Abstract
Positron emission tomography (PET) imaging studies in laboratory animals are almost always performed under isoflurane anesthesia to ensure that the subject stays still during the image acquisition. Isoflurane is effective, safe, and easy to use, and it is generally assumed to not have an impact on the imaging results. Motivated by marked differences observed in the brain uptake and metabolism of the PET tracer 3-[18F]fluoro-4-aminopyridine [(18F]3F4AP) between human and nonhuman primate studies, this study investigates the possible effect of isoflurane on this process. Mice received [18F]3F4AP injection while awake or under anesthesia and the tracer brain uptake and metabolism was compared between groups. A separate group of mice received the known cytochrome P450 2E1 inhibitor disulfiram prior to tracer administration. Isoflurane was found to largely abolish tracer metabolism in mice (74.8 ± 1.6 vs. 17.7 ± 1.7 % plasma parent fraction, % PF) resulting in a 4.0-fold higher brain uptake in anesthetized mice at 35 min post-radiotracer administration. Similar to anesthetized mice, animals that received disulfiram showed reduced metabolism (50.0 ± 6.9 % PF) and a 2.2-fold higher brain signal than control mice. The higher brain uptake and lower metabolism of [18F]3F4AP observed in anesthetized mice compared to awake mice is attributed to isoflurane’s interference in the CYP2E1-mediated breakdown of the tracer, which was confirmed by reproducing the effect upon treatment with the known CYP2E1 inhibitor disulfiram. These findings underscore the critical need to examine the effect of isoflurane in PET imaging studies before translating tracers to humans that will be scanned without anesthesia.
Keywords: PET imaging, [18F]3F4AP, anesthesia, metabolism, radiometabolites, radiotracer
INTRODUCTION
Positron emission tomography (PET) is a molecular imaging technique that uses biologically active radiolabeled compounds to visualize and quantify biochemical processes inside the body (1). PET is widely used in oncology (2), neurology (3), cardiology (4), and drug discovery (5). Furthermore, in recent decades there has been remarkable growth in the development of novel tracers for emerging targets (6,7). By detecting high-energy photons produced in the positron-electron annihilation, PET can provide quantitative images of a radiotracer distribution deep inside the body with high biochemical specificity and sensitivity. Given these properties, new tracers can be tested in animal models with reasonable certainty that the results in will translate to humans with good reproducibility (8).
3-[18F]fluoro-4-aminopyridine ([18F]3F4AP) is a radiolabeled derivative of the FDA-approved multiple sclerosis drug 4-aminopyridine (4AP, dalfampridine) developed for imaging demyelinated axons in the central nervous system by PET (9) (Fig. 1). Similar to 4AP, [18F]3F4AP binds to voltage-gated K+ channels in the brain, including exposed Kv channels in demyelinated axons. Previous work has shown that [18F]3F4AP is very sensitive to demyelinated lesions in rodents and nonhuman primates (NHPs) (9,10). Preclinical evaluation in NHPs presented fast brain uptake (SUV > 3 at 4 min) and washout, high plasma availability, and high metabolic stability (>90% parent fraction up to 2h post-injection)(10). Due to its potential for detecting demyelination this tracer has recently advanced into human studies (clinicaltrials.gov: NCT04699747, NCT04710550). First-in-human imaging with [18F]3F4AP revealed widespread biodistribution and high brain penetration, but also exhibited faster than expected clearance rate and greater metabolism of the radiotracer in blood (less than 50% parent remaining 1h post-injection) (11). Given that the metabolic breakdown of the tracer may lead to a reduced brain uptake, introduce background signal, and create variability across subjects, further studies are warranted. Moreover, the unforeseen difference in metabolic stability between humans and NHPs, which is normally similar (12), is intriguing.
Figure 1.

Chemical structures of 4AP and [18F]3F4AP.
The pharmacokinetics of 4AP have been carefully evaluated in a variety of species including rats (13), dogs (14), horses (15), cattle (16), and humans (17). Studies following oral administration of 4AP in rats, dogs and humans have shown rapid absorption and renal clearance of the drug primarily as an unchanged compound (13,18,19). Characterization across species has reported limited metabolism of 4AP. In humans 24h after oral administration of 14C-labeled 4AP ~90% of the radioactive species recovered in urine corresponded to the unchanged drug, with the remaining corresponding to the 3-hydroxy derivative and its sulfate conjugate (20). Investigation of the metabolic pathways of 4AP points to an initial step catalyzed by members of the cytochrome P450 family, where CYP2E1 has been identified as the key metabolic enzyme.
CYP2E1 is the most abundant isoform of cytochrome P450 in the human liver (21). P450 enzymes are responsible for the oxidation of over 90% of drugs (22). CYP2E1 plays an integral part in the breakdown and clearance of a variety of small molecules including acetaminophen and halogenated anesthetics (23). Recent in vitro studies from our laboratory have shown that CYP2E1 is also able to metabolize (nonradioactive) 3F4AP very efficiently (24). In an in vitro competition assay the affinity of CYP2E1 for 3F4AP was 50 times higher than the affinity for 4AP, suggesting a primary role for CYP2E1 in metabolizing 3F4AP. Moreover, CYP2E1 is the predominant cytochrome P450 isoform responsible for human clinical isoflurane metabolism in vivo (25). Given these observations, we postulated that isoflurane anesthesia could play an interfering role in the observed differences in uptake, clearance, and metabolic breakdown of [18F]3F4AP between monkeys and humans. The goals of this study were to (i) determine whether isoflurane affects the in vivo stability of [18F]3F4AP; (ii) examine the formation and presence of radioactive metabolites of [18F]3F4AP in the brain; and (iii) inhibit the metabolism of [18F]3F4AP in vivo by introducing clinically approved CYP2E1 inhibitors, such as disulfiram.
MATERIALS AND METHODS
Animals and Compliance:
A total of 54 adult male C57BL/6J mice (9-12 weeks-old, Jackson Laboratory, RRID:IMSR_JAX:000664) were used for this work. Mice were group-housed (4 per cage) in standard hanging cages on a 12h light/dark cycle, constant temperature (22 ± 1 °C), 40 % relative humidity, and were provided standard rodent food and water ad libitum. No exclusion criteria were pre-determined, and no animals were excluded. No statistical randomization was performed to allocate subjects in the study. Instead, mice for each anesthesia condition group were selected arbitrarily and radiotracer injections of subsets within anesthesia condition groups were performed in an alternating manner (i.e. 3 anesthetized mice, followed by 3 awake mice, then 3 anesthetized mice, and so on). Additionally, tissue collection, processing and radioactivity measurements were carried out blinded to the experimental group. All rodent procedures were approved by the Institutional Animal Care and Use Committee at the Massachusetts General Hospital (IACUC approval # 2019N000088, PI: Brugarolas). All animal studies were conducted in compliance with the ARRIVE guidelines for reporting animal experiments.
