ABSTRACT
Influenza A viruses pose a significant threat to global health, impacting both humans and animals. Zoonotic transmission, particularly from swine and avian species, is the primary source of human influenza outbreaks. Notably, avian influenza viruses of the H5N1, H7N9, and H9N2 subtypes are of pandemic concern through their global spread and sporadic human infections. Preventing and controlling these viruses is critical due to their high threat level. Vaccination remains the most effective strategy for influenza prevention and control in humans, despite varying vaccine efficacy across strains. This review focuses specifically on pandemic preparedness for avian influenza viruses. We delve into vaccines tested in animal models and summarize clinical trials conducted on H5N1, H7N9, and H9N2 vaccines in humans.
KEYWORDS: Vaccines, vaccine development, avian influenza, pandemic preparedness, pandemic viruses, influenza a viruses, wild birds
Introduction
Influenza A viruses (FLUAVs) are members of the Orthomyxoviridae family characterized by a segmented, single-stranded, negative-sense RNA genome enveloped in pleomorphic particles.1 The antigenic diversity of these viruses arises from two surface glycoproteins: hemagglutinin (HA) and neuraminidase (NA). Combinations of these proteins create numerous subtypes; currently, 19 HA and 9 NA subtypes are recognized, with 17 HA and 9 NA subtypes identified in birds alone.2,3 Among these, H5Nx, H7Nx, and H9N2 subtypes have achieved global spread in poultry.4–6 Beyond classification, HA and NA play crucial roles in viral replication. The trimeric HA protein mediates attachment to host cells by recognizing sialic acid receptors, making it the primary target for neutralizing antibodies.7 The tetrameric NA cleaves sialic acid linkages, facilitating the release of newly formed virions from infected cells.8
FLUAVs are found in wild birds, especially waterfowl and seabirds, which serve as natural reservoirs.9 Spillover events, particularly due to contact between wild and domestic birds, can lead to severe poultry outbreaks.10 Based on the HA protein’s molecular signatures and disease severity in chickens, FLUAVs are classified as high pathogenic avian influenza (HPAIV) or low pathogenic avian influenza (LPAIV) viruses.11 HPAIVs possess a polybasic cleavage site in HA, allowing cleavage by ubiquitous proteases, leading to systemic infection and high morbidity/mortality.12 Conversely, LPAIVs possess a monobasic site only recognized by extracellular trypsin-like proteases, causing mild disease with variable morbidity but low mortality.13
While H1N1 and H3N2 FLUAVs cause seasonal human epidemics, viruses circulating in animals pose a zoonotic and pandemic threat, as evidenced by reported human cases of avian H5N1, H7N9, and H9N2 subtypes.14–17 Vaccines are the primary defense against influenza in humans, and most infections can be prevented through vaccination. This underscores the need to develop vaccines for improved pandemic preparedness against avian influenza viruses.18
This review summarizes the importance of avian influenza, its pandemic potential, and control strategies focused on vaccination for enhanced pandemic preparedness and management. We primarily discuss vaccines tested in mice, ferrets, and non-human primates as established animal models for influenza research,19 and additionally, we address clinical trials using avian influenza vaccines in humans.
Avian influenza: a closer look at a complex threat
While avian influenza viruses of the H5N1, H7N9, and H9N2 subtypes remain prevalent in poultry worldwide, sporadic human infections have highlighted their public health concern. We primarily discuss these three widely prevalent subtypes. However, we should not underestimate the significance of other zoonotic avian influenza subtypes such as H3N8, H6Nx, and H10Nx viruses.20
In 1996, the strain A/Goose/Guangdong/1996 (H5N1) was initially detected in Southern China when several outbreaks were reported associated with mortality and neurological dysfunction in domestic waterfowl.21 This highly pathogenic strain has since claimed the lives of millions of birds and remains the ancestor of current H5 viruses circulating in Asia, Europe, Africa, and, more recently, The Americas.22 Since 1997, there have been around 900 H5N1 human cases worldwide with a case fatality rate (CFR) exceeding 50% (Figure 1), demonstrating the dual threat to animal and human health.23 While sustained human-to-human transmission has not been observed, the severity of the disease highlights the need for continued vigilance and robust public health measures.24 H5 viruses have undergone continuous evolution over time, branching out into multiple genetic clades. Among these, clade 2.3.4.4.b has become predominant globally, particularly in Asia, Africa, Europe, and the Middle East.25 This clade poses a significant threat due to its ability to jump from poultry to mammals, including raccoons, cats, red foxes, bears, minks, and sea mammals.26
Figure 1.
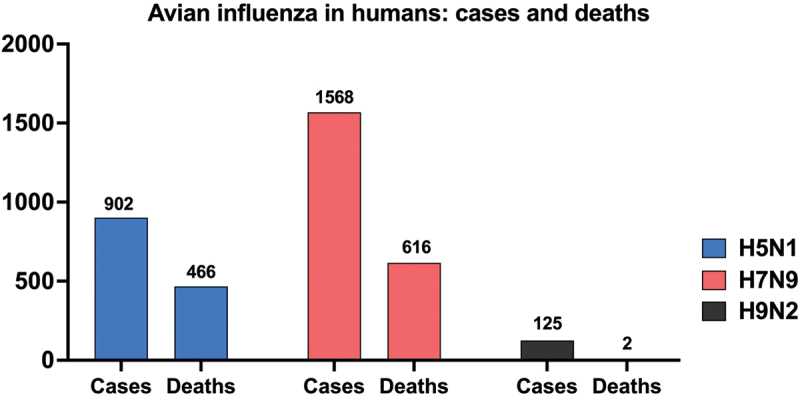
Cumulative global cases and deaths of human infections with H5N1, H7N9, and H9N2 avian influenza viruses reported to the World Health Organization (WHO). Cases and deaths are cumulative from the first reported case of each virus subtype in humans until February 22, 2024. Numbers on top of the bars indicate how many cases and deaths have been reported to the WHO. Note: as of February 22, 2024, two deaths have been reported from H9N2 infections according to the WHO. Due to the limited scale of the figure, these deaths are not visually represented, but the number is included in the graph.
Among H7Nx viruses, H7N9 is the one most frequently identified in human infections.27 First detected in China in 2013, H7N9 caused severe respiratory illness and fatalities in humans.28 Since 2013, there have been over 1500 human cases reported globally with a CFR of approximately 40% (Figure 1). While human cases have been linked to close contact with poultry, and sustained human-to-human transmission has not been observed,29 H7N9 human isolates readily transmit in ferrets.30 This alarmingly efficient transmission in ferrets highlights the potential for H7N9 to evolve into a pandemic influenza strain.28
H9N2 viruses remain enzootic among poultry species in Asia, the Middle East, and parts of Africa.31 Though classified as LPAIVs, H9N2 strains are notorious for donating internal gene segments to other influenza subtypes, playing a key role in the emergence of pandemic threats such as H5Nx, H7N9, and H10Nx .32–37 Worryingly, there has been sufficient evidence of interspecies transmission of H9N2 from poultry to mammalian species.38 This combination of high prevalence in poultry and the ability to infect mammals raises concerns about H9N2‘s potential to trigger a pandemic.39 Since 1998, over 100 cases of H9N2 infection in humans have been documented, resulting in two deaths (Figure 1). Close contact with poultry has been identified as the primary risk factor.40 The most recent case was reported on February 23, 2024, in a 22-month-old girl with relatively mild disease.41 No evidence of sustained human-to-human transmission exists so far (Figure 2a).42 However, due to the zoonotic potential, the World Health Organization (WHO) considers H9N2 a strain of pandemic concern.43
Figure 2.
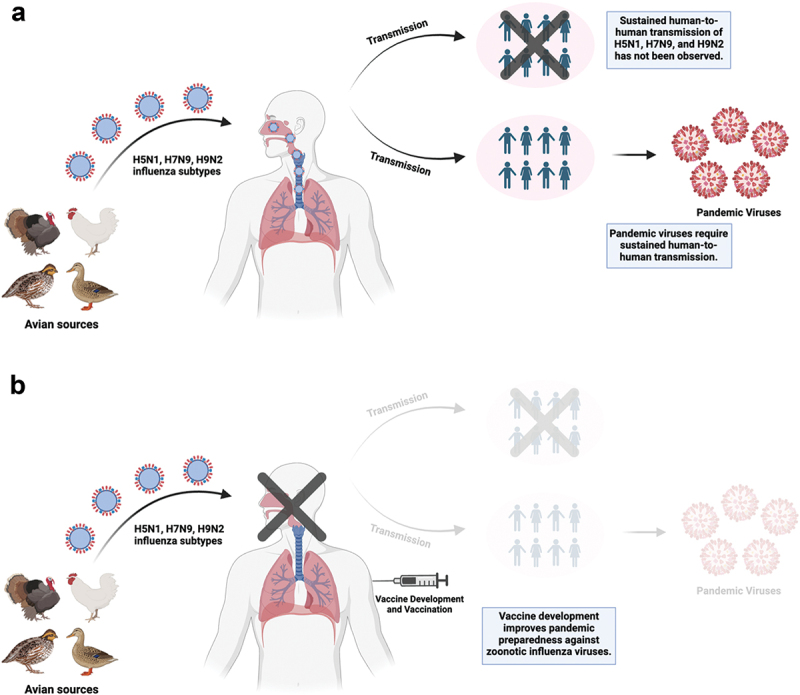
(a) H5N1, H7N9, and H9N2 influenza subtypes from avian sources can infect humans and cause disease. Sustained human-to-human transmission of these subtypes has not been observed but is required to the emergence of pandemic viruses which could further spread within the human population. (b) vaccine development and vaccination of humans can prevent infections and the generation of pandemic viruses, improving pandemic preparedness and management against avian influenza viruses. This figure was created with BioRender.com.
