Abstract
Background
Although nutritional treatment is an established pillar of multidisciplinary care provided in critical illness, there are many concerns regarding this issue in severe COVID-19. This observational, retrospective, multicentre study aimed to analyse the approach to nutritional treatment among selected intensive care units (ICUs) in Poland.
Methods
The medical records of 129 patients hospitalized in five units due to respiratory failure following COVID-19 were analysed in terms of nutritional management on the eighth day of the ICU stay. The Harris-Benedict equation (HB), Mifflin St. Jeor equation (MsJ) and ESPEN formula (20 kcal kg–1 body weight) were used to estimate the energy target for each patient, and two ESPEN formulas determined the protein target (1 g kg–1 body weight and 1.3 g kg–1 body weight).
Results
Evaluation of nutritional therapy was performed in 129 subjects. The fulfilment of caloric requirement considering the HB, MsJ and ESPEN formula was 66%, 66.7% and 62.5%, respectively. Two clinical centres managed to provide 70% or more of daily caloric requirements. According to the ESPEN formula, the implementation of the protein target was 70%; however, one of the investigated units provided a median of 157% of the protein demand. The nutritional management varied in the preferred route of nutrition administration. Neither method nor grade of nutrition supply influenced biochemical parameters on the 8th day of ICU stay.
Conclusions
Significant differences in nutritional treatment of critically ill COVID-19 patients in Polish ICUs were noted, which underlines the importance of setting up clear guidelines regarding this issue.
Keywords: intensive care, mechanical ventilation, prone position, COVID-19, SARS-CoV-2, nutrition
In Poland, 108 000 patients were treated in intensive care units (ICUs) in 2021 [1]. At that time, the main medical challenge was the SARS-CoV-2 pandemic, and the largest group of patients comprised those with COVID-19. Treatment in the ICU requires the implementation of multidisciplinary approaches adjusted to the main cause of deterioration of health, as well as therapy targeting comorbidities [2]. A personalised approach is an important determinant of treatment effectiveness.
Clinical nutrition is a significant component of therapy [3]. An aspect potentially influencing the inadequate fulfilment of nutritional needs and erroneous setting of therapeutic goals may be the high variability of the population treated in the ICU, which is mainly due to clinical reasons [4]. Furthermore, there is still a lack of definitive studies and diagnostically accurate predictors regarding clinical nutrition in critically ill patients [5]. Measuring the impact of a nutritional intervention on a patient’s condition is complicated by the fact that most clinical research excludes patients with malnutrition [3]. In clinical practice, this often occurs at the time of ICU admission because of the course of the underlying disease or recent hospitalisation. In addition, the ICU stay itself is a common cause of the development of malnutrition [6].
All the above factors are present in patients with COVID-19 [7]. Moreover, COVID-19 can be accompanied by nausea, vomiting and diarrhoea, impairing appropriate food intake and absorption [8]. COVID-19 could directly induce inflammatory responses and poor nutrient intake and absorption, leading to undernutrition with micronutrient deficiencies, which impairs immune system function with subsequent amplified risk of infection and disease severity [9]. Older individuals and those with comorbidity are more prone to suffer from severe COVID-19, are at higher risk of malnutrition, and hence deserve special attention when planning nutritional support [10, 11]. Patients with COVID-19 and high nutritional risk at ICU admission have significantly higher 28-day ICU mortality [12], as well as twice the probability of ICU 28-day mortality than those with low nutritional risk [11]. Difficulties in providing adequate medical nutrition therapy in patients with SARS-CoV-2 include, among others, presence of shock with a high lactate level, increased risk of regurgitations during non-invasive ventilation, prone positioning as an obstacle to delivery of an adequate number of enteral formulas, between-ward or between-hospital transfers with a shortage of food delivery, and severe dysphagia that may occur post-extubation. The initial lack of studies describing the course of SARS-CoV-2 infection, as well as studies dedicated to nutritional interventions in COVID-19 patients, presented healthcare professionals with the challenge of selecting appropriate clinical nutrition and modifying it adequately according to current needs and therapeutic options. ESPEN recommendations [3] only facilitate this task to a basic extent, leaving clinicians with many uncertainties.
The present study aimed to compare the mode of nutrition therapy by comparing the fulfilment of caloric and protein demands among patients on the 8th day of the ICU stay in critically ill patients with COVID-19 treated in selected Polish ICUs.
