Abstract
Nature is aglow with numerous captivating examples of UV-fluorescence in the animal kingdom. Despite a putative role as a visual signal, exploration of UV-fluorescence in plants and its role in plant-animal interactions is lagging in comparison. Almost 50 years ago, UV-fluorescence of floral nectar, a crucial reward for pollinators, was reported for 23 flowering plant species. Since this intriguing discovery, UV-fluorescent nectar has only seldom been addressed in the scientific literature and has not been scrutinized in a phylogenetic or ecological context. Here, we report the prevalence of vibrant UV-fluorescent floral nectar across the family Cleomaceae, including the first photographic documentation in vivo colour for flowering plants. Though Cleomaceae flowers are morphologically diverse varying in colour, nectary prominence, and nectar volume, UV-fluorescent floral nectar may be a ubiquitous characteristic of the family. Fluorescence spectra show that the identity and number of fluorescent compounds in floral nectar may differ among Cleomaceae species. As Cleomaceae pollinators range from insects to bats and birds, we suggest that the UV-fluorescent floral nectar not only functions as a visual cue for the diurnal pollinators but also for the nocturnal/crepuscular pollinators in low light settings.
Subject terms: Plant sciences, Plant signalling
Introduction
From vibrant colours to striking patterns, flowering plants display an astonishing array of characteristics that act as visual signals for pollinators1. The innate and learned preferences of pollinators to these suites of visual cues encourage visitation2,3, with flowering plants commonly offering rewards such as nectar in exchange for pollen transfer. Given the intricate ties among pollinators and floral displays, flower colour research is key to shedding light on plant-pollinator interactions and the evolution and diversity of floral form. Ultraviolet (UV)-fluorescence, a floral feature that may function as a visual signal for pollinator attraction, represents a significant gap in our knowledge of flower colour.
UV-fluorescence is a type of luminescence in which UV radiation is absorbed and longer wavelength light is emitted. Unlike the well-known UV nectar guides (i.e., patterns of UV-absorbance and -reflectance) that can only be perceived by animals with UV-receptors (e.g., insects and birds)1,4, the lower energy light emitted via UV-fluorescence can occur in the spectral range visible to humans (and many other animals)1,5. Further, UV-fluorescence has not been studied as extensively as UV nectar guides1,6–8. However, UV-fluorescence is of growing interest with many recent discoveries of this phenomenon across the animal kingdom (e.g., the hair of nocturnal springhare and flying squirrels9,10, skin of salamanders and catsharks11,12, and the bones of toadlets and chameleons13,14). Behavioural studies within the animal kingdom suggest that UV-fluorescence may be more than a coincidental by-product of chemical structure15–17. For example, female jumping spiders (Cosmophasis umbratica, Salticidae) have appendages that fluoresce bright green under UV radiation; in the absence of UV radiation, male jumping spiders do not perform typical courtship behaviour with non-fluorescing females17. Similarly, budgerigars (Melopsittacus undulatus, Psittacidae) have UV-fluorescent yellow plumage on their crown and cheeks; both sexes prefer budgerigars of the opposite sex with fluorescent plumage over those with masked fluorescence (i.e., concealed with UV-absorbing chemicals)15. Despite numerous captivating examples and a putative function as a visual signal in animals, the prevalence and significance of UV-fluorescence across flowering plants has scarcely been investigated.
Nearly 50 years ago, Thorp et al.18 were among the first to report the brilliant UV-fluorescence of nectar in flowering plants and suggested that this phenomenon functions as a visual signal for bees. Out of the 102 flowering plant species examined, 23 had nectar that fluoresced yellow to blue with varying degrees of intensity and the majority pollinated by bees18. Apart from nectar fluorophore (i.e., fluorescent molecule) identification for three species19–21, our understanding of UV-fluorescent nectar has seen limited progress since its discovery. Suggestions that nectar fluorescence could be used as an indicator of secretory cells in vivo22,23 and conceptual arguments about its ecological importance24,25 have not significantly enriched our fundamental knowledge of UV-fluorescent nectar. Yet, the presence of UV-fluorescence was recently reported in the prey traps of several carnivorous plant species and in the anthers and pollen of numerous flowering plants species26–28. Like the UV-fluorescent animal studies, behavioural experiments with UV-fluorescent prey traps and an anther/pollen fluorophore show that this phenomenon plays a role in animal attraction26,27. Pitcher plants (Nepenthes khasiana, Nepenthaceae) with masked fluorescence catch significantly less insect prey26 and bees are attracted to filter paper containing a fluorescent compound identified from anthers and pollen27. Though UV-fluorescence presumably contributes to the array of visual cues involved in pollinator attraction, further exploration is needed to determine the prevalence of UV-fluorescent nectar across flowering plants, characterize its molecular basis, and to establish a link to pollinator interactions.
Though UV-fluorescence in nature is a fascinating phenomenon to observe, to the best of our knowledge, there are only four published photographs of UV-fluorescent nectar18,29,30 (Fig. 1). Unfortunately, the photographs are monochromatic, captured ex vivo or marred by blurriness, and fail to ignite curiosity parallel to that of UV-fluorescence research in the animal kingdom. Here, we present the first in vivo colour images of UV-fluorescent nectar in flowering plants. We show UV-fluorescent nectar across Cleomaceae, a relatively small family with morphologically diverse flowers and a broad range of diurnal and nocturnal/crepuscular pollinators31,32. Moreover, we discuss the current state of flower florescence research, including the potential phylogenetic and ecological implications of UV-fluorescent floral nectar.
Figure 1.
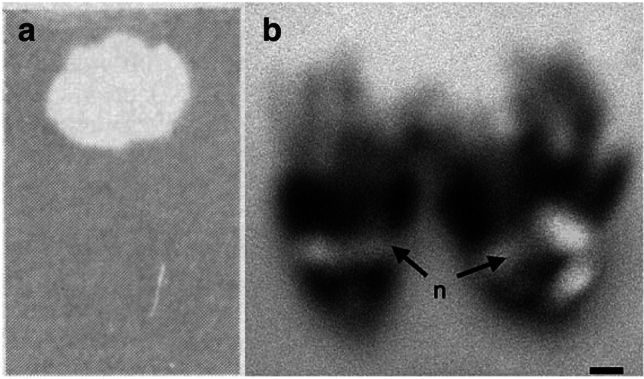
UV-fluorescent floral nectar of Prunus species. (a) A spot of P. amygdalus nectar on filter paper under long- and short-wavelength UV radiation. From Thorp et al.18. Reprinted with permission from AAAS. (b) A P. persica flower with nectar under UV radiation. From Radice and Galati29. Reprinted with permission from SNCSC. n, nectar. Scale bar, 2.5 mm (b).
