Abstract
Introduction:
Pelvic recurrence is a frequent pattern of relapse for women with endometrial cancer. A randomized trial compared progression-free survival following treatment with radiation therapy alone as compared to concurrent chemotherapy.
Methods:
Between February 2008 and August 2020, 165 patients were randomized 1:1 to receive either radiation treatment alone or a combination of chemotherapy and radiation treatment. The primary objective of the study was to determine whether chemoradiation therapy was more effective than radiation therapy alone at improving progression-free survival.
Results:
The majority of patients had low-grade (1 or 2) endometrioid histology (82%) and recurrences confined to the vagina (86%). External beam with either 3D or IMRT technique was followed by a boost delivered with brachytherapy or external beam. Patients randomized to receive chemotherapy were treated with weekly cisplatin (40 mg/m2). Rates of acute toxicity were higher in patients treated with chemoradiation as compared to radiation treatment alone. Median PFS was longer for patients treated with radiation therapy alone as compared to chemotherapy and radiation (Median PFS was not reached for RT vs 73 months for CRT, HR of 1.25 (CI: 0.75 – 2.07). At three years, 73% of patients treated definitively with radiation and 62% of patients treated with chemoradiation were alive and free of disease progression.
Conclusion:
Excellent outcomes can be achieved for women with localized recurrences of endometrial cancer when treated with radiation therapy. The addition of chemotherapy does not improve PFS for patients treated with definitive radiation therapy for recurrent endometrial cancer and increases acute toxicity. Patients with low-grade and vaginal recurrences who constituted the majority of those enrolled are best treated with radiation therapy alone.
INTRODUCTION
Endometrial cancer is the most common gynecologic cancer in the United States, predicted to affect 65,950 women in the United States annually.1 Hysterectomy is the standard of care for upfront treatment of endometrial cancer followed by adjuvant therapy based on pathologic findings. The majority of women are subsequently cured from their disease. However, for those women who do recur, isolated relapse in the lymph nodes and/or the vagina, is a frequent pattern of recurrence.2–4
Radiation therapy is established as a curative treatment modality for patients with endometrial cancer recurrences confined to the pelvis. Long term survival rates have been reported to range between 50–70%.3,5,6 The treatment approach requires delivering a definitive dose of radiation therapy to gross disease, comparable to doses delivered for definitive treatment of intact cervical cancer. This is accomplished with an initial external beam field that includes the vagina and the draining lymphatics and facilitates regression of the recurrent tumor. An additional boost is delivered to residual disease after external beam, which is ideally accomplished with brachytherapy, either intracavitary or interstitial. The added benefit of chemotherapy to this established radiation regimen has not been previously tested for patients with recurrences of endometrial cancer confined to the pelvis. Systemic therapy is efficacious in more advanced endometrial cancers, and chemotherapy has been shown to improve outcomes when combined with radiation treatment in other disease sites, such as cervical and head and neck cancer7,8 Thus, GOG238 was designed to determine if the addition of concurrent chemotherapy to radiation improved progression free survival. Patients with localized recurrences of endometrial cancer were randomized to radiation treatment including external beam radiation followed by a brachytherapy or external beam boost or the same regimen delivered with concurrent cisplatin.
MATERIALS/METHODS
Patient Selection and Accrual.
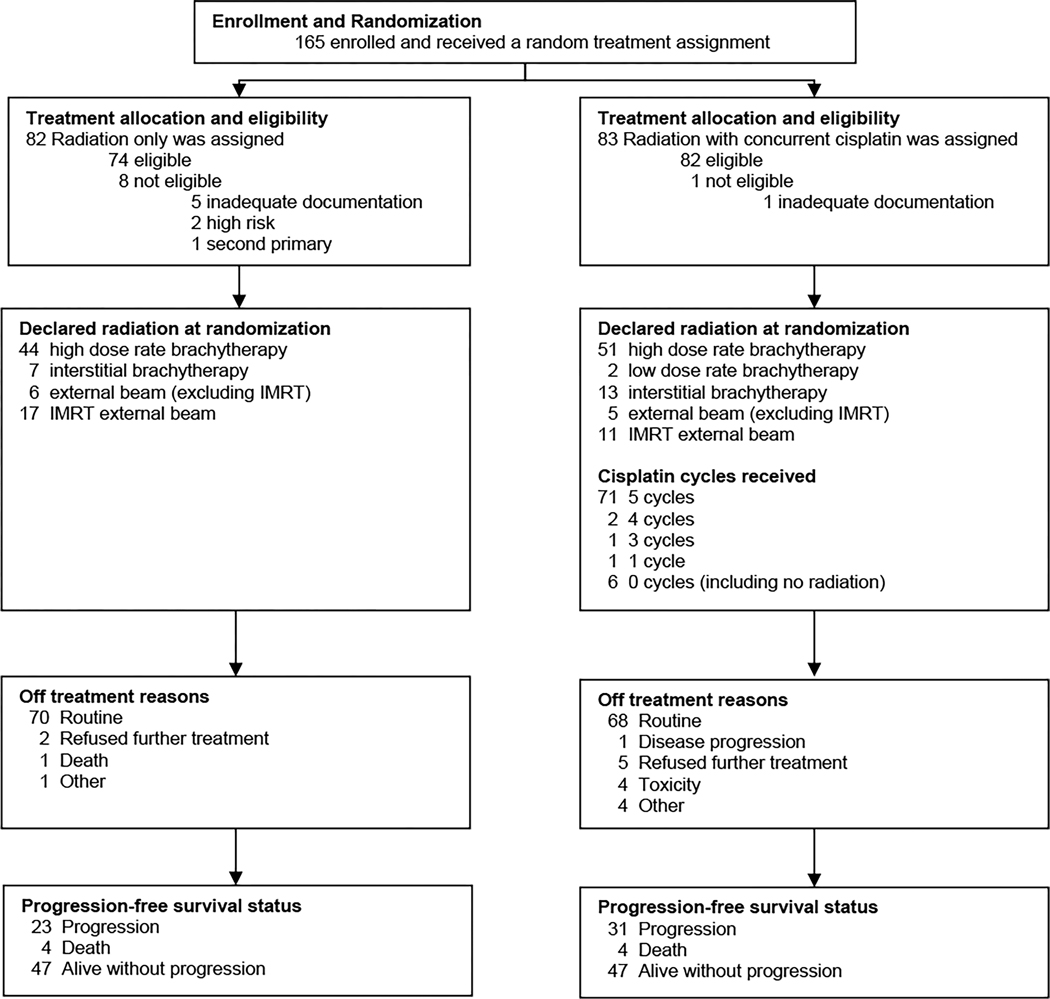