Radiochemistry:
[18F]3F4AP was produced in a GE TRACERlab Fx2N synthesizer according to previously reported procedure(26). Semipreparative HPLC separations were performed on a Sykam HPLC pump with the UV detector at 254 nm with a Waters C18 preparative column (XBridge BEH C18 OBD Prep Column 130 Å, 5 μm, 10 mm × 250 mm). The obtained [18F]3F4AP fraction (radiochemical purity > 99%, molar activity = 127 ± 29 GBq/μmol (3.4 ± 0.8 Ci/μmol) at end of synthesis (EoS); n = 5), in 95% 20 mM sodium phosphate buffer, pH 8.0, 5% EtOH solution) passed the QC (with Thermo-Scientific Dionex Ultimate-3000 UHPLC (RRID:SCR_019840) equipped with Waters XBridge BEH C18 analytical column [130 Å, 3.5 μm, 4.6 × 100 mm], 95% 10 mM sodium phosphate buffer, pH 8.0, 5% EtOH solution as mobile phase, tR = 4.7 min) and was ready for injection into animals.
[18F]3F4AP mouse PET/CT image acquisition, reconstruction, and analysis:
Mice (n = 2) were anesthetized with isoflurane gas and intravenously administered 7.4 MBq (200 μCi) [18F]3F4AP via the tail vein. PET was acquired in a Sedecal SuperArgus PET/CT scanner for a duration of 60 minutes under anesthesia (1.5 % isoflurane, O2 flow 2.0 L/min). CT was acquired following PET for anatomical reference. PET images were visualized and analyzed using AMIDE (RRID:SCR_005940). PET and CT images were co-registered and using CT for anatomical reference, a region of interest was drawn for the brain and time-activity curves (TACs) extracted.
Evaluation of anesthesia effects on the radioactivity concentration in whole blood and brain:
Two cohorts of mice were administered 1.12 – 4.7 MBq (30 – 130 μCi; corresponding to a mass of 4.9 – 16.1 ng 3F4AP) of [18F]3F4AP intravenously via tail vein injection. Radiotracer delivery was performed under isoflurane anesthesia (2 % isoflurane in O2 flow 2.0 L/min) for the first cohort (n = 12), which was kept anesthetized for the duration of the experiment. For the second cohort (n = 20), mice were temporarily physically restrained while awake for radiotracer administration and then allowed to move freely. For both groups, 35 minutes post-injection of the radioactive dose mice were euthanized by intraperitoneal (IP) injection of 200 mg/kg pentobarbital sodium and phenytoin sodium solution (Euthasol®). Brain tissue was harvested, and blood was collected via cardiac puncture to measure radioactivity concentration via gamma counting. Gamma-counting was performed in a Perkin-Elmer 2480 gamma-counter and calibration curve was produced with [18F]3F4AP standards to calculate radioactivity concentration in biological samples.
Evaluation of anesthesia effects on the in vivo metabolism of [18F]3F4AP:
Two cohorts of mice were administered 3.32 – 15.3 MBq (90 – 400 μCi; corresponding to a mass of 2.2 – 67.8 ng 3F4AP) of [18F]3F4AP intravenously under isoflurane anesthesia (n = 10) or temporary physical restriction while awake (n =11). For both groups, 35 minutes post-injection of the radioactive dose mice were euthanized by 200 mg/kg IP Euthasol®. Blood was collected via cardiac puncture to measure radioactivity concentration in whole blood (WB) and plasma (PL) via gamma counting. Brain tissue was harvested, mixed with 1.0 mL (~1:2 w/v) freshly prepared 0.4 N perchloric acid (HClO4) aqueous solution, and mechanically homogenized via ultrasonication at 30,000 rpm for 30 seconds at 4 °C with a Dremel® Moto-Tool. Homogenized lysates were centrifuged at 21,000 RCF (8 °C) for 7 min. Resulting supernatant was collected for radiometabolite analysis. Radioactivity concentrations of whole brain, homogenized lysates, supernatant, and residual pellet were measured via gamma-counting. All radioactivity concentrations were measured using a single well high-purity germanium detector. All blood, plasma and tissue samples were kept on ice at 0 °C for the duration of the experiment.
Radio-HPLC analysis of metabolites in plasma and brain samples:
Plasma samples and pH-neutralized brain supernatant samples were first filtrated through a 10K filter (Amicon™ Ultracel-10 regenerated cellulose membrane, 0.5 mL sample volume, Centrifugal Filter Unit) by centrifugation at 21,000 RCF (8 °C) for 15 min. The filtrates were injected into HPLC (Agilent 1260 Infinity II, along with Eckert & Ziegler FlowCount detector) through a C18 column (XBridge, BEH, 130 Å, 3.5 μm, 4.6 × 100 mm) with a vanguard cartridge (XBridge, BEH, 130 Å, 3.5 μm, 3.9 × 5 mm) using 10 mM NH4HCO3 aqueous solution (pH 8.0) and MeCN (96:4) as mobile phase.
In vivo inhibition of [18F]3F4AP metabolism by disulfiram treatment.
Preparation and administration of disulfiram sesame oil suspension:
Disulfiram (Sigma Aldrich) sesame oil suspension was prepared freshly as a 6 mg/mL stock solution. Each mouse was administered 40 mg/kg disulfiram via intraperitoneal injection 2 hours prior to the radioactive dose administration.
Analysis of the effects of disulfiram treatment on the in vivo uptake and metabolism of [18F]3F4AP:
Following pre-treatment of two cohorts of mice with disulfiram (n = 6) or sesame oil vehicle control (n = 4), 8.28 – 13.6 MBq (225 – 370 μCi; corresponding to a mass of 26.7 – 60.5 ng 3F4AP) of [18F]3F4AP were administered intravenously under temporary physical restriction while awake. Mice were euthanized 35 min post-tracer administration, blood and brain was collected, processed, and evaluated ex vivo for radioactivity content by gamma-counting and metabolite content by radio-HPLC.
