Abstract
Aims
This study aimed to evaluate the global research trend in the prevention and treatment of cardiotoxicity caused by anthracyclines from 2000 to 2023, and to explore international cooperation, research hotspots, and frontier trends.
Methods
The articles on the prevention and treatment of anthracycline-induced cardiotoxicity published from 2000 to 2023 were searched by Web of Science. The bibliometrics software CiteSpace was used for visual analysis of countries, institutions, journals, authors, cited authors, cited references, and keywords.
Results
This study analyzed the current status of global research on the prevention and treatment of cardiotoxicity caused by anthracyclines. A total of 3,669 papers were searched and 851 studies were included. The number of publications increased gradually throughout the years. Cardiovascular Toxicology (15) is the journal with the most publications. Circulation (547) ranked first among cited journals. In this field, the country with the most publications is the United States (229), and the institution with the most publications is Charles Univ Prague (18). In the analysis of the authors, Tomas S (10) ranked first. Cardinale D (262) ranked first among cited authors. In the ranking of cited literature frequency, the article ranked first is “Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy” (121). The keywords “heart failure” (215) and “oxidative stress” (212) were the most frequent. “Enalapril”, “inflammation”, “cell death”, “NF-κB” and “Nrf2” were the advanced research contents in 2019–2023.
Conclusions
This study provided valuable information for cardio-oncology researchers to identify potential collaborators and institutions, discover hot topics, and explore new research directions. The prevention and treatment of anthracycline-induced cardiotoxicity focuses on early detection and timely treatment. The results of the current clinical studies on the treatment of anthracycline-induced cardiotoxicity are contradictory, and more studies are needed to provide more reliable clinical evidence in the future.
Keywords: Anthracyclines, Cardiotoxicity, Bibliometric analysis, CiteSpace
1. Introduction
As global cancer survival rates improve, cardiovascular disease is now the second leading cause of long-term morbidity and mortality among cancer survivors [1]. Radiation therapy, especially chest radiation therapy, antineoplastic drugs such as anthracyclines, human epidermal growth factor receptor 2 (HER2) targeted agents, mitogen-activated protein kinase (MEK) inhibitors inhibitors, multitargeted kinase inhibitors, epidermal growth factor receptor (EGFR) inhibitors, immune checkpoint inhibitors, etc. all can cause cardiotoxicity in oncology patients [2,3]. Cardiovascular complications caused by antitumor therapy mainly include nine categories: myocardial insufficiency and heart failure (HF), coronary artery disease, valvular disease, arrhythmia, hypertension, embolic disease, peripheral vascular disease and stroke, pulmonary hypertension, and pericardial complications [4].
The chemotherapeutic drugs that most often induce cardiotoxicity in clinical practice are anthracyclines. Anthracyclines [5] are commonly used in the treatment of solid tumors, breast cancer, soft tissue sarcoma, lymphoma, leukemia, and other cancers. On the downside, the drug can damage the heart and its substructures with chemotherapy in a dose-dependent manner, increasing the incidence of congestive heart failure (CHF) by 4.7 %, 26 %, and 48 % at cumulative doses of 400, 550, and 700 mg/m2 of Doxorubicin (DOX), respectively [6], and their therapeutic efficacy is limited by cumulative cardiotoxicity [7]. The high morbidity and mortality of cardiotoxicity caused by antitumor therapy in patients with malignant tumors cannot be ignored [8]. Therefore, the prevention and treatment of cardiotoxicity caused by antineoplastic therapy is a common challenge for oncologists and cardiologists.
CiteSpace [9,10] is a visual bibliometric analysis tool under the Java language environment developed by Professor Chaomei Chen from Drexel University in the United States. The software contains various functions such as cooperation map, co-occurrence map, co-citation map, and burst words detection, which can discover and dig the research hotspot, evolution path, and development trend in a certain field. Currently, anthracycline-induced cardiotoxicity has received increasing attention. In recent years, the safety and efficacy of the prevention and treatment of cardiotoxicity caused by anthracyclines have been supported by clinical and experimental studies. There have been more than 7,000 studies of anthracycline-induced cardiotoxicity in the last 20 years, but such bibliometric studies have focused more on anthracycline-induced cardiotoxicity as a whole, with fewer descriptions of prevention and treatment. Our study explored the current status and trend of the prevention and treatment of cardiotoxicity induced by anthracyclines in the past 20 years through visualized bibliometric analysis, in order to provide a reference for further research.
2. Methods
2.1. Data collection
The bibliometric analysis relies on the Web of Science literature databases, including the Science Citation Index Expand (SCI-Expand), Social Sciences Citation Index (SSCI), and Conference Proceedings Citation Index-Science (CPCI-S). Search terms include “anthracycline*” and “cardiotoxicit*”, “cardiac toxicit*”, “toxicity, cardiac”, “cardiac damage*”, “cardiac injur*”, “heart damage”, “arrhythmia*”, “angina pectoris”, “myocardial ischemia”, “acute myocardial infarction”, “heart failure”, “left ventricular dysfunction”, “chronic cardiac failure” and “patient*”, “cell*”, “client*”, “rat*”, “mice”, “mouse”. The search period was from 2000 to 01-01 to 2023-02-12. The data was downloaded on February 12, 2023. Web of Science records the full record of the article and the references saved in plain text file format.
2.2. Inclusion criteria
Inclusion criteria were: (1) literature on the prevention and treatment of anthracycline cardiotoxicity, including clinical and basic research; (2) document type: articles and reviews; (3) literature published from 2000 to 2023.
2.3. Exclusion criteria
Exclusion criteria were: (1) duplicate articles; (2) non-public published articles; (3) data missing articles; (4) irrelevant articles.
2.4. Analysis tool
CiteSpace [9]contains a variety of bibliometric analysis methods, including co-authors, co-institutions, co-countries, co-occurring keywords, and co-citation analyses, and is capable of generating visualizations. The visual knowledge graph consists of nodes and links. Different nodes in the graph represent different elements, such as an author, an institution, a country, or a reference. Nodes are represented by circles. The size of the circle represents the frequency of node references. Different colors of nodes represent different years. The lighter the color, the earlier the reference year, and the lightest is white. The darker the color, the closer the reference year, and the darkest is red. Links between nodes represent co-cooperation, co-occurrence, or co-citation relationships. The centrality is an indicator to evaluate the importance of a node in the network. The value ranges from 0 to 1. It is generally considered that centrality >0.1 is a meaningful central media.
The CiteSpace version used in this study is 6.1 R6 (64-bit). The CiteSpace parameters are set as follows: time slicing (2000–2023), years per slice (1), term source (all selection), node types (choose one at a time), top n select (50), pruning (pathfinder).
3. Results
3.1. Annual publications
A total of 3,669 relevant literature were retrieved, and 851 studies were included; The research process of literature is shown in Fig. 1.
Fig. 1.
Literature search process.
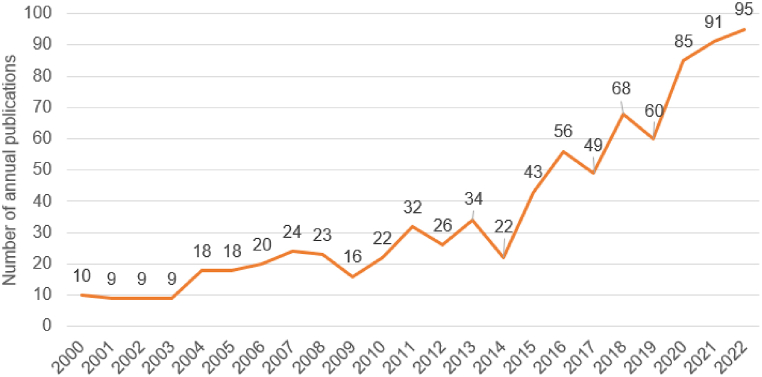
The annual volume of published literature is shown in Fig. 2. The number of literature increased from 10 in 2000 to 95 in 2022, showing a certain fluctuation. The published trend can be divided into three stages: the initial stage (2000–2003) had a steady increase; in the steady growth stage (2004–2008) the number of papers published began to increase gradually; in the rapid growth stage (2009–2022), the number of papers published first experienced a short-term decrease, followed by a zigzag rapid growth, and reached its peak in 2022.
Fig. 2.
The number of publications from 2000 to 2022.
4. Analysis of journals and cited journals
851 literature on the prevention and treatment of cardiotoxicity caused by anthracyclines were published in 381 journals. Most journals are classified as Pharmacology & Pharmacy (226). Others include Cardiac & Cardiovascular Systems (136), Oncology (120), Toxicology (68), Biochemistry & Molecular Biology (50). The top 5 journals ranked by number of publications are shown in Table 1. Cardiovascular Toxicology ranked first in the frequency of journals, and Biomedicine & Pharmacotherapy ranked first in impact factors of journals. Three journals had an impact factor greater than 5, with an average impact factor of about 5.58.
Table 1.
The top five journals with the highest frequency.
| Ranking | Frequency | Journal | IF 2021 |
|---|---|---|---|
| 1 | 15 | Cardiovascular Toxicology | 2.755 |
| 2 | 12 | Biomedicine & Pharmacotherapy | 7.419 |
| 3 | 12 | Frontiers in Cardiovascular Medicine | 5.846 |
| 4 | 12 | Oxidative Medicine and Cellular Longevity | 7.310 |
| 5 | 12 | Toxicology | 4.571 |
Co-citation journals refer to journals cited jointly by other researchers. Co-citation analysis can obtain the distribution of key knowledge sources in a certain field. Table 2, Table 3 lists the five cited journals with the highest frequency and centrality of research on the prevention and treatment of cardiotoxicity caused by anthracyclines. The most frequently cited Journal was Circulation, and the journal with the highest centrality was American Heart Journal.
Table 2.
