Abstract
Tracheal intubation in pediatric patients is a clinical scenario that can quickly become an emergency. Complication rates can potentially reach up to 60% in rapid sequence intubation. An alternate to this is delayed sequence intubation, which may reduce potential complications—mostly hypoxemia—and can be especially useful in non-cooperative children. This technique consists of the prior airway and oxygenation optimization. This is done through sedation using agents that preserve ventilatory function and protective reflexes and continuous oxygen therapy—prior and after the anesthetic induction—using nasal prongs. The objective of this narrative review is to provide a broader perspective on delayed sequence intubation by defining the concept and indications; reviewing its safety, effectiveness, and complications; and describing the anesthetic agents and oxygen therapy techniques used in this procedure.
Keywords: Airway management, apneic oxygenation, child, delayed sequence intubation, pediatric anesthesia, pediatric critical care, rapid sequence intubation, sedation
INTRODUCTION
Tracheal intubation in pediatrics is a common procedure indicated in patients requiring mechanical ventilation and/or airway protection.[1] It is a clinical scenario that can quickly become an emergency and a risk factor for morbidity and mortality, especially in young children (neonates and infants).[1] This procedure has very limited apnea times given the high oxygen consumption, reduced functional residual capacity and closure capacity, and high risk of airway collapse.[1] The experience of the operator (anesthesiologist, intensivist, or emergency doctor) is a protective factor of complications, reducing its incidence by 1%–2% for each year of clinical practice.[2,3] The incidence of difficult tracheal intubation—two failed attempts at direct laryngoscopy—can be up to 6% and the rate of first-attempt success can be up to 3% (first intubation attempts made by primary anesthesiologists can fall to 21%).[4,5,6] Moreover, two of three cases are unexpected and mostly related to unidentified dysmorphic facial features.[1,5]
The main complication associated with the tracheal intubation procedure is hypoxemia (incidence up to 40%), a risk factor for morbidity and mortality.[1,4,5] It is mainly related to the patient characteristics (age <2 years, prematurity, and ASA physical status ≥3), the number of laryngoscopy visualization (≥3), intubation trial attempts (≥2), and apnea time (>30 seconds).[1,2,3,6] Likewise, hypoxemia is the main cause of laryngoscopy or intubation interruption—for ventilation—increasing the number of attempts and, therefore, the risk of other complications.[5]
Rapid sequence intubation consists of urgent management (≤10 minutes) and control of an airway unable to maintain patency and/or protective reflexes and a potentially full stomach. Given the clinical condition and the lack of preparation of the patient, there is an increased risk of hypoxemia and bronchopulmonary aspiration, with complication rates of up to 60%.[7,8,9,10]
Delayed sequence intubation (DSI) may be an alternative to tracheal intubation. This procedure focuses on the prior optimization of oxygenation status and airway in potentially non-cooperative patients in whom standard preoxygenation with face mask can be challenging, and sometimes impossible. Its main target is to enhance safety by increasing the apnea time and by reducing the incidence of hypoxemia, the requirement for positive pressure ventilation, and, consequently, the risk of gastric insufflation and/or bronchopulmonary aspiration.[11]
The main objective of this narrative review is to provide a broader perspective on DSI by defining the concept and indications; reviewing its safety, effectiveness, and complications; and describing the anesthetic agents and oxygen therapy techniques used in this procedure.
A literature search was carried out in the following search engines and databases: Google, PubMed, Embase, and Cochrane Library. The search terms were as follows: airway management, child, rapid sequence intubation, DSI, procedural sedation, apneic oxygenation, complications, pediatric critical care, and pediatric anesthesia. All types of research articles published between January 2012 and March 2023 were included, with the only restriction of non-English publications.
DEFINITION
DSI was proposed by Weingart et al. as an alternative to rapid sequence intubation, which was defined as the optimization of oxygenation and airway status before intubation and consisting of two fundamental elements:[11,12,13,14,15,16,17]
Sedation—prior to anesthetic induction and muscle relaxation—that facilitates the administration of oxygen and/or nebulization, suctioning of oropharyngeal secretions, and/or placement of nasogastric tube, using anesthetic agents that preserve spontaneous breathing and patency and reflexes of the airway.
Oxygen therapy to prevent or delay the onset of hypoxemia and lessen its severity, especially in the moments before (preoxygenation) and after (apneic oxygenation) the anesthetic induction and muscle relaxation.
