Abstract
Background:
Leptin has a great effect on bone through direct or indirect involvement in bone remodeling. Considering the ambiguities that exist regarding the effect of leptin on bone and bone-related diseases including osteoporosis, in this study, we aimed to conduct a systematic review of various studies on the effect of leptin on osteoporosis, which may find an answer to the existing ambiguities.
Methods:
The search was performed to review studies on the effects of leptin on osteoporosis by using several databases including Scopus, PubMed, Web of Science, and Google Scholar. Electronic searches were conducted on 5 Jan 2023. There was no limit on the publication date of the articles. The risk of bias for the animal study was assessed with the CAMARADES checklist, and the study quality assessment was also assessed based on the guidelines for in vivo experiments (ARRIVE). In this study, the risk of bias (quality) of human studies was assessed using the quality assessment checklists by NHLBI.
Results:
Overall, 34 articles were included for data extraction and quality assessment. Overall, 27 human studies and seven animal studies were included in the article. The results of most of the studies conducted in this study showed that leptin has a physiological role in maintaining bone mass and better bone quality and reduces bone marrow adipogenesis and increases bone mineral density (BMD). As plasma leptin levels increased, BMD values or bone formation biomarkers increased.
Conclusion:
Leptin has an inhibitory role against bone resorption and increasing osteoprotegerin (OPG) levels, which, as a result, maintains bone density and reduces osteoclast activity, and has a positive relationship with increasing osteocalcin.
Keywords: Leptin, Bone losses, Bone resorption
Introduction
Osteoporosis is one of the most common noncommunicable diseases in today’s world. Hundreds of millions of people have osteoporosis, and many more are at risk (1). Osteoporosis is the most common metabolic bone disease in the world, the resulting fractures impose a huge medical and personal burden on the people who suffer from them and bring significant economic consequences (2). The most common fractures caused by osteoporosis are vertebral, proximal femur, and distal radius fractures, which severely affect a person’s quality of life (3). One of the most obvious symptoms of osteoporosis is fracture and pain caused by osteoporosis, which is one of the main reasons for reducing the quality of life of these patients (4). In osteoporosis, transient factors play a role in skeletal fragility, including defects in the properties of bone tissue materials, and defects in the microarchitecture of trabeculae (5).
Obesity may be harmful to bones. The process that induces osteoblastogenesis can inhibit adipogenesis and vice versa, for example, in menopause, there is a decrease in bone mass and an increase in fat at the same time (6). Factors that increase adipogenesis cause insufficient bone mass and osteoporosis because they both cause fatty marrow and inhibit osteoblastogenesis, which results in a decrease in the number of osteoblasts and bone formation (7). An increase in markers of bone resorption and a decrease in markers of bone formation in postmenopausal women with obesity were observed in studies (8). Leptin (obesity gene product), which is one of the most important cytokines known in fat tissue, is a 16 kDa protein that has a great effect on bone regeneration (9). The role of leptin in bone physiology is determined by the balance of direct and indirect effects on bone, such as anabolic effects on cartilage and osteoblasts and indirect effects on bone through the hypothalamus and sympathetic nervous system (10). However, it is not yet clear which effect of leptin (direct or indirect effect) regulates bone metabolism (9). Leptin can be an important biomarker for postmenopausal osteoporosis, so leptin can become a useful screening test for clinical follow-up of these patients (11).
Previous systematic review and meta-analysis results showed that leptin appears to be positively correlated with bone mineral density (BMD) in the whole body and with a higher pooled correlation in postmenopausal girls and women. However, serum leptin level was negatively correlated with BMD and bone mineral content (BMC) in boys. Conclusions may be difficult because the number of included studies on a specific population was small, and studies on different populations of different ethnicities were necessary to estimate the association between serum leptin levels and BMD and BMC values (12).
Given that the relationship between serum leptin levels and bone metabolism is complex, no definitive conclusions have been drawn on this issue (12, 13). As far as we know studies that have examined both human and animal studies to determine the relationship between leptin serum level and BMD is necessary to reach a more specific result.
Considering the ambiguities that exist regarding the effect of leptin on bone and bone-related diseases including osteoporosis, in this study, we decided to conduct a systematic review of various studies on the effect of leptin on osteoporosis, which may find an answer to the existing ambiguities.
Methods
Literature Search
A systematic literature search was performed to review studies on the effects of leptin on osteoporosis according to the Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines (14). Moreover, in this research, there was no limit on the publication date of the articles. The search was performed using several databases including Scopus, PubMed, web of science, and Google Scholar. Electronic searches were conducted on 5 Jan 2023.
This study was approved by the Ethics Committee of Abadan University of Medical Sciences (Ethical Code: IR.ABADANUMS.REC.1401.138).
