Abstract
Background
Pulpotomy procedures aiming to preserve and regenerate the dentin-pulp complex have recently increased exponentially due to developments in the field of biomaterials and tissue engineering in primary and permanent teeth. Although the number of studies in this domain has increased, there is still scarcity of evidence in the current literature.
Objectives
(1) Report the methods of outcome assessment of pulpotomy clinical trials in both primary and permanent teeth; (2) Identify the various bioactive agents and biodegradable scaffolds used in pulpotomy clinical trials in both primary and permanent teeth.
Materials and methods
A scoping review of the literature was performed, including a search of primary studies on PubMed, Scopus, Web of Science, ProQuest and Clinicaltrials.gov. A search for controlled trials or randomized controlled trials published between 2012 and 2023 involving primary or permanent teeth receiving partial or full pulpotomy procedures using bioactive/regenerative capping materials was performed.
Results
127 studies out of 1038 articles fulfilled all the inclusion criteria and were included in the current scoping review. More than 90% of the studies assessed clinical and radiographic outcomes. Histological, microbiological, or inflammatory outcomes were measured in only 9.4% of all included studies. Majority of the studies (67.7%) involved primary teeth. 119 studies used non-degradable bioactive cements, while biodegradable scaffolds were used by 32 studies, natural derivates and plant extracts studies were used in only 7 studies. Between 2012 (4 studies) and 2023 (11 studies), there was a general increase in the number of articles published. India, Egypt, Turkey, and Iran were found to have the highest total number of articles published (28, 28,16 and 10 respectively).
Conclusions
Pulpotomy studies in both primary and permanent teeth relied mainly on subjective clinical and radiographic outcome assessment methods and seldom analyzed pulpal inflammatory status objectively. The use of biodegradable scaffolds for pulpotomy treatments has been increasing with an apparent global distribution of most of these studies in low- to middle-income countries. However, the development of a set of predictable outcome measures as well as long-term evidence from well conducted clinical trials for novel pulpotomy dressing materials are still required.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12903-024-04221-w.
Keywords: Histological outcome, Clinical outcome, Pulpotomy, Bioactive agents, Biodegradable scaffolds, Primary teeth, Permanent teeth
Background
Pulpotomy is a minimally invasive vital pulp therapy in which a portion of an infected vital pulp is amputated or removed to preserve the vitality and function of the residual pulp tissue [1]. It is currently considered a common practice for asymptomatic, cariously exposed pulps of primary and young permanent teeth. This helps maintain the integrity of primary teeth that have inflammation limited to the coronal pulp, preserve the vitality of the radicular pulp, and ultimately retain the tooth until its normal exfoliation [2]. On the other hand, long-term preservation of permanent teeth requires a tooth with a favorable crown-to-root ratio and dentin walls thick enough to withstand normal function. Therefore, pulp preservation in immature permanent teeth with partial or full pulpotomy is also a paramount goal, since conventional root canal treatment inhibits the development of physiological dentin, exposing the thin canal walls to fracture of the root [2].
The clinical relevance of the inflammation-regeneration interplay has been further emphasized by the successful clinical and radiographic outcomes of pulpotomized mature teeth diagnosed with irreversible pulpitis in numerous studies. A treatment modality that was once considered an interim or emergency treatment at best is now being suggested as an alternative treatment modality to root canal treatment [3, 4]. Indeed, this has led to a plethora of clinical studies employing vital pulp therapy procedures as viable permanent therapeutic options for the mature permanent tooth with an inflamed dental pulp. In particular, pulpotomy procedures have been recently advocated as a viable treatment modality for mature permanent teeth diagnosed with irreversible pulpitis as yet another pillar of minimally invasive endodontics. While pulpotomy has been long utilized in pediatric dentistry for non-symptomatic primary teeth and in immature permanent teeth to preserve the radicular pulp and allow for apexogenesis to continue, this concept is relatively new for mature teeth. Several systematic reviews have indeed demonstrated significant success rates in pulpotomies of mature permanent teeth [3, 5, 6].
The outcome of primary teeth pulpotomy is commonly assessed clinically by the absence of pain, swelling, and sinus tract, or by clinical tests such as palpation, percussion, and mobility. Also assessed radiographically by the presence of normal periodontal ligament (PDL), absence of furcation or apical radiolucency, or evidence of internal/external resorption [7]. In addition to the forementioned criteria, the rationale in young permanent teeth undergoing partial or complete pulpotomy is that the remaining vital pulp enables the continuation of normal root development and apexogenesis, as determined by periodic radiographic evaluation [8].
Notwithstanding these recent revelations, current diagnostic terminology of pulpal status has been challenged as has the search for a consensus on how outcome assessment is interpreted following vital pulp therapy procedures in mature permanent teeth with inflamed pulps [9]. This in turn has triggered the search for more predictable assessors of pulpal inflammatory status, aiming to provide more predictable treatment outcomes. Another challenging area in endodontics is a lack of a universal core outcome set [10]. This is further complicated by the heterogeneity of reported outcomes and the lack of standardization particularly in the scope of vital pulp therapy modalities. Patient-reported outcomes are generally centred only around pain, ignoring other parameters such as tooth survival and oral health-related quality of life (OHRQoL). These outcomes, in addition to clinician-reported ones, should provide the basis for developing a set of core outcomes with consensus among clinicians [11].
One of the areas that has served to propel forward this new direction is the immense evolution of hydraulic cements, or calcium silicate cements, by providing bioactive, antimicrobial, and biocompatible dressings for the inflamed pulp in addition to providing an immediate seal [12]. The superior outcomes reported from clinical trials that used hydraulic calcium silicate cements are undeniable; however, well-controlled, long-term, high quality randomized clinical trials are still needed to make definitive selections on the best material to use [11]. In spite of the presence of numerous advanced hydraulic cements currently available in the market, none of these is capable of being completely replaced with new pulp tissue following a coronal pulpotomy, i.e. engineering a new functional dentin-pulp complex will remain the holy grail for any tissue regeneration strategy. Stemming from that, biomimetic biodegradable scaffolds whether in a cell-based or cell-free approach, have also received much attention as therapeutic agents following pulpotomy procedures [4].
Similarly, pulpotomy agents in primary teeth have evolved over the last century from the action of devitalization to preservation of the radicular pulp and ultimately to tissue regeneration. A variety of regenerative agents, including bioactive cements, biodegradable scaffolds, and natural derivatives, have also been used for regenerating the dentin-pulp complex in pulpotomized primary teeth [13]. The American Academy of Pediatric Dentistry’s clinical guidelines has recommended mineral trioxide aggregate (MTA) as the medicament of choice for teeth expected to be retained for 24 months or more. Other tricalcium silicates have conditional recommendations, and they recommended against the use of calcium hydroxide for pulpotomy of primary teeth [2]. However, the success of pulpotomy procedures depends on many factors other than the biological effect of the pulpotomy agent; these include the diagnosis of the preoperative and intraoperative pulp status, caries topography and extension, technique, final restoration, and the operator’s experience [14].
Although there has been a steep increase in the number of studies utilizing bioactive cements and tissue engineering approaches for dentin/pulp regeneration in primary and permanent teeth, there are still numerous gaps of knowledge, and the overall quality of evidence is low [11]. These gaps include the lack of objective tools for assessment of the true inflammatory status of the pulp as well as the absence of a clear core outcome set of measures for analyzing the results for both primary and permanent teeth. Moreover, the contribution of tissue engineering scaffolds to pulpotomy clinical trials is unclear for both dentitions. Hence, this scoping review aimed to map the existing clinical evidence on the outcome of pulpotomy procedures in both primary and permanent teeth using bioactive cements and biodegradable scaffolds. The objectives were to: (1) Report the methods of outcome assessment of pulpotomy clinical trials in both primary and permanent teeth; (2) Identify the different bioactive agents and biodegradable scaffolds employed in pulpotomy clinical trials in both primary and permanent teeth.
Materials and methods
This scoping review was carried out following the Joanna Briggs Institute (JBI) Methodology for Scoping Reviews [15]. The focused PCC question was: What is the available evidence on the outcome assessment methods of pulpotomy in primary and permanent teeth using bioactive agents and biodegradable scaffolds?
Literature search and study selection
An electronic literature search was conducted using MEDLINE (via PubMed), Web of Science, Scopus, ProQuest, and clinicaltrials.gov between the inception date and October 2023. The search strategy adopted for each database is presented in Table 1.
Table 1.
Search strategy for databases included in the review
| Database | Search terms | Initial number of results |
|---|---|---|
| PubMed | ((((((((Bioactive) OR (regenerative) OR (Pulp Capp*)) OR (pulp dress*)) OR (calcium silicate)) OR (non-degradable bioactive materials)) OR (degradable natural and synthetic tissue engineering scaffolds)) AND (pulpotomy)) AND ((“Tooth, Deciduous“[Mesh])) OR (“Dentition, Permanent“[Mesh]) AND (pulp) AND (“Histology“[MeSH Terms] OR “Clinical Trial“[Publication Type])) (y_10[Filter]) | 40 |
| Scopus | ( ( bioactive ) OR ( regenerative ) OR ( calcium AND silicate ) ) AND ( pulpotomy ) AND ( ( primary ) OR ( permanent ) AND ( teeth ) ) AND ( clinical ) AND ( LIMIT-TO ( SRCTYPE , “j" ) ) AND ( LIMIT-TO ( DOCTYPE , “ar" ) ) AND ( LIMIT-TO ( SUBJAREA , “DENT" ) ) AND ( LIMIT-TO ( PUBYEAR , 2023 ) OR LIMIT-TO ( PUBYEAR , 2022 ) OR LIMIT-TO ( PUBYEAR , 2021 ) OR LIMIT-TO ( PUBYEAR , 2020 ) OR LIMIT-TO ( PUBYEAR , 2019 ) OR LIMIT-TO ( PUBYEAR , 2018 ) OR LIMIT-TO ( PUBYEAR , 2017 ) OR LIMIT-TO ( PUBYEAR , 2016 ) OR LIMIT-TO ( PUBYEAR , 2015 ) OR LIMIT-TO ( PUBYEAR , 2014 ) OR LIMIT-TO ( PUBYEAR , 2013 ) OR LIMIT-TO ( PUBYEAR , 2012 ) ) AND ( LIMIT-TO ( LANGUAGE , “English" ) ) AND ( LIMIT-TO ( EXACTKEYWORD , “Human" ) OR LIMIT-TO ( EXACTKEYWORD , “Humans" ) ) | 372 |
| Web of science |
(((((((((((ALL=(Bioactive)) OR ALL=(regenerative)) OR ALL=(pulp capping)) OR ALL=(pulp dressing)) OR ALL=(calcium silicate)) OR ALL=(non-degradable bioactive materials))) OR ALL=(degradable natural and synthetic tissue engineering scaffolds))) AND ALL=(pulpotomy)) AND ALL=((Primary OR Permanent) AND (teeth))) AND ALL=((Histology OR Clinical)) Filters: from 2012–2023 |
171 |
| ProQuest |
(Bioactive OR regenerative) AND (pulpotomy) AND (primary OR permanent teeth) AND (clinical OR histological) Applied filters: Last 10 years |
365 |
| Clinicaltrials.gov |
Pulpotomy (as the condition) Applied filters: Recruiting, Not yet recruiting, Active, not recruiting, Completed, Terminated Studies | Interventional Studies | pulpotomy | Start date from 01/01/2012 to 10/31/2023 |
90 |
After exporting the search results from the databases to Zotero, duplicates were removed. Next, a title-and-abstract and a full-text screening phase were performed by two reviewers in an independent and duplicated manner to identify potentially eligible studies. References of all included articles were also screened to avoid any missing eligible studies. Records retrieved from ProQuest and Clinicaltrials.gov with published results in peer-reviewed journals were considered as duplicates. Regarding the date of the status of studies retrieved from Clinicaltrials.gov, the “actual completion date” was considered for completed studies while the “last update posted” was considered for studies that had either an unknown status, active, or recruiting. Agreement between reviewers in the selection process was calculated by the Cohen’s Kappa statistics (k = 0.8). Any discrepancies were resolved by a third reviewer.
Eligibility criteria
Inclusion criteria
Time: 2012–2023.
Age: no filter.
Study type: primary research (Controlled trials, randomized controlled trials).
Studies executing partial/full pulpotomy procedures.
Studies done on primary and/or permanent teeth.
Studies including bioactive/regenerative capping materials.
Exclusion criteria
No abstract available.
Not in English language.
Published prior to 2012.
Vital pulp therapy modalities including indirect/direct pulp capping.
Studies conducted on non-vital teeth.
Studies comparing pulpotomy with other vital pulp therapy procedures.
Studies assessing success and failure outcomes of pulpotomy that are not dependent on the type of pulp dressing material.
Case reports and case series.
Secondary research; reviews whether systematic or otherwise and surveys.
Position statements and clinical guidelines.
Papers that cannot be fully accessed.
Single arm studies.
Data collection and analysis
Two independent reviewers extracted data from the included studies into a standardized data extraction table, which was then subsequently counter-checked by another two reviewers. Data extracted for each paper included: study reference (author(s), year of publication, title, name of journal, and country where the study was conducted), study design, follow-up period, initial diagnosis representing pulp exposure type, arms of the study, outcome measures assessed, type of material used, sample size, and whether the study involved primary or permanent teeth. Details of the studies included are presented in Table 2.
Table 2.
