Abstract
This narrative review describes genomic characteristic, serotyping, immunogenicity, and vaccine development of Streptococcus pneumoniae capsular polysaccharide (CPS). CPS is a primary virulence factor of S. pneumoniae. The genomic characteristics of S. pneumoniae CPS, including the role of biosynthetic gene and genetic variation within cps (capsule polysaccharide) locus which may lead to serotype replacement are still being investigated. One hundred unique serotypes of S. pneumoniae have been identified through various methods of serotyping using phenotypic and genotypic approach. The advantages and limitations of each method are various, emphasizing the need for accurate and comprehensive serotyping for effective disease surveillance and vaccine targeting. In addition, we elaborate the critical role of CPS in vaccine development by providing an overview of immunogenicity, ongoing research of pneumococcal vaccines, and the impact on disease burden.
Keywords: Streptococcus pneumoniae, Capsular polysaccharide, Serotyping, Immunogenicity, Vaccine development
Introduction
Streptococcus pneumoniae is an opportunistic bacterium which cause various clinical manifestation such as otitis media, meningitis, septicemia, sinusitis, and pneumonia [1]. Primarily affected individuals are children, the elderly, and the immunocompromised [2]. In the United States, pneumococcal meningitis and bacteremia killed approximately 3,250 people in 2019 [3]. The prevalence of at least one chronic condition was 11% in adults aged 18–49 years, 25% in adults aged 50–64 years, and 51% in adults aged >65 years or older in the United States from 2013 to 2015 [4]. Based on Centers for Disease Control and Prevention in 2022, the number of deaths caused by pneumonia was 41,309 [5].
Capsular polysaccharide (CPS) has been regarded as the main virulence factor of S. pneumoniae. The basis of CPS are polymers with repeat units composed of more than two monosaccharides [6]. CPS also classifies S. pneumoniae into numerous serotypes. Several S. pneumoniae serotypes are implicated with severe infection, but only a few serotypes cause pneumococcal infections [7]. Serotypes associated with high prevalence of case fatality rate in various countries are 3, 11A, 9N, 19A, 16F, 19F, and 6A in adult’s cases and 6A, 3, and 19F in children’s cases [8]. However, the serotype distribution of S. pneumoniae has shown dynamic changes over the years. A study in Southampton, United Kingdom analyzed the serotype distribution of S. pneumoniae in children between 2006 and 2018. The introduction of pneumococcal conjugate vaccine (PCV), PCV13 in 2010 had a significant impact on serotype prevalence. The study found that proportion of PCV13 serotypes decreased, while non-PCV13 serotypes increased over time [9]. Recent study in Urumqi, China analyzed the serotype distribution and drug resistance of S. pneumoniae in children aged 8 years and under between 2010 and 2021. The introduction of PCV13 in 2013 led to a significant decrease in PCV13 serotypes and an increase in non-PCV13 serotypes [10].
In general, pneumococcus CPS are synthesized via a Wzx/Wzy-dependent pathway, except for serotypes 3 and 37 which use synthase-dependent pathway [11]. The capsule polysaccharide (cps) locus contains the majority of the genes for S. pneumoniae capsular biosynthesis. cps locus is flanked between dexB and aliA genes. The upstream region (5’ end) of cps locus started with the main regulatory genes, including wzh, wzg, wze, wzd, and (cpsABCD). These genes were found highly conserved in all pneumococcus serotypes [12].
Pneumococcal capsular serotyping helps to determine the pathogenicity of S. pneumoniae and important for evaluation and monitoring the emergence of serotype replacement, non-vaccine type (NVT), and novel serotype [12]. The Quellung reaction method is known as the gold standard for serotyping S. pneumoniae. However, it can only be performed on viable isolates and relatively expensive. Bentley et al. [11] in 2006 found that the cps locus of S. pneumoniae associated to biochemical structure and immunological patterns and made a breakthrough for molecular serotyping assays. Afterwards, molecular typing methods continue to performed, including multiplex polymerase chain reaction (PCR), whole-genome sequencing, and microarray [13]. Recently, there are 101 serotypes based on diversity of capsular types [14]. As the study of pneumococcal strains progressed, distinction among serotypes emerged as an essential key for the development of the vaccines [15].
Pneumococcal vaccinations aim to reduce number of cases, complications, and mortality from pneumonia and invasive pneumococcal disease (IPD) [16]. Recently, the available vaccines for prevention of S. pneumoniae infections are PCV7, PCV10, PCV13, PCV15, and PCV20 and 23-valent pneumococcal polysaccharide vaccine (PPSV23) [17]. The introduction of PCV had a positive impact on reducing the burden of pneumococcal disease worldwide, including in low-income and middle-income countries [18]. However, current vaccine provide protection against many, but not all, types of pneumococcal bacteria. The high number of serotypes and the emergence of non-vaccine serotypes pose challenges in achieving broad coverage [19].
In this review, we focused on overview of literature related to S. pneumoniae including genomic features, gene expression in biosynthesis of CPS, serotyping method of S. pneumoniae, and current insight of pneumococcal vaccine development. We also explore epidemiology and pathogenesis of pneumococcus infection related to the diversity of its serotypes. Furthermore, we would discuss the medications to treat and prevent pneumococcal disease and the implications of pneumococcal disease in the future.
Pneumococcus and Its Capsular Role in Pathogenesis
S. pneumoniae is one of the commensal floras. Unfortunately, this bacterium can turn into pathogen when it invades internal organs [20]. The pneumococcus is responsible for several infection disease including pneumonia, meningitis, otitis media, sinusitis, and bronchitis [21,22]. Infections of pneumococcal are thought to spread via aerosol or droplets. A naive immune system tends to make young infants more vulnerable to infection. Otherwise, in healthy young children and adults, mutations in genes cause innate or adaptive immune system defects that can lead to severe recurring infections [23].
The beginning of life cycle S. pneumoniae in the host usually precedes progression to invasive disease. In the colonization phase, pneumococci are threatened not only by the host’s immunological defenses but also by the other members of the nasopharyngeal flora. Avoiding mucosal clearance and adhering to the epithelium are crucial elements in the formation of carriage conditions [23]. The pathogenicity of pneumococcus is primarily caused by CPS, a polysaccharide layer that surrounds the bacterial cell [6].
S. pneumoniae CPS have a major influence in pneumococcal pathogenesis [11,24]. Most CPS serotypes has positive charge to aid colonization by preventing these bacteria from mucus trap [25]. CPS S. pneumoniae permitting bacteria to escape from nasal mucus and inhibit innate immune cells, avoids bacteria recognition by host receptors, and evade phagocytosis by host immune cells [26]. Moreover, it can inhibit complement and recognition by immunoglobulins and elude the neutrophil traps [27].
The immunochemical differences between CPS classifies them into many serotypes [11]. Recently, at least 100 serotypes have been identified [15]. Serotype S. pneumoniae plays an important role in monitoring and evaluating the pathogenesis of pneumococcal infection. Recent study has also shown that S. pneumoniae can persist intracellular, which may contribute to its ability to evade host immune responses and cause chronic infections [23]. Monitoring the prevalence and distribution of different S. pneumoniae serotypes is also important for evaluating the effectiveness of pneumococcal vaccines, which target specific serotypes [20].
Genomic Characteristics of Pneumococcus Capsular Polysaccharide: Capsular Regulatory Genes in S. pneumoniae
S. pneumoniae CPS plays critical role for colonization and immune evasion [28]. To avoid being trapped by the mucus in the nasopharynx, bacteria must express CPS during initial colonization. Pneumococcal capsule expression levels have been shown to vary greatly between sites, suggesting that S. pneumoniae regulates capsule expression to achieve optimal fitness [29]. The regulation of capsular production is essential for S. pneumoniae to survive in different niches of their hosts [30]. Elevated production of capsule is necessary for systemic virulence, but decrement of capsule production is beneficial for the adherence and colonization in the nasopharyngeal tract of the host [6].