Protecting against potential avian influenza pandemics: a vaccine approach
The local innate immune response is the first line of defense against influenza viruses, with alveolar macrophages, natural killer cells, and neutrophils playing critical roles in activating the adaptive immune system, limiting viral spread, and reducing disease severity.44 Most current influenza vaccines primarily target the HA protein, the primary immunogen of influenza viruses.45,46 While these vaccines are effective in inducing antibody responses against the HA protein, they often overlook other arms of the immune system, limiting their ability to offer complete protection.47 A truly ideal influenza vaccine should therefore stimulate the innate immune responses and the humoral and cellular arms of the adaptive immune response.48 This means inducing not only virus-specific antibodies against the HA protein but also activating CD4+ T lymphocytes and CD8+ T cytotoxic lymphocytes.49 These T cells help clear the virus and provide broader, cross-protective immunity against different influenza strains.50
Inactivated influenza vaccines come in various forms, including whole virus, split virus, subunit vaccines, and others. Briefly, inactivated whole virus vaccines are produced using pathogen-free embryonated chicken eggs. The viruses are then inactivated using formaldehyde or β-propiolactone.50 In split virus vaccines, the virus envelope is disrupted using diethyl ether or detergent treatment, exposing all viral proteins.47 Subunit vaccines are made using additional purification steps to separate the nucleocapsid and lipids from the surface proteins HA and NA.50,51 These vaccines dominate the influenza vaccine landscape in humans for several reasons: their low production costs, safety profile, and effectiveness.50 Split virus and subunit vaccines are a mainstay in seasonal vaccines for humans, thanks to their ability to induce strong antibody responses after vaccination.51 However, despite their wide use, high antibody levels alone may not be enough to guarantee complete protection against influenza. This is because inactivated vaccines often trigger an incomplete immune response, lacking robust T-cell and mucosal immunity.52 Live attenuated influenza vaccines (LAIVs), on the other hand, mimic a natural infection and can therefore stimulate cell-mediated and mucosal immunity, especially by increasing immunoglobulin A (IgA) levels in the respiratory tract, leading to a broader and more durable immune response.49 Several types of LAIVs exist, including cold-adapted, protein truncations, and rearranged-genome vaccines. Most LAIVs discussed in this review consist of reassortant viruses carrying the internal genes from cold-adapted, temperature-sensitive mutations of A/Ann Arbor/6/60 (a master donor virus) with the surface genes of the target virus (e.g., H5 HA and N1 NA).50 These mutations prevent the growth of these LAIVs at higher temperatures and restrict virus replication to the upper respiratory tract.53 However, these vaccines are not safe for immunocompromised individuals, and the potential for vaccine strain reversion and recombination with circulating strains is a concern.50
Influenza viruses present a major challenge for vaccine development, not only in humans but also in other animal species. The influenza virus polymerase complex lacks proofreading activity, resulting in a high mutation rate, and leading to a rapid accumulation of these mutations.47 This constant evolution in the HA and NA, called antigenic drift, allows the virus to escape from earlier immune responses.54 Furthermore, the ability of different influenza strains to reassort and exchange gene segments through antigenic shift adds another layer of complexity to vaccine development.55–57 Despite these challenges, vaccination remains the most powerful weapon for preventing and controlling influenza (Figure 2b).
To enhance pandemic preparedness, the WHO routinely selects strains derived from the animal reservoir as vaccine candidates and analyzes genetic sequences and the antigenic profiles of viruses from human cases (and related viruses from the animal reservoir). These vaccine candidates are selected based on relevance and/or incidence in poultry, past or current zoonotic infections, and antigenic profiles. WHO analyses focus on understanding the genetic and antigenic characteristics of these viruses, allowing for informed vaccine development. The latest report on the Northern Hemisphere influenza seasons 2024–2025 summarizes the available candidate vaccine viruses for various zoonotic influenza subtypes and lineages (Table 1).
Table 1.
Available candidate vaccine viruses for pandemic preparedness against the H5N1, H7N9, and H9N2 subtypes for the northern hemisphere influenza seasons 2024–2025.
| Candidate vaccine virus | Antigenic prototype | Subtype | Clade | Institution responsible |
|---|---|---|---|---|
| SJRG-161052 | A/Vietnam/1203/2004 | H5N1 | 1 | SJCRH, CDC |
| IDCDC-RG34B | A/Cambodia/X0810301/2013 | H5N1 | 1.1.1 | CDC |
| SJRG-166614 | A/duck/Hunan/795/2002 | H5N1 | 2.1 | SJCRH |
| IDCDC-RG2 | A/Indonesia/5/2005 | H5N1 | 2.1.3.2 | CDC |
| IBCDC-RG7 | A/chicken/India/NIV33487/2006 | H5N1 | 2.2 | CDC |
| IDCDC-RG11 | A/Egypt/2321-NAMRU3/2007 | H5N1 | 2.2.1 | CDC |
| IDCDC-RG13 | A/Egypt/3300-NAMRU3/2008 | H5N1 | 2.2.1.1 | CDC |
| IDCDC-RG30 | A/Hubei/1/2010 | H5N1 | 2.3.2.1a | CDC |
| SJ0009 | A/chicken/Guiyang/1153/2016 | H5N1 | 2.3.2.1d | SJCRH |
| IDCDC-RG6 | A/Anhui/1/2005 | H5N1 | 2.3.4 | CDC |
| IDCDC-RG35 | A/Guizhou/1/2013 | H5N1 | 2.3.4.2 | CDC |
| SJRG-165396 | A/goose/Guiyang/337/2006 | H5N1 | 4 | SJCRH |
| IDCDC-RG25A | A/chicken/Vietnam/NCVD-016/2008 | H5N1 | 7.1 | CDC |
| IDCDC-RG63A | A/duck/Bangladesh/17D1012/2018 | H5N1 | 2.3.2.1a | CDC |
| IDCDC-RG78A | A/American wigeon/South Carolina/22000345-001/2021-like | H5N1 | 2.3.4.4b | CDC |
| NIID-002 | A/Ezo red fox/Hokkaido/1/2022-like | H5N1 | 2.3.4.4b | NIID, Japan |
| IDCDC-RG56N | A/Guangdong/17SF003/2016 | H7N9 | n/a* | CDC |
| IDCDC-RG56B | A/Hong Kong/125/2017 | H7N9 | n/a* | CDC |
| IDCDC-RG32A | A/Shanghai/2/2013 | H7N9 | n/a* | CDC |
| IDCDC-RG33A | A/Anhui/1/2013 | H7N9 | n/a* | CDC |
| IDCDC-RG64A | A/Gansu/23277/2019-like | H7N9 | n/a* | CDC |
| IBCDC-2 | A/chicken/Hong Kong/G9/97 | H9N2 | Y280/G9 | CDC |
| IBCDC-RG26 | A/Hong Kong/33982/2009 | H9N2 | G1 | CDC |
| IDCDC-RG31 | A/Bangladesh/994/2011 | H9N2 | G1 | CDC |
| SJ008 | A/Hong Kong/308/2014 | H9N2 | Y280/G9 | SJCRH |
| IDCDC-RG61A | A/Anhui-Lujiang/39/2018 | H9N2 | Y280/G9 | CDC |
| IDCDC-RG66A | A/Oman/2747/2019 | H9N2 | G1 | CDC |
Only one candidate vaccine virus per clade is shown for H5N1 viruses, except clade 2.3.4.4b. * n/a: not available. CDC: Centers for Disease Control and Prevention; SJCRH: St. Jude Children’s Research Hospital; NIID: National Institute of Infectious Diseases, Japan.
Due to ongoing public health concerns and the zoonotic potential of avian influenza viruses, this discussion will focus on analyzing and summarizing the data on H5N1, H7N9, and H9N2 influenza vaccines for humans. We will specifically examine inactivated vaccines, LAIVs, and alternative vaccine platforms that have been tested in animal models and clinical trials. This focus on multiple vaccine platforms reflects the ongoing search for the most effective and safe approach to protect against the pandemic potential posed by avian influenza viruses.