Methods
Plant material
The following 10 Cleomaceae and one Brassicaceae species were grown from seed in professional growing mix (Sun Gro Horticulture, Agawam, MA, USA): Arivela viscosa (L.) Raf. accession 815 from Hortus Botanicus, Cleome amblyocarpa Barratte & Murb. accession 151485 from Royal Botanic Gardens Kew, Cleome violacea L. accession 813 from Hortus Botanicus, Gynandropsis gynandra (L.) Briq. accession TOT8917 kindly provided by M. Eric Schranz, Melidiscus giganteus (L.) Raf. accession 814 from Hortus Botanicus, Polanisia dodecandra (L.) DC. accession 68456 from B & T World Seeds, Sieruela hirta (Klotzsch) Roalson & J. C. Hall accession 74520 from B & T World Seeds, Sieruela monophylla (L.) Roalson & J. C. Hall accession 353278 from Royal Botanic Gardens Kew, Sieruela rutidosperma (DC.) Roalson & J. C. Hall accession 512496 from B & T World Seeds, Tarenaya houtteana (Schltdl.) Soares Neto & Roalson (see Neto et al.33 for the recent taxonomic revision) accession FL2400 from West Coast Seeds, and Brassica rapa L. Wisconsin Fast Plants cultivar. Cleomaceae species were grown in CMP3244 growth chambers (Environmental Growth Chambers, Chagrin Falls, OH, USA) set to a 28 °C 12 h light and 22 °C 12 h dark regime and B. rapa was grown in a greenhouse (Greenhouse Complex, Department of Biological Sciences, University of Alberta). Jocelyn C. Hall and Brandi Zenchyzen formally identified the plant material. Voucher specimens were deposited in the University of Alberta Vascular Plant Herbarium (ALTA; Supplementary Table S1).
Nectar volume measurements
Nectar was extracted from flowers with microcapillary tubes (0.4 mm i.d., 75 mm length; Drummond Scientific, Broomall, PA, USA) between 10:00 and 12:00. The height of nectar drawn into the microcapillary tube was measured to the nearest 0.5 mm and used to calculate nectar volume. For species with nectar too viscous to draw into a microcapillary tube (i.e., M. giganteus and T. houtteana) the following protocol was used: the flower was removed from the plant at the base of the pedicel, water was added to the nectar with a pipette, the pipette tip was used to manually swirl the nectar solution without aspirating, the nectar solution was extracted from the nectary with microcapillary tubes, and the amount of added water was subtracted from the total nectar solution volume. For each Cleomaceae species, nectar was collected from a minimum of three plants (i.e., biological replicates) and 1–30 flowers per plant. The five species with the highest nectar volumes were selected for in vivo photography and fluorescence spectra analysis.
Photography and fluorescence spectroscopy
Floral nectar was extracted with microcapillary tubes or a pipette between 10:00 and 12:00 and pooled from multiple flowers and plants for each species. Samples were stored at 4 °C between extractions and until photography or fluorescence spectroscopy analysis. Flowering plants and floral nectar in microcapillary tubes were photographed in a dark room against a black background while illuminated with an iLED gooseneck illuminator (i.e., white light; Laxco, Mill Creek, WA, USA) or C8 Convoy 365 nm UV flashlights (Yooperlites, Brimley, MI, USA). Photographs were captured using a D80 DLSR camera (Nikon, Tokyo, Japan) with an AF Micro-NIKKOR 60 mm f/2.8 D or AF Micro-NIKKOR 105 mm f/2.8 AI-S lens (Nikon) or a D500 DLSR camera (Nikon) with AF-S Teleconverter TC-14 III and AF-S Micro-NIKKOR 105 mm f/2.8 IF-ED lenses (Nikon). Photoshop (Adobe; San Jose, CA, USA) was used to darken the black backgrounds. As Brassicaceae is sister family to Cleomaceae, the nectar of B. rapa was photographed and compared to that of the Cleomaceae species.
Fluorescence excitation and emission spectra were measured using quartz cuvettes and a PTI QM-8075-11 spectrofluorometer (Horiba, Kyoto, Japan) for pooled nectar samples, each totaling approximately 120 µL, and a genistein standard (Toronto Research Chemicals, Toronto, ON, Canada) in ethanol. Highly viscous samples were diluted with ultrapure water for ease of transfer. Cleomaceae nectar fluorescence spectra were compared to that of genistein and hydroxycinnamate derivatives (reported by Mori et al.27).
Literature review
A literature review was conducted using Google Scholar to determine the phylogenetic distribution and possible ecological implications of UV-fluorescent nectar. As Thorp et al.18 is the key article that brought awareness to UV-fluorescent nectar, scientific literature citing this work was examined for mentions of additional species exhibiting this phenomenon and evaluations of its function. Further, we extended our review by searching for scientific literature containing the terms “ultraviolet”, “fluorescent”, and “nectar”. Studies that examined nectar for fluorescence after extraction from a pollinator or introduction of a solvent or stain were excluded due to the possibility of nectar modification. Examples of UV-fluorescence in other plant features were identified through review of the abovementioned literature. All methods were performed in compliance with the relevant guidelines and legislation.
