by RT-qPCR
Six key enzyme isoforms involved in KA biosynthesis with large differences in expression levels between tissues detected by RNA-sequencing were selected for RT-qPCR validation in the three tissues: isoform_53458 (GGPPS), isoform_76283 (GGPPS), isoform_30303 (GPPS), isoform_33932 (MK), isoform_305763 (CPS), and isoform_7162 (CPS). Their expression levels in the leaves, roots, and stems of A. gracilistylus were further analyzed by RT-qPCR. Isoform_53458 and isoform_30303 had the highest expression levels in the leaves; isoform_76283, isoform_305763, and isoform_7162 had the highest expression levels in the roots; whereas isoform_33932 had higher expression in the stems but lower expression in the leaves and roots. These results were consistent with the trend of expression levels of corresponding isoforms obtained in the transcriptome data of A. gracilistylus (Fig. 9).
Fig. 9.
Gene expression analysis of six isoforms related to KA biosynthesis in A. gracilistylus (error bars indicate standard error from three biological replicates). n = 3. L, leaf; R, root; S, stem. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; ns, p > 0.05
Discussion
At present, second-generation sequencing is widely used to study gene expression, gene function and structure, alternative splicing, and for the prediction of new transcripts (Hu et al. 2021). However, in a single reaction, second-generation sequencing can measure only hundreds of bases, whereas third-generation sequencing can measure thousands of bases (van Dijk et al. 2018). The emergence of third-generation sequencing avoids the introduction error of PCR amplification and overcame the problem of incomplete information of species without a reference genome (Li et al. 2022). In this study, both the DNB-seq and PacBio iso-seq platforms were used to sequence the transcriptome of the leaves, roots, and stems of A. gracilistylus. A total of 358,103 genes were detected using the DNB-seq platform and the average comparison rate of the genes set was 84.20%. In addition, 505,880 isoforms were obtained through the PacBio iso-seq platform, with an average length of 948 bp, N50 length of 1552 bp, and N90 length of 586 bp. Compared with traditional transcriptome research, the transcriptome database of A. gracilistylus obtained in this study has higher reliability and better assembly quality.
Correlation analysis showed that the expression levels of eight genes encoding HMGR, MK, DXR, IDI, CPS, and KO were highly correlated with the content of KA in the tissues of A. gracilistylus. Except for isoform_292476 and isoform_33932, the expression levels of the other six genes were higher in roots than in the other tissues. This could explain the higher content of KA in roots than in the leaves and stems of A. gracilistylus. Therefore, these results provide molecular-level support that the roots are the most important medicinal parts of A. gracilistylus.
Terpenoids are biosynthesized in plants through the MVA and MEP pathways. HMGR is the rate-limiting enzyme of the MVA pathway (Lichtenthaler 2000), and DXS and DXR are the key enzymes of the MEP pathway (Jomaa et al. 1999). Yang et al. inhibited the activities of HMGR and DXR in S. miltiorrhiza with highly specific inhibitors and found that cell growth in the hairy root of S. miltiorrhiza was mainly influenced by the MVA pathway, while tanshinone was mainly synthesized by the MEP pathway (Yang et al. 2012). In the KA biosynthesis pathway of A. gracilistylus, we found eight isoforms related to HMGR and six isoforms related to DXR. Among them, isoform_13399 and isoform_292476 of HMGR were highly correlated with KA content, with correlation coefficients of 0.87 and 0.89, respectively. The isoform_20225 of DXR was significantly correlated with KA content, with a correlation coefficient of 0.70.
MK catalyzes MVA to generate mevalonate-5-phosphate, which is the key enzyme of the MVA pathway. Niu et al. cloned an MK gene (SaMK) from Santalum album. Experiments using several elicitors showed that the expression of SaMK was up-regulated by stimulation with methyl jasmonate (Niu et al. 2021). Lluch et al. analyzed the functional promoter of the MK gene in A. thaliana and found that the regulatory elements between the MK 5′-flanking regions at positions −194 and −295 were crucial for high-level expression of the gene (Lluch et al. 2000).