INDICATIONS
Indications of DSI include the following:[12,14,18]
Healthy non-cooperative children.
Children with special educational needs, such as cerebral palsy, autism spectrum disorder, attention deficit disorder, or certain genetic diseases.
Children with altered mental status (confusion, agitation, or obtundation) associated with clinical condition, such as respiratory failure, encephalopathy, or shock.
PHYSIOLOGICAL RATIONALE
DSI can increase (1) oxygen saturation prior to anesthetic induction and muscle relaxation by up to 10%; (2) safe apnea time by up to 100%; and (3) the success rate at first intubation attempt by up to 100%.[11,19,20,21,22,23,24]
DSI can also reduce (1) the incidence of hypoxemia by up to 30% (even studies that have shown no significant difference have reported a reduction in hypoxemia incidence by >15%); (2) intubation attempts by up to 50%; (3) intubation time by up to 75%; and (4) rates of intubation-related complications. Likewise, no adverse effects associated with DSI have been found to the best of our knowledge.[19,24,25,26,27,28]
SEDATION
Sedation is one of the pillars of DSI. Its main objective is to facilitate the oxygen therapy initiation and its tolerance prior to the anesthetic induction and muscle relaxation (preoxygenation), as well as the administration of nebulized drugs, suctioning of oropharyngeal secretions, and/or insertion of nasogastric tube. DSI may not be possible without sedation in non-cooperative children or children with altered mental status. To effectively optimize the oxygenation status, anesthetic agents preserving spontaneous ventilation and airway patency and reflexes—without the need for positive pressure ventilation—throughout the sedation and preoxygenation procedure are indicated. The following subsections detail the anesthetics with a better safety and effectiveness profile for DSI; of note, only the most limiting and potentially dangerous adverse effects at the moment of DSI are discussed, and not other known and more common effects after emergence from anesthesia.[11,12,13,14,15,16,17,18]
Ketamine
Ketamine is the agent of choice in pediatric airway management—especially in urgent or emergent procedures—and is the most commonly used drug with DSI.[11,12,13,14,15] It is an antagonist of the N-methyl-D-Aspartate receptor with anxiolytic, amnestic, hypnotic, anticonvulsant, analgesic, sympathomimetic, sialogogue, anti-inflammatory, and immunomodulatory effects, allowing multiple routes of administration. Furthermore, it has a dissociative effect (thalamo-cortical inhibition and limbic system activation, without any effect on the brainstem) with disconnection from external stimuli but maintaining brain activity. It also preserves spontaneous ventilation and airway tone/reflexes and increases hemodynamic tone, and thus is an agent of choice in heart diseases and shocks.[29,30]
Pharmacokinetics of ketamine show high lipid solubility and low plasma protein binding; an onset of action of ≤1 min, distribution half-life of 5–15 min, and elimination half-life of 1–2 h; and with hepatic biotransformation and renal excretion.[29,30] The recommended doses for sedation in DSI are 1–2 mg/kg in slow bolus (1 min) to avoid potential transient respiratory depression. Maintenance, if required, can be performed with boluses of 0.2–0.5 mg/kg repeated every 10 min or continuous infusion at 5–20 μg/kg/min [Table 1].[29,30]
Table 1.
