Summary
B and T cells collaborate to drive autoimmune disease (AID). Historically, B- and T-cell (B–T cell) co-interaction was targeted through different pathways such as alemtuzumab, abatacept, and dapirolizumab with variable impact on B-cell depletion (BCD), whereas the majority of patients with AID including rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, and organ transplantation benefit from targeted BCD with anti-CD20 monoclonal antibodies such as rituximab, ocrelizumab, or ofatumumab. Refractory AID is a significant problem for patients with incomplete BCD with a greater frequency of IgD−CD27+ switched memory B cells, CD19+CD20− B cells, and plasma cells that are not directly targeted by anti-CD20 antibodies, whereas most lymphoid tissue plasma cells express CD19. Furthermore, B–T-cell collaboration is predominant in lymphoid tissues and at sites of inflammation such as the joint and kidney, where BCD may be inefficient, due to limited access to key effector cells. In the treatment of cancer, chimeric antigen receptor (CAR) T-cell therapy and T-cell engagers (TCE) that recruit T cells to induce B-cell cytotoxicity have delivered promising results for anti-CD19 CAR T-cell therapies, the CD19 TCE blinatumomab and CD20 TCE such as mosunetuzumab, glofitamab, or epcoritamab. Limited evidence suggests that anti-CD19 CAR T-cell therapy may be effective in managing refractory AID whereas we await evaluation of TCE for use in non-oncological indications. Therefore, here, we discuss the potential mechanistic advantages of novel therapies that rely on T cells as effector cells to disrupt B–T-cell collaboration toward overcoming rituximab-resistant AID.
Keywords: systemic lupus erythematosus, rheumatoid arthritis, rituximab, CAR T-cell therapy, T-cell engagers
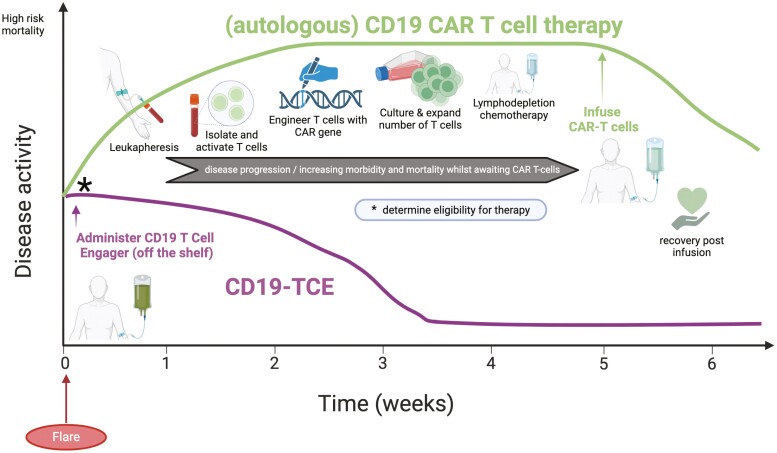
This graphical abstract compares the hypothetical timelines of CD19 CAR T-cell therapy (green line) and CD19-TCE (purple line) in the context of autoimmune disease flare. CAR T-cell therapy is often limited to specialized centers due to its high-production costs, labor-intensive process, delayed effector T-cell pool establishment, and tolerability issues with prerequisite toxic lymphodepleting chemotherapy, potentially restricting its use and impacting patient outcomes. In contrast, CD19-TCB, readily available and immediately deployable upon disease flare detection, mitigates the risks associated with disease progression due to delays in CAR T-cell therapy access.
Graphical Abstract
Graphical Abstract.
Introduction
B–T-cell collaboration in the pathogenesis of autoimmune disease
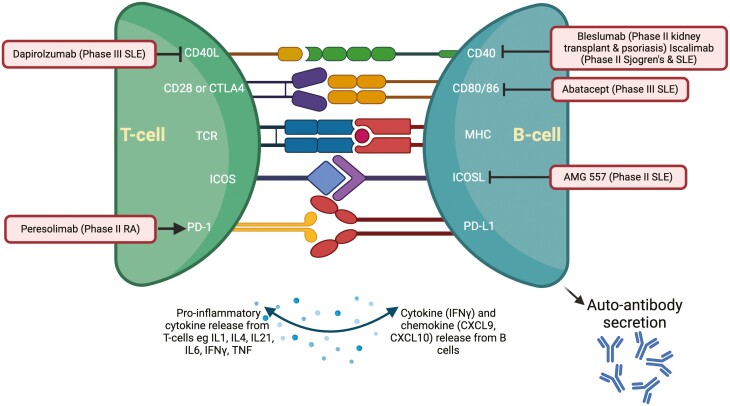
B- and T-cell (B–T-cell) collaboration perpetuates chronic inflammation in a range of autoimmune diseases (AID) including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and multiple sclerosis (MS) [1, 2]. This cellular collaboration may occur through contact-dependent or -independent pathways through cytokines and other immune stimuli. Within lymphoid aggregates and the germinal center, B–T-cell interactions involve an array of molecular pairings [3], summarized in Fig. 1 and Table 1. These signals stimulate T-cell secretion of cytokines and promote differentiation of naïve to memory B cells and plasma cells (PCs), Fig. 1. Some of these pathways have been targeted, as discussed later, whereas others are the subject of novel therapeutic strategies.
Figure 1.
Pathways of B–T-cell co-stimulation and trials of therapeutic agents. Molecular pairings are explained in Table 1. Drugs that target co-stimulation are outlined here. Dapirolizumab is an anti-CD40L mAb, currently in phase III study in SLE (NCT04294667). Bleslumab is an IgG4 mAb that targets CD40 which underwent phase II trial in plaque psoriasis with no clinical improvement compared to placebo [4], and demonstrated non-inferiority compared with standard of care for acute rejection in renal transplant recipients [5]. Iscalimab is another anti-CD40 mAb which is undergoing phase II trial in SLE and Sjogren’s Syndrome (NCT03656562, NCT04541589). Abatacept inhibits CD80/86 to prevent engagement with CD28 and is approved for use in RA but failed to meet the primary endpoint in the lupus nephritis phase III trial. AMG 557, anti-ICOSL antibody, underwent phase II trial in SLE and a newer therapy inhibiting ICOSL and BAFF is undergoing phase II trial (NCT04058028). PD-1 agonist, Peresolimab demonstrated modest improvement in disease activity in a phase II trial for patients with RA. Image created using Biorender.com
Table 1.
Overview of CD antigens and other molecules involved in B- and T-cell collaboration along with their function/utility
| Marker (± ligand/receptor) | Meaning/function/application |
|---|---|
| CD3 (TCR) | T-cell activation signaling and regulation of TCR expression |
| CD4 (MHC II) | T-helper cell |
| CD8 (MHC I) | Cytotoxic T cell |
| CD19 (co-receptor for BCR) | Pan B cell marker. Regulates B-cell development, activation, and differentiation |
| CD20 | B-cell activation and proliferation. Also present on a minority of T cells |
| CD27 (CD70) | Marker of B- and T-cell memory |
| CD28 (CD80/86) | Co-stimulation between B and T cells |
| CD40 (CD40L) | Co-stimulation between B and T cells |
| BAFF-R (BAFF) or BLyS | B-cell activating factor enhances B-cell survival |
| PD-1 (PD-L1 and PD-L2) | Programmed cell death, down-regulates the immune response |
| CXCL-10 (CXCR3) | Recruitment of monocytes, T cells, NK cells |
| CXCL-13 (CXCR5) | B-cell chemoattractant |
| CCR2 (CCL-2 also known as MCP-1) | Trafficking of monocytes to inflammatory sites |
| ICOS-ICOSL | ICOS part of the CD28 superfamily, provides co-stimulatory signal to activated T cells upon binding to ICOS-L |
| IL21-IL21R | Promotes proliferation and function of T and B cells, enhances cytotoxicity of CD8+ T cells and NK cells |
| TCR-MHCII | MHC displays peptides to the TCR, and TCR can discriminate foreign from self-peptides |
CXCL: CXC chemokine ligand; CCR: C-C motif chemokine receptor; ICOS, MCP: monocyte chemoattractant protein; MHC: major histocompatibility complex; TCR: T-cell receptor.
In this context of an ongoing immune response, an appreciation of B-cell biology is helpful. B cells originate from hematopoietic stem cells in the bone marrow and undergo differentiation in secondary lymphoid organs [6]. Differential expression of various cell surface markers, including cluster of differentiation (CD) molecules and immunoglobulin isotypes help to define classical subpopulations including naïve B cells (IgD+CD27−), unswitched memory B cells (IgD+CD27+), switched memory B cells (IgD−CD27+) and double negative memory B cells (IgD−CD27−) [6]. Naïve B cells have not yet encountered antigen, whereas switched memory B cells are primed to respond to antigen and double negative memory B cells increase with aging, autoimmunity, and chronic infectious diseases [7]. Until recently, the focus of B-cell depletion therapy has been on rituximab, an anti-CD20 monoclonal antibody that is widely used in hematological malignancies and AID (discussed in more detail below). The first FDA approved targeted biologic therapy for SLE was Belimumab, a mAb directed at B-cell activating factor (BAFF, also known as BLyS) [8], however, real-world data demonstrates variable success [9, 10]. BAFF is a B-cell survival and differentiation factor and is elevated in the serum of patients with SLE [11].
B–T-cell interactions in the peripheral inflammatory sites of various AID including RA SLE, type I diabetes mellitus, and celiac disease exhibit a population of T cells which are termed T-peripheral helper cells [1, 12, 13]. Rao et al. identified these cells, adjacent to B cells in lymphoid aggregates of the synovium in patients with RA as PD-1hiCXCR5−CD4+ which lack Bcl6 but produce IL-21 and CXCL13, resulting in B-cell differentiation into plasmablasts (PBs) [14]. This perpetuates B–T-cell networking in inflamed tissues, where ectopic lymphoid structures [15] are formed. Thus, B–T-cell collaboration occurs in both lymphoid tissues and at sites of inflammation.
