Abstract
In the present narrative review, we have summarized evidence on the pharmacological treatment of non-alcoholic fatty liver disease (NAFLD)/metabolic dysfunction-associated steatotic liver disease (MASLD). We start by reviewing the epidemiology of the condition and its close association with obesity and type 2 diabetes. We then discuss how randomized–controlled trials are performed following guidance from regulatory agencies, including differences and similarities between requirements of the US Food and Drug Administration and the European Medicine Agency. Difficulties and hurdles related to limitations of liver biopsy, a large number of screening failures in recruiting patients, as well as unpredictable response rates in the placebo group are evaluated. Finally, we recapitulate the strategies employed for potential drug treatments of this orphan condition. The first is to repurpose drugs that originally targeted T2DM and/or obesity, such as pioglitazone, glucagon-like peptide 1 receptor agonists (liraglutide and semaglutide), multi-agonists (tirzepatide and retatrutide), and sodium-glucose transporter 2 inhibitors. The second is to develop drugs specifically targeting NAFLD/MASLD. Among those, we focused on resmetirom, fibroblast growth factor 21 analogs, and lanifibranor, as they are currently in Phase 3 of their clinical trial development. While many failures have characterized the field of pharmacological treatment of NAFLD/MASLD in the past, it is likely that approval of the first treatments is near. As occurs in many chronic conditions, combination therapy might lead to better outcomes. In the case of non-alcoholic steatohepatitis, we speculate that drugs treating underlying metabolic co-morbidities might play a bigger role in the earlier stages of disease, while liver-targeting molecules will become vital in patients with more advanced disease in terms of inflammation and fibrosis.
Keywords: MASLD, NASH, MASH, GLP1-RA, resmetirom
Introduction
Non-alcoholic fatty liver disease (NAFLD) represents one of the most common chronic conditions in the general population. According to a recent systematic review by Younossi et al., its pooled prevalence among adults worldwide is 30.0% (95% confidence interval [CI] 27.9%–32.2%), with peaks as high as 44.4% in Latin America [1]. Moreover, the same study showed that its prevalence has increased significantly in the last three decades, ranging from 25.3% in studies performed in 1990–2006 to 38.2% in studies performed in 2016–2019. This growth has been paralleled with increasing rates of obesity and type 2 diabetes mellitus (T2DM) [2, 3].
While the association between liver fat and cirrhosis was hypothesized in the mid-1900s [4, 5], the term “non-alcoholic steatohepatitis” (NASH) was coined by Ludwig et al. in 1980 [6]. The authors described histologic findings that were indistinguishable from those typically found in alcoholic liver disease in a sample of overweight patients or patients who consumed little or no alcohol. Given the indistinguishable histologic features and the absence of an evident cause for its development, the exclusion of significant alcohol consumption became necessary to diagnose this clinical entity from the very beginning [7]. In subsequent years, it became clear that NAFLD and NASH occurred much more frequently in people with obesity [8], T2DM [9, 10], arterial hypertension [11, 12], and the metabolic syndrome [13], suggesting a central role of insulin resistance in its development and progression [14]. Indeed, its prevalence is more than doubled in patients with T2DM, for whom estimates frequently exceed 60%–70% [15, 16]. Concomitantly, it became clear that NAFLD was a heterogeneous disease, with differing histologic and prognostic features [17]. While many patients display only signs of liver steatosis (the so-called non-alcoholic fatty liver (NAFL)), some may progress to leucocyte infiltration and hepatocellular ballooning (NASH) with different degrees of liver fibrosis. Of note, several histologic cohort studies as well as meta-analyses identified the stage of liver fibrosis as the major determinant of the future development of liver-related events, such as cirrhosis decompensation, hepatocellular carcinoma (HCC), and liver-related mortality [18–20].
The evolving knowledge on its pathophysiology and natural history, as well as the desire to find a positive rather than negative definition of the condition, led a panel of experts to propose a new terminology—metabolic dysfunction-associated fatty liver disease (MAFLD) in 2020 and related diagnostic criteria in both adult [21] and pediatric [22] populations. With this new definition, MAFLD could be diagnosed in the presence of liver steatosis and metabolic dysfunction (defined as the presence of overweight/obesity, T2DM, or at least three criteria similar to those of the metabolic syndrome) without the need to exclude different forms of chronic liver disease or significant alcohol consumption. Several studies showed a good degree of overlap between NAFLD and MAFLD, even though differences exist according to the overall prevalence of metabolic dysfunction and other forms of liver disease in the studied population [23–26]. While this definition has been well received by several international societies, more recently, a Delphi panel proposed a new nomenclature—metabolic dysfunction-associated steatotic liver disease (MASLD), with a relative set of diagnostic criteria, that aimed to eliminate the potential stigma related to alcohol and fat [27]. Interestingly, NAFLD and MASLD are virtually interchangeable in epidemiologic terms, as suggested by recent studies [28, 29]. While we align with the recent MASLD definition, in the present review, the term “NASH” is still applied as it is the terminology used by regulatory agencies to define the criteria for patient recruitment as well as those of the primary end-point. Here, we started by summarizing requirements of regulatory agencies for approval of pharmacological treatment in the MASLD field and related difficulties and hurdles; we then review available evidence on the proposed pharmacologic agents, with a particular interest in drugs that are now entering Phase 3 of their clinical development.
What benefits should drugs demonstrate to receive approval?
Generally, the aim of pharmacological treatments is to confer clinical benefits to patients. In the field of chronic liver disease, this might be represented by demonstration of a significant reduction in hard clinical outcomes, such as development of cirrhosis and its complications (ascites, hepatic encephalopathy, upper gastro-intestinal bleeding) as well as all-cause mortality. Given the low incidence of these outcomes, regulatory agencies conceded that a drug might be conditionally approved based on its effect on liver histology; nonetheless, once the drug has reached the market, in order to obtain definitive approval, it must demonstrate benefits on clinical outcomes [30]. In particular, for NASH, the US Food and Drug Administration (FDA) stated that, for conditional approval, at least one of the following histologic end-points should be reached: (i) resolution of NASH (defined as an inflammation score of 0 or 1 and a ballooning score of 0) without worsening of fibrosis or (ii) improvement in fibrosis by one stage or more, without worsening of NASH (Table 1) [31]. On the other hand, the European Medicine Agency (EMA) requires that both NASH resolution and improvement in fibrosis are demonstrated. This approach was developed to accelerate conditional drug approval while outcome studies are being performed to test the drug on clinical outcomes. Because recent studies identified NASH with significant fibrosis (so-called “at-risk NASH”) as a predictor of future development of liver-related events [32, 33], this became the major inclusion criterion for Phase 3 randomized–controlled trials (RCTs). While this is certainly a reasonable approach, it poses great emphasis on liver histology and, therefore, liver biopsy as a gold-standard technique [34]. Potential drawbacks related to this strategy are the high number of screening failures (i.e. patients who are biopsied but do not display NASH with significant liver fibrosis, which might reach 70%), the highly variable placebo response rates (in part due to variability in liver biopsy itself), and the need for a relatively long time frame to obtain significant changes [35]. Moreover, while fibrosis has been robustly identified as an independent predictor of events [19], data are scarce on the relationship between NASH resolution and reduction in clinical outcomes. Finally, two histologic scoring systems can be applied: the NAFLD activity score (NAS) developed by the NASH Clinical Research Network in 2005 [36] and the Steatosis, Activity and Fibrosis (SAF) score developed by Bedossa et al. in 2012 [37]. For this reason, in parallel with generation of data on the efficacy of drug treatment, ongoing RCTs are also evaluating whether treatment response might be estimated through non-invasive modalities [38–40]. This would become crucial in clinical practice. On the one hand, liver biopsy cannot be offered to all patients with NAFLD/MASLD to identify “at-risk NASH”, limiting the opportunity of pharmacological treatment to only a fraction of affected individuals; on the other, it cannot be routinely performed during follow-up to monitor drug response.
