Abstract
Rationale
Data on risk factors for chronic hypoxemia in low- and middle-income countries are lacking.
Objectives
We aimed to quantify the association between potential risk factors and chronic hypoxemia among adults hospitalized in Kenya.
Methods
A hospital-based, case–control study was conducted at Moi Teaching and Referral Hospital in Eldoret, Kenya. Adult inpatients were screened on admission and enrolled in a 1:2 case-to-control ratio. Cases were patients with chronic hypoxemia, defined as resting oxygen saturation as measured by pulse oximetry (SpO2) ⩽ 88% on admission and either 1-month postdischarge SpO2 ⩽ 88% or, if they died before follow-up, documented SpO2 ⩽ 88% in the 6 months before enrollment. Control subjects were randomly selected, stratified by sex, among nonhypoxemic inpatients. Data were collected using questionnaires and structured chart review. Regression was used to assess the associations between chronic hypoxemia and age, sex, smoking status, biomass fuel use, elevation, and self-reported history of tuberculosis and human immunodeficiency virus diagnosis. Odds ratios (ORs) and 95% confidence intervals (CIs) are reported.
Results
We enrolled 108 chronically hypoxemic cases and 240 nonhypoxemic control subjects into our Chronic Hypoxemia among Kenyan Adults (CHAKA) cohort. In multivariable analysis, compared with control subjects, chronically hypoxemic cases had significantly higher odds of older age (OR, 1.2 per 5-year increase [95% CI, 1.1–1.3]), female sex (OR, 3.6 [95% CI, 1.8–7.2]), current or former tobacco use (OR, 4.7 [95% CI, 2.3–9.6]), and prior tuberculosis (OR, 11.8 [95% CI, 4.7–29.6]) but no increase in the odds of human immunodeficiency virus diagnosis and biomass fuel use.
Conclusions
These findings highlight the potential impact of prior tuberculosis on chronic lung disease in Kenya and the need for further studies on posttuberculosis lung disease.
Keywords: posttuberculosis lung disease, sex differences, ambulatory oxygen, chronic hypoxemia, global health
Chronic hypoxemia is a major cause of morbidity and mortality in patients with chronic lung diseases, which are ranked as the third leading cause of death by the World Health Organization (1–3). Major etiologies of chronic hypoxemia in high-income countries include chronic obstructive pulmonary disease (COPD) and interstitial lung disease, with tobacco use being a key risk factor (4). However, sparse data are available on risk factors for chronic hypoxemia among patient populations in low- and middle-income countries (LMICs) such as Kenya, where smoking rates are lower, but mortality from lung disease remains high (5).
For example, studies in Uganda and Kenya demonstrated higher rates of obstructive lung disease among women and patients younger than 40 years compared with high-income countries. This suggests that there may unique risk factors for chronic lung disease in these settings (6). Indoor air pollution from biomass fuel use is a frequently cited risk factor for chronic lung disease, as is human immunodeficiency virus (HIV) infection and prior lower respiratory tract infections such as tuberculosis (TB) (7, 8). However, to our knowledge, none of these studies focused on chronic hypoxemia, which characterizes advanced or end-stage lung disease and may be influenced by other health conditions such as heart disease. Furthermore, most studies describe cohorts of people living with HIV infection.
Given the paucity of epidemiologic data on chronic hypoxemia and its specific risk factors in LMICs, the aim of this study was to characterize patients with chronic hypoxemia admitted to a national referral hospital in Kenya and assess demographic, environmental, and clinical risk factors for chronic hypoxemia. Oxygen prescription and use among participants with chronic hypoxemia was also assessed. Strong epidemiologic data would provide support for strategies to curb the burden of hypoxemic lung disease in Kenya and direction for future research and funding efforts.
Methods
Study Design and Patients
All study procedures were approved by the ethical review boards at Duke University (Pro00100397) and Moi University (IREC/2019/72).
A hospital-based case–control study was conducted at Moi Teaching and Referral Hospital (MTRH) in Eldoret, Kenya, between September 2019 and March 2022. MTRH is one of two national referral hospitals in the country, offering tertiary care services to a catchment population of more than 24 million people across western Kenya. MTRH consists of several different wards, with the general medicine wards being among the largest in the hospital, responsible for caring for adult patients with a wide range of nonsurgical issues.
All hospitalized patients in the general medicine wards were eligible for participation in the study regardless of admission diagnosis. Eligible patients included those ⩾18 years with chronic hypoxemia, which was defined as resting oxygen saturation as measured by pulse oximetry (SpO2) ⩽ 88% on admission and either SpO2 ⩽ 88% at the 1-month postdischarge follow-up study visit or, if the patient died before follow-up, documented SpO2 ⩽ 88% during a previous outpatient visit or on hospital discharge examination in the 6 months before the current admission. As participants were recruited in the hospital but followed after discharge, patients who were hypoxemic on admission to the hospital and remained hypoxemic for three or more days were enrolled as suspected cases. Case confirmation occurred at time of death or at the 1-month postdischarge follow-up visit.
Control subjects were selected in a 2:1 ratio to suspected cases using survivor sampling among inpatients without hypoxemia ⩾18 years of age. Because hospital wards are separated by sex, control subjects were selected by numbering admission logs for each ward and using a random-number generator to select potential control subjects to approach for enrollment on the day of admission in each of the two hospital wards. Control subjects were then enrolled within three days of admission. Equal allocation of control subjects by sex was intended to capture the sex distribution of the population, which was assumed to be roughly equal (i.e., 50:50 male:female).
We powered the study on HIV infection as a primary exposure. We estimated a 10% prevalence of HIV infection among control subjects (9). With 112 cases and 224 control subjects, we estimated 80% power to detect a minimum odds ratio (OR) of 2.41, with an α value of 0.05 for greater odds of HIV infection among chronically hypoxemic cases.
Data Collection
Research assistants used structured paper forms to collect participant eligibility information. SpO2 was assessed among all participants (cases and control subjects) on room air using a Masimo Rad-5v pulse oximeter with at least two readings. For participants who were on clinically prescribed oxygen supplementation, a standardized operating procedure was used to safely assess SpO2 on both oxygen supplementation and room air.