Studies included in the review (arranged alphabetically according to author name)
| Author, year | Country | Study design | Sample size | Follow-up duration | Outcome measures | Materials used | Type of exposure | Primary/ Permanent |
Pre-operative Pulp Status |
|---|---|---|---|---|---|---|---|---|---|
| Abdelwahab D, 2023 [54] | Egypt | RCT | 60 | 1,3,6,12 months | Clinical and radiographic | Totalfill® BC RRM™ Fast Set Putty and MTA WHITE | Carious | Primary | Reversible pulpitis |
| Abd Al Gawad R and Hanafy R. 2021 [55] | Egypt | RCT | 72 | 3,6,12 months | Clinical and radiographic | NHA (Straumann Bone Ceramic), MTA, Formocresol | Carious OR traumatic | Primary | Reversible pulpitis |
| Abdel Maksoud E, 2023 [56] | Egypt | RCT | 36 | 3,6,9,12 months | Clinical, radiographic, microbiological | Hyaluronic Acid, Amniotic Membrane Allograft, Mineral Trioxide Aggregate | Carious | Primary | Reversible pulpitis |
| Aboul Kheir M et al., 2020 [57] | Egypt | RCT | 30 | 12 months | Clinical and radiographic | Chitosan scaffold, MTA | Carious | Permanent | Irreversible pulpitis |
| Abuelniel G et al., 2020 [58] | Egypt | RCT | 50 | 18 months | Clinical and radiographic | MTA, Biodentine | Traumatic | Immature anterior permanent teeth | Reversible pulpitis |
| Abuelniel G et al., 2021 [59] | Egypt | RCT | 60 | 6, 12 and 18 months | Clinical and radiographic | MTA, Biodentine | Carious | Immature permanent teeth | Reversible pulpitis |
| Airen P et al., 2012 [60] | India | NRS | 70 | 24 months | Clinical and radiographic | MTA, Formocresol | Carious | Primary | Reversible pulpitis |
| Airsang A et al., 2022 [61] | India | RCT | 60 | 6 months and 1 year | Clinical and radiographic | NeoMTA, Biodentine | Carious | Mature permanent | Irreversible pulpitis |
| Akcay M et al., 2014 [62] | Turkey | RCT | 128 | 12 months | Clinical and radiographic | Calcium hydroxide, MTA | Carious | Primary | Reversible pulpitis |
| Aksoy B et al., 2022 [63] | Turkey | RCT | 105 | 2 years (6,12,18 and 24) | Clinical and radiographic | Zinc oxide–eugenol, Calcium hydroxide, MTA | Carious | Primary | Reversible pulpitis |
| Alacam A, 2017 [64] | Turkey | RCT | 54 | 12 months | Clinical and radiographic | Biodentine, Calcium hydroxide, MTA | Carious | Young permanent molars | Reversible pulpitis |
| Alajaji N, 2021 [65] | Iran | NRS | 469 | 4 years | Clinical and radiographic | MTA, Ferric sulfate, Biodentine | Carious | Primary | Reversible pulpitis |
| Alamoudi N et al., 2018 [66] | KSA | RCT | 106 | 3, 6 and 12 months | Clinical and radiographic | Low-level laser, Formocresol | Carious | Primary | Reversible pulpitis |
| Alamoudi N, 2016 [67] | KSA | RCT | 112 | 6 and 12 months | Clinical and radiographic | Biodentine, Formocresol | Carious | Primary | Reversible pulpitis |
| Aljabban et al., 2021 [133] | Syria | RCT | 24 | 8 weeks | Clinical and histological | MTA, PRF | Sound premolar teeth scheduled for orthodontic extraction | Permanent | Normal pulp |
| Alnassar I et al., 2023 [68] | Syria | RCT | 40 | 1 week, 3 months, 6 months, 9 months, and 1 year | Clinical and radiographic | MTA, Bioceramic putty | Carious | Primary | Reversible pulpitis |
| Alzoubi H et al., 2021 [69] | Syria | NRS | 35 | 3, 6, 12 months histological evaluation after 3 months | Clinical and radiographic | Portland cement, MTA | Carious | Primary | Reversible pulpitis |
| Anandan V et al., 2021 [70] | India | NRS | 30 | 2,4 and 6 months | Clinical and radiographic | Formocresol BioFil-AB Collagen Particles | Carious | Primary | Reversible pulpitis |
| Aripirala M et al., 2021 [71] | India | RCT | 100 | 12 months | Clinical and radiographic | Simvastatin gel, 940 nm diode laser | Carious | Primary | Reversible pulpitis |
| Asgary S et al., 2012 [72] | Iran | RCT | 413 | 12 months | Clinical and radiographic | MTA, calcium enriched cement (CEM) | Carious | Permanent | Irreversible pulpitis |
| Asgary S et al., 2022 [73] | Iran | RCT. | 154 | 2 years and pain was assessed upto one week | Clinical and radiographic | Proroot MTA, CEM | Carious | Permanent | Reversible pulpitis OR Irreversible pulpitis |
| Awad S, 2021 [74] | Egypt | RCT | 17 | 2 years | Clinical and radiographic | Biodentine, Calcium Hydroxide, PRF | Carious | Infected immature permanent molars | NOT MENTIONED |
| Awawdeh L et al., 2018 [75] | Jordan | RCT | 68 | 3 years | Clinical and radiographic | Biodentine, MTA | Carious | Permanent | Reversible pulpitis |
| Bakhtiar H et al., 2017 [152] | Iran | RCT | 27 | 8 weeks | Clinical, radiographic, histological | Theracal, Biodentine, proroot MTA | Traumatic | Permanent third molars | Normal pulp |
| Bakhtiar H et al., 2018 [153] | Iran | RCT | 22 | 1 and 8 weeks | Clinical and histological | Retro-MTA, pro-root MTA | Sound teeth scheduled for extraction | Permanent | Normal pulp |
| Bani M et al., 2022 [76] | Turkey | RCT | 62 | 24 months | Clinical and radiographic | MTA, Biodentine | Carious | Primary molars | Reversible pulpitis |
| Bayoumi N, 2022 [77] | Egypt | RCT | 40 | 12 months | Clinical and radiographic | Sterile medicated collagen particles, Biofil-AB, Biodentine | Carious | Primary | Reversible pulpitis |
| Bhagat D et al., 2017 [154] | India | RCT | 30 | 6 months | Histological | MTA, Portland cement | Traumatic | Premolars scheduled for extraction | Normal pulp |
| Brar K et al., 2020 [78] | USA | NRS | 102 | 3 years | Clinical and radiographic | Ferric sulfate, Biodentine | Carious | Primary | Reversible pulpitis |
| Carti O and Oznurhan F, 2017 [79] | Sivas, Turkey | RCT | 50 | 12 months | Clinical and radiographic | Biodentine, MTA | Carious | Primary | Reversible pulpitis |
| Caruso S et al., 2018 [80] | Italy | NRS | 400 | 9 and 12 months | Clinical and radiographic | Biodentine, Calcium hydroxide | Carious | Primary | Reversible pulpitis |
| Celik B et al., 2013 [81] | Turkey | RCT | 139 | 24 months | Clinical and radiographic | MTA, Calcium hydroxide | Carious | Primary | Reversible pulpitis |
| Celik B et al., 2019 [82] | Turkey | RCT | 44 | 3,6,12, 18 and 24 months | Clinical and radiographic | MTA, Biodentine | Carious | Primary | Reversible pulpitis |
| Chailertvanitkul P et al., 2014 [83] | Thailand | RCT | 84 | 24 months | Clinical and radiographic | MTA, Calcium hydroxide | Carious | Permanent | Reversible pulpitis |
| Chak R et al., 2022 [84] | India | RCT | 60 | 3, 6, 9, and 12 months | Clinical and radiographic | 3Mixtatin, MTA | Carious | Primary | Reversible pulpitis |
| Chen J, 2017 [85] | USA | RCT | 56 | 6, 9 and 12 months | Clinical and radiographic | MTA, Ferric Sulfate | Carious | Primary molars | Reversible pulpitis |
| Clancy M, 2018 [86] | USA | RCT | 60 | 2 years with follow-up every 6 months | Clinical and radiographic | Biodentine, Formocresol | Carious | Primary | Reversible pulpitis |
| Cogulu D, 2019 [87] | Turkey | RCT | 57 | 6, 12,18 months | Expression levels of MMP-2, 8 and 9; and clinical and radiographic | MTA, Biodentine | Carious | Primary | Reversible pulpitis |
| Cordell S, 2019 [88] | USA | RCT | 50 | 6 and 12 months | Clinical and radiographic | NeoMTA, 15.5% ferric sulfate solution | Carious | Primary | Reversible pulpitis |
| de Lima S et al., 2020 [89] | Brazil | RCT | 70 | seven days, and at 1, 3, 6 and 12 months | Clinical and radiographic | Bio-C Pulpo, MTA | Carious | Primary | Reversible pulpitis |
| Eid A et al., 2022 [90] | Syria | RCT | 63 | 12 months | Clinical and radiographic | MTA (MM-MTA), nano-hydroxyapatite, platelet-rich fibrin | Carious | Young permanent molars | Reversible pulpitis |
| El Meligy O et al., 2016 [155] | KSA | RCT | 112 | 6 months | Clinical and radiographic | Biodentine, Formocresol | Carious | Primary | Reversible pulpitis |
| El Meligy O et al., 2019 [91] | Saudi Arabia | RCT | 112 | 3,6,12 months | Clinical and radiographic | Biodentine, Formocresol | Carious | Primary | Reversible pulpitis |
| Elbardissy A, 2018 [92] | Egypt | RCT | 43 | 3,6,9,12 months | Clinical and radiographic | Biodentine, Formocresol | Carious | Primary | Reversible pulpitis |
| El-desouky S, 2023 [93] | Egypt | RCT | 30 | 6,12 months | Clinical and radiographic | Eggshell Powder freshly mixed with Tea Tree Oil, Biodentine, MTA | Carious | Primary | Reversible pulpitis |
| Elhamouly Y et al., 2021 [18] | Egypt | Interim analysis /terminated RCT | 19 | 12 months | Clinical, radiographic, histological | Biodentine, bioactive glass | Carious | Primary | Reversible pulpitis |
| Elheeny A, 2023 [94] | Egypt | RCT | 128 | 12, 18 months | Clinical and radiographic | Simvastatin, MTA | Carious | Immature permanent teeth | Reversible pulpitis |
| Elsayed S, 2023 [136] | Egypt | RCT | 40 | 3,6 months | Clinical and radiographic | Biofil-AB, Biodentine | Carious | Primary | Reversible pulpitis |
| Elshaer N, 2021 [95] | Egypt | RCT | 40 | 12 months | Clinical and radiographic | Protooth MTA, MTA | Carious | Primary | Reversible pulpitis |
| Elshamy SH, 2022 [96] | Egypt | RCT | 38 | 12 months | Clinical and radiographic | Calcium hydroxide, Biodentine | Carious | Young permanent molars | Reversible pulpitis |
| Eltantawy W, 2023 [97] | Egypt | RCT | 96 | 18 months | Clinical and radiographic | Biodentine, hyaluronic acid, Formocresol | Carious | Primary | Reversible pulpitis |
| Eshghi A et al., 2022 [98] | Iran | RCT | 52 | 3,6, 9, 12 months | Clinical and radiographic | MTA, Biodentine | Carious | Primary | Reversible pulpitis |
| Fernandez C et al., 2013 [99] | Spain | RCT | 100 | 24 months | Clinical and radiographic | Formocresol, MTA, ferric sulphate, and NaOCl | Carious | Primary | Reversible pulpitis |
| Fouad W et al., 2019 [100] | Egypt | NRS | 84 | 12 months | Clinical and radiographic | Biodentine, MTA | Carious | Primary | Reversible pulpitis |
| Frenkel G et al., 2012 [101] | Israel | NRS | 86 | 47 months | Clinical and radiographic | Ferric Sulphate, MTA | Carious | Primary | Reversible pulpitis |
| Gaber R et al., 2022 [156] | Egypt | RCT | 20 | 6 months | Clinical and radiographic | MTA, Theracal | Carious | Primary | Reversible pulpitis |
| Gamal D, 2023 [103] | Egypt | RCT | 24 | 1 year | Clinical and radiographic | Wellroot PT and MTA | Carious | Primary | Reversible pulpitis |
| Ghent University, 2020 [104] | Belgium | RCT | 36 | 12 months | Clinical and radiographic | Totalfill Bioceramic Root Repair Material®, MTA | Carious | Primary teeth | Reversible pulpitis |
| Grewal N et al., 2016 [105] | India | RCT | 40 | 12 months | Clinical and radiographic | Biodentine, Calcium hydroxide | Carious | Primary molars | Reversible pulpitis |
| Guven Y et al., 2016 [106] | Turkey | RCT | 116 | 24 months | Clinical and radiographic | Proroot MTA, MTA-Plus, Biodentine, ferric sulfate | Carious | Primary molars | Reversible pulpitis |
| Hadassah Medical Organization, 2021 [107] | Israel | NRS | 60 | 36 months | Clinical and radiographic | MedCem MTA, Formocresol | Carious | Primary | Reversible pulpitis |
| Haideri S et al., 2021 [108] | India | NRS | 80 | 3,6,12 months | Clinical and radiographic | Formocresol, Mineral Trioxide Aggregate, Electrocautery, Bioactive Glass | Carious | Primary | Reversible pulpitis |
| Hugar S et al., 2017 [109] | India | RCT | 60 | 60 months | Clinical, radiographic, histological | MTA, Formocresol | Carious | Primary | Reversible pulpitis |
| Ildes G et al., 2022 [110] | Turkey | RCT | 130 | 1,3,6 and 12 months | Clinical and radiographic | 0.5% Hyaluronic Acid gel, Formocresol, 20% Ferric sulphate | Carious | Primary molars | Reversible pulpitis |
| Jayam C et al., 2018 [111] | India | RCT | 100 | 24 months | Clinical and radiographic | MTA, Formocresol | Carious | Primary | Reversible pulpitis |
| Jimeno F et al., 2022 [112] | Spain | RCT | 108 | 12 months | Clinical and radiographic | MTA ProRoot, MTA HP Repair, Biodentine | Carious | Primary | Reversible pulpitis |
| Joo Y et al., 2023 [113] | Korea | RCT | 153 | 3, 6, and 12 months | Clinical and radiographic | MTA, Wellroot PT, Proroot MTA | Carious | Primary | Reversible pulpitis |
| Juneja P and Kulkarni S, 2017 [114] | India | RCT | 51 | 18 months | Clinical and radiographic | MTA, Biodentine, Formocresol | Carious | Primary molars | Reversible pulpitis |
| Junqueira M et al., 2017 [115] | Brazil | NRS | 31 | 18 months | Clinical and radiographic | MTA and 15.5% Ferric Sulfate | Carious | Primary molars | Reversible pulpitis |
| Kakarla P et al., 2013 [102] | India | RCT | 40 | 24 months | Histological | Pulpotec, Biofil-AB | Retained sound and indicated for orthodontic extraction | Primary | Reversible pulpitis |
| Kalra M et al., 2017 [116] | India | RCT | 60 | 12 months | Clinical and radiographic | Fresh Aloe vera barbadensis plant extract, MTA | Carious | Primary molars | Reversible pulpitis |
| Kang C et al., 2015 [117] | Korea | RCT | 151 | 12 months | Clinical and radiographic | Proroot MTA, OrthoMTA, RetroMTA | Carious | Primary molars | Reversible pulpitis |
| Kang C et al., 2017 [118] | Korea | RCT | 104 | 1,3,6, and 12 months | Clinical and Radiographic | Proroot MTA, OrthoMTA, RetroMTA | Carious OR traumatic | Permanent | Reversible pulpitis |
| Kang C et al., 2021 [119] | Korea | RCT | 104 | 1, 3, 6, and 12 months and at 48–78 months | Clinical and radiographic | ProRoot MTA, OrthoMTA, RetroMTA | Carious | Mature permanent teeth | Reversible pulpitis |
| Kathal S et al., 2017 [120] | India | RCT | 40 | 12 months | Clinical and radiographic | MTA, antioxidant mix | Carious | Primary molars | Reversible pulpitis |
| Keles S, 2018 [121] | Turkey | RCT | 96 | 3,6, 9 and 18 months | Clinical and radiographic | OrthoMTA, RetroMTA and Ferric sulfate | Carious | Primary | Reversible pulpitis |
| Keswani D et al., 2014 [122] | India | RCT | 70 | 24 months | Clinical and radiographic | MTA, PRF | Carious | Immature Permanent | Reversible pulpitis |
| Koruyucu M, 2016 [123] | Turkey | RCT | 200 | 3 years | Clinical and radiographic | ProRoot MTA, Biodentine | Carious | Primary | Reversible pulpitis |
| Kumar V et al., 2016 [124] | India | RCT | 54 | 12 months | Clinical and radiographic | Calcium hydroxide, MTA, platelet-rich fibrin | Carious | Permanent molars | Irreversible pulpitis |
