Abstract
Purpose
Pertussis bacteria have many pathogenic and virulent antigens and severe adverse reactions have occurred when using inactivated whole-cell pertussis vaccines. Therefore, inactivated acellular pertussis (aP) vaccines and genetically detoxified recombinant pertussis (rP) vaccines are being developed. The aim of this study was to assess the safety profile of a novel rP vaccine under development in comparison to commercial diphtheria-tetanus-acellular pertussis (DTaP) vaccines.
Materials and Methods
The two positive control DTaP vaccines (two- and tri-components aP vaccines) and two experimental recombinant DTaP (rDTaP) vaccine (two- and tri-components aP vaccines adsorbed to either aluminum hydroxide or purified oat beta-glucan) were used. Temperature histamine sensitization test (HIST), indirect Chinese hamster ovary (CHO) cell cluster assay, mouse-weight-gain (MWG) test, leukocytosis promoting (LP) test, and intramuscular inflammatory cytokine assay of the injection site performed for safety assessments.
Results
HIST results showed absence of residual pertussis toxin (PTx) in both control and experimental DTaP vaccine groups, whereas in groups immunized with tri-components vaccines, the experimental tri-components rDTaP absorbed to alum showed an ultra-small amount of 0.0066 IU/mL. CHO cell clustering was observed from 4 IU/mL in all groups. LP tests showed that neutrophils and lymphocytes were in the normal range in all groups immunized with the two components vaccine. However, in the tri-components control DTaP vaccine group, as well as two- and tri-components rDTaP with beta-glucan group, a higher monocyte count was observed 3 days after vaccination, although less than 2 times the normal range. In the MWG test, both groups showed changes less than 20% in body temperature and body weight before the after the final immunizations. Inflammatory cytokines within the muscle at the injection site on day 3 after intramuscular injection revealed no significant response in all groups.
Conclusion
There were no findings associated with residual PTx, and no significant differences in both local and systemic adverse reactions in the novel rDTaP vaccine compared to existing available DTaP vaccines. The results suggest that the novel rDTaP vaccine is safe.
Keywords: Safety, Diphtheria-tetanus-acellular pertussis vaccines, Recombinant DTaP vaccine, Murine model study, Bordetella pertussis
Introduction
Whooping cough is mainly caused by the gram-negative bacteria Bordetella pertussis and Bordetella parapertussis [1]. B. pertussis, the main causative agent of pertussis, has more than 20 pathogenic virulence antigens, including pertussis toxin (PTx), fimbriae, pertactin (PRN), filamentous hemagglutinin (FHA), lipooligosaccharide, and adenylate cyclase toxin. In particular, PTx is a major epitope associated with pertussis and can induce immunogenic cells by attaching to macrophages and epithelial cells, like FHA and PRN, and thus plays a role as a major immunogenic antigen of pertussis vaccines [2,3,4]. As such, pertussis bacteria have many pathogenic and virulent antigens and severe adverse reactions have occurred when using inactivated whole-cell pertussis (wP) vaccines. Therefore, since the 1990s, the inactivated acellular pertussis (aP) vaccine, which primarily contains purified and separated major virulence antigens, has been predominantly used [5]. In addition, genetically detoxified recombinant pertussis (rP) vaccines, detoxified via genetic modifications, are being developed as future vaccines that consider pertussis polymorphism.
The PTx, the major immunogenic antigen of the pertussis vaccine, is a typical bacterial AB type toxin consisting of an A promotor (S1) and B oligomer complex (S2, S3, two copies of S4, and S5) [6,7]. The A promoter’s subunit peptide (S1) is separated from the B oligomer in the endoplasmic reticulum and secreted into the cytoplasm, exhibiting toxicity. The A promoter increases the adenosine diphosphate (ADP)-ribosylation of the α subunit of inhibitory/other/transducin G protein [8,9]. The α subunit blocks the inhibition of adenylate cyclase, leading to an increase in messenger cyclic adenosine monophosphate (cAMP), which in turn triggers a series of biological downstream effects such as leukocytosis, histamine sensitization, hypoglycemia due to insulinemia, exocrine secretion, inhibition of lymphocyte and neutrophil migration [10,11], and ERK/MAP kinase pathways alteration, resulting in various biological side effects. However, the B oligomer induces PTx to enter the cell by binding with the cell membrane protein, and the B oligomer itself exhibits reactions such as proliferation of T cells, glucose oxidation in adipocytes, and activation of TLR4 [12,13]. Due to the toxic side effects of PTx, the residue or reversion of PTx in aP or rP vaccines must be checked during the pertussis vaccine development stage and before shipment of each production after development.