Data from questionnaires and chart review were collected using Research Electronic Data Capture, hosted at Duke University (10, 11). Demographic and self-reported medical history data included education, occupation, tobacco use, biomass fuel use, electricity availability, self-reported medical comorbidities, and prior hospitalizations. Structured chart review data included admission and discharge characteristics, medical and surgical history as listed on admission or discharge forms or physician notes, and laboratory results. Medical comorbidities are presented as aggregates of participant self-report of medical history to research staff members and clinician-reported medical history within the patient charts. TB history was categorized and reported as prior TB for participants who reported histories of TB for which they were treated versus active TB for those who were receiving anti-TB treatment during the study period. Laboratory results closest to participant’s initial admission date were used.
A measure of participants’ primary residence elevation was constructed using the NASA Shuttle Radar Topographic Mission’s 90-m Digital Elevation Database (12). Each participant was geocoded to the ward of residence, and their residential ward (third administrative division), and the average elevation of each residential ward was calculated with raster-based geographic information system tools in R (R version 4.0.1, http://www.r-project.org) (13). Average elevation was used because coordinates for study participants were unavailable, and it partially accounts for variability in elevation exposure due to individual local mobility patterns.
Statistical Analysis
Standard summary statistics were used to describe the study population. Univariable logistic regressions and univariate statistical tests, such as the Wilcoxon rank sum and chi square test, were used to assess the associations between chronic hypoxemia and cohort characteristics, as appropriate.
A multivariable logistic regression model was then used to determine the association between chronic hypoxemia and TB adjusting for age, sex, smoking status, biomass fuel use, elevation, and history of HIV infection, which were selected a priori. We did not adjust for factors that are believed to be on the causal pathway (mediators) between TB and chronic hypoxemia, such as COPD. Model assumptions were assessed and a spline for elevation was included in the model, split at <2,000 m, the median elevation for the entire cohort. Model results are presented as ORs with 95% confidence intervals (CIs). Analyses were conducted using SAS version 9.4 (SAS Institute), and a P value <0.05 was considered to indicate statistical significance.
Results
Baseline Descriptive Characteristics
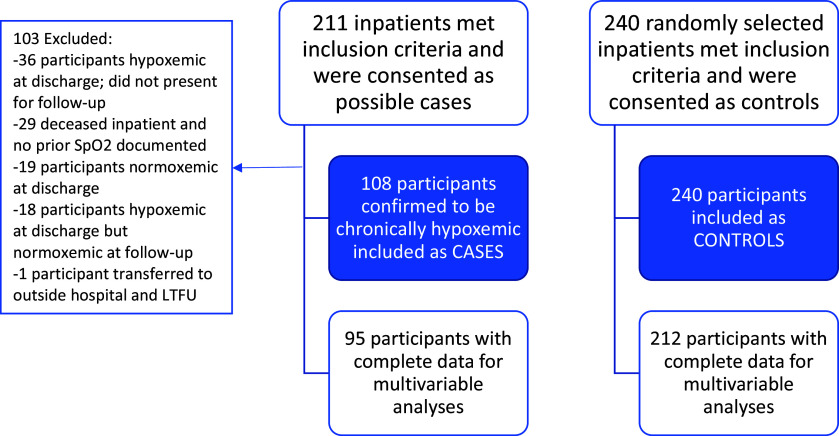
We enrolled 211 potential cases, of whom 108 (51.2%) were confirmed to be chronically hypoxemic and included in the analysis cohort as cases, and 240 nonhypoxemic control subjects into the Chronic Hypoxemia among Kenyan Adults (CHAKA) cohort (Figure 1). Of the 108 cases, 70 (65%) met the case definition on the basis of SpO2 ⩽ 88% at the postdischarge follow-up visit and 38 (35%) on the basis of SpO2 ⩽ 88% at a prior outpatient visit or on a discharge examination in the 6 months before admission.
Figure 1.
Study Flow Chart. LTFU = lost to follow-up; SpO2 = oxygen saturation as measured by pulse oximetry.
Demographics and medical history are shown in Table 1. Chronically hypoxemic cases were significantly older, with a median age of 64 years (interquartile range, 44–75 yr) compared with 42 years (interquartile range, 28–63 yr) among nonhypoxemic control subjects (OR, 1.04 [95% CI, 1.02–1.05]). They were also predominantly female compared with control subjects (61% vs. 44%; OR, 1.99 [95% CI, 1.25–3.16]). A significantly higher proportion of cases had no history of attending school (OR, 10.28 [95% CI, 2.63–29.13]) or had completed only primary school (OR, 3.41 [95% CI, 1.34–8.67]) compared with control subjects. In addition, cases had higher odds of being employed as farmers (OR, 2.35 [95% CI, 1.14–4.83]) or in small businesses (OR, 2.33 [95% CI, 1.04–5.25]) compared with control subjects. The odds of chronic hypoxemia increased 1.33 times for every 100-m increase in the mean elevation of a participant’s primary residence up to 2,000 m (95% CI, 1.12–1.58) using regression splines; however, there were no significant differences in the odds of chronic hypoxemia for increasing elevation above 2,000 m. Chronically hypoxemic cases also had a higher rate of enrollment in the National Health Insurance Fund, which is Kenya’s universal health coverage insurance plan, compared with control subjects (OR, 1.74 [95% CI, 1.06–2.85]).
Table 1.
