Abstract
The medicinal plant Portulaca oleraceae has a long history of usage in traditional medicine. Plant extracts have several interesting pharmacological effects but have some drawbacks that can be addressed via capsulation with chitosan. This work set out to do just that tally up the antioxidant effects of a polyphenol-rich P. olerace extract and see how capsulation affected them. The reflux extraction and response surface methodology (RSM) were carried out to optimize the phenolic and flavonoid content of P. oleraceae extract. Additionally, high-resolution mass spectrometry was employed to determine the secondary metabolite present in the extract. The microcapsules of extract-loaded chitosan were prepared using the ionic gelation method and characterized in terms of size, encapsulation efficiency (EE), and morphology of microcapsules. Fourier transform infrared (FTIR) was used to observe the successful production of microcapsules with a principal component analysis (PCA) approach. The antioxidant activity of microcapsules was established using the radical scavenging method. According to RSM, the highest amounts of TPC and TFC were obtained at 72.894 % ethanol, 2.031 h, and 57.384 °C. The compounds were employed from the optimized extract of P. oleraceae including phenolics and flavonoids. The microcapsules were secured with a %EE of 43.56 ± 2.31 %. The characteristics of microcapsules were approved for the obtained product's successful synthesis according to the PCA. The microcapsules have antioxidant activity in a concentration-dependent manner (p < 0.0001). The findings of this study underscored the benefits of employing chitosan as a nanocarrier for extract, offering a promising approach to enhance plant-derived therapies.
Keywords: Portulaca olearacea, Response surface methodology, Ionic gelation, Antioxidant activity
1. Introduction
Currently, the use of extracts in disease treatment and prevention has increased rapidly [1]. This can be seen from the large number of studies that use extracts as subjects for more in-depth exploration regarding their function in health [2]. Several studies reported that extracts have properties and health benefits because they contain secondary metabolite compounds that have certain activities on the physiology of living things [3]. The increase in pharmacological activity will be directly influenced by the large content of secondary metabolites in the extracts tested and the response of the body of living creatures in processing these secondary metabolite compounds. Therefore, these factors must be considered by researchers in developing extracts as traditional medicines to provide the expected pharmacological effects [[4], [5], [6]].
Indonesia is one of the countries that uses many medicinal plants for the treatment and prevention of disease [7]. This is supported by various factors, including the Indonesian people's long-standing habit of using natural medicines and the abundant natural resources in Indonesia [8]. It is reported that Portulaca oleraceae is a medicinal plant that is often used traditionally to treat and prevent various diseases [9]. This herb is highly abundant in vital nutrients, primarily minerals, vitamins A, C, E, and B, and omega-3 fatty acids. It also includes bioactive phytochemicals like carotenoids and phenolic antioxidants, scientifically proven to provide significant health advantages [10]. This herb exhibits many activities, including antioxidant [11], anti-inflammatory [12], anticancer [13], antidiabetic [14], hepatoprotective [15], analgesic [16], skeletal muscle-relaxant [17], gastroprotective [18], neuroprotective [19], wound-healing [20], and antibacterial properties [21]. However, the effect is considered not optimal and can still be optimized through various methods such as optimizing the polyphenol content in the extract and increasing its bioavailability.
The positive effects mostly arise from the presence of polyphenols, such as flavonoids (particularly quercetin and kaempferol glycosides), phenolic acids, and proanthocyanidins [22]. It has been reported that increasing the amount of polyphenols in the extract will have a positive influence on the biological activity of the extract [[23], [24], [25], [26], [27]]. To obtain the optimal amount of polyphenols in the extract, several approaches need to be taken, such as choosing the right extraction method and monitoring extraction factors [28]. It has been reported that the extraction method has a significant influence on the withdrawal of active plant compounds. This is followed by the influence of factors in the process of producing optimal polyphenol levels [29]. One method that is often used to produce extracts with high amounts of polyphenols is reflux. Reflux is an extraction method at a certain temperature to speed up the extraction process and increase extraction efficiency [30,31]. However, time, temperature, and solvent used will have their influence and interact with each other. Therefore, efforts are needed to determine the best conditions in the extraction process that can produce extracts with optimum polyphenol levels using the Response Surface Methodology (RSM) [32].
Response Surface Methodology (RSM) is a combined method of mathematical and statistical approaches that can predict the best conditions of a process to produce products with certain conditions [33]. This response will illustrate how each variable influences the polyphenol content of the extract and the interaction effect of these factors [34]. The results of the analysis will describe the best conditions for producing extracts that have optimum polyphenolic compounds [35]. In this study, polyphenolic compounds will be described by the total phenol content and total flavonoid content of the extract as gallic acid equivalents and quercetin equivalents. As stated above, the amount of secondary metabolite compounds in an extract is not the only thing that influences the biological activity of the extract. The interaction of the extract with the body is another important factor for assessing the biological effectiveness of the extract [30]. The primary hindrance to the ineffective usage of these essential properties is the propensity of polyphenols to deteriorate during storage under varying temperatures, humidity levels, light exposure, and pH conditions [36]. In addition to their limited ability to be absorbed in the gastrointestinal tract, these substances also have low release from the food matrix, are unstable during digestion, and have limited diffusion across cell membranes. Therefore, microencapsulation was intended to safeguard these constituents [37].
The utilization of various techniques for microencapsulation has emerged as a viable solution to address the above limitations and enable the incorporation of polyphenols into diverse functional products [38]. The spray-drying process has been a widely used method for many years to encapsulate natural materials due to its cost-effectiveness and the abundance of equipment accessible for this purpose [39]. The process involves the conversion of a liquid substance into powder form through the utilization of carrier agent ingredients [40]. Chitosan, a chitin derivative that has undergone partial deacetylation and is made up of N-acetylglucosamine, has gained recognition as a noteworthy biomaterial and pharmaceutical excipient for delivering drugs [41]. This is due to its capacity to interact well with living organisms, break down naturally, provoke minimal immune response, and be obtained at a reasonable price [42]. Furthermore, its pharmaceutical applications are enhanced by its moderate toxicity as well as its hemostatic and bacteriostatic abilities [43]. This research focuses on developing microencapsulates from chitosan and polyphenol-rich extracts from P. oleraceae which will be reflected in the percentage of encapsulate efficiency. In addition, the resulting microencapsulates will be tested for their antioxidant activity, thereby increasing confidence in the resulting microencapsulates.
2. Materials and methods
2.1. Materials
The following chemicals were supplied by Sigma-Aldrich Corporation (St. Louis, MO, USA): chitosan extracted from shrimp shells, sodium tripolyphosphate, methyl alcohol, ethyl alcohol, glacial acetic acid, DPPH (2,2-diphenyl-1-picryhydrazyl), Folin-ciocalteu, aluminum chloride, gallic acid (3,4,5-trihydroxybenzoic acid), sodium citrate, and quercetin (3,3′,4’,5,6-pentahydroxyflavone). All of these compounds were of the utmost purity grade.
2.2. Sample preparation and extraction
The P. oleraceae was collected from Sidodadi Regency, North Sumatera, Indonesia. The plant was found at 98°48′1.548″ East longitude and 3°38′36.225″ North latitude. The plant was identified as P. oleraceae by Medanese Herbarium, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara Indonesia (Voucher Number: 305/UN5.2.1.1.2.4/Hmed/2023). The entire plant excluding a root was dried at 50 °C for 48 h. Approximately 500 g of the final dry matter content was ground into a fine powder and stored in a vacuum bag for preservation.
The extraction process was carried out using conventional reflux. All factors in extraction were prepared based on the results of Design-Expert V.13. Three factors were conducted for this extraction including extraction time (X1), temperature (X2), and solvent concentration (X3). Seventeen experimental from three factors combinations were obtained for the extraction process [44]. Briefly, 20 g dry powder of P. oleraceae was soaked with 200 mL solvents in the bottom round flask. The extraction conditions were followed in Table 1. After the extraction process for each condition, the crude extract was filtrated with filter paper (4–12 μm). All collected samples were placed into a glass flask and concentrated using a rotary evaporator vacuum with slow speed at 110 rpm, at 65 °C, for about 3 h.
