Summary
Alarm substances signal imminent predation thread and enable anti-predation strategies. In shoaling fish, alarm cues diffuse from injured skins that induce intense fear and anti-predation behaviors in other members. While these “fear substances” are shown to be present in numerous fishes and thought to exist in roughly 8,000 Ostariophysan species, their chemical nature remains largely unknown. We posited that fish alarm cues comprise small compounds and induce specific behaviors characteristic of fish exposed to skin extracts. Using the behaviors as bioassays, we tracked the alarm function of zebrafish skin extract to two compounds, 24-methyl-5α-cholestane-3α,7α,12α,24,28-pentahydroxy 28-sulfate, an oxysterol sulfate, and 5α-cyprinol sulfate. At concentrations of less than one nanomolar, each compound induced anti-predator behaviors and increased cortisol levels in zebrafish. Their mixture, at the natural ratio, replicated the skin extract in eliciting the full suite of anti-predator behavior patterns. Our findings reveal a molecular mechanism whereby fish escape predation danger.
Subject areas: Biological sciences, Biomolecules, Neuroscience, Behavioral neuroscience
Graphical abstract

Highlights
-
•
Two compounds were tracked as the alarm substances of zebrafish in the skin extract
-
•
Each compound induces anti-predator responses in zebrafish at nanomolar concentrations
-
•
Their mixture replicates the effects of skin extract at its natural ratio
Biological sciences; Biomolecules; Neuroscience; Behavioral neuroscience
Introduction
Many animals use alarm cues in defense against predators. These cues may be visual, auditory, or chemical. The chemical cues, known as alarm pheromones or alarm substances, are essential to survival.1,2 Alarm pheromones warn conspecifics and sometimes other members of the group of the danger of predation,2 and are common among group-living insects and shoaling fishes. Insects and fish differ in their alarm pheromone production and anti-predator behavior strategies. Many social insects, upon the detection of predators, release alarm pheromones from their mandibular or pygidial glands that recruit group members to attack and, in this process, releases more alarm pheromone.3 Identified insect alarm pheromones are volatile compounds that dissipate efficiently in the air.3,4 In contrast, shoaling fish release alarm substances from their skin, and possibly other tissues, from injuries inflicted by predators.5 These passively released alarm substances appear to become “public information cues” that warn group members of imminent predation threats.5 Fish alarm substances trigger highly stereotypical behaviors to avoid predation or detection (or both) by predators. While the behavioral mechanism for fish to escape predation is well demonstrated and defined in many species,5 the molecules that carry the information of danger have not been elucidated.
There have been numerous efforts to define the chemical nature of fish alarm substances since 1938, when von Frisch proposed the existence of alarm substances in fish, namely schreckstoff (also known as "fear substance"), from the behavioral responses of minnows to injured conspecifics.6 Subsequently, extensive studies have shown that these substances elicit stereotypical behaviors and physiological responses in Ostariophysan,7 a superorder that includes over 10,000 fish species. In addition, several fish species are found to release disturbance cues when threatened (but not injured).5,8 There is also evidence that fish detect the odorants of potential predators and consequently become disturbed.5,9 It appears that fish, the largest group of vertebrate animals, rely heavily on chemical cues from various sources to assess predation risk. For decades, a great number of researchers have sought to identify the chemicals that cause “fear” or disturbance in fish.10 In black tetra and giant danio, hypoxanthine-N-oxide (H3NO) was postulated as the alarm substances at excessively high concentrations;11 however, it has not been detected reliably in the skin.12 Mathuru et al. (2012)13 noticed that vigorous shaking causes zebrafish to release substances that induce a mild fright reaction. They further showed that, through the fractionation of skin extract and behavioral assays, glycosaminoglycan chondroitins, major components of mucus, induce some components of alarm behavior, although the activation level is lower than those induced by skin extracts.13 Their evidence indicated that alarm cues and possibly disturbance cues are present in zebrafish skin tissues and that the full complement of alarm pheromones has yet to be characterized.
We sought to identify alarm substances that induce the same anti-predator responses induced by skin extract in zebrafish. When exposed to skin extracts, zebrafish show distinct and conspicuous locomotion patterns, including high-velocity erratic swimming, bottom-dwelling, and freezing.14 Protocols for measuring these behaviors have been well established.15 Low concentrations of conspecific blood (0.01%) also elicit robust defensive responses in adult zebrafish, indicating that damage-released chemical cues in the blood and epidermis mediate anti-predator behaviors.16 In addition, cortisol levels are elevated in zebrafish exposed to chemical cues from injured or disturbed individuals of the same species.17 Based on the characteristics of alarm substances examined in numerous fish species,1,6,9 we reasoned that the zebrafish alarm substances should be composed of a chemical or a mixture of chemicals that is found in skin tissues, induce a full complement of anti-predator behaviors and a stress response, and show expected functions at concentrations consistent with the abundance found in skin extract.18
Using a fractionation strategy guided by behavioral assays, we tracked the alarm substances activities of skin extract to two molecules. These two candidate components of alarm substances were isolated by chromatography and characterized unequivocally by nuclear magnetic resonance spectroscopy (NMR) and ultra-high performance liquid chromatography–high-resolution mass spectrometry (UPLC–HRMS). When mixed at the ratio found in the skin extract and diluted to concentrations expected from the skin extract, the two compounds induced the full suite of anti-predator behaviors and increased cortisol levels in adult zebrafish.
Results
Skin extract-induced anti-predator behaviors
To develop a reproducible bioassay that measures the full suite of anti-predator behaviors, we validated the known behavioral responses elicited by skin extract in zebrafish using a novel tank diving paradigm (Figure S1) as described by Cachat et al.15 Naive zebrafish 3–5 months old, were each used once in the assay. Skin extract (SKE) was prepared from skin samples of ∼800 zebrafish. 5% of the SKE was dissolved in 0.5 mL water to make a stock solution equivalent to the skins of 80 fish/mL. This stock solution was serially diluted by 10, 100, 1000, 104, 105, and 106-fold, respectively (denoted as 10, 100, 1000, 104, 105, and 106 × SKE, respectively), and subsequently tested for their behavioral activities (Figure S2).14,19,20,21 Twelve potential anti-predatory behavior parameters and derived indices of zebrafish were recorded and analyzed (Table S13). The results showed that four parameters characteristic of predator avoidance behaviors in zebrafish (Figure S3),14,22 namely erratic movements duration (%), time in bottom half (%), freezing duration (%), and latency to upper half (s), increased in fish exposed to the test stimulants. These four parameters were therefore selected to track the anti-predator activities of SKE fractions and to confirm the function of purified components. Recorded examples of erratic movement and freezing behaviors were given in Videos S1 and S2. Notably, the 1000 × SKE solution induced each of the four selected behaviors, consistent with previous findings.14 Thus, we used 1000 × SKE as the benchmark (Figures 1B−1E) to track the active components in bioassay-guided experiments. Because the behavior assay was conducted by adding 1 mL test stimulant to 1 L water in a test tank (Figure S2), the 1000 × SKE solution provided the equivalent of skin extract from 0.08 fish/L in the test tank.
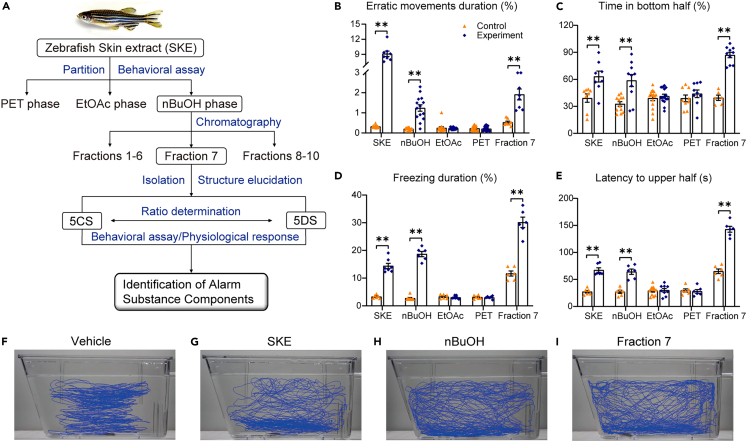
Figure 1.
Bioassay-guided fractionation of alarm substance
(A) Bioassay-guided fractionation to identify alarm substance components. Boxed fractions replicated the activity of skin extract (SKE) in inducing changes in four behavioral phenotypes, including (B) erratic movements duration (%), (C) time in bottom half (%), (D) freezing duration (%), and (E) latency to upper half (s). Fractions of successive separation by petroleum (PET), ethyl acetate (EtOAc), n-butanol (nBuOH), and fraction 7 of nBuOH, were reconstituted in water to equivalents of a 1000-fold dilution of SKE. Representative motion traces of females exposed to (F) vehicle, (G) SKE, (H) nBuOH, and (I) fraction 7, respectively. ∗p < 0.05; ∗∗p < 0.01. See Figure S2 for detailed fractionation procedures. In B-E, the exact sizes of biologically independent samples and the results of independent sample t-tests or Mann-Whitney U tests are summarised in Table S1. Data are represented as mean ± SEM.
The polar fraction of skin extract contained the alarm substance
To fractionate the active components (Figure 1A), SKE (80% of the sample) was successively extracted with petroleum ether (PET), ethyl acetate (EtOAc), and n-butanol (nBuOH) to segregate compounds of increasing polarity. Each extract was reconstituted in water at a concentration equivalent to 1000 × SKE and assayed for its behavioral activities (Figures 1B−1E and S1). When presented to adult zebrafish, the nBuOH extract replicated SKE in inducing erratic movements duration (%) (Figure 1B), time in bottom half (%) (Figure 1C), freezing duration (%) (Figure 1D), and latency to upper half (s) (Figure 1E), whereas neither EtOAc nor PET extract induced these behaviors (Figures 1B−1E). These results confirmed that the active components are relatively hydrophilic.
