Abstract
Objective
Vitamins and homocysteine (Hcy) are involved in liver metabolism and related to the pathogenesis of autoimmune liver disease (AILD), but consensus is lacking. This study aims to systematically summarize relevant evidence to clarify the association of serum vitamins and Hcy levels with AILD.
Methods
The English and Chinese literature was searched until August 29, 2023. Studies were included if they were observational studies of investigating serum vitamins and Hcy levels in patients with AILD and their healthy comparisons. Quality assessment was performed by using the Newcastle–Ottawa Scale, and a meta‐analysis was conducted using ReviewManager 5.3. The protocol was registered in the international prospective register of systematic reviews (PROSPERO), with registration number CRD42023455367.
Results
A total of 25 case–control studies comprising 3487 patients (1673 patients and 1814 healthy controls) were included for analysis. There were 548 autoimmune hepatitis (AIH) cases, 1106 primary biliary cholangitis (PBC) cases, and 19 primary sclerosing cholangitis (PSC) cases. We found that serum A and E were decreased in both AIH and PBC/PSC; but vitamin C was reduced only in patients with PBC, not AIH. In addition, decreased content of 25(OH)D3 was found in both AIH and PBC. However, levels of 25(OH)D did not differ between the patients and controls, and were independent of disease types and the country. Only one study that met the inclusion criteria reported vitamin B6, B9, B12, and Hcy changes, and found that vitamin B6 and B9 were significantly decreased in patients with PBC, while serum vitamin B12 and Hcy levels were significantly elevated in them. One eligible study each confirmed a reduction in plasma vitamin K1 and 1,25(OH)2D3 in patients with PBC.
Conclusion
Most vitamins are deficient in AILD, so appropriate vitamin supplementation should be necessary. Further studies with larger sample sizes are needed to validate these findings.
Keywords: autoimmune hepatitis, autoimmune liver disease, meta‐analysis, primary biliary cholangitis, vitamins
-
1.
Serum vitamin A, E, and 25(OH)D3 were decreased in both autoimmune hepatitis (AIH) and primary biliary cholangitis (PBC)/primary sclerosing cholangitis.
-
2.
Serum vitamin C was reduced only in patients with PBC but not in AIH.
-
3.
Most vitamins are deficient in autoimmune liver disease, and it is necessary to supplement vitamins in moderation.
1. INTRODUCTION
Autoimmune liver disease (AILD) is a common type of potentially life‐threatening chronic liver disease with autoimmune etiology, which is characterized serologically by elevated transaminases, gammaglobulinemia, and specific autoantibodies, and histologically by interfce heptitis, massive lymphoid and plasma cells infiltration in the portal tract and lobules, as well as sometimes hepatic rosette formation and emperipolesis. 1 Based on histopathological features and differences in autoantibody expression, AILD can be classified as autoimmune hepatitis (AIH), primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC), and overlap syndrome. AILD may initially be asymptomatic. With the progression of the disease, it can develop into liver fibrosis, cirrhosis, even acute fulminant hepatitis or end‐stage liver disease. 2 Some cryptogenic hepatocellular carcinoma may also be attributed to ALID. 2 Currently, AILD is increasingly occurring with contemporary global incidence per 100,000 for PBC ranging from 0.84 to 2.75, for PSC between 0.1 and 4.39, and for AIH from 0.4 to 2.39. And AILD accounted for 24% of liver transplantation cases in Europe and the United States. 3 But, up to 40% of AILDs inevitably recur after liver transplantation despite preoperative immunosuppressive therapy. 4 , 5 In addition, AILD is often accompanied by autoimmune diseases of other systems, like inflammatory bowel disease, Sjogren's syndrome. 6 , 7 Therefore, AILD has become a serious chronic liver disease, causing huge economic burden to the society. 4 , 5 , 8
AILD may occur due to genetic predisposition, environmental factors (smoking, drug, and xenobiotic exposure, various hepatitis virus infections), loss of immune tolerance to self‐antigens, immune disorders, as well as alterations of commensal intestinal flora. 9 , 10 But, the exact etiology and pathogenesis of AILD is still unclear. Recently, there is a certain amount of studies reporting vitamin deficiency in PBC or AIH. 11 , 12 , 13 Since vitamins are trace organic substances necessary to maintain normal physiological functions of the body, thus vitamins deficiency may play a prominent role in the development of AILD.
Each vitamin has a unique role in liver disease. Vitamin A, in the form of retinol, is mainly stored in hepatocyte and hepatic stellate cells (HSC), especially in HSC lipid droplets. 14 It has been reported that the rate of vitamin A deficiency in chronic liver disease is as high as 62.4%, and the degree of serum retinol deficiency was positively correlated with the severity and progression of liver disease. 12 B vitamins are a kind of water‐soluble small molecule compounds, which are widely involved in various physiological processes in the form of co‐enzymes. These following B vitamins may be associated with AILD: B6, B9 (folic acid), and B12, which work together to participate in the folic acid cycle and methionine metabolism, and promote the production and maturation of red blood cells. Deficiencies such as vitamin B9, vitamin B6, or vitamin B12 can cause hyperhomocysteine. 15 , 16 Homocysteine (Hcy) is shown to be decreased in a variety of liver diseases. 17 , 18
Vitamin C, also known as ascorbic acid, is a powerful antioxidant with the ability to scavenge many physiological free radicals, promote iron absorption, detoxification, etc. 19 Compared with healthy controls, serum vitamin C levels were found significantly decreased in PBC patients, 20 but did not change in AIH patients, as demonstrated in another study. 21
Vitamin D is a class of important fat‐soluble ring‐opening sterol, which main functions include the regulation of blood calcium and phosphorus concentration, new bone formation and calcification, promotion of the growth and differentiation of skin cells, and immune function modulation. 22 Vitamin D deficiency in patients with chronic liver disease has been reported in a number of studies. 11 , 23 , 24 It has been indicated that severe vitamin D deficiency can be an important prognostic biomarker in AIH or PBC, which is closely associated with incomplete response to ursodeoxycholic acid, progression to cirrhosis, and liver‐related death or even need for liver transplantation. 24 , 25 However, some studies have found no change or even a slight increase in vitamin D levels in patients with AILD.
Vitamin E is also an indispensable fat‐soluble multivitamin and exerts strong antioxidant and antiaging ability. 26 For AILD, most studies have reported lower serum vitamin E levels in patients. 21 , 27 , 28 But some research works have found no difference in vitamin E levels between AILD patients and healthy counterparts. 20
Vitamin K is an important fat‐soluble vitamin in the body and plays a critical role in the detoxification of xenobiotics, synthesis of coagulation factors, and metabolism of bile acid (BA). 29 As a coenzyme of γ‐hydroxylase, vitamin K participates in the synthesis of coagulation factors II., VII., IX., X., anticoagulant protein C, and anticoagulant S in the liver. 30 Thus, vitamin K deficiency is common in different forms of liver disease, including cholestasis, and is significantly associated with the severity of liver disease, as demonstrated by prolonged prothrombin time (PT). 30 , 31 However, the use of vitamin K supplementation for the treatment of patients with liver disease is somewhat controversial. 32 , 33
Due to the above controversy, there is no consensus on whether vitamin supplements should be taken, or what type of AILD should be treated with vitamin. In addition, few meta‐analyses or systematic review studies have been reported on the effects of serum vitamins and Hcy changes on AILD. Thus, we systematically summarized relevant evidence and conducted this meta‐analysis to clarify the association between serum vitamins and Hcy levels with AILD, with a view to contributing to the clinical prevention and treatment of AILD.
2. MATERIALS AND METHODS
2.1. Search strategy
This meta‐analysis was performed in strict accordance with the preferred reporting items for systematic reviews and meta‐analyses (PRISMA) statement. 34 Eight commonly used Chinese and English electronic databases were searched for available studies up to August 29, 2023 with no language restriction: PubMed, Embase (including conference abstracts), the Cochrane Library, Core collection in Web of Science, Chinese National Knowledge Infrastructure (CNKI), Chinese Biological Medicine (CBM), Wanfang and CQVIP databases. We established the following search strategy in PubMed: ((“autoimmune liver disease”[Mesh]) OR “autoimmune hepatitis” OR “AIH” OR “primary biliary cholangitis” OR “primary biliary cirrhosis” OR “PBC” OR “primary sclerosing cholangitis” OR “PSC” OR “autoimmune cholangitis” OR “autoimmune sclerosing cholangitis”) AND (“vitamin” OR “vitamin A” OR “retinol” OR “retinoic acid” OR “B‐vitamins” OR “vitamin B complex” OR “vitamin B6” OR “pyridoxamine” OR “pyridoxal” OR “vitamin B9” OR “folic acid” OR “folate” OR “folic acid, (DL)‐Isomer” OR “cyanocobalamin” OR “cobalamin” OR “vitamin B12” OR “ascorbic acid” OR “vitamin C” OR “vitamin D” OR “1,25(OH)(2) vitamin D”OR “25(OH) vitamin D” OR “25(OH) vitamin D3” OR “ergocalciferol” OR “calcitriol” OR “vitamin E” OR “tocopherols” OR “vitamin K” OR “vitamin K1” OR “phylloquinone” OR “vitamin K2” OR “menaquinone”). The same retrieval strategy was also applied in other databases. In addition, the reference lists of the relevant studies were reviewed to avoid missing any publication.
