Abstract
Introduction:
Divergent recommendations for periprocedural management of GLP-1 receptor agonist (GLP-1 RA) medications rely on limited evidence. We performed a systematic review and meta-analysis to provide quantitative measures of gastric emptying relevant to mechanisms of weight loss and to periprocedural management of GLP-1 RA. We hypothesized that the magnitude of gastric emptying delay would be low and of limited clinical significance to procedural sedation risks.
Methods:
A protocolized search identified studies on GLP-1 RA that quantified gastric emptying measures. Pooled estimates using random effects were presented as weighted mean difference with 95% confidence intervals (CI). Univariate meta-regression was performed to assess the influence of GLP-1 RA type, short- vs long-acting mechanism of action, and duration of treatment on gastric emptying.
Results:
Fifteen studies met inclusion criteria. Five studies (n=247) utilized scintigraphy (GES). Mean T1/2 was 138.4 minutes (CI:74.5-202.3) for GLP-1 RA versus 95.0 minutes (CI:54.9-135.0) for placebo, with pooled mean difference of 36.0 minutes (CI:17.0-55.0, p<0.01, I2=79.4%). Ten studies (n=411) utilized the acetaminophen absorption test (AAT), with no significant delay in gastric emptying measured by Tmax, AUC4hr, and AUC5hr with GLP-1 RA (p>0.05). On meta-regression, type of GLP-1 RA, mechanism of action, and treatment duration did not impact gastric emptying (p>0.05).
Conclusions:
While a gastric emptying delay of ~36 minutes is quantifiable on GLP-1 RA medications, it is of limited magnitude relative to standard periprocedural fasting periods. There were no substantial differences in gastric emptying on modalities reflective of liquid emptying (AAT), particularly at time points relevant to periprocedural care.
Keywords: GLP-1 receptor agonist, gastric emptying delay, gastric emptying scintigraphy, procedural sedation, pre-procedure care
Graphical Abstract

INTRODUCTION
Use of glucagon-like peptide-1 (GLP-1) receptor agonists (GLP-1 RA) have surged dramatically alongside an increasing body of evidence substantiating their diverse cardiometabolic benefits1-6. While clinical trials have suggested gastric emptying delay associated with these medications, real-world post-market pharmacoepidemiology studies have reiterated these concerns with reports of greater rates of gastroparesis7-11. These concerns have extended to the periprocedural management of these medications prior to administration of anesthesia or sedation, given the potential risk of aspiration from retained gastric contents.
Recent limited case series and retrospective case control studies suggest that these medications may be associated with higher incidence of retained food residue in the stomach found during upper endoscopy, thereby potentially increasing the risk of pulmonary aspiration12-14. These hypothetical concerns have driven considerable debates among different specialties regarding the optimal periprocedural management of GLP-1 RA and pre-procedural dietary recommendations for patients receiving anesthesia or sedation, particularly for elective procedures or operations. A consensus-based guidance from the American Society of Anesthesiologists (ASA) in 2022 recommends holding GLP-1 RA prior to elective surgeries and procedures, with consideration of delay of the procedure based on presence of gastrointestinal symptoms or if the medications were not stopped15. In contrast, the American Gastroenterological Association (AGA) has recently released a rapid clinical practice update that suggests to proceed with endoscopic procedures as planned for those who followed standard pre-procedure precautions16. This contrasting guidance was primarily established by expert consensus and based on mostly case reports/series with limited empirical data, often through the lens of each specific specialty, thereby further contributing to confusion and debates in clinical practice.
While prior studies and series have suggested prolonged gastric emptying associated with GLP-1 RA, the true magnitude and impact of delay, particularly with regards to aspiration risks during anesthesia, remain unclear. Published literature was limited by considerable heterogeneity in study designs, measures of gastric emptying, formulation of GLP-1 RA, and patient populations. The primary aim of this study was to quantify the duration of gastric emptying delay associated with GLP-1 RA therapies in ways relevant to guide periprocedural management decisions. Secondary aims included comparing short-acting to long-acting GLP-1 RA therapy on gastric emptying delay and assessing whether estimates differed based on duration of therapy and diagnostic modality of gastric emptying assessment. We hypothesized that while there may be comparative delay in gastric emptying associated with GLP-1 RA versus placebo or non-use, the difference is small and likely of limited significance in the context of procedural sedation risks. To accomplish these aims, we performed a systemic review and meta-analysis of published clinical trials to calculate pooled estimates of gastric emptying delay with GLP-1 RA compared to placebo or non-GLP-1 comparators in adult patients with diabetes mellitus and/or excess body weight.
METHODS
Overview of Search Strategy
A protocolized search strategy was conducted using PubMed, EMBASE, and the Cochrane Library databases from 2003 to November of 2023. The search strategy was conducted according to the Meta-Analysis Of Observational Studies in Epidemiology (MOOSE) and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRIMSA) guidelines (Supplemental Table 1). The search was conducted on November 1st 2023. The search protocol included a combination of GLP-1 RA or GLP-1 RA formulations (e.g. “glucagon-like peptide 1”, “GLP-1”, “semaglutide”, “lixisenatide”, “liraglutide”, “dulaglutide”, “albiglutide”), indication for GLP-1 agonist (diabetes mellitus [“diabetes”, “diabetes mellitus”, “blood glucose”, “type 1 diabetes”, “type 2 diabetes”], overweight, or obesity [“weight loss”, “overweight”, “obesity”]), and measures of gastric emptying (e.g. “gastric emptying”, “scintigraphy”, “breath test”, “acetaminophen”, “paracetamol”, “endoscopy”, “capsule”, “capsule endoscopy” as search terms and/or MeSH terms. (Supplemental Table 2)
Study selection and data collection
Selected titles and abstracts were then imported into the Covidence systemic review software package (Veritas Health Innovation, Melbourne, Australia). Screening of titles and abstracts suitable for full text review was performed using Covidence by two independent reviewers (BH and NL). Studies were eligible for inclusion if they assessed gastric emptying after treatment of diabetes or excess body weight with GLP-1 RA therapies. Modalities of gastric emptying assessment included scintigraphic measurement, stable isotope breath testing, acetaminophen-based absorption testing (AAT), and wireless motility capsule. GLP-1 RA formulations included liraglutide, semaglutide, lixisenatide, albiglutide, dulaglutide, and tirzepatide. Intravenous formulations were excluded from this analysis. Other exclusion criteria were studies with patients <18 years of age, lack of quantitative reporting of gastric emptying parameters, or lack of a comparator arm.
Full-text review was conducted for final inclusion into the study. Disagreements were resolved by review from a third independent reviewer when required. Study features and outcome measures were extracted prior to statistical analysis in Stata (StataCorp, College Station, TX). Relevant study characteristics recorded included study year, country of publication, methodology, treatment indication, GLP-1 RA formulation, sample size, cohort characteristics [age, sex, body mass index (BMI)], and gastric emptying diagnostic modality as well as emptying measure reported. Metrics of gastric emptying reported may include: (1) the time required for 50% of ingested gastric contents to empty (t1/2), (2) the time elapsed until reaching peak level of isotope exhalation on breath testing or acetaminophen blood concentration on AAT (tmax), (3) concentration of acetaminophen absorbed on AAT at 1, 4 or 5 hours, measured as area under the curve (AUC, μmol*hr/L), and (4) proportion of ingested gastric content emptied or retained at 4 hours on scintigraphy.
The primary analysis focused on quantification of gastric emptying half-time on scintigraphy (T1/2), as this was the most commonly reported measure, and the secondary outcome was Tmax on AAT. A commonly used method in pharmacokinetic study, AAT provides a surrogate measure of gastric emptying in clinical trials. Acetaminophen absorption, calculated as area under the curve (AUC) at specific time points (1 hour, 4 hours, 5 hours), was also analyzed as a secondary outcome. We also conducted systematic review of scintigraphy and AAT-based gastric emptying measures that were less consistently reported, such as emptying at specific time points. Additional systematic reviews were also performed for outcomes using other less frequently reported modalities of gastric emptying assessment [retention of food residue on esophagogastroduodenoscopy (EGD) and wireless motility capsule] and measures reported with significant heterogeneity (stable isotope breath testing).
Statistical Analysis
This systematic review and meta-analysis was performed by calculating pooled data using study provided mean, standard deviation, and sample size. When studies reported median, range, and sample size, estimation of the mean and variance was calculated in accordance with validated methodology.17 After data abstraction, mean difference and standardized difference in means were calculated between GLP-1 RA and placebo groups. Once appropriate studies were identified through systematic literature search and review, pooled rates were estimated using random effects models and presented as point estimates (rates) with 95% confidence intervals.18,19 Measured outcomes comparing cohorts or group T1/2 (scintigraphy), Tmax (AAT) and AUC at 1, 4, and 5 hours (AAT) were obtained. From this, weighted difference in means and standardized mean difference were calculated and transformed to the natural logarithm before pooling, and the variance was calculated.
