Abstract
A comprehensive study of the overall flavor and taste profile of different radishes is lacking. This study systematically compared the volatile profile of six radish varieties using HS-GC-IMS and their correlation with the E-nose analysis. Organic acids and amino acids were quantified, and their association with the E-tongues analysis was explored. A total of 73 volatile compounds were identified, with diallyl sulfide and dimethyl disulfide being the primary sulfides responsible for the unpleasant flavor in radish. Compared to other varieties, cherry radishes boast a significantly higher concentration of allyl isothiocyanate, which likely contributes to their characteristic radish flavor. Moreover, oxalic acid was identified as the most abundant organic acid in radish, accounting for over 97% of its content, followed by malic acid and succinic acid. In conclusion, the distinct flavor and taste characteristics of different radish varieties partially explain their suitability for diverse culinary preferences.
Keywords: Radish, GC/IMS, E-nose, E-tongue, Volatile compounds
Highlights
-
•
diallyl sulfide and dimethyl disulfide could be the primary sulfides responsible for the unpleasant flavor in radish.
-
•
Allyl isothiocyanate may contribute to the characteristic radish flavor.
-
•
Oxalic acid, malic acid and succinic acid play significant roles in shaping the taste of radishes.
1. Introduction
Radish (Raphanus sativus) is a root vegetable of the Brassicaceae family widely consumed worldwide (Hara, Torazawa, Asai, & Takahashi, 2011; Radovich, 2018). It gains great popularity for its low-calorie count and explosion of flavor. Beyond its refreshing bite, radishes boast an impressive nutritional profile, featuring potassium, vitamin C, folate, and fiber (Drost & Bitner, 2020). Meanwhile, packed with bioactive compounds like anthocyanins and sulforaphane, radishes offer a range of health benefits, from potentially lowering blood pressure to protecting against disease (Drost & Bitner, 2020; Gamba et al., 2021; Sevindik, Onat, Mohammed, Uysal, & Koçer, 2023).
Radishes possess a unique flavor profile characterized by pungency, sweetness, and crispness. This profile exhibits considerable variability depending on the variety (Mei, He, & Zhang, 2022). There are many radish varieties with different root shapes, sizes, and colors for sale in the grocery market (Drost & Bitner, 2020). Some varieties or cultivars have stronger pungent flavor than others, which is due to the high content of volatile alkaloid isothiocyanate (trans-4-methyl thiobutenyl isothiocyanate: MTB-ITC) (Gamba et al., 2021). Radishes can be consumed in salads, processed as dried or canned pickles, or salted, baked, or cooked, depending on not only the individual's preference but also the flavor, texture, taste, or even color of different varieties(Gamba et al., 2021), in which the flavor and taste can play an important role. Different flavor of radishes might be suitable for different ways of consumption. For example, some varieties have a spicier taste and stronger pungent flavor than others, making them more suitable for cooking, pickling, and fermentation than eating them raw. Some varieties have less off-flavor or more sweetness, which can be consumed as fruit (Kobayashi et al., 2020), or made into salad to maximize the benefit of consumers from undamaged active compounds.
Sulfur and nitrogen-containing compounds, such as isothiocyanates and nitriles, seem to play an important role in the characteristic aroma of radishes (Blažević & Mastelić, 2009). The importance of taste and flavor perceptions resulting from such compounds has been stressed by previous studies. For example, some specific glucosinolates (GSLs) impart bitterness, and many isothiocyanates (ITCs) art pungency, but not true for all GSLs and ITCs (Bell, Oloyede, Lignou, Wagstaff, & Methven, 2018). The influence of radish variety on the quality and flavor of processed radish-based foods remains unclear due to limited knowledge about the sensory profile of raw radish flesh (Zhou et al., 2023). So far, few comparative studies on the characterization of flavor compounds in varieties of radish have been carried out using UHPLC-MS/MS-based metabolome analysis (Mei et al., 2022; Wang et al., 2023). However, the correlation between different varieties and flavors has not been studied, and the compounds that have the most significant influence on the flavor and taste profile of different radishes have not been studied yet.
In this study, volatile compounds in different radish varieties were characterized by headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS) and E-nose. Free amino acids and organic acids that contribute to the tastes of different radishes were studied and analyzed by E-tongue. Main compounds and their contribution to flavor and taste were studied.
2. Materials and methods
2.1. Samples and treatment
Six different radish varieties were purchased from local market (Shandong, China), including white-skinned, white-fleshed and long-rooted radish (WW), red-skinned, white-fleshed and long-rooted radish (RW), red-skinned, red-fleshed and long-rooted radish (RR), green-skinned, green-fleshed and long-rooted radish (GG), green-skinned, green-fleshed and round-rooted radish (GR), cherry radish (CR). The radishes were stored at a 4 °C refrigerator for subsequent testing.
2.2. Energy and nutrients analysis
100 g of sample minces were loaded into the injection tray and analyzed for energy, protein, fat, carbohydrate and water content using the vegetable mode of the Calory Answer CA-HA (JWP, Hiragawa, Japan). The instrument irradiated food with near-infrared light (1100–2200 nm), and based on the different ingredients in food, the characteristics of reflected light and transmitted light will change. By detecting these changes, the composition analysis of food can be carried out. To ensure performance stability, the device was preheated for over 30 min before testing. Each radish sample was measured five times.
2.3. Texture analyses
The hardness and chewiness values of each sample were determined using a Texture Analyzer (TMS-PRO, FTC, USA.). A cube (2 × 2 × 2 cm3) was used as the radish model and each measurement was performed in quintuplicate. The setting parameters of the analyzer were as follows: the probe type was 12.7 mm aluminum cylindrical probe; pretest speed was 1 mm/s, test speed was 1 mm/s, posttest speed was 1 mm/s, distance was 50% compression, and trigger force value was 0.375 N, pause time was 2 s.
2.4. Color parameters analysis
The color of radish samples was determined based on Commission International Eclairage (CIE) using a 3nh Chroma Meter (Sunchi Technology Co., Ltd., Shenzhen, China). The instrument was preheated for 30 min and then calibrated with a white standard plate. The measurement model was a 2 × 2 × 2 cm3 cylinder, and the colors were expressed as CIE L*, b*, a*, C, and H values. L* value indicates the lightness of sample ranging from 0 (black) to 100 (white), while the values of a* and b* represent the degree of greenness (negative)-redness (positive) and blueness (negative)-yellowness (positive) of sample, respectively. The C value represents color saturation, indicating the distance covered by the central achromatic gray axis of the color space The C value quantifies chroma, the intensity of color relative to the gray axis in the color space, while H value delineates the hue angle, with a range of 0° (red)-90° (yellow)-180° (green)-270° (blue)-360° (red) (Jin et al., 2024; Long et al., 2024). A total of 5 measurements per treatment were obtained.