Data analysis:
Statistical analysis was performed using GraphPad Prism 10 software (RRID:SCR_002798). No sample size was calculated a priori. Sample size was selected based on previous studies of similar nature.(27–30) Data were not assessed for normality. Descriptive statistics including mean, standard deviation (SD) and standard error of the mean (SEM) were calculated for each group. Data was analyzed using two-tailed unpaired t tests with Welch correction and Holm-Šídák correction for multiple comparisons and one-way ANOVA with Tukey’s multiple comparison test with a significance level of α = 0.05 to assess differences among groups. Specific statistical analyses performed per experiment are described in figure captions. No tests for outliers were conducted. Grouped data are reported as mean ± SEM.
RESULTS
Study design
To test our hypothesis that isoflurane anesthesia interferes with the in vivo breakdown of [18F]3F4AP, we wanted to investigate metabolism of the tracer in awake and anesthesia conditions within the same species. Given the challenges and ethical considerations to test awake rhesus macaques or anesthetized humans, we opted to do the investigation in awake and anesthetized mice. Awake mice received the radiotracer dose while momentarily restrained and were returned to their home cage until the time of euthanasia whereas anesthetized mice received the dose under isoflurane and were kept anesthetized for the duration of the experiment (Fig. 2). Euthanasia at 35 min post-tracer administration was empirically chosen as an approximation to an equivalent time point of 90 min post-injection in NHPs and humans based on the time-activity curves (TACs) (Fig. 3A). After euthanasia, blood was collected via cardiac puncture and the brain dissected to measure radioactivity concentration and analyze radiometabolites present in these tissues. Although not perfusing the animals meant that blood would be present in the brain sample (around 5 % of brain volume corresponds to blood) we decided to not perfuse to avoid washing out some of the loosely bound metabolites within the brain. To measure the metabolites in blood, the plasma was separated from the cellular components using standard centrifugation techniques and the proteins and other large macromolecules filtered out using 10 kDa centrifugal filter prior to injection onto the radio-HPLC. From our experience with [18F]3F4AP blood radiometabolite analysis, this method is superior to trapping the radioactive species in a solid phase extraction cartridge followed by elution and injection onto the HPLC. To measure the radiometabolites in brain, the brain was mechanically homogenized in 2 volumes of perchloric acid solution and centrifuged at high speed to separate solid components from soluble components. In our tests, this method was superior to precipitating and extracting the organic components with acetonitrile and resulted in extraction of 69 ± 2% of radioactivity in the supernatant and 30 ± 2% in the wet pellet (n = 31, n = number of mice). Since the supernatant represents 68 ± 2% of the total mass, this extraction was considered quantitative. The supernatant was then filtered using the same 10 kDa centrifugal filter and the pass-through neutralized with base prior to injection on the HPLC. These methods had the added advantage that no organic solvent was introduced which would compromise the retention of the tracer and any polar radiometabolites on the HPLC column.
Figure 2. Experimental design.

Young adult mice are assigned to two groups (isoflurane and awake) arbitrarily. 35 min after intravenous administration of [18F]3F4AP the animals are euthanized, their blood (WB) and brain collected, and radioactivity concentration assessed. Tissue samples are processed, and plasma (PL) and brain homogenates analyzed for radiometabolite content.
Figure 3. [18F]3F4AP uptake in whole blood and brain of anesthetized and awake subjects.
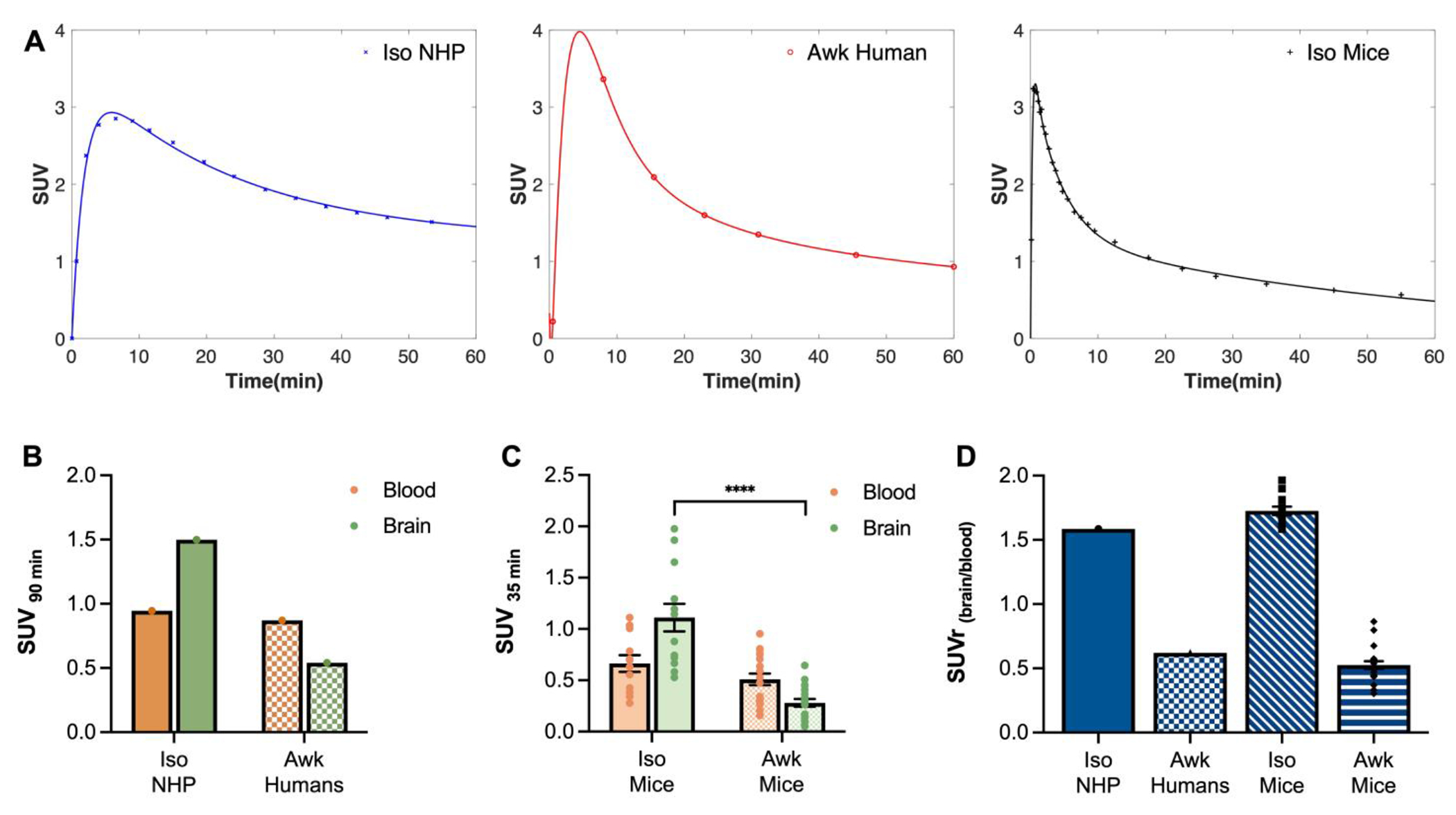
A) Representative brain time-activity curves (TACs) for anesthetized non-human primates (Iso NHP), awake humans (Awk Humans), and anesthetized mice (Iso Mice) extracted from PET imaging. (B) Tracer uptake in anesthetized NHPs and awake humans in whole blood and brain at 90 min post-tracer administration. Values were calculated from previous reported experiments(10,11). C) Tracer uptake in whole blood and brain samples of anesthetized (n = 12) and awake (n = 20) mice at 35 min post-tracer administration. Values were calculated from ex vivo gamma counting of collected tissue. n = number of mice. D) Normalization by brain-blood ratios in anesthetized and awake subjects calculated from data in panels B and C. Statistical analysis was performed using two-tailed unpaired t test with Welch correction and Holm-Šídák correction for multiple comparisons. * denotes direct comparison of groups (****p < 0.0001, df = 13.82, t = 5.956). Data are means ± SEM.