The histamine sensitization test (HIST), the most important test for safety evaluation, checks the residue or reversion of PTx. This method includes in vivo model studies such as lethal dose tests, temperature tests, leukocytosis promoting (LP) tests, mouse-weight-gain (MWG), or mouse-weight-decrease tests. Ex vivo tests include agglutination assays, while in vitro assays include enzymatic high-performance liquid chromatography-coupled assays, fetuin enzyme-linked immunosorbent assay, carbohydrate-binding assays, Chinese hamster ovary (CHO) cell clustering assays, cAMP-PTx reporter assays, ADP ribosylation assays, and islet-activating protein tests [13]. Among them, the CHO cell clustering assay, an alternative method that improves the problems of HIST, is a test that must be performed in the pertussis vaccine development stage along with HIST, after development, and before shipment after each production to check the residue or reversion of PTx. In addition, safety assessments such as gross inspection of the inoculation site, histologic evaluation, and intramuscular inflammatory cytokine analyses must be performed to evaluate local adverse reactions after immunization by vaccine recipients in the pertussis vaccine development stage.
The researchers in this study recently published results of an in vivo model immunogenic study of a tri-components rP vaccine (produced by a WHEP vector developed at Yonsei University), including recombinant PTx, FHA, and PRN antigens, which is being developed for the first time in Korea [14]. The primary aim of this study was to assess the safety profile of a novel rP vaccine under development in comparison to commercial diphtheria-tetanus-acellular pertussis (DTaP) vaccines by performing temperature HIST, indirect CHO cell cluster assay, MWG test, lymphocytosis-promoting factor (LPF) test, and intramuscular inflammatory cytokine assay of the injection site.
Materials and Methods
Vaccines
The two positive control DTaP vaccines and two experimental recombinant DTaP vaccines used were as follows: bivalent DTaP-inactivated poliovirus vaccine (IPV) (Sanofi, Paris, France) as control two-components aP vaccine (PTx, FHA) and trivalent DTaP-IPV/Haemophilus influenzae type b (Hib) vaccine (GSK, Rixensart, Belgium) as control tri-components aP vaccine (PTx, FHA, PRN). The bivalent DTaP-IPV vaccine comprised 25 flocculation units (Lf) diphtheria toxoid, 10 Lf tetanus toxoid (TT), 25 µg PTx, and 25 µg FHA adsorbed to aluminum hydroxide. The trivalent DTaP-IPV/Hib vaccine comprised 25 Lf diphtheria toxoid, 10 Lf TT, 25 µg PTx, 25 µg FHA, and 8 µg PRN adsorbed to aluminum hydroxide. The experimental recombinant DTaP vaccine was composed of 25 Lf diphtheria toxoid, 10 Lf TT, pertussis toxin subunit 1, and FHA as the two components experimental vaccine, and additional PRN domains as the experimental tri-components experimental vaccine (genetically fused with the WHEP domain and expressed as a soluble form, purified by Ni-affinity chromatography) adsorbed to either aluminum hydroxide or purified oat beta-glucan (donated by CLIPS BnC, Seoul, Korea). The mock-vaccinated group (negative control) was inoculated with 0.85% physiological saline.
Temperature histamine sensitization test
The temperature HIST was performed according to the World Health Organization’s (WHO’s) Expert Committee on Biological Standardization guidelines [15]. A reference linear line (including 95% confidence interval [CI]) for temperature relative to the concentration of the new standard pertussis toxin National Institute for Biological Standards and Control (NIBSC) 15/126 (1,881 IU/ampoule) was set (Fig. 1A). After dividing into the two components DTaP vaccination group and tri-components DTaP vaccination group, each group was classified into the negative control (saline group), positive control (bivalent or trivalent DTaP vaccination group), and experimental (DTaP two- or tri-components vaccine with alum adjuvant or beta-glucan adjuvant) groups. In each group, 10 female BALB/c mice aged 4–5 weeks were inoculated with the vaccine intraperitoneally, and 0.8% histamine solution (320 mg histamine dihydrochloride diluted in 40 mL normal saline) was injected intraperitoneally on day 4 of vaccination for histamine sensitization. After 30 minutes, the rectal temperature was measured at a depth of 15–20 mm of the mouse’s anus. The measured rectal temperature was then inserted into the equation of the established reference linear line relative to the concentration of NIBSC 15/126 (1,881 IU/ampoule) to measure residual PTx.
Fig. 1. Temperature histamine sensitization test results showing (A) the linear line of reference pertussis toxin (PTx) (NIBSC 15/126) Standard, (B) the results of bivalent control or experimental diphtheria-tetanus-acellular pertussis (DTaP) vaccines, and (C) the results of trivalent control or experimental DTaP vaccines. NIBSC, National Institute for Biological Standards and Control; rP, recombinant pertussis; CI, confidence interval; SEM, standard error of mean.