The top five cited journals with the highest frequency.
| Ranking | Frequency | Cited journals | IF 2021 |
|---|---|---|---|
| 1 | 547 | Circulation | 39.918 |
| 2 | 467 | Journal of Clinical Oncology | 50.717 |
| 3 | 405 | Journal of the American College of Cardiology | 27.203 |
| 4 | 398 | Journal of Molecular and Cellular Cardiology | 5.763 |
| 5 | 382 | Cancer research | 13.312 |
Table 3.
The top five cited journals with the highest centrality.
| Ranking | Centrality | Cited journals | IF 2021 |
|---|---|---|---|
| 1 | 0.08 | American Heart Journal | 5.099 |
| 2 | 0.06 | Biochimica et Biophysica Acta - Molecular Basis of Disease | 6.633 |
| 3 | 0.05 | Analytical Biochemistry | 3.191 |
| 4 | 0.05 | Annals of the New York Academy of Sciences | 6.499 |
| 5 | 0.05 | American Journal of Clinical Oncology - CANCER CLINICAL TRIALS | 2.787 |
4.1. Distribution of countries and institutions
CiteSpace was used to generate the country distribution map, and 64 nodes and 138 links were obtained (Fig. 3). Nodes represent countries, and links between nodes represent cooperation between countries. The larger the node, the more the number of published articles. Similarly, the wider the link, the closer the cooperation between countries. 851 articles were published by research teams from 64 countries. The top five countries for the frequency and centrality of publications are shown in Table 4. China published the most articles in Asia, and the United States published the most in North America. The centrality of the top five countries was all greater than 0.1, indicating that these countries have a certain influence in the study of the prevention and treatment of cardiotoxicity caused by anthracyclines.
Fig. 3.
A country cooperation map from 2000 to 2023.
Table 4.
The top five countries with the highest frequency and centrality.
| Ranking | Frequency | Country | Ranking | Centrality | Country |
|---|---|---|---|---|---|
| 1 | 229 | USA | 1 | 0.68 | USA |
| 2 | 178 | People R China | 2 | 0.12 | France |
| 3 | 81 | Italy | 3 | 0.11 | Canada |
| 4 | 62 | Canada | 4 | 0.11 | Egypt |
| 5 | 34 | Egypt | 5 | 0.11 | Saudi Arabia |
The research institutions for the prevention and treatment of anthracycline cardiotoxicity are mainly concentrated in universities. The number of publications and the ranking of centrality suggested that Charles University and Boston Children's Hospital were the main research institutions (Table 5).
Table 5.
The top five institutions with the highest frequency and centrality.
| Ranking | Frequency | Institution | Ranking | Centrality | Institution |
|---|---|---|---|---|---|
| 1 | 18 | Charles University | 1 | 0.04 | Boston Children's Hospital |
| 2 | 15 | University of Manitoba | 2 | 0.03 | University of Toronto |
| 3 | 10 | Beijing University of Chinese Medicine | 3 | 0.03 | Harvard University |
| 4 | 8 | China Medical University | 4 | 0.03 | University of Florida |
| 5 | 7 | University of Toronto | 5 | 0.03 | Baylor College Medicine |
4.2. Analysis of authors and cited authors
CiteSpace was used to construct the co-author map of literature, including 697 nodes and 965 links (Fig. 4). The size of nodes represents the number of authors' published articles, and the thickness of links between nodes represents the cooperation intensity between authors. The top five authors for publications are shown in Table 6. The author with the highest number of publications is Tomas Simunek, who together with Martin S and Vladimir G found that iron-mediated reactive oxygen species (ROS) is a key cause of chronic cardiotoxicity. Dexpropylenimine acts as an iron chelator shielding intracellular free iron thus exerting cardioprotective effects [11]. Michaela A found that the strong iron chelator o-108 can protect the cardiotoxicity caused by daunorubicin, but increasing the dose of o-108 failed to do so, suggesting that there are more complex mechanisms other than iron-mediated mechanisms for anthracycline-induced cardiotoxicity [12].
Fig. 4.
A co-author map from 2000 to 2023.
Table 6.
The top five authors with the highest frequency.
| Ranking | Frequency | Author | Institution | Country |
|---|---|---|---|---|
| 1 | 10 | Tomas S | Charles University | Czech Republic |
| 2 | 9 | Michaela A | Charles University | Czech Republic |
| 3 | 7 | Steven EL | Wayne State University | USA |
| 4 | 7 | Martin S | Charles University | Czech Republic |
| 5 | 6 | Vladimir G | Charles University | Czech Republic |
Generating a co-citation author graph with 869 nodes and 3507 links by CiteSpace (Fig. 5). The most frequently cited author was Cardinale D (Table 7), and the top centrality author is Ewer MS (Table 8). They focus on the early detection and timely treatment of anthracycline-induced cardiotoxicity. Lipshultz SE and Minotti G were core researchers. Lipshultz SE mainly engaged in the toxic effects of anthracyclines in childhood cancer patients. His research proves that cumulative dose increase of DOX is an independent risk of late cardiotoxicity factors, with increased incidence and severity of cardiac abnormalities over time [13]. Minotti G summarizes the role of iron and free radicals in anthracycline-induced cardiotoxicity on apoptosis or other forms of cardiac damage. He also described the pharmacology and clinical recommendations of cardioprotectants such as antioxidants and iron chelators, and advocated the development of targeted anthracyclines to reduce cardiotoxicity [14].
Fig. 5.
A cited author map from 2000 to 2023.
Table 7.
The top five cited authors with the highest frequency.
| Ranking | Frequency | Cited author | Institution | Country |
|---|---|---|---|---|
| 1 | 262 | Cardinale D | European Institute of Oncology | Italy |
| 2 | 239 | Swain SM | Comprehensive Breast Center | USA |
| 3 | 214 | Lipshultz SE | Department of Cardiology, Children's Hospital | USA |
| 4 | 206 | Minotti G | G. d'Annunzio University School | Italy |
| 5 | 163 | Zhang S | The University of Texas MD Anderson Cancer Center | USA |
Table 8.
The top five cited authors with the highest centrality.
| Ranking | Centrality | Cited author | Institution | Country |
|---|---|---|---|---|
| 1 | 0.13 | Ewer MS | The University of Texas | USA |
| 2 | 0.10 | Olson RD | University of Washington | USA |
| 3 | 0.09 | Vonhoff DD | National Cancer Institute Bethesda | USA |
| 4 | 0.08 | Minotti G | G. d'Annunzio University School | Italy |
| 5 | 0.07 | Lipshultz SE | Department of Cardiology, Children's Hospital | USA |
4.3. Analysis of cited references
The top 5 cited references in terms of frequency and centrality are listed in Table 9, Table 10. Sorted by frequency of cited references, most of them are original research literature, and sorted by centrality of cited references, most of them are review articles.
Table 9.
The top five cited references for the highest frequency.
| Ranking | Cited reference | Frequency | Representative author | Publication year | Journal | Research content |
|---|---|---|---|---|---|---|
| 1 | Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy | 121 | Cardinale D | 2015 | Circulation | Cardiotoxicity after anthracycline treatment occurs mostly within 1 year, is related to anthracycline dose, that early detection and timely treatment are essential for recovery of cardiac function [15]. |
| 2 | Identification of the molecular basis of doxorubicin-induced cardiotoxicity | 105 | Zhang S | 2012 | Nature Medicine | The specific knockout of topoisomerase-IIβ in cardiomyocytes can protect cardiomyocytes from doxorubicin-induced DNA double-strand breaks and transcriptome changes, which are the cause of mitochondrial ROS production [16]. |
| 3 | Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial | 73 | Bosch X | 2013 | Journal of the American College of Cardiology | Combined treatment with angiotensin-converting enzyme inhibitors (ACEI) and beta-blockers may prevent left ventricular systolic function in patients with malignant hemopathies treated with anthracycline [17]. |
| 4 | Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol | 72 | Gulati G | 2016 | European Heart Journal | In patients treated for early breast cancer with anthracycline, concomitant treatment with angiotensin receptor blocker (ARB) provides protection against an early decline in global left ventricular function [18]. |
| 5 | Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities | 67 | Vejpongsa P | 2014 | Journal of the American College of Cardiology | Topoisomerase-IIβ may serve as a major molecular target for cardioprotection [19]. |
Table 10.
The top five cited references for the highest centrality.
| Ranking | Cited reference | Centrality | Representative author | Publication year | Journal | Research content |
|---|---|---|---|---|---|---|
| 1 | Role of cardioprotective therapy for prevention of cardiotoxicity with chemotherapy: a systematic review and meta-analysis | 0.18 | Kalam K | 2013 | European Journal of Cancer | Dexrazoxane, β-blockers, statins and ACEI can reduce cardiac events and have similar efficacy [20]. |
| 2 | American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants | 0.18 | Hensley ML | 2009 | Journal of Clinical Oncology | Hensley ML defined the conditions for the use of dexrazoxane in breast cancer and other malignant tumors in the American Society of Clinical Oncology 2008 clinical practice guideline [21]. |
| 3 | Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation | 0.15 | Ichikawa Y | 2014 | The Journal of Clinical Investigation | The cardiotoxic effects of doxorubicin develop from mitochondrial iron accumulation and that reducing mitochondrial iron levels protects against doxorubicin-induced cardiomyopathy [22]. |
| 4 | Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity | 0.14 | Minotti G | 2004 | Pharmacological Reviews | A review of progress that may serve as a framework for reappraising the activity and toxicity of anthracyclines on basic and clinical pharmacology grounds [14]. |
| 5 | Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction | 0.13 | Pacher P | 2003 | Circulation | Targeted inhibition of peroxynitrite synthesis may be a new cardioprotective strategy [23]. |
“Burst words” refer to the key cited references whose citations change dramatically in a certain period of time, which represents the development trend of a certain scientific research field. It can be used to analyze different nodes such as cited references and keywords, the parameter γ value can limit the result of the emergence; the smaller the value, the more the result, generally choose between 0.5 and 0.8; minimum duration is the time of the emergence of the burst, the smaller the value the more the result, the default is two years.
Burst words analysis was performed on the cited references of the prevention and treatment of cardiotoxicity caused by anthracyclines, and the top 5 literature ranked by Strength were listed, as shown in Table 11.