Baseline characteristics of nonhypoxemic control subjects and chronically hypoxemia cases
| Variable | Nonhypoxemic Control Subjects (n = 240) | Chronically Hypoxemic Cases (n = 108) | Unadjusted Odds Ratio (95% CI) |
|---|---|---|---|
| Age, yr | 42.2 (28.1, 63.2) | 63.6 (43.8, 74.9) | 1.20 (1.13–1.27) per 5-yr increase |
| Sex | |||
| Male | 134 (55.8) | 42 (38.9) | Reference |
| Female | 106 (44.2) | 66 (61.1) | 1.99 (1.25–3.16) |
| BMI, kg/m2 | (n = 187) | (n = 91) | |
| 21.3 (18.9–23.5) | 20.8 (16.8–25.5) | 1.02 (0.97–1.07) per 1 kg/m2 increase | |
| Insured via NHIF | 143 (59.6) | 77 (72.0) | 1.74 (1.06–2.85) |
| Marital status | |||
| Single | 69 (28.8) | 14 (13.0) | Reference |
| Married | 130 (54.2) | 58 (53.7) | 2.20 (1.14–4.22) |
| Divorced/separated | 10 (4.2) | 6 (5.6) | 2.96 (0.92–9.47) |
| Widowed | 31 (12.9) | 30 (27.8) | 4.77 (2.22–10.23) |
| Highest level of education | |||
| None | 18 (7.5) | 30 (27.8) | 10.28 (3.63–29.13) |
| Primary school | 85 (35.4) | 47 (43.5) | 3.41 (1.34–8.67) |
| Secondary school | 96 (40.0) | 22 (20.4) | 1.41 (0.53–3.76) |
| College/university | 37 (15.4) | 6 (5.6) | Reference |
| Unknown | 4 (1.7) | 3 (2.8) | — |
| Occupation/type of work | (n = 240) | (n = 107) | |
| Farmer | 69 (28.8) | 49 (45.8) | 2.35 (1.14–4.83) |
| Small business/self-employed | 34 (14.2) | 24 (22.4) | 2.33 (1.04–5.25) |
| Unemployed | 43 (17.9) | 13 (12.1) | Reference |
| Skilled labor | 28 (11.7) | 10 (9.3) | 1.18 (0.46–3.06) |
| Student | 21 (8.8) | 1 (0.9) | 0.16 (0.02–1.29) |
| Driver (bus, car, motorcycle) | 13 (5.4) | 2 (1.9) | 0.51 (0.10–2.55) |
| Casual labor | 12 (5.0) | 5 (4.7) | 1.38 (0.41–4.64) |
| Teacher | 6 (2.5) | 1 (0.9) | 0.55 (0.06–5.01) |
| Healthcare worker | 4 (1.7) | 0 (0.0) | — |
| Retired | 10 (4.2) | 2 (1.9) | 0.66 (0.13–3.41) |
| Tobacco use | |||
| Never smoked | 192 (80.0) | 58 (53.7) | Reference |
| Current or former | 48 (20.0) | 50 (46.3) | 3.45 (2.11–5.65) |
| If yes, amount, sticks/d | 7.5 (2.5–20.0) | 7.0 (4.0–20.0) | — |
| Marijuana use | |||
| No | 230 (95.8) | 103 (95.4) | Reference |
| Yes | 10 (4.2) | 5 (4.6) | 1.12 (0.37–3.35) |
| Primary cook for household | |||
| No | 35 (14.6) | 21 (19.4) | 1.41 (0.78–2.57) |
| Yes | 205 (85.4) | 87 (80.6) | Reference |
| Cooking fuel type | |||
| Firewood | 175 (72.9) | 87 (80.6) | 1.66 (0.83–3.32) |
| Charcoal | 25 (10.4) | 9 (8.3) | 1.20 (0.44–3.26) |
| Other | 40 (16.7) | 12 (11.1) | Reference |
| Gas | 38 (15.8) | 11 (10.2) | — |
| Kerosene or electricity | 2 (0.8) | 1 (0.9) | — |
| Elevation of ward of primary residence (meters) | (n = 212) | (n = 95) | |
| 1,944.2 (1,768.2–2,114.8) | 2,015.3 (1,905.8–2,242.7) |
100-m increase (<2,000 m): 1.33 (1.12–1.58) 100-m increase (>2,000 m): 0.99 (0.87–1.13) |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; NHIF = National Health Insurance Fund.
Data are presented as n (%) or median (interquartile range). Boldface type indicates statistical significance. Baseline characteristics stratified by sex are available in the data supplement.
Compared with control subjects, chronically hypoxemic cases had higher odds of current or former tobacco use (OR, 3.45 [95% CI, 2.11–5.65]). Marijuana use was uncommon (<5%) and did not significantly differ between cases and control subjects (OR, 1.12 [95% CI, 0.37–3.35]). In terms of biomass use, firewood was the most common source of cooking fuel among participants, followed by gas and charcoal (Table 1). Compared with control subjects, biomass fuel use was higher in the chronic hypoxemic cases, but not statistically significantly (firewood: OR, 1.66 [95% CI, 0.83–3.32]).
Clinical characteristics including medical history and laboratory values on admission are shown in Table 2. Among cases, 34 (31.5%) participants reported histories of TB, and 6 (5.6%) were being treated for active TB, while among control subjects, 13 (5.4%) participants reported prior TB and 14 (5.8%) were being treated for active TB. Without adjusting for potential confounders, chronically hypoxemic cases had 8.19 times the relative odds of prior TB diagnosis (95% CI, 4.09–16.41) but no significantly increased odds of active TB (OR, 1.34 [95% CI, 0.50–3.63]). Among cases, other reported medical comorbidities with significantly higher odds included asthma (OR, 13.49 [95% CI, 2.94–62.01]), COPD (OR, 81.97 [95% CI, 24.70–272.04]), lung fibrosis (OR, 41.56 [95% CI, 5.43–317.91]), self-reported pulmonary embolism treated with blood thinners (OR, 4.82 [95% CI, 1.18–19.64]), pulmonary hypertension (OR, 52.53 [95% CI, 20.05–137.60]), heart disease including heart failure or arrythmias (OR, 3.54 [95% CI, 2.16–5.81]), and pneumonia (OR, 2.80 [95% CI, 1.37–5.73]). Cases also had higher odds of prior hospitalization (OR, 2.17 [95% CI, 1.25–3.79]). There were significantly lower odds of anemia (OR, 0.24 [95% CI, 0.09–0.61]), kidney disease (OR, 0.38 [95% CI, 0.18–0.78]), and cancer (OR, 0.38 [95% CI, 0.19–0.76]) among chronically hypoxemic cases compared with control subjects. There was no significant association between HIV diagnosis and chronic hypoxemia (OR, 1.06 [95% CI, 0.52–2.17]).
Table 2.