This prospective clinical trial enrolled patients with a diagnosis of locally recurrent endometrial cancer that was confined to the vagina and/or lymph nodes and who were planned to undergo definitive therapy with radiation. Participants were recruited from the members of NRG Oncology after institutional review board approval at each center. All participants provided written informed consent before registration and were to receive protocol-specified care and follow-up at a member site. A total of 165 patients were enrolled between February 2008 and August 2020. Nine patients were excluded for ineligibility (Figure 1), resulting in an intent-to-treat sample of 156 patients for analysis. Six eligible patients on the radiation with concurrent cisplatin arm were never treated (Figure 1). Four patients on the radiation with concurrent cisplatin arm came off the study due to toxicity (Figure 1).
Figure 1. CONSORT diagram.
CONSORT diagram depicting the flow of participants through the study. The study design and report are in accordance with the Consolidate Standard of Reporting Trials (CONSORT) statement.
Treatment.
All patients who received treatment in the study received external beam radiation therapy to the whole pelvis followed by a boost to the recurrent tumor. External beam was prescribed to 4500 cGy in 25 fractions (180 cGy/fraction) to target gross disease including vagina and pelvic lymph nodes. Patients with tumors involving the distal vagina also received radiation to the bilateral inguino-femoral lymph node regions. Concurrent cisplatin was delivered at 40 mg/m2 for those on the chemotherapy arm for a planned total of 5 cycles. In all 87% of patients on the chemotherapy arm received 5 cycles with the rest receiving less than 5 (Supplementary Table 1).
Radiation treatment boost:
Following initial external beam radiation, a boost was delivered with either brachytherapy or external beam based on the extent of disease. Boost technique was declared at the time of enrollment based on the criteria described in the protocol. The most common brachytherapy approach was HDR intracavitary brachytherapy (Table 2). The smallest volume recurrences, those which were limited to less than 5 mm depth were recommended to be treated with LDR or HDR intracavitary brachytherapy. Intracavitary brachytherapy was performed by placing an applicator within the vagina, using vaginal ovoids or vaginal cylinders without the use of interstitial needles. LDR intracavitary was prescribed to 30 Gy to a depth of 0.5 cm. This prescription was intended to deliver a dose to the GTV, as defined by physical exam and/or CT scan, of a minimum of 75 Gy including the external beam dose. A minimum mucosal margin of 3 cm from the tumor was recommended. Lesions of less than 5 mm thickness could also be treated with HDR intracavitary techniques. Three fractions of 7 Gy were prescribed to a depth of 5 mm with at least 3 cm of distal margin covering the extent of the disease to achieve a similar effective dose as was prescribed for LDR intracavitary.
Table 2.
Radiation Boost Technique.
| Treatment Assignment | |||
|---|---|---|---|
| Declared Radiation Boost Type | Radiation Only | Radiation+Cisplatin | Chi-squared Test P-value |
| High dose rate brachytherapy | 44 (59.5%) | 51 (62.2%) | 0.26 |
| Low dose rate brachytherapy | 0 (0.0%) | 2 (2.4%) | |
| Interstitial brachytherapy | 7 (9.5%) | 13 (15.9%) | |
| External beam (not IMRT) | 6 (8.1%) | 5 (6.1%) | |
| IMRT external beam | 17 (22.9%) | 11 (13.4%) | |
|
| |||
| Total | 74 (100.0%) | 82 (100.0%) | |
For lesions of depth greater than 5 mm, interstitial brachytherapy with needles using either LDR or HDR (HDR interstitial was an option only following amendment implemented in Aug of 2019) was recommended when the full extent of the lesions could be treated to an adequate dose with brachytherapy. Volumetric planning was recommended with CT or magnetic resonance (MR) imaging after protocol revision in 2014. With an HDR interstitial approach, five fractions of 4–6 Gy were prescribed to the gross disease with the D90 (dose to 90% of the target) being greater than or equal to the prescription dose.
For all brachytherapy cases treated with volumetric planning, the combined external beam and brachytherapy EQD2 for the rectal and sigmoid dose was recommended to be kept ≤ 70Gy, small bowel ≤ 65 Gy and bladder dose ≤ 90 Gy including the contribution from external beam (45 Gy) and intracavitary brachytherapy. For recurrent cases that were not amenable to brachytherapy because distribution of disease made it not technically feasible to safely implant the tumor volume, 3D conformal radiotherapy or IMRT techniques to deliver a minimal total dose of 65 Gy to the GTV (2000 cGy boost at a dose/fraction of 200 cGy in 10 fractions) were recommended.