Table 1.
Guidance from the US Food and Drug Administration on the conduction of Phase 3 randomized clinical trials in non-alcoholic steatohepatitis
| Study type | Enrollment rules | Patient population | Recommended end-point |
|---|---|---|---|
| Non-cirrhotic NASH | Histological diagnosis of NASH with liver fibrosis ≤6 months before enrollment | NAS ≥ 4 with at least one point each in inflammation and ballooning + NASH (CRN) fibrosis score F1–F3 |
OR
OR
For NASH drugs approved on the basis of liver histology under the “accelerated approval pathway,” randomized, double-blind, placebo-controlled clinical trials designed to describe and verify the “clinical benefit” of the drug should be underway at the time of submission of the marketing application |
| NASH with compensated cirrhosis | Careful exclusion of patients with decompensated cirrhosis (Child B and C) |
|
Time to development of a composite end-point:
|
NASH = non-alcoholic steatohepatitis, CRN = Clinical Research Network.
Repurposing drugs for obesity and T2MD
In the last two decades, pharmacological treatment for T2DM has undergone an unprecedented revolution [41]. This was made possible by two events. First, following a controversial meta-analysis showing possible cardiovascular harms of rosiglitazone (a thiazolidinedione), regulatory agencies requested that, in order for a diabetes treatment to reach the market, it had to demonstrate cardiovascular safety in a dedicated RCT. This led most drug companies to promote a large number of cardiovascular outcome trials, in which some drugs originally developed to treat hyperglycemia also showed benefits in cardiovascular and renal outcomes. Second, the development of incretin-based therapies and, to a lesser extent, sodium-glucose transporter 2 inhibitors gave clinicians safe and effective tools to treat not only hyperglycemia per se, but also the underlying metabolic abnormalities that have their roots in obesity, insulin resistance, and NAFLD/MASLD. Indeed, the promising results obtained in patients with T2DM on body weight, biomarkers of insulin resistance, and liver damage led to the development of dedicated trials in overweight and obese people without diabetes and in patients with NASH. In the next paragraphs, we will first review the data related to the drug classes originally developed for T2DM that showed beneficial effects on NAFLD/MASLD. Drugs originally developed for the treatment of NASH targeting the liver as the primary organ are subsequently considered.
Pioglitazone
Pioglitazone activates the peroxisome proliferator-activated receptor gamma (PPARγ) and, to a lesser extent, PPAR-α [42]. These nuclear receptors play a significant role in the pathophysiology of NAFLD/MASLD. Pioglitazone addresses insulin resistance, modulates lipid and glucose metabolism, and diminishes hepatic and gastro-intestinal inflammation [43]. Pioglitazone treatment is also able to induce significant modifications in body fat distribution. Specifically, it leads to a reduction in the visceral-to-subcutaneous fat ratio; among biochemical changes, it promotes elevation in plasma adiponectin levels. These changes reflect the mechanism underlying the reduction in steatosis and necroinflammation in NASH patients [44]. Numerous RCTs in NAFLD/MASLD have been conducted globally. They involved patients with or without T2DM and confirmed NAFLD/MASLD evaluated through biopsy. Musso et al. published a Phase 2 meta-analysis that encompasses eight RCTs, encompassing ∼500 patients with biopsy-confirmed NASH, treated with thiazolidinediones [45]. Among these trials, five RCTs assessed pioglitazone and three RCTs evaluated rosiglitazone, with treatment durations of 6–24 months. In this analysis, pioglitazone treatment resulted in a higher proportion of patients achieving NASH resolution. No individual study indicated an effect on fibrosis improvement; nonetheless, the meta-analytic approach revealed that thiazolidinedione therapy was associated with improvements in fibrosis, even in patients without diabetes. Major side effects related to pioglitazone use are increase in body weight, fluid retention (potentially leading to a higher rate of heart failure hospitalizations), macular edema, and fragility fractures [46]. On the other hand, it showed protective effects on some cardiovascular end-points in the PROACTIVE and IRIS studies [47, 48].
Recently, in order to overcome the side effects associated with pioglitazone use, a deuterium-stabilized enantiomer of the molecule was developed (PXL065). The molecule lacks PPARγ activity but retains non-genomic activity. In the Phase 2 DESTINY-1 trial, which included 117 patients with “at-risk NASH”, it met its primary end-point of reduction of liver fat content by magnetic resonance imaging (MRI) compared with placebo [49]. On histology, fibrosis improvement occurred in 40% (7.5 mg), 50% (15 mg, P = 0.06), and 35% (22.5 mg) vs 17% for placebo. Moreover, ≤50% of PXL065-treated patients achieved NASH improvement without worsening of fibrosis vs 30% with placebo. Importantly, as expected by the modified mechanism of action, there was no increase in body weight or increased risk of peripheral edema.
GLP1-receptor agonists and multi-agonists
GLP1-RA (receptor agonists) are synthetic analogs of the endogenous GLP1 peptide, which is secreted by the endocrine L cells located in the ileus and colonic mucosa. They initially entered the market as glucose-lowering medications and some of them are now approved for pharmacological treatment of both T2DM and obesity. Their mechanisms include glucose-dependent stimulation of insulin secretion, inhibition of glucagon secretion, appetite reduction, and slowing of gastric emptying [50]. This results in significant improvements in glycemic control, body weight, and blood pressure [51]. On the other hand, most studies did not identify GLP1 receptors on hepatocytes [52, 53]. Therefore, it is believed that their effects on liver fat and inflammation are mediated by their favorable metabolic and weight-related actions [54]. Indeed, a recent meta-analysis including 11 RCTs on GLP1-RA in NAFLD/MASLD showed a linear correlation between the percentage reduction in hepatic fat content on MRI obtained with the drugs and the reduction in body mass index (BMI), with an r2 value of 0.791 [55]. While most compounds in the class demonstrated efficacy in reducing aminotransferase levels and liver fat content on MRI [56], few studies are available on histologic end-points, specifically with liraglutide and semaglutide.