2.2. Selection criteria
In this step, two authors (JH Li and S Tian) independently assessed the potentially eligible studies. The following inclusion criteria were used in this meta‐analysis: (1) observational studies of human beings diagnosed with AILD; (2) patients in the control group were matched healthy comparisons; (3) studies providing sufficient data about serum vitamin (A, B, C, D, E, K, etc.) and Hcy levels. The exclusion criteria were: (1) adolescents (under 18 years of age) and pregnant women; (2) studies with less than 10 patients in the control group or the case group; (3) animal and cell experiments; (4) reviews, comments, case reports, expert consensus, duplicated reports, studies with incomplete data and no full text available; (5) other liver diseases: including liver transplantation, liver cancer, fatty liver, alcoholic liver disease, etc.; (6) other autoimmune diseases: autoimmune atrophic gastritis, inflammatory bowel disease, and so forth; (7) articles published in the non‐Chinese core journals. Any disagreements regarding the inclusion or exclusion of the articles were resolved by consensus or adjudicated by the third reviewer (XL Deng).
2.3. Quality assessment
Quality assessment of all studies was conducted by two independent reviewers (B Ci and YW Xi) using the Newcastle–Ottawa Scale. 35 In brief, each included study was evaluated from three aspects (selection, comparability control for important factors, and exposure), with a total of 9 stars. Studies with six or more stars are considered high quality. Any discrepancies regarding the quality assessment were discussed or resolved by another experienced researcher (XL Deng).
2.4. Data extraction
Two investigators (JH Li and S Tian) independently extracted available data from each study and double‐checked all information. The following details were recorded: authors, year of publication, country, sample sizes of case/control groups, patient gender and mean age, research type, method of assay, type of AILD (AIH, PBC, or PSC), type and levels of vitamins and Hcy in the patient serum.
2.5. Statistical analysis
The ReviewManager 5.3 software was used to analyze differences in serum vitamin and Hcy levels between healthy controls and patients with AILD. Subgroup analyses were performed based on the type of AILD (AIH, PBC, and PSC), country (China and other countries). The mean differences (MD) with 95% confidence intervals (CIs) were calculated for continuous variables with uniform measurement methods and unit, otherwise, standardized mean difference (SMD) was selected. Differences of p < .05 were accepted as significant. Fixed effects models or random effects models were conducted for low heterogeneity (I 2 < 50%) or significant heterogeneity (I 2 ≥ 50%) across the studies, respectively. When the number of papers was greater than or equal to 10, funnel plot was used to detect publication bias. Sensitivity analysis was used to test the impact of individual studies by Stata 15.1 software (StataCorp).
2.6. Register name and registration number
The protocol was registered in the international prospective register of systematic reviews (PROSPERO) by JH Li, with registration number CRD42023455367. Our meta‐analysis is consistent with the registration information.
3. RESULTS
3.1. Characteristics of the enrolled studies
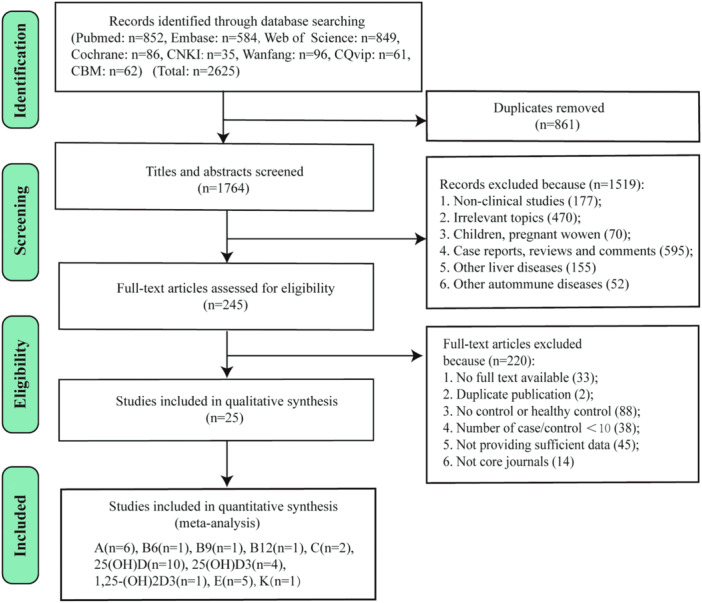
As shown in Figure 1, a total of 2625 records were originally obtained from eight databases. After removing 861 duplicates, 1519 records were excluded in turn by screening the titles and abstracts for the following reasons: no‐clinical studies (n = 177); irrelevant topics (n = 470); children and pregnant women (n = 70); case reports, reviews and comments (n = 595); other liver diseases (n = 155); and other autoimmune diseases (n = 52). Then, 245 full‐text articles were carefully reviewed for eligibility, and 220 of these were subsequently excluded for no full text available (n = 33), not core journals (n = 14), duplicate publication (n = 2), number of cases or controls < 10 (n = 38), no control or no healthy control (n = 88), and not providing sufficient data (n = 45). Ultimately, 25 studies 11 , 20 , 21 , 23 , 24 , 27 , 28 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 with a total of 3487 patients (1673 patients, 1814 healthy controls) were enrolled in the meta‐analysis. All included studies were case–control studies, 10 of which were conducted in China, and the remaining research works were from other countries, including Italy, Israel, Spain, Turkey, England, Sweden, UK, and USA. There were 548 AIH cases, 1106 PBC cases, and 19 PSC cases. Most cases were female. The detailed characteristics of all studies are summarized in Table 1. The detection methods of vitamins vary widely, including radioimmunoassay (RIA), high‐pressure liquid chromatography (HPLC), chemical luminescence immunoassay (CLIA), enzyme‐linked immunosorbent assay (ELISA), liquid chromatography‐tandem mass spectrometry, and microfluorometric method. The changes of serum vitamins and Hcy levels per study are depicted in Table 2.
Figure 1.
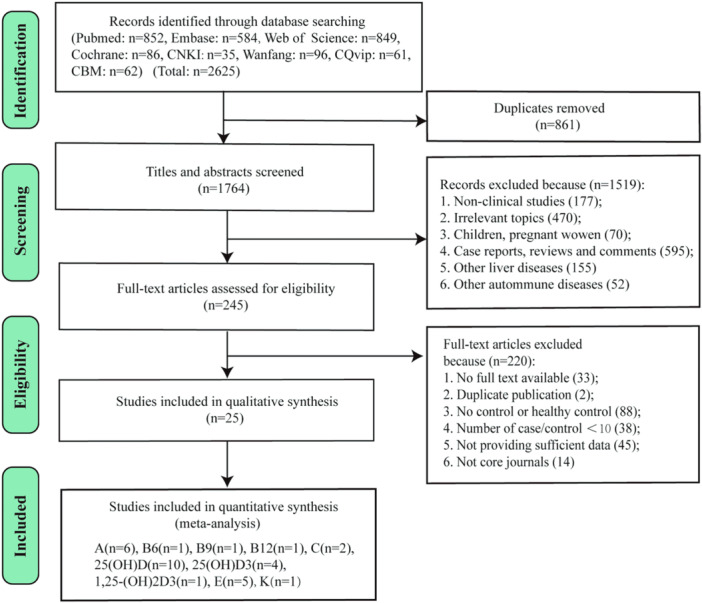
PRISMA flow diagram of the study selection process.
Table 1.