Statistical Analysis:
The primary endpoint of the study was to determine whether pelvic radiation therapy with concurrent cisplatin is more promising with respect to progression-free survival than pelvic radiation therapy alone in the treatment of recurrent uterine carcinoma limited to the pelvis and vagina. Progression-free survival (PFS) was defined as the time from study entry until disease progression, death, or date of last contact. The null hypothesis assessed whether the hazard of progression or death (PFS-hazard) in patients treated with pelvic radiation with concurrent cisplatin is not better than for those treated with pelvic radiation alone. At maturity, the study was planned to accumulate 94 PFS events to have 80% power with a one-sided 0.10 level test to detect a hazard ratio (HR) of 0.645 comparing pelvic radiation with concurrent cisplatin vs. pelvic radiation alone. The accrual target was 164 patients. The study design also included one planned interim futility analysis of the primary objective indicated when approximately 60 progression or death events were reported. At the time of the interim analysis, there were 57 reported PFS events across the two treatment arms (33 events in the pelvic radiation with concurrent cisplatin arm and 24 events in the pelvic radiation arm). The estimated hazard ratio comparing pelvic radiation with concurrent cisplatin vs. pelvic radiation only at the time of the interim analysis was λR+C/λR = 1.40 (95% CI: 0.82 – 2.40). This HR estimate >1 indicates worse PFS prognosis among patients in the experimental arm. Based on this finding, it was recommended to the DMC that the interim results be released, and that follow-up be suspended, due to the interim hazard ratio indicating worst prognosis among patients who received cisplatin and concerns about future data maturity.
The primary null hypothesis was tested by assessing the equality of hazard rates estimated with a Cox proportional hazards model stratified by initial performance status (0 vs 1) and type of recurrence at entry (vaginal only vs all others). All eligible patients were included in the analysis, regardless of the amount of study treatment received. Median follow-up was estimated with the reverse Kaplan-Meier method.
Secondary analysis included overall survival (OS), toxicity, and to estimate the prognostic and predictive significance for factors for PFS and OS including location (central pelvis versus vagina), histological subtype, grade, patient age, race, performance status, and the presence of lymph-vascular space involvement.
The stratified hazard ratio for OS was estimated with a Cox proportional hazards model stratified by initial performance status (0 vs 1) and type of recurrence at entry (vaginal only vs all others). All eligible patients were included in the analysis, regardless of the amount of study treatment received.
Adverse events were tabulated by any grade and by grades 3–5 for each treatment. P-values for the between-group comparison of the maximum grade of adverse events were calculated with a Kruskal-Wallis test corrected for ties.
Separate adjusted Cox proportional hazards models (stratified by performance status and tumor location, adjusted for treatment arm) were used to assess for prognostic associations between baseline factors and PFS and OS. Separate adjusted Cox proportional hazards (stratified by performance status and tumor location and including main effects of treatment and baseline factor plus an interaction term for treatment and baseline factor) were used to assess for predictive associations between outcomes and baseline factors with treatment allocation. Hazard ratios, 95% confidence intervals, and p-values from the Wald test were displayed in forest plots for both prognostic models and predictive models.
RESULTS
Patient Characteristics:
The patient and tumor characteristics of the 156 eligible patients included in the primary analysis are presented in Table 1. Most participants were between the ages of 60 and 79 years with a median age of 66 years. Most patients were white (89%) and non-Hispanic (89%). The most common histology was endometroid (89%) and most tumors were in the vagina only without involvement of other pelvic lymph nodes (86%). Radiation treatment was delivered with pelvic radiation followed by additional boost dose. The treatment technique for the boost dose was declared prior to randomization, based on tumor characteristics. There was no significant difference in the proportion of patients in each arm treated with each boost approach (p=0.26, Table 2).
Table 1.