Isoflurane anesthesia results in higher [18F]3F4AP brain uptake
Analysis of the TACs in Figure 3A shows an SUV 90 min post-injection of ~1.50 in anesthetized NHPs and 0.54 in awake humans. Blood sampling performed during these studies shows comparable radioactivity concentration in whole blood (WB) for both anesthetized NHPs and awake humans (SUV90 min-WB (NHP) 0.946 vs. SUV90 min-WB (human) 0.87) (Fig. 3B). To investigate whether isoflurane anesthesia could be playing a role in the observed difference in brain uptake and blood concentration of [18F]3F4AP, we compared radioactivity concentration in WB and brain of mice that had been administered the radioactive tracer while anesthetized or awake. As observed in Figure 3C mice that had received the dose under isoflurane anesthesia and were kept anesthetized showed higher radioactivity concentration in brain when compared to awake mice. At 35 min post-injection [18F]3F4AP brain uptake was 4.0-fold lower in awake mice in comparison to anesthetized mice (SUV35 min-br (iso mice) 1.14 ± 0.14, n = 12 vs. SUV35 min-br (awk mice) 0.28 ± 0.04, n = 20; p < 0.0001). In contrast, no significant differences were found in WB SUV values between the two groups (SUV35 min-WB (iso mice) 0.66 ± 0.08 vs. SUV35 min-WB (awk mice) 0.51 ± 0.05; p = 0.1373). Interestingly, the observed brain-blood ratio (SUVr(brain/blood)) in anesthetized and awake mice at 35 min post-injection is remarkably similar to that of anesthetized NHPs and awake humans at 90 min (Fig. 3D). Furthermore, awake and anesthetized mice that were euthanized at 60 sec post-tracer injection showed no significant differences in WB and brain SUV values, which strongly suggests that the difference at 35 min is not due to an effect of isoflurane on perfusion. (Fig. S1)
Isoflurane anesthesia reduces [18F]3F4AP metabolism
Upon confirmation that isoflurane anesthesia plays a role in the in vivo brain uptake of [18F]3F4AP, we set to evaluate the presence of radiometabolites in the plasma and brain of mice with and without isoflurane. We posited that the lower concentration in brain may be driven by metabolism of the tracer. As hypothesized, higher metabolism was observed in the plasma and brain of awake mice in comparison to anesthetized, revealed by the percent of parent fraction (%PF) remaining at 35 min post-tracer administration. Figure 4A shows representative radio-HPLC traces of plasma and brain samples for each anesthesia condition. Based on the HPLC elution, all the radiometabolites were more polar than the parent compound. In plasma, anesthetized animals showed 74.8 ± 1.6 % of unmetabolized [18F]3F4AP remaining in circulation, while awake animals presented only a 17.7 ± 1.7 %. In brain, anesthetized animals showed 98.4 ± 0.2 %PF, while awake animals showed 80.3 ± 2.7 %, indicating limited brain penetration of the radiometabolites (Fig. 4B).
Figure 4. Radio-HPLC analysis of plasma and brain of anesthetized and awake mice.

A) Representative radio-HPLC traces of plasma (orange) and brain (green) samples of anesthetized and awake mice. [18F]3F4AP parent peak signal is shown in yellow shaded box. Radioactive metabolites signals are shown in blue shaded box. B) Quantification of remaining percent parent fraction in plasma (%PFiso-pl 74.8 ± 1.6 %, n = 10; %PFawk-pl 17.7 ± 1.7 %, n = 11) and brain (%PFiso-br 98.4 ± 0.2 %, n = 5; %PFawk-br 80.3 ± 2.7 %, n = 8) after 35 min of [18F]3F4AP administration. Statistical analysis was performed using two-tailed unpaired t test with Welch correction and Holm-Šídák correction for multiple comparisons. * denotes direct comparison of groups (***p = 0.0004, df = 7.098, t = 6.181; ****p < 0.0001 df =18.98, t = 24.10). n = number of plasma or brain samples. Data are means ± SEM.
Disulfiram treatment can partially rescue metabolic stability of [18F]3F4AP
The observed lower in vivo metabolism of [18F]3F4AP under isoflurane and our recent findings that 3F4AP is a good substrate for CYP2E1-mediated oxidation in vitro suggests that isoflurane and [18F]3F4AP can act as competing substrates for CYP2E1 in vivo. We postulated that in anesthetized conditions, isoflurane saturates CYP2E1 activity preventing metabolic breakdown of [18F]3F4AP by the P450 enzyme and resulting in an inflated metabolic stability of the radiotracer. Therefore, we sought to investigate if pharmacological inhibition of CYP2E1 could serve as a potential strategy to rescue [18F]3F4AP’s apparent metabolic stability observed under isoflurane anesthesia in awake subjects.