Characteristics of included studies in the meta‐analysis.
| Study | Country | Research type | Disease type | Method of assay | Sample size (Case/Control) | Age (year) | Gender (male/female) | Observation index | Quality assessment | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Case | Control | ||||||||
| Huang and Hu 36 | China | Case–control | AIH | ELISA | 187/150 | 52.37 ± 8.04 | 53.42 ± 8.11 | 52/135 | 27/123 | 25‐(OH)D3 | 8 |
| Wang et al. 11 | China | Case–control | PBC | CLIA | 185/141 | 56.06 ± 11.48 | 54.41 ± 10.04 | 15/170 | 18/123 | 25(OH)D3 | 8 |
| Sen et al. 23 | China | Case–control | AIH | Liquid chromatography‐tandem mass spectrometry | 80/80 | NA | NA | NA | NA | 25‐(OH)D | 6 |
| Li et al. 37 | China | Case–control | PBC | CLIA | 74/66 | 59.4 ± 6.8 | 57.1 ± 5.3 | 32/42 | 31/35 | 25‐(OH)D | 8 |
| Lin et al. 38 | China | Case–control | PBC | Electrochemiluminescence immunoassay | 67/50 | 52.66 ± 11.47 | 46.37 ± 10.20 | 8/59 | 21/29 | 25‐(OH)D | 8 |
| Zhang 39 | China | Case–control | PBC | ELISA | 80/80 | 56.74 ± 6.83 | 55.65 ± 5.81 | 12/68 | NA | 25‐(OH)D | 8 |
| Tian and Wang 40 | China | Case–control | AIH | CLIA | 30/18 | 31.4 ± 22.5 | 36.4 ± 23.1 | 17/13 | 9/9 | 25‐(OH)D3 | 8 |
| Li and Song 41 | China | Case–control | AIH | ELISA | 150/70 | 47.4 ± 11.9 | 45.5 ± 11.1 | 14/136 | 10/60 | 25‐(OH)D | 8 |
| Yang et al. 42 | China | Case–control | PBC | HPLC | 17/300 | 54.29 ± 13.29 | 50.0 ± 11.0 | 3/14 | 185/115 | 25‐(OH)D | 7 |
| Agmon‐Levin et al. 24 | Italy, Israel and Spain | Case–control | PBC | CLIA | 79/70 | NA | NA | NA | NA | 25‐(OH)D | 8 |
| Efe et al. 43 | Turkey | Case–control | AIH | Liquid chromatography‐mass spectrometry | 68/34 | 41.9 ± 13.1 | 43.8 ± 11.1 | 19/49 | 7/27 | 25‐(OH)D3 | 8 |
| Cash et al. 20 | England | Case–control | PBC | HPLC | 51/34 | 56.2 ± 10.8 | 54.0 ± 12.2 | 2/49 | 7/27 | Vitamin C, Vitamin A, Vitamin E | 8 |
| Zhang et al. 44 | China | Case–control | PBC | RIA | 10/10 | 47–58 | NA | NA | NA | 1,25‐(OH)2D3 | 7 |
| Biagini 45 | Italy | Case–control | PBC | RIA, HPLC, and fluorescence detecting | 51/102 | 63 ± 13.9 | 63 ± 13 | 8/43 | 16/86 | Folic Acid, Vitamin B12, Vitamin B6,Homocysteine | 8 |
| Floreani 46 | Italy | Case–control | PBC | RIA | 35/33 | 52.5 ± 10 | 51.8 ± 2.22 | 0/35 | 0/33 | 25‐(OH)D | 7 |
| Pemberton et al. 21 | England | Case–control | AIH | Enhanced chemiluminescent technique | 33/35 | 46.5 ± 17.2 | 52.2 ± 12.9 | 5/28 | 7/28 | Vitamin A, Vitamin C, Vitamin E | 6 |
| Aboutwerat et al. 47 | England | Case–control | PBC | Enhanced chemiluminescent technique | 41/34 | 57.9 ± 9.0 | 53.1 ± 11.9 | 4/37 | 7/27 | Vitamin A, Vitamin E | 8 |
| Verma et al. 48 | England | Case–control | PBC | RIA | 37/21 | 60 (45–78) | 58 (36–74) | 0/37 | 0/21 | 25‐(OH)D | 8 |
| Floreani et al. 28 | Italy | Case–control | PBC/PSC | HPLC | 105/105 | 51.8 ± 13.5 | 51.35 ± 10 | 17/88 | 17/88 | Vitamin A, Vitamin E | 8 |
| Kowdley et al. 53 | USA | Case–control | PBC | HPLC | 77/255 | 51 ± 1.1 | NA | 3/74 | NA | Vitamin K1 | 6 |
| Janczewska et al. 49 | Sweden | Case–control | PBC | HPLC | 14/10 | 55 ± 6 | NA | NA | NA | Vitamin A | 6 |
| Nyberg et al. 50 | Sweden | Case–control | PBC | Fluorometry | 44/25 | NA | NA | 7/37 | 6/19 | Vitamin A | 8 |
| Jeffrey et al. 27 | UK | Case–control | PBC | Coiorimetrically | 80/26 | NA | NA | NA | NA | Vitamin E | 6 |
| Fonseca et al. 51 | UK | Case–control | PBC | RIA | 36/40 | 58 (49‐70) | 20‐60 | 4/32 | 20/20 | 25‐(OH)D | 6 |
| Sokol et al. 52 | USA | Case–control | PBC | Microfluorometric method | 42/25 | 49.5 ± 9.1 | 31.6 ± 3.1 | 2/40 | NA | Vitamin E | 6 |
Abbreviations: AIH, autoimmune hepatitis; CLIA, chemical luminescence immunoassay; ELISA, enzyme‐linked immunosorbent assay; HPLC, high‐pressure liquid chromatography; NA, not available; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; RIA, radioimmunoassay.
Table 2.
Results of studies included in the meta‐analysis.
| Year | A | B6 | B9 | B12 | C | 25(OH)D | 25(OH)D3 | 1,25(OH)2D3 | E | Hcy | K1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Huang and Hu 36 | ↓ | ||||||||||
| Wang et al. 11 | ↓ | ||||||||||
| Sen et al. 23 | ↓ | ||||||||||
| Li and Zhou 37 | ↑ | ||||||||||
| Lin et al. 38 | ↓ | ||||||||||
| Zhang 39 | ↓ | ||||||||||
| Tian and Wang 40 | ↓ | ||||||||||
| Li and Song 41 | ↓ | ||||||||||
| Yang et al. 42 | ↓ | ||||||||||
| Agmon‐Levin et al. 24 | ↓ | ||||||||||
| Efe et al. 43 | ↓ | ||||||||||
| Cash et al. 20 | ↓ | ↓ | N | ||||||||
| Zhang et al. 44 | ↓ | ||||||||||
| Biagini 45 | ↓ | ↓ | ↑ | ↑ | |||||||
| Floreani 46 | ↑ | ||||||||||
| Pemberton et al. 21 | ↓ | N | ↓ | ||||||||
| Aboutwerat et al. 47 | ↓ | N | N | ||||||||
| Verma et al. 48 | N | ||||||||||
| Floreani et al. 28 | ↓ | ↓ | |||||||||
| Kowdley et al. 53 | ↓ | ||||||||||
| Janczewska et al. 49 | ↓ | ||||||||||
| Nyberg et al. 50 | ↓ | ||||||||||
| Jeffrey et al. 27 | ↓ | ||||||||||
| Fonseca et al. 51 | N | ||||||||||
| Sokol et al. 52 | N |
Note: N represents no change.
All studies were of high quality, with scores greater than 6 stars. However, none of these studies described the no‐response rate. The quality assessment details for all studies are presented in Supporting Information S1: Table S1.
3.2. Changes of serum vitamins and Hcy levels in patients with AILD
3.2.1. Vitamin A
Six included studies 20 , 21 , 28 , 47 , 49 , 50 investigated vitamin A, with 274 patients in case group and 233 healthy individuals in the control group. As shown in Figure 2, vitamin A levels were lower in AILD patients compared with the control group (SMD = −1.45, 95% CI = [−2.22, −0.67], p = .0002) with high heterogeneity (Q = 69.93, I 2 = 93%, p < .00001; Figure 2). Further subgroup analysis based on disease types showed that serum vitamin A content was decreased by 1.58 (95% CI = [−2.47, −0.70], p = .0005) in PBC/PSC patients. However, heterogeneity is still high across studies (Q = 60.57, I 2 = 93%, p < .00001; Figure 2). Only study 47 reported serum vitamin A changes in AIH patients, and found that serum A content was decreased by 0.79 in them.
Figure 2.