METHODS
An observational, retrospective, multicentre study was conducted involving 233 patients hospitalised in five Polish university clinical centres (Katowice, Gdansk, Opole, Zabrze, Krakow) in anaesthesiology and intensive care units due to COVID-19 during the second and third waves of the SARS-CoV-2 pandemic in Poland. The centres were asked to include randomly selected adult patients admitted to the ICU from 2020 to 2021 due to COVID-19 (with the main diagnosis of acute respiratory failure), with a documented result of a molecular test for SARS-CoV-2 infection. Exclusion criteria were patient’s death before the 8th day of ICU stay, clinical nutrition prior to ICU admission, home mechanical ventilation, eating disorders, patients in the postoperative period, other chronic psychiatric or neurological diseases with cachexia, and burns. No upper or lower limit on the number of enrolled patients was established. This was at the discretion of the principal investigator at the centre.
The bioethics committee of the Pomeranian Medical University (KB-0012/42/03/2021/Z) gave permission to conduct the study due to the retrospective and non-experimental nature of the study, and the patients’ informed consent was waived.
Demographic, anthropometric (sex, age, weight, height, BMI) and laboratory data were collected from patients’ medical records at three time points: the day of ICU admission, the eighth day of ICU stay and the day of ICU discharge (or death). In addition, the study protocol required the collection of information on the method of respiratory support (non-invasive/invasive ventilation), the supply of catecholamines and the initiation of renal replacement therapy and ventilation in the prone position. On admission to the ICU, the following scales were also assessed: Nutrition Risk Score (NRS) 2002, Sequential Organ Failure Assessment (SOFA) and Clinical Frailty Score (CFS).
Clinical nutrition data including the method of diet supply and the caloric content of the diet (excluding calories delivered in non-dietary forms, e.g. propofol for sedation, glucose as a drug solvent or citrates during continuous renal replacement therapy) were only analysed on the eighth day of hospitalisation. The Harris-Benedict equation (HB) [13], Mifflin St. Jeor equation (MsJ) [14] and the formula recommended by ESPEN (20 kcal kg–1 body weight (b.w.)) [3] were used to calculate energy requirements. Protein requirements were calculated according to ESPEN guidelines (1.3 g kg–1 b.w. and 1 g kg–1 b.w.) [3, 7]. Information on complications of clinical nutrition observed in the study group throughout the eight day of ICU stay was also collected. These included regurgitation, vomiting, diarrhoea, retention of residual volume in the probe, flatulence and abdominal pain, and hyperglycaemia.
Statistical analysis
Statistical analysis was performed using MedCalc v.18 software (MedCalc Software, Ostend, Belgium). Quantitative variables were presented using medians and interquartile ranges (IQR). Qualitative data were expressed as frequencies and percentages. The Kruskal-Wallis test was used to verify differences between groups for independent continuous variables. The χ2 test or Fisher’s exact test was used to verify differences for qualitative variables. The correlation between variables was calculated using the Spearman coefficient. All tests were two-sided. The level of statistical significance was P < 0.05.
RESULTS
From the total group of 233 recruited subjects, 129 patients met the inclusion criteria and underwent analysis of nutritional therapy. Out of the 233 patients assessed on day one, 95 patients died before the 8th day, and 9 were transferred to another ward. Table 1 shows the demographic and clinical characteristics of the patients. Detailed characteristics of laboratory parameters assessed on the day of ICU admission are presented in Table 2.
TABLE 1.
