Abstract
Purpose:
To explore the clinicopathological characteristics of immunoglobulin G4 (IgG4)-positive ocular adnexal marginal zone B-cell lymphoma (OAML) and associated patient treatment outcomes.
Methods:
Medical records from patients diagnosed with IgG4-positive OAML treated at the West China Hospital between January 2016 and August 2023 were retrospectively analyzed.
Results:
This study included data from 22 patients (11 males, 11 females), aged between 36 and 83 years, with disease durations from 1 month to 30 years. Sixteen cases exhibited unilateral ocular involvement (ten left eyes, six right eyes), while six exhibited bilateral involvement. Common clinical symptoms included ocular masses, eyelid swelling, and proptosis, with the orbit and lacrimal gland being the most commonly impacted sites. Among the 22 patients, 13 who were clinically suspected of having IgG4-related ophthalmic disease (IgG4-ROD) underwent serum IgG4 testing pre-operatively, revealing elevated IgG4 levels in 11 of these patients. The use of computed tomography and magnetic resonance imaging facilitated the evaluation of the location and size of lesions. All 22 patients received surgical treatment. Subsequently, 14 of these patients underwent local radiotherapy, five received post-operative chemotherapy, and three were closely observed. The follow-up period of patients in this study was 3–77 months, with an average follow-up time of 36 months. Except for one patient who died of disease progression, all others showed favorable prognoses with significant improvements.
Conclusions:
These results support the classification of IgG4-positive OAML as a distinct OAML sub-type with clinical features that partially overlap with IgG4-ROD. Therefore, accurate differentiation between OAML and IgG4-ROD is imperative, necessitating timely surgical intervention and precise pathological diagnosis to prevent diagnostic errors and inappropriate treatment. Currently, no standardized treatments for IgG4-positive OAML exist, but our results suggest that standard OAML therapies are generally efficacious.
Keywords: Clinical characteristics, IgG4, marginal zone B-cell lymphoma, ocular adnexa, treatment outcomes
Ocular adnexal marginal zone B-cell lymphoma (OAML), which predominantly involves the orbit, lacrimal gland, conjunctiva, and eyelids, is the most frequently diagnosed lymphoma in the ocular adnexal region.[1] Immunoglobulin G4-related ophthalmic disease (IgG4-ROD), a chronic auto-immune inflammatory disorder, is characterized by the presence of ocular adnexal masses, elevated serum IgG4 levels, and infiltration of IgG4-positive plasma cells.[2] Ocular adnexal lymphoproliferative disorders, such as OAML and IgG4-ROD, share similarities in their pathogenesis. In recent years, an increasing number of reports have identified OAML patients exhibiting IgG4-positive plasma cell infiltration to the extent that some of these patients meet the criteria for IgG4-ROD diagnosis.[3,4,5,6] As such, some researchers have proposed the classification of IgG4-positive OAML cases as a distinct OAML sub-type more closely related to IgG4-ROD.[7]
Despite these findings, IgG4-positive OAML is still rare and inadequately characterized in medical literature, with limited information on its clinicopathological attributes and treatment outcomes. In order to bridge this gap, the present retrospective study was conducted focusing on the clinical characteristics and prognostic outcomes of patients treated for IgG4-positive OAML.
Methods
The case histories of all OAML patients treated at the West China Hospital from January 2016 to August 2023 were reviewed to identify relevant patients. Patients were included if they (1) had undergone prior surgery with pathologically confirmed OAML, (2) showed more than 10 IgG4-positive cells per high-powered field (HPF) in immunohistochemical analyses, and (3) had available follow-up data. Based on these criteria, 22 patients were identified for whom data including demographic characteristics, clinical features, serum IgG4 levels, imaging manifestations, histopathological characteristics, and treatment methods and results were recorded. The length of follow-up, patient survival, and occurrences of disease progression or recurrence were among the follow-up outcomes for these patients. From the time of diagnosis until August 2023, patient prognosis was monitored through out-patient clinics from the time of diagnosis until August 2023, with the exception of telephone-based follow-ups during the coronavirus disease 2019 (COVID-19) pandemic. Pathological slides for included patients were obtained from the hospital pathology department, and all diagnoses were re-confirmed using the World Health Organization (WHO) 2016 classification standards for Hematopoietic and Lymphoid Tissue Tumors.[8] Hematoxylin and eosin (H and E) staining, immunohistochemical (IHC) staining, and other molecular methods were used to confirm the pathological diagnosis of OAML. The present investigation was conducted in accordance with the Declaration of Helsinki and received approval from the institutional ethics review committee. Informed consent was obtained from all patients.
