Keywords: acid/base balance, cell migration, colon cancer, pHi regulation, sodium bicarbonate cotransporter
Abstract
NBCn1 (SLC4A7) is one of the two major Na+-HCO3− cotransporters in the human colonic epithelium, expressed predominantly in the highly proliferating colonocytes at the cryptal base. Increased NBCn1 expression levels are reported in tumors, including colorectal cancer. The study explores its importance for maintenance of the intracellular pH (pHi), as well as the proliferative, adhesive, and migratory behavior of the self-differentiating Caco2BBe colonic tumor cell line. In the self-differentiating Caco2BBe cells, NBCn1 mRNA was highly expressed from the proliferative stage until full differentiation. The downregulation of NBCn1 expression by RNA interference affected proliferation and differentiation and decreased intracellular pH (pHi) of the cells in correlation with the degree of knockdown. In addition, a disturbed cell adhesion and reduced migratory speed were associated with NBCn1 knockdown. Murine colonic Nbcn1–/– enteroids also displayed reduced proliferative activity. In the migrating Caco2BBe cells, NBCn1 was found at the leading edge and in colocalization with the focal adhesion markers vinculin and paxillin, which suggests that NBCn1 is involved in the establishment of cell-matrix adhesion. Our data highlight the physiological significance of NBCn1 in modulating epithelial pH homeostasis and cell-matrix interactions in the proliferative region of the colonic epithelium and unravel the molecular mechanism behind pathological overexpression of this transporter in human colorectal cancers.
NEW & NOTEWORTHY The transporter NBCn1 plays a central role in maintaining homeostasis within Caco2BBe colonic epithelial cells through its regulation of intracellular pH, matrix adhesion, migration, and proliferation. These observations yield valuable insights into the molecular mechanism of the aberrant upregulation of this transporter in human colorectal cancers.
INTRODUCTION
Cell metabolism causes a production of acid moieties that need to be constantly removed from the cytoplasm. In addition, many cellular events require highly dynamic pH regulation for their proper function. One such event in the intestinal epithelium is the WNT-dependent proliferation-associated cell signaling (1). Another local pH-regulatory event occurs at the focal adhesions during cell migration (2). The colonic epithelium is particularly prone to experience large intracellular acid loads. During proliferation, the high metabolic activity will generate acid moieties. During the secretion of anions and fluid, HCO3− ions are exported into the intestinal lumen, and during short-chain fatty acid (SCFA) and electrolyte absorption in the surface region, protons are absorbed and HCO3− exchanged for Cl– and SCFA anions. The intestinal epithelial cells therefore express a multitude of ion transporters that are able to remove acid moieties from or import base moieties into the enterocytes, with a differential expression profile along the crypt/villous axis (3–5).
Among these acid/base transporters are several isoforms of the SLC4 family of Na+-dependent and Na+-independent Cl–/HCO3− exchangers and Cl–-independent Na+-coupled HCO3− transporters. Early studies in the duodenum, which could not well distinguish between the different molecular species because of overlapping sensitivities of different anion transporters to pharmacological inhibitors, suggested a role of Na+-HCO3− cotransport for enterocyte import of HCO3− ions destined for secretion into the lumen (6) or for neutralizing acid loads during high luminal acidity (7). The expression of both NBCn1 (SLC4A7) and NBCe1 (SLC4A4) in intestinal tissue, including human intestine, was documented (8–10), but the involvement of the NBC isoforms in the different physiological functions of the epithelium could only be tested in gene-deleted mice (11–13) and remained incomplete. The NBCe1-deficient mice die during weaning (13, 14), and the separation of NBCe1 and NBCn1 expression and function based on pharmacological strategies is not exact.
Independent GWAS studies confirmed that polymorphisms in NBCn1 were associated with an increased risk for breast cancer (15–17). NBCn1 protein expression was upregulated in primary breast cancer tissue relative to normal breast epithelium, and functional studies suggested an important pathophysiological role of NBCn1 for the aggressiveness of breast cancer cells (18). It is known that colorectal cancer cells also have a particular need for pHi maintenance (19, 20) and that their ability to maintain an alkaline pHi in the face of an acidic microenvironment increases their aggressiveness (21–23).
We had previously observed high expression levels of NBCn1 in the human self-differentiating Caco2BBe intestinal cell line, which were >10-fold higher than those for NHE1 (24). When we explored the expression of acid/base transporters in stem cell-rich, highly proliferating, nondifferentiated human colonic organoids, resembling the enterocytes in the cryptal base, we found NBCn1 to be the most abundantly expressed base loader in the proliferative compartment of the human colon, and to be strongly downregulated during differentiation (5). The high NBCn1 expression in the highly proliferative colonic enterocyte compartment, as well as in a colonic tumor cell line (24) led us to explore the role of NBCn1 in enterocyte intracellular (pHi) regulation, the adaptation of enterocyte anion transport to NBCn1 silencing, as well as the proliferative and migratory behavior of NBCn1-silenced enterocytes. Also, the molecular mechanisms for the reduced adhesion and migratory behavior of the NBCn1-silenced cells were investigated.
METHODS
Animals
Transcription of Slc4a7 (gene for Nbcn1) was interrupted by insertion of a gene trap-cassette in a CG-rich region upstream of exon 1. Details of the mouse generation and general characterization are given elsewhere (25). Mice were bred in the animal facilities of Hannover Medical School on a congenic C57Bl/6 background and genotyped as previously described (11). Care was taken to gender-match littermates. The mice were housed under standard temperature and light conditions (12:12 h light-dark cycle; temperature, 21–22°C) and were allowed free access to food and water. All experiments involving animals were approved by the Hannover Medical School Committee on investigations involving animals and an independent committee assembled by the local authorities.
Cell Culture and Establishing NBCn1 Knockdown Cell Clones
Caco2BBe1 cells (CRL-2102, ATCC) were cultured in Dulbecco’s Modified Eagle Medium (DMEM; Gibco) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich), 1% nonessential amino acids (NEAA; Gibco), penicillin-streptomycin (Sigma-Aldrich), and maintained in humidified incubator at 37°C in a 5% CO2 atmosphere. For downregulation of NBCn1 using short hairpin RNA (shRNA) technique, forward and reverse oligonucleotides against NBCn1 or mock coding sequences were designed (Table 1), separately annealed and cloned into “pLenti CMV Hygro DEST (w117-1)” (Addgene 17454) plasmid, and used to produce lentiviral particles in HEK293T cells according to Campeau et al. (26). The viral supernatant was then mixed with protamine sulfate at a final concentration of 4 µg/mL and incubated with Caco2BBe cultures overnight. Two days after infection, the cultures were selected with final concentration of 150 µg/mL hygromycin B in complete culture medium for 2 wk or longer, until the cells were used for the experiments. The cultures were regularly characterized by RT-quantitative PCR (qPCR) for NBCn1 mRNA expression.
Table 1.
Sequence of the oligonucleotides used for downregulation of NBCn1 by short hairpin RNA approach
| Name | Sequence | |
|---|---|---|
| NBCn1-KD#1 | Fwd | 5′-CCGGACTGCTAGATGGCTGAAATTTCTCGAGAAATTTCAGCCATCTAGCAGTTTTTTG |
| Rev | 5′-AATTCAAAAAACTGCTAGATGGCTGAAATTTCTCGAGAAATTTCAGCCATCTAGCAGT | |
| NBCn1-KD#2 | Fwd | 5′-CCGGAGCAGCCTTGTGTGTTATATTCTCGAGAATATAACACACAAGGCTGCTTTTTTG |
| Rev | 5′-AATTCAAAAAAGCAGCCTTGTGTGTTATATTCTCGAGAATATAACACACAAGGCTGCT | |
| NBCn1-KD#3 | Fwd | 5′-CCGGTGGTCATCATGGACCTTATATCTCGAGATATAAGGTCCATGATGACCATTTTTG |
| Rev | 5′-AATTCAAAAATGGTCATCATGGACCTTATATCTCGAGATATAAGGTCCATGATGACCA | |
| NBCn1-KD#4 | Fwd | 5′-CCGGTATGGGAGTTTCCTCATTAAACTCGAGTTTAATGAGGAAACTCCCATATTTTTG |
| Rev | 5′-AATTCAAAAATATGGGAGTTTCCTCATTAAACTCGAGTTTAATGAGGAAACTCCCATA | |
| NBCn1-KD#5 | Fwd | 5′-CCGGCGCTCTGGAAGGTCCATATTTCTCGAGAAATATGGACCTTCCAGAGCGTTTTTG |
| Rev | 5′-AATTCAAAAACGCTCTGGAAGGTCCATATTTCTCGAGAAATATGGACCTTCCAGAGCG | |
| Universal scrambled (mock) | Fwd | 5′-CCGGCCTAAGGTTAAGTCGCCCTCGCTCGAGCGAGGGCGACTTAACCTTAGGTTTTTG |
| Rev | 5′-AATTCAAAAACCTAAGGTTAAGTCGCCCTCGCTCGAGCGAGGGCGACTTAACCTTAGG | |
Cell Proliferation and Adhesion Assays
Cell were seeded in multiple wells of 12-well or 6-well flat-bottom adherent culture vessels at 9,000 cells per cm2 culture area and maintained up to 18 days with regular medium change. Over the culture period, wells from each culture type were trypsinized at regular intervals and the total number of the yielded cells was visualized by trypan blue and counted with a Luna II automated cell counter (Logos Biosystems).