Indirect Chinese hamster ovary cell cluster assay
In the direct CHO cell clustering assay, the aluminum hydroxide component used as an adjuvant induces cytotoxic effects on CHO cells. To address the problems in evaluating test results, we conducted the indirect CHO clustering assay, recommended by the Biological Standardization Program of the European Directorate for the Quality of Medicines & HealthCare (EDQM) [16,17]. In this study, the clustering of CHO cells in the spiked vaccine was checked by gradual dilution of standard pertussis toxin NIBSC 15/126 (680 IU/ampoule) obtained from NIBSC (Table 1). The NIBSC 15/126 PTx was aliquoted after referring to stability data from the PTx data distributed by WHO. Only NIBSC 15/126 PTx stored at -20℃ for 1 and 2 weeks was used. Additionally, to prevent direct contact of the vaccine with cells, 0.4 µm polycarbonate tissue culture inserts were inserted during the procedure.
Table 1. Concentration test of vaccine spiked with NIBSC 15/126.
| Group no. | Saline (IU/mL) | Control (IU/mL) | rP Alum (IU/mL) | rP Beta (IU/mL) |
|---|---|---|---|---|
| 1 | 4 | 4 | 4 | 4 |
| 2 | 2 | 2 | 2 | 2 |
| 3 | 1 | 1 | 1 | 1 |
| 4 | 0 | 0 | 0 | 0 |
Five spiked samples were tested with each dilution unit in each group.
NIBSC, National Institute for Biological Standards and Control; rP, recombinant pertussis.
Leukocytosis promoting test
Female BALB/c mice 4–5 weeks of age were injected with the commercialized vaccines (bivalent and trivalent DTaP vaccines) for the control group and the recombinant vaccine (two- and tri-components DTaP vaccines) for the experimental group on the leg muscles twice at intervals of 7 days. After the final inoculation, blood samples were collected on days 1 and 3, and leukocytes in the blood were analyzed using MAXCOT Hematology (DREW Scientific Inc., Miami Lakes, FL, USA) to evaluate whether they deviated from the normal mouse range.
Mouse-weight-gain test and temperature changes
To assess endotoxin adverse reactions in the DTaP vaccine and its systemic reactions, body weight and body temperature changes were tracked before and after vaccinations. After dividing into the two components DTaP vaccine group and tri-components DTaP vaccination group, each group was classified into the negative control (saline group), positive control (bivalent or trivalent DTaP vaccine group), and experimental (two- or tri-components recombinant DTaP vaccine with alum adjuvant or beta-glucan adjuvant) groups. Ten female BALB/c mice aged 4–5 weeks in each group were vaccinated with allocated vaccines intraperitoneally at intervals of 2 weeks. Based on the temperature and weight before the last DTaP vaccination, changes in body weight and temperature, as well as local adverse reactions (redness and induration) and activity at the injection site, were visually checked daily for 1 week after vaccination. Body temperature was comparatively corrected by measuring the dermal temperature and rectal temperature using a non-contact infrared thermometer (HANSUNG, Gimhae, Korea).
Inflammatory muscular cytokines in the injection site
The leg muscles of the mice subjected to the LP test were extracted on 3 days and 1 week after the second vaccination. These samples were homogenized with a protease inhibitor cocktail, and the distribution of inflammatory cytokines was checked and compared by group using a mouse inflammation antibody array-membrane kit (40 targets; Abcam, Cambridge, UK).
Ethics statement
The animal studies were performed after receiving approval of the Institutional Animal Care and Use Committee in Catholic University (IACUC approval no., 2022-0260-04).
Results
Confirming residual pertussis toxin using temperature histamine sensitization tests
In a study conducted with histamine desensitization of less than or equal to 0.4 HSU/mL, the residual PTx was checked by comparing the temperature of mice to the reference linear line and equation set which was obtained from NIBSC 15/126 PTx concentration by temperature, as shown in Fig. 1A. For the groups immunized with control and experimental two components’ vaccines, all groups showed values below the reference line, indicating absence of residual PTx (Fig. 1B). In the case of tri-components DTaP vaccines, a PTx of 0.07 ng/mL was identified in the experimental recombinant DTaP vaccine containing alum, which was confirmed as an ultra-small amount of 0.0066 IU/mL when converted to an IU unit of 1,881 IU/mL (per 20 µg), which is the dose of NIBSC 15/126, the standard PTx (Fig. 1C).
CHO cell clustering
Both bivalent and trivalent DTaP vaccine groups showed CHO cell clustering from 4 IU/mL in all groups (Table 2).
Table 2. CHO cell clustering concentration of NIBSC 15/126 PTx in control and study groups.
| Variable | Saline (IU/mL) | Control (IU/mL) | rP Alum (IU/mL) | rP Beta (IU/mL) |
|---|---|---|---|---|
| CHO cells clustering concentration of NIBSC 15/126 PTx in two components DTaP vaccine | 4 | 4 | 4 | 4 |
| CHO cells clustering concentration of NIBSC 15/126 PTx in three components DTaP vaccine | 4 | 4 | 4 | 4 |
CHO, Chinese hamster ovary; NIBSC, National Institute for Biological Standards and Control; PTx, pertussis toxin; rP, recombinant pertussis; DTaP, diphtheria-tetanus-acellular pertussis.