Statistical analyses were carried out using GraphPad Prism 8 software. Demographic and clinical data are presented using descriptive statistics, including percentages, means, and medians. The Kaplan–Meier method determined progression-free survival (PFS). PFS was calculated from the date of diagnosis to the date of first relapse or disease progression or the date of last contact or death from any cause, whichever occurred first.
Results
A total of 308 cases of ocular adnexal MALT lymphoma were diagnosed and treated in our hospital from 2016 to 2023, among which 22 (7%) cases were diagnosed as IgG4-positive OAML. The clinical characteristics of the 22 patients (11 males, 11 females) enrolled in this study are summarized in Table 1. Of these cases, 16 exhibited unilateral ocular involvement (ten left eyes, six right eyes), while six exhibited bilateral involvement. The ages of the patients ranged from 36 to 83 years (mean, 60 years). The most common clinical manifestations were ocular mass (14/22), eyelid swelling (12/22), proptosis (9/22), epiphora (2/22), decreased visual acuity (2/22), ptosis (1/22), and ocular pain (1/22). The most commonly affected areas were the orbit (16/22) and the lacrimal gland (9/22), followed by the extra-ocular muscles (5/12), eyelid (2/22), conjunctiva (1/22), and lacrimal sac (1/22). The duration of ocular symptoms ranged from 1 month to 30 years (median 12 months). Pre-operative serum IgG4 testing was performed in 13 cases, of which 11 exhibited elevated IgG4 levels (≥ 1.5 g/L; reference range: 0.035–1.5 g/L).
Table 1.
Clinical and pathological characteristics of IgG4-positive OAML patients in the study cohort (22 patients)
| Characteristics | No. of Patients |
|---|---|
| Mean age at diagnosis (range), year | 60 (36 to 83) |
| Sex (male/female) | 11/11 |
| Laterality | |
| Unilateral (left/right) | 16 (10/6) |
| Bilateral | 6 |
| Ophthalmic Symptoms and signs | |
| Ocular mass/Periorbital swelling/Proptosis | 14/12/9 |
| Decreased visual acuity/Epiphora/Ocular pain/Ptosis | 2/2/1/1 |
| Ocular involvement sites | |
| Orbit/Lacrimal gland/Extraocular muscles | 16/9/5 |
| Eyelid/Conjunctiva/Lacrimal sac | 2/1/1 |
| Histopathological features | |
| Lymphocyte infiltration/Lymphoid follicles/Fibrosis | 22/20/20 |
| IgG4-positive cells/HPF No. | |
| 10-50 IgG4-positive cells per HPF | 8 |
| More than 50 IgG4-positive cells per HPF | 14 |
| IgH/IgK gene Monoclonal rearrangement positive | 19 |
A magnetic resonance imaging (MRI) or computed tomography (CT) scan of the orbits was performed on all 22 patients. While both CT and MRI were effective in delineating the morphology, size, and extent of lesions, MRI offered superior imaging quality [Figs. 1 and 2]. The tumor morphology in 18 cases exhibited a diffuse pattern, while four cases presented nodular masses. Notably, the borders of the tumors were indistinct in 20 cases, with clear demarcation observed only in two cases. Of the cases studied, 14 involved the intra-orbital soft tissue and deep orbit. Notably, six of these cases exhibited a ‘cast’ appearance around the eyeball, and five demonstrated extra-ocular muscle involvement. Importantly, neither optic nerve involvement nor orbital wall bone destruction was observed in any of the cases. Moreover, when using the extra-ocular muscles as reference points, enhanced scans consistently revealed lesions with mild to moderate enhancement, exhibiting a uniform pattern across all patients.
Figure 1.
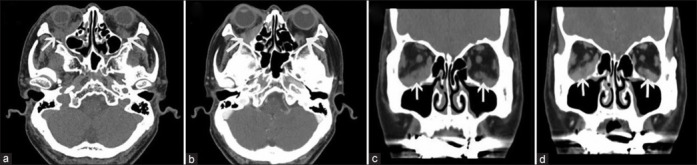
Orbital CT scans of IgG4-positive OAML in patient 5. (a) Axial CT images reveal bilateral orbital tumors (arrow) located inside the muscle cone. (b) Axial CT-enhanced images reveal moderate tumor enhancement (arrow) visible inside the orbits of both eyes. (c) Conical CT images reveal bilateral orbital tumors (arrow), with the tumor in the right eye being located at the bottom surrounding the inferior rectus muscle, while the tumor in the left eye is located at the lower outer corner. (d) Coronal CT-enhanced images suggest uniform tumor enhancement (arrow) in the orbits of both eyes, with signal levels comparable to that of the extraocular muscles
Figure 2.