The conversion of isopentenyl diphosphate to dimethylallyl diphosphate (DMAPP) catalyzed by IDI is the initial step of isoprenoid biosynthesis. DMAPP loses its inorganic pyrophosphate to form isoprene and then forms short-chain isoprenoid with IPP. Chen et al. found that overexpression of IDI in Eucommia ulmoides increased the accumulation of trans-polyisoprene (Chen et al. 2012). In a study of carotenoid-deficient mutants in the fruits of Solanum lycopersicum, Pankratov et al. showed that the lack of IDI1 reduced the concentration of carotenoids in the cotyledons, fruits, and flowers, but did not decrease the concentration of carotenoids in the mature leaves (Pankratov et al. 2016). These findings highlighted the role of IDI in plastids in optimizing the ratio of IPP and DMAPP as precursors of different downstream isoprenoid pathways. The current findings further indicate that increasing the expression level of IDI in plastids may promote the synthesis of KA in A. gracilistylus.
Misra et al. identified two forms of CPS (ApCPS1 and ApCPS2) in a study of the ent-labdane-related diterpene biosynthesis pathway of Andrographis paniculata (Misra et al. 2015). Their findings suggested that ApCPS1 may play a role in primary metabolism, possibly by providing a precursor for the biosynthesis of the plant hormone GA. ApCPS2 was suggested to participate in the tissue-specific accumulation of ent-labdane-related diterpene-specific metabolites. There are two CPS genes in rice. OsCPS1 is involved in the biosynthesis of GA, while OsCPS2 is involved in the biosynthesis of the plant antitoxins phytocassanes A-E and oryzalexins A-F. Using RT-qPCR analysis, Toyomasu et al. showed that the transcripts of OsCPS1 were mainly located in the vascular bundle tissues, while OsCPS2 transcripts were mainly located in epidermal cells. The biological functions of these two CPS genes are different, with OsCPS1 and OsCPS2 required for rice growth and defense, respectively, resulting in different positions of their transcripts in rice (Toyomasu et al. 2015).
KO is a key enzyme involved in three consecutive oxidations to obtain the final product during the synthesis of KA (Hu et al. 2014). Mutations in rice seeds encoding KO genes attenuated the enzymatic activity of the reaction converting ent-kaurene to KA, leading to reduced KA synthesis (Zhang et al. 2020). Itoh et al. isolated five KO-like (KOL) genes from the genome of the semi-dwarf cultivar Tan-Ginnbozu (d35Tan-Ginnbozu) of rice (Itoh et al. 2004). Sequence analysis and complementary experiments showed that OsKOL2 corresponded to D35, while the homozygous cells with D35 allele deletion showed a serious dwarf phenotype. KO in A. gracilistylus belongs to the P450 lineage of the cytochrome superfamily and has a series of characteristic domains: ETLR (E376-R379) is the substrate-binding domain, PERF (P431-F434) is the redox site of eukaryotic CYP450, and FGGGKRVCAG (F450-G459) is the CYP450 cysteine (C) heme ferro ligand-binding site. This was consistent with the reported characteristic domains in Carya cathayensis (Liang et al. 2020), Pyrus pyrifolia (Li et al. 2010), and Scoparia dulcis (Yamamura et al. 2018), indicating that KO is highly conserved in vascular plants.
In summary, the discovery of candidate isoforms related to the biosynthesis pathways of KA is meaningful for improving the effective components of A. gracilistylus through gene regulation. Among these candidate isoforms, HMGR (isoform_13399, isoform_292476), IDI (isoform_58522), and KO (isoform_18099) showed the highest correlation coefficients between the FPKM value and the relative content of KA. This means that high expression of these four genes may promote KA synthesis. The FHA TF family has a typical FHA domain, which is the only phosphoprotein binding domain with strict specificity for phosphothreonine (Durocher et al. 1999). FHA is closely related to stress adaptation and hormone transduction during plant growth and development (Wang 2023). The PPI network showed that DXR and DXS had high interactions with FHA, indicating that DXR and DXS may also play a role in plant stress resistance.
