Highlights
-
•
Different tumor types have different epidemiological manifestations in terms of morbidity and mortality and have shown different trends in recent years, and these variables provide guidance for further allocation of medical resources.
-
•
The study found that the incidence of chondrosarcoma patients decreased while the incidence of chordoma patients increased between 2000 and 2017.
-
•
The incidence of all kinds of primary bone cancer with WHO grade I and II decreased.
Keywords: Primary malignant bone cancer, Incidence, Mortality, Trends, Surveillance Epidemiology and End Results, Annual percentage change
Abstract
Background
Primary malignant bone cancers have extremely low incidence, resulting in poor evaluation of their epidemiological characteristics. The objective of this study was to investigate trends in the incidence of primary malignant bone cancers and related mortality.
Materials and methods
Data from patients diagnosed with malignant bone cancers from 2000 to 2017 in the Surveillance Epidemiology and End Results database were retrospectively analyzed. Annual age-adjusted incidence and mortality were calculated, and the annual percentage change analyzed. Further, characteristics including patient age and sex, as well as the primary site and stage of different tumor types, were analyzed.
Results
The overall age-adjusted incidence rate of primary malignant bone cancers was 7.70 per million people per year, and incidence rates had increased in patients between 60 and 79 years old, or with tumor size ≥ 8 cm. The incidence of chordoma increased significantly (annual percentage change (APC), 3.0 % per year), while those of WHO grade I and II primary bone cancers decreased. During 2000–2017, the mortality rate attributable to malignant bone cancers across the entire United States was 4.41 per million people per year. A positive mortality trend was observed during the study period (APC = 0.7 %, 95 % confidence interval: 0.0 %–1.5 %). Patients with osteosarcoma, and those who were female or of white ethnicity showed significant increasing trends in mortality rate.
Conclusions
Different tumor types have variable epidemiological manifestations, in terms of incidence and mortality, and exhibited altered trends over recent years. These variables can provide guidance to inform allocation of medical resources.
1. Introduction
Malignant primary bone cancers are extremely rare, comprising only 0.2 % of all neoplasms. In 2024, an estimated 3970 new cases will be diagnosed and 2050 individuals will die because of bone and joints cancers in the USA [1]. Bone tumors include osteosarcoma (OS), chondrosarcoma (CS), Ewing sarcoma, chordomas, and malignant giant cell tumor of bone (GCTB). OS, CS, and Ewing sarcoma are the most common types, comprising 35 %, 30 %, and 16 % of primary malignant bone cancers, respectively, while chordomas and GCTB are relatively rare, accounting for fewer than 5 % [2].
Malignant primary bone tumors are aggressive and can damage surrounding muscle, soft tissue, and neurovascular structures, leading to patient deaths [3], [4], [5]. Further, these tumors impose a substantial economic burden on society and families. A study of 49 centers found that the mean ± standard deviation cost of cervical corpectomy for spinal tumors was $9134 (± $10034), while that of thoracic laminectomy for neoplasm excision was $5382 (± $5502), and superficial bone biopsy cost $1853 (± $1717) [6]. Hence, prompt diagnosis is crucial to improve the survival rates of patients with these diseases. Unfortunately, rates of missed diagnosis are often high, due to the exceedingly low incidence rates of such tumors. Furthermore, accurate epidemiologic data are lacking, because most previous studies have been limited by small data samples or use of older data, due to the rarity of these cancers, resulting in poor understanding of the presentation and survival prospects of patients with these tumors.
In our study, we extracted recent data from the Surveillance Epidemiology and End Results (SEER) database, which covers approximately 26 % of the US population [7]. The purpose of our study was to analyze the presentation and epidemiology of patients with CS, OS, Ewing sarcomas, chordomas, and malignant GCTB. Our findings represent a systematic and comprehensive overview of malignant primary bone cancers, which can offer guidance to clinicians.
2. Methods
2.1. Data source
Age-adjusted incidence data from 2000 to 2017 were extracted from the SEER-18 registry database [8], which is derived from 18 high-quality, population-based registries, distributed in 18 states, covering approximately 26 % of the U.S. population. Demographic and cancer diagnostic information was extracted from the medical records of each case. Age-adjusted rates were calculated as weighted averages of crude rates, where the weights were the proportions of persons in corresponding age groups in a standard population; this approach minimizes the effect of differences in age distributions when comparing rates.