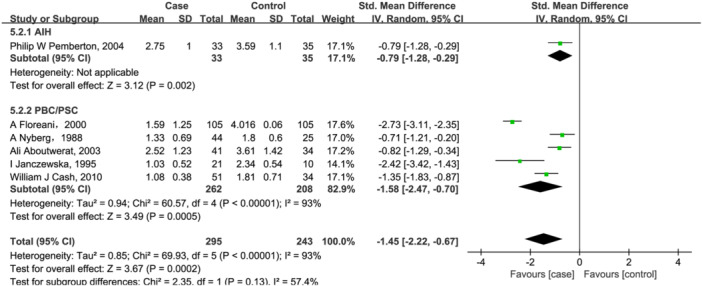
Forest plot of the meta‐analysis on serum vitamin A levels in patients with autoimmune liver disease (AILD). AIH, autoimmune hepatitis; CI, confidence interval; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; SD, standard deviation.
3.2.2. Vitamin B6, B9, B12, and Hcy
Only one study 45 involving 51 PBC patients and 102 healthy individuals examined vitamin B6, B9, B12, and Hcy together. In the study of Biagini et al., 45 compared with the control group, vitamin B6 and B9 were significantly decreased in patients with PBC (vitamin B6: 6.6 [1–20] vs. 10.0 [3–17] pg/mL, p < .001; vitamin B9: 5.3 [1.2‐13.4] vs. 10.7 [5.4‐18.5] ng/mL, p < .001), while serum vitamin B12 levels were significantly elevated in them (335 [201‐977] vs. 304.9 [176‐427.1] pg/mL, p < .05). For Hcy, the content of its fasting state in plasma was also significantly higher in patients than in controls (12.1 ± 8.76 [1.5‐58.8] vs. 9.9 ± 1.7 [6.4‐18.0] µmol/L, p < .001).
3.2.3. Vitamin C
Two studies 20 , 21 evaluated serum vitamin C levels in 84 cases and 69 controls. There is no significant difference between two groups (SMD = −0.58, 95% CI = [−1.34, 0.18], p = .13) with high heterogeneity (Q = 5.28, I 2 = 81%, p = .02; Figure 3). Notably, compared with controls, serum vitamin C was decreased in PBC patients, but not in patients with AIH.
Figure 3.
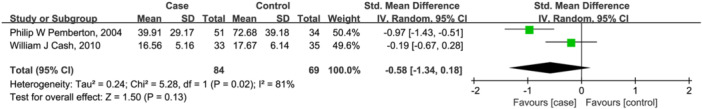
Forest plot of the meta‐analysis on serum vitamin C levels in patients with autoimmune liver disease (AILD). CI, confidence interval; SD, standard deviation.
3.2.4. 25(OH)D, 25(OH)D3, and 1,25(OH)2D3
A total of 10 studies 23 , 24 , 37 , 38 , 39 , 41 , 42 , 46 , 48 , 51 investigated 25(OH)D (655 case/810 controls), and four research works reported 25(OH)D3 11 , 36 , 40 , 43 (470 cases/343 controls) changes in AILD patients. For 25(OH)D, there were no significant differences between two groups both in total analysis (SMD = −1.07, 95% CI = [−2.35, 0.20], p = .1; Figure 4) and subgroup analysis based on disease types (AIH: SMD = −6.34, 95% CI = [−16.97, 4.28], p = .24; PBC: SMD = 0.13, 95% CI = [−1.12, 1.38], p = .84; Figure 4). For PBC patients, we further performed a subgroup analysis based on country. But, as shown in Supporting Information S1: Figure S1, no significant changes of 25(OH)D levels were observed both in studies conducted in China (SMD = −0.34, 95% CI = [−2.76, 2.08], p = .78) and other countries (SMD = 0.59, 95% CI = [−0.55, 1.73], p = .31) in PBC patients. And subgroup analysis also did not reduce heterogeneity between groups, suggesting that confounding factors of unknown origin may be existed.
Figure 4.
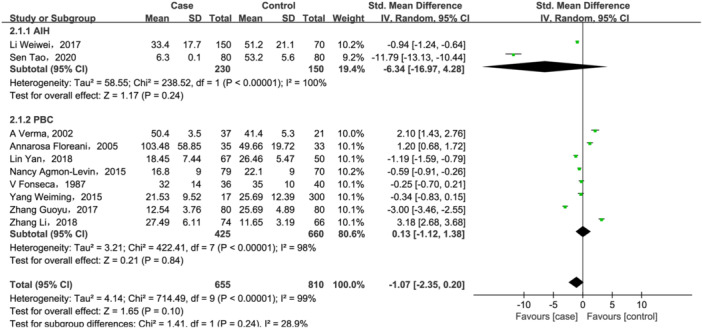
Forest plot of the meta‐analysis on serum vitamin 25(OH)D levels in patients with autoimmune liver disease (AILD). AIH, autoimmune hepatitis; CI, confidence interval; PBC, primary biliary cholangitis; SD, standard deviation.
Nevertheless, as shown in Figure 5, compared with healthy controls, 25(OH)D3 levels were significant lower in AILD (SMD = −2.09, 95% CI = [−2.49, −1.68], p < .00001) with high heterogeneity (Q = 12.84, I 2 = 77%, p = .005). In addition, 25(OH)D3 levels also decreased in both PBC and AIH (SMD = −1.88, 95% CI = [−2.09, −1.66], p < .00001, Figure 5) subgroups, and heterogeneity was totally eliminated after subgroup analysis.
Figure 5.
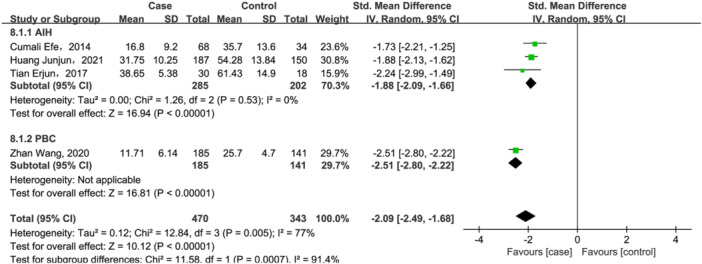
Forest plot of the meta‐analysis on serum vitamin 25(OH)D3 levels in patients with autoimmune liver disease (AILD). AIH, autoimmune hepatitis; CI, confidence interval; PBC, primary biliary cholangitis; SD, standard deviation.
Only one study 44 reported the serum 1,25(OH)2D3 change in ten PBC patients and equivalent controls and found that 1,25(OH)2D3 level was significant lower in patients with PBC (33.28 ± 4.07 vs. 48.25 ± 5.10 ng/ml, p < .01).
3.2.5. Vitamin E
Five studies 20 , 21 , 27 , 28 , 52 were for vitamin E (311 cases/225 controls). But, a significant association was found between lower vitamin E level (SMD = −1.01, 95% CI = [−1.86, −0.34], p = .005, Figure 6) and AILD. While evidence of heterogeneity was observed with vitamin E (Q = 58.35, I 2 = 93%, p < .00001, Figure 6). Further subgroup analysis based on disease types showed that for PBC/PSC patients, serum vitamin E content decreased by 1.19 (95% CI = [−2.12, −0.27], p = .01). Only one study 20 reported serum vitamin E changes in AIH patients, and found that serum vitamin E content decreased by 0.72 in them. However, heterogeneity was not significantly eliminated despite the subgroup analysis described above.
Figure 6.
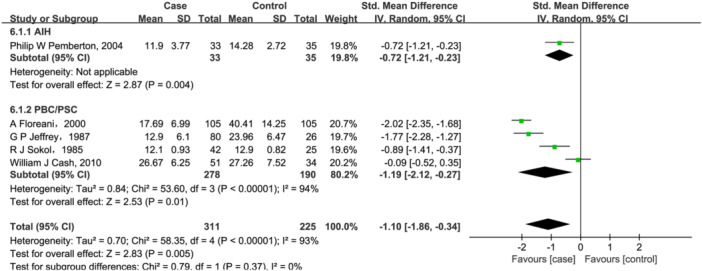
Forest plot of the meta‐analysis on serum vitamin E levels in patients with autoimmune liver disease (AILD). AIH, autoimmune hepatitis; CI, confidence interval; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; SD, standard deviation.
3.2.6. Vitamin K1
Only one eligible study 53 discussed the difference between vitamin K1 in 77 PBC patients and 255 healthy controls. It reported that median plasma vitamin K1 level was significantly lower in PBC patients (0.65 [0.05‐4.13] vs. 0.95 [0.2‐ 4.92] nmol/L; p < .0001). However, more studies and evidence are needed to verify this conclusion.