Table 11.
The top five cited references by the strength of burst words analysis.
| Ranking | Cited reference | Strength | Representative author | Publication year | Journal | Research content |
|---|---|---|---|---|---|---|
| 1 | Carvedilol for Prevention of Chemotherapy-Related Cardiotoxicity: The CECCY Trial | 15.25 | Avila MS | 2018 | Journal of the American College of Cardiology | Carvedilol has no effect on reducing the incidence of premature LVEF, but can reduce anthracycline-induced troponin levels and diastolic dysfunction [24]. |
| 2 | Anthracycline Chemotherapy and Cardiotoxicity | 11.13 | McGowan JV | 2017 | Cardiovascular Drugs and Therapy | Mechanism of cardiotoxicity induced by Anthracycline [25]. |
| 3 | Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy | 10.89 | Cardinale D | 2015 | Circulation | Cardiotoxicity after anthracycline treatment occurs mostly within 1 year, is related to anthracycline dose, that early detection and timely treatment are essential for recovery of cardiac function [15]. |
| 4 | Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline | 10.50 | Armenian SH | 2017 | Journal of Clinical Oncology | A guideline on the prevention and monitoring of cardiac function in adult cancer patients [26]. |
| 5 | Oxidative stress injury in doxorubicin-induced cardiotoxicity | 10.35 | Songbo M | 2019 | Toxicology Letters | Summarize the different pathogenesis of DOX-induced cardiotoxicity, and cardioprotective therapies that address these mechanisms [27]. |
4.4. Analysis of keywords
CiteSpace was used to generate a co-occurrence map of keywords, which included 526 nodes and 2,533 links (Fig. 6). “Heart failure” and “oxidative stress” were the most frequently used keywords (Table 12). Anthracycline therapy enhances survival in cancer patients, but also increases the risk of cardiac dysfunction, with the most common type of cardiotoxicity being HF, and oxidative stress has been identified as a major cause of anthracycline cardiotoxicity.
Fig. 6.
A keyword co-occurrence map from 2000 to 2023.
Table 12.
The top 10 keywords with the highest frequency and centrality.
| Ranking | Frequency | Keyword | Ranking | Centrality | Keyword |
|---|---|---|---|---|---|
| 1 | 215 | Heart failure | 1 | 0.11 | Anthracycline cardiotoxicity |
| 2 | 212 | Oxidative stress | 2 | 0.11 | Congestive heart failure |
| 3 | 140 | Breast cancer | 3 | 0.10 | Cardiotoxicity |
| 4 | 123 | Anthracycline cardiotoxicity | 4 | 0.10 | Adriamycin induced cardiomyopathy |
| 5 | 106 | Doxorubicin | 5 | 0.09 | Adriamycin |
| 6 | 104 | Cardiotoxicity | 6 | 0.09 | Activation |
| 7 | 101 | Chemotherapy | 7 | 0.09 | Acute lymphoblastic leukemia |
| 8 | 97 | Induced cardiotoxicity | 8 | 0.08 | Doxorubicin |
| 9 | 91 | Therapy | 9 | 0.08 | Apoptosis |
| 10 | 89 | Induced cardiomyopathy | 10 | 0.08 | Doxorubicin induced cardiotoxicity |
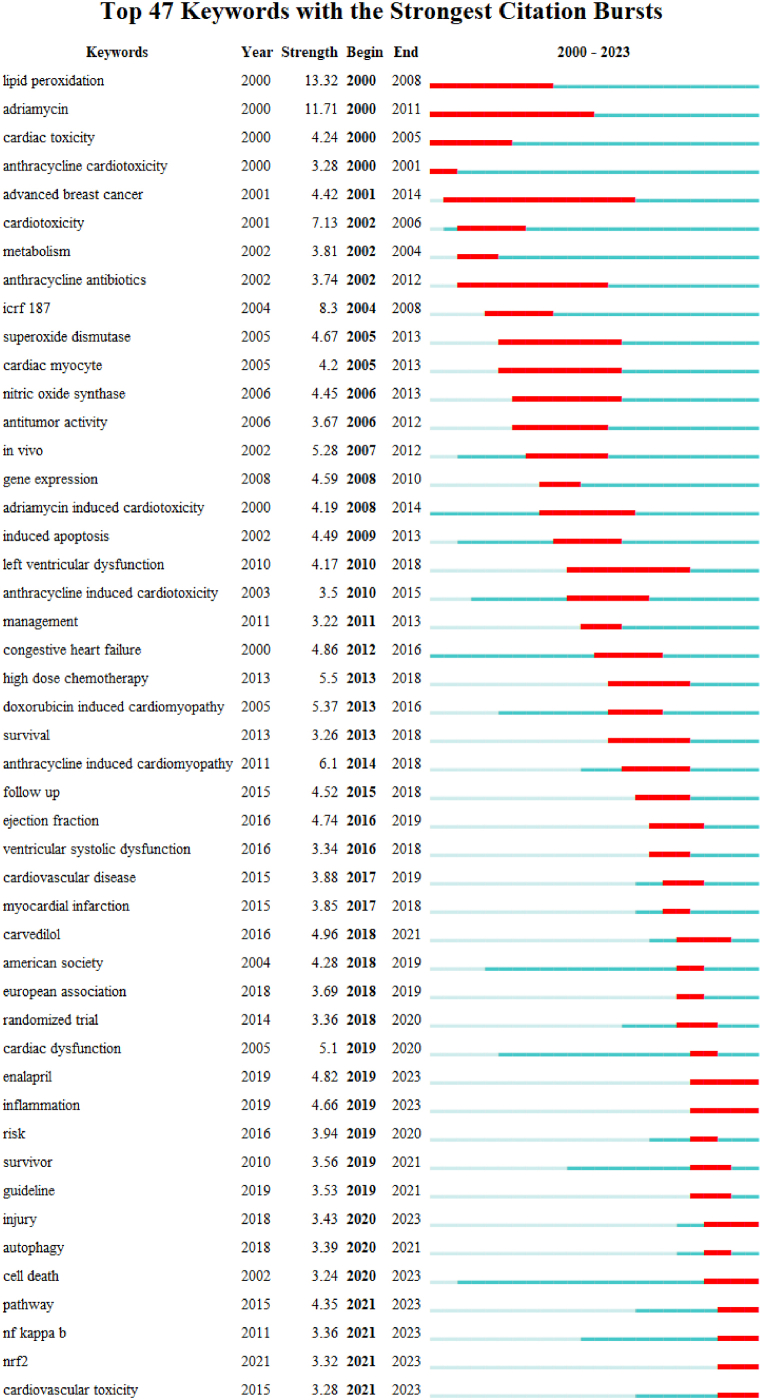
Burst words are keywords that show a sharp change in citations over a period of time, and which represent the trend of a particular subject. In Burst words analysis, the top 47 keywords with strongly cited outbreaks during 2000–2023 were shown in Fig. 7. “Enalapril”, “inflammation”, “cell death”, “Nuclear factor-kappa B (NF-κB)” and “nuclear factor erythroid-2-related factor 2 (Nrf2)” are the research trend of the future.
-
1.
Enalapril: Enalapril is an angiotensin-converting enzyme inhibitor, mainly used for the treatment of hypertension. It was found that Enalapril reduced anthracycline-induced cardiotoxicity, that Enalapril application reduced elevated Troponin I (TnI) and Creatine kinase isoenzyme MB (CKMB), preserved left ventricular ejection fraction (LVEF) in patients [17,28,29], and reduced the combined event of death and HF, maintain systolic blood pressure and diastolic function of the heart. Cancer patients with elevated troponin may benefit from Enalapril therapy. Therefore, it is better to use Enalapril during chemotherapy or in patients with elevated troponin [30]. Compared with acute cardiotoxicity, Enalapril mainly prevents cardiac dysfunction in chronic cardiotoxicity. However, we still need to weigh the benefits of ACEIs against the potential side effects in clinical applications [[31], [32], [33]]. In addition, the study samples of enalapril were small and have not been verified in prospective randomized controlled trials, so larger studies are needed to verify the relevance of the results and the clinical benefits of ACEI on LVEF.
-
2.
Inflammation: Cardiotoxicity caused by DOX involves many factors, and the activation of pro-inflammatory mediators is one of the mechanisms leading to cardiomyocyte injury [34]. Studies have shown that the levels of inflammatory factors such as tumor necrosis factor-alpha (TNF-α), interleukin-1beta (IL-1β) and interleukin-6 (IL-6) in DOX-administered rats were significantly increased [[35], [36], [37]]. DOX exposure stimulates the release of ROS and increases oxidative stress, thereby enhancing the expression of NF-κB in myocardial tissue, initiating an inflammatory cascade, and promoting the release of TNF-α and IL's [38,39]. TNF-α/TNFR1 pathway is a key factor in the inflammatory response to DOX [40,41]. Cardioprotective agents such as fisetin, Achillea fragrantissima and Alamandine can exert cardioprotective effects by inhibiting inflammatory factors NF-κB, TNF-α, IL-1β, IL-6 and cyclooxygenase (COX) 2 pathways [[42], [43], [44]].
-
3.
Cell death: Cell death is one of the main manifestations of cardiotoxicity, and cell death mechanisms such as apoptosis, autophagy, and necroptosis have been described to explain the cardiotoxicity of DOX [[45], [46], [47]]. Mitochondrial perturbation induced by excess oxygen radicals is the underlying cause of cardiomyocyte death induced by cardiotoxic chemotherapeutic agents [48]. Bnip3 was found to be a key effector of DOX-induced mitochondrial damage and cardiomyocyte necrosis, and it was highly enriched in DOX-treated cardiomyocytes, and knockdown or reduction of Bnip3 prevented DOX-induced cell necrosis [49]. Cardiomyocyte apoptosis is a key process in the occurrence and development of DOX-induced cardiotoxicity [50,51]. Inhibition of apoptosis may provide a new way to alleviate DOX-related cardiotoxicity [52]. In addition, DOX can also induce cell death through ferroptosis [53,54]. Ferroptosis manifested by elevated levels of 4-hydroxynonenal (4-HNE) and Malondialdehyde (MDA) [[55], [56], [57]]. After DOX treatment, the levels of MDA and 4-HNE increased, and the antioxidant pathway was activated to protect cardiomyocytes from DOX-induced ferroptosis [58].