Disrupting the B–T-cell networking in AID, historical perspectives
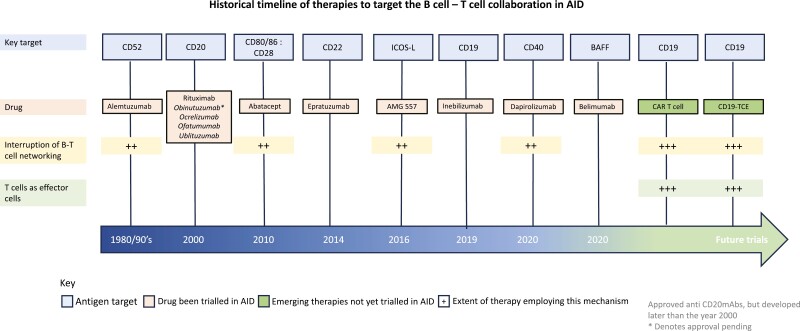
B–T-cell collaboration is a dominant source of chronic inflammation in AID. Hence, disrupting this network is an appealing therapeutic strategy. Over the past four decades, B–T-cell co-stimulation was targeted through different pathways such as alemtuzumab (anti-CD52 monoclonal antibody, CAMPATH-1H), abatacept (cytotoxic T-lymphocyte antigen 4 immunoglobulin), and dapirolizumab (anti-CD40L) with variable impact on B-cell depletion (BCD), Fig. 2. In the 1980s, alemtuzumab was used to deplete CD52 expressing cells including B and T cells, providing the first insights into disrupting B–T-cell networking. The 1990s trials of alemtuzumab in RA were terminated due to suboptimal therapeutic index probably owing to prolonged depletion of regulatory T cells [16], although it continues to be used to treat MS (albeit at lower doses). Abatacept inhibits the co-stimulatory CD28-CD80/86 pathway and is approved for RA [17] although the ALLURE trial of abatacept in lupus nephritis (LN) did not meet its primary endpoint [18]. Attempts have been made to block other key co-stimulatory signaling pathways including the CD40-CD40L axis. Second-generation agents have been developed including dapirolizumab-pegol which had favorable biomarker and safety response in SLE [19]; phase III results are awaited (NCT04294667). Therefore, despite these advances, there remains a great unmet need for disrupting B–T-cell collaboration in refractory patients with AID.
Figure 2.
Historical timeline of therapies that target B–T-cell collaboration in autoimmune disease. These agents were designed either to deplete B cells and/or disrupt the B–T-cell collaboration. The top row denotes the target antigen, the second row demonstrates the drugs that have undergone clinical trial (later two, t are yet to undergo clinical trial in AID). The third row represents therapies that interrupt B–T-cell networking and the fourth row represents treatments that employ T cells as effector cells. Text in italics under CD20 represents other approved anti-CD20 mAbs, *denotes pending approval
BCD with rituximab in RA and SLE; why is it suboptimal?
In the past three decades, BCD therapy with the CD20 monoclonal antibody rituximab, has revolutionized the treatment of severe or refractory AID and has been approved for use in RA [20], ANCA vasculitis [21], and pemphigus vulgaris (PV) [22] and is prescribed widely “off-licence” in SLE [23] and in immune thrombocytopenic purpura (ITP) [24]. Data from the Lupus Nephritis Assessment with Rituximab (LUNAR) study reported complete BCD with complete response, as defined in the study [25]. However, there remains a significant proportion of patients, up to 30%, who have disease refractory to rituximab, particularly in the context of incomplete BCD [23] and/or repopulation with PB and switched memory B cells (IgD−CD27+, SwMBC) [26].
How do memory B cells and CD19+CD20− PBs evade rituximab?
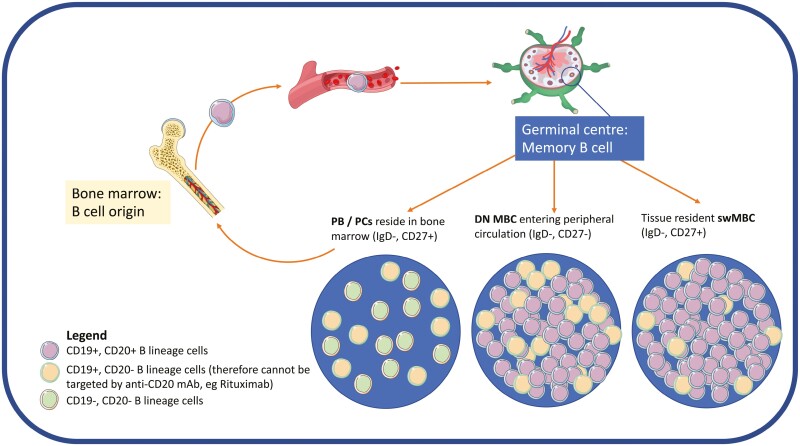
B cells can evade rituximab’s effects either through intrinsic mechanisms (lacking CD20 expression and antigenic modulation) or extrinsic mechanisms such as restricted vascular access to effector cells as discussed previously [27]. Upon activation, naïve B cells solicit T-cell co-stimulation in lymphoid tissues and at sites of inflammation such as the joint and the kidney to differentiate into memory B cells and antibody-secreting cells including short-lived CD19+CD20− PBs and long-lived CD20− PCs [14, 28]. In RA, rituximab fails to completely deplete SwMBC and CD19+CD20− PCs in lymphoid tissues [29], joints, and bone marrow [30–32] contributing to poor response. In patients with ITP with poor response to rituximab, autoreactive splenic memory B cells down-regulate their BCR and up-regulate anti-apoptotic proteins and evades rituximab while retaining the capacity to reactivate and differentiate into autoantibody secreting CD19+CD20− PBs [24]. In muscle-specific kinase myasthenia gravis, autoreactive SwMBC evades rituximab and differentiate into autoantibody secreting CD19+CD20− PBs contributing to relapse [33]. Further, rituximab has no direct effect on CD19+CD20− PBs and PCs, as they do not express CD20 [34, 35]. Thus, SwMBCs, CD19+CD20− PBs and CD19+CD20− PCs evade rituximab through distinct mechanisms, Fig. 3.
Figure 3.
Life cycle of B lineage cells. B cells originate in the bone marrow and migrate through peripheral circulation into lymphoid tissues such as lymph nodes and the spleen. Naïve B cells mature into memory B cells which then differentiate into switched memory B cells, SwMBC (IgD−,CD27+), or double negative memory B cells (DN MBC; IgD−, CD27−) entering the peripheral circulation or plasma blasts (PBs) and plasma cells (PCs) a majority of which reside in the bone marrow, tissues, and inflammatory sites. Proportions of CD19+CD20+ versus CD19+CD20− B cells are demonstrated pictorially within each subpopulation. Anti-CD20 monoclonal antibodies such as rituximab may not completely deplete CD19+CD20+ B cells in tissue and do not target CD19+CD20− B cells, therefore, alternative strategies of depletion including CD19 targeting approaches may help to overcome rituximab resistance in autoimmunity
Broadly, anti-CD20 mAbs can be grouped into types I and II, where type I mAbs such as rituximab, are more efficient at clustering CD20 compared to type II anti-CD20 mAbs [36]. This enables efficient complement activation and therefore enhanced complement-dependent cytotoxicity (CDC), however, it also increases the propensity for internalization of CD20:CD20 mAb complexes by B cells [37]. In addition, incomplete BCD with rituximab may be related to its internalization of rituximab [38]. Type II anti-CD20 mAbs such as obinutuzumab may, at least in part, overcome this resistance mechanism [27]. In a pivotal phase II study, obinutuzumab was shown to improve clinical response in LN [39] and phase III studies are ongoing. However, CD19+CD20− PBs and CD19+CD20− PCs are still not directly targeted. Furthermore, disease-associated macrophage phagocytic defects [40] and vascular access limitations may compromise the ability of anti-CD20 mAbs (and other B-cell depleting mAbs, such as those directed to CD19) to evoke antibody-dependent cellular phagocytosis (ADCP) [27, 41] as they rely on FcγR-bearing effector cells. In addition, NK cells are also scarce in tissues, limiting antibody dependent cellular cytotoxicity (ADCC). For example, we have previously reported that incomplete depletion and/ or persistent infiltration of B cells in the kidneys was associated with active LN refractory to rituximab [42].
Through histological analysis of kidney [43] and skin [44] of patients with AID, and the synovium in patients with RA [14], we know that B cells interact with T cells in lymphoid tissues and at sites of inflammation, to differentiate into autoantibody secreting PBs and PCs. At these sites, limited access to rituximab’s key effector cells, macrophages, and NK cells, may compromise depletion. Thus, antigen expression, modulation, and access to effector cells influence the efficiency of rituximab-mediated BCD. Therefore, it is important to consider both alternative target antigens and therapies that recruit other effector cells to improve BCD.
Approaches to overcome rituximab resistance in AID
Is CD19 an ideal target?
CD19 regulates the threshold for B-cell activation as a co-receptor of the BCR complex [45] with consequent implications for influencing autoimmunity [46]. CD19 deficiency impairs humoral immunity, at least in part, due to an increased threshold for B-cell activation [47] whereas overexpression is associated with AID such as SLE [28]. When compared with CD19−CD20− PCs, CD19+CD20− PCs accumulate more mutations and retain greater proliferative capacity, at least in vitro [34]. These observations implicate a significant role for CD19 in B-cell differentiation and activation.
When compared with CD20, B lineage cells express CD19 at an earlier stage in development and retain expression through all stages of differentiation into CD19+CD20− PBs and some CD19+CD20− PCs [28]. CD19hiCD11c+ memory B cells in humans were shown to respond robustly to antigen challenge, in vitro [48]. More recent evidence suggests that double negative (IgD−CD27−) DN B cells which express the transcription factor T-box expressed in T cells (T-bet) encoded by Tbx21, termed DN-T-bet+ B cells are expanded in aging, are associated with higher mortality from COVID-19 infection and disease activity in SLE as well as disease pathogenesis in RA. Therefore they are of great interest in the field of B-cell research [49].
Further, they demonstrate increased expression of CD19 which strengthens the argument to target CD19 in AID (Shah et al., in preparation). Considering the availability of newer therapies that target CD19, particularly in the field of oncology, we reappraise the concept of targeting CD19, put forward over a decade ago, to treat AID [28]. In addition, evidence from oncology highlights that cancers refractory to monoclonal antibodies have been effectively treated with CD19-targeted chimeric antigen receptor (CAR) T cells, probably owing to the deeper depletion of B cells which provides promise for patients with AID resistant to current mAb therapy, highlighted by the published case series in SLE [50]. These mechanistic considerations indicate that targeting CD19, particularly in AID, may overcome anti-CD20 mAb resistance.
How to target CD19-T-cell engagement as a mechanism of action?
Therapeutic options to target CD19+ B cells and PCs include (i) anti-CD19 mAbs; (ii) CD19-targeted CAR T cells; and (iii) CD19-directed T-cell engagers (TCE). The anti-CD19 mAb inebilizuzmab is approved for the treatment of neuromyelitis optica spectrum disorder [51] and showed initial promising results in a clinical trial in systemic sclerosis [52]. BCD with inebilizumab was greater in transgenic mice blood and spleen as well as in an in vitro ADCC assay using human PBMCs when compared to rituximab [53]. However, similar to rituximab, anti-CD19 mAbs are also disposed to internalization [54] and would be limited by disease-associated macrophage phagocytic defects [40] and vascular access limitations. Therefore, CD19-directed CAR T cells and CD19 TCE may be of greater utility in AID and will be discussed in the following sections.