Results
For the nine Cleomaceae species examined here, nectar is secreted by receptacular nectaries located between the perianth and stamens, or perianth and androgynophore (i.e., stalk-like structure subtending the reproductive organs; e.g., G. gynandra)32,34. The nectaries are diverse in form, ranging from adaxial protrusions or concavities to annular disks, and from inconspicuous to a prominent component of the flower32. The average nectar volume secreted varied from 0.01 to 19.73 µL with the highest average nectar volumes (> 0.5 µL) secreted by M. giganteus, T. houtteana, P. dodecandra, S. hirta, and C. violacea and lowest (< 0.5 µL) by S. rutidosperma, C. amblyocarpa, S. monophylla, and A. viscosa (in descending order; Table 1). Of note, Lunau et al.35 mentioned S. monophylla as an example of a species with a false nectary (i.e., a glossy surface mimicking nectar); however, we show that its nectary is functional. Arivela viscosa was excluded from subsequent fluorescence analyses due to insufficient nectar volumes. For the remaining nine Cleomaceae species, the floral nectar is colourless under white light but exhibits vibrant blue fluorescence when illuminated by UV-A radiation with peak intensity at 365 nm (Fig. 2).
Table 1.
Mean (± s.d.) floral nectar volume of nine Cleomaceae species.
| Species | Nectar volume (µL) |
|---|---|
| Arivela viscosa | 0.01 ± 0.02 (N = 58) |
| Cleome amblyocarpa | 0.19 ± 0.10 (N = 93) |
| Cleome violacea | 0.55 ± 0.20 (N = 130) |
| Gynandropsis gynandra | 0.44 ± 0.25 (N = 78) |
| Melidiscus giganteus | 19.73 ± 11.00 (N = 42) |
| Polanisia dodecandra | 2.78 ± 0.59 (N = 90) |
| Sieruela hirta | 2.12 ± 0.57 (N = 81) |
| Sieruela monophylla | 0.07 ± 0.08 (N = 67) |
| Sieruela rutidosperma | 0.31 ± 0.16 (N = 100) |
| Tarenaya houtteana | 6.20 ± 2.07 (N = 68) |
Figure 2.
UV-fluorescent floral nectar of Cleome violacea and other Cleomaceae species. (a, b) Cleome violacea under white light (a) and UV-A radiation (b). (c) Close up of C. violacea flower under UV-A radiation. (d) Nectar of five Cleomaceae species and water in microcapillary tubes under white light (top) and UV-A radiation (bottom). Cv, Cleome violacea; Pd, Polanisia dodecandra; Th, Tarenaya houtteana; Sh, Sieruela hirta; Mg, Melidiscus giganteus. Scale bar, 1 cm (a, b).
With nectaries and nectar that are not obscured by the perianth or stamens, C. violacea, P. dodecandra, and T. houtteana have the most visually striking examples of UV-fluorescent nectar. Under white light, the nectar of C. violacea is challenging to discern from the green three-lobed nectary (Fig. 2a). Yet, when excited with UV-A radiation, the vividly fluorescent nectar droplets are easily distinguished from the nectary and contrast the less intense red fluorescence of chlorophyll36 (Fig. 2b,c). Similarly, under white light, the nectar of P. dodecandra and T. houtteana accumulates on top of an orange cup-shaped nectary and light green ‘V’-shaped nectary, respectively (Fig. 3a,c). Under UV-A radiation, the nectar of both species intensely fluoresces (Figs. 2d, 3b,d). In addition, the vasculature within the petals of P. dodecandra fluoresces blue and the petals of T. houtteana fluoresce bright pink.
Figure 3.
UV-fluorescent floral nectar of Polanisia dodecandra and Tarenaya houtteana. (a, b) Polanisia dodecandra under white light (a) and UV-A radiation (b). (c, d) Tarenaya houtteana under white light (c) and UV-A radiation (d). Scale bars, 1 cm.
Cleomaceae species with nectaries and nectar partially or entirely concealed by the perianth and stamens include C. amblyocarpa, M. giganteus, S. hirta, S. monophylla, and S. rutidosperma. For instance, the perianth of S. hirta and M. giganteus partially obscure the adaxially secreted nectar (Fig. 4a,c). Though both species secrete UV-fluorescent nectar (Fig. 2d), the blue fluorescence of the nectar does not appear as vibrant against the intense fluorescence of the other floral structures (Fig. 4b,c). As C. amblyocarpa, G. gynandra, S. monophylla, and S. rutidosperma have obscured nectaries and/or low nectar volumes (< 0.5 µL), nectar was pooled from multiple flowers and observed in microcapillary tubes. Whether exposed or obscured, the nectar of the nine Cleomaceae species fluoresces blue under UV-A radiation (Fig. 2d, Supplementary Fig. S1). The intensity of fluorescence varies between and within genera and can differ within species (Supplementary Fig. S1). Of note, the pollen fluorescence can also be rather vivid though the nectar fluorescence often steals the show (i.e., P. dodecandra) (Fig. 3b). Like the Cleomaceae taxa, B. rapa has receptacular nectaries between the perianth and stamens and UV-fluorescent nectar (Supplementary Fig. S1).
Figure 4.
UV-fluorescent floral nectar of Sieruela hirta and Melidiscus giganteus. (a, b) Sieruela hirta under white light (a) and UV-A radiation (b). (c, d) Melidiscus giganteus under white light (c) and UV-A radiation (d). Arrowheads are pointing to the partially exposed nectar. Scale bars, 1 cm.
For the five species that secreted the greatest volumes of nectar, the nectar fluorescence excitation maxima ranged from 309 to 396 nm and the emission maxima varied from 416 to 476 nm (Fig. 5, Supplementary Table S2). The nectar fluorescence spectra of C. violacea, M. giganteus, and T. houtteana each had one set of emission and excitation peaks, while P. dodecandra and S. hirta had two sets of peaks. No two species had the same nectar fluorescence spectra. The Cleomaceae nectar fluorescence spectra maxima did not correspond to that of genistein (Supplementary Table S2), which fluoresced green instead of blue, but more closely resembled the fluorescence spectra of hydroxycinnamate derivatives27.
Figure 5.

Fluorescence spectra for the floral nectar of five Cleomaceae species. Excitation spectra, dotted lines; emission spectra, solid lines. Colours correspond to the human perceived colours for the emission spectra maximum wavelengths. Cv, Cleome violacea; Pd, Polanisia dodecandra; Th, Tarenaya houtteana; Sh, Sieruela hirta; Mg, Melidiscus giganteus.
Discussion
To our knowledge, our study is the first report of widespread UV-fluorescent floral nectar across a flowering plant family and the only photographic documentation of this phenomenon in vivo colour. Adding to the numerous reports of diverse animals exhibiting UV-fluorescence, we show that the floral nectar of nine representatives from six Cleomaceae genera fluoresces vibrant blue when illuminated with UV radiation.