The Phase 2 LEAN trial marked the initial RCT aimed at assessing the impact of a GLP1-RA on histologic liver end-points. In this Phase 2 study, 26 patients were randomly assigned to receive liraglutide, while another 26 were assigned to the placebo group. Despite its relatively small scale, the trial yielded positive outcomes, with 39% (9 out of 23) of patients in the liraglutide group experiencing NASH resolution, compared with 9% (2 out of 22) in the placebo group (relative risk 4.3 [95% CI 1.0–17.7]; P = 0.019). Additionally, the progression of fibrosis was observed in 9% (2 out of 23) of liraglutide recipients and 36% (8 out of 22) of those in the placebo group (relative risk 0.2 [95% CI 0.1–1.0]; P = 0.04) [57].
Semaglutide is a long-acting GLP1-RA available both as a weekly subcutaneous injection and as a daily tablet. To date, both formulations are approved for the treatment of T2DM, while only the subcutaneous formulation (at higher doses) is approved for the treatment of obesity. Among GLP1 mono-agonists, semaglutide demonstrated the highest efficacy in both glycemic control and body weight [58]. In patients with T2DM, the SUSTAIN-6 (subcutaneous formulation) and the PIONEER-6 (oral formulation) have shown considerable trends towards reduction in the incidence of cardiovascular events and mortality [59, 60]. The ongoing RCT is testing oral semaglutide in ∼9,600 patients with T2DM and previous cardiovascular events (SOUL, NCT03914326). Given its superior design, the larger number of patients recruited, and their high cardiovascular risk, it will provide more definitive evidence on the potential cardiovascular benefits of oral semaglutide in patients with T2DM. Furthermore, the SELECT trial, which recruited 17,604 patients with obesity and cardiovascular disease but without T2DM, showed that once-weekly semaglutide (at a dose of 2.4 mg) was associated with a 20% reduction in the primary end-point major adverse cardiovascular events (MACE) as well as a 19% reduction in all-cause mortality [61]. Given that cardiovascular disease remains the leading cause of death in patients with NAFLD/MASLD, these data are reassuring for potential future use in this patient population. Two Phase 2 RCTs specifically evaluated the effect of semaglutide on NASH. The first, which included 320 patients with NASH and F2–F3 fibrosis, showed a significant effect of semaglutide on NASH improvement without worsening of fibrosis at 72 weeks (which occurred in 40%, 36%, and 59% of patients treated with doses of 0.1, 0.2, and 0.4 mg/day, respectively, compared with 17% in the placebo group; P < 0.001) [62]. No significant differences were found in the secondary end-point of fibrosis improvement without worsening of NASH, which occurred in a similar proportion of patients in all treatment arms. Lack of improvement in fibrosis might have been related to the relatively short follow-up, the different dosing schedule, as well as a high response rate in the placebo group.
The second trial tested the safety of semaglutide 2.4 mg once-weekly in 71 patients with biopsy-proven NASH–cirrhosis [63]. The primary end-point was the proportion of patients with an improvement in liver fibrosis of one stage or more without worsening of NASH at 48 weeks. Even though semaglutide was well tolerated and associated with significant weight loss and improvement in metabolic parameters, no significant differences were found in the primary end-point (which occurred in 11% of patients in the semaglutide group and 29% of those in the placebo group) as well as on the secondary end-point of NASH resolution. Again, the relative short follow-up time for a treatment that does not directly target fibrogenesis likely played a key role in explaining these findings. Nonetheless, based on benefits on NASH and potential benefits in fibrosis with longer follow-up, the drug entered Phase 3 of its clinical development. The Phase 3 “Effect of Semaglutide in Subjects With Non-cirrhotic Non-alcoholic Steatohepatitis” is going to further evaluate the efficacy of once-weekly semaglutide 2.4 mg in the treatment of NASH (ESSENCE, NCT04822181). Importantly, the dosing regimen differs from the Phase 2 NASH trial and is aligned with the dose currently approved for the treatment of obesity. The study is currently actively recruiting patients with NASH, a NAS score of >3, and significant-advanced liver fibrosis (F2–F3). It plans to enroll ∼1,200 patients. The 72-week primary end-points are resolution of NASH without worsening of fibrosis and improvement in liver fibrosis with no worsening of NASH. After the 72-week end-point analysis, the trial will continue with its second part, which aims to evaluate the time to first liver-related clinical event (composite end-point) at Week 240. Histological (72 weeks) results are expected by the end of 2024, while data on clinical outcomes are expected in 2028.
More recently, molecular engineering has enabled the creation of single molecules that are able to activate multiple incretin and hormonal receptors. In particular, tirzepatide—an agonist for both glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 receptors administered once-weekly—has exhibited superiority in comparison with GLP1-RA concerning both glycemic control and weight loss [64]. Additionally, it has demonstrated favorable effects on hepatic end-points. In a sub-study of the SURPASS-3 trial exclusively involving patients with T2DM, tirzepatide displayed a significantly greater reduction in liver fat content (∼47% relative reduction in liver fat), as well as in the volume of visceral adipose tissue and abdominal subcutaneous adipose tissue, when compared with insulin degludec [65]. Based on these promising findings, the drug entered a Phase 2 NASH program. The ongoing Phase 2 study will enroll ∼200 patients with NASH and Stage 2 or 3 fibrosis. The primary end-point will be NASH resolution without worsening of fibrosis at Week 52 (SYNERGY-NASH, NCT04166773).
Finally, retatrutide (RETA)—an injectable triple hormone agonist targeting the GIP, GLP-1, and glucagon receptors administered on a once-weekly basis—has displayed promising results in the treatment of obesity. During a Phase 2 obesity trial, RETA treatment yielded substantial reductions in body weight, reaching ≤24% [66]. A sub-study within the same trial that focused on participants with NAFLD/MASLD revealed that all doses of RETA led to significantly greater reductions in liver fat content compared with the placebo. Specifically, RETA doses of 8 and 12 mg resulted in hepatic steatosis resolution (LFC < 5%) in >85% of participants at Week 48 [67]. These unprecedented results on weight loss show great promise for the multi-agonists in the treatment of metabolic dysfunction-associated steatohepatitis (MASH) in patients with overweight and obesity in the near future.