Inclusion criteria
The inclusion criteria for this study included the following: All experimental studies involving human participants or animal subjects that investigated the association of leptin with osteoporosis, BMD, bone fractures, bone resorption or bone formation and whose data were available were considered eligible. Besides, one of the conditions for the inclusion of articles was that the full text of the article was available in English. These studies included little published empirical research.
The primary outcome was the investigation of leptin concentration in people with osteoporosis and the secondary outcome was the relationship between leptin with osteoporosis and BMD.
Exclusion criteria
In this study, the exclusion criteria were qualitative studies, insufficient information to extract the method and results of the study, studies without data of interest and inadequate design, poor methodological quality, not having the full text of the article in English, book chapters, review articles, case studies, or discussion articles, meta-analysis, theses, conference abstract, etc.
Study Selection
In this study, screening was performed by searching the terms “leptin”, “osteoporosis”, and “bone losses “. Studies were eligible for inclusion if they examined osteoporosis, leptin, or bone losses. Duplicate articles were identified and removed. Studies that investigated leptin and bone losses or osteoporosis were considered. The inclusion and exclusion criteria for screening the titles and abstracts of the remaining articles in this study were reviewed. Then the full-text screening of the eligible articles was done carefully. Finally, for more relevant studies, the list of references and citations of eligible articles were checked manually.
Data Extraction
Data was extracted from selected studies and prepared two types of standard data extraction forms, including for human and animal studies, the following items were mentioned in the form: characteristics of the study, participant characteristics (for human studies) and characteristics of the animals used (for animal studies), study design, and the main result of the study.
The performance and reporting quality of the articles presented for the evaluation of leptin and osteoporosis studies were evaluated by two independent reviewers (NS; TB), and the overall evaluation included recommendations for designing and conducting an evaluation of the role of leptin as a biomarker for osteoporosis. The extraction forms were compared by another reviewer (SMM) and differences were reviewed, discussed, and edited.
Quality Assessment
The risk of bias (quality) of human studies using quality assessment checklists for observational and cross-sectional cohort studies by the National Heart, Lung, and Blood Institute (NHLBI), which defines quality based on a set of 14 criteria, was evaluated (15). Quality was rated according to the NHLBI instrument as 0 for poor (0–4 out of 14 questions), I for fair (5–10 out of 14 questions), or II for good (11–14 out of 14 questions) (15). Moreover, the risk of bias for the animal study was assessed by the Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADES) checklist (16). In addition, since part of the studies in this research was animals, the study quality assessment was also reviewed according to the Animal Research: Reporting of in Vivo Experiments (ARRIVE) guidelines (17).
Results
Results of Study Selection
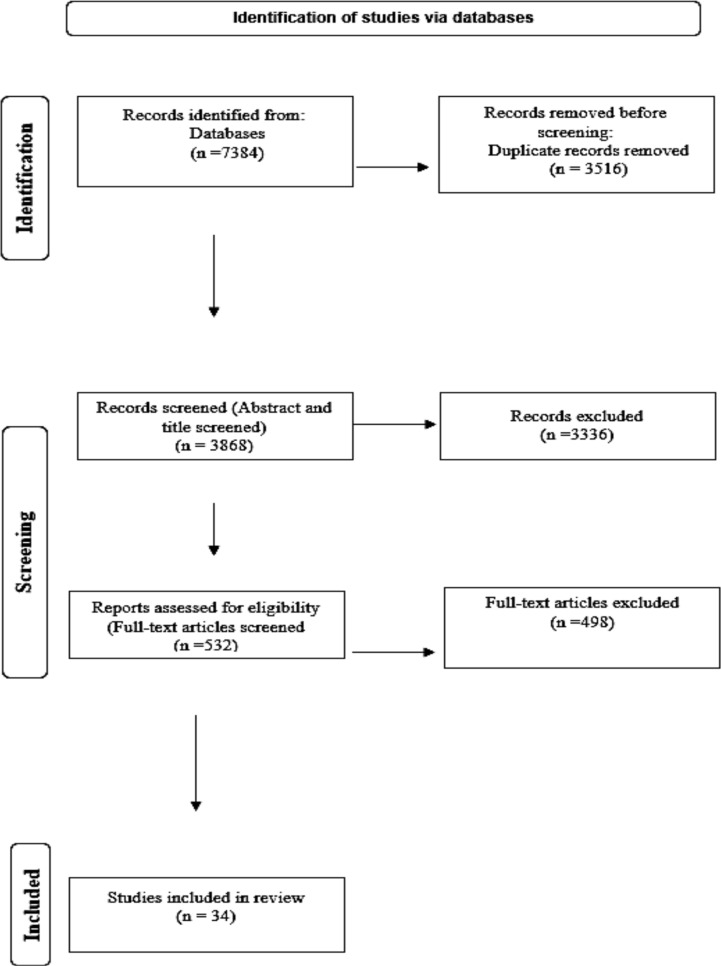
The database search retrieved 7384 records (PubMed=1980, Scopus=2208, Web of Science=3140, and Google scholar=56), of which 3516 were duplicated and removed in the first step. Titles and abstracts of the remaining 3868 studies were reviewed. We excluded 3336 articles that did not meet the inclusion criteria in this study. Upon review of the full text of the remaining 532 articles, 498 articles were excluded in the screening stage after the full-text review of the articles, and 34 articles were included in this study for data extraction and quality assessment. A PRISMA flow chart is shown in Fig. 1 to identify studies.