| Lourenco N et al., 2016 [157] | Brazil | RCT | 25 | 3 months | Histological and immunohistochemistry | Formocresol, Calcium hydroxide, MTA, Portland cement | Carious | Primary | Reversible pulpitis |
| Madan K et al., 2020 [125] | India | NRS | 40 | 3,6,12 months | Clinical and radiographic | MTA, propolis | Carious | Primary | Reversible pulpitis |
| Magdy M, 2020 [126] | Egypt | RCT | 36 | 18 months | Clinical and radiographic | MTA, Biodentine | Carious | Primary | Reversible pulpitis |
| Mahmoud S, 2022 [127] | Egypt | RCT | 130 | 12 months | Clinical and radiographic | MTA, Formocresol | Carious | Primary teeth | Reversible pulpitis |
| Manhas M et al., 2019 [135] | India | RCT | 30 | 1,3,6, months | Clinical and radiographic | MTA, Calcium hydroxide, PRF | Carious | Primary | Reversible pulpitis |
| Manohar S et al., 2022 [21] | India | NRS | 120 | 6, 12, 18, and 24 months | Clinical and radiographic | Biodentine, MTA Plus, Retro MTA, CEM cement | Carious | Primary | Reversible pulpitis |
| Mehrvarzfar P et al., 2017 [134] | Iran | RCT with histologic assessment | 39 | 6 weeks | Clinical, radiographic, histological | MTA, Treated dentin matrix scaffold | Traumatic | Permanent third molars | Normal pulp |
| Mentes A, 2020 [22] | Turkey | RCT | 120 | 1,3,6,12 months | Clinical and radiographic | Formocresol, ferric sulphate, and 0.5% hyaluronic acid | Carious | Primary | Reversible pulpitis |
| Nageh M, 2021 [23] | Egypt | RCT | 120 | 24 h, 48 h, 1 week, every 3 months for 12 months | Clinical and radiographic | Biodentine, PRF, MTA, Portland cement | Carious | Permanent | Irreversible pulpitis |
| Nagy P, 2017 [24] | Egypt | RCT | 22 | 12 months | Clinical and radiographic | MTA, theracal | Carious | Permanent | NOT MENTIONED |
| Najmi N, 2022 [25] | Pakistan | RCT | 114 | 12 months | Clinical and radiographic | PRF, MTA, calcium hydroxide | Carious | Mature permanent | Irreversible pulpitis |
| Neto J, 2017 [167] | Brazil | RCT | 30 | 6 months | Clinical and radiographic | Formocresol, PBS CIMMO cement, Zinc oxide | Carious | Primary | Reversible pulpitis |
| Nguyen T et al.,2014 [26] | Canada | RCT | 48 | 40 months | Clinical and radiographic | MTA, Ferric sulfate | Carious | Primary | Reversible pulpitis |
| Nosrat A et al., 2012 [27] | Iran | RCT | 51 | 12 months | Clinical and radiographic | MTA, CEM cement | Carious | Permanent | Irreversible pulpitis |
| Oliveira T et al., 2013 [28] | Brazil | RCT | 45 | 6,12 and 24 months | Clinical, radiographic, histological | MTA, Calcium hydroxide, Portland cement | Carious | Primary | Reversible pulpitis |
| Özgür B, et al. 2017 [29] | Turkey | RCT | 80 | 6, 12, 18, and 24 months. | Clinical and radiographic | MTA, calcium hydroxide | Carious | Immature permanent | Reversible pulpitis |
| Patidar S et al., 2017 [138] | India | RCT | 50 | 6 months | Clinical and radiographic | PRF, MTA | Carious | Primary molars | Reversible pulpitis |
| Perea M et al., 2017 [30] | Spain | RCT | 212 | 48 months | Clinical and radiographic | Formocresol, MTA | Carious | Primary molars | Reversible pulpitis |
| Petel R et al., 2021 [31] | Israel | RCT | 136 | 24–48 months | Clinical and radiographic | Formocresol, Portland cement | Carious | Primary | Reversible pulpitis |
| Prasad M et al., 2017 [139] | India | NRS | 30 | 9 months | Clinical and radiographic | Amniotic, Formocresol | Carious | Primary | Reversible pulpitis |
| Pratima B et al., 2018 [32] | India | NRS | 40 | 6, 12 months | Clinical and radiographic | Diode laser, MTA | Carious | Primary | Reversible pulpitis |
| Rajasekharan S et al., 2017 [33] | Belgium | RCT | 82 | 18 months | Clinical and radiographic | Biodentine, proroot MTA | Carious | Primary | Reversible pulpitis |
| Rao Q et al., 2020 [34] | China | NRS | 205 | 6–8 weeks, 1 year then yearly for 5 years | Clinical and radiographic | iRoot BP Plus, calcium hydroxide | Traumatic | Mature permanent teeth | Reversible pulpitis |
| Rojaramya K et al., 2022 [35] | India | RCT | 60 | 2 years | Clinical and radiographic | MTA, propolis | Carious | Primary | Reversible pulpitis |
| Rubanenko M, et al. 2019 [36] | Israel | RCT | 72 | 48 months | Clinical and radiographic | biodentine, Formocresol | Carious | Primary | Reversible pulpitis |
| Sajadi F et al., 2021 [168] | Iran | RCT | 38 | 3,6, months for clinical and radiographic. pain was evaluated up to 10 days after treatment | Clinical and radiographic | Ferric Sulfate, Calcium-Enriched Mixture Cement (CEM) | Carious | Primary | Reversible pulpitis |
| Sharaan M and Ali A, 2022 [37] | Egypt | RCT | 40 | 7 days and 3, 6 and 12 months | Clinical and radiographic | MTA, CEM | Carious | Permanent | Irreversible pulpitis |
| Sharaf R et al., 2021 [150] | Egypt | RCT | 90 | 6 months | Clinical and radiographic | Turmeric extract, Thymus Vulgaris extract, Nigella Sativa extract, aloe vera extract, Formocresol | Carious | Primary | Reversible pulpitis |
| Sherif R, 2019 [131] | Egypt | RCT | 38 | 12 months | Clinical and radiographic | PRF, Biodentine, diode laser | Carious | Permanent Molars | Irreversible pulpitis |
| Silva L et al., 2019 [151] | Brazil | RCT | 45 | 3,6 and 12 months | Clinical and radiographic | MTA, Calcium hydroxide, polyethylene glycol | Carious | Primary | Reversible pulpitis |
| Singh R et al., 2020 [38] | India | NRS | 60 | 12 months | Clinical and radiographic | Calcium hydroxide, MTA, PRF | Carious | Permanent | Irreversible pulpitis |
| Singh, D et al., 2023 [39] | India | RCT | 64 | 1, 3, 6, and 12 months | Clinical and radiographic | MTA, premixed bioceramic putty | Carious | Mature permanent | Reversible pulpitis |
| Suez Canal University, 2022 [40] | Egypt | RCT | 60 | 12 months | Clinical and radiographic | Biodentine, Simvastatin | Carious | Primary | Reversible pulpitis |
| Surinder et al.,2021 [132] | India | RCT | 60 | 9 months | Clinical and radiographic | MTA, Biodentine, Platelet Rich Fibrin | Carious | Permanent | Irreversible pulpitis |
| Taha N et al., 2022 [41] | Jordan | RCT | 164 | 6 and 12 months | Clinical and radiographic | Proroot MTA, Biodentine, totalfill | Carious | Permanent | Reversible pulpitis OR Irreversible pulpitis |
| Taha N, 2017 [42] | Jordan | RCT | 150 | 6 m, 1 year then yearly for 5 years | Clinical and radiographic | MTA, Biodentine, non-specified Bioceramic | Carious | Permanent | Reversible pulpitis OR Irreversible pulpitis |
| Togaru H et al., 2016 [43] | India | RCT | 90 | 12 months | Clinical and radiographic | Biodentine, MTA | Carious | Primary molars | Reversible pulpitis |
| Tozar K and Almaz M, 2019 [44] | Turkey | RCT | 90 | 12 months | Clinical and radiographic | Laser, MTA | Carious | Immature permanent | Reversible pulpitis |
| Tzanetakis G et al., 2023 [45] | Greece | RCT | 137 | 7days-2 years | Clinical and radiographic | MTA, Total Fill BC | Carious | Mature permanent | Irreversible pulpitis |
| Uesrichai N et al., 2019 [46] | Thailand | Non-inferiority RCT | 69 | every 6 m for mean follow up of 32.2 +/-17.9 months | Clinical and radiographic | Biodentine, Proroot MTA | Carious | Permanent | Irreversible pulpitis |
| Université de Montréal, 2016 [47] | Canada | RCT | 180 | 12 months | Clinical and radiographic | Biodentine, Formocresol | Carious | Primary | Reversible pulpitis |
| Vafaeia A et al., 2022 [48] | Iran | NRS | 316 | 28.2 ± 2.7 months | Clinical and radiographic | Protooth calcium silicate cement, MTA | Carious | Immature permanent teeth | Reversible pulpitis |
| Venugopal N et al., 2019 [137] | India | RCT | 90 | 6 months | Clinical, radiographic, histological | Formocresol, propolis, Platelet derived growth factor (PDGF)/scaffold | Carious | Primary molars | Reversible pulpitis |
| Vilella-Pastor S et al., 2021 [49] | Spain | RCT | 84 | 6,12,18,24 months | Clinical and Radiographic | MTA, Biodentine | Carious OR traumatic | Primary | Reversible pulpitis |
| Vu T et al., 2020 [50] | Vietnam | NRS | 50 | 12 months | Clinical and radiographic | Acemannan, MTA | Carious OR traumatic | Permanent | Reversible pulpitis |
| Wassel M, 2019 [51] | Egypt | RCT | 60 | 12 months | Clinical and radiographic | Theracal, Formocresol | Carious | Primary | Reversible pulpitis |
| Yang Y et al., 2020 [52] | China | RCT | 110 | 1, 3, 6, 12, 18 and 24 months | Clinical and Radiographic | iRoot BP Plus, Calcium hydroxide | Traumatic | Immature permanent | Reversible pulpitis |
| Yildirim C et al., 2016 [53] | Turkey | NRS | 140 | 24 months | Clinical and radiographic | Formocresol, MTA, Portland cement, enamel matrix derivative | Carious | Primary | Reversible pulpitis |
Descriptive statistics were used to characterize the included papers, and narrative synthesis was undertaken to explain the results. Categorical data were summarized in frequencies and percentages, and numerical data in means and SD. Statistical analysis was performed using SPSS V.25.0 for Mac (IBM).
Results
The search identified 1038 potentially relevant records from all databases included. After removing the duplicates, 982 articles were screened by two independent reviewers to assess eligibility, and any conflict was resolved by a third reviewer. A total of 127 studies fulfilled all the inclusion criteria and were included in the current scoping review while 843 articles were excluded due to lack of adherence to the inclusion criteria. The flow chart of the review process is shown in Fig. 1 [16].
Fig. 1.
Flow chart of the reviewing process
Study sample, design, and outcome assessment method
Table 3 presents the general characteristics of the included studies. The sample size ranged from 17 to 469 participants with mean 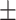 SD: 84.
SD: 84. 71.6. Most of the studies (84.3%) were randomized controlled trials. The highest percentage of the studies had follow-up duration for 12 months (48%) followed by those who had follow-up duration more than 1 year (37.8%). The outcome measured in most of the studies (90.6%) were clinical and radiographic, few studies (5.5%) measured both clinical and histological outcomes. Majority of the studies (67.7%) involved primary teeth as compared to 32.3% for the permanent teeth. Among those, 22.8% of the studies used mature teeth and only 9.4% of the studies used immature teeth. Carious exposure was the most common type of pulp exposure accounting for 89.8% of the studies. Regarding the pre-operative pulpal status, the majority of the teeth in the screened studies had a diagnosis of reversible pulpitis (81.9%) while a small number were diagnosed with irreversible pulpitis (10.2%).
71.6. Most of the studies (84.3%) were randomized controlled trials. The highest percentage of the studies had follow-up duration for 12 months (48%) followed by those who had follow-up duration more than 1 year (37.8%). The outcome measured in most of the studies (90.6%) were clinical and radiographic, few studies (5.5%) measured both clinical and histological outcomes. Majority of the studies (67.7%) involved primary teeth as compared to 32.3% for the permanent teeth. Among those, 22.8% of the studies used mature teeth and only 9.4% of the studies used immature teeth. Carious exposure was the most common type of pulp exposure accounting for 89.8% of the studies. Regarding the pre-operative pulpal status, the majority of the teeth in the screened studies had a diagnosis of reversible pulpitis (81.9%) while a small number were diagnosed with irreversible pulpitis (10.2%).
Interventions
Figure 2 presents the number of studies for different groups of bioactive pulpotomy agents of both primary and permanent teeth, and it shows that 78 studies used non-degradable bioactive cements in primary teeth as compared to 41 in permanent teeth, while biodegradable scaffolds were used by 19 studies involving primary teeth and 13 studies in permanent teeth, natural derivates and plant extracts studies presented six studies in primary teeth and only one study in permanent teeth.
Fig. 2.

Distribution of studies among different groups of bioactive pulpotomy agents
Among the studies that used non-degradable bioactive cements in primary teeth, the majority (58 studies) used MTA, followed by biodentine (32 studies) and calcium hydroxide (9 studies). Other materials like Portland cement, calcium-enriched mixture cement (CEM), Theracal, Totalfill, pre-mixed Bio Ceramic putty, PBS CIMMO cement and bio-c pulpo were used less frequently, as shown in Fig. 3.
Fig. 3.
Number of studies of different non-degradable bioactive cements in primary teeth
Figure 4 presents the number of publications included which used non-degradable bioactive cements in permanent teeth. It shows that the majority (36 studies) used MTA, followed by biodentine (14 studies) and calcium hydroxide (10 studies). Other materials like calcium-enriched mixture cement (CEM), Totalfill, Portland cement, TheraCal, iRoot BP plus, pre-mixed Bio ceramic putty, Protooth and non-specified calcium silicate cement were used less frequently.
Fig. 4.

Number of studies of different non-degradable bioactive cements in permanent teeth
Among the included studies which used biodegradable scaffolds in primary teeth, the most common materials used were 0.5% Hyaluronic acid gel (4 studies), Biofill-AB (4 studies), Simvastatin gel (3 studies), platelet-rich fibrin (2 studies), Bioactive glass (2 studies) and Amniotic (2 studies). While in studies involving permanent teeth, platelet-rich fibrin (PRF) was the most common material used (10 studies), other materials like chitosan scaffold, nano-hydroxyapatite, treated dentin matrix scaffold and Simvastatin gel were used with less frequency (Fig. 5). The coronal sealing materials used in direct contact with these biodegradable scaffolds included glass ionomer cement (8 studies) and zinc oxide eugenol (12 studies) mainly for primary teeth. On the other hand, calcium hydroxide (1 study), Portland cement (1study), MTA (6 studies), and biodentine (3 studies) were mainly used in permanent teeth. In 5 of the studies, the coronal sealing material was not mentioned. Additionally, some studies used different sealing materials for different arms of the same study (Table 4).
Fig. 5.

Number of studies of different biodegradable scaffolds in primary and permanent teeth
Table 4.