Sensitivity analyses including “one study removed” or “leave-one-out” analyses were conducted by removing each study individually and re-calculating the pooled results to evaluate the impact of each single included study on the overall effect estimate. When possible, univariable meta-regression was performed to assess the influence of the type of GLP-1 RA (formulation and short versus long-acting) and trial duration on gastric emptying. These variables were chosen a priori based on patient relevant outcomes and in effort to guide clinical decision making. Additional regression analyses were performed for average study body mass index (BMI) and impact of obesity (stratified as BMI < or ≥ 30 kg/m2). All calculated p-values were 2-sided, and p<0.05 was considered statistically significant. Tabular and graphical analyses were performed using the Comprehensive Meta-Analysis software, version 3 (BioStat, Englewood, NJ).
Risk of Bias and Quality Assessment
Risk of bias and study quality were evaluated using the Revised Cochrane risk-of-bias tool (RoB 2) for randomized trials and Risk Of Bias In Non-randomized Studies - of Interventions (ROBINS-I) tool for non-randomized studies20,21. These assessments were independently performed by two investigators (BH and TRM). Randomized trials were assessed for risk of bias in five domains: bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome, and bias in selection of the reported result. For non-randomized studies, assessment was performed for the following domains: confounding, selection of participants, classification of interventions, deviation from intended interventions, missing data, measurement of outcomes, and selection of the reported result. Risk of bias for each included study measured by either tool was further classified into low, moderate, or high risk. Any disagreements were resolved by discussion and consensus, and in consultation with a third reviewer (WWC).
Investigations of Heterogeneity
Heterogeneity was assessed for the individual meta-analyses using the chi-squared test for the Cochran Q and the I2 statistic.22 Significant heterogeneity was defined as p>0.05 on the Cochran Q test or I2 >50%. Further quantification of heterogeneity was categorized based upon the I2 value, with 0-25%, 25-50%, 50-75% and >75% indicating very low, low, moderate, and high amounts of heterogeneity, respectively.
Publication Bias
To assess for publication bias, a funnel plot was created. It was visually inspected for asymmetry and quantitatively assessed using Egger regression testing.23,24 The trim and fill method was used to correct for funnel plot asymmetry and provide an adjusted effect when necessary, as well as for confirmation of funnel plot symmetry.25
RESULTS
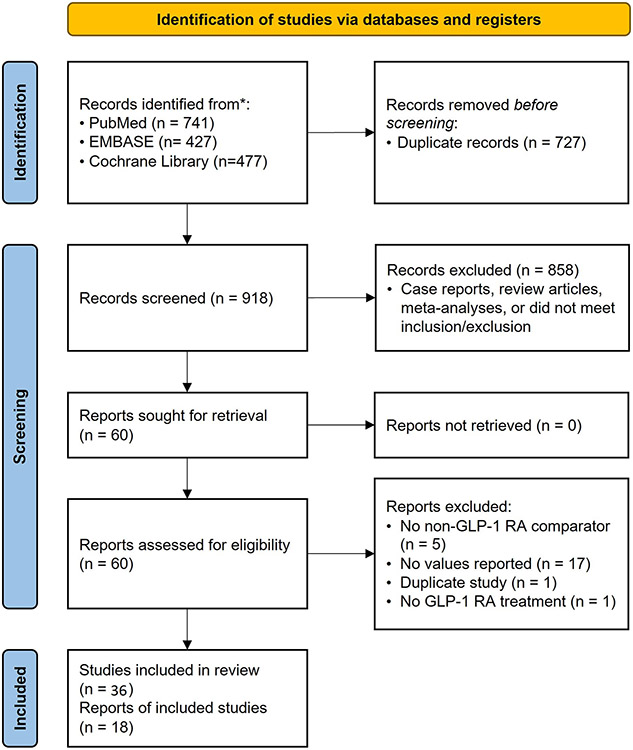
Our search protocol identified a total of 918 potentially relevant studies using PubMed, EMBASE, and the Cochrane Database. After completion of title and abstract screening, 60 articles were assessed for full-text review. With application of inclusion/exclusion criteria and removal of duplicate studies and/or duplicate study cohorts, 36 unique studies reflecting a total of 1,574 patients were included in our analyses (Figure 1)8-14,26-55. Among these, gastric emptying was assessed using scintigraphy in 7 studies, AAT in 19 studies, stable isotope breath testing in 5 studies, EGD in 3 studies, and wireless motility capsule in 2 studies. The majority of studies were performed in the United States (19) or Europe (12), with a smaller number in Asia (2), Australia (2), and Canada (1). The primary indication for GLP-1 RA agents was overweight or obesity (BMI >25 kg/m2) in 17% of the studies and diabetes mellitus in 67%. In addition, 6% of studies enrolled healthy volunteers and 8% did not report specific indications.
Figure 1:
Overview of Study Selection (PRISMA Flow Chart).
Scintigraphic Measurement of Gastric Emptying
For the primary outcome of quantified gastric emptying by scintigraphy reported as t1/2, five studies reflecting a total of 247 patients were analyzed (Table 1), with a mean age of 43.8 ±11.9 years, BMI of 33.1 ±4.2 kg/m2, and 81.7% female. This cohort included 122 patients treated with a GLP-1 RA agent and 125 receiving placebo. All studies were randomized controlled trials (RCT) or randomized crossover trials, with low risk of bias. The pooled T1/2 was 138.4 minutes (95% CI: 74.5-202.3) for the GLP-1 RA group versus 95.0 minutes (95% CI: 54.9-135.0) for the placebo group, resulting in a pooled mean difference of 36.0 minutes (95% CI: 17.0-55.0, I2=79.4%, Q-value=19.4, p<0.01) (Figure 2). The pooled standardized mean difference for patients on GLP-1 RA versus placebo was 1.03 (95% CI: 0.7-1.4, I2=22.0%, Q-value=5.1, p<0.01) (Supplemental Figure 1). Subgroup analyses stratified by GLP-1 RA mechanism of action with pooled estimates for t1/2 on scintigraphy and levels of heterogeneity are summarized in Table 4.
Table 1:
Overview of Studies Measuring Gastric Emptying by Scintigraphy
| Study | Year | Study Region |
Study Design |
GLP-1 Indication |
Medication | N | Age (SD) | BMI (SD, IQR) | Primary emptying measure |
Emptying at 4 hours |
|---|---|---|---|---|---|---|---|---|---|---|
| Jensterle | 2022 | Europe | RCT | Weight loss | Semaglutide | 19 | Not reported | 37.9 (IQR 33.2-41.4) | T1/2 | Yes |
| Jones | 2020 | Australia | RCT | Weight loss | Exenatide | 32 | 59.9 (0.9) | 29.6 (0.6) | T1/2 | No |
| Halawi | 2017 | US | RCT | Weight loss | Liraglutide | 40 | 42 (IQR 32-51) | 37.2 (IQR 33.6-41.0) | T1/2 | No |
| Maselli | 2022 | US | RCT | Weight loss | Liraglutide | 136 | 42 (IQR 32-51) | 35.9 (IQR 32.6-40.2) | T1/2 | No |
| Rayner | 2020 | US | RCT | Diabetes mellitus | Lixisenatide | 30 | 61.7 (6.2) | 32.0 (4.1) | Retention at 4 hours | Yes |
| Jalleh * | 2020 | Australia | Crossover trial | Mix of healthy volunteers, diabetes mellitus | Lixisenatide | 60 | Healthy group: 67.2 (2.3) ; T2D 61.9 (2.3) | Healthy: 25.4 (0.8); T2DM: 30.3 (0.7) | Retention at 3 hours | No |
| Acosta | 2015 | US | RCT | Weight loss | Exenatide | 20 | 34 (IQR 33-46.8) | 33.5 (32.9-36.7) | T1/2 | No |
Indicates study was not included in quantitative meta-analyses due to inadequate reporting of measure details or measure reported not being comparable to other studies
Figure 2:
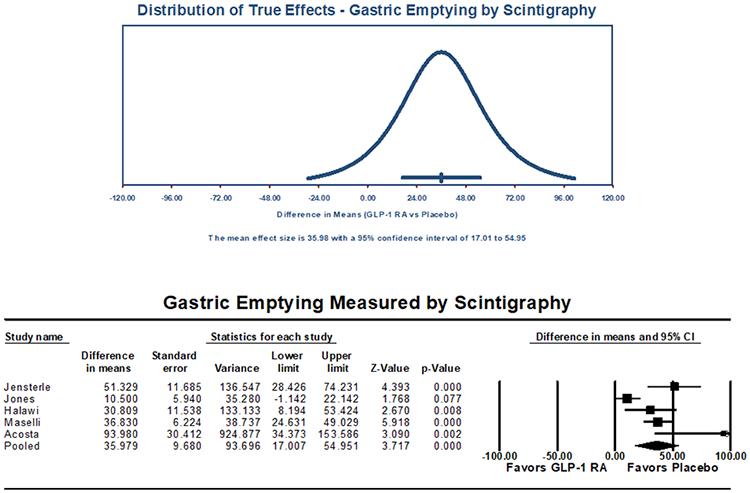
Gastric Emptying Study (Scintigraphy) Primary Outcome (T1/2, minutes), Pooled Mean Difference
Table 4:
Overview of gastric emptying measures by test modality with assessment of heterogeneity
| Scintigraphy | T1/2 (min) | 95% CI | Heterogeneity |
|---|---|---|---|
| GLP-1 (5 studies, n=122) | 138.4 | 74.5-202.3 | I2=98.9, Q-value=379.7 |
| Long Acting (4 studies, n=112) | 127.0 | 56.5-197.6 | I2=99.1, Q-value=368.1 |
| Short Acting (one study, n=10) | 189.6 | 136.8-242.4 | I2=0.0, Q-value=0.0 |
| Placebo | 95.0 | 54.9-135.0 | I2=98.6, Q-value=295.8 |
| Acetaminophen Absorption | Tmax (min) | 95% CI | Heterogeneity |