Fig. 1:
The Diagram of the literature search and selection process was limited to studies that investigated the relationship between osteoporosis and leptin
Overview of Included Studies
The articles included in this study were from seventeen countries, most of conducted in China (n=8) (18–24). The date of these studies was from 2000 to 2022. Human studies were 27 studies (18–21, 25–46), of which fifteen studies (11, 19–20, 25–36) were exclusively related to women, nine studies were conducted on both women and men (18, 21, 37–42), and three studies were exclusively conducted on men (43–46) (Table 1).
Table 1:
Characteristics of published human articles on the relationship of leptin to osteoporosis
| Authors | Year | Age (year)/sex | Leptin Dosage/measurement | Participants | Relationship of leptin with OP | Quality assessment |
|---|---|---|---|---|---|---|
| Shaarawy et al. (25) | 2003 | 58.8± 0.51/F | RIA | 90 POMP osteoporotic women and 30 healthy premenopausal women | Not associated | Good |
| Nakamura et al. (26) | 2020 | 65.9/F | RIA | 1167 POMP | Related | Good |
| Ye et al. (18) | 2022 | over 45 yr old/F/M | PCR | 738 Mulao minority people | Related | Fair |
| Crabbe et al. (43) | 2006 | 71–86/M | RIA | A sample of 352 ambulatory men | Related | Good |
| Saber et al. (37) | 2015 | 7.55± 3.34 F/M | RIA | 60 obese children and 36 lean children | Related | Good |
| Anagnostis et al. (44) | 2012 | 45.4±15/M | ELISA | Eighty-one male patients | Related | Good |
| Kocyigit et al. (27) | 2013 | 58.2±6.4/F | ELISA | 42 POMP women with osteoporosis and 37 healthy POMP women | Not associated | Good |
| Odabas et al. (28) | 2000 | 61.2±6 6.5/F | RIA | 50 POMP women with osteoporosis and 30 healthy POMP women | Not associated | Good |
| Prouteau et al. (38) | 2006 | 20±3.0/F/M | ELISA | 54 elite judoists | Related | Good |
| Lee et al. (29) | 2014 | 48 to 65/F | ELISA | 592 postmenopausal women aged | Related | Fair |
| Hess et al. (30) | 2005 | 65–86/F | 50 pM I125leptin | control (six) or osteoporotic (six) | Related | Fair |
| Lorentzon et al. (45) | 2006 | 18.9± 0.6/M | ELISA | 1068 men | Related | Fair |
| Yamauch et al. (31) | 2001 | 48–78/F | RIA | 139 postmenopausal women | Related | Fair |
| Pobeha (46) | 2011 | 62.2±7.3/F/M | ELISA | 21 patients without, and 18 with osteoporosis | Related | Fair |
| Mpalaris et al. (32) | 2016 | 46–80/F | RIA | 110 post-menopausal women | Related | Good |
| Zhang et al. (11) | 2022 | 45 to 80 /F | Multiplex kits | 1055 postmenopausal women | Related | Good |
| Rhie et al. (33) | 2010 | 8.3±1.2/F | RIA | 48 pre pubertal girls | Related | Fair |
| Zhang et al. (19) | 2009 | 70–86/F | 0.6 mg/ml | 17 postmenopausal women | Related | Fair |
| Hipmair et al. (39) | 2010 | 53–85 / F/M | RIA | 44 patients with osteoporosis | Related | Fair |
| Wang et al. (20) | 2022 | 61.00 /F | ECL | 75 Premenopausal females | Related | Good |
| Dennison et al. (40) | 2004 | 60–75/F/M | RIA | 170 men and 132 women | Related | Good |
| Sahin et al. (34) | 2003 | 55.1±6.3 /F | EIA | 100 POMP women | Not associated | Fair |
| Ardekani et al. (41) | 2014 | 54.5±15.5/F/M | ELISA | 81 with osteoporosis, 120 controls | Not associated | Fair |
| Ye et al. (21) | 2013 | 55.3±4.7/F/M | ELISA | 192 with osteoporosis, 221 control | Related | Good |
| Thomas et al. (42) | 2001 | F (21–93), M(23–90) | RIA | 137 premenopausal women, 165 POMP women, and 343 men | Related (women) | Fair |
| Roux et al. (35) | 2003 | 53 (45–68)/F | RIA | 121 postmenopausal women | Related | Good |
| Głogowska-Szeląg et al. (36) | 2019 | N/R/F | ELISA | 80 women with osteoporosis | Related | Fair |
Seven animal studies (22–24, 47–50) were included in the article, three studies were conducted only on females (23, 47, 48) and 4 studies were conducted on both females and males (22, 24, 49, 50). Four studies were conducted on rats (22–23, 47, 48), two studies on mice (49, 50), and one study on both (24) (Table 2).