Leukocytosis after immunization
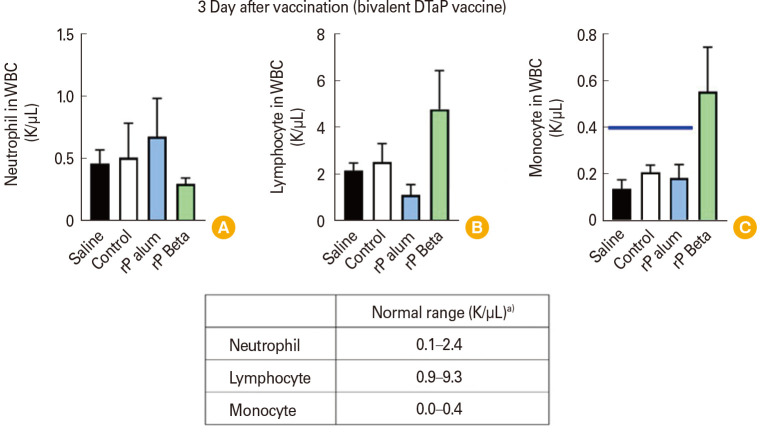
LP tests were performed to assess the expression of leukocytosis. The results showed that neutrophils and lymphocytes were in the normal range in both the control and experimental groups immunized with two components DTaP vaccines. However, in the group immunized with the two components experimental DTaP vaccine absorbed to beta-glucan adjuvant, monocytes were higher compared to other groups that were within the normal range (Fig. 2). In the case of tri-components DTaP vaccines, leukocytes were in the normal range after 1 day of vaccination in both the control and experimental groups. However, 3 days after vaccination, in the control group as well as the group immunized with the tri-components recombinant DTaP vaccine absorbed to beta-glucan adjuvant, higher monocyte counts were observed compared to other groups which were within the normal range (Fig. 3). Nevertheless, all elevated monocyte counts described were below 2 times the normal range.
Fig. 2. Leukocytes distributions at 3 days after immunization with bivalent diphtheria-tetanus-acellular pertussis (DTaP) vaccines. (A) Neutrophil concentration. (B) Lymphocyte concentration. (C) Monocyte concentration. Horizontal bold blue line indicates upper limit of monocyte normal ranges. WBC, white blood cell; rP, recombinant pertussis. a)The normal range of mouse blood cell counts was provided by Charles River Laboratories (Wilmington, MA, USA).
Fig. 3. (A, B) Leukocytes distributions at 1 and 3 days after immunization with trivalent diphtheria-tetanus-acellular pertussis (DTaP) vaccines. WBC, white blood cell; rP, recombinant pertussis. a)The normal range of mouse blood cell counts was provided by Charles River Laboratories (Wilmington, MA, USA).
Weight and temperature changes after immunizations
In the groups immunized with two components DTaP vaccine, both the control and experimental groups showed a body weight decreased by day 4 and then increased afterwards. Body temperature also dropped by day 4, then rose again, and fell again on day 6 (Fig. 4). However, in the groups immunized with tri-components DTaP vaccines, both the control and experimental groups did not show any clear changes in body weight and body temperature before and after immunization (Fig. 5). Nevertheless, both two- and tri-components DTaP vaccine control and experimental groups showed changes less than 20% in body temperature and body weight before the after the final immunizations. Thus, showing no serious adverse reactions. Furthermore, upon visual inspection, no abnormalities in physical activity were detected in all mice, confirming a healthy state overall.
Fig. 4. Weight and temperature changes after immunization with bivalent diphtheria-tetanus-acellular pertussis (DTaP) vaccines. (A) Weight changes in grams versus days after post-vaccination. (B) Weight changes in percentages versus days after post-vaccination. (C) Temperature changes in Celsius versus days after post-vaccination. (D) Temperature changes in percentages versus days after post-vaccination. WBC, white blood cell; rP, recombinant pertussis; SEM, standard error of mean; CI, confidence interval.
Fig. 5. Weight and temperature changes after immunization with trivalent diphtheria-tetanus-acellular pertussis (DTaP) vaccines. (A) Presenting weight changes in grams versus days after post-vaccination. (B) Weight changes in percentages versus days after post-vaccination. (C) Temperature changes in Celsius versus days after post-vaccination. (D) Temperature changes in percentages versus days after post-vaccination. WBC, white blood cell; rP, recombinant pertussis; SEM, standard error of mean; CI, confidence interval.
Muscular cytokines in the injection site
Regarding the bivalent and trivalent control and experimental vaccines, the confirmation of inflammatory cytokines within the muscle at the injection site on day 3 after intramuscular injection revealed a local inflammatory response and a response promoting the activation of immune cells through chemokines. However, no significant response was observed for innate cytokines such as interleukin (IL)-1β, IL-10, IL-17, and tumor necrosis factor-α (TNF-α). In the groups immunized with bivalent DTaP vaccines, no significant difference was observed between the experimental and control groups (Fig. 6A). However, in the groups immunized with trivalent DTaP vaccines, particularly in the control group, there was a notably higher inflammatory cytokine response compared to the experimental trivalent DTaP groups (Fig. 6B).