Clinical variables for chronically hypoxemic cases and nonhypoxemic control subjects
| Clinical Variable | Nonhypoxemic Control Subjects (n = 240) | Chronically Hypoxemic Cases (n = 108) | Unadjusted Odds Ratio (95% CI) |
|---|---|---|---|
| Diagnosis of tuberculosis | |||
| No | 213 (88.8) | 68 (63.0) | Reference |
| Yes, current active tuberculosis | 14 (5.8) | 6 (5.6) | 1.34 (0.50–3.63) |
| Yes, prior tuberculosis | 13 (5.4) | 34 (31.5) | 8.19 (4.09–16.41) |
| HIV testing results | |||
| Never done/unavailable | 100 (41.7) | 37 (34.3) | 0.77 (0.37–1.61) |
| Negative | 111 (46.3) | 57 (52.8) | 1.06 (0.52–2.17) |
| Positive | 29 (12.1) | 14 (13.0) | Reference |
| On antiretroviral therapy | 25 (86.2) | 14 (100) | |
| Anemia | 41 (17.1) | 5 (4.6) | 0.24 (0.09–0.61) |
| Asthma | 2 (0.8) | 11 (10.2) | 13.49 (2.94–62.01) |
| Chronic obstructive pulmonary disease/emphysema | 3 (1.3) | 55 (50.9) | 81.97 (24.70–272.04) |
| Cancer | 55 (22.9) | 11 (10.2) | 0.38 (0.19–0.76) |
| Diabetes | 36 (15.0) | 8 (7.4) | 0.45 (0.20–1.01) |
| Heart disease (including heart failure, rheumatic disease, congenital, arrythmias, etc.) | 47 (19.6) | 50 (46.3) | 3.54 (2.16–5.81) |
| Hypertension | 66 (27.5) | 32 (29.6) | 1.11 (0.67–1.83) |
| Kidney disease | 51 (21.3) | 10 (9.3) | 0.38 (0.18–0.78) |
| Lung fibrosis | 1 (0.4) | 16 (14.8) | 41.56 (5.43–317.91) |
| Pneumonia | 16 (6.7) | 18 (16.7) | 2.80 (1.37–5.73) |
| Pulmonary embolus, treated | 3 (1.3) | 6 (5.6) | 4.82 (1.18, 19.64) |
| Pulmonary hypertension/cor pulmonale | 5 (2.1) | 57 (52.8) | 52.53 (20.05–137.60) |
| Sickle-cell anemia | 2 (0.8) | 0 (0.0) | — |
| Stroke | 16 (6.7) | 5 (4.6) | 0.68 (0.24–1.91) |
| Thyroid disease | 5 (2.1) | 3 (2.8) | 1.34 (0.32–5.72) |
| Prior hospitalization | (n = 239) | (n = 108) | |
| Yes | 160 (66.9) | 88 (81.5) | 2.17 (1.25–3.79) |
| Number in the past year | 1 (0–2) | 1 (1–3) | — |
| Same problem as current admission | 104 (65.4) | 71 (80.7) | — |
| Laboratory values (continuous) | P value | ||
| WBCs, ×109/L | (n = 236) | (n = 105) | 0.26 |
| 7.7 (5.4–11.2) | 7.0 (5.3–9.6) | ||
| Hemoglobin, g/dl | 236 | 105 | <0.001 |
| 11.1 (8.6–13.6) | 14.2 (12.4–16.4) | ||
| Hematocrit, % | (n = 236) | (n = 104) | <0.001 |
| 32.9 (24.9–40.0) | 42.5 (37.5–49.7) | ||
| Platelets, per mm3 | (n = 235) | (n = 104) | 0.11 |
| 244.0 (164.0–345.0) | 220.0 (159.0–298.5) | ||
| Creatinine, mg/dl | (n = 234) | (n = 106) | 0.011 |
| 0.9 (0.6–1.5) | 0.8 (0.6–1.1) | ||
| Albumin, g/dl | (n = 195) | (n = 88) | 0.39 |
| 3.3 (2.7–3.8) | 3.4 (3.0–3.7) | ||
| PaCO2, mm Hg | (n = 39) | (n = 37) | <0.001 |
| 25.0 (20.3–32.2) | 48.6 (34.0–55.9) |
Definition of abbreviations: CI = confidence interval; HIV = human immunodeficiency virus; PaCO2 = partial pressure of carbon dioxide; WBC = white blood cell.
Data are presented as n (%) or median (interquartile range). Boldface type indicates statistical significance. Baseline characteristics stratified by sex are available in the data supplement.
Chronically hypoxemic cases had a significantly higher median hemoglobin concentration (14.2 vs. 11.1 g/dl) than control subjects but a lower mean creatinine concentration (0.8 vs. 0.9 mg/dl). Among the subset of participants in whom blood gases were assessed, chronically hypoxemic cases also had a significantly higher partial pressure of carbon dioxide (48.6 vs. 25.0 mm Hg). Other laboratory values, including white blood cell count, platelets, and albumin, did not different significantly between cases and control subjects (Table 3).
Table 3.
Association of chronic hypoxemia with discharge and mortality outcomes
| Outcome | Nonhypoxemic Control Subjects (n = 240) | Chronically Hypoxemic Cases (n = 108) | P Value |
|---|---|---|---|
| Admission length of stay, d | 8 (4–15) | 11 (6–18.5) | 0.014 (1) |
| Mortality rate at discharge | 33 (13.8) | 18 (16.7) | 0.48 (1) |
| Outcome at 1-mo phone follow-up | (n = 206)* | (n = 90) | 0.002 (2) |
| Alive and at home | 175 (84.9) | 61 (67.8) | |
| Alive but rehospitalized | 10 (4.9) | 14 (15.6) | |
| Deceased | 21 (10.2) | 15 (16.7) | |
| Time to phone follow-up from discharge, d | 34 (29–46) | 32 (28–38) | 0.033 (1) |
Definition of abbreviation: 1-mo = one month. Data are presented as median (interquartile range) or n (%). Boldface type indicates statistical significance.
One participant was missing follow-up status because of lack of phone or contacts.