The short-term proliferation of Caco2BBe control and KD#4 cells was assessed by crystal violet method. The cells were seeded in 96-well plates at a density of 20,000 cells and maintained in cell culture incubator for 18 h. Blank wells containing only medium were also included. After incubation, the wells were washed with PBS, fixed with 4% paraformaldehyde at room temperature for 20 min and then blocked with 0.1% BSA in DPBS for 5 min. After two washes with DPBS, the cells were stained with 50 µL of 0.1% crystal violet solution for 15 min, washed with distilled water to remove excess dye, and lysed with 100 µL of 1% SDS in dH2O for 30 min with gentle shaking. The optical density of samples at 590 nm was measured using BioTek Epoch Microplate Reader (Agilent). Data were illustrated as ratio of the control samples after subtraction of the blank values. Cell adhesion assay was performed similarly to crystal violet, except that 100,000 cells were seeded in a 96-well plate that was additionally coated with poly-l-lysine and the incubation time of the cells in culture incubator was limited to 2 h.
Murine Mid-Colonic Organoid Generation and Proliferation Assay
Murine mid-colon organoids were isolated from Nbcn1–/– mice and wild-type (WT) littermates. Genotyping was performed as previously described (11). Mice at the age of 12 wk were killed by cervical dislocation after light isoflurane anesthesia. Colon was extracted, washed in ice-cold PBS, and 1 cm of the mid-colon (about 3–6 cm away from the rectum) was used for crypt isolation. Crypt isolation and establishing three-dimensional colonoid cultures in Cultrex (R&D Systems) were performed as previously described (4). Colonoids at passage number 5 were used for BrdU proliferation assay. For this purpose, after the initial growth for 2 days in stemness medium (50% L-WRN-conditioned medium, 1× B-27 supplement, 1× N2 supplement, 1 mM N-acetyl-l-cysteine, 50 ng/mL murine EGF, 3 μM CHIR99021, 10 μM Y27632), colonoids were labeled with 10 µM BrdU in expansion medium (stemness medium without CHIR99021 and Y27632) for 12 h. The colonoids were isolated from Cultrex by three consequent washes and pipetting in ice-cold DPBS followed by centrifugation at 100g at 4°C for 5 min. Treatment with TrypLE Express Enzyme (ThermoFisher Scientific) for 3–5 min at 37°C was used to dissociate colonoids into single cells. Samples were washed with excessive medium and centrifuged to remove TrypLE reagent. After resuspension, cell number in each sample was determined, and 10,000 cells/well in a 96-well plate were used for the subsequent BrdU assay. BrdU cell proliferation assay (Millipore, #2750) was performed according to the manufacturer’s protocol for suspension cells. The BrdU incorporation into the newly synthesized DNA of proliferating cells was assessed by measuring the absorbance at 450 nm using a plate reader (Epoch; BioTek Instruments).
RNA Isolation and Quantitative Real-Time PCR
Total RNA was isolated from the cells using the RNeasy Mini Kit (Cat. No. 74106; Qiagen GmbH, Germany), according to the manufacturer’s instructions. The complementary DNA (cDNA) was synthesized from isolated RNA samples with the RevertAid First Strand cDNA Synthesis Kit (Cat. No. K1622; ThermoFisher Scientific). All cDNA samples were diluted to 2.5 ng·µL−1 final concentration with DNase/RNase-free water. For quantitative PCR, reaction mixture containing 4 μL diluted cDNA samples, 0.5 μL each of 10 μM forward and reverse primers (Table 2), and 5 μL qPCRBIO SyGreen Mix ROX (Nippon Genetics) was prepared. RPLP0 (ribosomal protein lateral stalk P0) mRNA in human samples and Gapdh (glyceraldehyde-3-phosphate dehydrogenase) mRNA in mouse samples were used as reference genes. The primer sequences used for qPCR analysis of mouse Nbcn1 were obtained from Choi et al. (27). Quantitative real-time PCR analysis was performed in triplicates using a Qiagen Rotor Gene Q real-time PCR detector system.
Table 2.
Sequence of the primers for RT-qPCR
| Target mRNA | Sequence | |
|---|---|---|
| NBCn1 (SLC4A7; Human) | Fwd | 5′-CTGCTATTCCTGCTTTGCTTTG |
| Rev | 5′-GTGATAGCCAGCTCCTTTCTT | |
| SLC26A3 (DRA; Human) | Fwd | 5′-CCAGCGTCTATTCCCTCAAAT |
| Rev | 5′-TCCCAGCAAATCCTCTGAATAC | |
| RPLP0 (reference gene; Human) | Fwd | 5′-ACGGATTACACCTTCCCACT |
| Rev | 5′-CGACTCTTCCTTGGCTTCAAC | |
| MEAD-Nbcn1 (mouse) | Fwd | 5′-CGAGCAGATGAGACCGCT |
| Rev | 5′-ACGCCGACGACTCTCTTTAC | |
| MERF-Nbcn1 (mouse) | Fwd | 5′-GAAAGATTTCAGCTGGCGAG |
| Rev | 5′-ACGCCGACGACTCTCTTTAC | |
| Total-Nbcn1 (mouse) | Fwd | 5′-ACAGAAGGCAGAATAAGTGCAATAGA |
| Rev | 5′-AGGTTGCCCAGCAAACAATG | |
| Gapdh (reference gene; mouse) | Fwd | 5′-TTTGCACTGGTACGTGTTGAT |
| Rev | 5′-AATGGATTTGGACGCATTGGT | |
Western Blot
Cells cultured in flasks or six-well plates were processed for Western blot analysis as outlined in a previous paper (5). Mock-transduced control cultures and NBCn1-KD#4 cultures were harvested at 1 wk post-confluence and NBCn1-KD#2 cultures were harvested 1 wk after the initial 14 days of antibiotic selection. Primary antibodies included mouse anti-SLC26A3 at a 1:500 dilution (sc-376187; Santa Cruz Biotechnology), anti-SLC4A7 at 1:2,000 dilution (ab82335; Abcam), and anti-β-actin at 1:2,000 dilution (3700; Cell Signaling Technology). Horseradish peroxidase-conjugated secondary antibodies (G-21040 or G-21234; Invitrogen) were used at a dilution of 1:10,000. Band visualization was achieved using an ECL solution (RPN2209; Cytiva) and the signals were captured and analyzed using a Fusion FX device, equipped with Fusion-Capt Advance software (Vilber Lourmat).
Measurement of Steady-State pHi in Caco2BBe Cells
Unlike NBCn1-KD#4 cells with moderate NBCn1 downregulation, NBCn1-KD#2 cells with substantial NBCn1 downregulation failed to be expanded after the KD was established in Caco2BBe cells. Therefore, we measured the steady-state pHi for NBCn1-KD#4 with double perfusion fluorometry and for NBCn1-KD#2 with single perfusion fluorometry. Control cells were prepared and analyzed for each approach similar to the corresponding KD cells. For double perfusion fluorometry, 10,000 cells were seeded on polyester transwell inserts with 6.5-mm diameter and 3-µm pore size, and maintained for few days to establish a subconfluent monolayer. For fluorometry, the membrane was sliced from insert and mounted in a custom-made chamber, which allows continuous perfusion of apical and basolateral compartments during fluorescent microscopy. For single perfusion, wild-type Caco2BBe cells were seeded on glass cover slips coated with poly-l-lysine in a six-well plate culture vessel, transduced and selected with hygromycin B for 2 wk to establish NBCn1-KD#2 cultures. Established control cells, which were transduced with scrambled construct, were seeded on coated coverslip and maintained for few days to represent subconfluent state of NBCn1-KD#2 cultures at the time of assay. The coverslips were mounted in a custom-made open chamber, which enables perfusion of the surface of the cells. This chamber was placed inside a closed cassette on the microscope stage, which maintained 5% CO2 and 37°C temperature during the assay.