Disulfiram (DSF; Antabuse) has been used in the treatment of alcoholism for more than 60 years due to its ability to inhibit acetaldehyde dehydrogenase, which causes the build-up of acetaldehyde after ethanol consumption and causes an aversive reaction(31). Resulting from its capability to inhibit several pharmacologically relevant target enzymes, a range of other therapeutic uses for DSF have been identified. CYP2E1 is one of the enzyme targets that can be inhibited by DSF. It has been shown that DSF administration inhibits CYP2E1 activity via mechanism-based inactivation of the P450 by an oxidized product of DSF formed in vivo(32). DSF has been widely used as an inhibitor of CYP2E1 in various in vivo studies. For example, single-dose DSF has been proven to be an effective in vivo inhibitor of 2E1 in the hydroxylation of chlorzoxazone – a specific noninvasive probe of hepatic 2E1 activity(33), and in the metabolism of volatile anesthetics such as enflurane(34), sevoflurane(35) and halothane(36) in humans. Clinical isoflurane metabolism in humans has also been shown to be prevented by 80–90% with DSF treatment(37). Preclinical work in rodents using CYP2E1 inhibition to reduce metabolic breakdown of pharmacologically active molecules has also been studied. For example, CYP2E1-mediated defluorination of the serotonin 1A receptor PET tracer [18F]FCWAY has been mitigated in rats with the use of miconazole – an antifungal agent and potent CYP2E1 inhibitor(38). Inhibition of CYP2E1 to prevent defluorination of [18F]FCWAY was later extended in human imaging studies using DSF treatment, as a less toxic alternative to miconazole(39).
To test the effect of DSF we selected a dose of 40 mg/kg, equivalent to 195 mg for a 60 kg human based on body surface area, which is within the clinical dose range for DSF (125 – 500 mg q.d.). A single dose of DSF 2h prior to radiotracer administration showed a 2.2-fold increase in the brain to blood concentration when compared to untreated awake mice (Fig. 5A). Additionally, presence of radioactive metabolites in the plasma of DSF-treated mice was reduced when compared to their untreated counterparts, evidenced by 50.0 ± 6.9 %PF at 35 min after tracer administration. As expected, the amount of radiometabolites in brain was also reduced (%PFiso-br 98.4 ± 0.2 % vs. %PFDSF-br 95.0 ± 0.5 %) (Fig. 5C). Additionally, CYP2E1 inhibition was confirmed to be mediated by DSF treatment, with no significant differences observed between awake animals with and without 2h pre-treatment with sesame oil vehicle.
Figure 5. Inhibition of [18F]3F4AP metabolism in vivo with disulfiram treatment.
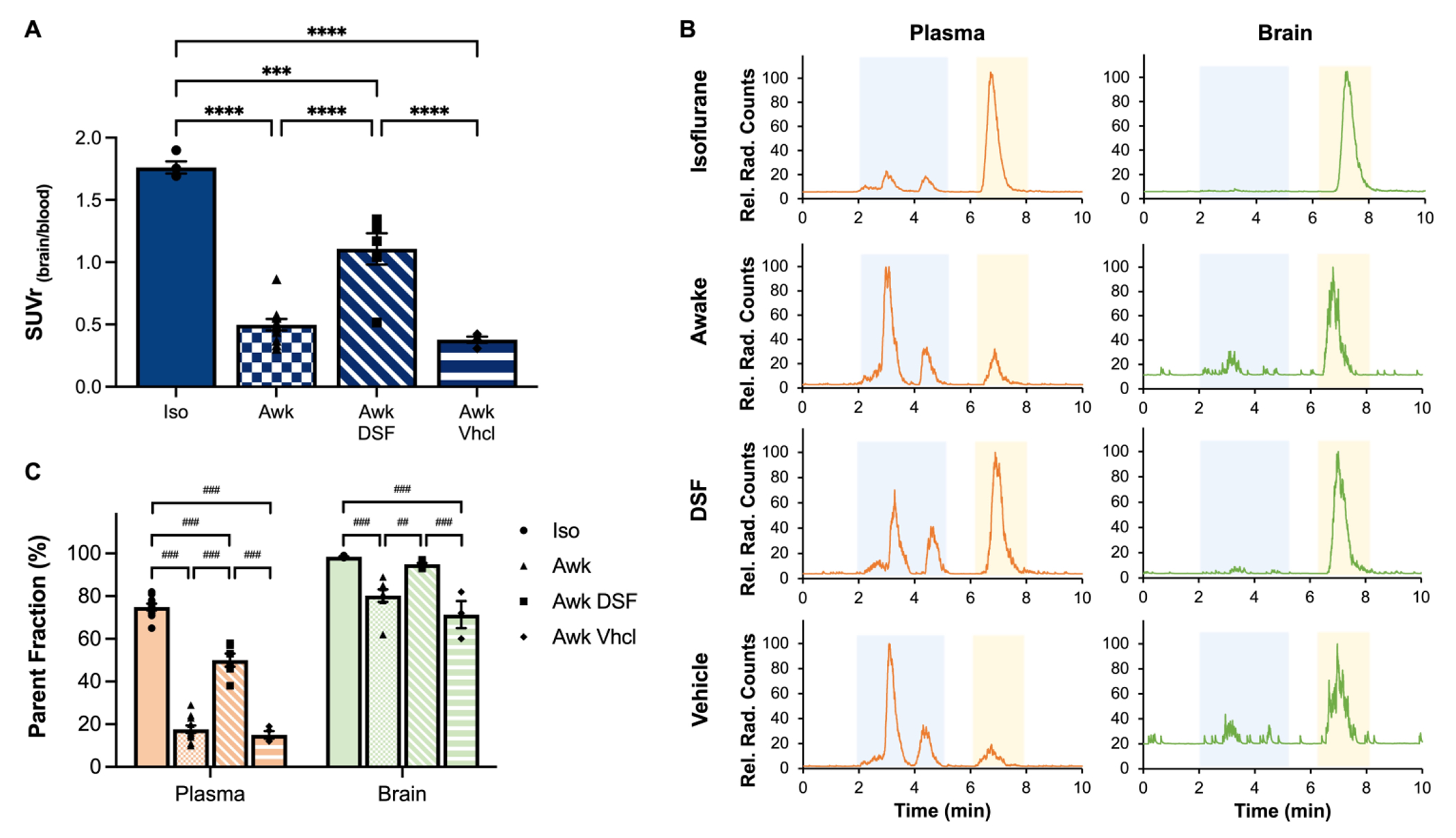
A) Brain-blood ratio normalization of tracer uptake in whole blood and brain of anesthetized (Iso, n = 4), awake (Awk, n = 11), disulfiram-treated (Awk DSF, n = 6) and vehicle control (Awk Vhcl, n = 4) mice. n = number of mice. Statistical analysis was performed using a one-way ANOVA (F = 55.78, degrees of freedom in the numerator, DFn = 3, degrees of freedom in the denominator, DFd = 21, p < 0.0001) and Tukey’s multiple comparison test. * denotes direct comparison of groups (***p = 0.0001, ****p < 0.0001) B) Representative radio-HPLC traces of plasma (orange) and brain (green) samples of anesthetized, awake, and disulfiram-treated and vehicle control mice. [18F]3F4AP parent peak signal is shown in yellow shaded box. Radioactive metabolites signals are shown in blue shaded box. C) Quantification of remaining percent parent fraction in anesthetized, awake, disulfiram-treated and vehicle control mice in plasma (%PFiso-pl 74.8 ± 1.6 %, n = 10; %PFawk-pl 17.7 ± 1.7 %, n = 11; %PFawk DSF-pl 50.0 ± 2.8 %, n = 6; %PFawk Vhcl-pl 15.0 ± 1.5 %, n = 4) and brain (%PFiso-br 98.4 ± 0.2 %, n = 5; %PFawk-br 80.3 ± 2.7 %, n = 8; %PFawk DSF-br 95.0 ± 0.5 %, n = 6; %PFawk Vhcl-br 71.3 ± 5.2 %, n = 3) after 35 min of [18F]3F4AP administration. n = number of plasma or brain samples. Statistical analysis was performed using a one-way ANOVA (plasma: F = 208.1, DFn = 3, DFd = 27, p < 0.001; brain: F = 17.46, DFn = 3, DFd = 18, p < 0.001) and Tukey’s multiple comparison test. # denotes direct comparison of groups (##p = 0.002, ###p < 0.001). Data are means ± SEM.
DISCUSSION
Our study demonstrates an artificially beneficial impact of isoflurane, the most commonly used anesthetic agent in animal PET studies, on the metabolism and brain uptake of the demyelination tracer [18F]3F4AP. Notably, the concentration of radioactive species in the brains of anesthetized animals was approximately 3.3 times higher than that in awake subjects. This effect is unexpected based on previous studies with [18F]FDG and a few other tracers that reported higher brain uptake in awake vs. anesthetized animals(40). Furthermore, the high brain uptake in anesthetized monkeys and rodents had caused the expectation of higher brain uptake in humans than what was later found(9–11). This unforeseen result prompts important questions about the underlying mechanisms responsible for this phenomenon.
A possible explanation for the increased brain uptake in anesthetized subjects is an increase in cerebral blood flow (CBF) under isoflurane. Even though it has been reported that isoflurane anesthesia can lead to an increase in CBF (41), the effects of isoflurane on CBF are dose-dependent and low concentrations of isoflurane like the concentrations used in this study tend to maintain CBF close to awake levels(42). This was confirmed by measuring a similar brain uptake in anesthetized and awake mice at 1 min post-injection. Therefore, it is more likely that an elevated bioavailability of the unmetabolized [18F]3F4AP in plasma, due to interference of isoflurane in the CYP2E1-mediated breakdown of the tracer, resulted in a greater transfer across the blood-brain barrier (BBB).
Our investigation into the metabolic stability of [18F]3F4AP yielded interesting outcomes. Although there was no significant difference in total radioactivity in the blood between anesthetized and awake mice, we detected substantial disparities in radiometabolite content in plasma. The higher concentration of metabolites in the plasma of awake compared to anesthetized mice (82.3% vs. 25.2%) strongly suggests that isoflurane has an inhibitory effect on the metabolic enzymes involved in [18F]3F4AP breakdown. Our research points towards CYP2E1 as a potential candidate enzyme, which has been previously reported to metabolize isoflurane and 4AP in vivo and has exhibited a high affinity for 3F4AP in vitro. Our results suggest that metabolites are not cleared from circulation more rapidly than the parent compound, or alternatively, the 35-minute time frame may not be sufficient to produce and eliminate metabolites.
We noted a substantially lower proportion of radiometabolites in the brain compared to blood, both in awake (18.3% vs. 82.3%) and anesthetized (1.6% vs. 25.2%) mice suggesting that metabolites do not enter the brain to the same extent as the parent compound. These findings align with a prior study that identified 5-hydroxy-3F4AP and 3F4AP N-oxide, as the major and minor products of CYP2E1-mediated oxidation of 3F4AP in vitro and showed that these compounds exhibit low permeability across an artificial brain membrane. Another possibility is the production of these metabolites within the brain, though CYP2E1 expression in the brain seems to be negligible in basal conditions(43). Finally, while we cannot entirely exclude the possibility of some metabolites crossing the BBB, a large fraction of the detected brain metabolites is likely a result of metabolites present in remaining blood in the vascular space of the brain, since perfusion was not performed, and minimal metabolism occurs within the brain.
Our findings suggest disulfiram as a potential avenue for mitigating the in vivo metabolic breakdown of [18F]3F4AP. We observed that a single dose of disulfiram, a known CYP2E1 inhibitor, could partially rescue the metabolic stability of the tracer. Importantly, the dose of disulfiram we used was equivalent to that employed in humans.
One limitation of the study was that it did not evaluate the effect of isoflurane or DSF using in vivo PET imaging. Assessing this effect would be challenging, as it would require conducting PET imaging in awake rodents or using an anesthetic that does not inhibit CYP2E1. Nevertheless, these experiments would be valuable as they could provide insights into whether the increase in SUV under anesthesia is regional or global. Alternatively, it may be possible to examine this effect in humans by scanning human subjects under sevoflurane anesthesia or following DSF administration.