Fig. 6. Inflammatory muscular cytokines at vaccine injection sites 3 days after immunizations with bivalent diphtheria-tetanus-acellular pertussis (DTaP) vaccines (A) and trivalent DTaP vaccines (B). rP, recombinant pertussis; IL, interleukin; IFN-γ, interferon-γ; TNF-α, tumor necrosis factor-α; SDF-1, stromal cell-derived factor-1; NEG, negative control; I-TAC, interferon-inducible T cell α chemoattractant; TCA-3, T-cell activation gene-3; KC, keratinocyte-derived cytokine; TECK, thymus-expressed chemokine; LIX, lipopolysaccharide-induced CXC chemokine; TIMP, tissue inhibitors of metalloproteinase; BLC, B-lymphocyte chemoattractant; MCP-1, monocyte chemotactic protein-1; MCSF, macrophage-colony stimulating factor; MIG, monokine induced by gamma interferon; MIP, macrophage inflammatory protein; GCSF, granulocyte-colony stimulating factor.
Discussion
Adverse effects of vaccines are generally caused by vaccine antigens, excipients, adjuvants, containers, and other components included in the vaccine. Serious adverse events have been reported after immunization with pertussis vaccines attributed to the toxicity of antigens contained in the wP vaccines. Therefore, there has been a transition to aP vaccines or genetically detoxified recombinant aP vaccines, where immunogenic pertussis antigens are purified and then inactivated. Nonetheless, residue or reversion pertussis toxoid (PTxd) have known to occur from the development stage, therefore basic safety assessments must be carried out in parallel. In this regard, we conducted a safety evaluation of a novel rP vaccine being developed in Korea to confirm its safety and assess the feasibility for subsequent human studies.
Murine HIST has been utilized to verify PTx, where histamine-resistant mice exhibit over 100 times more sensitivity symptoms when exposed to PTx [18,19,20]. This method confirms PTx toxin activity (binding, translocation, and enzymatic activities) with high sensitivity and minimal interference from other substances included in the vaccine. Moreover, the bioavailability observed in test mice is similar to that in humans, making it advantageous for the safety verification of wP vaccines primary and continued use even after the development of aP vaccines [21,22]. However, due to differences in sensitivity based on the strain, age, and gender of the experimental mice, as well as variations related to the inoculation site and the dosage of histamine used, there is a lack of standardized evaluation. Moreover, ethical concerns arise due to the many animals sacrificed for this purpose. Therefore, there is an increasing recognition of the need for modified HIST evaluation methods and alternative tests [23,24]. In this regard, the various modified HIST have been developed. PTx, a substance formerly known as LPF, causes leukocytosis in mice [25]. Considering these characteristics of PTx, the LP test was undergone for verification of residual PTx. No residual PTx was defined when peripheral blood leukocyte count did not increase by more than 10 times from pre-inoculation levels to 3 days post-inoculation, which indicates no exposure to PTx. This test was once considered a basic safety evaluation test for the wP vaccine.
However, due to variations between laboratories and differences in the number of experimental animals and the timing of peripheral blood collection after inoculation, it is not recommended as a basic test for aP vaccines [26]. In this study, residual PTx was validated by performing temperature HIST [27,28,29] and LP tests [30,31] developed to evaluate the properties and mechanisms of binding, internalization, and ADP ribosylation of PTx. In the temperature HIST results of this study, no evidence of residual PTx was found in any of the control groups or experimental vaccines, except for negligible traces in the rP with alum adjuvant (Fig. 1). In addition, in the LP test for the bivalent DTaP vaccine, neither the control group nor the experimental group showed an increase in leukocyte count by more than 10 times due to PTx on day 3 post-inoculation. Most of the counts were within the normal range, confirming the absence of PTx residue. Higher levels of monocytes were observed in the two- and tri-components recombinant DTaP vaccines with beta-glucan adjuvant and the trivalent DTaP vaccine of the control group, compared to other groups. They exceeded the normal range (Figs. 2, 3), potentially due to the innate immune response elicited by the adjuvants of these vaccines following inoculation.
