Abstract
Ewing’s sarcoma of the kidney is a rare tumor. Although renal carcinomas are known to involve the inferior cava, extension of the tumor up to the right atrium is not common. In the majority of cases when the tumor extends into the infrahepatic part of the inferior vena cava, it can be removed from the abdominal approach. Few patients require the use of cardiopulmonary bypass for removal of the tumor in the inferior vena cava and right atrium. The management of patients requiring resection of kidney tumors and right atrial mass is more complicated and requires a team approach consisting of oncosurgeons, cardiac surgeons, and cardiac anesthetists. The resection of the kidney tumor with a mass in the right atrium is usually done concomitantly. The cardiopulmonary bypass cannulation strategy needs to be modified in such cases.
Keywords: Kidney tumor, Ewing’s sarcoma, Right atrial mass, Partial sternotomy, Cardiopulmonary bypass, Total circulatory arrest
Introduction
Ewing’s sarcoma of the kidney is a rare tumor [1, 2]. The tumor arises from neuroectodermal cells and the usual sites of involvement are bone and soft tissues. The tumor occurs in children and young adults [2]. Tumor extension into the inferior vena cava (IVC) and right atrium (RA) worsens the prognosis in such cases [3]. Management of patients with renal tumors extending into IVC and RA becomes complicated.
A case of Ewing’s sarcoma extending up to the IVC and RA is discussed. Written informed consent was taken from the patient for publication of the clinical data.
Case report
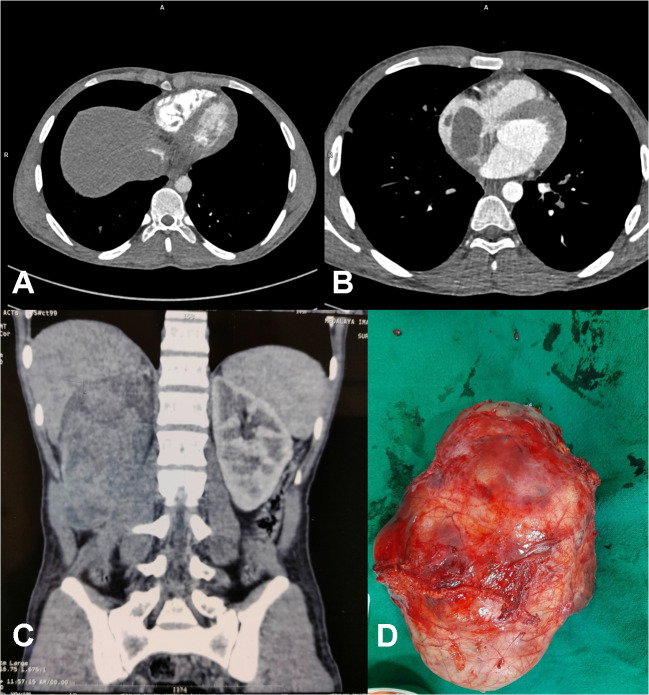
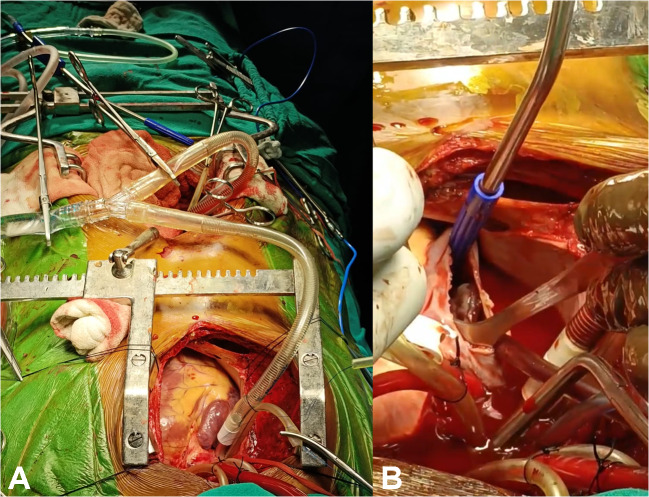
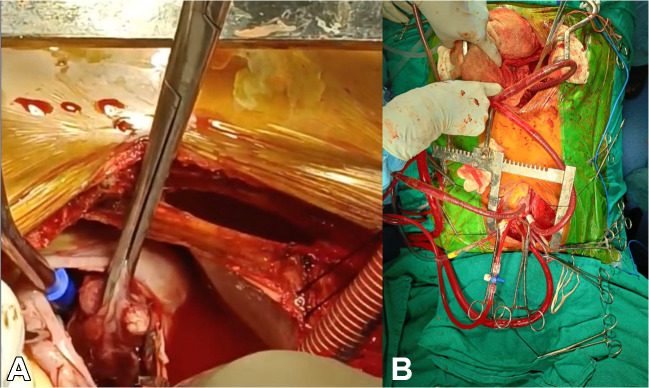
A 20-year-old male patient suffering from a large right renal mass was referred to us. Contrast-enhanced computed tomography (CECT) of the abdomen and chest revealed a large mass arising from the right kidney with extension into the IVC and RA (Fig. 1A, B, C). CECT chest also revealed embolism to the bilateral lower lobe pulmonary artery branches. The biopsy of the renal mass revealed it to be Ewing’s sarcoma. Operative intervention in the form of simultaneous resection of the kidney tumor through the midline laparotomy and excision of RA mass on cardiopulmonary bypass (CPB) through partial median sternotomy was planned. The platelet count of the patient was also low (60,000/µL). Preoperatively eight units of platelets along with six units of packed red blood cells (PRBC) and fresh frozen plasma (FFP) were arranged. Renal mass was approached through a midline laparotomy. A transperitoneal approach was used. The ascending colon was mobilized medially along with mobilization of the duodenum. The tumor was dissected from the surrounding structures and the fat planes were found to be preserved, without any infiltration into surrounding structures, apart from extension into IVC. After the mobilization of the right kidney with the tumor, IVC was also mobilized in the suprarenal and infrarenal segments. The right renal artery was ligated and divided first, followed by the division of the ureter and lastly renal vein was also divided. IVC was found to be dilated, thickened, and fibrotic. Removal of the large renal mass gave ample space in the abdomen for adequate proximal and distal control of IVC (Fig. 1D). A longitudinal incision was then given in the IVC and the tumor from the infrarenal part of the IVC could be extracted but the tumor in the suprarenal part could not be extracted completely. The cardiac team then did a partial upper median