Table 1.
Box-Behnken design (BBD) for independent variables and corresponding response values (experimental).
| Run | Independent variables |
Responses |
|||
|---|---|---|---|---|---|
| X1 (%) | X2 (°C) | X3 (hours) | TPC (mg GAE/ g sample) | TFC (mg QE/ g sample) | |
| 1 | 90 | 60 | 1 | 6.90 ± 0.21 | 20.97 ± 0.92 |
| 2 | 90 | 50 | 2 | 7.32 ± 0.28 | 21.57 ± 0.85 |
| 3 | 70 | 60 | 2 | 9.52 ± 0.35 | 26.95 ± 0.95 |
| 4 | 70 | 60 | 2 | 9.01 ± 0.31 | 28.01 ± 0.79 |
| 5 | 90 | 70 | 2 | 5.12 ± 0.15 | 19.96 ± 0.63 |
| 6 | 90 | 60 | 3 | 5.82 ± 0.14 | 18.36 ± 0.92 |
| 7 | 70 | 70 | 3 | 6.72 ± 0.27 | 25.91 ± 1.13 |
| 8 | 50 | 50 | 2 | 4.78 ± 0.12 | 17.03 ± 0.82 |
| 9 | 50 | 60 | 1 | 3.20 ± 0.09 | 17.73 ± 0.87 |
| 10 | 70 | 60 | 2 | 8.78 ± 0.30 | 27.21 ± 1.67 |
| 11 | 50 | 70 | 2 | 2.97 ± 0.08 | 24.72 ± 1.21 |
| 12 | 70 | 60 | 2 | 8.90 ± 0.31 | 27.35 ± 1.07 |
| 13 | 50 | 60 | 3 | 3.98 ± 0.09 | 17.95 ± 0.82 |
| 14 | 70 | 50 | 1 | 5.82 ± 0.15 | 26.31 ± 1.08 |
| 15 | 70 | 50 | 3 | 7.95 ± 0.40 | 25.31 ± 1.10 |
| 16 | 70 | 60 | 2 | 8.58 ± 0.43 | 28.62 ± 0.97 |
| 17 | 70 | 70 | 1 | 4.14 ± 0.11 | 23.85 ± 0.90 |
2.3. Determination of total phenolic and flavonoid contents
The crude extract's total phenolic content (TPC) was measured using a spectrophotometric method that has been previously published. In summary, a total of 100 μL of extract (used as a solvent for the blank), 200 μL of Folin–Ciocalteu reagent, 2 mL of distilled water, and 1 mL of Na2CO3 were combined in a reaction tube. The mixture was then incubated at a temperature of 50 °C for 25 min. Subsequently, the absorbance of the mixture was measured at a wavelength of 765 nm using a VWR UV-1600PC Spectrophotometer (VWR, Wayne, PA, USA), with gallic acid serving as the standard. The samples were subjected to triplicate analysis, and the total phenolic content (TPC) was quantified as the average value of gallic acid equivalents (GAE) in milligrams per gram of sample, with the standard deviation indicated [45].
In addition, the calorimetric method was employed to determine the total flavonoid content in the samples, building upon prior research. Quercetin was employed to establish standard calibration curves for the quantification of flavonoids. The samples were dissolved in 5 mL of distilled water and subjected to incubation at a temperature of 40 °C for 45 min. Subsequently, the samples were centrifuged at a speed of 1000 rpm for 10 min 1.2 mL of the produced solution was combined with an equal volume of 2 % aluminium chloride, resulting in a total volume of 2.4 mL. Following the mixing process, the solution was left to incubate for 60 min at room temperature. The solution's absorbance was quantified at a wavelength of 420 nm. The overall concentration of flavonoid content in the test sample was determined by analyzing the quercetin standard curve plot and reported as milligrams of quercetin equivalent (QE) per gram [45].
2.4. Conception of experiment and statistical analysis
This experiment was carried out utilizing independent variables, specifically X1, X2, and X3, each set at three different levels. The experimental design employed for this purpose was the BBD. The comprehensive design comprised a total of 17 experimental points. The encodings and actual values of the experimental design components are shown in Table 1.
Multiple regression analysis was applied to the BBD's experimental data to find a good fit with the second-order polynomial model, as shown below (Eq. (1)).
| (1) |
in the given equation, Y is the response function which comprises TPC (Y1) and TFC (Y2). Z0 is a constant term, while Zi, Zii, and Zij indicate the coefficients of the linear, quadratic, and interaction factors, respectively. Xi and Xj are the independent variables in the equation [46]. An analysis of variance (ANOVA) was conducted to assess the lack of fit, determination coefficient (R2), and the impact of linear, quadratic, and interaction terms on each response variable. The RSM was employed to construct three-dimensional charts representing the response surface. The experimental design, analysis of experimental data, model fitting, and optimization procedure were conducted using Design Expert V. 13 Software. To assess the distinction between the mean and the predicted value and observation value (by a one-sample t-test), as well as the Pearson correlation, the statistical software IBM SPSS Statistics (IBM Co., Ltd., America, United States) was employed [46].
2.5. Phytochemical analysis of polyphenol-rich extract
The analysis was conducted via liquid chromatography with the Thermo Scientific™ Vanquish™ UHPLC Binary Pump, coupled with Orbitrap high-resolution mass spectrometry using the Thermo Scientific™ Q Exactive™ Hybrid Quadrupole-Orbitrap™ High-Resolution Mass Spectrometer. The liquid chromatography analysis was conducted using a Thermo Scientific™ Accucore™ Phenyl-Hexyl analytical column with dimensions of 100 mm length, 2.1 mm inner diameter, and 2.6 μm particle size. The mobile phases employed in the experiment were MS-grade water with 0.1 % formic acid (A) and MS-grade methanol with 0.1 % formic acid (B). The experiment utilized a gradient approach with a flow rate of 0.3 mL/min. Initially, the mobile phase B was adjusted to a concentration of 5 % and then systematically raised to 90 % over a period of 16 min. Subsequently, it remained at a steady level of 90 % for a duration of 4 min, and then returned to the original state of 5 % B until 25 min had elapsed. The temperature of the column was adjusted to 40 °C, and the volume of the injection was 3 μL. The unspecific screening was conducted using complete MS/dd-MS2 acquisition mode in positive polarity [47].
2.6. Formulation and characterization of microencapsulate
2.6.1. Microencapsulate synthesis using ionic gelation method
The polyphenol-rich extract was encapsulated using the ionic gelation method in combination with sodium tripolyphosphate, following the approach outlined by Vonghirundecha et al. (2022) [48] with minor adjustments. In summary, the wall-material components of chitosan at a concentration of 0.1 % (w/v) were dissolved in a solution of 1 % (v/v) acetic acid and blended with sodium tripolyphosphate at a concentration of 2 % (w/v). The mixture was then agitated at a temperature of 65 °C until it became uniform. The process of encapsulation was accomplished by incorporating the polyphenol-rich extract into the wall material at a ratio of 1:1 (v/v) while continuously stirring at room temperature for the duration of one night.
2.6.2. Particle size and morphological analysis
The microcapsules of P. oleraceae extract with chitosan were analyzed for particle size using the CORDOUAN Technologies Particle Size Analyzer at room temperature [49]. Additionally, the surface morphology and structure of the microcapsules were analyzed using a scanning electron microscope (SEM) model TM 3000 Hitachi. The examination was conducted at magnifications 2500 × to accurately identify the particles' morphology [50].