| GLP-1 (6 studies, n=143) | 83.5 | 51.3-115.5 | I2=96.8, Q-value=154.7 |
| Long Acting (5 studies, n=123) | 66.0 | 36.0-96.1 | I2=96.5, Q-value=114.2 |
| Short Acting (one study, n=20) | 200.0 | 148.1-251.8 | I2=0.0, Q-value=0.0 |
| Placebo (6 studies, n=141) | 76.6 | 49.8-103.4 | I2=97.6, Q-value=204.4 |
| Acetaminophen Absorption | AUC 5 Hour | 95% CI | Heterogeneity |
| GLP-1 (4 studies, n=70) | 144.9 | 48.0-241.8 | I2=98.7, Q-value=229.5 |
| Placebo (4 studies, n=70) | 186.4 | 69.2-303.6 | I2=98.9, Q-value=274.9 |
| Acetaminophen Absorption | AUC 4 Hour | 95% CI | Heterogeneity |
| GLP-1 (4 studies, n=108) | 911.6 | 678.2-1145.0 | I2=99.3, Q-value=460.4 |
| Placebo (4 studies, n=107) | 496.5 | 363.6-629.4 | I2=98.9, Q-value=283.4 |
| Acetaminophen Absorption | AUC One Hour | 95% CI | Heterogeneity |
| GLP-1 (5 studies, n=97) | 46.1 | 22.0-70.1 | I2=97.2, Q-value=144.0 |
| Placebo (5 studies, n=97) | 63.6 | 44.4-82.8 | I2=94.6, Q-value=73.5 |
Sensitivity analyses performed by removing one study at a time did not reveal any statistically significant differences from the primary outcome (Supplemental Figure 2). When univariable meta-regression was performed to evaluate the impact of specific GLP-1 RA formulation with respect to gastric emptying on scintigraphy, there was no significant association between GLP-1 RA formulation and T1/2 (p=0.770). Additionally, there was no significant difference in emptying via scintigraphy for short versus long-acting formulations (p=0.076). Univariable regression assessing the effect of duration of the trial on gastric emptying also revealed no significant correlation (p=0.744) (Supplemental Table 3). This latter finding would suggest no significant GLP-1 RA tachyphylactic effect on gastric emptying by scintigraphy. Regression analysis evaluating the effect of obesity demonstrated a greater delay in gastric emptying on scintigraphy (T1/2) for patients with a BMI ≥30 kg/m2 [coefficient 31.5 (95% CI: 5.5-57.6, p=0.018)] (Supplemental Table 3).
Two studies reported outcomes of gastric emptying scintigraphy measures at 4 hours. The RCT by Jensterle et al. using weekly semaglutide noted a 4-hour gastric retention of 7% (IQR 0.8-14.5) before versus 37% (IQR 17-47.5) after GLP-1 RA treatment for 13 weeks. The other study by Rayner et al. reported an adjusted geometric means ratio of the AUC of 2.19 (95% CI: 1.82-2.64) for the lixisenatide group relative to the placebo group. Overall, there were substantial variations in the reporting of outcome measures, and the studies demonstrated heterogenous effects of GLP-1 RA on solid-phase gastric emptying by scintigraphy at 4 hours.
Acetaminophen Absorption-Based Measurement of Gastric Emptying
Nineteen studies reported outcomes of gastric emptying measures by AAT (Table 2), with 18 RCT or randomized crossover trials and 1 prospective interventional trial. Of these, six studies (all randomized) utilized the most commonly reported measure, Tmax, reflecting 284 patients (GLP-1 RA n=143, placebo n=141) with a mean age of 45.5±8.6 years, BMI of 29.9±3.9 kg/m2, and lower prevalence of female (36.6%). On meta-analysis, no significant difference in gastric emptying was demonstrated between GLP-1 RA and placebo, with a pooled mean difference in Tmax of 1.6 minutes (95% CI: −2.4-5.5, I2=0.0%, Q-value=3.2, p=0.432) (Figure 3) and a pooled standardized mean difference of 0.1 (95% CI: −0.1-0.4, I2=0.0%, Q-value=2.3, p=0.230) (Supplemental Figure 3). High levels of heterogeneity were again noted and are summarized in Table 4.
Table 2:
Overview of Studies Measuring Gastric Emptying by Acetaminophen Absorption Test
| Study | Year | Study Region |
Study Design |
GLP-1 Indication |
Medication | N | Age (SD) | BMI (SD, IQR) | Primary emptying measure |
AUC at 4 hours |
|---|---|---|---|---|---|---|---|---|---|---|
| Degn | 2004 | US | Crossover trial | DM | Liraglutide | 26 | 56.4 (9.2) | Not reported | Tmax | Yes |
| Frandsen | 2017 | Europe | RCT | DM | Liraglutide | 17 | 36 (9) | 24.8 (2.8) | Tmax | Yes |
| Hermansen | 2013 | Europe | RCT | DM | Liraglutide | 40 | 62.7 (IQR 53.0-73.0) | 30.8 (IQR 24.2-39.4) | AUC at 1, 8 hrs, Cmax | No |
| Kolterman | 2005 | US | RCT | DM | Exenatide | 16 | 52 (8) | 28.6 (5.0) | AUC at 2 hours, Cmax | No |
| Flint | 2011 | US | Crossover trial | DM | Liraglutide | 36 | 58.6 (6.9) | 29.7 (4.2) | AUC at 1, 5 hrs, Cmax | No |
| Ghazi | 2014 | US | Prospective cohort | DM | Exenatide | 34 | Not reported | Not reported | AUC at 5 hrs | No |
| Langeskov; Hjerpsted * | 2022 | US | Crossover trial | Healthy volunteers | Semaglutide | 56 | 42 (IQR 21-65) | 33.2 (IQR 30.5-42.8) | T1/2, AUC at 1, 5 hrs | No |
| Dejgaard | 2016 | Europe | RCT | DM, Weight loss | Liraglutide | 100 | 47 (13) | 30.3 (3.5) | Tmax | Yes |
| Kapitza | 2011 | US | Crossover trial | DM | Liraglutide | 36 | 59 (7) | 29.7 (4.2) | AUC at 8 hrs | No |
| Saxena * | 2021 | US | RCT | Weight loss | Liraglutide | 61 | 43 (10.9) | 34.18 (2.62) | AUC at 1, 6 hrs | No |
| Friedrichsen | 2021 | Europe | RCT | Weight loss | Semaglutide | 72 | 40.7 (12.2) | 34.2 (3.0) | Tmax, AUC at 1, 5 hrs | No |
| Defronzo * | 2008 | US | Crossover trial | DM | Exenatide | 61 | 54 (9) | 32.6 (5.1) | AUC at 4 hrs | Yes |
| Cersosimo | 2011 | US | Prospective cohort | DM | Exenatide | 17 | 43 (2) | 33.8 (1.5) | AUC at 6 hrs | No |
| Urva * | 2020 | US | RCT | Healthy volunteers, DM | Dulaglutide, Tirzepatide | 15 | Healthy: 40.3 (10.9); DM: 56.8 (6.9) | Healthy: 24.3 (2.7) ; DM 31.2 (4) | Tmax | No |
| Dahl | 2021 | Europe | Crossover trial | DM | Semaglutide | 30 | 58.2 (9.8) | 30.8 (2.4) | Tmax | No |
| Tonneijck * | 2018 | Europe | RCT | DM | Lixisenatide | 34 | 62 (7) | 30.6 (IQR 27.5-35.6) | Tmax, AUC at 3 hrs | No |
| Becker | 2015 | Europe | Crossover trial | Healthy volunteers | Lixisenatide | 40 | 31.0 (7.3) | 22.8 (2.7) | Tmax | No |
| Dupre | 2004 | Canada | Crossover trial | DM | Exenatide | 8 | 4.3 (4.7) | 25.7 (1.2) | AUC at 1 hr | No |
| Kolterman | 2003 | US | RCT | DM | Exenatide | 40 | 55.8 (2.1) | 28.8 (0.8) | AUC at 1, 2, 3, 5 hrs | No |
Indicates study was not included in quantitative meta-analyses due to inadequate reporting of measure details or measure reported not being comparable to other studies
Figure 3:
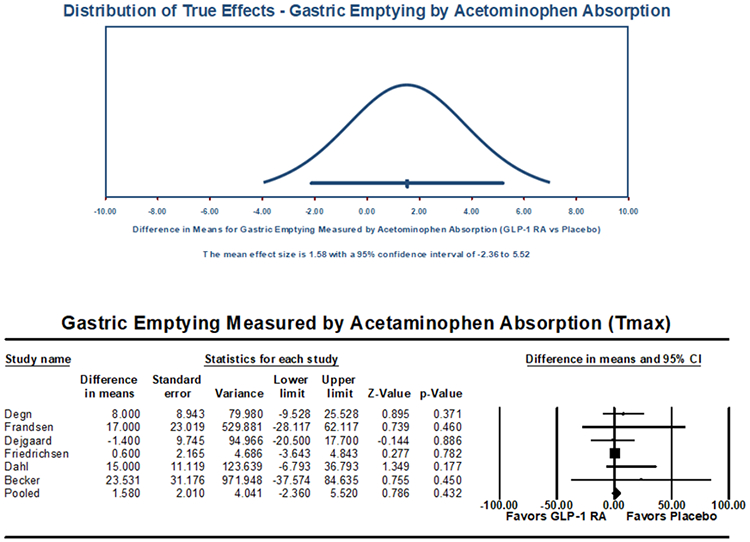
Acetaminophen Absorption-Based Measurement of Gastric Emptying Primary Outcome (Tmax, minutes), Pooled Mean Difference
One study removed sensitivity analysis revealed no overinfluence by any individual study on the overall effect estimate (Supplemental Figure 4). When univariable meta-regression was performed to evaluate the impact of specific GLP-1 RA formulation with respective to gastric emptying via AAT, there was no significant association between GLP-1 RA formulations and Tmax (p=0.675) (Supplemental Table 2). Short versus long acting GLP-1 RA medications did not impact emptying (p=0.481). Univariable meta-regression analyses revealed no significant effect on the rate of emptying by trial duration (p=0.143) or BMI (p=0.326).