Sedation and oxygen therapy in delayed sequence intubation
| Sedation | ||||
|---|---|---|---|---|
|
| ||||
| Anesthetic | Initial dose | Maintenance dose | Continuous infusion | |
| Ketamine | 1–2 mg/kg | 0.2–0.5 mg/kg | 5–20 µg/kg/min | |
| Dexmedetomidine | 0.5–1 µg/kg | 0.2–0.5 µg/kg | 0.2–1 µg/kg/h | |
| Ketodex1 | 0.5–1 mL/kg | 0.2–0.5 mL/kg | 0.2–0.5 mL/kg/h | |
| Sevoflurane | 2%–5% | |||
|
| ||||
| Oxygen therapy2 | ||||
|
| ||||
| Interface | Flows (L/kg/min) | Positive pressure (cmH2O) | O2 concentration (%) | |
|
| ||||
| Low | High | |||
|
| ||||
| Nasal cannula | 0.2–1 | - | - | 100 |
| HHFNC3 | - | ≥2 | ≥4 | 100 |
1Dilution with ketamine 1 mg + dexmedetomidine 1 mg per ml. 2Includes preoxygenation and apneic oxygenation. 3Humidified high-flow nasal cannula
Significant adverse effects (tachycardia, hypertension, or desaturation) are usually uncommon, transient, and without major clinical impact or need for therapeutic measures and have been associated with high doses and/or the patient’s age being <3 months; however, this association is controversial. Finally, it is worth mentioning sialorrhea/bronchorrhea, which can compromise airway patency (via obstruction or spasm), although it is not a serious adverse effect.[18,29,30]
Dexmedetomidine
The use of dexmedetomidine in pediatric airway management is currently booming, although the US Food and Drug Administration has not yet approved its use in children or its administration via any other route except the intravenous route.[31]
It is an alpha-2 adrenergic agonist with anxiolytic, amnestic, hypnotic, anticonvulsant, analgesic, sympatholytic, antisialogogue, anti-inflammatory, and immunomodulatory effects, allowing multiple routes of administration. It mimics the non-rapid eye movement phase of physiological sleep, preserving spontaneous ventilation and airway tone/reflexes, and causing minimal hemodynamic depression.[32,33,34]
Pharmacokinetics of dexmedetomidine show high lipid solubility and plasma protein binding; an onset of action of ≤5 min, distribution half-life of 5–10 min, and elimination half-life of 1–3 h; and with hepatic biotransformation and renal excretion.[32,33,34] The recommended doses for sedation in DSI are 0.5–1 μg/kg in slow bolus (2–5 min) to avoid potential transient arterial hypertension (biphasic hemodynamic response). Maintenance, if required, can be performed with boluses of 0.2–0.5 μg/kg repeated every 10 min or continuous infusion at 0.2–1 μg/kg/h [Table 1].[32,33,34]
Significant adverse effects (bradycardia, hypotension, or desaturation) are usually uncommon, transient, self-limited and without clinical repercussion, mostly associated with a previous cardiorespiratory pathology and/or prolonged infusions in pediatric intensive care unit.[32,33,34]
Ketodex (ketamine + dexmedetomidine)
Ketodex is a synergistic combination of ketamine and dexmedetomidine that improves the sedative action and airway/ventilation protective effects with a lower effective dose and reduces side effects.[35] Likewise, both agents have antagonistic pharmacological properties, highlighting the rapid versus slow onset of action, sympathomimetic versus sympatholytic effect, and sialogogue versus antisialogogue effects.[35]
The recommended doses (dilution with ketamine 1 mg + dexmedetomidine 1 μ/ml) for sedation in DSI are 0.5–1 ml/kg in slow bolus (1–2 min). Maintenance, generally less required, is performed with boluses of 0.2–0.5 ml/kg repeated every 15–20 min or continuous infusion of 0.2–0.5 ml/kg/h [Table 1].[35,36,37] Adverse effects, especially cardiorespiratory, are minimized, making this combination a very safe option for DSI.[35,36,37]
Sevoflurane
Sevoflurane is the reference inhalational anesthetic in pediatric anesthesia. Its use through nasal goggles for sedation in airway management can be very useful and effective. It is a halogenated agent—the exact mechanism of action is not yet well known—with amnesic, hypnotic, analgesic (minor), bronchodilator, and immunomodulatory effect, preserving spontaneous ventilation and airway tone/reflexes, and causing little hemodynamic depression.[38]
Pharmacokinetics of sevoflurane show low lipid solubility and plasma protein binding; an onset of action of <2 min, distribution half-life of 5–10 min, and elimination half-life of <1 h; and with pulmonary elimination (hepatic biotransformation is minimal).[38] The recommended doses for sedation in DSI (0.5–1 CAM) vary, depending on the flows, from 2% (high flows) to 4% (low flows). It may be necessary to initiate sedation with a Mapleson circuit to quickly reach these concentrations, as this can be more difficult due to nasal prong leaks through the nose and mouth [Table 1].[39,40,41]
Significant adverse effects (bradycardia, hypotension, or desaturation) are rare and well-tolerated without treatment, related to the use of high concentrations (induction), and/or cardiorespiratory pathology or dysautonomia. However, the use of sevoflurane is absolutely contraindicated in children with a history of or susceptibility to malignant hyperthermia.[39,40,41]
Finally, it should be noted that leaks can favor environmental contamination despite the short duration of the procedure and that the use of the anesthesia machine (sevoflurane vaporizer) is incompatible with the use of a high-flow generator system with humidifier.