3.3. Sensitivity analysis
The results of sensitivity analysis demonstrated that the heterogeneity among the included studies did not reverse the overall meta‐analysis results of vitamin A (Figure 7A), vitamin C (Figure 7B), 25(OH)D (Figure 7C), 25(OH)D3 (Figure 7D), and vitamin E (Figure 7E). In addition, there was no obvious influence of one individual study on the pooled SMDs.
Figure 7.
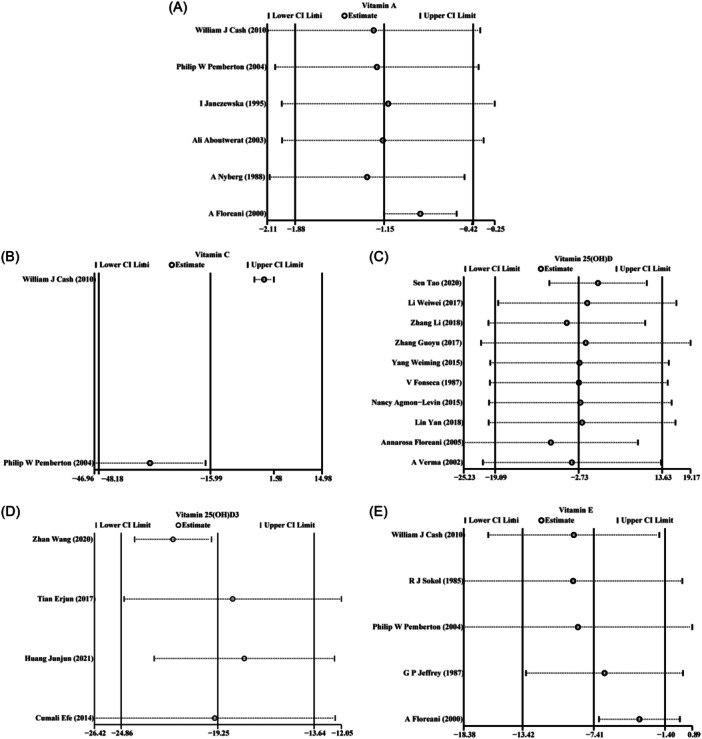
Sensitivity analysis of the meta‐analysis on vitamins levels in patients with autoimmune liver disease (AILD): (A) vitamin A; (B) vitamin C; (C) vitamin 25(OH)D; (D) vitamin 25(OH)D3; (E) vitamin E.
3.4. Publication bias
Only the number of studies about 25(OH)D more than 10, thus a funnel plot, Begg's and Egger's tests were employed to investigate publication bias. However, the shape of the funnel plot for this indicator did not seem symmetrical (Figure 8). Notably, Begg's tests (p = 1.0) and Egger's tests (p = .337) of the meta‐analysis suggested no exsited significant publication bias. These results indicated a potential bias other than publication bias, like the limited involved studies and enrolled patients.
Figure 8.
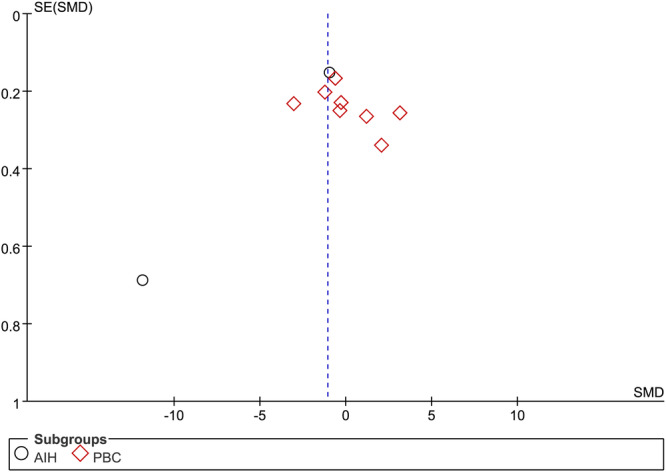
Funnel plot of 10 included studies in this meta‐analysis for serum vitamin 25(OH) D levels in patients with autoimmune liver disease (AILD). AIH, autoimmune hepatitis; PBC, primary biliary cholangitis; SMD, standardized mean difference.
4. DISCUSSION
To the best of our knowledge, this is the first meta‐analysis to systematically explore the association of serum vitamins and Hcy levels with AILD. We found that serum A and E were decreased in both AIH and PBC/PSC, but vitamin C was decreased only in patients with PBC, not AIH. In addition, the content of 25(OH)D3 was decreased both in AIH and PBC. However, 25(OH)D is shown no difference between the two and independent of the country. Only one study that met the inclusion criteria reported B vitamins and Hcy changes, and found that vitamin B6 and B9 were significantly decreased in patients with PBC, while serum vitamin B12 and Hcy levels were significantly elevated in them. One eligible study each confirmed a decrease in plasma vitamin K1 and 1,25(OH)2D3 in patients with PBC. Thus, most vitamins seem to be deficient in AILD.
Our meta‐analysis shows that serum vitamin A is generally decreased in all types of AILD. Vitamin A is mainly stored in the liver. For patients with cholestasis, the major circulating primary BAs and formation of sulfate‐conjugated BAs are abnormally increased, then BAs required for physiological metabolism in the gut are relatively insufficient. It impairs intestinal uptake of fat‐soluble vitamins, including vitamin A, resulting in less retinol stored in the liver and thus less retinol released into the blood. Bile duct injury was found in both PBC and PSC, which was histologically characterized by cholestasis. Therefore, it is not difficult to explain the decrease in serum vitamin A levels in PBC and PSC. For AIH, Pemberton et al. suggested that the decline in vitamin A levels might be associated with decreased vitamin A storage capacity of impaired hepatocytes and activated HSCs. 21 In the liver, vitamin A is involved in many important physiological functions. Vitamin A and its metabolites activate retinoic acid receptor (RAR) and retinoid X receptor (RXR) to generate RAR/RXR heterodimers and regulate BA synthesis and metabolism. 54 In addition, it has been shown that vitamin A affects the expression of fibroblast growth factor 19 (FGF19), and then inhibits the expression of CYP7A1, which is a rate‐limiting enzyme of bile salt synthesis. 55 Vitamin A supplementation may be beneficial for inducing bile excretion, alleviating cholestasis, and inhibiting HSC activation. However, excessive intake of vitamin A leads to acute and chronic toxic reactions, even causes cirrhosis and veno occlusive disease. 56 Thus, the drug dosage of vitamin A needed to be further studied.
B vitamins are essential for promoting metabolism in the liver by converting sugars, fats and proteins into calories. Among them, vitamin B6, B9 and B12 are involved in methionine metabolism. 57 Biagini et al. 45 found a decrease in vitamin B6 and B9, particularly vitamin B9, which was considered to be associated with insufficient dietary intake and impairment of the folate enterohepatic circulation in PBC patients. Hcy is an important intermediate product of methionine and cysteine metabolism, and its metabolism is dependent on sufficient supplies of vitamin B9 and B12. These B vitamins deficiencies block the Hcy remethylation pathway, resulting in increased Hcy level. 58 As a vascular damaging amino acid, Hcy is a risk factor for many diseases by regulating the coagulation and anticoagulation disfunction of endothelial cells, low density lipoprotein cholesterol oxidation, vascular sclerosis, inflammatory response, and abnormal metabolism of sulfur compounds. 59 Amounts of evidence show that Hcy level is positively correlated with the severity of liver injury, 17 , 18 which is also supported by our conclusion.
Evidence have shown that vitamin C improves liver damage, possibly by suppressing inflammatory response and oxidative damage. 60 , 61 Our study found that vitamin C was decreased only in patients with PBC, but did not differ in patients with AIH. But the exact reason is not clear. In addition, with limited involved studies, such conclusion may not be very reliable.