Table 2:
Characteristics of published animal articles on the relationship of Leptin to osteoporosis
| Authors | Year | Model/sex | Leptin Dosage/measurement | Groups | Relationship of leptin with OP | Score |
|---|---|---|---|---|---|---|
| Ahmed et al. (47) | 2012 | SD rats/F | 400 μg kg b.wt.−1) three times weekly for six months | 40 (5 groups each comprised 8 rats ) | Related | 6 |
| Stavropoulou et al. (48) | 2005 | Wistar rats/F | EIO | 40 Ovariectomy (OVX) | Related | 7 |
| Sun et al. (22) | 2020 | SD rats/F/M | ELISA | 162 healthy, SPF-grade 6-month-old | Related | 7 |
| Zhang et al. (23) | 2013 | SD rats/F | ELISA | 30, 6-month-old: Sham, OVX, and PRN | Related | 7 |
| Zheng et al. (24) | 2017 | SD rats/BALB/c nude mice/F/M | ELISA | 50 SD/F, and 6 male BALB/c nude mice | Related | 6 |
| Reich et al. (49) | 2008 | Mice/F/M | N/R | 16 LGXSM recombinant inbred (RI) mouse strains | Related | 5 |
| McCabe et al. (50) | 2019 | Mice/F/M | ELISA | LepRb Δ65, LL and SS mice on the C57/Bl6background | Related | 6 |
Relationship between leptin and osteoporosis in the Human study
In most human studies, there was an association between plasma leptin levels and BMD values or with biomarkers of bone formation or bone resorption, including the studies mentioned. Among the important risk factors for the occurrence of long bone fractures are higher adiponectin levels and low leptin levels, and in the higher quartiles of serum leptin levels, the rate of long bone fractures was significantly lower (26). There is an association between susceptibility to osteoporosis and single nucleotide polymorphism (SNP) of the leptin receptor gene, and three of the six SNPs were different between the osteoporosis and control groups (18). In elderly men, the possible role of leptin in bone homeostasis independently of free estradiol and body composition was reported in studies (43). Increased leptin and parathyroid hormone (PTH) were observed in obese children, associated with 25(OH) D deficiency and bone turnover markers, and could play an important role in the pathogenesis of obesity and bone-related diseases such as fractures and osteoporosis (37). In men, there is a negative correlation between leptin levels and bone mass independent of body mass index (BMI) and body weight (44). In healthy adults, in the regulation of bone metabolism, leptin can play an effective role and can potentially play a role in preventing osteoporosis (38). In postmenopausal Korean women, the leptin receptor (LEPR) polymorphism can be one of the factors affecting femoral neck BMD, and polymorphism analysis can be effective in identifying women at risk of osteoporosis (29). Leptin binding capacity was lower in osteoporotic mesenchymal stem cells compared to control cells during adipogenic and osteogenic differentiation, so they showed less sensitivity to leptin action during early differentiation (30). Leptin can be an independent negative predictor of areal BMD in several measured locations and bone parameters that can reflect cortical bone size (45).
In postmenopausal women, leptin is associated with BMD and vertebral fractures, and leptin may play an important role in better bone quality and maintaining bone mass (31). Significantly, lower serum levels and expression of leptin in adipose tissue were observed in patients with osteoporosis compared to patients without osteoporosis (46). In women with osteoporosis, compared to women who did not have osteoporosis, the concentration of adiponectin is higher, but the concentration of leptin, BMI, and body weight are lower (32).
Leptin and IFG-1 have the potential to become important biomarkers related to postmenopausal osteoporosis (PMOP) and suitable and useful screening tests for clinical follow-up of these patients (11). Femur BMD, serum leptin was identified as a positive independent predictor (33). Leptin up regulated the expression of OPG (19). In postmenopausal women with osteoporosis, circulating leptin may cause bone loss and correlates with high-turnover serum bone markers (39). Leptin and IL-6 are predictive biomarkers of low bone mass in postmenopausal women (20).