Exploring the potential of inactivated influenza vaccines through animal models
The animal data discussed below is summarized in Figure 3. Inactivated H5N1 vaccines demonstrated promising immunogenicity and protective efficacy in various animal models of relevance to humans.58 Mice vaccinated intramuscularly with an inactivated whole-virus H5N1 vaccine alone, or in combination with aluminum, were fully protected when given the combination which generated higher levels of neutralizing antibodies.58 In addition, intranasal administration with adjuvants like immune stimulating complexes (ISCOMs) and inmunair (INM) further enhanced systemic immune responses, including cell-mediated immunity.59–61 In ferrets, an inactivated whole virus H5N1 vaccine provided complete protection against mortality, even when challenged a year after vaccination.62 In addition, cynomolgus macaques vaccinated subcutaneously with a whole virus inactivated H5N1 vaccine developed antigen-specific immunoglobulin G (IgG) antibodies in plasma, nasal swabs, and tracheal swabs, leading to reduced viral propagation after challenge.63
Figure 3.
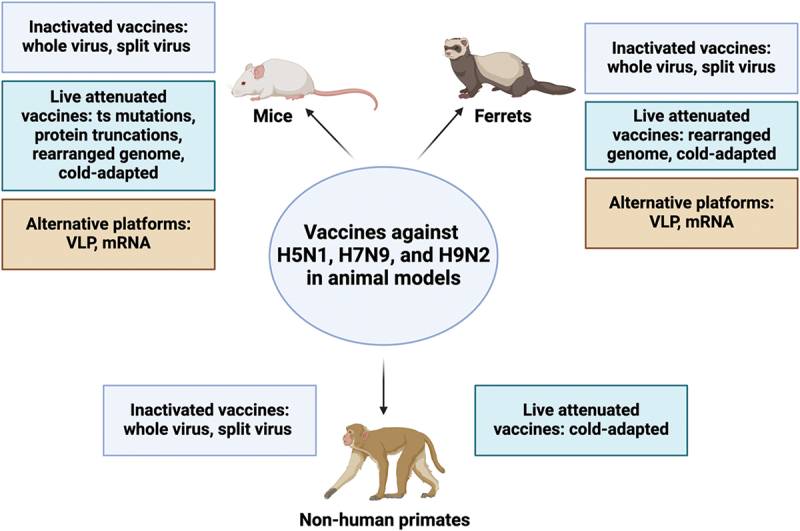
Summary of animal data for H5N1, H7N9, and H9N2 vaccines tested in mice, ferrets, and non-human primates. The figure categorizes vaccines by platform (inactivated, live attenuated, and alternative) and uses color-coded boxes for each category. It is important to note that only data from vaccines discussed in this review for each animal model are included. While other platforms exist, they are not discussed in detail within this manuscript. This figure was created with BioRender.com.
Mice vaccinated with a whole virus inactivated H7N9 vaccine via intramuscular injection showed distinct responses when administered either a single dose or two doses.64 While a single dose protected against lethal infection, the virus lingered in the lungs. However, two doses completely shielded the mice from both disease and detectable lung virus, coinciding with high IgG levels in their serum and lungs.64 Similarly, mice vaccinated with a single dose of the same vaccine with MF59 adjuvant exhibited specific IgM and IgG in the sera.65 The adjuvant enhanced antibody titers and reduced lung viral loads post-challenge.65 Ferrets vaccinated with a whole virus inactivated H7N9 vaccine, alone or with MF59 adjuvant, developed high levels of antibody titers as measured by the hemagglutination inhibition (HI) assay.66 Both vaccine combinations effectively protected against challenge. However, while no viral replication was detected in the lower respiratory tract or brain after the challenge, the virus persisted in the upper respiratory tracts of these ferrets.66 Similar results were observed with an H7N9 inactivated whole-virus vaccine.67 Importantly, vaccinated-contact ferrets still became infected when placed in direct contact with unvaccinated-infected ferrets, demonstrating that vaccination did not prevent transmission.67 In rhesus macaques immunized twice intramuscularly, inactivated whole-virus adjuvanted vaccines have also been demonstrated to be immunogenic and induce HI titers.68
Mice were intranasally vaccinated with an H9N2 inactivated whole-virus vaccine,69 with or without polyethyleneimine (PEI), a mucosal adjuvant.70 The combination of PEI and the inactivated vaccine enhanced antigen-specific IgA levels in the nasal cavity, trachea, and lung, as well as IgG levels in the serum. Additionally, the combination increased antigen uptake, improved cross-presentation, and enhanced dendritic cell maturation compared to the inactivated vaccine given alone.69 In a similar study, mice vaccinated intramuscularly twice with two inactivated whole-virus aluminum hydroxide adjuvanted vaccines against Y280 or Y439 H9N2 lineages were fully protected upon H9N2 homologous challenge, but only partially protected after a heterologous challenge.71 Similarly, cynomolgus macaques vaccinated subcutaneously with a formalin-inactivated H9N2 whole-virus vaccine developed antigen-specific IgG and neutralizing antibodies, which correlated with a reduction in viral titers after challenge.72
Researchers have also explored the potential of split vaccines for avian influenza. Studies in mice have consistently demonstrated that these vaccines elicit a strong immune response after vaccination, as evidenced by high HI titers.73 Ferrets vaccinated intramuscularly with a split virus oil-in-water adjuvanted H5N1 vaccine showed increased cellular immunity in lungs and peripheral blood, promoting earlier viral clearance, particularly through interferon-gamma (IFN-γ)+ CD8− T cells in the airways compared to mock vaccination.74 In macaques, adjuvanted split virus H5N1 vaccines induced high immune responses and protected against homologous H5N1 challenge.75 Similarly, mice and ferrets received intramuscular injections twice with an H7N9 split vaccine.76 In both species, HI and IgG titers increased after prime, and a boost significantly enhanced IgG titers. Notably, vaccinated mice survived the lethal challenge, regained weight quickly, and showed decreased lung virus titers compared to controls.76 Another similar study found that ferrets vaccinated with a split virion H7N9 vaccine, both alone and with adjuvant system 03 (AS03) adjuvant, developed heterologous antibody titers against H7N7 and H7N3 viruses and were protected from the homologous virus, with no detectable virus in the lungs after challenge.77
Preclinical evaluation of live attenuated influenza vaccines in animal models
The advent of reverse genetics has been instrumental in the development of alternative LAIVs.78 Employing this technique, researchers used an avian influenza virus backbone engineered with temperature-sensitive mutations in its internal polymerase basic 2 (PB2) and polymerase basic 1 (PB1) genes, along with the HA and NA from an H5N1 virus.79 Mice were vaccinated intranasally with varying doses and were protected against a lethal H5N1 challenge. Notably, a booster dose significantly reduced viral replication and accelerated virus clearance compared to prime-only.79 Non-structural protein 1 (NS1) and matrix protein (M) truncations have emerged as promising candidates for H5N1 vaccine development. C-terminal truncation of the NS1 protein, combined with mutations in PB2 for increased attenuation in mammals, resulted in an attenuated replication phenotype in a mouse model.80 Mice vaccinated intranasally with this H5N1 vaccine were fully protected from death upon lethal challenge.80 Similarly, deletions in the M2 protein’s C-terminus also led to an attenuated phenotype in mice.81 Intranasal vaccination with the truncated H5N1 M2 protein completely protected mice against lethal homologous and heterologous H5N1 challenges. Notably, vaccinated mice exhibited significantly higher IgG and IgA levels in the trachea and lungs compared to controls.81
LAIVs with rearranged genomes have emerged as promising candidates for influenza vaccines.82 Researchers successfully rearranged the H9N2 virus genome by expressing the H5 open reading frame (ORF) from the NS gene segment. This rearranged virus, administered intranasally in mice and ferrets, protected against both H5N1 and H9N2 viruses.83 Similarly, another vaccine featured the H5 gene segment flanked by NA (H9) packaging signals at the 3’ and 5’ ends, while the H9 HA was still expressed from segment 4.84 Intranasal vaccination of mice with this candidate vaccine induced a robust antibody response against both subtypes, strong proinflammatory cytokine response, and robust IFN-γ production. Notably, vaccinated mice were fully protected against both subtypes when challenged with the homologous viruses.84 These studies demonstrate the potential of rearranged genomes as effective vaccine platforms for influenza.
A cold-adapted H5N1 vaccine was used to vaccinate mice intranasally.85 Overall, a single immunization induced HI and neutralizing antibody titers, and IgA antibodies in the respiratory tract which protected mice against lethal challenge.85 In a similar study, a cold-adapted H5N1 vaccine induced IFN-γ-secretion and interleukin 4 (IL-4) secretion by the mouse splenocytes, protecting against lethal challenge.53 In ferrets vaccinated intranasally, 4 weeks apart, a similar approach fully protected against virus replication of homologous and heterologous H5N1 viruses.86 Furthermore, in non-human primates, robust neutralizing antibody responses and HA-specific CD4+ T cell responses were observed when rhesus macaques were immunized intranasally with an H5N1 cold-adapted vaccine.87 Moreover, vaccinated macaques were fully protected from antigenically identical and drifted H5N1 viruses.87
Cold-adapted vaccines have also shown promise for the H7N9 influenza subtype. Studies in mice have demonstrated their effectiveness.88 A single intranasal dose offered protection against disease and death but allowed replication of a heterologous challenge virus in the respiratory tract.88 However, two doses provided complete protection, with minimal viral shedding observed after challenge. This protection is likely due to enhanced mucosal antibody responses, particularly IgA, and IFN-y- and IL-4-producing T cells, as found in separate studies.89 Intranasal vaccination with two doses of two separate H7N9 cold-adapted vaccines proved safe and immunogenic in ferrets.90 Both vaccines induced at least a 4-fold or greater increase in HI titers against antigenically identical and drifted H7N9 viruses, indicating broad protection. They also triggered serum IgG and upper respiratory tract IgA responses.90 Intriguingly, a single dose of an H7N9 cold-adapted vaccine given intranasally even prevented transmission from vaccinated to naive ferrets in direct contact.91
Cold-adapted LAIVs have shown promise in the mouse model for H9N2 influenza virus.92 This vaccine candidate administered intranasally in mice showed high HI titers against the homologous H9N2 virus, but low HI titers against heterologous viruses, suggesting limited cross-protection against other influenza strains. Upon challenge, vaccinated mice were significantly less susceptible to infection compared to the control group.92 These cold-adapted H9N2 vaccines generated high antibody titers against the homologous virus in ferrets and African green monkeys.92,93 Additionally, vaccinated monkeys were completely protected from the challenge virus in their respiratory tracts, demonstrating the vaccine’s efficacy in a non-human primate model.93 These findings suggest that cold-adapted vaccines are a promising strategy for the control of avian influenza. Their ability to induce both protective antibodies and T-cell responses, along with their potential to prevent transmission, highlights their potential impact on future vaccine development.
