ABSTRACT
Mycobacterium abscessus (Mab) affects patients with immunosuppression or underlying structural lung diseases such as cystic fibrosis (CF). Additionally, Mab poses clinical challenges due to its resistance to multiple antibiotics. Herein, we investigated the synergistic effect of dual β-lactams [sulopenem and cefuroxime (CXM)] or the combination of sulopenem and CXM with β-lactamase inhibitors [BLIs—avibactam (AVI) or durlobactam (DUR)]. The sulopenem-CXM combination yielded low minimum inhibitory concentration (MIC) values for 54 clinical Mab isolates and ATCC19977 (MIC50 and MIC90 ≤0.25 µg/mL). Similar synergistic effects were observed in time-kill studies conducted at concentrations achievable in clinical settings. Sulopenem-CXM outperformed monotherapy, yielding ~1.5 Log10 CFU/mL reduction during 10 days. Addition of BLIs enhanced this antibacterial effect, resulting in an additional reduction of CFUs (~3 Log10 for sulopenem-CXM and AVI and ~4 Log10 for sulopenem-DUR). Exploration of the potential mechanisms of the synergy focused on their interactions with L,D-transpeptidases (Ldts; LdtMab1–LdtMab4), penicillin-binding-protein B (PBP B), and D,D-carboxypeptidase (DDC). Acyl complexes, identified via mass spectrometry analysis, demonstrated the binding of sulopenem with LdtMab2–LdtMab4, DDC, and PBP B and CXM with LdtMab2 and PBP B. Molecular docking and mass spectrometry data suggest the formation of a covalent adduct between sulopenem and LdtMab2 after the nucleophilic attack of the cysteine residue at the β-lactam carbonyl carbon, leading to the cleavage of the β-lactam ring and the establishment of a thioester bond linking the LdtMab2 with sulopenem. In conclusion, we demonstrated the biochemical basis of the synergy of sulopenem-CXM with or without BLIs. These findings potentially broaden the selection of oral therapeutic agents to combat Mab.
IMPORTANCE
Treating infections from Mycobacterium abscessus (Mab), particularly those resistant to common antibiotics like macrolides, is notoriously difficult, akin to a never-ending struggle for healthcare providers. The rate of treatment failure is even higher than that seen with multidrug-resistant tuberculosis. The role of combination β-lactams in inhibiting L,D-transpeptidation, the major peptidoglycan crosslink reaction in Mab, is an area of intense investigation, and clinicians have utilized this approach in the treatment of macrolide-resistant Mab, with reports showing clinical success. In our study, we found that cefuroxime and sulopenem, when used together, display a significant synergistic effect. If this promising result seen in lab settings, translates well into real-world clinical effectiveness, it could revolutionize current treatment methods. This combination could either replace the need for more complex intravenous medications or serve as a “step down” to an oral medication regimen. Such a shift would be much easier for patients to manage, enhancing their comfort and likelihood of sticking to the treatment plan, which could lead to better outcomes in tackling these tough infections. Our research delved into how these drugs inhibit cell wall synthesis, examined time-kill data and binding studies, and provided a scientific basis for the observed synergy in cell-based assays.
KEYWORDS: sulopenem, oral carbapenem, Mycobacterium abscessus, dual β-lactams
INTRODUCTION
Mycobacterium abscessus (Mab), a nontuberculous mycobacterium (NTM), is well known for its recalcitrance to treatment, presenting a formidable challenge for clinicians (1). Eradication of Mab infection is challenging, given its innate resistance to most antituberculous medications, proclivity to form biofilms, need for a multidrug regimen with associated toxicity, longer duration of therapy, predilection for infecting immunocompromised patients with underlying lung diseases, and the challenge to mount an effective immune response to clear the infection (2). In the presence of macrolide resistance genes (erm41 and rrl) in subsp. abscessus, induced or acquired, respectively, treatment failure rates can soar up to 70% in some reports, exceeding those observed with multidrug-resistant tuberculosis (MDR-TB) and even extensively drug-resistant TB (XDR-TB) (3, 4). In light of these challenges, significant research efforts have focused on identifying essential genes and targets for drug development, including the repurposing of β-lactams alone or in combination therapies.
Mab contains a class A β-lactamase enzyme, known as BlaMab (5), which is capable of hydrolyzing penicillins and cephalosporins. Penems appear to be more resistant to hydrolysis (5). Despite the presence of BlaMab, current treatment guidelines do not address this issue (6). Older β-lactamase inhibitors (BLIs), such as clavulanic acid and sulbactam, are ineffective against BlaMab (5). However, recent research has identified that the newly developed diazabicyclooctane (DBO) class of BLIs [avibactam (AVI), nacubactam, and zidebactam] exhibit activity against BlaMab; some DBO’s even demonstrate intrinsic antimicrobial activity but, the significance of this is unclear (7–12). Durlobactam (DUR) is a DBO compound with an expanded spectrum of activity compared with other DBOs, demonstrating robust inhibition against class A, C, and D serine β-lactamases. DUR’s potential to enhance in vitro susceptibility when combined with β-lactams, accompanied by the elucidation of a biochemical rationale underlying its mode of action, was previously reported (7).
The peptidoglycan structure of Mab is notably divergent from that of most bacterial species, primarily owing to its synthesis involving both L,D-transpeptidases and penicillin-binding proteins (PBPs), which include the following two protein classes: D,D-transpeptidases and D,D-carboxypeptidase. D,D-transpeptidases play a pivotal role in the synthesis of peptidoglycan, whereas D,D-carboxypeptidases are responsible for catalyzing the removal of terminal amino acids from peptidoglycan sidechains. Several essential PBPs, including PBP-lipo (MAB_3167 c), PBP B (MAB_2000), DacB1, and DDC, were investigated, revealing that their “knockout” resulted in a synergistic growth-inhibition effect (8–10).
Most recently, the role of combination β-lactams in inhibiting L,D-transpeptidation, the major peptidoglycan crosslink reaction in Mab, has become an area of intense investigation. The five L,D-transpeptidases (LDTMab1-5) (11), which are essential enzymes for catalyzing transpeptidation during cell wall synthesis, were previously studied and were found to be inhibited by carbapenems and cephalosporins, but not penicillins (12). These reports suggested that by combining two β-lactams with or without a β-lactamase inhibitor, multiple targets in the cell wall synthesis pathway can be inactivated (7, 11, 13), leaving the cell wall in a compromised state. The synergistic effects of dual β-lactams (imipenem and ceftaroline combination) (11) were achieved through the inhibition of multiple target proteins. Emerging clinical data further corroborate the effectiveness of dual β-lactam combinations (14–16). However, the coadministration of imipenem and cefoxitin, which are the sole two β-lactams recommended in the guidelines for Mab treatment, has undergone clinical trials with outcomes lacking clear evidence of efficacy (17). These observations indicate the necessity for an optimized β-lactam treatment strategy and lend support to the hypothesis that achieving optimal β-lactam therapy may entail the simultaneous targeting of multiple proteins within the intricate and highly redundant network of enzymes participating in peptidoglycan biosynthesis.