CAR T-cell therapy
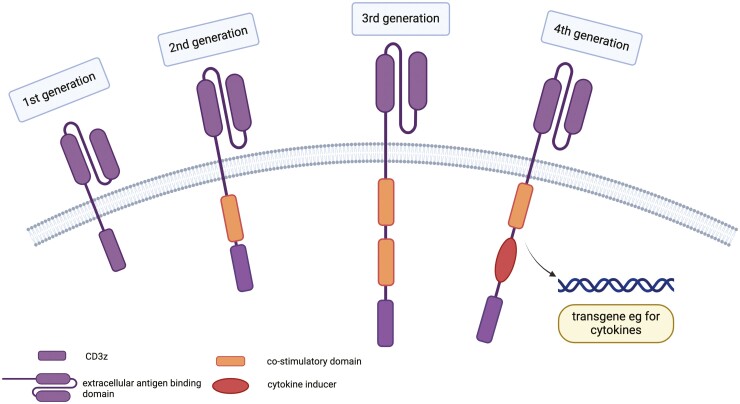
The introduction of CAR T cells to treat cancer has been instrumental in providing individualized, targeted treatment through genetically engineered T cells that express a CAR specific to a tumor-associated antigen, such as CD19 in B cell [55] malignancies. Recognition of the target antigen-bearing B cells activates CAR T cells to proliferate and selectively eliminate the target B cells. The basic structure of a CAR includes an extracellular surface domain for antigen recognition (typically derived from an antibody fragment), a transmembrane domain, and an intracellular signaling domain that activates T cells (typically derived from CD3z chain). The evolution of CAR from first to fourth generation includes the addition of co-stimulatory domains (one in second generation and two in third generation CARs) as well as co-expression of additional transgenes for cytokine secretion (fourth generation) [56], Fig. 4.
Figure 4.
Evolution of CARs across the generations. All CARs have a single chain variable region of a mAb. (A) first-generation CARs contain an intracellular signaling domain of CD3 zeta chain alone; (B) second-generation includes a single co-stimulatory domain (CD28 or 4-1BB); (C) third-generation CARs combine two of the above co-stimulatory domains; and (D) fourth-generation CARs are diversified in that they can express cytokines. Image created using BioRender.com
Once administered, CAR T cells can also expand and establish immune memory, thus providing long-term surveillance of disease as described in malignancy [57]. CAR T-cell therapy has been approved for the treatment of B-cell acute lymphoblastic leukemia (ALL), lymphoma, and multiple myeloma [55]. Factors such as antigen overload are considered to contribute to undesirable effects including cytokine release syndrome (CRS) and neurotoxicity, leading to newer generation therapies with fewer toxicities being developed [58]. Complete remission for at least 3 years, of various relapsed B-cell malignancies was demonstrated in 51% of patients treated with CAR T-cell therapies, with few late-onset side effects [59]. This success led to CAR T cells being explored for treating refractory AID.
CAR T-cell therapy in AID
The success of using CAR T-cell therapy for the management of B-cell malignancies inspired its research in a range of AID including SLE, myasthenia gravis, and type 1 diabetes mellitus, as outlined in Table 1. In animal models of SLE, anti-CD19 CAR T-cell treatment resulted in profound and sustained BCD with low circulating PCs and increased survival rates [60]. This data provided the basis for the use of anti-CD19 CAR T-cell therapy in the treatment of five patients with refractory multiorgan lupus which was well tolerated leading to serological and clinical remission at relatively short follow-up [50]. Probably owing to lower antigen load, the first cohort of patients with SLE-treated with anti-CD19 CAR T-cell therapy experienced only low-grade CRS [61], of which tocilizumab (anti-IL-6 receptor mAb) was used (successfully) in only one patient owing to persistent fevers for 3 days [50]. Thus, current preliminary evidence suggests that CD19 targeting CAR T-cell therapy seems a safe and effective therapeutic strategy in AID such as SLE. Anti-CD19 CAR T-cell therapy was associated with a reduction in autoantibodies and pro-inflammatory cytokines including IL-6 and TNF-α [62]. Intriguingly, despite excellent clinical responses, the authors demonstrated an increase in serum BAFF levels.
With regard to other autoimmune diseases, single case studies of anti-CD19 CAR T-cell therapy indicate a potential use of the approach also in anti-synthetase syndrome [63] and systemic sclerosis [64]. To note, an important potential confounder when appraising the mechanisms of response to CAR T-cell therapy is the use of lymphocyte depletion with fludarabine that may have contributed to the response. Several studies exploring the safety, tolerability, and preliminary efficacy of anti-CD19 CAR T-cell therapy in AID have been initiated (NCT05938725, NCT05869955, NCT03030976, NCT05798117, and NCT05930314).
Limitations of CAR T-cell therapy
Although the case examples of anti-CAR T cells in AID are promising, it is also important to understand the limitations. Two of the five patients treated with anti-CD19 CAR T-cell therapy had persistence of clonotypic IgG in follow-up samples, demonstrating suboptimal depletion and/or rapid repopulation of memory B cells [50]. Remarkably, despite lower antigen overload, three of five SLE patients treated with anti-CD19 CAR T-cell therapy repopulated their B cells by day 50 after treatment [50] when compared with prolonged BCD achieved in B-cell malignancies up to several years post-infusion [55]. Potential explanations for incomplete depletion and/or relatively early repopulation of B cells include (i) complete depletion of target cells removing the sustained stimulus needed to maintain an optimal pool of CAR T cells, as CAR T cells had disappeared at week 4 after treatment; (ii) higher proportion of senescent and/or exhausted SLE CAR T cells; and (iii) potential inhibition of CAR T-cell expansion due to the persistent effects of immunosuppression such as mycophenolate mofetil beyond cessation of therapy [65].
Implications of lymphodepletion in AID
Patients with AID, particularly SLE, are often lymphopenic owing to the underlying disease process and the effects of immunosuppression, which may impact the process of leukapheresis required to generate the CAR T cells. Nevertheless, patients with active SLE in the previously discussed case series [50] were successfully leukapheresed before CAR T-cell therapy and concurrent treatment with steroids and immunosuppressive agents [66]. The process of lymphodepletion itself increases the likelihood of infections and is an additional step preceding CAR T-cell therapy, compared to “off the shelf” TCE therapy.
Risks of hypogammaglobulinemia
A major consideration with CAR T-cell therapy is the risk of hypogammaglobulinemia; this may be observed with TCE but likely to a lesser extent. In the treatment of cancer, approximately a third of patients develop hypogammaglobulinemia following CAR T-cell infusion [67], owing to potent and persistent depletion of normal CD19+ B cells. Very low IgG levels can arise from 9 weeks after treatment and continue beyond 4 years [67]. This poses a risk of serious life-threatening infections, necessitating intravenous immunoglobulin infusions as a prevention strategy, as per the majority of trials [68], however, this can be expensive and not readily accessible for all patients.
Importantly, B-cell aplasia and hypogammaglobulinemia result in suboptimal vaccine responses, which is also a significant concern especially in the current era of SARS-CoV-2 infection with only 29% of patients who receive CAR T-cell therapy for lymphoma/myeloma mounting a clinically relevant antibody response to vaccination [69]. Reassuringly, vaccine responses were stable following CAR T-cell therapy in the SLE case series [50], likely related to the remaining pool of CD19− plasma cells which are able to secrete antibodies 2 years post-treatment [70]. These aspects also need to be accounted for during TCE trial design in AID.
Logistical limitations of CAR T cell therapy
Logistical limitations are also considerable. For example, in patients with rapidly progressing cancer or AID, the practical feasibility of CAR T-cell therapy may be limited as there is typically a protracted vein-to-vein time of approximately 6–8 weeks, due to the time required for producing, transporting, and ensuring quality control of the personalized cell therapy, as illustrated in the graphical abstract. This process is typical for most CAR T-cell therapies, although the novel YTB323 omits the ex vivo expansion stage (NCT05798117).
Further disadvantages of CAR T-cell therapy include the high cost involved with engineering and storage of CAR T cells and the specialist training required to administer treatment as detailed in Table 2. Therefore, readily available and effective novel treatments are required while awaiting CAR T-cell therapy [79]. One approach to obviate the limiting factor of individual custom-made CAR T cells is the generation of “universal CAR T cells” as reviewed by Zhao et al. [56]. These can serve as “off the shelf” therapies to treat a wide range of clinical indications as they are engineered to target multiple antigens. Further gene editing work is underway to ensure universal CAR T cells are not depleted by the recipient’s immune system and are able to expand without causing harmful effects [80].
Table 2.
Evidence for the use of CAR T-cell therapies in non-malignant settings
| Specialty | Indication | Study phase/type | Outcome | Ref |
|---|---|---|---|---|
| Neurology | Multiple sclerosis (murine model = experimental autoimmune encephalomyelitis) | Murine model | Depleted B cells in peripheral blood and CNS Improved clinical scores of EAE |
[71] |
| Myasthenia Gravis (using anti-B-cell maturation antigen CAR T cells) | Phase 1b/2a (human) | Safe, well-tolerated, and clinical improvement Phase IIb ongoing (NCT04146051) |
[72] | |
| Transplant medicine | Post-transplant lymphoproliferative disorder (PTLD) post-renal transplant | Case series (n = 3) (human) | Demonstrated safety and feasibility (with regard to stopping immunosuppression) however only one of three patients maintained in remission at 3 months follow-up |
[73] |
| Case series of three patients with refractory PTLD post solid organ transplants (cardiac transplant, kidney transplant, and pancreas transplant) | Case series (n = 3) (human) | Poor outcomes, multiple complications including CRS, immune effector cell-associated neurotoxicity syndrome (ICANS), acute kidney injury, lack of response to CAR T-cell therapy, and mortality | [74] | |
| Refractory PTLD post heart and kidney transplant | Case report (human) | Six months post CAR T-cell infusion, clinically well, and normal ejection fraction on echocardiography | [75] | |
| Rheumatology | Systemic lupus erythematosus | Case series (n = 5) (human) | Deep depletion of B cells, clinical improvement, normalization of anti-ds-DNA antibodies and all achieved remission after 3 months. Three patients repopulated B cells less than 50 days post CAR T-cell therapy (although mainly naïve B cells) |
[50] |
| Systemic sclerosis (diffuse cutaneous) | Case report (human) | Extensive fibrosis (skin, heart, and lung)—all showing improvement post treatment Well tolerated, mild CRS (Grade 1), no signs of ICANS. |
[64] | |
| Anti-synthetase syndrome (myositis and interstitial lung disease) | Case report (n = 2) (human) | Treated with CD19-targeting CAR T cells. Excellent outcome with biochemical, serological, and radiological resolution of myositis and improvement in pulmonary function tests/CT chest. | [63, 76] | |
| Dermatology | Pemphigus vulgaris—target antigen desmoglein 3 | Preclinical study, ex vivo (human) | Depletion of Dsg3 cells and antibodies in human pemphigus vulgaris model | [77] |
| Endocrinology | Type I diabetes Mellitus—target antigen Insulin | Murine model | Delayed onset of diabetes but no long-term protection | [78] |
To this end, we consider alternative strategies, with the potential of TCE bispecific antibodies as a novel therapeutic option to disrupt B-T cell collaboration in AID. Table 2 outlines the major differences and similarities of using CAR T-cell therapy and TCEs.