From animal models to human trials: evaluating safety and immunogenicity of avian influenza vaccines
Clinical trials have been extensively conducted to evaluate vaccines against avian influenza viruses. The results of these trials are summarized in Table 2. A phase 1 randomized trial in healthy adults demonstrated that an adjuvanted whole-virus H5N1 vaccine was safe and well-tolerated, with the most common side effects being pain at the site of vaccination and fever.94 The study also found the vaccine to be immunogenic, with two doses generating high levels of neutralizing antibodies. Supporting this finding, a separate study showed that a single dose of a non-adjuvanted whole-virus H5N1 vaccine generated a seroconversion rate (SCR) exceeding 90%.95 Phase 2/3 trials with inactivated whole-virus adjuvanted H5N1 vaccines in adults demonstrated good tolerability with mild adverse effects, and antibody titers greater than 1:40 after vaccination indicated protection.96 For H7N9, an alum-adjuvanted H7N9 whole virus inactivated vaccine was safe and immunogenic in healthy adults, with two doses inducing an HI titer above 40 in 98.2% of participants.97 Researchers have also evaluated inactivated whole-virus H9N2 influenza vaccines in clinical trials.98 A phase 1/2 randomized study assessed a nonadjuvanted, whole-virus H9N2 vaccine in healthy adults. The vaccine was found to be safe and well-tolerated, and 52.8% to 88.9% of participants developed antibody levels predictive of protection, indicating immunogenicity.98 Another phase 1 randomized study compared whole virus and subunit H9N2 vaccines in adults. Both vaccines were well-tolerated, and antibody levels were similar between the two groups.99
Table 2.
Summary of clinical trials for H5N1, H7N9, and H9N2 avian influenza vaccines.
| Subtype | Vaccine Description | Doses (Ag) | Phase | Outcomes | Trial ID | Reference |
|---|---|---|---|---|---|---|
| H5N1 | Inactivated whole virus adjuvanted (aluminum hydroxide) | 1.25, 2.5, 5, 10, 15, and 30 μg | 1, 2/3 | Safe and well-tolerated; two doses generated high levels of neutralizing antibodies |
NCT00356798 NCT02612909 |
94 96 |
| H7N9 | Inactivated whole virus adjuvanted (aluminum hydroxide) | 7.5, 15, and 30 μg | 1/2 | Safe and immunogenic; two doses induced an HI titer above 40 in 98.2% of participants | NCT03369808 | 97 |
| H9N2 | Inactivated whole virus nonadjuvanted | 3.75, 7.5, 15, 30, and 45 µg | 1/2 | Safe and well-tolerated; 52.8% to 88.9% of participants developed antibody levels predictive of protection | NCT01320696 | 98 |
| H5N1* | Split virus | 90 µg is approved. Tested: 7.5, 15, and 30 µg |
1/2, 3 | Safe and well-tolerated in all age groups; elicited neutralizing and HI titers; licensed in the US |
NCT00457509 NCT00415129 |
102 103 105 |
| H5N1* | Split virus adjuvanted (AS03) | 3.75 µg is approved. | 1/2, 3, 4 | Safe and well-tolerated; high neutralizing seroconversion rates; elicited robust antibody responses against homologous and heterologous strains; licensed in the US |
NCT00510874 NCT00616928 NCT01730378 NCT01788228 NCT01310413 |
107 108 109 |
| H5N1* | Subunit vaccine adjuvanted (MF59) | 7.5 µg is approved. Tested: 3.75 µg |
1/2, 3, 4 | Safe and well-tolerated; high seroconversion rates, elicited neutralizing antibodies and HI titers; licensed in the US |
NCT01776554 NCT02839330 NCT00812019 NCT01776541 |
111 112 113 114 |
| H7N9 | Subunit vaccine adjuvanted | 3.75, 7.5, and 15 µg | 1 | Safe; adjuvant was necessary to increase HI titers |
NCT01928472 NCT03682120 |
n/a |
| H9N2 | Subunit vaccine adjuvanted |
3.75, 7.5, 15, and 30 µg | 1 | Safe and well-tolerated; prime induced protective levels of antibodies | NCT00133471 | 99 |
| H7N9 | Split virus adjuvanted (oil-in-water IB160 or SE) | 3.75, 7.5, and 15 µg | 1 | Safe and well-tolerated; adjuvanted formulations elicited a significantly greater increase in antibody titers | NCT03330899 | 115 |
| H7N9 | Split virus adjuvanted (AS03) | 2.78, 3.75, and 5.08 µg | 1, 2 | Enhanced immune responses and good tolerability were observed compared to unadjuvanted or alum-adjuvanted formulations |
NCT01999842 NCT03318315 |
116 117 |
| H9N2 | Split virus adjuvanted (AS03) | 1.9, 3.75, and 15 µg | 1/2 | Safe and well-tolerated; the adjuvanted vaccine generated a seroconversion rate of ≥ 98.1%, both higher than the unadjuvanted vaccine | NCT01659086 | 118 |
| H5N1 | Live attenuated cold-adapted | 106.7 and 107.5 TCID50 | 1/2 | Safe, restricted in replication; IgA and IgG were detected by ELISA in the serum |
NCT00347672 NCT00488046 |
121 |
| H7N9 | Live attenuated cold-adapted | 107.5 TCID50 | 1 | Safe and well-tolerated; increase in neutralizing antibodies, serum IgG and IgA, mucosal IgA, CD4+, and CD8+ virus-specific T cells |
NCT02480101 NCT03739229 NCT01995695 NCT02274545 |
125 126 127 |
| H9N2 | Live attenuated cold-adapted | 107 and 2 × 107 TCID50 | 1 | Well-tolerated, exhibited an attenuated phenotype; Following two doses, 92% of participants showed greater than a 4-fold increase in HI titers, with 79% having a similar increase in neutralizing antibody titers | NCT00110279 | 128 |
| H5N1 | Virus-like particle | 5, 10, and 20 µg | 1, 2 | Well-tolerated; nearly 96% of individuals in the higher dose groups developed neutralizing antibody responses |
NCT00984945 NCT01991561 |
140 147 |
| H7N9 | Virus-like particle | 5, 15, and 30 µg | 1 | Well-tolerated; robust HI and neutralizing antibody responses | NCT01897701 | 141 |
| H7N9 | mRNA | 10, 25, and 50 µg | 1 | Safe and well-tolerated; 100% of participants seroconverted against the H7N9 strain. |
NCT03076385 NCT03345043 |
146 |
| H5N1 | Recombinant, protein-based adjuvanted (SE) Recombinant, protein-based adjuvanted (GLA/SE) |
3.8, 7.5 and 15 µg 3.8, 7.5, 15, 45, 135 µg |
1/2 1/2 |
Well-tolerated; 70% of participants receiving adjuvant seroconverted. Well-tolerated and immunogenic; GLA/SE improved serum antibody responses |
NCT01612000 NCT01147068 |
148 149 |
| H7N9 | Recombinant, protein-based adjuvanted (SE or MF59) | 7.5, 15, and 30 µg | 1/2 | Strong induction of anti-H7 antibodies; lacking neutralizing antibodies |
NCT02464163 NCT05608005 |
150 |
This table includes clinical trials, across all phases, with published results or results posted online. Trial IDs can be found at clinicaltrials.gov. * Vaccines that have been licensed for use in the United States by the United States Food and Drug Administration (FDA). Ag: antigen. AS03: adjuvant system 03; IB160: oil-in-water adjuvant emulsion; SE: stable emulsion; GLA/SE: glucopyranosyl lipid A/stable emulsion. n/a: a publication related to the clinical trial was not available.