In terms of hospital duration and mortality, chronically hypoxemic cases had a longer median length of stay (11 vs. 8 days; P = 0.014), but there was no significant difference in inpatient mortality (16.7% vs. 13.8%; P = 0.48) compared with control subjects (Table 3). However, phone follow-up at one month after discharge showed significantly higher mortality rates (16.7% vs. 10.2%) and rehospitalization (15.6% vs. 4.9%) among cases who survived initial hospitalization compared with control subjects (P = 0.002).
Multivariable Analysis
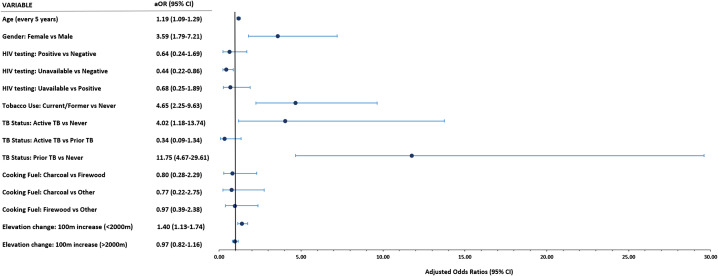
In multivariable analysis, we found that cases had nearly 12 times higher relative odds of having prior TB (OR, 11.75 [95% CI, 4.67–29.61]) and 4 times higher odds (OR, 4.02 [95% CI, 1.18–13.74]) of having active TB compared with nonhypoxemic control subjects (Figure 2). In addition, after controlling for other factors, hypoxemic cases were significantly older (OR, 1.19 per 5-year increase [95% CI, 1.09–1.29]), more frequently female (OR, 3.59 [95% CI, 1.79–7.21]), and more often current or former tobacco users (OR, 4.65 [95% CI, 2.25–9.63]) compared with control subjects.
Figure 2.
Forest plot showing results of multivariable logistic regression model estimating the odds of each exposure, comparing chronically hypoxemic cases with nonhypoxemic control subjects. *Model includes age, gender, HIV testing results, tobacco use, TB status, cooking fuel type, and elevation change. aOR = adjusted odds ratio; CI = confidence interval; HIV = human immunodeficiency virus; TB = tuberculosis.
The average elevation of the ward of primary residence was associated with chronic hypoxemia at elevations <2,000 m above sea level (OR, 1.40 per 100-m increase [95% CI, 1.13–1.74]) but was not meaningfully associated with chronic hypoxemia for participants living above 2,000 m (OR, 0.97 [95% CI, 0.82–1.16]). Chronically hypoxemic cases did not have significant differences in cooking fuel type (OR, 0.80 for charcoal vs. firewood [95% CI, 0.28–2.29]) or positive HIV test results (OR, 0.64 [95% CI, 0.24–1.69]) compared with control subjects. Adding chronic lung disease and chronic heart disease to the model had limited effect on the strengths of these associations (see the data supplement).
Follow-Up Characteristics and Resource Availability of Chronically Hypoxemic Patients
Oxygen use, home oxygen prescription and availability, and hypoxemia on follow-up among the subset of chronically hypoxemic cases were also analyzed (Table 4). Their mean SpO2 on room air was 75.9 ± 9.0% on admission, 80.2 ± 7.2% at discharge, and 79.2 ± 6.3% at follow-up. At the time of screening and enrollment in the study, only 63 (58.3%) chronically hypoxemic participants were receiving supplemental oxygen therapy, with a median of 6 L/min (interquartile range, 5–10 L/min). In terms of home oxygen use, only 10 (9.3%) of chronically hypoxemic cases were using oxygen at home at the time of admission. At discharge, 41 (45.6%) cases had home oxygen discussed with them by someone from their care teams, of whom only 13 (31.7%) were able to obtain home oxygen before discharge.
Table 4.
Follow-up characteristics and resource availability of chronically hypoxemic cases
| Variable | Chronically Hypoxemic Cases (n = 108) |
|---|---|
| Receiving supplemental oxygen on admission | |
| No | 45 (41.7) |
| Yes | 63 (58.3) |
| Amount of supplemental oxygen, L/min | |
| Yes | 6 (5–10) |
| Oxygen saturation as measured by pulse oximetry on room air | |
| On admission | (n = 108) |
| 75.9 ± 9.0 | |
| At discharge | (n = 84) |
| 80.2 ± 7.2 | |
| At follow-up visit | (n = 70) |
| 79.2 ± 6.3 | |
| Using home oxygen on admission | 10 (9.3) |
| Did anyone from your care team discuss home oxygen with you? | (n = 90) |
| No | 49 (54.4) |
| Yes | 41 (45.6) |
| Ability to obtain home oxygen | (n = 41) |
| No, cannot afford oxygen | 7 (17.1) |
| Yes, it has been purchased | 13 (31.7) |
| Maybe, will try to obtain | 16 (39.0) |
| Other | 5 (12.2) |
| Electricity in participant’s home | |
| No | 60 (55.6) |
| Yes | 48 (44.4) |
| Power outages per week | |
| None | 3 (6.3) |
| One or two times per week | 36 (75.0) |
| Three or four times per week | 4 (8.3) |
| Five or six times per week | 5 (10.4) |
| Every day | 0 (0.0) |
| Average duration of power outages | (n = 45) |
| <1 h | 10 (22.2) |
| 1–4 h | 15 (33.3) |
| 4–8 h | 7 (15.6) |
| >8 h | 13 (28.9) |
| Owns generator | 3 (2.8) |
Data are presented as n (%), median (interquartile range), or mean ± standard deviation.
To assess the feasibility of home oxygen concentrators in this population, the availability of electricity was surveyed. Only 48 (44.4%) of chronically hypoxemic cases had electricity in their homes, among whom 45 (93.7%) participants reported at least one or two power outages per week. The majority of participants (55.5%) reported an average outage duration of ⩽4 hours, though 13 (28.9%) had an average duration >8 hours.
Discussion
In this cohort of adults admitted to a tertiary referral hospital in Kenya, TB and female sex were strongly associated with chronic hypoxemia in addition to more well-established risk factors such as older age, tobacco use, and altitude. Chronically hypoxic cases were at nearly 4-fold higher odds of being female and nearly 12-fold higher odds of having prior TB compared with control subjects. These findings highlight important areas of future investigation regarding post-TB lung disease (PTLD) and sex disparities in lung health.
PTLD is defined as evidence of a “chronic respiratory abnormality with or without symptoms, attributable at least in part to previous tuberculosis” and encompasses obstructive lung disease, restrictive lung disease, and pulmonary hypertension, all of which may lead to chronic hypoxemia (14). The size and strength of the association between both active and prior TB in this study underscore the important role that TB plays in chronic hypoxemia among this population. This is in contrast to patient populations in the United States and Europe receiving home oxygen for chronic hypoxemia, among whom age and tobacco use are the major risk factors (4, 15). Our findings are congruent with the current understanding of how TB disease, and the immune response to TB, likely leads to lung injury and remodeling and therefore various phenotypes of chronic lung disease (16). The estimates of higher odds for asthma, COPD, lung fibrosis, and pulmonary hypertension align with current understanding of the pathophysiology of chronic hypoxemia in this population. Indeed, clinical and epidemiologic studies of patients treated for TB have found increased risk of obstructive airway disease, bronchiectasis, pulmonary hypertension, and low forced vital capacity pattern on spirometry compared with those without histories of TB (17–19). Further prospective studies are needed to better understand how the pathophysiology, risk factors, and outcomes of these varying phenotypes of lung disease may be similar or different in individuals with prior TB. These studies will also need to account for the increased risk of TB disease in individuals with COPD (20).
In addition, chronically hypoxemic cases had significantly higher odds of being female. In fact, identifying eligible female control subjects for enrollment into the study was difficult because many women identified by the random generator were hypoxemic on initial screening and thus ineligible to be selected as part of the control group. Future studies on chronic hypoxemia and PTLD should explore potential biological, social, and environmental mechanisms for sex differences in chronic lung disease, in line with ongoing work on sex differences in asthma, COPD, pulmonary hypertension, and cystic fibrosis (21, 22). Given that biomass fuel use and type (i.e., firewood vs. charcoal vs. gas and/or kerosene) were not significantly different between our cases and control subjects, future studies should also move beyond differential exposure to indoor air pollution and biomass fuel use as the primary rationale for sex differences in lung disease across East Africa or include more precise individual indoor and outdoor air pollution exposure assessments such as particulate matter measurements (6, 23, 24).
The odds of living at higher elevations up to 2,000 m were increased among chronically hypoxemic cases, but the odds were not different between cases and control subjects above 2,000 m. The impact of increasing elevation on hypoxemia is well established and helps explain the findings up to 2,000 m. The lack of an association beyond 2,000 m may be due to residual confounding or selection bias, as patients with chronic hypoxemia may move to lower elevations to help with symptoms.
Interestingly, cases did not show a significantly higher prevalence of HIV infection compared with control subjects, despite the known independent association between HIV infection and chronic lung disease (25–27). The prevalence of HIV infection among both cases and control subjects is consistent with known prevalence rates of HIV infection among the general inpatient population at MTRH, and the majority of both cases (100%) and control subjects (86.2%) were on antiretroviral therapy, suggesting that unknown testing status or major differences in viral suppression likely do not explain this negative finding (9). Possible explanations include that data regarding the association between HIV infection and lung disease come from studies in the United States and Europe, where rates of TB are significantly lower, rates of HIV infection from intravenous drug use are higher, and environmental exposures differ compared with Kenya, which may have a differential impact on HIV infection and lung disease (28, 29). Also, many of the initial studies on HIV infection and lung disease were conducted among populations that were not universally on treatment from the time of diagnosis, an era in which TB was a major cause of death among people living with HIV infection (30, 31). Our lack of an association may reflect the beneficial impact of advancements in HIV treatment on severity of chronic lung disease (30).
Other notable findings include significantly higher odds of less than secondary school education and employment as farmers or small business owners/self-employed among chronically hypoxemic cases in univariable analysis. This suggests that occupational lung disease, certain environmental exposures, or socioeconomic status may play an important role in this population. Furthermore, the higher hemoglobin and hematocrit concentrations among chronically hypoxemic cases can be readily explained by normal physiologic responses to hypoxemia. However, the higher creatinine concentrations and relative anemia among control subjects is noteworthy and may be explained by high rates of chronic kidney disease and cancer among control subjects. These high rates of kidney disease and cancer among our nonhypoxemic control subjects are most likely because MTRH is a tertiary referral hospital with some of the only publicly available dialysis and cancer services in the region.
Finally, healthcare use among chronically hypoxemic cases is high, with cases having higher rates of prior hospitalizations in the past year, longer admission length of stay, and higher rates of rehospitalization at one-month follow-up. Although there was no difference in mortality at discharge, mortality was higher among chronically hypoxemic cases after discharge. The lack of difference in mortality rates at discharge may be influenced by our case definition, in that we were not able to confirm chronic hypoxemia among 29 participants who died before discharge because of a lack of prior documentation of SpO2. Higher mortality rates at follow-up may be linked to our findings that fewer than 10% of chronically hypoxemic participants used home oxygen before admission, fewer than half were prescribed home oxygen by the care team before discharge, and only about one-third were able to maintain existing or obtain new home oxygen at discharge. Given that long-term oxygen therapy is a well-established cornerstone in the management of chronic hypoxemia and improves mortality and morbidity, it will be critical to increase availability and use of home oxygen among patients in western Kenya (32, 33). This task will be further complicated by limited access to electricity and frequent power outages, as seen in our study.
Strengths and Limitations
Strengths of this study include a rigorous epidemiologic study design with a hospital-based control group and comprehensive data collection, including measurements of the elevation of study participants’ residences. There was also a high rate of phone and in-person follow-up among patients who survived past hospital discharge, which limits potential selection bias of the OR due to attrition.
However, the study is not without limitations. First, although MTRH covers a large catchment area in Kenya, this was a single-center study, and the measures of association may not be generalizable to other clinical contexts. Furthermore, as MTRH is a large referral hospital seeing high-acuity patients, the findings regarding mortality and other outcomes may also not be generalizable to lower acuity settings.
Second, individual air pollution exposures were imprecisely assessed, and there may be recall bias in participants’ reporting of clinical history, such as prior TB, both of which could lead to exposure misclassification, though this was likely to be nondifferential between cases and control subjects, which would lead to an attenuation of the OR estimate toward the null. Self-reported history of TB may also not be clinically accurate, given the potential for misdiagnosis or erroneous attribution of respiratory symptoms or abnormal chest imaging to TB by clinicians (34). Future studies should consider including more definitive TB diagnosis and history verification, as well as more precise measures of individual-level indoor and outdoor air pollution exposures.
Third, missing elevation data may have affected the precision of estimates in the multivariable model, especially for the odds of active TB among cases and control subjects. We previously reported the results of a multivariable model that does not include elevation for comparison (35).
Fourth, our study was powered on HIV infection as a primary exposure, which may have contributed to the wide CIs we found. A larger sample size may have provided more precise estimates. Finally, as we used survival sampling, there was potential for Berkson’s bias (36, 37). For example, our estimate of the association between sex and chronic hypoxemia may be an overestimate if the true odds of being female in the cohort are closer to 1. If the true odds of being female are 1, then our estimated OR for female sex would be attenuated from 1.99 to 1.57.
Conclusions
In this hospital-based case–control study of adults admitted to a tertiary referral hospital in Kenya, TB, female sex, age, tobacco use, and altitude were significantly associated with chronic hypoxemia, even when accounting for HIV status and biomass fuel use. Despite high rates of hospital admission and readmission, longer length of stay, and high mortality rates after discharge, participants with chronic hypoxemia face significant challenges in obtaining long-term oxygen therapy, which could improve their morbidity and mortality. Future directions for research include prospective studies on PTLD and sex disparities in lung health, as well as further work and implementation studies on home oxygen therapy in Kenya and other resource-limited settings.
Acknowledgments
Acknowledgment
The authors express immense gratitude to the individuals who were willing to participate in this study and provide their time and energy during and after their hospitalization. The authors thank the incredible nursing and medical records staff on the inpatient medicine wards at MTRH, who made this study possible. The authors also thank the Duke Hubert-Yeargan Center for Global Health, the Academic Model Providing Access to Healthcare Consortium, and the Academic Model Providing Access to Healthcare Research Office for their support. Finally, we thank Chani Sra for his support of this study.
Footnotes
Supported by a Vanderbilt-Emory-Cornell-Duke Consortium Global Health Fellowship, funded by the National Heart, Lung, and Blood Institute and the Fogarty International Center of the National Institutes of Health Division of Intramural Research (grant D43 TW009337), as well as the CHEST Foundation (N.N.). The Duke Biostatistics, Epidemiology and Research Design (BERD) Methods Core support of this project was made possible (in part) by grant UL1TR002553 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The views expressed are solely those of the authors and do not necessarily represent the views of the NIH. The funders of this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Author Contributions: N.N. contributed to study conceptualization, funding acquisition, methodology, data curation, formal analysis, writing the original draft, and revising and editing the manuscript. J.R.E. contributed to the methodology, formal analysis, and revising and editing the manuscript. E.B., S.K., and M.M.J. contributed to data collection and revising and editing the manuscript. A.P., C.L.G., and K.W.-K. contributed to the analysis and editing the manuscript. N.M.T., L.D., P.S.K., D.L., and L.G.Q. contributed to the conceptualization, funding acquisition, methodology, supervision of the study and revising and editing manuscript. The authors have no conflicts of interest to disclose.
This article has a data supplement, which is accessible at the Supplements tab.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet . 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. GBD 2019 Diseases and Injuries Collaborators Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet . 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Cause-specific mortality, 2000–2019. Geneva, Switzerland: World Health Organization; 2019. https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death [Google Scholar]
- 4. Jacobs SS, Krishnan JA, Lederer DJ, Ghazipura M, Hossain T, Tan AM, et al. Home oxygen therapy for adults with chronic lung disease: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med . 2020;202:e121–e141. doi: 10.1164/rccm.202009-3608ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. WHO global report on trends in prevalence of tobacco use 2000–2025. 3rd ed. Geneva, Switzerland: World Health Organization; 2019. https://apps.who.int/iris/handle/10665/330221 [Google Scholar]
- 6. van Gemert F, Kirenga B, Chavannes N, Kamya M, Luzige S, Musinguzi P, et al. Prevalence of chronic obstructive pulmonary disease and associated risk factors in Uganda (FRESH AIR Uganda): a prospective cross-sectional observational study. Lancet Glob Health . 2015;3:e44–e51. doi: 10.1016/S2214-109X(14)70337-7. [DOI] [PubMed] [Google Scholar]
- 7. Brakema EA, van Gemert FA, van der Kleij RMJJ, Salvi S, Puhan M, Chavannes NH. COPD’s early origins in low-and-middle income countries: what are the implications of a false start? NPJ Prim Care Respir Med . 2019;29:6. doi: 10.1038/s41533-019-0117-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zifodya JS, Temu TM, Masyuko SJ, Nyale G, Kinuthia J, Page ST, et al. HIV, pulmonary infections, and risk of chronic lung disease among Kenyan adults. Ann Am Thorac Soc . 2021;18:2090–2093. doi: 10.1513/AnnalsATS.202103-251RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karwa R, Maina M, Mercer T, Njuguna B, Wachira J, Ngetich C, et al. Leveraging peer-based support to facilitate HIV care in Kenya. PLoS Med . 2017;14:e1002355. doi: 10.1371/journal.pmed.1002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform . 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. REDCap Consortium The REDCap Consortium: building an international community of software platform partners. J Biomed Inform . 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarvis A, Reuter H, Nelson A, Guevara E. The Shuttle Radar Topography Mission (SRTM) 90m Digital Elevation Database v4.1. Consortium for Spatial Information; 2008. https://csidotinfo.wordpress.com/data/srtm-90m-digital-elevation-database-v4-1/ [Google Scholar]
