Abstract
Currently, 30% of the global population is overweight or obese, with projections from the World Obesity Federation suggesting that this figure will surpass 50% by 2035. Adipose tissue dysfunction, a primary characteristic of obesity, is closely associated with an increased risk of metabolic abnormalities, such as hypertension, hyperglycemia, and dyslipidemia, collectively termed metabolic syndrome. In particular, visceral fat accretion is considered as a hallmark of aging and is strongly linked to higher mortality rates in humans. Adipokines, bioactive peptides secreted by adipose tissue, play crucial roles in regulating appetite, satiety, adiposity, and metabolic balance, thereby rendering them key players in alleviating metabolic diseases and potentially extending health span. In this review, we elucidated the role of adipokines in the development of obesity and related metabolic disorders while also exploring the potential of certain adipokines as candidates for longevity interventions.
Keywords: adipose tissue, adipokine, metabolic dysfunction, aging, health span
1. Introduction
Obesity is characterized by an excessive accumulation of adipose tissue, and the major causes of metabolic diseases are a disproportionate increase in adipose tissue and insulin resistance. Adequate amounts of adipose tissue are crucial in mammals, serving not only as an energy storage depot but also as an endocrine organ that regulates metabolic function through the secretion of numerous adipokines. However, excessive accumulation of adipose tissue is associated with metabolic dysfunction and increased susceptibility to obesity, diabetes, and cancer [1]. Adipose tissue, commonly known as fat tissue, comprises two main types: white adipose tissue (WAT) and brown adipose tissue (BAT). White adipose tissue acts as an energy reservoir for other organs, whereas brown adipose tissue functions in cold-induced adaptive thermogenesis. Histologically, white adipose tissue is subdivided into two forms, visceral and subcutaneous. The enlargement of visceral adipose tissue, often termed visceral obesity, is strongly linked to inflammation and insulin resistance [2,3]. The expansion of adipose tissue triggers adipocyte death through mechanical and oxidative stresses, as well as hypoxic conditions, leading to the recruitment of proinflammatory macrophages to adipose tissues [4,5,6]. The infiltration of macrophages contributes to adipose tissue dysfunction, inducing inflammation and insulin resistance in individuals with obesity [7]. Obesity-induced cell inflammation accelerates adipose tissue dysfunction, disrupting overall energy homeostasis and increasing susceptibility to age-related diseases [8]. Uncontrolled secretion of adipokines and the senescence-associated secretory phenotype (SASP) resulting from adipose tissue dysfunction are well-recognized features of aging and metabolic diseases [9] (Figure 1). Several epidemiological cohort studies have shown that obesity increases all-cause mortality and reduces life expectancy in humans [10,11,12]. This has been demonstrated by studies using rodents, which show that suppression of obesity through the removal of visceral adipose tissue results in improved insulin action and prolongs lifespan [13,14]. As a depot of energy storage, adipose tissue is now recognized as an endocrine tissue that regulates metabolic diseases, including obesity, and aging through the regulation of various hormones known as adipokines [15]. Since the identification of adiponectin and leptin as representative adipokines that regulate obesity, numerous types of adipokines have been discovered, prompting extensive research into their roles in health and metabolic diseases [16].
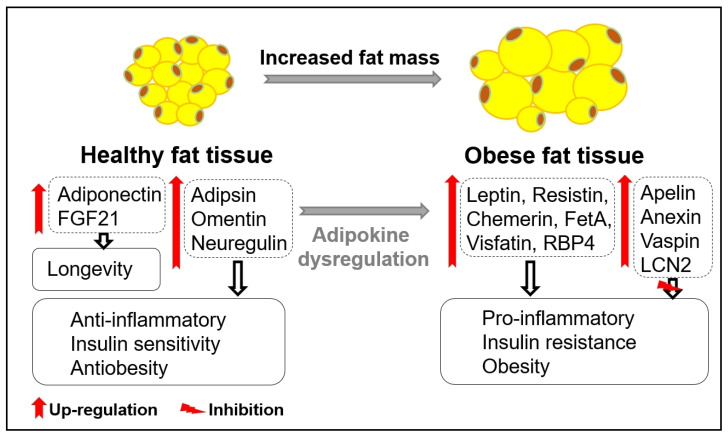
Figure 1.
Adipokines in healthy and obese fat tissue. The secretion of beneficial adipokines (adiponectin, FGF21, adipsin, omentin, neuregulin) from healthy fat tissue and detrimental adipokines (leptin, resistin, chemerin, FetA, visfatin, RBP4) from obese fat tissue plays important roles in inflammation, insulin sensitivity, and obesity. Obese-related secretion of other adipokines, such as apelin, annexin, vaspin, and LCN2, plays compensatory roles in inhibiting inflammation, insulin sensitivity, and obesity.
Aging is a process in which tissue function gradually deteriorates as the ability to maintain metabolic homeostasis decreases over time, rendering the body vulnerable to external stress and increasing susceptibility to metabolic diseases, such as obesity, type 2 diabetes, and cardiovascular disease [17]. Although the exact mechanism remains unclear, caloric-restriction-induced suppression of oxidative stress and improvement in energy metabolism contribute to lifespan extension and the reduction of age-related metabolic diseases. Furthermore, the enhancement of adipose tissue function through the modulation of adiponectin and fibroblast growth factor 21 (FGF21), a caloric-restriction-induced adipokine, promotes an extension of health span [18,19].
In this review, we elucidate the role of adipokines in regulating metabolic function and discuss their implications for metabolic diseases and health (Table 1).
Table 1.
Biological effects of adipokines on health and diseases.
| Adipokines | Roles |
|---|---|
| Adiponectin | Improves glucose homeostasis; has antidiabetic, anti-inflammatory, and antiatherogenic effects |
| FGF21 | Improves age-related tissue dysfunctions; extends lifespan; |
| positively associated with longevity | |
| Adipsin | Improves glucose tolerance and beta-cell functions; |
| stimulates triacylglycerol synthesis and storage in adipose tissue; | |
| positively associated with longevity; increases cell survival and SIRT1 activity and has neuroprotective effects | |
| Apelin | Regulates food intake; improves glucose disposal |
| Omentin | Improves insulin sensitivity; has an anti-inflammatory effect |
| Annexin | Regulates inflammation, lipolysis, lipogenesis, and adiposity |
| Neuregulin | Regulates cell proliferation, survival, migration, and differentiation; |
| reduces hepatic glucose production and lipogenesis; | |
| stimulates thermogenesis in brown adipose tissue | |
| Leptin | Regulates appetite and energy expenditure; |
| negatively associated with longevity | |
| Resistin | Positively associated with obesity and insulin resistance; accelerates inflammation; |
| positively correlated with cellular senescence and aging | |
| Visfatin | Stimulates triacylglycerol synthesis and storage in adipose tissue; |
| positively associated with longevity; increases cell survival and SIRT1 activity and has neuroprotective effects | |
| Chemerin | Regulates cell proliferation, differentiation, and energy metabolism; |
| negatively associated with longevity | |
| Vaspin | Regulates insulin sensitivity, adipocyte differentiation, and angiogenesis; inhibits inflammation |
| Lipocalin-2 | Regulates dyslipidemia and insulin resistance; inhibits inflammation |
| RBP4 | Positively associated with obesity and insulin resistance; |
| impairs mitochondrial fatty acid β-oxidation | |
| Fetuin A | Positively associated with insulin resistance and inflammation |
The adipokines discussed in this review are summarized in this table.
2. Adipokines in the Regulation of Health and Diseases
2.1. Adiponectin
Adiponectin, predominantly secreted by adipocytes, is the most abundant adipokine in plasma [20,21]. Adiponectin is associated with insulin secretion and energy expenditure and is negatively correlated with metabolic disease parameters such as body mass index (BMI), as well as glucose, insulin, triglyceride, and visceral fat levels [22]. Adiponectin exhibits antiatherogenic, antidiabetic, anti-inflammatory, and anti-apoptotic effects by inhibiting monocyte adhesion to endothelial cells and suppressing macrophage transformation into foam cells by suppressing the tumor necrosis factor alpha (TNFα)—nuclear factor kappa B (NF-κB) signaling pathway [23]. Circulating adiponectin and TNFα levels are inversely correlated in both lean and obese individuals [24]. Adiponectin was also shown to increase tissue inhibitor of metalloproteinases (TIMP-1) in human monocyte-derived macrophages through IL-10 induction, which plays an important role in the regulation of vascular inflammation [25]. Recently, protective characteristics of adiponectin that preserve β-cell function have also been reported [26]. Additionally, studies using adiponectin transgenic mice identify it as a longevity gene, demonstrating resistance to metabolic effects, improvement in glucose homeostasis, and amelioration of age-related tissue dysfunctions with extension of health span [4]. Elevated adiponectin level is detected in many longevity model mice, such as fat-specific insulin receptor knockout mice, the Ames dwarf mice (df/df), and GHRKO mice [27,28]. Studies in humans also indicate that higher adiponectin levels are considered crucial parameters in caloric-restricted humans and centenarians [29,30,31]. Thus, adiponectin and its related pathways are promising targets for the treatment of metabolic diseases and aging.
2.2. Fiboblast Growth Factor 21 (Fgf21)
FGF21, a subfamily of FGF, is produced by the liver, adipose tissue, and skeletal muscle [32]. FGF21 can diffuse away from the tissue of expression and function as an endocrine hormone due to a lack of the FGF heparin-binding domain [33]. FGF21 is predominantly induced in the liver under fasting conditions through peroxisome proliferator-activated receptor alpha (PPARa) [34]. Fgf21 exhibits suppressive effects on hyperglycemia and atherogenic activity [35]. It reduces plasma triglyceride levels by accelerating lipoprotein lipase (LPL)- and cluster of differentiation 36 (CD36)-mediated triglyceride disposal processes in the liver and adipose tissue, along with the thermogenesis-mediated lipid catabolic process in brown adipose tissue [36]. The metabolic effects of FGF21 require co-expression of fibroblast growth factor receptor 1c (FGFR1c) and b-klotho [37,38]. The growth reduction by FGF21 has also been demonstrated by several studies using genetically modified mouse models, in which transgenic mice overexpressing FGF21 are smaller than wild-type mice, and FGF21-knockout mice grow more than wild-type mice under food-restricted conditions [39,40]. Furthermore, the longevity-related effects of FGF21 have been indicated by findings related to the increased lifespan of transgenic Fgf21-overexpressing mice [5]. The specific mechanisms underlying the beneficial effects of FGF21 are unclear but may involve the suppression of the growth hormone (GH)/insulin-like growth factor 1 (IGF-1) signaling axis in the liver, along with adiponectin [5,41,42].