While undergoing testing in both animal models and human trials, inactivated whole virus vaccines are known for their increased reactogenicity compared to other options like split virus or subunit vaccines.50 As a result, these latter types are preferred for humans due to their reduced tendency to cause side effects.100 The United States Food and Drug Administration (FDA) has licensed three H5N1 vaccines: two split virus vaccines and one subunit vaccine. These vaccines meet the FDA’s criteria for safety, purity, and potency. Specifically, they demonstrate sufficient immunogenicity, as measured by HI assay, with pre-defined success thresholds. The FDA requires that the lower limit of the 95% confidence interval for SCR must be at least 40% and that 70% or more of subjects must achieve an HI titer of at least 40 (seroprotection rate).101 Several studies analyzed the safety and immunogenicity of split-virus H5N1 vaccines in healthy adults.102,103 Overall, the vaccine was considered well-tolerated and induced an immune response, reflected by high levels of neutralizing antibodies detected after vaccination.102 Additionally, other studies found the vaccine to be well-tolerated and immunogenic in all age groups, including young and elderly adults and children, leading to its approval by the FDA in 2007.104–106 Similarly, phases 1/2, 3, and 4 studies have been conducted with a split virus AS03-adjuvanted H5N1 vaccine.107 In adults, the vaccine was immunogenic and elicited robust antibody responses against different strains.108,109 The AS03-adjuvanted H5N1 vaccine has been extensively studied in clinical trials across continents and various age groups (children, adults, and elderly), and induced broad and persistent immune responses with no reported safety concerns, leading to its approval by the FDA in 2013.107,108,110 In 2020, an MF59-adjuvanted, cell culture-derived H5N1 subunit vaccine was licensed in the United States.111 Overall, clinical trials demonstrated the vaccine’s safety and tolerability across various age groups. In addition, the vaccine also induced neutralizing antibodies and HI titers, resulting in high SCRs.112–114 Additional information on subunit vaccines for H7N9 and H9N2 is provided in Table 2. Research has also explored the safety and effectiveness of split virus H7N9 vaccines, both alone and with adjuvants, through clinical trials.115 A randomized phase 1 study investigated a split virus H7N9 vaccine alone and in combination with an oil-in-water adjuvant.115 As anticipated, adjuvanted formulations elicited a significantly greater increase in antibody titers. Notably, the vaccine was well-tolerated in healthy adults, with pain at the injection site and headache being the most frequent side effects.115 Similar findings emerged from studies using AS03 adjuvant, where enhanced immune responses and good tolerability were observed compared to unadjuvanted or alum-adjuvanted H7N9 vaccine formulations in healthy adults.97,116,117 A phase 1/2 study analyzed an H9N2 split-virus vaccine with and without an adjuvant (AS03).118,119 The adjuvanted vaccine generated protective levels of antibodies in 100% of individuals and a SCR of ≥98.1%, both higher than the unadjuvanted vaccine. Notably, the safety profile of both vaccines was acceptable.118,119 Overall, these clinical trials suggest that inactivated vaccines have potential for pandemic preparedness, although further research is needed to optimize their immunogenicity and duration of protection.
Two distinct approaches have been explored for developing H5N1 LAIVs in humans. A phase 1 study in healthy adults investigated the safety and immunogenicity of an H5N1 LAIV lacking the interferon antagonist NS1.120 Delivered intranasally, the vaccine was safe and well-tolerated, inducing significant antibody titers and local IgA responses.120 Furthermore, several studies tested cold-adapted H5N1 LAIVs in humans. A phase 1/2 trial demonstrated safety and immunogenicity after two intranasal doses, with robust neutralizing antibody and antigen-specific IgA responses.121 However, another trial showed no HI or neutralizing antibody responses, although IgA and IgG were detected by ELISA in the serum.122 Despite this, another study using a cold-adapted H5N1 vaccine found long-lasting memory responses and strong B- and T-cell immunity,123,124 highlighting potential advantages over inactivated vaccines.
A phase 1 trial in healthy adults evaluated the immunogenicity and safety of two doses of a cold-adapted H7N9 LAIV administered intranasally.125 No serious adverse effects were observed, and the vaccine was considered safe and well-tolerated. Importantly, the vaccine induced a robust immune response, as evidenced by the increase in levels of neutralizing antibodies, serum IgG and IgA, mucosal IgA, CD4+, and CD8+ virus-specific T cells.125 Supporting these findings, similar studies also reported increased mucosal and cell-mediated immunity upon vaccination with an H7N9 cold-adapted vaccine.126 Additionally, another H7N9 cold-adapted vaccine was safe and increased levels of H7-specific antibody-dependent cellular cytotoxicity mediating antibodies in healthy adults.127
In a human open-label study, healthy adults received an H9N2 cold-adapted vaccine via nasal drops.128 The vaccine was well-tolerated, exhibiting an attenuated phenotype in humans. Following two doses, 92% of participants showed greater than a 4-fold increase in HI titers, with 79% having a similar increase in neutralizing antibody titers. This suggests that two doses of the vaccine effectively induced immune responses in humans.
Beyond traditional approaches: exploring alternative vaccine platforms for H5N1, H7N9, and H9N2 avian influenza viruses
A major research effort is underway to develop a universal influenza vaccine. These vaccines target conserved viral epitopes, aiming to overcome the challenges posed by the highly mutable nature of influenza viruses.129 By targeting these conserved regions, the vaccines elicit cross-protective, broadly neutralizing immunity, offering protection against a wider range of influenza strains.130 Several vaccine platforms hold promise as a universal vaccine, including vectored, nucleic acid-based, protein-based, virus-like particle (VLP), and even LAIVs.50 While this discussion focuses on VLP and messenger RNA (mRNA) vaccines due to their current advancements in avian influenza research, it is crucial to acknowledge the potential of other platforms and not discount their contributions. Several of these platforms are listed in Table 2.
VLPs hold promise for influenza vaccines due to their ability to incorporate multiple HA subtypes into their envelopes, potentially inducing broader immunity against several subtypes and leading to more effective vaccines.131,132 Mice immunized intramuscularly with an H5N1 VLP expressing the HA, NA, and M1 exhibited stronger humoral and cellular immune responses, which translated to improved survival after a lethal challenge.133 A similar approach also conferred protection in mice, evidenced by decreased virus shedding and milder pulmonary lesions after H7N9 challenge.134 Similarly, mice and ferrets were vaccinated intramuscularly with a VLP expressing the HA, NA, and M of an H9N2 virus, with or without an adjuvant.135 The H9N2 VLP-induced antibody levels that correlated with reduced virus replication in both species after challenge.135 Notably, the adjuvant enhanced immunogenicity and protective efficacy.136 Another study explored the potential of VLPs co-expressing H5, H7, and H9 HAs.137 Ferrets immunized intranasally with these VLPs showed the highest levels of virus-neutralizing antibodies against the H9 subtype. Furthermore, vaccinated animals exhibited less weight loss and lower viral titers compared to the control group.137
Although extensively tested in humans for seasonal influenza viruses with proven safety and immunogenicity, including both humoral and cellular immune responses,138,139 VLPs have limited clinical trial data regarding their immunogenicity against avian influenza strains. A phase 1 study investigated the safety and immunogenicity of an alum-adjuvanted plant-derived H5N1 VLP vaccine in healthy adults.140 The vaccine was remarkably well-tolerated at all doses, and nearly 96% of individuals in the higher dose groups developed neutralizing antibody responses.140 Consistent with these findings, another phase 1 trial demonstrated that subjects vaccinated with an adjuvanted H7N9 VLP vaccine mounted robust HI and neutralizing antibody responses.141 Additionally, compared to the unadjuvanted group, these participants exhibited significantly higher total binding antibody levels and enhanced antibody affinity maturation.
The remarkable success of mRNA vaccines in tackling the COVID-19 pandemic has sparked their exploration for other infectious diseases, including influenza.142 An mRNA encapsulated in lipid nanoparticles (LNP) against clade 2.3.4.4b H5 viruses was developed and tested in mice and ferrets.143 The mRNA vaccine induced high levels of neutralizing antibodies and HA-specific CD8+ T cell responses in mice, and protected ferrets from lethal challenge with an H5N1 virus.143 Similarly, an mRNA-LNP H7N9 vaccine generated a rapid and strong immune response in mice and ferrets, and a single dose protected against lethal challenge and reduced virus replication in the lungs compared to mock.144 Another study explored an mRNA-LNP multi-antigen vaccine targeting three conserved influenza antigens (M2 ion channel, alpha helix of the HA stalk region, and the nucleoprotein).145 Administered intramuscularly, this H9N2 vaccine induced neutralizing antibodies and cross-reactive CD8+ T cells in mice, ultimately protecting them against an H9N2 virus challenge.145
While data on mRNA vaccines for avian influenza in humans is scarce, a phase 1 study has shed light on their potential.146 This study assessed the safety and immunogenicity of an H7N9 mRNA vaccine in healthy humans. The results were encouraging: the vaccine generated a robust protective immune response, adverse effects were mostly mild or moderate, and 100% of participants achieved seroconversion against the H7N9 strain.146 These early findings highlight the exciting potential of mRNA vaccines in the fight against influenza. Their ability to trigger diverse immune responses paves the way for developing universal influenza vaccines, a long-sought goal in public health.