Sulopenem, formerly known as CP-70,429, is a novel broad-spectrum thiopenem β-lactam compound with a structure similar to that of other carbapenems, but with modifications that provide enhanced stability and activity against several β-lactamase enzymes (18). The molecule has a small molecular weight (345 Da) and contains a β-lactam ring with a hydroxyethyl group attached at C6 and a thiazolidine ring fused to it (Fig. 1). The side chain attached to the C2 possesses a sulfoxide group that enhances the compound’s activity against gram-negative bacteria such as the Enterobacterales (18, 19). These structural features are unique and make it a potential new treatment option for drug-resistant bacterial infections. Sulopenem safety and efficacy are currently being evaluated in phase three clinical trial [NCT03357614, entitled “Sulopenem for the Treatment of Complicated Urinary Tract Infections” (SUNSHINE)]. In 2020, sulopenem received a Qualified Infectious Disease Product (QIDP) designation from the US Food and Drug Administration. Due to its availability in oral formulation, sulopenem offers clear advantages for the patient and compliance with the dosing regimen; however, it necessitates coadministration with probenecid to mitigate renal clearance, as its oral bioavailability typically falls within the range of 30% to 40% (18).
FIG 1.
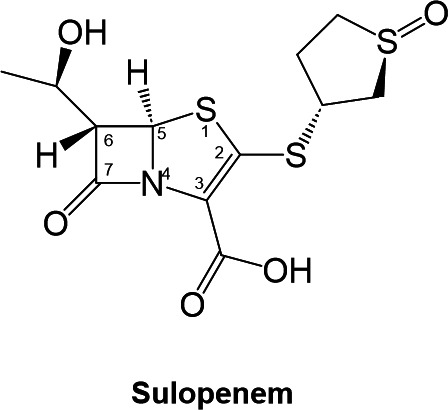
Sulopenem chemical representation.
In this study, we used cell-based and in vitro static concentration time-kill (SCTK) assays, along with other biochemical techniques, to extend the previous investigation that examined imipenem and ceftaroline. We assessed the synergistic activities of sulopenem and cefuroxime, and we also tested whether the presence or absence of β-lactamase inhibition would further demonstrate improved susceptibility. Our hypothesis is that sulopenem, an oral penem antibiotic, targets multiple L,D-transpeptidases, LdtMab2–LdtMab4, DDC, and PBP B. The conserved active site His333 activates the catalytic cystine (Cys351) to drive acyl enzyme formation with sulopenem, resulting in significant improvements in microbial killing of Mab both in the presence or absence of β-lactamase inhibition.
RESULTS
Sulopenem exhibits in vitro activity comparable with imipenem
An initial evaluation of the in vitro antimicrobial activity of sulopenem was conducted on the Mab ATCC 19977 strain, using Middlebrook 7H9 broth supplemented with 10% (vol/vol) oleic albumin dextrose catalase and 0.05% (vol/vol) Tween 80. The minimal inhibitory concentration (MIC) for sulopenem alone was found to be 1 µg/mL. Further susceptibility testing with sulopenem against a set of 54 previously characterized Mab subsp. abscessus clinical isolates demonstrated MICs similar to that of ATCC 19977, with a MIC50 of 2 µg/mL and a MIC90 of 4 µg/mL (Tables 1 and 2). These values are comparable with the previously reported MIC50 and MIC90 values of imipenem (11).
TABLE 1.
Comparative MIC50s and MIC90s for 54 Mab clinical strains and ATTCC 19977: evaluating sulopenem (SUL), cefuroxime (CXM), and combined sulopenem with 4 µg/mL cefuroxime
| Antibiotics (μg/mL) | MIC range (μg/mL) | MIC50 (μg/mL) | MIC90 (μg/mL) |
|---|---|---|---|
| CXM | 8–64 | 16 | 32 |
| SUL | 1–8 | 2 | 4 |
| SUL +CXM (4 µg/mL) | ≤ 0.25–8 | ≤ 0.25 | ≤ 0.25 |
TABLE 2.
MIC50s for Mab ATTCC 19977: evaluating sulopenem (SUL), cefuroxime (CXM), combination of sulopenem and cefuroxime, and addition of β-lactamase inhibitors [1 µg/mL of dudrlobactam (DUR) +sulbactam (SULB) or 4 µg/mL of avibactam (AVI)]
| MIC50 (μg/mL) | ||||
|---|---|---|---|---|
| Antibiotics (μg/mL) | Alone | + DUR + SULB (1:1) |
+ DUR + SULB (1 µg/mL) |
+ AVI (4 µg/mL) |
| DUR + SULB (1:1 ratio) | 8 | –a | – | – |
| AVI | >128 | – | – | – |
| SUL | 1 | 0.25 | 1 | 0.5 |
| CXM | 4 | 1 | 2 | 4 |
| SUL (0.25 µg/mL) +CXM | 1 | – | – | 0.5 |
| SUL +CXM (2 µg/mL) | ≤0.0625 | ≤0.0625 | ≤0.0625 | ≤0.0625 |
| SUL +CXM (4 µg/mL) | ≤0.0625 | ≤0.0625 | ≤0.0625 | ≤0.0625 |
"–” Means ”emphasis”.
Checkerboard assay reveals 4 µg/mL CXM concentration as synergistic in combination with sulopenem
A checkerboard assay was conducted to assess the potential synergistic effect of sulopenem and CXM against Mab strain ATCC 19977. The checkerboard assay was designed to simultaneously evaluate the inhibitory activities of both sulopenem and CXM in a two-dimensional matrix, where serial dilutions of sulopenem were combined with CXM. The MIC of CXM against Mab ATCC 19977 was determined to be in the range of 16–32 µg/mL. Based on this MIC value, a concentration of 4 µg/mL CXM was selected for inclusion in the assay to evaluate its potential synergy with sulopenem.
Synergistic effects of sulopenem and CXM combination against Mab subsp. abscessus clinical isolates
CXM susceptibility against the 54 clinical isolates was subsequently tested. As anticipated, CXM demonstrated diminished activity against Mab clinical isolates in comparison to sulopenem, with a MIC range of 8–64 µg/mL. The MIC50 and MIC90 values were 16 µg/mL and 32 µg/mL, respectively. The addition of CXM at a fixed concentration of 4 µg/mL significantly improved the potency of sulopenem by lowering the MICs. In fact, 52 of 54 isolates demonstrated an MIC of sulopenem less than 0.25 µg/mL in the presence of CXM, indicating a pronounced synergistic effect between the two antimicrobial agents (Fig. 2).
FIG 2.
Minimal inhibitory concentration distribution: comparison for sulopenem (SUL), cefuroxime (CXM), and combined sulopenem with 4 µg/mL cefuroxime against 54 Mab clinical strains and the ATTCC 19977 strain (See Table 1 for MIC data).