2.3. Adipsin
Adipsin, the first adipokine discovered in 1987 [43], is predominantly expressed in white adipose tissue, especially in subcutaneous adipose tissue, and is implicated in the development of obesity and type 2 diabetes [44]. Adipsin is mainly produced by adipocytes via PPARγ [45,46], and its circulating levels are decreased in obese mice [44]. Depletion of adipsin induces glucose intolerance resulting from beta-cell failure, whereas replenishment of adipsin decreases blood glucose levels through appropriate insulin secretion in obese mice, highlighting its crucial role in maintaining glucose homeostasis and beta-cell function [47]. However, a recent study showed that mice lacking adipsin suppress the expansion of marrow adipose tissue (MAT), thereby inhibiting bone loss during obesity and aging [48], indicating that adipsin has a positive association with glucose-insulin homeostasis but has a negative association with bone remodeling.
2.4. Apelin
Apelin, a regulatory peptide identified as an endogenous ligand of the G protein-coupled receptor (APJ) [49], is widely distributed in the body, including adipose tissue (mainly adipocytes), the central nervous system, the heart, skeletal muscle, and the stomach [50]. Apelin is cleaved by the cells to produce endogenous peptides such as apelin-12, -13, -17, and -36 [51]. Apelin levels in the serum and adipose tissue are upregulated in obese and insulin-resistant mice, and apelin contributes to the regulation of food intake, cell proliferation, blood pressure, lipolysis, and glucose metabolism [49,52,53]. Apelin was shown to suppress insulin resistance by increasing AMP-activated protein kinase (AMPK)-mediated glucose utilization and stimulating glucose transporter (Glut) 4, involved in the PI3K and Akt signaling pathways. Comprehensive research using apelin-knockout mice has shown it to induce hyperinsulinemia and insulin resistance [54,55], while studies using apelin treatment mice have shown beneficial functions in obesity and insulin resistance, indicating that apelin could serve as a therapeutic target for treating obesity and related diseases [56,57]. Additionally, apelin has been shown to exert protective effects against bone metabolism through proliferation of osteoblasts via the APJ/PI3k/Akt pathway. Apelin and APJ are also expressed in vascular smooth muscle cells, endothelial cells, and myocardial cells, and low apelin levels are reported in patients with heart failure, suggesting that they are involved in the myocardial response to infarction and ischemia [58,59,60]. The function of apelin in aging has been reported to be that it regulates inflammation, apoptosis, and oxidative stress, which increases during the aging process [61].
2.5. Omentin
Omentin, also known as intelectin-1, is primarily produced by visceral adipose tissue and is another potential regulator of insulin sensitivity [62,63]. Encoded by omentin-1 and omentin-2 genes, particularly omentin-1, the main circulating form is positively correlated with adiponectin and high-density lipoprotein levels and negatively correlated with BMI, insulin resistance, triglycerides, and leptin levels [64,65]. Omentin exerts anti-inflammatory effects by inhibiting TNF-α-induced cyclooxygenase-2 (COX-2) expression and Jun N-terminal kinase signaling via activation of AMPK and endothelial nitric oxide synthase [66,67]. Omentin also enhances the stability of atherosclerotic plaque by modulating macrophage viability and inflammation [68]. Furthermore, several studies have indicated a decrease in omentin levels in obesity, cancer, and various cardiovascular diseases, including carotid atherosclerosis, coronary artery disease, heart failure, and dilated cardiomyopathy [64,69,70].
2.6. Annexin
Annexins constitute 12 structurally related Ca2+- and membrane-binding proteins (AnxA1-AnxA111 and AnxA13) [71,72]. ANXA1, the first identified and extensively studied member of the annexin family, is abundantly expressed in macrophages and neutrophils, and its expression is increased in obesity [73,74]. ANXA1 has been proposed as an anti-inflammatory protein that regulates peripheral leukocyte migration and is a promoter of macrophage phagocytosis in apoptotic neutrophils [75]. Furthermore, ANXA1 is involved in protecting hepatic function, as well as regulating various adipose tissue functions, including those related to inflammation, lipolysis, lipogenesis, and adiposity [76,77]. A study employing ANXA1-knockout mice revealed accelerated hepatic inflammation and fibrosis, elevated glucose and insulin levels, increased adiposity, and decreased insulin sensitivity, emphasizing the significance of ANXA1 in these processes [73,76]. ANXA1 has also been reported to exert a protective effect in resolving inflammation and maintaining vascular homeostasis [78].
Overall, the studies on annexin 1 mentioned above show that annexin 1 alleviates metabolic and vascular diseases by regulating adipose tissue metabolism and inflammation.
2.7. Neuregulin (Nrg)
Neuregulin, a member of the epidermal growth factor (EGF) family of extracellular ligands, comprises four isoforms, namely Nrg1–4 [79]. Nrg1, an extensively studied and ubiquitously expressed protein in endothelial and mesenchymal cells, is implicated in cell proliferation, survival, migration, and differentiation [80]. Rodent studies have shown that Nrg1 reduces hepatic glucose production via the ErbB3/Akt signaling pathway [81], indicating its involvement in the regulation of glucose homeostasis. Research across various rodent species and naked mole rats, characterized by longevity, has demonstrated higher Nrg1 levels in longer-lived rodents, suggesting a potential link between Nrg1 and the longevity pathway.
Nrg4, secreted by white and brown adipose tissues, is involved in the regulation of tissue development and tumorigenesis and has been recently discovered in comparison to other adipokines [82]. Nrg4 expression in adipose tissue is lower in obese individuals but increases upon exposure to cold temperatures or epinephrine, suggesting that Nrg4 is involved in the regulation of adipose tissue innervation. However, a study using Nrg4-knockout mice showed insulin resistance under a high-fat diet, but the rectal temperature and expression of the representative thermogenic genes UCP1 and Dio2 did not change under cold stimulation, indicating that Nrg4 is not directly linked to thermogenesis in brown adipose tissue [82]. A binding assay to identify the target of Nrg4 showed that Nrg4 specifically binds to the liver and improves diet-induced fatty liver disease by attenuating the hepatic lipogenic pathway [82], suggesting that circulating Nrg4 from adipose tissue ameliorates the severity of fatty liver and insulin resistance by modulating hepatic lipogenesis.
2.8. Leptin
Leptin, a 16 kDa adipocyte-derived adipokine, is considered a potential marker for obesity-related complications such as atherosclerosis [83] and neuropathy [84]. The obese phenotype observed in ob/ob mice, characterized by leptin deletion, is associated with hyperglycemia and insulin resistance [85]. Circulating leptin levels are positively correlated with BMI and adiposity, and their levels are significantly higher in obesity [86,87]. Leptin regulates appetite and energy expenditure by inhibiting neuropeptide Y (NPY), pro-opiomelanocortin (POMC), and corticoliberin (CRH) [88,89] and enhances insulin sensitivity by increasing glucose uptake and oxidation in skeletal muscle and free fatty acid oxidation [90]. However, leptin fails to inhibit appetite and body weight in obese people due to leptin resistance, suggesting that improvement of leptin sensitivity is important for clinical treatment [91]. Owing to the opposing effects of leptin and adiponectin on inflammation and insulin resistance, their ratio has been proposed as a marker of adipose tissue dysfunction [92]. Furthermore, leptin plays a pivotal role in the regulation of satiety, fertility, puberty, activity, and fetal growth [93,94]. The function of leptin in aging has been reported to be that it enhances the vascular aging by calcification of vascular cells [95].
2.9. Resistin
Resistin was originally discovered as an adipocyte-specific hormone in rodents and was named for its ability to resist insulin action [96]. This leads to the development of obesity and type 2 diabetes mellitus [96]. Unlike rodents, human resistin is mainly expressed in peripheral blood mononuclear cells, bone marrow cells, and macrophages other than adipocytes, and it accelerates the inflammatory response via NF-κB-mediated activation of TNFa, IL16, and MCP1, classifying it as a proinflammatory molecule [97,98,99,100]. The functional variabilities between mice and human resistin may result from the difference in the 3′ introns. Mouse resistin carries a very large intron in the 3′ UTR, which has a number of regulatory sequences, including the PPAR/RXR binding element [101]. Moreover, resistin levels increase in patients with metabolic syndrome, including obese individuals, and positively correlate with BMI and white adipose tissue mass [102,103]. Resistin has also been involved in age and age-related diseases [104] and is a risk factor for all-cause mortality in elderly people, based on the Finnish cohort study [105]. The inhibition of AMPK and SIRT1, which are crucial in cellular senescence and metabolic regulation, by resistin has been proposed as a conserved mechanism underlying cellular senescence and aging in both humans and mice, despite species diversity [106,107]. These observations suggest that resistin plays a central role not only in the development of insulin resistance and inflammation but also in age and age-related diseases.
2.10. Visfatin/NAMPT
Visfatin, also known as nicotinamide phosphate ribosyltransferase (NAMPT), is a product of the pancreatic beta-cell growth factor (PBEF) gene and is predominantly produced by adipocytes and macrophages in visceral adipose tissue [108,109]. The insulin-mimetic activity of visfatin by binding to the insulin receptor, but in a distinct site from insulin, was first demonstrated by Fukuhara et al. [109]. Elevated in obesity, insulin resistance, and type 2 diabetes, visfatin stimulates triacylglycerol synthesis and storage in adipose tissue through activation of glucose uptake and lipogenesis [109,110]. Additionally, visfatin induces the expression of proinflammatory cytokines, such as TNFa, IL1b, and IL6, thereby increasing monocyte–endothelial cell adhesion [111]. The role of visfatin in health is controversial and remains unclear. Despite its positive association with obesity under calorie excess [111], visfatin reportedly improves longevity by enhancing cell survival and SIRT1 activity, as well as through its neuroprotective effects [112,113]. This discrepancy may be attributed to the existence of two distinct forms of visfatin: intravisfatin (iNAMPT), which is positively correlated with obesity under caloric excess, and circulating extravisfatin (eNAMPT), which is associated with anti-aging and longevity effects induced by the suppression of age-related physiological decline through SIRT1-mediated deacetylation of iNAMPT [114,115]. Further studies are required to clarify these controversial findings.
2.11. Chemerin
Chemerin, also known as tazarotene-induced gene (TIG)2 and retinoic acid receptor responder (RARRES)2, is primarily secreted by adipose tissue, liver, and immune cells. Chemerin regulates biological processes, such as cell proliferation and differentiation, angiogenesis, and energy metabolism [116,117,118]. Pro-chemerin is produced by the N-terminal cleavage of pre-pro-chemerin, and chemerin is formed by the C-terminal processing of pro-chemerin [119,120,121]. It was initially reported as a chemotactic factor for immune cells, including dendritic cells and macrophages [119]. Subsequently, chemerin was reported to function as an adipokine related to obesity and inflammation [116]. Chemerin was reported to be elevated in the blood, adipose tissue, and liver from obese rodents [122,123], and it is necessary for adipogenesis due to its interaction with PPARγ [118]. The angiogenic action of chemerin supports the notion that chemerin enhances adipose tissue growth by inducing angiogenesis and vascularization [124]. Although chemerin is positively correlated with inflammation and obesity [116], its role, including processing, isoforms, and biological activity in obesity, remains unclear [125]. Furthermore, chemerin acts as a ligand activator of chemokine-like receptor (CMKLR)-1 and as an initiator of innate and adaptive immune responses [126]. Human studies involving obesity and centenarians have suggested that serum chemerin levels are negatively associated with successful aging and health [127,128].