Coronal sealing materials used in studies employing biodegradable scaffolds
| Author, Year | Type of Exposure | Type of teeth used | Materials used | Coronal Sealing Materials | |
|---|---|---|---|---|---|
| 1 | Abd Al Gawad R and Hanafy R. 2021 [55] | Carious OR traumatic | Primary | NHA (Straumann Bone Ceramic), MTA, Formocresol | stainless crown cemented with glass ionomer |
| 2 | Abdel Maksoud E, 2023 [56] | Carious | Primary | Hyaluronic Acid, Amniotic Membrane Allograft, Mineral Trioxide Aggregate | zinc oxide eugenol |
| 3 | Aboul Kheir M et al., 2020 [57] | Carious | Permanent | Chitosan scaffold, MTA | MTA |
| 4 | Aljabban et al., 2021 [133] | Sound premolar teeth scheduled for orthodontic extraction | Permanent | MTA, PRF | MTA |
| 5 | Anandan V et al., 2021 [70] | Carious | Primary | Formocresol, BioFil-AB Collagen Particles | zinc oxide eugenol and glass ionomer cement |
| 6 | Aripirala M et al., 2021 [71] | Carious | Primary | Simvastatin gel, 940 nm diode laser | resin-modified glass ionomer cement |
| 7 | Awad S, 2021 [74] | Carious | Infected immature permanent molars | Biodentine, Calcium Hydroxide, PRF | NOT MENTIONED |
| 8 | Bayoumi N, 2022 [77] | Carious | Primary | Sterile medicated collagen particles, Biofil-AB, Biodentine | NOT MENTIONED |
| 9 | Chak R et al., 2022 [84] | Carious | Primary | 3Mixtatin, MTA | glass ionomer cement and stainless crowns |
| 10 | Eid A et al., 2022 [90] | Carious | Immature permanent molars | MTA (MM-MTA), nano-hydroxyapatite, platelet-rich fibrin | IRM for nano-hydroxyapatite and zinc oxide eugenol for the PRF group |
| 11 | Elhamouly Y et al., 2021 [18] | Carious | Primary | Biodentine, bioactive glass | glass ionomer |
| 12 | Elheeny A, 2023 [94] | Carious | Immature permanent teeth | Simvastatin, MTA | NOT MENTIONED |
| 13 | Elsayed S, 2023 [136] | Carious | Primary | Biofil-AB, Biodentine | glass ionomer |
| 14 | Eltantawy W, 2023 [97] | Carious | Primary | Biodentine, hyaluronic acid, Formocresol | zinc oxide eugenol |
| 15 | Haideri S et al., 2021 [108] | Carious | Primary | Formocresol, Mineral Trioxide Aggregate, Electrocautery, Bioactive Glass | IRM and stainless-steel crown |
| 16 | Ildes G et al., 2022 [110] | Carious | Primary | 0.5% Hyaluronic Acid gel, Formocresol, 20% Ferric sulphate | zinc oxide eugenol and composite/stainless steel crown |
| 17 | Kakarla P et al., 2013 [102] | Sound and indicated for extraction | Primary | Pulpotec, Biofil-AB | zinc oxide eugenol and glass ionomer cement |
| 18 | Keswani D et al., 2014 [122] | Carious | Immature Permanent | MTA, PRF | zinc oxide eugenol and amalgam |
| 19 | Kumar V et al., 2016 [124] | Carious | Permanent | Calcium hydroxide, MTA, platelet-rich fibrin | MTA |
| 20 | Manhas M et al., 2019 [135] | Carious | Primary | MTA, Calcium hydroxide, PRF | either calcium hydroxide or MTA |
| 21 | Mehrvarzfar P et al., 2017 [134] | Traumatic | Permanent third molars | MTA, Treated dentin matrix scaffold | resin-modified glass ionomer cement |
| 22 | Mentes A, 2020 [22] | Carious | Primary | Formocresol, ferric sulphate, and 0.5% hyaluronic acid | zinc oxide eugenol, composite and stainless-steel crown |
| 23 | Nageh M, 2021 [23] | Carious | Permanent | Biodentine, PRF, MTA, Portland cement | PRF covered with portland cement or MTA or biodentine |
| 24 | Najmi N, 2022 [25] | Carious | Permanent | PRF, MTA, calcium hydroxide | NOT MENTIONED |
| 25 | Patidar S et al., 2017 [138] | Carious | Primary | PRF, MTA | zinc oxide eugenol and glass ionomer cement, stainless steel crown |
| 26 | Prasad M et al., 2017 139] | Carious | Primary | Amniotic, Formocresol | zinc oxide eugenol |
| 27 | Sherif R, 2019 [131] | Carious | Permanent | PRF, Biodentine, diode laser | biodentine, glass ionomer and composuite |
| 28 | Singh R et al., 2020 [38] | Carious | Permanent | Calcium hydroxide, MTA, PRF | NOT MENTIONED |
| 29 | Suez Canal University, 2022 [40] | Carious | Primary | Biodentine, Simvastatin | glass ionomer cement and stainless-steel crown |
| 30 | Surinder et al.,2021 [132] | Carious | Permanent | MTA, Biodentine, Platelet Rich Fibrin | PRF/MTA, PRF/Biodentine |
| 31 | Venugopal N et al., 2019 [137] | Carious | Primary | Formocresol, propolis, Platelet derived growth factor (PDGF)/scaffold | collagen membrane then glass ionomer and stainless-steel crown |
| 32 | Yildirim C et al., 2016 [53] | Carious | Primary | Formocresol, MTA, Portland cement, enamel matrix derivative | zinc oxide eugenol and glass ionomer cement |
Table 3.
Characteristics and outcomes of the included studies
| Mean (SD) | ||
|---|---|---|
| Sample size | 84.9 (71.6) | |
| n (%) | ||
| Study design | ||
| Randomized controlled trial | 107 (84.3%) | |
| Non-randomized trial | 20 (15.7%) | |
| Follow-up duration | ||
| Less than 6 months | 5 (3.9%) | |
| 6 months | 11 (8.7%) | |
| 9 months | 2 (1.6%) | |
| 12 months | 61 (48%) | |
| More than 1 year | 48 (37.8%) | |
| Outcome measured | ||
| Clinical and radiographic | 115 (90.6%) | |
| Clinical, radiographic and histological | 7 (5.5%) | |
| Histological | 2 (1.6%) | |
| Clinical, radiographic and inflammatory | 1 (0.8%) | |
| Histological and immunohistochemistry | 1 (0.8%) | |
| Clinical, radiographic and microbiological | 1 (0.8%) | |
| Primary/permanent teeth | ||
| Primary teeth | 86 (67.7%) | |
| Permanent teeth | ||
| Mature teeth | 29 (22.8%) | |
| Immature teeth | 12 (9.4%) | |
| Pulp exposure type | ||
| Carious | 114 (89.8%) | |
| Traumatic | 6 (4.7%) | |
| Carious or traumatic | 4 (3.1%) | |
| Sound tooth indicated for extraction | 3 (2.4%) | |
| Pre-operative pulp status | ||
| Normal pulp | 5 (3.9%) | |
| Reversible pulpitis | 104 (81.9%) | |
| Irreversible pulpitis | 13 (10.2%) | |
| Reversible or Irreversible pulpitis | 3 (2.36%) | |
| NOT MENTIONED | 2 (1.57%) | |
Natural derivates and plant extracts presented the least number of studies. Concerning primary teeth, three studies used propolis, two studies used fresh aloe vera barbadensis plant extract and only one study was found for each of these materials: turmeric extract, nigella sativa extract, thymus vulgaris extract and egg-shell powder mixed with tea tree oil. As for permanent teeth, only one study was reported, and it used acemannan (Fig. 6).
Fig. 6.
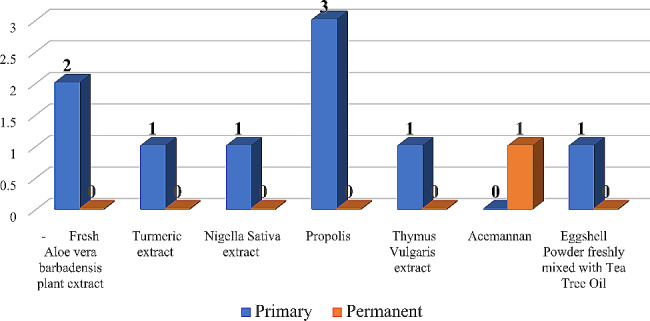
Number of natural derivates and plant extracts studies in primary and permanent teeth
Time distribution of the included studies
The number of articles published increased generally between 2012 (4 studies) and 2023 (11 studies), indicating a growing interest in and expansion of the research field of bioactive pulpotomy agents. The peak of the studies was in 2017 and 2022, accounting for 20 studies (Fig. 7).
Fig. 7.
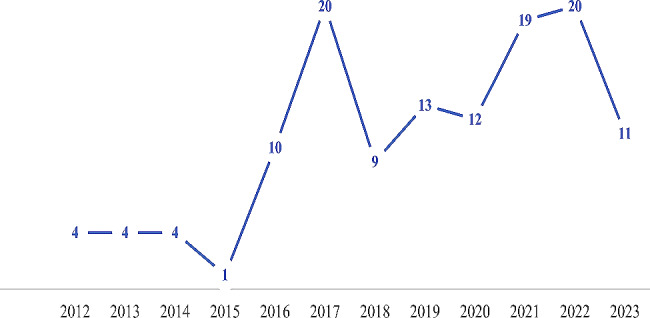
Trend in the number of publications using bioactive cements and biodegradable scaffolds from 2012 to 2023
Global distribution of the included studies
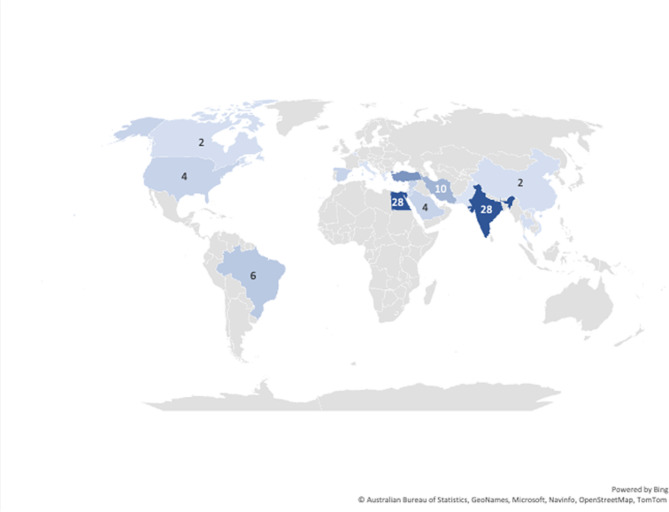
Concerning publishing countries; India, Egypt, Turkey, and Iran were found to have the highest total number of published articles (28, 28, 16 and 10 studies, respectively). Other studies were conducted in smaller numbers in Brazil (6 studies), United States (4 studies), Syria (4 studies), Spain (4 studies), Israel (4 studies), Korea (4 studies), Kingdom of Saudi Arabia (4 studies), Jordan (3 studies), Thailand (2 studies), Belgium (2 studies), Canada (2 studies), China (2 studies), Italy (1 study), Pakistan (1 study), Greece (1 study) and Vietnam (1 study) (Fig. 8). Nineteen articles from the total number included in the review are registered clinical trials that are still in the recruitment phase; fourteen of them are being conducted in Egypt, two in India, one in Spain, one in Jordan and one in Pakistan.
Fig. 8.
Map of the studies included in the scoping review
Discussion
Over the past decade, there has been a paradigm shift in the realization that an inflamed pulp may be worth saving. Advancements in the fields of tissue engineering and biomaterials have made preservation and regeneration of the dentin-pulp complex the most sought-after goals of vital pulp therapy strategies. Although the evolution of biomaterials since the discovery of calcium hydroxide has been immense and revolutionary, the unique spatiotemporal nature of the dentin-pulp complex poses multiple challenges. This is further complicated by the inherent anatomical, physiological, and biological differences between the primary and the permanent dental pulps [17]. Furthermore, while the required outcome may be the same, indications and outcome assessment methods for pulpotomy procedures in primary and permanent teeth may be quite different. Indeed, in an era where personalized patient care will represent the future of medicine, bioactive vital pulp therapy agents that aim to regenerate anatomical and functional tissues like the native tissue are continuously being developed. These agents and strategies must therefore be carefully tailored not only to whether the tooth is primary or permanent but also according to the developmental and inflammatory status of the tooth in question [18].
Although pulpotomy procedures for primary teeth have long been practiced, the concept of a pulpotomy for a mature permanent tooth has only recently been addressed. Hence, we aimed to focus more on the last 10 years in which a peak in the publication of these papers was noted. Additionally, the use of bioactive cements and biodegradable scaffolds in randomized clinical trials focusing on pulpotomy is relatively new. Therefore, the goal of this scoping review was to elucidate the present knowledge gap and highlight the need for clear decision-making guidelines regarding outcome assessment methods of pulpotomy procedures utilizing regenerative agents in primary and permanent teeth. It was designed and reported with reference to the recently updated JBI scoping review guidelines [15, 19] and Preferred Reporting Items for Systematic Reviews and Meta-Analyses Scoping Review extension (PRISMA-ScR) [2, 20], and was reinforced by the diverse expertise of the authors who include methodologists, analysts, and clinicians sharing an intrigue in evidence-based health care.
While planning this review, the language was restricted to English only to avoid potential confusion in interpretation of data during translation of full text articles. As for exclusion of studies comparing different vital pulp therapy techniques, we wanted to focus the attention of this review on different materials without having the confounding variables of different procedural parameters. Furthermore, we intended to target randomized and non-randomized controlled clinical trials only to provide an overview of the available highest level of clinical evidence to answer the research question, and to determine where further research may be indispensable in this field. We did not set limits for the follow-up periods to include short- and long-term clinical, radiographic, as well as histological and inflammatory assessments. Most of the screened clinical trials (85.8%) comprised 12 months and longer follow-up intervals [18, 21–127]. As the objective of this review was focused on outcome assessment rather than treatment success, which is highly dependent on the initial inflammatory pulp status, we did not restrict our search according to the type of exposure being carious or traumatic to retrieve as many trials as possible in our search in primary and permanent teeth. Additionally, included studies did not stratify the outcomes according to the type of exposure. Remarkably, the pre-operative pulpal status was mainly distinguished as “reversible pulpitis” for both primary and permanent teeth [18, 21, 22, 26, 28–36, 39, 40, 43, 44, 47–56, 58–60, 62–71, 75–123, 125–127, 135–139, 150, 151, 155–157, 167, 168]. On the other hand, teeth categorized with “irreversibly inflamed pulps” were indicated for pulpotomy only for mature permanent teeth [23, 25, 27, 37, 38, 45, 46, 57, 61, 72, 124, 131, 132]. The small percentage of studies performed in mature permanent teeth with irreversible pulpitis highlights this new trend in treatment of the inflamed pulp.
With regards to the total number of studies, the fact that the studies in primary teeth represented almost double those in permanent teeth again clearly reflects that the pulpotomy trend for mature permanent teeth is a new direction in treatment. This is owing to the fact that the pulpotomy procedure is the preferred treatment for preserving the vitality of an asymptomatic cariously exposed primary or immature permanent tooth as dictated by the American Academy of Pediatric Dentistry [128] and is a newly prospective substitute for root canal treatment in managing mature or immature permanent teeth with carious pulp exposures, even with irreversible pulpitis [129]. This was also augmented by the recent position statement from the European Society of Endodontology [130] who recommended minimally invasive vital pulp therapy (VPT) for permanent teeth.
Interestingly, while studies on both primary and permanent teeth displayed a high tendency to use bioactive agents, more than 31.7% (13/41) of studies on permanent teeth implemented biodegradable scaffolds [23, 25, 38, 57, 74, 90, 94, 122, 131–134]versus only 22.1% (19/86) of the studies conducted on primary teeth [18, 22, 40, 53, 55, 56, 70, 71, 77, 84, 97, 102, 108, 110, 135–139]. This could reflect the recent nature of the use of pulpotomy procedures as a permanent treatment modality in mature permanent teeth, which coincides with the recent boom in the development and optimization of a wide variety of bioactive agents [3, 5, 6]. This could also be attributed to the higher need for retaining the permanent teeth throughout life of the patients. Furthermore, it might highlight the differences in outcome assessment methods and follow up duration required following pulpotomy in primary or permanent teeth.