2.5. E-nose analysis
An electronic nose (FOX 4000, Alpha M.O.S., Toulouse, France) was used to analyze the flavor profile of the radishes. The detection of the gases was performed with an array made of 18 different metal oxide semi-conductor (MOS) sensors corresponding based on different types of volatile substances. A 10 mL headspace glass sampling vial containing 1.5 g radish flesh was incubated at 50 °C for 5 min. The analytical parameters were as follows: the system washing duration was 180 s, and the duration of collection and analysis was 120 s, which was the same as the preparation time. The gaseous sample injection volume was 1 mL, and the injection speed was set at 500 μL/s. Each sample was measured ten times, and three sets of stable data were selected for further analysis (Wu et al., 2023).
2.6. E-tongue analysis
E-tongue analysis was conducted using the α-ASTREE instrument (A MOS, Toulouse, France), which featured a sixteen autosampler carousel for sample handling. The instrument was equipped with seven sensors for detecting sourness (AHS), saltiness (CTS), umami (NMS), and four non-sensitive electrodes (PKS, ANS, SCS, and CPS) (Zhao et al., 2023). To extract the taste substances, 100 g of samples were mixed with 200 mL of deionized water, subjected to 40 min of ultrasonic treatment, and then filtered. An 80 mL portion of the filtrate was collected into a specific beaker for E-tongue analysis. Each data collection period lasted 120 s, and the detection head was washed with distilled water for 180 s before and after each test (Duan, Dong, Dong, & Gao, 2021). All analyses were performed ten times.
2.7. Organic acids
To measure the contents of organic acid, a previous method by Lin et al. (2018) was referred to. Briefly, the radish was homogenized (the mass ratio of radish to ultra-pure water was 1:1) using a wall breaker, and ultrasonic was carried out at 70 °C for 20 min, then the mixture was centrifuged at 12,000 rpm/min for 10 min. The supernatant was further filtered through a 0.22-μm membrane filter. Organic acid contents in the filtrate were determined by HPLC (Agilent 1260, AGILENT, California, USA), and all samples were analyzed in triplicate. Chromatographic conditions: mobile phase A: 0.1% phosphoric acid solution, mobile phase B: methanol solution; flow rate of 1 mL/min; column temperature of 40 °C; detection wavelength of 210 nm; injection volume of 20 μL.
2.8. Free amino acids
Referring to Qiao et al. (2024), The contents of free amino acids (FAAs) were measured as follows: the radish was homogenized (the mass ratio of radish to ultra-pure water was 1:1) using a wall breaker, then 20 g homogenate was added in 10 mL of 7% sulfosalicylic acid, and ultrasonic was carried out at 40 °C for 40 min. The mixture was centrifuged at 12,000 rpm for 10 min. The supernatant was further filtered through a 0.22-μm membrane filter. FAAs contents in the filtrate were determined by an amino acid analyzer (S433D, SYKAM, Munich, Germany). Analysis conditions: LCA K07/Li chromatographic column (150 mm × 4.6 mm); detection wavelengths of 570 and 440 nm; reactor temperature of 13 °C; column temperature of 38–74 °C; injection volume of 50 μL; analysis time of 108 min, the flow rate of ninhydrin reagent was 0.25 mL/min. FAAs content was determined through systematic analysis in triplicate.
2.9. Headspace gas chromatography-ion mobility spectrometry (HS-GC-IMS) analysis
The volatile flavor compounds were determined by HS-GC-IMS (G.A.S. Instrument, Germany) according to the method described by Wu B (Wu et al., 2023). Briefly, 1.5 g of radish flesh was weighed into a headspace glass sampling vial, inoculating at 50 °C for 20 min, next, 500 μL of the gaseous sample was automatically injected into the GC-IMS system by a syringe at 85 °C. The volatile gas was separated by a chromatographic column (WAX 30 m × 0. 53 mm, 1 μm film thickness, RESTEK, USA) and combined with IMS at 45 °C (Li et al., 2023). N2 (99.999% purity) was used as the carrier gas and had a flow rate of 150 mL/min. Then, the gas fraction was detected through GC using an IMS instrument. The drift tube temperature was 45 °C for 30 min with the following gradient mobility program: 2 mL/min for 2 min, an increase to 10 mL/min within 8 min, an increase to 100 mL/min within 10 min, and finally an increase to 150 mL/min within 10 min. Last, IMS data were collected in positive mode and analyzed using VOC software (v.1.0.7, G.A.S., Germany). The National Institute of Standards and Technology (NIST) library combined with the retention index (RI), IMS database, and drift time (Dt) were used for qualitative analysis of (volatile organic compounds (VOCs) (Shen et al., 2023). The intensities of VOCs were based on the peak volume of the selected signal peak. All analyses were performed in triplicates.
2.10. Statistical analysis
Laboratory Analytical Viewer (LAV) processing software, GC-IMS Library Search, the Reporter, and Gallery plot were used to analyze the data from GC-IMS. Statistical analyses for the differences were performed using the SPSS 23.0 software (IBM, Armonk, NY, USA). Radar map-based visual analysis and principal component analysis (PCA) were conducted by Origin 2021 (Origin Lab Corporation, Northampton, MA, USA). Correlation analysis, clustering analysis, and graphic presentations were generated using the tools from https://www.genescloud.cn. SIMCA 14.1 (Umetrics, Umea, Sweden) was used to analyze the orthogonal partial least squares discriminant analysis (OPLS-DA) of the volatile compounds. The variables important in the projection (VIP) were performed on the website https://www.metaboanalyst.ca/.
3. Results and discussion
3.1. Nutritional compositions, texture, and coloration of different varieties
Nutritional quality is critical for radishes, a previous study reported that the protein content differed from 42 radish varieties, ranging from 0.34 to 1.15 g.kg−1 fresh weight (Lu et al., 2008), and carbohydrate content of 5.835 g.kg−1 and 4.04 g.kg−1 in white and red radishes, respectively (Gamba et al., 2021). As shown in Table 1, among the six radish varieties, RW and CR are particularly low in calories, containing only 42.67 and 47.33 KJ/100 g, respectively, compared to other radishes. In contrast, GG has the highest calories, with 160.33 KJ/100 g, which is mainly due to the higher carbohydrates than other varieties (86.70 g.kg−1). RW contains relatively low levels of fat (0.33 g.kg−1) and carbohydrates (16.30 g.kg−1), resulting in lower energy content (42.67 KJ/100 g) than other varieties. Overall, RW has a relatively high protein content (10.67 g.kg−1) and lower levels of carbohydrates, fat, and energy. Texture, especially hardness and chewiness, plays a critical role in consumer acceptance of fermented radishes. This makes certain varieties, such as WW, RR, and GG, more suitable for this type of product (Zheng et al., 2023; Zhou et al., 2023). In addition, GG has a higher hardness and chewiness than other radish varieties, with values of 33.71 N and 30.44 mJ, respectively. GR has a lower hardness and chewiness, with values of 4.38 N and 4.46 mJ, respectively (Table 1). These results suggested that there were significant differences between different radish varieties in terms of their composition and texture.