Synergistic effect of sulopenem in combination with CXM or BLIs in a time-kill study
Bactericidal activity was assessed through the static-concentration time-kill experiment. At four times, the MIC (4 × MIC of sulopenem and CXM), sulopenem (8 µg/mL), and CXM (64 µg/mL) exhibited a 2-log reduction in colony-forming units (CFU) after 2–3 days against ATCC 19977 (Fig. 3). However, at 2 µg/mL of sulopenem and 8–12 µg/mL of CXM, both agents only achieved growth inhibition (stasis) without significant bactericidal effect. When combined, sulopenem and CXM demonstrated a slight synergistic effect, resulting in a 1–2 log CFU difference compared with monotherapy. Combining a fixed concentration of 8 µg/mL CXM with sulopenem or a fixed concentration of 2 µg/mL of sulopenem with CXM led to a reduction in bacterial load comparable with monotherapy. Nevertheless, during the course of 10 days, regrowth of bacteria was observed in most concentration ranges for both monotherapy and combination treatments.
FIG 3.
Time-kill curves of sulopenem (A) in monotherapy, cefuroxime (CXM) in monotherapy (B), the combination of sulopenem and CXM (C and D), sulopenem in the presence of avibactam (AVI) (E), and the combination of sulopenem and sulbactam with or without DUR (F) against ATCC 19977. To counteract thermal degradation, 10% of sulopenem and 20% of cefuroxime were supplemented every 24 h.
Addition of BLIs, AVI (4 µg/mL) or DUR (12 µg/mL), enhanced the bactericidal activity of sulopenem. The combination of sulopenem + AVI resulted in a decrease of up to 2-log CFU, whereas sulopenem + DUR exhibited a 3.5-log CFU reduction. Although 4 µg/mL of AVI alone did not lead to significant killing, the combination of AVI + sulopenem yielded a 2.7-log reduction in Log10 CFU unit. At 12 µg/mL (6-fold of MIC of DUR), DUR monotherapy achieved a 2-log reduction in bacterial load, suggesting possible intrinsic antimicrobial activity in addition to its BLI activity against Mab, which was more effective compared with AVI. Although sulopenem + AVI showed regrowth on day 4, sulopenem + DUR inhibited regrowth successfully for up to 10 days.
Considering that DUR is commercially available with sulbactam, we also investigated the bacterial effect of triple combination of sulopenem + sulbactam + DUR. Sulbactam had no bactericidal effect either alone or in combination with sulopenem or DUR. However, when combined with sulopenem and DUR, sulbactam exhibited a similar synergistic effect on bacterial killing. Furthermore, SCTK studies were conducted using two randomly selected clinical isolates (122 and 686). Comparable results were obtained for isolate 122 (Fig. S2), whereas less killing was observed for isolate 686 (Fig. S3).
Timed electrospray ionization mass spectrometry (ESI/MS) captured covalent adduct formation between LdtMab2-4, D,D-carboxypeptidase, PBP B, and BlaMab with sulopenem and cefuroxime
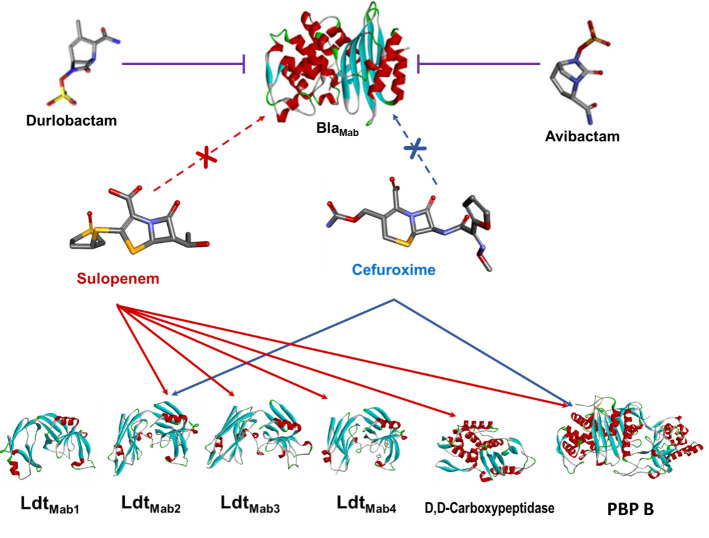
To explore the potential mechanism for transpeptidation inhibition by sulopenem, we investigated if sulopenem and CXM could form an acyl complex with LdtMab1–LdtMab4, D,D-carboxypeptidase, and PBP B. Ten micrograms of Ldts, D,D-carboxypeptidase, and PBP B were incubated with sulopenem and CXM at room temperature in a molar ratio of 1:20 (enzyme to sulopenem) for 5 minutes, 2 hours, and 24 hours in 50 mM Tris-HCl (pH 7.5) and 300 mM sodium chloride for a total reaction volume of 20 µL. Sulopenem underwent reaction with LdtMab2, LdtMab3, LdtMab4, D,D-carboxypeptidase (Fig. 4 and 5; Fig. S1), and PBP B. The resultant covalent drug adduct was measured using intact-protein ultra-performance liquid chromatography (UPLC) coupled with ESI/MS analysis. A peak corresponding to the mass of LdtMab2 or LdtMab4 plus + 86 Da mass shift was captured. This mass difference corresponds to the addition of the sulopenem, followed by postacylation changes and fragmentation of the compound. This phenomenon stems from the distinctive cleavage of the C5-C6 bond, a mechanism previously reported in penems and carbapenems (20–23). Sulopenem formed a + 349 Da adduct with LdtMab3, D,D-carboxypeptidase, and PBP B. In contrast to sulopenem, CXM engaged solely with LdtMab2 and PBP B, resulting in the formation of a + 381 Da adduct for LdtMab2 and a + 364 Da adduct for PBP B (Fig. 4).
FIG 4.
Capturing covalent adduct formation between sulopenem and LdtMab2 (A) and LdtMab3 (B) using timed electrospray ionization mass spectrometry. After 5 minute incubation of sulopenem with LdtMab2, the adduct formed is 86 Da (A). When incubated with LdtMab3, a 349 Da (sulopenem Mw) adduct is preserved after 2.5 hours (B).
FIG 5.
Interaction between BlaMab, L,D-transpeptidases (LdtMab1–LdtMab4), D,D-carboxypeptidase, and PBP B with β-lactams (sulopenem and cefuroxime) and β-lactamase inhibitors (durlobactam and avibactam).
We subsequently examined the covalent binding of sulopenem and CXM to BlaMab. Following coincubation for time intervals of 5 seconds, 15 seconds, and 2 hours, we were unable to detect any interaction between sulopenem and/or CXM with BlaMab. At these experimental conditions, BlaMab (28,433.5 Da) demonstrated binding with AVI (+265.5 Da) and DUR (+277 Da), as previously reported (7, 11).
Computational modeling and molecular docking of sulopenem with LdtMab2 and LdtMab3
For the molecular docking of sulopenem, the following two different models of LdtMab2 enzyme were used (11): (i) closed cavity, observed in apoenzyme structure, and (ii) the model with the opened active site, observed in crystal structures with the compounds trapped into the active site.