For primary teeth, the main objective of pulpotomy procedures is to keep the tooth symptom-free until the successor tooth erupts [7]. Hence, it is seldom required to aim to regenerate the damaged tissue but rather sustain the condition of the vital pulp until the time of shedding. Indeed, many studies in primary teeth do not consider minor radiographic changes as a reason for further intervention since the tooth can function and the patient has no signs or symptoms [140–143]. However, this concept fails to consider the duration of time that this failure may require. Additionally, it has been shown that the inflammatory milieu within the pulp may be influenced by the active conditions of physiologic tooth resorption, and the contrary may also be true [144–147]. Root resorption is one of the most frequently reported reasons of failure in primary teeth which may again highlight a continued inflammatory trigger even following the pulpotomy procedure [33, 99, 148]. Aiming to regenerate the lost dentin-pulp tissue and restore nociception and immune defense within the pulp may create an inflammation-free environment, allowing the natural process of shedding and eruption. On the other hand, the goal of partial or complete pulpotomy procedures in permanent teeth is to remove the coronally inflamed or infected pulp and preserve the remaining normal or reversibly inflamed radicular pulp. It also aims to promote healing and repair of the remaining vital tissue, not as a temporary treatment but rather as a long-term predictable treatment like conventional root canal treatment [130, 149].
The recent rise in the implementation of biodegradable scaffolds for pulpotomy procedures demonstrates the rapid transition in knowledge and understanding of the dentin-pulp complex from preservation to regeneration. There are also a handful of studies that have used natural derivatives and plant extracts indicating a tendency towards using readily available, naturally healing materials that not only have therapeutic potential but are also cost-effective and environment friendly [35, 50, 93, 116, 125, 137, 150]. Indeed, the use of these extracts in primary teeth has long preceded their use as palliative and healing agents in the permanent dentition.
The evolution of bioactive cements is clearly demonstrated by the results of this study in that most clinical studies utilized either MTA or more recently biodentine [18, 21, 23–30, 32, 33, 35–50, 53–66, 68, 69, 72–101, 103–109, 111–127, 131–136, 138, 151–157]. MTA has been advocated as the new gold standard for pulpotomy procedures. Certainly, almost all of the included studies used MTA for one of the control arms [21, 23–30, 32, 33, 35, 37–39, 41–46, 48–50, 53–70, 72, 73, 75, 76, 78, 79, 81–85, 87–90, 92–96, 98–104, 106–109, 111–127, 132–136, 138, 151–154, 156, 157]. It has excellent potential as a pulpotomy medicament, as it is highly biocompatible, with regenerative potential and effective induction of dentinal bridge formation. Furthermore, a recent study suggested MTA to be a useful material in both infected and uninfected pulp tissue [158] with low toxicity [159] and no adverse effects on permanent successors [160]. The years of clinical experience have revealed some disadvantages of MTA that occur in practice, such as long setting time, potential of discoloration and lengthy procedure. Biodentine seems to have superior properties in that it is more biocompatible, has better handling properties, produces more predictable dentin bridges, and shows comparable treatment outcomes to MTA [59, 82, 98, 161].
Out of the 13 studies that used biodegradable scaffolds in permanent teeth, the majority were conducted using PRF [23, 25, 38, 74, 122, 124, 131–133, 138]. On the other hand, the 19 studies conducted on primary teeth utilized a variety of scaffolds with 0.5% hyaluronic acid gel being the most implemented [22, 56, 97, 110]. This could be due to the rising concept of using regenerative agents for pulpotomy procedures in primary teeth. The use of PRF as a scaffold for permanent tooth pulpotomy stems from the rise in the use of PRF and other platelet-derived concentrates as scaffolds for regenerative endodontics [162, 163]. Platelet rich fibrin is a second-generation platelet-rich concentrate that relies on the body’s own clotting mechanisms without the addition of extrinsic factors to trigger coagulation. The clinical protocol for producing autologous PRF is relatively simple, cost-effective, and reproducible. In comparison to platelet-rich plasma (PRP) and other platelet-derived concentrates, PRF can provide a more sustained release of growth factors which can aid in stem cell recruitment, angiogenesis, and cell proliferation and differentiation [164, 165]. One drawback which may limit the use of PRF and other concentrates for primary teeth pulpotomy is that its procurement may be considered an invasive procedure from children often requiring withdrawing 5–10 cc of blood [165, 166].
A notable observation in this study was that most pulpotomized primary teeth as well as young permanent teeth, capped with biodegradable scaffolds in the screened studies, were covered with zinc oxide eugenol and/or glass ionomer cement followed by stainless steel crowns. This could be because zinc oxide eugenol is regarded as a preservative material not capable of initiating a reparative process in addition to being the material of choice for standard of care procedures in pulpotomized primary teeth [128]. On the other hand, studies in mature permanent teeth that used biodegradable scaffolds/agents mainly utilized mineral trioxide aggregate, biodentine or other calcium silicate cements for sealing. These materials have also been recommended by recent guidelines as vital pulp capping materials [130]. Undoubtedly, the choice of sealing material may have a profound negative effect on the outcome of healing, further advancing the inflammatory process and eventually contributing to failure of the procedure [18].
Although this review clearly shows that numerous well-conducted clinical studies have evaluated pulpotomy outcomes with bioactive agents both in primary and permanent teeth, more than 90% of the screened trials [18, 21–101, 103–115, 125–127, 131–139, 150–153, 155, 156, 167, 168] assessed the pulpotomy treatment outcome via subjective clinical and radiographic parameters.However, recent data has shown that the initial inflammatory status of the pulp is perhaps the only true determining factor that affects the outcome of treatment. Around only 10% of the studies mapped in this review performed histological analysis or attempted to measure inflammatory biomarkers [18, 28, 56, 87, 102, 109, 133, 134, 137, 152, 154, 157]. While histological analysis is of course not possible and, in fact, unwarranted in most clinical trials, it remains the only measure of the actual condition of the pulp [169].
Several recent studies have shown that dentinal fluid and pulpal blood of teeth with inflamed pulps may contain elevated levels of pro-inflammatory markers that can determine the inflammatory status of the pulp [170, 171]. Whilst numerous efforts have been made recently to link biological markers of inflammation (quantitative measure of inflammatory cytokines) to the status of pulp, scarce evidence was identified among the screened trials in this regard. Only one published study [18] and one completed registered clinical trial [87] assessed the relationship between markers of pulp inflammation and the outcome of pulpotomy treatment. Therefore, this review highlighted the gap in the literature with respect to inflammatory assessment of the preoperative pulpal status and its correlation with the pulpotomy outcomes. This has triggered the search for specific pulpal markers and the development of chair-side detection kits that may better help in assessing the eligibility of teeth for pulpotomy procedures and thereby provide the basis for better diagnosis and predictable treatment outcomes.
Another important fact to take into consideration is the duration of follow-up. Pulpotomy procedures have been long practiced in primary teeth, thus providing long term data. However, there are very few studies in permanent teeth with more than 2-4-year follow-up, which is considered moderate-term follow-up at best. Most of the studies were content to hit the 12-month recall [18, 22–25, 27, 32, 37–44, 47, 50, 51, 54–57, 61, 62, 64, 66–72, 77, 79, 80, 84, 85, 88, 90–93, 95, 96, 98, 100, 103–105, 108, 110, 112, 113, 116–118, 120, 124, 125, 127, 131, 132, 151]. This, however, does not allow ample time to assess important parameters such as incidence of root resorption, pulp canal obliteration, tooth survival or impact on quality of life [10, 11].
Two intriguing findings from the current scoping review are the trend of publications from 2012 to 2023 as well as the global distribution of studies. The increased implementation of vital pulp treatment strategies in the last ten years has started as a result of a global effort to diagnose accurately the pulp status of permanent teeth, to preserve pulp vitality, and to increase pulp survival. Regarding the number of publications, there appear to be two peaks: in 2017 and in 2022. The first peak appears to coincide with the initial surge of clinical trials published deeming pulpotomy as a permanent treatment modality for mature permanent teeth with symptomatic irreversible pulpitis, as evidenced by several studies published in that period (20/127) [24, 29, 30, 33, 42, 64, 79, 85, 109, 114–116, 118, 120, 134, 138, 139, 152, 154, 167]. The second peak seems to correspond to the immediate post-pandemic phase. During the COVID-19 outbreak, clinical researchers were impaired by reduced access to health care, and clinical trials were suspended and postponed. Scientific research was then resumed as the world was vaccinated and access to health care was restored [172].
At the beginning of the pandemic and later throughout the rest of 2020 to 2021, most clinical recommendations for the emergency treatment of “hot” teeth or teeth with symptomatic irreversible pulpitis were to employ pulpotomy procedures when possible as a permanent treatment [173, 174]. The reduced invasive nature of the procedure and the ability to perform a pulpotomy in one visit, in addition to the reduced operative time, reduced the risks of infection with COVID-19 due to dental exposure. This, coupled with the apparent reduced cost of pulpotomies, seemed to help in convincing more practitioners to attempt this treatment, although it is still new and lacking long-term evidence. This behavior corresponds perfectly with the sharp peak in the number of publications and clinical trials reported in 2022 (20/127) [21, 25, 35, 37, 41, 48, 61, 63, 73, 76, 77, 84, 90, 96, 98, 110, 112, 127, 156]. The other remarkable finding that coincides with the publication trends is the global distribution, where most studies are distributed in low-to-middle-income countries in the middle east and Asia, particularly in Egypt and India. This global distribution again reflects how adopting pulpotomy procedures as permanent treatments, especially in mature permanent teeth, can present multiple benefits especially where resources are limited.
A significant strength of this scoping review is the demonstration of compliance with the recently updated JBI scoping review guidelines [15, 19] and PRISMA-ScR [2, 20]. Moreover, to our knowledge, this is the first scoping review to address the outcome assessment methods of pulpotomy procedures in both primary and permanent teeth using regenerative non-degradable bioactive cements and biodegradable tissue engineering scaffolds. Another point of strength in this review is that it included grey literature, especially registered clinical trials in the National Institute of Health (NIH) database. The inclusion of grey literature allows a more objective perspective on the status of the evolution of new concepts in treatment.
On the other hand, some limitations related to the methodology were encountered while conducting this review. Only primary research (controlled trials, randomized controlled trials) was included while uncontrolled trials, case reports, case series, systematic reviews, position statements, and clinical guidelines were not. Despite our search being guided by an expert librarian, our electronic literature search was bounded to MEDLINE (via Pubmed), Web of Science, Scopus, Proquest, and clinicaltrials.gov in an attempt to limit the number of articles being scanned; however, this might have led to missing some evidence that address the review questions and objectives present in other search engines.
Our review also revealed significant knowledge gaps, including a scarcity of studies conducted on permanent teeth and a dearth of studies establishing a correlation between actual inflammatory status of the pulp and treatment outcomes. Therefore, long term evidence from well-conducted clinical trials is still needed, as well as the development of a set of predictable outcome measures and the interpretation of outcomes in terms of both treatment success and tooth survival. It is also crucial when designing new biodegradable scaffolds, for promoting tissue regeneration following pulpotomy procedures, to tailor their properties according to the inflammatory milieu and whether they will be designed for usage in primary or permanent teeth.
Conclusions
Within the limitations of this scoping review, the findings underscored that evaluation methods of pulpotomy procedures using regenerative agents in primary and permanent teeth, over the past decade, primarily focused subjectively on clinical and radiographic outcomes. On the other hand, there are few studies that objectively assessed the pulpal inflammatory status. Among various materials, MTA emerged as the most frequently utilized capping material followed by biodentine. However, a limited number of studies incorporating biodegradable scaffolds for pulpotomy procedures were found. Furthermore, the results indicated a recent surge in publications originating in low-to-middle-income countries; hence, indicating a widespread implementation potential for pulpotomy procedures in both dentitions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- OHRQoL
Oral health-related quality of life
- MTA
Mineral trioxide aggregate
- JBI
Joanna Briggs Institute
- CEM
Calcium-enriched mixture
- PRF
Platelet-rich fibrin
- PRISMA-ScR
Preferred Reporting Items for Systematic Reviews and Meta-Analyses Scoping Review extension
- VPT
Vital pulp therapy
- PRP
Platelet-rich plasma
- NIH
National Institutes of Health
- RCT
Randomized clinical trial
- NRS
Non-randomized study
Author contributions
YE: Conceptualization (equal); Methodology (supporting); Investigation (equal); Writing – original draft (equal); writing – review and editing (equal); Visualization (equal). MMA: Methodology (lead); data curation (lead); Investigation (equal); Formal analysis (lead); Validation (lead); Visualization (equal). KMLD: Conceptualization (equal); Methodology (supporting); Investigation (equal); Supervision (lead), Visualization (equal). RME Conceptualization (equal); Methodology (supporting); Investigation (equal); Writing – original draft (equal); writing – review and editing (equal); Visualization (equal). All authors contributed to critical revision and approval of the final manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lin GSS, Yew YQ, Lee HY, Low T, Pillai MPM, Laer TS, et al. Is pulpotomy a promising modality in treating permanent teeth? An umbrella review. Odontology. 2022;110(2):393–409. doi: 10.1007/s10266-021-00661-w. [DOI] [PubMed] [Google Scholar]
- 2.Tricco AC, Zarin W, Ghassemi M, Nincic V, Lillie E, Page MJ, et al. Same family, different species: methodological conduct and quality varies according to purpose for five types of knowledge synthesis. J Clin Epidemiol. 2018;96:133–42. doi: 10.1016/j.jclinepi.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Cushley S, Duncan HF, Lappin MJ, Tomson PL, Lundy FT, Cooper P, et al. Pulpotomy for mature carious teeth with symptoms of irreversible pulpitis: a systematic review. J Dent. 2019;88:103158. doi: 10.1016/j.jdent.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 4.El karim Ikhlas A, CPR, About Imad, Tomson Phillip L, Lundy Fionnuala T. Duncan Henry F. Deciphering Reparative Processes in the Inflamed Dental Pulp. 2021;2.