Conclusion
In this study, the full-length transcriptome database of the leaves, roots, and stems of A. gracilistylus was constructed by combining the DNB-seq and PacBio iso-seq techniques for the first time. In total, 505,880 full-length isoforms were obtained and 408,954 of the isoforms were successfully annotated in seven databases. Sixty isoforms encoding 11 key enzymes involved in the biosynthesis pathway of KA in A. gracilistylus were identified. The isoforms encoding the key enzymes HMGR (isoform_13399, isoform_292476), IDI (isoform_58522), and KO (isoform_18099) might play key roles in the accumulation of KA in A. gracilistylus. Moreover, putative TFs involved in KA biosynthesis in A. gracilistylus were discovered. In conclusion, this study will facilitate further research on the A. gracilistylus functional genome and provides important insights into the mechanism of KA biosynthesis in A. gracilistylus.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the Beijing Genomics Institute for assistance with experiments.
Author contributions
Project design: QY and JW. Experiments and data analysis: BH, TS, JX, XZ, JZ and RH. Manuscript preparation: BH. Manuscript revision: QY and JW. Sample preparation: BH. All authors read and approved the final manuscript.
Funding
This work was supported by the Investigation and Development of Commonly Used Traditional Chinese Medicine Resources in the Dabie Mountain Area (Grant Number 2021HZ035, RH2200001421), the Natural Science Foundation of Anhui Province of China (Grant Number 2008085MH268), and the Natural Science Research Grant of Higher Education of Anhui Province (Grant Number 2022AH050474).
Data availability
The RNA-seq datasets from three A. gracilistylus tissues were deposited in the NCBI Sequence Read Archive (SRA) database (Accession: PRJNA945217).
Declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Qingshan Yang, Email: yangqingshan2011@ahtcm.edu.cn.
Jiawen Wu, Email: wujiawen@ahtcm.edu.cn.
References
- Ambrosio SR, Tirapelli CR, Coutinho ST, de Oliveira DC, de Oliveira AM, Da Costa FB. Role of the carboxylic group in the antispasmodic and vasorelaxant action displayed by kaurenoic acid. J Pharm Pharmacol. 2010;56:1407. doi: 10.1211/0022357044715. [DOI] [PubMed] [Google Scholar]
- Barker R, Fernandez Garcia MN, Powers SJ, Vaughan S, Bennett MJ, Phillips AL, Thomas SG, Hedden P. Mapping sites of gibberellin biosynthesis in the Arabidopsis root tip. New Phytol. 2021;229:1521–1534. doi: 10.1111/nph.16967. [DOI] [PubMed] [Google Scholar]
- Cavalcanti BC, Costa-Lotufo LV, Moraes MO, Burbano RR, Silveira ER, Cunha KM, Rao VS, Moura DJ, Rosa RM, Henriques JA, Pessoa C. Genotoxicity evaluation of kaurenoic acid, a bioactive diterpenoid present in Copaiba oil. Food Chem Toxicol. 2006;44:388. doi: 10.1016/j.fct.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Chen R, Harada Y, Bamba T, Nakazawa Y, Gyokusen K. Overexpression of an isopentenyl diphosphate isomerase gene to enhance trans-polyisoprene production in Eucommia ulmoides Oliver. BMC Biotechnol. 2012;12:78. doi: 10.1186/1472-6750-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi RJ, Shin EM, Jung HA, Choi JS, Kim YS. Inhibitory effects of kaurenoic acid from Aralia continentalis on LPS-induced inflammatory response in RAW264.7 macrophages. Phytomedicine. 2011;18:677–682. doi: 10.1016/j.phymed.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Cock PJ, Fields CJ, Goto N, Heuer ML, Rice PM. The sanger FASTQ file format for sequences with quality scores, and the Solexa/Illumina FASTQ variants. Nucleic Acids Res. 2010;38:1767–1771. doi: 10.1093/nar/gkp1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durocher D, Henckel J, Fersht AR, Jackson SP. The FHA domain is a modular phosphopeptide recognition motif. Mol Cell. 1999;4:387–394. doi: 10.1016/s1097-2765(00)80340-8. [DOI] [PubMed] [Google Scholar]