Eight studies (n=355) included outcomes of gastric emptying estimates at 4 or 5 hours on AAT. There were no significant differences in gastric emptying between GLP-1 RA and placebo as measured by the area under the curve (AUC) of acetaminophen absorption at 4 hours [7.6 μmol*hr/L (95% CI: −29.4-44.5; I2=58.0%, Q-value=7.1, p=0.688)] or 5 hours [−38.3 μmol*hr/L (95% CI: −13.0-6.42; I2=90.1%, Q-value=30.3, p=0.164)] (Figure 4). Five studies (n=194) reported outcomes of gastric emptying at 1 hour on AAT. A small, statistically significant decrease in AUC was noted with GLP-1 RA compared to placebo, with a pooled mean difference of [−16.3 μmol*hr/L (95% CI: −24.4 to −8.2; I2=42.2%, Q-value=6.9, p<0.001)] (Figure 4). The pooled standardized mean difference in AUC again showed a small decrease in acetaminophen absorption at 1 hour, but no significant delay at 4 or 5 hours for GLP-1 RA (Supplemental Figure 5).
Figure 4:
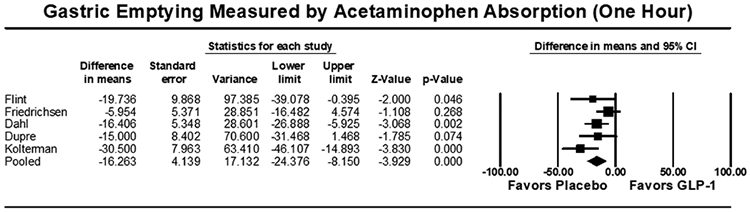
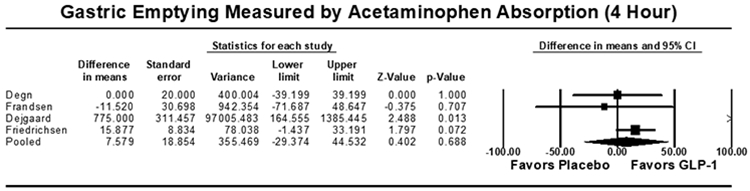
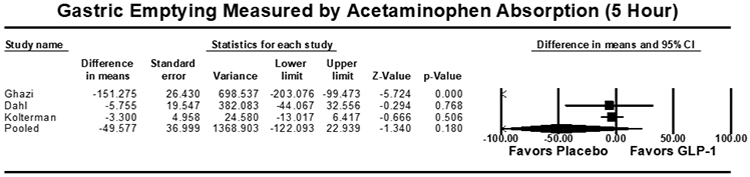
Acetaminophen Absorption Studies Secondary Outcome [Area Under the Curve (AUC, μmol*hr/L)], Pooled Mean Difference. (A) AUC at 1 hour, (B) AUC at 4 hours, (C) AUC at 5 hours
Stable Isotope Breath Test Measurement of Gastric Emptying
Five studies included outcomes of gastric emptying assessment on stable isotope breath testing, although heterogeneity in reported outcome measures limited our ability to perform meta-analysis (Table 3A). All studies were RCT or randomized crossover studies, with low risk of bias. Of these, four studies reported T1/2 as the gastric emptying outcome on breath testing (Table 3A). One RCT of cotadutide with a 49-day treatment duration did not find a significant difference in T1/2 compared to placebo50. Two studies (one crossover trial using lixisenatide and one prospective trial using exenatide) demonstrated T1/2 delays of approximately 80 minutes by GLP-1 RA agents52,53, while the RCT by Lorenz et al. using lixisenatide for 28 days found a larger difference of nearly 200 minutes compared to the placebo49. No studies reported gastric emptying measures at 4 hours. Overall gastric emptying delay outcomes were mixed using breath testing as the modality of assessment with the largest delays noted with lixisenatide.
Table 3A:
Overview of Studies Measuring Gastric Emptying by Stable Isotope Breath Test
| Study | Year | Study Region |
Study Design |
GLP-1 Indication |
Medication | N | Age (SD) | BMI (SD, IQR) | Primary emptying measure |
Emptying at 4 hours |
|---|---|---|---|---|---|---|---|---|---|---|
| Lorenz | 2013 | Europe | RCT | DM | Lixisenatide | 43 | 53.7 (7.1) | 31.4 (4.1) | t1/2 | No |
| Parker | 2020 | US | RCT | DM | Cotadutide | 22 | 61.9 (6.0) | 31.1 (3.5) | t1/2 | No |
| Inaishi | 2022 | Asia | Crossover trial | DM | Exenatide | 29 | 55 (12) | 33.3 (10.6) | tmax | No |
| Meier | 2019 | Europe | Crossover trial | DM | Lixisenatide | 44 | Lixisenatide 1st: 61 (6) ; Insulin 1st: 57 (11) | 30.4 (3.5); 31.5 (3.8) | t1/2 | No |
| Beti | 2018 | Europe | Prospective cohort | DM | Exenatide | 30 | Baseline normal emptying group: 50.6 (11.9); baseline delayed emptying group: 59.5 (8.2) | 41.5 (9.7); 36.8 (5.6) | t1/2 | No |
Wireless Motility Capsule Gastric Transit Time
Two studies were included that utilized the wireless motility capsule to assess gastric emptying time (Table 3B). One study by Nakatani et al. was a prospective crossover study which included 14 patients. The other study by Wegeberg was a RCT which included a total of 39 patients. Both studies utilized liraglutide with a trial duration of 3 weeks or longer. Neither study showed a significant difference in gastric transit time on liraglutide.
Table 3B:
Overview of Studies Measuring Gastric Emptying by Wireless Motility Capsule
Retained Gastric Contents on Esophagogastroduodenoscopy
Three studies evaluated the effect of GLP-1 RA on retained gastric contents during EGD (Table 3C). All were retrospective cohort studies that included a total of 297 patients on GLP-1 RA therapy and 694 controls who had not received GLP-1 RA. Two studies demonstrated a significant difference in retention of gastric contents on EGD, ranging from ~5% to 24% in the GLP-1 RA group compared to 0.5-5% in the placebo group, while one study showed no significant difference between treatment groups. There was notable heterogeneity in the definition of the primary outcome of retained gastric contents, ranging from “food retention” to a combination of solids and predefined fluid volumes in the suction canister documented on endoscopy reports. Moreover, the presence of retained gastric content was generally reported as dichotomized outcomes that may have included residue volume of unclear clinical significance, and the data was obtained from review of written endoscopy report, which may serve as a source of potential bias.