The automatic CDOCKER module did not generate any conformations with the sulopenem intact. The Michaelis-Menten complex generated when the open active site LdtMab2 conformation was chosen (Fig. 7A and C) shows that His333 positioned very close to the catalytic Cys351:HG, (His333:NE2 less than 2.5 Å). The imidazole ring of His333 is held into this productive conformation through a network of hydrophobic interactions by Val319 and Ala335. Once sulopenem is docked into the active site, the His333 imidazole ring is further stabilized by the hydroxyethyl moiety that interacts with Trp337. This proximity is consistent with previous studies (11), which show that the His333:NE2 is necessary to activate Cys351 for the nucleophilic attack on the lactam bond of sulopenem (Fig. 6). The carbonyl group of sulopenem is positioned into the oxyanion hole created by Cys351:NH and Gly350:NH (Fig. 7). After the acyl formation complex, the His333 imidazole ring moves outside (≈5.6–6 Å) from the oxyanion hole (Fig. 7B). The generated molecular docking complex of the acyl sulopenem and LdtMab2 shows the sulopenem carbonyl positioned outside of the oxyanion hole (Fig. 7B and C).
FIG 6.
Proposed mechanism of action between sulopenem and LdtMab transpeptidases. The covalent adduct formation between sulopenem and LdtMab2 can be explained through the nucleophilic attack of the cysteine residue at the carbonyl carbon of the β-lactam ring in sulopenem, with the help of activation from His333 (1). This nucleophilic attack results in the opening of the β-lactam ring and the formation of a thioester bond between the enzyme and sulopenem (2). Steps (3) and (4) are intermediate steps in the reaction mechanism suggested by the MDS results, where three water molecules were recruited into the active site. The end adduct of 87 Da (5) was observed after 5 minute incubation of LdtMab2 with sulopenem. When sulopenem is incubated with LdtMab3, after 2.5 h, the adduct with the intact sulopenem (2) was observed in the MS. The molecular modeling of sulopenem and LdtMab3 does not result in water molecules recruited into the active site. This may suggest that the reaction mechanism of sulopenem with LdtMab3 ends after step 2, with the release of the intact sulopenem.
FIG 7.
LdtMab2 and sulopenem as Michaelis-Menten (A) and acyl enzyme (B) complexes. During MDS simulation of the acyl-enzyme (C, D) and sulopenem fragment (E) complexes, three water molecules are recruited into the active site of LdtMab2. Initially, (A) H333 is at H-bond distance from Cys351 and ready to activate it for acyl-enzyme formation. After the acyl formation (B), the His333 is moving away from Cys351, and sulopenem carbonyl is positioned outside of the oxyanion hole. The potential H-bonds interactions are represented with green, hydrophobic interaction with pink, and sulfur with yellow.
To further understand the potential interactions of sulopenem and explore/explain the adduct formation of +87 Da seen on the ESI/MS data (Fig. 4) when LdtMab2 is incubated for 5 minutes with sulopenem, the molecular dynamic simulation was run for the acyl complex (Fig. 7C and D) and potential fragment of sulopenem (Fig. 7E). The model shows three water molecules recruited into the active site during the MDS. The first water molecule is positioned H-bond distance from thiazolidine ring N4, (Fig. 6 and 7C) making possible interactions with moieties on the sulopenem fragment in the active site. Two more water molecules, which are recruited, are less than 3 Å from sulfa groups from the acyl and/or fragmented sulopenem (Fig. 7D and E).
The mass spectrometry data show that LdtMab3 forms an intact adduct with sulopenem, after 3 hours of incubation (Fig. 4B). The molecular modeling and the docking of sulopenem into the active site of LdtMab3 show that the carbonyl is positioned toward the oxyanion hole formed by Cys351:NH and Gly350:NH, ready for acylation. However, His333:NE2 is more than 5 Å away from the catalytic Cys351 (Fig. 8A and B), and the sulopenem carbonyl makes H-bonds with Gly329. This suggests that the acyl enzyme complex formation may take longer to form (slow acylation). When the acyl enzyme is formed, the complex is held and stabilized in the active site of LdtMab3 through a network of H-bonds and hydrophobic interactions (Fig. 6 and 8C and D).
FIG 8.

LdtMab3 and sulopenem molecular docking as Michaelis-Menten (A, B) and acyl enzyme (C, D) complexes. The sulopenem carbonyl is positioned toward the LdtMab3 oxyanion hole formed by Cys351:NH and Gly350:NH, ready for acylation. However, His333:NE2 is more than 5 Å away from the catalytic Cys351, and the sulopenem carbonyl makes H-bonds with Gly329 (A, B). This suggests that the acyl enzyme complex may take longer to form. When the acyl enzyme is formed (C), the complex is held and stabilized in the active site of LdtMab3 through a network of H-bonds (green) and hydrophobic interactions (pink) (C, D).
This unique behavior of sulopenem can be explained due to the residues variability in the active sites of LdtMab2 and LdtMab3 (Fig. S4). The variability is observed in the sequence alignment (Fig. S4F), and the size and shape of the “outside” and ”inside” active site cavities (Fig.S4A and B). The entrance of the active site cavity is similar for both enzymes, with an approximatively 12–13 Å opening (Fig. S4A and B). However, the inside cavity increases from 5 to 6 Å in LdtMab2 to up to 14 Å in LdtMab3 (Fig. S4A and B), mostly due to the changes from Tyr to Ala and Tyr331 to Phe. The most important changes are observed at the entrance on the active site, with the Tyr305 in LdtMab2 replaced by alanine and proline 310, and His339 replaced by Asn349 (Fig. S4). The hairpin loops in LdtMab3 have a 7 amino acids deletion [Fig. S4 A, B (yellow representation), and F]. This deletion and the Pro310 in the middle of the alpha helix changes dramatically the architecture of the active site (Fig. S4C and D), making it smaller and more restrained in LdtMab3. The LdtMab3 is closer in overall topology to LdtMt5 (24) and also structurally and functionally distinct.
DISCUSSION
In recent years, the repurposing and reintroduction of β-lactams as a potential treatment for Mab infections has gained renewed interest. This interest has been augmented by the introduction of new DBO β-lactamase inhibitors. DBOs, such as AVI, relebactam, and the recently FDA-approved DUR, have demonstrated the ability to inhibit the enzymatic activity of BlaMab and restore in vitro susceptibility to β-lactams in Mab (7, 25–27). For example, the addition of AVI was found to increase Mab susceptibility to ceftaroline, with even further reductions in MICs observed upon the addition of ceftazidime (26). Similarly, DUR enhanced the susceptibilities for CXM and imipenem in a large collection of clinical isolates of Mab subspecies abscessus (7). Relebactam was also found to enhance susceptibility when combined with amoxicillin and imipenem against Mab isolates (25).
The precise dynamics of these DBOs in BlaMab inhibition and inhibition of Ldts are complex. In fact, previous studies have demonstrated that dual β-lactam combinations can significantly reduce MICs, without the need for BlaMab enzyme inhibition. In time-kill profiles of sulopenem in the presence of AVI (Fig. S2), we found a 1–1.5 Log10 reduction in CFU units compared with the absence of DBOs, whereas AVI did not show any killing against ATCC 19977 and clinical isolates Mab122. This suggests that the synergistic effect has been driven by their activity to inhibit BlaMab. However, DUR yielded significant killing showing two log10 decrease of bacterial load by itself and three log10 reduction by combination with sulopenem, suggesting that DUR enhanced in vitro bacterial killing by both inhibition of BlaMab and transpeptidation. Combination β-lactams was also found highly active in vitro in prior studies; this is exemplified by various combinations such as ceftaroline and imipenem (11), doripenem and cefdinir (12), ceftazidime and ceftaroline (28), imipenem and cefdinir, imipenem and cefoxitin (26), and sulopenem with CXM (29, 30), tebipenem, and CXM combined with amoxicillin (30), questioning the need for BlaMab inhibition. Clinicians have utilized this approach in treating macrolide-resistant Mab infections with reports of clinical success (14–16).