Demographic and clinical characteristics of patients on day of ICU admission
| Category | Katowice n = 43 |
Gdansk n = 42 |
Krakow n = 14 |
Zabrze n = 11 |
Opole n = 19 |
In total N = 129 |
|
|---|---|---|---|---|---|---|---|
| Men, n (%) | 27 (63) | 27 (64) | 6 (43) | 9 (82) | 17 (89) | 86 (66.67) | |
| Age (years) | 61 (52.5–69.5) | 67 (53.5–73.0) | 66.5 (54.0–72.0) | 55 (48.0–63.5) | 48 (42.5–49.5) | 61 (49.0–70.0) | |
| BMI (kg m–2) | 29 (25–34) | 28.6 (26–32.5) | 30.9 (26.5–35) | 28 (25–29) | 29 (26–33.6) | 28.9 (26–33) | |
| NRS 2002 (points) | 4 (3–4) | 3 (3–4) | 3 (3–4) | 4 (4–4) | 3 (3–3) | 3 (3–4) | |
| CFS (points) | 3 (2–4) | 6 (4–9) | – | 2 (2–4) | – | 4 (3–5) | |
| SOFA (points) | 7 (5–12) | 7 (5–10) | – | 8 (6–9) | 7 (5–7) | 7 (5–10) | |
| Mechanical ventilation, n (%) | 28 (65.12) | 41 (97.62) | 13 (92.86) | 8 (72.73) | 19 (100) | 109 (84.50) | |
| Non-invasive mechanical ventilation, n (%) | 16 (37.21) | 4 (9.52) | 1 (7.14) | 0 (0.00) | 0 (0.00) | 21 (16.28) | |
| Use of catecholamines, n (%) | 29 (67.44) | 35 (83.33) | 9 (64.29 | 3 (27.28) | 13 (68.42) | 89 (68.99) | |
| Renal replacement therapy, n (%) | 7 (16.28) | 2 (4.76) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 9 (6.98) | |
| Comorbidities, n (%) | |||||||
| DM | 10 (23.26) | 12 (28.57) | 6 (42.86) | 3 (27.28) | 3 (15.79) | 31 (24.03) | |
| COPD | 3 (6.98) | 3 (7.14) | 2 (14.29) | 1 (9.09) | 0 (0.00) | 9 (6.98) | |
| Asthma | 2 (4.65) | 3 (7.14) | 1 (7.14) | 0 (0.00) | 0 (0.00) | 6 (4.65) | |
| Obesity | 20 (46.51) | 24 (57.14) | 8 (57.14) | 4 (36.37) | 9 (47.37) | 65 (50.39) | |
| Nicotine dependence | 4 (9.30) | 1 (2.38) | 0 (0.00) | 2 (18.19) | 0 (0.00) | 7 (5.43) | |
BMI – body mass index, NRS 2002 – Nutritional Risk Score, CFS – Clinical Frailty Scale, SOFA – Sequential Organ Failure Assessment Score, PaO2 – partial pressure of oxygen in arterial blood, FiO2 – fraction of inspired oxygen, ARDS – acute respiratory distress syndrome, DM – diabetes mellitus, COPD – chronic obstructive pulmonary disease
TABLE 2.
Results of selected laboratory tests on day of admission
| Category | Katowice n = 43 |
Gdansk n = 42 |
Krakow n = 14 |
Zabrze n = 11 |
Opole n = 19 |
In total N = 129 |
|---|---|---|---|---|---|---|
| Glucose (mg dL–1) | 214 (143.5–272) | 141 (109–217) | 216.9 (148–264) | 115.6 (94.7–140) | 180.5 (146.0–234.5) | 174 (129–241.5) |
| Total protein (g L–1) | 55 (50.8–59) | 53 (49–58) | 60.2 (54–66.6) | 56.5 (50–65) | 54.5 (51.4–58.7) | 53.8 (46.6–59) |
| Albumin (mg mL–1) | 27.8 (25–31.7) | 23 (21–25) | 30.6 (28.05–32) | 26 (25–27.5) | 28 (23–30) | 25 (21–28) |
| C-reactive protein (mg L–1) | 126.8 (69–159) | 107 (47–229) | 167 (110–196) | 160 (43–202) | 68 (29.0–166.0) | 117 (48–191.8) |
| Total cholesterol (mg dL–1) | 131 (107–179) | 132 (108–154) | 137 (121–168) | 147 (136.5–178) | – | 134 (111–167) |
| Triglycerides (mg dL–1) | 182 (148.5–397) | 155 (117–201) | 185.5 (149.8–276.8) | 253 (225–379) | – | 185 (134–281.5) |
| Urea (mg dL–1) | 66.6 (45.7–101.75) | 31 (16–44) | 78 (53.5–84) | 44 (26.43–97) | 56 (43–76) | 46 (30–76) |
| Creatinine (mg dL–1) | 1.12 (0.76–1.74) | 0.93 (0.72–1.26) | 0.87 (0.7–1.55) | 0.66 (0.53–1.08) | 0.68 (0.59–1) | 0.91 (0.65–1.3) |
| Sodium (mmol L–1) | 140 (134.6–144) | 140 (137–146) | 145.5 (140–150) | 137 (133–139) | 142 (139–144) | 140 (137–144) |
| Potassium (mmol L–1) | 4.27 (3.97–4.6) | 4.4 (4–4.9) | 4.2 (4.15–4.8) | 4.68 (4.35–5.14) | 4 (3.65–4.63) | 4.3 (4–4.8) |
| Phosphorus (mg dL–1) | 4 (3.35–5.30) | 3.8 (3–4.8) | 3.75 (3.56–5.64) | 4.37 (2.71–5.25) | 3.3 (1.65–4.7) | 3.8 (3–4.98) |
The patients included in the study spent an average of 5 (IQR 1–10) days in hospital in other wards before ICU admission. Mortality during the entire hospitalisation was 45.7% (n = 59).