We fractionated the nBuOH extract by semi-preparative HPLC with a reverse-phase column, on which the major components coeluted in fraction 7. Fraction 7 induced the same four behaviors (Figures 1B−1E), replicating the behavioral activities of 1000 × SKE and the nBuOH extract.
The behavioral traces (Figures 1F−1I) also showed SKE, nBuOH, and fraction 7 induced changes in the movement trajectories. Behavioral trajectories stimulated by SKE (Figure 1G) showed an apparent bottom-dwelling (time in the bottom half) response as compared to those induced by the vehicle (Figure 1F), a pattern that was also observed in fish exposed to nBuOH and fraction 7 (Figures 1H and 1I). Compared to those induced by the vehicle, the traces induced by SKE, nBuOH, and fraction 7 suggested the test fish touched the tank side walls with increasing frequencies (Figures 1G−1I). This may be because the components in the alarm substance were gradually purified as the remaining interfering substances were eliminated, causing the zebrafish to respond more strongly to the stimuli. Touching the side walls likely reflects evasive behaviors upon exposure to the treatment stimulants. Taken together, fraction 7 contained the alarm substance candidates.
Alarm substance candidates were identified as two oxysterols
We identified a predominant peak consisting of two compounds within fraction 7 (Figure 2A and insets). Using HPLC-HRMS, we further isolated and detected them. The NMR spectra and HRMS spectra indicated the compound at a mass-to-charge ratio (m/z) of 531 was isolated to a purity of 97.5% and identified it as 5α-cyprinol sulfate (5CS), a known cholanoid (Figure 2A insets).23,24
Figure 2.
Identification of alarm substance in the n-butanol extract
(A) Chromatography of the n-butanol extract, which shows a predominant peak at retention time 7.05 min that includes 5α-cyprinol (5CS) and 5α-daniol sulfate (5DS) (insets, elucidated chemical structure, ion chromatogram, and mass spectrum, respectively). BPI, base peak ion; m/z, mass-to-charge ratio.
(B and C) Key HMBC correlations (arrows) of putative analogs of 5DS. The dashed arrows represent unreasonable correlations.
The compound with m/z of 545 was obtained with a purity of 99.1%. The molecular formula was determined to be C28H50O8S based on the HRMS data (m/z 545.3149, [M – H]−) (Figure 2A insets) and the carbon count observed in the 13C NMR data. The constitution of the compound was determined to be an oxysterol with a sulfate half ester (−OSO3Na) based on the analysis of 1D and 2D-NMR data. The connectivity of the tetracyclic steroidal backbone was determined through analyses of 1H–1H COSY and HMBC correlations (Figure S6). The 13C NMR spectra exhibited distinct resonances corresponding to a 3α,7α,12α-trihydroxy-5α-cholane structure. These results suggested that the compound and 5CS shared a tetracyclic structure.
No evidence was observed for additional oxidation in the tetracyclic steroid framework of the molecule with m/z 545 in the NMR spectra. Thus, we assumed that the side chain at C-17 consisted of a C9H19O5S subunit. Three methyl groups were identified, each with a single neighboring proton, based on the presence of three three-proton doublets with a coupling constant (J) ranging from 6.5 to 6.9 Hz. The COSY and HMBC correlations provided evidence for the presence of a methyl branch at C-20 and C-25, as well as an oxygenated methylene group attached to C-24, which is an oxygenated quaternary carbon. The two-proton doublet (J = 2.5 Hz) at 3.62 ppm and an oxygenated quaternary carbon at 73.5 ppm suggested the presence of a sulfate adjacent to C-2425 or C-2826 (Figures 2B and 2C, respectively). Based on these data, we introduced the side chain as analog B, shown in Figure 2. Notably, the singlet signal at 4.03 ppm, which can be attributed to the hydroxyl either on C-24 or C-28 of the side chain in the COSY spectrum, displayed long-range correlations to C-23 and C-25 in the HMBC spectra. These spectra strongly supported the hydroxy substituent at C-25 and sulfation at C-28, respectively. This side chain subunit is present in a sulfated polyhydroxysteroid isolated from starfish, which possesses a different four-ring core backbone.26 All side chain 1H and 13C NMR shift data strongly support this constitutional assignment.
The configuration of C-24 could not be assigned because of the high degree of similarity of both the proton and carbon NMR spectra between the C-24R and C-24S isomers.26 We are attempting to give an unambiguous answer by using the Mosher reaction when additional quantities become available in the future. The structure was assigned as 24-methyl-5α-cholestane-3α,7α,12α,24,28-pentahydroxy-28-sulfate (Figure 2A insets), a novel oxysterol. Herein, we name this compound 5α-daniol sulfate (5DS), acknowledging its origin from Danio and the presence of a sulfate ester.
Skin extract contains nanomolars of 5α-cyprinol sulfate and 5α-daniol sulfate
If 5CS and 5DS were alarm substance components, they should, at the concentrations found in 1000 × SKE, replicate 1000 × SKE in inducing changes in all four behavior parameters. We developed and validated a UPLC-MS/MS method with excellent reliability and reproducibility to quantify 5CS and 5DS in SKE. With this method, we found that the average concentrations of 5CS and 5DS were 1.01 × 10−7 mol/mL and 1.02 × 10−9 mol/mL, respectively, in the 1000 × SKE solution. Since the test solution was diluted by 1000-fold in the behavioral bioassay (Figure S1), the final concentrations of 5CS and 5DS in the 1000 × SKE treatment were 1.01 × 10−7 M and 1.02 × 10−9 M, respectively, after being introduced to the test tank. The 5CS concentrations are approximately 100 (99.19 ± 2.3) times those of 5DS.
Mixtures of 5α-cyprinol sulfate and 5α-daniol sulfate induced anti-predator behaviors
We measured zebrafish behavioral responses to 5CS or 5DS, each at concentrations ranging from 10−6 to 10−12 M. When exposed to 5CS, the zebrafish showed a sharp rise in erratic movement duration at a concentration of 10−10 M (Figure 3A). The time spent in the bottom half showed a significant increase at 10−12 M (Figure 3B). The freezing duration showed no significant changes compared to the vehicle (Figure 3C). The latency to the upper half was elevated remarkably at 10−10 M of 5CS (Figure 3D). Although there was no change in the freezing behavioral phenotype, we tentatively concluded that the threshold for 5CS to induce a partial anti-predator response was 10−10 M. After being exposed to 5DS, zebrafish showed increases in all four behavioral parameters at 10−12 M (Figures 3A−3D). Thus, the threshold for 5DS to induce an anti-predator response was 10−12 M or lower. The locomotion traces showed that 5CS caused more erratic movements (Figures 3A and 3I), while 5DS is more likely to trigger freezing (Figures 3C and 3J), indicating that distinct components of the alarm substance are associated with different behavioral characteristics.
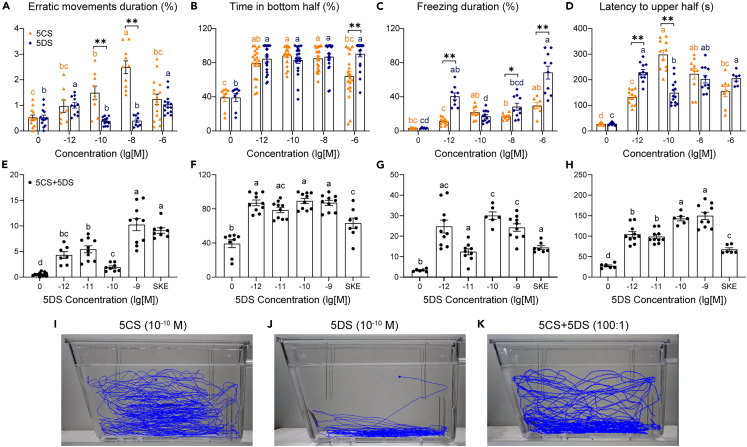
Figure 3.
Anti-predator behaviors induced by 5CS and 5DS with representative trajectories
5α-cyprinol (5CS) and 5α-daniol sulfate (5DS) each induced certain anti-predator behaviors: (A) erratic movement duration (%), (B) time in the bottom half (%), (C) freezing duration (%), and (D) latency to the upper half (s). The mixture of 5CS and 5DS at a ratio of 100:1 induced all four behaviors (E‒H; 5DS concentrations labeled on the X axis; 5CS concentrations are 100 times those of 5DS). Skin extract (SKE) was tested at a 1000-fold dilution as a positive control. Representative female motion traces triggered by (I) 10−10 M of 5CS, (J) 10−10 M of 5DS, and (K) a mixture of 5CS (10−8 M) and 5DS (10−10 M). ∗p < 0.05, ∗∗p < 0.01. Letters above the line indicate significant differences between groups and ∗ or ∗∗ indicate a difference in behaviors induced by different compounds at the same concentration. The exact sizes of biologically independent samples and results of ANOVA followed by Bonferroni multiple comparisons ((A) two-sided Kruskal–Wallis test (B and F) and Tamhane test (C, D, E, G, and H)) are summarised in Table S9. In addition, statistical results of behavior comparison caused by different compounds at a specific concentration are summarised in Table S10 with independent samples t-test (A, C, and D) and Mann-Whitney U test (B). Data are represented as mean ± SEM.