The central question that persists is whether the additive or synergistic impact of DBOs on Mab susceptibility arises from their ability to inhibit BlaMab, their inherent antimicrobial properties in hindering L,D-transpeptidases, or a combination of both factors. In our mass spectrometry data, we did not observe any evidence of binding between sulopenem and CXM with BlaMab (Fig. 5). This observation aligns with previous reports indicating that the kinetic parameters for the interaction between CXM and BlaMab are exceptionally high, making it challenging to determine, thus suggesting that BlaMab may not hydrolyze CXM (5). Moreover, it is noteworthy that AVI and DUR exhibit inhibitory effects not only on BlaMab but also on LDTs (7). This multifaceted inhibitory activity may elucidate the observed synergistic effects of β-lactam antibiotics combined with β-lactamases, which stem from the additional role of DBOs in inhibiting LDTs. This is one possible explanation and further research will be necessary to elucidate the question.
LdtMab2 belongs to a family of enzymes that catalyze the formation of 3→3 cross-links between peptidoglycan layers in the cell wall (20). In Mab, LdtMab2 plays a pivotal role in maintaining cell wall integrity and is essential for bacterial survival. The active site of LdtMab2 contains a highly conserved cysteine residue, which acts as a nucleophile during the catalytic process (11). The covalent adduct formation between sulopenem and LdtMab2 can be explained by the nucleophilic attack of the cysteine residue at the carbonyl carbon of the β-lactam ring in sulopenem (Fig. 6 and 7A). This nucleophilic attack results in the opening of the β-lactam ring and the formation of a thioester bond between the enzyme and sulopenem (Fig. 6 and 7B).
Similarly, previous studies have demonstrated that tebipenem, another oral carbapenem, exhibits binding and activity against LdtMab2 (12). Like sulopenem, tebipenem is a penem antibiotic with a similar bicyclic ring system composed of a β-lactam ring fused to a five-membered ring, which is connected to an azetidine ring at C2. Based on the tebipenem molecular modeling and its binding to LdtMab2, the value of theoretical free energy of binding for LdtMab2 and tebipenem was higher when compared with doripenem free energy of binding, suggesting that tebipenem is a better inhibitor (12).
The range of MIC values of sulopenem against clinical isolates in this cohort, which ranged from 1 to 8 μg/mL, is quite intriguing despite the absence of CLSI (Clinical Laboratory Standards Institute) breakpoints for sulopenem activity against Mab. Currently, the only carbapenem included in the guidelines for treating Mab infections is imipenem, which has a CLSI breakpoint for susceptibility at <4 µg/mL, intermediate activity at 8–16 μg/mL, and resistance at >32 µg/mL (31). Sulopenem in vitro activity was comparable with imipenem in this study. Moreover, the addition of 4 µg/mL of CXM led to a significant further reduction in MICs in over 90% of the clinical isolates. These data suggest its potential use as an oral step-down therapy following an initial parenteral induction phase. Notably, both sulopenem and CXM are available in oral formulations. Similar synergy with combinations of tebipenem and avibactam was observed in a recent study (30).
The time-kill analysis provided further support for the MIC outcomes, corroborating the in vitro efficacy of sulopenem in killing of Mab for both ATCC 19977 and the clinical isolate Mab122. Augmentation of the potency of sulopenem was observed by the addition of CXM or DBOs such as AVI or DUR. The synergistic impact of the dual β-lactam combination (sulopenem and CXM) in the SCTK was somewhat diminished compared with that observed in the MIC test. This discrepancy might be attributed to the compensatory action of thermally stable drugs counteracting the effects of thermally unstable drugs during the MIC test. Both sulopenem and CXM exhibit limited thermal stability (with CXM being relatively more stable) (30). In the susceptibility testing, we were unable to counterbalance the thermal degradation over the 3–7-day testing period, unlike in the time-kill study where such supplementation was feasible. The practice of supplementing unstable agents to counteract chemical degradation has implications for MIC data interpretation. Although the CLSI does not currently stipulate supplementation in susceptibility testing for rapidly growing mycobacteria, pertinent effects on MIC testing in Mab have been reported (32).
Macrolides, which historically found common utility in treating Mab infections, exhibit notable efficacy against susceptible Mab strains, albeit primarily manifesting bacteriostatic effects. In the context of our time-kill assay, a majority of drug regimens, encompassing monotherapies, dual β-lactams, and β-lactam combined with AVI, elicited bacteriostatic effects, with an exception observed upon the addition of DUR, culminating in a bactericidal effect. This combination strategy, yielding a bactericidal impact, holds promise for mitigating elevated relapse rates and suboptimal treatment outcomes. Given that certain antibiotics exhibit limited penetration into bronchial secretions (2), particularly pertinent in Mab lung infections, opting for interventions demonstrating bactericidal activities aligns logically.
The assessment of β-lactam efficacy in the SCTK study encompassed an examination involving one wild-type isolate (ATCC 19977) alongside randomly selected two distinct clinical isolates (Mab 122 and Mab 686). Paralleled patterns of bacterial killing efficacy were discerned in both ATCC 19977 and Mab 122. In contrast, growth inhibition was the primary outcome exhibited by Mab 686, yielding much lower bacterial killing. Consistently, a high MIC value of this isolate was observed. Furthermore, the addition of BLIs failed to elicit enhancements in bacterial killing. Plausible explanations for this phenomenon encompass potential mutations in peptidoglycan synthesis proteins, including Ldts and PBPs, or a plausible reduction in cell envelope permeability. To gain deeper insights, comprehensive investigations are warranted, involving genome sequencing and subsequent comparative analyses between the ATCC reference strain and these clinically derived isolates.
MICs of Mab 686 isolates were 2-fold to 4-fold higher than those of ATCC 19977 and Mab 122 (Table S1), consistent with the findings of the time-kill experiments (Fig. S2 and S3). We observed a wide MIC range among the clinical isolates (Table 1). This finding underscores the importance of tailored treatment approaches based on individual strain susceptibility profiles, highlighting the necessity of implementing comprehensive drug susceptibility testing (DST) protocols prior to initiating therapy. Implementing protocols for routine DST, along with strategies for appropriate antibiotic selection and dosing, becomes imperative in combating antimicrobial resistance and ensuring optimal patient outcomes. Additionally, the isolate that did not demonstrate sufficient bacterial killing raises concerns regarding the potential development of antibiotic resistance or alternative mechanisms of bacterial survival. This highlights the complexity of antimicrobial resistance mechanisms and emphasizes the need for continual surveillance and monitoring of bacterial populations to detect emerging resistance patterns.