SGLT2-inhibitors
SGLT2-inhibitors (SGLT2-I) lead to significant improvements in the glycemic profile, reduction of visceral adipose tissue, elevation of plasma adiponectin levels, and a decrease in uric acid levels. Additionally, they mitigate oxidative stress and systemic inflammation while increasing glucagon levels [68]. They have also shown clear benefits in heart failure and chronic kidney disease outcomes for patients both with and without T2DM [69, 70]. Therefore, potential benefits are anticipated in the field of NAFLD/MASLD. While, to date, no data are available of their effects on histologic end-points, 12 RCTs investigating the use of these drugs specifically for treating NAFLD/MASLD were conducted in diverse regions globally [71]. The SGLT2-I examined in these RCTs comprise dapagliflozin, empagliflozin, ipragliflozin, and canagliflozin administered over a median period of 24 weeks. The study participants, 90% of whom had T2DM, were diagnosed with NAFLD/MASLD through imaging techniques. Meta-analysis of these studies reveals that SGLT2-I, in comparison with the control group, significantly reduced the hepatic fat percentage as assessed by using MRI–proton density fat fraction (PDFF). Future RCTs, if ever performed, might provide evidence on a possible effect of these compounds on histologic severity.
Specific liver-targeting molecules
A large series of molecules have been tested for the treatment of NASH and, to date, none has received formal approval from regulatory agencies. While the reasons for this are multiple, as recently reviewed [72], limited efficacy and/or an unfavorable side-effect profiles are among the most relevant. The following paragraphs do not provide a comprehensive evaluation of all strategies that are being employed for the treatment of NAFLD and NASH; rather, the aim is to focus on those drugs that are in later stages of their clinical program (e.g. Phase 3) and for which a decision on a possible approval might be reached within a few months or years. Major features of ongoing Phase 3 trials are shown in Table 2.
Table 2.
Features of ongoing Phase 3 randomized–controlled trials in NAFLD/MASLD
| Trial name | Drug | Inclusion criteria | Estimated enrollment | Primary end-point | Available results | Expected completion |
|---|---|---|---|---|---|---|
| MAESTRO-NAFLD 1 | Resmetirom | Combination of non-invasive methods or liver biopsy | 1,143 patients (actual) | Treatment-emergent adverse events at Week 52 | The drug was well tolerated with the major side effects being transient nausea and diarrhea—adverse events led to drug discontinuation in <4% of patients in all arms | Completed |
| MAESTRO-OLE | Resmetirom | Combination of non-invasive methods or liver biopsy | 1,143 patients (actual) | Treatment-emergent adverse events at Week 104 | NA | 2025 |
| MAESTRO-NASH | Resmetirom | Biopsy-proven “at-risk NASH” | 1,759 patients (actual) |
|
NASH resolution
Fibrosis improvement:
|
2026 (clinical outcomes) |
| MAESTRO-NASH-OUTCOMES | Resmetirom | Compensated liver cirrhosis | 700 patients | Any event of all-cause mortality, liver transplant, ascites, hepatic encephalopathy, gastroesophageal variceal hemorrhage, and confirmed increase in MELD score from <12 to ≥15 due to liver disease | NA | 2026 |
| ESSENCE | Semaglutide | Biopsy-proven “at-risk NASH” | 1,200 patients |
|
NA |
|
| NATIV3 | Lanifibranor | Biopsy-proven “at-risk NASH” | 1,000 patients |
|
NA |
|
NA = not available.
Resmetirom and other TRβ agonists
Thyroid hormones exert their effect through the activation of thyroid hormone receptors (TRs) located in the cell nucleus. There are two different subtypes of TR (TRα and TRβ) with different tissue expression. While TRα are the major isoforms present in the heart and bone, TRβ are highly expressed in the liver and mediate most of the metabolic effects of the thyroid hormones [73]. This differential expression has been exploited to develop selective TRβ agonists that can provide beneficial metabolic effects without side effects related to activation of TRα at the level of the heart (potentially leading to tachycardia and arrhythmias) and bone (potentially leading to bone loss and fractures) [74]. At the level of the liver, activation of the TR receptor leads to reduction in de novo lipogenesis, promoting fatty acid oxidation, modulating mitophagy and mitochondrial biogenesis as well as cholesterol metabolism, and potentially exerting direct anti-inflammatory and anti-fibrotic effects [75]. While several compounds within this class are being studied, resmetirom is further along the clinical trial pathway and has reached the Phase 3 stage. In a Phase 2 study involving 84 patients with NASH and F1–F3 fibrosis, it demonstrated superiority compared with placebo in the relative reduction of liver fat on MRI at Week 12 (∼32.9% resmetirom vs ∼10.4% placebo), which served as the primary end-point [76]. Moreover, at Week 36, NASH resolution without worsening of fibrosis was achieved by 6.5% of patients in the placebo group and 24.7% of patients in the resmetirom group (odds ratio 4.75, P = 0.032), while no difference was found in improvement of liver fibrosis. Based on these promising results, the drug entered the Phase 3 MAESTRO clinical trial program [77]. The MAESTRO-NAFLD-1 was designed to evaluate the safety profile of resmetirom and treatment-emergent adverse events over 52 weeks were the primary end-point [78]. It recruited 1,143 patients with NAFLD/MASLD diagnosed either through non-invasive methods or liver biopsy. The drug was well tolerated, with the major side effects being transient nausea and diarrhea. Adverse events led to drug discontinuation in <4% of patients in all arms. Significant reductions were found in LDL-C (–12.6%), apoB (–18.0%), triglycerides (–20.4%), 16-week hepatic fat based on MRI (–38.6%), liver stiffness on Fibroscan (–1.70 kPa), and 52-week hepatic fat (–33.9%) [78]. Reductions in atherogenic lipid particles of this entity might also be relevant for cardiovascular prevention. An open-label extension of the study is ongoing and will follow participants for an additional 52 weeks (MAESTRO-OLE, NCT04951219).
The MAESTRO-NASH trial recruited 1,759 patients with biopsy-proven NASH and F2–F3 fibrosis [77]. The trial had a 52-week primary end-point of NASH resolution without worsening of fibrosis and fibrosis improvement without worsening of NASH, as well as a 54-month primary end-point of clinical outcomes (all-cause mortality, liver transplant, liver-related events, histological progression to cirrhosis, and confirmed increase in Mayo End stage Liver Disease (MELD) score from <12 to ≥15). Results from an interim analysis performed on 966 patients have been recently reported [79]. In the intention-to-treat analysis in Week 52, a higher proportion of patients reached NASH resolution without worsening of fibrosis in the resmetirom (25.9% and 29.9% in those on 80 and 100 mg/day, respectively) compared with placebo (9.7%, P < 0.001). The drug also demonstrated improvement in liver fibrosis without worsening of NASH (achieved by 24.2%, 25.9%, and 14.2% of patients in the groups on 80 and 100 mg of resmetirom and in the placebo group, respectively). Results were consistent in subgroup analyses based on age, sex, presence of T2DM, fibrosis stage, and NAS score. The trial confirmed a good tolerability profile, with <8% of patients experiencing side effects that led to treatment discontinuation. Patients in the resmetirom groups experienced marked increases in sex hormone-binding globulin levels and total estradiol and testosterone. While free testosterone levels remained unchanged, future studies are needed to evaluate the impact of the drug on the pituitary–gonadal axis. Similarly, the drug seems to affect the conversion of T4 to T3. Within the trial, free T4 levels decreased by 17%–21% and the mean thyroid stimulating hormone (TSH) levels also decreased. Importantly, the mean plasma free T3 levels remained unchanged [80].