Patient and Treatment Characteristics at Time of Study Enrollment
| Radiation (n=74) | Radiation+Cisplatin (n=82) | Total (n=156) | |
|---|---|---|---|
| Age (in years) | |||
| Mean | 66.5 | 65.0 | 65.7 |
| Less than 60 years | 16 (21.6%) | 19 (23.2%) | 35 (22.4%) |
| 60–80 years | 49 (66.2%) | 59 (72.0%) | 108 (69.2%) |
| Greater than 80 years | 9 (12.2%) | 4 (4.9%) | 13 (8.3%) |
| Ethnicity | |||
| Hispanic or Latino | 4 (5.4%) | 6 (7.3%) | 10 (6.4%) |
| Non-Hispanic | 65 (87.8%) | 74 (90.2%) | 139 (89.1%) |
| Not Specified | 5 (6.8%) | 2 (2.4%) | 7 (4.5%) |
| Race | |||
| White | 65 (87.8%) | 74 (90.2%) | 139 (89.1%) |
| Black | 5 (6.8%) | 1 (1.2%) | 6 (3.8%) |
| Asian | 0 (0.0%) | 3 (3.7%) | 3 (1.9%) |
| Other | 4 (5.4%) | 4 (4.9%) | 8 (5.1%) |
| Performance Status | |||
| 0 | 66 (89.2%) | 71 (86.6%) | 137 (87.8%) |
| 1 | 8 (10.8%) | 11 (13.4%) | 19 (12.2%) |
| Histology/Grade | |||
| Endometrioid, grade 1 | 48 (64.9%) | 42 (51.2%) | 90 (57.7%) |
| Endometrioid, grade 2 | 16 (21.6%) | 22 (26.8%) | 38 (24.4%) |
| Endometrioid, grade 3 | 2 (2.7%) | 8 (9.8%) | 10 (6.4%) |
| Endometrioid, grade unknown | 1 (1.4%) | 0 (0.0%) | 1 (0.6%) |
| Serous | 2 (2.7%) | 4 (4.9%) | 6 (3.8%) |
| Clear Cell | 1 (1.4%) | 0 (0.0%) | 1 (0.6%) |
| Mixed Epithelial | 3 (4.1%) | 2 (2.4%) | 5 (3.2%) |
| Adenocarcinoma, not specified | 1 (1.4%) | 3 (3.7%) | 4 (2.6%) |
| Other | 0 (0.0%) | 1 (1.2%) | 1 (0.6%) |
| Location | |||
| Both pelvis and vagina | 4 (5.4%) | 7 (8.5%) | 11 (7.1%) |
| Pelvis only | 7 (9.5%) | 4 (4.9%) | 11 (7.1%) |
| Vagina only | 63 (85.1%) | 71 (86.6%) | 134 (85.9%) |
| Pelvic Lymph Node Involvement | |||
| No | 73 (98.6%) | 77 (93.9%) | 150 (96.2%) |
| Yes | 1 (1.4%) | 5 (6.1%) | 6 (3.8%) |
| Lymph-Vascular Invasion | |||
| Yes | 13 (17.6%) | 12 (14.6%) | 25 (16.0%) |
| No | 46 (62.2%) | 57 (69.5%) | 103 (66.0%) |
| Not evaluated | 15 (20.3%) | 13 (15.9%) | 28 (17.9%) |
Q1=first quartile; Q3=third quartile.
Toxicity:
A toxicity was higher in patients receiving combined modality treatment. Overall, 57% of patients in chemoradiation arm developed grade 3 toxicity, as compared to 31% of patients treated with radiation therapy alone. Acute toxicity rates were higher in patients receiving chemotherapy for auditory events (p=0.012), constitutional symptoms (p=0.016), blood/bone marrow events (p<0.001), musculoskeletal (0.032), metabolic events (p=0.001), ocular events, neurologic events (p= 0.05) and vascular events (p=0.014) in patients treated with cisplatin (Table 3). Only gastrointestinal events were higher in the patients treated with radiation alone (p=0.042) (Table 3) There were no significant differences between treatment groups for late adverse events (Supplementary Table 2).
Table 3.
Summary of Acute Adverse Events
| Radiation (N=74) | Radiation+Cisplatin (N=76) | ||||
|---|---|---|---|---|---|
| Adverse Event (%) | Any Grade | Grade 3–5 | Any Grade | Grade 3–5 | p-value |
| 2.8 | 1.4 | 14.4 | 1.3 | 0.012 | |
| Allergy/Immunology | 0.0 | 0.0 | 2.6 | 1.3 | 0.16 |
| Coagulation | 0.0 | 0.0 | 1.3 | 0.0 | 0.32 |
| Constitutional Symptoms | 64.9 | 2.7 | 82.9 | 1.3 | 0.016 |
| Cardiac | 15.0 | 4.1 | 23.7 | 7.9 | 0.16 |
| Dermatology/Skin | 28.4 | 0.0 | 38.2 | 2.6 | 0.28 |
| Endocrine | 4.2 | 1.4 | 6.6 | 0.0 | 0.52 |
| Gastrointestinal | 79.7 | 9.5 | 71.0 | 9.2 | 0.042 |
| Renal/Genitourinary | 32.4 | 2.7 | 31.6 | 3.9 | 0.99 |
| Hemorrhage/Bleeding | 10.8 | 0.0 | 9.2 | 1.3 | 0.76 |
| Blood/Bone Marrow | 67.6 | 12.2 | 89.5 | 36.9 | <0.001 |
| Infection | 8.1 | 1.4 | 18.4 | 1.3 | 0.07 |
| Lymphatics | 10.8 | 0.0 | 19.7 | 1.3 | 0.11 |
| Musculoskeletal | 4.2 | 0.0 | 14.5 | 2.6 | 0.032 |
| Metabolic/Laboratory | 40.5 | 2.7 | 61.8 | 18.4 | 0.001 |
| Neurology | 23.0 | 0.0 | 36.8 | 3.9 | 0.05 |
| Ocular | 0.0 | 0.0 | 6.6 | 0.0 | 0.025 |
| Pulmonary | 16.2 | 0.0 | 27.6 | 2.6 | 0.08 |
| Pain | 47.3 | 5.4 | 57.9 | 0.0 | 0.89 |
| Sexual/Reproductive | 21.7 | 0.0 | 11.8 | 0.0 | 0.10 |
| Vascular | 0.0 | 0.0 | 7.9 | 2.6 | 0.014 |
P-values for the between-group comparison of the maximum grade of adverse events were calculated with a Kruskal-Wallis test corrected for ties.
Outcome:
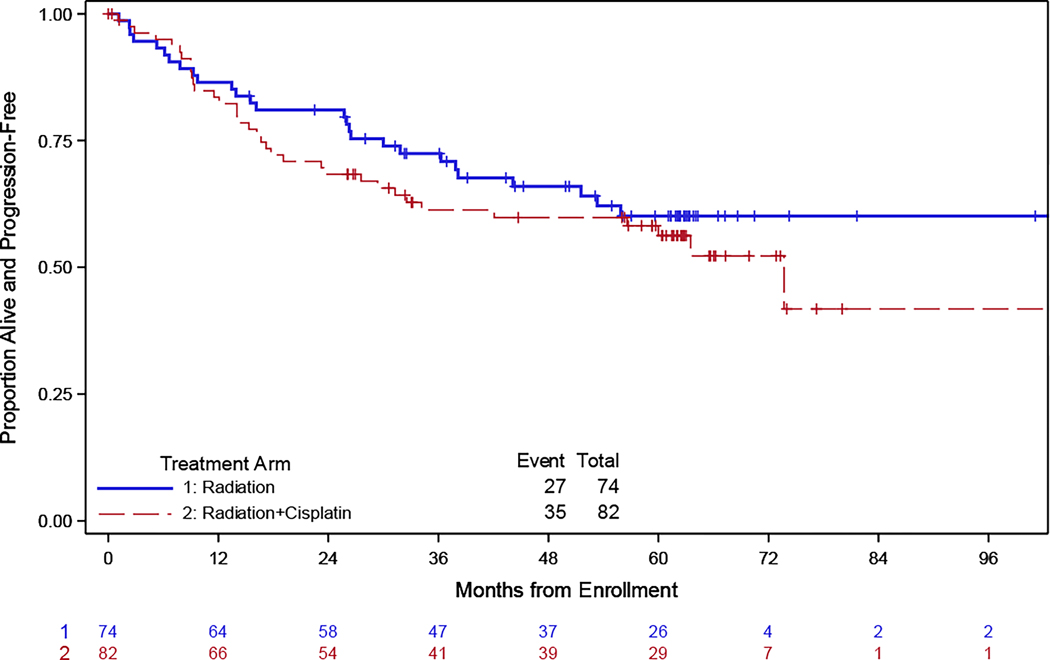
At the time of the final analysis, 62 patients progressed across the two treatment arms: 35 in the group receiving pelvic radiation with concurrent cisplatin and 27 in the group receiving pelvic radiation only. The median follow-up for the PFS endpoint was 62 months, with a maximum of 128 months. The median PFS was not reached in patients treated with radiation only due to an insufficient number of events. In comparison, patients treated with concurrent cisplatin had a 73.7 month median progression free survival. There was no significant difference in progression-free survival between patients treated with radiation alone as compared to chemoradiation (Figure 2A). The estimated hazard ratio comparing the two treatment arms was 1.25 (95% CI: 0.75 – 2.07), indicating no significant difference in the PFS of the group receiving pelvic radiation as compared to patients receiving concurrent cisplatin.
Figure 2. Progression free and overall survival. Kaplan-Meier curve comparing progression-free survival (PFS) and overall survival (OS) for patients treated with chemoradiation (CRT) versus radiation alone (RT).
The x-axis represents time in months, and the y-axis represents the probability of remaining progression-free or alive overall. The solid line represents the PFS for the RT group, and the dashed line represents the PFS for the CRT group.
In terms of overall survival (OS), there were 39 deaths in the two treatment arms: 18 in the group receiving pelvic radiation only and 21 in the group receiving pelvic radiation with concurrent cisplatin. The Kaplan-Meier estimates for the cumulative probabilities of OS for each treatment arm are shown in Figure 2B. Median overall survival was 97 months (95% CI: 66.9, NR) in the radiation only arm and 99.6 months (95% CI: 73.7, NR) in the concurrent cisplatin arm. The stratified hazard ratio for death comparing pelvic radiation with concurrent cisplatin vs. pelvic radiation was 1.10 (95% CI: 0.57–2.12).
The sites of recurrence are listed in Supplementary Table 3 with most common sites of recurrence being vagina, pelvis, abdomen, and lung. The causes of death were cancer-related in 13 (61.9%) of patients treated with chemoradiation as compared to 7 (38.9%) in patients treated with radiation therapy alone (Supplementary Table 4). One treatment related death was reported in both study arms.
Prognostic factors:
To identify prognostic factors associated with outcome in all patients, multivariate analysis was performed. Worse performance status and vaginal tumor location only were associated with lower progression-free survival (Figure 3A). Other clinical and disease features at the time of enrollment, including younger age, LVSI (present vs absent), histology (grade 1 vs other) or radiation boost technique were not associated with improved progression-free survival. With regard to analysis of prognostic factors for overall survival, only age at the time of enrollment (Supplementary Figure 1) was associated with lower risk of death.
Figure 3. Forest plot showing the analysis for potential prognostic (3A) and predictive factors per treatment arm (3B) for progression-free survival (PFS).
The x-axis represents the odds ratio (OR) for each predictive factor. The y-axis lists the predictive factors, and the horizontal lines extending from the squares represent the 95% CIs. Separate adjusted Cox proportional hazards models (stratified by performance status and tumor location, adjusted for treatment arm) were used to assess for prognostic associations between baseline factors and PFS. Separate adjusted Cox proportional hazards (stratified by performance status and tumor location, and including main effects of treatment and baseline factor plus an interaction term for treatment and baseline factor) were used to assess for predictive associations between PFS and baseline factors with treatment allocation.
Predictive factors for treatment benefit:
Clinical and pathological factors were assessed to determine if they were associated with benefit from CRT. There was no evidence that any of the clinical factors at time of enrollment, including age, histology (grade 1 vs other), tumor location (vaginal only vs other), boost technique (brachytherapy vs external) were associated with progression-free survival (Figure 3B) or overall survival (Supplementary Figure 2).