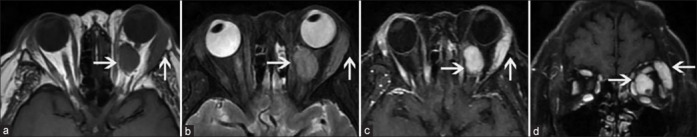
Orbital MRI images of IgG4-positive OAML in patient 8. (a) T1-weighted (T1W) images suggest the location of an orbital tumor (arrow) in the left eye in the lacrimal gland area and surrounding the optic nerve inside the orbital muscle cone, presenting with a low signal level. (b) T2-weighted images reveal the iso intensity of the orbital tumor (arrow) signal in the left eye with that of the extraocular muscle. (c, d) Axial and coronal T1W fat suppression enhanced images suggesting the uniform enhancement of the left-sided orbital tumor (arrow) with signal levels comparable to those for the extraocular muscle
The pathological characteristics of all 22 patients are summarized and presented in Table 1. Tumor tissue samples resembled fish flesh, with solid cut surfaces and firm textures [Fig. 3a]. Histological examination revealed high levels of small infiltrating B-cells in all patients, with 20 patients exhibiting reactive lymph follicles and 20 exhibiting evidence of fibrosis [Fig. 3b]. IHC staining was performed for all 22 patients in this study cohort. OAML was diagnosed per the WHO pathological classification standard, primarily based on CD20 and CD79 positivity and negative CD3, CD5, CD10, and cyclin D1 staining. PCR + GENSCAN analyses of IgK/IgH gene rearrangement further confirmed the monoclonality of B-cells in these patients, with 19 (86%) exhibiting confirmed IgH and/or IgK gene rearrangement. IgG4 immunostaining was positive in all patients’ pathological tissue samples, with counts of ≥ 50 IgG4-positive cells per HPF in 14 patients and 10–50 per HPF in eight patients [Fig. 3c]. Ki-67 positivity rates for patients in this study ranged from 3.0% to 35.0% (median: 11%, mean 14%) [Fig. 3d].
Figure 3.
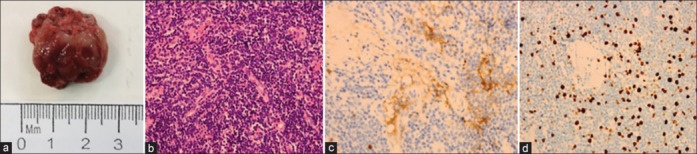
Histological and immunohistochemical analysis of IgG4-positive OAML in patient 8. (a) Gross tumor tissue. (b) Histological H and E staining revealing dense lymphocytic proliferation with sclerosis (Magnification: 400x). (c) IgG4 immunostaining reveals the presence of IgG4-positive plasma cells in the lymphoplasmacytic lesions (Magnification: 400x). (d) Representative Ki-67 staining results for biopsy tissue sections (Magnification: 400x)
Specifically, two patients in our study cohort presented with recurrent disease after prior orbital mass excision surgery. Patient 22, initially diagnosed with ‘bilateral orbital IgG4-positive lymphoid tissue atypical hyperplasia’ 1 year post surgery, was re-diagnosed with ‘bilateral IgG4-positive OAML’ at this visit. Patient 5, previously diagnosed with ‘left orbital IgG4-negative OAML’ 2 years ago and treated with radiotherapy, achieved complete remission. The patient was pathologically diagnosed as having “right orbital IgG4-positive OAML” during this visit.
All patients in this study cohort underwent surgical tumor resection, with the extent of surgery varying based on tumor localization. Two cases with localized lesions underwent complete macroscopic excision, while 20 cases with tumors extending into the deep orbit were excised to the maximum extent feasible to preserve ocular structure and function. All patients underwent standard staging examinations and assessment of systemic disease status after ophthalmic surgery. Systemic involvement of lymphoma was found in three patients, affecting cervical, thoracic, and abdominal lymph nodes, as well as the ureter. In this study, 14 patients received surgery and external-beam radiotherapy (EBRT), five patients received surgery and chemotherapy, and three patients only received surgery treatment. Of 14 patients who received EBRT, the median radiation dose was 26 Gy (range 24–30 Gy in 12–15 fractions) with 2.0 Gy daily fraction size. Among the five individuals who underwent chemotherapy, three patients received four cycles of bendamustine and rituximab (BR) regimen; one patient received four cycles of rituximab, cyclophosphamide, vindesine, and prednisone (R-COP) regimen; and one patient received four cycles of rituximab, fludarabine, and cyclophosphamide (R-FC) regimen. During the follow-up period, except for telephone-based follow-up during the COVID-19 pandemic, we mainly monitor the prognosis of patients through out-patient follow-up. The average follow-up duration was 36 months. At the last follow-up, 20 patients had achieved complete remission, one patient was in partial remission following radiotherapy, and one patient had died due to disease progression post chemotherapy. Overall, the cohort demonstrated favorable prognoses, evidenced by a 5-year PFS rate of 95% [Fig. 4].
Figure 4.
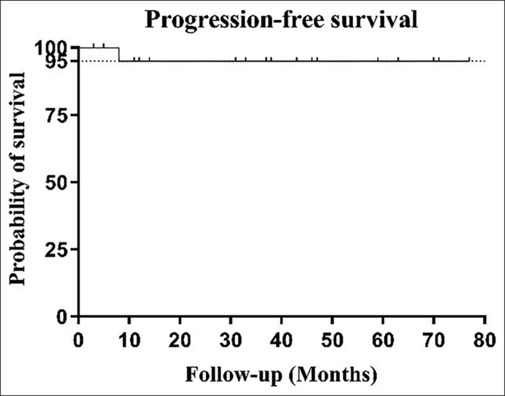
Kaplan–Meier survival analysis of IgG4-positive OAML patients in the study cohort. The PFS rate was 95%
Discussion
In recent years, there has been an increased focus among medical professionals and academics on the strong correlation between OAML and IgG4-ROD. Evidence indicates that a fraction of individuals with OAML demonstrate a historical presence of IgG4-ROD or manifest high levels of IgG4 in both serum and tissue. This information supports the hypothesis that IgG4 may play a pathogenic role in at least certain cases of OAML.[5,9,10,11] IgG4-positive OAML is increasingly recognized as a unique pathological sub-type of OAML closely related to IgG4-ROD. Furthermore, previous research has suggested that IgG4 negatively regulates immune responses, leading to poorer prognostic outcomes, as seen in various cancers, including melanoma,[12] gastric cancer,[13] and pancreatic cancer.[14] However, it is unclear whether the positive expression of IgG4 is related to the poor prognosis in OAML. This retrospective analysis of 7 years of clinical data on IgG4-positive OAML patients treated at our hospital lays a foundational evidence base. This work supports future studies aimed at elucidating the clinicopathological features and prognosis of this enigmatic disease.
These analyses revealed that IgG4-positive OAML frequently affects the orbit or lacrimal gland and can be accompanied by elevated serum and tissue IgG4 levels, consistent with the clinicopathological characteristics of IgG4-ROD. This finding is consistent with those of several previous reports.[7,15,16] The present study found that serum IgG4 levels were not elevated in all patients, with normal levels observed in some patients. This observation indicates that the increased levels of serum IgG4 may lack specificity, thereby highlighting the significance of pathological examination in the diagnosis of IgG4-positive OAML. When a patient presents with clinical signs that align with IgG4-related disease, it is advisable to proceed with surgical intervention immediately. This will facilitate a comprehensive pathological examination, confirming the diagnosis and initiating appropriate treatment.
Accurately diagnosing IgG4-positive OAML depends on identifying characteristic pathological changes that can help distinguish between this condition and IgG4-ROD. The present study revealed that IgG4-positive MALT lymphoma has histopathological features similar to those of IgG4-ROD, both of which have the background of small lymphocyte infiltration and fibrosis. Differentiating these two diseases depends on additional immunohistochemical and molecular pathology tests to identify monoclonal B-cell proliferation. IHC staining revealed CD20 and CD79 positivity, with negative staining for other T- and B-cell markers. Molecular pathological examination can facilitate the detection of amplification peaks indicating IgH and/or IgK gene rearrangement events, providing additional confirmation of B-cell clonal expansion.[17] In this study, 86% of these IgG4-positive OAML patients were positive for IgH/IgK gene rearrangement, which can serve as a specific biomarker for diagnosing lymphoma. However, negative IgH/IgK gene rearrangement results do not rule out this diagnosis, and an accurate pathological diagnosis depends on a comprehensive combined analysis of morphological, immunohistochemical, and clinical features.