Vitamin D plays a role in the body metabolism in different forms. Neither endogenous cholecalciferol converted by 7‐dehydrocholesterol stored in human epidermal keratinocytes, nor vitamin D2 and D3 ingested from food, are biologically active. They must work through converting to 25(OH)D2 and 25(OH)D3 by the 25‐hydroxylase system in the hepatocyte microsomes and then to 1,25(OH)2D3 under catalysis of the 25‐hydroxyvitamin D‐1 alpha‐hydroxylase in the proximal convoluted tubules of the kidney. 62 Both 25(OH)D2 and 25(OH)D3 are collectively known as 25(OH)D, but 25(OH)D2 content is relatively low and difficult to be distinguished. Compared with 25(OH)D3, 1,25(OH)2D3 possesses the strongest activity against rickets, and the role of its metabolites in regulating calcium and phosphorus is higher than 25(OH)D3 200 times. 63 Nevertheless, 1,25(OH)2D3 is homeostatic regulated by serum calcium and phosphorus concentration, parathyroid hormone, as well as calcitonin, and it has a short half‐life (4‐8 h). 63 Thus, 25(OH)D3 is the main storage form and commonly used to reflect the nutritional status of vitamin D in the body. Since 25‐hydroxylase is mainly present in the hepatocyte microsomes, vitamin D metabolism is closely related to liver diseases. Vitamin D receptor has been found on the surface of almost all cells of the immune system, which may partly explain why abnormal vitamin D metabolism affect the development of some autoimmune diseases. It has been demonstrated that the polymorphisms of vitamin D receptor gene are significantly associated with increased individual susceptibility to develop chronic AILD. 64 , 65 For PBC, lacking bile salts affects the absorption of fat‐soluble vitamins and fat digestion, which may be one of the causes of vitamin D deficiency. AILD tends to occur in female. Compared with men, female are more likely to suffer from vitamin D deficiency due to the enhancement of vitamin D receptor gene expression and transcription by estrogen. 66 In the PSC, vitamin D could reverse CD28‐T cells mediated inflammatory response, thereby reducing bile ducts damage caused by high levels of TNFα and IFNγ cytokines. 67 The degree of vitamin D deficiency is positively correlated with the severity and progression of chronic liver disease. Paternostro et al. 68 found that when vitamin D ≤ 10 ng/ml, the risk of adverse events, such as ascites, portal hypertension and primary hepatocarcinoma as well as the mortality in patients with cirrhosis increased. Ebadi et al. 69 also indicated that severe vitamin D deficiency was independently associated with a higher risk of developing liver cirrhosis (HR = 3.40, 95% CI: 1.30–8.87; p = .01) and liver‐related mortality or the need for liver transplantation (HR = 5.26, 95% CI 1.54–18.0; p = .008) in AIH. Vitamin D supplementation may improve vitamin D levels and reduce bone loss in patients with AILD, 70 but the long‐term prognosis is uncertain because of lack of evidence.
The main active substance of vitamin E is alpha‐tocopherol, which is an important antioxidant against reactive oxygen species (ROS) in the body. Overloaded ROS can cause oxidative stress‐related damage, which is one of the pathogenic bases for chronicity and progression of liver diseases. Vitamin E deficiency reduces free radical scavenging, thus accelerating liver disease deterioration. 26 , 61 Moreover, vitamin E is involved in regulation of the expression of genes controlling cholesterol homeostasis, phospholipid metabolism, and lipid uptake. 71 The vitamin E also plays a critical role in regulating the inflammatory response by inhibiting the expressions of IL‐1β, MCP and IL‐6, thus attenuating liver fibrosis. 72 Some PBC patients with low serum vitamin E levels exhibit clinically significant psychomotor dysfunction. 13 In addition, vitamin E supplementation contributes to inhibiting neutrophil chemotaxis and may improve digestive function in children with chronic cholestasis. 73 Consistent with previous results, our study confirms that vitamin E deficiency is prevalent in AILD patients. But the jury is still out on how to supplement vitamin E in them, thus further research is necessarily needed.
There are two main forms of vitamin K: phytoformine or phylloquinone (vitamin K1) and menaquinone (vitamin K2). Vitamin K1 is primarily derived from the diet, while vitamin K2 is synthesized by the intestinal bacterial flora. Vitamin K1 can be converted to vitamin K2. 74 As with other fat‐soluble vitamins, vitamin K deficiency is quite common in patients with cholestasis due to reduced BAs in the gut. And the decrease in vitamin K1 was accompanied by reductions in serum vitamin A and E levels in PBC patients, which may be partly attributed to decreased intestinal absorption. In addition, since patients with liver diseases are prone to peritonitis, the increased use of antibiotics can lead to a decrease in the production of vitamin K2 by the gastrointestinal flora. 75 Vitamin K levels are closely related to the stage of liver disease, which is owing to a significant decrease in the ability of hepatocytes to synthesize coagulation factors in severe liver diseases. Therefore, vitamin K is recommended for the correction of prolonged PT and the prevention of bleeding in patients with cirrhosis or liver failure. 76 , 77 However, Strople et al. 31 found that despite vitamin K supplementation, ongoing vitamin K deficiency was still common in cholestatic liver disease. Aldrich et al. 33 reviewed amounts of studies and concluded that routine use of vitamin K had no benefit in the correction of cirrhosis‐related coagulation dysfunction. Therefore, the level of vitamin K in AILD and whether supplementation of vitamin K is worthwhile remains a matter of discussion and clinical practice.
This meta‐analysis has several strengths. Our study systematically explores the association of multiple vitamins and Hcy levels with AILD, with subgroup analyses as far as possible. Secondly, we have included domestic and foreign studies on the premise of quality assurance, which is relatively representative. In addition, our control groups were healthy individuals, so as to minimize the interference of other diseases.
However, these limitations should not be ignored in our meta‐analysis. First, the limited number of studies and small sample size of some studies may increase heterogeneity. Second, despite the subgroup analysis, the heterogeneity of some overall effect results is still high, and the possible sources of heterogeneity may include severe stages of AILD, gender, and so forth. It has been demonstrated that the level of 25(OH)D3 in peripheral blood of AIH patients in the active inflammatory stage was significantly lower than that in the remission stage. 36 Besides, the mean level of 25(OH)D in advanced stage of PBC patients was lower than that in early stage patients. 38 However, due to the limited data, it cannot be quantitatively analyzed. Third, there are no data on the association between other vitamins and AILD due to no studies that meet our requirements. Finally, some data are obtained from combination and transformation, and certain errors may be unavoidable. Therefore, more studies with large sample even RCT studies of vitamins supplementation, are needed in the future to verify the association between vitamins and AILD.
n conclusion, the current study shows that most vitamins seem to be deficient in AILD. Vitamins, like A, E, and 25(OH)D3, were decreased in both AIH and PBC/PSC, but vitamin C was decreased only in patients with PBC, not AIH. Vitamin B6, B9, K1, and 1,25(OH)2D3 were also significantly decreased in patients with PBC, but not supported by sufficient data. Serum vitamin B12 and Hcy levels were significantly elevated in them. Further clinical trials involving more patients are needed to verify these findings.
AUTHOR CONTRIBUTIONS
The protocol of this meta‐analysis was designed by Jiahuan Li and Xiaoling Deng. Literature retrieval, data extraction and analysis were performed by Li Jiahuan and Shan Tian. Data confirmation and quality assessment of included studies were conducted by Bai Ci and Yuwen Xi. The manuscript was written by Li Jiahuan and revised by Xiaoling Deng. Any disagreement regarding the formation process of this meta‐analysis was resolved by consensus or adjudicated by Xiaoling Deng. All authors approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
None declared.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
This work was financially supported the Natural Science Foundation of Hubei Province (nos. 2020CFB774).
Li J, Tian S, Ci B, Xi Y, Deng X. Serum vitamins and homocysteine levels in autoimmune liver disease: a systematic review and meta‐analysis. Immun Inflamm Dis. 2024;12:e1258. 10.1002/iid3.1258
Jiahuan Li and Shan Tian contributed equally to this work.