2.12. Vaspin
Visceral adipose tissue-derived serpin (vaspin), a member of the serine protease inhibitor family, is highly expressed in adipose tissue [129]. Elevated vaspin levels in rodents and humans are correlated with obesity [129,130,131]. Vaspin regulates insulin sensitivity, preadipocyte differentiation, and angiogenesis [132]. The role of vaspin in suppressing inflammation and insulin resistance was also demonstrated by a study in which administration of vaspin improved glucose tolerance and insulin sensitivity, inhibited proinflammatory cytokines, such as TNFa, resistin, and leptin, and increased levels of adiponectin and GLUT4 in the white adipose tissue of obese mice [129]. The elevated adipocytes differentiation by vaspin was also proven by a study using 3T3-L1 adipocytes in which treatment with vaspin increased expression of PPARg, CEBPa, and CEBPb [133]. Furthermore, vaspin promotes glucose uptake to skeletal muscle through GLTU4 in obese humans [134]. These findings indicate that vaspin appears to be a useful therapeutic candidate for metabolic diseases, including obesity and type 2 diabetes mellitus.
2.13. Lipocalin-2
Lipocalin-2 (LCN2), also known as neutrophil gelatinase-associated lipocalin, was initially identified as a secretory protein mainly produced by activated astrocytes and microglia [135]. LCN2 is considered an important regulator of the immune response caused by high expression during infection [136]. Recently, LCN2 has been reported as a new adipokine that is upregulated in obese mice and humans [137,138]. The critical role of LCN2 in metabolic disorders has been demonstrated by studies using LCN2-knockout mice that gained more weight and developed dyslipidemia and insulin resistance [139,140,141]. LCN2 is also involved in the regulation of TNF-mediated inflammatory signaling [142,143] and thermogenesis [144]. Furthermore, LCN2 is secreted by the bone marrow, inhibits food intake in a melanocortin 4 receptor (MC4R)-dependent manner, maintains glucose homeostasis by increasing insulin secretion, and improves glucose tolerance [145]. LCN2 is increased in aging-related brain diseases such as Alzheimer’s disease, Parkinson’s disease, and vascular dementia and is reported to play a role in suppressing neurodegenerative processes [146]. These data demonstrate that Lcn2 is regulated by metabolic stress and inflammatory and nutrient signals, suggesting a pivotal role for LCN2 in metabolic disorders and inflammatory diseases.
2.14. RBP4
Retinol-binding protein 4 (RBP4), a member of the lipocalin protein family, is the only known specific transport protein responsible for delivering retinol (vitamin A) in the circulatory system [147,148,149]. RBP4 is primarily produced by the liver and adipose tissue, and its expression is elevated in insulin-resistant mice and humans with obesity and type 2 diabetes [150,151,152]. A study using genetically modified mice showed that transgenic overexpression of RBP4 caused insulin resistance, whereas genetic deletion of RBP4 enhanced insulin sensitivity [152]. The mechanisms of RBP4 involved in insulin sensitization have been demonstrated to be that it alters insulin sensitivity by affecting insulin signaling in muscles through modulation of tyrosine phosphorylation of IRS1 and PI3K activation [152]. The effects of RBP4 on whole-body glucose metabolism were further proven by studies using muscle-specific RBP4 transgenic mice with glucose intolerance and insulin resistance [153]. Furthermore, the proinflammatory effects of RBP4 have shown that RBP4 primes the NLRP3 inflammasome partially through toll-like receptor 4 (TLR4) and TLR2 in macrophages, which impairs insulin signaling in adipocytes [154,155]. The elevated circulating RBP4 level is also associated with hepatic lipid accumulation and liver steatosis in humans [151,156], The study using the NAFLD model mice further showed that the acceleration of NAFLD in RBP4 transgenic mice was mainly attributed to reduced mitochondrial content and impaired mitochondrial fatty acid β-oxidation [157]. Thus, RBP4 contributes to the development of obesity and its associated diseases, including NAFLD.
Overall, the regulation of RBP4 is a novel therapeutic approach for the deterioration of lipid metabolism.
2.15. Fetuin A
Fetuin A (FetA), also known as alpha-2-Heremans-Schmid glycoprotein, is mainly produced by the liver, but it is extensively expressed by multiple tissues, such as adipose tissue, kidneys, the brain, and skin [158,159]. FetA was initially identified as an inhibitor of insulin receptor tyrosine kinase in the muscles and liver [160,161]. As such, FetA, which is involved in the formation of insulin receptors, induces insulin resistance with inflammation, causing metabolic disorders, including type 2 diabetes mellitus and nonalcoholic fatty liver disease [162,163]. The FetA/adiponectin ratio has been proposed as a sensitive indicator for evaluating metabolic syndrome in the elderly [164]. FetA also plays a role in anti-apoptotic action by inhibiting proteolytic cleavage and caspase activity [165]. Furthermore, FetA has been reported to regulate PPARγ phosphorylation at serine 273 through the RAas-MEK-ERK pathway, which inhibits the insulin-sensitizing and anti-inflammatory effects of adiponectin [166,167]. Inhibitory phosphorylation of PPARγ by FetA has been shown to inhibit adipogenesis and impair adipocyte function through crosstalk with CD36 [168,169]. The effects of FetA on brain function, including brain development, neuroprotection, and innate immunity, have also been reported [170,171]. Taken together, FetA may have therapeutic and diagnostic roles in the treatment of metabolic diseases.
3. Age-Related Changes in Adipose Tissue and Adipokines
The redistribution of adipose tissue in aging with increased visceral adipose tissue and decreased subcutaneous adipose tissue [172] results in an increase in inflammatory cytokines, which trigger metabolic disorders, such as obesity and type 2 diabetes mellitus [15]. Age-related accumulation of visceral adipose tissue also negatively affects cardiac and brain functions [173,174]. The dysregulation of adipokines caused by abnormal accumulation of visceral fat has been shown in the phenotypes of metabolic diseases, as well as aging. An age-related increase in adipokines (adiponectin, leptin, adipsin, vaspin, resistin, and chemerin) [31,175,176,177,178,179] and age-related decrease in adipokines (FGF21, annexin A1, and visfatin) [78,180,181] have been reported in humans. The construction of an aging adipokine profile based on these human studies of adipokines that changed with aging will contribute to extending health span through regulation of adipose tissue function.
4. Adipokines Viewed from Caloric Restriction and Centenarian Studies
Caloric restriction (CR), a decreased calorie intake with maintenance of adequate nutrition, not only reduces the risk of metabolic syndrome, including obesity and diabetes, but also extends the lifespan of numerous species, ranging from yeast to primates [182,183,184]. These beneficial functions of CR have also been gradually proven by human caloric restriction and centenarian studies [185,186,187]. It is known that the beneficial functions of CR in metabolic homeostasis and lifespan extension are due to increased insulin sensitivity and improved adipose tissue function; however, several studies place greater emphasis on the importance of adipose tissue function because even in mTORC2-knockout mice with induced insulin resistance, the beneficial functions of CR are maintained, and some long-lived mice do not show an increase in insulin sensitivity [188,189].
CR improves energy efficiency by increasing the utilization of fat, which has higher calories per gram than carbohydrates, leading to metabolic homeostasis being maintained and lifespan being extended by suppressing adiposity and maintaining adipose tissue function. It has been reported that adipokines secreted by adipose tissue, in particular adiponectin, which positively correlates with CR, and leptin and resistin, which negatively correlate with CR, play an important role in adipose tissue function and other health benefits including maintenance of glucose homeostasis [190,191,192] in humans. Furthermore, in studies of centenarians, CR is established as an eating habit of the majority centenarians, and increased adiponectin levels were considered as their common phenotype [31,193]. Although the detailed mechanism of how improved fat regulation contributes to lifespan extension has not yet been accurately reported, adipokine regulation is likely to be at least partially involved.
5. Conclusions
Since the discovery of leptin in 1994, numerous bioactive molecules have been discovered in adipose tissue. Adipokines play crucial roles in glucose homeostasis, fat metabolism, and inflammation. Their discovery emphasized the significance of adipose tissue as a representative endocrine organ that regulates obesity and obesity-related metabolic diseases. In particular, adiponectin and FGF21, which are induced by fasting or caloric restriction, have diverse roles in various tissues controlling metabolic diseases, as well as in delaying aging and promoting longevity (Figure 2). They are anticipated to act as vital mediators for extending health span, which has consistently been a focus area of global research. Notably, studies on centenarians have revealed high adiponectin levels and decreased adiposity, indicating the existence of protective phenotypes associated with longevity and healthy aging in humans. Establishing in-depth research and profiling of adipokines through human studies of caloric restriction and centenarians will help uncover new mechanisms for obesity and anti-aging and develop treatments for them.
Figure 2.
Regulation of physiological functions by adiponectin and FGF21. Adiponectin and FGF21, which are induced by fasting or caloric restriction (CR), have diverse roles in various tissues controlling metabolic diseases and promoting longevity.