TCE: clinical trial experience and technical aspects
TCE represents a novel class of targeted therapeutics that recruit T cells [81]. From a clinical perspective, in the late 1990s, the potential for bispecific antibodies as therapeutic interventions became clearer for cancers such as breast, leukemia, and lung [82], which led to a surge of interest in their use and FDA approval of catumaxomab for malignant ascites [83] and blinatumomab for refractory B-ALL [84] More recently, three CD20 T-cell engagers, mosunetuzumab, glofitamab, and epcoritamab have been approved for treatment of refractory/relapsed follicular lymphoma and refractory/relapsed diffuse large B-cell lymphoma [85]. Technological advancements over time have enabled a range of modifications to enhance the flexibility and number of binding sites, half-life, production yield, and potency of these therapeutics [86].
TCE technologies
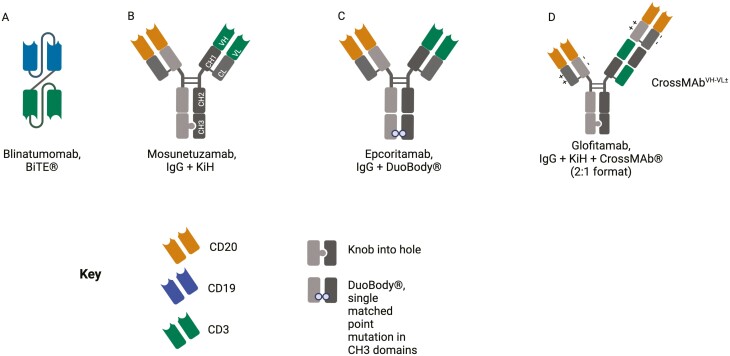
TCEs can be broadly categorized into (i) small, short half-life bispecific antibody fragments (single chain variable fragments) such as bispecific T-cell engagers (BiTE®s) which require repeated administration (Fig. 5A); and (ii) larger IgG-based T-cell bispecific antibodies (TCBs) with extended half-lives (Fig. 5B and C). The development of TCBs has evolved from single chain variable fragments in the early 1990s [87], to the development of “knobs into holes” (KiH) technology in the late 1990s [88] to the more advanced technologies including CrossMab to engineer bispecific antibodies [89, 90], Fig. 5.
Figure 5.
Selected TCE formats in a schematic representation used for T-cell redirecting therapies. (A) Blinatumomab, tandem scFv (single chain variable fragment) (BiTE) format. (B) Mosunetuzumab, IgG-based-TCE with monovalent binding using a native antibody structure with 1 Fab arm to bind CD20 (target antigen) and 1 Fab arm to bind CD3 on T cells, combined with the KiH technology as demonstrated in the CH3 domain to achieve heavy chain heterodimerization. (C) Epcoritamab, IgG-based TCE with point mutations in each Fc region (CH3 domain) to allow controlled Fab-arm exchange, termed DuoBody®. (D) Glofitamab, bivalent binding to increase the avidity of TCE binding to the target antigen, CD20, with additional KiH and CrossMabVH-VL with charge interactions using variable regions. Image created using Biorender.com
CD19-TCE
Blinatumomab, a BiTE® composed of two single-chain antibodies targeting CD19 on B cells and CD3ε on T cells fused via a flexible linker (Fig. 5A), is approved for B-cell ALL [85]. It is engineered to have a short half-life of 2 h to enable tight control of serum levels in case of adverse events. Blinatumomab relies on the presence of CD19+ target cells to activate T cells, with sensitive response from CD8+ T cells to induce lysis of tumor cells as demonstrated in video-assisted microscopy studies [91]. In vitro studies of human B-lymphoma cells demonstrated a higher degree of tumor cell elimination with blinatumomab compared to rituximab [92]. Interestingly, the combination of blinatumomab and rituximab was synergistically more efficient, especially at low effector-to-target cell ratios and low Blinatumomab concentrations [92]. This combined effect was found to be due to potent activation of pro-caspases 3 and 7 in target cells, which is instrumental in triggering granzyme-mediated apoptosis. The BiTE subtype is potent with regard to target cell killing. Regardless, the requirement for repeat dosing of Blinatumomab may limit its routine use in clinical practice.
CD20-TCE
Three CD20 TCE have been approved for refractory B cell lymphomas: mosunetuzumab, glofitamab, and epcoritamab [85], Fig. 5. Mosunetuzumab is an IgG-based TCE with 1:1 binding to CD20 and CD3; it uses KiH technology and in vitro assembly to overcome incorrect light chain association [93]. Epcoritamab is also IgG-based, although employs the unique DuoBody® technology with point mutations in each Fc region (CH3 domain) to allow controlled Fab-arm exchange [94]. Recent IgG-based TCEs have been developed for increased avidity. Glofitamab has two Fab regions which bind CD20, one Fab region which binds CD3 (so-called 2:1 format), and a longer half-life of 10 days, owing to its Fc region and interaction with FcRn [90]. The Fc also includes the P329G LALA mutations [81], which abolish conventional effector functions and therefore it employ a different mechanism of action compared to rituximab. The 2:1 format (Fig. 5C) enables greater potency with regard to B-cell cytotoxicity compared to 1:1 antibodies, thought to be due to the close proximity of the CD20 binder and CD3 binder, resulting in a more stable T cell to target B-cell synapse induced by the head-to-tail fusion design [95].
Effector mechanisms of TCEs: lessons learnt from treating malignant disease
Bispecific antibodies can redirect the effector function of various immune cells. T cells are promising as effector cells as they are abundant, able to expand rapidly, and have potent cytotoxic capacity. TCE are designed to by-pass the normal major histocompatibility complex–T-cell receptor (MHC–TCR) interaction usually required between antigen presenting cells and T cells, and instead co-engage the CD3 molecules on the T cell and form an immunological synapse via the target antigen such as CD19 or CD20 on the surface of B cells that helps redirect co-stimulation to cytotoxicity [96, 97], Fig. 6. This synapse is similar to that formed during cytotoxicity with CAR T cells. The CD20-TCE recruitment of T cells is evident in in vitro culture assays demonstrating that tumor lysis is dependent on T-cell recruitment, activation, and expansion of CD4+ and more profoundly CD8+ subsets [81]. Importantly, CD20-TCE depleted B cells in the spleen and lymph nodes, efficiently [81]. These findings may be of relevance to AID where inefficient BCD in lymphoid tissues and inflammatory sites, as discussed earlier, contributes to refractory disease.
Figure 6.
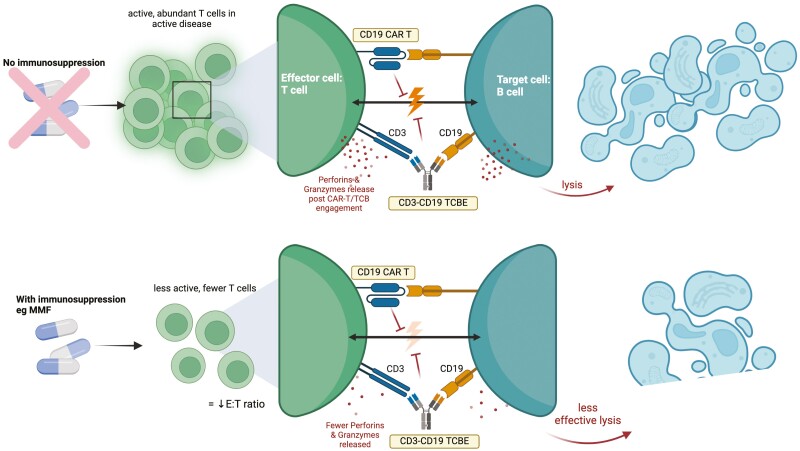
The potential effect of immunosuppressive treatments on T-cell effector function. Mycophenolate mofetil (MMF) as per the bottom panel, results in fewer T cells to serve as effector cells for therapies such as CD19 TCE and CD19 CAR T cells. MMF can directly reduce the number of T cells and impair their activation and reduce their cytotoxicity against target B cells with lower release of perforin and granzyme molecules. Image created using Biorender.com
Employing T cells to disrupt the B–T collaboration: CAR T and TCE
As discussed above, in AID, B and T cells colocalize in lymphoid tissues and at inflammatory sites. Therefore, using CAR T cells or TCE that employ T cells as effector cells to deplete B cells may provide a distinct advantage over rituximab-mediated BCD that relies on macrophages and/or NK cells as the dominant effector mechanism. The key differences and similarities between CAR T-cell therapy and TCE therapy are described in Table 3.
Table 3.
Mechanistic differences and similarities between CAR T and TCE: experience in oncology
| CAR T-cell therapy | TCE | |
|---|---|---|
| Side effect profile | Variable between CAR T regimens. In some oncological indications, about 80% suffer CRS, longer lasting and at a higher grade Neurotoxicity: immune effector cell-associated neurotoxicity syndrome (ICANS) occurs in approximately 13–21% of patients, lasting 4–5 times longer than with TCE. |
Variable between different TCE and indications. Approx. 50% suffer CRS, earlier onset but shorter duration. Obinutuzumab (anti-CD20mAb) pre-treatment limits CRS Neurologic side effects e.g. headache but less severe than ICANS, much less frequent than CAR T cells. |
| Efficacy | Higher rates of complete response in hematological malignancies | Dose-dependent response, but can be up to 30% less effective than CAR T cell therapy |
| Pre-conditioning | Leukodepletion so higher rates of infection and risk of rejection in transplant patients. | No preconditioning, but pre-medication with dexamethasone to reduce cytokine production and with obinutuzumab for glofitamab |
| Hypogammaglobulinemia | Persistence of engineered T cells in vivo resulting in sustained B-cell aplasia and hypogammaglobulinemia may require IVIg | TCB can deplete normal B cells and plasma precursor cells leading to a higher risk of hypogammaglobulinemia, but therapeutic regimen could be personalized according to clinical need |
| Effector cell type | Engineered T cells Less differentiated T cells (naïve and memory) show better efficacy than effector T cells |
Endogenous T cells Antigen-experienced T cells mediate TCE-induced cell death, whereas naïve T cells are not activated |
| Logistical differences/similarities | ||
| Cost | +++ (~£300 000 in the UK) [98] | ++ (~£56,000 per cycle UK) [99] |
| Production | Personalized therapy requiring individual engineering of patient’s T cells—labor intensive, time-consuming (resulting in disease progression), and higher risk of a production error. Also requires the patient to have sufficient peripheral T-cell counts for successful isolation of T cells from leukapheresis. |
“Off the shelf” medication, so technically less delay to administration than CAR T-cell therapy. Can be manufactured in large quantities. Can be used independently of peripheral lymphocyte counts |
| Administration | Single intravenous administration, however, from decision to treat to administering therapy can be 6–8 weeks when disease may progress. Specialist training of staff required to administer CAR T-cell therapy and monitor for complications during infusion |
Shorter half-life so may need repeat dosing. Quick to administer so can treat patient promptly and halt progression of disease. No additional specialist training required, similar administration to routine mAbs used such as rituximab. |
| Approval for use | ALL, large B-cell lymphoma, mantle cell lymphoma, multiple myeloma (FDA approval) | Blinatumomab (CD3-CD19) for ALL, epcoritamab-bysp and glofitamab (CD3-CD20) for DLBCL (FDA approval), mosunetuzumab (CD3-CD20) for follicular lymphoma |
| Repeat treatment | Complicated due to maintenance of T-cell pool, patient factors (risk of infection). | More convenient and standardized |
Aside from requiring lymphodepletion, an important aspect to highlight is that the expansion of CARs in vivo cannot be controlled, demonstrated by the rapid rise in circulating CARs, reaching up to 59% by day nine post-infusion [50].