In 1983, it was reported by Hewlett et al. [32] that exposure of CHO cells to PTx for 48 hours resulted in morphological changes in cluster formations. These CHO cell changes are highly sensitive and can be tested in vitro on microplates; therefore, they have been applied to measuring residual PTx and PTx-neutralizing antibodies [33]. However, CHO cell morphological changes can be caused by alum salts and adjuvants contained in the vaccine, and the test results differ depending on the cells and reagents used for the test. There are significant differences in protocols and subjective evaluations between laboratories [34,35,36]; therefore, a modified method has been developed to compensate for this. In 2013, through EDQM and Biological Reference Preparation batch 1 collaborative joint study [37,38], direct and indirect modified CHO cell clustering assays were developed using the newly proposed standardized PTx (NIBS code 15/126) by WHO to avoid alum adjuvant interference effects and reduce inter-laboratory variations. In other words, both a direct method for diluting alum adjuvant-contained aP vaccines and an indirect method using a semi-permeable membrane to prevent contact between CHO cells and alum adjuvant were developed. It is suggested that verifying residual PTx through the detection of spiked PTx in test aP vaccines using the indirect method can yield results similar to the HIST sensitivity [16]. In this experiment, vaccines spiked with 1–4 IU/mL PTx, which is a considered method for residue PTx testing of genetically detoxified PTx [16,39,40]. In this study, experiments conducted using a similar method yielded comparable results. CHO cell clustering was consistent at a PTx level of 4 IU/mL in both the control and experimental vaccines, similar to previous studies [37,38,39] (Table 2).
The MWG test was previously performed by WHO as a routine lot-releasing test prior to the release of DTwP (diphtheria, tetanus, and whole cell pertussis) vaccines, based on the fact that weight loss when wP was administered to mice was associated with systemic adverse reactions in children vaccinated with wP vaccines [41]. The MWG test, associated with general toxicity caused by components like heat-labile toxin, lipopolysaccharide, and adenylate-cyclase toxin in wP vaccines, involves monitoring weight loss following wP vaccine administration from day 3 to 7 after inoculation to assess changes in body weight. Because such toxins are not present in aP or rP vaccines, conducting the MWG test is not typically recommended as a standard procedure. However, it can be performed in conjunction with the LP test during the pertussis vaccine development stage to assess the presence of endotoxins that may be included in the vaccine and to evaluate systemic reactions following inoculation. The results of the post-vaccination weight change in this study showed almost identical weight change patterns in both the control with the bivalent and trivalent DTaP vaccines and experimental groups. Similarly, the concurrently measured temperature also exhibited similar patterns, suggesting they were likely due to immune responses following vaccination (Figs. 4, 5). However, the evaluation of local reactions of the developed vaccine is done through visual observation of the inoculation site, organizational changes, and changes in inflammatory cytokine responses within the intramuscular inoculation site. In general, local reactions to vaccines vary depending on the antigens, molecular weight, and adjuvants contained in the vaccine [42]. In the evaluation of muscle tissue at the vaccination site conducted 3 days after vaccination, the trivalent DTaP control vaccine exhibited significantly higher TNF-α levels than other control and experimental vaccines (Fig. 6). However, no pronounced differences were observed among other cytokines.
In fact, for chemically detoxified aP vaccines, verification of residual PTx should be conducted for each batch. Additionally, to confirm the inactivation of PTxd, verification of PTx reversion should be conducted for each batch after incubating at 37℃ for 4 weeks. However, in the case of genetically modified PTx, the need for PTx reversion verification is theoretically unnecessary, and only residual PTxd verification is required. In this regard, we report on the safety assessment related to residual PTxd validation as described above.
The supply of DTaP monovalent vaccines poses a limitation to this study as they are very limited in the domestic market in South Korea (there is no single tri-components DTaP vaccine, and only two components DTaP vaccine is limited available). Therefore, a combination vaccine of bivalent and trivalent DTaP was used in this study. The beta-glucan adjuvant used has not yet been certified in South Korea; therefore, steps must be taken before it can be applied in human studies. It was used to assess the immunogenic suitability and matching of the recombinant antigen and for comparison with the alum adjuvant.
In conclusion, as a result of modified HIST, indirect CHO cell clustering assays, and LP tests on the three components recombinant DTaP vaccine under development for the first time in Korea, there were no findings associated with residual PTxd, and it was confirmed that there were no significant differences compared to existing available DTaP vaccines. Furthermore, safety has been demonstrated in both local and systemic reactions, indicating the potential for future clinical studies involving human subjects.
Footnotes
No potential conflict of interest relevant to this article was reported.
This research was supported by grant from Ministry of Food and Drug Safety in 2023 (22183MFDS448).