- 5.Alqaderi H, Lee CT, Borzangy S, Pagonis TC. Coronal pulpotomy for cariously exposed permanent posterior teeth with closed apices: a systematic review and meta-analysis. J Dent. 2016;44:1–7. doi: 10.1016/j.jdent.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Lin GSS, Hisham ARB, Ch Er CIY, Cheah KK, Ghani N, Noorani TY. Success rates of coronal and partial pulpotomies in mature permanent molars: a systematic review and single-arm meta-analysis. Quintessence Int. 2021;0(0):0. doi: 10.3290/j.qi.b912685. [DOI] [PubMed] [Google Scholar]
- 7.Fuks AB. Current concepts in vital primary pulp therapy. Eur J Paediatr Dent. 2002;3(3):115–20. [PubMed] [Google Scholar]
- 8.American Association of Endodontists. Guide to Clinical Endodontics 6th ed. Chicago, Ill.: American Association of Endodontists2013. https://www.aae.org/specialty/clinical-resources/guide-clinical-endodontics/
- 9.Rechenberg DK, Zehnder M. Call for a review of diagnostic nomenclature and terminology used in endodontics. Int Endod J. 2020;53(10):1315–7. doi: 10.1111/iej.13374. [DOI] [PubMed] [Google Scholar]
- 10.Cushley S, Duncan HF, Lundy FT, Nagendrababu V, Clarke M, El Karim I. Outcomes reporting in systematic reviews on vital pulp treatment: a scoping review for the development of a core outcome set. Int Endod J. 2022;55(9):891–909. doi: 10.1111/iej.13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duncan HF. Present status and future directions-vital pulp treatment and pulp preservation strategies. Int Endod J. 2022;55(3):497–511. doi: 10.1111/iej.13688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjorndal L, Simon S, Tomson PL, Duncan HF. Management of deep caries and the exposed pulp. Int Endod J. 2019;52(7):949–73. doi: 10.1111/iej.13128. [DOI] [PubMed] [Google Scholar]
- 13.Jha S, Goel N, Dash BP, Sarangal H, Garg I, Namdev R. An update on newer Pulpotomy agents in primary teeth: a Literature Review. J Pharm Bioallied Sci. 2021;13(Suppl 1):S57–61. doi: 10.4103/jpbs.JPBS_799_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Igna A. Vital Pulp Therapy in primary dentition: Pulpotomy-A 100-Year challenge. Child (Basel). 2021;8(10). [DOI] [PMC free article] [PubMed]
- 15.Peters M, Godfrey C, McInerney P, Munn Z, Tricco A, Khalil H. In: Chapter 11: scoping reviews (2020 version) Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis: JBI; 2020. [Google Scholar]
- 16.Page M, McKenzie J, Bossuyt P, Boutron I, Hoffman T, Mulrow C et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372. [DOI] [PMC free article] [PubMed]
- 17.Herlesová J. Histology of dental pulp in clinically intact deciduous and permanent teeth in children. Acta Universitatis Carol Med. 1968;14(6):229–34. [PubMed] [Google Scholar]
- 18.Elhamouly Y, El Backly RM, Talaat DM, Omar SS, El Tantawi M, Dowidar KML. Tailored 70S30C bioactive glass induces severe inflammation as pulpotomy agent in primary teeth: an interim analysis of a randomised controlled trial. Clin Oral Invest. 2021;25(6):3775–87. doi: 10.1007/s00784-020-03707-5. [DOI] [PubMed] [Google Scholar]
- 19.Peters MDJ, Marnie C, Tricco AC, Pollock D, Munn Z, Alexander L, et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth. 2020;18(10):2119–26. doi: 10.11124/JBIES-20-00167. [DOI] [PubMed] [Google Scholar]
- 20.Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for scoping reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169(7):467–73. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 21.Manohar S, Bazaz N, Neeraja G, Subramaniam P, Sneharaj N. A comparative evaluation of four regenerative materials for pulpotomy in primary molars: an in vivo study. Dent Res J. 2022;19:102. doi: 10.4103/1735-3327.363532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.NCT04115358. Evaluation of hyaluronic acid pulpotomies in primary molars. https://clinicaltrials.gov/study/NCT04115358. Completed. 2021-08–03.
- 23.NCT04784949. PRF pulpotomy using different bioceramic materials in permanent molars. https://clinicaltrials.gov/study/NCT04784949. Completed. 2021-03–5.
- 24.NCT03119779. Effect of pulpotomy using theracal versus mta on survival rate of cariously-exposed vital permanent molars. https://clinicaltrials.gov/study/NCT03119779. Completed. 2017-07-25.
- 25.NCT05266859. Efficacy of PRF and MTA as compared to calcium hydroxide for pulpotomy in human irreversibly inflamed permanent teeth. https://clinicaltrials.gov/study/NCT05266859. Completed. 2022-08-17.
- 26.Nguyen TD, Judd PL, Barrett EJ, Sidhu N, Casas MJ. Comparison of Ferric Sulfate Combined Mineral Trioxide Aggregate Pulpotomy and Zinc Oxide Eugenol Pulpectomy of primary Maxillary incisors: an 18-month Randomized, Controlled Trial. Pediatr Dent. 2017;39(1):34–8. [PubMed] [Google Scholar]
- 27.Nosrat A, Seifi A, Asgary S. Pulpotomy in caries-exposed immature permanent molars using calcium-enriched mixture cement or mineral trioxide aggregate: a randomized clinical trial. Int J Pediatr Dent. 2013;23(1):56–63. doi: 10.1111/j.1365-263X.2012.01224.x. [DOI] [PubMed] [Google Scholar]
- 28.Oliveira TM, Moretti AB, Sakai VT, Lourenço Neto N, Santos CF, Machado MA, et al. Clinical, radiographic and histologic analysis of the effects of pulp capping materials used in pulpotomies of human primary teeth. Eur Arch Paediatr Dent. 2013;14(2):65–71. doi: 10.1007/s40368-013-0015-x. [DOI] [PubMed] [Google Scholar]
- 29.Ozgur B, Uysal S, Gungor HC. Partial pulpotomy in Immature Permanent molars after Carious exposures using different Hemorrhage Control and Capping materials. Pediatr Dent. 2017;39(5):364–70. [PubMed] [Google Scholar]
- 30.Perea MB, Mendoza BS, Garcia-Godoy F, Mendoza AM, Iglesias-Linares A. Clinical and radiographic evaluation of white MTA versus formocresol pulpotomy: a 48-month follow-up study. Am J Dent. 2017;30(3):131–6. [PubMed] [Google Scholar]
- 31.Petel R, Ziskind K, Bernfeld N, Suliman H, Fuks AB, Moskovitz M. A randomised controlled clinical trial comparing pure Portland cement and formocresol pulpotomies followed from 2 to 4 years. Eur Arch Paediatr Dent. 2021;22(4):547–52. doi: 10.1007/s40368-020-00578-y. [DOI] [PubMed] [Google Scholar]
- 32.Pratima B, Chandan GD, Nidhi T, Nitish I, Sankriti M, Nagaveni S, et al. Postoperative assessment of diode laser zinc oxide eugenol and mineral trioxide aggregate pulpotomy procedures in children: a comparative clinical study. J Indian Soc Pedod Prev Dent. 2018;36(3):308–14. doi: 10.4103/JISPPD.JISPPD_1132_17. [DOI] [PubMed] [Google Scholar]
- 33.Rajasekharan S, Martens LC, Vandenbulcke J, Jacquet W, Bottenberg P, Cauwels RG. Efficacy of three different pulpotomy agents in primary molars: a randomized control trial. Int Endod J. 2017;50(3):215–28. doi: 10.1111/iej.12619. [DOI] [PubMed] [Google Scholar]
- 34.Rao Q, Kuang J, Mao C, Dai J, Hu L, Lei Z, et al. Comparison of iRoot BP Plus and Calcium Hydroxide as Pulpotomy materials in Permanent incisors with complicated Crown fractures: a retrospective study. J Endod. 2020;46(3):352–7. doi: 10.1016/j.joen.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 35.RojaRamya KS, Chandrasekhar R, Uloopi KS, Vinay C. Treatment outcomes of Pulpotomy with Propolis in comparison with MTA in human primary molars: a 24-month follow-up Randomized Controlled Trial. Int J Clin Pediatr Dentistry. 2022;15(Suppl 1):S3–7. doi: 10.5005/jp-journals-10005-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubanenko M, Petel R, Tickotsky N, Fayer I, Fuks AB, Moskovitz M. A randomized controlled clinical trial comparing Tricalcium Silicate and Formocresol pulpotomies followed for two to four years. Pediatr Dent. 2019;41(6):446–50. [PubMed] [Google Scholar]
- 37.Sharaan M, Ali A. Mineral Trioxide Aggregate vs calcium-enriched mixture pulpotomy in Young Permanent molars with a diagnosis of irreversible pulpitis: a Randomized Clinical Trial. Iran Endod J. 2022;17(3):106–13. doi: 10.22037/iej.v17i3.35706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh R, Singh R, Kavita K, Kommula A, Kulkarni G, Jois HS. To compare Mineral Trioxide Aggregate, platelet-rich fibrin, and Calcium Hydroxide in Teeth with irreversible pulpitis: a clinical study. J Pharm Bioallied Sci. 2020;12(Suppl 1):S436–9. doi: 10.4103/jpbs.JPBS_130_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh DVV, Taneja S, Fatima S. Comparative evaluation of treatment outcome of partial pulpotomy using different agents in permanent teeth-a randomized controlled trial. Clin Oral Invest. 2023;27(9):5171–80. doi: 10.1007/s00784-023-05136-6. [DOI] [PubMed] [Google Scholar]
- 40.NCT05582317. Efficacy of combination of biodentine and simvastatin as a pulp capping materials in vital pulpotomy of primary molars. https://clinicaltrials.gov/study/NCT05582317. Completed. 2022-10-17.
- 41.Taha NA, Al-Rawash MH, Imran ZA. Outcome of full pulpotomy in mature permanent molars using 3 calcium silicate-based materials: a parallel, double blind, randomized controlled trial. Int Endod J. 2022;55(5):416–29. doi: 10.1111/iej.13707. [DOI] [PubMed] [Google Scholar]
- 42.NCT04345263. Outcome of full pulpotomy using calcium silicate based materials. https://clinicaltrials.gov/study/NCT04345263. Unknown. 2021-09-22.
- 43.Togaru H, Muppa R, Srinivas N, Naveen K, Reddy VK, Rebecca VC. Clinical and radiographic evaluation of success of two commercially available Pulpotomy agents in primary teeth: an in vivo study. J Contemp Dent Pract. 2016;17(7):557–63. doi: 10.5005/jp-journals-10024-1889. [DOI] [PubMed] [Google Scholar]
- 44.NCT04010929. Efficacy of Er,Cr:YSGG laser in partial pupotomy. https://clinicaltrials.gov/study/NCT04010929. Completed. 2019-07-08.
- 45.Tzanetakis GN, Koletsi D, Georgopoulou M. Treatment outcome of partial pulpotomy using two different calcium silicate materials in mature permanent teeth with symptoms of irreversible pulpitis: a randomized clinical trial. Int Endod J. 2023;56(10):1178–96. doi: 10.1111/iej.13955. [DOI] [PubMed] [Google Scholar]
- 46.Uesrichai N, Nirunsittirat A, Chuveera P, Srisuwan T, Sastraruji T, Chompu-Inwai P. Partial pulpotomy with two bioactive cements in permanent teeth of 6- to 18-year-old patients with signs and symptoms indicative of irreversible pulpitis: a noninferiority randomized controlled trial. Int Endod J. 2019;52(6):749–59. doi: 10.1111/iej.13071. [DOI] [PubMed] [Google Scholar]
- 47.NCT02201498. Randomized clinical trial for primary molar pulpotomy, biodentine vs formocresol-ZOE. https://clinicaltrials.gov/study/NCT02201498. Completed. 2018-01-12.
- 48.Vafaei A, Nikookhesal M, Erfanparast L, Løvschall H, Ranjkesh B. Vital pulp therapy following pulpotomy in immature first permanent molars with deep caries using novel fast-setting calcium silicate cement: a retrospective clinical study. J Dent. 2022;116:103890. doi: 10.1016/j.jdent.2021.103890. [DOI] [PubMed] [Google Scholar]
- 49.Vilella-Pastor S, Sáez S, Veloso A, Guinot-Jimeno F, Mercadé M. Long-term evaluation of primary teeth molar pulpotomies with Biodentine and MTA: a CONSORT randomized clinical trial. Eur Arch Paediatr Dent. 2021;22(4):685–92. doi: 10.1007/s40368-021-00616-3. [DOI] [PubMed] [Google Scholar]
- 50.Vu TT, Nguyen MT, Sangvanich P, Nguyen QN, Thunyakitpisal P. Acemannan used as an Implantable Biomaterial for Vital Pulp Therapy of Immature Permanent Teeth Induced continued Root formation. Pharmaceutics. 2020;12(7):644. doi: 10.3390/pharmaceutics12070644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.NCT04397094. Theracal pulpotomy in primary molars. https://clinicaltrials.gov/study/NCT04397094. Completed. 2020-07–29.
- 52.Yang Y, Xia B, Xu Z, Dou G, Lei Y, Yong W. The effect of partial pulpotomy with iRoot BP plus in traumatized immature permanent teeth: a randomized prospective controlled trial. Dent Traumatology: Official Publication Int Association Dent Traumatol. 2020;36(5):518–25. doi: 10.1111/edt.12563. [DOI] [PubMed] [Google Scholar]
- 53.Yildirim C, Basak F, Akgun OM, Polat GG, Altun C. Clinical and radiographic evaluation of the effectiveness of Formocresol, Mineral Trioxide Aggregate, Portland Cement, and Enamel Matrix Derivative in primary Teeth pulpotomies: a Two Year Follow-Up. J Clin Pediatr Dent. 2016;40(1):14–20. doi: 10.17796/1053-4628-40.1.14. [DOI] [PubMed] [Google Scholar]
- 54.NCT04795830. Bioactive materials in pulp therapy of primary teeth. https://clinicaltrials.gov/study/NCT04795830. Completed. 2023-07-21.
- 55.Abd Al Gawad RY, Hanafy RMH. Success rate of three capping materials used in pulpotomy of primary molars: a randomized clinical trial. Saudi Dent J. 2021;33(7):560–7. doi: 10.1016/j.sdentj.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.NCT05981352. Evaluation of different materials in pulpotomy of primary molars. https://clinicaltrials.gov/study/NCT05981352. Not yet recruiting. 2023.
- 57.NCT04308863. Evaluation of chitosan scaffold and mineral trioxide aggregate pulpotomy in mature permanent molars with irreversible pulpitis. https://clinicaltrials.gov/study/NCT04308863. Completed 2022-05-19.
- 58.Abuelniel GM, Duggal MS, Kabel N. A comparison of MTA and Biodentine as medicaments for pulpotomy in traumatized anterior immature permanent teeth: a randomized clinical trial. Dent Traumatology: Official Publication Int Association Dent Traumatol. 2020;36(4):400–10. doi: 10.1111/edt.12553. [DOI] [PubMed] [Google Scholar]
- 59.Abuelniel GM, Duggal MS, Duggal S, Kabel NR. Evaluation of Mineral Trioxide Aggregate and Biodentine as pulpotomy agents in immature first permanent molars with carious pulp exposure: a randomised clinical trial. Eur J Paediatr Dent. 2021;22(1):19–25. doi: 10.23804/ejpd.2021.22.01.04. [DOI] [PubMed] [Google Scholar]
- 60.Airen P, Shigli A, Airen B. Comparative evaluation of formocresol and mineral trioxide aggregate in pulpotomized primary molars–2 year follow up. J Clin Pediatr Dent. 2012;37(2):143–7. doi: 10.17796/jcpd.37.2.h427vr8157444462. [DOI] [PubMed] [Google Scholar]
- 61.Airsang AJ, Shankaregowda AM, Meena N, Lingaiah U, Lakshminarasimhaiah V, Harti S. Comparative Assessment of Complete Pulpotomy in mature permanent teeth with Carious exposure using calcium Silicate Cement: a Randomized Clinical Trial. World J Dentistry. 2022;13(S2):S135–43. doi: 10.5005/jp-journals-10015-2145. [DOI] [Google Scholar]
- 62.Akcay M, Sari S. The effect of sodium hypochlorite application on the success of calcium hydroxide and mineral trioxide aggregate pulpotomies in primary teeth. Pediatr Dent. 2014;36(4):316–21. [PubMed] [Google Scholar]
- 63.Aksoy B, Güngör HC, Uysal S, Gonzales CD, Ölmez S. Ferric sulfate pulpotomy in primary teeth with different base materials: a 2-year randomized controlled trial. Quintessence Int. 2022;53(9):782–9. doi: 10.3290/j.qi.b3149429. [DOI] [PubMed] [Google Scholar]
- 64.NCT03426046. Treatment of immature permanent teeth with three different pulp capping materials with partial pulpotomy. https://clinicaltrials.gov/study/NCT03426046. Completed. 2018-02-12.
- 65.Alajaji N. Comparing the Outcomes of Three Pulpotomy Agents in Primary Molars: A Retrospective Study. University of Illinois at Chicago. Thesis. 2021.