Plasma leptin has a strong and positive correlation with volumetric BMD, and BMC, among elderly men and women (40). Polymorphism in LEPR and the leptin genes is associated with an increased risk of osteoporosis through increased expression of pro-inflammatory cytokines (21). A significant correlation between serum leptin level and BMD was observed in women, but not in men (42). Leptin correlated positively with the total hip and spine (35). Leptin can be considered a bone tissue protective factor in postmenopausal women (36). In a small number of studies, no correlation was observed between BMD, bone resorption and formation biomarkers, and leptin (25, 27, 28, 34, 41).
Relationship between leptin and osteoporosis in the animal study
Leptin provided a promising effect on bone through its direct action on bone and matrix mineralization (47). In estrogen-deficient adult mice, serum leptin levels showed a strong positive correlation with bone formation and markers of bone resorption, indicating that leptin is effective in maintaining bone mass (50). It has been observed that with increasing age, and with a decrease in the level of serum sex hormones, an increase in the expression of serum LEP and a decrease in the expression of leptin receptor occurs, which causes a decrease in bone quality, a disturbance in bone metabolism, a decrease in the ratio of OPG/RANKL, and also causes bone resorption may be more than bone formation (22). An increase in peripheral leptin was observed in the ovariectomized group, and treatment with propranolol could regulate the level of peripheral leptin (23).
Stimulation of periodontal regeneration in osteoporosis can occur by overexpression of human LEP in bone marrow stromal cells (BMSCs) (24). Gene effects associated with increased leptin levels are associated with stronger and larger femurs (49). The leptin receptor (LepRb) mutations cause obesity, osteoporosis, and stunting, similar to the phenotype of db/db and ob/ob mice, which completely lack leptin receptor signaling (50).
Quality assessment
In this study, the recommendations of the quality evaluation results in 27 human studies, 14 human studies were good and 13 were fair. Each animal study was given a quality score out of 10 possible points, and the median of the group was calculated as 6.
Discussion
This systematic review summarizes 27 human studies and seven animal studies that were conducted around the world on the relationship between leptin, and osteoporosis. High quality in human evidence (14 human studies were good and 13 were fair) suggests that leptin levels were low in people with osteoporosis. Besides, the moderate quality of the animal studies in this study also supports these results.
In postmenopausal women, a significant correlation was observed between plasma leptin level and BMD. Leptin level was significantly lower in women with vertebral fractures than in women without fractures. As a result, leptin can play an important role in better bone quality and maintaining bone mass (31). Findings from a longitudinal study suggest a possible role of leptin as a determinant of bone homeostasis in elderly men. The strengths of this study are the detailed clinical assessment of a community-dwelling study population and the prospective longitudinal BMD data and the possibility of adjustment for major confounders. However, these observations are limited to elderly men and should not be easily generalized to other age groups. Moreover, they seem to differ from the findings for women (43). Another longitudinal study has investigated POMP women, IL-6 and serum leptin can be predictors of the risk of osteoporosis-related fractures for postmenopausal women with low bone mass. This study also has certain limitations. First, only people from the Beijing community were included in this study and only two-year follow-up interviews were conducted, but the strength of this follow-up study was the relatively large sample size and the establishment of a fracture risk prediction model suitable for the clinical characteristics of Chinese postmenopausal women with low bone mass (20).
Another human study showed that serum leptin levels were significantly associated with BMD in women and that leptin regulation moderated the relationship between fat mass and bone density, but these results were not observed in men (42). Leptin can be considered a protective factor for bone tissue in postmenopausal women (36).
In the few human studies included in this study, no association between leptin and BMD was observed (25, 27, 28, 34, 41).
There is an association between decreased levels of leptin and BMD and thus the risk of developing osteoporosis in animal studies but the quality of the articles is moderate, and moderate-level evidence supports this response (22–24, 47–50).
A meta-analysis has been performed regarding the relationship between leptin and BMD. Blood leptin levels were significantly lower and adiponectin levels were significantly higher in subjects with PMOP compared to healthy subjects with normal BMD. Because this study lacked effective longitudinal cohort studies, the causality of the association between adipokines levels and the occurrence of PMOP was not inferred. There was considerable heterogeneity of results in this meta-analysis, which may significantly weaken the validity of the results. However, this meta-analysis had strong points, such as the relationships between three common adipokines with the occurrence of PMOP were investigated, and at the same time, the differences in the levels of adipokines and the relationships of different adipokines with BMD and BMI were estimated, and the quality of the included studies was average (13). Evidence from another systematic review and meta-analysis showed that leptin had a significant positive association with BMD and BMC in all populations, except in boys, especially in girls and postmenopausal women. There were limitations to this study, such as the sample size was not large enough and the number of studies included on a specific population was small. In this systematic review and meta-analysis, studies and the number of boy patients were very few. Moreover, studies related to different populations of different ethnicities were necessary to estimate the relationship between leptin serum levels and BMD values (12).