-
4.
NF kappa B: NF-κB is one of the main signal transduction pathways in response to DOX-induced oxidative stress, involved in inflammation, apoptosis, anti-oxidation and other pathophysiology processes, and plays a key role in DOX-induced cardiotoxicity. Oxidative stress can lead to an inflammatory response by activating NF-κB, up-regulate inflammatory factors in the heart, including IL-1β, IL-6, interleukin-17 (IL-17) and TNF-α [[59], [60], [61]], and promote inflammatory mediator expression [62]. The NF-κB and mitogen-activated protein kinases (MAPK) signaling pathways are the main intermediates of oxidative stress-induced apoptosis. In response to DOX stimulation, NF-κB and MAPKs are activated in cardiomyocytes, thereby promoting cardiomyocyte apoptosis [63]. Taurine blocks NF-κB activation and apoptosis by preventing the c-Jun N-terminal kinase (JNK) and p-38 phosphorylation [64]. NF-κB has a negative regulatory effect on Nrf2. Nrf2 is the main regulator of oxidative stress. Inhibition of NF-κB and activation of Nrf2 signaling can protect the heart by antioxidant and scavenging free radicals [65].
-
5.
Nrf2: Nrf2 is considered a major antioxidant regulator by increasing the transcription of antioxidant genes [[66], [67], [68]]. In addition, Nrf2 also reduces inflammatory injury by regulating inflammatory cytokines and antioxidant enzymes [69], and up-regulates the B-cell lymphoma-2 (BCL-2) to enhance resistance to apoptotic stimuli [70]. Nrf2-deficient mice are more prone to DOX-induced cardiotoxicity and cardiac dysfunction [71]. Tripartite motif-containing protein 21 (TRIM21) negatively regulates the p62-kelch-like ECH-associated protein 1 (Keap1) -Nrf2 antioxidant pathway, and TRIM21 ablation may serve as a potential therapeutic target to reduce DOX-induced cardiotoxicity [58]. Sirtuin 1 (SIRT1) can activate Nrf2 through deacetylation [72]. Fisetin inhibits ferroptosis by activating the SIRT1/Nrf2 signaling pathway to play an anti-DOX-induced cardiomyopathy effect [73].
Fig. 7.
Top 47 keywords with the strongest citation bursts.
5. Discussion
The bibliometric analysis of the prevention and treatment of anthracycline-induced cardiotoxicity over the past 20 years shows that, in general, the number of published literature is increasing. In this study, Cardiovascular Toxicology ranked first in the frequency of journals, indicating that the journal has a certain influence on the research in this field. Most journals are mainly in the field of Pharmacology & Pharmacy, other fields include Cardiac & Cardiovascular Systems, Oncology and Toxicology. The United States, China and Italy are major research powers with high publication rates, while the United States, France and Canada are major research countries with high publication centrality. The cooperation between countries is mainly pairwise cooperation, and there is a lack of large-scale communication between countries. Charles Univ Prague has published the most articles. Most institutions are universities and only a few are hospitals. The centrality of the institutions was low, indicating that the cooperation among the institutions was not close and mainly internal cooperation. At present, the research strength of the prevention and treatment of anthracycline-induced cardiotoxicity has not yet formed a joint force, which restricts its long-term development to some extent. Exchanges and cooperation between multiple countries and institutions should be strengthened to promote the continuous development of research. Based on the bibliometric analysis, the results show that the research hotspots can be summarized as follows.
Firstly, HF is the most important complication of anthracycline treatment. The treatment of anthracycline-induced heart failure is similar to other types of HF. The main drugs include ACEIs, ARBs, β-blockers and statins [74]. It has been found that combined treatment with ACEI and β-blockers may prevent left ventricular systolic dysfunction in patients with malignant hemopathies treated with intensive chemotherapy. Candesartan can prevent early LVEF decline caused by anthracyclines [18]. But there is also reported that β-blockers carvedilol had no effect on the incidence of premature LVEF reduction [24], while statins may reduce the risk of HF [75]. A recent randomized controlled trial study has also shown that statins reduced the incidence of cardiac dysfunction in anthracycline-treated lymphoma patients [76]. And the incidence of cardiotoxicity can be reduced regardless of the type of statin used, suggesting that there is no difference between types of statins [77]. However, it has also been pointed out that statins do not have a significant role in changing LVEF, which may be related to the short observation time [78]. In conclusion, statins are effective in preventing anthracycline-induced cardiotoxicity, but the selection of the appropriate dose and duration of treatment during statin therapy still needs to be explored in large-sample clinical trials.
Secondly, the mechanism of anthracycline-induced cardiotoxicity is unclear, but oxidative stress, inflammation and apoptosis play a crucial role in cardiovascular events. Oxidative stress has been identified as the main cause of anthracycline cardiotoxicity. It is generally believed that DOX activates a variety of signaling molecules through ROS, including MAPK family members composed of extracellular-signal regulated protein kinase (ERK) and c-JNK and p-38, and then regulates Bcl-2 and Bax [79]. However, interventions to reduce oxidative stress were unsuccessful in reducing the incidence of cardiotoxicity in DOX-treated patients. This may be related to individual differences in response to DOX [80]. Inflammation is also an important mechanism of cardiotoxicity after DOX treatment. DOX promotes the release of pro-inflammatory factors TNF-α, IL-1β, and IL-6 by activating the NF-κB signaling pathway, regulates the inflammatory enzymes COX 2 and inducible nitric oxide synthase (iNOS), and enhances the inflammatory response [81,82]. DOX also triggers the release of pro-inflammatory cytokines, activates MAPK signaling pathways to trigger apoptosis [83,84]. Autophagy plays an important role in DOX-induced cardiotoxicity, and the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway is a key negative regulator of autophagy [85,86]. The Akt phosphorylation level decreased and autophagy markers increased in the heart tissue of DOX-treated mice [87,88].
Lastly, the evolution of these keywords reveals the history of research on the prevention and treatment of anthracycline-induced cardiotoxicity. Lipid peroxidation and heart failure have always been the main trends of research, lipid peroxidation mainly flourished in the early stage of research, and heart failure mainly flourished in the middle of research. The middle stage of research is mainly divided into clinical research and molecular mechanism research. Clinical research mainly focuses on complications such as cardiac myocyte, left ventricular dysfunction, and myocardial infarction caused by anthracyclines, as well as patient survival rate and follow-up. Molecular mechanism research focuses on oxidative stress, apoptosis, gene expression, etc. In the past 5 years, more attention has been paid to the molecular mechanism research of anthracycline-induced cardiotoxicity and the clinical observation of drug treatment of anthracycline-induced cardiotoxicity. It is expected that research on inflammation, cell death, NF-κB and their relationship will be the mainstream research trend in this field.
Cardiotoxicity is the most common and serious complication of anthracycline therapy, manifesting as dose accumulation, progression, and irreversibility [89,90]. Patients with different types of tumors have differences in age, sex, comorbidities, anthracycline dose and infusion regimen, and clinical characteristics. It is difficult to recommend guidelines for the preventive management of anthracycline-induced cardiotoxicity in all cancer types [91]. The current approach to prevent cardiotoxicity is the use of liposomal doxorubicin and the cardioprotective agent dexrazoxane. However, its clinical use is limited due to the risk of secondary malignancy and myelosuppression. Although there are many other drugs that may have cardioprotective effects in clinical practice, such as ACEI, ARBs, and β-blockers, however there are not enough clinical research data [92], and long-term studies are lacking [93]. At present, more research is needed to determine the best prevention options. Furthermore, there has been a growing recognition of the interaction between cancer and CVD, leading to the emergence of the cardio-oncology (CO) field and cardio-oncology programs (COP) to tackle existing gaps in the care of these interconnecting conditions [94]. COP offered comprehensive multidisciplinary care and address the cardiovascular needs through the journey of cancer, including before (prevention and risk assessment), during (surveillance and early detection of cardiotoxicity), and after (survivorship). COP expanded the workplace to community hospitals, not just academic centers.
Monitoring and managing cardiovascular (CV) risk factors are essential for safe cancer therapy. The Kurume-CREO registry was a single-center, prospective observational study from Japan [95]. This study focused on the incidence of cancer treatment-related cardiovascular toxicity (CTR-CVT) during chemotherapy and performed risk stratification with the Heart Failure Association and International Cardio-Oncology Society (HFA-ICOS) risk assessment tools to analyze the relationship between different stratified populations and the incidence of CTR-CVT to determine prognosis. It found that patients with high/very high risk were well predicted by the HFA-ICOS risk assessment tool, demonstrating the importance of monitoring patients with cancer for potential CV risks and emphasizing the need for further research to improve treatment protocols for those at higher risk. The CARDIOTOX registry was a prospective multicenter study from Spain to identify the factors related with risk of cancer therapy cardiotoxicity and assessing the utility of clinical biochemical, and echocardiographic (ECHO) parameters for the early detection of cardiovascular disease in patients treated with cancer therapies as well as the possible factors related with prognosis and the recovery of left ventricular function [96]. The Systemic Coronary Risk Estimation (SCORE) was significantly associated with higher rates of severe cardiotoxicity (CTox) and all-cause mortality. Global collaboration in cardio-oncology is needed to understand the prevalence of cancer therapy-related cardiovascular toxicity in different risk groups, practice settings, and geographic locations. The Global Cardio Oncology Registry (G-COR) was a multicenter international observational cohort study [97]. The objectives were to identify the risk factors of CTR-CVT and to develop risk score model; to characterize geographic and regional on biomarkers, imaging modalities, socioeconomic and demographic variables; and to assess their impact on care, surveillance strategies, treatments, and outcomes.