Evolution of UV-fluorescent nectar
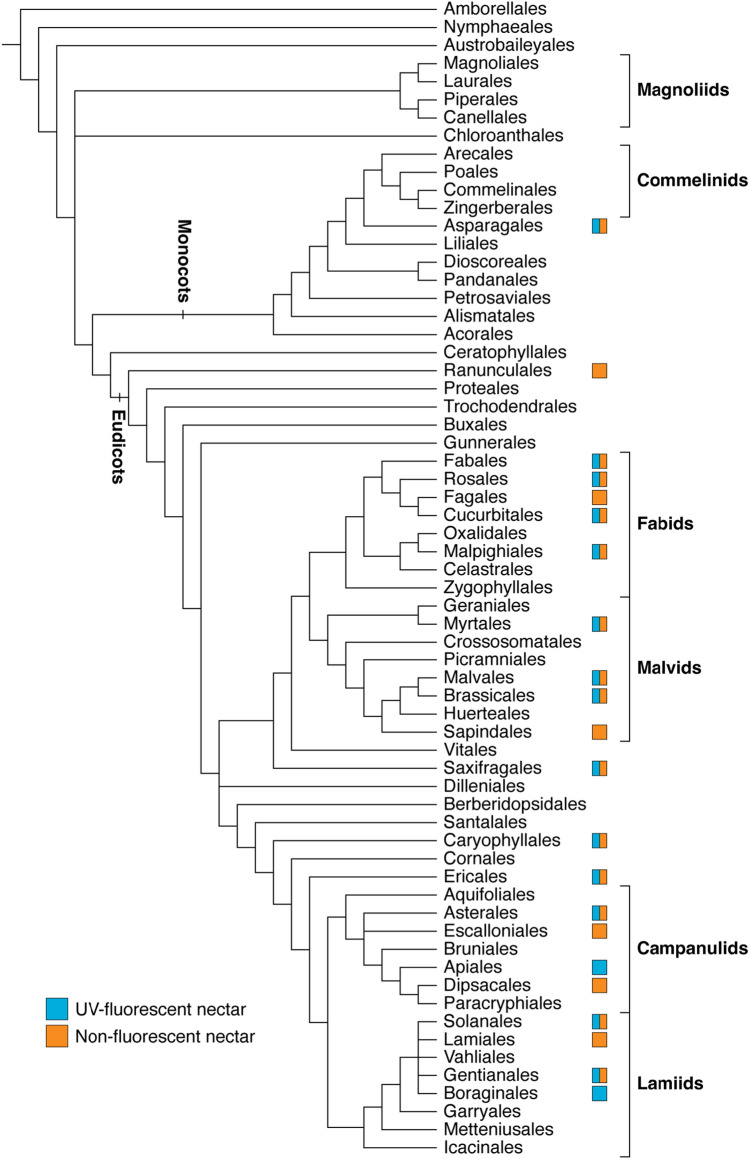
Though there is limited evidence about its prevalence, the scattered distribution of UV-fluorescent nectar suggests that this phenomenon arose multiple times across flowering plants (Fig. 6). Including the taxa from our investigation, UV-fluorescent nectar has been documented for 41 species (19 families), predominantly belonging to the eudicots18,21,22,30,37 (Table 2). For most of these families, the occurrence of UV-fluorescent nectar varies within family, genus, or even species (Table 2; Supplementary Table S3). For instance, Thorp et al.18 noted the presence of UV-fluorescent nectar in five Prunus (Rosaceae) species and absence in four, including UV-fluorescent nectar in peach but not nectarine flowers (i.e., varieties of P. persica). The variability between closely related individuals suggests that the genetic mechanisms governing fluorophore biosynthesis are conserved but differentially expressed within lineages. While nectar fluorescence is variable in Brassicaceae (sister family to Cleomaceae), this phenomenon may be a unifying feature of Cleomaceae as all ten species investigated, which span multiple genera and clades, exhibit nectar fluorescence to varying degrees of intensity18 (Supplementary Fig. S1).
Figure 6.
Phylogenetic distribution of UV-fluorescent and non-fluorescent floral nectar across flowering plants. A box is present for orders that have data for at least one species. The phylogeny is adapted from that of the Angiosperm Phylogeny Group67 and data is summarized from Thorp et al.18, Scogin21, Roshchina et al.22, Nakanishi30, Davis et al.37, and the present study.
Table 2.
Flowering plant species with UV-fluorescent floral nectar.
Adapted from the data of Thorp et al. 18, Scogin21, Roshchina et al. 22, Nakanishi30, Davis et al.37, and the present study (indicated by an asterisks).