Pneumococcus CPS is synthesized by genes located in cps locus. Genes that are involved for the capsular biosynthesis regulation are located at cps locus, between dexB and aliA (except serotype 37 and serotype 3 which regulated by tts gene) which ranges from 2.2 to 30 kb. Diversity in coding region of the cps locus is responsible for the enormous diversity of CPSs in chemical structure and antigenic property [28]. This arrangement facilitates genetic exchange of CPS genes by homologous recombination, particularly under stress environment such as antibiotic treatments [22,31].
The cps locus in S. pneumoniae is co-transcribed as an operon from a common promoter upstream of cpsA gene. The core promoter of the capsule operon of S. pneumoniae is necessary for colonization and invasive disease. The approximately 250 bp region located immediately upstream of cpsA is relatively conserved in most S. pneumoniae isolates synthesizing their capsular through a Wzx/Wzy-polymerase-dependent pathway [29,30].
Except for serotypes 3 and 37, which are regulated by the synthase-pathway, most biosynthesis of S. pneumoniae using Wzx/Wzy-dependent mechanism. Highly conserved genes (cpsA, cpsB, cpsC, cpsD, and cpsE/wchA) are located upstream of cps loci in the Wzx/Wzy-dependent pathway [32]. In contrast to serotype 3, which has its tts gene in the cps locus, serotype 37 has its tts synthase genes located near gpmA [32,33].
CPS biosynthetic transcription may be regulated by the cpsA genes [1]. The phosphoregulatory system is comprised of cpsBCD genes. The N-terminal integral membrane activation domain and the C-terminal cytoplasmic kinase domain, respectively, are found in cpsCD [34]. Transphosphorylation between CpsD proteins does not necessitate CpsC, although CpsC is needed for the first tyrosine autophosphorylation of CpsD. Dephosphorylating CpsD necessitates the presence of CpsB, a manganese-dependent phosphotyrosine protein phosphatase that is a group of the PHP. Transphosphorylation of CpsD proteins is inhibited by CpsB, which has been shown to bind CpsD [35].
Several transcriptional regulators have been identified that control the expression of the cps operon in S. pneumoniae [29,32]. These regulators include CpsR, CpsY, and CpsEm among others. They bind to specific regions of the cps operon and either activate or repress its transcription, thereby influencing capsule synthesis and expression [36].
The cps loci are thought to be serotype specific since the downstream areas do not demonstrate similar sequence conservation or grouping as the upstream regions. These domains encode enzymes involved in polymer-specific processes like flippase (wzx), polymerase (wzy), glycosyltransferases, and O-acetylases. These genes encode proteins like glycosyltransferases and acetyltransferases, as well as enzymes involved in the synthesis of nucleotide-activated sugar precursors, and are serotype-specific. Wzy polymerase activity, modification enzymes that impact O-acetylation, or substitution of glycosyltransferases are all possible causes of cross-reactive but different serotypes [1]. Capsule production relies on the activity of sugar-synthesis genes (e.g., rhamnose gene) located at the very ends of the locus. Comparing the DNA of different serotypes, however, revealed that the formation of numerous serotypes was due to the acquisition and loss of genes in the capsid [31].
Several recent findings about S. pneumoniae capsular synthesis have been investigated at genomic and proteomic levels. Recent research by Glanville et al. [36] showed that two highly conserved factor of transcription, CpsR and SpxR. Pneumococcus can be rendered avirulent by changing three nucleotides in that sequence, which raises the possibility that molecular switch plays a significant role in the development of serotype-specific illness consequences [36].
In 2020, Zheng et al. [37] discovered that multiple differentially expressed proteins, including, Cps2D Cps2L, Cps2M Cps2E, Cps2T, and Cps2C, contribute in CPS synthesis. A study by Zheng et al. [30] also discovers comE plays critical role in capsular production level. Additionally, a study revealed that ComX, RegM, CopY, GlnR, CpsR, ComE, and RitR are a number of potential transcriptional regulators of the cps gene [30]. Through horizontal gene transfer mediated by natural transformation, the locus of cps undergoes significant changes. According to Wen et al. [28], the cps promoter sequence polymorphisms indicate a unique method for regulating the degree of encapsulation and pathogenicity in S. pneumoniae strains.
Serotype Replacement of S. pneumoniae
Serotype is an important factor in determining the potential and prevalence of IPD. In early 2000, PCV7 was released, and surveillance data revealed a decrement of vaccine-type (VT) S. pneumoniae while nasopharyngeal carriage and disease rise in NVT S. pneumoniae. This phenomenon is known as serotype replacement. Serotype replacement can occur due to selective pressures such as vaccination or antibiotic use that influence changes in prevalence of certain serotypes over others [38].
The key genomic factors influencing serotype replacement within cps loci are mainly caused by high diversity of genetic makeup within these loci. The presence of these diversity and low percentage of G+C content of the region suggests that these gene have been acquired into pneumococci (or their ancestors) on multiple occasions [11]. S. pneumoniae may acquires new genetic material through horizontal gene transfer, which can lead to the emergence of novel serotypes. The emergence of novel serotypes may influence the prevalence of circulating serotypes, leading to serotype replacement. This genetic event can occur through transformation, transduction, or conjugation, mediated by bacteriophages or conjugative plasmids [38]. In addition, minor genetic changes such as point mutation, deletion, and insertion within cps loci may alter the CPS chemical structure to change serotype and alter sensitivity to complement [39,40].
Genomic factors may contribute to the development of effective pneumococcal vaccines by highlighting the need for vaccines that target a broader range of serotypes and protein antigens of the pneumococci since the introduction of the PCV may eliminated VT, thus NVT are subsequently increased in carriage and disease [41].
Genomic structure modification within cps loci may result changes in antigenicity of the bacteria, which leads to the emergence of novel serotype or serotype prevalence alteration within population. Different serotypes of S. pneumoniae CPSs may have varying immunogenicity, even when conjugated to identical carrier proteins, since the host-immune system recognizes and responds specific to each serotype, leading to different antibody titers and memory T cell responses [42].
Capsular switching also refers to the process by which pneumococcal bacteria can change the type of capsule surrounding them. This can occur through the acquisition of new genetic material from other bacteria, which can result in changes in the serotype distribution of pneumococcal populations. Both serotype replacement and capsular switching can contribute to changes in the serotype distribution of pneumococcal populations over time [38].
Wyres et al. [38] analyzed serotype and multilocus sequence typing data for 426 pneumococci dated from 1937 through 2007 to investigate the contribution of serotype replacement. The researchers identified 36 independent capsular switch events, 18 of which were explored in detail with whole-genome sequence data. Recombination fragment lengths were estimated for 11 events and ranged from approximately 19.0 kb to ≥58.2 kb. Recombination of large DNA fragments sometimes including the cps locus and penicillin-binding protein genes, predated both vaccine introduction and widespread antibiotic use. This type of recombination has likely been an intrinsic feature throughout the history of pneumococcal evolution [38].
Serotype replacement can reduce overall effectiveness of pneumococcal vaccines. If the replacing serotypes are more virulent or antibiotic-resistant, they may pose challenges in maintaining long-term protection against pneumococcal disease [43]. This phenomenon also highlights the importance of developing vaccines that provide broader serotype coverage [44].
Biosynthesis Pathway of CPS
In order to create a lipid-linked repeat unit, CPSs are typically made by first transferring a monosaccharide phosphate from a nucleotide diphosphate sugar to a lipid carrier coupled with a membrane. The flippase then transports this to the outer membrane, polymerizes it to create the mature CPS, and attaches it to the pneumococcal cell wall [11].
Wzx/Wzy-dependent pathway
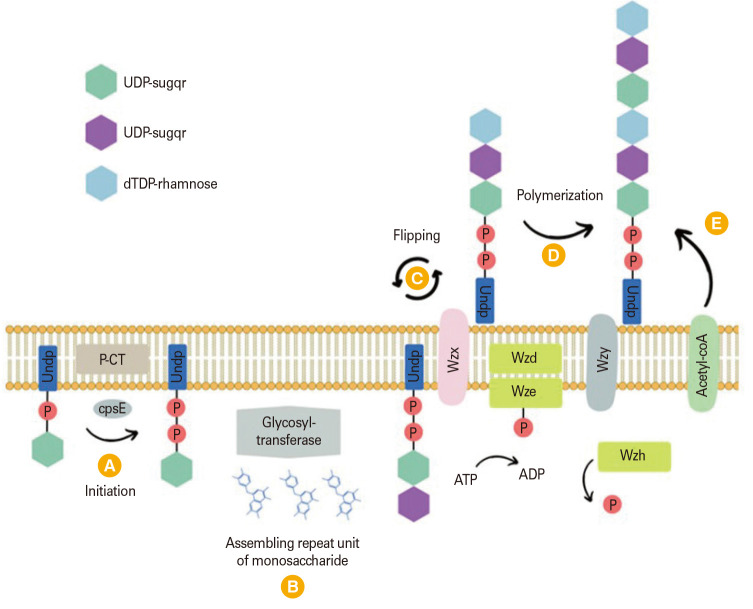
Beginning with the assembly of sugar repeat units on the inner side of the cell membrane, Wzx/Wzy-dependent synthesis involves the flippase transporting those units to the outer side of the membrane and the Wzy polymerase polymerizing them. The reversible conversion of sugar phosphate to undecaprenol phosphate through pyrophosphate bond leads to the creation of an undecaprenyl phosphate (UndP)-sugar (Fig. 1) [45]. UndP involved in peptidoglycan synthesis as well. The initial sugar in most pneumococcal serotypes is Glc-1-P, and the process is catalyzed by the membrane-integral glycosyltransferase CpsE. The oligosaccharide chain expands when more monosaccharide subunits, either identical or different, are added by other glycosyltransferases encoded by the cps locus. The expanding chain is subsequently transferred to the outer membrane by flippase (Wzx). In conclusion, Wzx/Wzy-dependent pathway needed cpsE for initiation, Wzy for polymerization, and Wzx for transport to produce capsule. Additionally, discrete repeat units are added block by block before the capsule is covalently bonded to the cell wall [1].
Fig. 1. The Wzx/Wzy-dependent pathway of capsular polysaccharide biosynthesis [45]. Biosynthesis begins with the synthesis of the O-antigen repeat unit in the cytoplasm. The repeat unit is then transferred to a lipid carrier undecaprenyl phosphate (UndP) (A). Glycosyl-transferase will be assembling the repeat unit of monosaccharide (B) before the UndP flipped across the inner membrane to the periplasmic side by Wzx/flippase (C). Wzy polymerase catalyzes the polymerization of the repeat units to form the polysaccharide chain (D), Wzy polymerase adds repeat units one by one to the growing chain and acetyl-coA will be added (E). UDP, uridine diphosphate; dTDP, deoxythymidine diphosphate.
Synthase-dependent pathway
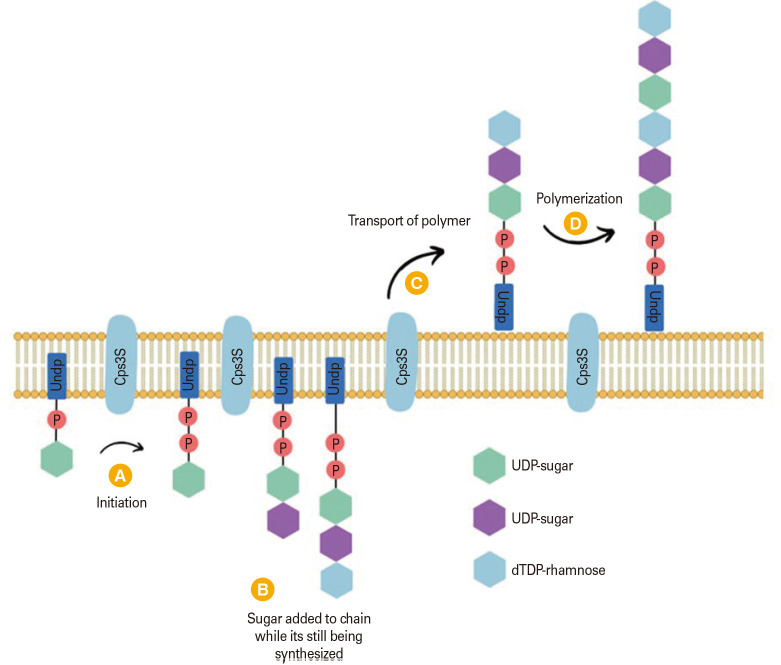
Serotypes 3 and 37 use a synthase-dependent biosynthetic mechanism that is very different from the Wzx/Wzy-dependent pathway [1]. In the synthase-dependent pathway, capsular production is initiate by a single enzyme that transfers carbs to expand the polysaccharide [45]. Instead of being attached to the cell wall through a covalent bond, CPSs are found connected to the cell via a phosphatidylglycerol membrane anchor or interactions with the synthase enzyme (Fig. 2) [45].
Fig. 2. Synthase-dependent biosynthesis pathway [45]. Serotype 3 PS is created by the Cps3S synthase (structure indicated in the inset). The synthesis is started by Cps3S that transferring glucose (Glc) from uridine diphosphate (UDP)-glucose to a phosphatidyl glycerol (PG) acceptor (A), glucuronic acid (GlcUA) from UDP-GlcUA to the PG-linked Glc (B), Cps3S transport the polysaccharide chain to the outer membrane (C) and added chain length through polymerization (D). UndP, undecaprenyl phosphate; dTDP, deoxythymidine diphosphate.
Transferring glucose from UDP (uridine diphosphate)-Glc to the production of serotype 3 CPS began with the production of a phosphatidylglycerol membrane anchor. The following section describes how the synthesis proceeds by alternating the addition of glucuronic acid and glucose from their respective NDP-sugars. The rate of this reaction is determined by the accessibility of the sugars. Around eight sugars in length, a tipping point occurs where lipid-linked sugars are bound to the synthase. The polysaccharide is reoriented and undergoes processive polymerization at high molecular weight after binding, allowing it to pass through the cell membrane. This is yet another difference from Wzy-reliant capsule synthesis. The capsule stays attached to the cell without being linked to the peptidoglycan, instead interacting with phosphatidylglycerol or the synthase [1].
Capsular Typing of S. pneumoniae
Traditional serotyping methods classify pneumococci into subgroups defined by the type of capsule [46]. Neufeld first characterized the capsular reaction test, also known as the Quellung reaction, in 1902 as the gold standard method for pneumococcal typing [47]. A type-specific of antibodies will bind to the pneumococcus cell wall and change the refractile index of light and make the capsule look enlarged or “swollen” under a microscope if they recognize a certain capsule epitope [48].
The sequencing of an individual’s entire genome is becoming increasingly commonplace for medical purposes. When compared to the conventional procedures, this diagnostic technology’s advantageous characteristics—including its low price and quick response time—make it a viable and promising option. In comparison to more traditional approaches to DNA sequencing, next-generation sequencing is distinguished by its better throughput, longer read lengths, decreased sequencing times, and lower total costs [46]. Whole-genome sequencing has emerged as an alternative to multiplex PCR for serotyping. Although the primer specificity in multiplex PCR is adequate for serotyping in clinical samples, the development of primer pairs has been hindered by high sequence homology in the pneumococcal capsule region, resulting in insufficient characterization of the isolates [48].
Kapatai et al. [13] and Public Health England devised the PneumoCaT (https://github.com/ukhsa-collaboration/PneumoCaT), an automated bioinformatics pipeline in 2016 to predict the serotype level for 94 types of S. pneumoniae. PneumoCaT was able to predict 99.0% of non-UK and 99.1% of typable UK isolates, according to their research. In addition, the results of retesting demonstrated an increase in concordance from 91.5% to 99.3% [13].
The quick, high-throughput approach of serotyping S. pneumoniae called SeroBA (https://github.com/sanger-pathogens/seroba) was developed by Epping et al. [49] in 2018. By adopting a k-mer-based technique, SeroBA can accurately predict serotypes from raw whole-genome sequencing read data with a concordance of 98%, proceed 10,000 samples in less than a day on a typical server, and designate serotypes at coverages as low as 15–20x. SeroBA’s cps locus sequence assembly results are also very helpful for downstream analysis. To explore the evolution of the cps locus across a collection of S. pneumoniae samples, or to find novel mutations within a serogroup, a phylogenetic tree can be constructed using this data. Using negative S. mitis and S. pseudopneumoniae control samples, Epping et al. [49] showed that SeroBA had a 100% specificity while PneumoCaT only had a 92% specificity. SeroBA and PneumoCaT can identify serotypes in sequencing data reads using Illumina platform [13,49]. These algorithms will report “mixed” for sample that contains multiple serotypes. To determine the serotype composition from mixed samples, Knight et al. [50] develop SeroCall (https://github.com/knightjimr/SeroCall). SeroCall is a software tool that can identify and quantitate the serotypes present in samples, even when several serotypes are present. It is designed to work with samples that may contain multiple serotypes, which is a common occurrence in pneumococcal infections [50].
Although SeroBA provides faster serotyping, it has no advantage over PneumoCaT in mixed serotype detection and may be more limited in detection of mixed types due to the partial assembly-based approaches used. SeroCall provides very accurate mixed serotype detection, the use of mapping for serotype screening lengthens the run time compared to SeroBA’s k-mer-screening method. As a result to help determine multiple serotypes in pneumococcal carriage samples in a lightweight tool, Sheppard et al. [51] develop PneumoKITy in 2022 (https://github.com/CarmenSheppard/PneumoKITy/actions).
PneumoKITy uses the effective mash k-mer for serotyping that is significantly faster than PneumoCaT. When compared to the current FastQ version of PneumoCaT, PneumoKITy uses up to 11 MB less RAM and is up to 29 times faster. In situations when many serotypes are present, such as in nasopharyngeal carriage studies [51].
The bioinformatic challenge in serotyping is based on the similarity of segments of the capsular biosynthetic cassette in various serotypes. A bioinformatic pipeline offers a significant benefit in obtaining detailed information on serotype drift and might be used to target future phenotypic investigation of potential novel serotypes [13,49].
Capsular Immunogenicity
S. pneumoniae CPS is a T-cell-independent antigen, which means it is poorly immunogenic in young children. Vaccination with PCVs has shown effectiveness in reducing pneumococcal disease in children. However, multiple colonization events in an individual lifetime result in serum antibody responses to the CPS. Antibodies play a crucial role in protecting against pneumococcal infections. CPS inhibits both the classic and alternative complement pathways by limiting binding of immunoglobulins, complement components, and C-reactive protein to the bacterial surface. This inhibition helps S. pneumoniae evade the host immune system [6].
Natural acquired immunity to pneumococcal CPSs develops during childhood, robust in young adults and deteriorates in the elderly. The reduction in natural acquired immunity also results in suboptimal functional antibody responses to current pneumococcal vaccines. While PCV13 has overcome some of the immunological limitations of PPSV23, reduced antibody functionality combined with limited serotype coverage means that pneumococcal vaccination in the elderly does not deliver as substantial a benefit as would be expected [52].
Adler et al. [52] also found that improvements in the functionality of aged antibodies, particularly immunoglobulin M, will need to be induced if anti-CPS antibodies remain the mediator of protection. A mucosal vaccine, with an appropriate adjuvant, might be an alternative strategy. Furthermore, vaccination strategies also focused to exploit noncapsular antigens or T cell-mediated immunity that have shown a degree of promise in early-phase studies of young adults. Future studies should investigate the dynamics of colonization and mechanisms of natural acquired immunity in the elderly in greater detail, as well as explore the nature of respiratory mucosal immunity in the elderly to better inform vaccine development for this growing and vulnerable population [52].
A systematic review and meta-analysis found that pneumococcal vaccines are immunogenic in patient co-infected with human immunodeficiency virus (HIV) [53]. HIV-infected children have a markedly increased risk for pneumococcal infection compared to those who are not HIV-infected [54]. A study found that HIV-infected infants had poorer immune responses to pneumococcal conjugate vaccine compared to HIV-uninfected infants. However, the study also found that the immune response improved with antiretroviral treatment [55].
Current research on capsular immunogenicity is focused on understanding the immune response to CPS and developing effective vaccines against encapsulated bacteria. Thanawastien et al. [56] discover that the protein capsular matrix vaccine (PCMV) produced using poly-gamma-D-glutamic acid (PGA) and the carrier/matrix protein dominant negative inhibitor (DNI), derived from Bacillus anthracis has potential as a vaccine candidate. The PCMV was found to contain higher molecular weight material than the DNI control, indicating that the DNI in the PCMV was cross-linked into higher molecular weight forms. The slower electrophoretic mobility of both the PGA and DNI components in the PCMV sample indicates that the two components are likely associated in a macromolecular complex. A capture-based enzyme-linked immunosorbent assay was used to demonstrate that PGA was entrapped in the DNI protein matrix. These data indicate that PGA, in the PCMV sample, was associated with the bound DNI matrix [56].
Pneumococcal Vaccine Development
Pneumococcal vaccines have been developed to provide protection against S. pneumoniae. Vaccines targeting specific serotypes have substantially decreased the incidence of pneumococcal disease. The first pneumococcal vaccine was developed in the 1980s and contained purified CPS antigen from 14 different types of pneumococci. PPSV23 was later developed in 1983 to provide protection against 80%–90% of the pneumococcal capsular serotypes. This review describes development of currently available pneumococcal vaccines, provides summary tables of current pneumococcal vaccine recommendations in children and adults, and describes new potential vaccine antigens in the pipeline. S. pneumoniae, the bacteria responsible for pneumonia, otitis media, meningitis, and bacteremia, remains a cause of morbidity and mortality in both children and adults. Introductions of unconjugated and conjugated pneumococcal polysaccharide vaccines have each reduced the rate of pneumococcal infections caused by the organism S. pneumoniae. The first vaccine developed, the 23-valent pneumococcal polysaccharide vaccine (PPSV23), protected adults and children older than 2 years of age against invasive disease caused by the 23 capsular serotypes contained in the vaccine. Because PPSV23 did not elicit a protective immune response in children younger than 2 years of age, the 7-valent pneumococcal conjugate vaccine (PCV7) containing seven of the most common serotypes from PPSV23 in pediatric invasive disease was developed for use in children younger than 2 years of age. The last vaccine to be developed, the 13-valent pneumococcal conjugate vaccine (PCV13), contains the seven serotypes in PCV7, five additional serotypes from PPSV23, and a new serotype not contained in PPSV23 or PCV7. Serotype replacement with virulent strains that are not contained in the polysaccharide vaccines has been observed after vaccine implementation and stresses the need for continued research into novel vaccine antigens. We describe eight potential protein antigens that are in the pipeline for new pneumococcal vaccines PPSV23 (covering serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9V, 9N, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 33F) [57].
In England and Wales, PPSV23 was 27% effective against the vaccine serotypes causing IPD in older adults, irrespective of time since vaccination [58]. A study conducted in Denmark estimated the effectiveness of PPSV23 against all-serotype IPD and PPSV23 serotype IPD in individuals ≥65 years. The adjusted vaccine effectiveness was 42% for all-serotype IPD and 58% for PPSV23-serotype IPD [59].
PCV is a newer type of vaccine that consists of CPSs covalently bound to a protein carrier, which is highly immunogenic but non-toxic. PCVs have been developed to provide broader protection against pneumococcal serotypes and have been integrated into national childhood immunization programs worldwide. The first PCV, PCV7 was licensed for use in the United States in 2000 and provided protection against serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F. Since then, PCV and PPSV vaccines has already been adopted into national vaccination programs in developed nations across Europe and the United States, significantly decreasing IPD caused by serotypes covered by these vaccines [60].
The effect PCV7 has been studied in various populations. In California, PCV7 protected more than nine in 10 babies from invasive disease caused by vaccine serotypes. The vaccinated children also had fewer ear infections. Vaccination with PCV7 was 88% effective in preventing IPD due to vaccine serotypes. The incidence of IPD caused by vaccine serotypes decreased by approximately 80% among children <2 years However, other studies shown that vaccination with PCV7 results in a decline in nasopharyngeal carriage of penicillin-resistant S. pneumoniae, in carriage of VT S. pneumoniae, and serotype replacement by NVT.
Since then, PCVs have progressed to PCV10 (covering PCV7 serotypes and serotypes 6A, 3, and 19A). Studies demonstrated that direct effectiveness of PCV10 against all vaccine serotypes was found to be 97% in Canada and 84% in Brazil [61]. PCV10 effectiveness of at least one dose was 84.8% against PCV10 serotypes and showed varying effectiveness against specific serotypes such as 1, 7F, 19A, and 6C [62].
In 2019, PCV15 was developed to provide protection against an expanded range of pneumococcal serotypes compared to PCV10 (including serotypes 22F and 33F). PCV15 has been shown to be highly immunogenic and induces both IgG and opsonophagocytic activity to all 15 serotypes included in the vaccine at levels comparable to PCV13 for shared serotypes and superior for serotypes unique to PCV15 [63].
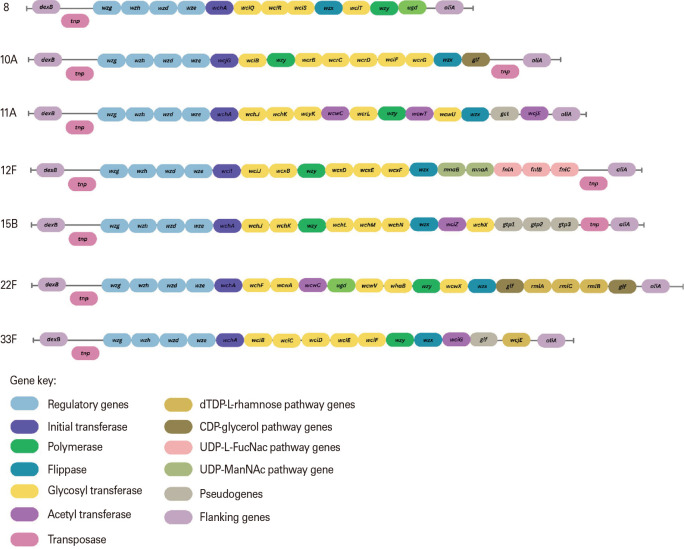
The prevalence of serotype 8, 10A, 11A, 12F, 15B, 22F, and 33F causing IPD varies by region and time. In high-income countries, some of these serotypes have been shown to cause more severe clinical manifestations such as meningitis [64]. In Europe, a systematic review found that these seven serotypes have an increasing trend in IPD incidence prevalence in adults [65]. PCV20 covers serotype 8, 10A, 11A, 12F, 15B, 22F, and 33F was licensed for adults >18 years in 2021 (Fig. 3) [11]. Unlike previous conjugate vaccine formulations, PCV20 was licensed for adults before submission for licensure in children. However, the Food and Drug Administration has accepted a supplemental biologics license application for pediatric use of PCV20.
Fig. 3. Represent genetic differences of vaccine-type cps (capsule polysaccharide) locus among serotypes that found high in prevalence after introduction of pneumococcal conjugate vaccine (PCV)13, leading to development of new PCV [11]. dTDP, deoxythymidine diphosphate; CDP, cytidine diphosphate; UDP, uridine diphosphate.
PCV20 has been shown to induce antibody levels comparable to those induced by PCV13. The immunogenicity and safety of PCV20 have been evaluated in phase II and phase III clinical trials in adults aged 18 years and older. Phase II and phase III study compared the immunogenicity of PCV20 adults aged 18–49 years compared to adults aged 60–64 years. The study found that PCV20 was well-tolerated and induced robust immune responses for all 20 serotypes included in the vaccine [66]. Comparison table of pneumococcal vaccine product, composition, serotype coverage, and recommendation usage are provided in (Table 1) [57,67].
Table 1. Comparison table of pneumococcal vaccine product, composition, serotype coverage, and recommendation usage [57,67].
| Product | Vaccine based | Serotypes coverage | Age group recommended use |
|---|---|---|---|
| PPSV23 (Pneumovax 23) | Purified capsular polysaccharide antigens | 1, 2, 3, 4, 5, 6B, 7F, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F | • Adults ≥65 yr |
| • Children 2–18 yr | |||
| PCV7 (Prevnar 7) | Purified capsular polysaccharide antigens conjugated to carrier protein (CRM197, a non-toxic variant of diphteria toxin) | 4, 6B, 9V, 14, 18C, 19F, and 23F | • Children <2 yr |
| • Children 2–4 yr | |||
| PCV10 (Synflorix) | Purified capsular polysaccharide antigens conjugated to carrier protein (CRM197, a non-toxic variant of diphteria toxin) | 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F | • Children <5 yr |
| PCV10 (Pneumosil) | Purified capsular polysaccharide antigens conjugated to carrier protein (CRM197, a non-toxic variant of diphteria toxin) | 1, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A,19F, and 23F | • Children <5 yr |
| PCV13 (Prevnar 13) | Purified capsular polysaccharide antigens conjugated to carrier protein (CRM197, a non-toxic variant of diphteria toxin) | 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F | • Children <5 yr |
| • Adult ≥19 yr with certain medical conditions (HIV infection, chronic heart, lung, or liver disease) | |||
| • Adults ≥65 yr | |||
| PCV15 (Vaxneuvance) | Purified capsular polysaccharide antigens conjugated to carrier protein (CRM197, a non-toxic variant of diphteria toxin) | 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F | • Children <2 yr |
| • Adults ≥65 yr | |||
| PCV20 (Prevnar 20) | Purified capsular polysaccharide antigens conjugated to carrier protein (CRM197, a non-toxic variant of diphteria toxin) | 1, 3, 4, 5, 6A, 6B, 7F, 8, 9V, 10A, 11A, 12F, 14, 15B, 18C, 19A, 19F, 22F, 23F, and 33F | • Adults ≥65 yr |
HIV, human immunodeficiency virus.
The development of pneumococcal vaccines faces challenges related to the complex nature of the pneumococcal pathogen, the need to identify the most appropriate protein antigens to target, and the need to develop a vaccine that is effective against most serotypes. Human challenge testing is a complex and controversial issue that raises ethical concerns. Further research is needed to evaluate the effectiveness of pneumococcal vaccines in different populations and to develop a vaccine that will retain its efficacy for most pneumococcal serotypes [68]. However, current research on pneumococcal vaccine is addressing serotype-independent vaccines, which has been a challenging and elusive endeavor [69].
Furthermore, S. pneumoniae characteristic is capable of intra- and interspecies DNA recombination, which modifies capsule composition, and virulence factor, antibiotic resistance, and molecular typing [70]. Therefore, pneumococcal disease remains a formidable challenge in terms of both prevention and treatment.
Current Research on Serotype 3 against Pneumococcal Vaccine
Following the introduction of PCV13 and PPSV23, serotype 3 remains a major cause of severe clinical manifestations such as bacteremia-induced septicemia, meningitis, and pneumonia. In some countries, serotype 3 has also been associated with empyema [71]. Serotype 3 considered as a challenging target for vaccines due to its ability to evade host’s immune system through antigenic variation, clonal relationship, and vaccine escape recombinants [71]. Study also found that Global Pneumococcal Sequencing Clusters (GPSC), GPSC12 strain of serotype 3 S. pneumoniae is predominantly associated with vaccine escape. The poor effectiveness of PCVs against serotype 3 is likely due to the mucoid nature of the capsule, which hinders the immune response [72].
The extent to which PCV13 protects against serotype 3 capsule formation has been explored. According to Choi et al. [73], the protective capacity of the antibody elicited by the vaccine is overwhelmed by the serotype 3 capsule produced and released, and even a small amount of serotype 3 culture supernatant containing the respective capsule was found to abolish the vaccine’s antibody-mediated protection. Based on this finding, it was estimated that approximately eight times more antibodies were needed to provide protection against serotype 3 compared to other serotypes. In addition to inhibiting interactions of the bacteria with phagocytes, serotype 3 can evade capsular antibody because the capsule is not covalently attached to the bacterial surface. Instead, the capsule antibody binds to shed capsule and neutralized in its capacity to opsonize the bacteria itself. Antibodies bound to the capsule on the bacterium’s surface would also be eventually released. Hence, the mechanism of vaccine escapes by serotype 3 highlights the challenges in developing effective vaccines against pneumococcal disease [71,73].
Conclusion
This intensive review concludes by emphasizing the complex relationship between the genomic characteristics of S. pneumoniae CPSs, serotyping methods, immunogenicity, and vaccine development. The diversity and plasticity of the pneumococcal genome have contributed to the diversity of capsular serotypes, posing challenges for disease surveillance and vaccine effectiveness. However, advances in serotyping method, including molecular and genomic approaches, have facilitated the identification of pneumococcus serotype precisely.
Extensive research has been conducted on the function of CPSs in the pathogenesis of pneumococcal infections and their immunogenicity. The development and use of polysaccharide-based and conjugate vaccines have substantially decreased the global incidence of pneumococcal diseases. Nonetheless, the ongoing emergence of non-vaccine serotypes underscores the need for sustained vigilance and the development of next-generation vaccines able to provide broader protection.
Future research should concentrate on elucidating the intricate genetic mechanisms underlying CPS synthesis and serotype switching. A greater comprehension of these processes will facilitate the creation of novel strategies to combat the adaptability of S. pneumoniae. In addition, efforts should be made to increase vaccine coverage by incorporating a wider range of serotypes and investigating alternative immunization approaches, for instance, protein-based vaccine.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Brown J, Hammerschmidt S, Orihuela C. Streptococcus pneumoniae: molecular mechanisms of host-pathogen interactions. Amsterdam: Elsevier/Academic Press; 2015. [Google Scholar]
- 2.Masomian M, Ahmad Z, Gew LT, Poh CL. Development of next generation Streptococcus pneumoniae vaccines conferring broad protection. Vaccines (Basel) 2020;8:132. doi: 10.3390/vaccines8010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Pneumococcal disease [Internet] Atlanta (GA): Centers for Disease Control and Prevention; 2023. [cited 2023 Aug 29]. Available from: https://www.cdc.gov/pneumococcal/about/facts.html . [Google Scholar]
- 4.Grant LR, Meche A, McGrath L, et al. Risk of pneumococcal disease in US adults by age and risk profile. Open Forum Infect Dis. 2023;10:ofad192. doi: 10.1093/ofid/ofad192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. FastStats: pneumonia [Internet] Atlanta (GA): Centers for Disease Control and Prevention; 2022. [cited 2023 Sep 27]. Available from: https://www.cdc.gov/nchs/fastats/pneumonia.htm . [Google Scholar]
- 6.Paton JC, Trappetti C. Streptococcus pneumoniae capsular polysaccharide. Microbiol Spectr. 2019;7 doi: 10.1128/microbiolspec.gpp3-0019-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gierke R, Wodi AP, Kobayashi M. Pneumococcal disease [Internet] Atlanta (GA): Centers for Disease Control and Prevention; 2021. [cited 2023 Sep 27]. Available from: https://www.cdc.gov/vaccines/pubs/pinkbook/pneumo.html . [Google Scholar]
- 8.Müller A, Kleynhans J, de Gouveia L, et al. Streptococcus pneumoniae serotypes associated with death, South Africa, 2012-2018. Emerg Infect Dis. 2022;28:166–179. doi: 10.3201/eid2801.210956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cleary DW, Jones J, Gladstone RA, et al. Changes in serotype prevalence of Streptococcus pneumoniae in Southampton, UK between 2006 and 2018. Sci Rep. 2022;12:13332. doi: 10.1038/s41598-022-17600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo MY, Shi XH, Gao W, et al. The dynamic change of serotype distribution and antimicrobial resistance of pneumococcal isolates since PCV13 administration and COVID-19 control in Urumqi, China. Front Cell Infect Microbiol. 2023;13:1110652. doi: 10.3389/fcimb.2023.1110652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bentley SD, Aanensen DM, Mavroidi A, et al. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2006;2:e31. doi: 10.1371/journal.pgen.0020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagaraj G, Ganaie F, Govindan V, Ravikumar KL. Development of PCRSeqTyping: a novel molecular assay for typing of Streptococcus pneumoniae. Pneumonia (Nathan) 2017;9:8. doi: 10.1186/s41479-017-0032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapatai G, Sheppard CL, Al-Shahib A, et al. Whole genome sequencing of Streptococcus pneumoniae: development, evaluation and verification of targets for serogroup and serotype prediction using an automated pipeline. PeerJ. 2016;4:e2477. doi: 10.7717/peerj.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amari S, Warda K, Elkamouni Y, Arsalane L, Bouskraoui M, Zouhair S. Serotype distribution and antimicrobial resistance of Streptococcus pneumoniae among children with acute otitis media in Marrakech, Morocco. Iran J Microbiol. 2022;14:47–55. doi: 10.18502/ijm.v14i1.8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganaie F, Saad JS, McGee L, et al. A new pneumococcal capsule type, 10D, is the 100th serotype and has a large cps fragment from an oral Streptococcus. mBio. 2020;11:e00937–e00920. doi: 10.1128/mBio.00937-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Pneumococcal vaccination: what everyone should know [Internet] Atlanta (GA): Centers for Disease Control and Prevention; 2023. [cited 2023 Sep 27]. Available from: https://www.cdc.gov/vaccines/vpd/pneumo/public/index.html . [Google Scholar]
- 17.Centers for Disease Control and Prevention. Pneumococcal vaccination: summary of who and when to vaccinate [Internet] Atlanta (GA): centers for disease control and prevention; 2023. [cited 2023 Sep 27]. Available from: https://www.cdc.gov/vaccines/vpd/pneumo/hcp/who-when-to-vaccinate.html . [Google Scholar]
- 18.Dhoubhadel BG, Morimoto K. Prevention of pneumococcal diseases: the challenge remains. Lancet Glob Health. 2022;10:e1375–e1376. doi: 10.1016/S2214-109X(22)00374-6. [DOI] [PubMed] [Google Scholar]
- 19.Licciardi P, Papadatou I. Pneumococcal vaccines: challenges and prospects. Vaccines (Basel) 2019;7:25. doi: 10.3390/vaccines7010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiser JN, Ferreira DM, Paton JC. Streptococcus pneumoniae: transmission, colonization and invasion. Nat Rev Microbiol. 2018;16:355–367. doi: 10.1038/s41579-018-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mousavi SF, Nobari S, Rahmati Ghezelgeh F, et al. Serotyping of Streptococcus pneumoniae isolated from Tehran by multiplex PCR: are serotypes of clinical and carrier isolates identical? Iran J Microbiol. 2013;5:220–226. [PMC free article] [PubMed] [Google Scholar]
- 22.Domenech A, Moreno J, Ardanuy C, Linares J, de la Campa AG, Martin-Galiano AJ. A novel typing method for Streptococcus pneumoniae using selected surface proteins. Front Microbiol. 2016;7:420. doi: 10.3389/fmicb.2016.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subramanian K, Henriques-Normark B, Normark S. Emerging concepts in the pathogenesis of the Streptococcus pneumoniae: from nasopharyngeal colonizer to intracellular pathogen. Cell Microbiol. 2019;21:e13077. doi: 10.1111/cmi.13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henriques-Normark B, Tuomanen EI. The pneumococcus: epidemiology, microbiology, and pathogenesis. Cold Spring Harb Perspect Med. 2013;3:a010215. doi: 10.1101/cshperspect.a010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson AL, Roche AM, Gould JM, Chim K, Ratner AJ, Weiser JN. Capsule enhances pneumococcal colonization by limiting mucus-mediated clearance. Infect Immun. 2007;75:83–90. doi: 10.1128/IAI.01475-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammerschmidt S, Wolff S, Hocke A, Rosseau S, Muller E, Rohde M. Illustration of pneumococcal polysaccharide capsule during adherence and invasion of epithelial cells. Infect Immun. 2005;73:4653–4667. doi: 10.1128/IAI.73.8.4653-4667.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brooks LRK, Mias GI. Streptococcus pneumoniae’s virulence and host immunity: aging, diagnostics, and prevention. Front Immunol. 2018;9:1366. doi: 10.3389/fimmu.2018.01366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wen Z, Liu Y, Qu F, Zhang JR. Allelic variation of the capsule promoter diversifies encapsulation and virulence in Streptococcus pneumoniae. Sci Rep. 2016;6:30176. doi: 10.1038/srep30176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shainheit MG, Mule M, Camilli A. The core promoter of the capsule operon of Streptococcus pneumoniae is necessary for colonization and invasive disease. Infect Immun. 2014;82:694–705. doi: 10.1128/IAI.01289-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng Y, Zhang X, Wang X, Wang L, Zhang J, Yin Y. ComE, an essential response regulator, negatively regulates the expression of the capsular polysaccharide locus and attenuates the bacterial virulence in Streptococcus pneumoniae. Front Microbiol. 2017;8:277. doi: 10.3389/fmicb.2017.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mostowy RJ, Croucher NJ, De Maio N, et al. Pneumococcal capsule synthesis locus cps as evolutionary hotspot with potential to generate novel serotypes by recombination. Mol Biol Evol. 2017;34:2537–2554. doi: 10.1093/molbev/msx173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varvio SL, Auranen K, Arjas E, Makela PH. Evolution of the capsular regulatory genes in streptococcus pneumoniae. J Infect Dis. 2009;200:1144–1151. doi: 10.1086/605651. [DOI] [PubMed] [Google Scholar]
- 33.Nakamoto R, Kwan JM, Chin JF, et al. The bacterial tyrosine kinase system CpsBCD governs the length of capsule polymers. Proc Natl Acad Sci U S A. 2021;118:e2103377118. doi: 10.1073/pnas.2103377118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geno KA, Hauser JR, Gupta K, Yother J. Streptococcus pneumoniae phosphotyrosine phosphatase CpsB and alterations in capsule production resulting from changes in oxygen availability. J Bacteriol. 2014;196:1992–2003. doi: 10.1128/JB.01545-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morona JK, Morona R, Miller DC, Paton JC. Streptococcus pneumoniae capsule biosynthesis protein CpsB is a novel manganese-dependent phosphotyrosine-protein phosphatase. J Bacteriol. 2002;184:577–583. doi: 10.1128/JB.184.2.577-583.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glanville DG, Gazioglu O, Marra M, et al. Pneumococcal capsule expression is controlled through a conserved, distal cis-regulatory element during infection. PLoS Pathog. 2023;19:e1011035. doi: 10.1371/journal.ppat.1011035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng YD, Pan Y, He K, et al. SPD_1495 contributes to capsular polysaccharide synthesis and virulence in Streptococcus pneumoniae. mSystems. 2020;5:e00025–e00020. doi: 10.1128/mSystems.00025-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wyres KL, Lambertsen LM, Croucher NJ, et al. Pneumococcal capsular switching: a historical perspective. J Infect Dis. 2013;207:439–449. doi: 10.1093/infdis/jis703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azarian T, Grant LR, Arnold BJ, et al. The impact of serotype-specific vaccination on phylodynamic parameters of Streptococcus pneumoniae and the pneumococcal pan-genome. PLoS Pathog. 2018;14:e1006966. doi: 10.1371/journal.ppat.1006966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown JS. Single-nucleotide polymorphisms within the cps loci: another potential source of clinically important genetic variation for Streptococcus pneumoniae? Infect Immun. 2021;89:e0037421. doi: 10.1128/IAI.00374-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yokota SI, Tsukamoto N, Sato T, Ohkoshi Y, Yamamoto S, Ogasawara N. Serotype replacement and an increase in non-encapsulated isolates among community-acquired infections of Streptococcus pneumoniae during post-vaccine era in Japan. IJID Reg. 2023;8:105–110. doi: 10.1016/j.ijregi.2023.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamboj KK, Kirchner HL, Kimmel R, Greenspan NS, Schreiber JR. Significant variation in serotype-specific immunogenicity of the seven-valent Streptococcus pneumoniae capsular polysaccharide-CRM197 conjugate vaccine occurs despite vigorous T cell help induced by the carrier protein. J Infect Dis. 2003;187:1629–1638. doi: 10.1086/374785. [DOI] [PubMed] [Google Scholar]
- 43.Lo SW, Gladstone RA, van Tonder AJ, et al. Pneumococcal lineages associated with serotype replacement and antibiotic resistance in childhood invasive pneumococcal disease in the post-PCV13 era: an international whole-genome sequencing study. Lancet Infect Dis. 2019;19:759–769. doi: 10.1016/S1473-3099(19)30297-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanquet G, Krizova P, Dalby T, et al. Serotype replacement after introduction of 10-valent and 13-valent pneumococcal conjugate vaccines in 10 countries, Europe. Emerg Infect Dis. 2022;28:137–138. doi: 10.3201/eid2801.210734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geno KA, Gilbert GL, Song JY, et al. Pneumococcal capsules and their types: past, present, and future. Clin Microbiol Rev. 2015;28:871–899. doi: 10.1128/CMR.00024-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia-Garcia S, Perez-Arguello A, Henares D, Timoneda N, Munoz-Almagro C. Rapid identification, capsular typing and molecular characterization of Streptococcus pneumoniae by using whole genome nanopore sequencing. BMC Microbiol. 2020;20:347. doi: 10.1186/s12866-020-02032-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Habib M, Porter BD, Satzke C. Capsular serotyping of Streptococcus pneumoniae using the Quellung reaction. J Vis Exp. 2014;(84):e51208. doi: 10.3791/51208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jauneikaite E, Tocheva AS, Jefferies JM, et al. Current methods for capsular typing of Streptococcus pneumoniae. J Microbiol Methods. 2015;113:41–49. doi: 10.1016/j.mimet.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Epping L, van Tonder AJ, Gladstone RA, et al. SeroBA: rapid high-throughput serotyping of Streptococcus pneumoniae from whole genome sequence data. Microb Genom. 2018;4:e000186. doi: 10.1099/mgen.0.000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knight JR, Dunne EM, Mulholland EK, et al. Determining the serotype composition of mixed samples of pneumococcus using whole-genome sequencing. Microb Genom. 2021;7:mgen000494. doi: 10.1099/mgen.0.000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sheppard CL, Manna S, Groves N, et al. PneumoKITy: a fast, flexible, specific, and sensitive tool for Streptococcus pneumoniae serotype screening and mixed serotype detection from genome sequence data. Microb Genom. 2022;8:mgen000904. doi: 10.1099/mgen.0.000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adler H, Ferreira DM, Gordon SB, Rylance J. Pneumococcal capsular polysaccharide immunity in the elderly. Clin Vaccine Immunol. 2017;24:e00004–e00017. doi: 10.1128/CVI.00004-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garrido HM, Schnyder JL, Tanck MW, et al. Immunogenicity of pneumococcal vaccination in HIV infected individuals: a systematic review and meta-analysis. EClinicalMedicine. 2020;29-30:100576. doi: 10.1016/j.eclinm.2020.100576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nunes MC, Madhi SA. Safety, immunogenicity and efficacy of pneumococcal conjugate vaccine in HIV-infected individuals. Hum Vaccin Immunother. 2012;8:161–173. doi: 10.4161/hv.18432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Madhi SA, Adrian P, Cotton MF, et al. Effect of HIV infection status and anti-retroviral treatment on quantitative and qualitative antibody responses to pneumococcal conjugate vaccine in infants. J Infect Dis. 2010;202:355–361. doi: 10.1086/653704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thanawastien A, Cartee RT, Griffin TJ, 4th, Killeen KP, Mekalanos JJ. Conjugate-like immunogens produced as protein capsular matrix vaccines. Proc Natl Acad Sci U S A. 2015;112:E1143–E1151. doi: 10.1073/pnas.1425005112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daniels CC, Rogers PD, Shelton CM. A review of pneumococcal vaccines: current polysaccharide vaccine recommendations and future protein antigens. J Pediatr Pharmacol Ther. 2016;21:27–35. doi: 10.5863/1551-6776-21.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Djennad A, Ramsay ME, Pebody R, et al. Effectiveness of 23-valent polysaccharide pneumococcal vaccine and changes in invasive pneumococcal disease incidence from 2000 to 2017 in those aged 65 and over in England and Wales. EClinicalMedicine. 2019;6:42–50. doi: 10.1016/j.eclinm.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nielsen KF, Nielsen LB, Lomholt FK, et al. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease among 948,263 individuals ≥ 65 years of age: a Danish cohort study. Eur J Clin Microbiol Infect Dis. 2022;41:1473–1477. doi: 10.1007/s10096-022-04513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Richter SS, Heilmann KP, Dohrn CL, Riahi F, Diekema DJ, Doern GV. Pneumococcal serotypes before and after introduction of conjugate vaccines, United States, 1999-2011(1.) Emerg Infect Dis. 2013;19:1074–1083. doi: 10.3201/eid1907.121830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peckeu L, van der Ende A, de Melker HE, Sanders EA, Knol MJ. Impact and effectiveness of the 10-valent pneumococcal conjugate vaccine on invasive pneumococcal disease among children under 5 years of age in the Netherlands. Vaccine. 2021;39:431–437. doi: 10.1016/j.vaccine.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 62.Savulescu C, Krizova P, Valentiner-Branth P, et al. Effectiveness of 10 and 13-valent pneumococcal conjugate vaccines against invasive pneumococcal disease in European children: SpIDnet observational multicentre study. Vaccine. 2022;40:3963–3974. doi: 10.1016/j.vaccine.2022.05.011. [DOI] [PubMed] [Google Scholar]
- 63.Stacey HL, Rosen J, Peterson JT, et al. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine (PCV-15) compared to PCV-13 in healthy older adults. Hum Vaccin Immunother. 2019;15:530–539. doi: 10.1080/21645515.2018.1532249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grant LR, Slack MP, Theilacker C, et al. Distribution of serotypes causing invasive pneumococcal disease in children from high-income countries and the impact of pediatric pneumococcal vaccination. Clin Infect Dis. 2023;76:e1062–e1070. doi: 10.1093/cid/ciac475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Teixeira R, Kossyvaki V, Galvez P, Mendez C. Pneumococcal serotype evolution and burden in European adults in the last decade: a systematic review. Microorganisms. 2023;11:1376. doi: 10.3390/microorganisms11061376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hurley D, Griffin C, Young M, et al. Safety, tolerability, and immunogenicity of a 20-valent pneumococcal conjugate vaccine (PCV20) in adults 60 to 64 years of age. Clin Infect Dis. 2021;73:e1489–e1497. doi: 10.1093/cid/ciaa1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Campling J, Vyse A, Liu HH, et al. A review of evidence for pneumococcal vaccination in adults at increased risk of pneumococcal disease: risk group definitions and optimization of vaccination coverage in the United Kingdom. Expert Rev Vaccines. 2023;22:785–800. doi: 10.1080/14760584.2023.2256394. [DOI] [PubMed] [Google Scholar]
- 68.Collins AM, Wright AD, Mitsi E, et al. First human challenge testing of a pneumococcal vaccine: double-blind randomized controlled trial. Am J Respir Crit Care Med. 2015;192:853–858. doi: 10.1164/rccm.201503-0542OC. [DOI] [PubMed] [Google Scholar]
- 69.Musher DM, Anderson R, Feldman C. The remarkable history of pneumococcal vaccination: an ongoing challenge. Pneumonia (Nathan) 2022;14:5. doi: 10.1186/s41479-022-00097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Varghese R, Neeravi A, Subramanian N, et al. Clonal similarities and sequence-type diversity of invasive and carriage Streptococcus pneumoniae in India among children under 5 years. Indian J Med Microbiol. 2019;37:358–362. doi: 10.4103/ijmm.IJMM_19_348. [DOI] [PubMed] [Google Scholar]
- 71.Luck JN, Tettelin H, Orihuela CJ. Sugar-coated killer: serotype 3 pneumococcal disease. Front Cell Infect Microbiol. 2020;10:613287. doi: 10.3389/fcimb.2020.613287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kwun MJ, Ion AV, Cheng HC, et al. Post-vaccine epidemiology of serotype 3 pneumococci identifies transformation inhibition through prophage-driven alteration of a non-coding RNA. Genome Med. 2022;14:144. doi: 10.1186/s13073-022-01147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Choi EH, Zhang F, Lu YJ, Malley R. Capsular polysaccharide (CPS) release by serotype 3 pneumococcal strains reduces the protective effect of anti-type 3 CPS antibodies. Clin Vaccine Immunol. 2015;23:162–167. doi: 10.1128/CVI.00591-15. [DOI] [PMC free article] [PubMed] [Google Scholar]