References
- 1.Melvin JA, Scheller EV, Miller JF, Cotter PA. Bordetella pertussis pathogenesis: current and future challenges. Nat Rev Microbiol. 2014;12:274–288. doi: 10.1038/nrmicro3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorji D, Mooi F, Yantorno O, Deora R, Graham RM, Mukkur TK. Bordetella pertussis virulence factors in the continuing evolution of whooping cough vaccines for improved performance. Med Microbiol Immunol. 2018;207:3–26. doi: 10.1007/s00430-017-0524-z. [DOI] [PubMed] [Google Scholar]
- 3.Corbel MJ, Xing DK. Toxicity and potency evaluation of pertussis vaccines. Expert Rev Vaccines. 2004;3:89–101. doi: 10.1586/14760584.3.1.89. [DOI] [PubMed] [Google Scholar]
- 4.Higgs R, Higgins SC, Ross PJ, Mills KH. Immunity to the respiratory pathogen Bordetella pertussis. Mucosal Immunol. 2012;5:485–500. doi: 10.1038/mi.2012.54. [DOI] [PubMed] [Google Scholar]
- 5.Gustafsson L, Hallander HO, Olin P, Reizenstein E, Storsaeter J. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. N Engl J Med. 1996;334:349–355. doi: 10.1056/NEJM199602083340602. [DOI] [PubMed] [Google Scholar]
- 6.Sekura RD, Fish F, Manclark CR, Meade B, Zhang YL. Pertussis toxin: affinity purification of a new ADP-ribosyltransferase. J Biol Chem. 1983;258:14647–14651. [PubMed] [Google Scholar]
- 7.Tamura M, Nogimori K, Yajima M, Ase K, Ui M. A role of the B-oligomer moiety of islet-activating protein, pertussis toxin, in development of the biological effects on intact cells. J Biol Chem. 1983;258:6756–6761. [PubMed] [Google Scholar]
- 8.Bokoch GM, Katada T, Northup JK, Hewlett EL, Gilman AG. Identification of the predominant substrate for ADP-ribosylation by islet activating protein. J Biol Chem. 1983;258:2072–2075. [PubMed] [Google Scholar]
- 9.Katada T, Ui M. ADP ribosylation of the specific membrane protein of C6 cells by islet-activating protein associated with modification of adenylate cyclase activity. J Biol Chem. 1982;257:7210–7216. [PubMed] [Google Scholar]
- 10.US Code of Federal Regulations. Washington (DC): Government Printing Office; 1983. Title 21: pertussis vaccine 620.1; pp. 58–61. [Google Scholar]
- 11.Munoz J. In: Pertussis toxin. Sekura RD, Moss J, Vaughan M, editors. London: Academic Press Inc.; 1985. Biological activities of pertussigen (pertussis toxin) pp. 1–18. [Google Scholar]
- 12.Markey K, Asokanathan C, Feavers I. Assays for determining pertussis toxin activity in acellular pertussis vaccines. Toxins (Basel) 2019;11:417. doi: 10.3390/toxins11070417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoonakker ME. In vivo models and in vitro assays for the assessment of pertussis toxin activity. Toxins (Basel) 2021;13:565. doi: 10.3390/toxins13080565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang KR, Kim JA, Cho GW, et al. Comparative evaluation of recombinant and acellular pertussis vaccines in a murine model. Vaccines (Basel) 2024;12:108. doi: 10.3390/vaccines12010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. WHO Expert Committee on Biological Standardization. World Health Organ Tech Rep Ser. 2013;(979):1–366. [PubMed] [Google Scholar]
- 16.Isbrucker R, Daas A, Wagner L, Costanzo A. Transferability study of CHO cell clustering assays for monitoring of pertussis toxin activity in acellular pertussis vaccines. Pharmeur Bio Sci Notes. 2016;2015:97–114. [PubMed] [Google Scholar]
- 17.Gray MC, Guerrant RL, Hewlett EL. The CHO cell clustering response to pertussis toxin: history of its discovery and recent developments in its use. Toxins (Basel) 2021;13:815. doi: 10.3390/toxins13110815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carbonetti NH. Pertussis toxin and adenylate cyclase toxin: key virulence factors of Bordetella pertussis and cell biology tools. Future Microbiol. 2010;5:455–469. doi: 10.2217/fmb.09.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamura M, Nogimori K, Murai S, et al. Subunit structure of islet-activating protein, pertussis toxin, in conformity with the A-B model. Biochemistry. 1982;21:5516–5522. doi: 10.1021/bi00265a021. [DOI] [PubMed] [Google Scholar]
- 20.Sato Y, Sato H. Development of acellular pertussis vaccines. Biologicals. 1999;27:61–69. doi: 10.1006/biol.1999.0181. [DOI] [PubMed] [Google Scholar]
- 21.Kind LS. The altered reactivity of mice after inoculation with Bordetella pertussis vaccine. Bacteriol Rev. 1958;22:173–182. doi: 10.1128/br.22.3.173-182.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoonakker M, Arciniega J, Hendriksen C. Safety testing of acellular pertussis vaccines: use of animals and 3Rs alternatives. Hum Vaccin Immunother. 2017;13:2522–2530. doi: 10.1080/21645515.2017.1349585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner LD, Corvette LJ, Ngundi MM, Burns DL. Towards replacement of the acellular pertussis vaccine safety test: comparison of in vitro cytotoxic activity and in vivo activity in mice. Vaccine. 2017;35:7160–7165. doi: 10.1016/j.vaccine.2017.10.082. [DOI] [PubMed] [Google Scholar]