Incidence-based mortality data were obtained from the SEER-9 registry database [9], which includes information from around 10 % of the US population. Death data for each case was extracted from death certificates. Unlike general mortality rate, incidence-based mortality allows partitioning of mortality according to variables associated with cancer onset; its accuracy depends on high-quality population-based registry data and high-quality follow-up information. The analysis included cases diagnosed during 1975–2017 and deaths during 2000–2017, which ensured the maximum number of deaths and avoided underestimation of mortality in the earlier years.
2.2. Demographic and cancer characteristics
Demographic information analyzed in this study were primarily sex, race, and age. Age at diagnosis and age at death were extracted from medical records and death certificates, respectively, and divided into age groups (0–19, 20–39, 40–59, 60–79, and 80+ years).
Primary malignant bone tumors were defined according to the International Classification of Diseases for Oncology, Third Edition (ICD-O-3). The site code was ICD-O-3 topography code, C40.0-C41.9. Histological types included: OS (ICD-O-3 histologic code: 91809194), CS (9220-9243), Ewing sarcoma (9260), chordoma (9370-9372), and GCTB (9250-9252). Only bone cancers with malignant behavior (ICD-O-3 behavior code, /3) were included.
Pathological grade of malignant bone cancer was defined by grade, as follows: Grade I, well differentiated; Grade II, moderately differentiated; Grade III, poorly differentiated; and unknown, grade cannot be assessed. The stages of malignant bone cancer for cases diagnosed between 2000 and 2015 were coded using SEER Historic Stage A, and divided into localized, regional, distant, and unknown. The American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 6th Edition, was used to classify cases diagnosed between 2004 and 2015 into stage I–IV or unknown. In addition, continuously updated schemes to define tumor size have been available since 1983: extent of disease-4 code (1983–1987), extent of disease-10 code (1988–2003), collaborative staging code (2004–2015), and tumor size summary (2016 and later); these codes were used in combination to determine bone tumor size.
2.3. Statistical analyses
Age-adjusted incidence and incidence-based mortality rates were calculated using SEER Stat 8.3.5. All rates are expressed per million person-years. Age-adjusted rates were calculated based on the 2000 US standard population. Joinpoint regression analysis program (version 4.0.2) was used to determine the significance of changes in incidence and mortality trends. Annual percentage change (APC) and 95 % confidence interval (CI) values were calculated to quantify incidence and mortality trends. Statistical significance was determined using the Monte Carlo permutation method. Two-sided P < 0.05 was considered statistically significant.
3. Results
3.1. Incidence of malignant bone cancer
During 2000–2017, 11,793 patients newly-diagnosed with malignant bone cancer were registered in the SEER-18 database. The majority of patients were male (6609 [56.0 %]) and white (9722 [82.4 %]). According to age distribution, the proportion of children and adolescents (<20 years old) was highest, reaching 3584 cases (30.4 %). Common histologic types of malignant bone tumor were OS (4603 [39.0 %]) and CS (CS, 3897 [33.0 %]); however, OS and CS showed completely different age distributions, with OS tending to occur in children and adolescents (<20 years old: 2204 cases [47.9 %]), while CS was more common in middle-aged and older individuals (≥40 years old; 2932 cases [75.2 %]).
According to cancer characteristics, the number of patients diagnosed with grade III malignant bone cancer was largest, and OS was the dominant histologic type. In patients diagnosed with grade I or II cancer, CS was the dominant histologic type. Although the majority of patients had localized (4036 [38.9 %]) or regional (3847 [37.0 %]) stage cancers, cases with distant metastases (1842 [17.7 %]) were not uncommon. Distant metastasis occurred in more cases of OS than CS (870 [21.4 %] vs. 325 [9.4 %]). According to AJCC/TNM stage, OS was dominated by stage II and IV, while CS was dominated by stage I. Patients with tumor size < 8 cm were more common across all cases, while tumors > 8 cm were more common in patients with OS.