- Fernandes VC, Pereira SI, Coppede J, Martins JS, Rizo WF, Beleboni RO, Marins M, Pereira PS, Pereira AM, Fachin AL. The epimer of kaurenoic acid from Croton antisyphiliticus is cytotoxic toward B-16 and HeLa tumor cells through apoptosis induction. Genet Mol Res GMR. 2013;12:1005–1011. doi: 10.4238/2013.April.2.16. [DOI] [PubMed] [Google Scholar]
- Flügge UI, Gao W. Transport of isoprenoid intermediates across chloroplast envelope membranes. Plant Biol (stuttg) 2005;7:91–97. doi: 10.1055/s-2004-830446. [DOI] [PubMed] [Google Scholar]
- Hai G, Zhang H, Guo L. Research progress in pharmacological effects of diterpenoids. J Xinxiang Med Univ. 2015;32:77–80. [Google Scholar]
- Hedden P. The current status of research on gibberellin biosynthesis. Plant Cell Physiol. 2020;61:1832–1849. doi: 10.1093/pcp/pcaa092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A, Jayakumar V, Nitasaka E, et al. Genome sequence and analysis of the Japanese morning glory Ipomoea nil. Nat Commun. 2016;7:13295. doi: 10.1038/ncomms13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Gao W, Liu Y, Cheng Q, Su P, Liu Y, Chen M. Cloning and bioinformatics analysis of ent-kaurene oxidase synthase gene in Salvia miltiorrhiza. China J Chin Materia Med. 2014;39:4174–4179. [PubMed] [Google Scholar]
- Hu T, Chitnis N, Monos D, Dinh A. Next-generation sequencing technologies: an overview. Hum Immunol. 2021;82:801–811. doi: 10.1016/j.humimm.2021.02.012. [DOI] [PubMed] [Google Scholar]
- Huang Y, Yang W, Pei Z, Guo X, Liu D, Sun J, Zhang A. The genes for gibberellin biosynthesis in wheat. Funct Integr Genom. 2012;12:199–206. doi: 10.1007/s10142-011-0243-2. [DOI] [PubMed] [Google Scholar]
- Itoh H, Tatsumi T, Sakamoto T, Otomo K, Toyomasu T, Kitano H, Ashikari M, Ichihara S, Matsuoka M. A rice semi-dwarf gene, Tan-Ginbozu (D35), encodes the gibberellin biosynthesis enzyme, ent-kaurene oxidase. Plant Mol Biol. 2004;54:533–547. doi: 10.1023/B:PLAN.0000038261.21060.47. [DOI] [PubMed] [Google Scholar]
- Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ. Multiple sequence alignment with Clustal X. Trends Biochem Sci. 1998;23:403–405. doi: 10.1016/s0968-0004(98)01285-7. [DOI] [PubMed] [Google Scholar]
- Jomaa H, Wiesner J, Sanderbrand S, Altincicek B, Weidemeyer C, Hintz M, Türbachova I, Eberl M, Zeidler J, Lichtenthaler HK, Soldati D, Beck E. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science. 1999;285:1573–1576. doi: 10.1126/science.285.5433.1573. [DOI] [PubMed] [Google Scholar]
- Kasahara H, Hanada A, Kuzuyama T, Takagi M, Kamiya Y, Yamaguchi S. Contribution of the mevalonate and methylerythritol phosphate pathways to the biosynthesis of gibberellins in Arabidopsis. J Biol Chem. 2002;277:45188–45194. doi: 10.1074/jbc.M208659200. [DOI] [PubMed] [Google Scholar]
- Kuang C (2015) Study on the protective effect of the leaves of Acanthopanax gracilistylus extract on acute liver injury in rats and its content determination. Dissertation, Hunan University of Chinese Medicine
- Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Quilantang NG, Hahm DH, Kang KS, Jacinto SD, Choi YJ, Lee SC, Lee S. Optimization of extraction conditions of continentalic and kaurenoic acids from Aralia continentalis by HPLC/UV and their validation. J Chromatogr Sci. 2020;58:672–677. doi: 10.1093/chromsci/bmaa019. [DOI] [PubMed] [Google Scholar]
- Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Tian Y, Wang C, Tian W, Song W, Yin H. Cloning and bioinformatics analysis of ent-kaurene oxidase gene PpKO in pear (Pyrus pyrifolia Nakai) Acta Horticulturae. Sinica. 2010;37:1575–1582. [Google Scholar]
- Li Q, Song J, Zhou Y, Chen Y, Zhang L, Pang Y, Zhang B. Full-length transcriptomics reveals complex molecular mechanism of salt tolerance in Bromus inermis L. Front Plant Sci. 2022;13:917338. doi: 10.3389/fpls.2022.917338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B, Zhang J, Ren F, Hu H, Xu C, Hu Y, Huang Y, Lou H, Zhang Q. Cloning and expression analysis of ent-kaurene oxidase gene CcKo in Carya cathayensis. Scientia Silvae Sinicae. 2020;56:70–82. [Google Scholar]
- Lichtenthaler HK. Non-mevalonate isoprenoid biosynthesis: enzymes, genes and inhibitors. Biochem Soc Trans. 2000;28:785–789. doi: 10.1042/bst0280785. [DOI] [PubMed] [Google Scholar]
- Liu H, Luo C, Chen D, Wang Y, Guo S, Chen X, Bai J, Li M, Huang X, Cheng X, Huang C. Whole-transcriptome analysis of differentially expressed genes in the mutant and normal capitula of Chrysanthemum morifolium. BMC Genom Data. 2021;22:2. doi: 10.1186/s12863-021-00959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lluch MA, Masferrer A, Arró M, Boronat A, Ferrer A. Molecular cloning and expression analysis of the mevalonate kinase gene from Arabidopsis thaliana. Plant Mol Biol. 2000;42:365–376. doi: 10.1023/a:1006325630792. [DOI] [PubMed] [Google Scholar]
- Lu MX, Yang Y, Zou QP, Luo J, Hwang EH. Anti-diabetic effects of acankoreagenin from the leaves of Acanthopanax gracilistylus herb in RIN-m5F cells via suppression of NF-κB activation. Molecules. 2018;23:958. doi: 10.3390/molecules23040958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu JH, Lee GS, Kim KH, Kim HW, Cho SI, Jeong SI, Kim HJ, Ju YS, Kim HK, Sadikot RT, Christman JW, Oh SR, Lee HK, Ahn KS, Joo M. ent-kaur-16-en-19-oic acid, isolated from the roots of Aralia continentalis, induces activation of Nrf2. J Ethnopharmacol. 2011;137:1442. doi: 10.1016/j.jep.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magi A, Pippucci T, Sidore C. XCAVATOR: accurate detection and genotyping of copy number variants from second and third generation whole-genome sequencing experiments. BMC Genom. 2017;18:747. doi: 10.1186/s12864-017-4137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra RC, Garg A, Roy S, Chanotiya CS, Vasudev PG, Ghosh S. Involvement of an ent-copalyl diphosphate synthase in tissue-specific accumulation of specialized diterpenes in Andrographis paniculata. Plant Sci. 2015;240:50–64. doi: 10.1016/j.plantsci.2015.08.016. [DOI] [PubMed] [Google Scholar]
- Morrone D, Chen X, Coates RM, Peters RJ. Characterization of the kaurene oxidase CYP701A3, a multifunctional cytochrome P450 from gibberellin biosynthesis. Biochem J. 2010;431:337–344. doi: 10.1042/BJ20100597. [DOI] [PubMed] [Google Scholar]
- Ndom JC, Mbafor JT, Meva’a LM, Kakam Z, Phanuel AS, Ndongo E, Harwood LM, Mpondo TN. New alkamide and ent-kaurane diterpenoid derivatives from Senecio erechtitoides (Asteraceae) Phytochem Lett. 2010;3:201. doi: 10.1016/j.phytol.2010.07.007. [DOI] [Google Scholar]