Subsequently, the mixture of 10−7 M of 5CS and 10−9 M of 5DS (approximately the same concentrations as those in the 1000 × SKE treatment) was prepared with isolated compounds and serially diluted to complete the bioassays. The stock solution induced significant changes, in comparison to the vehicle control, in all four behavioral parameters. The prepared stock solution and SKE (1000-fold dilution) induced the same levels of erratic movements duration (Figure 3E). Compared to 1000 × SKE, the mixture induced large increases in time in the bottom half (Figure 3F), freezing duration (Figure 3G), and latency in the upper half (Figure 3H). The results indicated that the mixture replicated SKE in evoking anti-predator behavioral responses from zebrafish.
5α-cyprinol sulfate and 5α-daniol sulfate elevated cortisol levels
We further assessed whether 5CS or 5DS induce physiological changes characteristic of zebrafish exposed to alarm or disturbance cues,17 as in these fish endocrine responses often correlate with behavioral endpoints.27 Upon exposure to alarm substances28 or washings of conspecific individuals that had spotted predators,29 zebrafish show a rise in cortisol levels. We sacrificed zebrafish immediately after they were recorded for behavioral responses to 5CS and 5DS and prepared samples for cortisol analyses.30 A significant elevation of cortisol occurred upon exposure to 5CS and 5DS (Figure 4) at concentrations of 10−10 M and 10−8 M, respectively.
Figure 4.

Whole-body cortisol level (ng g−1 body mass) in adult zebrafish exposed to 5CS and 5DS at concentrations from 10−12 M to 10−6 M
Vertical bars, one SEM. For each compound, responses that do not share a letter are different. Asterisk indicates different cortisol levels induced by different compounds at the same concentration (∗p < 0.05; ∗∗p < 0.01). The exact sizes of biologically independent samples and the results of the two-sided Kruskal–Wallis test followed by a multiple comparison of mean ranks are summarised in Table S11. Independent samples t-test (concentration (lg[M]) = −12, −10 and −6) and Mann-Whitney U test (concentration (lg[M]) = −8) of the comparison at the same concentration are summarised in Table S12. Data are represented as mean ± SEM.
Discussion
Our experimental evidence indicates that 5CS and 5DS compose the alarm substance of zebrafish. These two compounds resulted from a stepwise fractionation where the active fraction from each step replicated the skin extract in the induction of the conspicuous anti-predatory behaviors. As expected, 5CS and 5DS each induced components of anti-predator behaviors and an elevation of cortisol levels in zebrafish. Of note, individual components of alarm odorants often do not elicit the full behavioral response.16 The 5CS and 5DS mixture, at the concentrations and ratios found in the skin extract, induced the same set of behaviors as the skin extract. The compounds were effective in stimulating behavioral and physiological responses at sub-nanomolar concentrations. These results demonstrate that 5CS and 5DS are components of the zebrafish alarm substance, and are characteristic of the “fear substance.”6
When the fish were exposed to alarm substance component(s) that had been further fractionated, they showed incremental increases in freezing duration, from SKE (14.4%; Figure 1) to nBuOH (18.7%; Figure 1) and Fraction 7 (30.2%; Figure 1), and finally, to 5DS (17.1%, 10−10 M; 28.4%, 10−8 M, Figure 3). The fish also exhibited the same pattern in latency to the upper half (Figures 1 and 3). SKE consists of unknown constituents, while 5CS and 5DS are isolated and purified compounds. It appears that when the active components become further fractionated from the mixture, they become more effective in stimulating freezing duration and latency. The impact of other skin substances on the efficacy of the alarm substances in SKE needs further investigation. In our experiments, we examined the overall antipredator behavior elicited by isolated alarm substances in four main behavioral phenotypes (Figure 3). The dose-response patterns of the examined behavior parameters are not linear, as has also been shown in the previous report,14 with the high dose inducing a similar or lower response than the medium dose.
We designed our bioassay to measure innate behavioral responses and used each test subject only once in the behavioral experiments. The fright reaction in fish is thought to be innate, although prey fish are known to learn to associate odorants with predation or the risk of predation.1,5 We assessed the innate response to reduce variations that could be introduced by associative learning, allowing a consistent measurement of behavioral activities across fractions throughout the stepwise fractionation process. The test was based on the unconditioned model novel tank diving paradigm, which uses the animal’s locomotion activity in an unfamiliar setting to assess the fear and anxiety response caused by alarm compounds or synthetic chemicals.15,17 The behavior phenotypes (e.g., freezing, bottom-dwelling, and erratic movement) and total locomotion are well documented in the model and thought to indicate anxiety, fear, and stress.31 Our findings demonstrated that zebrafish exposed to skin extract display increased bottom-dwelling behavior, reduced the exploration of the upper region of the tank, and a higher frequency of freezing and erratic movement. Compounds 5CS and 5DS, identified using this bioassay-guided approach, replicated the behavioral effects of skin extracts. The behavioral phenotype indicators observed in our experiments were in line with previous findings.14,15,32
Our results implicate an additional function of oxysterols, a large family of cholesterol metabolites with broad tissue distribution and physiological function in animals.33 Oxysterols have been found in human34 and rat35 skins and zebrafish embryos.36 Oxysterol sulfation derivatives 5CS and 5DS are likely to partition into cell membranes such as cholesterol37 because oxysterols move more rapidly between intracellular membrane organelles due to the greatly increased hydrophilicity of additional hydroxyls and sulfate.38 5CS is surface active, increasing the permeability of water-soluble compounds across the mucosal membrane.39,40 We anticipate that 5DS also has hydrophilic-lipophilic properties because of its structural similarity to 5CS. These chemical characteristics enable 5CS and 5DS to exude from injured skin and disperse rapidly in water. Notably, known insect alarm pheromones are thought to dissipate rapidly, which is a characteristic essential to efficient warning of impending danger.41 As alarm substance chemicals, 5CS and 5DS have the advantage of being released from any injured tissues, including blood, which is also known to contain alarm substances.16 Moreover, 5CS and 5DS can be considered derivatives of bile alcohols, a group of metabolites that mediate intraspecific communication and regulate a variety of behaviors in fish, such as foraging, mating, homing, spawning, and migrating.42 Sulfation is a critical conjugation reaction of bile acid and alcohol, which substantially increases their water solubility and facilitates accessibility to fish’s olfactory organs.43
Both 5CS and 5DS appear to possess the biological and chemical features expected of fish alarm substances.2,44,45 Because alarm substances are not species-specific and often elicit equivalent alarm responses in other species, they are postulated to be “public information cues” to which behavior responses have evolved.5 The survival benefits of these responses have been confirmed in numerous species.1 Zebrafish are known to shoal with other Cyprinidae fish in their native habitats46 and are highly social with other Danio species in laboratory culture systems.47 5CS is the dominant bile alcohol sulfate of cypriniform fish (including zebrafish) and is likely released from injured individuals of this taxonomic group,48 which is known to use alarm substances. Interestingly, 5CS has been shown to act as a kairomone that induces diel vertical migration and the morphological defenses of Daphnia to avoid predators.49,50 Likewise, chondroitin sulfates derived from the tissues of sharks, sturgeon, and C. elegans all induced weak fear responses in zebrafish.13 5DS has the same bile salt backbone, albeit with an unprecedented adduct of sulfated methylene on C-24. As a novel compound, the biological function and the presence of 5DS in other fish species remain to be discovered. If confirmed to be alarm cues in other cypriniform species, 5CS, and possibly 5DS, could become molecular templates to study how related species that face shared predation threads respond to shared alarm cues. Alarm substances are likely less specialized than pheromones,41 which may be useful for fish that shoal together to warn and detect shared predation threats. It would be interesting to determine if 5CS or 5DS (or both) induce anti-predation behaviors in other species, particularly those that shoal with zebrafish. Such studies may provide useful information to infer how alarm substances evolved as a result of the evolutionary arms race between prey and predators.
The alarm substances identified in this study provide a molecular model to examine the broad function of danger signaling in fish. The zebrafish is extensively used as a model organism in studies of behavior,51 physiology,52 and development.53 In this species, the alarm substance elicits innate fear, manifested by changes in conspicuous behaviors, anxiety levels, and early development. Zebrafish embryos are known to alter their development progress when exposed to adult alarm substances,54 while crushed embryos release alarm substances that induce anti-predator behaviors in adults.55 5CS and 5DS would enable us to interrogate how the olfactory receptor neurons and the central nervous system mediate responses to danger and incite fear throughout life history, which will further support the utility of zebrafish as a model animal in studies related to human anxiety.56,57
Limitations of the study
One limitation of our study is that we only examined the respective effects of 5CS and 5DS on cortisol levels. The experiment on the effects of cortisol elevation from the combination of both compounds could not be conducted due to the insufficient quantity of the remaining 5DS after the study. The function of each compound indicated that both 5CS and 5DS could increase cortisol levels (Figure 4). The combination of 5CS and 5DS may induce an increase in stress hormone levels, as evidenced by the significant anti-predator behavioral responses observed in response to this mixture. Antipredator behaviors, such as erratic movement and freezing, are believed to be closely associated with levels of stress hormones.14,15,17 Despite the limitation, we have sufficient evidence to support the hypothesis that the two alarm pheromone components elevate stress hormone levels in zebrafish. We intend to investigate the endocrine impacts of the 5CS and 5DS mixtures once 5DS becomes available in the future.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Ethanol | Merck, China | CAS#64-17-5 |
| Petroleum ether | Merck, China | CAS#8032-32-4 |
| Ethyl acetate | Merck, China | CAS#141-78-6 |
| n-Butanol | Merck, China | CAS#71-36-3 |
| Methanol (LC-MS grade) | Merck, China | CAS#67-56-1 |
| Methanol-d4 | Cambridge Isotope Laboratories, Inc., American | CAS#811-98-3 |
| Formic acid | Merck, China | CAS#64-18-6 |
| Tricaine | Merck, China | CAS#886-86-2 |
| Cortisol | Sigma-Aldrich (Shanghai) Trading Co.Ltd., China | CAS#50-23-7 |
| Cortisol-d4 | Sigma-Aldrich (Shanghai) Trading Co.Ltd., China | CAS#73565-87-4 |
| Acetic acid | Merck, China | CAS#64-19-7 |
| Ammonium acetate (LC-MS grade) | Merck, China | CAS#631-61-8 |
| Deposited data | ||
| CB raw data | Harvard Dataverse Repository | https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi%3A10.7910%2FDVN%2FU1EIA8&version=DRAFT |
| Experimental models: Organisms/strains | ||
| Zebrafish | Qingfeng Fisheries, Shanghai, China |
N/A |
| Software and algorithms | ||
| SPSS 22.0 | IBM | https://www.ibm.com/support/pages/spss-statistics-220-available-download |
| Graph Pad Prism 9.0.0 | GraphPad Software | https://www.graphpad.com/ |
| EthoVision XT10 | Noldus Information Technology | https://www.noldus.com.cn/ethovision-xt/ |
| ChemDraw 19.0 | PerkinElmer | http://www.chemdraw.net.cn/ |
| MestReNova 14.0.0 | Mestrelab Research | https://mestrelab.com/download/mnova/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Ke Li (kli@yic.ac.cn).