DATA AVAILABILITY STATEMENT
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
REFERENCES
- 1. Carbone M, Neuberger JM. Autoimmune liver disease, autoimmunity and liver transplantation. J Hepatol. 2014;60:210‐223. [DOI] [PubMed] [Google Scholar]
- 2. Czaja AJ. Hepatocellular carcinoma and other malignancies in autoimmune hepatitis. Dig Dis Sci. 2013;58:1459‐1476. [DOI] [PubMed] [Google Scholar]
- 3. Adam R, Karam V, Delvart V, et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J Hepatol. 2012;57:675‐688. [DOI] [PubMed] [Google Scholar]
- 4. Duclos‐Vallee JC, Sebagh M. Recurrence of autoimmune disease, primary sclerosing cholangitis, primary biliary cirrhosis, and autoimmune hepatitis after liver transplantation. Liver Transpl. 2009;15(suppl 2):S25‐S34. [DOI] [PubMed] [Google Scholar]
- 5. Carbone M, Mells GF, Alexander GJ, et al. Calcineurin inhibitors and the IL12A locus influence risk of recurrent primary biliary cirrhosis after liver transplantation. Am J Transplant (AJT). 2013;13:1110‐1111. [DOI] [PubMed] [Google Scholar]
- 6. Deng X, Li J, Hou S, Ci B, Liu B, Xu K. Prevalence and impact of sjögren's syndrome in primary biliary cholangitis: a systematic review and meta‐analysis. Ann Hepatol. 2022;27:100746. [DOI] [PubMed] [Google Scholar]
- 7. Fraga M, Fournier N, Safroneeva E, et al. Primary sclerosing cholangitis in the Swiss inflammatory bowel disease cohort study: prevalence, risk factors, and long‐term follow‐up. Eur J Gastroenterol Hepatol. 2017;29:91‐97. [DOI] [PubMed] [Google Scholar]
- 8. Lamba M, Ngu JH, Stedman CAM. Trends in incidence of autoimmune liver diseases and increasing incidence of autoimmune hepatitis. Clin Gastroenterol Hepatol. 2021;19:573‐579.e1. [DOI] [PubMed] [Google Scholar]
- 9. Czaja AJ. Global disparities and their implications in the occurrence and outcome of autoimmune hepatitis. Dig Dis Sci. 2017;62:2277‐2292. [DOI] [PubMed] [Google Scholar]
- 10. Sucher E, Sucher R, Gradistanac T, Brandacher G, Schneeberger S, Berg T. Autoimmune hepatitis‐immunologically triggered liver pathogenesis‐diagnostic and therapeutic strategies. J Immunol Res. 2019;2019:1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Z, Peng C, Wang P, et al. Serum vitamin D level is related to disease progression in primary biliary cholangitis. Scand J Gastroenterol. 2020;55:1333‐1340. [DOI] [PubMed] [Google Scholar]
- 12. Chaves GV, Peres WAF, Gonçalves JC, Ramalho A. Vitamin A and retinol‐binding protein deficiency among chronic liver disease patients. Nutrition. 2015;31:664‐668. [DOI] [PubMed] [Google Scholar]
- 13. Arria A, Tarter R, Warty V, Van Thiel D. Vitamin E deficiency and psychomotor dysfunction in adults with primary biliary cirrhosis. Am J Clin Nutr. 1990;52:383‐390. [DOI] [PubMed] [Google Scholar]
- 14. Wallace M, Friedman S, Mann D. Emerging and disease‐specific mechanisms of hepatic stellate cell activation. Semin Liver Dis. 2015;35:107‐118. [DOI] [PubMed] [Google Scholar]
- 15. Katre P, Bhat D, Lubree H, et al. Vitamin B12 and folic acid supplementation and plasma total homocysteine concentrations in pregnant Indian women with low B12 and high folate status. Asia Pac J Clin Nutr. 2010;19:335‐343. [PubMed] [Google Scholar]
- 16. El‐Khodary NM, Dabees H, Werida RH. Folic acid effect on homocysteine, sortilin levels and glycemic control in type 2 diabetes mellitus patients. Nutr Diabetes. 2022;12:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pastore A, Alisi A, di Giovamberardino G, et al. Plasma levels of homocysteine and cysteine increased in pediatric NAFLD and strongly correlated with severity of liver damage. Int J Mol Sci. 2014;15:21202‐21214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tripathi M, Singh BK, Zhou J, et al. Vitamin B(12) and folate decrease inflammation and fibrosis in NASH by preventing syntaxin 17 homocysteinylation. J Hepatol. 2022;77:1246‐1255. [DOI] [PubMed] [Google Scholar]
- 19. Padayatty S, Levine M. Vitamin C: the known and the unknown and Goldilocks. Oral Dis. 2016;22:463‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cash WJ, Mccance DR, Young IS, et al. Primary biliary cirrhosis is associated with oxidative stress and endothelial dysfunction but not increased cardiovascular risk. Hepatol Res. 2010;40:1098‐1106. [DOI] [PubMed] [Google Scholar]
- 21. Pemberton PW, Aboutwerat A, Smith A, Burrows PC, McMahon RFT, Warnes TW. Oxidant stress in type I autoimmune hepatitis: the link between necroinflammation and fibrogenesis? Biochim Biophys Acta (BBA) ‐ Mol Basis Dis. 2004;1689:182‐189. [DOI] [PubMed] [Google Scholar]
- 22. Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diab Endocrinol. 2014;2:76‐89. [DOI] [PubMed] [Google Scholar]
- 23. Shen T, Hong Z, Zhao Q, et al. Correlation of vitamin D with inflammatory factors, oxidative stress and T cell subsets in patients with autoimmune hepatitis. Exp Ther Med. 2020;19:3419‐3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Agmon‐Levin N, Kopilov R, Selmi C, et al. Vitamin D in primary biliary cirrhosis, a plausible marker of advanced disease. Immunol Res. 2015;61:141‐146. [DOI] [PubMed] [Google Scholar]
- 25. Guo GY, Shi YQ, Wang L, et al. Serum vitamin D level is associated with disease severity and response to ursodeoxycholic acid in primary biliary cirrhosis. Aliment Pharmacol Ther. 2015;42:221‐230. [DOI] [PubMed] [Google Scholar]
- 26. Bril F, Biernacki DM, Kalavalapalli S, et al. Role of vitamin E for nonalcoholic steatohepatitis in patients with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2019;42:1481‐1488. [DOI] [PubMed] [Google Scholar]
- 27. Jeffrey GP, Muller DPR, Burroughs AK, et al. Vitamin E deficiency and its clinical significance in adults with primary biliary cirrhosis and other forms of chronic liver disease. J Hepatol. 1987;4:307‐317. [DOI] [PubMed] [Google Scholar]
- 28. Floreani A, Baragiotta A, Martines D, Naccarato D'O. Plasma antioxidant levels in chronic cholestatic liver diseases. Aliment Pharmacol Ther. 2000;14:353‐358. [DOI] [PubMed] [Google Scholar]
- 29. Sultana H, Komai M, Shirakawa H. The role of vitamin K in cholestatic liver disease. Nutrients. 2021;13:2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Negrev NN, Radev RZ, Velikova MS, Anogeianaki A. Effects of the hormones of the thyroid axis on the vitamin k‐dependent plasma factors of blood coagulation (II, VII, IX, and X). Int J Immunopathol Pharmacol. 2008;21:221‐226. [DOI] [PubMed] [Google Scholar]
- 31. Strople J, Lovell G, Heubi J. Prevalence of subclinical vitamin K deficiency in cholestatic liver disease. J Pediatr Gastroenterol Nutr. 2009;49:78‐84. [DOI] [PubMed] [Google Scholar]
- 32. Saja MF, Abdo AA, Sanai FM, Shaikh SA, Gader AGMA. The coagulopathy of liver disease: does vitamin K help? Blood Coagul Fibrinolysis. 2013;24:10‐17. [DOI] [PubMed] [Google Scholar]
- 33. Aldrich SM, Regal RE. Routine use of vitamin K in the treatment of Cirrhosis‐Related coagulopathy: is it A‐O‐K? Maybe not, we say. P & T. 2019;44:131‐136. [PMC free article] [PubMed] [Google Scholar]
- 34. Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25:603‐605. [DOI] [PubMed] [Google Scholar]
- 36. Huang JJ, Hu D. The level of 25‐dihydroxyvitamin D3 in peripheral blood and its correlation with liver function in patients with autoimmune hepatitis. J North Sichuan Med Coll. 2021;36:1657‐1660. [Google Scholar]
- 37. Zhang Li, Yu M, Zhou Y, et al. Diagnostic effect of 25‐(OH)D on patients with biliary cirrhosis. Hainan Med J. 2018;29:3411‐3413. [Google Scholar]
- 38. Lin Y, Fu HY, Zheng W, et al. The potential clinical value of serum bone metabolism level in the monitoring of primary biliary cirrhosis with osteoporosis. J Pract Med. 2018;34:2236‐2240. [Google Scholar]
- 39. Zhang GY. Study on clinical significance of combined detection of LBP, 25‐(OH)D and MIP‐3 αin diagnostic process of PBC. Shaanxi Med J\. 2017;46:1659‐1661. [Google Scholar]