Table 3C:
Overview of Studies Measuring Gastric Emptying by Retention of Gastric Contents on Endoscopy
| Study | Year | Study Region |
Study Design | GLP-1 Indication |
Medication | N (GLP) |
Age (SD) | BMI (SD, IQR) |
Primary emptying measure |
|---|---|---|---|---|---|---|---|---|---|
| Silveira | 2023 | US | Retrospective cohort | Unspecified | Semaglutide | 33 | Not reported | Not reported | Residual gastric content on endoscopy (solid content in esophagus or stomach, or >0.8 mL/Kg of fluid in suction canister) |
| Kobori | 2023 | Asia | Retrospective cohort | Unspecified | Unspecified | 205 | 70 (NR) | Not reported | Gastric residue on endoscopy defined as solids in stomach |
| Stark | 2022 | US | Retrospective cohort | Unspecified | Unspecified | 59 | 64 (10) | 33 (6) | Documented food retention on endoscopy report |
Study Quality and Risk of Bias Assessment
All studies including randomized trials (RoB 2) and non-randomized studies (ROBINS-I) were assessed for quality and risk of bias. Among those included for meta-analyses, all were high quality with low risk of bias (Supplemental Figure 6A-C). Publication bias was also assessed, with visual inspection of the funnel plot for scintigraphy and AAT studies. There was no evidence of publication bias for scintigraphy studies, although mild asymmetry was noted on funnel plot review for AAT studies. Egger testing was performed and revealed no evidence of significant publication bias (scintigraphy studies: p=0.200 and AAT studies: p=0.056) (Figure 5A-B). Given the funnel plot asymmetry for AAT studies, the Duval and Tweedie’s trim and fill method was applied with 3 studies trimmed, although no significant differences in outcomes were noted [0.9 minutes (95% CI: −3.0-4.8)] (Supplemental Figure 7).
Figure 5A:
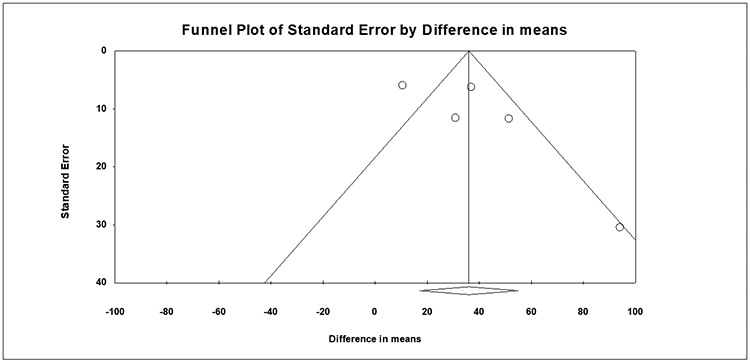
Funnel Plot Assessment of Publication Bias, Gastric Emptying study (Scintigraphy)
Figure 5B:
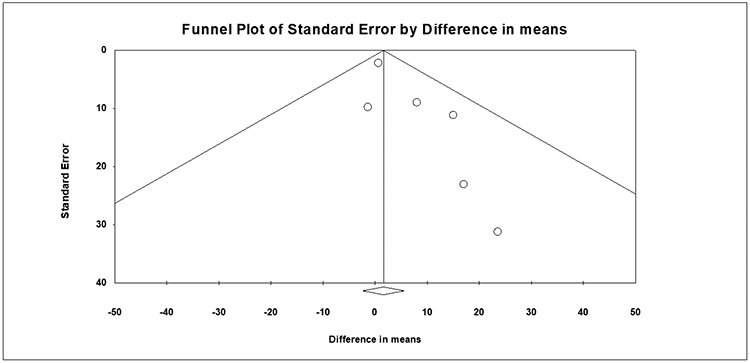
Funnel Plot Assessment of Publication Bias, Acetaminophen Absorption Studies
DISCUSSION
Agonists for receptors of the incretin hormone GLP-1 are increasingly used for the treatment of type 2 diabetes mellitus and obesity. These agents achieve glycemic control and weight reduction through a variety of mechanisms, including impact on pancreatic islet cells and insulin production56, centrally-mediated appetite/satiety modulation57, and effects on the motility and accommodation of the gastrointestinal tract58. The clinical impact of GLP-1 RA agents on the symptoms and function of the gastrointestinal tract has been described, particularly with regards to gut motility and transit. In particular, concerns for delayed gastric emptying associated with GLP-1 RA agents use have led to debates on the optimal periprocedural care when anesthesia or sedation is needed. While altered gastric motility was described with GLP-1 RA, the overall magnitude and impact of the delayed emptying has not been well quantified across agents and published clinical trials. To our knowledge, this meta-analysis and systematic review represents the first effort to provide pooled, quantified measures of gastric emptying associated with GLP-1 RA, with particular implications on periprocedural management and dietary recommendations.
Our study found that while GLP-1 RA was associated with longer T1/2 on scintigraphy than placebo, the magnitude of delay was relatively modest (approximately 36 minutes), especially when compared to the typical recommended durations of pre-anesthesia fasting (usually 6-8 hours). Two published clinical trials performed the 4-hour scintigraphy, with only one reporting the percent retention at 4 hours - the current gold standard in radiographic measure of gastric emptying. In this particular study, mild-to-moderate retention (37%) of the solid test meal was noted. Systematic review of stable isotope breath testing revealed heterogenous findings, ranging from no difference to 80-200 minutes of delay in T1/2, with the longest delay associated with lixisenatide49-53. Studies using a wireless motility capsule did not demonstrate significant delays in gastric transit time with both short and long-acting liraglutide formulations54,55.
AAT was the most reported method for gastric emptying assessment due to its widespread use in clinical trials for pharmacokinetic evaluation. We did not find significant differences in time to peak acetaminophen absorption (Tmax) between GLP-1 RA and placebo on AAT. Using the AUC analyses for acetaminophen absorption, a minor decrease of questionable clinical significance was noted with GLP-1 RA at 1 hour, but this was no longer significant at 4 or 5 hours. There are important considerations in interpretating these results, as the AAT depend on both gastric emptying and intestinal absorption, and are more reflective of liquid, rather than solid, phase emptying59,60. While scintigraphy remains the gold standard diagnostic test, acetaminophen absorption is a widely used surrogate assessment of liquid phase emptying in pharmacokinetic studies61. Our results would, therefore, suggest no clinically significant delay in liquid phase gastric emptying with GLP-1 RA use, especially at time points relevant to pre-procedural fasting, despite mildly prolonged solid emptying noted on scintigraphy. Potential decrease in gastric emptying delay by GLP-1 RA over time has also been suggested, as tachyphylaxis has been observed in some agents and endogenous GLP-128,62-65. However, our subgroup analyses revealed no significant differences in both solid phase and liquid phase gastric emptying between long-acting and short-acting GLP-1 RA agents. Moreover, meta-regression by study duration did not show significant impact on gastric emptying by treatment duration. Therefore, with regards to periprocedural management of patients taking GLP-1 RA, distinction between long- and short-acting agents and between long and short duration of use do not appear to be necessary.
Three recent retrospective studies utilized endoscopic data to report evidence of increase in retained gastric contents as primary outcomes12-14. The definitions of gastric retention varied among these studies, but they mostly reflected subjective descriptions of any retained food residue on the written procedural report, which may range from presence of small particles to more substantial contents. While gastric retention on endoscopy would theoretically raise concerns for pulmonary complications, the risk and clinical significance would likely depend on the size, consistency, and volume of the retained food. It is not clear from these retrospective chart review studies alone whether the increased presence of food residue in the stomach among GLP-1 RA users were of clinically relevant volume. Indeed, only one case of aspiration during endoscopy by a patient taking GLP-1 RA agent has been reported in the literature, who belonged to the GLP-1 RA arm of the retrospective study by Silveira et al12 – although it is unclear if retained food was noted during this patient’s endoscopy. These studies also had notable limitations with regard to confounding, with one study solely reporting unadjusted data and the other two utilizing propensity score matching, which did not fully capture the full spectrum of potential contributors to gastric dysmotility (e.g. medications affecting gastric emptying, surgical history, diabetes severity, etc.).
Among GLP-1 RA agents available in the U.S., semaglutide has been rapidly gaining popularity in recent years, with widespread use among patients for diabetes control and for weight management. Of five total studies involving semaglutide with quantitative gastric emptying assessments, three utilized the AAT, with two demonstrating mild delays in liquid emptying with semaglutide within 1-2 hours27,37,40,44. However, these mild liquid emptying delays normalized by 4-5 hours after ingestion in both studies. One study examined gastric retention using 4-hour emptying scintigraphy10, which showed increased solid phase retention of 72% and 37% at 2 and 4 hours, respectively, in the semaglutide arm. These observations for semaglutide were consistent with the findings of our primary meta-analyses, with notable, although non-severe, solid emptying delay and mild/negligible early liquid emptying delay that normalized within 4-5 hours.