Nutrition assessment on the eighth day of hospitalization was carried out in 129 patients. Enteral nutrition was the preferred method of diet supply in 3 of the 5 centres (Gdansk, Krakow, Zabrze). Enteral nutrition and combined nutrition (supplementary parenteral nutrition) were used in the centre in Opole. In only one of the analysed ICUs (Katowice), three forms of daily diet supply were used. This centre also observed the highest percentage of patients ventilated in the prone position on the eighth day of hospitalisation (58%, n = 26). In all centres, the highest proportion of calories was supplied in the enteral form. Compared to the centres in Gdansk, Krakow, Zabrze and Opole, parenteral nutrition was more frequently chosen in the centre in Katowice (Table 3). Two of the observed centres described complications of nutrition therapy on the eighth day of hospitalisation. The Katowice clinic reported adverse events during clinical nutrition in 21 patients (49%), while in the centre in Gdansk, complications were observed in 27 hospitalised patients (64%).
TABLE 3.
Type of nutrition therapy applied to ICU patients on the eighth day of stay
| Category | Katowice n = 43 |
Gdansk n = 42 |
Krakow n = 14 |
Zabrze n = 11 |
Opole n = 19 |
In total n = 129 |
|---|---|---|---|---|---|---|
| EN, n (%) | 9 (21) | 42 (100) | 13 (93) | 9 (82) | 5 (26) | 78 (61) |
| PN, n (%) | 18 (42) | 0 (0) | 1 (7) | 2 (18) | 0 (0) | 21 (16.5) |
| SPN, n (%) | 16 (37) | 0 (0) | 0 (0) | 0 (0) | 14 (74) | 30 (23.5) |
EN – enteral nutrition, PN – parenteral nutrition, SPN – supplemental parenteral nutrition.
On the eighth day of the ICU stay proper caloric fulfilment according to ESPEN guidelines was implemented in 14.8% of the included patients. The patients were provided with 66% of their calorie requirements, considering the indications according to the Harris-Benedict equation (HB), 66.7% considering the Mifflin-St. Jeor equation (MSJ), and 62.5% considering the formula recommended by ESPEN. Only two wards managed to provide patients with a calorie supply exceeding 70% of the calculated calorie requirements (Krakow and Opole). The fulfilment of calorie requirements did not differ significantly between the centres in Katowice, Gdansk, Zabrze and Krakow, regardless of the formula considered (HB: P = 0.1, MSJ: P = 0.09, ESPEN: P = 0.1). Calorie supply was significantly higher in the centre in Opole compared to the centres in Katowice, Gdansk and Zabrze (HB: 93, IQR 114–78, P = 0.01, MSJ: 102, IQR 120–84, P = 0.01, ESPEN: 94, IQR 117–75, P = 0.006).
On the eighth day of the ICU stay the proper fulfilment of the 1 g kg–1 b.w. ESPEN guideline protein threshold was implemented among 33.8% of all included patients and the 1.3 g kg–1 b.w. threshold among 15% of the whole study population. The fulfilment of protein requirements calculated according to the formula recommended by ESPEN (1 g kg–1 b.w.) was 70%. The centre in Krakow achieved a protein supply of 63% on the eighth day while maintaining a calorie supply of 80-90% depending on the formula considered. The highest protein supply was 157% of daily requirements and was observed in the ICU in Opole. Compared to the other centres, the hospital in Opole provided patients with significantly more protein on the eighth day of the ICU stay (ESPEN 1 g kg–1 b.w.: 159, IQR 188–113, P < 0.01; ESPEN 1.3 g kg–1 b.w.: 122, IQR 144–87, P < 0.01).