Materials availability
There are restrictions to the availability of 5DS due to almost entirely being used for experiments.
Data and code availability
All data generated in the study have been deposited to the Harvard Dataverse Repository and are publicly available as of the date of publication. Accession numbers are listed in the key resources table.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and study participant details
Zebrafish housing
The fish were housed in a recirculating system (Haisheng, Shanghai, China), with water that had been treated through a water filtration system (Haier, Qingdao, China) and supplemented with sea salt to maintain a concentration of approximately 750 ppm. Each tank (10 liters) contained 10–20 fish. The water temperatures were kept within the range of 25–27°C. Ceiling-mounted fluorescent light tubes were used for illumination on a 12-hour cycle, with the lights turning on at 08:00 and turning off at 20:00. Adult zebrafish were fed daily with brine shrimp (BESSN, Beijing, China).
Experimental models
Adult wild zebrafish (3–5 months old; average weight, 0.2 g; average body length, 3.1 cm) were acquired from a commercial distributor (Qingfeng Fisheries, Shanghai, China). All zebrafish were randomly grouped according with the principle of randomization. All experiments were performed following the guidelines of the Animal Care Ethics Committee of the Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences (2021R001).
Method details
Behavioral bioassay
The behavioral bioassay, a novel tank diving test, was modified from Cachat et al.15 The experiment was conducted using a trapezoidal prism aquarium tank with dimensions of 27 × 7 cm (upper), 22 × 7 cm (lower), and a depth of 15 cm. This tank, referred to as a "novel tank," was supplemented by a pretreatment beaker (Figure S1) with a capacity of 1 L. Zebrafish were initially exposed to alarm substances for 5 minutes in the pretreatment beaker before being moved to a novel tank for behavioral observation and recording.17 The control group underwent the same procedure and was exposed to the appropriate vehicle stimulus for each experiment. The fish behaviors were documented using a Canon EOS Rebel T3 camera immediately when the fish was transferred into the novel tank. The recording lasted for 7 minutes. The fish motion was tracked using an EthoVision XT10 (Noldus Information Technology, Wageningen, the Netherlands) based on recorded videos. The tracking analysis commenced once the subject fish was detected for a duration exceeding 1 s. The detection settings were chosen to precisely capture the alarm behavior of zebrafish.15 The movement tracks were smoothed and analyzed for abnormalities using EthoVision XT10. Next, a standard two-dimensional swim track was generated.
Analysis of behavioral data
The behavioral data of zebrafish were analyzed using computer-aided analysis. Two trained observers conducted the analysis using a double-blind procedure. We evaluated 12 behavioral parameters: time in bottom half (%), latency to upper half (s), freezing duration (%), erratic movements duration (%), number of entries to the bottom, distance traveled in bottom (m), average velocity (cm/s), total distance traveled (m), time spent (bottom/top), entries (bottom/top), average bottom entry duration (s), and distance traveled (bottom/top). Commercial software offered an efficient method for quantifying behavioral endpoints, including time spent at the bottom, latency to enter the top, number of entries to the bottom or top, distance traveled, and average velocity. In addition, manual analysis was conducted to examine freezing and erratic movements.15 In this study, freezing refers to a state of immobility in fish, characterized by limited movement in the gills and the eyes. This behavior is predominantly observed when the fish is positioned near the bottom, in a corner, or just beneath the water's surface.58 The erratic movement referred to is characterized by a high swim speed of more than 3 cm/s and a seemingly aimless zig-zag pattern with frequent changes in swimming direction.58 This behavior is commonly observed at the bottom of the tank, but can also occur in midwater. The remaining parameters can be quantified through computerize video tracking and automatic recording, and subsequently determined using the formula.59 The data were plotted using GraphPad Prism software (GraphPad, NJ, USA) and statistically analyzed using SPSS (version 11.0, SPSS Inc, IL, USA).
Extraction, fractionation, and bioassay of compounds from the skin
The zebrafish (approximately 800 fish) were caught using a net and anesthetized with tricaine spray (Sigma-Aldrich, St. Louis, MO, USA) on an ice plate. The skin of each fish was peeled off using dissecting scissors and forceps on the ice plate. The harvested skin samples (46.2 g) were homogenized (60 Hz, 90 s) and extracted by ethanol three times (95%, 3 × 300 mL, vortex 10 min each, Merck, Germany). The three batches of extracts were pooled together and centrifuged for 20 min at 10000g and 4oC (Bruker, Rheinstetten, Germany). The supernatants were concentrated using a roto-evaporator (model R-210, Buchi Rotavapor, Flawil, Switzerland). The residue (skin extract; SKE, ca. 7.5 g) was separated into three parts, with 5% (0.38 g) of the residue used for behavioral assays, 80% (6.0 g) used for compound isolation, and the remaining 15% (1.1 g) archived (Figure S2).
We tracked the alarm substance activities in each fractionation step and used the results to guide our search for the active compounds (Figure S2). The residue (5%) was dissolved in 0.5 mL of water to acquire a stock solution equivalent to 80 fish/mL (designated as SKE stock solution), diluted serially from 10 to 106-fold, resulting in “stimulus solutions”. Each stimulus solution was assayed by adding 1 mL of stimulus solution to the 1 L pretreatment beaker. The 10 to 106 fold stimulus solutions in the pretreatment beaker corresponded to 8 fish extract/L to 8 × 10–5 fish extract/L (Figure S2). This dilution scheme is similar to the alarm substance extract protocol14,19,20 and the odorant concentration applied in alarm response experiments (1 × 10–3 fish extract/L).21
Subsequently, a portion of the residue (80%; 6.0 g) was suspended in 100 mL of water and subjected to sequential partitioning with petroleum ether (PET), ethyl acetate (EtOAc), and n-butanol (nBuOH) (3 × 100 mL each, Merck, China). The extract of each phase was concentrated in vacuo, resulting in extracts of PET (1.2 g), EtOAc (0.3 g), and nBuOH (4.1 g). These fractions of skin extract, namely, PET, EtOAc, and nBuOH, were each reconstituted into 8 mL of water (Figure S2). From each fraction, we took 0.1 mL from the 8 mL stock solution and mixed it with 99.9 mL water (1000 fold dilution), resulting in a test solution equivalent to 1000 × SKE (0.08 fish/mL) and subjected these solutions to the behavioral bioassay (Figure S2). The test solutions were assayed as described for SKE stimulus solutions. The final treatment concentration in the baker was 0.08 fish/L. The behavioral outcomes were compared to those elicited by the 0.08 fish extract (1000 × SKE treatment) to determine which fraction or fractions contained the components of alarm substance.
The n-butanol test solution replicated the potency of 1000 × SKE (0.08 fish/mL) in the bioassay. Hence, the residue of nBuOH extract (4.05 g, 98.7% of n-butanol extract) was chromatographed by high-performance liquid chromatography (HPLC) coupled with a quaternary gradient pump, a photodiode array detector (Waters, Milford, MA, USA). We used a reverse phase column (Waters Xbridge, BEH C18 10 × 250 mm, 5 μm) at room temperature and eluted fractions with a mobile phase consisting of water (A) and methanol (B) at 4.0 mL/min. Separation was achieved using the following gradient program for 50 min: 95% A for 5 min, decreased to 5% A from 5 to 40 min, and then maintained at 5% A from 40.0 to 50.0 min. The manual injection volume was 1 mL. The five-minute eluents contained a large amount of water and methanol and were lyophilized. Fraction 7 was reconstituted to 7.9 mL (Figure S2), from which 0.1 mL was taken and diluted with 99.9 mL of water, resulting in a 103-fold diluted solution that was equivalent to 0.08 fish/mL (1000 × Fraction 7), and 1 mL of this solution was added into 1 L of water in the pretreatment beaker and proceeded to be assessed for its behavioral activities.
Fraction 7 (the remaining 7.8 mL) was concentrated to obtain a residue (24 mg) that was further purified using Sephadex LH-20 (Sigma-Aldrich, Shanghai, China), eluted first by CH2Cl2-MeOH (1:1) and then by MeOH (100%), which resulted in compounds 1 (7.8 mg, 5CS) and 2 (1.8 mg, 5DS).
Compound 5CS (1 mg) was reconstituted in 1.8 mL of water to make a 10–3 M stock solution and, subsequently, diluted serially to 10–9 M. Similarly, 1 mg of 5DS was reconstituted in water to form 1.8 mL of 10–3 M stock solution and diluted to prepare a treatment solution ranging from 10–6 to 10–9 M (Figure S2). We also combined 100 μL of 5CS (10–3 M) and 100 μL of 5DS (10–5 M) and diluted this combined solution to 1 mL to make a stock solution of the mixture (5CS, 10–4 M, and 5DS, 10–6 M). The solution was serially diluted. The novel tank diving tests were carried out on 5CS, 5DS, and the mixture, respectively. In each trial, 1 mL of test solution was dissolved in 1 L of water to prepare a treatment with concentrations as shown in Figure S2.