The overall age-adjusted incidence rate (AAIR) during the study period was 7.70 (95 % CI, 7.56 to 7.84) per 1,000,000 person-years. The AAIR values for OS and CS were 3.04 (95 % CI, 2.95 to 3.12) and 2.51 (95 % CI, 2.43 to 2.59) per 1,000,000 person-years, respectively. In general, AAIR was higher in males than in females, and in people with white than those with black or other ethnicity; however, AAIR of OS was higher in the population with black ethnicity than that in those with white ethnicity (3.67 [95 % CI, 3.40 to 3.96] vs. 3.04 [95 % CI, 2.94 to 3.15] per 1,000,000 person-years). In addition, the AAIR of CS increased with age, while that of OS peaked at 5.19 (95 % CI, 4.97 to 5.41) per 1,000,000 person-years in people younger than 20 years.
3.2. Mortality attributable to malignant bone cancer
There were 2,210 deaths recorded in the SEER-9 database from 2000 to 2017. Among these, 1,284 (58.1 %) were male and 1,811 (81.9 %) were patients with white ethnicity. According to age distribution, the proportions of mortality in middle-aged and older patients were slightly higher than those of young patients. In addition, there were relatively large numbers of deaths in patients with OS of all age groups, while deaths in young patients with CS were rare. Most deaths occurred in patients with OS (858 [38.8 %]) and CS (806 [36.5 %]), while other tumor types were responsible for relatively fewer deaths. Regarding WHO grade, the majority of deaths were of patients diagnosed with OS grade III, but the number of deaths of patients diagnosed with CS did not differ among those diagnosed with different grade tumors. Among patients diagnosed with OS, deaths were most common in those with regional stage disease, while deaths were most frequent in patients diagnosed with localized stage CS. In terms of tumor size, numbers of deaths were similar between patients with tumor > 8 cm and ≤ 8 cm.
During 2000–2017, 25,229 patients died of malignant bone tumors, according to data from across the entire United States, representing a mortality rate of 4.41 (95 % CI, 4.36 to 4.47) per 1,000,000 person-years. Male (5.48 per 1,000,000 person-years) had a higher mortality rate than female (3.50 per 1,000,000 person-years) patients. For the population covered by the SEER-9 database, overall incidence-based mortality (IBM) was 4.21 (95 % CI, 4.04 to 4.39) per 1,000,000 person-years, while the IBM of males was higher than that of females, reaching almost double in patients with CS. In addition, the IBM rates of patients with CS and OS were 1.65 (95 % CI, 1.54 to 1.77) and 1.51 (95 % CI, 1.41 to 1.62) per 1,000,000 person-years, respectively, which was much higher than those for other tumor types. Patients with WHO grade III tumors had higher IBM, reaching 1.50 (95 % CI, 1.40 to 1.61) per 1,000,000 person-years, particularly individuals with OS. Among all patients with malignant bone tumors, those with regional lesions had the highest IBM, at 1.53 (95 % CI, 1.42 to 1.65) per 1,000,000 person-years. IBM rates were similar between patients with tumor size > 8 cm and ≤ 8 cm.