In conclusion, this study unveils the in vitro synergy of sulopenem against Mab, shedding light on its potential as a therapeutic agent. Additionally, we have postulated a plausible mechanism underpinning its efficacy and have illustrated its synergistic interplay with CXM. These discoveries augment the ever-expanding roster of β-lactam compounds demonstrating remarkable effectiveness against Mab. Given the growing prevalence and therapeutic challenges posed by NTM infections, particularly Mab, the imperative for novel drug development looms large, as healthcare professionals worldwide grapple with the dearth of definitive treatment protocols. Remarkably, most of the synergistic β-lactams scrutinized herein boast well-established pharmacokinetic/pharmacodynamics profiles and a decades-long safety record. Thus, initiating an adaptive clinical trial to scrutinize the comparative efficacy of dual β-lactam therapy against the current standard of care assumes paramount significance.
MATERIALS AND METHODS
Bacterial strains, antibiotics, and reagents
The 54 clinical isolates analyzed in this study were obtained from deidentified patients. To ensure consistency and reliability, we specifically selected isolates from our well-characterized whole genome-sequenced clinical isolates belonging to the Mycobacterium abscessus subspecies abscessus. Of these, 34 isolates were sourced from National Jewish Health, whereas 11 and 9 isolates were obtained from University Hospitals Cleveland Medical Center and Cleveland Metrohealth, respectively. Additionally, ATCC 19977 was acquired from the American Type Culture Collection (ATCC). The active ingredient cefuroxime (CXM) salts and avibactam (AVI) were purchased from AchemBlock, sulopenem was sourced from Iterum Therapeutics, and durlobactam (DUR) was provided from Entasis Therapeutics. All β-lactams and BLIs were prepared in sterile, distilled water or in broth and filter-sterilized with a 0.22 µm PES syringe filter.
In vitro susceptibility testing and combination studies. Minimum inhibitory concentration (MIC) determination
Minimum inhibitory concentrations (MICs) of sulopenem and CXM were determined using microdilution. Approximately 5 × 105 colony-forming units (CFU) per milliliter were inoculated into Middlebrook 7H9 broth supplemented with 10% (vol/vol) oleic albumin dextrose catalase (OADC) and 0.05% (vol/vol) Tween 80. When CXM is combined with sulopenem, the CXM is added at a fixed concentration of 4 µg/mL to serial dilutions of sulopenem. Isolates were incubated with test agents at 30°C for 3–7 days, and MIC was defined as the lowest antibiotic concentration that prevented visible bacterial growth. We use active moiety compounds, like cefuroxime salt, and not cefuroxime axetil.
Cloning and purification of LDTs, DDC, PBP B, and BlaMab
Cloning and purification of Ldts (LdtMab2–LdtMab4), DDC, PBP B, and BlaMab were performed as previously described (7). Briefly, truncated sequences of Ldts, DDC, and PBP B (Δ1–41) were generated by Celtek Biosciences and cloned into the pET28(a) + vector with a TEV (tobacco etch virus) protease cleavage site prior to the start codon of the target protein sequences. Clones were transformed into Escherichia coli BL2 (DE3) and grown to reach 0.6–0.8 at an OD600. Protein expression was induced with 0.25 mM isopropyl β-d-1-thiogalactopyranoside (IPTG). After incubation for 18 hours at 18°C, cells were harvested and stored at −20°C overnight. When ready to harvest, cell pellets were resuspended in a buffer containing 50 mM Tris (pH 8.0), 400 mM sodium chloride, and 1 mM Tris (2-carboxyethyl) phosphine hydrochloride (TCEP), followed by sonication and centrifugation. The supernatant was passed through a His Prep FF 16/10 column (GE Healthcare) and washed with five column volumes of buffer, and bound protein was eluted with 500 mM imidazole. Eluted protein was subjected to dialysis overnight at 4°C in a buffer containing 50 mM Tris (pH 8.0), 150 mM sodium chloride, and 0.5 mM TCEP in the presence of His-tagged TEV protease (ratio of TEV protease to protein was 1:3). To remove the His tag, uncleaved fusion proteinand His-tagged TEV protease, passage over the His Prep FF 16/10 column was performed. Fractions containing LdtMab2 were pooled, concentrated, and stored in 20% glycerol at −20°C.
Static drug-concentration time-kill assay
The SCTK studies were conducted over a period of 10 days in Middlebrook 7H9 broth, enriched with 10% (vol/vol) OADC, 0.2% (vol/vol) glycerol, and 0.05% (vol/vol) Tween 80 in duplicate. The efficacy of β-lactams and BLIs was evaluated using ATCC 19977 and two clinical isolates obtained from National Jewish Health (Mab122 and Mab686). The concentrations of β-lactams and BLIs were carefully chosen, considering MIC and clinically attainable levels in patients based on predicted average unbound steady-state plasma concentrations. To offset thermal degradation, a small supplementary dose of sulopenem and CXM was added daily, guided by stability data on β-lactams provided by our collaborator (data not shown) or previously reported (30), 10% of sulopenem and 20% of cefuroxime. The supplemental dose was determined via the following equation. This approach ensures the maintenance of steady-state antibiotic concentrations within the broth (sulopenem 2 mg/L and cefuroxime 8 mg/L). The broth was exchanged with fresh broth containing the appropriate drug concentration every 3 days. The bacterial suspension was centrifuged at 4,500 × g, and the supernatant broth containing antibiotics was discarded. Fresh broth with antibiotics was then added accordingly.
where F (%) is the redosing fraction. This is consistent with the degradation rates at 24 h, 10% for sulopenem, 20% for cefuroxime, and 100% for all antibiotics when exchanging broth. Vdrug,day0 is antibiotic stock volume on the first day, VBroth,day0 (mL) is broth volume on the first day, and VBroth,dayX (mL) is broth volume at X day.
Throughout the SCTK studies, an initial inoculum of 105.6–106.3 CFU/mL was used. Viable counts were assessed immediately before dosing (referred to as “0 h“) and subsequently at 24 hour intervals for a total of 10 days. To eliminate any carry-over of antibiotics, all samples were thoroughly washed twice with sterile saline and subjected to serial 10-fold dilutions to determine viable counts. The viable counting procedure involved subculturing 100 µL of either an undiluted sample or an appropriately diluted sample on Middlebrook 7H10 agar plates supplemented with 1% (vol/vol) OADC, 0.2% glycerol, and 0.05% (vol/vol) Tween 80. This method resulted in a counting limit of 1.0 log10 CFU/mL, equivalent to a single colony per agar plate.
Mass spectrometry analysis of Ldts, D,D-carboxypeptidase, PBP B, and BlaMab
Ten micrograms of LdtMab1-4, DDC, PBP B, and BlaMab were incubated at room temperature with sulopenem or cefuroxime at a molar ratio of 1:20 for 5 min, 3 hours, and 24 hours in 50 mM Tris-HCl, pH 7.5, and 300 mM sodium chloride for a total reaction volume of 20 µL. Reactions were quenched with 10 µL acetonitrile and added to 1 mL 0.1% formic acid in water. Samples were analyzed using a quadrupole time-of-flight (Q-TOF) Waters Synapt-G2-Si electrospray ionization mass spectrometer (ESI-MS) and Waters Acquity H class ultra-performance liquid chromatography (UPLC) with a BEH C18 1.7 µm column (2.1 × 50 mm). The Synapt G2-Si was calibrated with sodium iodide with a 50–2000 m/z mass range. MassLynx V4.1 was used to deconvolute protein peaks. The tune settings for each sample were as follows: capillary voltage at 3 kV, sampling cone at 35 V, source offset at 35, source temperature of 100°C, desolvation temperature of 500°C, cone gas at 100 L/h, desolvation gas at 800 L/h, and 6.0 nebulizer. Mobile phase A was 0.1% formic acid in water. Mobile phase B wa-s 0.1% formic acid in 100% acetonitrile. Mass accuracy for this system is ±5 Da.