The FDA has granted Priority Review to the drug and resmetirom became the first drug to receive conditional approval for treating fibrotic NASH in March 2024. Finally, the MAESTRO-NASH-OUTCOMES trial plans to recruit ∼700 adults with well-compensated (Child–Pugh A) NASH–cirrhosis. It is an event-driven study in which the primary outcome is represented by clinical events, such as cirrhosis decompensation and development of HCC. It is presumed to last for 2–3 years (NCT05500222).
Fibroblast growth factor 21 analogs
Fibroblast growth factor 21 (FGF21) is a protein hormone that acts as a regulator of energy metabolism, glucose homeostasis, and lipid levels [81]. Since native FGF21 has a very short half-life, molecular engineering has been performed to inhibit its rapid cleavage and inactivation. While the first molecule to report Phase 2 results was pegbelfermin, its development was terminated as it did not show improvement in liver fibrosis in the FALCON 1 and FALCON 2 randomized clinical trials [82, 83]. Currently, efruxifermin (EFX) and pegozafermin have shown promise in Phase 2 clinical trials and are being moved to Phase 3.
EFX is a subcutaneous, long-acting FGF21 analog fused with a Fc IgG portion. In the Phase 2b HARMONY trial involving 128 patients with NASH and F2–F3 fibrosis, it showed efficacy in both NASH resolution without worsening of fibrosis (76% and 47% of patients treated with EFX at 50 and 28 mg, respectively, compared with 15% in the placebo group) and fibrosis improvement without worsening of NASH (41% and 39% of patients compared with 20% in the placebo group) [84]. Based on these positive results, it was recently announced that the SYNCHRONY Phase 3 program will soon be initiated [85]. Within the program, SYNCHRONY Histology will evaluate the efficacy of EFX in patients with “at-risk NASH” on fibrosis improvement and resolution of NASH. SYNCHRONY Real-World will assess the safety and tolerability of EFX in patients with non-invasively diagnosed NASH or NAFLD, and it is expected to report important data on the change in biomarkers for fibrosis and other established non-invasive end-points. Finally, pending the results of the Phase 2b SYMMETRY trial involving patients with cirrhosis, SYNCHRONY Outcomes is going to evaluate patients with cirrhosis with a primary focus on clinical outcomes (NCT05039450).
Pegozafermin is a subcutaneous FGF21 analog with structural features similar to EFX. In the Phase 2b ENLIVEN trial involving 219 patients with “at-risk NASH,” it showed efficacy at 24 weeks on fibrosis improvement (22%, 26%, and 27% in the groups taking 15, 30, and 44 mg of pegozafermin compared with 7% in the placebo group) as well as on NASH resolution (37%, 23%, and 26% in the groups taking 15, 30, and 44 mg of pegozafermin compared with 2% in the placebo group) [86]. Based on these promising results, a Phase 3 program was recently announced [87]. It will include the ENLIGHTEN-Cirrhosis trial, enrolling patients with compensated cirrhosis, and the ENLIGHTEN-Fibrosis trial, enrolling patients with “at-risk NASH.” They are expected to start by the end of 2024. Side effects of both drugs are mainly gastro-intestinal in nature and characterized by nausea and diarrhea.
Lanifibranor
Lanifibranor is a Pan-PPAR agonist [88]. In the Phase 2b NATIVE trial involving 247 patients with “at-risk NASH,” it showed significant improvements in NASH resolution without worsening of fibrosis at Week 24 (achieved by 49% and 39% of patients treated with 1,200 and 800 mg of lanifibranor, respectively, compared with 22% of patients in the placebo group) [89]. Improvement in fibrosis without worsening of NASH (48% and 34%, respectively, vs 29%) and resolution of NASH plus improvement in fibrosis (35% and 25%, respectively, vs 9%) occurred more frequently in the lanifibranor groups compared with the placebo group. The drug showed favorable effects on insulin resistance estimated through the homeostatic model assessment of insulin resistance (HOMA-IR) and glycemic control. Patients in the lanifibranor groups gained weight (∼2.5 kg). Consistently with data obtained with pioglitazone, gastro-intestinal adverse events, peripheral edema, and anemia occurred more frequently with lanifibranor than with placebo. Of note, the study used the SAF histologic system and not the NASH Clinical Research Network. Based on these positive results, the Phase 3 clinical program was initiated. In particular, the NATIV3 trial aims to recruit ∼1,000 patients with “at-risk NASH” (NCT04849728). The primary end-point is a composite of resolution of NASH and improvement of fibrosis at Week 72, making it a good candidate for approval by the EMA and therefore to reach the European market.
Combined approach and final considerations
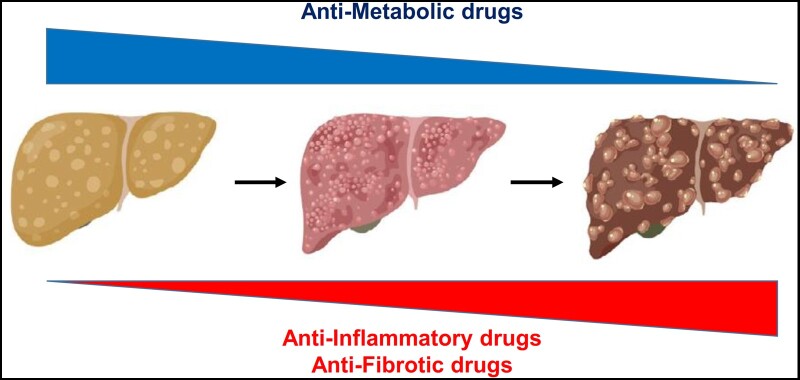
NAFLD/MASLD is a complex disease in which different genetic, lifestyle-related, and even environment-related factors play significant roles [90]. It is therefore highly likely that patients with this condition might have heterogeneous pathophysiological alterations that might be counteracted by targeting different molecular pathways. Lack of identification of possible subgroups of patients harboring different metabolic insults (together with limitations of liver biopsy itself) might account for the suboptimal response rates seen in clinical trials on histologic end-points. Indeed, in all available trials, resolution of NASH and fibrosis improvement are met by 30%–40% of patients in the active treatment group. While this is a significant improvement compared with lifestyle changes in the placebo arm, the residual hepatic risk seems to be substantial. Given the complex pathophysiology of NAFLD/MASLD, it is reasonable to assume that, as occurs in many chronic conditions such as arterial hypertension and T2DM, combination therapy might lead to higher response rates and better results. The rationale for combination therapy is to prevent compensatory pathways that may limit the efficacy of a single agent and targeting concomitant injury pathways, thereby expanding the biological response [91]. Based on available data on the natural history of NAFLD/MASLD and on results of Phase 2 clinical trials, we speculate that drugs targeting underlying metabolic abnormalities (such as obesity and insulin resistance) could play a more significant role in the earlier stages of disease, when liver fibrosis is less predominant. On the contrary, drugs specifically targeting liver inflammation and collagen deposition are needed when significant damage has already occurred (Figure 1).