OXYGEN THERAPY
Oxygen therapy is another pillar of DSI. Its main objectives are to increase the safe apnea time, to prevent or delay the onset of hypoxemia, and to lessen its severity, if it occurs. This can be more challenging in children aged <2 years with systemic inflammatory response syndrome and cardiac or respiratory disorders; the latter is the main indication for urgent intubation in pediatrics. Oxygen therapy should be started well in advance and maintained uninterrupted throughout the procedure, especially in the moments immediately before and after the anesthetic induction and muscle relaxation (apnea).[25,27] Coinciding with these moments, there are two types of oxygen therapy techniques applied to DSI, which are detailed in the following subsections.
Preoxygenation
It consists of the continuous administration of 100% oxygen prior to the cessation of spontaneous breathing to increase the intrapulmonary oxygen reserve (denitrogenation). The lower the airway permeability, the lower the functional residual capacity, and the higher the oxygen consumption, the higher the intrapulmonary shunt.
Preoxygenation with 20° head-up tilt can be performed in two ways: breathing at tidal volume and low flows (<1 L/kg/min) for 2 minutes or breathing at vital capacity and high flows (≥2 L/kg/min) for 30–60 seconds. High flows can provide variable positive pressure (≥4 cmH2O) to counteract potential airway collapse and atelectasis during sedation [Table 1].[42,43,44]
Apneic oxygenation
It consists of the continuous administration of 100% oxygen after the cessation of spontaneous breathing to delay the onset of hypoxemia. The effectiveness of this technique lies on: (1) a preoxygenation that increases the alveolar oxygen partial pressure and the alveolar–arterial oxygen gradient (maintained later by continuous oxygen therapy); (2) a permeable airway that allows continuous oxygen flow to the alveolus; and (3) a minimal alveolar derecruitment to reduce intrapulmonary shunt.[25,28]
The apnea time can reach >180 seconds in children aged 0–24 months and >300 seconds in children aged 2–10 years. This means prolonging the safe apnea time (without continuous oxygen delivery) by >100%.[19,20,21,22,23]
The apneic oxygenation technique can be performed using low-flow (<1 L/kg/min) or high-flow (≥2 L/kg/min) therapies. High flows can generate a variable positive pressure in the airway (≥4 cmH2O) that can enhance oxygenation and apnea time (although there seems to be no major difference between both types of therapy, provided that 100% oxygen is used). However, high flows appear to have no effect on CO2 clearance or THRIVE (Transnasal Humidified Rapid Insufflation Ventilatory Exchange) in children aged <10 years (not even at 4 L/kg/min flows).[18,19,20,28,44]
In patients for whom 100% oxygen may be contraindicated (such as premature children or those with a congenital heart disease), high-flow oxygen therapy with the highest acceptable FiO2 is recommended, to be maintained during preoxygenation and apneic oxygenation (assuming shorter apnea times compared with the use of 100% oxygen).[18,19,20,28,44]
The recommended oxygen delivery devices in DSI are nasal cannulas. These do not interfere with laryngoscopy and intubation (as can occur with the use of face masks and pharyngeal tubes) and can be maintained throughout the whole procedure without interrupting the oxygen administration. They also allow gas leaks through the nose and mouth that can protect the airway and stomach against overpressure. There are two types of nasal cannula interfaces, as detailed in the following subsections.[18,19,20,28,44]
Conventional nasal cannulas
They are more accessible, cheaper, and have a low profile, but do not humidify/heat the gas and high flows can worsen airway conditions (although rare) due to the short duration of the procedure. They can be connected to the auxiliary common gas outlet port of the anesthesia machine (with vaporizer) for sevoflurane sedation.
High-flow nasal cannulas or humidified high-flow nasal cannulas
High-flow nasal cannulas or humidified high-flow nasal cannulas allow greater flows, humidify/heat the gas, and protect the airway, but are less accessible, more expensive, have a higher profile that can hinder the intubation maneuver, and pose a greater risk of gastric insufflation (a nasogastric tube placement can be considered in case of full stomach confirmed by imaging test) or barotrauma (although this is very rare). The high-flow generator system with humidifier is not compatible with the anesthesia machine (and vaporizers), and thus sevoflurane cannot be used as the sedation agent.
Adverse effects associated with oxygen therapy in DSI are uncommon, as they are usually short procedures with short exposure to high oxygen concentrations and high flows (provided that both can be reduced after intubation). The most significant effects are detailed in the following subsections.[42,44]
Gastric insufflation/barotrauma
They may be related to high flows and positive airway pressure and can be counteracted by nasal prongs leaks.[42,44]
Airway injury
High flows of 100% oxygen can cause airway dryness, inflammation, and bronchoconstriction, favoring the increase of airflow resistance. This can be counteracted by flow and oxygen concentration reduction and/or inhaled gas humidification and heating after intubation.[42,44]
Absorption atelectasis
They can be associated with the use of 100% oxygen and may lead to hypoxemia. However, they can be counteracted with the positive pressure generated by high flows and also by recruitment maneuvers and oxygen concentration reduction after intubation.[42,44]
Bradycardia
It may appear as a vasovagal response (by chemoreceptor activation) triggered by hyperoxia and usually has no clinical significance or need for treatment.[42,44]
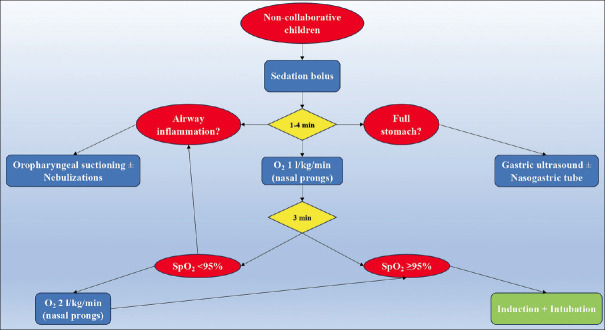
In practice, it is recommended to always start a low-flow (0.2–1 L/kg/min) oxygen therapy. If oxygen saturation reaches optimal levels (95–100%), such flow regime will be maintained until the end of the procedure. If oxygen saturation does not increase or decreases (<92%), flows will be increased progressively (up to 2 L/kg/min) testing the patient’s tolerance and response to oxygen therapy and may result in potential complications.[20,23,28,44] Figure 1 provides a flow chart of the DSI algorithm.
Figure 1.
Flow chart of delayed sequence intubation algorithm
COMPLICATIONS
Airway management in healthy children, especially those aged <2 years, presents some particularities for anatomical and physiological reasons. Furthermore, critically ill children requiring urgent intubation and/or with difficult airways also have unfavorable conditions (cardiopulmonary disorders, full stomach, and/or excessive manipulation) that might potentially lead to adverse effects in up to 60% of patients. The major serious complications of DSI are detailed in the following subsections.[1,2,3,4,5,6]
Hypoxemia
Defined as a PaO2 <60 mmHg or SpO2 <90%, it is the main complication in urgent intubations (up to 40%) and also the main trigger for other complications. It is mainly associated with the laryngoscopy and intubation maneuver (time and number of attempts) but can also occur with laryngospasm/bronchospasm (1.2%) and bronchopulmonary aspiration (0.1%).[1,2,3,4,5,6]
Bronchopulmonary aspiration is described in more detail, as it is very specific to the moment of urgent intubation and—although extremely rare—life threatening. It consists of the passage of gastric contents to the airway and lungs. Hydrochloric acid damage can cause bronchospasm, airway and pulmonary edema, surfactant destruction, atelectasis, intrapulmonary shunt, and pulmonary hypertension; large particle aspiration can also obstruct the airway, further aggravating hypoxemia. The main risk factors are emergency intubation (without fasting), altered level of consciousness, pain, paralytic ileus, and bowel obstruction. Given that the only treatment is supportive, prevention through risk factor identification, ultrasound assessment of gastric contents, and nasogastric tube placement, if necessary, is essential.[1,2,3,4,5,6]
Bradycardia
Bradycardia is defined as a heart rate <60 bpm in children, <80 bpm in infants, and <100 bpm in neonates. Its incidence can reach up to 8% and is mainly associated with severe and sustained hypoxemia, and also with laryngocardiac reflex or the use of sedatives and analgesics. Bradycardia may lead to other serious complications such as hypotension, cardiac arrest, and/or neurological injury.[1,2,3,4,5,6]
Hypotension
Hypotension is defined as mean arterial pressure <60 mmHg in children, <45–50 mmHg in infants, and mean arterial pressure below postmenstrual age (in weeks) in neonates. The incidence rate of hemodynamic instability can reach up to 2% and the immediate evolution can be poor in 5.5% of the patients.[1,2,3,4,5,6]
Cardiac arrest
Cardiac arrest is defined as the need to apply chest compressions >1 minute during intubation or within 20 minutes thereafter. The incidence rate is estimated at about 0.03%. Cardiac arrest is mostly related to cardiorespiratory instability as well as to a history of heart disease or polymalformative syndrome with airway difficulty.[1,2,3,4,5,6]
CONCLUSION
DSI is an easy and affordable procedure that might be a useful and effective alternative to rapid sequence intubation in non-cooperative children. Apneic oxygenation enhances the conditions, safety, and success of DSI (and any other intubation). This enhancement may be achieved to a greater or lesser extent with the simple placement of conventional nasal cannula. Therefore, its use should be considered as a safety standard for any intubation that carries a certain risk of hypoxemia, such as urgent intubations or those in neonates/infants or children with cardiorespiratory disorders. However, future studies providing new evidence in certain aspects and applications are required.
Peer review
This article was peer-reviewed by two independent and anonymous reviewers.
Data availability statement
Data sharing is not applicable for this article, as no new data were created or analyzed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Disma N, Virag K, Riva T, Kaufmann J, Engelhardt T, Habre W, et al. Difficult tracheal intubation in neonates and infants. Neonate and children audit of anaesthesia practice in Europe (NECTARINE): A prospective European multicentre observational study. Br J Anaesth. 2021;126:1173–81. doi: 10.1016/j.bja.2021.02.021. [DOI] [PubMed] [Google Scholar]
- 2.Habre W, Disma N, Virag K, Becke K, Hansen TG, Jöhr M, et al. Incidence of severe critical events in paediatric anaesthesia (APRICOT): A prospective multicentre observational study in 261 hospitals in Europe. Lancet Respir Med. 2017;5:412–25. doi: 10.1016/S2213-2600(17)30116-9. [DOI] [PubMed] [Google Scholar]
- 3.Engelhardt T, Virag K, Veyckemans F, Habre W. APRICOT Group of the European Society of Anaesthesiology Clinical Trial Network. Airway management in paediatric anaesthesia in Europe-insights from APRICOT (anaesthesia practice in children observational trial): A prospective multicentre observational study in 261 hospitals in Europe. Br J Anaesth. 2018;121:66–75. doi: 10.1016/j.bja.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 4.King MR, Jagannathan N. Best practice recommendations for difficult airway management in children-is it time for an update? Br J Anaesth. 2018;121:4–7. doi: 10.1016/j.bja.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Jagannathan N, Asai T. Difficult airway management: Children are different from adults, and neonates are different from children! Br J Anaesth. 2021;126:1086–8. doi: 10.1016/j.bja.2021.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Fiadjoe JE, Nishisaki A, Jagannathan N, Hunyady AI, Greenberg RS, Reynolds PI, et al. Airway management complications in children with difficult tracheal intubation from the pediatric difficult intubation (PeDI) registry: A prospective cohort analysis. Lancet Respir Med. 2016;4:37–48. doi: 10.1016/S2213-2600(15)00508-1. [DOI] [PubMed] [Google Scholar]
- 7.Rinderknecht AS, Mittiga MR, Meinzen-Derr J, Geis GL, Kerrey BT. Factors associated with oxyhemoglobin desaturation during rapid sequence intubation in a pediatric emergency department: Findings from multivariable analyses of video review data. Acad Emerg Med. 2015;22:431–40. doi: 10.1111/acem.12633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerrey BT, Rinderknecht AS, Geis GL, Nigrovic LE, Mittiga MR. Rapid sequence intubation for pediatric emergency patients: Higher frequency of failed attempts and adverse effects found by video review. Ann Emerg Med. 2012;60:251–9. doi: 10.1016/j.annemergmed.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett BL, Scherzer D, Gold D, Buckingham D, McClain A, Hill E, et al. Optimizing rapid sequence intubation for medical and trauma patients in the pediatric emergency department. Pediatr Qual Saf. 2020;5:e353. doi: 10.1097/pq9.0000000000000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long E, Cincotta DR, Grindlay J, Sabato S, Fauteux-Lamarre E, Beckerman D, et al. A quality improvement initiative to increase the safety of pediatric emergency airway management. Paediatr Anaesth. 2017;27:1271–7. doi: 10.1111/pan.13275. [DOI] [PubMed] [Google Scholar]
- 11.Weingart SD, Trueger NS, Wong N, Scofi J, Singh N, Rudolph SS. Delayed sequence intubation: A prospective observational study. Ann Emerg Med. 2015;65:349–55. doi: 10.1016/j.annemergmed.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 12.Schneider ED, Weingart SD. A case of delayed sequence intubation in a pediatric patient with respiratory syncytial virus. Ann Emerg Med. 2013;62:278–9. doi: 10.1016/j.annemergmed.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 13.Merelman AH, Perlmutter MC, Strayer RJ. Alternatives to rapid sequence intubation: Contemporary airway management with ketamine. West J Emerg Med. 2019;20:466–71. doi: 10.5811/westjem.2019.4.42753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miescier MJ, Bryant RJ, Nelson DS. Delayed sequence intubation with ketamine in 2 critically ill children. Am J Emerg Med. 2016;34:2–e1-2. doi: 10.1016/j.ajem.2015.11.053. [DOI] [PubMed] [Google Scholar]