Molecular modeling, docking, and analysis
The crystal structure of LdtMab2 (PDB:5UWV) was used to model the enzyme. The missing loop (D301–D313) was reconstructed using the LdtMt2 (PDB: 6IYW) structure as a template and SWISS-MODEL homology-modeling server accessible via the ExPASy web server (33). The structure was future-minimized and prepared as previously described (11) using Discovery Studio software (BIOVIA DS Client 2020).
The structural model of LdtMab3 was similarly generated, using LdtMtb5 (PDB:4Z7A) as the template. The structure was minimized using a Conjugate Gradient method, with an RMS gradient of 0.001 kcal/(mol × Å). Generalized Born with a simple Switching (GBSW) solvation model was used, and long-range electrostatics were treated using a Particle Mesh Ewald method with periodic boundary conditions. The SHAKE algorithm was applied.
The intact, acyl and fragmented sulopenem was built and docked into the active site of LdtMab2 and LdtMab3 transpeptidases structures. The CDOCKER protocol was used to dock the compounds into the active sites of LdtMab2 and LdtMab3. The protocol uses a CHARMm-based molecular dynamics (MD) scheme to dock ligands into a receptor binding site as previously described (11). The generated poses were analyzed, and the best-ranked ones were used to create the Michaelis-Menten and acyl-enzyme complexes and were further minimized. To equilibrate the structure, a medium-long molecular dynamic simulation (1 ns) was performed, using a NAMD protocol (11, 34).
Contributor Information
Robert A. Bonomo, Email: Robert.bonomo@va.gov.
Alejandro J. Vila, IBR (CONICET) University of Rosario, Rosario, Santa Fe, Argentina
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/mbio.00609-24.
Table S1 and Fig. S1 to S4.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Nessar R, Cambau E, Reyrat JM, Murray A, Gicquel B. 2012. Mycobacterium abscessus: a new antibiotic nightmare. J Antimicrob Chemother 67:810–818. doi: 10.1093/jac/dkr578 [DOI] [PubMed] [Google Scholar]
- 2. Johansen MD, Herrmann JL, Kremer L. 2020. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat Rev Microbiol 18:392–407. doi: 10.1038/s41579-020-0331-1 [DOI] [PubMed] [Google Scholar]
- 3. Griffith DE, Daley CL. 2022. Treatment of Mycobacterium abscessus pulmonary disease. Chest 161:64–75. doi: 10.1016/j.chest.2021.07.035 [DOI] [PubMed] [Google Scholar]
- 4. Dousa KM, Kurz SG, Bark CM, Bonomo RA, Furin JJ. 2020. Drug-resistant tuberculosis: a glance at progress and global challenges. Infect Dis Clin North Am 34:863–886. doi: 10.1016/j.idc.2020.06.001 [DOI] [PubMed] [Google Scholar]
- 5. Soroka D, Dubée V, Soulier-Escrihuela O, Cuinet G, Hugonnet J-E, Gutmann L, Mainardi J-L, Arthur M. 2014. Characterization of broad-spectrum Mycobacterium abscessus class A beta-lactamase. J Antimicrob Chemother 69:691–696. doi: 10.1093/jac/dkt410 [DOI] [PubMed] [Google Scholar]
- 6. Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ Jr, Andrejak C, Böttger EC, Brozek J, Griffith DE, Guglielmetti L, Huitt GA, Knight SL, Leitman P, Marras TK, Olivier KN, Santin M, Stout JE, Tortoli E, van Ingen J, Wagner D, Winthrop KL. 2020. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J 56:2000535. doi: 10.1183/13993003.00535-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dousa KM, Nguyen DC, Kurz SG, Taracila MA, Bethel CR, Schinabeck W, Kreiswirth BN, Brown ST, Boom WH, Hotchkiss RS, Remy KE, Jacono FJ, Daley CL, Holland SM, Miller AA, Bonomo RA. 2022. Inhibiting Mycobacterium abscessus cell wall synthesis: using a novel diazabicyclooctane beta-lactamase inhibitor to augment beta-lactam action. mBio 13:e0352921. doi: 10.1128/mbio.03529-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kurepina N, Chen L, Composto K, Rifat D, Nuermberger EL, Kreiswirth BN. 2022. CRISPR inhibition of essential peptidoglycan biosynthesis genes in Mycobacterium abscessus and its impact on beta-lactam susceptibility. Antimicrob Agents Chemother 66:e0009322. doi: 10.1128/aac.00093-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Akusobi C, Benghomari BS, Zhu J, Wolf ID, Singhvi S, Dulberger CL, Ioerger TR, Rubin EJ. 2022. Transposon mutagenesis in Mycobacterium abscessus identifies an essential penicillin-binding protein involved in septal peptidoglycan synthesis and antibiotic sensitivity. Elife 11:e71947. doi: 10.7554/eLife.71947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Galanis C, Maggioncalda EC, Kumar P, Glby LG. 2022. Encoded by MAB_3167c, is required for in vivo growth of Mycobacteroides abscessus and exhibits mild beta-lactamase activity. J Bacteriol 204:e0004622. doi: 10.1128/jb.00046-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dousa KM, Kurz SG, Taracila MA, Bonfield T, Bethel CR, Barnes MD, Selvaraju S, Abdelhamed AM, Kreiswirth BN, Boom WH, Kasperbauer SH, Daley CL, Bonomo RA. 2020. Insights into the L,D-transpeptidases and d,d-carboxypeptidase of Mycobacterium abscessus: ceftaroline, imipenem, and novel diazabicyclooctane inhibitors. Antimicrob Agents Chemother 64:e00098-20. doi: 10.1128/AAC.00098-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kumar P, Chauhan V, Silva JRA, Lameira J, d’Andrea FB, Li S-G, Ginell SL, Freundlich JS, Alves CN, Bailey S, Cohen KA, Lamichhane G. 2017. Mycobacterium abscessus L,D-transpeptidases are susceptible to inactivation by carbapenems and cephalosporins but not penicillins . Antimicrob Agents Chemother 61:10. doi: 10.1128/AAC.00866-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nguyen DC, Dousa KM, Kurz SG, Brown ST, Drusano G, Holland SM, Kreiswirth BN, Boom WH, Daley CL, Bonomo RA. 2021. “One-two punch”: synergistic ss-lactam combinations for Mycobacterium abscessus and target redundancy in the inhibition of peptidoglycan synthesis enzymes. Clin Infect Dis 73:1532–1536. doi: 10.1093/cid/ciab535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alahmdi B, Dousa KM, Kurz SG, Kaufman A, Bonomo RA, Taimur S. 2023. Eradicating pulmonary Mycobacterium abscessus: the promise of dual beta-lactam therapy. Open Forum Infect Dis 10:ofad312. doi: 10.1093/ofid/ofad312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wolf AB, Money KM, Chandnani A, Daley CL, Griffith