Currently, most of the studies on the prevention and treatment of cardiotoxicity underway were observational trials, and more prospective randomized clinical trials are expected afterwards. Research collaboration must be facilitated in the future through the establishment of registries to characterize these patients, assess resource utilization, and monitor their long-term outcomes to better understand the burden of cardiovascular disease in cancer patients, as well as risk stratification tools and global registries.
This study is based on the prevention and treatment of anthracycline-induced cardiotoxicity and explores the international cooperation, research hotspots and frontier trends of such studies in the last 20 years through bibliometric methods. In the method of bibliometric analysis, compared with other studies, this study analyses the centrality ranking of country, institution, author, cited literature and keyword analysis, in addition to their frequency ranking. Meanwhile, on the basis of author and journal analysis, cited author and cited journal content analysis are added. In addition, unlike previous studies that only used burst words analysis for keyword analysis, this study also conducted burst words analysis for cited references, which was used to find out the particularly popular literature in different time periods during the last 20 years. However, there are some limitations of this study. It was possible that there are other databases with significant articles that may not have been analyzed as the CiteSpace analysis only supported the Web of Science database; Only English literature was included in this study, which may have overlooked high-quality literature in other languages, such as Chinese and Spanish. These limitations need to be addressed at a later stage.
6. Conclusions
In conclusion, the comprehensive analysis with reference to multiple studies mainly focuses on the following aspects: early detection and treatment, molecular mechanism, drug selection and therapeutic effect, and cardioprotective strategies.
The prevention and treatment of anthracycline-induced cardiotoxicity focuses on early detection and timely treatment. Generally, the cardiotoxicity of anthracycline treatment mostly appears within 1 year, so early detection of anthracycline-induced cardiotoxicity through different means is the basis of treatment. Currently, there are many therapeutic drugs for anthracycline-induced cardiotoxicity, such as ACEIs, ARBs, statins, etc. However, the samples of the clinical studies of these drugs are small, and the observation period is short, mostly observational, which makes the results of the current clinical studies contradictory to each other. Therefore, more large-scale clinical studies with large samples, multicenter, long-term follow-up, and randomized control are needed to provide more reliable clinical evidence on the prevention and treatment of anthracycline-induced cardiotoxicity in the future. Meanwhile, there are great difficulties in recommending cardiotoxicity treatment due to the different types of tumor patients and the different doses of anthracyclines used. In addition, the molecular mechanisms of anthracycline-induced cardiotoxicity are complex and targeted therapies against these molecular mechanisms would be a potential application for the prevention and treatment of anthracycline cardiotoxicity.
This study provides useful information for potential collaborators and institutions, and provides a direction for researchers to identify the development trend of the prevention and treatment of anthracycline-induced cardiotoxicity and further research.
Data availability statement
The data generated in the present study may be requested from the first author upon reasonable request.
Funding
This research was supported by the National Science Fund for Distinguished Young Scholars (81725024).
CRediT authorship contribution statement
Yifan Kong: Writing – original draft, Formal analysis, Data curation, Conceptualization. Xiaohong Wei: Writing – review & editing, Conceptualization. Di Zhang: Software, Methodology, Formal analysis, Data curation. Hongyuan Lin: Investigation. Mengqi Peng: Investigation. Hongcai Shang: Supervision, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Yifan Kong, Email: 20210941135@bucm.edu.cn.
Hongcai Shang, Email: Shanghongcai@126.com.
References
- 1.Henson K.E., Reulen R.C., Winter D.L., Bright C.J., Fidler M.M., Frobisher C., Guha J., Wong K.F., Kelly J., Edgar A.B., McCabe M.G., Whelan J., Cutter D.J., Darby S.C., Hawkins M.M. Cardiac mortality among 200 000 five-year survivors of cancer diagnosed at 15 to 39 Years of age: the teenage and Young adult cancer survivor study. Circulation. 2016;134(20):1519–1531. doi: 10.1161/CIRCULATIONAHA.116.022514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellahham S., Khalouf A., Elkhazendar M., Dababo N., Manla Y. An overview of radiation-induced heart disease. Radiat. Oncol. J. 2022;40(2):89–102. doi: 10.3857/roj.2021.00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herrmann J., Lenihan D., Armenian S., Barac A., Blaes A., Cardinale D., Carver J., Dent S., Ky B., Lyon A.R., Lopez-Fernandez T., Fradley M.G., Ganatra S., Curigliano G., Mitchell J.D., Minotti G., Lang N.N., Liu J.E., Neilan T.G., Nohria A., O'Quinn R., Pusic I., Porter C., Reynolds K.L., Ruddy K.J., Thavendiranathan P., Valent P. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur. Heart J. 2022;43(4):280–299. doi: 10.1093/eurheartj/ehab674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zamorano J.L., Lancellotti P., Rodriguez M.D., Aboyans V., Asteggiano R., Galderisi M., Habib G., Lenihan D.J., Lip G., Lyon A.R., Lopez F.T., Mohty D., Piepoli M.F., Tamargo J., Torbicki A., Suter T.M. ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur. Heart J. 2016;37(36):2768–2801. doi: 10.1093/eurheartj/ehw211. 2016. [DOI] [PubMed] [Google Scholar]
- 5.Narezkina A., Nasim K. Anthracycline cardiotoxicity. Circ Heart Fail. 2019;12(3) doi: 10.1161/CIRCHEARTFAILURE.119.005910. [DOI] [PubMed] [Google Scholar]
- 6.Swain S.M., Whaley F.S., Ewer M.S. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer-Am. Cancer Soc. 2003;97(11):2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 7.Sala V., Della S.A., Hirsch E., Ghigo A. Signaling pathways underlying anthracycline cardiotoxicity. Antioxidants Redox Signal. 2020;32(15):1098–1114. doi: 10.1089/ars.2020.8019. [DOI] [PubMed] [Google Scholar]
- 8.Salas-Mera D., Deiros-Bronte L., Uceda-Galiano A., Mozo-Del C.Y., Garcia-Guereta L., Gutierrez-Larraya F. Chemotherapy-induced cardiotoxicity in adolescent after heart transplant: do not forget the right ventricle. Pediatr. Cardiol. 2019;40(8):1756–1758. doi: 10.1007/s00246-019-02170-8. [DOI] [PubMed] [Google Scholar]
- 9.Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc. Natl. Acad. Sci. U. S. A. 2004;101(Suppl 1):5303–5310. doi: 10.1073/pnas.0307513100. Suppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C., Song M. Visualizing a field of research: a methodology of systematic scientometric reviews. PLoS One. 2019;14(10) doi: 10.1371/journal.pone.0223994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sterba M., Popelova O., Vavrova A., Jirkovsky E., Kovarikova P., Gersl V., Simunek T. Oxidative stress, redox signaling, and metal chelation in anthracycline cardiotoxicity and pharmacological cardioprotection. Antioxidants Redox Signal. 2013;18(8):899–929. doi: 10.1089/ars.2012.4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sterba M., Popelova O., Simunek T., Mazurova Y., Potacova A., Adamcova M., Kaiserova H., Ponka P., Gersl V. Cardioprotective effects of a novel iron chelator, pyridoxal 2-chlorobenzoyl hydrazone, in the rabbit model of daunorubicin-induced cardiotoxicity. J. Pharmacol. Exp. Ther. 2006;319(3):1336–1347. doi: 10.1124/jpet.106.111468. [DOI] [PubMed] [Google Scholar]
- 13.Lipshultz S.E., Lipsitz S.R., Mone S.M., Goorin A.M., Sallan S.E., Sanders S.P., Orav E.J., Gelber R.D., Colan S.D. Female sex and higher drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N. Engl. J. Med. 1995;332(26):1738–1743. doi: 10.1056/NEJM199506293322602. [DOI] [PubMed] [Google Scholar]
- 14.Minotti G., Menna P., Salvatorelli E., Cairo G., Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004;56(2):185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 15.Cardinale D., Colombo A., Bacchiani G., Tedeschi I., Meroni C.A., Veglia F., Civelli M., Lamantia G., Colombo N., Curigliano G., Fiorentini C., Cipolla C.M. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131(22):1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 16.Zhang S., Liu X., Bawa-Khalfe T., Lu L.S., Lyu Y.L., Liu L.F., Yeh E.T. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012;18(11):1639–1642. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 17.Bosch X., Rovira M., Sitges M., Domenech A., Ortiz-Perez J.T., de Caralt T.M., Morales-Ruiz M., Perea R.J., Monzo M., Esteve J. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies) J. Am. Coll. Cardiol. 2013;61(23):2355–2362. doi: 10.1016/j.jacc.2013.02.072. [DOI] [PubMed] [Google Scholar]