Among the limitations of this systematic review, the following can be mentioned: First, animal studies were few. Second, more human studies with a high sample size are needed. Third, most of the studies were reported on women, so there is a need to conduct studies on men as well as compare women and men with osteoporosis. Forth, the limitation of this study is the small number (4 studies) of prospective longitudinal studies, which suggests that more longitudinal studies should be conducted in both women and men in the future. Fifth, in this study, most of the studies were cross-sectional, prone to bias. Sixth, we did not register the formal protocol of this study in any public platform, but we followed the methodological framework recommended by the Cochrane Manual to reduce the risk of bias. Lastly, because the conditions or prerequisites for a statistical or quantitative combination were not available in this study, including the fact that the data of the articles were heterogeneous and did not have the ability to be combined and statistically analyzed, therefore meta-analysis was not performed. Studies with long-term follow-up and large sample sizes should be conducted to find higher quality evidence. The threshold of leptin level below which can cause osteoporosis was not known and more studies in this regard are necessary. Animal and human studies are needed for the therapeutic role of leptin and its effective dose. Among the strengths of this systematic review is the high quality of human studies reviewed from different parts of the world.
Conclusion
In most high-quality human studies, there was an association between plasma leptin levels and BMD values or with biomarkers of bone formation. There is moderate evidence in animal studies that leptin has a promising effect on bone through its direct effect on bone formation and matrix mineralization. The peripheral pathway of leptin of regulation bone metabolism is dominant and that leptin has a physiological role in maintaining bone mass and better bone quality. Leptin reduces bone marrow adipogenesis and increases BMD. As a result, leptin is effective in maintaining bone mass. Moreover, no correlation between leptin and BMD was observed. As a result, more studies are needed to determine whether leptin has the potential to become a useful screening test for osteoporosis. More studies with a larger sample size are needed to investigate the cellular and molecular mechanisms of the effect of leptin on osteoporosis.
Journalism Ethics considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Footnotes
Funding
This research was supported by Abadan University of Medical Sciences.
Conflict of interest
The authors declared no competing interest.
References
- 1.Carey JJ, Chih-Hsing Wu P, Bergin D. (2022). Risk assessment tools for osteoporosis and fractures in 2022. Best Pract Res Clin Rheumatol, 36(3):101775. [DOI] [PubMed] [Google Scholar]
- 2.LeBoff MS, Greenspan SL, Insogna KL, et al. (2022). The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int, 33(10):2049–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eastell R, O’Neill TW, Hofbauer LC, et al. (2016). Postmenopausal osteoporosis. Nat Rev Dis Primers, 2:16069. [DOI] [PubMed] [Google Scholar]
- 4.Men Z, Huang C, Xu M, Ma J, Wan L, Huang J, et al. (2022). Zhuanggu Zhitong Capsule alleviates postmenopausal osteoporosis in ovariectomized rats by regulating autophagy through AMPK/mTOR signaling pathway. Ann Transl Med, 10(16):900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armas LA, Recker RR. (2012). Pathophysiology of osteoporosis: new mechanistic insights. Endocrinol Metab Clin North Am, 41(3):475–86. [DOI] [PubMed] [Google Scholar]
- 6.Colaianni G, Brunetti G, Faienza MF, Colucci S, Grano M. (2014). Osteoporosis and obesity: Role of Wnt pathway in human and murine models. World J Orthop, 5(3):242–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadie-Van Gijsen H, Crowther NJ, et al. (2013). The interrelationship between bone and fat: from cellular see-saw to endocrine reciprocity. Cell Mol Life Sci, 70(13):2331–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.López-Gómez JJ, Pérez-Castrillón JL, García de Santos I, et al. (2022). Influence of Obesity on Bone Turnover Markers and Fracture Risk in Postmenopausal Women. Nutrients, 14(8):1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen XX, Yang T. (2015). Roles of leptin in bone metabolism and bone diseases. J Bone Miner Metab, 33(5):474–85. [DOI] [PubMed] [Google Scholar]
- 10.Reid IR, Baldock PA, Cornish J. (2018). Effects of Leptin on the Skeleton. Endocr Rev, 39(6):938–59. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Huang X, Sun K, Li M, Wang X, Han T, et al. (2022). The Potential Role of Serum IGF-1 and Leptin as Biomarkers: Towards Screening for and Diagnosing Postmenopausal Osteoporosis. J Inflamm Res, 15:533–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu K, Liu P, Liu R, Wu X, Cai M. (2015). Relationship between serum leptin levels and bone mineral density: a systematic review and meta-analysis. Clin Chim Acta, 444:260–263. [DOI] [PubMed] [Google Scholar]
- 13.Shu L, Fu Y, Sun H. (2022). The association between common serum adipokines levels and postmenopausal osteoporosis: A meta-analysis. J Cell Mol Med, 26(15):4333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page MJ, McKenzie JE, Bossuyt PM, et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ, 372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institutes for Health (2021). Study quality assessment tool for observational cohort and cross-sectional studies.