2.6.3. Fourier-transform infrared (FT-IR) and principle component analysis
Chitosan powder, extract, and microcapsules were subjected to Fourier Transform Infra-Red (FT-IR) analysis using KBr pellets. The analysis was conducted in the region of 500–4000 cm−1 using a Shimadzu instrument. Following the measurements, a graph depicting the percentage of light transmission in relation to the wavelength was created for each sample. The distinctive peak corresponding to the chemical bond was identified [51]. The samples' FTIR spectra were subsequently analyzed using principal component analysis (PCA) in Minitab v.16 software (Minitab Inc., State College, PA). PCA operates by transforming the spectrum of an n-dimensional variable (spectra) into a PC, where each spectrum in the dataset is represented by a single score in a lower-dimensional space. The PCA are arranged in order of their eigenvalues, which measure the extent to which each PC captures variance. Subsequently, the PCA exhibiting the highest eigenvalues, which correspond to the most substantial changes in the FTIR spectra, are selected and preserved. Conversely, the PCA that solely encompass noise are rejected, since they possess the lowest eigenvalues. PCA score plots were employed to objectively classify samples based on their measured attributes [52].
2.6.4. Encapsulation efficiency
The encapsulation efficiency (EE) is the proportion of active chemicals that are enclosed within the polymer solution, represented as a percentage. The efficiency of shielding materials in trapping the extract is shown by this value. The calculation of the encapsulation efficiency was performed using Eq. (2) [53].
| (2) |
2.7. Antioxidant activity of microencapsulate using the DPPH method
The antioxidant activity of extract and microcapsules can be assessed by their ability to scavenge DPPH. The samples were generated at different concentrations, specifically 15.625, 31.25, 62.5, 125.00, 250.00, and 500.00 μg/mL. Subsequently, 100 μL of each sample were combined with 125 μL of DPPH methanolic solution and allowed to react at room temperature for a duration of 30 min. The spectrophotometric measurement of absorbance at 517 nm was conducted using a UVmini-1240 spectrophotometer (Shimadzu, Japan), with a blank sample as the reference. The trials were conducted three times in order to ensure accuracy, with ascorbic acid being utilized as a reference for comparison. The calculation of the free radical scavenging activity was performed using Eq. (3) [54].
| (3) |
3. Results and discussion
3.1. Fitting of the RSM
Table 1 describes the experimental conditions and conclusions for each extraction condition. All response variables were converted into second-order quadratic polynomial equations to accommodate variations in answers based on extraction factors. The statistical significance of the fitted second-order quadratic model equations was assessed using ANOVA. The regression coefficient (β), the adjusted correlation coefficient (R2), coefficient of variation (CV), and sufficient precision were employed to quantify the degree of fit of the model (Table 2). The terms with p-values greater than 0.05 were removed from the models to enhance the accuracy and predictive power. The p-values were utilized to determine the significance of each coefficient. When the p-values were less than 0.05, 0.01, and 0.001, the model terms were statistically significant, very significant, and extremely significant, respectively.
Table 2.
ANOVA for quadratic model.
| ANOVA for quadratic model for TPC | |||||||
|---|---|---|---|---|---|---|---|
| Source | RC | SS | DF | MS | F-value | p-value | |
| Model | 70.81 | 9 | 7.87 | 14.21 | 0.0010 | Significant | |
| Intercept | 8.96 | ||||||
| Linear terms | |||||||
| X1 | 1.28 | 13.08 | 1 | 13.08 | 23.63 | 0.0018 | Significant |
| X2 | −0.8650 | 5.99 | 1 | 5.99 | 10.81 | 0.0133 | Significant |
| X3 | 0.5513 | 2.43 | 1 | 2.43 | 4.39 | 0.0744 | Not significant |
| Interaction terms | |||||||
| X1X2 | −0.0975 | 0.0380 | 1 | 0.0380 | 0.0687 | 0.8008 | Not significant |
| X1X3 | −0.4650 | 0.8649 | 1 | 0.8649 | 1.56 | 0.2515 | Not significant |
| X2X3 | 0.1125 | 0.0506 | 1 | 0.0506 | 0.0914 | 0.7711 | Not significant |
| Quadratic terms | |||||||
| X12 | −2.55 | 27.30 | 1 | 27.30 | 49.32 | 0.0002 | Significant |
| X22 | −1.36 | 7.83 | 1 | 7.83 | 14.15 | 0.0071 | Significant |
| X32 | −1.44 | 8.69 | 1 | 8.69 | 15.69 | 0.0055 | Significant |
| Lack of fit | 3.38 | 3 | 1.13 | 9.07 | 0.0694 | Not significant | |
| Pure error | 0.4965 | 4 | 0.1241 | ||||
| R2 | 0.9481 | ||||||
| Adjusted R2 | 0.8814 | ||||||
| Adeq precision | 11.0009 | ||||||
| C.V.% | 11.55 | ||||||
| ANOVA for quadratic model for TFC | |||||||
|---|---|---|---|---|---|---|---|
| Source | RC | SS | DF | MS | F-value | p-value | |
| Model | 247.42 | 9 | 27.49 | 14.71 | 0.0009 | Significant | |
| Intercept | 8.96 | ||||||
| Linear terms | |||||||
| X1 | 1.28 | 1.47 | 1 | 1.47 | 0.7869 | 0.4045 | Not significant |
| X2 | −0.8650 | 2.23 | 1 | 2.23 | 1.19 | 0.3112 | Not significant |
| X3 | 0.5513 | 0.2211 | 1 | 0.2211 | 0.1183 | 0.7410 | Not significant |
| Interaction terms | |||||||
| X1X2 | −0.0975 | 21.62 | 1 | 21.62 | 11.57 | 0.0114 | Significant |
| X1X3 | −0.4650 | 2.00 | 1 | 2.00 | 1.07 | 0.3351 | Not significant |
| X2X3 | 0.1125 | 2.34 | 1 | 2.34 | 1.25 | 0.3000 | Not significant |
| Quadratic terms | |||||||
| X12 | −2.55 | 189.02 | 1 | 189.02 | 101.14 | <0.0001 | Significant |
| X22 | −1.36 | 0.0489 | 1 | 0.0489 | 0.0262 | 0.8761 | Not significant |
| X32 | −1.44 | 19.92 | 1 | 19.92 | 10.66 | 0.0138 | Significant |
| Lack of fit | 11.24 | 3 | 3.75 | 8.14 | 0.0754 | Not significant | |
| Pure error | 1.84 | 4 | 0.4604 | ||||
| R2 | 0.9498 | ||||||
| Adjusted R2 | 0.8852 | ||||||
| Adeq precision | 9.6226 | ||||||
| C.V.% | 5.84 | ||||||
Table 2 demonstrates that the model terms have a substantial impact, with a p-value of less than 0.001. The R2 values of the constructed regression models exceed 0.94, indicating a substantial degree of statistical significance. An optimal signal-to-noise ratio is indicated by a suitable level of precision, with a value greater than 4 being considered desirable according to Choi et al. (2022) [55]. The Adeq accuracy ratio for TPC and TFC is 11.00 and 9.62, respectively, indicating a strong signal and appropriateness for this approach. The CV quantifies the degree of consistency in a model, with TPC and TFC yielding values of 11.55 and 5.48, respectively. These values suggest that the model is highly replicable. Three-dimensional surfaces and contour plots were generated using several linear regression equations to illustrate the interactions between independent variables (Fig. 1. A and B).
Fig. 1.
The three-dimensional (3D) response surface plots of P. oleraceae extraction for TPC (A) and TFC (B) for solvent concentration, temperature, and extraction time as a function of key interaction factors for RSM.
3.2. Influence of variables on TPC and TFC
The TPC and TFC were determined for 17 extracts according to the experimental design outlined in Table 1. The extracts included TPC and TFC in the range of 2.97 ± 0.25 to 9.52 ± 0.35 mg GAE/g and 17.03 ± 0.64 to 28.62 ± 0.74 mg QE/g, respectively. The equations presented in Eqs. (4), (5)) are second-order polynomials that demonstrate the connections between TPC, TFC, and their respective variables.
| Y TPC (mg GAE/g) = 8.96 + 1.28 x X1 - 0.8650 x X2 + 0.5513 x X3 – 0.0975 x X1 x X2 – 0.4650 x X1 x X3 + 0.1125 x X2 x X3 - 2.55 x X12 – 1.36 x X22 – 1.44 x X32 | (4) |
| Y TFC (mg QE/g) = 27.63 + 0.4287 x X1 + 0.5275 x X2 – 0.1663 x X3 – 2.32 x X1 x X2 – 0.7075 x X1 x X3 + 0.7650 x X2 x X3 – 6.71 x X12 – 0.1078 x X22 - 2.18 x X32 | (5) |
The lack of fit values for TPC and TFC were not statistically significant (p = 0.0694 and 0.0754, respectively), indicating that the model successfully predicted R2 values of 0.9481 (TPC) and 0.9498 (TFC), as well as Adj. R2 values of 0.8814 (TPC) and 0.8852 (TFC) (Table 2). The RSM model precisely forecasted the effects of the parameters on the TPC and TFC of the extract. According to Fig. 1 A and B, the combination of 70 % ethanol and a temperature of 60 °C resulted in the highest TPC and TFC after 2 h. Sedraoui et al., found that medium-concentration ethanol increases the polarity of the solvent, resulting in the dissolution of phenolic compounds that are polar or moderately polar [56]. The presence of a moderate amount of ethanol in water can have an impact on the arrangement and composition of phospholipids in cell membranes. This phenomenon impacts the permeability of plant cells, resulting in increased extraction and diffusion of polyphenols [57]. The previous comparative study conducted experiments that revealed that the extraction of phenolic compounds from green tea leaves was enhanced by high hydrostatic pressure when ethanol was present in the solvent. The extraction reached its maximum efficiency at a concentration of 50 % ethanol and decreased thereafter [58].
3.3. Model validation
The desirability function concurrently optimizes TPC and TFC. The desirability function was employed to predict the parameters, enabling a multivariate analysis to determine the optimal value for all responses in a single extraction [59]. The study utilized the following conditions: X1 at 72.894 %, X2 at 57.384 °C, and X3 at 2.031 h, to attain the highest possible overall attractiveness, which was measured at D = 0.929. Given the ideal conditions, the expected values for TPC and TFC are 9.23 mg GAE/g and 27.476 mg QE/g, respectively. In order to establish the adequacy of the model equations, a replicated experiment was carried out in the optimal conditions projected by Derringer's desire model. The obtained results are as follows: TPC is 10.97 ± 0.74 mg GAE/g and TFC is 29.86 ± 0.94 mg QE/g. The model effectively optimized the shared extraction parameters for all answers, as demonstrated by the strong correspondence between experimental and anticipated values. The identification of phenolic and flavonoid components in the extract was conducted using liquid chromatography-orbitrap high-resolution mass spectrometry (LC-Orbitrap HRMS). Table 3 demonstrates the identification of 9 compounds as phenolic and flavonoid in positive mode using comprehensive MS/dd-MS2 acquisition data. This data includes the precursor ion mass, fragments, recognized fragmentation patterns specific to the compounds, neutral mass loss, and information obtained from literature and online databases.
Table 3.
The evaluation of phenolic and flavonoid compounds using LC-HRMS.
| Plant secondary metabolite | Chemical formula | m/z (M + H+) | Retention time (min) | Chemical structure |
|---|---|---|---|---|
| Trans-p-Coumaraldehyde | C9 H8 O2 | 149.05960 | 1.445 | 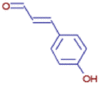 |
| 5-Pentylresorcinol | C11 H16 O2 | 181.12209 | 9.250 | 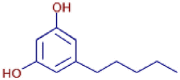 |
| (3R,4R)-4-(3,4-Dihydroxybenzyl)-3-(3-hydroxy benzyl)dihydro-2(3H)-furanone | C18 H18 O5 | 315.12219 | 10.207 | 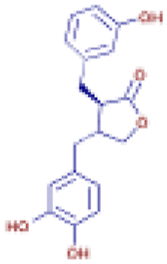 |
| 2-(2,4-Dimethoxyphenyl)-7-hydroxy-5-methoxy-2,3-dihydro-4H-chromen-4-one | C18 H18 O6 | 331.11710 | 10.910 | 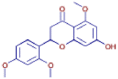 |
| 4-Vinylcatechol | C8 H8 O2 | 137.05966 | 0.806 | 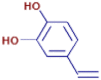 |
| Ophiopogonone B | C18 H16 O5 | 313.10651 | 10.109 | 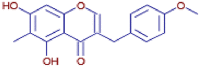 |
| 1-(5-Acetyl-2-hydroxyphenyl)- 3-methyl-1-butanone | C13 H16 O3 | 223.13261 | 5.913 | 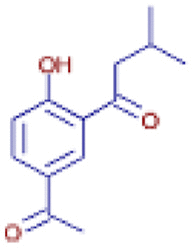 |
| 3-[(2E)-3,7-Dimethyl-2,6-octadien-1-yl]-5-methoxy-2-methyl-1,4-benzenediol | C18 H26 O3 | 331.28378 | 15.085 | 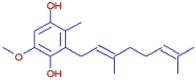 |
| m-Cresol | C7 H8 O | 194.15388 | 7.974 |  |
3.4. Characteristics and morphology of extract-chitosan microencapsulate
The ionic gelation process was utilized to create a microencapsulated extract of P. oleraceae. The synthesis of microcapsules containing P. oleraceae extract was successfully achieved by using pH 4, 90 min of stirring time, and 0.1 % (w/v) chitosan. The percentage of encapsulation efficiency (%EE) is reported as 43.56 ± 2.31 %. The amino group in chitosan can undergo protonation in an acidic environment, resulting in a neutral solution. This protonation process is influenced by the pKa value of the amino group, which is approximately 6.5 [60]. Despite the protonation of the amino group in an acidic solution, it is possible to enhance the solubility of chitosan [61]. The utilization of this characteristic holds significant significance in biological applications, particularly when chitosan is employed for drug delivery to sites in acidic environments [62]. At a pH of 4, chitosan attracts a greater number of proton donors, leading to the protonation of more amine groups (NH2) into NH+3 ions. These ions then form ionic cross-links with NaTPP, resulting in the formation of microcapsules [63]. The abundance of protonated chitosan will enhance chitosan's capacity to absorb the bioactive chemicals from the extract, hence augmenting the encapsulation efficiency [64].
This observation closely resembled a prior study by Khorshidian et al. [65], which showed that pH 4 was the optimal pH for formulating microcapsules containing chitosan. The charge densities of biopolymers with opposite signs were stoichiometrically balanced at a pH of 4. If the pH was below the optimal level, the degree of ionization of amine groups on the chitosan molecular chain hindered the creation of uniform particles in the solution [66]. At pH values over 4, the degree of ionization and solubility of chitosan reduced, likely leading to variability in size distribution [67].
The microcapsules that were obtained were then described based on their dimensions and surface morphology. An examination of the size distribution of particles can provide valuable insights into the behavior of microcapsules and assist in predicting their stability [68]. The average size of the particles, as seen in Fig. 2A, was 19.89 ± 0.76 μm and Fig. 2B displays the SEM picture. Concerning the SEM images (Fig. 2B), the microcapsules exhibit a greater abundance of spherical and uniform forms. Nevertheless, the outside of the microcapsules exhibited some degree of unevenness [69]. However, the study did not evaluate the viscosity of the microcapsules, which is a shortcoming.
Fig. 2.
The characteristic of P. oleraceae extract-chitosan microcapsule. Particle size analysis (A) and Morphological analysis using SEM with a magnification of 2500 (B).
The sizes and surfaces of the microcapsules containing P. oleraceae extract seem to be comparable to those of the typical microcapsules. Other authors have also achieved comparable outcomes using the ionic gelation approach, producing microcapsules that are tiny, spherical, and exhibit some imperfections and roughness [70,71]. However, the microcapsule product already fell into the range of microcapsule sizes, with an average diameter of 19.89 ± 0.76 μm.
The FTIR analysis was performed to further investigate the microcapsules. Fig. 3., presents the FTIR findings, and the identification of the significant peaks is listed in Table 4. The prominent peaks of the bioactive compounds in P. oleraceae were found at specific wavenumbers: 3471.87 cm−1 (corresponding to O–H stretching), 1515.12 cm−1 (corresponding to C C stretching), 1417.68 cm−1 (corresponding to C–C stretching), and 1020.34 cm−1 (corresponding to C–O–C stretching) (Fig. 3) [72]. A subset of these peaks were detected within microcapsules. The FTIR spectra of the microcapsules exhibit a displacement in the O–H functional group at the encapsulate peak. Initially, the peak was observed at a wavenumber of 3471.87 cm−1 and subsequently relocated to 3394.72 cm−1. The detection of a novel absorption peak at 2926.01 cm−1 and 1257.57 cm−1 indicates the existence of a stretching motion of C–H bonds in an alkane and the presence of a N–H group from an amine. This small peak is attributed to chitosan, which was employed as a covering material for the microcapsules [73]. The presence of a phosphate group (P O) is indicated by the absorption peak observed at 1055.06 cm−1. This suggests that the peak originated from NaTPP, which was used as a crosslinker during the manufacture of microcapsules [74].
Fig. 3.
The FTIR spectrum of P. oleraceae extract, microcapsules, and chitosan in a wavenumber of 4000–500 cm−1..
Table 4.
Assignment of the FTIR spectra.
| No |
P. oleraceae Extract |
Microcapsules |
||
|---|---|---|---|---|
| Wavenumber (cm−1) | Functional groups | Wavenumber (cm−1) | Functional groups | |
| 1 | 3471.87 | O–H stretch | 3394.72 | O–H stretch |
| 2 | 1515.12 | C C stretch | 2926.01 | C–H stretch |
| 3 | 1417.68 | C–C stretch | 1257.57 | N–H stretch |
| 4 | 1020.34 | C–O–C stretch | 1055.06 | P O stretch |
FTIR measurement indicates that chitosan microcapsules effectively incorporated bioactive compounds from the extracts through ionic crosslinking using the microprecipitation method. The detection of distinctive peaks corresponding to bioactive molecules (1514.12 cm−1 for C C aromatics) on the microcapsules serves as conclusive proof that the bioactive molecules were effectively crosslinked to the chitosan microcapsules [72]. In addition, the microcapsule exhibited distinct peaks at 1257.57 cm−1 for N–H amines and 1055.06 cm−1 for P O, but these peaks were not present in the extract [74]. This outcome is corroborated by the PCA data obtained from the extraction process, microcapsules, and chitosan.
The PCA was used to ensure further differentiation of microcapsules based on the FTIR transmittance of each sample. Fig. 4 shows the PCA score plot using two principal components accounting for 100 % of the total variance with PC1 and PC2, explaining 99.8 % and 0.02 % of the total variance, respectively. Based on the score plot, all samples were clustered into their respective groups. This plot clarifies the similarities and differences in the FTIR spectrum of the microcapsule; the closer one group is to another, the greater the similarity in the composition of each sample [75]. It shows that the microcapsule is separated from the extract and chitosan. All data shows that the microcapsule of P. oleraceae extract and chitosan has succeeded in producing.
Fig. 4.
The distribution of samples based on % transmittance in the wavenumber of fingerprint area. Chitosan (A), P. oleraceae extract (B), and Microcapsules (C).
3.5. Antioxidant activity of microcapsule
The DPPH technique was employed to evaluate the antioxidant activity of both the microcapsule and extract. The outcomes are illustrated in Fig. 5. The microcapsule demonstrates activity in scavenging DPPH radicals that varies depending on their concentration. At a dose of 500.00 μg/mL, the microcapsules exhibited a significant ability to inhibit 60.75 ± 3.85 % of DPPH radicals. The activity diminishes as the concentration drops from 250.00 μg/mL to 15.625 μg/mL. The activity decreases by 57.31 ± 3.56 %, 45.72 ± 3.25 %, 36.85 ± 2.94 %, 25.23 ± 2.75 %, and 21.07 ± 2.25 % respectively. The efficacy of microcapsules is significantly lower than that of extracts, having a p-value of less than 0.0001. The extract, administered at a concentration of 500.00 μg/mL, exhibited a notable capacity to suppress DPPH radicals by 85.36 ± 3.75 %. The inhibition found with microcapsules was significantly distinct from this (p < 0.001). The extract demonstrated increased activity at different concentrations, with values of 74.27 ± 3.17 %, 62.78 ± 3.51 %, 53.85 ± 2.86 %, 44.68 ± 2.52 %, and 32.68 ± 2.21 %, respectively (p < 0.001).
Fig. 5.
The antioxidant activity of P. oleracea extract and microcapsules against DPPH radical in a variation of concentrations. The result showed significant differences between extract and microcapsules using a t-test with p < 0.0001.
The results indicate that the microcapsules of P. oleraceae demonstrate diminished biological efficacy in comparison to the non-microencapsulated extract. The current study demonstrates a comparable outcome to the previous research, indicating that the antioxidant activity of microcapsules is inferior to that of the extract [76]. This phenomenon may occur due to the partial liberation of the bioactive substances enclosed in the microcapsules, leading to the retention of certain molecules within the microcapsules [77]. Furthermore, the main purpose of microencapsulation is not to increase biological activity, but rather to protect and control the release of active chemicals. Nevertheless, microcapsules containing extracts derived from P. oleraceae remain efficacious for utilization as antioxidants. The utilization of microcapsules containing the P. oleraceae extract exhibits potential as a natural antioxidant agent.
4. Conclusions
This study provides the optimization models to produce a polyphenol-rich extract of P. oleraceae using the RSM approach. The optimum condition was validated in X1: 72.894 %, X2: 57.384 °C: and X3, 2.031 h. The experimental data showed TPC and TFC are not significantly different from the predicted data of 10.97 ± 0.74 mg GAE/g and 29.86 ± 0.94 mg QE/g, respectively. The microcapsules of the extract under optimum conditions were successfully produced using chitosan and NaTPP with a % EE of 43.56 ± 2.31 %. The FTIR measurement revealed the formation of cross-linking between chitosan and NaTPP. The scanning electron microscopy (SEM) examination revealed that the surface of the encapsulated extracts was characterized by a rough texture and predominantly spherical shape. However, the particle size analysis (PSA) demonstrated that the micro-sized particles were obtained with an average diameter of 19.89 ± 0.76 μm. The microcapsules retained the antioxidant properties but at a lesser level compared to the extract. This work facilitates the crucial role of microencapsulation in producing the polyphenol-rich extract of P. oleraceae.
Funding
This research was funded by the Directorate General Higher Education, Ministry of Education, Culture, Research, and Technology of Indonesia through “Penelitian Kerja Sama Dalam Negeri” research grant 2023 (Contract No: 167/E5/PG.02.00.PL/2023).
Data availability
Data will be made available on request.
CRediT authorship contribution statement
Lokot Donna Lubis: Supervision, Funding acquisition, Conceptualization. Arya Tjipta Prananda: Writing – original draft, Validation, Project administration. Nur Aira Juwita: Investigation, Funding acquisition, Conceptualization. Muhammad Amin Nasution: Writing – original draft, Funding acquisition, Conceptualization. Rony Abdi Syahputra: Writing – review & editing, Project administration, Investigation. Sumaiyah Sumaiyah: Software, Methodology, Investigation, Data curation. Rodiah Rahmawaty Lubis: Writing – review & editing, Supervision. Muhammad Fauzan Lubis: Writing – original draft, Software, Investigation, Funding acquisition, Conceptualization. Ririn Astyka: Validation, Resources, Project administration, Methodology. Jihan Firyal Atiqah: Software, Project administration, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors wish to thank Universitas Sumatera Utara for facilitating this research and Directorate General Higher Education, Ministry of Education, Culture, Research, and Technology of Indonesia, for providing research grant.
References
- 1.Lubis M.F., Hasibuan P.A.Z., Syahputra H., Surbakti C., Astyka R. Saurauia vulcani (Korth.) as herbal medicine potential from North Sumatera, Indonesia: a literature review. Heliyon. 2022;8(4) doi: 10.1016/j.heliyon.2022.e09249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altemimi A., Lakhssassi N., Baharlouei A., Watson D.G., Lightfoot D.A. Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants. 2017;6(4):42. doi: 10.3390/plants6040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adrian R.A. Syahputra, Juwita N.A., Astyka R., Lubis M.F. Andaliman (Zanthoxylum acanthopodium DC.) a herbal medicine from North Sumatera, Indonesia: phytochemical and pharmacological review. Heliyon. 2023;9(5) doi: 10.1016/j.heliyon.2023.e16159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Twaij B.M., Hasan M.N. Bioactive secondary metabolites from plant sources: types, synthesis, and their therapeutic uses. Int. J. Plant Biol. 2022;13(1):4–14. [Google Scholar]
- 5.Gandhi S.G., Mahajan V., Bedi Y.S. Changing trends in biotechnology of secondary metabolism in medicinal and aromatic plants. Planta. 2015;241(2):303–317. doi: 10.1007/s00425-014-2232-x. 2015. [DOI] [PubMed] [Google Scholar]
- 6.Lubis M.F., Sumaiyah S., Lubis L.D., Fitri K., Astyka R. Application of Box-Behnken design for optimization of Vernonia amygdalina stem bark extract in relation to its antioxidant and anti-colon cancer activity. Arab. J. Chem. 2024 [Google Scholar]
- 7.Arifah F.H., Nugroho A.E., Rohman A., Sujarwo W. A review of medicinal plants for the treatment of diabetes mellitus: the case of Indonesia. South Afr. J. Bot. 2022;149:537–558. [Google Scholar]
- 8.SuwardiMardudi A.B., NaviaBaihaqi Z.I., Muntaha Documentation of medicinal plants used by Aneuk Jamee tribe in Kota Bahagia sub-district, South Aceh, Indonesia. Biodiversitas. 2021;22(1):6–15. [Google Scholar]
- 9.Iranshahy M., Javadi B., Iranshahi M., Jahanbakhsh S.P., Mahyari S., Hassani F.V., Karimi G. A review of traditional uses, phytochemistry and pharmacology of Portulaca oleracea L. J. Ethnopharmacol. 2017;205:158–172. doi: 10.1016/j.jep.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Li K., Xia T., Jiang Y., Wang N., Lai L., Xu S., Yue X., Xin H. A review on ethnopharmacology, phytochemistry, pharmacology and potential uses of Portulaca oleracea L. J. Ethnopharmacol. 2024;319(Pt 2) doi: 10.1016/j.jep.2023.117211. [DOI] [PubMed] [Google Scholar]
- 11.Uddin MdK., Juraimi A.S., Ali Md E., Ismail M.R. Evaluation of antioxidant properties and mineral composition of purslane (Portulaca oleracea L.) at different growth stages. Int. J. Mol. Sci. 2012;13(8):10257–10267. doi: 10.3390/ijms130810257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li C., Meng Y., Ying Z., Xu N., Hao D., Gao M., Zhang W., Xu L., Gao Y., Ying X. Three novel alkaloids from Portulaca oleracea L. and their anti-inflammatory effects. J. Agric. Food Chem. 2016;64(29):5837–5844. doi: 10.1021/acs.jafc.6b02673. [DOI] [PubMed] [Google Scholar]
- 13.Al-Sheddi E.S., Farshori N.N., Al-Oqail M.M., Musarrat J., Al-Khedhairy A.A., Siddiqui M.A. Portulaca oleracea seed oil exerts cytotoxic effects on human liver cancer (HepG2) and human lung cancer (A-549) cell lines. Asian Pac. J. Cancer Prev. APJCP. 2015;16(8):3383–3387. doi: 10.7314/apjcp.2015.16.8.3383. [DOI] [PubMed] [Google Scholar]
- 14.Bai Y., Zhang X., Ma J., Xu G. Anti-diabetic effect of Portulaca oleracea L. Polysaccharide and its mechanism in diabetic rats. Int. J. Mol. Sci. 2016;17(8):1201. doi: 10.3390/ijms17081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eidi A., Mortazavi P., Moghadam J.Z., Mardani P.M. Hepatoprotective effects of Portulaca oleracea extract against CCl4-induced damage in rats. Pharm. Biol. 2015;53(7):1042–1051. doi: 10.3109/13880209.2014.957783. [DOI] [PubMed] [Google Scholar]
- 16.Chan K., Islam M.W., Radhakrishnan R., Zakaria M.N., Habibullah M., Attas A. The analgesic and anti-inflammatory effects of Portulaca oleracea L. subsp. sativa (Haw.) Celak. J. Ethnopharmacol. 2000;73(3):445–451. doi: 10.1016/s0378-8741(00)00318-4. [DOI] [PubMed] [Google Scholar]
- 17.Rafieian-Kopaei M., Alesaeidi S. Portulaca oleracea: a review study with anti-inflammatory and muscle relaxant perspective. Indian J Med Res Pharm Sci. 2016;3(11):50–56. [Google Scholar]
- 18.Jan M.S.Z., AhmadAbdullah W., Kamil A., Khan M.A., Rehman M.U., Ullah I., Jan M.S. Protective effect of the solvent extracts of Portulacca oleracea against acidified ethanol induced gastric ulcer in rabbits.Drug. Chem. Toxicol. 2022;45(1):301–310. doi: 10.1080/01480545.2019.1691584. [DOI] [PubMed] [Google Scholar]
- 19.Moneim A.E.A., Dkhil M.A., Al-Quraishy S. The potential role of Portulaca oleracea as a neuroprotective agent in rotenone-induced neurotoxicity and apoptosis in the brain of rats. Pestic Biochem Phys. 2013;105(3):203–212. [Google Scholar]
- 20.Guo J., Peng J., Han J., Wang K., Si R., Shan H., Wang X., Zhang J. Extracts of Portulaca oleracea promote wound healing by enhancing angiology regeneration and inhibiting iron accumulation in mice. Chin Herb Med. 2022;26(2):263–272. doi: 10.1016/j.chmed.2021.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei X., Li J., Liu B., Zhang N., Liu H. Separation and identification of Four New compounds with antibacterial activity from Portulaca oleracea L. Molecules. 2015;20(9):16375–16387. doi: 10.3390/molecules200916375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Lorenzo C., Colombo F., Biella S., Stockley C., Restani P. Polyphenols and human health: the role of bioavailability. Nutrients. 2021;13(1):273. doi: 10.3390/nu13010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barakat A.Z., Bassuiny R.I., Abdel-Aty A.M., Mohamed S.A. Diabetic complications and oxidative stress: the role of phenolic-rich extracts of saw palmetto and date palm seeds. J. Food Biochem. 2020;44(11) doi: 10.1111/jfbc.13416. [DOI] [PubMed] [Google Scholar]
- 24.Messaoudi M., Rebiai A., Sawicka B., Atanassova M., Ouakouak H., Larkem I., Egbuna C., Awuchi C.G., Boubekeur S., Ferhat M.A., Begaa S., Benchikha N. Effect of extraction methods on polyphenols, flavonoids, mineral elements, and biological activities of essential oil and extracts of Mentha pulegium L. Molecules. 2021;27(1):11. doi: 10.3390/molecules27010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lubis M.F., Hasibuan P.A.Z., Syahputra H., Astyka R., Baruna I. Phytochemical profile and pharmacological activity of Vernonia amygdalina delile stem bark extracts using different solvent extraction. OAMJMS. 2022;10(A):860–866. [Google Scholar]
- 26.Abdel-Aty A.M., Elsayed A.M., Salah H.A., Bassuiny R.I., Mohamed S.A. Egyptian chia seeds (Salvia hispanica L.) during germination: upgrading of phenolic profile, antioxidant, antibacterial properties and relevant enzymes activities. Food Sci. Biotechnol. 2021;30:723–734. doi: 10.1007/s10068-021-00902-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lubis M.F., Hasibuan P.A.Z., Syahputra H., Kelait J.M., Kaban V.E., Astyka R. Duku (Lansium domesticum) leaves extract induces cell cycle arrest and apoptosis of HepG2 cells via PI3K/Akt Pathways. Trends Sci. 2023;20(2):6437. 6437. [Google Scholar]
- 28.Lefebvre T., Destandau E., Lesellier E. Selective extraction of bioactive compounds from plants using recent extraction techniques: a review. J. Chromatogr. A. 2021;1635 doi: 10.1016/j.chroma.2020.461770. [DOI] [PubMed] [Google Scholar]
- 29.Oreopoulou A., Tsimogiannis D., Oreopoulou V. Extraction of polyphenols from aromatic and medicinal plants: an overview of the methods and the effect of extraction parameters. Polyphenols in plants. 2019:243–259. [Google Scholar]
- 30.Alonso-Carrillo N., Aguila-Santamaria M.D., Vernon-Carter E.J., Jimenez-Alvarado R., Cruz-Sosa F., Roman-Guerrero A. Extraction of phenolic compounds from Satureja macrostema using microwave-ultrasound assisted and reflux methods and evaluation of their antioxidant activity and cytotoxicity. Ind. Crops Prod. 2017;103:213–221. [Google Scholar]
- 31.Ma Y., Meng A., Liu P., Chen Y., Yuan A., Dai Y., Ye K., Yang Y., Wang Y., Li Z. Reflux extraction optimization and antioxidant activity of phenolic compounds from Pleioblastus amarus (Keng) shell. Molecules. 2022;27(2):362. doi: 10.3390/molecules27020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moldovan M.L., Iurian S., Puscas C., Silaghi-Dumitrescu R., Hanganu D., Bogdan C., Vlase L., Oniga I., Benedec D. A design of experiments Strategy to enhance the recovery of polyphenolic compounds from Vitis vinifera by-products through heat reflux extraction. Biomolecules. 2019;9(10):529. doi: 10.3390/biom9100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bezerra M.A., Santelli R.E., Oliveira E.P., Villar L.S., Escaleira L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta. 2008;76(5):965–977. doi: 10.1016/j.talanta.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 34.Yolmeh M., Jafari S.M. Applications of response surface methodology in the food industry processes. Food Bioprocess Technol. 2017;10:413–433. [Google Scholar]
- 35.Liyana-Pathirana C., Shahidi F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005;93(1):47–56. [Google Scholar]
- 36.Difonzo G., Squeo G., Pasqualone A., Summo C., Paradiso V.M., Caponio F. The challenge of exploiting polyphenols from olive leaves: addition to foods to improve their shelf-life and nutritional value. J. Sci. Food Agric. 2021;101(8):3099–3116. doi: 10.1002/jsfa.10986. [DOI] [PubMed] [Google Scholar]
- 37.Dias M.I., Ferreira I.C., Barreiro M.F. Microencapsulation of bioactives for food applications. Food Funct. 2015;6(4):1035–1052. doi: 10.1039/c4fo01175a. [DOI] [PubMed] [Google Scholar]
- 38.Bora A.F.M., Ma S., Li X., Liu L. Application of microencapsulation for the safe delivery of green tea polyphenols in food systems: review and recent advances. Food Res. Int. 2018;105:241–249. doi: 10.1016/j.foodres.2017.11.047. [DOI] [PubMed] [Google Scholar]
- 39.Banožić M., Vladić J., Banjari I., Velić D., Aladić K., Jokić S. Spray drying as a method of choice for obtaining high quality products from food wastes– A review. Food Rev. Int. 2023;39(4):1953–1985. [Google Scholar]
- 40.Drosou C.G., Krokida M.K., Biliaderis C.G. Encapsulation of bioactive compounds through electrospinning/electrospraying and spray drying: a comparative assessment of food-related applications. Dry. Technol. 2017;35(2):139–162. [Google Scholar]
- 41.de Sousa V.R., Marcelo da Cunha Santos A., Viana de Sousa B., de Araújo Neves G., Navarro de Lima Santana L., Menezes R.R. A review on chitosan's uses as biomaterial: tissue engineering, drug delivery systems and cancer treatment. Materials. 2020;13(21):4995. doi: 10.3390/ma13214995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abourehab M.A.S., Pramanik S., Abdelgawad M.A., Abualsoud B.M., Kadi A., Ansari M.J., Deepak A. Recent advances of chitosan formulations in biomedical applications. Int. J. Mol. Sci. 2022;23(18) doi: 10.3390/ijms231810975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alzandi A.A., Naguib D.M., Abas A.M. Onion extract encapsulated on nano chitosan: a promising anticancer agent. J. Gastrointest. Cancer. 2021;53(1):211–216. doi: 10.1007/s12029-020-00561-2. [DOI] [PubMed] [Google Scholar]
- 44.Jorge A.J., Heliodoro D.L.G.T., Alejandro Z.C., Ruth B.C., Noé A.C. The optimization of phenolic compounds extraction from cactus pear (Opuntia ficus-indica) skin in a reflux system using response surface methodology. Asian Pac. J. Trop. Biomed. 2013;3(6):436–442. doi: 10.1016/S2221-1691(13)60093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lubis M.F., Syahputra H., Illian D.N., Kaban V.E. Antioxidant activity and nephroprotective effect of Lansium parasiticum leaves in doxorubicin-induced rats. J Res Pharm. 2022;26(3):565–573. [Google Scholar]
- 46.Siddiqui N., Aeri V. Optimization of Betulinic acid extraction from Tecomella undulata bark using a Box-Behnken design and its Densitometric validation. Molecules. 2016;21(4):393. doi: 10.3390/molecules21040393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trifan A., Zengin G., Sinan K.I., Wolfram E., Skalicka-Woźniak K., Luca S.V. LC-HRMS/MS phytochemical profiling of Symphytum officinale L. and Anchusa ochroleuca M. Bieb. (Boraginaceae): unveiling their multi-biological potential via an integrated approach. J. Pharm. Biomed. Anal. 2021;10(204) doi: 10.1016/j.jpba.2021.114283. [DOI] [PubMed] [Google Scholar]
- 48.Vonghirundecha P., Chusri S., Meunprasertdee P., Kaewmanee T. Microencapsulated functional ingredients from a Moringa oleifera leaf polyphenol-rich extract: characterization, antioxidant properties, in vitro simulated digestion, and storage stability. LWT. 2022;154 [Google Scholar]
- 49.Ghobadi M., Koocheki A., Varidi M.J., Varidi M. Encapsulation of curcumin using Grass pea (Lathyrus sativus) protein isolate/Alyssum homolocarpum seed gum complex nanoparticles. Innovat. Food Sci. Emerg. Technol. 2021;72 [Google Scholar]
- 50.da Silva Soares B., Siqueira R.P., de Carvalho M.G., Vicente J., Garcia-Rojas E.E. Microencapsulation of sacha inchi oil (Plukenetia volubilis L.) using complex coacervation: formation and structural characterization. Food Chem. 2019;15(298) doi: 10.1016/j.foodchem.2019.125045. [DOI] [PubMed] [Google Scholar]
- 51.Cichocki W., Czerniak A., Smarzynski K., Jezowski O., Kmiecik D., Baranowska H.M., Walkowiak K., Ostrowska-Ligeza E., Rozanska M.B., Lesiecki M., Kowalczewski P.L. Physicochemical and morphological study of the Saccharomyces cerevisiae cell-based microcapsules with novel cold-pressed oil blends. Appl. Sci. 2022;12(13):6577. [Google Scholar]
- 52.Widodo H., Sismindari S., Asmara W., Rohman A. Antioxidant activity, total phenolic and flavonoid contents of selected medicinal plants used for liver diseases and its classification with chemometrics. J. Appl. Pharmaceut. Sci. 2019;9(6):99–105. [Google Scholar]
- 53.Soltanzadeh M., Peighambardoust S.H., Ghanbarzadeh B., Mohammadi M., Lorenzo J.M. Chitosan nanoparticles as a promising nanomaterial for encapsulation of pomegranate (Punica granatum L.) peel extract as a natural source of antioxidants. Nanomaterials. 2021;11(6):1439. doi: 10.3390/nano11061439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harahap U., Dalimunthe A., Hertiani T., MuhammadNasri M., Satria D. Antioxidant and antibacterial activities of ethanol extract of Vernonia amygdalina Delile. Leaves. AIP Conf. Proc. 2021;2342(1) [Google Scholar]