The impact of our study likely goes beyond [18F]3F4AP. Most PET tracers are metabolized to some degree and CYP2E1 is the most abundant P450 enzyme in the liver and likely responsible for metabolizing other small molecule tracers. Even though isoflurane has been reported to be metabolized by CYP2E1, the ability of isoflurane to saturate the enzyme thus preventing tracer metabolism has not been previously recognized. Instead, differences encountered between animal and human studies have commonly been vaguely ascribed to species differences(12). Auspiciously, this work describes a simple experimental method that can be used to test the effect of anesthesia on tracer metabolism and brain uptake before translating new PET tracers to humans.
Supplementary Material
ACKNOWLEDGEMENTS
We thank David Lee and Kyle Stewart at the MGH Gordon PET cyclotron facility for producing fluorine-18. Funding: This study was supported by R01NS114066 (PB), S10OD018035, K99EB033407 (YPZ) and MGH-ECOR PSDA award (KMRT).
ABBREVIATIONS
- 3F4AP
3-fluoro-4-aminopyrdine
- 4AP
4-aminopyridine
- CBF
cerebral blood flow
- CT
computed tomography
- CYP2E1
Cytochrome P450 Family 2 Subfamily E Member 1
- DSF
disulfiram
- EoS
end of synthesis
- HPLC
high-performance liquid chromatography
- IP
intraperitoneal
- IV
intravenous
- NHP
nonhuman primate
- PET
positron emission tomography
- RRID
research resource identifier
- SD
standard deviation
- SEM
standard error of the mean
- SUV
standardized uptake value
- TAC
time activity curve
- WB
whole brain
- %PF
percent parent fraction
Footnotes
DISCLAIMER
PB has a financial interest in Fuzionaire Diagnostics and the University of Chicago. PB is a named inventor on patents related to [18F]3F4AP owned by the University of Chicago and licensed to Fuzionaire Diagnostics. Dr. Brugarolas’ interests were reviewed and are managed by MGH and Mass General Brigham in accordance with their conflict-of-interest policies. The other authors declare no conflict of interests.
DATA AVAILABILITY
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request. A preprint version of this article was posted on bioRxiv on December 14, 2023: https://doi.org/10.1101/2023.12.14.571690
REFERENCES
- 1.Phelps ME. Positron emission tomography provides molecular imaging of biological processes. Proc Natl Acad Sci U S A. 2000;97(16):9226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bomanji JB, Costa DC, Ell PJ. Clinical role of positron emission tomography in oncology. Lancet Oncol. 2001;2(3):157–64. [DOI] [PubMed] [Google Scholar]
- 3.Politis M, Piccini P. Positron emission tomography imaging in neurological disorders. J Neurol. 2012;259(9):1769–80. [DOI] [PubMed] [Google Scholar]
- 4.Bengel FM, Higuchi T, Javadi MS, Lautamaki R. Cardiac positron emission tomography. J Am Coll Cardiol. 2009;54(1):1–15. [DOI] [PubMed] [Google Scholar]
- 5.Matthews PM, Rabiner EA, Passchier J, Gunn RN. Positron emission tomography molecular imaging for drug development. Br J Clin Pharmacol. 2012;73(2):175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pike VW. PET radiotracers: crossing the blood-brain barrier and surviving metabolism. Trends Pharmacol Sci. 2009;30(8):431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fowler JS, Ding YS, Volkow ND. Radiotracers for positron emission tomography imaging. Semin Nucl Med. 2003;33(1):14–27. [DOI] [PubMed] [Google Scholar]
- 8.Rowland DJ, Cherry SR. Small-animal preclinical nuclear medicine instrumentation and methodology. Semin Nucl Med. 2008;38(3):209–22. [DOI] [PubMed] [Google Scholar]
- 9.Brugarolas P, Sanchez-Rodriguez JE, Tsai HM, et al. Development of a PET radioligand for potassium channels to image CNS demyelination. Sci Rep. 2018;8(1):607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guehl NJ, Ramos-Torres KM, Linnman C, et al. Evaluation of the potassium channel tracer [(18)F]3F4AP in rhesus macaques. J Cereb Blood Flow Metab. 2021;41(7):1721–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brugarolas P, Wilks MQ, Noel J, et al. Human biodistribution and radiation dosimetry of the demyelination tracer [(18)F]3F4AP. Eur J Nucl Med Mol Imaging. 2023;50(2):344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw RC, Tamagnan GD, Tavares AAS. Rapidly (and Successfully) Translating Novel Brain Radiotracers From Animal Research Into Clinical Use. Front Neurosci. 2020;14(871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caggiano A, Blight A, Parry TJ. Identification of metabolites of dalfampridine (4-aminopyridine) in dog and rat. J Drug Assess. 2013;2(1):72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rupp SM, Shinohara Y, Fisher DM, Miller RD, Castagnoli N Jr. Pharmacokinetics and pharmacodynamics of 4-aminopyridine in anesthetized dogs. J Pharmacol Exp Ther. 1983;225(2):351–4. [PubMed] [Google Scholar]
- 15.Kitzman JV, Wilson RC, Booth NH, Hendricks HL, Bush PB. Pharmacokinetics of 4-aminopyridine in horses. Am J Vet Res. 1984;45(7):1333–5. [PubMed] [Google Scholar]
- 16.Kitzman JV, Wilson RC, Booth NH, Zahner JM, Hendricks HL, Bush PB. Pharmacokinetics of 4-aminopyridine in cattle. Am J Vet Res. 1984;45(12):2625–7. [PubMed] [Google Scholar]
- 17.Blight AR, Henney HR 3rd. Pharmacokinetics of 14C-radioactivity after oral intake of a single dose of 14C-labeled fampridine (4-aminopyridine) in healthy volunteers. Clin Ther. 2009;31(2):328–35. [DOI] [PubMed] [Google Scholar]
- 18.Evenhuis J, Agoston S, Salt PJ, de Lange AR, Wouthuyzen W, Erdmann W. Pharmacokinetics of 4-aminopyridine in human volunteers. A preliminary study using a new GLC method for its estimation. Br J Anaesth. 1981;53(6):567–70. [DOI] [PubMed] [Google Scholar]
- 19.Uges DR, Sohn YJ, Greijdanus B, Scaf AH, Agoston S. 4-Aminopyridine kinetics. Clin Pharmacol Ther. 1982;31(5):587–93. [DOI] [PubMed] [Google Scholar]