- Niu M, Xiong Y, Yan H, Zhang X, Li Y, da Silva JAT, Ma G. Cloning and expression analysis of mevalonate kinase and phosphomevalonate kinase genes associated with the MVA pathway in Santalum album. Sci Rep. 2021;11:16913. doi: 10.1038/s41598-021-96511-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou YJ, Ren QQ, Fang ST, Wu JG, Jiang YX, Chen YR, Zhong Y, Wang DD, Zhang GX. Complete genome insights into Lactococcus petauri CF11 isolated from a healthy human gut using second- and third-generation sequencing. Front Genet. 2020;11:119. doi: 10.3389/fgene.2020.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankratov I, McQuinn R, Schwartz J, Bar E, Fei Z, Lewinsohn E, Zamir D, Giovannoni JJ, Hirschberg J. Fruit carotenoid-deficient mutants in tomato reveal a function of the plastidial isopentenyl diphosphate isomerase (IDI1) in carotenoid biosynthesis. Plant J. 2016;88:82–94. doi: 10.1111/tpj.13232. [DOI] [PubMed] [Google Scholar]
- Park SH, Nhiem NX, Kiem PV, Choi EM, Kim JA, Kim YH. A new norlupane triterpene from the leaves of Acanthopanax koreanum increases the differentiation of osteoblastic MC3T3-e1 cells. Arch Pharmacal Res. 2010;33:75. doi: 10.1007/s12272-010-2228-4. [DOI] [PubMed] [Google Scholar]
- Ragasa CY, Alimboyoguen AB, Raga UD. A bioactive diterpene from Smallanthus sonchifolius. Nat Prod Commun. 2008;3:1663–1666. [Google Scholar]
- Rhoads A, Au KF. PacBio sequencing and its applications. Genom Proteom Bioinf. 2015;13:278–289. doi: 10.1016/j.gpb.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto T, Miura K, Itoh H, Tatsumi T, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Agrawal GK, Takeda S, Abe K, Miyao A, Hirochika H, Kitano H, Ashikari M, Matsuoka M. An overview of gibberellin metabolism enzyme genes and their related mutants in rice. Plant Physiol. 2004;134:1642–1653. doi: 10.1104/pp.103.033696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan C, Wang C, Shi Y, Zhang S, Zhao L, Wu J. Identification of key enzyme genes involved in biosynthesis of steroidal saponins and analysis of biosynthesis pathway in Polygonatum cyrtonema China. J Chin Materia Medica. 2020;45:2847–2857. doi: 10.19540/j.cnki.cjcmm.20200329.108. [DOI] [PubMed] [Google Scholar]
- Sithisarn P, Jarikasem S, Thisayakorn K. Anti-inflammatory and antioxidative effects of leaf extract from Acanthopanax trifoliatus. Planta Med. 2009;75:891. doi: 10.1055/s-0029-1234285. [DOI] [Google Scholar]
- Su P, Tong Y, Cheng Q, Hu Y, Zhang M, Yang J, Teng Z, Gao W, Huang L. Functional characterization of ent-copalyl diphosphate synthase, kaurene synthase and kaurene oxidase in the Salvia miltiorrhiza gibberellin biosynthetic pathway. Sci Rep. 2016;6:23057. doi: 10.1038/srep23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirapelli CR, Ambrosio SR, Costa FBd, Coutinho ST, Oliveira DCRd, Oliveira AMd. Analysis of the mechanisms underlying the vasorelaxant action of kaurenoic acid in the isolated rat aorta European. J Pharmacol. 2004;492:233. doi: 10.1016/j.ejphar.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Toyomasu T, Usui M, Sugawara C, Kanno Y, Sakai A, Takahashi H, Nakazono M, Kuroda M, Miyamoto K, Morimoto Y, Mitsuhashi W, Okada K, Yamaguchi S, Yamane H. Transcripts of two ent-copalyl diphosphate synthase genes differentially localize in rice plants according to their distinct biological roles. J Exp Bot. 2015;66:369–376. doi: 10.1093/jxb/eru424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk EL, Jaszczyszyn Y, Naquin D, Thermes C. The third revolution in sequencing technology. Trends Genet TIG. 2018;34:666–681. doi: 10.1016/j.tig.2018.05.008. [DOI] [PubMed] [Google Scholar]
- Wang Q. The role of forkhead-associated (FHA)-domain proteins in plant biology. Plant Mol Biol. 2023;111:455–472. doi: 10.1007/s11103-023-01338-4. [DOI] [PubMed] [Google Scholar]