- 16.Macleod MR, O’Collins T, Howells DW, et al. (2004). Pooling of animal experimental data reveals influence of study design and publication bias. Stroke, 35(5):1203–8. [DOI] [PubMed] [Google Scholar]
- 17.Percie du Sert N, Ahluwalia A, Alam S, et al. (2020). Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol, 18(7):e3000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye G, Huang Y, Yin L, Wang J, Huang X, Bin X. (2022). Association between LEPR polymorphism and susceptibility of osteoporosis in Chinese Mulao people. Artif Cells Nanomed Biotechnol, 50(1):10–16. [DOI] [PubMed] [Google Scholar]
- 19.Zhang ZM, Jiang LS, Jiang SD, Dai LY. (2009). Osteogenic potential and responsiveness to leptin of mesenchymal stem cells between postmenopausal women with osteoarthritis and osteoporosis. J Orthop Res, 27(8):1067–73. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Zhang Y, Qi B, et al. (2022). IL-6 and Leptin Are Potential Biomarkers for Osteoporotic Fracture Risk Assessment and Prediction of Postmenopausal Women with Low Bone Mass: A Follow-Up Study Using a Regional Sample Cohort. Oxid Med Cell Longev, 2022:8691830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye XL, Lu CF. (2013). Association of polymorphisms in the leptin and leptin receptor genes with inflammatory mediators in patients with osteoporosis. Endocrine, 44(2):481–8. [DOI] [PubMed] [Google Scholar]
- 22.Sun P, Zhang Y, Wei Z, Wang Z, Guo S, Lin Y. (2020). Effect of Qing’e Decoction on Leptin/Leptin Receptor and Bone Metabolism in Naturally Aging Rats. Evid Based Complement Alternat Med, 2020:2532081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Xiaofeng LV, Zhang Y, Jiao X, Chen B. (2013). Propranolol prevents osteoporosis and up-regulates Leptin in ovariectomized rats. Iran J Pharm Res, 12(3):557–62. [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng B, Jiang J, Chen Y, et al. (2017). Leptin Overexpression in Bone Marrow Stromal Cells Promotes Periodontal Regeneration in a Rat Model of Osteoporosis. J Periodontol, 88(8):808–818. [DOI] [PubMed] [Google Scholar]
- 25.Shaarawy M, Abassi AF, Hassan H, Salem ME. (2003). Relationship between serum leptin concentrations and bone mineral density as well as biochemical markers of bone turnover in women with postmenopausal osteoporosis. Fertil Steril, 79(4):919–24. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura Y, Nakano M, Suzuki T, et al. (2020). Two adipocytokines, leptin and adiponectin, independently predict osteoporotic fracture risk at different bone sites in postmenopausal women. Bone, 137:115404. [DOI] [PubMed] [Google Scholar]
- 27.Koçyiǧit H, Bal S, Atay A, et al. (2013). Plasma leptin values in postmenopausal women with osteoporosis. Bosn J Basic Med Sci, 13(3):192–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Odabaşi E, Ozata M, Turan M, et al. (2000). Plasma leptin concentrations in postmenopausal women with osteoporosis. Eur J Endocrinol, 142(2):170–3. [DOI] [PubMed] [Google Scholar]
- 29.Lee HJ, Kim H, Ku SY, et al. (2014). Association between polymorphisms in leptin, leptin receptor, and β-adrenergic receptor genes and bone mineral density in postmenopausal Korean women. Menopause, 21(1):67–73. [DOI] [PubMed] [Google Scholar]
- 30.Hess R, Pino AM, Ríos S, Fernández M, Rodríguez JP. (2005). High affinity leptin receptors are present in human mesenchymal stem cells (MSCs) derived from control and osteoporotic donors. J Cell Biochem, 94(1):50–7. [DOI] [PubMed] [Google Scholar]
- 31.Yamauchi M, Sugimoto T, Yamaguchi T, et al. (2001). Plasma leptin concentrations are associated with bone mineral density and the presence of vertebral fractures in postmenopausal women. Clin Endocrinol (Oxf), 55(3):341–7. [DOI] [PubMed] [Google Scholar]
- 32.Mpalaris V, Anagnostis P, Anastasilakis AD, et al. (2016). Serum leptin, adiponectin and ghrelin concentrations in post-menopausal women: Is there an association with bone mineral density? Maturitas, 88:32–6. [DOI] [PubMed] [Google Scholar]