- 13.The raster package. 2021. https://rspatial.org/raster/pkg/index.html
- 14. Allwood BW, van der Zalm MM, Amaral AFS, Byrne A, Datta S, Egere U, et al. Post-tuberculosis lung health: perspectives from the First International Symposium. Int J Tuberc Lung Dis . 2020;24:820–828. doi: 10.5588/ijtld.20.0067. [DOI] [PubMed] [Google Scholar]
- 15. Hardinge M, Annandale J, Bourne S, Cooper B, Evans A, Freeman D, et al. British Thoracic Society Home Oxygen Guideline Development Group British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for home oxygen use in adults. Thorax . 2015;70:i1–i43. doi: 10.1136/thoraxjnl-2015-206865. [DOI] [PubMed] [Google Scholar]
- 16. Ravimohan S, Kornfeld H, Weissman D, Bisson GP. Tuberculosis and lung damage: from epidemiology to pathophysiology. Eur Respir Rev . 2018;27:170077. doi: 10.1183/16000617.0077-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Allwood BW, Myer L, Bateman ED. A systematic review of the association between pulmonary tuberculosis and the development of chronic airflow obstruction in adults. Respiration . 2013;86:76–85. doi: 10.1159/000350917. [DOI] [PubMed] [Google Scholar]
- 18. Ivanova O, Hoffmann VS, Lange C, Hoelscher M, Rachow A. Post-tuberculosis lung impairment: systematic review and meta-analysis of spirometry data from 14 621 people. Eur Respir Rev . 2023;32:220221. doi: 10.1183/16000617.0221-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Louw E, Baines N, Maarman G, Osman M, Sigwadhi L, Irusen E, et al. The prevalence of pulmonary hypertension after successful tuberculosis treatment in a community sample of adult patients. Pulm Circ . 2023;13:e12184. doi: 10.1002/pul2.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hamada Y, Fong CJ, Copas A, Hurst JR, Rangaka MX. Risk for development of active tuberculosis in patients with chronic airway disease-a systematic review of evidence. Trans R Soc Trop Med Hyg . 2022;116:390–398. doi: 10.1093/trstmh/trab122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lachowicz-Scroggins ME, Vuga LJ, Laposky AD, Brown M, Banerjee K, Croxton TL, et al. The intersection of women’s health, lung health, and disease. Am J Physiol Lung Cell Mol Physiol. 2021;321:L624–L627. doi: 10.1152/ajplung.00333.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pinkerton KE, Harbaugh M, Han MK, Jourdan Le Saux C, Van Winkle LS, Martin WJ, II, et al. Women and lung disease: sex differences and global health disparities. Am J Respir Crit Care Med . 2015;192:11–16. doi: 10.1164/rccm.201409-1740PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fallahzadeh A, Sharifnejad Tehrani Y, Sheikhy A, Ghamari SH, Mohammadi E, Saeedi Moghaddam S, et al. The burden of chronic respiratory disease and attributable risk factors in North Africa and Middle East: findings from Global Burden of Disease Study (GBD) 2019. Respir Res . 2022;23:268. doi: 10.1186/s12931-022-02187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Siddharthan T, Grigsby MR, Goodman D, Chowdhury M, Rubinstein A, Irazola V, et al. Association between household air pollution exposure and chronic obstructive pulmonary disease outcomes in 13 low- and middle-income country settings. Am J Respir Crit Care Med . 2018;197:611–620. doi: 10.1164/rccm.201709-1861OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Crothers K, Huang L, Goulet JL, Goetz MB, Brown ST, Rodriguez-Barradas MC, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med . 2011;183:388–395. doi: 10.1164/rccm.201006-0836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Besutti G, Santoro A, Scaglioni R, Neri S, Zona S, Malagoli A, et al. Significant chronic airway abnormalities in never-smoking HIV-infected patients. HIV Med . 2019;20:657–667. doi: 10.1111/hiv.12785. [DOI] [PubMed] [Google Scholar]
- 27. Bigna JJ, Kenne AM, Asangbeh SL, Sibetcheu AT. Prevalence of chronic obstructive pulmonary disease in the global population with HIV: a systematic review and meta-analysis. Lancet Glob Health . 2018;6:e193–e202. doi: 10.1016/S2214-109X(17)30451-5. [DOI] [PubMed] [Google Scholar]
- 28. Cock KM, Weiss HA. The global epidemiology of HIV/AIDS. Trop Med Int Health . 2000;5:A3–A9. doi: 10.1046/j.1365-3156.2000.00590.x. [DOI] [PubMed] [Google Scholar]
- 29. Fettig J, Swaminathan M, Murrill CS, Kaplan JE. Global epidemiology of HIV. Infect Dis Clin North Am . 2014;28:323–337. doi: 10.1016/j.idc.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Drummond MB, Kunisaki KM, Huang L. Obstructive lung diseases in HIV: a clinical review and identification of key future research needs. Semin Respir Crit Care Med . 2016;37:277–288. doi: 10.1055/s-0036-1578801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med . 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 32. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet . 1981;1:681–686. [PubMed] [Google Scholar]
- 33. Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med . 1980;93:391–398. doi: 10.7326/0003-4819-93-3-391. [DOI] [PubMed] [Google Scholar]
- 34. Houben RMGJ, Lalli M, Kranzer K, Menzies NA, Schumacher SG, Dowdy DW. What if they don’t have tuberculosis? The consequences and trade-offs involved in false-positive diagnoses of tuberculosis. Clin Infect Dis . 2019;68:150–156. doi: 10.1093/cid/ciy544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Navuluri N, Egger J, Thielman N, Murdoch D, Parish A, Green C, et al. Prior tuberculosis and female sex are major risk factors for chronic hypoxemia in Kenyan adults [abstract] Am J Respir Crit Care Med . 2023;207:A1215. [Google Scholar]
- 36. Berkson J. Limitations of the application of fourfold table analysis to hospital data. Biometrics . 1946;2:47–53. [PubMed] [Google Scholar]
- 37. Feinstein AR, Walter SD, Horwitz RI. An analysis of Berkson’s bias in case-control studies. J Chronic Dis . 1986;39:495–504. doi: 10.1016/0021-9681(86)90194-3. [DOI] [PubMed] [Google Scholar]