Author Contributions
Conceptualization and writing, S.P.; review and funding acquisition, I.S. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
Author Isao Shimokawa was employed by SAGL, Limited Liability Company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The SAGL, Limited Liability Company in affiliation had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
This research was funded by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science, grant number 23H03331 and 19H04033.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Chouchani E.T., Kajimura S. Metabolic adaptation and maladaptation in adipose tissue. Nat. Metab. 2019;1:189–200. doi: 10.1038/s42255-018-0021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawai T., Autieri M.V., Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021;320:C375–C391. doi: 10.1152/ajpcell.00379.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longo M., Zatterale F., Naderi J., Parrillo L., Formisano P., Raciti G.A., Beguinot F., Miele C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019;20:2358. doi: 10.3390/ijms20092358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan T., Muise E.S., Iyengar P., Wang Z.V., Chandalia M., Abate N., Zhang B.B., Bonaldo P., Chua S., Scherer P.E. Metabolic dysregulation and adipose tissue fibrosis: Role of collagen VI. Mol. Cell. Biol. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furukawa S., Fujita T., Shimabukuro M., Iwaki M., Yamada Y., Nakajima Y., Nakayama O., Makishima M., Matsuda M., Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sung H.K., Doh K.O., Son J.E., Park J.G., Bae Y., Choi S., Nelson S.M., Cowling R., Nagy K., Michael I.P., et al. Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab. 2013;17:61–72. doi: 10.1016/j.cmet.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Lumeng C.N., Bodzin J.L., Saltiel A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frasca D., Blomberg B.B., Paganelli R. Aging, Obesity, and Inflammatory Age-Related Diseases. Front. Immunol. 2017;8:1745. doi: 10.3389/fimmu.2017.01745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frasca D., Blomberg B.B. Adipose tissue, immune aging, and cellular senescence. Semin. Immunopathol. 2020;42:573–587. doi: 10.1007/s00281-020-00812-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdelaal M., le Roux C.W., Docherty N.G. Morbidity and mortality associated with obesity. Ann. Transl. Med. 2017;5:161. doi: 10.21037/atm.2017.03.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhaskaran K., Dos-Santos-Silva I., Leon D.A., Douglas I.J., Smeeth L. Association of BMI with overall and cause-specific mortality: A population-based cohort study of 3.6 million adults in the UK. Lancet Diabetes Endocrinol. 2018;6:944–953. doi: 10.1016/S2213-8587(18)30288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhana K., Nano J., Ligthart S., Peeters A., Hofman A., Nusselder W., Dehghan A., Franco O.H. Obesity and Life Expectancy with and without Diabetes in Adults Aged 55 Years and Older in the Netherlands: A Prospective Cohort Study. PLoS Med. 2016;13:e1002086. doi: 10.1371/journal.pmed.1002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barzilai N., She L., Liu B.Q., Vuguin P., Cohen P., Wang J., Rossetti L., Barzilai N., She L., Liu B.Q., et al. Surgical removal of visceral fat reverses hepatic insulin resistance. Diabetes. 1999;48:94–98. doi: 10.2337/diabetes.48.1.94. [DOI] [PubMed] [Google Scholar]
- 14.Muzumdar R., Allison D.B., Huffman D.M., Ma X., Atzmon G., Einstein F.H., Fishman S., Poduval A.D., McVei T., Keith S.W., et al. Visceral adipose tissue modulates mammalian longevity. Aging Cell. 2008;7:438–440. doi: 10.1111/j.1474-9726.2008.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ou M.Y., Zhang H., Tan P.C., Zhou S.B., Li Q.F. Adipose tissue aging: Mechanisms and therapeutic implications. Cell Death Dis. 2022;13:300. doi: 10.1038/s41419-022-04752-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zorena K., Jachimowicz-Duda O., Ślęzak D., Robakowska M., Mrugacz M. Adipokines and Obesity. Potential Link to Met abolic Disorders and Chronic Complications. Int. J. Mol. Sci. 2020;21:3570. doi: 10.3390/ijms21103570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. Hallmarks of aging: An expanding universe. Cell. 2023;186:243–278. doi: 10.1016/j.cell.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Li N., Zhao S., Zhang Z., Zhu Y., Gliniak C.M., Vishvanath L., An Y.A., Wang M.Y., Deng Y., Zhu Q., et al. Adiponectin preserves metabolic fitness during aging. eLife. 2021;10:e65108. doi: 10.7554/eLife.65108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y., Xie Y., Berglund E.D., Coate K.C., He T.T., Katafuchi T., Xiao G., Potthoff M.J., Wei W., Wan Y., et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife. 2012;1:e00065. doi: 10.7554/eLife.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scherer P.E., Williams S., Fogliano M., Baldini G., Lodish H.F. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 21.Ouchi N., Kihara S., Funahashi T., Matsuzawa Y., Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr. Opin. Lipidol. 2003;14:561–566. doi: 10.1097/00041433-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Freitas Lima L.C., Braga V.A., do Socorro de França Silva M., Cruz J.C., Sousa Santos S.H., de Oliveira Monteiro M.M., Balarini C.M. Adipokines, diabetes and atherosclerosis: An inflammatory association. Front. Physiol. 2015;6:304. doi: 10.3389/fphys.2015.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ouchi N., Walsh K. Adiponectin as an anti-inflammatory factor. Clin. Chim. Acta. 2007;380:24–30. doi: 10.1016/j.cca.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hajri T., Tao H., Wattacheril J., Marks-Shulman P., Abumrad N.N. Regulation of adiponectin production by insulin: Interactions with tumor necrosis factor-α and interleukin-6. Am. J. Physiol. Endocrinol. Metab. 2011;300:E350–E360. doi: 10.1152/ajpendo.00307.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumada M., Kihara S., Ouchi N., Kobayashi H., Okamoto Y., Ohashi K., Maeda K., Nagaretani H., Kishida K., Maeda N., et al. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109:2046–2049. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- 26.Munhoz A.C., Serna J.D.C., Vilas-Boas E.A., Caldeira da Silva C.C., Santos T.G., Mosele F.C., Felisbino S.L., Martins V.R., Kowaltowski A.J. Adiponectin reverses β-Cell damage and impaired insulin secretion induced by obesity. Aging Cell. 2023;22:e13827. doi: 10.1111/acel.13827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blüher M., Kahn B.B., Kahn C.R. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 28.Bartke A., Wright J.C., Mattison J.A., Ingram D.K., Miller R.A., Roth G.S. Extending the lifespan of long-lived mice. Nature. 2001;414:412. doi: 10.1038/35106646. [DOI] [PubMed] [Google Scholar]
- 29.Ott B., Skurk T., Hastreiter L., Lagkouvardos I., Fischer S., Büttner J., Kellerer T., Clavel T., Rychlik M., Haller D., et al. Effect of caloric restriction on gut permeability, inflammation markers, and fecal microbiota in obese women. Sci. Rep. 2017;7:11955. doi: 10.1038/s41598-017-12109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pareja-Galeano H., Santos-Lozano A., Sanchis-Gomar F., Fiuza-Luces C., Garatachea N., Gálvez B.G., Lucia A., Emanuele E. Circulating leptin and adiponectin concentrations in healthy exceptional longevity. Mech. Ageing Dev. 2017;162:129–132. doi: 10.1016/j.mad.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 31.Arai Y., Kamide K., Hirose N. Adipokines and Aging: Findings from Centenarians and the Very Old. Front. Endocrinol. 2019;10:142. doi: 10.3389/fendo.2019.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Itoh N. FGF21 as a Hepatokine, Adipokine, and Myokine in Metabolism and Diseases. Front. Endocrinol. 2014;5:107. doi: 10.3389/fendo.2014.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishimura T., Nakatake Y., Konishi M., Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim. Biophys. Acta. 2000;1492:203–206. doi: 10.1016/S0167-4781(00)00067-1. [DOI] [PubMed] [Google Scholar]
- 34.Inagaki T., Dutchak P., Zhao G., Ding X., Gautron L., Parameswara V., Li Y., Goetz R., Mohammadi M., Esser V., et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Gimeno R.E., Moller D.E. FGF21-based pharmacotherapy--potential utility for metabolic disorders. Trends Endocrinol. Metab. 2014;25:303–311. doi: 10.1016/j.tem.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Schlein C., Talukdar S., Heine M., Fischer A.W., Krott L.M., Nilsson S.K., Brenner M.B., Heeren J., Scheja L. FGF21 Lowers Plasma Triglycerides by Accelerating Lipoprotein Catabolism in White and Brown Adipose Tissues. Cell Metab. 2016;23:441–453. doi: 10.1016/j.cmet.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Ogawa Y., Kurosu H., Yamamoto M., Nandi A., Rosenblatt K.P., Goetz R., Eliseenkova A.V., Mohammadi M., Kuro-o M. BetaKlotho is required for metabolic activity of fibroblast growth factor. Proc. Natl. Acad. Sci. USA. 2007;104:7432–7437. doi: 10.1073/pnas.0701600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kharitonenkov A., Dunbar J.D., Bina H.A., Bright S., Moyers J.S., Zhang C., Ding L., Micanovic R., Mehrbod S.F., Knierman M.D., et al. FGF-21/FGF-21 receptor interaction and activation is determined by betaKlotho. J. Cell Physiol. 2008;215:1–7. doi: 10.1002/jcp.21357. [DOI] [PubMed] [Google Scholar]
- 39.Inagaki T., Lin V.Y., Goetz R., Mohammadi M., Mangelsdorf D.J., Kliewer S.A. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 2008;8:77–83. doi: 10.1016/j.cmet.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kubicky R.A., Wu S., Kharitonenkov A., De Luca F. Role of fibroblast growth factor 21 (FGF21) in undernutrition-related attenuation of growth in mice. Endocrinology. 2012;153:2287–2295. doi: 10.1210/en.2011-1909. [DOI] [PubMed] [Google Scholar]