- 15.Löllgen RM, Webster P, Lei E, Weatherall A. Delayed sequence intubation for management of respiratory failure in a 6-year-old child in a paediatric emergency department. Emerg Med Australas. 2014;26:308–9. doi: 10.1111/1742-6723.12196. [DOI] [PubMed] [Google Scholar]
- 16.Kaur A, Ray S, Dias R, Rajan K. Facilitation of delayed sequence intubation with oxygen reserve index monitoring in a child with esophageal perforation. Can J Anaesth. 2021;68:1826–7. doi: 10.1007/s12630-021-02092-1. [DOI] [PubMed] [Google Scholar]
- 17.de Oliveira Castro BM, de Souza RL. Advantages of delayed sequence intubation in selected patients with COVID-19. Anesth Analg. 2020;131:e133–4. doi: 10.1213/ANE.0000000000004977. [DOI] [PubMed] [Google Scholar]
- 18.Ciccozzi A, Pizzi B, Vittori A, Piroli A, Marrocco G, Della Vecchia F, et al. The perioperative anesthetic management of the pediatric patient with special needs: An overview of literature. Children (Basel) 2022;9:1438. doi: 10.3390/children9101438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Else SD, Kovatsis PG. A narrative review of oxygenation during pediatric intubation and airway procedures. Anesth Analg. 2020;130:831–40. doi: 10.1213/ANE.0000000000004403. [DOI] [PubMed] [Google Scholar]
- 20.Riva T, Préel N, Theiler L, Greif R, Bütikofer L, Ulmer F, et al. Evaluating the ventilatory effect of transnasal humidified rapid insufflation ventilatory exchange in apnoeic small children with two different oxygen flow rates: A randomised controlled trial. Anaesthesia. 2021;76:924–32. doi: 10.1111/anae.15335. [DOI] [PubMed] [Google Scholar]
- 21.Riva T, Pedersen TH, Seiler S, Kasper N, Theiler L, Greif R, et al. Transnasal humidified rapid insufflation ventilatory exchange for oxygenation of children during apnoea: A prospective randomised controlled trial. Br J Anaesth. 2018;120:592–9. doi: 10.1016/j.bja.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Humphreys S, Lee-Archer P, Reyne G, Long D, Williams T, Schibler A. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) in children: A randomized controlled trial. Br J Anaesth. 2017;118:232–8. doi: 10.1093/bja/aew401. [DOI] [PubMed] [Google Scholar]
- 23.Soneru CN, Hurt HF, Petersen TR, Davis DD, Braude DA, Falcon RJ. Apneic nasal oxygenation and safe apnea time during pediatric intubations by learners. Paediatr Anaesth. 2019;29:628–34. doi: 10.1111/pan.13645. [DOI] [PubMed] [Google Scholar]
- 24.Bruckner M, Morris NM, Pichler G, Wolfsberger CH, Heschl S, Mileder LP, et al. In newborn infants a new intubation method may reduce the number of intubation attempts: A randomized pilot study. Children (Basel) 2021;8:553. doi: 10.3390/children8070553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.George S, Wilson M, Humphreys S, Gibbons K, Long E, Schibler A. Apnoeic oxygenation during paediatric intubation: A systematic review. Front Pediatr. 2022;10:918148. doi: 10.3389/fped.2022.918148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Napolitano N, Laverriere EK, Craig N, Snyder M, Thompson A, Davis D, et al. Apneic oxygenation as a quality improvement intervention in an academic PICU. Pediatr Crit Care Med. 2019;20:e531–7. doi: 10.1097/PCC.0000000000002123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aroonpruksakul N, Sangsungnern P, Kiatchai T. Apneic oxygenation with low-flow oxygen cannula for rapid sequence induction and intubation in pediatric patients: A randomized-controlled trial. Transl Pediatr. 