In addition, the expansion and duration of CAR T-cell action is not easily controlled, whereas a TCE can be given at a specific dose and the half-life of the molecule is expected to determine its duration of action. Overall, treatment with TCE may potentially overcome some of these limitations of CAR T-cell therapy such as a lag time from decision to treatment to allow for engineering of CAR T cells, prior leukapheresis, and requirement for specialist centers with experience of cell-based immunotherapies.
Immunological/biological pitfalls in recruiting T cells as effector cells
Despite the undoubted promise of CAR T cells and TCE, there remain potential hurdles. Both CAR T cells and TCE may evoke “bystander killing” of antigen-negative cells directly in contact with antigen-positive cells [100]. While this local bystander effect is desirable in the treatment of solid tumors to prevent the escape of antigen-negative cancer cells, the potential implications of this in AID are unknown.
More recently, there are an increasing number of reports of macrophage activation syndrome (MAS)/hemophagocytic lymphohistiocytosis (HLH) as a complication of CAR T-cell therapy given for hematological malignancies, possibly as a distinct variant of CRS [101]. MAS/HLH is a serious condition of hyperinflammation, fevers, and cytopenias, and can be life-threatening. Patients with autoimmune disease such as SLE are already predisposed to developing secondary MAS/HLH [102], therefore initiation of CAR T-cell therapy in this cohort needs careful consideration.
Another potential pitfall with recruiting T cells as effector cells is a possible reduction in T-cell counts, which may increase the risk of infection, due to apoptosis noted with first-generation CAR T-cell treatments [103]. Reassuringly, in studies with CD20-TCB, peripheral T-cell counts decreased in the first 24 h of drug administration before returning to baseline by 72 h [81], considered to reflect an activation-induced marginalization. Therefore, the risk in the short term with these agents seems low but will need monitoring in the long term.
Impact of the tissue microenvironment
An additional consideration is the tissue microenvironment, which is known to influence T-cell cytotoxicity. AID-related T-cell subpopulations with features of anergy, exhaustion, and senescence may compromise the efficiency of TCE [104]. In addition, resistance to TCEs may arise from immune escape, through the expression of immune checkpoint molecules such as PD-1. In this context, combination treatment with checkpoint inhibitors, already explored in cancer immunotherapy may be limited by the potential activation of autoreactive T cells [105]. Alternatively, next generation trispecific TCEs to additionally provide co-stimulation may be beneficial [106]. As CD3 is a pan T-cell marker, TCEs can recruit all T-cell populations including naïve, regulatory T cells, and exhausted T cells as effector cells. In AID, regulatory and exhausted T cells are associated with disease remission and improved prognosis [107]. Mechanistic clinical studies will help us understand the clinical relevance of these potential limitations.
Clinical adverse effects of recruiting T cells as effector cells
The main adverse effect associated with both types of T-cell therapy is CRS, which is the rapid systemic release of pro-inflammatory cytokines including IL-6, IL-10, TNF-α, and IFN-γ, upon activation of the T cells [108]. CRS manifests as fever, fatigue, and vasodilation, and can lead to multi-organ failure. Pre-treatment with corticosteroids such as dexamethasone may reduce the risk of CRS. Anti-IL-6 receptor antibody, tocilizumab, has been approved for use prior to CAR T-cell therapy to attenuate CRS [109]. In murine models, combination treatment with Janus Kinase (JAK) inhibitors or mammalian target of rapamycin (mTOR) inhibitor, restricted CD19-TCB-related CRS while retaining their efficacy [110].
Immune effector cell-associated neurotoxicity syndrome (ICANS) is another dose-dependent unwanted side effect unique to patients receiving T-cell engaging treatments, through adherence of T cells to cerebral microvascular endothelium and migration across the blood-brain barrier [111]. In ALL, ICANS, characterized by headache, dizziness, tremor, confusion, and encephalopathy, was associated with high-dose blinatumomab given in the first treatment cycle, probably owing to the higher tumor burden. As the target cell load is much lower in AID, the required dose of TCEs will be lower, consequently, the risk of CRS and ICANS should be lower than that reported for cancer immunotherapy.
What is the impact of immunosuppressive therapy on T-cell cytotoxicity in the context of TCE and CAR T cells?
Other important considerations include AID-specific concurrent drug regimens. For example, transplant recipients and patients with AID and transplant recipients receive immunosuppressants to regulate immune response. In the context of T-cell-based therapy, concurrent use of immunosuppressants may inhibit the effector function of the T cells, thereby, compromising the efficiency of CAR T cells and TCEs. For example, mycophenolate mofetil (MMF) can induce apoptosis in activated human T cells [112]; and in a murine model, mycophenolic acid, the active form of MMF has shown dose-dependent reduction in the generation of cytotoxic T cells [113]. Fig. 6 illustrates the potential impact of immunosuppressants on T-cell cytotoxicity in the context of TCE and CAR T-cell therapies. Therefore withholding immunosuppressants for a period of time to allow for T-cell recovery to enhance performance may be considered in prospective trial design [114].
In a case series of renal transplant recipients requiring CAR T-cell therapy for post-transplant lymphoproliferative disorders (PTLD), MMF was discontinued at the time of PTLD diagnosis (with DLBCL), and tacrolimus was stopped 2 weeks prior to leukapheresis for production of CAR T cells [73]. Similarly, a report of CAR T-cell infusion for anti-synthetase syndrome involved tapering azathioprine and steroids 7 days before leukapheresis and starting MMF 35 days after CAR T-cell infusion [76], which allowed for harvesting of fully functional T cells. This aligns with our proposition of correct sequencing of immunosuppressive treatments including the use of corticosteroids to allow full efficacy of TCE and/or CAR T therapies.
Developing personalized B cell targeting regimens
Where pathogenic B-cell identity is well described, CAR T therapy can potentially enhance the prospects for personalized therapy. For example, desmoglein 3 targeting CAR T cells were engineered to selectively eliminate Dsg3 specific B cells, in vitro and in vivo in animal models [115] toward developing therapies for PV. Currently, a phase I study of BCMA CAR T therapy (NCT04561557) is ongoing for the treatment of neurological disorders including Aquaporin-related neuromyelitis optica spectrum disorder (NMOSD). However, the identity of pathogenic B cells remains elusive for the majority of AID, where non-selective BCD therapy remains the current standard strategy.
In routine practice of managing AID, rituximab induction therapy incorporates two doses of 1 g, given 2 weeks apart. Retreatment with the same or lower dose of rituximab, is usually at 6 months or longer for optimal management of disease activity [17]. Current evidence highlights that response can be improved with better depletion with a lower frequency of memory B cells and PB in RA and SLE [27]. As discussed previously, presumably due to more efficient BCD, obinutuzumab treatment seems to be effective in LN [39]. To this end, targeting CD19 and disrupting the B–T-cell networking in AID, with CD19/CD3 TCEs or CAR T cells would be expected to provide mechanistic advantages. For example, targeting CD19, expressed on memory B cells, CD19+CD20−PBs, and CD19+CD20−PCs should help deplete these “rituximab-resistant cells” whereas the use of TCEs would help direct T cells from B-cell “co-stimulation to cytotoxicity” to disrupt B–T networking. Key lessons from previous SLE rituximab trials include (i) patient selection with regard to disease manifestations, severity of disease activity, serological parameters, and previous treatment are important to consider so as not to exclude the most active patients, (ii) defining standard concomitant therapy in the comparator and placebo arms as variable usage of glucocorticoid and immunosuppressants such as MMF can impact outcomes, (iii) defining endpoints in particular the steroid sparing effect, (iv) selecting the right disease activity index, and (v) defining follow-up duration and side effects. These serve as a reminder of the importance of optimal trial design to evaluate the “real” potential of TCE [25, 116].
Optimizing co-therapies with immunosuppressants, and sequential therapy with rituximab
Co-therapy with immunosuppressants and/or rituximab therapy may influence the efficacy and safety of TCEs. As demonstrated in Fig. 6, patients with AID are often being treated with immunosuppression such as MMF and corticosteroids. Therefore, considering discontinuation of MMF for 3–6 weeks [50] may optimize the effector function of T cells to disrupt the B–T-cell network in AID. Thereafter, a delayed introduction of MMF may be considered as needed for optimal control of disease activity.
Sequential therapy with rituximab, which is already competitively priced as a biosimilar, followed by CD19-TCE will enable targeting of B–T-cell networks in ectopic lymphoid tissue within peripherally inflamed tissues in AID, Fig. 3. A potential limitation of this sequence is that rituximab therapy may result in lower expression of CD19 [24], probably through internalization as shown in vitro [38], thus, compromising the efficiency of CD19-TCE or CD19-CAR T therapy. Therefore, treatment with CD19-TCE first followed by rituximab, as needed, could be considered as an alternative strategy for those with poor depletion with CD19-TCE alone. In this context, it would be important to have strategies to detect B cells using novel antibodies that bind an alternative epitope to the therapeutic mAbs, less challenging for CD19 as it is a bigger antigen than CD20.
Conclusions
CD19 CAR T-cell or CD19-TCE therapy to convert B- and T-cell co-stimulation into conflict and disrupt their networking could prove to be a paradigm shift in treating AID. TCE, designed and developed through advanced antibody engineering methods, offers a mechanistically sound, logistically convenient, and favorable alternative therapeutic strategy in the management of refractory AID. To this end, mechanistic studies of TCE in AID, particularly during early-phase clinical trials, are of critical importance to optimize the use of TCE in combination with standard-of-care therapy as an alternative strategy to deplete B-lineage cells to improve outcomes for people with refractory AID.
Acknowledgements
Not applicable.
Contributor Information
Kavina Shah, Centre for Rheumatology, UCLH, London, UK.
Maria Leandro, Centre for Rheumatology, UCLH, London, UK; Department of Rheumatology, University College London Hospital, London, UK.
Mark Cragg, University of Southampton Faculty of Medicine, Antibody and Vaccine Group, Centre for Cancer Immunology, University of Southampton, Southampton, UK.
Florian Kollert, Roche Innovation Center Basel, Early Development Immunology, Infectious Diseases & Ophthalmology, Basel, Switzerland.
Franz Schuler, Roche Innovation Center Basel, Roche Pharma Research and Early Development, Schlieren, Switzerland.
Christian Klein, Roche Innovation Center Zurich, Cancer Immunotherapy Discovery, Oncology Discovery & Translational Area, Schlieren, Switzerland.
Venkat Reddy, Centre for Rheumatology, UCLH, London, UK; Department of Rheumatology, University College London Hospital, London, UK.
Ethical approval
Not applicable.
Conflict of interests
None declared.
Funding
Funders include Cancer Research UK (DRCRPG-May23/100001), VR's work is funded by MRC-CARP Fellowship from the UK Research and Innovation, Medical Research Council (MR/T024968/1), research grant from UCLH Biomedical Research Centre, National Institute for Health and Care Research, and Roche Innovation Center, Zurich.