IgG4-positive plasma cell infiltration is a key characteristic of IgG4-positive OAML, distinguishing this disease from general OAML cases.[18] This study revealed that infiltration of IgG4-positive cells can occur in primary and recurrent OAML cases, with considerable variability among patients concerning the degree of IgG4-positive plasma cell infiltration in tumor tissue. Notably, patients with high IgG4-positive cells exhibit more pronounced clinical similarities to IgG4-ROD, potentially leading to misdiagnosis in clinical practice. Furthermore, a unique case was observed in this study, where recurrent lymphoma, stemming from aberrant lymphocyte proliferation, was consistently associated with elevated IgG4-positive plasma cells. As mentioned above, the finding raises the question of whether the invasion of IgG4-positive cells could potentially be associated with the malignant transformation of both benign and malignant lymphoproliferative diseases.
Given its recent recognition as a distinct disease entity, limited information is available on the prognosis and optimal treatment of IgG4-positive OAML. All patients in this study cohort were treated using approaches appropriate for managing general OAML cases, including the initial surgical removal of as much tumor tissue as possible while preserving ocular function. Imaging was conducted following the surgical procedure to evaluate individuals for potential systemic implications. Radiotherapy was the preferred initial treatment modality for patients in cases where ocular lesions were not fully excised but lacked evidence of systemic involvement. Conversely, chemotherapy was the preferred approach in cases where indications of systemic involvement accompanied incomplete ocular lesion excision. All patients in this study cohort exhibited positive prognostic outcomes, with a remarkable PFS rate of 95% during the follow-up period. As such, we believe that standard OAML treatment approaches also apply to managing IgG4-positive OAML.
This study has several limitations, notably the small sample size and retrospective design, which may introduce potential reporting bias. Moreover, due to the restricted number of cases within our cohort, our statistical power was constrained, emphasizing the need for additional studies on the diagnosis and therapy of this ailment. Despite these limitations, this case series offers valuable insights into the clinical characteristics and treatment outcomes of IgG4-positive OAML, aiding medical professionals in more effective detection and management.
Conclusion
In conclusion, IgG4-positive OAML, a unique sub-type of OAML, exhibits certain clinicopathological features that share similarities with IgG4-ROD. This similarity raises the risk of misdiagnosis and inappropriate treatment. For patients clinically diagnosed with IgG4-ROD, we recommend timely surgical intervention and thorough pathological evaluation to ascertain the presence of OAML tumor components, enabling appropriate subsequent treatment.
Patient consent
Informed consent was obtained from the patients or relatives to publish their cases.
Consent for publication
All the authors have approved the manuscript.
Author contributions
Design and conduct of the study: YY, XL, WM; Data collection, interpretation and analysis: YY, MH, XL; Preparation of the manuscript: YY; Review of the manuscript: WM; Final approval of the manuscript: YY, XL, MH, WM.
Ethics
The study adhered to tenets of the Declaration of Helsinki, and it was approved by the ethics review board of the West China Hospital of Sichuan University.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank all the reviewers who participated in the review and MJEditor (www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript.