Figure 1.
Relative contribution of antimetabolic and anti-inflammatory/anti-fibrotic drugs in the treatment of NAFLD/MASLD. For NAFLD/MASLD, we believe that medications addressing metabolic issues may be more important in the initial disease stages, while treatments specifically targeting the liver will become crucial for patients with advanced inflammation and fibrosis.
Authors’ Contributions
S.C. and G.P. wrote, reviewed, and edited the manuscript. All authors approved the final version of the manuscript to be published. S.C. is the guarantor of this work.
Contributor Information
Stefano Ciardullo, Department of Medicine and Rehabilitation, Policlinico di Monza, Monza, Italy; Department of Medicine and Surgery, University of Milano Bicocca, Milan, Italy.
Emanuele Muraca, Department of Medicine and Rehabilitation, Policlinico di Monza, Monza, Italy.
Michela Vergani, Department of Medicine and Rehabilitation, Policlinico di Monza, Monza, Italy; Department of Medicine and Surgery, University of Milano Bicocca, Milan, Italy.
Pietro Invernizzi, Division of Gastroenterology, Center for Autoimmune Liver Diseases, Department of Medicine and Surgery, University of Milano Bicocca, Monza, Italy; European Reference Network on Hepatological Diseases (ERN RARE-LIVER) San Gerardo Hospital, Monza, Italy.
Gianluca Perseghin, Department of Medicine and Rehabilitation, Policlinico di Monza, Monza, Italy; Department of Medicine and Surgery, University of Milano Bicocca, Milan, Italy.
Funding
The authors received no specific funding for this work.
Conflicts of Interest
None declared.
References
- 1. Younossi ZM, Golabi P, Paik JM. et al. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology 2023;77:1335–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saeedi P, Petersohn I, Salpea P. et al. ; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract 2019;157:107843. [DOI] [PubMed] [Google Scholar]
- 3. Li M, Gong W, Wang S. et al. Trends in body mass index, overweight and obesity among adults in the USA, the NHANES from 2003 to 2018: a repeat cross-sectional survey. BMJ Open 2022;12:e065425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Connor CL. Fatty infiltration of the liver and the development of cirrhosis in diabetes and chronic alcoholism. Am J Pathol 1938;14:347–64.9. [PMC free article] [PubMed] [Google Scholar]
- 5. Ayonrinde OT. Historical narrative from fatty liver in the nineteenth century to contemporary NAFLD—Reconciling the present with the past. JHEP Rep 2021;3:100261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ludwig J, Viggiano TR, McGill DB. et al. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc 1980;55:434–8. [PubMed] [Google Scholar]
- 7. Åberg F, Färkkilä M.. Drinking and obesity: alcoholic liver disease/nonalcoholic fatty liver disease interactions. Semin Liver Dis 2020;40:154–62. [DOI] [PubMed] [Google Scholar]
- 8. Ciardullo S, Pizzi M, Pizzi P. et al. Prevalence of elevated liver stiffness among potential candidates for bariatric surgery in the United States. Obes Surg 2022;32:712–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ciardullo S, Monti T, Perseghin G.. High prevalence of advanced liver fibrosis assessed by transient elastography among U.S. adults with type 2 diabetes. Diabetes Care 2021;44:519–25. [DOI] [PubMed] [Google Scholar]
- 10. Mantovani A, Petracca G, Beatrice G. et al. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated meta-analysis of 501 022 adult individuals. Gut 2021;70:962–9. [DOI] [PubMed] [Google Scholar]
- 11. Ciardullo S, Monti T, Grassi G. et al. Blood pressure, glycemic status and advanced liver fibrosis assessed by transient elastography in the general United States population. J Hypertens 2021;39:1621–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oikonomou D, Georgiopoulos G, Katsi V. et al. Non-alcoholic fatty liver disease and hypertension: coprevalent or correlated? Eur J Gastroenterol Hepatol 2018;30:979–85. [DOI] [PubMed] [Google Scholar]
- 13. Marchesini G, Brizi M, Bianchi G. et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes 2001;50:1844–50. [DOI] [PubMed] [Google Scholar]
- 14. Marchesini G, Brizi M, Morselli-Labate AM. et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med 1999;107:450–5. [DOI] [PubMed] [Google Scholar]
- 15. Younossi ZM, Golabi P, de Avila L. et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol 2019;71:793–801. [DOI] [PubMed] [Google Scholar]
- 16. Ajmera V, Cepin S, Tesfai K. et al. A prospective study on the prevalence of NAFLD, advanced fibrosis, cirrhosis and hepatocellular carcinoma in people with type 2 diabetes. J Hepatol 2023;78:471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sanyal AJ, Harrison SA, Ratziu V. et al. The natural history of advanced fibrosis due to nonalcoholic steatohepatitis: data from the simtuzumab trials. Hepatology 2019;70:1913–27. [DOI] [PubMed] [Google Scholar]
- 18. Ekstedt M, Hagström H, Nasr P. et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015;61:1547–54. [DOI] [PubMed] [Google Scholar]
- 19. Taylor RS, Taylor RJ, Bayliss S. et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology 2020;158:1611–25.e12. [DOI] [PubMed] [Google Scholar]
- 20. Dulai PS, Singh S, Patel J. et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology 2017;65:1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eslam M, Newsome PN, Sarin SK. et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 2020;73:202–9. [DOI] [PubMed] [Google Scholar]
- 22. Eslam M, Alkhouri N, Vajro P. et al. Defining paediatric metabolic (dysfunction)-associated fatty liver disease: an international expert consensus statement. Lancet Gastroenterol Hepatol 2021;6:864–73. [DOI] [PubMed] [Google Scholar]
- 23. Ciardullo S, Perseghin G.. Prevalence of NAFLD, MAFLD and associated advanced fibrosis in the contemporary United States population. Liver Int 2021;41:1290–3. [DOI] [PubMed] [Google Scholar]
- 24. Ciardullo S, Carbone M, Invernizzi P. et al. Impact of the new definition of metabolic dysfunction-associated fatty liver disease on detection of significant liver fibrosis in US adolescents. Hepatol Commun 2022;6:2070–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ciardullo S, Vergani M, Ronchetti C. et al. Shaping the future of pediatric liver health: unraveling the impact of the new metabolic-associated fatty liver disease definition. Hepatobiliary Surg Nutr 2023;12:611–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamamura S, Eslam M, Kawaguchi T. et al. MAFLD identifies patients with significant hepatic fibrosis better than NAFLD. Liver Int 2020;40:3018–30. [DOI] [PubMed] [Google Scholar]