Conclusion
The H5N1, H7N9, and H9N2 subtypes of avian influenza virus pose a dual threat, not only causing significant economic losses to the global poultry industry but also presenting a pressing public health concern due to documented spillover events and human cases. Vaccination remains the primary defense against the spread of these viruses. This review delves deep into the landscape of avian influenza vaccines for humans, exploring both established platforms and promising new directions. Inactivated and LAIVs have been the standard platforms, tested in animal models and humans. Inactivated vaccines, while safe and cost-effective, elicit primarily humoral immunity. LAIVs, on the other hand, induce a broader immune response, encompassing humoral, mucosal, and cell-mediated immunity. This makes them potentially more protective, despite concerns about their safety. More recently, promising alternatives such as VLPs and mRNA-LNP vaccines have emerged. Exploring and employing a diverse range of vaccine platforms is crucial for enhancing pandemic preparedness and mitigating the threat of avian influenza viruses.
Acknowledgments
We would like to express our sincere gratitude to all investigators who contributed to the articles reviewed here. We acknowledge and apologize to those whose valuable work could not be included due to space limitations.
Funding Statement
The work was supported by the National Institute of Allergy and Infectious Diseases National Institute of Food and Agriculture. Funding for this work includes grants, contracts, and subawards to D.R.P. including National Institute of Food and Agriculture (NIFA), U.S. Department of Agriculture (USDA) Grant award numbers 2020-67015-31539 and 2021-67015-33406, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH) Grant award number R21AI146448 and R01AI154894, Contract number 75N93021C00014 and Options 15A, 15B and 17A. Additional funds were provided to D.R.P. by the Georgia Research Alliance and the Caswell S Eidson Chair in Poultry Medicine endowment funds.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Dadonaite B, Gilbertson B, Knight ML, Trifkovic S, Rockman S, Laederach A, Brown LE, Fodor E, Bauer DLV.. The structure of the influenza a virus genome. Nat Microbiol. 2019;4(11):1781–14. doi: 10.1038/s41564-019-0513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krammer F, Smith GJD, Fouchier RAM, Peiris M, Kedzierska K, Doherty PC, Palese P, Shaw ML, Treanor J, Webster RG. et al. Influenza. Nat Rev Dis Primers. 2018;4(1):3. doi: 10.1038/s41572-018-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fereidouni S, Starick E, Karamendin K, Genova CD, Scott SD, Khan Y, Harder T, Kydyrmanov A. Genetic characterization of a new candidate hemagglutinin subtype of influenza a viruses. Emerg Microbes Infect. 2023;12(2):2225645. doi: 10.1080/22221751.2023.2225645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berhane Y, Hisanaga T, Kehler H, Neufeld J, Manning L, Argue C, Handel K, Hooper-McGrevy K, Jonas M, Robinson J. et al. Highly pathogenic avian influenza virus a (H7N3) in domestic poultry, Saskatchewan, Canada, 2007. Emerg Infect Dis. 2009;15(9):1492–5. doi: 10.3201/eid1509.080231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banet-Noach C, Perk S, Simanov L, Grebenyuk N, Rozenblut E, Pokamunski S, Pirak M, Tendler Y, Panshin A. H9N2 influenza viruses from Israeli poultry: a five-year outbreak. Avian Dis. 2007;51(s1):290–6. doi: 10.1637/7590-040206R1.1. [DOI] [PubMed] [Google Scholar]
- 6.Sonnberg S, Phommachanh P, Naipospos TS, McKenzie J, Chanthavisouk C, Pathammavong S, Darnell D, Meeduangchanh P, Rubrum AM, Souriya M. et al. Multiple introductions of avian influenza viruses (H5N1), laos, 2009–2010. Emerg Infect Dis. 2012;18(7):1139–43. doi: 10.3201/eid1807.111642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kosik I, Yewdell JW. Influenza hemagglutinin and neuraminidase: Yin–Yang Proteins coevolving to thwart immunity. Viruses. 2019;11(4):346. doi: 10.3390/v11040346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu C, Eichelberger MC, Compans RW, Air GM. Influenza type a virus neuraminidase does not play a role in viral entry, replication, assembly, or budding. J Virol. 1995;69(2):1099–106. doi: 10.1128/JVI.69.2.1099-1106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olsen B, Munster VJ, Wallensten A, Waldenstrom J, Osterhaus AD, Fouchier RA. Global patterns of influenza a virus in wild birds. Science. 2006;312(5772):384–8. doi: 10.1126/science.1122438. [DOI] [PubMed] [Google Scholar]
- 10.Normile D. Avian influenza. Evidence points to migratory birds in H5N1 spread. Science. 2006;311(5765):1225. doi: 10.1126/science.311.5765.1225. [DOI] [PubMed] [Google Scholar]
- 11.Spackman E. A brief introduction to avian influenza virus. Methods Mol Biol. 2020;2123:83–92. doi: 10.1007/978-1-0716-0346-8_7. [DOI] [PubMed] [Google Scholar]
- 12.Steinhauer DA. Role of hemagglutinin cleavage for the pathogenicity of influenza virus. Virology. 1999;258(1):1–20. doi: 10.1006/viro.1999.9716. [DOI] [PubMed] [Google Scholar]
- 13.Bosch FX, Garten W, Klenk HD, Rott R. Proteolytic cleavage of influenza virus hemagglutinins: primary structure of the connecting peptide between HA1 and HA2 determines proteolytic cleavability and pathogenicity of Avian influenza viruses. Virology. 1981;113(2):725–35. doi: 10.1016/0042-6822(81)90201-4. [DOI] [PubMed] [Google Scholar]
- 14.Zhang G, Xu L, Zhang J, Fang Q, Zeng J, Liu Y, Ke C. A H9N2 human case and surveillance of avian influenza viruses in live poultry markets — Huizhou City, Guangdong Province, China, 2021. China CDC Wkly. 2022;4(1):8–10. doi: 10.46234/ccdcw2021.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K. et al. Human infection with a novel avian-origin influenza a (H7N9) virus. N Engl J Med. 2013;368(20):1888–97. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 16.Gu W, Shi J, Cui P, Yan C, Zhang Y, Wang C, Zhang Y, Xing X, Zeng X, Liu L. et al. Novel H5N6 reassortants bearing the clade 2.3.4.4b HA gene of H5N8 virus have been detected in poultry and caused multiple human infections in China. Emerg Microbes Infect. 2022;11(1):1174–85. doi: 10.1080/22221751.2022.2063076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J. et al. Characterization of an avian influenza a (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279(5349):393–6. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 18.Kim H, Webster RG, Webby RJ. Influenza virus: dealing with a drifting and shifting pathogen. Viral Immunol. 2018;31:174–83. doi: 10.1089/vim.2017.0141. [DOI] [PubMed] [Google Scholar]
- 19.Caceres CJ, Seibert B, Cargnin Faccin F, Cardenas-Garcia S, Rajao DS, Perez DR. Influenza antivirals and animal models. FEBS Open Bio. 2022;12(6):1142–65. doi: 10.1002/2211-5463.13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qi L, Pujanauski LM, Davis AS, Schwartzman LM, Chertow DS, Baxter D, Scherler K, Hartshorn KL, Slemons RD, Walters K-A. et al. Contemporary avian influenza a virus subtype H1, H6, H7, H10, and H15 hemagglutinin genes encode a mammalian virulence factor similar to the 1918 pandemic virus H1 hemagglutinin. mBio. 2014;5(6):e02116. doi: 10.1128/mBio.02116-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu Z, Song Y, Zhou H, Xu X, Hu Q, Wu H, Zhang A, Zhou Y, Chen J, Dan H. et al. Avian influenza (H5N1) virus in waterfowl and chickens, central China. Emerg Infect Dis. 2007;13(5):772–5. doi: 10.3201/eid1305.061209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Vries E, Guo H, Dai M, Rottier PJ, van Kuppeveld FJ, de Haan CA. Rapid emergence of highly pathogenic avian influenza subtypes from a subtype H5N1 hemagglutinin variant. Emerg Infect Dis. 2015;21(5):842–6. doi: 10.3201/eid2105.141927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wan XF. Lessons from emergence of A/goose/Guangdong/1996-like H5N1 highly pathogenic avian influenza viruses and recent influenza surveillance efforts in southern China. Zoonoses Public Health. 2012;59(Suppl2):32–42. doi: 10.1111/j.1863-2378.2012.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruno A, Alfaro-Nunez A, de Mora D, Armas R, Olmedo M, Garces J, Garcia-Bereguiain MA. First case of human infection with highly pathogenic H5 avian influenza a virus in South America: a new zoonotic pandemic threat for 2023? J Travel Med. 2023;30(5). doi: 10.1093/jtm/taad032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Li Q, Li B, Guo Y, Xing J, Xu Q, Liu L, Zhang J, Qi W, Jia W. et al. Continuous reassortment of clade 2.3.4.4 H5N6 highly pathogenetic avian influenza viruses demonstrating high risk to public health. Pathogens. 2020;9(8):670. doi: 10.3390/pathogens9080670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imai M, Herfst S, Sorrell EM, Schrauwen EJ, Linster M, De Graaf M, Fouchier RAM, Kawaoka Y. Transmission of influenza A/H5N1 viruses in mammals. Virus Res. 2013;178(1):15–20. doi: 10.1016/j.virusres.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banks J, Speidel EC, McCauley JW, Alexander DJ. Phylogenetic analysis of H7 haemagglutinin subtype influenza a viruses. Arch Virol. 2000;145(5):1047–58. doi: 10.1007/s007050050695. [DOI] [PubMed] [Google Scholar]