Our study provided insights into two important aspects of GLP-1 RA use. First, the overall delay in gastric emptying associated with GLP-1 RA agents appears, at best, mild to moderate in magnitude, with unclear clinical significance. Prior studies of the gastroparesis population have found poor correlations between measures of gastric emptying and clinical symptoms66,67. Therefore, the adverse gastrointestinal symptoms and the therapeutic effect of weight loss associated with GLP-1 RA may be related to factors other than altered motility and transit alone. For example, GLP-1 RA may affect the appetite/satiation and neurosensory input of the gastrointestinal tract through central or vagally-mediated pathways, while regulation of insulin/glucagon release may be impacted by activation of GLP-1 receptors in the pancreatic beta-cells. Hence, management of gastrointestinal symptoms with GLP-1 RA use should leverage diverse therapeutic approaches including neuromodulation, rather than focusing on amending motor dysfunction alone.
Second, as previously mentioned, concerns for procedural pulmonary complications due to retained gastric contents has fueled cross-disciplinary debates and driven divergent consensus-based recommendations from organizations such as ASA and AGA15,16. Overall, ASA recommends a more conservative approach, including holding GLP-1 RA pre-procedure and consideration of delaying elective procedures if the medications were not held. Conversely, recent AGA practice updates do not recommend deviations from standard periprocedural precautions or stopping GLP-1 RA prior to elective endoscopy due to insufficient evidence. Current ASA preoperative fasting guidelines for healthy individuals recommend minimal fasting periods of 2 hours for clear liquids and 6-8 hours for solids68. For patients with gastroparesis, clinical judgment is advised, although in clinical practice there are typically no routine deviations from standard precautions. Our study results suggest that patients who remain on GLP-1 RA would not be at increased risk of retained liquid contents at standard procedural fasting time points. While the small magnitude of solid emptying delay found on scintigraphy is reassuring, the amount of available literature using scintigraphy assessment remains modest. Considerations for holding GLP-1 RA agents must be counterbalanced by the potential for worsening glycemic control which may also worsen gastric emptying delay in patients with diabetes. Nevertheless, the modest delay per T1/2 (~40 minutes) and 4-hour retention (~37%) would imply that avoiding solid foods for the day prior to procedure should be sufficient in preventing significant gastric retention. Therefore, based on our study results, we propose the following precaution for users of GLP-1 RA undergoing endoscopic procedures regardless of formulation or duration of use: (1) continuation of GLP-1 RA therapy, (2) liquid-only diet on the day prior to procedure, and (3) standard pre-anesthesia fasting periods.
Our study had several strengths. This comprehensive review was largely consisted of RCT and crossover-based trials. We included both short and long-acting GLP-1 RA, with representation from all current, commercially available formulations. Extensive sensitivity and subgroup analyses and meta-regression allowed thorough comparisons across different formulations, treatment durations, and testing modalities. There were also several limitations. The small number of studies utilizing some diagnostic modalities such as breath testing precluded formal meta-analysis of these subgroups. In addition, stratification in the meta-analyses by indication for GLP-1 RA (diabetes vs obesity) was also not possible due to the number of studies included. While higher doses of GLP-1 RA are typically used for weight loss indications, systematic review of studies using semaglutide for weight loss indications did not demonstrate substantial deviations from the main results. There were also few studies that employed the optimal scintigraphy test per current consensus guidelines (4-hour study to estimate % retention). Lastly, AAT is a surrogate measure of gastric emptying that also depends on intestinal absorption, although it is widely used and still valuable for liquid phase emptying.
In conclusion, we found a mild gastric emptying delay (~36 minutes per T1/2) on solid phase scintigraphy and no significant differences on modalities reflective of liquid emptying with GLP-1 RA use. No evidence of tachyphylaxis by duration of use or formulation (long vs short acting) was noted. Our findings do not support withholding GLP-1 RA agents in patients anticipated to remain on a liquid diet prior to procedure. While our results demonstrated modest delays relative to the 8 hour pre-procedural fasting periods on solid phase testing, more studies quantifying gastric retention on scintigraphy at 4 hours are needed. Based on current evidence, a conservative approach with liquid diet on the day prior to procedures while continuing GLP-1 RA therapy would represent the most sensible approach until more conclusive data on solid diet is available.
Supplementary Material
Supplemental Table 1: PRISMA Checklist
Supplemental Table 2: Search Strategy
Supplemental Table 3: Univariable Meta-Regression Outcomes
Supplemental Figure 1: Gastric Emptying Study (Scintigraphy) Primary Outcome (T1/2), Pooled Standardized Mean Difference
Supplemental Figure 2: Sensitivity Analysis for Scintigraphy studies, One Study Removed Analysis
Supplemental Figure 3: Acetaminophen Absorption Studies Primary Outcome (Tmax), Pooled Standardized Mean Difference
Supplemental Figure 4: Sensitivity Analysis for Acetaminophen Absorption Studies (Tmax), One Study Removed Analysis
Supplemental Figure 5: Acetaminophen Absorption Studies Secondary Outcome [Area Under the Curve (AUC)], Pooled Standardized Mean Difference. (A) AUC at 1 hour, (B) AUC at 4 hours, (C) AUC at 5 hours
Supplemental Figure 6: (A) Risk of Bias for Included Scintigraphy Studies with Revised Cochrane Risk-of-Bias Tool for Randomized Trials. (B) Risk of Bias for Included Acetaminophen Absorption Studies with Revised Cochrane Risk-of-Bias Tool for Randomized Trials. (C) Risk of Bias for Included Acetaminophen Absorption Studies with Risk of Bias In Non-randomized Studies - of Interventions (ROBINS-I) Tool for Non-Randomized Studies
Supplemental Figure 7: Assessment of Publication Bias, Trim-and-Fill Method
Funding:
BH is supported by the NIH/NIDDK award 1T32DK135449-01.
Footnotes
Potential Conflicts of Interest:
Walter Chan served on the advisory board for Phathom Pharmaceuticals, Sanofi Pharmaceuticals, and Regeneron Pharmaceuticals. No other authors have potential conflicts of interest to disclose.
All authors approve of final submission.
Data Availability:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Ussher JR, Drucker DJ. Glucagon-like peptide 1 receptor agonists: cardiovascular benefits and mechanisms of action. Nat Rev Cardiol. Jul 2023;20(7):463–474. doi: 10.1038/s41569-023-00849-3 [DOI] [PubMed] [Google Scholar]
- 2.Sattar N, Lee MMY, Kristensen SL, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. Oct 2021;9(10):653–662. doi: 10.1016/S2213-8587(21)00203-5 [DOI] [PubMed] [Google Scholar]
- 3.Nogueiras R, Nauck MA, Tschöp MH. Gut hormone co-agonists for the treatment of obesity: from bench to bedside. Nat Metab. Jun 2023;5(6):933–944. doi: 10.1038/s42255-023-00812-z [DOI] [PubMed] [Google Scholar]
- 4.Elmaleh-Sachs A, Schwartz JL, Bramante CT, Nicklas JM, Gudzune KA, Jay M. Obesity Management in Adults: A Review. JAMA. Nov 28 2023;330(20):2000–2015. doi: 10.1001/jama.2023.19897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michos ED, Lopez-Jimenez F, Gulati M. Role of Glucagon-Like Peptide-1 Receptor Agonists in Achieving Weight Loss and Improving Cardiovascular Outcomes in People With Overweight and Obesity. J Am Heart Assoc. Jun 06 2023;12(11):e029282. doi: 10.1161/JAHA.122.029282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Targher G, Mantovani A, Byrne CD. Mechanisms and possible hepatoprotective effects of glucagon-like peptide-1 receptor agonists and other incretin receptor agonists in non-alcoholic fatty liver disease. Lancet Gastroenterol Hepatol. Feb 2023;8(2):179–191. doi: 10.1016/S2468-1253(22)00338-7 [DOI] [PubMed] [Google Scholar]