Figure 1 shows the energy supply and Figure 2 shows the protein supply on the eighth day of ICU hospitalisation, presented as a percentage of calculated daily requirements. There was no correlation between the method and degree of implementation of clinical nutrition and patients’ biochemical test results on the eighth day. The supply of calories and protein did not differ significantly depending on the type of respiratory support. Three centres described in the study used ventilation in the prone position on the eighth day of ICU hospitalization. In Katowice, mechanical ventilation in the prone position was used in 26 patients (58%), in Gdansk in 5 patients (12%) and in Krakow in 2 patients (14%). Patients ventilated in the prone position (irrespective of the type of respiratory support) received fewer calories, assessed using both the Harris-Benedict and Mifflin-St. Jeor equations, and less protein, determined both according to the 1 g kg–1 b.w. formula and the 1.3 g kg–1 b.w. formula (Table 4).
FIGURE 1.
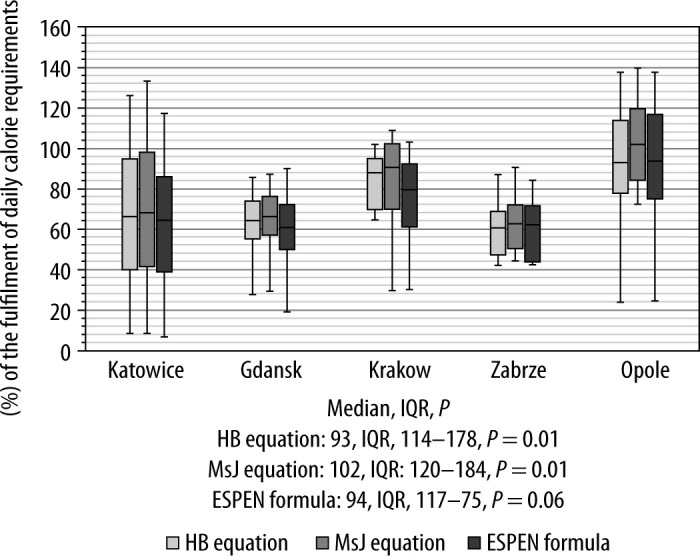
Percentage of fulfilment of daily calorie requirements in patients on the eighth day of ICU hospitalisation. HB formula – calorie requirement fulfilment according to the Harris-Benedict equation, MsJ formula – calorie requirement fulfillment according to the Mifflin St. equation, ESPEN formula – calorie requirement fulfilment according to the formula recommended by ESPEN (20 kcal kg–1 body weight)
FIGURE 2.
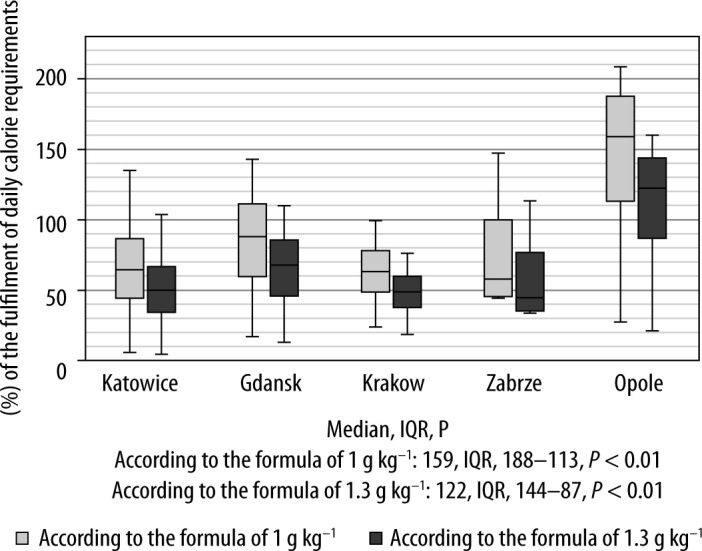
Percentage of the fulfilment of daily protein requirement in patients on the eighth day of ICU hospitalisation. According to the formula of 1 g kg–1 – fulfilment of the protein requirements in relation to the recommendation of 1 g kg–1 of body weight (light grey), according to the formula of 1.3 g kg–1 – fulfilment of the protein requirements in relation to the recommendation of 1.3 g kg–1 of body weight (dark grey)
TABLE 4.