DE, Chauhan L, Coffman N, Piquet AL, Tyler KL, Zimmer SM, Montague BT, Mann S, Pastula DM. 2023. Mycobacterium abscessus meningitis associated with stem cell treatment during medical tourism. Emerg Infect Dis 29:1655–1658. doi: 10.3201/eid2908.230317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moguillansky N, DeSear K, Dousa KM. 2023. A 40-year-old female with Mycobacterium abscessus successfully treated with a dual beta-lactam combination. Cureus 15:e40993. doi: 10.7759/cureus.40993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Story-Roller E, Maggioncalda EC, Cohen KA, Lamichhane G. 2018. Mycobacterium abscessus and beta-lactams: emerging insights and potential opportunities. Front Microbiol 9:2273. doi: 10.3389/fmicb.2018.02273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhanel GG, Pozdirca M, Golden AR, Lawrence CK, Zelenitsky S, Berry L, Schweizer F, Bay D, Adam H, Zhanel MA, Lagacé-Wiens P, Walkty A, Irfan N, Naber K, Lynch JP 3rd, Karlowsky JA. 2022. Sulopenem: an intravenous and oral penem for the treatment of urinary tract infections due to multidrug-resistant bacteria. Drugs 82:533–557. doi: 10.1007/s40265-022-01688-1 [DOI] [PubMed] [Google Scholar]
- 19. Okamoto K, Gotoh N, Nishino T. 2001. Pseudomonas aeruginosa reveals high intrinsic resistance to penem antibiotics: penem resistance mechanisms and their interplay. Antimicrob Agents Chemother 45:1964–1971. doi: 10.1128/AAC.45.7.1964-1971.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kumar P, Kaushik A, Lloyd EP, Li S-G, Mattoo R, Ammerman NC, Bell DT, Perryman AL, Zandi TA, Ekins S, Ginell SL, Townsend CA, Freundlich JS, Lamichhane G. 2017. Non-classical transpeptidases yield insight into new antibacterials. Nat Chem Biol 13:54–61. doi: 10.1038/nchembio.2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lohans CT, Chan HTH, Malla TR, Kumar K, Kamps JJAG, McArdle DJB, van Groesen E, de Munnik M, Tooke CL, Spencer J, Paton RS, Brem J, Schofield CJ. 2019. Non-hydrolytic beta-lactam antibiotic fragmentation by l,d-transpeptidases and serine beta-lactamase cysteine variants. Angew Chem Int Ed Engl 58:1990–1994. doi: 10.1002/anie.201809424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gupta R, Al-Kharji NMSA, Alqurafi MA, Nguyen TQ, Chai W, Quan P, Malhotra R, Simcox BS, Mortimer P, Brammer Basta LA, Rohde KH, Buynak JD. 2021. Atypically modified carbapenem antibiotics display improved antimycobacterial activity in the absence of beta-lactamase inhibitors. ACS Infect Dis 7:2425–2436. doi: 10.1021/acsinfecdis.1c00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Batchelder HR, Zandi TA, Kaushik A, Naik A, Story-Roller E, Maggioncalda EC, Lamichhane G, Nuermberger EL, Townsend CA. 2022. Structure-activity relationship of penem antibiotic side chains used against mycobacteria reveals highly active compounds. ACS Infect Dis 8:1627–1636. doi: 10.1021/acsinfecdis.2c00229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brammer Basta LA, Ghosh A, Pan Y, Jakoncic J, Lloyd EP, Townsend CA, Lamichhane G, Bianchet MA. 2015. Loss of a functionally and structurally distinct ld-transpeptidase, LdtMt5, compromises cell wall integrity in Mycobacterium tuberculosis. J Biol Chem 290:25670–25685. doi: 10.1074/jbc.M115.660753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lopeman RC, Harrison J, Rathbone DL, Desai M, Lambert PA, Cox JAG. 2020. Effect of amoxicillin in combination with imipenem-relebactam against Mycobacterium abscessus. Sci Rep 10:928. doi: 10.1038/s41598-020-57844-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pandey R, Chen L, Manca C, Jenkins S, Glaser L, Vinnard C, Stone G, Lee J, Mathema B, Nuermberger EL, Bonomo RA, Kreiswirth BN. 2019. Dual beta-lactam combinations highly active against Mycobacterium abscessus complex in vitro. mBio 10:e02895-18. doi: 10.1128/mBio.02895-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dubée V, Bernut A, Cortes M, Lesne T, Dorchene D, Lefebvre A-L, Hugonnet J-E, Gutmann L, Mainardi J-L, Herrmann J-L, Gaillard J-L, Kremer L, Arthur M. 2015. Beta-lactamase inhibition by avibactam in Mycobacterium abscessus. J Antimicrob Chemother 70:1051–1058. doi: 10.1093/jac/dku510 [DOI] [PubMed] [Google Scholar]
- 28. Story-Roller E, Maggioncalda EC, Lamichhane G. 2019. Select beta-lactam combinations exhibit synergy against Mycobacterium abscessus in vitro. Antimicrob Agents Chemother 63:e02613-18. doi: 10.1128/AAC.02613-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dousa KM, Nguyen DC, Kurz SG, Taracila MA, Bethel O, Bonomo RA. 2020. 1572. Combination cefuroxime and sulopenem is active in vitro against Mycobacterium abscessus. Open Forum Infect Dis 7:S785. doi: 10.1093/ofid/ofaa439.1752 [DOI] [Google Scholar]
- 30. Negatu DA, Zimmerman MD, Dartois V, Dick T. 2022. Strongly bactericidal all-oral beta-lactam combinations for the treatment of Mycobacterium abscessus lung disease. Antimicrob Agents Chemother 66:e0079022. doi: 10.1128/aac.00790-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fröberg G, Maurer FP, Chryssanthou E, Fernström L, Benmansour H, Boarbi S, Mengshoel AT, Keller PM, Viveiros M, Machado D, et al. 2023. Towards clinical breakpoints for non-tuberculous mycobacteria - determination of epidemiological cut off values for the Mycobacterium avium complex and Mycobacterium abscessus using broth microdilution. Clin Microbiol Infect 29:758–764. doi: 10.1016/j.cmi.2023.02.007 [DOI] [PubMed] [Google Scholar]
- 32. Rominski A, Schulthess B, Müller DM, Keller PM, Sander P. 2017. Effect of beta-lactamase production and beta-lactam instability on MIC testing results for Mycobacterium abscessus. J Antimicrob Chemother 72:3070–3078. doi: 10.1093/jac/dkx284 [DOI] [PubMed] [Google Scholar]
- 33. Guex N, Peitsch MC, Schwede T. 2009. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: a historical perspective. Electrophoresis 30 Suppl 1:S162–S173. doi: 10.1002/elps.200900140 [DOI] [PubMed] [Google Scholar]
- 34. Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kalé L, Schulten K. 2005. Scalable molecular dynamics with NAMD. J Comput Chem 26:1781–1802. doi: 10.1002/jcc.20289 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 and Fig. S1 to S4.