- 7.Sodhi M, Rezaeianzadeh R, Kezouh A, Etminan M. Risk of Gastrointestinal Adverse Events Associated With Glucagon-Like Peptide-1 Receptor Agonists for Weight Loss. JAMA. Nov 14 2023;330(18):1795–1797. doi: 10.1001/jama.2023.19574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halawi H, Khemani D, Eckert D, et al. Effects of liraglutide on weight, satiation, and gastric functions in obesity: a randomised, placebo-controlled pilot trial. Lancet Gastroenterol Hepatol. Dec 2017;2(12):890–899. doi: 10.1016/S2468-1253(17)30285-6 [DOI] [PubMed] [Google Scholar]
- 9.Jones KL, Huynh LQ, Hatzinikolas S, et al. Exenatide once weekly slows gastric emptying of solids and liquids in healthy, overweight people at steady-state concentrations. Diabetes Obes Metab. May 2020;22(5):788–797. doi: 10.1111/dom.13956 [DOI] [PubMed] [Google Scholar]
- 10.Jensterle M, Ferjan S, Ležaič L, et al. Semaglutide delays 4-hour gastric emptying in women with polycystic ovary syndrome and obesity. Diabetes Obes Metab. Apr 2023;25(4):975–984. doi: 10.1111/dom.14944 [DOI] [PubMed] [Google Scholar]
- 11.Acosta A, Camilleri M, Burton D, et al. Exenatide in obesity with accelerated gastric emptying: a randomized, pharmacodynamics study. Physiol Rep. Nov 2015;3(11)doi: 10.14814/phy2.12610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silveira SQ, da Silva LM, de Campos Vieira Abib A, et al. Relationship between perioperative semaglutide use and residual gastric content: A retrospective analysis of patients undergoing elective upper endoscopy. J Clin Anesth. Aug 2023;87:111091. doi: 10.1016/j.jclinane.2023.111091 [DOI] [PubMed] [Google Scholar]
- 13.Kobori T, Onishi Y, Yoshida Y, et al. Association of glucagon-like peptide-1 receptor agonist treatment with gastric residue in an esophagogastroduodenoscopy. J Diabetes Investig. Jun 2023;14(6):767–773. doi: 10.1111/jdi.14005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stark JE, Cole JL, Ghazarian RN, Klass MJ. Impact of Glucagon-Like Peptide-1 Receptor Agonists (GLP-1RA) on Food Content During Esophagogastroduodenoscopy (EGD). Ann Pharmacother. Aug 2022;56(8):922–926. doi: 10.1177/10600280211055804 [DOI] [PubMed] [Google Scholar]
- 15.Anesthesiologists ASo. American Society of Anesthesiologists consensus-based guidance on preoperative management of patients (adults and children) on glucagon-like peptide-1 (GLP-1) receptor agonists. https://www.asahq.org/about-asa/newsroom/news-releases/2023/06/american-society-of-anesthesiologists-consensus-based-guidance-on-preoperative [Google Scholar]
- 16.Hashash JG, Thompson CC, Wang AY. AGA Rapid Clinical Practice Update on the Management of Patients Taking GLP-1 Receptor Agonists Prior to Endoscopy: Communication. Clin Gastroenterol Hepatol. Nov 07 2023;doi: 10.1016/j.cgh.2023.11.002 [DOI] [PubMed] [Google Scholar]
- 17.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. Apr 20 2005;5:13. doi: 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gingrich RA, Awe WC, Boyden AM, Peterson CG. Cholecystostomy in acute cholecystitis. Factors influencing morbidity and mortality. Am J Surg. Aug 1968;116(2):310–5. doi: 10.1016/0002-9610(68)90509-6 [DOI] [PubMed] [Google Scholar]
- 19.Overton RC. A comparison of fixed-effects and mixed (random-effects) models for meta-analysis tests of moderator variable effects. Psychological Methods 1998: 3(3):354–79. [Google Scholar]
- 20.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. Aug 28 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 21.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. Oct 12 2016;355:i4919. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamata K, Takenaka M, Kitano M, et al. Endoscopic ultrasound-guided gallbladder drainage for acute cholecystitis: Long-term outcomes after removal of a self-expandable metal stent. World J Gastroenterol. Jan 28 2017;23(4):661–667. doi: 10.3748/wjg.v23.i4.661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dollhopf M, Larghi A, Will U, et al. EUS-guided gallbladder drainage in patients with acute cholecystitis and high surgical risk using an electrocautery-enhanced lumen-apposing metal stent device. Gastrointest Endosc. Oct 2017;86(4):636–643. doi: 10.1016/j.gie.2017.02.027 [DOI] [PubMed] [Google Scholar]
- 25.Higa JT, Sahar N, Kozarek RA, et al. EUS-guided gallbladder drainage with a lumen-apposing metal stent versus endoscopic transpapillary gallbladder drainage for the treatment of acute cholecystitis (with videos). Gastrointest Endosc. Sep 2019;90(3):483–492. doi: 10.1016/j.gie.2019.04.238 [DOI] [PubMed] [Google Scholar]
- 26.Stevens JE, Horowitz M, Deacon CF, Nauck M, Rayner CK, Jones KL. The effects of sitagliptin on gastric emptying in healthy humans - a randomised, controlled study. Aliment Pharmacol Ther. Aug 2012;36(4):379–90. doi: 10.1111/j.1365-2036.2012.05198.x [DOI] [PubMed] [Google Scholar]
- 27.Langeskov EK, Kristensen K. Population pharmacokinetic of paracetamol and atorvastatin with co-administration of semaglutide. Pharmacol Res Perspect. Aug 2022;10(4):e00962. doi: 10.1002/prp2.962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maselli D, Atieh J, Clark MM, et al. Effects of liraglutide on gastrointestinal functions and weight in obesity: A randomized clinical and pharmacogenomic trial. Obesity (Silver Spring). Aug 2022;30(8):1608–1620. doi: 10.1002/oby.23481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rayner CK, Watson LE, Phillips LK, et al. Effects of Sustained Treatment With Lixisenatide on Gastric Emptying and Postprandial Glucose Metabolism in Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care. Aug 2020;43(8):1813–1821. doi: 10.2337/dc20-0190 [DOI] [PubMed] [Google Scholar]
- 30.Jalleh R, Pham H, Marathe CS, et al. Acute Effects of Lixisenatide on Energy Intake in Healthy Subjects and Patients with Type 2 Diabetes: Relationship to Gastric Emptying and Intragastric Distribution. Nutrients. Jul 01 2020;12(7)doi: 10.3390/nu12071962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Degn KB, Juhl CB, Sturis J, et al. One week's treatment with the long-acting glucagon-like peptide 1 derivative liraglutide (NN2211) markedly improves 24-h glycemia and alpha- and beta-cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes. May 2004;53(5):1187–94. doi: 10.2337/diabetes.53.5.1187 [DOI] [PubMed] [Google Scholar]
- 32.Frandsen CS, Dejgaard TF, Andersen HU, et al. Liraglutide as adjunct to insulin treatment in type 1 diabetes does not interfere with glycaemic recovery or gastric emptying rate during hypoglycaemia: A randomized, placebo-controlled, double-blind, parallel-group study. Diabetes Obes Metab. Jun 2017;19(6):773–782. doi: 10.1111/dom.12830 [DOI] [PubMed] [Google Scholar]
- 33.Hermansen K, Bækdal TA, Düring M, et al. Liraglutide suppresses postprandial triglyceride and apolipoprotein B48 elevations after a fat-rich meal in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, cross-over trial. Diabetes Obes Metab. Nov 2013;15(11):1040–8. doi: 10.1111/dom.12133 [DOI] [PubMed] [Google Scholar]
- 34.K OG, K DD,S L, et al. Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. January/15/2005 2005;62(2)doi: 10.1093/ajhp/62.2.173 [DOI] [PubMed] [Google Scholar]
- 35.Flint A, Kapitza C, Hindsberger C, Zdravkovic M. The once-daily human glucagon-like peptide-1 (GLP-1) analog liraglutide improves postprandial glucose levels in type 2 diabetes patients. Adv Ther. Mar 2011;28(3):213–26. doi: 10.1007/s12325-010-0110-x [DOI] [PubMed] [Google Scholar]
- 36.Ghazi T, Rink L, Sherr JL, Herold KC. Acute metabolic effects of exenatide in patients with type 1 diabetes with and without residual insulin to oral and intravenous glucose challenges. Diabetes Care. 2014;37(1):210–6. doi: 10.2337/dc13-1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hjerpsted JB, Flint A, Brooks A, Axelsen MB, Kvist T, Blundell J. Semaglutide improves postprandial glucose and lipid metabolism, and delays first-hour gastric emptying in subjects with obesity. Diabetes Obes Metab. Mar 2018;20(3):610–619. doi: 10.1111/dom.13120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dejgaard TF, Frandsen CS, Hansen TS, et al. Efficacy and safety of liraglutide for overweight adult patients with type 1 diabetes and insufficient glycaemic control (Lira-1): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. Mar 2016;4(3):221–232. doi: 10.1016/S2213-8587(15)00436-2 [DOI] [PubMed] [Google Scholar]
- 39.Saxena AR, Banerjee A, Corbin KD, Parsons SA, Smith SR. Energy intake as a short-term biomarker for weight loss in adults with obesity receiving liraglutide: A randomized trial. Obes Sci Pract. Jun 2021;7(3):281–290. doi: 10.1002/osp4.486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friedrichsen M, Breitschaft A, Tadayon S, Wizert A, Skovgaard D. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating, and gastric emptying in adults with obesity. Diabetes Obes Metab. Mar 2021;23(3):754–762. doi: 10.1111/dom.14280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin. Oct 2008;24(10):2943–52. doi: 10.1185/03007990802418851 [DOI] [PubMed] [Google Scholar]