- 33.Rhie YJ, Lee KH, Chung SC, et al. (2010). Effects of body composition, leptin, and adiponectin on bone mineral density in prepubertal girls. J Korean Med Sci, 25(8):1187–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Şahin G, Polat G, Baoiş S, et al. (2003). Body composition, bone mineral density, and circulating leptin levels in postmenopausal Turkish women. Rheumatol Int, 23(2):87–91. [DOI] [PubMed] [Google Scholar]
- 35.Roux C, Arabi A, Porcher R, Garnero P. (2003). Serum leptin as a determinant of bone resorption in healthy postmenopausal women. Bone, 33(5):847–52. [DOI] [PubMed] [Google Scholar]
- 36.Głogowska-Szeląg J, Kos-Kudła B, Marek B, et al. (2019). Assessment of selected adipocytokines in obese women with postmenopausal osteoporosis. Endokrynol Pol, 70(6):478–83. [DOI] [PubMed] [Google Scholar]
- 37.Saber LM, Mahran HN, Baghdadi HH, et al. (2015). Interrelationship between bone turnover markers, calciotropic hormones and leptin in obese Saudi children. Eur Rev Med Pharmacol Sci, 19(22):4332–43. [PubMed] [Google Scholar]
- 38.Prouteau S, Benhamou L, Courteix D. (2006). Relationships between serum leptin and bone markers during stable weight, weight reduction and weight regain in male and female judoists. Eur J Endocrinol, 154(3):389–95. [DOI] [PubMed] [Google Scholar]
- 39.Hipmair G, Böhler N, Maschek W, et al. (2010). Serum leptin is correlated to high turnover in osteoporosis. Neuro Endocrinol Lett, 31(1):155–60. [PubMed] [Google Scholar]
- 40.Dennison EM, Syddall HE, Fall CH, et al. (2004). Plasma leptin concentration and change in bone density among elderly men and women: the Hertfordshire Cohort Study. Calcif Tissue Int, 74(5):401–6. [DOI] [PubMed] [Google Scholar]
- 41.Mohiti-Ardekani J, Soleymani-Salehabadi H, Owlia MB, Mohiti A. (2014). Relationships between serum adipocyte hormones (adiponectin, leptin, resistin), bone mineral density and bone metabolic markers in osteoporosis patients, J Bone Miner Metab, 32(4):400–4. [DOI] [PubMed] [Google Scholar]
- 42.Thomas T, Burguera B, Melton Iii LJ, et al. (2001). Role of serum leptin, insulin, and estrogen levels as potential mediators of the relationship between fat mass and bone mineral density in men versus women. Bone, 29(2):114–20. [DOI] [PubMed] [Google Scholar]
- 43.Crabbe P, Goemaere S, Zmierczak H, et al. (2006). Are serum leptin and the Gln223Arg polymorphism of the leptin receptor determinants of bone homeostasis in elderly men? Eur J Endocrinol, 154(5):707–14. [DOI] [PubMed] [Google Scholar]
- 44.Anagnostis P, Vakalopoulou S, Charizopoulou M, et al. (2013). Is there any association between leptin levels and bone mineral density in haemophiliac men? Arch Med Sci, 9(3):459–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lorentzon M, Landin K, Mellström D, Ohlsson C. (2006). Leptin is a negative independent predictor of areal BMD and cortical bone size in young adult Swedish men. J Bone Miner Res, 21(12):1871–8. [DOI] [PubMed] [Google Scholar]
- 46.Pobeha P, Ukropec J, Skyba P, et al. (2011). Relationship between osteoporosis and adipose tissue leptin and osteoprotegerin in patients with chronic obstructive pulmonary disease. Bone, 48(5):1008–14. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed HH, Morcos NY, Eskander EF, et al. (2012). Potential role of leptin against glucocorticoid-induced secondary osteoporosis in adult female rats. Eur Rev Med Pharmacol Sci, 16(10):1446–52. [PubMed] [Google Scholar]
- 48.Stavropoulou A, Christopoulou GE, Anastassopoulos G, et al. (2005). Alteration in serum leptin correlates with alterations in serum N-telopeptide of collagen type I and serum osteocalcin during the progression of osteoporosis in ovariectomized rats. Clin Chem Lab Med, 43(12):1359–65. [DOI] [PubMed] [Google Scholar]
- 49.Reich MS, Jarvis JP, Silva MJ, Cheverud JM. (2008). Genetic relationships between obesity and osteoporosis in LGXSM recombinant inbred mice. Genet Res (Camb), 90(5):433–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCabe IC, Fedorko A, Myers MG, Jr., Leinninger G, Scheller E, McCabe LR. (2019). Novel leptin receptor signaling mutants identify location and sex-dependent modulation of bone density, adiposity, and growth. J Cell Biochem, 120(3):4398–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]