- 28.Li C, Chen H. H7N9 Influenza Virus in China. Cold Spring Harb Perspect Med. 2021;11(8):a038349. doi: 10.1101/cshperspect.a038349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Wu P, Pei Y, Tsang TK, Gu D, Wang W, Zhang J, Horby PW, Uyeki TM, Cowling BJ. et al. Assessment of human-to-human transmissibility of avian influenza A(H7N9) virus across 5 waves by analyzing clusters of case patients in Mainland China, 2013–2017. Clin Infect Dis. 2019;68(4):623–31. doi: 10.1093/cid/ciy541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belser JA, Gustin KM, Pearce MB, Maines TR, Zeng H, Pappas C, Sun X, Carney PJ, Villanueva JM, Stevens J. et al. Pathogenesis and transmission of avian influenza a (H7N9) virus in ferrets and mice. Nature. 2013;501(7468):556–9. doi: 10.1038/nature12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peacock THP, James J, Sealy JE, Iqbal M. A global perspective on H9N2 avian influenza virus. Viruses. 2019;11(7):620. doi: 10.3390/v11070620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Niu S, Zhang B, Yang C, Zhou Z. WITHDRAWN: the whole genome analysis for the first human infection with H10N3 influenza virus in China. J Infect. 2021. doi: 10.1016/j.jinf.2021.06.021. [DOI] [PubMed] [Google Scholar]
- 33.Guan Y, Shortridge KF, Krauss S, Webster RG. Molecular characterization of H9N2 influenza viruses: were they the donors of the “internal” genes of H5N1 viruses in Hong Kong? Proc Natl Acad Sci USA. 1999;96:9363–7. doi: 10.1073/pnas.96.16.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li F, Liu J, Yang J, Sun H, Jiang Z, Wang C, Zhang X, Yu Y, Zhao C, Pu J. et al. H9N2 virus-derived M1 protein promotes H5N6 virus release in mammalian cells: mechanism of avian influenza virus inter-species infection in humans. PLOS Pathog. 2021;17(12):e1010098. doi: 10.1371/journal.ppat.1010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pu J, Wang S, Yin Y, Zhang G, Carter RA, Wang J, Xu G, Sun H, Wang M, Wen C. et al. Evolution of the H9N2 influenza genotype that facilitated the genesis of the novel H7N9 virus. Proc Natl Acad Sci USA. 2015;112(2):548–53. doi: 10.1073/pnas.1422456112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, Zhang J, Huang F, Zhang Y, Luo H, Zhang H. Complex reassortment of polymerase genes in Asian influenza a virus H7 and H9 subtypes. Infect Genet Evol. 2014;23:203–8. doi: 10.1016/j.meegid.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 37.Zhang M, Zhao C, Chen H, Teng Q, Jiang L, Feng D, Li X, Yuan S, Xu J, Zhang X. et al. Internal gene cassette from a human-origin H7N9 influenza virus promotes the pathogenicity of H9N2 avian influenza virus in mice. Front Microbiol. 2020;11:1441. doi: 10.3389/fmicb.2020.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu H, Hua RH, Wei TC, Zhou YJ, Tian ZJ, Li GX, Liu T-Q, Tong G-Z. Isolation and genetic characterization of avian origin H9N2 influenza viruses from pigs in China. Vet Microbiol. 2008;131(1–2):82–92. doi: 10.1016/j.vetmic.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 39.Zhang C, Xuan Y, Shan H, Yang H, Wang J, Wang K, Li G, Qiao J. Avian influenza virus H9N2 infections in farmed minks. Virol J. 2015;12(1):180. doi: 10.1186/s12985-015-0411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peiris M, Yuen KY, Leung CW, Chan KH, Ip PL, Lai RW, Orr WK, Shortridge KF. Human infection with influenza H9N2. The Lancet. 1999;354(9182):916–7. doi: 10.1016/s0140-6736(99)03311-5. [DOI] [PubMed] [Google Scholar]
- 41.The Government of the Hong Kong Special Administrative Region . CHP reports investigation progress of case of influenza a (H9) infection. 2024. https://www.info.gov.hk/gia/general/202402/23/P2024022300566.htm#:~:text=The%20Centre%20for%20Health%20Protection,Services%20Branch%20of%20the%20CHP.
- 42.Uyeki TM, Chong YH, Katz JM, Lim W, Ho YY, Wang SS, Tsang TH., Au, WWY, Chan, S.C., Rowe T, et al. Lack of evidence for human-to-human transmission of avian influenza a (H9N2) viruses in Hong Kong, China 1999. Emerg Infect Dis. 2002;8(2):154–9. doi: 10.3201/eid0802.010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alexander PE, De P, Rave S. Is H9N2 avian influenza virus a pandemic potential? Can J Infect Dis Med Microbiol. 2009;20:e35–6. doi: 10.1155/2009/578179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mifsud EJ, Kuba M, Barr IG. Innate immune responses to influenza virus infections in the upper respiratory tract. Viruses. 2021;13(10):2090. doi: 10.3390/v13102090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nichol KL, Treanor JJ. Vaccines for seasonal and pandemic influenza. J Infect Dis. 2006;194(2):S111–8. doi: 10.1086/507544. [DOI] [PubMed] [Google Scholar]
- 46.Shi J, Zeng X, Cui P, Yan C, Chen H. Alarming situation of emerging H5 and H7 avian influenza and effective control strategies. Emerg Microbes Infect. 2023;12(1):2155072. doi: 10.1080/22221751.2022.2155072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boivin S, Cusack S, Ruigrok RW, Hart DJ. Influenza a virus polymerase: structural insights into replication and host adaptation mechanisms. J Biol Chem. 2010;285:28411–7. doi: 10.1074/jbc.R110.117531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harvey AG, Graves AM, Uppalapati CK, Matthews SM, Rosenberg S, Parent EG, Fagerlie MH, Guinan J, Lopez BS, Kronstad LM. et al. Dendritic cell-natural killer cell cross-talk modulates T cell activation in response to influenza A viral infection. Front Immunol. 2022;13:1006998. doi: 10.3389/fimmu.2022.1006998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Janssens Y, Joye J, Waerlop G, Clement F, Leroux-Roels G, Leroux-Roels I. The role of cell-mediated immunity against influenza and its implications for vaccine evaluation. Front Immunol. 2022;13:959379. doi: 10.3389/fimmu.2022.959379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rajao DS, Perez DR. Universal vaccines and vaccine platforms to protect against influenza viruses in humans and agriculture. Front Microbiol. 2018;9:123. doi: 10.3389/fmicb.2018.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen J, Wang J, Zhang J, Ly H. Advances in development and application of influenza vaccines. Front Immunol. 2021;12:711997. doi: 10.3389/fimmu.2021.711997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lai L, Rouphael N, Xu Y, Sherman AC, Edupuganti S, Anderson EJ, Lankford-Turner P, Wang D, Keitel W, McNeal MM. et al. Baseline levels of influenza-specific B cells and T cell responses modulate human immune responses to swine variant influenza A/H3N2 vaccine. Vaccines. 2020;8(1):126. doi: 10.3390/vaccines8010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun W, Wang Z, Sun Y, Li D, Zhu M, Zhao M, Wang Y, Xu J, Kong Y, Li Y. et al. Safety, immunogenicity, and protective efficacy of an H5N1 chimeric cold-adapted attenuated virus vaccine in a mouse model. Viruses. 2021;13(12):2420. doi: 10.3390/v13122420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boni MF. Vaccination and antigenic drift in influenza. Vaccine. 2008;26(3):C8–14. doi: 10.1016/j.vaccine.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steel J. New strategies for the development of H5N1 subtype influenza vaccines: progress and challenges. BioDrugs. 2011;25(5):285–98. doi: 10.1007/BF03256169. [DOI] [PubMed] [Google Scholar]
- 56.Morens DM, Fauci AS. Emerging infectious diseases in 2012: 20 years after the institute of medicine report. mBio. 2012;3(6). doi: 10.1128/mBio.00494-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Claas EC, de Jong JC, van Beek R, Rimmelzwaan GF, Osterhaus AD. Human influenza virus A/HongKong/156/97 (H5N1) infection. Vaccine. 1998;16(9–10):977–8. doi: 10.1016/s0264-410x(98)00005-x. [DOI] [PubMed] [Google Scholar]