NMR analysis
The NMR spectra of the compounds were obtained at 23°C using a Bruker Avance III 500 NMR spectrometer with a 5 mm tube. The spectrometer operated at 500 MHz for 1H and 125 MHz for 13C. The 13C DEPT spectra (135°, 90°, and 45°) were employed to distinguish between the resonance signals of methine and methylene groups. The research employed 1H and 13C resonances, along with a set of two-dimensional (2D) homonuclear (1H-1H) and heteronuclear (1H-13C) shift-correlated approaches. The employed methodologies encompassed 1H-detected heteronuclear multiple quantum correlation (HMQC), 1H-detected heteronuclear multiple bond correlation (HMBC), 1H-1H correlation spectroscopy (COSY), and 1H-1H nuclear Overhauser effect spectroscopy (NOESY) experiments. The 2D-NMR spectra were obtained using recommended pulse sequences and parameters provided by the manufacturer.
Define the predator avoidance behavior phenotype
Although the predator avoidance response elicited by injured skin has been observed in zebrafish in numerous studies, we verified the behaviors in our experimental paradigm (Figure S1). A total of 12 possible antipredatory behavior parameters and derived indices of zebrafish triggered by skin extracts were recorded using a novel tank diving test (Figure S3) and statistically analyzed. The results showed that four parameters increased in fish exposed to SKE, namely erratic movements duration (%) (Figure S3A; p < 0.01, Mann-Whitney U test with Bonferroni correction), time in bottom half (%) (Figure S3B; p < 0.01, Mann-Whitney U test with Bonferroni correction), freezing duration (%) (Figure S3C; p < 0.01, independent samples t-test with Bonferroni correction) and latency to upper half (s) (Figure S3D; p < 0.01, independent samples t-test with Bonferroni correction). These four parameters were selected to track the alarm substance activities in bioassays used to guide the fractionation process and to confirm the function of purified alarm substance components. Notably, the 1000-fold dilution of SKE (1000xSKE) induced responses in each selected phenotype (Figures S3A‒S3D). Thus, we chose a 1000xSKE as a positive control to evaluate the behavioral responses in bioassay-guided experiments.
Chemical structure elucidation of 5CS
Compound 5CS was obtained as a pale amorphous powder. The high resolution negative ion mass spectral data gave a pseudomolecular ion of m/z 531.3019 [M – H]–, corresponding to the molecular formula of C27H48O8S, (cal. 531.2992 [M – H]–, Δ = 5.1 ppm) (Figure S10). The collision-induced fragmentation of this ion resulted in the detection of a product ion at m/z 452, which coincided with that of grass carp bile alcohol;60 the latter was attributed to the monoisotopic mass of hydrogen sulfate anion (HSO4−, m/z 98). The latter ion indicated the presence of a sulfate group and implied four degrees of unsaturation.
The constitution of 5CS was determined by 1D- and 2D-NMR analyses (Figures S4 and S5; Table S2). The 1H, 13C, and HSQC NMR spectra (Figures S11-S15) of 5CS in methanol-d4 showed signals for two methyl singlets representing the methyl groups C-18 and C-19 and a doublet for the methyl groups C-21. These methyl group signals and the predicted molecular formula indicated a classic 27-carbon steroid skeleton. Among the HMBC correlations (Figure S4) for 5CS was a long-range communication between the methyl protons for Me-19 to a CH (δC 32.9). The COSY spectrum (Figure S14) showed correlations between CH-CH2-CH-CH2-CH. The proton on C-5 also showed HMBC correlations to the CH at δC 67.4 (Figure S16) and CH at δC 68.9, indicating the oxygenation on C-3 and C-7. The long-range communication between the methyl protons for Me-18 to carbinol methine proton at δC 74.1 allowed us to locate another site of oxygenation on C-12. These relationships were highly reminiscent of the hydroxylation pattern in petromyzonol.61 The chemical shifts of all five carbons C-3–C-7 matched well with those of petromyzonol (Table S2).
Accounting for the molecular formula deduced by HR-MS, we assumed the composition of the C17 side chain to be a C8H17O5S subunit. A CH2 resonance at δH 4.03 was attached to a carbon having a chemical shift of δC 69.1 (HSQC). This is a characteristic set of chemical shifts for sulfated primary alcohol,23 in addition to another primary alcohol CH2 resonance at δH 3.60 and δC 63.3, which accounted for all heteroatoms present in 5CS. In the HMBC spectrum (Figure S16), the CH3 resonance at δH 1.02 showed a long-range correlation with δC 48.5 (C-17), which also exhibited a strong correlation with Me-18. In addition, the COSY correlation between H-21 and H-20 and the correlation between H-20 and H-17 were supportive of the connectivity between the side chain and tetracyclic steroidal backbone. Therefore, the planar structure was assigned as 3,7,12-trihydroxy-cholestan-27 sulfate, consistent with that of cyprinol sulfate.24,62
The relative configuration of 5CS was supported by NOESY (Figure S5) and coupling constant analysis. The NOESY correlations observed from H-19 to H-8β, from H-8β to H-18, from H-18 to H-21, as well as on the counterpart, the correlations from H-5α to H-9α, from H-9α to H-14α, and from H-14α to H-17α, indicated the relative configuration for each ring junction to be trans. Furthermore, the NOESY spectrum (Figure S17) indicated that the following characteristics: the correlations from H-3 to H-1β and from H-1β to H-19; the H-7 displayed a correlation with H-8β; the H-12 showed a correlation with H-11β, which also present correlation with H-8β. These features indicated the hydroxyls at C-3, C-7, and C-12 have an axial orientation.24 The chemical shifts of 5CS showed similarity to those of 5α-cyprinol sulfate and a significant difference from those of the 5β analog (Figure S5; Tables S2 and S3).
In addition, the optical rotation of 5CS ([α]25D +38.0, c 0.20, MeOH) was consistent with that of isolated 5α-cyprinol sulfate.24 Therefore, we confirmed that the chemical structure is 5α-cyprinol sulfate.
Chemical structure elucidation of 5DS
Compound 5DS was obtained as a white amorphous powder. The molecular formula of 5DS was established as C28H50O8S from its HRESIMS (m/z 545.3149, [M – H]−) (Figure S18) in conjunction with the carbon count indicated by the 13C NMR data (Figure S20). This indicated four degrees of unsaturation. The constitution of 5DS was deduced to be an oxysterol with a sulfate half ester (−OSO3Na) through analysis of 1D- (Table S4) and 2D-NMR (Figures S22 and S23) data. The connectivity of most of the tetracyclic steroidal backbone was established by interpretating of the 1H–1H COSY and HMBC correlations (Figures S22 and S25). The 13C NMR spectrum showed characteristic resonances for a 3α,7α,12α-trihydroxy-5α-cholane skeleton (Figures S20 and S21). These results suggested that 5DS shared a tetracyclic structure common to that deduced earlier to be present in 5CS.
There was no evidence in the NMR spectra for additional oxidation in the tetracyclic steroid framework. Therefore, we assumed that the composition of the C17 side chain was a C9H19O5S subunit. Three of the three-proton doublets (J = 6.5−6.9 Hz) indicated three methyl groups, each having a single vicinal proton. The COSY and HMBC correlations supported the methyl branching at C20 and C25, and an oxygenated methylene substituent attached to C24, an oxygenated quaternary carbon. The two-proton doublet (J = 2.5) at 3.62 ppm and an oxygenated quaternary carbon at 73.5 ppm suggested the presence of a sulfated adjacent to C2425 or C2826 (Figures 2B and 2C, respectively). To accommodate these facts, we proposed the side chain as analog B shown in Figure 2. Notably, the singlet signal at 4.03 ppm, which can be attributed to the hydroxyl either on C-24 or C-28 of the side chain in the COSY spectrum, displayed long-range correlations to C-23 and C-25 in HMBC spectrum which strongly supporting the hydroxy substituent at C-25 and sulfated on C-28, correspondingly. This substructural unit is present in the sulfated polyhydroxy steroid isolated from starfish, which possesses a different steroid backbone.26 The 1H and 13C NMR shift data for all side chain protons strongly support this constitutional assignment for 5DS.
While it is tempting to propose that 5DS and reference compound26 also share the same configuration at C-24 (Figure S7), we have been unable to locate any reported examples that are epimeric at C-24 to assess whether chemical shift differences were distinctive. The 24R configuration in reference compound26 was assigned by the enantioselective synthesis of (24R-) and (24S-)-24-hydroxymethyl-24-hydroxycholesterols. The comparison presented a high degree of similarity of the proton and carbon NMR spectra for each of the C-24-epimers, and an unambiguous answer to this question must await resolution by synthesis, which we are pursuing to solve by Mosher reaction when more amounts of 5DS become available in future.26
Quantitative analysis of 5CS and 5DS in the AS stock solution and skin
LC-MS/MS has been widely used in the quantification of oxysterols due to its selectivity and specificity. After optimization of chromatographic conditions, the analytes 5CS and 5DS were well separated on a Thermo Hypersil Gold C18 column as shown in Figure S8. Using the optimized SIM parameters displayed in Table S5, the target analytes in the present study could be quantified reproducibly and accurately.