3.3. Incidence trends of malignant bone cancers.
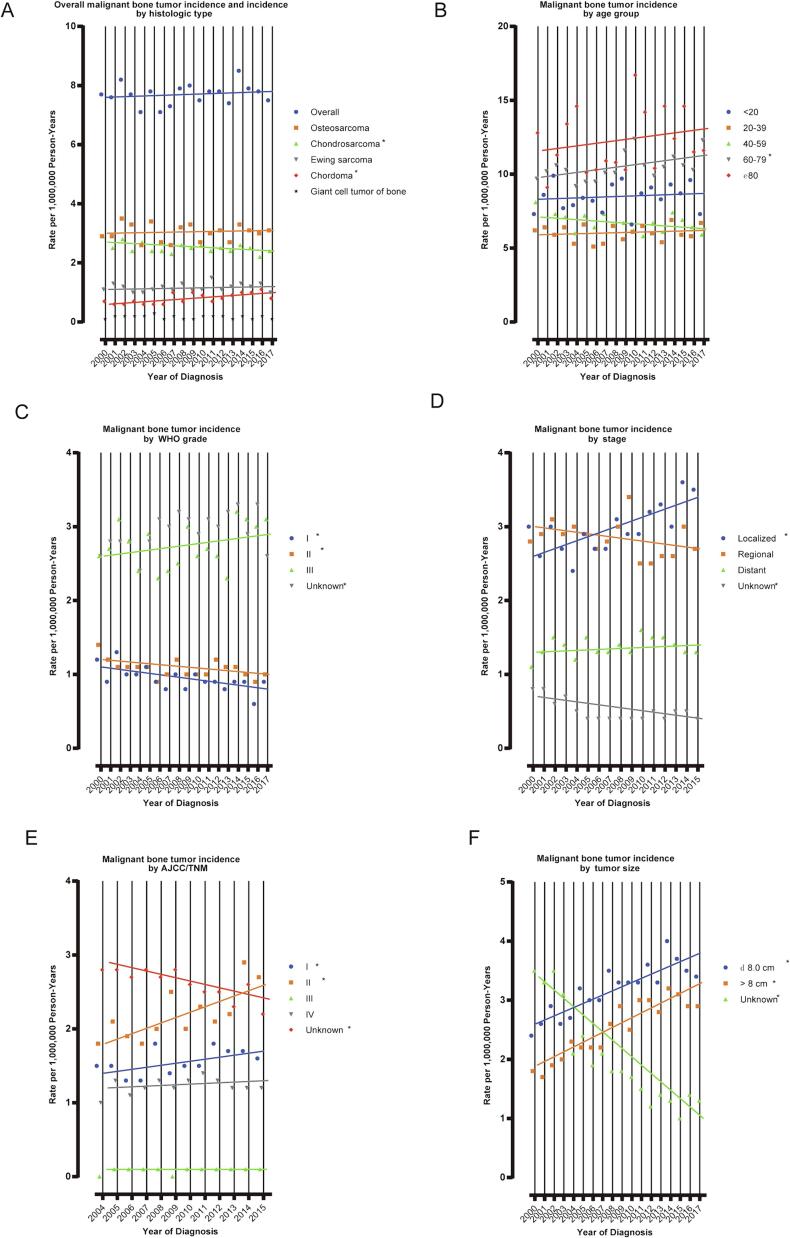
Among all patients with malignant primary bone cancers included, an increasing overall incidence rate was observed between 2000 and 2017, but was not significant (APC = 0.2 %, 95 % CI: –0.3 % to 0.6 %) (Fig. 1A). A significant increase in the incidence of malignant bone cancer was detected only in patients aged 60–79 years (APC = 0.9 %, 95 % CI: 0.1 % to 1.6 %) (Fig. 1B). The incidence of chordoma increased significantly, at a rate of 3.0 % (95 % CI: 1.5 % to 4.5 %) per year. During the research period, incidence rates increased significantly for localized, stage I, stage II, and all size categories of malignant bone cancers, while they decreased significantly for WHO grade I and II malignant bone cancer (Fig. 1C and D).
Fig. 1.
Trends in Annual Primary Malignant Bone Cancer Incidence Rates. (A) represents primary malignant bone cancer incidence (2000–2017), overall and by histologic type; (B) incidence (2000–2017) according to age group; (C) incidence (2000–2017) according to WHO grade; (D) incidence (2000–2015) according to stage; (E) incidence (2004–2015) according to AJCC/TNM; (F) incidence (2000–2017) according to tumor size. Each point represents the observed incidence rates (1,000,000 person-years). The slope of the lines represents the annual percent change (APC). All rates are age-adjusted to the 2000 US standard population.
Among patients with OS, incidence was stable during the study period (APC = 0.95 % CI: –0.9 % to 0.9 %). Significant increases in incidence rates were detected only in patients with stage II (APC = 3.1 %, 95 % CI: 0.6 % to 5.7 %) and tumor size > 8 cm (APC = 4.1 %, 95 % CI: 2.7 % to 5.6 %) (Fig. 1E). Among patients with CS, a significant decrease in the incidence rate was observed during the study period (APC = –0.6 %, 95 % CI: –1.2 % to –0.1 %). The incidence rate of CS decreased significantly in female patients between 2000 and 2017 (APC = –1.0 %, 95 % CI: –1.5 % to –0.5 %). Among different age groups, incidence of CS only decreased significantly in patients aged 40–59 years (APC = –1.7 %, 95 % CI: –2.7 % to –0.6 %). Significant increases in incidence rate of malignant bone cancer were observed for WHO grade III, stage I, and all tumor size categories, while significant decreases in the incidence rate were observed for WHO grade I and regional malignant bone cancer (Fig. 1F).