- 18.Gulati G., Heck S.L., Ree A.H., Hoffmann P., Schulz-Menger J., Fagerland M.W., Gravdehaug B., von Knobelsdorff-Brenkenhoff F., Bratland A., Storas T.H., Hagve T.A., Rosjo H., Steine K., Geisler J., Omland T. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 x 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur. Heart J. 2016;37(21):1671–1680. doi: 10.1093/eurheartj/ehw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vejpongsa P., Yeh E.T. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J. Am. Coll. Cardiol. 2014;64(9):938–945. doi: 10.1016/j.jacc.2014.06.1167. [DOI] [PubMed] [Google Scholar]
- 20.Kalam K., Marwick T.H. Role of cardioprotective therapy for prevention of cardiotoxicity with chemotherapy: a systematic review and meta-analysis. Eur. J. Cancer. 2013;49(13):2900–2909. doi: 10.1016/j.ejca.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 21.Hensley M.L., Hagerty K.L., Kewalramani T., Green D.M., Meropol N.J., Wasserman T.H., Cohen G.I., Emami B., Gradishar W.J., Mitchell R.B., Thigpen J.T., Trotti A.R., von Hoff D., Schuchter L.M. American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J. Clin. Oncol. 2009;27(1):127–145. doi: 10.1200/JCO.2008.17.2627. [DOI] [PubMed] [Google Scholar]
- 22.Ichikawa Y., Ghanefar M., Bayeva M., Wu R., Khechaduri A., Naga P.S., Mutharasan R.K., Naik T.J., Ardehali H. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J. Clin. Invest. 2014;124(2):617–630. doi: 10.1172/JCI72931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pacher P., Liaudet L., Bai P., Mabley J.G., Kaminski P.M., Virag L., Deb A., Szabo E., Ungvari Z., Wolin M.S., Groves J.T., Szabo C. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation. 2003;107(6):896–904. doi: 10.1161/01.cir.0000048192.52098.dd. [DOI] [PubMed] [Google Scholar]
- 24.Avila M.S., Ayub-Ferreira S.M., de Barros W.M.J., Das D.C.F., Goncalves B.S., Rigaud V., Higuchi-Dos-Santos M.H., Hajjar L.A., Kalil F.R., Hoff P.M., Sahade M., Ferrari M., de Paula C.R., Mano M.S., Bittencourt V.C.C., Abduch M.C., Lofrano A.M., Guimaraes G.V., Issa V.S., Bittencourt M.S., Bocchi E.A. Carvedilol for prevention of chemotherapy-related cardiotoxicity: the CECCY trial. J. Am. Coll. Cardiol. 2018;71(20):2281–2290. doi: 10.1016/j.jacc.2018.02.049. [DOI] [PubMed] [Google Scholar]
- 25.McGowan J.V., Chung R., Maulik A., Piotrowska I., Walker J.M., Yellon D.M. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc. Drugs Ther. 2017;31(1):63–75. doi: 10.1007/s10557-016-6711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armenian S.H., Lacchetti C., Barac A., Carver J., Constine L.S., Denduluri N., Dent S., Douglas P.S., Durand J.B., Ewer M., Fabian C., Hudson M., Jessup M., Jones L.W., Ky B., Mayer E.L., Moslehi J., Oeffinger K., Ray K., Ruddy K., Lenihan D. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2017;35(8):893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 27.Songbo M., Lang H., Xinyong C., Bin X., Ping Z., Liang S. Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol. Lett. 2019;307:41–48. doi: 10.1016/j.toxlet.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Cardinale D., Colombo A., Sandri M.T., Lamantia G., Colombo N., Civelli M., Martinelli G., Veglia F., Fiorentini C., Cipolla C.M. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114(23):2474–2481. doi: 10.1161/CIRCULATIONAHA.106.635144. [DOI] [PubMed] [Google Scholar]
- 29.Janbabai G., Nabati M., Faghihinia M., Azizi S., Borhani S., Yazdani J. Effect of enalapril on preventing anthracycline-induced cardiomyopathy. Cardiovasc. Toxicol. 2017;17(2):130–139. doi: 10.1007/s12012-016-9365-z. [DOI] [PubMed] [Google Scholar]
- 30.Cardinale D., Ciceri F., Latini R., Franzosi M.G., Sandri M.T., Civelli M., Cucchi G., Menatti E., Mangiavacchi M., Cavina R., Barbieri E., Gori S., Colombo A., Curigliano G., Salvatici M., Rizzo A., Ghisoni F., Bianchi A., Falci C., Aquilina M., Rocca A., Monopoli A., Milandri C., Rossetti G., Bregni M., Sicuro M., Malossi A., Nassiacos D., Verusio C., Giordano M., Staszewsky L., Barlera S., Nicolis E.B., Magnoli M., Masson S., Cipolla C.M. Anthracycline-induced cardiotoxicity: a multicenter randomised trial comparing two strategies for guiding prevention with enalapril: the International CardioOncology Society-one trial. Eur. J. Cancer. 2018;94:126–137. doi: 10.1016/j.ejca.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Silber J.H., Cnaan A., Clark B.J., Paridon S.M., Chin A.J., Rychik J., Hogarty A.N., Cohen M.I., Barber G., Rutkowski M., Kimball T.R., Delaat C., Steinherz L.J., Zhao H. Enalapril to prevent cardiac function decline in long-term survivors of pediatric cancer exposed to anthracyclines. J. Clin. Oncol. 2004;22(5):820–828. doi: 10.1200/JCO.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 32.Totzeck M., Mincu R.I., Heusch G., Rassaf T. Heart failure from cancer therapy: can we prevent it? ESC Heart Fail. 2019;6(4):856–862. doi: 10.1002/ehf2.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang K., Zhang Y., Liu W., He C. Effects of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use on cancer therapy-related cardiac dysfunction: a meta-analysis of randomized controlled trials. Heart Fail. Rev. 2021;26(1):101–109. doi: 10.1007/s10741-019-09906-x. [DOI] [PubMed] [Google Scholar]
- 34.Chen J.Y., Hu R.Y., Chou H.C. Quercetin-induced cardioprotection against doxorubicin cytotoxicity. J. Biomed. Sci. 2013;20(1):95. doi: 10.1186/1423-0127-20-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vyas D., Deshpande K., Chaturvedi L., Gieric L., Ching K. Rapid extensive recurrence of triple negative breast cancer: are both therapy and cancer Biology the culprit? J. Clin. Med. Res. 2016;8(2):162–167. doi: 10.14740/jocmr2365w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdel-Daim M.M., Kilany O.E., Khalifa H.A., Ahmed A. Allicin ameliorates doxorubicin-induced cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Cancer Chemother. Pharmacol. 2017;80(4):745–753. doi: 10.1007/s00280-017-3413-7. [DOI] [PubMed] [Google Scholar]
- 37.Narikawa M., Umemura M., Tanaka R., Hikichi M., Nagasako A., Fujita T., Yokoyama U., Ishigami T., Kimura K., Tamura K., Ishikawa Y. Doxorubicin induces trans-differentiation and MMP1 expression in cardiac fibroblasts via cell death-independent pathways. PLoS One. 2019;14(9) doi: 10.1371/journal.pone.0221940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abd E.T., Mohamed R.H., Pasha H.F., Abdel-Aziz H.R. Catechin protects against oxidative stress and inflammatory-mediated cardiotoxicity in adriamycin-treated rats. Clin. Exp. Med. 2012;12(4):233–240. doi: 10.1007/s10238-011-0165-2. [DOI] [PubMed] [Google Scholar]
- 39.Sun Z., Yan B., Yu W.Y., Yao X., Ma X., Sheng G., Ma Q. Vitexin attenuates acute doxorubicin cardiotoxicity in rats via the suppression of oxidative stress, inflammation and apoptosis and the activation of FOXO3a. Exp. Ther. Med. 2016;12(3):1879–1884. doi: 10.3892/etm.2016.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaczmarek A., Krysko O., Heyndrickx L., Love A.T., Delvaeye T., Bachert C., Leybaert L., Vandenabeele P., Krysko D.V. TNF/TNF-R1 pathway is involved in doxorubicin-induced acute sterile inflammation. Cell Death Dis. 2013;4(12) doi: 10.1038/cddis.2013.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vyas D., Laput G., Vyas A.K. Chemotherapy-enhanced inflammation may lead to the failure of therapy and metastasis. OncoTargets Ther. 2014;7:1015–1023. doi: 10.2147/OTT.S60114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma T., Kandhare A.D., Mukherjee-Kandhare A.A., Bodhankar S.L. Fisetin, a plant flavonoid ameliorates doxorubicin-induced cardiotoxicity in experimental rats: the decisive role of caspase-3, COX-II, cTn-I, iNOs and TNF-alpha. Mol. Biol. Rep. 2019;46(1):105–118. doi: 10.1007/s11033-018-4450-y. [DOI] [PubMed] [Google Scholar]
- 43.Hijazi M.A., Jambi H.A., Aljehany B.M., Althaiban M.A. Potential protective effect of Achillea fragrantissima against adriamycin-induced cardiotoxicity in rats via an antioxidant and anti-inflammatory pathway. BioMed Res. Int. 2019;2019 doi: 10.1155/2019/5269074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hekmat A.S., Navabi Z., Alipanah H., Javanmardi K. Alamandine significantly reduces doxorubicin-induced cardiotoxicity in rats. Hum. Exp. Toxicol. 2021;40(10):1781–1795. doi: 10.1177/09603271211010896. [DOI] [PubMed] [Google Scholar]
- 45.Lu L., Wu W., Yan J., Li X., Yu H., Yu X. Adriamycin-induced autophagic cardiomyocyte death plays a pathogenic role in a rat model of heart failure. Int. J. Cardiol. 2009;134(1):82–90. doi: 10.1016/j.ijcard.2008.01.043. [DOI] [PubMed] [Google Scholar]