- 20.Caggiano A, Blight A. Identification of metabolites of dalfampridine (4-aminopyridine) in human subjects and reaction phenotyping of relevant cytochrome P450 pathways. J Drug Assess. 2013;2(1):117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drozdzik M, Busch D, Lapczuk J, et al. Protein Abundance of Clinically Relevant Drug-Metabolizing Enzymes in the Human Liver and Intestine: A Comparative Analysis in Paired Tissue Specimens. Clin Pharmacol Ther. 2018;104(3):515–24. [DOI] [PubMed] [Google Scholar]
- 22.Rendic S, Guengerich FP. Survey of Human Oxidoreductases and Cytochrome P450 Enzymes Involved in the Metabolism of Xenobiotic and Natural Chemicals. Chem Res Toxicol. 2015;28(1):38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guengerich FP. Cytochrome P450 2E1 and its roles in disease. Chem Biol Interact. 2020;322(109056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Y, Ramos-Torres K, Brugarolas P. Metabolic Stability of the Demyelination Positron Emission Tomography Tracer [(18)F]3-Fluoro-4-Aminopyridine and Identification of Its Metabolites. J Pharmacol Exp Ther. 2023;386(1):93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kharasch D, Evan Hankins C, Douglas Cox K. Clinical Isoflurane Metabolism by Cytochrome P450 2E1. Anesthesiology. 1999;90(3):766–71. [DOI] [PubMed] [Google Scholar]
- 26.Basuli F, Zhang X, Brugarolas P, Reich DS, Swenson RE. An efficient new method for the synthesis of 3-[(18) F]fluoro-4-aminopyridine via Yamada-Curtius rearrangement. J Labelled Comp Radiopharm. 2018;61(2):112–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou YP, Sun Y, Takahashi K, et al. Development of a PET radioligand for α2δ-1 subunit of calcium channels for imaging neuropathic pain. Eur J Med Chem. 2022;242(114688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aarnio R, Alzghool OM, Wahlroos S, et al. Novel plasma protein binding analysis method for a PET tracer and its radiometabolites: A case study with [(11)C]SMW139 to explain the high uptake of radiometabolites in mouse brain. J Pharm Biomed Anal. 2022;219(114860. [DOI] [PubMed] [Google Scholar]
- 29.Seneca N, Zoghbi SS, Shetty HU, et al. Effects of ketoconazole on the biodistribution and metabolism of [11C]loperamide and [11C]N-desmethyl-loperamide in wild-type and P-gp knockout mice. Nucl Med Biol. 2010;37(3):335–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Celen S, Koole M, De Angelis M, et al. Preclinical evaluation of 18F-JNJ41510417 as a radioligand for PET imaging of phosphodiesterase-10A in the brain. J Nucl Med. 2010;51(10):1584–91. [DOI] [PubMed] [Google Scholar]
- 31.Zhou YP, Wilks MQ, Dhaynaut M, et al. Radiosynthesis automation, non-human primate biodistribution and dosimetry of K (+) channel tracer [ (11) C]3MeO4AP. bioRxiv. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pratt-Hyatt M, Lin HL, Hollenberg PF. Mechanism-based inactivation of human CYP2E1 by diethyldithocarbamate. Drug Metab Dispos. 2010;38(12):2286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peter R, Böcker R, Beaune PH, Iwasaki M, Guengerich FP, Yang CS. Hydroxylation of chlorzoxazone as a specific probe for human liver cytochrome P-450IIE1. Chem Res Toxicol. 1990;3(6):566–73. [DOI] [PubMed] [Google Scholar]
- 34.Kharasch ED, Thummel KE, Mautz D, Bosse S. Clinical enflurane metabolism by cytochrome P450 2E1. Clin Pharmacol Ther. 1994;55(4):434–40. [DOI] [PubMed] [Google Scholar]
- 35.Kharasch ED, Karol MD, Lanni C, Sawchuk R. Clinical sevoflurane metabolism and disposition. I. Sevoflurane and metabolite pharmacokinetics. Anesthesiology. 1995;82(6):1369–78. [DOI] [PubMed] [Google Scholar]
- 36.Kharasch ED, Hankins D, Mautz D, Thummel KE. Identification of the enzyme responsible for oxidative halothane metabolism: implications for prevention of halothane hepatitis. Lancet. 1996;347(9012):1367–71. [DOI] [PubMed] [Google Scholar]
- 37.Kharasch ED, Hankins DC, Cox K. Clinical isoflurane metabolism by cytochrome P450 2E1. Anesthesiology. 1999;90(3):766–71. [DOI] [PubMed] [Google Scholar]
- 38.Tipre DN, Zoghbi SS, Liow JS, et al. PET imaging of brain 5-HT1A receptors in rat in vivo with 18F-FCWAY and improvement by successful inhibition of radioligand defluorination with miconazole. J Nucl Med. 2006;47(2):345–53. [PubMed] [Google Scholar]
- 39.Ryu YH, Liow JS, Zoghbi S, et al. Disulfiram inhibits defluorination of (18)F-FCWAY, reduces bone radioactivity, and enhances visualization of radioligand binding to serotonin 5-HT1A receptors in human brain. J Nucl Med. 2007;48(7):1154–61. [DOI] [PubMed] [Google Scholar]
- 40.Miranda A, Bertoglio D, Stroobants S, Staelens S, Verhaeghe J. Translation of Preclinical PET Imaging Findings: Challenges and Motion Correction to Overcome the Confounding Effect of Anesthetics. Front Med (Lausanne). 2021;8(753977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rakymzhan A, Li Y, Tang P, Wang RK. Differences in cerebral blood vasculature and flow in awake and anesthetized mouse cortex revealed by quantitative optical coherence tomography angiography. J Neurosci Methods. 2021;353(109094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masamoto K, Kanno I. Anesthesia and the quantitative evaluation of neurovascular coupling. J Cereb Blood Flow Metab. 2012;32(7):1233–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agúndez JA, Jiménez-Jiménez FJ, Alonso-Navarro H, García-Martín E. Drug and xenobiotic biotransformation in the blood-brain barrier: a neglected issue. Front Cell Neurosci. 2014;8(335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request. A preprint version of this article was posted on bioRxiv on December 14, 2023: https://doi.org/10.1101/2023.12.14.571690