- 42.Cersosimo E, Gastaldelli A, Cervera A, et al. Effect of exenatide on splanchnic and peripheral glucose metabolism in type 2 diabetic subjects. J Clin Endocrinol Metab. Jun 2011;96(6):1763–70. doi: 10.1210/jc.2010-2146 [DOI] [PubMed] [Google Scholar]
- 43.Urva S, Coskun T, Loghin C, et al. The novel dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 (GLP-1) receptor agonist tirzepatide transiently delays gastric emptying similarly to selective long-acting GLP-1 receptor agonists. Diabetes Obes Metab. Oct 2020;22(10):1886–1891. doi: 10.1111/dom.14110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dahl K, Brooks A, Almazedi F, Hoff ST, Boschini C, Baekdal TA. Oral semaglutide improves postprandial glucose and lipid metabolism, and delays gastric emptying, in subjects with type 2 diabetes. Diabetes Obes Metab. Jul 2021;23(7):1594–1603. doi: 10.1111/dom.14373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tonneijck L, Muskiet MHA, Twisk JW, et al. Lixisenatide Versus Insulin Glulisine on Fasting and Postbreakfast Systemic Hemodynamics in Type 2 Diabetes Mellitus Patients. Hypertension. Aug 2018;72(2):314–322. doi: 10.1161/HYPERTENSIONAHA.117.10740 [DOI] [PubMed] [Google Scholar]
- 46.Becker RH, Stechl J, Steinstraesser A, Golor G, Pellissier F. Lixisenatide reduces postprandial hyperglycaemia via gastrostatic and insulinotropic effects. Diabetes Metab Res Rev. Sep 2015;31(6):610–8. doi: 10.1002/dmrr.2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dupré J, Behme MT, McDonald TJ. Exendin-4 normalized postcibal glycemic excursions in type 1 diabetes. J Clin Endocrinol Metab. Jul 2004;89(7):3469–73. doi: 10.1210/jc.2003-032001 [DOI] [PubMed] [Google Scholar]
- 48.Kolterman OG, Buse JB, Fineman MS, et al. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab. Jul 2003;88(7):3082–9. doi: 10.1210/jc.2002-021545 [DOI] [PubMed] [Google Scholar]
- 49.Lorenz M, Pfeiffer C, Steinsträsser A, et al. Effects of lixisenatide once daily on gastric emptying in type 2 diabetes--relationship to postprandial glycemia. Regul Pept. Aug 10 2013;185:1–8. doi: 10.1016/j.regpep.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 50.Parker VER, Robertson D, Wang T, et al. Efficacy, Safety, and Mechanistic Insights of Cotadutide, a Dual Receptor Glucagon-Like Peptide-1 and Glucagon Agonist. J Clin Endocrinol Metab. Mar 01 2020;105(3)doi: 10.1210/clinem/dgz047 [DOI] [PubMed] [Google Scholar]
- 51.Inaishi J, Saisho Y, Watanabe Y, et al. Changes in glycemic variability, gastric emptying and vascular endothelial function after switching from twice-daily to once-weekly exenatide in patients with type 2 diabetes: a subpopulation analysis of the twin-exenatide study. BMC Endocr Disord. Jan 11 2022;22(1):20. doi: 10.1186/s12902-022-00932-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meier JJ, Menge BA, Schenker N, et al. Effects of sequential treatment with lixisenatide, insulin glargine, or their combination on meal-related glycaemic excursions, insulin and glucagon secretion, and gastric emptying in patients with type 2 diabetes. Diabetes Obes Metab. Apr 2020;22(4):599–611. doi: 10.1111/dom.13935 [DOI] [PubMed] [Google Scholar]
- 53.Beti C, Stratmann B, Bokman G, et al. Exenatide Delays Gastric Emptying in Patients with Type 2 Diabetes Mellitus but not in Those with Gastroparetic Conditions. Horm Metab Res. Apr 2019;51(4):267–273. doi: 10.1055/a-0818-6374 [DOI] [PubMed] [Google Scholar]
- 54.Nakatani Y, Maeda M, Matsumura M, et al. Effect of GLP-1 receptor agonist on gastrointestinal tract motility and residue rates as evaluated by capsule endoscopy. Diabetes Metab. Oct 2017;43(5):430–437. doi: 10.1016/j.diabet.2017.05.009 [DOI] [PubMed] [Google Scholar]
- 55.Wegeberg AL, Hansen CS, Farmer AD, et al. Liraglutide accelerates colonic transit in people with type 1 diabetes and polyneuropathy: A randomised, double-blind, placebo-controlled trial. United European Gastroenterol J. Jul 2020;8(6):695–704. doi: 10.1177/2050640620925968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boer GA, Holst JJ. Incretin Hormones and Type 2 Diabetes-Mechanistic Insights and Therapeutic Approaches. Biology (Basel). Dec 16 2020;9(12)doi: 10.3390/biology9120473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shah M, Vella A. Effects of GLP-1 on appetite and weight. Rev Endocr Metab Disord. Sep 2014;15(3):181–7. doi: 10.1007/s11154-014-9289-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deane AM, Nguyen NQ, Stevens JE, et al. Endogenous glucagon-like peptide-1 slows gastric emptying in healthy subjects, attenuating postprandial glycemia. J Clin Endocrinol Metab. Jan 2010;95(1):215–21. doi: 10.1210/jc.2009-1503 [DOI] [PubMed] [Google Scholar]
- 59.Marathe CS, Rayner CK, Jones KL, Horowitz M. Relationships between gastric emptying, postprandial glycemia, and incretin hormones. Diabetes Care. May 2013;36(5):1396–405. doi: 10.2337/dc12-1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. J Nucl Med Technol. Mar 2008;36(1):44–54. doi: 10.2967/jnmt.107.048116 [DOI] [PubMed] [Google Scholar]
- 61.Camilleri M, Kuo B, Nguyen L, et al. ACG Clinical Guideline: Gastroparesis. Am J Gastroenterol. Aug 01 2022;117(8):1197–1220. doi: 10.14309/ajg.0000000000001874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Umapathysivam MM, Lee MY, Jones KL, et al. Comparative effects of prolonged and intermittent stimulation of the glucagon-like peptide 1 receptor on gastric emptying and glycemia. Diabetes. Feb 2014;63(2):785–90. doi: 10.2337/db13-0893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodbard HW, Lingvay I, Reed J, et al. Semaglutide Added to Basal Insulin in Type 2 Diabetes (SUSTAIN 5): A Randomized, Controlled Trial. J Clin Endocrinol Metab. Jun 1 2018;103(6):2291–2301. doi: 10.1210/jc.2018-00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nauck MA, Kemmeries G, Holst JJ, Meier JJ. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes. May 2011;60(5):1561–5. doi: 10.2337/db10-0474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. Oct 04 2008;372(9645):1240–50. doi: 10.1016/S0140-6736(08)61206-4 [DOI] [PubMed] [Google Scholar]
- 66.Jalleh RJ, Jones KL, Rayner CK, Marathe CS, Wu T, Horowitz M. Normal and disordered gastric emptying in diabetes: recent insights into (patho)physiology, management and impact on glycaemic control. Diabetologia. Dec 2022;65(12):1981–1993. doi: 10.1007/s00125-022-05796-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parkman HP, Van Natta ML, Maurer AH, et al. Postprandial symptoms in patients with symptoms of gastroparesis: roles of gastric emptying and accommodation. Am J Physiol Gastrointest Liver Physiol. Jul 1 2022;323(1):G44–G59. doi: 10.1152/ajpgi.00278.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Practice Guidelines for Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration: Application to Healthy Patients Undergoing Elective Procedures: An Updated Report by the American Society of Anesthesiologists Task Force on Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration. Anesthesiology. Mar 2017;126(3):376–393. doi: 10.1097/ALN.0000000000001452 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: PRISMA Checklist
Supplemental Table 2: Search Strategy
Supplemental Table 3: Univariable Meta-Regression Outcomes
Supplemental Figure 1: Gastric Emptying Study (Scintigraphy) Primary Outcome (T1/2), Pooled Standardized Mean Difference
Supplemental Figure 2: Sensitivity Analysis for Scintigraphy studies, One Study Removed Analysis
Supplemental Figure 3: Acetaminophen Absorption Studies Primary Outcome (Tmax), Pooled Standardized Mean Difference
Supplemental Figure 4: Sensitivity Analysis for Acetaminophen Absorption Studies (Tmax), One Study Removed Analysis
Supplemental Figure 5: Acetaminophen Absorption Studies Secondary Outcome [Area Under the Curve (AUC)], Pooled Standardized Mean Difference. (A) AUC at 1 hour, (B) AUC at 4 hours, (C) AUC at 5 hours
Supplemental Figure 6: (A) Risk of Bias for Included Scintigraphy Studies with Revised Cochrane Risk-of-Bias Tool for Randomized Trials. (B) Risk of Bias for Included Acetaminophen Absorption Studies with Revised Cochrane Risk-of-Bias Tool for Randomized Trials. (C) Risk of Bias for Included Acetaminophen Absorption Studies with Risk of Bias In Non-randomized Studies - of Interventions (ROBINS-I) Tool for Non-Randomized Studies
Supplemental Figure 7: Assessment of Publication Bias, Trim-and-Fill Method
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.