- 41.Lin Z., Tian H., Lam K.S., Lin S., Hoo R.C., Konishi M., Itoh N., Wang Y., Bornstein S.R., Xu A., et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013;17:779–789. doi: 10.1016/j.cmet.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Holland W.L., Adams A.C., Brozinick J.T., Bui H.H., Miyauchi Y., Kusminski C.M., Bauer S.M., Wade M., Singhal E., Cheng C.C., et al. An FGF21-Adiponectin-Ceramide Axis Controls Energy Expenditure and Insulin Action in Mice. Cell Metab. 2013;17:790–797. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cook K.S., Min H.Y., Johnson D., Chaplinsky R.J., Flier J.S., Hunt C.R., Spiegelman B.M. Adipsin: A circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science. 1987;237:402–405. doi: 10.1126/science.3299705. [DOI] [PubMed] [Google Scholar]
- 44.Flier J.S., Cook K.S., Usher P., Spiegelman B.M. Severely impaired adipsin expression in genetic and acquired obesity. Science. 1987;237:405–408. doi: 10.1126/science.3299706. [DOI] [PubMed] [Google Scholar]
- 45.Choy L.N., Rosen B.S., Spiegelman B.M. Adipsin and an endogenous pathway of complement from adipose cells. J. Biol. Chem. 1992;267:12736–12741. doi: 10.1016/S0021-9258(18)42338-1. [DOI] [PubMed] [Google Scholar]
- 46.Tontonoz P., Hu E., Spiegelman B.M. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-X. [DOI] [PubMed] [Google Scholar]
- 47.Lo J.C., Ljubicic S., Leibiger B., Kern M., Leibiger I.B., Moede T., Kelly M.E., Chatterjee Bhowmick D., Murano I., Cohen P., et al. Adipsin is an adipokine that improves β cell function in diabetes. Cell. 2014;158:41–53. doi: 10.1016/j.cell.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aaron N., Kraakman M.J., Zhou Q., Liu Q., Costa S., Yang J., Liu L., Yu L., Wang L., He Y., et al. Adipsin promotes bone marrow adiposity by priming mesenchymal stem cells. eLife. 2021;10:e69209. doi: 10.7554/eLife.69209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tatemoto K., Hosoya M., Habata Y., Fujii R., Kakegawa T., Zou M.X., Kawamata Y., Fukusumi S., Hinuma S., Kitada C., et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem. Biophys. Res. Commun. 1998;251:471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 50.Boucher J., Masri B., Daviaud D., Gesta S., Guigné C., Mazzucotelli A., Castan-Laurell I., Tack I., Knibiehler B., Carpéné C., et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology. 2005;146:1764–1771. doi: 10.1210/en.2004-1427. [DOI] [PubMed] [Google Scholar]
- 51.Cirillo P., Ziviello F., Pellegrino G., Conte S., Cimmino G., Giaquinto A., Pacifico F., Leonardi A., Golino P., Trimarco B. The adipokine apelin-13 induces expression of prothrombotic tissue factor. Thromb. Haemost. 2015;113:363–372. doi: 10.1160/TH14-05-0451. [DOI] [PubMed] [Google Scholar]
- 52.Castan-Laurell I., Masri B., Valet P. The apelin/APJ system as a therapeutic target in metabolic diseases. Expert Opin. Ther. Targets. 2019;23:215–225. doi: 10.1080/14728222.2019.1561871. [DOI] [PubMed] [Google Scholar]
- 53.Hu G., Wang Z., Zhang R., Sun W., Chen X. The Role of Apelin/Apelin Receptor in Energy Metabolism and Water Homeostasis: A Comprehensive Narrative Review. Front. Physiol. 2021;12:632886. doi: 10.3389/fphys.2021.632886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li L., Yang G., Li Q., Tang Y., Yang M., Yang H., Li K. Changes and relations of circulating visfatin, apelin, and resistin levels in normal, impaired glucose tolerance, and type 2 diabetic subjects. Exp. Clin. Endocrinol. Diabetes. 2006;114:544–548. doi: 10.1055/s-2006-948309. [DOI] [PubMed] [Google Scholar]
- 55.Soriguer F., Garrido-Sanchez L., Garcia-Serrano S., Garcia-Almeida J.M., Garcia-Arnes J., Tinahones F.J., Garcia-Fuentes E. Apelin levels are increased in morbidly obese subjects with type 2 diabetes mellitus. Obes. Surg. 2009;19:1574–1580. doi: 10.1007/s11695-009-9955-y. [DOI] [PubMed] [Google Scholar]
- 56.Attané C., Foussal C., Le Gonidec S., Benani A., Daviaud D., Wanecq E., Guzmán-Ruiz R., Dray C., Bezaire V., Rancoule C., et al. Apelin treatment increases complete Fatty Acid oxidation, mitochondrial oxidative capacity, and biogenesis in muscle of insulin-resistant mice. Diabetes. 2012;61:310–320. doi: 10.2337/db11-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li M., Fang H., Hu J. Apelin-13 ameliorates metabolic and cardiovascular disorders in a rat model of type 2 diabetes with a high-fat diet. Mol. Med. Rep. 2018;18:5784–5790. doi: 10.3892/mmr.2018.9607. [DOI] [PubMed] [Google Scholar]
- 58.Maguire J.J., Kleinz M.J., Pitkin S.L., Davenport A.P. [Pyr1]apelin-13 identified as the predominant apelin isoform in the human heart: Vasoactive mechanisms and inotropic action in disease. Hypertension. 2009;54:598–604. doi: 10.1161/HYPERTENSIONAHA.109.134619. [DOI] [PubMed] [Google Scholar]
- 59.Yu X.H., Tang Z.B., Liu L.J., Qian H., Tang S.L., Zhang D.W., Tian G.P., Tang C.K. Apelin and its receptor APJ in cardiovascular diseases. Clin. Chim. Acta. 2014;428:1–8. doi: 10.1016/j.cca.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 60.Chong K.S., Gardner R.S., Morton J.J., Ashley E.A., McDonagh T.A. Plasma concentrations of the novel peptide apelin are decreased in patients with chronic heart failure. Eur. J. Heart Fail. 2006;8:355–360. doi: 10.1016/j.ejheart.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 61.Zhou Q., Chen L., Tang M., Guo Y., Li L. Apelin/APJ system: A novel promising target for anti-aging intervention. Clin. Chim. Acta. 2018;487:233–240. doi: 10.1016/j.cca.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 62.Yue P., Jin H., Aillaud M., Deng A.C., Azuma J., Asagami T., Kundu R.K., Reaven G.M., Quertermous T., Tsao P.S. Apelin is necessary for the maintenance of insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010;298:E59–E67. doi: 10.1152/ajpendo.00385.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang R.Z., Lee M.J., Hu H., Pray J., Wu H.B., Hansen B.C., Shuldiner A.R., Fried S.K., McLenithan J.C., Gong D.W. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: Possible role in modulating insulin action. Am. J. Physiol. Endocrinol. Metab. 2006;290:E1253–E2361. doi: 10.1152/ajpendo.00572.2004. [DOI] [PubMed] [Google Scholar]
- 64.Pan H.Y., Guo L., Li Q. Changes of serum omentin-1 levels in normal subjects and in patients with impaired glucose regulation and with newly diagnosed and untreated type 2 diabetes. Diabetes Res. Clin. Pract. 2010;88:29–33. doi: 10.1016/j.diabres.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 65.de Souza Batista C.M., Yang R.Z., Lee M.J., Glynn N.M., Yu D.Z., Pray J., Ndubuizu K., Patil S., Schwartz A., Kligman M., et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 2007;56:1655–1661. doi: 10.2337/db06-1506. [DOI] [PubMed] [Google Scholar]
- 66.Tan B.K., Adya R., Farhatullah S., Lewandowski K.C., O’Hare P., Lehnert H., Randeva H.S. Omentin-1, a novel adipokine, is decreased in overweight insulin-resistant women with polycystic ovary syndrome: Ex vivo and in vivo regulation of omentin-1 by insulin and glucose. Diabetes. 2008;57:801–808. doi: 10.2337/db07-0990. [DOI] [PubMed] [Google Scholar]
- 67.Yamawaki H., Kuramoto J., Kameshima S., Usui T., Okada M., Hara Y. Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochem. Biophys. Res. Commun. 2011;408:339–343. doi: 10.1016/j.bbrc.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 68.Lin X., Sun Y., Yang S., Yu M., Pan L., Yang J., Yang J., Shao Q., Liu J., Liu Y., et al. Omentin-1 Modulates Macrophage Function via Integrin Receptors αvβ3 and αvβ5 and Reverses Plaque Vulnerability in Animal Models of Atherosclerosis. Front. Cardiovasc. Med. 2021;8:757926. doi: 10.3389/fcvm.2021.757926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shibata R., Takahashi R., Kataoka Y., Ohashi K., Ikeda N., Kihara S., Murohara T., Ouchi N. Association of a fat-derived plasma protein omentin with carotid artery intima-media thickness in apparently healthy men. Hypertens. Res. 2011;34:1309–1312. doi: 10.1038/hr.2011.130. [DOI] [PubMed] [Google Scholar]
- 70.Shibata R., Ouchi N., Kikuchi R., Takahashi R., Takeshita K., Kataoka Y., Ohashi K., Ikeda N., Kihara S., Murohara T. Circulating omentin is associated with coronary artery disease in men. Atherosclerosis. 2011;219:811–814. doi: 10.1016/j.atherosclerosis.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 71.Gerke V., Creutz C.E., Moss S.E. Annexins: Linking Ca2+ signalling to membrane dynamics. Nat. Rev. Mol. Cell. Biol. 2005;6:449–461. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- 72.Moss S.E., Morgan R.O. The annexins. Genome Biol. 2004;5:219. doi: 10.1186/gb-2004-5-4-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Akasheh R.T., Pini M., Pang J., Fantuzzi G. Increased adiposity in annexin A1-deficient mice. PLoS ONE. 2013;8:e82608. doi: 10.1371/journal.pone.0082608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aguilera C.M., Gomez-Llorente C., Tofe I., Gil-Campos M., Cañete R., Gil Á. Genome-wide expression in visceral adipose tissue from obese prepubertal children. Int. J. Mol. Sci. 2015;16:7723–7737. doi: 10.3390/ijms16047723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perretti M., D’Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat. Rev. Immunol. 2009;9:62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- 76.Locatelli I., Sutti S., Jindal A., Vacchiano M., Bozzola C., Reutelingsperger C., Kusters D., Bena S., Parola M., Paternostro C., et al. Endogenous annexin A1 is a novel protective determinant in nonalcoholic steatohepatitis in mice. Hepatology. 2014;60:531–544. doi: 10.1002/hep.27141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grewal T., Enrich C., Rentero C., Buechler C. Annexins in Adipose Tissue: Novel Players in Obesity. Int. J. Mol. Sci. 2019;20:3449. doi: 10.3390/ijms20143449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.You Q., Ke Y., Chen X., Yan W., Li D., Chen L., Wang R., Yu J., Hong H. Loss of Endothelial Annexin A1 Aggravates Inflammation-Induched Vascular Aging. Adv. Sci. 2024:e2307040. doi: 10.1002/advs.202307040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Falls D.L. Neuregulins: Functions, forms, and signaling strategies. Exp. Cell Res. 2003;284:14–30. doi: 10.1016/S0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 80.Meyer D., Yamaai T., Garratt A., Riethmacher-Sonnenberg E., Kane D., Theill L.E., Birchmeier C. Isoform-specific expression and function of neuregulin. Development. 1997;124:3575–3586. doi: 10.1242/dev.124.18.3575. [DOI] [PubMed] [Google Scholar]
- 81.Caillaud K., Boisseau N., Ennequin G., Chavanelle V., Etienne M., Li X., Denis P., Dardevet D., Lacampagne A., Sirvent P. Neuregulin 1 improves glucose tolerance in adult and old rats. Diabetes Metab. 2016;42:96–104. doi: 10.1016/j.diabet.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 82.Wang G.X., Zhao X.Y., Meng Z.X., Kern M., Dietrich A., Chen Z., Cozacov Z., Zhou D., Okunade A.L., Su X., et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat. Med. 2014;20:1436–1443. doi: 10.1038/nm.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.83 Csongrádi É., Káplár M., Nagy B., Jr., Koch C.A., Juhász A., Bajnok L., Varga Z., Seres I., Karányi Z., Magyar M.T., et al. Adipokines as atherothrombotic risk factors in obese subjects: Associations with haemostatic markers and common carotid wall thickness. Nutr. Metab. Cardiovasc. Dis. 2017;27:571–580. doi: 10.1016/j.numecd.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 84.Jung C.H., Kim B.Y., Mok J.O., Kang S.K., Kim C.H. Association between serum adipocytokine levels and microangiopathies in patients with type 2 diabetes mellitus. J. Diabetes Investig. 2014;5:333–339. doi: 10.1111/jdi.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J.M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 86.Flehmig G., Scholz M., Klöting N., Fasshauer M., Tönjes A., Stumvoll M., Youn B.S., Blüher M. Identification of adipokine clusters related to parameters of fat mass, insulin sensitivity and inflammation. PLoS ONE. 2014;9:e99785. doi: 10.1371/journal.pone.0099785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chandra A., Neeland I.J., Berry J.D., Ayers C.R., Rohatgi A., Das S.R., Khera A., McGuire D.K., de Lemos J.A., Turer A.T. The relationship of body mass and fat distribution with incident hypertension: Observations from the Dallas Heart Study. J. Am. Coll. Cardiol. 2014;64:997–1002. doi: 10.1016/j.jacc.2014.05.057. [DOI] [PubMed] [Google Scholar]
- 88.Guan X.M., Yu H., Van der Ploeg L.H. Evidence of altered hypothalamic pro-opiomelanocortin/neuropeptide Y mRNA expression in tubby mice. Brain Res. Mol. Brain Res. 1998;59:273–279. doi: 10.1016/S0169-328X(98)00150-8. [DOI] [PubMed] [Google Scholar]
- 89.Ge T.T., Yao X.X., Zhao F.L., Zou X.H., Yang W., Cui R.J., Li B.J. Role of leptin in the regulation of food intake in fasted mice. J. Cell. Mol. Med. 2020;24:4524–4532. doi: 10.1111/jcmm.15110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shimabukuro M., Koyama K., Chen G., Wang M.Y., Trieu F., Lee Y., Newgard C.B., Unger R.H. Direct antidiabetic effect of leptin through triglyceride depletion of tissues. Proc. Natl. Acad. Sci. USA. 1997;94:4637–4641. doi: 10.1073/pnas.94.9.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Savage D.B., O’Rahilly S. Leptin: A novel therapeutic role in lipodystrophy. J. Clin. Investig. 2002;109:1285–1286. doi: 10.1172/JCI0215326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Frühbeck G., Catalán V., Rodríguez A., Gómez-Ambrosi J. Adiponectin-leptin ratio: A promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte. 2018;7:57–62. doi: 10.1080/21623945.2017.1402151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blüher M., Mantzoros C.S. From leptin to other adipokines in health and disease: Facts and expectations at the beginning of the 21st century. Metabolism. 2015;64:131–145. doi: 10.1016/j.metabol.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 94.Mantzoros C.S., Magkos F., Brinkoetter M., Sienkiewicz E., Dardeno T.A., Kim S.Y., Hamnvik O.P., Koniaris A. Leptin in human physiology and pathophysiology. Am. J. Physiol. Endocrinol. Metab. 2011;301:E567–E584. doi: 10.1152/ajpendo.00315.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Parhami F., Tintut Y., Ballard A., Fogelman A.M., Demer L.L. Leptin enhances the calcification of vascular cells: Artery wall as a target of leptin. Circ. Res. 2001;88:954–960. doi: 10.1161/hh0901.090975. [DOI] [PubMed] [Google Scholar]
- 96.Steppan C.M., Bailey S.T., Bhat S., Brown E.J., Banerjee R.R., Wright C.M., Patel H.R., Ahima R.S., Lazar M.A. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 97.Lehrke M., Reilly M.P., Millington S.C., Iqbal N., Rader D.J., Lazar M.A. An inflammatory cascade leading to hyperresistinemia in humans. PLoS Med. 2004;1:e45. doi: 10.1371/journal.pmed.0010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Silswal N., Singh A.K., Aruna B., Mukhopadhyay S., Ghosh S., Ehtesham N.Z. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem. Biophys. Res. Commun. 2005;334:1092–1101. doi: 10.1016/j.bbrc.2005.06.202. [DOI] [PubMed] [Google Scholar]
- 99.Fain J.N., Cheema P.S., Bahouth S.W., Hiler M.L. Resistin release by human adipose tissue explants in primary culture. Biochem. Biophys. Res. Commun. 2003;300:674–678. doi: 10.1016/S0006-291X(02)02864-4. [DOI] [PubMed] [Google Scholar]
- 100.Patel L., Buckels A.C., Kinghorn I.J., Murdock P.R., Holbrook J.D., Plumpton C., Macphee C.H., Smith S.A. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem. Biophys. Res. Commun. 2003;300:472–476. doi: 10.1016/S0006-291X(02)02841-3. [DOI] [PubMed] [Google Scholar]
- 101.Ghosh S., Singh A.K., Aruna B., Mukhopadhyay S., Ehtesham N.Z. The genomic organization of mouse resistin reveals major differences from the human resistin: Functional implications. Gene. 2003;305:27–34. doi: 10.1016/S0378-1119(02)01213-1. [DOI] [PubMed] [Google Scholar]
- 102.Filková M., Haluzík M., Gay S., Senolt L. The role of resistin as a regulator of inflammation: Implications for various human pathologies. Clin. Immunol. 2009;133:157–170. doi: 10.1016/j.clim.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 103.Gnacińska M., Małgorzewicz S., Stojek M., Łysiak-Szydłowska W., Sworczak K. Role of adipokines in complications related to obesity: A review. Adv. Med. Sci. 2009;54:150–157. doi: 10.2478/v10039-009-0035-2. [DOI] [PubMed] [Google Scholar]
- 104.Cardoso A.L., Fernandes A., Aguilar-Pimentel J.A., de Angelis M.H., Guedes J.R., Brito M.A., Ortolano S., Pani G., Athanasopoulou S., Gonos E.S., et al. Towards frailty biomarkers: Candidates from genes and pathways regulated in aging and age-related diseases. Ageing Res. Rev. 2018;47:214–277. doi: 10.1016/j.arr.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 105.Parkkila K., Kiviniemi A., Tulppo M., Perkiömäki J., Kesäniemi Y.A., Ukkola O. Resistin is a risk factor for all-cause mortality in elderly Finnish population: A prospective study in the OPERA cohort. PLoS ONE. 2021;16:e0248015. doi: 10.1371/journal.pone.0248015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yu A., Zheng Y., Zhang R., Huang J., Zhu Z., Zhou R., Jin D., Yang Z. Resistin impairs SIRT1 function and induces senescence-associated phenotype in hepatocytes. Mol. Cell. Endocrinol. 2013;377:23–32. doi: 10.1016/j.mce.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 107.Ruderman N.B., Xu X.J., Nelson L., Cacicedo J.M., Saha A.K., Lan F., Ido Y. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010;298:E751–E760. doi: 10.1152/ajpendo.00745.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Samal B., Sun Y., Stearns G., Xie C., Suggs S., McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol. Cell. Biol. 1994;14:1431–1437. doi: 10.1128/mcb.14.2.1431-1437.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fukuhara A., Matsuda M., Nishizawa M., Segawa K., Tanaka M., Kishimoto K., Matsuki Y., Murakami M., Ichisaka T., Murakami H., et al. Visfatin: A protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–430. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 110.Chang Y.H., Chang D.M., Lin K.C., Shin S.J., Lee Y.J. Visfatin in overweight/obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases: A meta-analysis and systemic review. Diabetes Metab. Res. Rev. 2011;27:515–527. doi: 10.1002/dmrr.1201. [DOI] [PubMed] [Google Scholar]
- 111.Lin Y.T., Chen L.K., Jian D.Y., Hsu T.C., Huang W.C., Kuan T.T., Wu S.Y., Kwok C.F., Ho L.T., Juan C.C. Visfatin Promotes Monocyte Adhesion by Upregulating ICAM-1 and VCAM-1 Expression in Endothelial Cells via Activation of p38-PI3K-Akt Signaling and Subsequent ROS Production and IKK/NF-κB Activation. Cell Physiol. Biochem. 2019;52:1398–1411. doi: 10.33594/000000098. [DOI] [PubMed] [Google Scholar]
- 112.Araki T., Sasaki Y., Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- 113.Pillai J.B., Isbatan A., Imai S., Gupta M.P. Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2alpha deacetylase activity. J. Biol. Chem. 2005;280:43121–43130. doi: 10.1074/jbc.M506162200. [DOI] [PubMed] [Google Scholar]
- 114.Nielsen K.N., Peics J., Ma T., Karavaeva I., Dall M., Chubanava S., Basse A.L., Dmytriyeva O., Treebak J.T., Gerhart-Hines Z. NAMPT-mediated NAD+ biosynthesis is indispensable for adipose tissue plasticity and development of obesity. Mol. Metab. 2018;11:178–188. doi: 10.1016/j.molmet.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yoshida M., Satoh A., Lin J.B., Mills K.F., Sasaki Y., Rensing N., Wong M., Apte R.S., Imai S.I. Extracellular Vesicle-Contained eNAMPT Delays Aging and Extends Lifespan in Mice. Cell Metab. 2019;30:329–342.e5. doi: 10.1016/j.cmet.2019.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ernst M.C., Sinal C.J. Chemerin: At the crossroads of inflammation and obesity. Trends Endocrinol. Metab. 2010;21:660–667. doi: 10.1016/j.tem.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 117.Muruganandan S., Roman A.A., Sinal C.J. Role of chemerin/CMKLR1 signaling in adipogenesis and osteoblastogenesis of bone marrow stem cells. J. Bone Miner. Res. 2010;25:222–234. doi: 10.1359/jbmr.091106. [DOI] [PubMed] [Google Scholar]
- 118.Muruganandan S., Parlee S.D., Rourke J.L., Ernst M.C., Goralski K.B., Sinal C.J. Chemerin, a novel peroxisome proliferator-activated receptor gamma (PPARgamma) target gene that promotes mesenchymal stem cell adipogenesis. J. Biol. Chem. 2011;286:23982–23995. doi: 10.1074/jbc.M111.220491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wittamer V., Franssen J.D., Vulcano M., Mirjolet J.F., Le Poul E., Migeotte I., Brézillon S., Tyldesley R., Blanpain C., Detheux M., et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J. Exp. Med. 2003;198:977–985. doi: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Goralski K.B., McCarthy T.C., Hanniman E.A., Zabel B.A., Butcher E.C., Parlee S.D., Muruganandan S., Sinal C.J. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J. Biol. Chem. 2007;282:28175–28188. doi: 10.1074/jbc.M700793200. [DOI] [PubMed] [Google Scholar]