- 66.NCT03782714. Low-level laser therapy versus formocresol in primary molar pulpotomies. https://clinicaltrials.gov/study/NCT03782714. Completed. 2018-12-20.
- 67.NCT03779698. BiodentineTM versus formocresol pulpotomy technique in primary molars. https://clinicaltrials.gov/study/NCT03779698. Completed. 2018-12-19. [DOI] [PMC free article] [PubMed]
- 68.Alnassar I, Altinawi M, Rekab MS, Alzoubi H, Abdo A. Evaluation of the efficacy of mineral trioxide aggregate and bioceramic putty in primary molar pulpotomy with symptoms of irreversible pulpitis (a randomized-controlled trial) Clin Exp Dent Res. 2023;9(2):276–82. doi: 10.1002/cre2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.NCT04634123. The use of Portland cement in primary anterior teeth pulpotomy. https://clinicaltrials.gov/study/NCT04634123. Completed. 2021-12-09.
- 70.Anandan V, Inbanathan J, Saket P, Krishnamoorthy V, Gandhi S, Chandrababu VK. Assessment of Clinical and Radiographic Success Rate of Formocresol-based Pulpotomy versus Collagen-based Pulpotomy: an in vivo study. J Contemp Dent Pract. 2021;22(6):680–5. doi: 10.5005/jp-journals-10024-3117. [DOI] [PubMed] [Google Scholar]
- 71.Aripirala M, Bansal K, Mathur VP, Tewari N, Gupta P, Logani A. Comparative evaluation of diode laser and simvastatin gel in pulpotomy of primary molars: a randomized clinical trial. J Indian Soc Pedod Prev Dent. 2021;39(3):303–9. doi: 10.4103/jisppd.jisppd_60_21. [DOI] [PubMed] [Google Scholar]
- 72.Asgary S, Eghbal MJ. Treatment outcomes of pulpotomy in permanent molars with irreversible pulpitis using biomaterials: a multi-center randomized controlled trial. Acta Odontol Scand. 2012;71(1):130–6. doi: 10.3109/00016357.2011.654251. [DOI] [PubMed] [Google Scholar]
- 73.Asgary S, Eghbal MJ, Shahravan A, Saberi E, Baghban AA, Parhizkar A. Outcomes of root canal therapy or full pulpotomy using two endodontic biomaterials in mature permanent teeth: a randomized controlled trial. Clin Oral Invest. 2022;26(3):3287–97. doi: 10.1007/s00784-021-04310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.NCT05347160. Evaluation of different pulpotomy agents used for treatment of immature molars. https://clinicaltrials.gov/study/NCT05347160. Active not recruiting. 2022-04-26.
- 75.Awawdeh L, Al-Qudah A, Hamouri H, Chakra RJ. Outcomes of vital pulp therapy using Mineral Trioxide Aggregate or Biodentine: a prospective Randomized Clinical Trial. J Endod. 2018;44(11):1603–9. doi: 10.1016/j.joen.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 76.Bani M, Aktaş N, Çınar Ç, Odabaş ME. The clinical and radiographic success of primary molar pulpotomy using Biodentine™ and Mineral Trioxide Aggregate: a 24-Month Randomized Clinical Trial. Pediatr Dent. 2022;39(4):284–8. [PubMed] [Google Scholar]
- 77.NCT05479877. Evaluation of hyaluronic acid pulpotomies in primary molars. https://clinicaltrials.gov/study/NCT04115358. Completed. 2021-08–03.
- 78.Brar K. Comparing Biodentine and Ferric Sulfate as Pulpotomy agents in primary molars: a retrospective study. University of Illinois at Chicago; 2020.
- 79.Carti O, Oznurhan F. Evaluation and comparison of mineral trioxide aggregate and biodentine in primary tooth pulpotomy: clinical and radiographic study. Niger J Clin Pract. 2017;20(12):1604–9. doi: 10.4103/1119-3077.196074. [DOI] [PubMed] [Google Scholar]
- 80.Caruso S, Dinoi T, Marzo G, Campanella V, Giuca MR, Gatto R, et al. Clinical and radiographic evaluation of biodentine versus calcium hydroxide in primary teeth pulpotomies: a retrospective study. BMC Oral Health. 2018;18:1–7. doi: 10.1186/s12903-018-0522-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Celik B, Ataç AS, Cehreli ZC, Uysal S. A randomized trial of mineral trioxide aggregate cements in primary tooth pulpotomies. J Dentistry Child (Chicago Ill) 2013;80(3):126–32. [PubMed] [Google Scholar]
- 82.Celik BN, Mutluay MS, Arikan V, Sari S. The evaluation of MTA and Biodentine as a pulpotomy materials for carious exposures in primary teeth. Clin Oral Invest. 2019;23(2):661–6. doi: 10.1007/s00784-018-2472-4. [DOI] [PubMed] [Google Scholar]
- 83.Chailertvanitkul P, Paphangkorakit J, Sooksantisakoonchai N, Pumas N, Pairojamornyoot W, Leela-Apiradee N, et al. Randomized control trial comparing calcium hydroxide and mineral trioxide aggregate for partial pulpotomies in cariously exposed pulps of permanent molars. Int Endod J. 2014;47(9):835–42. doi: 10.1111/iej.12225. [DOI] [PubMed] [Google Scholar]
- 84.Chak RK, Singh RK, Mutyala J, Killi NK. Clinical Radiographic evaluation of 3Mixtatin and MTA in primary Teeth pulpotomies: a randomized controlled. Int J Clin Pediatr Dentistry. 2022;15(Suppl 1):S80–6. doi: 10.5005/jp-journals-10005-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.NCT02783911. Comparison of mineral trioxide aggregate (MTA) & ferric sulfate (FS) pulpotomies. https://clinicaltrials.gov/study/NCT02783911. Terminated. 2021-06-11.
- 86.Clancy MR. Comparing the success of BiodentineTM vs. Ferric Sulfate in primary molar pulpotomies. University of Illinois at Chicago; 2018.
- 87.NCT05145686. The role of matrix metalloproteinases on the primary teeth pulpotomy treatments. https://clinicaltrials.gov/study/NCT05145686. Completed. 2022-01-03.
- 88.Cordell SC. Randomized Controlled Clinical Trial Comparing the Success of Two Pulpotomy Agents for Primary Molars. PhD Thesis: University of Illinois at Chicago; 2019.
- 89.Lima SPR, Santos GLD, Ferelle A, Ramos SP, Pessan JP, Dezan-Garbelini CC. Clinical and radiographic evaluation of a new stain-free tricalcium silicate cement in pulpotomies. Brazilian oral Res. 2020;34:e102. doi: 10.1590/1807-3107bor-2020.vol34.0102. [DOI] [PubMed] [Google Scholar]
- 90.Eid A, Mancino D, Rekab MS, Haikel Y, Kharouf N. Effectiveness of three agents in Pulpotomy Treatment of Permanent Molars with Incomplete Root Development: a Randomized Controlled Trial. Healthc (Basel Switzerland) 2022;10(3):431. doi: 10.3390/healthcare10030431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.El Meligy O, Alamoudi NM, Allazzam SM, El-Housseiny AAM. Biodentine(TM) versus formocresol pulpotomy technique in primary molars: a 12-month randomized controlled clinical trial. BMC Oral Health. 2019;19(1):3. doi: 10.1186/s12903-018-0702-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.NCT03582319. Clinical and radiographic evaluation of biodentine versus formocresol. https://clinicaltrials.gov/study/NCT03582319. Completed. 2018-07–11.
- 93.NCT05812053. Comparative evaluation of eggshell powder in primary teeth pulpotomy. https://clinicaltrials.gov/study/NCT05812053. Recruiting. 2023-12-07.
- 94.NCT05878158. Simvastatin versus MTA in pulpotomy of immature permanent molars. https://clinicaltrials.gov/study/NCT05878158. Recruiting. 2023-05–26.
- 95.NCT04710160. Clinical and radiographic evaluation in pulpotomy of primary molars using protooth vs. MTA. https://clinicaltrials.gov/study/NCT04710160. Unknown. 2021-08-05.
- 96.NCT04989036. Biodentine vital pulpotomy in immature molars. https://clinicaltrials.gov/study/NCT04989036. Completed. 2021-08–04.
- 97.NCT05937100. New vital pulpotomy medications in primary molars. https://clinicaltrials.gov/study/NCT05937100. Active not recruiting. 2023-07-10.
- 98.Eshghi A, Hajiahmadi M, Nikbakht MH, Esmaeili M. Comparison of clinical and radiographic success between MTA and Biodentine in Pulpotomy of primary Mandibular Second molars with irreversible pulpitis: a Randomized double-blind clinical trial. Int J Dent. 2022;2022:6963944. doi: 10.1155/2022/6963944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fernández CC, Martínez SS, Jimeno FG, Lorente Rodríguez AI, Mercadé M. Clinical and radiographic outcomes of the use of four dressing materials in pulpotomized primary molars: a randomized clinical trial with 2-year follow-up. Int J Pediatr Dent. 2013;23(6):400–7. doi: 10.1111/ipd.12009. [DOI] [PubMed] [Google Scholar]
- 100.Fouad WA, Abd Al Gawad RY. Is biodentine, as successful as, mineral trioxide aggregate for pulpotomy of primary molars? A split-mouth clinical trial. Tanta Dent J. 2019;16(2):115–9. doi: 10.4103/tdj.tdj_35_18. [DOI] [Google Scholar]
- 101.Frenkel G, Kaufman A, Ashkenazi M. Clinical and radiographic outcomes of pulpotomized primary molars treated with white or gray mineral trioxide aggregate and ferric sulfate–long-term follow-up. J Clin Pediatr Dent. 2012;37(2):137–41. doi: 10.17796/jcpd.37.2.j3h27p624u163868. [DOI] [PubMed] [Google Scholar]
- 102.Kakarla P, Avula JS, Mellela GM, Bandi S, Anche S. Dental pulp response to collagen and pulpotec cement as pulpotomy agents in primary dentition: a histological study. J Conserv Dent. 2013;16(5):434–8. doi: 10.4103/0972-0707.117525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.NCT05747300. Wellroot PT versus MTA in pulpotomy of primary molars. https://clinicaltrials.gov/study/NCT05747300. Not Yet Recruiting. 2023-02–28.
- 104.NCT05149651. Efficacy of Totalfill® and ProRoot MTA® as Pulpotomy Agent in primary molars. https://clinicaltrials.gov/study/NCT05149651. Completed. 2023-11-22.
- 105.Grewal N, Salhan R, Kaur N, Patel HB. Comparative evaluation of calcium silicate-based dentin substitute (Biodentine((R))) and calcium hydroxide (pulpdent) in the formation of reactive dentin bridge in regenerative pulpotomy of vital primary teeth: triple blind, randomized clinical trial. Contemp Clin Dent. 2016;7(4):457–63. doi: 10.4103/0976-237X.194116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.NCT03135626. Comparison of the success rates of four different pulpotomy techniques. https://clinicaltrials.gov/study/NCT03135626. Completed. 2017-05-01.
- 107.NCT01962077. Comparison of MedCem MTA and formocresol used in pulpotomies in primary teeth. https://clinicaltrials.gov/study/NCT01962077. Completed. 2021-07-06.
- 108.Haideri S, Koul M, Raj R, Salam SA, Kalim MS, Gupta V. To evaluate and compare the clinical and radiographic outcomes of Formocresol, Mineral Trioxide Aggregate, Electrocautery, and Bioactive Glass when used for Pulpotomy in Human Primary Teeth. J Pharm Bioallied Sci. 2021;13(Suppl 2):S1251–8. doi: 10.4103/jpbs.jpbs_23_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hugar SM, Reddy R, Deshpande SD, Shigli A, Gokhale NS, Hugar SS. In vivo comparative evaluation of Mineral Trioxide Aggregate and Formocresol Pulpotomy in primary molars: a 60-month follow-up study. Contemp Clin Dent. 2017;8(1):122–7. doi: 10.4103/ccd.ccd_849_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ildeş GÇ, Sezgin BI, Vieira AR, Mentes A. A randomized clinical trial of hyaluronic acid gel pulpotomy in primary molars with 1 year follow-up. Acta Odontol Scand. 2022;80(4):273–80. doi: 10.1080/00016357.2021.1998612. [DOI] [PubMed] [Google Scholar]
- 111.Jayam C, Mitra M, Mishra J, Bhattacharya B, Jana B. Evaluation and comparison of white mineral trioxide aggregate and formocresol medicaments in primary tooth pulpotomy: clinical and radiographic study. J Indian Soc Pedod Prev Dent. 2018;32(1):13–8. doi: 10.4103/0970-4388.127043. [DOI] [PubMed] [Google Scholar]
- 112.NCT04902495. Clinical and radiographic evaluation of pulpotomies in primary molars using tricalcium silicate cements. https://clinicaltrials.gov/study/NCT04902495. Recruiting. 2022-11-03.
- 113.Joo Y, Lee T, Jeong SJ, Lee JH, Song JS, Kang CM. A randomized controlled clinical trial of premixed calcium silicate-based cements for pulpotomy in primary molars. J Dent. 2023;137:104684. doi: 10.1016/j.jdent.2023.104684. [DOI] [PubMed] [Google Scholar]
- 114.Juneja P, Kulkarni S. Clinical and radiographic comparison of biodentine, mineral trioxide aggregate and formocresol as pulpotomy agents in primary molars. Eur Arch Paediatr Dent. 2017;18(4):271–8. doi: 10.1007/s40368-017-0299-3. [DOI] [PubMed] [Google Scholar]
- 115.Junqueira MA, Cunha NNO, Caixeta FF, Marques NCT, Oliveira TM, Moretti A, et al. Clinical, radiographic and histological evaluation of primary teeth pulpotomy using MTA and ferric sulfate. Braz Dent J. 2017;29(2):159–65. doi: 10.1590/0103-6440201801659. [DOI] [PubMed] [Google Scholar]
- 116.Kalra M, Garg N, Rallan M, Pathivada L, Yeluri R. Comparative evaluation of Fresh Aloe barbadensis Plant Extract and Mineral Trioxide Aggregate as Pulpotomy agents in primary molars: a 12-month follow-up study. Contemp Clin Dent. 2017;8(1):106–11. doi: 10.4103/ccd.ccd_874_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kang CM, Kim SH, Shin Y, Lee HS, Lee JH, Kim GT, et al. A randomized controlled trial of ProRoot MTA, OrthoMTA and RetroMTA for pulpotomy in primary molars. Oral Dis. 2015;21(6):785–91. doi: 10.1111/odi.12348. [DOI] [PubMed] [Google Scholar]
- 118.Kang CM, Sun Y, Song JS, Pang NS, Roh BD, Lee CY, et al. A randomized controlled trial of various MTA materials for partial pulpotomy in permanent teeth. J Dent. 2017;60:8–13. doi: 10.1016/j.jdent.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 119.Kang CM, Seong S, Song JS, Shin Y. The role of hydraulic silicate cements on Long-Term properties and Biocompatibility of partial pulpotomy in Permanent Teeth. Mater (Basel) 2021;14(2):305. doi: 10.3390/ma14020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kathal S, Gupta S, Bhayya DP, Rao A, Roy AP, Sabhlok A. A comparative evaluation of clinical and radiographic success rate of pulpotomy in primary molars using antioxidant mix and mineral trioxide aggregate: an in vivo 1-year follow-up study. J Indian Soc Pedod Prev Dent. 2017;35(4):327–31. doi: 10.4103/JISPPD.JISPPD_255_16. [DOI] [PubMed] [Google Scholar]
- 121.NCT03718676. Pulpotomy with various MTA materials and ferric sulphate. https://clinicaltrials.gov/study/NCT03718676. Completed. 2019-03–5.