sternotomy, which was curved into the right fourth intercostal space. After opening the pericardium, the patient was heparinized (4 mg/kg). The cannulation circuit for CPB included an ascending aortic and superior vena cava (SVC) cannula along with another venous cannula in infrarenal IVC through the abdomen (Fig. 2A). A separate venous line was also included for the return of suprarenal IVC blood during the opening of RA. Cardioplegia was given through a cannula in the ascending aorta. The patient was cooled to 18 °C in anticipation of the need for total circulatory arrest. Head cooling was also started by placing ice packs around the head. An incision was made in the RA after cardioplegia; there was excess blood flow in the opened up RA making visualization of the tumor difficult. Total circulatory arrest (TCA) was then initiated and the tumor in the RA and IVC was mobilized and removed using blunt dissection (Fig. 2B). After ensuring no macroscopic tumor was left in the IVC and RA, a cannula was inserted into the IVC through the RA (Fig. 3A, B). Circulation through CPB was restored after 8 min and the incision in the RA was closed. Bilateral pulmonary embolectomy was done thereafter and it also required a period of TCA of 8 min. Circulation through CPB was restarted along with the re-warming of the patient to normal body temperature. Standard de-airing of the heart and weaning from CPB were started. The cannula from infrarenal IVC was clamped and removed, and the abdominal part of the IVC was repaired. The patient came off the bypass smoothly. Standard sternotomy closure was done after placing a mediastinal and right pleural drain. Lastly, the abdominal incision was closed after placing an abdominal drain. Three separate biopsy samples, the first renal mass, second IVC and RA mass, and third tissue/thrombus extracted from the pulmonary artery were sent for histopathological examination. Total surgical time was approximately 7 h, including 90 min of CBP time and 45 min of aortic cross-clamp. The patient was ventilated overnight and extubated the next day without any evidence of neurological deficit. Inotropes were given in mild doses and the need for pulmonary vasodilators did not arise. Adrenaline infusion was given in a dose of 0.1 μg/kg/min for the first 2 days and then tapered off. Anticoagulation with warfarin was started on the second postoperative day. There was serous discharge from the abdominal drain for 6 days post-operatively which gradually decreased. Total blood loss was approximately 800 mL. Four units of PRBC, six units of platelets, and one unit of FFP were given in the perioperative period. The patient was discharged on the eighth postoperative day. The patient came for a follow-up 15 days after the procedure and was doing well. The biopsy report of the kidney mass and mass in RA was consistent with Ewing’s sarcoma, whereas the tissue extracted from the pulmonary arteries was thrombus only. The patient was thereafter attached to the medical oncology and radiotherapy department for further management and advised follow-up after 1 month.
Fig. 1.
A CT (computed tomographic) scan (abdomen) axial section showing a mass in the IVC (inferior vena cava); B CT scan (thorax) axial section showing a mass in RA (right atrium); C CT scan (abdomen), coronal section showing a mass in the right kidney; D resected specimen of the renal mass
Fig. 2.
A Operative image of infrarenal IVC (inferior vena cava) cannulation and CPB (cardiopulmonary bypass) circuit; B operative image of mass in RA (right atrium)
Fig. 3.