Author contributions
Florian Kollert, Roche: Employment and Stock Ownership; Franz Schuler, Roche: Employment, patents, stock ownership; Christian Klein, Roche: Employment, patents, stock ownership; Venkat R Reddy, Research grants from Roche.
References
- 1. Rao DA. The rise of peripheral T helper cells in autoimmune disease. Nat Rev Rheumatol 2019, 15, 453–4. doi: 10.1038/s41584-019-0241-7 [DOI] [PubMed] [Google Scholar]
- 2. van Langelaar J, Rijvers L, Smolders J, van Luijn MM.. B and T cells driving multiple sclerosis: identity, mechanisms and potential triggers. Front Immunol 2020, 11, 760. doi: 10.3389/fimmu.2020.00760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Petersone L, Edner NM, Ovcinnikovs V, Heuts F, Ross EM, Ntavli E, et al. T cell/B cell collaboration and autoimmunity: an intimate relationship. Front Immunol 2018, 9, 1941. doi: 10.3389/fimmu.2018.01941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anil Kumar MS, Papp K, Tainaka R, Valluri U, Wang X, Zhu T, et al. Randomized, controlled study of bleselumab (ASKP1240) pharmacokinetics and safety in patients with moderate-to-severe plaque psoriasis. Biopharm Drug Dispos 2018, 39, 245–55. doi: 10.1002/bdd.2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harland RC, Klintmalm G, Jensik S, Yang H, Bromberg J, Holman J, et al. Efficacy and safety of bleselumab in kidney transplant recipients: a phase 2, randomized, open-label, noninferiority study. Am J Transplant 2020, 20, 159–71. doi: 10.1111/ajt.15591 [DOI] [PubMed] [Google Scholar]
- 6. Leandro MJ. B-cell subpopulations in humans and their differential susceptibility to depletion with anti-CD20 monoclonal antibodies. Arthritis Res Therapy 2013, 15, S3. doi: 10.1186/ar3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sachinidis A, Garyfallos A.. Double negative (DN) B cells: a connecting bridge between rheumatic diseases and COVID-19? Mediterr J Rheumatol 2021, 32, 192–9. doi: 10.31138/mjr.32.3.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Horowitz DL, Furie R.. Belimumab is approved by the FDA: what more do we need to know to optimize decision making? Curr Rheumatol Rep 2012, 14, 318–23. doi: 10.1007/s11926-012-0256-4 [DOI] [PubMed] [Google Scholar]
- 9. Venturelli V, Isenberg DA.. Targeted therapy for SLE-what works, what doesn’t, what’s next. J Clin Med 2023, 12, 3198. doi: 10.3390/jcm12093198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parodis I, Vital EM, Hassan S-U, Jönsen A, Bengtsson AA, Eriksson P, et al. De novo lupus nephritis during treatment with belimumab. Rheumatology 2020, 60, 4348–54. doi: 10.1093/rheumatology/keaa796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guerreiro Castro S, Isenberg DA.. Belimumab in systemic lupus erythematosus (SLE): evidence-to-date and clinical usefulness. Ther Adv Musculoskelet Dis 2017, 9, 75–85. doi: 10.1177/1759720X17690474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Christophersen A, Lund EG, Snir O, Sola E, Kanduri C, Dahal-Koirala S, et al. Distinct phenotype of CD4(+) T cells driving celiac disease identified in multiple autoimmune conditions. Nat Med 2019, 25, 734–7. doi: 10.1038/s41591-019-0403-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ekman I, Ihantola E-L, Viisanen T, Rao DA, Näntö-Salonen K, Knip M, et al. Circulating CXCR5(-)PD-1(hi) peripheral T helper cells are associated with progression to type 1 diabetes. Diabetologia 2019, 62, 1681–8. doi: 10.1007/s00125-019-4936-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rao DA, Gurish MF, Marshall JL, Slowikowski K, Fonseka CY, Liu Y, et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 2017, 542, 110–4. doi: 10.1038/nature20810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pitzalis C, Jones GW, Bombardieri M, Jones SA.. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol 2014, 14, 447–62. doi: 10.1038/nri3700 [DOI] [PubMed] [Google Scholar]
- 16. Cooles FAH, Anderson AE, Drayton T, Harry RA, Diboll J, Munro L, et al. Immune reconstitution 20 years after treatment with alemtuzumab in a rheumatoid arthritis cohort: implications for lymphocyte depleting therapies. Arthritis Res Ther 2016, 18, 302. doi: 10.1186/s13075-016-1188-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. NICE. Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a TNF inhibitor. Technology Appraisal Guidance [TA195] 2010. [Google Scholar]
- 18. Dooley MA, Appel G, Furie R, Wofsy D, Takeuchi T, Malvar A, Doria A, et al. A phase III randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of abatacept or placebo on standard of care in patients with active class III or IV lupus nephritis. Arthritis Rheumatol. 2018, 70, 1–3584. [Google Scholar]
- 19. Furie RA, Bruce IN, Dörner T, Leon MG, Leszczyński P, Urowitz M, et al. Phase 2, randomized, placebo-controlled trial of dapirolizumab pegol in patients with moderate-to-severe active systemic lupus erythematosus. Rheumatology (Oxford, England) 2021, 60, 5397–407. doi: 10.1093/rheumatology/keab381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Norris-Grey C, Cambridge G, Moore S, Reddy V, Leandro M.. Long-term persistence of rituximab in patients with rheumatoid arthritis: an evaluation of the UCL cohort from 1998 to 2020. Rheumatology 2021, 61, 591–6. doi: 10.1093/rheumatology/keab248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith KGC, Jones RB, Burns SM, Jayne DRW.. Long-term comparison of rituximab treatment for refractory systemic lupus erythematosus and vasculitis: remission, relapse, and re-treatment. Arthritis Rheumatism 2006, 54, 2970–82. doi: 10.1002/art.22046 [DOI] [PubMed] [Google Scholar]
- 22. Werth VP, Joly P, Mimouni D, Maverakis E, Caux F, Lehane P, et al.; PEMPHIX Study Group. Rituximab versus mycophenolate mofetil in patients with Pemphigus vulgaris. N Engl J Med 2021, 384, 2295–305. doi: 10.1056/NEJMoa2028564 [DOI] [PubMed] [Google Scholar]
- 23. Shah K, Cragg M, Leandro M, Reddy V.. Anti-CD20 monoclonal antibodies in systemic lupus erythematosus. Biologicals 2021, 69, 1–14. doi: 10.1016/j.biologicals.2020.11.002 [DOI] [PubMed] [Google Scholar]
- 24. Crickx E, Chappert P, Sokal A, Weller S, Azzaoui I, Vandenberghe A, et al. Rituximab-resistant splenic memory B cells and newly engaged naive B cells fuel relapses in patients with immune thrombocytopenia. Sci Transl Med 2021, 13, eabc3961. doi: 10.1126/scitranslmed.abc3961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gomez Mendez LM, Cascino MD, Garg J, Katsumoto TR, Brakeman P, Dall’Era M, et al. Peripheral blood B cell depletion after rituximab and complete response in lupus nephritis. Clin J Am Soc Nephrol 2018, 13, 1502–9. doi: 10.2215/CJN.01070118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vital EM, Dass S, Buch MH, Henshaw K, Pease CT, Martin MF, et al. B cell biomarkers of rituximab responses in systemic lupus erythematosus. Arthritis Rheum 2011, 63, 3038–47. doi: 10.1002/art.30466 [DOI] [PubMed] [Google Scholar]
- 27. Reddy V, Dahal LN, Cragg MS, Leandro M.. Optimising B-cell depletion in autoimmune disease: is obinutuzumab the answer? Drug Discov Today 2016, 21, 1330–8. doi: 10.1016/j.drudis.2016.06.009 [DOI] [PubMed] [Google Scholar]
- 28. Mei HE, Schmidt S, Dorner T.. Rationale of anti-CD19 immunotherapy: an option to target autoreactive plasma cells in autoimmunity. Arthritis Res Ther 2012, 14, S1. doi: 10.1186/ar3909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramwadhdoebe TH, van Baarsen LGM, Boumans MJH, Bruijnen STG, Safy M, Berger FH, et al. Effect of rituximab treatment on T and B cell subsets in lymph node biopsies of patients with rheumatoid arthritis. Rheumatology (Oxford) 2019, 58, 1075–85. doi: 10.1093/rheumatology/key428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thurlings RM, Vos K, Wijbrandts CA, Zwinderman AH, Gerlag DM, Tak PP.. Synovial tissue response to rituximab: mechanism of action and identification of biomarkers of response. Ann Rheum Dis 2008, 67, 917–25. doi: 10.1136/ard.2007.080960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Teng YK, Levarht EW, Toes RE, Huizinga TW, van Laar JM.. Residual inflammation after rituximab treatment is associated with sustained synovial plasma cell infiltration and enhanced B cell repopulation. Ann Rheum Dis 2009, 68, 1011–6. doi: 10.1136/ard.2008.092791 [DOI] [PubMed] [Google Scholar]
- 32. Nakou M, Katsikas G, Sidiropoulos P, Bertsias G, Papadimitraki E, Raptopoulou A, et al. Rituximab therapy reduces activated B cells in both the peripheral blood and bone marrow of patients with rheumatoid arthritis: depletion of memory B cells correlates with clinical response. Arthritis Res Ther 2009, 11, R131. doi: 10.1186/ar2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stathopoulos P, Kumar A, Nowak RJ, O’Connor KC.. Autoantibody-producing plasmablasts after B cell depletion identified in muscle-specific kinase myasthenia gravis. JCI Insight 2017, 2, e94263. doi: 10.1172/jci.insight.94263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mei HE, Wirries I, Frolich D, Brisslert M, Giesecke C, Grun JR, et al. A unique population of IgG-expressing plasma cells lacking CD19 is enriched in human bone marrow. Blood 2015, 125, 1739–48. doi: 10.1182/blood-2014-02-555169 [DOI] [PubMed] [Google Scholar]
- 35. Halliley JL, Tipton CM, Liesveld J, Rosenberg AF, Darce J, Gregoretti IV, et al. Long-lived plasma cells are contained within the CD19(−)CD38(hi)CD138(+) subset in human bone marrow. Immunity 2015, 43, 132–45. doi: 10.1016/j.immuni.2015.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cragg MS, Morgan SM, Chan HTC, Morgan BP, Filatov AV, Johnson PWM, et al. Complement-mediated lysis by anti-CD20 mAb correlates with segregation into lipid rafts. Blood 2003, 101, 1045–52. doi: 10.1182/blood-2002-06-1761 [DOI] [PubMed] [Google Scholar]
- 37. Lim SH, Vaughan AT, Ashton-Key M, Williams EL, Dixon SV, Chan HTC, et al. Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy. Blood 2011, 118, 2530–40. doi: 10.1182/blood-2011-01-330357 [DOI] [PubMed] [Google Scholar]