Linearity was determined by analysis of a 9-point calibration curve. Charcoal-stripped serum after the removal of bile acids was used as the blank serum matrix for the preparation of calibrators. The linear correlation coefficients (r2) of the calibration curves were 0.9992 for 5CS and 0.9996 for 5DS, respectively, suggesting excellent linearity for the quantification. A high level of linearity in responses over a sufficient dynamic concentration range was observed and summarized in Table S6. The intra- and inter-day accuracy and precision were evaluated using 3 QC concentrations distributed throughout the calibration range for each analyte (Table S7). Intra-day accuracy (n = 5) ranged from 100.60 to 105.03%. For precision of analytes, intra-day variation (n = 5) ranged from 1.18% to 4.15%. Inter-day accuracy (n = 3) ranged from 97.59 to 108.03%. For precision of analytes, inter-day variation (n = 3) ranged from 2.51% to 4.32%. The precision and accuracy for all analytes were more than 85%, which was within the interval set by the US Food and Drug Administration (FDA) concerning the validation of bioanalytical methods. Therefore, the developed method yields excellent reliability and reproducibility.
The method has been used for quantitative analysis of 1000 × SKE stock solution (Table S8). The concentrations of 5CS and 5DS in the sample were 535.64 ± 8.33 ng/mL and 5.54 ± 0.14 ng/mL (n = 5), respectively. According to our dilution procedure of concentration determination, we deduced that the concentrations of 5CS and 5DS were 1.03×10–7 mol/mL and 1.12×10–9 mol/mL. After adding 1 mL to 1 L water, the final concentrations of 5CS and 5DS in 1000x AS treatment were 1.03×10–7 M and 1.12×10–9 M, respectively. The mixture of 10–7 M of 5CS and 10–9 M of 5DS (approximately the same concentrations as those in the 1000xSKE treatment) was prepared with isolated compounds and serially diluted to complete the bioassays.
Behavior response triggered by 5CS
After being exposed to 5CS, zebrafish showed a sharp rise in the erratic movement duration at the concentration of 10–10 M (control n = 13 and experiment n = 9, p = 0.024) (Figure 3A). The time spent in the bottom half showed a significant increase at 10–12 M (control n = 11 and experiment n = 20, p = 0.013) (Figure 3B). The freezing duration showed no significant changes with control (Figure 3C). Latency to upper half elevated remarkably at 10–10 M of 5CS (control n = 6 and experiment n = 11, p < 0.001; Figure 3D). Thus, we concluded that the threshold for 5CS to induce anti-predation response was at 10–10 M.
Behavior response triggered by 5DS
Zebrafish exposed to 5DS at 10–12 M showed remarkable increases in all four behavioral parameters, erratic movements (control n = 13 and experiment n = 11, p = 0.005), bottom behaviors (control n = 11 and experiment n = 20, p = 0.003), freezing duration (control n = 8 and experiment n = 9, p = 0.001), and latency to upper half (control n = 6 and experiment n = 11, p < 0.001; Figures 3A–3D). Thus, the threshold for 5DS to induce anti-predator response was 10–12 M or lower.
Behavior response triggered by the mixture of 5CS and 5DS
For the mixture of 5CS and 5DS, the stock solution was prepared by isolated compounds with purities higher than 97.0%. The serial dilution of the stock solution was tested in the behavioral assays. The stock solution induced significant changes, in comparison to the vehicle control, in all four behavioral parameters (p < 0.05 for all parameters). The mixture stock solution and SKE (1000-fold dilution) induced the same levels of erratic movements duration (stock solution n = 10 and SKE n = 8, p = 1.000) (Figure 3E). Compared to 1000xSKE, the mixture induced large increases in time in the bottom half (stock solution n = 10 and SKE n = 8, p = 0.081) (Figure 3F), freezing duration (stock solution n = 10 and SKE n = 7, p = 0.005) (Figure 3G), and latency to upper half (stock solution n = 10 and SKE n = 6, p = 0.001) (Figure 3H). The results indicated that the mixture replicated SKE in evoking antipredatory behavioral responses from zebrafish.
Notably, the locomotion traces showed that 5CS caused more erratic movements while 5DS is more likely to trigger freezing (Figures 3I and 3J), indicating that different components of the alarm substance contribute to different aspects of the behavior.
Cortisol in the whole zebrafish
According to the cortisol elevation time frame,30,63 each fish has been sacrificed and prepared to assess cortisol levels immediately after 5 min-recording behavioral measurements. The significant elevation of cortisol upon exposure to 5CS and 5DS (Figure 4) occurred at concentrations of 10–10 M (control n = 15 and experiment n = 12, p = 0.002) and 10–8 M (control n = 15 and experiment n = 13, p = 0.001), respectively.
Quantification and statistical analysis
Quantitative analysis of 5CS and 5DS
We developed and validated a LC-MS/MS method to quantify 5CS and 5DS, in zebrafish skin. Quantitative analysis was conducted using a UPLC system (UltiMate 3000, Thermo Fisher Scientific, Waltham, MA, USA) coupled with a TSQ Quantiva mass spectrometer (Thermo Fisher Scientific). A Thermo Hypersil Gold C18 column with dimensions of 2.1 × 50 mm and particle size of 1.9 μm was employed for the separation process. The mobile phase comprised solvent A, which was water containing 0.1% formic acid (FA), and solvent B, which was methanol. Gradient elution was performed using the following conditions: 0–0.2 min with 10% B, 0.21–2.0 min with a linear increase from 10% B to 90% B, 2.0–4.0 min with 90% B, 4.01–4.5 min with a linear decrease from 90% B to 10% B, and finally, the column was re-equilibrated with 10% B for 1.0 min. The flow rate was 0.3 mL/min. The column temperature was set to 40°C, and the sample injection volume was 1.0 μL. The autosampler syringe was washed with methanol during the intervals between injections.
The TSQ Quantiva was operated in negative mode. The selected reaction monitoring transitions for determination were monitored under optimal conditions. The monitoring ion pairs used for 5CS and 5DS were 531.3→97.0 and 545.3→97.0, respectively. The collision energies for 5CS and 5DS were optimized to 55 V, while the RF lens was set at 190 V for both analytes. The following mass parameters were configured: negative ion at 2500 V, sheath gas at 35 arb, auxiliary gas at 15 arb, sweep gas at 2 arb, ion transfer tube temperature at 350°C, vaporizer temperature at 300°C, and CID gas at 1.5 mTorr. The dwell times for both analytes were 100 ms. The Xcalibur software, developed by Thermo Fisher Scientific, controlled the MS system. The 1000 × SKE solution was diluted and analyzed using UPLC-MS/MS to quantify the absolute concentration of 5CS and 5DS (Figure S9).
Quantitative analysis of cortisol
The method developed and validated by our laboratory was used to extract and determine the whole-body cortisol of zebrafish.64 Briefly, the fish were euthanized using tricaine at a concentration of 500 mg/L in the ice bath, removed of excessive water using paper towels, frozen in liquid nitrogen, and stored at –80°C. To process the samples, the fish were thawed, weighed, diced, and then homogenized at 60 Hz for 90 seconds. The sample was subsequently centrifuged at 10,000 g and 4°C for 10 minutes. The stable isotope internal standard cortisol-d4 (500 ng, Sigma-Aldrich Corp.) was added to the fish supernatant. Solid-phase extraction (SPE) with the Waters Oasis HLB Vac Cartridge that had been pre-conditioned with 10 mL of methanol and 10 mL of water was used to extract the cortisol from the supernatant. Samples were processed using a 1 mL/min Supelco vacuum manifold (Sigma-Aldrich Corp.), which can extract 12 samples concurrently. To reduce interference, loaded cartridges were rinsed with a solution consisting of 30% methanol/water and 2% acetic acid (5 mL). Subsequently, the cartridges were washed with a solution of 75% methanol and 2% acetic acid in water (5 mL). Eluates were collected and concentrated using a Cold Trap with Vacuum Centrifuge Concentrator (JM Technology Co., Beijing, China). The concentrated eluates were then reconstituted in 100 μL of methanol/H2O (55:45, v:v) for UPLC-MS analysis. The UPLC-MS method validated following FDA guidelines was used to accurately measure cortisol concentration in whole-body homogenates of zebrafish.64
Statistical analysis
All behavior and endocrine data were tested for normality. For sets of data that conformed to a normal distribution, an independent samples t-test (for data from two treatments) or one-way ANOVA (for data from three or more treatments) was used, with Bonferroni correction where needed.
To define the parameters that describe the full spectrum of behaviors induced by all components of AS, or SKE, data from all 12 measured behavior parameters were pooled and analyzed. The data did not conform to normality, and various transformations did not correct the issue. We, therefore, decided not to perform a MANOVA on the pooled data. Data from each of the 12 parameters were subjected to independent sample t-tests if the data conformed to the normal distribution and the Mann-Whitney U test if the data did not conform to the normal distribution. All tests were subjected to Bonferroni correction (with total tests = 12).
Bioassay data from the products of each fractionation step were treated as the results of an independent experiment. To account for possible block effects due to the lengthy process of fractionation and the different solvents used, blank control tests using appropriate vehicle stimuli were performed for each set of experiments. The four parameters determined to represent anti-predation behavior were analyzed with either an independent sample t-test or a Mann-Whitney U test, with Bonferroni correction. For data that do not conform to a normal distribution, a non-parametric test is used. For each assay, tests were repeated ten times (n = 10). A P-value < 0.05 was considered to indicate significance.
Bioassay data for purified compounds were also analyzed with one ANOVA or Kruskal-Wallis test, and with post hoc tests. Cortisol data were analyzed similarly.
Acknowledgments
We thank Ying Kong in Yantai Branch, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, for assistance with quantitative analysis method development and validation for 5CS and 5DS. This study received funding from the National Natural Science Foundation of China (32270533) and the seed project of Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences (YICE351010101). K. L. expresses gratitude for the Taishan Scholar Program provided by the Shandong Province of China (tsqn20190403), as well as the support received from the Yantai Municipal City's Shuangbai Plan (2018020). W. L. is supported by the Great Lakes Fishery Commission.