- Winand R, Bogaerts B, Hoffman S, Lefevre L, Delvoye M, Braekel JV, Fu Q, Roosens NH, Keersmaecker SC, Vanneste K. Targeting the 16S rRNA gene for bacterial identification in complex mixed samples: comparative evaluation of second (Illumina) and third (Oxford nanopore technologies) generation sequencing technologies. Int J Mol Sci. 2019;21:298. doi: 10.3390/ijms21010298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Wei M, Lu Y, Zheng S, Xu Y, Yan D. Research progress of Eleutherococcus nodiflorus(Dunn) J Anhui Agric Sci. 2014;42:7391–7394. [Google Scholar]
- Xie X, Cheng Z, Chen D. TLC ldentification and HPLC-ELSD Determination of ent-Kaur-16-en-19-oic acid in acanthopanacis cortex. Lishizhen Med Materia Medica. 2013;24:2425–2427. [Google Scholar]
- Xie S, Huang C, Huang S (2003) An experimental study of the antisenility effect of total glycosides of Acanthopanax Bark. Herald Med 226–228
- Xu HB, Yang TH, Xie P, Tang ZS, Song X, Xu HL, Li YH, Zhang DB, Liu YR, Liang YN, Zhang Y, Liu SJ, Wei SM, Sun C, Liu HB, Deng C, Wang W. LC-MS guided isolation of gracilistones A and B, a pair of diastereomeric sesquiterpenoids with an unusual tetrahydrofuran-fused tricyclic skeleton from Acanthopanax gracilistylus and their potential anti-inflammatory activities. Fitoterapia. 2018;130:265–271. doi: 10.1016/j.fitote.2018.09.012. [DOI] [PubMed] [Google Scholar]
- Yamamura Y, Taguchi Y, Ichitani K, Umebara I, Ohshita A, Kurosaki F, Lee JB. Characterization of ent-kaurene synthase and kaurene oxidase involved in gibberellin biosynthesis from Scoparia dulcis. J Nat Med. 2018;72:456–463. doi: 10.1007/s11418-017-1168-4. [DOI] [PubMed] [Google Scholar]
- Yang YL, Chang FR, Wu CC, Wang WY, Wu YC. New ent-kaurane diterpenoids with anti-platelet aggregation activity from Annona squamosa. J Nat Prod. 2002;65:1462. doi: 10.1021/np020191e. [DOI] [PubMed] [Google Scholar]
- Yang D, Du X, Liang X, Han R, Liang Z, Liu Y, Liu F, Zhao J. Different roles of the mevalonate and methylerythritol phosphate pathways in cell growth and tanshinone production of Salvia miltiorrhiza hairy roots. PLoS ONE. 2012;7:e46797. doi: 10.1371/journal.pone.0046797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Cai W, Li M, Li N, Ma S, Cheng X, Wei F. Progress in chemical and pharmacological research of Acanthopanax gracilistylus. Modern Chin Med. 2020;22:652–662. [Google Scholar]
- Ye G, Zhang H, Chen B, Nie S, Liu H, Gao W, Wang H, Gao Y, Gu L. De novo genome assembly of the stress tolerant forest species Casuarina equisetifolia provides insight into secondary growth. Plant J Cell Mol Biol. 2019;97:779–794. doi: 10.1111/tpj.14159. [DOI] [PubMed] [Google Scholar]
- Zhang H, Li M, He D, Wang K, Yang P. Mutations on ent-kaurene oxidase 1 encoding gene attenuate its enzyme activity of catalyzing the reaction from ent-kaurene to ent-kaurenoic acid and lead to delayed germination in rice. PLoS Genet. 2020;16:e1008562. doi: 10.1371/journal.pgen.1008562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z (2012) Studies on chemical constituents, pharmacological effects and determination of contents of the fruits of Acanthopanaxgracilistylus. Dissertation, Nanjing University of Chinese Medicine
- Zheng J, Zhang G, Wei M, Lu Y, Qu L, Guo Y, Wu Y, Gao X. Research progress of original base, chemical components and pharmacological effects on cortex acanthopanacis. J Liaoning Univ Tradit. 2015;17:104–107. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-seq datasets from three A. gracilistylus tissues were deposited in the NCBI Sequence Read Archive (SRA) database (Accession: PRJNA945217).