- 38. Reddy V, Cambridge G, Isenberg DA, Glennie MJ, Cragg MS, Leandro M.. Internalization of rituximab and the efficiency of B cell depletion in rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheumatol 2015, 67, 2046–55. doi: 10.1002/art.39167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Furie RA, Aroca G, Cascino MD, Garg JP, Rovin BH, Alvarez A, et al. B-cell depletion with obinutuzumab for the treatment of proliferative lupus nephritis: a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis 2022, 81, 100–7. doi: 10.1136/annrheumdis-2021-220920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mok CC, Lau CS.. Pathogenesis of systemic lupus erythematosus. J Clin Pathol 2003, 56, 481–90. doi: 10.1136/jcp.56.7.481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gong Q, Ou Q, Ye S, Lee WP, Cornelius J, Diehl L, et al. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol 2005, 174, 817–26. doi: 10.4049/jimmunol.174.2.817 [DOI] [PubMed] [Google Scholar]
- 42. Reddy VR, Pepper RJ, Shah K, Cambridge G, Henderson SR, Klein C, et al. Disparity in peripheral and renal B-cell depletion with rituximab in systemic lupus erythematosus: an opportunity for obinutuzumab? Rheumatology 2021, 61, 2894–904. doi: 10.1093/rheumatology/keab827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chang A, Henderson SG, Brandt D, Liu N, Guttikonda R, Hsieh C, et al. In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J Immunol (Baltimore, Md. : 1950) 2011, 186, 1849–60. doi: 10.4049/jimmunol.1001983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fetter T, Niebel D, Braegelmann C, Wenzel J.. Skin-associated B cells in the pathogenesis of cutaneous autoimmune diseases—implications for therapeutic approaches. Cells 2020, 9, 2627. doi: 10.3390/cells9122627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carter RH, Fearon DT.. CD19: lowering the threshold for antigen receptor stimulation of B lymphocytes. Science 1992, 256, 105–7. doi: 10.1126/science.1373518 [DOI] [PubMed] [Google Scholar]
- 46. Tedder TF, Inaoki M, Sato S.. The CD19-CD21 complex regulates signal transduction thresholds governing humoral immunity and autoimmunity. Immunity 1997, 6, 107–18. doi: 10.1016/s1074-7613(00)80418-5 [DOI] [PubMed] [Google Scholar]
- 47. van Zelm MC, Reisli I, van der Burg M, Castaño D, van Noesel CJM, van Tol MJD, et al. An antibody-deficiency syndrome due to mutations in the CD19 gene. N Engl J Med 2006, 354, 1901–12. doi: 10.1056/NEJMoa051568 [DOI] [PubMed] [Google Scholar]
- 48. Glass DR, Tsai AG, Oliveria JP, Hartmann FJ, Kimmey SC, Calderon AA, et al. An integrated multi-omic single-cell atlas of human B cell identity. Immunity 2020, 53, 217–32.e5. doi: 10.1016/j.immuni.2020.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wing E, Sutherland C, Miles K, Gray D, Goodyear CS, Otto TD, et al. Double-negative-2 B cells are the major synovial plasma cell precursor in rheumatoid arthritis. Front Immunol 2023, 14, 1241474. doi: 10.3389/fimmu.2023.1241474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mackensen A, Müller F, Mougiakakos D, Böltz S, Wilhelm A, Aigner M, et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat Med 2022, 28, 2124–32. doi: 10.1038/s41591-022-02017-5 [DOI] [PubMed] [Google Scholar]
- 51. Cree BAC, Bennett JL, Kim HJ, Weinshenker BG, Pittock SJ, Wingerchuk DM, et al.; N-MOmentum study investigators. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet 2019, 394, 1352–63. doi: 10.1016/S0140-6736(19)31817-3 [DOI] [PubMed] [Google Scholar]
- 52. Schiopu E, Chatterjee S, Hsu V, Flor A, Cimbora D, Patra K, et al. Safety and tolerability of an anti-CD19 monoclonal antibody, MEDI-551, in subjects with systemic sclerosis: a phase I, randomized, placebo-controlled, escalating single-dose study. Arthritis Res Ther 2016, 18, 131. doi: 10.1186/s13075-016-1021-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Herbst R, Wang Y, Gallagher S, Mittereder N, Kuta E, Damschroder M, et al. B-cell depletion in vitro and in vivo with an afucosylated anti-CD19 antibody. J Pharmacol Exp Ther 2010, 335, 213–22. doi: 10.1124/jpet.110.168062 [DOI] [PubMed] [Google Scholar]
- 54. Reddy V, Cambridge G, Isenberg DA, Glennie MJ, Cragg MS, Leandro M.. Internalization of rituximab and the efficiency of B cell depletion in rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheumatol 2015, 67, 2046–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cappell KM, Kochenderfer JN.. Long-term outcomes following CAR T cell therapy: what we know so far. Nat Rev Clin Oncol 2023, 20, 359–71. doi: 10.1038/s41571-023-00754-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhao J, Lin Q, Song Y, Liu D.. Universal CARs, universal T cells, and universal CAR T cells. J Hematol Oncol 2018, 11, 132. doi: 10.1186/s13045-018-0677-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Feins S, Kong W, Williams EF, Milone MC, Fraietta JA.. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. Am J Hematol 2019, 94, S3–9. doi: 10.1002/ajh.25418 [DOI] [PubMed] [Google Scholar]
- 58. Mullard A. FDA approves fourth CAR-T cell therapy. Nat Rev Drug Discov 2021, 20, 166. doi: 10.1038/d41573-021-00030-w [DOI] [PubMed] [Google Scholar]
- 59. Cappell KM, Sherry RM, Yang JC, Goff SL, Vanasse DA, McIntyre L, et al. Long-term follow-up of anti-CD19 chimeric antigen receptor T-cell therapy. J Clin Oncol: Off J Am Soc Clin Oncol 2020, 38, 3805–15. doi: 10.1200/JCO.20.01467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kansal R, Richardson N, Neeli I, Khawaja S, Chamberlain D, Ghani M, et al. Sustained B cell depletion by CD19-targeted CAR T cells is a highly effective treatment for murine lupus. Sci Transl Med 2019, 11, eaav1648. doi: 10.1126/scitranslmed.aav1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Taubmann J, Müller F, Boeltz S, Völkl S, Aigner M, Kleyer A, et al. OP0141 Long term safety and efficacy of CAR-T cell treatment in refractory systemic lupus erythematosus—data from the first seven patients. Ann Rheum Dis 2023, 82, 93–4. [Google Scholar]
- 62. Nunez D, Patel D, Volkov J, Wong S, Vorndran Z, Müller F, et al. Cytokine and reactivity profiles in SLE patients following anti-CD19 CART therapy. Mol Ther Methods Clin Dev 2023, 31, 101104. doi: 10.1016/j.omtm.2023.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Müller F, Boeltz S, Knitza J, Aigner M, Völkl S, Kharboutli S, et al. CD19-targeted CAR T cells in refractory antisynthetase syndrome. Lancet (London, England) 2023, 401, 815–8. doi: 10.1016/S0140-6736(23)00023-5 [DOI] [PubMed] [Google Scholar]
- 64. Bergmann C, Müller F, Distler JHW, Györfi A-H, Völkl S, Aigner M, et al. Treatment of a patient with severe systemic sclerosis (SSc) using CD19-targeted CAR T cells. Ann Rheum Dis 2023, 82, 1117–20. doi: 10.1136/ard-2023-223952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Griffin J, Xue S, Carpenter B, Velica P, Holler A, Nicholson E, et al. T cells engineered for resistance to mycophenolate mofetil demonstrate enhanced expansion and improved tumour control in immunosuppressed hosts. Mol Ther 2014, 22, S58. [Google Scholar]
- 66. Kretschmann S, Völkl S, Reimann H, Krönke G, Schett G, Achenbach S, et al. Successful generation of CD19 chimeric antigen receptor T cells from patients with advanced systemic lupus erythematosus. Transplant Cell Ther 2023, 29, 27–33. doi: 10.1016/j.jtct.2022.10.004 [DOI] [PubMed] [Google Scholar]
- 67. Wat J, Barmettler S.. Hypogammaglobulinemia after chimeric antigen receptor (CAR) T-cell therapy: characteristics, management, and future directions. J Allergy Clin Immunol Pract 2022, 10, 460–6. doi: 10.1016/j.jaip.2021.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hill JA, Giralt S, Torgerson TR, Lazarus HM.. CAR-T—and a side order of IgG, to go? Immunoglobulin replacement in patients receiving CAR-T cell therapy. Blood Rev 2019, 38, 100596. doi: 10.1016/j.blre.2019.100596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wiedmeier-Nutor JE, Iqbal M, Rosenthal AC, Bezerra ED, Garcia-Robledo JE, Bansal R, et al. Response to COVID-19 vaccination post-CAR T therapy in patients with non-hodgkin lymphoma and multiple myeloma. Clin Lymphoma Myeloma Leuk 2023, 23, 456–62. doi: 10.1016/j.clml.2023.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bhoj VG, Arhontoulis D, Wertheim G, Capobianchi J, Callahan CA, Ellebrecht CT, et al. Persistence of long-lived plasma cells and humoral immunity in individuals responding to CD19-directed CAR T-cell therapy. Blood 2016, 128, 360–70. doi: 10.1182/blood-2016-01-694356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gupta S, Simic M, Sagan SA, Shepherd C, Duecker J, Sobel RA, et al. CAR-T cell–mediated B-cell depletion in central nervous system autoimmunity. Neurology - Neuroimmunology Neuroinflammation 2023, 10, e200080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Granit V, Benatar M, Kurtoglu M, Miljković MD, Chahin N, Sahagian G, et al.; MG-001 Study Team. Safety and clinical activity of autologous RNA chimeric antigen receptor T-cell therapy in myasthenia gravis (MG-001): a prospective, multicentre, open-label, non-randomised phase 1b/2a study. Lancet Neurol 2023, 22, 578–90. doi: 10.1016/S1474-4422(23)00194-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mamlouk O, Nair R, Iyer SP, Edwards A, Neelapu SS, Steiner RE, et al. Safety of CAR T-cell therapy in kidney transplant recipients. Blood 2021, 137, 2558–62. doi: 10.1182/blood.2020008759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Krishnamoorthy S, Ghobadi A, Santos RD, Schilling JD, Malone AF, Murad H, et al. CAR-T therapy in solid organ transplant recipients with treatment refractory posttransplant lymphoproliferative disorder. Am J Transplant 2021, 21, 809–14. doi: 10.1111/ajt.16367 [DOI] [PubMed] [Google Scholar]