- 24.Parfentjev IA, Goodline MA. Histamine shock in mice sensitized with Hemophilus pertussis vaccine. J Pharmacol Exp Ther. 1948;92:411–413. [PubMed] [Google Scholar]
- 25.Carbonetti NH. Pertussis leukocytosis: mechanisms, clinical relevance and treatment. Pathog Dis. 2016;74:ftw087. doi: 10.1093/femspd/ftw087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO Expert Committee on Biological Standardization; WHO Expert Committee on Biological Standardization, editors. WHO Expert Committee on Biological Standardization: sixty-second report. Geneva: WHO Press; 2013. Recommendations to assure the quality, safety and efficacy of acellular pertussis vaccines; pp. 187–260. (WHO technical report series; no. 979) [Google Scholar]
- 27.Jensen SE, Illigen KE, Badsberg JH, Hasløv KR. Specificity and detection limit of a dermal temperature histamine sensitization test for absence of residual pertussis toxin in vaccines. Biologicals. 2012;40:36–40. doi: 10.1016/j.biologicals.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Gaines-Das R, Ochiai M, Douglas-Bardsley A, et al. Transferability of dermal temperature histamine sensitization test for estimation of pertussis toxin activity in vaccines. Hum Vaccin. 2009;5:166–171. doi: 10.4161/hv.5.3.6615. [DOI] [PubMed] [Google Scholar]
- 29.Ochiai M, Yamamoto A, Kataoka M, Toyoizumi H, Arakawa Y, Horiuchi Y. Highly sensitive histamine-sensitization test for residual activity of pertussis toxin in acellular pertussis vaccine. Biologicals. 2007;35:259–264. doi: 10.1016/j.biologicals.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Morse SI, Morse JH. Isolation and properties of the leukocytosis- and lymphocytosis-promoting factor of Bordetella pertussis. J Exp Med. 1976;143:1483–1502. doi: 10.1084/jem.143.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta RK, Saxena SN, Sharma SB, Ahuja S. The effects of purified pertussis components and lipopolysaccharide on the results of the mouse weight gain test. J Biol Stand. 1988;16:321–331. doi: 10.1016/0092-1157(88)90020-0. [DOI] [PubMed] [Google Scholar]
- 32.Hewlett EL, Sauer KT, Myers GA, Cowell JL, Guerrant RL. Induction of a novel morphological response in Chinese hamster ovary cells by pertussis toxin. Infect Immun. 1983;40:1198–1203. doi: 10.1128/iai.40.3.1198-1203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pertussis vaccines: WHO position paper. Wkly Epidemiol Rec. 2010;85:385–400. [PubMed] [Google Scholar]
- 34.Sato Y, Kimura M, Fukumi H. Development of a pertussis component vaccine in Japan. Lancet. 1984;1:122–126. doi: 10.1016/s0140-6736(84)90061-8. [DOI] [PubMed] [Google Scholar]
- 35.Pierce VM, Vazquez M. New combination vaccines: integration into pediatric practice. Pediatr Infect Dis J. 2007;26:1149–1150. doi: 10.1097/INF.0b013e31815dd80f. [DOI] [PubMed] [Google Scholar]
- 36.Hoonakker ME, Ruiterkamp N, Hendriksen CF. The cAMP assay: a functional in vitro alternative to the in vivo Histamine Sensitization test. Vaccine. 2010;28:1347–1352. doi: 10.1016/j.vaccine.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Markey K, Asokanathan C, Tierney S, Hockley J, Douglas-Bardsley A. Collaborative study: evaluation of proposed second international standard for pertussis toxin code: 15/126. Geneva: WHO Press; 2017. [Google Scholar]
- 38.Markey K, Douglas-Bardsley A, Hockley J, Le Tallec D, Costanzo A. Calibration of pertussis toxin BRP batch 1 in a standardised CHO cell-based clustering assay. Pharmeur Bio Sci Notes. 2018;2018:112–123. [PubMed] [Google Scholar]
- 39.Wagner L, Isbrucker R, Locht C, et al. In search of acceptable alternatives to the murine histamine sensitisation test (HIST): what is possible and practical? Pharmeur Bio Sci Notes. 2016;2016:151–170. [PubMed] [Google Scholar]
- 40.Yuen CT, Horiuchi Y, Asokanathan C, et al. An in vitro assay system as a potential replacement for the histamine sensitisation test for acellular pertussis based combination vaccines. Vaccine. 2010;28:3714–3721. doi: 10.1016/j.vaccine.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Pittman M, Cox CB. Pertussis vaccine testing for freedom-from-toxicity. Appl Microbiol. 1965;13:447–456. doi: 10.1128/am.13.3.447-456.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kashiwagi Y, Maeda M, Kawashima H, Nakayama T. Inflammatory responses following intramuscular and subcutaneous immunization with aluminum-adjuvanted or non-adjuvanted vaccines. Vaccine. 2014;32:3393–3401. doi: 10.1016/j.vaccine.2014.04.018. [DOI] [PubMed] [Google Scholar]