Author contributions
Conceptualization, K.L. and W.L.; methodology, K.L.; investigation, Y.L., Z.Y., K.L., A.L., X.L., and X.Y.; visualization, Y.L.; funding acquisition, K.L.; supervision, K.L.; writing − original draft, K.L. and Y.L.; writing − review and editing, K.L. and W.L.
Declaration of interests
The authors declare no competing interests.
Published: April 3, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.109660.
Supplemental information
References
- 1.Wyatt T.D. Cambridge University Press; 2014. Pheromones and Animal Behavior: Chemical Signals and Signatures. [Google Scholar]
- 2.Wyatt T.D. Pheromones and signature mixtures: defining species-wide signals and variable cues for identity in both invertebrates and vertebrates. J. Comp. Physiol. A. 2010;196:685–700. doi: 10.1007/s00359-010-0564-y. [DOI] [PubMed] [Google Scholar]
- 3.Kannan K., Galizia C.G., Nouvian M. Olfactory strategies in the defensive behaviour of insects. Insects. 2022;13:470. doi: 10.3390/insects13050470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verheggen F.J., Haubruge E., Mescher M.C. In: Vitamins & Hormones. Litwack G., editor. Academic Press; 2010. Alarm pheromones-chemical signaling in response to danger; pp. 215–239. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari M.C., Wisenden B.D., Chivers D.P. Chemical ecology of predator-prey interactions in aquatic ecosystems: a review and prospectus. Can. J. Zool. 2010;88:698–724. doi: 10.1139/z10-029. [DOI] [Google Scholar]
- 6.von Frisch K. Zur Psychologie des Fisch-Schwarmes. Naturwissenschaften. 1938;26:601–606. doi: 10.1007/BF01590598. [DOI] [Google Scholar]
- 7.Pfeiffer W. Schreckreaktion und Schreckstoffzellen bei Ostariophysi und Gonorhynchiformes. Z. Vergl. Physiol. 1967;56:380–396. doi: 10.1007/bf00298056. [DOI] [Google Scholar]
- 8.Chivers D.P., Smith R.J.F. Chemical alarm signalling in aquatic predator-prey systems: a review and prospectus. Ecoscience. 1998;5:338–352. doi: 10.1080/11956860.1998.11682471. [DOI] [Google Scholar]
- 9.Chivers D.P., Brown G.E., Ferrari M.C. In: Chemical Ecology in Aquatic Systems. Brönmark C., Hansson L.-A., editors. Oxford University Press; 2012. The evolution of alarm substances and disturbance cues in aquatic animals; pp. 127–139. [Google Scholar]
- 10.D∅ving K.B., Lastein S. The alarm reaction in fishes-odorants, modulations of responses, neural pathways. Ann. N. Y. Acad. Sci. 2009;1170:413–423. doi: 10.1111/j.1749-6632.2009.04111.x. [DOI] [PubMed] [Google Scholar]
- 11.Pfeiffer W., Riegelbauer G., Meier G., Scheibler B. Effect of hypoxanthine-3(N)-oxide and hypoxanthine-1(N)-oxide on central nervous excitation of the black tetra Gymnocorymbus ternetzi (Characidae, Ostariophysi, Pisces) indicated by dorsal light response. J. Chem. Ecol. 1985;11:507–523. doi: 10.1007/bf00989562. [DOI] [PubMed] [Google Scholar]
- 12.Johnston R.E., Müller-Schwarze D., Sorensen P.W. Springer; 2012. Advances in Chemical Signals in Vertebrates. [Google Scholar]
- 13.Mathuru A.S., Kibat C., Cheong W.F., Shui G., Wenk M.R., Friedrich R.W., Jesuthasan S. Chondroitin fragments are odorants that trigger fear behavior in fish. Curr. Biol. 2012;22:538–544. doi: 10.1016/j.cub.2012.01.061. [DOI] [PubMed] [Google Scholar]
- 14.Speedie N., Gerlai R. Alarm substance induced behavioral responses in zebrafish (Danio rerio) Behav. Brain Res. 2008;188:168–177. doi: 10.1016/j.bbr.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cachat J., Stewart A., Grossman L., Gaikwad S., Kadri F., Chung K.M., Wu N., Wong K., Roy S., Suciu C., et al. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat. Protoc. 2010;5:1786–1799. doi: 10.1038/nprot.2010.140. [DOI] [PubMed] [Google Scholar]
- 16.Kermen F., Darnet L., Wiest C., Palumbo F., Bechert J., Uslu O., Yaksi E. Stimulus-specific behavioral responses of zebrafish to a large range of odors exhibit individual variability. BMC Biol. 2020;18:66. doi: 10.1186/s12915-020-00801-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egan R.J., Bergner C.L., Hart P.C., Cachat J.M., Canavello P.R., Elegante M.F., Elkhayat S.I., Bartels B.K., Tien A.K., Tien D.H., et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009;205:38–44. doi: 10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loennstedt O.M., McCormick M.I. Chemical alarm cues inform prey of predation threat: the importance of ontogeny and concentration in a coral reef fish. Anim. Behav. 2011;82:213–218. doi: 10.1016/j.anbehav.2011.04.015. [DOI] [Google Scholar]
- 19.Fontana B.D., Alnassar N., Parker M.O. The zebrafish (Danio rerio) anxiety test battery: comparison of behavioral responses in the novel tank diving and light-dark tasks following exposure to anxiogenic and anxiolytic compounds. Psychopharmacology. 2022;239:287–296. doi: 10.1007/s00213-021-05990-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canzian J., Fontana B.D., Quadros V.A., Rosemberg D.B. Conspecific alarm substance differently alters group behavior of zebrafish populations: Putative involvement of cholinergic and purinergic signaling in anxiety- and fear-like responses. Behav. Brain Res. 2017;320:255–263. doi: 10.1016/j.bbr.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 21.Diaz-Verdugo C., Sun G.J., Fawcett C.H., Zhu P., Fishman M.C. Mating suppresses alarm response in zebrafish. Curr. Biol. 2019;29:2541–2546.e3. doi: 10.1016/j.cub.2019.06.047. [DOI] [PubMed] [Google Scholar]
- 22.Al Shuraiqi A., Al-Habsi A., Barry M.J. Time-dose- and transgenerational effects of fluoxetine on the behavioural responses of zebrafish to a conspecific alarm substance. Environ. Pollut. 2021;270 doi: 10.1016/j.envpol.2020.116164. [DOI] [PubMed] [Google Scholar]
- 23.Asakawa M., Noguchi T., Seto H., Furihata K., Fujikura K., Hashimoto K. Structure of the toxin isolated from carp (Cyprius carpio) bile. Toxicon. 1990;28:1063–1069. doi: 10.1016/0041-0101(90)90144-v. [DOI] [PubMed] [Google Scholar]
- 24.Hahn M., von Elert E., Bigler L., Díaz Hernández M.D., Schloerer N.E. 5 alpha-Cyprinol sulfate: Complete NMR assignment and revision of earlier published data, including the submission of a computer-readable assignment in NMReDATA format. Magn. Reson. Chem. 2018;56:1201–1207. doi: 10.1002/mrc.4782. [DOI] [PubMed] [Google Scholar]
- 25.Rao M.N., Shinnar A.E., Noecker L.A., Chao T.L., Feibush B., Snyder B., Sharkansky I., Sarkahian A., Zhang X., Jones S.R., et al. Aminosterols from the dogfish shark Squalus acanthias. J. Nat. Prod. 2000;63:631–635. doi: 10.1021/np990514f. [DOI] [PubMed] [Google Scholar]
- 26.Iorizzi M., Bryan P., McClintock J., Minale L., Palagiano E., Maurelli S., Riccio R., Zollo F. Chemical and biological investigation of the polar constituents of the starfish Luidia clathrata, collected in the gulf of Mexico. J. Nat. Prod. 1995;58:653–671. doi: 10.1021/np50119a003. [DOI] [PubMed] [Google Scholar]
- 27.Collier A.D., Kalueff A.V., Echevarria D.J. In: The rights and wrongs of zebrafish: behavioral phenotyping of zebrafish. Kalueff A.V., editor. Springer International Publishing; 2017. Zebrafish models of anxiety-like behaviors; pp. 45–72. [Google Scholar]
- 28.Abreu M.S., Giacomini A.C.V.V., Koakoski G., Piato A.L.S., Barcellos L.J.G. Divergent effect of fluoxetine on the response to physical or chemical stressors in zebrafish. PeerJ. 2017;5 doi: 10.7717/peerj.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barcellos L.J.G., Koakoski G., da Rosa J.G.S., Ferreira D., Barreto R.E., Giaquinto P.C., Volpato G.L. Chemical communication of predation risk in zebrafish does not depend on cortisol increase. Sci. Rep. 2014;4:5076. doi: 10.1038/srep05076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran S., Chatterjee D., Gerlai R. Acute net stressor increases whole-body cortisol levels without altering whole-brain monoamines in zebrafish. Behav. Neurosci. 2014;128:621–624. doi: 10.1037/bne0000005. [DOI] [PubMed] [Google Scholar]
- 31.Maximino C., de Brito T.M., da Silva Batista A.W., Herculano A.M., Morato S., Gouveia A., Jr. Measuring anxiety in zebrafish: A critical review. Behav. Brain Res. 2010;214:157–171. doi: 10.1016/j.bbr.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 32.Li Y., Yan Z., Lin A., Li X., Li K. Non-dose-dependent relationship between antipredator behavior and conspecific alarm substance in zebrafish. Fishes. 2023;8:76. doi: 10.3390/fishes8020076. [DOI] [Google Scholar]
- 33.Schroepfer G.J. Oxysterols: modulators of cholesterol metabolism and other processes. Physiol. Rev. 2000;80:361–554. doi: 10.1152/physrev.2000.80.1.361. [DOI] [PubMed] [Google Scholar]
- 34.Skrede S., Björkhem I., Kvittingen E.A., Buchmann M.S., Lie S.O., East C., Grundy S. Demonstration of 26-hydroxylation of C27-steroids in human-skin fibroblasts, and a deficiency of this activity in cerebrotendinous xanthomatosis. J. Clin. Invest. 1986;78:729–735. doi: 10.1172/jci112633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emmons G.T., Stpyrek J., Dam R., Martin M., Kudo K., Schroepfer G.J. 5-alpha-cholest-8(14)-en-3-beta-ol-15-one, a potent regulator of cholesterol metabolism: occurrence in rat skin. J. Lipid Res. 1988;29:1039–1054. doi: 10.1016/S0022-2275(20)38468-6. [DOI] [PubMed] [Google Scholar]
- 36.Fanti F., Merola C., Vremere A., Oliva E., Perugini M., Amorena M., Compagnone D., Sergi M. Quantitative analysis of oxysterols in zebrafish embryos by HPLC-MS/MS. Talanta. 2020;220 doi: 10.1016/j.talanta.2020.121393. [DOI] [PubMed] [Google Scholar]
- 37.Linsenbardt A.J., Taylor A., Emnett C.M., Doherty J.J., Krishnan K., Covey D.F., Paul S.M., Zorumski C.F., Mennerick S. Different oxysterols have opposing actions at N-methyl-D-aspartate receptors. Neuropharmacology. 2014;85:232–242. doi: 10.1016/j.neuropharm.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan D., Konen V.M. In: International Review of Cytology. Jeon K.W., editor. Academic Press; 2008. Characteristics of oxysterol binding proteins; pp. 253–285. [DOI] [PubMed] [Google Scholar]
- 39.Murakami T., Ohoku K., Yumoto R., Yoshii M., Une M., Kuramoto T., Hoshita T., Yata N. Enhancing effect of 5 alpha-cyprinol sulfate on mucosal membrane permeability to sodium ampicillin in rats. Eur. J. Pharm. Biopharm. 2000;49:111–117. doi: 10.1016/s0939-6411(99)00083-1. [DOI] [PubMed] [Google Scholar]
- 40.Goto T., Holzinger F., Hagey L.R., Cerrè C., Ton-Nu H.T., Schteingart C.D., Steinbach J.H., Shneider B.L., Hofmann A.F. Physicochemical and physiological properties of 5 alpha-cyprinol sulfate, the toxic bile salt of cyprinid fish. J. Lipid Res. 2003;44:1643–1651. doi: 10.1194/jlr.M300155-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Verheggen F.J., Haubruge E., Mescher M.C. In: Vitamins and Hormones: Pheromones. 239. Litwack G., editor. 2010. Alarm pheromones-chemical signaling in response to danger; p. 215. [DOI] [PubMed] [Google Scholar]
- 42.Buchinger T.J., Li W., Johnson N.S. Bile Salts as Semiochemicals in Fish. Chem. Senses. 2014;39:647–654. doi: 10.1093/chemse/bju039. [DOI] [PubMed] [Google Scholar]
- 43.Kurogi K., Krasowski M.D., Injeti E., Liu M.-Y., Williams F.E., Sakakibara Y., Suiko M., Liu M.-C. A comparative study of the sulfation of bile acids and a bile alcohol by the Zebra danio (Danio rerio) and human cytosolic sulfotransferases (SULTs) J. Steroid Biochem. Mol. Biol. 2011;127:307–314. doi: 10.1016/j.jsbmb.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jesuthasan S.J., Mathuru A.S. The alarm response in zebrafish: innate fear in a vertebrate genetic model. J. Neurogenet. 2008;22:211–228. doi: 10.1080/01677060802298475. [DOI] [PubMed] [Google Scholar]
- 45.Crane A.L., Bairos-Novak K.R., Goldman J.A., Brown G.E. Chemical disturbance cues in aquatic systems: a review and prospectus. Ecol. Monogr. 2022;92 doi: 10.1002/ecm.1487. [DOI] [Google Scholar]
- 46.Engeszer R.E., Patterson L.B., Rao A.A., Parichy D.M. Zebrafish in the wild: a review of natural history and new notes from the field. Zebrafish. 2007;4:21–40. doi: 10.1089/zeb.2006.9997. [DOI] [PubMed] [Google Scholar]
- 47.Saverino C., Gerlai R. The social zebrafish: behavioral responses to conspecific, heterospecific, and computer animated fish. Behav. Brain Res. 2008;191:77–87. doi: 10.1016/j.bbr.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hofmann A.F., Hagey L.R., Krasowski M.D. Bile salts of vertebrates: structural variation and possible evolutionary significance. J. Lipid Res. 2010;51:226–246. doi: 10.1194/jlr.R000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hahn M.A., Effertz C., Bigler L., von Elert E. 5α-cyprinol sulfate, a bile salt from fish, induces diel vertical migration in Daphnia. Elife. 2019;8 doi: 10.7554/eLife.44791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hahn M., von Elert E. One kairomone and multiple effects in daphnia Species-5 alpha-cyprinol sulfate induces morphological defenses in the invasive species Daphnia lumholtzi. Front. Ecol. Evol. 2022;10:170. doi: 10.3389/fevo.2022.804521. [DOI] [Google Scholar]
- 51.Piato Â.L., Capiotti K.M., Tamborski A.R., Oses J.P., Barcellos L.J.G., Bogo M.R., Lara D.R., Vianna M.R., Bonan C.D. Unpredictable chronic stress model in zebrafish (Danio rerio): behavioral and physiological responses. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2011;35:561–567. doi: 10.1016/j.pnpbp.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 52.Segner H. Zebrafish (Danio rerio) as a model organism for investigating endocrine disruption. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009;149:187–195. doi: 10.1016/j.cbpc.2008.10.099. [DOI] [PubMed] [Google Scholar]
- 53.Veldman M.B., Lin S. Zebrafish as a developmental model organism for pediatric research. Pediatr. Res. 2008;64:470–476. doi: 10.1203/PDR.0b013e318186e609. [DOI] [PubMed] [Google Scholar]
- 54.Wisenden B.D., Paulson D.C., Orr M. Zebrafish embryos hatch early in response to chemical and mechanical indicators of predation risk, resulting in underdeveloped swimming ability of hatchling larvae. Biol. Open. 2022;11 doi: 10.1242/bio.059229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao X., Li W. Embryonic substances induce alarm response in adult zebrafish (Danio rerio) J. Fish. Biol. 2020;97:225–230. doi: 10.1111/jfb.14354. [DOI] [PubMed] [Google Scholar]
- 56.Kalueff A.V., Stewart A.M., Gerlai R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014;35:63–75. doi: 10.1016/j.tips.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stewart A.M., Braubach O., Spitsbergen J., Gerlai R., Kalueff A.V. Zebrafish models for translational neuroscience research: from tank to bedside. Trends Neurosci. 2014;37:264–278. doi: 10.1016/j.tins.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blaser R., Gerlai R. Behavioral phenotyping in zebrafish: comparison of three behavioral quantification methods. Behav. Res. Methods. 2006;38:456–469. doi: 10.3758/bf03192800. [DOI] [PubMed] [Google Scholar]
- 59.Audira G., Lai Y.H., Huang J.C., Chen K.H.C., Hsiao C.D. Phenomics approach to investigate behavioral toxicity of environmental or occupational toxicants in adult zebrafish (Danio rerio) Curr. Protoc. 2021;1 doi: 10.1002/cpz1.223. [DOI] [PubMed] [Google Scholar]
- 60.Hwang D.F., Yeh Y.H., Lai Y.S., Deng J.F. Identification of cyprinol and cyprinol sulfate from grass carp bile and their toxic effects in rats. Toxicon. 2001;39:411–414. doi: 10.1016/s0041-0101(00)00134-3. [DOI] [PubMed] [Google Scholar]
- 61.Hoye T.R., Dvornikovs V., Fine J.M., Anderson K.R., Jeffrey C.S., Muddiman D.C., Shao F., Sorensen P.W., Wang J. Details of the structure determination of the sulfated steroids PSDS and PADS: New components of the sea lamprey (Petromyzon marinus) migratory pheromone. J. Org. Chem. 2007;72:7544–7550. doi: 10.1021/jo070957l. [DOI] [PubMed] [Google Scholar]
- 62.Ishida H., Nakayasu H., Miyamoto H., Nukaya H., Tsuji K. Study on the bile salts from sunfish, Mola mola L. I. The structures of sodium cyprinol sulfates, the sodium salt of a new bile acid conjugated with taurine, and a new bile alcohol and its new sodium sulfates. Chem. Pharm. Bull. 1998;46:12–16. doi: 10.1002/chin.199828229. [DOI] [Google Scholar]
- 63.Ramsay J.M., Feist G.W., Varga Z.M., Westerfield M., Kent M.L., Schreck C.B. Whole-body cortisol response of zebrafish to acute net handling stress. Aquaculture. 2009;297:157–162. doi: 10.1016/j.aquaculture.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Y., Yan Z., Li X., Yin X., Li K. UPLC-TOF-MS method for simultaneous quantification of steroid hormones in tissue homogenates of zebrafish with solid-phase extraction. Molecules. 2021;26:6213. doi: 10.3390/molecules26206213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated in the study have been deposited to the Harvard Dataverse Repository and are publicly available as of the date of publication. Accession numbers are listed in the key resources table.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.