3.4. Mortality trends among patients with malignant bone cancer
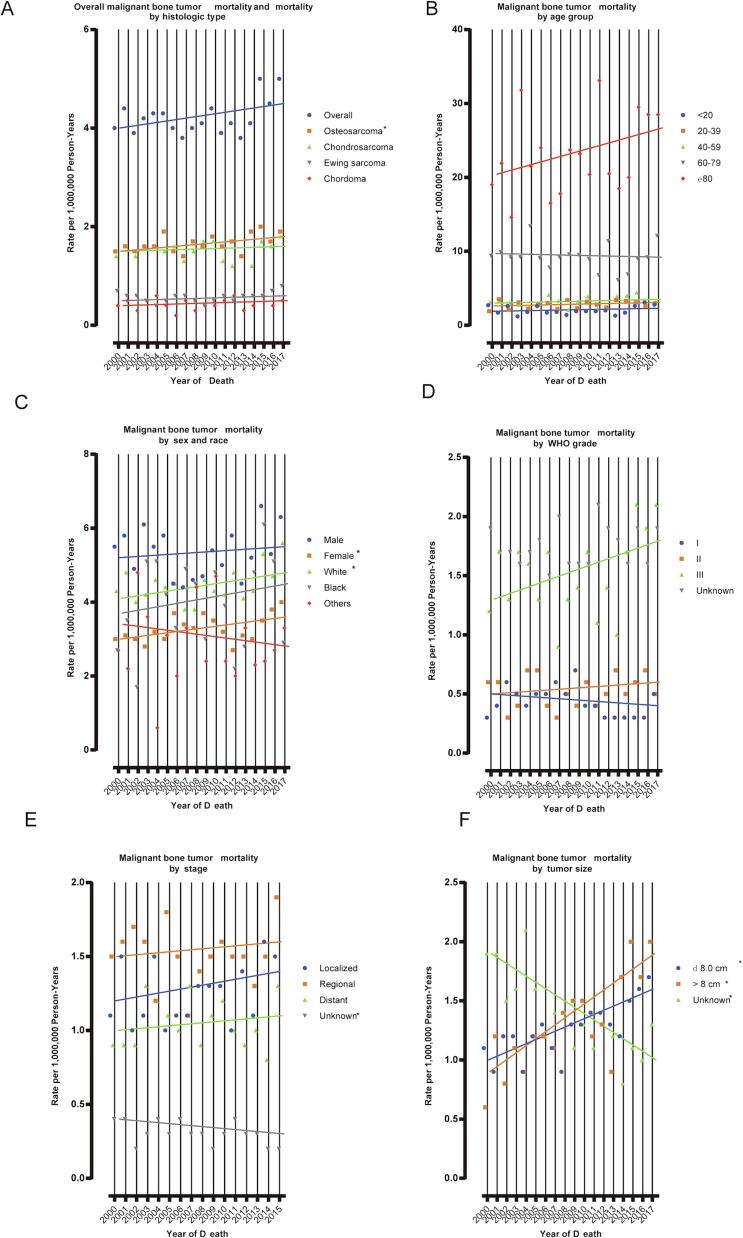
For the population covered by SEER-9, IBM attributable to malignant bone cancer rose significantly during the study period (APC = 0.7 %, 95 % CI: 0.0 % to 1.5 %), with OS the dominant disease (APC = 1.0 %, 95 % CI: 0.0 % to 1.9 %) (Fig. 2A and 2B). Significant increases in the IBM trend were detected among female patients (APC = 1.0 %, 95 % CI: 0.1 % to 2.0 %) and those of white ethnicity (APC = 0.9 %, 95 % CI: 0.0 % to 1.9 %) (Fig. 2C). Patients with WHO grade III malignant bone cancer had increasing IBM (APC = 2.0 %, 95 % CI: 0.0 % to 4.0 %) with a borderline P value (Fig. 2D). Among patients with OS, significantly increased IBM was observed for those with malignant bone cancers of WHO grade III, that were localized, or > 8 cm (Fig. 2E). Further, a significant increase in IBM of patients with CS was only detected among those with tumor size > 8 cm had (Fig. 2F).