DISCUSSION
This randomized trial comparing chemoradiation to the standard of care radiation therapy for the treatment of locally recurrent, non-metastatic endometrial cancer demonstrated that the addition of cisplatin to radiation therapy did not improve outcomes. Despite the prior lack of evidence, chemotherapy has been widely adopted in this setting as an adjuvant to radiation therapy based on demonstrated benefits in other disease sites and retrospective series suggesting a benefit.6–8 The results presented here demonstrate that at least for eligible patients in this trial, the addition of chemotherapy does not improve outcomes and predictably results in more toxicity during treatment without clinical benefit.
Patients on this trial who received definitive radiation had a 3-year progression free survival rate of 73%, marked better outcomes than patients with endometrial cancer recurrences in other sites which are typically treated with systemic therapy. Progression-free survival rates are traditionally less than 30% for patients with distant metastatic recurrences of endometrial cancer.10,11 Most recently, the addition of immune checkpoint blockade with chemotherapy was shown to substantially improve outcomes for all women with advanced and recurrent endometrial cancer.12,13 Notably, the most favorable subset of patients with dMMR who were treated with pembrolizumab had progression free survival at one year of 74% and just under 60% at three years. These rates are a substantial improvement over treatment with chemotherapy alone but are still lower than what is observed with radiation treatment for patients with small volume recurrences. This underscores the importance of identifying patients with local recurrences who can be treated with curative radiation therapy, rather than including them with other patients with advanced or recurrent disease in future clinical trials. By identifying and treating these patients with a distinct paradigm of curative radiation therapy and investigate the benefit of the addition of immune checkpoint blockade or other agents, it may be possible to achieve even better outcomes and further improve the overall prognosis for this group12.
The utilization of image-guided brachytherapy to delineate the treatment target has resulted in a simultaneous reduction of local recurrence and radiation toxicity for patients with locally advanced cervical cancer.14 The fundamental principles of image guided brachytherapy in cervical cancer have been adopted to inform the treatment of recurrent endometrial cancer in this trial. Treatment guidelines for how-to approach image guided brachytherapy for recurrent endometrial cancers have been developed and endorsed by the American Brachytherapy Society, Canadian Brachytherapy Group, and the GEC-ESTRO organization15,16 Over the course of enrollment on this trial, the practice of brachytherapy was evolving as these principals from cervical cancer were applied to recurrent endometrial cancers. Most patients in this trial had relatively small tumors and were treated with appropriately simple brachytherapy techniques where the advanced applicators and imaging used for cervical cancer would be expected to have less marked benefits that are predicted in larger and more complex disease. Although the disease in the vagina was relatively small volume, the rate of repeated recurrence in the vagina of 30% suggests that further refinements in brachytherapy treatment are needed. The relative benefits of external beam as compared to brachytherapy boosts, remain to be determined and were difficult to assess in this study given the small number of patients treated with each boost technique and the potentially confounding relationship between boost technique and tumor site. Ultimately, the use of MRI guided adaptive brachytherapy may to improve local control rates for recurrences of endometrial cancer as has been demonstrated in cervical cancer.
Enrollment in this study was slower than anticipated. This may have been due to a bias on the part of investigators in favor of adding chemotherapy based on the benefits seen in other settings including cervical cancer, head and neck cancer, and adjuvant therapy for node positive endometrial cancers.7,8,17 This same bias may have resulted in preferential enrollment of lower risk patients including those with low grade and small volume tumors on this trial as patients with more advanced disease were treated with chemotherapy off trial. As a result, the generalizability of these findings to patients with isolated nodal disease or other histologic subtypes of endometrial cancer may be limited.
Another potential reason for the slower-than-expected enrollment in the study may have been the constraints on the type of radiation therapy that could be administered. When this trial was initially written in 2007, CT-based brachytherapy planning and more advanced, HDR-driven techniques, were not widely used. The original protocol therefore relied heavily on LDR brachytherapy. Over the time course of the trial, multiple amendments were required to be made to adjust the protocol with the changing landscape of radiation oncology practice. This included a transition to CT-based planning, expanding the HDR dose schemes that could be delivered, and ultimately allowing the use of interstitial brachytherapy. Some of these amendments were able to be made in the earlier stages of the protocol, but the allowance for HDR interstitial brachytherapy was not completed and active until the final year of the protocol (Aug 2019). As a result, the type of brachytherapy offered throughout the study was mostly limited to vaginal brachytherapy using cylinders, and the overall number of patients undergoing an interstitial procedure made up a small proportion of all enrolled patients, again selecting for those with small volume and accessible recurrences. Additionally, patients requiring interstitial brachytherapy, who are more likely to have larger tumors and higher recurrence risk, may have not enrolled for preference to be treated with the addition of chemotherapy.
The PORTEC-3 study which analyzed the molecular subtypes of endometrial cancer found that patients with p53 mutations derived the greatest benefit from the addition of chemotherapy to radiation therapy.18 In contrast, patients with mismatch repair deficiency, POLE mutated, and non-significant molecular profile did not have significantly higher survival rates when treated with chemotherapy. Molecular subtype analysis was not performed on this trial as tumor specimens were not collected and are thus not available for analysis. It is possible that p53 mutated tumors may be more likely to benefit from the addition of chemotherapy in the recurrent setting as was observed in the adjuvant setting. Future studies may further define the relative benefits of systemic therapy in addition to radiation in this setting.
The reported toxicities in our study were generally consistent with the well-documented side effects associated with cisplatin chemotherapy. Delivery of concurrent chemotherapy resulted in increased constitutional symptoms, auditory, ocular, neurological, vascular and bone marrow toxicity. Late toxicity was similar between the two arms. However, it is important to acknowledge the potential underreporting of certain toxicities, particularly those related to musculoskeletal and sexual health. In our study, patient-reported measures of toxicity were not included, relying solely on physician-reported data. It is widely recognized that physician-reported toxicities may underestimate the true extent of adverse events experienced by patients.9.
Conclusion
In conclusion, this randomized clinical trial provides evidence that the addition of concurrent chemotherapy does not improve outcomes for patients with recurrent endometrial cancer treated with definitive radiation therapy. It is worth noting that many patients enrolled in the study had low-grade tumors and recurrences at the vaginal apex, and therefore the findings may be most applicable to these subgroups of patients. Radiation therapy remains the backbone of curative intent treatment for local recurrences. Future research should focus on identifying molecular subsets of tumors and defining the optimal adjuvant therapies for these subgroups, in order to improve treatment outcomes for all patients with recurrent endometrial cancer.
Supplementary Material
Supplementary Figure 1. Forest plot showing the analysis for potential prognostic (3A) and predictive factors per treatment arm (3B) for overall survival (OS). The x-axis represents the odds ratio (OR) for each predictive factor. The y-axis lists the predictive factors, and the horizontal lines extending from the squares represent the 95% CIs. Separate adjusted Cox proportional hazards models (stratified by performance status and tumor location, adjusted for treatment arm) were used to assess for prognostic associations between baseline factors and OS. Separate adjusted Cox proportional hazards (stratified by performance status and tumor location, and including main effects of treatment and baseline factor plus an interaction term for treatment and baseline factor) were used to assess for predictive associations between OS and baseline factors with treatment allocation.
Supplementary Table 4. Cause of Death by Treatment Regimen in Deceased Eligible Patients
Supplementary Table 1. Number of cycles of cisplatin received.
Supplementary Table 3. Site of Recurrence
Supplementary Table 2. Late Adverse Events.
CONTEXT SUMMARY.
Key Objective:
This study aimed to compare the efficacy of radiation therapy alone and concurrent chemotherapy with cisplatin in improving progression-free survival (PFS) for women with localized recurrences of endometrial cancer.
Knowledge Gained:
The study found that adding chemotherapy did not improve progression-free survival in patients with localized endometrial cancer recurrences. At three years, 73% of patients treated with radiation alone and 62% of those receiving chemoradiation were free from disease progression, with higher rates of acute toxicity rates in the chemoradiation group.
Relevance:
Based on these data, patients with vaginal-only recurrences of grade 1 or 2 endometrioid endometrial cancer should be treated with radiation therapy without the addition of chemotherapy.
Relevance statement written by Gini Fleming
ACKNOWLEDGEMENTS
This study was supported by National Cancer Institute grants to NRG Oncology (1U10 CA180822) and NRG Operations (U10CA180868).
The following NRG Oncology/Gynecologic Oncology Group member institutions participated in the primary treatment studies: University of Oklahoma Health Sciences Center, University of Iowa Hospitals and Clinics, Women’s Cancer Center of Nevada, Ohio State University Comprehensive Cancer Center, University of Massachusetts Memorial Health Care, Case Western Reserve University, University of Wisconsin Hospital and Clinics, Delaware/Christiana Care CCOP, Abington Memorial Hospital-Asplundh Cancer Pavilion, Maine Medical Center - Scarborough Campus, Roswell Park Comprehensive Cancer Center, University of Colorado Cancer Center - Anschutz Cancer Pavilion, Wake Forest University Health Sciences, University of New Mexico, Geisinger Medical Center, University of Cincinnati, Indiana University Hospital/Melvin and Bren Simon Cancer Center, University of California Medical Center at Irvine-Orange Campus, University of Kentucky, Huntsman Cancer Institute/University of Utah, Oncology Alliance-Glendale, University of Rochester, West Virginia University Healthcare, Loyola University Medical Center, Saint Vincent Hospital, University of New Mexico, Northwestern University, University of Chicago, Cancer Trials Support Unit, Aurora Women’s Pavilion of Aurora West Allis Medical Center, Aultman Health Foundation, Reading Hospital, Kaiser Permanente-San Diego Mission, Evanston CCOP-NorthShore University Health System, Kalamazoo CCOP, Michigan Cancer Research Consortium Community Clinical Oncology Program, Greenville Health System Cancer Institute/Greenville CCOP, Catholic Health Initiatives NCORP, University of Texas Southwestern Medical Center, Georgia Center for Oncology Research and Education (CORE) and Georgia2 Cares Minority Underserved NCORP.
Funding:
This study was supported by National Cancer Institute grants to NRG Oncology (1U10 CA180822) and NRG Operations (U10CA180868); NCT00492778
CONFLICT OF INTERESTS
The following authors have no conflicts of interest to disclose: Dr. Ann Klopp, Dr. Susan Zweizig, Dr. Steven Waggoner, Dr. Lana de Souza Lawrence, Dr. Parviz Hanjani, Dr. Higinia Cardenes, Dr. William Small and Dr. Jonathan Feddock.
Dr. Danielle Enserro wishes to disclose that NCI support was received as Cooperative Grant/NCTN Grant funding for all aspects of this trial including travel to group meetings, statistical analysis, study monitoring, etc.
Dr. Matthew Powell acknowledges receiving consulting fees from the following: GlaxoSmithKline GSK/Tesaro, Merck, AstraZeneca, Clovis Oncology, Seagen and Eisai.
Dr. Marcus Randall reports receiving support for attending GOG/NRG travel and meeting support as Co-Chair of the Uterine Corpus Committee.
Dr. Robert Mannel and Dr. Laura Holman report institutional support received from NCI clinical trials network in support of this manuscript.
Dr. David Bender has received grants from Tesaro, Inc., AbbVie, Inc., AstraZeneca, Merck Sharp & Dohme Company, Clovis Oncology, Inc. and Genentech.
Dr. Christina Kushnir received consulting fees from Intuitive Surgical and Ethicon Biosurgery.
Dr. Floor Backes acknowledges research grants to Institution from Merck, Eisai, Immunogen, Clovis, BeiGene, Natera and Tempus. Dr. Backes received personal fees from UptoDate. Additionally, Dr. Backes received consulting fees from Agenus, Merck, Clovis, Immunogen, Eisai, AstraZeneca, GlaxoSmithKline, Myriad and Genentech. Dr. Backes also received personal fees for CME lectures from Clinical Educational Concepts, Clinical Care Options, Medscape/WebMD, Med Learning, 13Health, CMR Institute, Global Learning Initiative/Prova, OncLive. Dr. Backes served as a Board Member for the Society of Gynecologic Oncology (unpaid), Co-Chair for both NRG Oncology Developmental Therapeutics Committee and IGCS Education360.
Dr. Kristin Bradley reports serving as Treasurer of the American Brachytherapy Society (unpaid position) and is on the Board of Directors for the same.
Dr. Christopher Darus reports serving on the AstraZeneca Advisory Board and has received payments for the same. He also reports owning stock in Regeneron and Humanigen.
Dr. David Miller reports serving as Chair, Uterine Corpus Committee for GOG then NRG Oncology, 2003-2018.
Data Sharing Statement:
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
REFERENCES
- 1.Siegel RL, Miller KD, Wagle NS & Jemal A. Cancer statistics, 2023. CA Cancer J Clin 73, 17–48 (2023). [DOI] [PubMed] [Google Scholar]
- 2.Keys HM, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 92, 744–751 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Creutzberg CL, et al. Survival after relapse in patients with endometrial cancer: results from a randomized trial. Gynecol Oncol 89, 201–209 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Creutzberg CL, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet 355, 1404–1411 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Jhingran A, Burke TW & Eifel PJ Definitive radiotherapy for patients with isolated vaginal recurrence of endometrial carcinoma after hysterectomy. Int J Radiat Oncol Biol Phys 56, 1366–1372 (2003). [DOI] [PubMed] [Google Scholar]
- 6.Francis SR, et al. Recurrent early stage endometrial cancer: Patterns of recurrence and results of salvage therapy. Gynecol Oncol 154, 38–44 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Eifel PJ, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90–01. J Clin Oncol 22, 872–880 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Al-Sarraf M, et al. Postoperative radiotherapy with concurrent cisplatin appears to improve locoregional control of advanced, resectable head and neck cancers: RTOG 88–24. Int J Radiat Oncol Biol Phys 37, 777–782 (1997). [DOI] [PubMed] [Google Scholar]
- 9.Yeung AR, et al. Improvement in Patient-Reported Outcomes With Intensity-Modulated Radiotherapy (RT) Compared With Standard RT: A Report From the NRG Oncology RTOG 1203 Study. J Clin Oncol 38, 1685–1692 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller DS, et al. Carboplatin and Paclitaxel for Advanced Endometrial Cancer: Final Overall Survival and Adverse Event Analysis of a Phase III Trial (NRG Oncology/GOG0209). J Clin Oncol 38, 3841–3850 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makker V, et al. Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. N Engl J Med 386, 437–448 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eskander RN, et al. Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. N Engl J Med (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirza MR, et al. Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer. N Engl J Med (2023). [DOI] [PubMed] [Google Scholar]
- 14.Potter R, et al. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): a multicentre prospective cohort study. Lancet Oncol 22, 538–547 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Kamrava M, et al. American Brachytherapy Society recurrent carcinoma of the endometrium task force patterns of care and review of the literature. Brachytherapy 16, 1129–1143 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Kamrava M, et al. GEC-ESTRO (ACROP)-ABS-CBG Consensus Brachytherapy Target Definition Guidelines for Recurrent Endometrial and Cervical Tumors in the Vagina. Int J Radiat Oncol Biol Phys 115, 654–663 (2023). [DOI] [PubMed] [Google Scholar]
- 17.de Boer SM, et al. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol 20, 1273–1285 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leon-Castillo A, et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit From Adjuvant Therapy. J Clin Oncol 38, 3388–3397 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Forest plot showing the analysis for potential prognostic (3A) and predictive factors per treatment arm (3B) for overall survival (OS). The x-axis represents the odds ratio (OR) for each predictive factor. The y-axis lists the predictive factors, and the horizontal lines extending from the squares represent the 95% CIs. Separate adjusted Cox proportional hazards models (stratified by performance status and tumor location, adjusted for treatment arm) were used to assess for prognostic associations between baseline factors and OS. Separate adjusted Cox proportional hazards (stratified by performance status and tumor location, and including main effects of treatment and baseline factor plus an interaction term for treatment and baseline factor) were used to assess for predictive associations between OS and baseline factors with treatment allocation.
Supplementary Table 4. Cause of Death by Treatment Regimen in Deceased Eligible Patients
Supplementary Table 1. Number of cycles of cisplatin received.
Supplementary Table 3. Site of Recurrence
Supplementary Table 2. Late Adverse Events.
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.