- 46.Zhu W., Soonpaa M.H., Chen H., Shen W., Payne R.M., Liechty E.A., Caldwell R.L., Shou W., Field L.J. Acute doxorubicin cardiotoxicity is associated with p53-induced inhibition of the mammalian target of rapamycin pathway. Circulation. 2009;119(1):99–106. doi: 10.1161/CIRCULATIONAHA.108.799700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang T., Zhang Y., Cui M., Jin L., Wang Y., Lv F., Liu Y., Zheng W., Shang H., Zhang J., Zhang M., Wu H., Guo J., Zhang X., Hu X., Cao C.M., Xiao R.P. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat. Med. 2016;22(2):175–182. doi: 10.1038/nm.4017. [DOI] [PubMed] [Google Scholar]
- 48.Singal P.K., Kirshenbaum L.A. A relative deficit in antioxidant reserve may contribute in cardiac failure. Can. J. Cardiol. 1990;6(2):47–49. [PubMed] [Google Scholar]
- 49.Dhingra A., Jayas R., Afshar P., Guberman M., Maddaford G., Gerstein J., Lieberman B., Nepon H., Margulets V., Dhingra R., Kirshenbaum L.A. Ellagic acid antagonizes Bnip3-mediated mitochondrial injury and necrotic cell death of cardiac myocytes. Free Radic. Biol. Med. 2017;112:411–422. doi: 10.1016/j.freeradbiomed.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 50.Arola O.J., Saraste A., Pulkki K., Kallajoki M., Parvinen M., Voipio-Pulkki L.M. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res. 2000;60(7):1789–1792. [PubMed] [Google Scholar]
- 51.Kumar D., Kirshenbaum L.A., Li T., Danelisen I., Singal P.K. Apoptosis in adriamycin cardiomyopathy and its modulation by probucol. Antioxidants Redox Signal. 2001;3(1):135–145. doi: 10.1089/152308601750100641. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y., Lei T., Yuan J., Wu Y., Shen X., Gao J., Feng W., Lu Z. GCN2 deficiency ameliorates doxorubicin-induced cardiotoxicity by decreasing cardiomyocyte apoptosis and myocardial oxidative stress. Redox Biol. 2018;17:25–34. doi: 10.1016/j.redox.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fang X., Wang H., Han D., Xie E., Yang X., Wei J., Gu S., Gao F., Zhu N., Yin X., Cheng Q., Zhang P., Dai W., Chen J., Yang F., Yang H.T., Linkermann A., Gu W., Min J., Wang F. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. U. S. A. 2019;116(7):2672–2680. doi: 10.1073/pnas.1821022116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koleini N., Nickel B.E., Edel A.L., Fandrich R.R., Ravandi A., Kardami E. Oxidized phospholipids in Doxorubicin-induced cardiotoxicity. Chem. Biol. Interact. 2019;303:35–39. doi: 10.1016/j.cbi.2019.01.032. [DOI] [PubMed] [Google Scholar]
- 55.Galluzzi L., Vitale I., Aaronson S.A., Abrams J.M., Adam D., Agostinis P., Alnemri E.S., Altucci L., Amelio I., Andrews D.W., Annicchiarico-Petruzzelli M., Antonov A.V., Arama E., Baehrecke E.H., Barlev N.A., Bazan N.G., Bernassola F., Bertrand M.J.M., Bianchi K., Blagosklonny M.V., Blomgren K., Borner C., Boya P., Brenner C., Campanella M., Candi E., Carmona-Gutierrez D., Cecconi F., Chan F.K., Chandel N.S., Cheng E.H., Chipuk J.E., Cidlowski J.A., Ciechanover A., Cohen G.M., Conrad M., Cubillos-Ruiz J.R., Czabotar P.E., D'Angiolella V., Dawson T.M., Dawson V.L., De Laurenzi V., De Maria R., Debatin K.M., DeBerardinis R.J., Deshmukh M., Di Daniele N., Di Virgilio F., Dixit V.M., Dixon S.J., Duckett C.S., Dynlacht B.D., El-Deiry W.S., Elrod J.W., Fimia G.M., Fulda S., García-Sáez A.J., Garg A.D., Garrido C., Gavathiotis E., Golstein P., Gottlieb E., Green D.R., Greene L.A., Gronemeyer H., Gross A., Hajnoczky G., Hardwick J.M., Harris I.S., Hengartner M.O., Hetz C., Ichijo H., Jäättelä M., Joseph B., Jost P.J., Juin P.P., Kaiser W.J., Karin M., Kaufmann T., Kepp O., Kimchi A., Kitsis R.N., Klionsky D.J., Knight R.A., Kumar S., Lee S.W., Lemasters J.J., Levine B., Linkermann A., Lipton S.A., Lockshin R.A., López-Otín C., Lowe S.W., Luedde T., Lugli E., MacFarlane M., Madeo F., Malewicz M., Malorni W., Manic G., Marine J.C., Martin S.J., Martinou J.C., Medema J.P., Mehlen P., Meier P., Melino S., Miao E.A., Molkentin J.D., Moll U.M., Muñoz-Pinedo C., Nagata S., Nuñez G., Oberst A., Oren M., Overholtzer M., Pagano M., Panaretakis T., Pasparakis M., Penninger J.M., Pereira D.M., Pervaiz S., Peter M.E., Piacentini M., Pinton P., Prehn J.H.M., Puthalakath H., Rabinovich G.A., Rehm M., Rizzuto R., Rodrigues C.M.P., Rubinsztein D.C., Rudel T., Ryan K.M., Sayan E., Scorrano L., Shao F., Shi Y., Silke J., Simon H.U., Sistigu A., Stockwell B.R., Strasser A., Szabadkai G., Tait S.W.G., Tang D., Tavernarakis N., Thorburn A., Tsujimoto Y., Turk B., Vanden Berghe T., Vandenabeele P., Vander Heiden M.G., Villunger A., Virgin H.W., Vousden K.H., Vucic D., Wagner E.F., Walczak H., Wallach D., Wang Y., Wells J.A., Wood W., Yuan J., Zakeri Z., Zhivotovsky B., Zitvogel L., Melino G., Kroemer G. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death. Cell Death Differ. 2018;25(3):486–541. doi: 10.1038/s41418-017-0012-4. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zou Y., Li H., Graham E.T., Deik A.A., Eaton J.K., Wang W., Sandoval-Gomez G., Clish C.B., Doench J.G., Schreiber S.L. Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nat. Chem. Biol. 2020;16(3):302–309. doi: 10.1038/s41589-020-0472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamada N., Karasawa T., Kimura H., Watanabe S., Komada T., Kamata R., Sampilvanjil A., Ito J., Nakagawa K., Kuwata H., Hara S., Mizuta K., Sakuma Y., Sata N., Takahashi M. Ferroptosis driven by radical oxidation of n-6 polyunsaturated fatty acids mediates acetaminophen-induced acute liver failure. Cell Death Dis. 2020;11(2):144. doi: 10.1038/s41419-020-2334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hou K., Shen J., Yan J., Zhai C., Zhang J., Pan J.A., Zhang Y., Jiang Y., Wang Y., Lin R.Z., Cong H., Gao S., Zong W.X. Loss of TRIM21 alleviates cardiotoxicity by suppressing ferroptosis induced by the chemotherapeutic agent doxorubicin. EBioMedicine. 2021;69 doi: 10.1016/j.ebiom.2021.103456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hwang J.W., Yao H., Caito S., Sundar I.K., Rahman I. Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic. Biol. Med. 2013;61:95–110. doi: 10.1016/j.freeradbiomed.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Z., Wang M., Liu J., Ye J., Jiang H., Xu Y., Ye D., Wan J. Inhibition of TRPA1 attenuates doxorubicin-induced acute cardiotoxicity by suppressing oxidative stress, the inflammatory response, and endoplasmic reticulum stress. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/5179468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quagliariello V., Vecchione R., Coppola C., Di Cicco C., De Capua A., Piscopo G., Paciello R., Narciso V., Formisano C., Taglialatela-Scafati O., Iaffaioli R.V., Botti G., Netti P.A., Maurea N. Cardioprotective effects of nanoemulsions loaded with anti-inflammatory nutraceuticals against doxorubicin-induced cardiotoxicity. Nutrients. 2018;10(9) doi: 10.3390/nu10091304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arunachalam S., Nagoor M.M., Azimullah S., Sharma C., Goyal S.N., Ojha S. Nerolidol attenuates oxidative stress, inflammation, and apoptosis by modulating Nrf2/MAPK signaling pathways in doxorubicin-induced acute cardiotoxicity in rats. Antioxidants. 2021;10(6) doi: 10.3390/antiox10060984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lou H., Danelisen I., Singal P.K. Involvement of mitogen-activated protein kinases in adriamycin-induced cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2005;288(4):H1925–H1930. doi: 10.1152/ajpheart.01054.2004. [DOI] [PubMed] [Google Scholar]
- 64.Das J., Ghosh J., Manna P., Sil P.C. Taurine suppresses doxorubicin-triggered oxidative stress and cardiac apoptosis in rat via up-regulation of PI3-K/Akt and inhibition of p53, p38-JNK. Biochem. Pharmacol. 2011;81(7):891–909. doi: 10.1016/j.bcp.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 65.Meeran M., Azimullah S., Mamoudh H.H., Sharma C., Kumar S., Goyal S.N., Ojha S. Nerolidol, a sesquiterpene from the essential oils of aromatic plants, attenuates doxorubicin-induced chronic cardiotoxicity in rats. J. Agric. Food Chem. 2021;69(26):7334–7343. doi: 10.1021/acs.jafc.0c05667. [DOI] [PubMed] [Google Scholar]
- 66.Moi P., Chan K., Asunis I., Cao A., Kan Y.W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. U. S. A. 1994;91(21):9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Minelli A., Bellezza I., Conte C., Culig Z. Oxidative stress-related aging: a role for prostate cancer? Biochim. Biophys. Acta. 2009;1795(2):83–91. doi: 10.1016/j.bbcan.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 68.Bellezza I., Giambanco I., Minelli A., Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018;1865(5):721–733. doi: 10.1016/j.bbamcr.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 69.Xue W.L., Bai X., Zhang L. rhTNFR:Fc increases Nrf2 expression via miR-27a mediation to protect myocardium against sepsis injury. Biochem. Biophys. Res. Commun. 2015;464(3):855–861. doi: 10.1016/j.bbrc.2015.07.051. [DOI] [PubMed] [Google Scholar]