| Group | Order | Family | Species |
|---|---|---|---|
| Monocots | Asparagales | Amaryllidaceae | Allium cepa |
| Asparagaceae | Muilla maritima | ||
| Orchidaceae | Maxillaria anceps | ||
| Eudicots | Fabales | Fabaceae | Glycine max |
| Robinia pseudoacacia | |||
| Rosales | Rosaceae | Photinia serratifolia (formerly P. serrulata) | |
| Prunus amygdalus (formerly P. dulcis) | |||
| Prunus ilicifolia (formerly P. ilicifolia and P. lyonii) | |||
| Prunus lusitanica | |||
| Prunus mume | |||
| Prunus persica | |||
| Prunus salicina | |||
| Cucurbitales | Cucurbitaceae | Cucumis sativus | |
| Malpighiales | Euphorbiaceae | Euphorbia pulcherrima | |
| Passifloraceae | Passiflora caerulea | ||
| Myrtales | Onagraceae | Ludwigia peploides | |
| Malvales | Malvaceae | Bombax ceiba (formerly B. malabaricum) | |
| Fremontodendron californicum | |||
| Fremontodendron mexicanum | |||
| Pseudobombax ellipticum | |||
| Brassicales | Cleomaceae | Cleome amblyocarpa* | |
| Cleome violacea* | |||
| Cleomella arborea (formerly Isomeris arborea) | |||
| Gynandropsis gynandra* | |||
| Melidiscus giganteus* | |||
| Polanisia dodecandra* | |||
| Sieruela hirta* | |||
| Sieruela monophylla* | |||
| Sieruela rutidosperma* | |||
| Tarenaya houtteana* | |||
| Brassicaceae | Brassica rapa* | ||
| Crambe maritima | |||
| Saxifragales | Crassulaceae | Kalanchoe daigremontiana (formerly Bryophyllum daigremontianum) | |
| Caryophyllales | Polygonaceae | Fagopyrum esculentum | |
| Ericales | Balsaminaceae | Impatiens balsamina | |
| Asterales | Asteraceae | Centaurea solstitialis | |
| Lasthenia californica (formerly L. chrysostoma) | |||
| Apiales | Apiaceae | Daucus carota | |
| Solanales | Convolvulaceae | Convolvulus arvensis | |
| Gentianales | Apocynaceae | Hoya carnosa | |
| Boraginales | Hydrophyllaceae | Phacelia viscida |
With the possibility of multiple independent origins of UV-fluorescent nectar, the question arises: are distinct fluorophores responsible for nectar fluorescence throughout the flowering plant phylogeny? Like nectar, species with anthers and pollen that emit blue fluorescence under UV radiation are dispersed across flowering plants27,28. Mori et al.27 identified the anther and pollen fluorophores of five species in four different eudicot families as hydroxycinnamate derivatives and suggested their widespread distribution and shared biosynthetic pathway for taxa with fluorescent blue anthers and pollen. Alternatively, UV-fluorescent compounds can be unique to specific clades and thus act as taxonomically informative characters. For example, ester-linked ferulic acid exclusively occurs in the cell walls of the monophyletic clades commelinid monocots and core Caryophyllales38,39. When observed with UV-fluorescence microscopy, cell walls with ester-linked ferulic acid fluoresce blue when in water and green when in acid38. For the five Cleomaceae species examined, the distinct fluorescence spectra suggest that different compound(s) are responsible for the nectar fluorescence in each species. Further, the two sets of excitation and emission maxima for P. dodecandra and S. hirta indicate two fluorophores may be present in the nectar of these species. Additional research is needed to identify the nectar fluorophores of Cleomaceae species and determine if the fluorescent compounds are from a shared biosynthetic class.
Though often perceived as a simple sugar solution, nectar consists of a complex array of biomolecules and microorganisms (i.e., bacteria and fungi)34,40,41. Major constituents such as water, carbohydrates, and amino acids reward pollinators; proteins can tailor nectar chemistry for pollinators and prevent microbial infections; and secondary metabolites including scented and coloured compounds can contribute to pollinator attraction40–44. Since biomolecules, including large macromolecules such as proteins, can act as fluorophores and microorganisms can contain fluorophores36, the complexity of nectar poses a challenge for the identification of UV-fluorescent components. Yet, in response to the fascinating finding of Thorp et al.18, Scogin19–21 identified the UV-fluorescent compounds in the nectar of three Malvaceae species as a genistein-related isoflavone and its glucoside (Fremontodendron californicum and F. mexicanum) and a hydroxycoumarin (Bombax ceiba). Since this discovery, there have been several reports of a hydroxycinnamate derivative (i.e., chlorogenic acid; pollen/anther fluorophore27), genistein-related isoflavone and glucosides, and a hydroxycoumarin (i.e., aesculetin) in the nectar of diverse taxa, including two species with known UV-fluorescent nectar18,45–50 (Robinia pseudoacacia, Fabaceae and Fagopyrum esculentum, Polygonaceae; Supplementary Table S4). However, these studies did not examine the nectar samples for UV-fluorescence.
Potential ecological function of fluorescent nectar
This seemingly prevalent phenomenon in Cleomaceae brings forth the question: does UV-fluorescent nectar serve as a visual cue for the array of Cleomaceae pollinators? The significance of UV-fluorescence for pollinator attraction has been debated. Thorp et al.18 posited that UV-fluorescent nectar functions as a visual signal for foraging bees. However, this hypothesis has been criticized due to concerns that the emitted fluorescence may be imperceptible to insects amid the reflected light24,25. While describing the colour of a flower may appear straightforward, providing an ecologically relevant description proves challenging as colour perception is dependent on the sensory and processing capabilities of the observer1. For instance, insects and birds have UV photoreceptors, while humans do not1,4. As a result, the perceived colour of a flower may drastically differ between humans and pollinators. Under sunlight, nectar fluorescence may not be conspicuous to the human eye, yet behavioural assays have shown that honeybees make fine colour discriminations and are attracted to a UV-fluorescent compound (i.e., chlorogenic acid)27,51. Further, honeybees have a blue photoreceptor maximally activated at 436 nm, lying within the range of Cleomaceae nectar fluorescence emission maxima (416–476 nm; Fig. 5, Supplementary Table S2), and an innate preference for blue colours52,53.
Unlike Thorp et al.18 that reported bees as the primary pollinators of taxa with UV-fluorescent nectar, Cleomaceae consists of both generalist species pollinated by a variety of insects and sometimes hummingbirds (e.g., A. viscosa, Cleomella arborea, P. dodecandra)54–56 and specialist species solely pollinated by bats (e.g., M. giganteus and T. houtteana)57,58. Though echolocation and olfaction play important roles in bat orientation and foraging59,60, all bat species have functional eyes61. For example, one of the bat pollinators of T. houtteana (i.e., Glossophaga soricine, Phyllostomidae) is colour-blind but able to perceive UV radiation as well as some human-visible light61. Further, Domingos-Melo et al.62 suggested that bats are attracted to the brightness of visible light reflecting off chiropterophilous (i.e., bat-pollinated) flowers at dusk. In addition to bats, nocturnal/crepuscular pollinators of Cleomaceae also include hawkmoths (e.g., G. gynandra)63. Investigations on animals show that UV-fluorescence is most prevalent and intense for nocturnal species and suggest that this phenomenon is an overlooked visual signal for nocturnal animals9,10,64–66. Likewise, a behavioural assay involving the UV-fluorescent pitcher plant N. khasiana revealed that UV-fluorescence may play a role in insect attraction in low light settings26. Prey capture of unmasked pitcher plants primarily occurred at night and masking of the fluorescent blue rim significantly reduced the capture of insect prey26. With evidence from the behavioral assays suggesting fluorescence aids in the attraction of both diurnal and nocturnal/crepuscular pollinators26,27, it is possible that fluorescent nectar in Cleomaceae not only acts as a visual signal for daytime pollinators but may also assist in the attraction of nocturnal/crepuscular pollinators during twilight. Alternatively, or in addition to a visual role, nectar fluorophores may impact pollinator health. For instance, high concentrations of chlorogenic acid significantly reduce infection by a gut pathogen in the bumblebee Bombus impatiens (Apidae)68.