Perfusion buffer for steady-state phase contained 1.5 mM CaCl2, 10 mM glucose, 10 mM HEPES, 2.4 mM K2HPO4, 0.4 mM KH2PO4, 1.5 mM KCl, 1 mM MgCl2, 116 mM NaCl, and 22 mM NaHCO3 and was warmed up to 37°C and continuously gassed with carbogen (5% CO2/95% O2 mix). Including the partial loss of CO2 in the perfusion tubing, 22-mM bicarbonate in the system was determined to provide a pH of 7.4 in the chamber. Calibration buffers contained 1.2 mM CaCl2, 10 mM glucose, 10 mM HEPES, 2.4 mM K2HPO4, 0.4 mM KH2PO4, 20 mM KCl, 110 mM K-gluconate, 1.2 mM MgCl2, and 20 mM NaCl, and their pH was adjusted to 6.8 or 7.6 with either HCl or NaOH solutions.
Cells were loaded with 5 µM BCECF-AM in steady-state buffer for about 30 min at room temperature. Then, chambers were mounted on the microscope stage and continuously perfused with steady-state buffer. Cells were sequentially excited at 445 nm (pH-insensitive) and 495 nm (pH-sensitive) wavelengths in 1-min intervals using a VisiChrome high-speed polychromator system (Visitron Systems), and the 530-nm emission with a 25-nm bandwidth was collected and recorded by a CCD digital camera (CoolSNAP HQ). After obtaining stable steady-state readouts for at least 30 min, cells were perfused with the first calibration buffer supplemented with 10 µM nigericin to clamp the pHi of the cells to the calibration solution followed by perfusion with the second calibration buffer. After subtracting the background values, the ratio of pH-sensitive/pH-insensitive intensities for each time point was calculated and used to determine the pHi using linear regression analysis of the two calibration points.
Wound Scratch Assay and Cell Migration
Control and NBCn1-KD#4 cells were cultured in Falcon® 12.5 cm2 culture flasks (Corning, NY). Cells were used as soon as they had reached confluence and formed a monolayer, that is, the control cells about 4 days and the Caco2BBe KD#4 cells about 10 days after seeding. Sterile 10-μL pipette tips were used to manually scratch a linear wound. After re-exposing the cultures to 5% CO2 for 30 min, the flasks were tightly closed and placed in heating chambers (37°C) mounted on AxioVert.A1 inverted microscopes (Carl Zeiss, Inc., Göttingen, Germany), and suitable regions were selected for imaging. Cell migration was videoed by time-lapse microscopy at 10-min intervals over a 12-h period using digital cameras (CCD, C8484-05G02) and the HCImageLive image acquisition software (both from Hamamatsu Photonics Deutschland GmbH, Herrsching, Germany). The changes in the wound area were quantified and analyzed using the NIH ImageJ software (NIH, Bethesda, MD). The cell-free area between the edges of the wound was measured and the migration rate was expressed as the percentage of wound closure over time, represented by the reduction of the cell-free area. This percentage was calculated dividing the wound area measured at various times post-scratching by the initial area at the beginning of the recording. An additional set of experiments with mitomycin was performed to rule out possible effects that could be caused by the observed differences in growth rate between the two clones.
Immunofluorescence and Confocal Microscopy
Wild-type Caco2BBe cells were seeded on poly-l-lysine-coated glass coverslips at low density (5,000 cells/cm2) and incubated for 24–48 h. The cells were washed in PBS, fixed in 2% PFA in PBS, washed again and blocked and permeabilized in 5% normal goat serum and 0.2% Triton X-100 solution in PBS for 30 min at room temperature. Samples were exposed to primary antibodies including anti-SLC4A7 (ab82335; Abcam), anti-vinculin (V9131; Sigma), and/or anti-paxillin (610051; BD Biosciences) diluted 1:500 in permeabilization buffer over night at 4°C with mild agitation. After few washes, the samples were exposed to a mixture of goat anti-mouse-AF488, goat anti-rabbit-AF568 (A11029 and A11011, respectively; Invitrogen), Phalloidin-iFluor 647 and DAPI in permeabilization buffer for 1 h at room temperature. The coverslips were finally washed and mounted on a glass slide using immunoselect antifading mounting medium (DIANOVA). Confocal microscopy was performed using a TCS SP8 microscope (LEICA Microsystems, Mannheim, Germany) with a 63× oil immersion objective. The images were analyzed and exported by LAS X (LEICA Microsystems) software. Analysis and visualization of colocalization were performed in Fiji (28) using the “Colocalization Finder” plugin.
Data Analysis and Statistics
Data analysis and visualization were performed with Microsoft Excel 2016 and GraphPad Prism version 8.0.2 (GraphPad Software Inc., San Diego, CA) software. Data values were presented as means ± SE. Statistical analysis was performed by calculating P values from unpaired, two-tailed parametric Student’s t test, or ANOVA. The statistical significances were indicated as ns (not significant) P > 0.05, *P ≤ 0.05, **P ≤ 0.01, or ***P ≤ 0.001.
RESULTS
Expression of NBCn1 (SLC4A7) mRNA in Human Colonic Mucosa, Colon Cancer, and in Caco2BBe Cells during the Different Growth Phases
The data from GENT2 database (29) show a significant upregulation of NBCn1 expression in colorectal cancer, as compared with healthy colonic tissue (Fig. 1A). Figure 1B shows that in contrast to the situation in colonoids from healthy human mucosa, where NBCn1 is highly expressed in the proliferative zone and is strongly downregulated during differentiation (5), the NBCn1 mRNA expression level remains relatively stable during the course of Caco2BBe culture from preconfluent state to 7-day postconfluency. In contrast, SLC26A3, a major apical Cl–/HCO3− exchanger, is strongly upregulated with increased differentiation of the Caco2BBe cells postconfluency (Fig. 1C). This suggested to us that the Caco2BBe cell line may be a useful model system to study the role of this transporter for pH homeostasis, the proliferative capacity, and the migratory behavior of colonic epithelial cells.
Figure 1.
Expression of NBCn1 in human tissue and Caco2BBe cells. A: gene expression of NBCn1 is upregulated in human colon cancer tissue compared with the healthy colon tissue. The Log2 mRNA expression data are provided by GENT2 database (29) available at http://gent2.appex.kr/gent2/ and are illustrated in Tukey box plot. B: expression of NBCn1 (Slc4A7) mRNA at different stages of Caco2BBe culture is relatively stable over the time of the culture. C: SLC26A3 encodes for an apical Cl–/HCO3– exchanger and is a differentiation marker of Caco2BBe cells. SLC26A3 mRNA expression is enhanced after the confluent monolayer starts to differentiate. Means ± SE, ANOVA. ns, not significant; preconfl., preconfluence cultures with 50% cell density. **P < 0.01, ***P < 0.001.
Different NBCn1 Knockdown Efficiency in Caco2BBe Cells
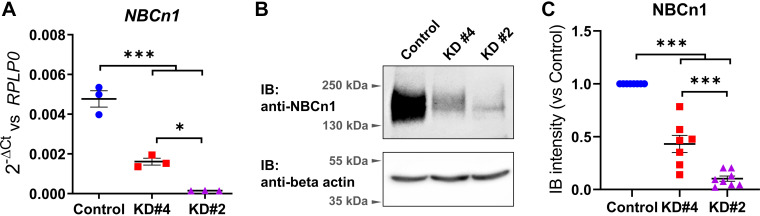
In Caco2BBe cells, NBCn1 expression was knocked down (KD) using five distinct shRNA constructs (Table 1), followed by hygromycin selection. Subsequent assessment of mRNA levels immediately post selection revealed that shRNA#2 achieved a 97% reduction and shRNA#4 a 66% reduction in NBCn1 mRNA expression (Fig. 2A). The reduction of NBCn1 expression by other shRNA sequences was suboptimal. Therefore, knockdown #2 and #4 were chosen for subsequent experimentation. At the protein level, KD#2 cells, harvested 1 wk after 14 days of selection, and KD#4 cells, obtained from passages 4 or 5 post-transduction and collected 7 days post-confluence, exhibited reductions of 90% and 57% in NBCn1 expression, respectively, compared with control cells transduced with a scrambled shRNA (Fig. 2, B and C). Caco2BBe NBCn1-KD#2 cells with the most substantial downregulation failed to grow after passaging the antibiotic selected cells. Therefore, the line could not be continued and for each set of experiments, the mother cells had to be transduced and selected with hygromycin. The KD#4 cells could be passaged and maintained in culture. However, over subsequent passages, the efficiency of NBCn1 downregulation in KD#4 cells gradually decreased despite continuous antibiotic selection. This suggests the drawback of NBCn1-KD on the expansion of the affected cells which results in overgrowth of antibiotic-resistant cells lacking the NBCn1 downregulation. Accordingly, subsequent experiments were performed with NBCn1-KD#4 passage numbers not exceeding 15, normally within 1 mo.