- 58.Lu X, Tumpey TM, Morken T, Zaki SR, Cox NJ, Katz JM. A mouse model for the evaluation of pathogenesis and immunity to influenza a (H5N1) viruses isolated from humans. J Virol. 1999;73(7):5903–11. doi: 10.1128/JVI.73.7.5903-5911.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suartha IN, Suartini GAA, Wirata IW, Dewi N, Putra GNN, Kencana GAY, Mahardika GN. Intranasal administration of inactivated avian influenza virus of H5N1 subtype vaccine-induced systemic immune response in chicken and mice. Vet World. 2018;11(2):221–6. doi: 10.14202/vetworld.2018.221-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sjolander A, van’t Land B, Lovgren Bengtsson K. Iscoms containing purified quillaja saponins upregulate both Th1-like and Th2-like immune responses. Cell Immunol. 1997;177:69–76. doi: 10.1006/cimm.1997.1088. [DOI] [PubMed] [Google Scholar]
- 61.Abel LC, Chen S, Ricca LG, Martins MF, Garcia M, Ananias RZ, Mussalem JS, Squaiella CC, Shaw RJ, Longo‐Maugéri IM. et al. Adjuvant effect of LPS and killed Propionibacterium acnes on the development of experimental gastrointestinal nematode infestation in sheep. Parasite Immunol. 2009;31(10):604–12. doi: 10.1111/j.1365-3024.2009.01132.x. [DOI] [PubMed] [Google Scholar]
- 62.Ducatez MF, Webb A, Crumpton JC, Webby RJ. Long-term vaccine-induced heterologous protection against H5N1 influenza viruses in the ferret model. Influenza other Respir Viruses. 2013;7(4):506–12. doi: 10.1111/j.1750-2659.2012.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakayama M, Shichinohe S, Itoh Y, Ishigaki H, Kitano M, Arikata M, Pham VL, Ishida H, Kitagawa N, Okamatsu M. et al. Protection against H5N1 highly pathogenic avian and pandemic (H1N1) 2009 influenza virus infection in cynomolgus monkeys by an inactivated H5N1 whole particle vaccine. PloS One. 2013;8(12):e82740. doi: 10.1371/journal.pone.0082740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seo SH, Kim HS. Inactivated antigen of the H7N9 influenza virus protects mice from its lethal infection. Viral Immunol. 2016;29(4):235–43. doi: 10.1089/vim.2015.0103. [DOI] [PubMed] [Google Scholar]
- 65.Chang H, Duan J, Zhou P, Su L, Zheng D, Zhang F, Fang F, Li X, Chen Z. Single immunization with MF59-adjuvanted inactivated whole-virion H7N9 influenza vaccine provides early protection against H7N9 virus challenge in mice. Microbes Infect. 2017;19(12):616–25. doi: 10.1016/j.micinf.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 66.Hatta M, Zhong G, Chiba S, Lopes TJS, Neumann G, Kawaoka Y. Effectiveness of whole, inactivated, low pathogenicity influenza A(H7N9) vaccine against antigenically distinct, highly pathogenic H7N9 virus. Emerg Infect Dis. 2018;24(10):1910–13. doi: 10.3201/eid2410.180403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wong SS, Jeevan T, Kercher L, Yoon SW, Petkova AM, Crumpton JC, Franks J, Debeauchamp J, Rubrum A, Seiler P. et al. A single dose of whole inactivated H7N9 influenza vaccine confers protection from severe disease but not infection in ferrets. Vaccine. 2014;32(35):4571–7. doi: 10.1016/j.vaccine.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pan W, Han L, Dong Z, Niu X, Li Z, Bao L, Li C, Luo Q, Yang Z, Li X. et al. Induction of neutralizing antibodies to influenza a virus H7N9 by inactivated whole virus in mice and nonhuman primates. Antiviral Res. 2014;107:1–5. doi: 10.1016/j.antiviral.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 69.Qin T, Yin Y, Huang L, Yu Q, Yang Q, Burns DL. H9N2 influenza whole inactivated virus combined with polyethyleneimine strongly enhances mucosal and systemic immunity after intranasal immunization in mice. Clin Vaccine Immunol. 2015;22(4):421–9. doi: 10.1128/CVI.00778-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wegmann F, Gartlan KH, Harandi AM, Brinckmann SA, Coccia M, Hillson WR, Kok WL, Cole S, Ho L-P, Lambe T. et al. Polyethyleneimine is a potent mucosal adjuvant for viral glycoprotein antigens. Nat Biotechnol. 2012;30(9):883–8. doi: 10.1038/nbt.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park SJ, Kang YM, Cho HK, Kim DY, Kim S, Bae Y, Kim J, Kim G, Lee Y-J, Kang H-M. et al. Cross-protective efficacy of inactivated whole influenza vaccines against Korean Y280 and Y439 lineage H9N2 viruses in mice. Vaccine. 2021;39(42):6213–20. doi: 10.1016/j.vaccine.2021.09.028. [DOI] [PubMed] [Google Scholar]
- 72.Nakayama M, Ozaki H, Itoh Y, Soda K, Ishigaki H, Okamatsu M, Sakoda Y, Park C-H, Tsuchiya H, Kida H. et al. Vaccination against H9N2 avian influenza virus reduces bronchus-associated lymphoid tissue formation in cynomolgus macaques after intranasal virus challenge infection. Pathol Int. 2016;66(12):678–86. doi: 10.1111/pin.12472. [DOI] [PubMed] [Google Scholar]
- 73.Miyaki C, Quintilio W, Miyaji EN, Botosso VF, Kubrusly FS, Santos FL, Iourtov D, Higashi HG, Raw I. Production of H5N1 (NIBRG-14) inactivated whole virus and split virion influenza vaccines and analysis of immunogenicity in mice using different adjuvant formulations. Vaccine. 2010;28(13):2505–9. doi: 10.1016/j.vaccine.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 74.Wong SS, Duan S, DeBeauchamp J, Zanin M, Kercher L, Sonnberg S, Fabrizio T, Jeevan T, Crumpton J-C, Oshansky C. et al. The immune correlates of protection for an avian influenza H5N1 vaccine in the ferret model using oil-in-water adjuvants. Sci Rep. 2017;7(1):44727. doi: 10.1038/srep44727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ruat C, Caillet C, Bidaut A, Simon J, Osterhaus AD. Vaccination of macaques with adjuvanted formalin-inactivated influenza a virus (H5N1) vaccines: protection against H5N1 challenge without disease enhancement. J Virol. 2008;82(5):2565–9. doi: 10.1128/JVI.01928-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duan Y, Gu H, Chen R, Zhao Z, Zhang L, Xing L, Lai C, Zhang P, Li Z, Zhang K. et al. Response of mice and ferrets to a monovalent influenza A (H7N9) split vaccine. PloS One. 2014;9(6):e99322. doi: 10.1371/journal.pone.0099322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stadlbauer D, Waal L, Beaulieu E, Strohmeier S, Kroeze E, Boutet P, Osterhaus ADME, Krammer F, Innis BL, Nachbagauer R. et al. AS03-adjuvanted H7N9 inactivated split virion vaccines induce cross-reactive and protective responses in ferrets. NPJ Vaccines. 2021;6(1):40. doi: 10.1038/s41541-021-00299-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nogales A, Martinez-Sobrido L. Reverse genetics approaches for the development of influenza vaccines. Int J Mol Sci. 2016;18(1):20. doi: 10.3390/ijms18010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hickman D, Hossain MJ, Song H, Araya Y, Solorzano A, Perez DR. An avian live attenuated master backbone for potential use in epidemic and pandemic influenza vaccines. J Gen Virol. 2008;89:2682–90. doi: 10.1099/vir.0.2008/004143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Steel J, Lowen AC, Pena L, Angel M, Solórzano A, Albrecht R, Perez DR, García-Sastre A, Palese P. Live attenuated influenza viruses containing NS1 truncations as vaccine candidates against H5N1 highly pathogenic avian influenza. J Virol. 2009;83(4):1742–53. doi: 10.1128/JVI.01920-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Watanabe T, Watanabe S, Kim JH, Hatta M, Kawaoka Y. Novel approach to the development of effective H5N1 influenza a virus vaccines: use of M2 cytoplasmic tail mutants. J Virol. 2008;82(5):2486–92. doi: 10.1128/JVI.01899-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Caceres CJ, Cardenas-Garcia S, Jain A, Gay LC, Carnaccini S, Seibert B, LM Ferreri, Geiger G, Jasinskas A, Nakajima R, et al. Development of a novel live attenuated influenza a virus vaccine encoding the IgA-inducing protein. Vaccines. 2021;9. doi: 10.3390/vaccines9070703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pena L, Sutton T, Chockalingam A, Kumar S, Angel M, Shao H, Chen H, Li W, Perez DR. Influenza viruses with rearranged genomes as live-attenuated vaccines. J Virol. 2013;87(9):5118–27. doi: 10.1128/JVI.02490-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ren W, Pei S, Jiang W, Zhao M, Jiang L, Liu H, Yi Y, Hui M, Li J. A replication-deficient H9N2 influenza virus carrying H5 hemagglutinin conferred protection against H9N2 and H5N1 influenza viruses in mice. Front Microbiol. 2022;13:1042916. doi: 10.3389/fmicb.2022.1042916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee YH, Jang YH, Seong BL. Cell-cultured, live attenuated, X-31ca-based H5N1 pre-pandemic influenza vaccine. Virology. 2017;504:73–8. doi: 10.1016/j.virol.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 86.Suguitan AL Jr., McAuliffe J, Mills KL, Jin H, Duke G, Lu B, Luke CJ, Murphy B, Swayne DE, