ABSTRACT
Purpose:
Lymphangiomas are benign hamartomas in the spectrum of lymphatic malformations, exhibiting multifaceted clinical features. Spinal involvement is exceedingly rare, with only 35 cases reported to date. Both due to their rarity and chameleonic radiologic features, spinal lymphangiomas (SLs) are usually misdiagnosed; postoperatively, surgeons are thus confronted with an unexpected histopathological diagnosis with sparse pertinent literature and no treatment guidelines available.
Methods:
Here, we report the case of a 67-year-old female who underwent surgery for a T6-T7 epidural SL with transforaminal extension, manifesting with spastic paraparesis. Then, we present the results of the first systematic review of the literature on this subject, delineating the clinical and imaging features and the therapeutic implications of this rare disease entity.
Results:
Our patient was treated with T6-T7 hemilaminectomy and resection of the epidural mass, with complete recovery of her neurological picture. No recurrence was evident at 18 months. In the literature, 35 cases of SL were reported that can be classified as vertebral SL (n = 18), epidural SL (n = 10), intradural SL (n = 3), or intrathoracic lymphangiomas with secondary spinal involvement (n = 4). Specific treatment strategies (both surgical and nonsurgical) were adopted in relation to each of these categories.
Conclusion:
Gathering knowledge about SL is fundamental to promote both correct preoperative identification and appropriate perioperative management of this rare disease entity. By reviewing the literature and discussing an exemplary case, we delineate a framework that can guide surgeons facing such an unfamiliar diagnosis.
Keywords: Cystic hygroma, lymphangioma, lymphatic malformation, review, spine surgery, spine tumor
INTRODUCTION
Lymphatic anomalies presenting as soft tissue masses are not uncommon and were probably first reported by Redenbacher in 1828; the name cystic hygroma was first used by Wernher in 1843. The terminology adopted in the literature (lymphangioma, cystic hygroma, and lymphangiomatosis) is confounding and is influenced by the traditional definitions employed by the specialties that deal with these lesions (otolaryngology, pediatric surgery, thoracic surgery, and plastic surgery). As a general rule, the term cystic hygroma is employed only for congenital, cystic lesion of the upper trunk, head and neck, which represent almost 50-60% of cases and are typically diagnosed in utero or at birth.[1,2] “Lymphangioma” is used instead to design mass lesions occurring elsewhere (thoracic and abdominal viscera, limbs, and bones). According to the 2015 guidelines on nomenclature of vascular malformations, the umbrella term “lymphatic anomaly” should be used, in order to stress the malformative origin of these lesions.[3]
Spinal involvement is exceedingly rare, with only 35 cases reported to date [Table 1]. The corresponding literature is limited and no comprehensive reviews have been published yet in order to better define clinical, imaging, and surgical aspects involved in the management of spinal lymphangiomas (SL). Herein, we present an original case of a thoracic spinal epidural lymphangioma and discuss the results of a systematic analysis of the pertinent literature.
Table 1.
Summary of the reported cases of spinal lymphatic anomalies
| Source | Age (years) | Sex | Location | MRI appearance | ||
|---|---|---|---|---|---|---|
|
| ||||||
| T2 | T1 | Contrast enhancement; other | ||||
| Our case, 2022 | 67 | Female | T6–T7 epidural; transforaminal extension | Hyperintense | Hypointense | Homogeneous |
| Chia et al., 2021[3] | 53 | Female | T4–T8 epidural; posterior epidural hematoma | Heterogeneous | Heterogeneous | Heterogeneous |
| Payne et al., 2019[4] | 23 | Male | C4–T5 mediastinal; transforaminal epidural extension | Isointense | Heterogeneous | NR, cystic |
| Fattahi et al., 2019[5] | 61 | Male | T8 vertebral body; posterior epidural extension | NR | NR | Homogeneous |
| Rali et al., 2017[6] | 23 | Male | C4–T7 mediastinal; transforaminal epidural extension | Heterogeneous | Heterogeneous | NR; calcifications |
| Pan et al., 2017[7] | 58 | Male | T3–T4 intradural | Heterogeneous | Hyperintense | Heterogeneous |
| Pan et al., 2017[7] | 60 | Female | T10–T12 epidural; transforaminal extension | Hyperintense | Hypointense | Heterogeneous |
| Wright et al., 2017[8] | 18 | Female | Skull base; C1–C2 vertebrae | NR | NR | NR |
| Kerolus et al., 2016[9] | 83 | Female | T5–T8 intradural | Hyperintense | NR | Homogeneous |
| Aslan et al., 2015[10] | 32 | Male | C2–T11 epidural; transforaminal extension | Hyperintense | Hypointense | Heterogeneous; cystic |
| Renjen et al., 2014[11] | 9 | Male | Multiple thoracic and lumbar vertebrae | Hyperintense | Hypointense | NR |
| Lee et al., 2011[12] | 43 | Female | C6–C7 epidural; transforaminal extension | Hyperintense | Isointense | NR; fluid levels, old hemorrhage |
| Ha 2010[13] | 16 | Male | T5–T7 epidural | Hyperintense | NR | NR |
| Sadrizadeh et al., 2009[14] | 32 | Male | T12–L4; L2 collapse; epidural extension | Hyperintense | NR | NR |
| McLoughlin et al., 2008[15] | 1 | Male | T2–T3 mediastinal; transforaminal extension; posterior epidural hematoma | Hyperintense | NR | NR, epidural hematoma |
| Ozeki et al., 2007[16] | 5 | Male | Multiple; T10 vertebral, other vertebrae | Hyperintense | NR | NR |
| Ozturk and Yousem, 2007[17] | 53 | Female | Multiple; T6, T8–L2 vertebral bodies and pedicles | Heterogeneous | Heterogeneous | Heterogeneous |
| Chu et al., 2007[18] | 61 | Female | C6–T1 epidural | Hyperintense | Hypointense | Heterogeneous |
| Pavanello et al., 2007[19] | 4 | Male | C0–C3 vertebral | Hyperintense | Hypointense | Homogeneous |
| Jiang et al., 2006[20] | 47 | Male | S2–S3 epidural | Hyperintense | Hypointense | NR |
| Jiang et al., 2006[20] | 12 | Male | L1–L5 epidural | Heterogeneous | Isointense | Heterogeneous |
| Wallace and Ross, 2005[21] | 31 | Male | Sacrum | NR | NR | NR |
| Jea et al., 2003[22] | 4 | Female | Skull base; C1–C4 vertebrae | Hyperintense | Hypointense | Homogeneous |
| Watkins et al., 2003[23] | 15 | Female | C5–T1 vertebrae | NR | NR | NR |
| Watkins et al., 2003[23] | 12 | Male | C4–T3 and L5 vertebrae | Hyperintense | NR | NR |
| Kanamori, 2004[24] | 56 | Male | Sacral intradural | Hyperintense | Hypointense | NR |
| Méndez et al., 2002[25] | 41 | NR | L4 vertebral; dorsal epidural extension | Heterogeneous | Hypointense | Homogeneous |
| Saito et al., 1999[26] | 56 | Female | L4–L5 epidural; transforaminal extension | Hyperintense | Isointense | Homogeneous |
| Maki et al., 1999[27] | 27 | Female | T12, L1, L3, L5 and sacral vertebrae | Hyperintense | Hypointense | NR |
| Maki et al., 1999[27] | 12 | Male | Cervical and thoracic spine | Hyperintense | Hypointense | NR |
| Forstner et al., 1998[28] | 41 | Female | T4 body; T10 spinous process | Hypointense | Hypointense | No |
| Whitley and Flannery, 1996[29] | 5 | Male | T3–T10 mediastinal; transforaminal epidural extension | NR | NR | NR, cystic |
| Keenen et al., 1995[30] | 12 | Female | L3 vertebra, contiguous abdominal cyst | NR | NR | NR |
| Edwards et al., 1983[31] | - | - | Same patient as Canady 1980 | - | - | - |
| Canady and Chou, 1980[32] | 8 | Male | Skull base; C1–T1 vertebrae | NA | NA | NA |
| Rogers and Chou, 1973[33] | 8 | Male | Skull base; C1–C2 vertebrae | NA | NA | NA |
| Reilly et al., 1972[34] | 7 | Female | T5–T6 vertebrae | NA | NA | NA |
|
| ||||||
| Source | Presenting symptoms (duration) | Treatment | Clinical outcome | Follow-up (months) | Notes | |
|
| ||||||
| Our case, 2022 | Spastic paraparesis and hypoestesia (1 month) | Surgical resection (complete) | Complete recovery (MRS 0). No residue | 18, no recurrence | ||
| Chia et al., 2021[3] | Acute paraparesis, lower extremity anesthesia, and urinary retention | T4–T7 laminectomy with hematoma evacuation and resection of lymphangioma | Good clinical recovery (MRS 1). No residue | 1 | ||
| Payne et al., 2019[4] | Quadriparesis (3 months) | C4–T10 hemilaminectomies, endoscopic cyst fenestration | Return to work (MRS 1). Residue | NR | Cystic | |
| Fattahi et al., 2019[5] | Progressive paraparesis and sphyncter dysfunction (5 months) | Resection of epidural component; posterior spinal decompression and stabilization | Complete recovery (MRS 0). Residue, stable | 6, residue stable | Severe intraoperative bleeding | |
| Rali et al., 2017[6] | Progressive paraparesis, hypoestesia (2 months) | C4–T5 hemilaminectomies and resection | Postoperative respiratory failure requiring rehab (MRS 4). Residue | NR | GLA | |
| Pan et al., 2017[7] | Progressive paraparesis, hypoestesia, back pain (NR) | Surgical resection (complete) | NR. Complete resection | NR | Intradural; hemangiolymphangioma | |
| Pan et al., 2017[7] | Leg monoparesis, hypoestesia (4 years) | Surgical resection (complete) | NR. Complete resection | NR | Hemangiolymphangioma | |
| Wright et al., 2017[8] | Severe occipital headache (NR) | Percutaneous transoral vertebroplasty | Complete pain relief (MRS 0) | 24, disease stable | ||
| Kerolus et al., 2016[9] | Posterior cord ataxia, lower thoracic band hypoestesia (8 months) | 1) Partial surgical resection; 2) Cysto-peritoneal shunt | Improvement of gait (MRS 2). Residue | 28, residue stable | Intradural, cystic, adherent to medullary pia. Cyst reaccumulation requiring revision shunt treatment | |
| Aslan et al., 2015[10] | Back pain (6 months) | TC-guided needle aspiration | NR. Residue | NR | Most extensive case reported | |
| Renjen et al., 2014[11] | Pleuritic chest pain related to pleural effusion | Sirolimus for visceral disease | NA | 6, NR | GLA | |
| Lee et al., 2011[12] | Progressive cervical radiculopathy (2 months) | Surgical resection (complete) | Complete recovery (MRS 0). No residue | 12, no recurrence | ||
| Ha 2010[13] | Progressive paraparesis (2 years) | Surgical resection (complete) | Recovery from paraparesis (MRS 1). No residue | NR | ||
| Sadrizadeh et al., 2009[14] | Back and chest pain; leg paresthesias (4 months) | Retroperitoneal L2 corpectomy with cage; T12–L4 posterior stabilization with transpedicular cement augmentation | Early return to deambulation (MRS 1). No residue | 6, no recurrence | Lymphatic leakage, causing chylothorax and complicating surgical management | |
| McLoughlin et al., 2008[15] | Acute ascending quadriparesis | Hematoma evacuation with C5–T2 laminectomy; elective mediastinal mass resection (partial) | Complete recovery (MRS 0). Residue | 48, residue stable | Cystic. Spinal epidural hematoma caused by intralesional bleeding | |
| Ozeki et al., 2007[16] | Back pain | Interferon alpha | NR; regression of some bone lesions | 9 | Generalized lymphatic anomaly | |
| Ozturk and Yousem, 2007[17] | Back pain (NR) | None | - | 72, disease stable | Generalized lymphatic anomaly | |
| Chu et al., 2007[18] | Cervical radiculopathy (2 years) | Surgical resection (complete) | Partial recovery (MRS 2). No residue | 12, no recurrence | Solitary dumbbell-shaped lesion | |
| Pavanello et al., 2007[19] | Headache, head tilt, recurrent osteomastoiditis (NR) | Local radiation therapy | NR | 18, no recurrence | Chiari 1 malformation | |
| Jiang et al., 2006[20] | Progressive paraparesis (1 month) | Surgical resection (complete) | Complete recovery (MRS 0). No residue | 3, no recurrence | Adherent to lumbosacral roots | |
| Jiang et al., 2006[20] | Progressive paraparesis, back pain (1 month) | Surgical resection (complete) | Complete recovery (MRS 0). No residue | 12, no recurrence | Intra-lesional hemorrhage | |
| Wallace and Ross, 2005[21] | Severe low back and hip pain (2 months) | Percutaneous vertebroplasty | Complete pain relief (MRS 0) | 3 | Immediate pain relief; short follow-up | |
| Jea et al., 2003[22] | Exertional headache, ear pain (NR) | Suboccipital decompression | Improved pain (MRS 1) | NR | Chiari 1 malformation, GLA | |
| Watkins et al., 2003[23] | Acute Brown-Séquard syndrome; cervico-thoracic deformity | C4–T2 corpectomy with fibular allograft; halo vest; plans for posterior stabilization | Death due to cardiorespiratory complications (postoperative day 14) | - | GLA with chylothorax causing respiratory complications | |
| Watkins et al., 2003[23] | Cervico-thoracic deformity; neck pain (1 year) | C6–T4 corpectomy with rib autgroaft and anterior plate fixation, C2–T7 posterior stabilization | Paraparesis with subsequent complete recovery (MRS 1) | 12, no recurrence | ||
| Kanamori, 2002[24] | Progressive sciatica (6 months) | Surgical resection (complete) | Complete pain relief (MRS 0). No residue | 66, no recurrence | Intradural | |
| Méndez et al., 2002[25] | Low back pain, intermittent sciatica (2+years) | Embolization, vertebroplasty (inefficacious), then surgical revision and L4 corpectomy | Partial pain relief (MRS 1). No residue | 24, no recurrence | Solitary lesion | |
| Saito et al., 1999[26] | Low back pain, sciatica (6 months) | Surgical resection (complete) | Persistent pain (MRS 2). No residue | 23, no recurrence | Dumbbell shaped, strongly adherent to nerve root dura | |
| Maki et al., 1999[27] | Paraparesis, back pain (NR) | Sacral laminectomy, interferon alpha | Limited pain relief (MRS 2) | 96 | GLA | |
| Maki et al., 1999[27] | Diffuse bone pain (NR) | Interferon alpha | Pain relief (MRS 0) | 48 | GLA | |
| Forstner et al., 1998[28] | Back pain (NR) | None | - | 84 | GLA; sclerotic variant. T4 lesion demonstrated initial rapid growth, then stable dimensions | |
| Whitley and Flannery, 1996[29] | None | T3–T10 laminectomy and resection of epidural mass | NR. No residue | NR | Proteus syndrome; cystic lesion | |
| Keenen et al., 1995[30] | Low back pain (1 year) | Transperitoneal partial corpectomy with iliac crest autograft; resection of abdominal cyst | Pain relief (MRS 1). No residue | 22, no recurrence | Solitary lesion | |
| Edwards et al., 1983[31] | - | - | - | Same patient from Canady 1980 | ||
| Canady and Chou, 1980[32] | Progressive cervical myelopathy (NR) | C1–C2 posterior decompression; C3–T1 posterior fusion; then C5–C7 decompression and C5–T1 stabilization with fibular autograft | Death during follow-up due to cardiopulmonary complications | 72 | Severe cervical instability due to massive osteolysis; bone chronic inflammation and necrosis | |
| Rogers and Chou, 1973[33] | Occipital headache (6 months) | Posterior cranio-cervical decompression | Pain relief (MRS 1). Residue | NR | ||
| Reilly et al., 1972[34] | Low back pain, claudication (1 year) | Radiation treatment | NR. Regression of lesions | 24, no recurrence | ||
GLA - Generalized lymphatic anomaly; MRS - Modified Rankin scale; NA - not applicable; NR - Not reported
CASE PRESENTATION
A 67-year-old female presented at our emergency department with a 3-week history of walking difficulties and impaired sensation in the lower extremities. Neurological examination revealed spastic paraparesis (Medical Research Council 3/5 in both legs) and moderate hypoesthesia below the level T6-T7. A magnetic resonance imaging (MRI) study revealed at T6-T7, a 26 mm × 12 mm extradural intracanal-intraforaminal lesion displacing the spinal cord anteriorly and to the left, without radiological signs of myelomalacia [Figure 1]. It extended into the right T6-T7 neural foramen, which was widened. The mass was hyperintense on T2-weighted sequences, hypointense on T1, and homogeneously enhancing. An unrelated subcutaneous lipoma was also evident. A right T6-T7 hemilaminectomy was performed; the lesion appeared dark red and prone to bleeding. Gross total resection was achieved, leading to an optimal decompression of the dural sac and the spinal cord. A histopathological diagnosis of lymphangioma was made [Figure 2].
Figure 1.
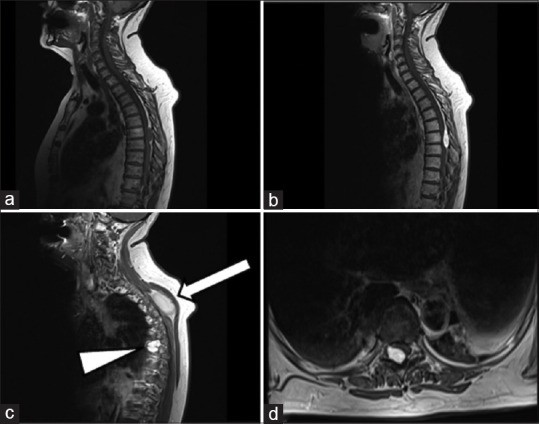
Preoperative magnetic resonance imaging. (a) T1, (b) postcontrast T1, (c) Paramedian postcontrast T1, showing transforaminal extension (arrowhead) and the unrelated subcutaneous lipoma (arrow), (d) axial postcontrast T1
Figure 2.
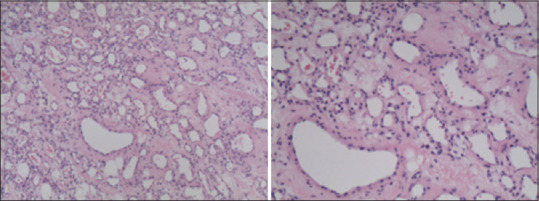
Magnification: 10x (left), 20x (right), H/E-stained microscopic sections of the extradural lesion, showing vascular structures of lymphatic and capillary appearance. The endothelium of these channels reacted extensively with D2-40 antibodies, which react with podoplanin and are used as a selective marker of lymphatic structures
Our patient had an uneventful postoperative course, characterized by an early and significant improvement in symptoms; she subsequently made a full recovery.
At her 18-month appointment, she appeared neurologically intact. Follow-up MRI did not show any recurrence of the lesion [Figure 3].
Figure 3.
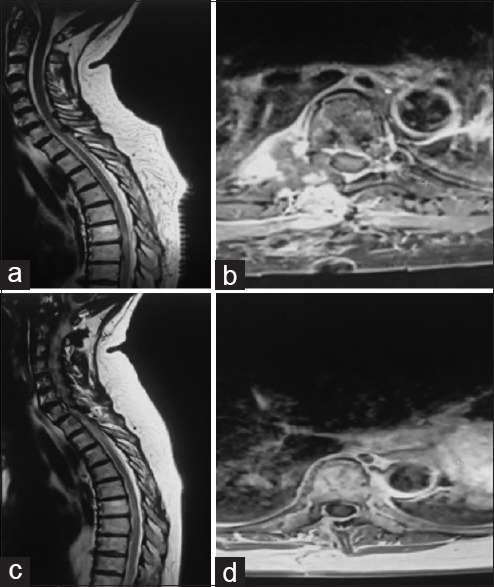
Early postoperative magnetic resonance imaging (MRI) (a and b), 18-month follow-up MRI (c and d)
In order to provide an overview on the subject, we performed a systematic review of the literature based on the NCBI database. The following search string was adopted: “(“lymphangioma” OR “cystic hygroma” OR “lymphatic malformation” OR “lymphangiomatosis”) AND (“spinal” OR “vertebral” or “spine”).”
For each pertinent article, we selected information regarding patient’s age and sex; lesion location, pathology, and MRI appearance; presenting symptoms, treatment, and clinical and surgical outcome; and follow-up duration.
RESULTS
Through our search, we identified 32 papers published between 1972 and 2019, presenting a total of 35 cases. Among the reported spinal pathologies, four main phenotypes were identified: (1) eighteen patients had single or multiple vertebral bony involvement (with an epidural extension in three cases); (2) ten patients presented a selective involvement of spinal epidural space, similar to our patient (with or without transforaminal extension); (3) four had a secondary spinal involvement by mediastinal masses extending into spinal epidural space through the intervertebral foramina; and (4) three patients presented intradural extramedullary lymphangiomas (two thoracic and one sacral) [Table 1].[3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]
DISCUSSION
Pathophysiology
General consensus is that lymphangiomas are hamartomas originating from the proliferation of abnormal lymphatic structures excluded from the systemic lymphatic circulation;[2,35] however, the detailed pathophysiological mechanisms remain a matter of debate. A distinction has been made between congenital (more frequent) and rarer, acquired lymphangiomas.[1,2] In the former case, failure of the lymphatic system to connect with or separate from the venous system and abnormal budding of the lymphatic system from the cardinal vein lead to the formation of lymphatic anomalies.[1] In acquired cases, focal abnormalities of lymphatic vessels are thought to originate from insults such as trauma, radiation, infections, and chronic inflammation. Interestingly, according to the literature, 90% of all soft tissue lymphangiomas are congenital and/or clinically evident by the 2nd year of age;[2] anyway, we found that the mean age at presentation for SL is 30 years. To justify adult presentations, most authors assume a two-hit hypothesis, stating that under normal conditions, small abnormalities of lymphatic drainage could be compensated in healthy subjects; clinically significant disease would arise in conditions associated with a local increase in lymphatic fluid volume, leading to lesion expansion.[1] Anyway, mechanisms of delayed cellular proliferation (triggered by inflammatory or traumatic mechanisms) cannot be excluded.[1]
Anatomopathological features
Lymphangiomas are characterized by stromal aggregates of lymphocytes and endothelial lining that usually stain positively with factor VIII related antigen or CD31 and that is often surrounded by a layer of smooth muscle tissue.[1] One of the most reliable markers of this pathology is represented by podoplanin, a transmembrane mucoprotein selectively expressed in lymphatic endothelium. Significant reactivity of the disease tissue with anti-podoplanin monoclonal antibodies, such as D2-40 murine antibodies, can thus reliably characterize it as a lymphatic anomaly.
The traditional histopathological classification, comprising capillary, cavernous, and cystic lymphangiomas, is mostly considered obsolete; we have thus decided to exclude it from our analysis. Currently, it has been replaced by the distinction between macrocystic (with cysts >1 cm in diameter) and microcystic (<1 cm) lymphangiomas,[1] which anyway has a limited applicability to spinal lymphatic anomalies.
In cases of bone involvement, up to the 1970s, no clear distinction was made between lymphatic and “simple” vascular anomalies, which were both included under the ambiguous definition of “angiomatosis.”[32] This generated confusion and complicated the analysis of the oldest reports and case series.
Epidemiology and clinical features
In the literature, the mean age at presentation of SL is 30.4 years (median 26, range 1–83). Sex distribution is 41% of females and 59% of males. The clinical picture related to spinal lymphatic anomalies is strongly dependent on the site and on the extent of involvement of spinal anatomical structures. The thoracic spine seems to be the most frequently affected segment, followed by the cervical spine and the lumbosacral spine.
Most commonly, a gradual onset of symptoms (especially back pain and/or signs and symptoms of spinal cord/root compression) is reported. An acute presentation has been described in relation to cord compression determined by intralesional bleeding (with formation of spinal epidural hematomas)[3,15] or spinal instability.[23] Our patient presented with progressive onset (3 weeks) of thoracic back pain with motor and sensitive impairment. According to the literature, the average time from symptom onset to surgery is 10 months (range, 0–48 months).
A good clinical and neurological outcome is generally reported, with the exception of cases presenting with extensive spinal and/or thoracic involvement. Anyway, data on long-term outcomes are lacking, as median follow-up in the literature (reported in 20 cases) is about 22 months.
Imaging
According to the available literature, SL involving the vertebrae tend to appear as osteolytic lesions on plain spine radiographs; cases with more extensive spinal involvement can present with vertebral collapse and remodeling and severe column deformities.[23] In some of the latter cases, there may be an apparent overlap with Gorham-Stout Syndrome (GSS), a rare disorder characterized by extensive osteolysis with lymphatic proliferation replacing the eroded bone.[19,22,32,33] The features that should characterize specifically GSS are the progressive and massive extent of the osteolysis and the involvement of cortical bone.[36,37]
On spine computed tomography (CT) scans, vertebral lymphangiomas tend to appear as osteolytic lesions that subvert the normal trabecular architecture, mimicking the CT behavior of vertebral hemangiomas (see below); calcifications have been reported.[6]
On MRI, the most consistently reported feature is T2 hyperintensity. On T1, lymphangiomas show a more variable behavior, displaying hypointensity (more frequent) or iso/hyperintensity. Contrast enhancement (CE) is generally present; among cases where postcontrast MRI sequences were acquired, only one was nonenhancing;[28] about half of the enhancing lesions showed intense, homogeneous CE, and the other half showed heterogeneous CE. MRI features seem not to be specific to the phenotype and location of the lesion (namely, vertebral, epidural, intradural, and mediastinal).
Macrocystic appearance of SL is not common;[4,6,9,10,29] this contrasts with the cystic macroscopic texture of most soft-tissue lymphangiomas.[2,38] In fact, in the majority of cases of SL with macrocystic features, spinal involvement was secondary, while the bulk of the lesion resided in the paravertebral viscera.[4,6,29]
Of note, SL can demonstrate signs of intralesional bleeding;[3,12,15] in two cases, spontaneous tumor bleeding resulted in a symptomatic spinal epidural hematoma.[3,15]
Diagnosis through imaging alone is virtually impossible, as lymphangiomas seem to be “great imitators,” mimicking the features of various and far more common pathologies. In particular, in case of vertebral involvement, lymphangiomas cannot be distinguished from hemangiomas (showing the same T2 hyperintensity, T1 variable intensity, and CE); on CT images, they can even mimic the pathognomonic features of the “polka dot” and “jail bar” sign.[25] When they are confined to the spinal canal, lymphangiomas can mimic spinal schwannomas or neurofibromas, potentially recreating the typical dumbbell shape[18,26] (in our case, transforaminal extension with widening of the neural foramen was deemed particularly suggestive of schwannoma). Other important and common differential diagnoses are spinal meningiomas (especially in cases with homogeneous CE), cystic lesions, and metastases.
A reliable diagnosis based on imaging alone can generally be formulated only in cases of secondary spinal involvement by a mediastinal/thoracic mass; in these cases, the patient often has a known history of systemic lymphangiomatosis. The same applies to spinal lesions that are incidentally discovered in patients with known diffuse lymphatic anomalies.[17,28]
Surgery: Indications, techniques, and outcomes
Surgery is the mainstay of treatment for symptomatic SL, except when pain is the only clinical manifestation. Gross total resection should be a goal, considering that (1) information on recurrence rate/residual growth is limited; (2) residual pathological tissue could keep producing/leaking chyle, generating symptomatic pseudocysts[9] or chyle leaks;[14] and (3) there is a bleeding risk associated with residual tumor.[3,5,15] Anyway, complete resection is not always achievable, whether due to the span of the lesion,[10] its deep/complex location, or its adherence to vulnerable structures. For example, intradural lymphangiomas can adhere to the spinal cord pia;[9] complex mediastinal and/or vertebral masses often show intimate relationships with thoracoabdominal vital structures.[14]
In the literature, the best surgical outcome is reported for intracanal epidural lesions. In these cases, with a typical extension of 2–3 vertebral levels, complete resection was generally achieved (9 of 10 patients). The only exception was a giant cystic lesion with cervicothoracic span, treated with CT-guided needle aspiration (view below).[10] Intraoperative reports consistently described the lesions as dark red; this was also our intraoperative impression (there are 3 exceptions, with reported clear or greyish texture).[13,20] Clinical outcome, when reported (8 of 10 patients), was generally good (75% complete recovery; 12.5% partial recovery; and 12.5% unchanged symptoms). In 4 additional cases,[4,6,15,29] the epidural mass was the “tip of the iceberg,” representing a transforaminal extension of voluminous mediastinal lesions; surgical management involved posterior decompression and, when feasible, resection of the epidural mass. In one case, the mediastinal mass was removed in a second surgical step,[15] while in other patients, it was left untouched. Of note, these cases appeared different from the “pure” epidural lymphangiomas described above, because they presented a macrocystic appearance and had a wider vertical span (5–11 levels).
Interestingly, in 2 cases, there was an acute onset of symptoms due to intralesional bleeding, producing a spinal epidural hematoma.[3,15]
Among 3 intradural lesions, 2 could be resected completely; one was adherent to the spinal cord pia and thus a residue was left.[9] In this case, residual tissue continued to produce fluid that accumulated in a pseudocyst with symptomatic cord compression. After an unsuccessful surgical revision, it was effectively managed with a cystoperitoneal shunt.
Cases of vertebral involvement were mainly approached with a preoperative diagnostic suspect of hemangioma. In particular, vertebral lymphangiomas show considerable similarities with the so-called “aggressive” hemangiomas.[39,40] Conservative treatments (embolization and percutaneous vertebroplasty) have been used for pain management,[8,21,25] while surgical treatment with posterior, anterior, or combined strategies was reserved for spinal instability or neurologic deterioration.
Intraoperative or postoperative chyle leaks from the involved vertebrae have been consistently reported.[8,21,22,31,33] In a case of L2 collapse with cord compression, a copious chyle leak was observed intraoperatively from the crushed vertebra; it stopped after somatectomy and ligation of the adjacent soft tissues. Furthermore, during T12-L4 posterior fixation, additional leaking was observed through the pedicular screw holes on the adjacent vertebrae (which showed signs of milder somatic involvement on MRI). This was managed with transpedicular cement augmentation through cannulated screws.[14]
Some reported cases of extensive osteolytic involvement of the craniocervical junction in children[19,22,32,33] cannot be reliably differentiated from Gorham-Stout disease (GSD), which is also in the spectrum of lymphatic anomalies.[37] According to the 2013 criteria published by Lala et al.,[36] GSD is characterized by extensive and progressive osteolysis involving mainly the axial skeleton with cortical erosion, while in generalized lymphatic anomalies, bone lesions are not progressive and tend to spare the cortex. In these reports, structural remodeling of the skull base and first cervical vertebra caused platybasia, basilar invagination and/or reduction of posterior fossa volume, which was associated with Chiari I malformation (CIM) in two cases;[19 22] we believe that in such cases the lymphatic anomaly, which was probably congenital, was responsible for the abnormal development of the skull base, producing the changes that are typically associated with CIM.[19,41] In spite of the risk of subsequent instability, two of these patients were managed with suboccipital decompression and C1 laminectomy.[22,33] No adequate follow-up is available for the assessment of postoperative instability and eventual disease progression. One other case was managed with rigid collar and radiation treatment, with apparent disease control and promotion of bone fusion at the craniocervical junction at the 2-year follow-up.[19] In another case, bone grafts were also attacked by the disease, with ongoing instability; radiation therapy was not effective in stopping disease progression.[32]
The bottom line is probably that these cases represent an individual subset in the spectrum of SL; it is interesting to compare them to the more “typical” and non-progressive craniocervical lymphatic anomaly reported by Wright et al.[8]
Establishing the risk of recurrence/regrowth of the lesion after surgical resection is difficult given the limited availability of information. The main data on the natural history of SL come from the follow-up of patients with a known systemic lymphangiomatosis, in which spinal involvement was noted during disease staging. There are only 2 available reports.[17,28] In one, the spinal lesion remained stable in the course of a 6 years of follow-up.[17] In the other case, the vertebral lesion showed an initial, rapid increase in size and then remained stable for the subsequent 7 years.[28] Both patients remained asymptomatic.
In the largest published series on common pediatric lymphangiomas,[2] 154 patients with various disease locations were treated with surgical resection (complete in 93% of cases). Recurrence rate was 17% during the 3-year mean follow-up for the complete resection group. Of note, recurrence rates depended strongly on the anatomical site, being 33% for head and neck lesions and 0% for visceral lesions. Thus, the translation of these data to SL should be done cautiously.
According to our literature analysis, in the 12 cases where complete resection was obtained and follow-up duration is reported, no recurrence was observed over a mean follow-up of 18.5 months (range, 3–66). In all 4 cases where a residue was left, the disease appeared stable after a mean follow-up of 26.5 months (range, 6–48).[5,8,9,15] Anyway, the concept of recurrence presents different nuances. For example, in one case, recurrence of symptoms was observed after reaccumulation of chyle in a pseudocyst.[9] It is worth noting that an aggressive disease course has been described only in relation to the aforementioned cases of extensive craniocervical involvement, which seem to represent an individual subset in the spectrum of spinal lymphatic anomalies.
To resume, available data indicate that complete resection should be pursued whenever possible, as this minimizes the risk of recurrence and complications during follow-up. Patients should be followed up with periodic imaging studies.
Other treatment modalities
Due to the virtual impossibility to formulate a diagnostic suspect based on imaging only, in real-life clinical situations, nonoperative management of SL is limited to the following circumstances: (1) patients with a known systemic lymphangiomatosis, where spinal lesions are discovered during disease staging or because of symptoms; (2) operated patients with incompletely resected or satellite spinal lesions; and (3) patients in which a diagnostic biopsy of the lesion was obtained. The main indications were refractory pain, diffuse symptomatic disease not amenable to surgical treatment, or as an adjunct to surgery.
The following nonsurgical strategies have been reported:
Vertebroplasty: Four reports are available. One[8] is the case of a patient with a previous diagnosis of generalized lymphatic anomaly, complaining of severe occipital headache. A craniocervical CT scan demonstrated an osteolytic involvement of the clivus and C1-C2 vertebrae with cortical sparing. The authors performed a transoral clivoplasty and C1-C2 vertebroplasty with excellent results (complete pain relief, no neurological complications and no signs of disease progression at a follow up of 2 years). In another case, after L4 percutaneous vertebroplasty, the patient presented again with cement migration requiring surgical revision; later, possible disease progression led to vertebral body collapse which was finally managed with L4 corpectomy.[25] An increased risk of cement migration could have been predicted preoperatively due to the extension of the vertebral lesion, which had also an epidural propagation. This recalls the complications reported with the same treatment for aggressive hemangiomas.[39] Unfortunately, there are no reports of balloon kyphoplasty, which could be useful in limiting cement leaks.[39] In a patient with systemic lymphomatosis, sacral vertebroplasty was successful in relieving pain on a 4-month follow-up.[21] Finally, cement augmentation represented an useful intraoperative aid in one case of multilevel somatic involvement,[14] where intraoperative vertebroplasty was performed through cannulated screws. This helped in stopping a troublesome transpedicular chyle leak and also was useful in consolidating the anterior column (view above), allowing for more stable instrumentation. Overall, percutaneous vertebroplasty can be considered in cases of refractory pain if the risk of cement diffusion is deemed to be low; cement augmentation can also be considered as a surgical aid to help in the management of collaterally involved bone. There are insufficient data to argue about the risk of cement diffusion in such cases. In one report, contrast medium was injected in the lesion and demonstrated spreading in the adjacent soft tissues,[21] suggesting a possible communication between the abnormal lymphatic structures and the drainage system of the nearby soft tissues
Percutaneous cyst aspiration: There is one report describing CTguided percutaneous aspiration of a giant epidural cyst. Unfortunately, no follow-up is reported. Theoretically, by considering the available data, this technique could carry a significant risk of cyst recurrence/reaccumulation[2,9]
Radiation therapy: The use of radiotherapy was limited to two cases of aggressive lymphatic anomalies of the craniocervical junction, in the attempt to control disease progression.[19,32] In one case,[19] the patient was managed with a rigid collar and the treatment (total dose: 3450 cGy) appeared effective in stopping the osteolytic process and in promoting bone fusion at 18 months; the collar was then withdrawn. In the other case,[32] the disease progressed in spite of radiation treatment (total dose: 4100 cGy), leading to uncontrollable craniocervical instability. Apparently, postmortem exam of the irradiated bone showed a regression of the lymphatic involvement.[31,32] As we noted previously, such cases of diffuse osteolytic involvement probably represent a parallel disease entity in the spectrum of lymphatic anomalies, closer to GSD
Systemic interferon alpha: The first report on the use of pegylated interferon alpha-2B for the treatment of diffuse lymphatic anomalies was published in 1991; since then, some case series have accumulated, for a total of 14 patients.[16] The rationale of this treatment comes from the known anti-angiogenic and antiproliferative effect of interferon-alpha probably mediated by vascular endothelial growth factor inhibition.[35] Unfortunately, data on treatment duration and dosage are incomplete and heterogeneous. The main part of these patients was treated because of symptomatic lung and pleural disease, with mixed results.[16] In three, there was a spinal involvement.[16,27] One patient (female, 30)[27] had multiple vertebral involvement with L3 compression fracture and refractory back pain. Due to the diffuse nature of her disease, she underwent a course of interferon alpha (dosage and duration not specified) with scarce benefit. Another patient (male, 12) presenting with bone pain due to diffuse cervico-thoracic involvement, was given a 42-month course of interferon-alpha (dose not specified); this was effective in halting disease progression first, and then promoting the regression of the bone lesions; bone pain resolved. Treatment was discontinued due to the occurrence of transient anemia and abnormal liver function tests; anyway, the effects of treatment persisted over the next 18 months of follow-up. Finally, another patient (male, 5)[16] with multiple visceral and vertebral involvement, leading to a T10 compression fracture, was given 1 µg/kg of subcutaneous pegylated interferon-alpha 2B once a week, later increased to 1.5 µg/kg weekly. Treatment was initiated in an attempt to control pulmonary disease. After a course of 3 months, he showed regression of pleural effusion, improvement of pulmonary function tests, and, collaterally, a partial regression of bone lesions. He reported common interferon side effects such as fatigue, headache, and a mild rise in body temperature. A total course of 9 months was well tolerated overall.
In contrast with lymphangiomas occurring in other anatomical districts (especially head, neck, and upper torso), which are managed in a significant portion of cases with sclerotherapy,[42,43] there are no reports of the use of this treatment for SL. This can be attributed to the theoretical risk of diffusion of the sclerosing agent, which could generate damage to nearby tissues and especially to the spinal cord and roots.[21,39] In one case, intralesional alcohol injection was hypothesized for pain control in a sacral lesion,[21] but after intralesional contrast injection showed evidence of soft tissue diffusion, treatment plan was shifted to vertebroplasty.
CONCLUSION
With this work, we aimed at gathering literature data on spinal lymphatic malformation, in order to eliminate ambiguities and categorize their peculiar clinical and radiologic features and treatment possibilities. In real-life clinical practice, two scenarios can be encountered. “in the first scenario, after an incorrect pre-operative diagnostic hypothesis, clinicians are confronted with the unexpected histopathological diagnosis of lymphatic malformation. In this case, during the initial postoperative follow-up, an additional focus should be put on anticipating and recognizing possible chyle leaks (especially in cases of vertebral lesions) and/or early pseudocyst formation, especially if a residue was left. A periodic follow-up imaging with MRI should be obtained, even if with gross total resection chances of recurrence should be reasonably low.
In the second scenario, a diagnosis of SL is formulated in a patient with a previously documented generalized lymphatic anomaly or after a biopsy of the lesion(s). When surgical treatment is advised, preoperative arteriography and transarterial embolization can be considered, if deemed safe, to reduce intraoperative bleeding.[5] Whenever possible, gross total resection should be pursued to prevent recurrence and due to the aforementioned complications related to residual disease.
Data on nonsurgical treatments are limited but show some promise. Additional experience is warranted to enrich the armamentarium available to clinicians dealing with this pathology.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that her name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Wiegand S, Eivazi B, Barth PJ, von Rautenfeld DB, Folz BJ, Mandic R, et al. Pathogenesis of lymphangiomas. Virchows Arch. 2008;453:1–8. doi: 10.1007/s00428-008-0611-z. [DOI] [PubMed] [Google Scholar]
- 2.Alqahtani A, Nguyen LT, Flageole H, Shaw K, Laberge JM. 25 years'experience with lymphangiomas in children. J Pediatr Surg. 1999;34:1164–8. doi: 10.1016/s0022-3468(99)90590-0. [DOI] [PubMed] [Google Scholar]
- 3.Chia KJ, Lin LH, Sung MT, Su TM, Huang JF, Lee HL, et al. Acute spontaneous thoracic epidural hematoma associated with intraspinal lymphangioma: A case report. World J Clin Cases. 2021;9:3411–7. doi: 10.12998/wjcc.v9.i14.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Payne C, Gigliotti MJ, Castellvi A, Yu A. Rare case of cystic hygroma in the epidural space resulting in multilevel spinal cord compression. BMJ Case Rep. 2019;12:e230326. doi: 10.1136/bcr-2019-230326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fattahi A, Taheri M, Majdi M. Lymphangioma of the thoracic spine with epidural compression: A case report. Iran J Med Sci. 2019;44:172–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Rali P, Gandhi V, Malik K. Recurring giant mediastinal cystic lymphangioma. Am J Respir Crit Care Med. 2017;196:e1–3. doi: 10.1164/rccm.201611-2388IM. [DOI] [PubMed] [Google Scholar]
- 7.Pan X, Dong Y, Yuan T, Yan Y, Tong D. Two cases of hemolymphangioma in the thoracic spinal canal and spinal epidural space on MRI: The first report in the literature. Medicine (Baltimore) 2017;96:e9524. doi: 10.1097/MD.0000000000009524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright CH, Kusyk D, Rosenberg WS, Sweet JA. Percutaneous transoral clivoplasty and upper cervical vertebroplasties for multifocal skeletal lymphangiomatosis resulting in complete resolution of pain: Case report. J Neurosurg Spine. 2017;26:171–6. doi: 10.3171/2016.8.SPINE1675. [DOI] [PubMed] [Google Scholar]
- 9.Kerolus MG, Patil J, Kurian A, Sani S. Intradural cavernous lymphangioma of the thoracic spine: Case report, technical considerations, and review of the literature. Spine J. 2016;16:e561–5. doi: 10.1016/j.spinee.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Aslan K, Bekci T, Gunbey HP, Incesu L, Turgut E. A rare cause of backache: Epidural lymphangioma of the cervicothoracic spine. Spine J. 2015;15:1900. doi: 10.1016/j.spinee.2015.04.022. [DOI] [PubMed] [Google Scholar]
- 11.Renjen P, Kovanlikaya A, Narula N, Brill PW. Importance of MRI in the diagnosis of vertebral involvement in generalized cystic lymphangiomatosis. Skeletal Radiol. 2014;43:1633–8. doi: 10.1007/s00256-014-1935-1. [DOI] [PubMed] [Google Scholar]
- 12.Lee SC, Moon SG, Kim NR, Choe WJ, Moon WJ. Cervical epidural lymphangioma presenting as a hemorrhagic cyst: A case report. Spine (Phila Pa 1976) 2011;36:E1117–20. doi: 10.1097/BRS.0b013e3181ffe9a7. [DOI] [PubMed] [Google Scholar]
- 13.Ha BY, Park JB, Kim YM, Lyo IU. Lymphangioma in the Epidural Space of the Thoracic Spine. J Korean Neurosurg Soc. 2010;47:403. doi: 10.3340/jkns.2010.47.5.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sadrizadeh A, Etemad Rezaie H, Soltani E. A rare case of lumbar vertebral lymphangioma presenting as chylothorax. Spine J. 2009;9:e1–5. doi: 10.1016/j.spinee.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 15.McLoughlin GS, Nuchtern JG, Dauser RC, Sciubba DM, Gokaslan ZL, Wolinsky JP. Mediastinal lymphangioma presenting as an acute epidural hematoma. J Neurosurg Pediatr. 2008;1:474–6. doi: 10.3171/PED/2008/1/6/474. [DOI] [PubMed] [Google Scholar]
- 16.Ozeki M, Funato M, Kanda K, Ito M, Teramoto T, Kaneko H, et al. Clinical improvement of diffuse lymphangiomatosis with pegylated interferon alfa-2b therapy: Case report and review of the literature. Pediatr Hematol Oncol. 2007;24:513–24. doi: 10.1080/08880010701533603. [DOI] [PubMed] [Google Scholar]
- 17.Ozturk A, Yousem DM. Magnetic resonance imaging findings in diffuse lymphangiomatosis: Neuroradiological manifestations. Acta Radiol. 2007;48:560–4. doi: 10.1080/02841850701352038. [DOI] [PubMed] [Google Scholar]
- 18.Chu M, Li G, Wei L, Lin Y, Qi J, Wang C, et al. Arare case of cavernous lymphangioma in the epidural space of the cervicothoracic spine. Spine (Phila Pa 1976) 2007;32:E48–51. doi: 10.1097/01.brs.0000250992.55556.fe. [DOI] [PubMed] [Google Scholar]
- 19.Pavanello M, Piatelli G, Ravegnani M, Consales A, Rossi A, Nozza P, et al. Cystic angiomatosis of the craniocervical junction associated with Chiari I malformation: Case report and review of the literature. Childs Nerv Syst. 2007;23:697–700. doi: 10.1007/s00381-006-0274-5. [DOI] [PubMed] [Google Scholar]
- 20.Jiang YG, Xiang J, Zhang L. Intraspinal lymphangioma: 2 case reports and literature review. Surg Neurol. 2006;66:430–6. doi: 10.1016/j.surneu.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 21.Wallace MJ, Ross M. Bone lymphangiomatosis: Treatment with percutaneous cementoplasty. Spine (Phila Pa 1976) 2005;30:E336–9. doi: 10.1097/01.brs.0000166510.95018.97. [DOI] [PubMed] [Google Scholar]
- 22.Jea A, McNeil A, Bhatia S, Birchansky S, Sotrel A, Ragheb J, et al. Arare case of lymphangiomatosis of the craniocervical spine in conjunction with a Chiari I malformation. Pediatr Neurosurg. 2003;39:212–5. doi: 10.1159/000072474. [DOI] [PubMed] [Google Scholar]
- 23.Watkins RG, 4th, Reynolds RA, McComb JG, Tolo VT. Lymphangiomatosis of the spine: Two cases requiring surgical intervention. Spine (Phila Pa 1976) 2003;28:E45–50. doi: 10.1097/01.BRS.0000042235.41817.04. [DOI] [PubMed] [Google Scholar]
- 24.Kanamori M. Cystic lymphangiomas of the cauda equina. Spine J. 2004;4:357–9. doi: 10.1016/j.spinee.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Méndez JA, Hochmuth A, Boetefuer IC, Schumacher M. Radiologic appearance of a rare primary vertebral lymphangioma. AJNR Am J Neuroradiol. 2002;23:1665–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Saito T, Terada K, Tsuchiya K, Oda Y, Tsuneyoshi M, Iwamoto Y. Lymphangioma presenting as a dumbbell tumor in the epidural space of the lumbar spine. Spine (Phila Pa 1976) 1999;24:74–6. doi: 10.1097/00007632-199901010-00018. [DOI] [PubMed] [Google Scholar]
- 27.Maki DD, Nesbit ME, Griffiths HJ. Diffuse lymphangiomatosis of bone. Australas Radiol. 1999;43:535–8. doi: 10.1046/j.1440-1673.1999.00726.x. [DOI] [PubMed] [Google Scholar]
- 28.Forstner R, Datz C, Dietze O, Rettenbacher L. Sclerotic variant of lymphangiomatosis of bone: Imaging findings at diagnosis and long-term follow-up. Skeletal Radiol. 1998;27:445–8. doi: 10.1007/s002560050415. [DOI] [PubMed] [Google Scholar]
- 29.Whitley JM, Flannery AM. Lymphangiolipoma of the thoracic spine in a pediatric patient with Proteus syndrome. Childs Nerv Syst. 1996;12:224–7. doi: 10.1007/BF00301256. [DOI] [PubMed] [Google Scholar]
- 30.Keenen TL, Buehler KC, Campbell JR. Solitary lymphangioma of the spine. Spine (Phila Pa 1976) 1995;20:102–5. doi: 10.1097/00007632-199501000-00018. [DOI] [PubMed] [Google Scholar]
- 31.Edwards WH, Jr, Thompson RC, Jr, Varsa EW. Lymphangiomatosis and massive osteolysis of the cervical spine. A case report and review of the literature. Clin Orthop Relat Res. 1983:222–9. [PubMed] [Google Scholar]
- 32.Canady AI, Chou SN. Cervical lymphangiomatosis with progressive craniospinal deformity. Neurosurgery. 1980;6:422–5. [PubMed] [Google Scholar]
- 33.Rogers HM, Chou SN. Lymphangioma of the craniocervical junction. Case report. J Neurosurg. 1973;38:510–3. doi: 10.3171/jns.1973.38.4.0510. [DOI] [PubMed] [Google Scholar]
- 34.Reilly BJ, Davidson JW, Bain H. Lymphangiectasis of the skeleton. A case report. Radiology. 1972;103:385–6. doi: 10.1148/103.2.385. [DOI] [PubMed] [Google Scholar]
- 35.Liu T, Basseri S, Mussari B, DaBreo D, SenGupta S, Villalobos D, et al. Generalized lymphatic anomalies and review of the current management landscape: A case report and review of the literature. J Med Case Rep. 2021;15:398. doi: 10.1186/s13256-021-02953-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lala S, Mulliken JB, Alomari AI, Fishman SJ, Kozakewich HP, Chaudry G. Gorham-stout disease and generalized lymphatic anomaly – Clinical, radiologic, and histologic differentiation. Skeletal Radiol. 2013;42:917–24. doi: 10.1007/s00256-012-1565-4. [DOI] [PubMed] [Google Scholar]
- 37.Wassef M, Blei F, Adams D, Alomari A, Baselga E, Berenstein A, et al. Vascular Anomalies Classification: Recommendations From the International Society for the Study of Vascular Anomalies. Pediatrics. 2015;136:e203–14. doi: 10.1542/peds.2014-3673. [DOI] [PubMed] [Google Scholar]
- 38.Bagrodia N, Defnet AM, Kandel JJ. Management of lymphatic malformations in children. Curr Opin Pediatr. 2015;27:356–63. doi: 10.1097/MOP.0000000000000209. [DOI] [PubMed] [Google Scholar]
- 39.Vasudeva VS, Chi JH, Groff MW. Surgical treatment of aggressive vertebral hemangiomas. Neurosurg Focus. 2016;41:E7. doi: 10.3171/2016.5.FOCUS16169. [DOI] [PubMed] [Google Scholar]
- 40.Gaudino S, Martucci M, Colantonio R, Lozupone E, Visconti E, Leone A, et al. Asystematic approach to vertebral hemangioma. Skeletal Radiol. 2015;44:25–36. doi: 10.1007/s00256-014-2035-y. [DOI] [PubMed] [Google Scholar]
- 41.Bianchi F, Benato A, Frassanito P, Tamburrini G, Massimi L. Functional and morphological changes in hypoplasic posterior fossa. Childs Nerv Syst. 2021;37:3093–104. doi: 10.1007/s00381-021-05193-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morgan P, Keller R, Patel K. Evidence-based management of vascular malformations. Facial Plast Surg. 2016;32:162–76. doi: 10.1055/s-0036-1581141. [DOI] [PubMed] [Google Scholar]
- 43.Elluru RG, Balakrishnan K, Padua HM. Lymphatic malformations: Diagnosis and management. Semin Pediatr Surg. 2014;23:178–85. doi: 10.1053/j.sempedsurg.2014.07.002. [DOI] [PubMed] [Google Scholar]