- 27. Rinella ME, Lazarus JV, Ratziu V. et al. ; NAFLD Nomenclature Consensus Group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Ann Hepatol 2024;29:101133. [DOI] [PubMed] [Google Scholar]
- 28. Ciardullo S, Carbone M, Invernizzi P. et al. Exploring the landscape of steatotic liver disease in the general US population. Liver Int 2023;43:2425–33. [DOI] [PubMed] [Google Scholar]
- 29. Hagström H, Vessby J, Ekstedt M. et al. 99% of patients with NAFLD meet MASLD criteria and natural history is therefore identical. J Hepatol 2024;80:e76–7. [DOI] [PubMed] [Google Scholar]
- 30. Anania FA, Dimick-Santos L, Mehta R. et al. Nonalcoholic steatohepatitis: current thinking from the division of hepatology and nutrition at the food and drug administration. Hepatology 2021;73:2023–7. [DOI] [PubMed] [Google Scholar]
- 31. Sanyal AJ, Brunt EM, Kleiner DE. et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology 2011;54:344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Younossi ZM, Stepanova M, Rafiq N. et al. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology 2011;53:1874–82. [DOI] [PubMed] [Google Scholar]
- 33. Harrison SA, Ratziu V, Boursier J. et al. A blood-based biomarker panel (NIS4) for non-invasive diagnosis of non-alcoholic steatohepatitis and liver fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol 2020;5:970–85. [DOI] [PubMed] [Google Scholar]
- 34. Rockey DC, Caldwell SH, Goodman ZD. et al. ; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology 2009;49:1017–44. [DOI] [PubMed] [Google Scholar]
- 35. Harrison SA, Allen AM, Dubourg J. et al. Challenges and opportunities in NASH drug development. Nat Med 2023;29:562–73. [DOI] [PubMed] [Google Scholar]
- 36. Kleiner DE, Brunt EM, Van Natta M. et al. ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–21. [DOI] [PubMed] [Google Scholar]
- 37. Bedossa P, Poitou C, Veyrie N. et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology 2012;56:1751–9. [DOI] [PubMed] [Google Scholar]
- 38. Newsome PN, Sasso M, Deeks JJ. et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol 2020;5:362–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rasmussen DGK, Anstee QM, Torstenson R. et al. NAFLD and NASH biomarker qualification in the LITMUS consortium—lessons learned. J Hepatol 2023;78:852–65. [DOI] [PubMed] [Google Scholar]
- 40. Pavlides M, Mózes FE, Akhtar S. et al. ; LITMUS Consortium Investigators. Liver Investigation: Testing Marker Utility in Steatohepatitis (LITMUS): Assessment & validation of imaging modality performance across the NAFLD spectrum in a prospectively recruited cohort study (the LITMUS imaging study): Study protocol. Contemp Clin Trials 2023;134:107352. [DOI] [PubMed] [Google Scholar]
- 41. Standl E, Schnell O.. Treatment paradigm shifting implications of recent cardiovascular outcome trials: core insights on the brink of the 2020ies. Diabetes Res Clin Pract 2020;161:108054. [DOI] [PubMed] [Google Scholar]
- 42. Gillies PS, Dunn CJ.. Pioglitazone. Drugs 2000;60:333–43. Discussion 44–5. [DOI] [PubMed] [Google Scholar]
- 43. Francque S, Szabo G, Abdelmalek MF. et al. Nonalcoholic steatohepatitis: the role of peroxisome proliferator-activated receptors. Nat Rev Gastroenterol Hepatol 2021;18:24–39. [DOI] [PubMed] [Google Scholar]
- 44. Gastaldelli A, Sabatini S, Carli F. et al. PPAR-γ-induced changes in visceral fat and adiponectin levels are associated with improvement of steatohepatitis in patients with NASH. Liver Int 2021;41:2659–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Musso G, Cassader M, Paschetta E. et al. Thiazolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis: a meta-analysis. JAMA Intern Med 2017;177:633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Viscoli CM, Inzucchi SE, Young LH. et al. Pioglitazone and risk for bone fracture: safety data from a randomized clinical trial. J Clin Endocrinol Metab 2017;102:914–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dormandy JA, Charbonnel B, Eckland DJ. et al. ; PROactive Investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005;366:1279–89. [DOI] [PubMed] [Google Scholar]
- 48. Kernan WN, Viscoli CM, Furie KL. et al. ; IRIS Trial Investigators. Pioglitazone after Ischemic Stroke or Transient Ischemic Attack. N Engl J Med 2016;374:1321–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Harrison SA, Thang C, Bolze S. et al. Evaluation of PXL065: deuterium-stabilized (R)-pioglitazone in patients with NASH: A phase II randomized placebo-controlled trial (DESTINY-1). J Hepatol 2023;78:914–25. [DOI] [PubMed] [Google Scholar]
- 50. Nauck MA, Quast DR, Wefers J. et al. The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: a pathophysiological update. Diabetes Obes Metab 2021;23(Suppl 3):5–29. [DOI] [PubMed] [Google Scholar]
- 51. Zelniker TA, Wiviott SD, Raz I. et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation 2019;139:2022–31. [DOI] [PubMed] [Google Scholar]
- 52. Pyke C, Heller RS, Kirk RK. et al. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 2014;155:1280–90. [DOI] [PubMed] [Google Scholar]
- 53. Dunphy JL, Taylor RG, Fuller PJ.. Tissue distribution of rat glucagon receptor and GLP-1 receptor gene expression. Mol Cell Endocrinol 1998;141:179–86. [DOI] [PubMed] [Google Scholar]
- 54. Ciardullo S, Vergani M, Perseghin G.. Nonalcoholic fatty liver disease in patients with type 2 diabetes: screening, diagnosis, and treatment. J Clin Med 2023;12:5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mantovani A, Petracca G, Beatrice G. et al. Glucagon-Like Peptide-1 Receptor Agonists for Treatment of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: An Updated Meta-Analysis of Randomized Controlled Trials. Metabolites 2021;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kuchay MS, Krishan S, Mishra SK. et al. Effect of dulaglutide on liver fat in patients with type 2 diabetes and NAFLD: randomised controlled trial (D-LIFT trial). Diabetologia 2020;63:2434–45. [DOI] [PubMed] [Google Scholar]
- 57. Armstrong MJ, Gaunt P, Aithal GP. et al. ; LEAN Trial Team. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016;387:679–90. [DOI] [PubMed] [Google Scholar]
- 58. Pratley RE, Aroda VR, Lingvay I. et al. ; SUSTAIN 7 Investigators. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol 2018;6:275–86. [DOI] [PubMed] [Google Scholar]
- 59. Marso SP, Bain SC, Consoli A. et al. ; SUSTAIN-6 Investigators. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med 2016;375:1834–44. [DOI] [PubMed] [Google Scholar]