- 75. Oren D, DeFilippis EM, Lotan D, Clerkin KJ, Fried J, Reshef R, et al. Successful CAR T cell therapy in a heart and kidney transplant recipient with refractory PTLD. JACC CardioOncol 2022, 4, 713–6. doi: 10.1016/j.jaccao.2022.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pecher AC, Hensen L, Klein R, Schairer R, Lutz K, Atar D, et al. CD19-targeting CAR T cells for myositis and interstitial lung disease associated with antisynthetase syndrome. JAMA 2023, 329, 2154–62. doi: 10.1001/jama.2023.8753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lee J, Lundgren DK, Mao X, Manfredo-Vieira S, Nunez-Cruz S, Williams EF, et al. Antigen-specific B cell depletion for precision therapy of mucosal pemphigus vulgaris. J Clin Invest 2020, 130, 6317–24. doi: 10.1172/JCI138416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhang L, Sosinowski T, Cox AR, Cepeda JR, Sekhar NS, Hartig SM, et al. Chimeric antigen receptor (CAR) T cells targeting a pathogenic MHC class II:peptide complex modulate the progression of autoimmune diabetes. J Autoimmun 2019, 96, 50–8. doi: 10.1016/j.jaut.2018.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Guidetti A, Perrone G, Coluccia P, Fumagalli L, Dodero A, Farina L, et al. The real life accessibility to CAR T-cell therapy: current experience in the only active center in Italy. Blood 2019, 134, 5619–5619. doi: 10.1182/blood-2019-125286 [DOI] [Google Scholar]
- 80. Jo S, Das S, Williams A, Chretien A-S, Pagliardini T, Le Roy A, et al. Endowing universal CAR T-cell with immune-evasive properties using TALEN-gene editing. Nat Commun 2022, 13, 3453. doi: 10.1038/s41467-022-30896-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bacac M, Colombetti S, Herter S, Sam J, Perro M, Chen S, et al. CD20-TCB with obinutuzumab pretreatment as next-generation treatment of hematologic malignancies. Clin Cancer Res 2018, 24, 4785–97. doi: 10.1158/1078-0432.CCR-18-0455 [DOI] [PubMed] [Google Scholar]
- 82. Ball ED, Guyre PM, Mills L, Fisher J, Dinces NB, Fanger MW.. Initial trial of bispecific antibody-mediated immunotherapy of CD15-bearing tumors: cytotoxicity of human tumor cells using a bispecific antibody comprised of anti-CD15 (MoAb PM81) and anti-CD64/Fc gamma RI (MoAb 32). J Hematother 1992, 1, 85–94. doi: 10.1089/scd.1.1992.1.85 [DOI] [PubMed] [Google Scholar]
- 83. Seimetz D, Lindhofer H, Bokemeyer C.. Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM x anti-CD3) as a targeted cancer immunotherapy. Cancer Treat Rev 2010, 36, 458–67. doi: 10.1016/j.ctrv.2010.03.001 [DOI] [PubMed] [Google Scholar]
- 84. Przepiorka D, Ko CW, Deisseroth A, Yancey CL, Candau-Chacon R, Chiu HJ, et al. FDA approval: blinatumomab. Clin Cancer Res 2015, 21, 4035–9. doi: 10.1158/1078-0432.CCR-15-0612 [DOI] [PubMed] [Google Scholar]
- 85. Tapia-Galisteo A, Álvarez-Vallina L, Sanz L.. Bi- and trispecific immune cell engagers for immunotherapy of hematological malignancies. J Hematol Oncol 2023, 16, 83. doi: 10.1186/s13045-023-01482-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kontermann RE, Brinkmann U.. Bispecific antibodies. Drug Discov Today 2015, 20, 838–47. doi: 10.1016/j.drudis.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 87. Gruber M, Schodin BA, Wilson ER, Kranz DM.. Efficient tumor cell lysis mediated by a bispecific single chain antibody expressed in Escherichia coli. J Immunol 1994, 152, 5368–74. [PubMed] [Google Scholar]
- 88. Ridgway JB, Presta LG, Carter P.. ‘Knobs-into-holes’ engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng 1996, 9, 617–21. doi: 10.1093/protein/9.7.617 [DOI] [PubMed] [Google Scholar]
- 89. Klein C, Sustmann C, Thomas M, Stubenrauch K, Croasdale R, Schanzer J, et al. Progress in overcoming the chain association issue in bispecific heterodimeric IgG antibodies. MAbs 2012, 4, 653–63. doi: 10.4161/mabs.21379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Surowka M, Schaefer W, Klein C.. Ten years in the making: application of CrossMab technology for the development of therapeutic bispecific antibodies and antibody fusion proteins. mAbs 2021, 13, 1967714. doi: 10.1080/19420862.2021.1967714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hoffmann P, Hofmeister R, Brischwein K, Brandl C, Crommer S, Bargou R, et al. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct. Int J Cancer 2005, 115, 98–104. doi: 10.1002/ijc.20908 [DOI] [PubMed] [Google Scholar]
- 92. d’Argouges S, Wissing S, Brandl C, Prang N, Lutterbuese R, Kozhich A, et al. Combination of rituximab with blinatumomab (MT103/MEDI-538), a T cell-engaging CD19-/CD3-bispecific antibody, for highly efficient lysis of human B lymphoma cells. Leuk Res 2009, 33, 465–73. doi: 10.1016/j.leukres.2008.08.025 [DOI] [PubMed] [Google Scholar]
- 93. Sun LL, Ellerman D, Mathieu M, Hristopoulos M, Chen X, Li Y, et al. Anti-CD20/CD3 T cell–dependent bispecific antibody for the treatment of B cell malignancies. Sci Transl Med 2015, 7, 287ra70–ra70. doi: 10.1126/scitranslmed.aaa4802 [DOI] [PubMed] [Google Scholar]
- 94. Engelberts PJ, Hiemstra IH, de Jong B, Schuurhuis DH, Meesters J, Beltran Hernandez I, et al. DuoBody-CD3xCD20 induces potent T-cell-mediated killing of malignant B cells in preclinical models and provides opportunities for subcutaneous dosing. EBioMedicine 2020, 52, 102625. doi: 10.1016/j.ebiom.2019.102625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Klein C, Neumann C, Fauti T, Weinzierl T, Freimoser-Grundschober A, Waldhauer I, et al. Abstract 3629: Engineering a novel asymmetric head-to-tail 2 + 1 T-cell bispecific (2 + 1 TCB) IgG antibody platform with superior T-cell killing compared to 1 + 1 asymmetric TCBs. Cancer Res 2017, 77, 3629–3629. doi: 10.1158/1538-7445.am2017-3629 [DOI] [Google Scholar]
- 96. Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, et al. The immunological synapse: a molecular machine controlling T cell activation. Science 1999, 285, 221–7. doi: 10.1126/science.285.5425.221 [DOI] [PubMed] [Google Scholar]
- 97. Martz E. Multiple target cell killing by the cytolytic T lymphocyte and the mechanism of cytotoxicity. Transplantation 1976, 21, 5–11. doi: 10.1097/00007890-197601000-00002 [DOI] [PubMed] [Google Scholar]
- 98. NICE. Axicabtagene ciloleucel for treating relapsed or refractory diffuse large B-cell lymphoma after first-line chemoimmunotherapy. Technology appraisal guidance [TA895]. 2023, 1–24.
- 99. NICE. Blinatumomab for treating acute lymphoblastic leukaemia in remission with minimal residual disease activity. Technology appraisal guidance [TA589]. 2019, 1–24.
- 100. Ross SL, Sherman M, McElroy PL, Lofgren JA, Moody G, Baeuerle PA, et al. Bispecific T cell engager (BiTE®) antibody constructs can mediate bystander tumor cell killing. PLoS One 2017, 12, e0183390. doi: 10.1371/journal.pone.0183390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rainone M, Ngo D, Baird JH, Budde LE, Htut M, Aldoss I, et al. Interferon-γ blockade in CAR T-cell therapy-associated macrophage activation syndrome/hemophagocytic lymphohistiocytosis. Blood Adv 2023, 7, 533–6. doi: 10.1182/bloodadvances.2022008256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lerkvaleekul B, Vilaiyuk S.. Macrophage activation syndrome: early diagnosis is key. Open Access Rheumatol 2018, 10, 117–28. doi: 10.2147/OARRR.S151013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhang C, Liu J, Zhong JF, Zhang X.. Engineering CAR-T cells. Biomarker Res 2017, 5, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Crespo J, Sun H, Welling TH, Tian Z, Zou W.. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol 2013, 25, 214–21. doi: 10.1016/j.coi.2012.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhang K, Kong X, Li Y, Wang Z, Zhang L, Xuan L.. PD-1/PD-L1 Inhibitors in patients with preexisting autoimmune diseases. Front Pharmacol 2022, 13, 854967. doi: 10.3389/fphar.2022.854967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wu L, Seung E, Xu L, Rao E, Lord DM, Wei RR, et al. Trispecific antibodies enhance the therapeutic efficacy of tumor-directed T cells through T cell receptor co-stimulation. Nat Cancer 2020, 1, 86–98. doi: 10.1038/s43018-019-0004-z [DOI] [PubMed] [Google Scholar]
- 107. McKinney EF, Lee JC, Jayne DRW, Lyons PA, Smith KGC.. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature 2015, 523, 612–6. doi: 10.1038/nature14468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Singh A, Dees S, Grewal IS.. Overcoming the challenges associated with CD3+ T-cell redirection in cancer. Br J Cancer 2021, 124, 1037–48. doi: 10.1038/s41416-020-01225-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Le RQ, Li L, Yuan W, Shord SS, Nie L, Habtemariam BA, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist 2018, 23, 943–7. doi: 10.1634/theoncologist.2018-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Leclercq G, Haegel H, Toso A, Zimmermann T, Green L, Steinhoff N, et al. JAK and mTOR inhibitors prevent cytokine release while retaining T cell bispecific antibody in vivo efficacy. J ImmunoTher Cancer 2022, 10, e003766. doi: 10.1136/jitc-2021-003766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zhou S, Liu M, Ren F, Meng X, Yu J.. The landscape of bispecific T cell engager in cancer treatment. Biomarker Res 2021, 9, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Nakamura M, Ogawa N, Shalabi A, Maley WR, Longo D, Burdick JF.. Positive effect on T-cell regulatory apoptosis by mycophenolate mofetil. Clin Transplant 2001, 15, 36–40. doi: 10.1034/j.1399-0012.2001.00006.x [DOI] [PubMed] [Google Scholar]
- 113. Eugui EM, Mirkovich A, Allison AC.. Lymphocyte-selective antiproliferative and immunosuppressive effects of mycophenolic acid in mice. Scand J Immunol 1991, 33, 175–83. doi: 10.1111/j.1365-3083.1991.tb03747.x [DOI] [PubMed] [Google Scholar]
- 114. Prémaud A, Rousseau A, Johnson G, Canivet C, Gandia P, Muscari F, et al. Inhibition of T-cell activation and proliferation by mycophenolic acid in patients awaiting liver transplantation: PK/PD relationships. Pharmacol Res 2011, 63, 432–8. doi: 10.1016/j.phrs.2011.01.005 [DOI] [PubMed] [Google Scholar]
- 115. Ellebrecht CT, Bhoj VG, Nace A, Choi EJ, Mao X, Cho MJ, et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science 2016, 353, 179–84. doi: 10.1126/science.aaf6756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Reddy V, Jayne D, Close D, Isenberg D.. B-cell depletion in SLE: clinical and trial experience with rituximab and ocrelizumab and implications for study design. Arthritis Res Ther 2013, 15, S2–16. doi: 10.1186/ar3910 [DOI] [PMC free article] [PubMed] [Google Scholar]