Funding Statement
This work was supported by the science and technology program of Chengdu city (grant numbers 2021-YF05-00844-SN)
References
- 1.Johansson P, Eckstein A, Küppers R. The biology of ocular adnexal marginal zone lymphomas. Cancers. 2022;14:1264. doi: 10.3390/cancers14051264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu WK, Tsai CC, Kao SC, Liu CJ. Immunoglobulin G4-related ophthalmic disease. Taiwan J Ophthalmol. 2018;8:9–14. doi: 10.4103/tjo.tjo_12_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato Y, Ohshima K, Takata K, Huang X, Cui W, Ohno K, et al. Ocular adnexal IgG4-producing mucosa-associated lymphoid tissue lymphoma mimicking IgG4-related disease. J Clin Exp Hematop. 2012;52:51–5. doi: 10.3960/jslrt.52.51. [DOI] [PubMed] [Google Scholar]
- 4.Lee MJ, Kim N, Choe JY, Khwarg SI, Jeon YK, Choung HK, et al. Clinicopathological analysis of ocular adnexal extranodal marginal zone B-Cell lymphoma with IgG4-positive cells. PLoS One. 2015;10:e0131458. doi: 10.1371/journal.pone.0131458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sohn EJ, Ahn HB, Roh MS, Jung WJ, Ryu WY, Kwon YH. Immunoglobulin G4 (IgG4)-positive ocular adnexal mucosa-associated lymphoid tissue lymphoma and idiopathic orbital inflammation. Ophthalmic Plast Reconstr Surg. 2018;34:313–9. doi: 10.1097/IOP.0000000000000965. [DOI] [PubMed] [Google Scholar]
- 6.Li KM, Xu MH, Wu X, He WM. The expression of IgG and IgG4 in orbital MALT lymphoma: The similarities and differences of IgG4-related diseases. OncoTargets Ther. 2020;13:5755–61. doi: 10.2147/OTT.S242852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohno K, Sato Y, Ohshima K, Takata K, Miyata-Takata T, Takeuchi M, et al. A subset of ocular adnexal marginal zone lymphomas may arise in association with IgG4-related disease. Sci Rep. 2015;5:13539. doi: 10.1038/srep13539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–90. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oleś K, Składzień J, Szczepański W, Okoń K, Leszczyńska J, Bojanowska E, et al. Immunoglobulin G4-related disease (IgG4-RD) in the orbit: Mucosa-associated lymphoid tissue (MALT)-type lymphomas. Med Sci Monit. 2015;21:1043–50. doi: 10.12659/MSM.893043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishida K, Sogabe Y, Makihara A, Senoo A, Morimoto H, Takeuchi M, et al. Ocular adnexal marginal zone lymphoma arising in a patient with IgG4-related ophthalmic disease. Mod Rheumatol. 2019;29:383–7. doi: 10.1080/14397595.2016.1216733. [DOI] [PubMed] [Google Scholar]
- 11.Liu R, Wang J, Wang N, Li J, Ge X, Zhang J, et al. Clinical features and prognoses of IgG4-positive and IgG4-negative lacrimal lymphomas. Front Oncol. 2021;11:622847. doi: 10.3389/fonc.2021.622847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crescioli S, Correa I, Karagiannis P, Davies AM, Sutton BJ, Nestle FO, et al. IgG4 characteristics and functions in cancer immunity. Curr Allergy Asthma Rep. 2016;16:7. doi: 10.1007/s11882-015-0580-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyatani K, Saito H, Murakami Y, Watanabe J, Kuroda H, Matsunaga T, et al. A high number of IgG4-positive cells in gastric cancer tissue is associated with tumor progression and poor prognosis. Virchows Arch. 2016;468:549–57. doi: 10.1007/s00428-016-1914-0. [DOI] [PubMed] [Google Scholar]
- 14.Liu Q, Niu Z, Li Y, Wang M, Pan B, Lu Z, et al. Immunoglobulin G4 (IgG4)-positive plasma cell infiltration is associated with the clinicopathologic traits and prognosis of pancreatic cancer after curative resection. Cancer Immunol Immunother. 2016;65:931–40. doi: 10.1007/s00262-016-1853-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubota T, Moritani S, Yoshino T, Nagai H, Terasaki H. Ocular adnexal marginal zone B cell lymphoma infiltrated by IgG4-positive plasma cells. J Clin Pathol. 2010;63:1059–65. doi: 10.1136/jcp.2010.082156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoon J, Kim KJ, Sa HS. Clinical analysis of ocular adnexal mucosa-associated lymphoid tissue lymphoma with IgG4-related ophthalmic disease. Orbit (Amsterdam, Netherlands) 2022;41:551–7. doi: 10.1080/01676830.2021.1962365. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, He H, Li Y, Huang Y, Li G, Yu Q, et al. The application of antigen receptor gene rearrangement of BIOMED-2 in the pathologic diagnosis of 348 cases with non-Hodgkin lymphoma in a single institution in Southwest of China. Pathol Res Pract. 2019;215:152615. doi: 10.1016/j.prp.2019.152615. [DOI] [PubMed] [Google Scholar]
- 18.Nishimura Y, Wien EA, Nishimura MF, Nishikori A, Sato Y, Otsuka F. Clinical characteristics and outcomes of IgG4-positive marginal zone lymphoma: Systematic scoping review. Pathol Int. 2022;72:361–70. doi: 10.1111/pin.13251. [DOI] [PubMed] [Google Scholar]