Fulfilment of calorie and protein requirements on the eighth day of ICU stay depending on the patient’s position.
| Category | Prone | No prone | P-value |
|---|---|---|---|
| HB (kcal) | 64.97 IQR 43.9-77.8 | 71.5 IQR 59.2-92.6 | < 0.042 |
| MSJ (kcal) | 66.7 IQR 46.8-80.0 | 75.4 IQR 51.5-96.3 | < 0.048 |
| Protein requirement (formula of 1 g kg-1 b.w.) | 59.6 IQR 43.6-84.0 | 86.9 IQR 58.1-120.5 | < 0.0002 |
| Protein requirement (formula of 1.3 g kg-1 b.w.) | 45.9 IQR 33.5-64.6 | 66.9 IQR 44.7-92.7 | < 0.0002 |
HB – fulfilment of requirements according to the Harris-Benedict equation, MsJ – fulfilment of requirements according to the Mifflin St. Jeor equation, according to the formula of 1 g kg–1 – fulfilment of the protein requirement in relation to the recommendation of 1 g kg–1 of b.w., according to the formula of 1.3 g kg–1 – fulfilment of the protein requirement in relation to the recommendation of 1.3 g kg–1 of b.w.
DISCUSSION
Appropriate nutritional intervention is an essential component of treatment, and the nutritional status of any SARS-CoV-2-infected patient should be assessed before applying general treatment [15]. Many factors, such as older age, multimorbidity, reduced oral calorie intake, prolonged ICU stay, increased catabolism and inflammation, lead to malnutrition, which may be associated with a poorer patient prognosis [7]. There are many factors that can contribute to body wasting in COVID-19; they include loss of appetite and taste, fever and inflammation, immobilisation, as well as general malnutrition, catabolic-anabolic imbalance, endocrine dysfunction, and organ-specific complications of COVID-19 such as cardiac and renal dysfunction [16]. Determining the direct impact of clinical nutrition is complicated by the fact that the severity of illnesses and the number of comorbidities in adult ICU patients are increasing [2]. Polish centres conducting clinical nutrition are mainly based on ESPEN guidelines, which allow for discretion in some aspects of patient nutrition due to the lack of adequate research on clinical nutrition in COVID-19 infection. This is probably the main reason why patients are fed differently depending on the centre.
For mechanically ventilated patients, indirect calorimetry should be the standard for determining calorie requirements [17]. However, its use is limited in daily practice both because of the low availability of highly specialised equipment and, in the case of infectious diseases, due to the potentially higher risk of exposure of medical professionals to COVID-19 [17]. In line with initial assumptions, calorie targets in this study were estimated using the Harris-Benedict equation, Mifflin St. Jeor equation and ESPEN recommendations (20 kcal kg–1) [3]. According to the specified guidelines, the estimated calorie requirements were met by the centres in Krakow and Opole. However, it is worth remembering that prediction equations have limitations and often lead to large errors in estimating the true needs of patients [18].
One of the first studies to analyse outcomes among COVID-19 patients in the Polish population showed lower mortality compared to this study [19]; however, in the present study the observational period was longer, and the analysed sample contained more individuals, which may have affected the outcomes.
Observational studies have shown that a higher protein supply is associated with lower mortality in critically ill patients [20, 21]. The use of high-protein formulas or additional protein supplementation to ensure target values may be beneficial when the basic composition of nutrients available in the ward armamentarium makes it difficult to provide the correct amount of protein [22]. Nevertheless, a recent study showed that the administration of too high doses of protein (2.2 g kg–1 b.w.) may worsen the treatment outcome in highly burdened patients [23]. ESPEN guidelines recommend a protein supply of 1.3 g kg–1 b.w., but in daily practice, it has been observed that the amount of protein provided to ICU patients is lower in relation to the large losses caused by proteolysis and muscle loss due to critical illness, which further hinders the achievement of protein targets [7, 24]. In the study presented here, protein requirements were met to the greatest extent by the centre in Opole.
Current ESPEN guidelines for clinical nutrition in the ICU show the superiority of enteral over parenteral nutrition, even among patients with ARDS ventilated in the prone position [7]. In the study described above, three centres based clinical nutrition mainly on the enteral supply of energy, macronutrients and micronutrients. In the centre in Katowice, almost half of the patients received calories only parenterally. It is worth noting that although the centres differed as to the percentage of parenteral nutrition in the history of patients’ stay (P < 0.05), the number of patients experiencing nutritional complications was comparable for the same group of patients from the centre in Gdansk, where all patients received enteral nutrition. All the complications described in the study could have led to the need to reduce the doses of nutritional preparations or, ultimately, to completely switch to parenteral nutrition. Nutritional complications are a major problem in daily medical practice and may also result in poorer compliance in patients rotated for better ventilation, as in the case of COVID-19 patients [25]. Current guidelines recommend that enteral nutrition should be maintained during ventilation in a prone position [26]. In cases of an increased number of complications, enteral nutrition is reduced or converted to parenteral nutrition to simultaneously provide patients with nutrients and ensure the best possible body position in the context of the underlying disease. In the study described, it was noted that in the case of centres that used the prone position more frequently, the inclusion of parenteral nutrition was also observed more often. This did not have a major impact on the degree of compliance with dietary recommendations. However, studies show that the use of ventilation in a prone position has a significant impact on the nutritional care of critically ill patients with SARS-CoV-2, reducing calorie and protein intake [27].
LIMITATIONS OF INFERENCE
Limitations of this study include its retrospective nature. It should be emphasised that the patients analysed constituted a specific group of patients in whom the severity of the condition and the additional physical barrier between the medical professionals and the patient may have affected the quality of the data obtained (e.g. body mass index assessment). Moreover, due to the multicentre nature of the study, medical records were kept by several therapeutic teams, resulting in differences in the form of data collection and affecting the number of properly recorded observations. Furthermore, the multicentre nature of the study carries the risk of individual selection of patients, which cannot be objectified, as in the case of a one-centre study, which increases the risk of selection and observational bias. Including a larger number of patients in the study would have optimised the analyses performed, but it is currently not possible to continue the study. Furthermore, due to organisational constraints, the study protocol contained three measurement points and did not describe in detail the patients’ conditions after the eighth day of hospitalisation. Including consecutive days of patients’ stay in the study could have contributed to further interesting results in long-term observation. Finally, no sample size calculations were performed, as it was impossible from a clinical point of view due to the novelty of the investigation, and from a statistical point of view due to the observational nature of this study. To our knowledge, there is no reference study to perform such a calculation. We were unable to make any reasonable comparison with previous data.
CONCLUSIONS
The clinical nutrition of critically ill patients with COVID-19 varied significantly among intensive care units. In this study, protein and energy requirements on the eighth day of ICU stay were not fulfilled in most of the participating centres. Although the baseline condition and the methods used to treat acute respiratory distress syndrome may affect the supply of energy and protein, standardisation of the nutritional procedure should also be sought in this specific patient group. The lack of multicentre studies describing the direct impact of specific nutritional interventions on the treatment outcomes of critically ill patients with COVID-19 leaves room for further analyses.
ACKNOWLEDGEMENTS
Assistance with the article
none.
Financial support and sponsorship
none.
Conflicts of interest
none.
Presentation
none.
References
- 1.https://stat.gov.pl/download/gfx/portalinformacyjny/pl/defaultaktualnosci/5513/1/12/1/zdrowie_i_ochrona_zdrowia_2021.pdf.
- 2.Singer P, Weinberger H, Tadmor B. Which nutritional regimen for the comorbid complex intensive care unit patient? World Rev Nutr Diet 2013; 105: 169-178. doi: 10.1159/000341294. [DOI] [PubMed] [Google Scholar]
- 3.Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr 2019; 38: 48-79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 4.Wischmeyer PE. Tailoring nutrition therapy to illness and recovery. Crit Care 2017; 21 (Suppl 3): 316. doi: 10.1186/s13054-017-1906-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Compher C, Bingham AL, McCall M, et al. Guidelines for the provision of nutrition support therapy in the adult critically ill patient: the American Society for Parenteral and Enteral Nutrition. JPEN J Parenter Enteral Nutr 2022; 46: 12-41. doi: 10.1002/jpen.2267. [DOI] [PubMed] [Google Scholar]
- 6.White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy Malnutrition Work Group; A.S.P.E.N. Malnutrition Task Force; A.S.P.E.N. Board of Directors . Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). JPEN J Parenter Enteral Nutr 2012; 36: 275-283. doi: 10.1177/0148607112440285. [DOI] [PubMed] [Google Scholar]
- 7.Barazzoni R, Bischoff SC, Breda J, et al.; endorsed by the ESPEN Council . ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr 2020; 39: 1631-1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507-513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antwi J, Appiah B, Oluwakuse B, Abu BAZ. The Nutrition-COVID-19 Interplay: a Review. Curr Nutr Rep 2021; 10: 364-374. doi: 10.1007/s13668-021-00380-2. Epub 2021 Nov 27. Erratum In: Curr Nutr Rep 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 2020; 395: 1763-1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang P, He Z, Yu G, et al. The modified NUTRIC score can be used for nutritional risk assessment as well as prognosis prediction in critically ill COVID-19 patients. Clin Nutr 2021; 40: 534-541. doi: 10.1016/j.clnu.2020.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leoni MLG, Moschini E, Beretta M, Zanello M, Nolli M. The modified NUTRIC score (mNUTRIC) is associated with increased 28-day mortality in critically ill COVID-19 patients: Internal validation of a prediction model. Clin Nutr ESPEN 2022; 48: 202-209. doi: 10.1016/j.clnesp.2022.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roza AM, Shizgal HM. The Harris Benedict equation reevaluated: resting energy requirements and the body cell mass. Am J Clin Nutr 1984; 40: 168-182. doi: 10.1093/ajcn/40.1.168. [DOI] [PubMed] [Google Scholar]
- 14.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 1990; 51: 241-247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 15.Short KR, Kedzierska K, van de Sandt CE. Back to the future: lessons learned from the 1918 influenza pandemic. Front Cell Infect Microbiol 2018; 8: 343. doi: 10.3389/fcimb.2018.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anker MS, Landmesser U, von Haehling S, Butler J, Coats AJS, Anker SD. Weight loss, malnutrition, and cachexia in COVID-19: facts and numbers. J Cachexia Sarcopenia Muscle 2021; 12: 9-13. doi: 10.1002/jcsm.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ochoa JB, Cárdenas D, Goiburu ME, Bermúdez C, Carrasco F, Correia MITD. Lessons learned in nutrition therapy in patients with severe COVID-19. JPEN J Parenter Enteral Nutr 2020; 44: 1369-1375. doi: 10.1002/jpen.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bendavid I, Lobo DN, Barazzoni R, et al. The centenary of the Harris-Benedict equations: How to assess energy requirements best? Recom-mendations from the ESPEN expert group. Clin Nutr 2021; 40: 690-701. doi: 10.1016/j.clnu.2020.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Piwowarczyk P, Szczukocka M, Kutnik P, et al. Risk factors and outcomes for acute respiratory failure in coronavirus disease 2019: an observational cohort study. Adv Clin Exp Med 2021; 30: 165-171. doi: 10.17219/acem/130603. [DOI] [PubMed] [Google Scholar]
- 20.Zusman O, Theilla M, Cohen J, Kagan I, Bendavid I, Singer P. Resting energy expenditure, calorie, and protein consumption in critically ill patients: a retrospective cohort study. Crit Care 2016; 20: 367. doi: 10.1186/s13054-016-1538-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song JH, Lee HS, Kim SY, et al. The influence of protein provision in the early phase of intensive care on clinical outcomes for critically ill patients on mechanical ventilation. Asia Pac J Clin Nutr 2017; 26: 234-240. doi: 10.6133/apjcn.032016.01. [DOI] [PubMed] [Google Scholar]
- 22.Taylor S, Dumont N, Clemente R, Allan K, Downer C, Mitchell A. Critical care: meeting protein requirements without overfeeding energy. Clin Nutr ESPEN 2016; 11: e55-e62. doi: 10.1016/j.clnesp.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Heyland DK, Patel J, Compher C, et al.; EFFORT Protein Trial team . The effect of higher protein dosing in critically ill patients with high nutritional risk (EFFORT Protein): an international, multicentre, pragmatic, registry-based randomised trial. Lancet 2023; 401: 568-576. doi: 10.1016/S0140-6736(22)02469-2. [DOI] [PubMed] [Google Scholar]
- 24.Wischmeyer PE. Tailoring nutrition therapy to illness and recovery. Crit Care 2017; 21 (Suppl 3): 316. doi: 10.1186/s13054-017-1906-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alves de Paula J, Rabito EI, Justino SR, et al. Administration of enteral nutrition and gastrointestinal complications in Covid-19 critical patients in prone position. Clin Nutr Open Sci 2022; 45: 80-90. doi: 10.1016/j.nutos.2022.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruni A, Garofalo E, Grande L, et al. Nursing issues in enteral nutrition during prone position in critically ill patients: a systematic review of the literature. Intensive Crit Care Nurs 2020; 60: 102899. doi: 10.1016/j.iccn.2020.102899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lew CCH, Ng PS, Wong KW, et al. Nutrition support practices for critically ill patients with severe acute respiratory syndrome coronavirus-2: a multicentre observational study in Singapore. Ann Acad Med Singap 2022; 51: 329-340. doi: 10.47102/annals-acadmedsg.202231. [DOI] [PubMed] [Google Scholar]