- 40. Tian EJ, Wang H. Vitamin D‐induced regulatory T cell differentiation and its role in the pathogenesis of autoimmune hepatitis. J Xinxiang Med Univ. 2017;34:592‐595. [Google Scholar]
- 41. Li WW, Song SW. Correiation between 25‐hydroxy vitamin D and autoimmune hepatitis. J Xinxiang Med Univ. 2017;34:1080‐1084. [Google Scholar]
- 42. Yang WM, Xin GJ, Ding SN. Levels and clinical significance of serum 25‐hydroxy vitamin D in patients with chronic liver disease. J Clin Hepatol. 2015;31:754‐757. [Google Scholar]
- 43. Efe C, Kav T, Aydin C, et al. Low serum vitamin D levels are associated with severe histological features and poor response to therapy in patients with autoimmune hepatitis. Dig Dis Sci. 2014;59:3035‐3042. [DOI] [PubMed] [Google Scholar]
- 44. Zhang XR, Cai HP, Tan LY, et al. Expression and significance of vitamin D3 receptor in peripheral blood lymphocytes of patients with primary biliary cirrhosis. Chin J Digest. 2006;26:638‐639. [Google Scholar]
- 45. Biagini MR. Hyperhomocysteinemia and hypercoagulability in primary biliary cirrhosis. World J Gastroenterol. 2006;12:1607‐1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Floreani A. Is osteoporosis a peculiar association with primary biliary cirrhosis? World J Gastroenterol. 2005;11:5347‐5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Aboutwerat A, Pemberton PW, Smith A, et al. Oxidant stress is a significant feature of primary biliary cirrhosis. Biochim Biophys Acta (BBA) ‐ Mol Basis Dis. 2003;1637:142‐150. [DOI] [PubMed] [Google Scholar]
- 48. Verma A, Maxwell JD, Ang L, et al. Ursodeoxycholic acid enhances fractional calcium absorption in primary biliary cirrhosis. Osteoporos Int. 2002;13:677‐682. [DOI] [PubMed] [Google Scholar]
- 49. Janczewska I, Ericzon BG, Eriksson LS. Influence of orthotopic liver transplantation on serum vitamin A levels in patients with chronic liver disease. Scand J Gastroenterol. 1995;30:68‐71. [DOI] [PubMed] [Google Scholar]
- 50. Nyberg A, Berne B, Nordlinder H, et al. Impaired release of vitamin A from liver in primary biliary cirrhosis. Hepatology. 1988;8:136‐141. [DOI] [PubMed] [Google Scholar]
- 51. Fonseca V, Epstein O, Gill DS, et al. Hyperparathyroidism and low serum osteocalcin despite vitamin D replacement in primary biliary cirrhosis. J Clinical Endocrinol Metab. 1987;64:873‐877. [DOI] [PubMed] [Google Scholar]
- 52. Sokol R, Balistreri W, Hoofnagle J, Jones E. Vitamin E deficiency in adults with chronic liver disease. Am J Clin Nutr. 1985;41:66‐72. [DOI] [PubMed] [Google Scholar]
- 53. Kowdley KV, Emond MJ, Sadowski JA, Kaplan MM. Plasma vitamin K1 level is decreased in primary biliary cirrhosis. Am J Gastroenterol. 1997;92:2059‐2061. [PubMed] [Google Scholar]
- 54. Li B, Cai SY, Boyer JL. The role of the retinoid receptor, RAR/RXR heterodimer, in liver physiology. Biochim Biophys Acta (BBA) ‐ Mol Basis Dis. 2021;1867:166085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jahn D, Sutor D, Dorbath D, et al. Farnesoid X receptor‐dependent and ‐independent pathways mediate the transcriptional control of human fibroblast growth factor 19 by vitamin A. Biochim Biophys Acta (BBA) ‐ Gene Regul Mech. 2016;1859:381‐392. [DOI] [PubMed] [Google Scholar]
- 56. Geubel A, De Galocsy C, Alves N, Rahier J, Dive C. Liver damage caused by therapeutic vitamin A administration: estimate of dose‐related toxicity in 41 cases. Gastroenterology. 1991;100:1701‐1709. [DOI] [PubMed] [Google Scholar]
- 57. Halsted CH. B‐Vitamin dependent methionine metabolism and alcoholic liver disease. Clin Chem Lab Med. 2013;51:457‐465. [DOI] [PubMed] [Google Scholar]
- 58. Brütting C, Hildebrand P, Brandsch C, Stangl GI. Ability of dietary factors to affect homocysteine levels in mice: a review. Nutr Metab. 2021;18:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kumar A, Palfrey HA, Pathak R, Kadowitz PJ, Gettys TW, Murthy SN. The metabolism and significance of homocysteine in nutrition and health. Nutr Metab. 2017;14:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Milošević MD, Paunović MG, Matić MM, Ognjanović BI, Saičić ZS. Role of selenium and vitamin C in mitigating oxidative stress induced by fenitrothion in rat liver. Biomed Pharmacother. 2018;106:232‐238. [DOI] [PubMed] [Google Scholar]
- 61. Ivancovsky‐Wajcman D, Fliss‐Isakov N, Salomone F, et al. Dietary vitamin E and C intake is inversely associated with the severity of nonalcoholic fatty liver disease. Dig Liver Dis. 2019;51:1698‐1705. [DOI] [PubMed] [Google Scholar]
- 62. Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21:319‐329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bikle D, Christakos S. New aspects of vitamin D metabolism and action ‐ addressing the skin as source and target. Nat Rev Endocrinol. 2020;16:234‐252. [DOI] [PubMed] [Google Scholar]
- 64. Tanaka A, Nezu S, Uegaki S, et al. Vitamin D receptor polymorphisms are associated with increased susceptibility to primary biliary cirrhosis in Japanese and Italian populations. J Hepatol. 2009;50:1202‐1209. [DOI] [PubMed] [Google Scholar]
- 65. Li Y, Tang Y, Shi Y, et al. Polymorphisms in the vitamin D receptor gene and risk of primary biliary cirrhosis: a meta‐analysis. J Gastroenterol Hepatol. 2014;29:706‐715. [DOI] [PubMed] [Google Scholar]
- 66. Nashold FE, Spach KM, Spanier JA, Hayes CE. Estrogen controls vitamin D3‐mediated resistance to experimental autoimmune encephalomyelitis by controlling vitamin D3 metabolism and receptor expression. The J Immunol. 2009;183:3672‐3681. [DOI] [PubMed] [Google Scholar]
- 67. Liaskou E, Jeffery LE, Trivedi PJ, et al. Loss of CD28 expression by liver‐infiltrating T cells contributes to pathogenesis of primary sclerosing cholangitis. Gastroenterology. 2014;147:221‐232.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Paternostro R, Wagner D, Reiberger T, et al. Low 25‐OH‐vitamin D levels reflect hepatic dysfunction and are associated with mortality in patients with liver cirrhosis. Wien Klin Wochenschr. 2017;129:8‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ebadi M, Bhanji RA, Mazurak VC, et al. Severe vitamin D deficiency is a prognostic biomarker in autoimmune hepatitis. Aliment Pharmacol Ther. 2019;49:173‐182. [DOI] [PubMed] [Google Scholar]
- 70. Floreani A, Carderi I, Ferrara F, et al. A 4‐year treatment with clodronate plus calcium and vitamin D supplements does not improve bone mass in primary biliary cirrhosis. Dig Liver Dis. 2007;39:544‐548. [DOI] [PubMed] [Google Scholar]
- 71. El Hadi H, Vettor R, Rossato M. Vitamin E as a treatment for nonalcoholic fatty liver disease: reality or myth? Antioxidants. 2018;7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Baig MT, Ghufran H, Mehmood A, Azam M, Humayun S, Riazuddin S. Vitamin E pretreated wharton's jelly‐derived mesenchymal stem cells attenuate CCl(4)‐induced hepatocyte injury in vitro and liver fibrosis in vivo. Biochem Pharmacol. 2021;186:114480. [DOI] [PubMed] [Google Scholar]
- 73. Sokol RJ, Heubi JE, Mcgraw C, Balistreri WF. Correction of vitamin E deficiency in children with chronic cholestasis. II. effect on gastrointestinal and hepatic function. Hepatology. 1986;6:1263‐1269. [DOI] [PubMed] [Google Scholar]
- 74. Mladěnka P, Macáková K, Kujovská Krčmová L, et al. Vitamin K ‐ sources, physiological role, kinetics, deficiency, detection, therapeutic use, and toxicity. Nutr Res. 2022;80:677‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chen LJ, Hsiao FY, Shen LJ, et al. Use of Hypoprothrombinemia‐inducing cephalosporins and the risk of hemorrhagic events: a nationwide nested case‐control study. PLoS One. 2016;11:e158407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shah NL, Northup PG, Caldwell SH. A clinical survey of bleeding, thrombosis, and blood product use in decompensated cirrhosis patients. Ann Hepatol. 2012;11:686‐690. [PubMed] [Google Scholar]
- 77. Xiong Z, Liu Y, Chang T, et al. Effect of vitamin K1 on survival of patients with chronic liver failure: a retrospective cohort study. Medicine. 2020;99:e19619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.