2022;11:427–37. doi: 10.21037/tp-21-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Napolitano N, Polikoff L, Edwards L, Tarquinio KM, Nett S, Krawiec C, et al. Effect of apneic oxygenation with intubation to reduce severe desaturation and adverse tracheal intubation-associated events in critically ill children. Crit Care. 2023;27:26. doi: 10.1186/s13054-023-04304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bali A, Dang AK, Gonzalez DA, Kumar R, Asif S. Clinical uses of ketamine in children: A narrative review. Cureus. 2022;14:e27065. doi: 10.7759/cureus.27065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simonini A, Brogi E, Cascella M, Vittori A. Advantages of ketamine in pediatric anesthesia. Open Med (Wars) 2022;17:1134–47. doi: 10.1515/med-2022-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Hoorn CE, Flint RB, Skowno J, Davies P, Engelhardt T, Lalwani K, et al. Off-label use of dexmedetomidine in paediatric anaesthesiology: An international survey of 791 (paediatric) anaesthesiologists. Eur J Clin Pharmacol. 2021;77:625–35. doi: 10.1007/s00228-020-03028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahmoud M, Barbi E, Mason KP. Dexmedetomidine: What's new for pediatrics?A narrative review. J Clin Med. 2020;9:2724. doi: 10.3390/jcm9092724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDonald D, Palsgraf H, Shah P. Dexmedetomidine –An emerging option for sedation in neonatal patients. J Perinatol. 2022;42:845–55. doi: 10.1038/s41372-022-01351-3. [DOI] [PubMed] [Google Scholar]
- 34.Freriksen JJ, van der Zanden TM, Holsappel IG, Molenbuur B, de Wildt SN. Best evidence-based dosing recommendations for dexmedetomidine for premedication and procedural sedation in pediatrics: Outcome of a risk-benefit analysis by the Dutch pediatric formulary. Paediatr Drugs. 2022;24:247–57. doi: 10.1007/s40272-022-00498-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li HP, Liu KP, Yao L. Dexmedetomidine in combination with ketamine for pediatric procedural sedation or premedication: A meta-analysis. Am J Emerg Med. 2021;50:442–8. doi: 10.1016/j.ajem.2021.08.073. [DOI] [PubMed] [Google Scholar]
- 36.Magoon R, Choudhary N, Wadhawan S. Ketodex for MRI sedation in syndromic children with congenital cardiac anomalies –A case series. Indian J Anaesth. 2022;66:456–9. doi: 10.4103/ija.ija_606_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vila Moutinho Tavares S, Tavares JC, Borges Marques J, Teixeira de Figueiredo J, de Souza Passos RL. Ketamine-dexmedetomidine combination for sedation in pediatric major surgery in a low-income country. Paediatr Anaesth. 2023;33:278–81. doi: 10.1111/pan.14465. [DOI] [PubMed] [Google Scholar]
- 38.De Hert S, Moerman A. Sevoflurane. F1000Res. 2015;4:626. doi: 10.12688/f1000research.6288.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Rover I, Wylleman J, Dogger JJ, Bramer WM, Hoeks SE, de Graaff JC. Needle-free pharmacological sedation techniques in paediatric patients for imaging procedures: A systematic review and meta-analysis. Br J Anaesth. 2023;130:51–73. doi: 10.1016/j.bja.2022.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Kim K, Kim S. Application of sevoflurane inhalation sedation in dental treatment: A mini review. J Dent Anesth Pain Med. 2021;21:321–7. doi: 10.17245/jdapm.2021.21.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim J, Yoo S, Kim J, Kim S. Using nasal cannula for sevoflurane deep sedation in emergency dental treatment. J Dent Anesth Pain Med. 2015;15:11–5. doi: 10.17245/jdapm.2015.15.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nimmagadda U, Salem MR, Crystal GJ. Preoxygenation: Physiologic basis, benefits, and potential risks. Anesth Analg. 2017;124:507–17. doi: 10.1213/ANE.0000000000001589. [DOI] [PubMed] [Google Scholar]
- 43.Azam Danish M. Preoxygenation and Anesthesia: A detailed review. Cureus. 2021;13:e13240. doi: 10.7759/cureus.13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lyons C, Callaghan M. Apnoeic oxygenation in paediatric anaesthesia: A narrative review. Anaesthesia. 2021;76:118–27. doi: 10.1111/anae.15107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable for this article, as no new data were created or analyzed.