- 60. Husain M, Birkenfeld AL, Donsmark M. et al. ; PIONEER 6 Investigators. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med 2019;381:841–51. [DOI] [PubMed] [Google Scholar]
- 61. Lincoff AM, Brown-Frandsen K, Colhoun HM. et al. ; SELECT Trial Investigators. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. N Engl J Med 2023;389:2221–32. [DOI] [PubMed] [Google Scholar]
- 62. Newsome PN, Buchholtz K, Cusi K. et al. ; NN9931-4296 Investigators. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N Engl J Med 2021;384:1113–24. [DOI] [PubMed] [Google Scholar]
- 63. Loomba R, Abdelmalek MF, Armstrong MJ. et al. ; NN9931-4492 Investigators. Semaglutide 2·4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: a randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol Hepatol 2023;8:511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Frías JP, Davies MJ, Rosenstock J. et al. ; SURPASS-2 Investigators. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med 2021;385:503–15. [DOI] [PubMed] [Google Scholar]
- 65. Gastaldelli A, Cusi K, Fernández Landó L. et al. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol 2022;10:393–406. [DOI] [PubMed] [Google Scholar]
- 66. Jastreboff AM, Kaplan LM, Frías JP. et al. ; Retatrutide Phase 2 Obesity Trial Investigators. Triple-hormone-receptor agonist retatrutide for obesity—a phase 2 trial. N Engl J Med 2023;389:514–26. [DOI] [PubMed] [Google Scholar]
- 67. Abbasi J. New weight loss drugs make headlines at diabetes meeting. JAMA 2023;330:399–400. [DOI] [PubMed] [Google Scholar]
- 68. Scheen AJ. Beneficial effects of SGLT2 inhibitors on fatty liver in type 2 diabetes: a common comorbidity associated with severe complications. Diabetes Metab 2019;45:213–23. [DOI] [PubMed] [Google Scholar]
- 69. McGuire DK, Shih WJ, Cosentino F. et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol 2021;6:148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Packer M, Anker SD, Butler J. et al. ; EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–24. [DOI] [PubMed] [Google Scholar]
- 71. Mantovani A, Byrne CD, Targher G.. Efficacy of peroxisome proliferator-activated receptor agonists, glucagon-like peptide-1 receptor agonists, or sodium-glucose cotransporter-2 inhibitors for treatment of non-alcoholic fatty liver disease: a systematic review. Lancet Gastroenterol Hepatol 2022;7:367–78. [DOI] [PubMed] [Google Scholar]
- 72. Lonardo A, Ballestri S, Mantovani A. et al. Endpoints in NASH clinical trials: are we blind in one eye? Metabolites 2024;14:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ortiga-Carvalho TM, Sidhaye AR, Wondisford FE.. Thyroid hormone receptors and resistance to thyroid hormone disorders. Nat Rev Endocrinol 2014;10:582–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Targher G. Editorial: Resmetirom—a promising treatment option for NASH and liver fibrosis. Aliment Pharmacol Ther 2024;59:128–9. [DOI] [PubMed] [Google Scholar]
- 75. Sinha RA, Singh BK, Yen PM.. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat Rev Endocrinol 2018;14:259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Harrison SA, Bashir MR, Guy CD. et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2019;394:2012–24. [DOI] [PubMed] [Google Scholar]
- 77. Harrison SA, Ratziu V, Anstee QM. et al. Design of the phase 3 MAESTRO clinical program to evaluate resmetirom for the treatment of nonalcoholic steatohepatitis. Aliment Pharmacol Ther 2024;59:51–63. [DOI] [PubMed] [Google Scholar]
- 78. Harrison SA, Taub R, Neff GW. et al. Resmetirom for nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled phase 3 trial. Nat Med 2023;29:2919–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Harrison SA, Bedossa P, Guy CD. et al. ; MAESTRO-NASH Investigators. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N Engl J Med 2024;390:497–509. [DOI] [PubMed] [Google Scholar]
- 80. Cusi K. Selective Agonists of Thyroid Hormone Receptor Beta for the Treatment of NASH. N Engl J Med 2024;390:559–61. [DOI] [PubMed] [Google Scholar]
- 81. Lee Y, Lim S, Hong ES. et al. Serum FGF21 concentration is associated with hypertriglyceridaemia, hyperinsulinaemia and pericardial fat accumulation, independently of obesity, but not with current coronary artery status. Clin Endocrinol (Oxf) 2014;80:57–64. [DOI] [PubMed] [Google Scholar]
- 82. Abdelmalek MF, Sanyal AJ, Nakajima A. et al. Pegbelfermin in patients with nonalcoholic steatohepatitis and compensated cirrhosis (FALCON 2): a randomized phase 2b study. Clin Gastroenterol Hepatol 2024;22:113–23.e9. [DOI] [PubMed] [Google Scholar]
- 83. Sanyal A, Charles ED, Neuschwander-Tetri BA. et al. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet 2019;392:2705–17. [DOI] [PubMed] [Google Scholar]
- 84. Harrison SA, Frias JP, Neff G. et al. ; HARMONY Study Group. Safety and efficacy of once-weekly efruxifermin versus placebo in non-alcoholic steatohepatitis (HARMONY): a multicentre, randomised, double-blind, placebo-controlled, Phase 2b trial. Lancet Gastroenterol Hepatol 2023;8:1080–93. [DOI] [PubMed] [Google Scholar]
- 85.<Akero Therapeutics Announces Positive End-of-Phase 2 Meeting with the FDA and SYNCHRONY Phase 3 Program for Efruxifermin in NASH.pdf> (22 March 2024, date last accessed).
- 86. Loomba R, Sanyal AJ, Kowdley KV. et al. Randomized, Controlled Trial of the FGF21 Analogue Pegozafermin in NASH. N Engl J Med 2023;389:998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.<89bio Reaches Alignment with the FDA and EMA on Phase 3 Program for Pegozafermin in Nonalcoholic Steatohepatitis (NASH); Program Initiation Planned in the First Half of 2024.pdf> (22 March 2024, date last accessed).
- 88. Yoneda M, Kobayashi T, Asako N. et al. Pan-peroxisome proliferator-activated receptor agonist lanifibranor as a dominant candidate pharmacological therapy for nonalcoholic fatty liver disease. Hepatobiliary Surg Nutr 2022;11:433–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Francque SM, Bedossa P, Ratziu V. et al. ; NATIVE Study Group. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. N Engl J Med 2021;385:1547–58. [DOI] [PubMed] [Google Scholar]
- 90. Romeo S, Sanyal A, Valenti L.. Leveraging Human Genetics to Identify Potential New Treatments for Fatty Liver Disease. Cell Metab 2020;31:35–45. [DOI] [PubMed] [Google Scholar]
- 91. Ratziu V, Charlton M.. Rational combination therapy for NASH: Insights from clinical trials and error. J Hepatol 2023;78:1073–9. [DOI] [PubMed] [Google Scholar]