Studies on the visual cues of flowers have predominately focused on pigmentation that is reflective in the visible range, or UV-absorptive or -reflective, while neglecting UV-fluorescence as a potential signal for pollinators. Yet, amid the recent wave of UV-fluorescence discoveries in the animal kingdom, UV-fluorescence has emerged as a budding area of research accompanied by many outstanding questions. Here, we add Cleomaceae floral nectar to the growing collection of UV-fluorescence findings in nature and suggest: (1) fluorescent blue nectar may be a taxonomically informative characteristic for Cleomaceae, (2) several compounds are responsible for nectar fluorescence across Cleomaceae, and (3) UV-fluorescent nectar not only serves as a visual signal for bees but may also function as a visual cue for nocturnal/crepuscular pollinators, such as bats and hawkmoths, in low light settings. Whether tied to pollinator interactions or merely an incidental consequence of chemical structure, we hope the visually striking UV-fluorescence of Cleomaceae floral nectar prompts further systematic, chemical, and ecological investigations into the UV-fluorescence of flowering plants.
Supplementary Information
Acknowledgements
The authors thank Rolf Vinebrooke for his thoughtful feedback on the manuscript, Jennifer Jones (Analytical and Instrumentation Laboratory, Department of Chemistry, University of Alberta) for their assistance with fluorescence spectroscopy, and Megan Ljubotina (Greenhouse Complex, Department of Biological Sciences, University of Alberta) for growing B. rapa. This research was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC) [funding reference number 5014131].
Author contributions
B.Z., K.M., and J.C.H. grew the Cleomaceae specimens. B.Z. and J.C.H. formally identified the plant material and deposited voucher specimens in the ALTA herbarium. B.Z. and K.M. measured the nectar volumes and collected nectar for photography and fluorescence spectroscopy. B.Z. and J.H.A. captured the photographs. B.Z. wrote the manuscript and created the figures with input from co-authors.
Data availability
All data generated during this study are included in this article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-62626-7.
References
- 1.Willmer P. Pollination and Floral Ecology. Princeton University Press; 2011. [Google Scholar]
- 2.Giurfa M, Núñez J, Chittka L, Menzel R. Colour preferences of flower-naive honeybees. J. Comp. Physiol. A. 1995;177:247–259. doi: 10.1007/BF00192415. [DOI] [Google Scholar]
- 3.Riffell JA, Lei H, Abrell L, Hildebrand JG. Neural basis of a pollinator’s buffet: olfactory specialization and learning in Manduca sexta. Science. 2013;339:200–204. doi: 10.1126/science.1225483. [DOI] [PubMed] [Google Scholar]
- 4.Kevan PG, Chittka L, Dyer AG. Limits to the salience of ultraviolet: lessons from colour vision in bees and birds. J. Exp. Biol. 2001;204:2571–2580. doi: 10.1242/jeb.204.14.2571. [DOI] [PubMed] [Google Scholar]
- 5.Gerl EJ, Morris MR. The causes and consequences of color vision. Evol.: Educ. Outreach. 2008;1:476–486. [Google Scholar]
- 6.Thompson WR, Meinwald J, Aneshansley D, Eisner T. Flavonols: pigments responsible for ultraviolet absorption in nectar guide of flower. Science. 1972;177:528–530. doi: 10.1126/science.177.4048.528. [DOI] [PubMed] [Google Scholar]
- 7.Koski MH, Ashman T-L. Dissecting pollinator responses to a ubiquitous ultraviolet floral pattern in the wild. Funct. Ecol. 2014;28:868–877. doi: 10.1111/1365-2435.12242. [DOI] [Google Scholar]
- 8.Silberglied RE. Communication in the ultraviolet. Annu. Rev. Ecol. Syst. 1979;10:373–398. doi: 10.1146/annurev.es.10.110179.002105. [DOI] [Google Scholar]
- 9.Olson ER, et al. Vivid biofluorescence discovered in the nocturnal Springhare (Pedetidae) Sci. Rep. 2021;11:1–8. doi: 10.1038/s41598-021-83588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohler AM, Olson ER, Martin JG, Anich PS. Ultraviolet fluorescence discovered in New World flying squirrels (Glaucomys) J. Mammal. 2019;100:21–30. doi: 10.1093/jmammal/gyy177. [DOI] [Google Scholar]
- 11.Gruber DF, et al. Biofluorescence in catsharks (Scyliorhinidae): fundamental description and relevance for elasmobranch visual ecology. Sci. Rep. 2016;6:24751. doi: 10.1038/srep24751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox JL, Fitzpatrick BM. Biofluorescent sexual dimorphism revealed in a southern Appalachian endemic salamander, Plethodon metcalfi. Sci. Rep. 2023;13:3588. doi: 10.1038/s41598-023-29051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goutte S, et al. Intense bone fluorescence reveals hidden patterns in pumpkin toadlets. Sci. Rep. 2019;9:5388. doi: 10.1038/s41598-019-41959-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prötzel D, et al. Widespread bone-based fluorescence in chameleons. Sci. Rep. 2018;8:698. doi: 10.1038/s41598-017-19070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnold KE, Owens IPF, Marshall NJ. Fluorescent signaling in parrots. Science. 2002;295:92. doi: 10.1126/science.295.5552.92. [DOI] [PubMed] [Google Scholar]
- 16.Mazel CH, Cronin TW, Caldwell RL, Marshall NJ. Fluorescent enhancement of signaling in a mantis shrimp. Science. 2004;303:51. doi: 10.1126/science.1089803. [DOI] [PubMed] [Google Scholar]