- 122.Keswani D, Pandey RK, Ansari A, Gupta S. Comparative evaluation of platelet-rich fibrin and mineral trioxide aggregate as pulpotomy agents in permanent teeth with incomplete root development: a randomized controlled trial. J Endod. 2014;40(5):599–605. doi: 10.1016/j.joen.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 123.NCT03395496. Comparison of biodentine and MTA pulpotomies in the primary molar teeth 3 year follow up. https://clinicaltrials.gov/study/NCT03395496. Completed. 2018-01–10.
- 124.Kumar V, Juneja R, Duhan J, Sangwan P, Tewari S. Comparative evaluation of platelet-rich fibrin, mineral trioxide aggregate, and calcium hydroxide as pulpotomy agents in permanent molars with irreversible pulpitis: a randomized controlled trial. Contemp Clin Dent. 2016;7(4):512–8. doi: 10.4103/0976-237X.194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Madan K, Baliga S, Deulkar P, Thosar N, Rathi N, Deshpande M, et al. A comparative evaluation between propolis and mineral trioxide aggregate as pulpotomy medicaments in primary molars. J Evol Med Dent Sci. 2020;9:1256–60. doi: 10.14260/jemds/2020/273. [DOI] [Google Scholar]
- 126.NCT05102318. Clinical and radiographic evaluation of mineral trioxide aggregate and biodentine as pulp medicaments in primary molars. https://clinicaltrials.gov/study/NCT05102318. Unknown 2022-02-11.
- 127.NCT05314842. Pulpotomy in primary molars treated with premixed bio-ceramic MTA versus formocresol. https://clinicaltrials.gov/study/NCT05314842. Not yet recruiting. 2022-04-06.
- 128.Dhar V, Marghalani AA, Crystal YO, Kumar A, Ritwik P, Tulunoglu O, et al. Use of Vital Pulp Therapies in primary teeth with deep caries lesions. Pediatr Dent. 2017;39(5):146–59. [PubMed] [Google Scholar]
- 129.Philip N, Suneja B. Minimally invasive endodontics: a new era for pulpotomy in mature permanent teeth. Br Dent J. 2022;233(12):1035–41. doi: 10.1038/s41415-022-5316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.European Society of Endodontology developed b. Duncan HF, Galler KM, Tomson PL, Simon S, El-Karim I, et al. European Society of Endodontology position statement: management of deep caries and the exposed pulp. Int Endod J. 2019;52(7):923–34. doi: 10.1111/iej.13080. [DOI] [PubMed] [Google Scholar]
- 131.NCT05427851. Diode laser pulpotomy of mature permanent molars with irreversible pulpitis. https://clinicaltrials.gov/study/NCT05427851. Active not recruiting. 2022-06-22.
- 132.NCT04773886. Effectiveness of mineral trioxide aggregate and platelet rich fibrin along with biodentine. https://clinicaltrials.gov/study/NCT04773886. Completed. 2021-03–16.
- 133.NCT04331964. The use of platelet-rich fibrin in partial pulpotomy procedure. https://clinicaltrials.gov/study/NCT04331964. Completed. 2022-02-14.
- 134.Mehrvarzfar P, Abbott PV, Mashhadiabbas F, Vatanpour M, Tour Savadkouhi S. Clinical and histological responses of human dental pulp to MTA and combined MTA/treated dentin matrix in partial pulpotomy. Aust Endod J. 2017;44(1):46–53. doi: 10.1111/aej.12217. [DOI] [PubMed] [Google Scholar]
- 135.Manhas M, Mittal S, Sharma AK, Gupta KK, Pathania V, Thakur V. Biological approach in repair of partially inflamed dental pulp using second-generation platelet-rich fibrin and mineral trioxide aggregate as a pulp medicament in primary molars. J Indian Soc Pedod Prev Dent. 2019;37(4):399–404. doi: 10.4103/JISPPD.JISPPD_133_19. [DOI] [PubMed] [Google Scholar]
- 136.NCT05829304. A comparative clinical and radiographic study of collagen based pulpotomy in cariously exposed vital primary molars. https://clinicaltrials.gov/study/NCT05829304. Not yet recruiting. 2023-04-25.
- 137.Reddy NV, Popuri SK, Velagala D, Reddy A, Puppala N. Comparative evaluation of Formocresol, Propolis and Growth factor as Pulpotomy medicaments in Deciduous Teeth-An Invivo Study. J Clin Diagn Res. 2019;13(8):29. [Google Scholar]
- 138.Patidar S, Kalra N, Khatri A, Tyagi R. Clinical and radiographic comparison of platelet-rich fibrin and mineral trioxide aggregate as pulpotomy agents in primary molars. J Indian Soc Pedod Prev Dent. 2017;35(4):367–73. doi: 10.4103/JISPPD.JISPPD_178_17. [DOI] [PubMed] [Google Scholar]
- 139.Prasad MG, Adiya PVA, Babu DN, Krishna ANR. Amniotic membrane versus formocresol as pulpotomy agents in human primary molars: an in vivo study. Pesqui Bras Odontopediatria Clin Integr. 2017;17(1):1–8. [Google Scholar]
- 140.Eidelman E, Holan G, Fuks AB. Mineral trioxide aggregate vs. formocresol in pulpotomized primary molars: a preliminary report. Pediatr Dent. 2001;23(1):15–8. [PubMed] [Google Scholar]
- 141.Fuks AB, Holan G, Davis JM, Eidelman E. Ferric sulfate versus dilute formocresol in pulpotomized primary molars: long-term follow up. Pediatr Dent. 1997;19(5):327–30. [PubMed] [Google Scholar]
- 142.Holan G, Eidelman E, Fuks AB. Long-term evaluation of pulpotomy in primary molars using mineral trioxide aggregate or formocresol. Pediatr Dent. 2005;27(2):129–36. [PubMed] [Google Scholar]
- 143.Kusum B, Rakesh K, Richa K. Clinical and radiographical evaluation of mineral trioxide aggregate, biodentine and propolis as pulpotomy medicaments in primary teeth. Restor Dent Endod. 2015;40(4):276–85. doi: 10.5395/rde.2015.40.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.de Araujo Junior RF, Souza TO, de Medeiros CA, de Souza LB, Freitas Mde L, de Lucena HF, et al. Carvedilol decrease IL-1beta and TNF-alpha, inhibits MMP-2, MMP-9, COX-2, and RANKL expression, and up-regulates OPG in a rat model of periodontitis. PLoS ONE. 2013;8(7):e66391. doi: 10.1371/journal.pone.0066391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kanzaki H, Chiba M, Shimizu Y, Mitani H. Periodontal ligament cells under mechanical stress induce osteoclastogenesis by receptor activator of nuclear factor kappaB ligand up-regulation via prostaglandin E2 synthesis. J Bone Min Res. 2002;17(2):210–20. doi: 10.1359/jbmr.2002.17.2.210. [DOI] [PubMed] [Google Scholar]
- 146.Wang L, Zhou Z, Chen Y, Yuan S, Du Y, Ju X, et al. The alpha 7 Nicotinic Acetylcholine receptor of Deciduous Dental Pulp Stem cells regulates Osteoclastogenesis during physiological Root Resorption. Stem Cells Dev. 2017;26(16):1186–98. doi: 10.1089/scd.2017.0033. [DOI] [PubMed] [Google Scholar]
- 147.Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. 2005;115(2):282–90. doi: 10.1172/JCI200523394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yaman E, Gorken F, Pinar Erdem A, Sepet E, Aytepe Z. Effects of folk medicinal plant extract Ankaferd Blood stopper((R)) in vital primary molar pulpotomy. Eur Arch Paediatr Dent. 2012;13(4):197–202. doi: 10.1007/BF03262870. [DOI] [PubMed] [Google Scholar]
- 149.AAE Position Statement on Vital Pulp Therapy J Endod. 2021;47(9):1340–4. doi: 10.1016/j.joen.2021.07.015. [DOI] [PubMed] [Google Scholar]
- 150.NCT04719247. Clinical and radiographic evaluation of turmeric, thymus vulgaris, nigella sativa and aloe vera as pulpotomy medicaments in primary teeth. https://clinicaltrials.gov/study/NCT04719247 Completed. 2021-12-15.
- 151.Cosme-Silva L, Sakai VT, Lopes CS, Silveira APPd, Moretti RT, Gomes-Filho JE, et al. Comparison between calcium hydroxide mixtures and mineral trioxide aggregate in primary teeth pulpotomy: a randomized controlled trial. J Appl Oral Sci. 2019;27:e20180030. doi: 10.1590/1678-7757-2018-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bakhtiar H, Nekoofar MH, Aminishakib P, Abedi F, Naghi Moosavi F, Esnaashari E, et al. Human pulp responses to partial pulpotomy treatment with TheraCal as compared with biodentine and ProRoot MTA: a clinical trial. J Endod. 2017;43(11):1786–91. doi: 10.1016/j.joen.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 153.Bakhtiar H, Aminishakib P, Ellini MR, Mosavi F, Abedi F, Esmailian S, et al. Dental Pulp response to RetroMTA after partial pulpotomy in Permanent Human Teeth. J Endod. 2018;44(11):1692–6. doi: 10.1016/j.joen.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 154.Bhagat D, Sunder RK, Devendrappa SN, Vanka A, Choudaha N. A comparative evaluation of ProRoot mineral trioxide aggregate and Portland cement as a pulpotomy medicament. J Indian Soc Pedod Prev Dent. 2017;34(2):172–6. doi: 10.4103/0970-4388.180448. [DOI] [PubMed] [Google Scholar]
- 155.El Meligy OA, Allazzam S, Alamoudi NM. Comparison between biodentine and formocresol for pulpotomy of primary teeth: a randomized clinical trial. Quintessence Int. 2016;47(7):571–80. doi: 10.3290/j.qi.a36095. [DOI] [PubMed] [Google Scholar]
- 156.NCT04617600. Survival rate after TheraCal PT pulpotomy versus MTA pulpotomy in children with vital primary molars. https://clinicaltrials.gov/study/NCT04617600. Not yet recruiting. 2022-03-04.
- 157.Lourenço Neto N, Marques NC, Fernandes AP, Rodini CO, Sakai VT, Abdo RC, et al. Immunolocalization of dentin matrix protein-1 in human primary teeth treated with different pulp capping materials. J Biomedical Mater Res Part B Appl Biomaterials. 2016;104(1):165–9. doi: 10.1002/jbm.b.33379. [DOI] [PubMed] [Google Scholar]
- 158.Xu D, Mutoh N, Ohshima H, Tani-Ishii N. The effect of mineral trioxide aggregate on dental pulp healing in the infected pulp by direct pulp capping. Dent Mater J. 2021;40(6):1373–9. doi: 10.4012/dmj.2020-393. [DOI] [PubMed] [Google Scholar]
- 159.de Menezes JV, Takamori ER, Bijella MF, Granjeiro JM. In vitro toxicity of MTA compared with other primary teeth pulpotomy agents. J Clin Pediatr Dent. 2009;33(3):217–21. doi: 10.17796/jcpd.33.3.cq7677j4l532r1rg. [DOI] [PubMed] [Google Scholar]
- 160.Mendoza-Mendoza A, Biedma-Perea M, Iglesias-Linares A, Abalos-Labruzzi C, Solano-Mendoza B. Effect of mineral trioxide aggregate (MTA) pulpotomies in primary molars on their permanent tooth successors. Am J Dent. 2014;27(5):268–72. [PubMed] [Google Scholar]
- 161.Bani M, Aktas N, Cinar C, Odabas ME. The clinical and radiographic success of primary molar pulpotomy using Biodentine and Mineral Trioxide Aggregate: a 24-Month Randomized Clinical Trial. Pediatr Dent. 2017;39(4):284–8. [PubMed] [Google Scholar]
- 162.Rahul M, Lokade A, Tewari N, Mathur V, Agarwal D, Goel S, et al. Effect of Intracanal scaffolds on the Success Outcomes of Regenerative Endodontic Therapy - A Systematic Review and network Meta-analysis. J Endod. 2023;49(2):110–28. doi: 10.1016/j.joen.2022.11.011. [DOI] [PubMed] [Google Scholar]
- 163.Ulusoy AT, Turedi I, Cimen M, Cehreli ZC. Evaluation of blood clot, platelet-rich plasma, platelet-rich fibrin, and platelet pellet as scaffolds in Regenerative Endodontic treatment: a prospective Randomized Trial. J Endod. 2019;45(5):560–6. doi: 10.1016/j.joen.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 164.Miron RJ, Zucchelli G, Pikos MA, Salama M, Lee S, Guillemette V, et al. Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clin Oral Invest. 2017;21(6):1913–27. doi: 10.1007/s00784-017-2133-z. [DOI] [PubMed] [Google Scholar]
- 165.Noor Mohamed R, Basha S, Al-Thomali Y. Efficacy of platelet concentrates in pulpotomy - a systematic review. Platelets. 2018;29(5):440–5. doi: 10.1080/09537104.2018.1445844. [DOI] [PubMed] [Google Scholar]
- 166.Gandhi K, Goswami P, Malhotra R. Phlebotomy for obtaining platelet-rich fibrin autograft in children for pediatric dental procedures: parental views, understanding, and acceptance. J Indian Soc Pedod Prev Dent. 2020;38(2):119–25. doi: 10.4103/JISPPD.JISPPD_4_20. [DOI] [PubMed] [Google Scholar]
- 167.NCT03200938. Clinical applicability of PBS® CIMMO cement in pulpotomies. https://clinicaltrials.gov/study/NCT03200938. Completed. 2020-12-31.
- 168.Sajadi FS, Jalali F, Khademi M. Ferric sulfate versus calcium-enriched mixture cement in pulpotomy of primary molars: a randomized clinical trial. Pesquisa Brasileira em Odontopediatria E Clínica Integrada. 2021;21:7. doi: 10.1590/pboci.2021.007. [DOI] [Google Scholar]
- 169.Mejare IA, Axelsson S, Davidson T, Frisk F, Hakeberg M, Kvist T, et al. Diagnosis of the condition of the dental pulp: a systematic review. Int Endod J. 2012;45(7):597–613. doi: 10.1111/j.1365-2591.2012.02016.x. [DOI] [PubMed] [Google Scholar]
- 170.Ballal NV, Duncan HF, Wiedemeier DB, Rai N, Jalan P, Bhat V, et al. MMP-9 levels and NaOCl Lavage in Randomized Trial on Direct Pulp Capping. J Dent Res. 2022;101(4):414–9. doi: 10.1177/00220345211046874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kaur B, Kobayashi Y, Cugini C, Shimizumi E. A Mini Review: the potential biomarkers for non-invasive diagnosis of Pulpal inflammation. Front Dent Med. 2021;2:1–13. doi: 10.3389/fdmed.2021.718445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Riccaboni M, Verginer L. The impact of the COVID-19 pandemic on scientific research in the life sciences. PLoS ONE. 2022;17(2):e0263001. doi: 10.1371/journal.pone.0263001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.El karim I, Duncan H. Reducing intervention in the COVID-19 era: opportunities for Vital Pulp Treatment. Front Dent Med. 2021;2:1–7. doi: 10.3389/fdmed.2021.686701. [DOI] [Google Scholar]
- 174.Asagry S, Shamszadeh S, Editorial COVID-19 and Endodontic emergencies. Iran Endod J. 2020;15(2):64. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.