Fig. 2.
Trends in Annual Primary Malignant Bone Cancer Mortality Rates. (A) represents primary malignant bone cancer mortality (2000–2017), overall and by histologic type; (B) mortality (2000–2017) according to age group; (C) mortality (2000––2017) according to sex and race; (D) mortality (2000–2017) according to WHO grade; (E) mortality (2000–2017) according to stage; (F) mortality (2000–2017) according to tumor size. Each point represents the observed mortality rates (1,000,000 person-years). The slope of the lines represents the annual percent change (APC). All rates are age adjusted to the 2000 US standard population.
4. Discussion
To the best of our knowledge, data suitable for investigating the incidence of primary bone tumors and related mortality are scarce, due to the relative rarity of these tumors. Here, we collected recent data from patients with primary bone tumors from the SEER database and conducted detailed analyses of incidence and mortality according to age, sex, race, histologic type, grade, stage, and tumor size. Our findings can potentially address the lack of data regarding primary brain tumors, by providing up-to-date estimates. To avoid potential selection bias, this study was conducted using a large, population-based sample.
First, we found that the overall AAIR of primary malignant bone tumors between 2000 and 2017 was 7.70 (95 % CI, 7.56 to 7.84) per 1,000,000 person-years, while the mortality attributable to malignant bone tumors across the entire United States was 4.41 (95 % CI, 4.36 to 4.47) per 1,000,000 person-years. The incidence of bone cancers reported in this study is similar to that found in other regions. A study in Taiwan [10] reported an age-standardized incidence rate of primary bone cancer between 2003 and 2010 of 6.70 per million person-years; the authors detected an upward trend of 4.8 % per year over the study period, which was more significant in females. Another study from Brazil [11] showed that the median incidence rates of bone tumors were 5.74 and 11.25 per million in children and young adults respectively, while the corresponding median mortality rates were 1.22 and 5.07 deaths per million. An analysis of epidemiological data related to bone tumors in Lebanon from 2000 to 2022 reported a total of 730 bone tumor diagnoses, of which 64 % were malignant and 40 % were metastases [12].
In the present study, we found that primary bone tumors, including OS, occurred more frequently in males than in females, with a ratio of 1.3:1, which is consistent with previous reports [13], [14]. We found that OS was the most common type of primary bone cancer followed by CS and Ewing’s sarcoma. Corresponding with the established consensus that OS has a bimodal age distribution, with an early peak between the ages of 10 and 19 years and a later peak among those over 60 years old [15], [16], [17], we observed two incidence peaks in the population categories younger than 20 years and older than 60 years. OS in older adulthood is generally considered to be a secondary neoplasm related to sarcomatous transformation of Paget’s bone disease [18], and there is significant geographic variation in the incidence of Paget’s disease, with high prevalence noted in the UK and North America [19]. This can partially explain the high incidence of OS observed in older adults in our research.
We also conducted subgroup analysis to investigate variations in the incidence of primary bone cancers and related mortality and identified an increase in the overall incidence among patients aged between 60 and 79 years, which may be attributable to several factors: (1) available data from older patients with primary bone cancer has gradually improved over recent years; (2) exposures, such as irradiation, which can lead to increased development of OS; (3) deficiency in sun exposure resulting in lack of vitamin D, as adequate vitamin D uptake is correlated with lower risk of OS [19], [20]. Further, we report a decrease in the incidence of patients with CS, while there was an increase in that of patients with chordomas, which are extremely rare tumors whose incidence increases with age. The rise in numbers of older patients included in the SEER database and progress in imaging techniques may have contributed to the increase in diagnosis of chordomas [21]. It is difficult to assess chondroid tumors at the histopathological level and to distinguish benign disease from CS [22], [23]. In recent years, more precise diagnosis of CS has actually prompted a decrease its in incidence. Furthermore, we demonstrated increased incidence of patients with TNM stage II OS and tumor size > 8 cm; however, the incidence of all kinds of primary bone cancer of WHO grade I and II was decreased.
Patients with primary bone cancer generally have a poor prognosis. Our findings showed no significant differences in overall mortality, although the mortality of patients with OS increased. Further, while previous studies have reported that the 5-year survival rate of patients with OS has increased significantly due to the use of chemotherapeutic agents, survival has not improved and new drugs have failed to reduce the mortality of patients with OS over the past 30 years for complex reasons [24], [25], [26]. First, the anatomical locations of OS vary according to sex and age and can lead to more difficulty in treatment. Second, drug resistance almost invariably occurs, limiting treatment efficacy [27]. Tumor size is an important prognostic factor influencing the survival of patients with OS [28]. Bao et al. reported that patients with tumor size ≥ 8 cm were prone to early metastasis, resulting in extremely poor prognosis. Here, our data suggest that the mortality of patients with tumor size ≥ 8 cm increased from 2000 to 2017.
This research had several limitations. First, due to the retrospective nature of our analyses using a large national database, we could only estimate incidence and mortality based on registry data. Thus, the accuracy of our conclusions largely depends on the precision of the raw data. Although we retrieved as much relevant data as possible from the SEER database, large quantities of information about patients with primary bone cancers were missing. Moreover, records of patients who died from these tumors are rare, because of their low incidence. Thus, it is difficult to distinguish meaningful results, since slight changes in the data can lead to significant fluctuations.
CRediT authorship contribution statement
Jie Yang: Software, Methodology, Data curation, Conceptualization. Suo Lou: Writing – original draft, Methodology. Teng Yao: Writing – original draft, Methodology, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported in-part by the Natural Science Foundation of Zhejiang Province of China (LY24H250001).
References
- 1.Siegel R.L., Giaquinto A.N., Jemal A. Cancer statistics, 2024. CA Cancer J. Clin. 2024;74(1):12–49. doi: 10.3322/caac.21820. [DOI] [PubMed] [Google Scholar]
- 2.Biermann J.S., Chow W., Reed D.R., et al. NCCN Guidelines Insights: Bone Cancer, Version 2.2017. J. Natl. Compr. Canc. Netw. 2017;15(2):155–167. doi: 10.6004/jnccn.2017.0017. [DOI] [PubMed] [Google Scholar]
- 3.Montgomery C., Couch C., Emory C.L., Nicholas R. Giant Cell Tumor of Bone: Review of Current Literature, Evaluation, and Treatment Options. J. Knee Surg. 2019;32(4):331–336. doi: 10.1055/s-0038-1675815. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson J.L., Turner S.P. Bone Cancer: Diagnosis and Treatment Principles. Am. Fam. Physician. 2018;98(4):205–213. [PubMed] [Google Scholar]
- 5.Chordomas Sundaresan N. Clin. Orthop. Relat. Res. 1986;204:135–142. [PubMed] [Google Scholar]
- 6.Kasthuri V.S., Alsoof D., Balmaceno-Criss M., et al. Variability in expenses related to spine oncology care: comparison of payer-negotiated rates at National Cancer Institute-Designated Cancer Centers. Spine J. 2024;24(2):304–309. doi: 10.1016/j.spinee.2023.10.008. [DOI] [PubMed] [Google Scholar]
- 7.The Surveillance, Epidemiology, and End Results (SEER) Program. https://seer.cancer.gov/.
- 8.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER Research Data, 18 Registries, Nov 2019 Sub (2000-2017) - Linked To County Attributes - Time Dependent (1990-2017) Income/Rurality, 1969-2018 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2020, based on the November 2019 submission.
- 9.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence-Based Mortality - SEER Research Data, 9 Registries, Nov 2019 Sub (1975-2017) - Linked To County Attributes - Time Dependent (1990-2017) Income/Rurality, 1969-2018 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2020, based on the November 2019 submission.
- 10.Hung G.Y., Horng J.L., Yen H.J., et al. Incidence patterns of primary bone cancer in taiwan (2003–2010): a population-based study. Ann. Surg. Oncol. 2014;21(8):2490–2498. doi: 10.1245/s10434-014-3697-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balmant N.V., Reis R.S., Santos M.O., Maschietto M., Camargo B. Incidence and mortality of bone cancer among children, adolescents and young adults of Brazil. Clinics (Sao Paulo) 2019;74:e858. doi: 10.6061/clinics/2019/e858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daher M., Zalaquett Z., Mekhael E., et al. Epidemiology of bone tumors in Lebanon: a retrospective study from 2000 to 2022 at a tertiary center. Future Sci. OA. 2023;9(9):FSO886. doi: 10.2144/fsoa-2023-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anfinsen K.P., Devesa S.S., Bray F., et al. Age-period-cohort analysis of primary bone cancer incidence rates in the United States (1976–2005) Cancer Epidemiol. Biomark. Prev. 2011;20(8):1770–1777. doi: 10.1158/1055-9965.EPI-11-0136. [DOI] [PubMed] [Google Scholar]
- 14.Xia L., Zheng R., Xu Y., et al. Incidence and mortality of primary bone cancers in China, 2014. Chin. J. Cancer Res. 2019;31(1):135–143. doi: 10.21147/j.issn.1000-9604.2019.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsson S.E., Lorentzon R. The incidence of malignant primary bone tumours in relation to age, sex and site. A study of osteogenic sarcoma, chondrosarcoma and Ewing's sarcoma diagnosed in Sweden from 1958 to 1968. J. Bone Joint Surg. Br. 1974;56B(3):534–540. [PubMed] [Google Scholar]
- 16.Dorfman H.D., Czerniak B. Bone cancers. Cancer. 1995;75(1 Suppl):203–210. doi: 10.1002/1097-0142(19950101)75:1+<203::aid-cncr2820751308>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 17.Franchi A. Epidemiology and classification of bone tumors. Clin. Cases Miner. Bone Metab. 2012;9(2):92–95. [PMC free article] [PubMed] [Google Scholar]
- 18.Price C.H. Osteogenic sarcoma; an analysis of the age and sex incidence. Br. J. Cancer. 1955;9(4):558–574. doi: 10.1038/bjc.1955.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirabello L., Troisi R.J., Savage S.A. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int. J. Cancer. 2009;125(1):229–234. doi: 10.1002/ijc.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee M.S., Li H.L., Hung T.H., Chang H.Y., Yang F.L., Wahlqvist M.L. Vitamin D intake and its food sources in Taiwanese. Asia Pac. J. Clin. Nutr. 2008;17(3):397–407. [PubMed] [Google Scholar]
- 21.Whelan J., McTiernan A., Cooper N., et al. Incidence and survival of malignant bone sarcomas in England 1979–2007. Int. J. Cancer. 2012;131(4):E508–E517. doi: 10.1002/ijc.26426. [DOI] [PubMed] [Google Scholar]
- 22.van Praag Veroniek V.M., Rueten-Budde A.J., Ho V., et al. Incidence, outcomes and prognostic factors during 25 years of treatment of chondrosarcomas. Surg. Oncol. 2018;27(3):402–408. doi: 10.1016/j.suronc.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Thorkildsen J., Taksdal I., Bjerkehagen B., et al. Chondrosarcoma in Norway 1990–2013; an epidemiological and prognostic observational study of a complete national cohort. Acta Oncol. 2019;58(3):273–282. doi: 10.1080/0284186X.2018.1554260. [DOI] [PubMed] [Google Scholar]
- 24.Restrepo D.J., Huayllani M.T., Boczar D., et al. Which Factors Affect Survival in Patients With Upper Limb Osteosarcoma? Anticancer Res. 2019;39(9):5027–5031. doi: 10.21873/anticanres.13693. [DOI] [PubMed] [Google Scholar]
- 25.Mirabello L., Troisi R.J., Savage S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115(7):1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu J., Sun H., Li J., et al. Increased survival of patients aged 0–29 years with osteosarcoma: A period analysis, 1984–2013. Cancer Med. 2018;7(8):3652–3661. doi: 10.1002/cam4.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye K., Wang S., Wang J., Han H., Ma B., Yang Y. Zebularine enhances apoptosis of human osteosarcoma cells by suppressing methylation of ARHI. Cancer Sci. 2016;107(12):1851–1857. doi: 10.1111/cas.13088. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Bao J., Zeng J., Song C., et al. A Retrospective Clinicopathological Study of Osteosarcoma Patients with Metachronous Metastatic Relapse. J. Cancer. 2019;10(13):2982–2990. doi: 10.7150/jca.30750. [DOI] [PMC free article] [PubMed] [Google Scholar]