- 17.Lim MLM, Land MF, Li D. Sex-specific UV and fluorescence signals in jumping spiders. Science. 2007;315:481. doi: 10.1126/science.1134254. [DOI] [PubMed] [Google Scholar]
- 18.Thorp RW, Briggs DL, Estes JR, Erickson EH. Nectar fluorescence under ultraviolet irradiation. Science. 1975;189:476–478. doi: 10.1126/science.189.4201.476. [DOI] [PubMed] [Google Scholar]
- 19.Scogin R. Nectar constituents in the genus Fremontia (Sterculiaceae): sugars, flavonoids, and proteins. Bot. Gaz. 1979;140:29–31. doi: 10.1086/337054. [DOI] [Google Scholar]
- 20.Scogin R. 5, 7-Dimethoxy-4’-hydroxyisoflavone from Fremontia (Sterculiaceae) nectar. Aliso: J. Syst. Florist. Bot. 1979;9:479–480. doi: 10.5642/aliso.19790903.06. [DOI] [Google Scholar]
- 21.Scogin R. Reproductve phytochemistry of Bombacaceae: Floral anthocyanins and nectar constituents. Aliso: J. Syst. Florist. Bot. 1986;11:377–385. doi: 10.5642/aliso.19861103.09. [DOI] [Google Scholar]
- 22.Roshchina VV, Melnikova EV, Mit’kovskaya LV, Karnaukhov VN. Microspectrofluorimetry for the study of intact plant secreting cells. J. Gen. Biol. 1998;59:531–553. [Google Scholar]
- 23.Roshchina VV. Autofluorescence of plant secreting cells as a biosensor and bioindicator reaction. J. Fluorescence. 2003;13:403–420. doi: 10.1023/A:1026164922760. [DOI] [Google Scholar]
- 24.Kevan PG. Fluorescent nectar. Science. 1976;194:341–342. doi: 10.1126/science.194.4262.341. [DOI] [PubMed] [Google Scholar]
- 25.Iriel A, Lagorio MG. Is the flower fluorescence relevant in biocommunication? Naturwissenschaften. 2010;97:915–924. doi: 10.1007/s00114-010-0709-4. [DOI] [PubMed] [Google Scholar]
- 26.Kurup R, et al. Fluorescent prey traps in carnivorous plants. Plant. Biol. 2013;15:611–615. doi: 10.1111/j.1438-8677.2012.00709.x. [DOI] [PubMed] [Google Scholar]
- 27.Mori S, et al. Biocommunication between plants and pollinating insects through fluorescence of pollen and anthers. J. Chem. Ecol. 2018;44:591–600. doi: 10.1007/s10886-018-0958-9. [DOI] [PubMed] [Google Scholar]
- 28.Mori S, Hasegawa Y, Moriguchi Y. Color strategies of camellias recruiting different pollinators. Phytochem. 2023;207:113559. doi: 10.1016/j.phytochem.2022.113559. [DOI] [PubMed] [Google Scholar]
- 29.Radice S, Galati BG. Floral nectary ultrastructure of Prunus persica (L.) Batch cv. Forastero (Newcomer), an Argentine peach. Plant Syst. Evol. 2003;238:23–32. doi: 10.1007/s00606-002-0279-9. [DOI] [Google Scholar]
- 30.Nakanishi T. Morphological and ultraviolet absorption differences between fertile and sterile anthers of Japanese apricot cultivars in relation to their pollination stimuli. Sci. Hortic. 1982;18:57–63. doi: 10.1016/0304-4238(82)90103-0. [DOI] [Google Scholar]
- 31.Bayat S, Schranz ME, Roalson EH, Hall JC. Lessons from Cleomaceae, the sister of crucifers. Trends Plant Sci. 2018;23:808–821. doi: 10.1016/j.tplants.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Zenchyzen B, et al. Comparative nectary morphology across Cleomaceae (Brassicales) Plants. 2023;12:1263. doi: 10.3390/plants12061263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neto RLS, Thomas WW, Roalson EH, Barbosa MRV. Taxonomic revision of Tarenaya (Cleomaceae) Ann. Mo. Bot. Gard. 2022;107:250–313. doi: 10.3417/2022705. [DOI] [Google Scholar]
- 34.Carey S, Zenchyzen B, Deneka AJ, Hall JC. Nectary development in Cleome violacea. Front. Plant Sci. 2023;13:1085900. doi: 10.3389/fpls.2022.1085900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lunau K, et al. Nectar mimicry: a new phenomenon. Sci. Rep. 2020;10:7039. doi: 10.1038/s41598-020-63997-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marshall J, Johnsen S. Fluorescence as a means of colour signal enhancement. Philos. Trans. R. Soc. B Biol. Sci. 2017;372:20160335. doi: 10.1098/rstb.2016.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis KL, Stpiczyńska M, Gregg A. Nectar-secreting floral stomata in Maxillaria anceps Ames & C. Schweinf. (Orchidaceae) Ann. Bot. 2005;96:217–227. doi: 10.1093/aob/mci182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris PJ, Trethewey JAK. The distribution of ester-linked ferulic acid in the cell walls of angiosperms. Phytochem. Rev. 2010;9:19–33. doi: 10.1007/s11101-009-9146-4. [DOI] [Google Scholar]
- 39.Baby S, et al. UV induced visual cues in grasses. Sci. Rep. 2013;3:2738. doi: 10.1038/srep02738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicolson SW, Thornburg RW. Nectaries and Nectar. Springer; 2007. pp. 215–264. [Google Scholar]
- 41.Roy R, Schmitt AJ, Thomas JB, Carter CJ. Review: nectar biology: From molecules to ecosystems. Plant Sci. 2017;262:148–164. doi: 10.1016/j.plantsci.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 42.Hansen DM, Olesen JM, Mione T, Johnson SD, Müller CB. Coloured nectar: distribution, ecology, and evolution of an enigmatic floral trait. Biol. Rev. 2007;82:83–111. doi: 10.1111/j.1469-185X.2006.00005.x. [DOI] [PubMed] [Google Scholar]
- 43.Parachnowitsch AL, Manson JS, Sletvold N. Evolutionary ecology of nectar. Ann. Bot. 2019;123:247–261. doi: 10.1093/aob/mcy132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raguso RA. Why are some floral nectars scented? Ecology. 2004;85:1486–1494. doi: 10.1890/03-0410. [DOI] [Google Scholar]