A Operative image of extraction of mass in RA (right atrium) using deep hypothermic circulatory arrest (DHCA); B operative image showing cannula in suprahepatic IVC (inferior vena cava) and infrarenal IVC after removal of a mass in the RA and IVC
Discussion
Renal cell carcinoma is the most common malignant tumor of the kidney and chances of IVC involvement are 4% and that of RA involvement are 1% [4]. Ewing’s sarcoma of the kidney is rare in comparison to the renal cell carcinoma. Diagnosis of Ewing’s sarcoma may require immunohistochemistry apart from histopathology examination. Casey et al. in their study of renal cell carcinomas mentioned 3-year survival of 20% in cases who required CPB, whereas it was 100% in cases who required laparotomy alone [4].
A multidisciplinary team approach is required for the management of renal tumors extending high into the IVC or RA [4, 5]. Median sternotomy as well as minimal access techniques have been used for the removal of the IVC or RA tumor. Wotkowicz et al., in their comparative study on renal cell tumors extending into the IVC or RA, concluded that the minimal access technique offers a significant advantage over the median sternotomy approach [6]. We also used a partial median sternotomy approach in this case. This way, a continuous long incision from the sternum to the symphysis pubis could be avoided. This decreased the risk of cross-infection from the peritoneal cavity to the pericardial cavity and vice versa. Respiratory complications also were minimized using this strategy and early mobilization and recovery were achieved.
Removal of tumors from RA and suprahepatic IVC requires CPB, preferably with deep hypothermic TCA to obtain a bloodless field for tumor extraction under vision [7]. Intraoperative use of transesophageal echocardiography may be helpful to check the adequacy of tumor extraction from the IVC. The venous cannulation strategy needs modification in such cases as a cannula in IVC cannot be inserted from RA initially [7, 8]. The cannulation strategy may need to be individualized in such cases depending upon the extent of pathology, availability of resources, and surgeon’s preference for the technique. The options in such cases include either placement of a femoral cannula or placement of an infrarenal IVC cannula through the abdomen, as was done in this case. Vacuum-assisted venous drainage may also be helpful in such cases. As there is a lot of space in the abdomen available after the removal of the tumor, placement of an infrarenal IVC cannula is easier.
Conclusion
Simultaneous removal of kidney tumor, IVC, and RA mass can be performed safely. Partial sternotomy gives good exposure for the removal of IVC and RA mass and prevents a continuous long incision from the sternum to the symphysis pubis in such cases. Periods of TCA arrest allow the removal of the tumor in a relatively bloodless field. Modification of the venous cannulation strategy for CPB with a cannula in infrarenal IVC provides an adequate return for CPB and can be done easily.
Funding
None.
Declarations
Ethics approval
Not required (as per institutional ethical committee case reports do not require prior approval).
Informed consent statement
Yes.
Conflict of interest
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hakky TS, Gonzalvo AA, Lockhart JL, Rodriguez AR. Primary Ewing sarcoma of the kidney: a symptomatic presentation and review of the literature. Ther Adv Urol. 2013;5:153–159. doi: 10.1177/1756287212471095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patra S, Trivedi P. Primary Ewing sarcoma of the kidney: a series of four cases. Malays J Pathol. 2022;44:93–99. [PubMed] [Google Scholar]
- 3.Tarek N, Said R, Andersen CR, Suki TS, Foglesong J, Herzog CE, et al. Primary Ewing sarcoma/primitive neuroectodermal tumor of the kidney: the MD Anderson Cancer Center experience. Cancers (Basel) 2020;12:2927. doi: 10.3390/cancers12102927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casey RG, Raheem OA, Elmusharaf E, Madhavan P, Tolan M, Lynch TH. Renal cell carcinoma with IVC and atrial thrombus: a single centre’s 10 year surgical experience. Surgeon. 2013;11:295–299. doi: 10.1016/j.surge.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Lawindy SM, Kurian T, Kim T, Mangar D, Armstrong PA, Alsina AE, et al. Important surgical considerations in the management of renal cell carcinoma (RCC) with inferior vena cava (IVC) tumour thrombus. BJU Int. 2012;110:926–939. doi: 10.1111/j.1464-410X.2012.11174.x. [DOI] [PubMed] [Google Scholar]
- 6.Wotkowicz C, Libertino JA, Sorcini A, Mourtzinos A. Management of renal cell carcinoma with vena cava and atrial thrombus: minimal access vs median sternotomy with circulatory arrest. BJU Int. 2006;98:289–297. doi: 10.1111/j.1464-410X.2006.06272.x. [DOI] [PubMed] [Google Scholar]
- 7.Gagné-Loranger M, Lacombe L, Pouliot F, Fradet V, Dagenais F. Renal cell carcinoma with thrombus extending to the hepatic veins or right atrium: operative strategies based on 41 consecutive patients. Eur J Cardiothorac Surg. 2016;50:317–321. doi: 10.1093/ejcts/ezw023. [DOI] [PubMed] [Google Scholar]
- 8.Yano D, Yokoyama Y, Tokuda Y, Kato M, Mashiko Y, Kuwabara F, et al. Multidisciplinary surgical approach for renal cell carcinoma with inferior vena cava tumor thrombus. Surg Today. 2022;52:1016–1022. doi: 10.1007/s00595-021-02415-1. [DOI] [PubMed] [Google Scholar]