- 70.Niture S.K., Jaiswal A.K. Nrf2 protein up-regulates antiapoptotic protein Bcl-2 and prevents cellular apoptosis. J. Biol. Chem. 2012;287(13):9873–9886. doi: 10.1074/jbc.M111.312694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li S., Wang W., Niu T., Wang H., Li B., Shao L., Lai Y., Li H., Janicki J.S., Wang X.L., Tang D., Cui T. Nrf2 deficiency exaggerates doxorubicin-induced cardiotoxicity and cardiac dysfunction. Oxid. Med. Cell. Longev. 2014;2014 doi: 10.1155/2014/748524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fan Z., Wirth A.K., Chen D., Wruck C.J., Rauh M., Buchfelder M., Savaskan N. Nrf2-Keap1 pathway promotes cell proliferation and diminishes ferroptosis. Oncogenesis. 2017;6(8) doi: 10.1038/oncsis.2017.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li D., Liu X., Pi W., Zhang Y., Yu L., Xu C., Sun Z., Jiang J. Fisetin attenuates doxorubicin-induced cardiomyopathy in vivo and in vitro by inhibiting ferroptosis through SIRT1/Nrf2 signaling pathway activation. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.808480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barry E., Alvarez J.A., Scully R.E., Miller T.L., Lipshultz S.E. Anthracycline-induced cardiotoxicity: course, pathophysiology, prevention and management. Expet Opin. Pharmacother. 2007;8(8):1039–1058. doi: 10.1517/14656566.8.8.1039. [DOI] [PubMed] [Google Scholar]
- 75.Seicean S., Seicean A., Plana J.C., Budd G.T., Marwick T.H. Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: an observational clinical cohort study. J. Am. Coll. Cardiol. 2012;60(23):2384–2390. doi: 10.1016/j.jacc.2012.07.067. [DOI] [PubMed] [Google Scholar]
- 76.Neilan T.G., Quinaglia T., Onoue T., Mahmood S.S., Drobni Z.D., Gilman H.K., Smith A., Heemelaar J.C., Brahmbhatt P., Ho J.S., Sama S., Svoboda J., Neuberg D.S., Abramson J.S., Hochberg E.P., Barnes J.A., Armand P., Jacobsen E.D., Jacobson C.A., Kim A.I., Soumerai J.D., Han Y., Friedman R.S., Lacasce A.S., Ky B., Landsburg D., Nasta S., Kwong R.Y., Jerosch-Herold M., Redd R.A., Hua L., Januzzi J.L., Asnani A., Mousavi N., Scherrer-Crosbie M. Atorvastatin for anthracycline-associated cardiac dysfunction: the STOP-CA randomized clinical trial. JAMA. 2023;330(6):528–536. doi: 10.1001/jama.2023.11887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Titus A., Cheema H.A., Shafiee A., Seighali N., Shahid A., Bhanushali K.B., Kumar A., Khan S.U., Khadke S., Thavendiranathan P., Hundley W.G., Scherrer-Crosbie M., Nohria A., Neilan T.G., Dani S.S., Nasir K., Ganatra S. Statins for attenuating cardiotoxicity in patients receiving anthracyclines: a systematic review and meta-analysis. Curr. Probl. Cardiol. 2023;48(10) doi: 10.1016/j.cpcardiol.2023.101885. [DOI] [PubMed] [Google Scholar]
- 78.Thavendiranathan P., Houbois C., Marwick T.H., Kei T., Saha S., Runeckles K., Huang F., Shalmon T., Thorpe K.E., Pezo R.C., Prica A., Maze D., Abdel-Qadir H., Connelly K.A., Chan J., Billia F., Power C., Hanneman K., Wintersperger B.J., Brezden-Masley C., Amir E. Statins to prevent early cardiac dysfunction in cancer patients at increased cardiotoxicity risk receiving anthracyclines. Eur. Heart J. Cardiovasc. Pharmacother. 2023;9(6):515–525. doi: 10.1093/ehjcvp/pvad031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ray P.D., Huang B.W., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012;24(5):981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Octavia Y., Tocchetti C.G., Gabrielson K.L., Janssens S., Crijns H.J., Moens A.L. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J. Mol. Cell. Cardiol. 2012;52(6):1213–1225. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 81.Kim D.H., Chung J.H., Yoon J.S., Ha Y.M., Bae S., Lee E.K., Jung K.J., Kim M.S., Kim Y.J., Kim M.K., Chung H.Y. Ginsenoside Rd inhibits the expressions of iNOS and COX-2 by suppressing NF-kappaB in LPS-stimulated RAW264.7 cells and mouse liver. J. Ginseng Res. 2013;37(1):54–63. doi: 10.5142/jgr.2013.37.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang J., Wang Z., Chen D.L. Shikonin ameliorates isoproterenol (ISO)-induced myocardial damage through suppressing fibrosis, inflammation, apoptosis and ER stress. Biomed. Pharmacother. 2017;93:1343–1357. doi: 10.1016/j.biopha.2017.06.086. [DOI] [PubMed] [Google Scholar]
- 83.Yu L., McPhee C.K., Zheng L., Mardones G.A., Rong Y., Peng J., Mi N., Zhao Y., Liu Z., Wan F., Hailey D.W., Oorschot V., Klumperman J., Baehrecke E.H., Lenardo M.J. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 2010;465(7300):942–946. doi: 10.1038/nature09076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sahu R., Dua T.K., Das S., De Feo V., Dewanjee S. Wheat phenolics suppress doxorubicin-induced cardiotoxicity via inhibition of oxidative stress, MAP kinase activation, NF-kappaB pathway, PI3K/Akt/mTOR impairment, and cardiac apoptosis. Food Chem. Toxicol. 2019;125:503–519. doi: 10.1016/j.fct.2019.01.034. [DOI] [PubMed] [Google Scholar]
- 85.Li L., Dong H., Song E., Xu X., Liu L., Song Y. Nrf2/ARE pathway activation, HO-1 and NQO1 induction by polychlorinated biphenyl quinone is associated with reactive oxygen species and PI3K/AKT signaling. Chem. Biol. Interact. 2014;209:56–67. doi: 10.1016/j.cbi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 86.Al-Taee H., Azimullah S., Meeran M., Alaraj A.M., Al J.R., Tariq S., Ab K.M., Adeghate E., Ojha S. beta-caryophyllene, a dietary phytocannabinoid attenuates oxidative stress, inflammation, apoptosis and prevents structural alterations of the myocardium against doxorubicin-induced acute cardiotoxicity in rats: an in vitro and in vivo study. Eur. J. Pharmacol. 2019;858 doi: 10.1016/j.ejphar.2019.172467. [DOI] [PubMed] [Google Scholar]
- 87.Zeng C., Duan F., Hu J., Luo B., Huang B., Lou X., Sun X., Li H., Zhang X., Yin S., Tan H. NLRP3 inflammasome-mediated pyroptosis contributes to the pathogenesis of non-ischemic dilated cardiomyopathy. Redox Biol. 2020;34 doi: 10.1016/j.redox.2020.101523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Z., Yao X., Jiang W., Li W., Zhu S., Liao C., Zou L., Ding R., Chen J. Advanced oxidation protein products induce microglia-mediated neuroinflammation via MAPKs-NF-kappaB signaling pathway and pyroptosis after secondary spinal cord injury. J. Neuroinflammation. 2020;17(1):90. doi: 10.1186/s12974-020-01751-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang J., Cui X., Yan Y., Li M., Yang Y., Wang J., Zhang J. Research progress of cardioprotective agents for prevention of anthracycline cardiotoxicity. Am. J. Transl. Res. 2016;8(7):2862–2875. [PMC free article] [PubMed] [Google Scholar]
- 90.Galan-Arriola C., Lobo M., Vilchez-Tschischke J.P., Lopez G.J., de Molina-Iracheta A., Perez-Martinez C., Aguero J., Fernandez-Jimenez R., Martin-Garcia A., Oliver E., Villena-Gutierrez R., Pizarro G., Sanchez P.L., Fuster V., Sanchez-Gonzalez J., Ibanez B. Serial magnetic resonance imaging to identify early stages of anthracycline-induced cardiotoxicity. J. Am. Coll. Cardiol. 2019;73(7):779–791. doi: 10.1016/j.jacc.2018.11.046. [DOI] [PubMed] [Google Scholar]
- 91.Chung W.B., Youn H.J. Pathophysiology and preventive strategies of anthracycline-induced cardiotoxicity. Korean J. Intern. Med. 2016;31(4):625–633. doi: 10.3904/kjim.2016.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park C.S., Youn H.J., Cho E.J., Oh Y.S., Baek S.H., Lee J.H., Kang J.H., Chung W.S., Chae J.S., Kim J.H. Cardioprotective effect of IGF-1 in mouse with adriamycin induced cardiomyopathy. Korean Circ. J. 2002;32(12):1116–1123. [Google Scholar]
- 93.Veeder J.A., Hothem L.N., Cipriani A.E., Jensen B.C., Rodgers J.E. Chemotherapy-associated cardiomyopathy: mechanisms of toxicity and cardioprotective strategies. Pharmacotherapy. 2021;41(12):1066–1080. doi: 10.1002/phar.2638. [DOI] [PubMed] [Google Scholar]
- 94.Manla Y., Franco F.X., Sadler D. Program building in cardio-oncology: bridging existing care gaps to improve cardiovascular and cancer outcomes. Curr. Treat. Options Cardiovasc. Med. 2024;26(3):47–67. [Google Scholar]
- 95.Shibata T., Nohara S., Morikawa N., Shibao K., Ito S., Shibata R., Toh U., Nagafuji K., Fukami K., Fukumoto Y. Cardiovascular adverse events and prognosis in patients with haematologic malignancies and breast cancer receiving anticancer agents: Kurume-CREO Registry insights. Eur. J. Prev. Cardiol. 2023;30(18):1941–1949. doi: 10.1093/eurjpc/zwad210. [DOI] [PubMed] [Google Scholar]
- 96.Caro-Codón J., López-Fernández T., Álvarez-Ortega C., Zamora Auñón P., Rodríguez I.R., Gómez Prieto P., Buño Soto A., Canales Albendea M., Albaladejo A., Mediavilla G., et al. Cardiovascular risk factors during cancer treatment. Prevalence and prognostic relevance: insights from the CARDIOTOX registry. Eur. J. Prev. Cardiol. 2022;29(6):859–868. doi: 10.1093/eurjpc/zwaa034. [DOI] [PubMed] [Google Scholar]
- 97.Teske A.J., Moudgil R., López-Fernández T., Barac A., Brown S.A., Deswal A., Neilan T.G., Ganatra S., Abdel Qadir H., Menon V., et al. Global cardio oncology registry (G-COR): registry design, primary objectives, and future perspectives of a multicenter global initiative. Circ. Cardiovasc. Qual. Outcomes. 2023;16(10) doi: 10.1161/CIRCOUTCOMES.123.009905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in the present study may be requested from the first author upon reasonable request.