Table 1.
Nutritional compositions and texture of different radish varieties.
| Sample | energy/KJ.100 g−1 | protein/g.kg−1 | fat/ g.kg−1 | carbohydrate/ g.kg−1 | moisture/g.100 g−1 | Hardness/N | Chewiness/mJ |
|---|---|---|---|---|---|---|---|
| WW | 97.67 ± 6.66c | 13.33 ± 6.51a | 2.67 ± 0.21 | 39.00 ± 8.85bc | 94.50 ± 0.17ab | 26.60 ± 4.04ab | 16.05 ± 7.92abc |
| RW | 42.67 ± 6.43d | 10.67 ± 5.03abc | 0.33 ± 0.06 | 16.30 ± 2.92d | 96.40 ± 0.46a | 16.33 ± 1.79c | 4.09 ± 1.53c |
| RR | 100.33 ± 11.15c | 11.00 ± 5.91ab | 1.33 ± 0.06 | 45.67 ± 7.85b | 93.60 ± 0.10b | 24.13 ± 6.36bc | 19.44 ± 11.41ab |
| GG | 160.33 ± 15.04a | 6.33 ± 0.17c | 1.00 ± 0.00 | 86.70 ± 7.00a | 89.60 ± 0.85c | 33.71 ± 8.37a | 30.44 ± 12.99a |
| GR | 121.00 ± 0.00b | 8.67 ± 3.79b | 1.00 ± 0.00 | 46.00 ± 12.44b | 93.77 ± 3.23ab | 4.38 ± 0.08d | 4.46 ± 0.00bc |
| CR | 47.33 ± 4.62d | 2.00 ± 2.00d | 1.33 ± 0.31 | 23.00 ± 5.61c | 96.30 ± 0.35a | 21.60 ± 3.36bc | 10.87 ± 2.96bc |
Note: different lower-case letters represent significant differences among samples as tested by Tukey's HSD test (P < 0.05).
Different radish varieties have different colors of their flesh (Fig. 1). And a wide variety of colors affect the appearance and nutritional quality of radishes (Zhang, Qiu, Tan, Xiao, & Mei, 2020). As shown in Table 2 Radishes having white flesh had a higher value of L(brightness) compared with others, as the L value of RW, WW, and CR are 81.68, 70.68, and 68.76, respectively. Red-fleshed radishes had significantly higher a* (redness/greenness) and C (chroma) values than other varieties, such as RR and GR, making them ideal for fresh consumption due to their appealing appearance. By contrast, the c values of white flesh radishes were low, at 4.59 and 4.86 for WW and RW, respectively. GG has the highest H value of 83.4, and red-fleshed radishes, RR, and GR, have the lower H values of 1.34 and 1.25, respectively (Table 2). Anthocyanins are water-soluble vacuolar pigments that are responsible for the red color of radish. Red radish color characteristics not only have health benefits because of their antioxidant activities (Matsufuji et al., 2007; Rahman, Ichiyanagi, Komiyama, Hatano, & Konishi, 2006), but also make them preferred by consumers when they give pink color in pickles. Previous research shows that white radishes were devoid of anthocyanin aglycones in either skin or flesh, whereas pigmented radish cultivars exhibit a presence of diverse anthocyanin aglycones, including pelargonidin, cyanidin, and malvidin, in varying concentrations (Lim, Kim, & Lee, 2023). Red radish cultivars represent a viable candidate for natural colorant extraction owing to their anthocyanin content (Li et al., 2023, Park et al., 2016).
Fig. 1.
Radish varieties with different shapes, skin colors, and flesh colors. Including white-skinned, white-fleshed and long-rooted radish (WW), red-skinned, white-fleshed and long-rooted radish (RW), red-skinned, red-fleshed and long-rooted radish (RR), green-skinned, green-fleshed and long-rooted radish (GG), green-skinned, green-fleshed and round-rooted radish (GR), cherry radish (CR). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Color parameters of different radish varieties.
| Sample | L* | a* | b* | C | H |
|---|---|---|---|---|---|
| WW | 70.68 ± 0.01b | 2.50 ± 0.01c | 3.85 ± 0.03d | 4.59 ± 0.03f | 57.00 ± 0.07d |
| RW | 81.68 ± 0.07a | 1.82 ± 0.00e | 4.50 ± 0.02c | 4.86 ± 0.02e | 68.01 ± 0.10c |
| RR | 42.38 ± 0.09f | 19.79 ± 0.10a | 0.47 ± 0.03e | 19.79 ± 0.10a | 1.34 ± 0.09e |
| GG | 57.32 ± 0.04d | 1.74 ± 0.02e | 15.02 ± 0.01a | 15.12 ± 0.01c | 83.40 ± 0.07a |
| GR | 45.63 ± 0.19e | 19.65 ± 0.12b | 0.43 ± 0.01f | 19.66 ± 0.11b | 1.25 ± 0.02f |
| CR | 68.76 ± 0.01c | 2.23 ± 0.01d | 6.74 ± 0.01b | 7.10 ± 0.01d | 71.72 ± 0.02b |
Note: different lower-case letters represent significant differences among samples as tested by Tukey's HSD test (P < 0.05).