- 121.Du X.Y., Zabel B.A., Myles T., Allen S.J., Handel T.M., Lee P.P., Butcher E.C., Leung L.L. Regulation of chemerin bioactivity by plasma carboxypeptidase N, carboxypeptidase B (activated thrombin-activable fibrinolysis inhibitor), and platelets. J. Biol. Chem. 2009;284:751–758. doi: 10.1074/jbc.M805000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ernst M.C., Issa M., Goralski K.B., Sinal C.J. Chemerin exacerbates glucose intolerance in mouse models of obesity and diabetes. Endocrinology. 2010;151:1998–2007. doi: 10.1210/en.2009-1098. [DOI] [PubMed] [Google Scholar]
- 123.Yun H., Dumbell R., Hanna K., Bowen J., McLean S.L., Kantamneni S., Pors K., Wu Q.F., Helfer G. The Chemerin-CMKLR1 Axis is Functionally important for Central Regulation of Energy Homeostasis. Front. Physiol. 2022;13:897105. doi: 10.3389/fphys.2022.897105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bozaoglu K., Curran J.E., Stocker C.J., Zaibi M.S., Segal D., Konstantopoulos N., Morrison S., Carless M., Dyer T.D., Cole S.A., et al. Chemerin, a novel adipokine in the regulation of angiogenesis. J. Clin. Endocrinol. Metab. 2010;95:2476–2485. doi: 10.1210/jc.2010-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Buechler C., Feder S., Haberl E.M., Aslanidis C. Chemerin Isoforms and Activity in Obesity. Int. J. Mol. Sci. 2019;20:1128. doi: 10.3390/ijms20051128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wittamer V., Bondue B., Guillabert A., Vassart G., Parmentier M., Communi D. Neutrophil-mediated maturation of chemerin: A link between innate and adaptive immunity. J. Immunol. 2005;175:487–493. doi: 10.4049/jimmunol.175.1.487. [DOI] [PubMed] [Google Scholar]
- 127.Sanchis-Gomar F., Pareja-Galeano H., Santos-Lozano A., Garatachea N., Fiuza-Luces C., Venturini L., Ricevuti G., Lucia A., Emanuele E. A preliminary candidate approach identifies the combination of chemerin, fetuin-A, and fibroblast growth factors 19 and 21 as a potential biomarker panel of successful aging. AGE. 2015;37:42. doi: 10.1007/s11357-015-9776-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stefanov T., Blüher M., Vekova A., Bonova I., Tzvetkov S., Kurktschiev D., Temelkova-Kurktschiev T. Circulating chemerin decreases in response to a combined strength and endurance training. Endocrine. 2014;45:382–391. doi: 10.1007/s12020-013-0003-2. [DOI] [PubMed] [Google Scholar]
- 129.Hida K., Wada J., Eguchi J., Zhang H., Baba M., Seida A., Hashimoto I., Okada T., Yasuhara A., Nakatsuka A., et al. Visceral adipose tissue-derived serine protease inhibitor: A unique insulin-sensitizing adipocytokine in obesity. Proc. Natl. Acad. Sci. USA. 2005;202:10610–10615. doi: 10.1073/pnas.0504703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Feng R., Li Y., Wang C., Luo C., Liu L., Chuo F., Li Q., Sun C. Higher vaspin levels in subjects with obesity and type 2 diabetes mellitus: A meta-analysis. Diabetes Res. Clin. Pract. 2014;106:88–94. doi: 10.1016/j.diabres.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 131.Youn B.S., Klöting N., Kratzsch J., Lee N., Park J.W., Song E.S., Ruschke K., Oberbach A., Fasshauer M., Stumvoll M., et al. Serum vaspin concentrations in human obesity and type 2 diabetes. Diabetes. 2008;57:372–377. doi: 10.2337/db07-1045. [DOI] [PubMed] [Google Scholar]
- 132.Kurowska P., Mlyczyńska E., Dawid M., Jurek M., Klimczyk D., Dupont J., Rak A. Review: Vaspin (SERPINA12) Expression and Function in Endocrine Cells. Cells. 2021;10:1710. doi: 10.3390/cells10071710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu P., Li G., Wu J., Zhou X., Wang L., Han W., Lv Y., Sun C. Vaspin promotes 3T3-L1 preadipocyte differentiation. Exp. Biol. Med. 2015;240:1520–1527. doi: 10.1177/1535370214565081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nicholson T., Church C., Tsintzas K., Jones R., Breen L., Davis E.T., Baker D.J., Jones S.W. Vaspin promotes insulin sensitivity of elderly muscle and is upregulated in obesity. J. Endocrinol. 2019;241:31–43. doi: 10.1530/JOE-18-0528. [DOI] [PubMed] [Google Scholar]
- 135.Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L., Jacobs A.H., Wyss-Coray T., Vitorica J., Ransohoff R.M., et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Flo T.H., Smith K.D., Sato S., Rodriguez D.J., Holmes M.A., Strong R.K., Akira S., Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 137.Yan Q.W., Yang Q., Mody N., Graham T.E., Hsu C.H., Xu Z., Houstis N.E., Kahn B.B., Rosen E.D. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes. 2007;56:2533–2540. doi: 10.2337/db07-0007. [DOI] [PubMed] [Google Scholar]
- 138.Wang Y., Lam K.S., Kraegen E.W., Sweeney G., Zhang J., Tso A.W., Chow W.S., Wat N.M., Xu J.Y., Hoo R.L., et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin. Chem. 2007;53:34–41. doi: 10.1373/clinchem.2006.075614. [DOI] [PubMed] [Google Scholar]
- 139.Guo H., Jin D., Zhang Y., Wright W., Bazuine M., Brockman D.A., Bernlohr D.A., Chen X. Lipocalin-2 deficiency impairs thermogenesis and potentiates diet-induced insulin resistance in mice. Diabetes. 2010;59:1376–1385. doi: 10.2337/db09-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Guo H., Bazuine M., Jin D., Huang M.M., Cushman S.W., Chen X. Evidence for the regulatory role of lipocalin 2 in high-fat diet-induced adipose tissue remodeling in male mice. Endocrinology. 2013;154:3525–3538. doi: 10.1210/en.2013-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jin D., Guo H., Bu S.Y., Zhang Y., Hannaford J., Mashek D.G., Chen X. Lipocalin 2 is a selective modulator of peroxisome proliferator-activated receptor-gamma activation and function in lipid homeostasis and energy expenditure. FASEB J. 2011;25:754–764. doi: 10.1096/fj.10-165175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Law I.K., Xu A., Lam K.S., Berger T., Mak T.W., Vanhoutte P.M., Liu J.T., Sweeney G., Zhou M., Yang B., et al. Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes. 2010;59:872–882. doi: 10.2337/db09-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang J., Wu Y., Zhang Y., Leroith D., Bernlohr D.A., Chen X. The role of lipocalin 2 in the regulation of inflammation in adipocytes and macrophages. Mol. Endocrinol. 2008;22:1416–1426. doi: 10.1210/me.2007-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Guo H., Foncea R., O’Byrne S.M., Jiang H., Zhang Y., Deis J.A., Blaner W.S., Bernlohr D.A., Chen X. Lipocalin 2, a Regulator of Retinoid Homeostasis and Retinoid-mediated Thermogenic Activation in Adipose Tissue. J. Biol. Chem. 2016;291:11216–11229. doi: 10.1074/jbc.M115.711556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mosialou I., Shikhel S., Liu J.M., Maurizi A., Luo N., He Z., Huang Y., Zong H., Friedman R.A., Barasch J., et al. MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature. 2017;543:385–390. doi: 10.1038/nature21697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dekens D.W., Eisel U.L.M., Gouweleeuw L., Schoemaker R.G., De Deyn P.P., Naudé P.J.W. Lipocalin 2 as a link between ageing, risk factor conditions and age-related brain diseases. Ageing Res. Rev. 2021;70:101414. doi: 10.1016/j.arr.2021.101414. [DOI] [PubMed] [Google Scholar]
- 147.Flower D.R., North A.C., Sansom C.E. The lipocalin protein family: Structural and sequence overview. Biochim. Biophys. Acta. 2000;1482:9–24. doi: 10.1016/S0167-4838(00)00148-5. [DOI] [PubMed] [Google Scholar]
- 148.O’Byrne S.M., Blaner W.S. Retinol and retinyl esters: Biochemistry and physiology. J. Lipid Res. 2013;54:1731–1743. doi: 10.1194/jlr.R037648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Quadro L., Blaner W.S., Salchow D.J., Vogel S., Piantedosi R., Gouras P., Freeman S., Cosma M.P., Colantuoni V., Gottesman M.E. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J. 1999;18:4633–4644. doi: 10.1093/emboj/18.17.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Blaner W.S. Vitamin A signaling and homeostasis in obesity, diabetes, and metabolic disorders. Pharmacol. Ther. 2019;197:153–178. doi: 10.1016/j.pharmthera.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lee S.A., Yuen J.J., Jiang H., Kahn B.B., Blaner W.S. Adipocyte-specific overexpression of retinol-binding protein 4 causes hepatic steatosis in mice. Hepatology. 2016;64:1534–1546. doi: 10.1002/hep.28659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yang Q., Graham T.E., Mody N., Preitner F., Peroni O.D., Zabolotny J.M., Kotani K., Quadro L., Kahn B.B. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 153.Moraes-Vieira P.M., Yore M.M., Dwyer P.M., Syed I., Aryal P., Kahn B.B. RBP4 activates antigen-presenting cells, leading to adipose tissue inflammation and systemic insulin resistance. Cell Metab. 2014;19:512–526. doi: 10.1016/j.cmet.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Moraes-Vieira P.M., Yore M.M., Sontheimer-Phelps A., Castoldi A., Norseen J., Aryal P., Simonyté Sjödin K., Kahn B.B. Retinol binding protein 4 primes the NLRP3 inflammasome by signaling through Toll-like receptors 2 and 4. Proc. Natl. Acad. Sci. USA. 2020;117:31309–31318. doi: 10.1073/pnas.2013877117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.McInnes K.J., Smith L.B., Hunger N.I., Saunders P.T., Andrew R., Walker B.R. Deletion of the androgen receptor in adipose tissue in male mice elevates retinol binding protein 4 and reveals independent effects on visceral fat mass and on glucose homeostasis. Diabetes. 2012;61:1072–1081. doi: 10.2337/db11-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen X., Shen T., Li Q., Chen X., Li Y., Li D., Chen G., Ling W., Chen Y.M. Retinol Binding Protein-4 Levels and Non-alcoholic Fatty Liver Disease: A community-based cross-sectional study. Sci. Rep. 2017;7:45100. doi: 10.1038/srep45100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu Y., Mu D., Chen H., Li D., Song J., Zhong Y., Xia M. Retinol-Binding Protein 4 Induces Hepatic Mitochondrial Dysfunction and Promotes Hepatic Steatosis. J. Clin. Endocrinol. Metab. 2016;101:4338–4348. doi: 10.1210/jc.2016-1320. [DOI] [PubMed] [Google Scholar]