- 55.Choi H.J., Naznin M., Alam M.B., Javed A., Alshammari F.H., Kim S., Lee S.H. Optimization of the extraction conditions of Nypa fruticans Wurmb. using response surface methodology and artificial neural network. Food Chem. 2022;381 doi: 10.1016/j.foodchem.2022.132086. [DOI] [PubMed] [Google Scholar]
- 56.Sedraoui S., Badr A., Barba M.G.M., Doyen A., Tabka Z., Desjardins Y. Optimization of the ultrahigh-pressure–assisted extraction of phenolic compounds and antioxidant activity from palm dates (Phoenix dactylifera L.) Food Anal. Methods. 2020;13(10):1556–1569. [Google Scholar]
- 57.Oreopoulou A., Tsimogiannis D., Oreopoulou V. Extraction of polyphenols from aromatic and medicinal plants: an overview of the methods and the effect of extraction parameters. Polyphenols in plants. 2019:243–259. [Google Scholar]
- 58.Xi J., He L., Yan L. Kinetic modeling of pressure-assisted solvent extraction of polyphenols from green tea in comparison with the conventional extraction. Food Chem. 2014;166:287–291. doi: 10.1016/j.foodchem.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 59.Swamy G.J., Sangamithra A., Chandrasekar V. Response surface modeling and process optimization of aqueous extraction of natural pigments from Beta vulgaris using Box–Behnken design of experiments. Dyes Pigments. 2014;111:64–74. [Google Scholar]
- 60.Ferreira L.M.B., Dos Santos A.M., Boni F.I., Dos Santos K.C., Robusti L.M.G., de Souza M.P.C., Ferreira N.N., Carvalho S.G., Cardoso V.M.O., Chorilli M., Cury B.S.F., de Godoi D.R.M., Gremião M.P.D. Design of chitosan-based particle systems: a review of the physicochemical foundations for tailored properties. Carbohydr. Polym. 2020;250 doi: 10.1016/j.carbpol.2020.116968. [DOI] [PubMed] [Google Scholar]
- 61.Fan M., Hu Q., Shen K. Preparation and structure of chitosan soluble in wide pH range. Carbohydr. Polym. 2009;78(1):66–71. [Google Scholar]
- 62.Ahmed T.A., Aljaeid B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. Ther. 2016;10:483–507. doi: 10.2147/DDDT.S99651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yousefi M., Khorshidian N., Mortazavian A.M., Khosravi-Darani K. Preparation optimization and characterization of chitosan-tripolyphosphate microcapsules for the encapsulation of herbal galactagogue extract. Int. J. Biol. Macromol. 2019;140:920–928. doi: 10.1016/j.ijbiomac.2019.08.122. [DOI] [PubMed] [Google Scholar]
- 64.Yousefi M., Mohammadi V.G., Shadnoush M., Khorshidian N., Mortazavian A.M. Zingiber officinale essential oil-loaded chitosan-tripolyphosphate nanoparticles: fabrication, characterization and in-vitro antioxidant and antibacterial activities. Food Sci. Technol. Int. 2022;28(7):592–602. doi: 10.1177/10820132211040917. [DOI] [PubMed] [Google Scholar]
- 65.Khorshidian N., Mahboubi A., Kalantari N., Hosseini H., Yousefi M., Arab M., da Cruz A.G., Mortazavian A.M., Mahdavi F.S. Chitosan-coated alginate microcapsules loaded with herbal galactagogue extract: formulation optimization and characterization. Iran. J. Pharm. Res. (IJPR) 2019;18(3):1180–1195. doi: 10.22037/ijpr.2019.1100776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hashad R.A., Ishak R.A., Fahmy S., Mansour S., Geneidi A.S. Chitosan-tripolyphosphate nanoparticles: optimization of formulation parameters for improving process yield at a novel pH using artificial neural networks. Int. J. Biol. Macromol. 2016;86:50–58. doi: 10.1016/j.ijbiomac.2016.01.042. [DOI] [PubMed] [Google Scholar]
- 67.Antoniou J., Liu F., Majeed H., Qi J., Yokoyama W., Zhong F. Physicochemical and morphological properties of size-controlled chitosan–tripolyphosphate nanoparticles. Colloids Surf., A: Physicochem Eng. 2015;465:137–146. [Google Scholar]
- 68.do Nascimento T.G., do Nascimento N.M., Ribeiro A.S., de Almeida C.P., dos Santos J.I.Z., Basilio-Junior I.D., Calheiros-Silva F.G., Lira G.M., Escodro P.B., de Mores Porto I.C.C., da Silva V.A., Dornelas C.B., dos Santos Sousa J., de Freitas J.D. Preparation and characterization of chitosanates loaded with Brazilian red propolis extract. J. Therm. Anal. Calorim. 2021;147:7837–7848. [Google Scholar]
- 69.Iannone M., Mare R., Paolino D., Gagliardi A., Froiio F., Cosco D., Fresta M. Characterization and in vitro anticancer properties of chitosan-microencapsulated flavan-3-ols-rich grape seed extracts. Int. J. Biol. Macromol. 2017;104(Pt A):1039–1045. doi: 10.1016/j.ijbiomac.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 70.de Moura S.C.S.R., Berling C.L., Germer S.P.M., Alvim I.D., Hubinger M.D. Encapsulating anthocyanins from Hibiscus sabdariffa L. calyces by ionic gelation: pigment stability during storage of microparticles. Food Chem. 2017;241(241):317–327. doi: 10.1016/j.foodchem.2017.08.095. [DOI] [PubMed] [Google Scholar]
- 71.Thuekeaw S., Buwjoom T., Maneewan B., Nuengjamnong C. Porous alginate microcapsule-containing sweet basil oil (Ocimum basilicum) generated by gas foaming with ionic gelation as an efficient antioxidant delivery in stimulated poultry gastrointestinal tract. ACS Food Sci Technol. 2023;3(12):2078–2084. [Google Scholar]
- 72.Shah T.K., Kumar A., Tandel R., Bhat R.A.H., Sarma D. Identification of functional groups in Thymus linearis ethanol leaf extract through fourier transform infrared spectroscopy (FTIR) J. Pharmacogn. Phytochem. 2019;8(4):3013–3015. [Google Scholar]
- 73.Parizel A.L., Stulzer H.K., Laranjeira M.C.M., Brighente I.M.d., de Souza T.C.R. Evaluation of chitosan microparticles containing curcumin and crosslinked with sodium tripolyphosphate produced by spray drying. Quím. Nova. 2012;35:1127–1132. [Google Scholar]
- 74.Chen K., Zhang M., Mujumdar A.S., Wang M. Quinoa protein isolate-gum Arabic coacervates cross-linked with sodium tripolyphosphate: characterization, environmental stability, and Sichuan pepper essential oil microencapsulation. Food Chem. 2023;404(Pt A) doi: 10.1016/j.foodchem.2022.134536. [DOI] [PubMed] [Google Scholar]
- 75.Boeing J.S., Barizão E.O., E Silva B.C., Montanher P.F., de Cinque Almeida V., Visentainer J.V. Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: application of principal component analysis. Chem. Cent. J. 2014;8(1):48. doi: 10.1186/s13065-014-0048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Phupaboon S., Matra M., Prommachart R., Totakul P., Supapong C., Wanapat M. Extraction, characterization, and chitosan microencapsulation of bioactive compounds from Cannabis sativa L., Cannabis indica L., and Mitragyna speiosa K. Antioxidants. 2022;11(11):2103. doi: 10.3390/antiox11112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abrahao F.R., Rocha L.C.R., Santos T.A., do Carmo E.L., Pereira L.A.S., Borges S.V., Pereira R.G.F.A., Botrel D.A. Microencapsulation of bioactive compounds from espresso spent coffee by spray drying. LWT. 2018;103(23):116–124. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.