- 45.Cai X-H, et al. Mystery revisited: is nocturnal colored nectar a nonadaptive floral trait? Ecology. 2022;103:e3663. doi: 10.1002/ecy.3663. [DOI] [PubMed] [Google Scholar]
- 46.Palmer-Young EC, et al. Chemistry of floral rewards: intra- and interspecific variability of nectar and pollen secondary metabolites across taxa. Ecol. Monogr. 2019;89:e01335. doi: 10.1002/ecm.1335. [DOI] [Google Scholar]
- 47.Gismondi A, et al. From Robinia pseudoacacia L. nectar to Acacia monofloral honey: biochemical changes and variation of biological properties. J. Sci. Food Agric. 2018;98:4312–4322. doi: 10.1002/jsfa.8957. [DOI] [PubMed] [Google Scholar]
- 48.Gao Y, et al. Analysis of chemical composition of nectars and honeys from Citrus by extractive electrospray ionization high resolution mass spectrometry. LWT. 2020;131:109748. doi: 10.1016/j.lwt.2020.109748. [DOI] [Google Scholar]
- 49.Nešović M, et al. Polyphenol profile of buckwheat honey, nectar and pollen. R. Soc. Open Sci. 2020;7:201576. doi: 10.1098/rsos.201576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fotirić Akšić M, et al. The morpho-anatomy of nectaries and chemical composition of nectar in pear cultivars with different susceptibility to Erwinia amlylovora. Horticulturae. 2023;9:424. doi: 10.3390/horticulturae9040424. [DOI] [Google Scholar]
- 51.Dyer AG, Neumeyer C. Simultaneous and successive colour discrimination in the honeybee (Apis mellifera) J. Comp. Physiol. A. 2005;191:547–557. doi: 10.1007/s00359-005-0622-z. [DOI] [PubMed] [Google Scholar]
- 52.Peitsch D, et al. The spectral input systems of hymenopteran insects and their receptor-based colour vision. J. Comp. Physiol. A. 1992;170:23–40. doi: 10.1007/BF00190398. [DOI] [PubMed] [Google Scholar]
- 53.Morawetz L, Svoboda A, Spaethe J, Dyer AG. Blue colour preference in honeybees distracts visual attention for learning closed shapes. J. Comp. Physiol. A. 2013;199:817–827. doi: 10.1007/s00359-013-0843-5. [DOI] [PubMed] [Google Scholar]
- 54.Higuera-Díaz M, Manson JS, Hall JC. Pollination biology of Cleomella serrulata and Polanisia dodecandra in a protected natural prairie in southern Alberta, Canada. Botany. 2015;93:745–757. doi: 10.1139/cjb-2015-0084. [DOI] [Google Scholar]
- 55.Saroop S, Kaul V. Tritrophism in mellitophylic Cleome viscosa L. Genet. Resour. Crop Evol. 2019;66:1367–1370. doi: 10.1007/s10722-019-00798-2. [DOI] [Google Scholar]
- 56.Grant V, Grant KA. Records of hummingbird pollination in the western American flora: II. additional California records. Aliso: J. Syst. Florist. Bot. 1967;6:103–105. doi: 10.5642/aliso.19670603.05. [DOI] [Google Scholar]
- 57.Machado IC, Lopes AV, Leite AV, Neves CB. Cleome spinosa (Capparaceae): polygamodioecy and pollination by bats in urban and Caatinga areas, northeastern Brazil. Bot. Jahrb. Syst. Pflanzengesch. Pflanzengeogr. 2006;127:69–82. doi: 10.1127/0006-8152/2006/0127-0069. [DOI] [Google Scholar]
- 58.Fleming TH, Geiselman C, Kress WJ. The evolution of bat pollination: a phylogenetic perspective. Ann. Bot. 2009;104:1017–1043. doi: 10.1093/aob/mcp197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gonzalez-Terrazas TP, et al. Finding flowers in the dark: nectar-feeding bats integrate olfaction and echolocation while foraging for nectar. R. Soc. Open Sci. 2016;3:160199. doi: 10.1098/rsos.160199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Domingos-Melo A, Milet-Pinheiro P, Navarro DMAF, Lopes AV, Machado IC. It’s raining fragrant nectar in the Caatinga: evidence of nectar olfactory signaling in bat-pollinated flowers. Ecology. 2020;101:e02914. doi: 10.1002/ecy.2914. [DOI] [PubMed] [Google Scholar]
- 61.Winter Y, López J, von Helversen O. Ultraviolet vision in a bat. Nature. 2003;425:612–614. doi: 10.1038/nature01971. [DOI] [PubMed] [Google Scholar]
- 62.Domingos-Melo A, et al. Shining bright in the dusk: how do bat-pollinated flowers reflect light? Ecology. 2021;102:e03416. doi: 10.1002/ecy.3416. [DOI] [PubMed] [Google Scholar]
- 63.Martins DJ, Johnson SD. Interactions between hawkmoths and flowering plants in East Africa: polyphagy and evolutionary specialization in an ecological context. Biol. J. Linn. Soc. 2013;110:199–213. doi: 10.1111/bij.12107. [DOI] [Google Scholar]
- 64.Travouillon KJ, Cooper C, Bouzin JT, Umbrello LS, Lewis SW. All-a-glow: spectral characteristics confirm widespread fluorescence for mammals. R. Soc. Open Sci. 2023;10:230325. doi: 10.1098/rsos.230325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lamb JY, Davis MP. Salamanders and other amphibians are aglow with biofluorescence. Sci. Rep. 2020;10:2821. doi: 10.1038/s41598-020-59528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anich PS, et al. Biofluorescence in the platypus (Ornithorhynchus anatinus) Mammalia. 2021;85:179–181. doi: 10.1515/mammalia-2020-0027. [DOI] [Google Scholar]
- 67.Angiosperm Phylogeny Group et al. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Biol. J. Linn. Soc.181, 1–20 (2016).
- 68.Egan PA, et al. Crop domestication alters floral reward chemistry with potential consequences for pollinator health. Front. Plant Sci. 2018;9:1357. doi: 10.3389/fpls.2018.01357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated during this study are included in this article.