- 158.Trepanowski J.F., Mey J., Varady K.A. Fetuin-A: A novel link between obesity and related complications. Int. J. Obes. 2015;39:734–741. doi: 10.1038/ijo.2014.203. [DOI] [PubMed] [Google Scholar]
- 159.Chekol Abebe E., Tilahun Muche Z., Behaile T/Mariam A., Mengie Ayele T., Mekonnen Agidew M., Teshome Azezew M., Abebe Zewde E., Asmamaw Dejenie T., Asmamaw Mengstie M. The structure, biosynthesis, and biological roles of fetuin-A: A review. Front. Cell Dev. Biol. 2022;10:945287. doi: 10.3389/fcell.2022.945287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Auberger P., Falquerho L., Contreres J.O., Pages G., Le Cam G., Rossi B., Le Cam A. Characterization of a natural inhibitor of the insulin receptor tyrosine kinase: cDNA cloning, purification, and anti-mitogenic activity. Cell. 1989;58:631–640. doi: 10.1016/0092-8674(89)90098-6. [DOI] [PubMed] [Google Scholar]
- 161.Goustin A.S., Derar N., Abou-Samra A.B. Ahsg-fetuin blocks the metabolic arm of insulin action through its interaction with the 95-kD β-subunit of the insulin receptor. Cell Signal. 2013;25:981–988. doi: 10.1016/j.cellsig.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 162.Dogru T., Kirik A., Gurel H., Rizvi A.A., Rizzo M., Sonmez A. The Evolving Role of Fetuin-A in Nonalcoholic Fatty Liver Disease: An Overview from Liver to the Heart. Int. J. Mol. Sci. 2021;22:6627. doi: 10.3390/ijms22126627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Reinehr T. Inflammatory markers in children and adolescents with type 2 diabetes mellitus. Clin. Chim. Acta. 2019;496:100–107. doi: 10.1016/j.cca.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 164.Zhou Z., Sun M., Jin H., Chen H., Ju H. Fetuin-a to adiponectin ratio is a sensitive indicator for evaluating metabolic syndrome in the elderly. Lipids Health Dis. 2020;19:61. doi: 10.1186/s12944-020-01251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Reynolds J.L., Skepper J.N., McNair R., Kasama T., Gupta K., Weissberg P.L., Jahnen-Dechent W., Shanahan C.M. Multifunctional roles for serum protein fetuin-a in inhibition of human vascular smooth muscle cell calcification. J. Am. Soc. Nephrol. 2005;16:2920–2930. doi: 10.1681/ASN.2004100895. [DOI] [PubMed] [Google Scholar]
- 166.Das S., Chattopadhyay D., Chatterjee S.K., Mondal S.A., Majumdar S.S., Mukhopadhyay S., Saha N., Velayutham R., Bhattacharya S., Mukherjee S. Increase in PPARγ inhibitory phosphorylation by Fetuin-A through the activation of Ras-MEK-ERK pathway causes insulin resistance. Biochim. Biophys. Acta Mol. Basis Dis. 2021;1867:166050. doi: 10.1016/j.bbadis.2020.166050. [DOI] [PubMed] [Google Scholar]
- 167.Sardana O., Goyal R., Bedi O. Molecular and pathobiological involvement of fetuin-A in the pathogenesis of NAFLD. Inflammopharmacology. 2021;29:1061–1074. doi: 10.1007/s10787-021-00837-4. [DOI] [PubMed] [Google Scholar]
- 168.Hotamisligil G.S., Johnson R.S., Distel R.J., Ellis R., Papaioannou V.E., Spiegelman B.M. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 1996;274:1377–1379. doi: 10.1126/science.274.5291.1377. [DOI] [PubMed] [Google Scholar]
- 169.Bonen A., Campbell S.E., Benton C.R., Chabowski A., Coort S.L., Han X.X., Koonen D.P., Glatz J.F., Luiken J.J. Regulation of fatty acid transport by fatty acid translocase/CD36. Proc. Nutr. Soc. 2004;63:245–249. doi: 10.1079/PNS2004331. [DOI] [PubMed] [Google Scholar]
- 170.Elsas J., Sellhaus B., Herrmann M., Kinkeldey A., Weis J., Jahnen-Dechent W., Häusler M. Fetuin-a in the developing brain. Dev. Neurobiol. 2013;73:354–369. doi: 10.1002/dneu.22064. [DOI] [PubMed] [Google Scholar]
- 171.Li W., Zhu S., Li J., Huang Y., Zhou R., Fan X., Yang H., Gong X., Eissa N.T., Jahnen-Dechent W., et al. A hepatic protein, fetuin-A, occupies a protective role in lethal systemic inflammation. PLoS ONE. 2011;6:e16945. doi: 10.1371/journal.pone.0016945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kuk J.L., Saunders T.J., Davidson L.E., Ross R. Age-related changes in total and regional fat distribution. Ageing Res. Rev. 2009;8:339–348. doi: 10.1016/j.arr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 173.Sawaki D., Czibik G., Pini M., Ternacle J., Suffee N., Mercedes R., Marcelin G., Surenaud M., Marcos E., Gual P., et al. Visceral Adipose Tissue Drives Cardiac Aging Through Modulation of Fibroblast Senescence by Osteopontin Production. Circulation. 2018;138:809–822. doi: 10.1161/CIRCULATIONAHA.117.031358. [DOI] [PubMed] [Google Scholar]
- 174.Shin J.A., Jeong S.I., Kim M., Yoon J.C., Kim H.S., Park E.M. Visceral adipose tissue inflammation is associated with age-related brain changes and ischemic brain damage in aged mice. Brain Behav. Immun. 2015;50:221–231. doi: 10.1016/j.bbi.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 175.Sánchez-Rodríguez M., García-Sánchez A., Retana-Ugalde R., Mendoza-Núñez V.M. Serum leptin levels and blood pressure in the overweight elderly. Arch. Med. Res. 2000;31:425–428. doi: 10.1016/S0188-4409(99)00079-X. [DOI] [PubMed] [Google Scholar]
- 176.Milek M., Moulla Y., Kern M., Stroh C., Dietrich A., Schön M.R., Gärtner D., Lohmann T., Dressler M., Kovacs P., et al. Adipsin Serum Concentrations and Adipose Tissue Expression in People with Obesity and Type 2 Diabetes. Int. J. Mol. Sci. 2022;23:2222. doi: 10.3390/ijms23042222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Xu X., Wen J., Lu Y., Ji H., Zhuang J., Su Y., Liu B., Li H., Xu Y. Impact of age on plasma vaspin concentration in a group of normal Chinese people. J. Endocrinol. Investig. 2017;40:143–151. doi: 10.1007/s40618-016-0533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Acquarone E., Monacelli F., Borghi R., Nencioni A., Odetti P. Resistin: A reappraisal. Mech. Ageing Dev. 2019;178:46–63. doi: 10.1016/j.mad.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 179.Schmid A., Bala M., Leszczak S., Ober I., Buechler C., Karrasch T. Pro-inflammatory chemokines CCL2, chemerin, IP-10 and RANTES in human serum during an oral lipid tolerance test. Cytokine. 2016;80:56–63. doi: 10.1016/j.cyto.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 180.Nakanishi K., Ishibashi C., Ide S., Yamamoto R., Nishida M., Nagatomo I., Moriyama T., Yamauchi-Takihara K. Serum FGF21 levels are altered by various factors including lifestyle behaviors in male subjects. Sci. Rep. 2021;11:22632. doi: 10.1038/s41598-021-02075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Olszanecka-Glinianowicz M., Owczarek A., Bożentowicz-Wikarek M., Brzozowska A., Mossakowska M., Zdrojewski T., Grodzicki T., Więcek A., Chudek J. Relationship between circulating visfatin/NAMPT, nutritional status and insulin resistance in an elderly population—Results from the PolSenior substudy. Metabolism. 2014;63:1409–1418. doi: 10.1016/j.metabol.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 182.Mattison J.A., Colman R.J., Beasley T.M., Allison D.B., Kemnitz J.W., Roth G.S., Ingram I.K., Weindruch R., de Cabo R., Anderson R.M. Caloric restriction improves health and survival of rhesus monkeys. Nat. Commun. 2017;8:14063. doi: 10.1038/ncomms14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lin S.J., Kaeberlein M., Andalis A.A., Sturtz L.A., Defossez P.A., Culotta V.C., Fink G.R., Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- 184.Longo V.D., Anderson R.M. Nutrition, longevity and disease: From molecular mechanisms to interventions. Cell. 2022;185:1455–1470. doi: 10.1016/j.cell.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Most J., Tosti V., Redman L.M., Fontana L. Calorie restriction in humans: An update. Ageing Res. Rev. 2017;39:36–45. doi: 10.1016/j.arr.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kraus W.E., Bhapkar M., Huffman K.M., Pieper C.F., Krupa Das S., Redman L.M., Villareal D.T., Rochon J., Roberts S.B., Ravussin E., et al. 2 years of calorie restriction and cardiometabolic risk (CALERIE): Exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7:673–683. doi: 10.1016/S2213-8587(19)30151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dakic T., Jevdjovic T., Vujovic P., Mladenovic A. The Less We Eat, the Longer We Live: Can Caloric Restriction Help Us Become Centenarians? Int. J. Mol. Sci. 2022;23:6546. doi: 10.3390/ijms23126546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yu D., Tomasiewicz J.L., Yang S.E., Miller B.R., Wakai M.H., Sherman D.S., Cummings N.E., Baar E.L., Brinkman J.A., Syed F.A., et al. Calorie-Restriction-Induced Insulin Sensitivity Is Mediated by Adipose mTORC2 and Not Required for Lifespan Extension. Cell Rep. 2019;29:236–248.e3. doi: 10.1016/j.celrep.2019.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chiba T., Yamaza H., Shimokawa I. Role of insulin and growth hormone/insulin-like growth factor-I signaling in lifespan extension: Rodent longevity models for studying aging and calorie restriction. Curr. Genom. 2007;8:423–428. doi: 10.2174/138920207783591726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hall K.D., Bemis T., Brychta R., Chen K.Y., Courville A., Crayner E.J., Goodwin S., Guo J., Howard L., Knuth N.D., et al. Calorie for Calorie, Dietary Fat Restriction Results in More Body Fat Loss than Carbohydrate Restriction in People with Obesity. Cell Metab. 2015;22:427–436. doi: 10.1016/j.cmet.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Weiss E.P., Racette S.B., Villareal D.T., Fontana L., Steger-May K., Schechtman K.B., Klein S., Holloszy J.O., Washington University School of Medicine CALERIE Group Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: A randomized controlled trial. Am. J. Clin. Nutr. 2006;84:1033–1042. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tam C.S., Covington J.D., Ravussin E., Redman L.M. Little evidence of systemic and adipose tissue inflammation in overweight individuals. Front. Genet. 2012;3:58. doi: 10.3389/fgene.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Angelino D., Pietrangeli F., Serafini M. Early Dinner Time and Caloric Restriction Lapse Contribute to the Longevity of Nonagenarians and Centenarians of the Italian Abruzzo Region: A Cross-Sectional Study. Front. Nutr. 2022;9:863106. doi: 10.3389/fnut.2022.863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.