3.2. E-nose and E-tongue analysis
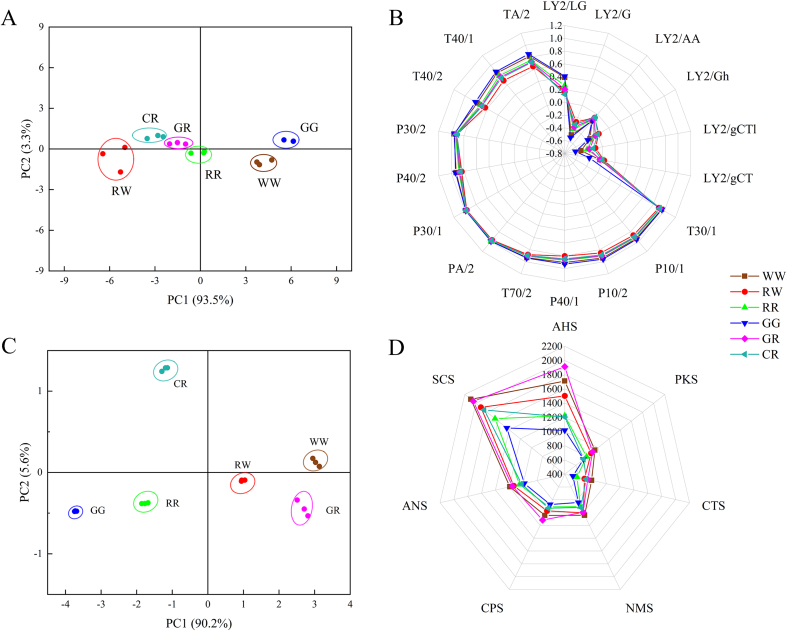
Electronic noses are widely employed in food research to characterize the overall flavor profiles (S. Li, Du, Wang, & Wei, 2022; Wei, Dan, Zhao, & Wang, 2023), such as distinguishing between different varieties of oolong tea (He et al., 2022), black olives (Sánchez et al., 2022), and coffees (Dong, Zhao, Hu, Dong, & Tan, 2017) based on their species. In this study, the E-nose was used to analyze the overall flavor of different radish varieties. PCA analysis was conducted, as shown in Fig. 2A, the proximity of GG and WW, GR and RR, and GR and CR in the PCA plot suggests overlapping flavor profiles for these pairs, respectively. PC1 accounts for 93.5% of the total variance and clearly distinguished the six radish varieties, indicating significant differences in their flavor profiles (Fig. 2A). WW displayed the highest overall response to sensors, while RW exhibited the lowest. Radish samples showed a minimal response to LY2/LG, LY2/G, LY2/AA, LY2/Gh, LY2/gCT1 and LY2/gCT, which indicates sensitivity to organic amines (Wu et al., 2023). All varieties responded strongly to P30/2, P30/1, and PA/2 sensors, which detect organic compounds. In contrast, the response intensity varied for sensors TA/2, T40/1, T40/2, P40/2, T70/2, P10/1, P10/2, and T30/1, sensitive to non-polar compounds such as propanol, fluorides, aldehydes, amines, ethanol, and oxidizing gases (WW > GG > RR > CR > GR > RW) (Fig. 2B) (Luo et al., 2024).
Fig. 2.
PCA analysis (A) and electronic nose radar (B), PCA analysis (C) and electronic tongue radar (D) of different radish varieties raw flesh.
The E-tongue analyzer equipped with seven sensors was used to identify different radish varieties and comprehensive taste characteristics. The original data could be dimensionally reduced and the samples were well classified by PCA in Fig. 2C, the accumulative variance contribution (95.8%) represented that the two principal components could reveal most information of the original data (Fig. 2C). The samples were separated well and divided into 6 groups in 4 regions according to their distributions, with closely positioned samples indicating correlation, for example, WW and RW, as well as RR and GG.
The taste characteristics were distinguished in the dimension of 7 sensors (Fig. 2D). The various radish cultivars exhibit distinct responses to the electronic tongue sensors. Overall, the higher response values from WW and GR suggest that these varieties have richer and more pronounced flavor, whereas the lower responses from GG indicate that it has overall lower taste than others. Specifically, radishes exhibit a higher response to the sourness sensor (AHS) compared to the umami sensor (NMS), with the saltiness sensor (CTS) responding next. Notably, different radish varieties exhibit significant differences in their responses to sourness sensor. Building on the work of Hong et al. (2021) who demonstrated the potential of electronic sensors for analyzing taste intensity in radishes, this study explores their application in differentiating between varieties. Additionally, Liu et al. (2024) utilized electronic tongues to evaluate the dynamic changes in taste quality during radish pickling. These studies suggest that electronic sensors offer a promising approach for radish variety discrimination.
3.3. Flavor contribution of organic acids
Organic acids, being one group of the primary metabolites of plants, play an important role in maintaining the nutritional value and sensory quality of foods (Shi, Pu, Zhou, & Zhang, 2022). Oxalic acid is the most enriched organic acid in radish flesh with above 97% of its content, followed by malic acid, accounting for 0.4% to 0.8% of total organic acid (Fig. 3A, B). Oxalic acid, with a low threshold value of 0.504 mg/g, is identified as the primary contributor to the sour taste in radishes due to its high intensity of sour perception (Qiao et al., 2024). As the previous study reported, oxalic acid is the major organic acid in raw radish and maintained one of the highest concentrations even across fermentation (Gutiérrez & Perez, 2004; Y. Yang et al., 2022), despite malic acid was also identified as primary organic acid in radish (Bae, Lee, & Lee, 2012). In this study, RW has the highest amount of total organic acid (6.33 mg/g) as well as the content of oxalic acid (6.19 mg/g), followed by WW, while GR has the least (Fig. 3A, B).
Fig. 3.
Organic acids (A) and organic acids without oxalate (B) in different radish varieties.
3.4. Flavor contribution of FAAs
Free amino acids, in addition to their role in protein structure, play critical roles in nutrition, metabolism, and signaling. Moreover, it is known that free amino acids enrich the flavor of the food by imparting sweet, bitter, umami and sour taste (Kobayashi, Kobayashi, Takahashi, Kumakura, & Matsuoka, 2021). A total of 20 FAAs were detected in different radish varieties. Glutamic (Glu), Serine (Ser), and Lysine (Lys) were the main FAAs in radishes (Fig. 4A).
Fig. 4.
Free amino acids (A) and taste-active amino acids (B) in different radish varieties.
Glutamate, primarily found in radish as its major amino acid, plays a dual role by engaging with taste receptor cells to impart the umami flavor (Brosnan & Brosnan, 2013) and acting as a precursor for the synthesis of alanine, proline, and GABA during radish dehydration (Kobayashi et al., 2021). Varietal differences are evident, with RW and RR radishes exhibiting higher glutamic acid levels at 4.69 and 4.25 mg/g, respectively, while WW contains the least at 3.13 mg/g. Notably, GR had a higher content of Asp (1.23 mg/g), which also has umami taste, compared to the other samples. Therefore, RW, RR and GR has a higher amount of hydrophilic amino acids including glutamate and aspartic acid, compared with other radishes, suggesting its more palatable tastes (Choi et al., 2010). Serine, recognized for its sweet taste (Schiffman, Sennewald, & Gagnon, 1981),predominates as the most abundant free amino acid in GG (4.39 mg/g), exceeding the levels in all other samples and intriguingly, surpassing that of Glu (3.80 mg/g). This finding suggests that GG's sweetness likely stems, in part, from its high serine content. Lysine, which has a bitter taste, is more abundant in RR (3.25 mg/g) and GR (3.54 mg/g) and less abundant in WW (0.94 mg/g) and CR (0.39 mg/g) (Fig. 4A). Despite imparting an unpleasant bitter taste to radishes, lysine enriches the flavor complexity and slightly boosts the freshness of radishes.
GR and GG had higher total free amino acid contents than the other samples, at 21.90 and 21.05 mg/g, respectively. The sweet amino acid content was higher than the bitter and umami amino acid contents. WW (8.85 mg/g) and CR (8.52 mg/g) had lower total free amino acid contents, but the umami amino acid content was higher than the bitter and sweet contents. The contents of umami and sweet amino acids were similar in RR and RW (Fig. 4B).
3.5. Aroma volatile compounds in radishes
In the 3D representation shown in Supplementary Fig. S1, clear visual distinctions can be observed among different radish varieties across a wide range of the GC-IMS spectrum. To further differentiate the volatile flavor substances, all detected compounds in the GC-IMS spectra were selected to generate fingerprints using the Gallery Plot plug-in, as illustrated in Fig. 5A, here each row represents a volatile compound and each column represents a sample. A total of 73 single compounds were identified in the samples (Fig. 5A), including 4 alcohols, 3 acids, 5 terpenes, 5 ketones, 14 esters, 10 aldehydes, 6 pyrazines, 2 sulfides, 2 phenols, and 6 others (Fig. 5B). The relative content of different category of volatile compounds in each radish varieties were shown in Fig. 5B, in general, radishes had high content of esters and aldehydes within the flesh. Specifically, WW has higher levels of pyrazines, ketones, alcohols, and sulfides than others, which coordinated with the stronger overall flavor revealed by E-nose and E-tongue analysis. In addition, WW variety has the highest ester content compared to other varieties, followed by CR. This suggests that WW may have sweeter and floral aromas. The top 3 enriched esters contributing to this difference were 1-methoxy-2-propyl acetate, dihydromyrcenyl acetate, and methyl 3-(methylthio) propanoate. RR has the highest aldehyde content and CR has the lowest, with nonanal being the main component, which provides citrus notes (Wang et al., 2022). Moreover, CR had significantly higher levels of acids by GC-IMS (Fig. 5B) which could contribute to taste. GR and RR have lower levels of both acids and sulfides.
Supplementary Fig. S1.
Three- (A) and two-dimensional (B) topographic spectra of volatile flavor compounds in different radish varieties detected by GC-IMS, and two-dimensional map of volatile flavor compounds of WW (C), RW (D), RR (E), GG (F), GR (G) and CR (H) was selected as a reference, respectively, while the plots of other samples were deducted from the reference.
Fig. 5.
HS-GC–IMS Fingerprint of VOCs (A), and aroma compounds ratio in radish varieties (B), OPLS-DA plot (C) and Variable Importance in Projection scores (VIP) diagram (D).
Two sulfides including dimethyl disulfide and diallyl sulfide, in which the latter is higher contained in all radish varieties than the former (Fig. 5A, Table S1). Among six different radish varieties, WW has the highest content of diallyl sulfide while CR has the highest dimethyl disulfide compared with others. As the main component of garlic extract, diallyl sulfide, with garlic-like aroma, is featured with strong anti-cancer, anti-virus, antibacterial activity, strong inhibition of platelet aggregation, and immunity improvement (Casella, Leonardi, Melai, Fratini, & Pistelli, 2013; Wargovich, 1987; C. S. Yang, Chhabra, Hong, & Smith, 2001). Although it has been considered to be the main component responsible for the unpleasant flavor in radish and its fermented product (Mi et al., 2023). A previous study showed that during the process of radish fermented by lactic acid bacteria, diallyl sulfide decreased as the fermentation period increased (Kim, Cho, Kim, Hurh, & Baek, 2019). Dimethyl disulfide is commonly found in radishes and other crucifers (Wallbank & Wheatley, 1976) and is well-known for its association with off-odors in radish roots (Hong et al., 2021). The substantial difference in dimethyl disulfide and diallyl sulfide content in radishes, as observed in this study, raises the possibility that aroma attribution might not be essential in every case, depending on the properties of the volatile compounds. Air-dried radishes have a stronger aroma than salted radishes, the main reason for which is their relatively high content of sulfur compounds (Coogan & Wills, 2008). Thermal processing can decompose sulfides, and the sulfur-containing compounds in heat-treated radish is relatively lower than that in unheated radish (Hong et al., 2021). Furthermore, the sulfide content markedly impacts the flavor profile of radishes fermented products, such as kimchi (Oerlemans, Barrett, Suades, Verkerk, & Dekker, 2006).
Isothiocyanates, constitute the major flavor characteristics of fresh radish,is the main contributor for radish's pungent flavor (Kjӕr, Øgaard Madsen, Maeda, Ozawa, & Uda, 1978), and it often derived from enzymatically breaking down of glucosinolates (McCormick, Unsicker, & Gershenzon, 2012; Singh & Singh, 2012). In this study, only one isothiocyanate was determined in the radish varieties, allyl isothiocyanate. The content of allyl isothiocyanate ranks in the six radishes as CR > GG > WW > RW > GR > RR. Not only as a typical vegetable VOCs, but also reported to be a potential antibacterial agent (Maruthupandy & Seo, 2019). This suggested that Isothiocyanates might be a contributor to the typical radish flavor of Cherry radish. Allyl isothiocyanate is further hydrolyzed into 3-butenenitrile, carbon disulfide and dimethyl disulfide during the fermentation of Paocai, and also could be decomposed during cooking, resulting in a milder radish flavor (Wang et al., 2022).
To identify volatile compounds that differentiate the aroma profiles of different radishes. A supervised OPLS-DA model was established. As shown in Fig. 5C, clear separation among groups was observed, except GR and RW. Based on the VIP calculated by the PLS-DA model, 1-phenylethanol, 2-ethyl-3-methylpyrazine, 3-heptanol, 1-methoxy-2-propyl acetate, methy 2-(methythio) propanoate, 3-hydroxy-2-butanone, 2-ethyl-5-methylpyrazine and dihydromyrcenyl acetate were significantly higher in WW than other radish varieties (Fig. 5D, VIP > 1). Aceti acid-D and 1,8-cineole are characterized volatile compounds for CR that distinguish it from other varieties (VIP > 1). Butanal is significantly higher concentrated in GG than others (VIP > 1). The results showed that the aroma profile among different radish varieties is distinguished from others.
3.6. Correlation analysis of flavor and taste
To further study the contribution of volatile compounds to flavor, and amino acids to tastes of different radish varieties, potential correlation analysis between E-nose sensor responses and volatile compound levels detected by GC-IMS, and between E-Tongue sensor responses and amino acids content were studied, respectively. As shown in Fig. 6A, sensors including LY2/LG, P10/1, P10/2, P40/1, P70/2, PA/2, P30/1, P40/2, P30/2, T40/2, T40/1, TA/2 were positively correlated to methyl hexanoate, Isoamyl hexanoate, ethy(S)-(−)-lactate, butyl acetate, 1-pentanol, (E)-2-hexenal-M, (E)-2-hexenal-D. Conversely, LY2/G, LY2/AA, LY2/Gh, LY2/gCT and T30/1 were negatively correlated to those compounds (Fig. 6A).
Fig. 6.
Correlation analysis heatmap illustrates the correlation between the levels of volatile compounds and the E-nose sensor responses (A), and between organic aids/amino acids and E-tongue sensor responses (B) and cluster analysis of samples (C). In the heatmap, colors represent correlation coefficients, with green indicating positive correlations and brown indicating negative correlations. ** indicates extremely significant differences (P < 0.01), while * denotes significant differences (P < 0.05). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Meanwhile, amino acids and organic acids are related to the E-tongue sensor response. For example, lactic acid and succinic acid were positively correlated to AHS, and CPS. Lactic acids were positively correlated to all the sensors of the E-tongue. Despite the low concentration of lactic acid and succinic acids in radish flesh, they contribute to the taste of radish tastes and can be distinguished by E-tongue sensors. Amino acids including Thr, Ser, Hy-pro, Val, Ile, Leu, and Phe were negatively correlated to CTS, NMS, and SCS sensors response (Fig. 6B). The overall flavor and taste differences of different radishes were determined by cluster analysis based on all data generated in this study, as shown in Fig. 6C, the volatile compounds, amino acids and organic acids significantly affect the overall flavor and taste in different radish varieties, which not only the electronic sensor evaluation has the potential to be used to distinguished different radish varieties, but also give insight of explanation of their suitability for different consuming preference (Fig. 6C). Different radish varieties exhibit distinct flavors and tastes, with RR and GG showing the closest resemblance.
4. Conclusion
The present study gave the first sight of the overall flavor and taste profile of six different radish varieties, revealing that diallyl sulfide and dimethyl disulfide as the major sulfides responsible for the unpleasant flavor in radishes, and exhibited the highest concentration in white skin flesh radishes than others. Allyl Isothiocyanate may be a contributor of typical radish flavor to cherry radish. Organic acids including oxalic acid, succinic acid, and lactic acids can significantly contribute to the taste of radishes. The distinguished flavor and taste characteristics of different radish varieties partially explained their suitability for different consuming preferences.
The following are the supplementary data related to this article.
Supplementary material 1
CRediT authorship contribution statement
Xuemei Cai: Writing – original draft, Data curation. Kaixian Zhu: Methodology, Formal analysis. Wanli Li: Validation, Resources. Yiqin Peng: Methodology. Yuwen Yi: Methodology. Mingfeng Qiao: Validation, Funding acquisition. Yu Fu: Visualization, Validation, Investigation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the Sichuan Science and Technology Program (No. 2023ZYD0079), the Sichuan Tourism University research team project (No. 20SCTUTG01 & No. 22SCTUTG01), the Multi-dimensional Data Sensing and Intelligent Information Processing Key Laboratory (No. DWSJ2308), and Key Laboratory of Sichuan Cuisine Artificial Intelligence (No. CR23Y08).
Contributor Information
Mingfeng Qiao, Email: mfqiao@sctu.edu.cn.
Yu Fu, Email: yufu@swun.edu.cn.
Data availability
Data will be made available on request.
References
- Bae R.-N., Lee Y.-K., Lee S.-K. Changes in nutrient levels of aqueous extracts from radish (Raphanus sativus L.) root during liquefaction by heat and non-heat processing. Horticultural Science & Technology. 2012;30(4):409–416. [Google Scholar]
- Bell L., Oloyede O.O., Lignou S., Wagstaff C., Methven L. Taste and flavor perceptions of Glucosinolates, Isothiocyanates, and related compounds. Molecular Nutrition & Food Research. 2018;62(18) doi: 10.1002/mnfr.201700990. [DOI] [PubMed] [Google Scholar]
- Blažević I., Mastelić J. Glucosinolate degradation products and other bound and free volatiles in the leaves and roots of radish (Raphanus sativus L.) Food Chemistry. 2009;113(1):96–102. doi: 10.1016/j.foodchem.2008.07.029. [DOI] [Google Scholar]
- Brosnan J.T., Brosnan M.E. Glutamate: a truly functional amino acid. Amino Acids. 2013;45:413–418. doi: 10.1007/s00726-012-1280-4. [DOI] [PubMed] [Google Scholar]
- Casella S., Leonardi M., Melai B., Fratini F., Pistelli L. The role of diallyl sulfides and dipropyl sulfides in the in vitro antimicrobial activity of the essential oil of garlic, Allium sativum L., and leek, Allium porrum L. Phytotherapy Research. 2013;27(3):380–383. doi: 10.1002/ptr.4725. [DOI] [PubMed] [Google Scholar]
- Choi S.-H., Ryu D.-K., Park S.-H., Ahn K.-G., Lim Y.-P., An G.-H. Composition analysis between kohlrabi (Brassica oleracea var. gongylodes) and radish (Raphanus sativus) Horticultural Science & Technology. 2010;28(3):469–475. [Google Scholar]
- Coogan R.C., Wills R.B.H. Flavour changes in Asian white radish (Raphanus sativus) produced by different methods of drying and salting. International Journal of Food Properties. 2008;11(2):253–257. doi: 10.1080/10942910701298093. [DOI] [Google Scholar]
- Dong W., Zhao J., Hu R., Dong Y., Tan L. Differentiation of Chinese robusta coffees according to species, using a combined electronic nose and tongue, with the aid of chemometrics. Food Chemistry. 2017;229:743–751. doi: 10.1016/j.foodchem.2017.02.149. [DOI] [PubMed] [Google Scholar]
- Drost D., Bitner W. 2020. Radishes in the garden. [Google Scholar]
- Duan Z., Dong S., Dong Y., Gao Q. Geographical origin identification of two salmonid species via flavor compound analysis using headspace-gas chromatography-ion mobility spectrometry combined with electronic nose and tongue. Food Research International. 2021;145 doi: 10.1016/j.foodres.2021.110385. [DOI] [PubMed] [Google Scholar]
- Gamba M., Asllanaj E., Raguindin P.F., Glisic M., Franco O.H., Minder B.…Muka T. Nutritional and phytochemical characterization of radish (Raphanus sativus): A systematic review. Trends in Food Science & Technology. 2021;113:205–218. [Google Scholar]
- Gutiérrez R.M.P., Perez R.L. Raphanus sativus (Radish): Their chemistry and biology. The Scientific World Journal. 2004;4:811. doi: 10.1100/tsw.2004.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M., Torazawa D., Asai T., Takahashi I. Variations in the soluble sugar and organic acid contents in radish (Raphanus sativus L.) cultivars. International Journal of Food Science & Technology. 2011;46(11):2387–2392. doi: 10.1111/j.1365-2621.2011.02761.x. [DOI] [Google Scholar]
- He C., Li Y., Zhou J., Yu X., Zhang D., Chen Y.…Yu Z. Study on the suitability of tea cultivars for processing oolong tea from the perspective of aroma based on olfactory sensory, electronic nose, and GC-MS data correlation analysis. Foods. 2022;11(18):2880. doi: 10.3390/foods11182880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S.J., Boo C.G., Lee J., Hur S.W., Jo S.M., Jeong H.…Shin E.-C. Chemosensory approach supported the analysis of wintering radishes produced in Jeju island by different processing methods. Food Science and Biotechnology. 2021;30(8):1033–1049. doi: 10.1007/s10068-021-00948-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S., Li M., Liu Z., Liu R., Li Y., Zhu Y., Yuan Y., Li P., Li P., Chen C., Sun Y. Study on the correlation between color and taste of beauty tea infusion and the pivotal contributing compounds based on UV–visible spectroscopy, taste equivalent quantification and metabolite analysis. Food Chemistry: X. 2024;21 doi: 10.1016/j.fochx.2024.101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B., Cho Y.-J., Kim M., Hurh B., Baek H.-H. Changes in volatile flavor compounds of radish fermented by lactic acid bacteria. Korean Journal of Food Science and Technology. 2019;51(4):324–329. [Google Scholar]
- Kjӕr A., Øgaard Madsen J., Maeda Y., Ozawa Y., Uda Y. Volatiles in distillates of processed radish of Japanese origin. Agricultural and Biological Chemistry. 1978;42(11):1989–1996. [Google Scholar]
- Kobayashi H., Shirasawa K., Fukino N., Hirakawa H., Akanuma T., Kitashiba H. Identification of genome-wide single-nucleotide polymorphisms among geographically diverse radish accessions. DNA Research. 2020;27(1) doi: 10.1093/dnares/dsaa001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi W., Kobayashi T., Takahashi A., Kumakura K., Matsuoka H. Metabolism of glutamic acid to alanine, proline, and γ-aminobutyric acid during takuan-zuke processing of radish root. Journal of Food Science. 2021;86(2):563–570. doi: 10.1111/1750-3841.15567. [DOI] [PubMed] [Google Scholar]
- Li M., Zhang J., Li L., Wang S., Liu Y., Gao M. Effect of enzymatic hydrolysis on volatile flavor compounds of Monascus-fermented tartary buckwheat based on headspace gas chromatography-ion mobility spectrometry. Food Research International. 2023;163 doi: 10.1016/j.foodres.2022.112180. [DOI] [PubMed] [Google Scholar]
- Li S., Du D., Wang J., Wei Z. Application progress of intelligent flavor sensing system in the production process of fermented foods based on the flavor properties. Critical Reviews in Food Science and Nutrition. 2022:1–30. doi: 10.1080/10408398.2022.2134982. [DOI] [PubMed] [Google Scholar]
- Li W., Zhang Y., Deng H., Yuan H., Fan X., Yang H., Tan S. In vitro and in vivo bioaccessibility, antioxidant activity, and color of red radish anthocyanins as influenced by different drying methods. Food Chemistry: X. 2023;18 doi: 10.1016/j.fochx.2023.100633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S.-H., Kim D.-H., Lee J.-Y. Molecular mechanism controlling anthocyanin composition and content in radish plants with different root colors. Plant Physiology and Biochemistry. 2023;204:108091. doi: 10.1016/j.plaphy.2023.108091. [DOI] [PubMed] [Google Scholar]
- Lin H., Yu X., Fang J., Lu Y., Liu P., Xing Y.…He Q. Flavor compounds in Pixian broad-bean paste: Non-volatile organic acids and amino acids. Molecules. 2018;23(6):1299. doi: 10.3390/molecules23061299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Zhang L., Karrar E., Wu D., Chen C., Zhang Z., Li J. A cooperative combination of non-targeted metabolomics and electronic tongue evaluation reveals the dynamic changes in metabolites and sensory quality of radish during pickling. Food Chemistry. 2024;446:138886. doi: 10.1016/j.foodchem.2024.138886. [DOI] [PubMed] [Google Scholar]
- Long P., Li Y., Han Z., Zhu M., Zhai X., Jiang Z., Wen M., Ho C.-T., Zhang L. Discovery of color compounds: Integrated multispectral omics on exploring critical colorant compounds of black tea infusion. Food Chemistry. 2024;432 doi: 10.1016/j.foodchem.2023.137185. [DOI] [PubMed] [Google Scholar]
- Lu Z., Liu L., Li X., Gong Y., Hou X., Zhu X.…Wang L. Analysis and evaluation of nutritional quality in Chinese radish (Raphanus sativus L.) Agricultural Sciences in China. 2008;7(7):823–830. doi: 10.1016/S1671-2927(08)60119-4. [DOI] [Google Scholar]
- Luo X., Huang K., Yu Y., Yang Q., Yang H., Xiong S.…Hu Y. Insights into the potential mechanism of liquid nitrogen spray freezing’s influence on volatile compounds in surimi gels with different cross-linking degrees: Focus on oxidation, protein structure, intermolecular force and free amino acid alterations. Food Chemistry. 2024;444 doi: 10.1016/j.foodchem.2024.138558. [DOI] [PubMed] [Google Scholar]
- Maruthupandy M., Seo J. Allyl isothiocyanate encapsulated halloysite covered with polyacrylate as a potential antibacterial agent against food spoilage bacteria. Materials Science and Engineering: C. 2019;105 doi: 10.1016/j.msec.2019.110016. [DOI] [PubMed] [Google Scholar]
- Matsufuji H., Kido H., Misawa H., Yaguchi J., Otsuki T., Chino M.…Yamagata K. Stability to light, heat, and hydrogen peroxide at different pH values and DPPH radical scavenging activity of acylated anthocyanins from red radish extract. Journal of Agricultural and Food Chemistry. 2007;55(9):3692–3701. doi: 10.1021/jf063598o. [DOI] [PubMed] [Google Scholar]
- McCormick A.C., Unsicker S.B., Gershenzon J. The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends in Plant Science. 2012;17(5):303–310. doi: 10.1016/j.tplants.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Mei S., He Z., Zhang J. Identification and analysis of major flavor compounds in radish taproots by widely targeted metabolomics. Frontiers in Nutrition. 2022;9 doi: 10.3389/fnut.2022.889407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi T., Jin Y., Che Y., Huang J., Zhou R., Wu C. Profiling the composition and metabolic functions of the microbial community in pellicle-forming radish paocai. International Journal of Food Microbiology. 2023;388 doi: 10.1016/j.ijfoodmicro.2023.110087. [DOI] [PubMed] [Google Scholar]
- Oerlemans K., Barrett D.M., Suades C.B., Verkerk R., Dekker M. Thermal degradation of glucosinolates in red cabbage. Food Chemistry. 2006;95(1):19–29. doi: 10.1016/j.foodchem.2004.12.013. [DOI] [Google Scholar]
- Park C., Baskar T., Park S.-Y., Kim S.-J., Valan Arasu M., Al-Dhabi N.…Park S. Metabolic profiling and antioxidant assay of metabolites from three radish cultivars (Raphanus sativus) Molecules. 2016;21(2):157. doi: 10.3390/molecules21020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao M., Xiong H., Cai X., Jiang Y., Zhao X., Miao B. Evaluation of loquat jam quality at different cooking times based on physicochemical parameters, GC-IMS and intelligent senses. Foods. 2024;13(2):340. doi: 10.3390/foods13020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radovich T.J.K. Biology and classification of vegetables. Handbook of Vegetables and Vegetable Processing. 2018:1–23. [Google Scholar]
- Rahman M.M., Ichiyanagi T., Komiyama T., Hatano Y., Konishi T. Superoxide radical-and peroxynitrite-scavenging activity of anthocyanins; structure-activity relationship and their synergism. Free Radical Research. 2006;40(9):993–1002. doi: 10.1080/10715760600815322. [DOI] [PubMed] [Google Scholar]
- Sánchez R., Martín-Tornero E., Lozano J., Fernández A., Arroyo P., Meléndez F., Martín-Vertedor D. Electronic nose application for the discrimination of sterilization treatments applied to Californian -style black olive varieties. Journal of the Science of Food and Agriculture. 2022;102(6):2232–2241. doi: 10.1002/jsfa.11561. [DOI] [PubMed] [Google Scholar]
- Schiffman S.S., Sennewald K., Gagnon J. Comparison of taste qualities and thresholds of D- and L-amino acids. Physiology & Behavior. 1981;27(1):51–59. doi: 10.1016/0031-9384(81)90298-5. [DOI] [PubMed] [Google Scholar]
- Sevindik M., Onat C., Mohammed F.S., Uysal İ., Koçer O. Antioxidant and antimicrobial activities of White Radish. Turkish Journal of Agriculture-Food Science and Technology. 2023;11(2):372–375. [Google Scholar]
- Shen C., Cai Y., Wu X., Gai S., Wang B., Liu D. Characterization of selected commercially available grilled lamb shashliks based on flavor profiles using GC-MS, GC× GC-TOF-MS, GC-IMS, E-nose, and E-tongue combined with chemometrics. Food Chemistry. 2023;423 doi: 10.1016/j.foodchem.2023.136257. [DOI] [PubMed] [Google Scholar]
- Shi Y., Pu D., Zhou X., Zhang Y. Recent Progress in the study of taste characteristics and the nutrition and health properties of organic acids in foods. Foods. 2022;11(21):3408. doi: 10.3390/foods11213408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S.V., Singh K. Cancer chemoprevention with dietary isothiocyanates mature for clinical translational research. Carcinogenesis. 2012;33(10):1833–1842. doi: 10.1093/carcin/bgs216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallbank B.E., Wheatley G.A. Volatile constituents from cauliflower and other crucifers. Phytochemistry. 1976;15(5):763–766. [Google Scholar]
- Wang D., Chen G., Tang Y., Ming J., Huang R., Li J., Ye M., Fan Z., Chi Y., Zhang Q., Zhang W. Study of bacterial community succession and reconstruction of the core lactic acid bacteria to enhance the flavor of paocai. International Journal of Food Microbiology. 2022;375 doi: 10.1016/j.ijfoodmicro.2022.109702. [DOI] [PubMed] [Google Scholar]
- Wang J., Wei Q., Wang W., Hu H., Yan Y., Wang Y.…Hu T. Understanding the nutraceutical diversity through a comparative analysis of the taproot metabolomes of different edible radish types via UHPLC–Q–TOF–MS. Food Chemistry. 2023;403 doi: 10.1016/j.foodchem.2022.134469. [DOI] [PubMed] [Google Scholar]
- Wargovich M.J. Diallyl sulfide, a flavor component of garlic (Allium sativum), inhibits dimethyihydrazine-induced colon cancer. Carcinogenesis. 1987;8(3):487–489. doi: 10.1093/carcin/8.3.487. [DOI] [PubMed] [Google Scholar]
- Wei G., Dan M., Zhao G., Wang D. Recent advances in chromatography-mass spectrometry and electronic nose technology in food flavor analysis and detection. Food Chemistry. 2023;405 doi: 10.1016/j.foodchem.2022.134814. [DOI] [PubMed] [Google Scholar]
- Wu B., Zhu C., Deng J., Dong P., Xiong Y., Wu H. Effect of Sichuan pepper (Zanthoxylum genus) addition on flavor profile in fermented Ciba chili (Capsicum genus) using GC-IMS combined with E-nose and E-tongue. Molecules. 2023;28(15):5884. doi: 10.3390/molecules28155884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.S., Chhabra S.K., Hong J.-Y., Smith T.J. Mechanisms of inhibition of chemical toxicity and carcinogenesis by diallyl sulfide (DAS) and related compounds from garlic. The Journal of Nutrition. 2001;131(3):1041S–1045S. doi: 10.1093/jn/131.3.1041S. [DOI] [PubMed] [Google Scholar]
- Yang Y., Fan Y., Li T., Yang Y., Zeng F., Wang H.…Zhang Y. Microbial composition and correlation between microbiota and quality-related physiochemical characteristics in Chongqing radish paocai. Food Chemistry. 2022;369 doi: 10.1016/j.foodchem.2021.130897. [DOI] [PubMed] [Google Scholar]
- Zhang J., Qiu X., Tan Q., Xiao Q., Mei S. A comparative metabolomics study of flavonoids in radish with different skin and flesh colors (Raphanus sativus L.) Journal of Agricultural and Food Chemistry. 2020;68(49):14463–14470. doi: 10.1021/acs.jafc.0c05031. [DOI] [PubMed] [Google Scholar]
- Zhao X., Feng J., Laghi L., Deng J., Dao X., Tang J., Ji L., Zhu C., Picone G. Characterization of Flavor Profile of “Nanx Wudl” Sour Meat Fermented from Goose and Pork Using Gas Chromatography–Ion Mobility Spectrometry (GC–IMS) Combined with Electronic Nose and Tongue. Foods. 2023;12(11):2194. doi: 10.3390/foods12112194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Zhou Q., Chen Q., Gao J., Wu Y., Yang F., Zhong K., Gao H. Improvement of physicochemical characteristics, flavor profiles and functional properties in Chinese radishes via spontaneous fermentation after drying. Journal of Food Science. 2023;88(4):1292–1307. doi: 10.1111/1750-3841.16486. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Zheng Z., Wu Y., Zhang X., Jia Z., Zhong K., Gao H. Unraveling the core bacterial community responsible for quality and flavor improvement of the radish paocai during spontaneous fermentation. Food Bioscience. 2023;55 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Data Availability Statement
Data will be made available on request.