Figure 2.
Generation of NBCn1 knockdown Caco2BBe cells. Caco2BBe cells were transduced using lentiviral particles to generate NBCn1 knockdown (KD) cells, selected with hygromycin B and then harvested for Western and qPCR analysis. The knockdown efficiency was analyzed by quantifying NBCn1 mRNA by RT-qPCR, using RPLP0 as a control gene (A) as well as by Western blot, using β-actin as loading control (B and C). For further analysis, we selected two clones, namely KD#2 clone with 97% mRNA and 90% protein and KD#4 with 66% mRNA and 57% protein downregulation in comparison with the control, which was transduced with universal scrambled shRNA. Means ± SE, ANOVA, *P < 0.05, ***P < 0.001.
After reaching confluency, Caco2BBe cells gradually differentiate into polarized enterocytes which is associated with increased expression of differentiation markers, including the apical Cl–/HCO3− exchanger, SLC26A3 (Fig. 1C). However, we observed a substantially lower SLC26A3 expression in the KD#4 and KD#2 cells, at the same time of culture as the control cells (Fig. 3, A and B). This suggests that the downregulation of NBCn1 not only reduces the proliferative activity of Caco2BBe cells, but may also interferes with cellular differentiation.
Figure 3.
NBCn1-KD affects expression of SLC26A3. Representative immunoblot image showing expression of SLC26A3 protein in control and NBCn1-KD clones of Caco2BBe. Control and NBCn1-KD#4 cultures were harvested at 1 wk post-confluence and NBCn1-KD#2 cultures were harvested 1 wk after the initial 14 days of antibiotic selection (A). β-Actin was used as a loading control. Relative SLC26A3 signals were quantified and illustrated in ratio to control after normalization to the β-actin signal (B). NBCn1-KD substantially reduces protein expression of SLC26A3. Means ± SE, ANOVA. ns, not significant. ***P < 0.001.
Steady-State pHi in Caco2BBe in Association with NBCn1 Knockdown
The steady-state pHi was analyzed in Caco2BBe NBCn1-KD#2, KD#4 and control cells that were loaded with pH-sensitive dye BCECF, perfused with NaHCO3-buffered solution and assayed at a pH close to 7.4 in a temperature- and CO2-controlled chamber. Since NBCn1 downregulation was found to affect the growth and expansion of the cultures, pHi measurements were conducted in subconfluent cultures.
Due to the inability of KD#2 cells to be passaged, different approaches were used for the two KD cell lines. KD#4 cells were established and then cultured at low passage numbers on transwell membranes to form expanding islets of cells. The pHi of KD#4 cells cultivated on transwell membranes was measured via double perfusion fluorometry (separate reservoirs for selective perfusion of apical and basolateral compartment), whereas the pHi of KD#2 cells, which were directly transduced and selected onto coverslips, was assessed using single perfusion fluorometry. Control cells were prepared for each approach separately and were assayed in a similar fashion in parallel to the KD samples. For measuring the steady-state pHi of the control and KD#4 cells in the dual perfusion chamber, the upper and the lower compartments have a similar buffer composition; therefore, lack of monolayer integrity does not affect this experiment. We maintained a similar flow rate on both sides to ensure balanced pressure on the cells. After recording a stable steady-state pHi in the presence of CO2/HCO3− for at least 30 min, the pHi of the cells was clamped to two sequential calibration buffers with pH values slightly higher or lower than the estimated pHi. The results showed that the pHi of control cells was not different when measured in the double or single perfusion system. Both KD#2 and KD#4 cells had significantly reduced pHi values compared with control cells, with KD#2 cells having significantly lower pHi values than KD#4 cells (Fig. 4, A and B). In comparison with wild-type Caco2BBe cells, the control cells transduced with a scrambled shRNA showed a significantly lower pHi (Fig. 4B), likely due to the culture with hygromycin.
Figure 4.
NBCn1 downregulation reduces steady-state pHi in Caco2BBe cells. A: steady-state intracellular pH was measured by single chamber perfusion fluorometry for NBCn1-KD#2 cells and control cells which were grown and transduced on cover slips, directly after 2 wk of selection with hygromycin. B: steady-state intracellular pH was measured by double perfusion fluorometry for NBCn1-KD#4 cells, control cells, and wild-type (WT) nontransduced Caco2BBe culture. For these samples, the cultures were established in flasks, then seeded on transwell inserts and assayed in subconfluent state. The same culture conditions and times were used for WT and KD cells. Both NBCn1-KD clones have a significantly lowered steady-state pHi compared with their paired controls. The control cultures grown in the presence of hygromycin have a lower steady-state pHi than the nontransduced WT cells. Means ± SE, Student’s t test or ANOVA. *P < 0.05, **P < 0.01.
Proliferation and Adhesion of Caco2BBe Cells upon NBCn1 Downregulation
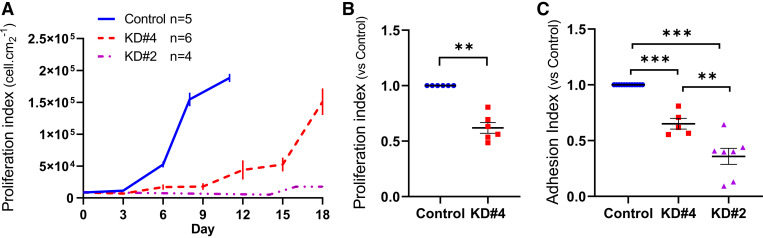
We compared long-term growth of the Caco2BBe-KD#2 and KD#4 cells with the control ones by counting cell number from parallel cultures for a period of up to 18 days. Although control cultures reached confluency after almost 11 days, the NBCn1-KD#4 cultures expanded slower and were unable to form confluent monolayers even after 18 days. Cell proliferation in NBCn1-KD#2 cells was strikingly perturbed. A slight increase in cell number was only detectable after 2 wk, coinciding with an unexpected increase in NBCn1 expression from baseline (Fig. 5A). The proliferation of control and KD#4 cells after 18 h of seeding was evaluated through crystal violet staining. The results showed that the short-term proliferation rate of KD#4 cells was significantly lower than that of the control cells (Fig. 5B). Short-term proliferation assessments were not conducted for KD#2 cells, as these cells exhibited no growth during the initial 2 wk of the long-term proliferation experiments.
Figure 5.
NBCn1 downregulation affects cell proliferation. A: NBCn1-KD#2 and KD#4 cells were seeded in multiple wells of culture plates at 9000 cells per cm2, and maintained for up to 18 days. Cell proliferation was determined by detaching and counting cells from selected culture wells. Control cultures reached confluency at day 11. B: short-term proliferation of Caco2BBe control and KD#4 cells was assessed by the crystal violet method 18 h after seeding in a 96-well plate. The results are given as ratio of the control samples. C: cell-matrix adhesion of NBCn1- KD#2 and KD#4 cells was determined separately in comparison with control cells. Per each well 100,000 cells were seeded in 96-well plates and incubated for 2 h. The ratio of the adherent cells was determined by crystal violet assay after removing nonadherent cells. Means ± SE, ANOVA. **P < 0.01, ***P < 0.001.
Similarly, we used crystal violet staining to study the effect of NBCn1 downregulation on cell-matrix adhesion of Caco2BBe cells. For this purpose, the incubation time after seeding was limited to 2 h to reflect the immediate capability of the resuspended cell in matrix adhesion. The results showed a significantly reduced matrix adhesion for both NBCn1-KD groups compared with the control group (Fig. 5C). Collectively, these data highlight the importance of NBCn1 for adhesion and proliferation of Caco2BBe cells.
Proliferation of Nbcn1–/– Colonoids
We have previously shown that nondifferentiated colonoid cultures from both murine and human express higher quantities of NBCn1 compared with differentiated ones (4, 5). However, the murine highly proliferative organoids expressed much more Nhe1 mRNA than the Caco2BBe cells did, in relation to Nbcn1 (4, 21). Since NHE1 has a similar function to NBCn1 and is also known to promote cell proliferation, the question arose: will the deletion of NBCn1 affect the proliferation of the murine nondifferentiated organoids? We generated colonoid cultures from mid-colon of Nbcn1–/– mice and wild-type littermates. The plating efficiency and the survival rate of the organoids were comparable up to passage number 10. Since it was known that the Nbcn1 “knockout” mouse strain that we studied is an incomplete knockout due to alternative splicing (27), we assessed the mRNA expression of both variants of Nbcn1. Cell proliferation was analyzed in nondifferentiated colonoids at passage number 5 using the BrdU assay. The results showed that the mRNA expression of the MEAD variant of Nbcn1 was strongly decreased in the Nbcn1–/–colonic organoids, whereas the MERF variant, transcribed from an alternative promoter, was expressed at very low levels and not significantly altered between WT and Nbcn1–/– organoids (Fig. 6A). A significantly reduced number of BrdU positive cells was observed in Nbcn1–/– colonoids compared to the control samples (Fig. 6B). The morphology (Fig. 6C) and size range (data not shown) of the colonic organoids were not different between WT and Nbcn1–/– organoids over the days of culture These data indicate that lack of Nbcn1 affects mucosal cell proliferation in mice and further confirms the results obtained in Caco2BBe model.
Figure 6.
BrdU proliferation assay of Nbcn1+/+ and Nbcn1–/– colonoids. A: RT-qPCR of Nbcn1 variants in the mouse colonoid cultures. MEAD-Nbcn1 (the expression of which is controlled by P1 promoter) is the primary variant of Nbcn1 in Nbcn1+/+ mice colonoid cultures. Expression of MEAD-Nbcn1 is significantly abolished in colonoid cultures from Nbcn1–/– mice. MERF-Nbcn1 (regulated by the P2 promoter), exhibits basal expression levels with no notable distinction between Nbcn1+/+ and Nbcn1–/– cultures; two-way ANOVA. B: mid-colon organoids from Nbcn1+/+ and Nbcn1–/– mice in day 2 culture were labeled with BrdU for 12 h and the signal intensity for incorporated BrdU was measured in resuspended cells. Organoids at passage 5 from three mice pairs; Student’s t test, *P < 0.05. C: representative light microscopy images of Nbcn1+/+ and Nbcn1–/– organoids which were used for BrdU labeling. The cultures are visually similar. Also, when the diameter of all organoids on the field of vision was assessed for all days and all organoid preparations, the average diameter of Nbcn1+/+ and Nbcn1–/– organoids was not significantly different (data not shown). Scale bar = 200 µm.
Migration Ability of NBCn1 Knockdown Cell
The wound healing scratch assay was used to evaluate sheet migratory rates in KD#4 and control cells. Caco2BBe NBCn1-KD#2 cells could not be maintained post passaging and therefore excluded here. We observed that the migration rate of Caco2BBe NBCn1-KD#4 cells was significantly lower than that of the control cells throughout the 12-h duration of the experiment (Fig. 7A). By the end of 12 h, control cells had migrated to cover 23.5% of the denuded area, in contrast to 13.1% covered by NBCn1 KD#4 cells (Fig. 7, A and B). The NBCn1 expression in KD#4 cells that were specifically used for migration measurements was only 26.4% lower compared with the control cells (Fig. 6C). This finding indicates that even a slight reduction in NBCn1 expression negatively influences the migratory speed of Caco2BBe cells.
Figure 7.
NBCn1 knockdown reduces Caco2BBe cell migration. A and B: wound scratch assay was used to determine the migratory activity of NBCn1 knockdown Caco2BBe cells and control cells. Representative images showing the extent of wound healing over 12-h periods from control and NBCn1-KD#4 cells. The dashed line indicates the initial scratch area at the beginning of the assay. Scale bar = 100 µm. All images are shown at the same magnification. C: the area that the cells covered was named wound gap closure and was calculated as a percentage of the initial wound area that was covered by the cells after 12 h. D: NBCn1 mRNA knockdown efficiency in the migration experiments. The experiment showed significantly reduced migratory activity in KD#4 cells. Student’s t test, *P < 0.05, ***P < 0.001.
Immunocytochemical Localization of NBCn1
Different aspects of epithelial cell homeostasis including matrix adhesion, proliferation, and migration are modulated by focal adhesions. Focal adhesions are macromolecular assemblies that anchor basal plasma membrane of the cells to the extracellular matrix (ECM) and provide a mechanical support for the cytoskeletal dynamics. We investigated the cellular localization of NBCn1 in subconfluent wild-type Caco2BBe cultures together with paxillin and vinculin as marker proteins for focal adhesions. We detected a predominant localization of NBCn1 at the leading edge of the migrating cells, where it modestly colocalized with both paxillin and vinculin markers (Fig. 8). These data suggest a role for NBCn1 function in formation of focal adhesions, likely similar to that of NHE1 in enhancing integrin/ECM interaction (2).
Figure 8.
NBCn1 localizes to the leading edge of the Caco2BBe cells. Localization of NBCn1 and two protein markers for focal adhesions, namely vinculin and paxillin, was investigated in subconfleunt Caco2BBe wild-type cells. At the plasma membrane, NBCn1 was mainly found at the leading edge, where it in part colocalized with focal adhesion markers paxillin or vinculin. Colocalization analysis and visualization (bottom) were performed in Fiji using the “Colocalization Finder” plugin. DAPI, nuclei; Phalloidin, F-actin.
DISCUSSION
This study shows a dramatic antiproliferative effect of NBCn1-silencing in the Caco2BBe self-differentiating intestinal cell line. These cells were selected for the study because of their high NBCn1 expression, their proliferative capacity, and their ability to self-differentiate (24). In these aspects, they have similarities to the nondifferentiated, highly proliferative human colonoids that resemble in many aspects the cells in the lower part of the colonic crypt (5). The relatively higher expression of NBCn1 compared with the other major base loader, namely NHE1, was not seen in the murine highly proliferative nondifferentiated organoids, where NHE1 mRNA expression was more than twofold higher than that for NBCn1 (4). Therefore, findings from the murine Nbcn1–/– colon (12) may only partly reflect the situation in the human colon, and in the Caco2BBe cells. In line with this assumption is the fact that we did observe a lower proliferative activity in the murine Nbcn1–/– organoids, but to a much lesser degree than that observed in NBCn1-silenced Caco2BBe cells. Although the Nbcn1–/– mouse strain that we used for our experiments was found to be an incomplete knockout established by promoter trap strategy with residual expression of NBCn1 variants (27), we had seen very significant loss of Nbcn1 immunostaining in the colon of this mouse (12) and found very low (but not absent) Nbcn1 mRNA expression in the colonic organoids (Fig. 6A).
We assessed the steady-state pHi of the NBCn1-silenced cell lines in comparison with the mock-silenced cells. Mock-silenced Caco2BBe cells also have a lower pHi than the WT Caco2BBe cells, likely because they are grown in the presence of hygromycin. However, the NBCn1 silenced cells have a more acidic pHi than the mock-silenced cells, with a correlation to the degree of NBCn1 silencing. Interestingly, the cells also displayed a reduction in the expression of the endogenous SLC26A3 apical anion exchanger. This transporter is strongly upregulated during differentiation and serves to import luminal Cl– in exchange for intracellular HCO3−, thus acts as an acid loader. An original purpose of our study was to assess the effect of a chronically low pHi (by knocking down a major base importer of the Caco2BBe cells) on the expression, membrane trafficking, and transport rate of SLC26A3. However, the low expression of SLC26A3 in NBCn1-silenced cells made this plan not feasible. The strongly reduced expression of SLC26A3 may also be due to a delayed differentiation of the KD#4 and KD#2 cells, secondary to the strong decrease in proliferative activity. A reduction in the expression of SLC26A3 could nevertheless have helped to mitigate the cellular acidosis inflicted by NBCn1 silencing.
The proliferative activity of cultured cells also depends on their ability to attach to the matrix and to migrate towards each other. This migratory behavior correlates with the invasiveness of cancer cells and is therefore under intense investigation (23, 30, 31). In this context, the role of isoform 1 of the Na+/H+ exchanger family (NHE1) has been recognized early (32, 33). A specific role of NHE1 in the assembly of the proteins required to form the focal adhesions was described more than two decades ago, which is at least in part independent of its regulatory function on the cytoplasmic pH of the whole cell (32). We had previously investigated the migratory behavior in Caco2BBe cells and had observed no significant effect of pharmacological NHE1 inhibition (A. Paehler von der Nolte, unpublished observations), whereas we did see an effect of NHE1 inhibition in a gastric cell line with an identical technical approach (34). The apparent lack of NHE1 involvement in Caco2BBe cell migration was in stark contrast to the behavior of a number of other cell types (32, 33, 35–37). One possibility for the differences was that another NHE may perform the function of NHE1 in these cells. However, we found that NHE2 silencing increased the migratory speed of Caco2BBe cells (38). In the vascular smooth muscle cells, NBCn1 was found to be highly expressed and to be involved in cell migration (39). We therefore studied the migratory speed of the NBCn1-silenced and mock-silenced cells with a similar wound healing assay used by this group. We had to use the NBCn1-silenced #4 cells, which display only a weak reduction in NBCn1 expression, because this wound healing assay requires monolayer formation and a comparable cell density between control and silenced cells. Even with the relatively mild reduction in NBCn1 mRNA level to approximately 74% of that in the mock-silenced controls (at the time of the wound closure experiment), a significant reduction in migratory speed was observed in the NBCn1-silenced cells.
Because this suggested a very pronounced effect of NBCn1 activity on cell migration, we searched for the potential molecular mechanism. Dynamic pH changes, both intracellular and the extracellular pH, at the focal adhesions may be required to allow the cells to attach to and detach from the matrix (2, 40, 41). Caco2BBe cells do not perform directional migration at the single cell level, but they do form lamellipodia and cytoskeletal remodeling. The lamellipodia were investigated by immunocytochemical staining and confocal microscopy of the focal adhesion proteins vinculin and paxillin, as well as NBCn1. NBCn1 accumulated at the leading edge, in close vicinity and in part colocalizing with vinculin and paxillin at the leading edge. This suggests that NBCn1 may play a dominant role in the pH dynamics of the leading edge of migrating cells, particularly in cells with a high expression of NBCn1 in contrast to NHE1, such as the Caco2BBe cells. Again, this is consistent with previous findings from vascular smooth muscle cells, where NBCn1 expression was required for filopodia formation and generation of pH gradients from the leading to the rear end of cells (39).
As stated in introduction, the NBCn1 gene was found as a susceptibility gene for breast cancer (15), a finding that was reproduced in other association studies (16, 42). The associated NBCn1 variant was linked to the transcriptional activity (16). Transcriptional upregulation of NBCn1 is linked to poor survival in patients with luminal A and triple-negative breast cancer (43). However, other oncogenic mechanisms can upregulate NBCn1 in breast cancer cells via nontranscriptional mechanisms (44). In breast cancer cells, the NBCn1 expression and the dependency on Na+, HCO3− cotransport for pHi regulation, measured in biopsies of human primary breast carcinomas, independently predicted the proliferative activity, the invasive behavior, and the patient survival (42), and a recent study by the same group demonstrated that antibodies directed against NBCn1 that were able to inhibit the transporter in a pH-dependent fashion reduced colony formation, caused G2/M-phase cell cycle accumulation, increased apoptosis of metastatic triple-negative breast cancer cells in vitro, and were able to reduce tumor growth in a xenograft murine model (45). On the other hand, the involvement of NBCn1 in cellular processes that are required for proliferation is not necessarily always the consequence of an NBCn1-mediated increase in the cellular pH. A recent study demonstrated that mTOR signaling resulted in increased expression of NBCn1 via transcriptional mechanisms. The NBCn1-mediated import of HCO3− enhanced nucleotide synthesis, and inhibition of NBCn1 resulted in a decrease of nucleotide synthesis and reduced proliferative activity, but did not cause a decrease in cellular pH (46).
In addition, NBCn1 imports Na+, and this feature may be as important, or more so, as the effect of NBCn1 on intracellular alkalinization for its growth-promoting effect (47). Early recognized was the effect of growth hormones on the activation of Na+/H+ exchange, which led to a cellular alkalinization only in the absence but not in the presence of CO2/HCO3− (48), suggesting that the growth-promoting consequence of Na+/H+ activation may be the import of Na+.
The role of pH dynamics in colorectal cancer cells has also been investigated for decades, and different pH-regulatory mechanisms were described in different colon cancer cell lines, including Na+-HCO3− cotransport (20, 49–51). The recent focus, however, was on the important roles of NHE1 and the carbonic anhydrase IX to allow survival of gastrointestinal cancer cells in the acidic and hypoxic tumor environment (19, 21, 52, 53). Given the significant overexpression of NBCn1 in colorectal cancer tissue compared with healthy colon (Fig. 1A) (29), it will become important to understand more about the physiological and pathophysiological regulation of NBCn1 in proliferating normal and malignant enterocytes. This may help obtain insight into the differential expression of different pHi-regulatory pathways in healthy enterocytes along the differentiation pathways, and in human colorectal cancer cells, design strategies for more efficient treatment, but also allow insight into the high intestinal burden of therapies, for example, the EGF-receptor (EGFR)-targeted therapies (54). The EGFR pathway has been shown to play a major role in the upregulation of NBCn1, but not of NHE1 expression, in ΔErbB2 expressing breast cancer cells (55, 56). The EGFR pathway plays a major role in both normal and malignant proliferation in the intestinal tract (57–59).
In summary, the data suggest that NBCn1 expression is required for optimal enterocyte proliferation, but is essential for the pH-regulatory, the proliferative and the migratory capacity of the colonic tumor cell line Caco2BBe, where it colocalizes with focal adhesion proteins at the basolateral membrane. It will be important to learn more about NBCn1 regulation and function in the healthy intestine and during malignant transformation.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This study was funded by the DFG Sachbeihilfen SE460/21-1 and 22-1.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.S., C.S., M.A., and U.E.S. conceived and designed research; M.J., A.S., C.S., K.N., and M.A. performed experiments; M.J., A.S., C.S., K.N., and M.A. analyzed data; A.S., C.S., K.N., M.A., and U.E.S. interpreted results of experiments; C.S., K.N., and M.A. prepared figures; M.J., M.A., and U.E.S. drafted manuscript; E.B., M.A., and U.E.S. edited and revised manuscript; M.J., A.S., C.S., K.N., E.B., M.A., and U.E.S. approved final version of manuscript.
REFERENCES
- 1. Strubberg AM, Liu J, Walker NM, Stefanski CD, MacLeod RJ, Magness ST, Clarke LL. Cftr modulates Wnt/β-catenin signaling and stem cell proliferation in murine intestine. Cell Mol Gastroenterol Hepatol 5: 253–271, 2018. doi: 10.1016/j.jcmgh.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ludwig FT, Schwab A, Stock C. The Na+/H+ -exchanger (NHE1) generates pH nanodomains at focal adhesions. J Cell Physiol 228: 1351–1358, 2013. doi: 10.1002/jcp.24293. [DOI] [PubMed] [Google Scholar]
- 3. Jakab RL, Collaco AM, Ameen NA. Physiological relevance of cell-specific distribution patterns of CFTR, NKCC1, NBCe1, and NHE3 along the crypt-villus axis in the intestine. Am J Physiol Gastrointest Liver Physiol 300: G82–G98, 2011. doi: 10.1152/ajpgi.00245.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nikolovska K, Cao L, Hensel I, Di Stefano G, Seidler AE, Zhou K, Qian J, Singh AK, Riederer B, Seidler U. Sodium/hydrogen-exchanger-2 modulates colonocyte lineage differentiation. Acta Physiol (Oxf) 234: e13774, 2022. doi: 10.1111/apha.13774. [DOI] [PubMed] [Google Scholar]
- 5. Salari A, Zhou K, Nikolovska K, Seidler U, Amiri M. Human colonoid-myofibroblast coculture for study of apical Na+/H+ exchangers of the lower cryptal neck region. Int J Mol Sci 24: 4266, 2023. doi: 10.3390/ijms24054266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jacob P, Rossmann H, Kretz A, Christiani S, Vielliar-Baron D, Gregor M, Seidler U. DRA expression and brush border membrane anion exchange characteristics in rat and rabbit duodenum. Gastroenterology 118: A599, 2000. doi: 10.1016/S0016-5085(00)84534-5. [DOI] [Google Scholar]
- 7. Flemström G, Isenberg JI. Gastroduodenal mucosal alkaline secretion and mucosal protection. News Physiol Sci 16: 23–28, 2001. doi: 10.1152/physiologyonline.2001.16.1.23. [DOI] [PubMed] [Google Scholar]
- 8. Praetorius J, Hager H, Nielsen S, Aalkjaer C, Friis UG, Ainsworth MA, Johansen T. Molecular and functional evidence for electrogenic and electroneutral Na+-HCO3− cotransporters in murine duodenum. Am J Physiol Gastrointest Liver Physiol 280: G332–G343, 2001. doi: 10.1152/ajpgi.2001.280.3.G332. [DOI] [PubMed] [Google Scholar]
- 9. Damkier HH, Nielsen S, Praetorius J. Molecular expression of SLC4-derived Na+-dependent anion transporters in selected human tissues. Am J Physiol Regul Integr Comp Physiol 293: R2136–R2146, 2007. doi: 10.1152/ajpregu.00356.2007. [DOI] [PubMed] [Google Scholar]
- 10. Boedtkjer E, Praetorius J, Fuchtbauer EM, Aalkjaer C. Antibody-independent localization of the electroneutral Na+-HCO3− cotransporter NBCn1 (slc4a7) in mice. Am J Physiol Cell Physiol 294: C591–C603, 2008. doi: 10.1152/ajpcell.00281.2007. [DOI] [PubMed] [Google Scholar]
- 11. Chen M, Praetorius J, Zheng W, Xiao F, Riederer B, Singh AK, Stieger N, Wang J, Shull GE, Aalkjaer C, Seidler U. The electroneutral Na+:HCO3− cotransporter NBCn1 is a major pHi regulator in murine duodenum. J Physiol 590: 3317–3333, 2012. doi: 10.1113/jphysiol.2011.226506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Singh AK, Xia W, Riederer B, Juric M, Li J, Zheng W, Cinar A, Xiao F, Bachmann O, Song P, Praetorius J, Aalkjaer C, Seidler U. Essential role of the electroneutral Na+-HCO3− cotransporter NBCn1 in murine duodenal acid-base balance and colonic mucus layer build-up in vivo. J Physiol 591: 2189–2204, 2013. doi: 10.1113/jphysiol.2012.247874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yu Q, Liu X, Liu Y, Riederer B, Li T, Tian DA, Tuo B, Shull G, Seidler U. Defective small intestinal anion secretion, dipeptide absorption, and intestinal failure in suckling NBCe1-deficient mice. Pflugers Arch 468: 1419–1432, 2016. doi: 10.1007/s00424-016-1836-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gawenis LR, Bradford EM, Prasad V, Lorenz JN, Simpson JE, Clarke LL, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE. Colonic anion secretory defects and metabolic acidosis in mice lacking the NBC1 Na+/HCO3− cotransporter. J Biol Chem 282: 9042–9052, 2007. doi: 10.1074/jbc.M607041200. [DOI] [PubMed] [Google Scholar]
- 15. Ahmed S, Thomas G, Ghoussaini M, Healey CS, Humphreys MK, Platte R , et al. Newly discovered breast cancer susceptibility loci on 3p24 and 17q23.2. Nat Genet 41: 585–590, 2009. doi: 10.1038/ng.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen W, Zhong R, Ming J, Zou L, Zhu B, Lu X, Ke J, Zhang Y, Liu L, Miao X, Huang T. The SLC4A7 variant rs4973768 is associated with breast cancer risk: evidence from a case-control study and a meta-analysis. Breast Cancer Res Treat 136: 847–857, 2012. doi: 10.1007/s10549-012-2309-9. [DOI] [PubMed] [Google Scholar]
- 17. Climente-Gonzalez H, Lonjou C, Lesueur F; GENESIS Study Group; Stoppa-Lyonnet D, Andrieu N, Azencott CA. Boosting GWAS using biological networks: a study on susceptibility to familial breast cancer. PLoS Comput Biol 17: e1008819, 2021. doi: 10.1371/journal.pcbi.1008819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boedtkjer E, Moreira JM, Mele M, Vahl P, Wielenga VT, Christiansen PM, Jensen VE, Pedersen SF, Aalkjaer C. Contribution of Na+,HCO3−-cotransport to cellular pH control in human breast cancer: a role for the breast cancer susceptibility locus NBCn1 (SLC4A7). Int J Cancer 132: 1288–1299, 2013. doi: 10.1002/ijc.27782. [DOI] [PubMed] [Google Scholar]
- 19. Parks SK, Cormerais Y, Durivault J, Pouyssegur J. Genetic disruption of the pHi-regulating proteins Na+/H+ exchanger 1 (SLC9A1) and carbonic anhydrase 9 severely reduces growth of colon cancer cells. Oncotarget 8: 10225–10237, 2017. doi: 10.18632/oncotarget.14379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ramirez MA, Toriano R, Parisi M, Malnic G. Control of cell pH in the T84 colon cell line. J Membr Biol 177: 149–157, 2000. doi: 10.1007/s002320001108. [DOI] [PubMed] [Google Scholar]
- 21. Swietach P, Patiar S, Supuran CT, Harris AL, Vaughan-Jones RD. The role of carbonic anhydrase 9 in regulating extracellular and intracellular ph in three-dimensional tumor cell growths. J Biol Chem 284: 20299–20310, 2009. doi: 10.1074/jbc.M109.006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gorbatenko A, Olesen CW, Boedtkjer E, Pedersen SF. Regulation and roles of bicarbonate transporters in cancer. Front Physiol 5: 130, 2014. doi: 10.3389/fphys.2014.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stock C. How dysregulated ion channels and transporters take a hand in esophageal, liver, and colorectal cancer. Rev Physiol Biochem Pharmacol 181: 129–222, 2021. doi: 10.1007/112_2020_41. [DOI] [PubMed] [Google Scholar]
- 24. Yu Y, Seidler A, Zhou K, Yuan Z, Yeruva S, Amiri M, Yun CC, Nikolovska K, Seidler U. Expression, localization and functional activity of the major Na+/H+ exchange isoforms expressed in the intestinal cell line Caco-2BBe. Cell Physiol Biochem 52: 1017–1038, 2019. doi: 10.33594/000000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boedtkjer E, Praetorius J, Matchkov VV, Stankevicius E, Mogensen S, Füchtbauer AC, Simonsen U, Füchtbauer EM, Aalkjaer C. Disruption of Na+,HCO3− cotransporter NBCn1 (slc4a7) inhibits NO-mediated vasorelaxation, smooth muscle Ca2+ sensitivity, and hypertension development in mice. Circulation 124: 1819–1829, 2011. doi: 10.1161/CIRCULATIONAHA.110.015974. [DOI] [PubMed] [Google Scholar]
- 26. Campeau E, Ruhl VE, Rodier F, Smith CL, Rahmberg BL, Fuss JO, Campisi J, Yaswen P, Cooper PK, Kaufman PD. A versatile viral system for expression and depletion of proteins in mammalian cells. PLoS One 4: e6529, 2009. doi: 10.1371/journal.pone.0006529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Choi I, Beedholm K, Dam VS, Bae S-H, Noble DJ, Garraway SM, Aalkjaer C, Boedtkjer E. Sodium bicarbonate cotransporter NBCn1/Slc4a7 affects locomotor activity and hearing in mice. Behav Brain Res 401: 113065, 2021. doi: 10.1016/j.bbr.2020.113065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park SJ, Yoon BH, Kim SK, Kim SY. GENT2: an updated gene expression database for normal and tumor tissues. BMC Med Genomics 12, Suppl 5: 101, 2019. doi: 10.1186/s12920-019-0514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stock C, Schwab A. Protons make tumor cells move like clockwork. Pflugers Arch 458: 981–992, 2009. doi: 10.1007/s00424-009-0677-8. [DOI] [PubMed] [Google Scholar]
- 31. Swietach P, Boedtkjer E, Pedersen SF. How protons pave the way to aggressive cancers. Nat Rev Cancer 23: 825–841, 2023. doi: 10.1038/s41568-023-00628-9. [DOI] [PubMed] [Google Scholar]
- 32. Tominaga T, Barber DL. Na-H exchange acts downstream of RhoA to regulate integrin-induced cell adhesion and spreading. Mol Biol Cell 9: 2287–2303, 1998. doi: 10.1091/mbc.9.8.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Klein M, Seeger P, Schuricht B, Alper SL, Schwab A. Polarization of Na+/H+ and Cl−/HCO3− exchangers in migrating renal epithelial cells. J Gen Physiol 115: 599–608, 2000. doi: 10.1085/jgp.115.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paehler Vor der Nolte A, Chodisetti G, Yuan Z, Busch F, Riederer B, Luo M, Yu Y, Menon MB, Schneider A, Stripecke R, Nikolovska K, Yeruva S, Seidler U. Na+/H+ exchanger NHE1 and NHE2 have opposite effects on migration velocity in rat gastric surface cells. J Cell Physiol 232: 1669–1680, 2017. doi: 10.1002/jcp.25758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schwab A, Rossmann H, Klein M, Dieterich P, Gassner B, Neff C, Stock C, Seidler U. Functional role of Na+-HCO3− cotransport in migration of transformed renal epithelial cells. J Physiol 568: 445–458, 2005. doi: 10.1113/jphysiol.2005.092957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Clement DL, Mally S, Stock C, Lethan M, Satir P, Schwab A, Pedersen SF, Christensen ST. PDGFRα signaling in the primary cilium regulates NHE1-dependent fibroblast migration via coordinated differential activity of MEK1/2-ERK1/2-p90RSK and AKT signaling pathways. J Cell Sci 126: 953–965, 2013. doi: 10.1242/jcs.116426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang Y, Li Y, Thompson KN, Stoletov K, Yuan Q, Bera K, Lee SJ, Zhao R, Kiepas A, Wang Y, Mistriotis P, Serra SA, Lewis JD, Valverde MA, Martin SS, Sun SX, Konstantopoulos K. Polarized NHE1 and SWELL1 regulate migration direction, efficiency and metastasis. Nat Commun 13: 6128, 2022. doi: 10.1038/s41467-022-33683-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nikolovska K, Seidler UE, Stock C. The role of plasma membrane sodium/hydrogen exchangers in gastrointestinal functions: proliferation and differentiation, fluid/electrolyte transport and barrier integrity. Front Physiol 13: 899286, 2022. doi: 10.3389/fphys.2022.899286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boedtkjer E, Bentzon JF, Dam VS, Aalkjaer C. Na+-HCO3− cotransporter NBCn1 increases pHi gradients, filopodia, and migration of smooth muscle cells and promotes arterial remodelling. Cardiovasc Res 111: 227–239, 2016. doi: 10.1093/cvr/cvw079. [DOI] [PubMed] [Google Scholar]
- 40. Stock C, Gassner B, Hauck CR, Arnold H, Mally S, Eble JA, Dieterich P, Schwab A. Migration of human melanoma cells depends on extracellular pH and Na+/H+ exchange. J Physiol 567: 225–238, 2005. doi: 10.1113/jphysiol.2005.088344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Srivastava J, Barreiro G, Groscurth S, Gingras AR, Goult BT, Critchley DR, Kelly MJ, Jacobson MP, Barber DL. Structural model and functional significance of pH-dependent talin-actin binding for focal adhesion remodeling. Proc Natl Acad Sci USA 105: 14436–14441, 2008. doi: 10.1073/pnas.0805163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fernandez-Navarro P, Pita G, Santamariña C, Moreno MP, Vidal C, Miranda-García J, Ascunce N, Casanova F, Collado-García F, Herráez B, González-Neira A, Benítez J, Pollán M. Association analysis between breast cancer genetic variants and mammographic density in a large population-based study (Determinants of Density in Mammographies in Spain) identifies susceptibility loci in TOX3 gene. Eur J Cancer 49: 474–481, 2013. doi: 10.1016/j.ejca.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 43. Toft NJ, Axelsen TV, Pedersen HL, Mele M, Burton M, Balling E, Johansen T, Thomassen M, Christiansen PM, Boedtkjer E. Acid-base transporters and pH dynamics in human breast carcinomas predict proliferative activity, metastasis, and survival. eLife 10: e68447, 2021. doi: 10.7554/eLife.68447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gorbatenko A, Olesen CW, Loebl N, Sigurdsson HH, Bianchi C, Pedraz-Cuesta E, Christiansen J, Pedersen SF. Oncogenic p95HER2 regulates Na+-HCO3−cotransporter NBCn1 mRNA stability in breast cancer cells via 3′UTR-dependent processes. Biochem J 473: 4027–4044, 2016. doi: 10.1042/BCJ20160054. [DOI] [PubMed] [Google Scholar]
- 45. Axelsen TV, Olesen C, Khan D, Mohammadi A, Bouzinova EV, Nielsen CJF, Mele M, Hauerslev KR, Pedersen HL, Balling E, Vahl P, Tramm T, Christiansen PM, Boedtkjer E. Antibodies toward Na+-HCO3−-cotransporter NBCn1/SLC4A7 block net acid extrusion and cause pH-dependent growth inhibition and apoptosis in breast cancer. Br J Cancer 130: 1206–1220, 2024. doi: 10.1038/s41416-024-02591-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ali ES, Lipońska A, O’Hara BP, Amici DR, Torno MD, Gao P, Asara JM, Yap M-NF, Mendillo ML, Ben-Sahra I. The mTORC1-SLC4A7 axis stimulates bicarbonate import to enhance de novo nucleotide synthesis. Mol Cell 82: 3284–3298.e7, 2022. doi: 10.1016/j.molcel.2022.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Orlov SN, Hamet P. Intracellular monovalent ions as second messengers. J Membr Biol 210: 161–172, 2006. doi: 10.1007/s00232-006-0857-9. [DOI] [PubMed] [Google Scholar]
- 48. Mills GB, Cragoe EJ Jr, Gelfand EW, Grinstein S. Interleukin 2 induces a rapid increase in intracellular pH through activation of a Na+/H+ antiport. Cytoplasmic alkalinization is not required for lymphocyte proliferation. J Biol Chem 260: 12500–12507, 1985. [PubMed] [Google Scholar]
- 49. Abrahamse SL, Bindels RJ, van Os CH. The colon carcinoma cell line Caco-2 contains an H+/K+-ATPase that contributes to intracellular pH regulation. Pflugers Arch 421: 591–597, 1992. doi: 10.1007/BF00375056. [DOI] [PubMed] [Google Scholar]
- 50. Köttgen M, Leipziger J, Fischer KG, Nitschke R, Greger R. pH regulation in HT29 colon carcinoma cells. Pflugers Arch 428: 179–185, 1994. doi: 10.1007/BF00374856. [DOI] [PubMed] [Google Scholar]
- 51. Bischof G, Cosentini E, Hamilton G, Riegler M, Zacherl J, Teleky B, Feil W, Schiessel R, Machen TE, Wenzl E. Effects of extracellular pH on intracellular pH-regulation and growth in a human colon carcinoma cell-line. Biochim Biophys Acta 1282: 131–139, 1996. doi: 10.1016/0005-2736(96)00050-8. [DOI] [PubMed] [Google Scholar]
- 52. Jakubicková L, Biesová Z, Pastoreková S, Kettmann R, Pastorek J. Methylation of the CA9 promoter can modulate expression of the tumor-associated carbonic anhydrase IX in dense carcinoma cell lines. Int J Oncol 26: 1121–1127, 2005. [PubMed] [Google Scholar]
- 53. Huntington KE, Louie A, Zhou L, Seyhan AA, Maxwell AW, El-Deiry WS. Colorectal cancer extracellular acidosis decreases immune cell killing and is partially ameliorated by pH-modulating agents that modify tumor cell cytokine profiles. Am J Cancer Res 12: 138–151, 2022. [PMC free article] [PubMed] [Google Scholar]
- 54. Li J, Xie J. Mucositis with anti-EGFR monoclonal antibody in cancer patients: a meta-analysis of randomized controlled trials. Jpn J Clin Oncol 48: 718–727, 2018. doi: 10.1093/jjco/hyy083. [DOI] [PubMed] [Google Scholar]
- 55. Gorbatenko A, Olesen CW, Mørup N, Thiel G, Kallunki T, Valen E, Pedersen SF. ErbB2 upregulates the Na+-HCO3−-cotransporter NBCn1/SLC4A7 in human breast cancer cells via Akt, ERK, Src, and Kruppel-like factor 4. FASEB J 28: 350–363, 2014. doi: 10.1096/fj.13-233288. [DOI] [PubMed] [Google Scholar]
- 56. Lauritzen G, Jensen MB, Boedtkjer E, Dybboe R, Aalkjaer C, Nylandsted J, Pedersen SF. NBCn1 and NHE1 expression and activity in DeltaNErbB2 receptor-expressing MCF-7 breast cancer cells: contributions to pHi regulation and chemotherapy resistance. Exp Cell Res 316: 2538–2553, 2010. doi: 10.1016/j.yexcr.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 57. Srivatsa S, Paul MC, Cardone C, Holcmann M, Amberg N, Pathria P, Diamanti MA, Linder M, Timelthaler G, Dienes HP, Kenner L, Wrba F, Prager GW, Rose-John S, Eferl R, Liguori G, Botti G, Martinelli E, Greten FR, Ciardiello F, Sibilia M. EGFR in tumor-associated myeloid cells promotes development of colorectal cancer in mice and associates with outcomes of patients. Gastroenterology 153: 178–190 e110, 2017. doi: 10.1053/j.gastro.2017.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang J, Ji JY, Yu M, Overholtzer M, Smolen GA, Wang R, Brugge JS, Dyson NJ, Haber DA. YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat Cell Biol 11: 1444–1450, 2009. doi: 10.1038/ncb1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL. Yap-dependent reprogramming of Lgr5+ stem cells drives intestinal regeneration and cancer. Nature 526: 715–718, 2015. doi: 10.1038/nature15382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.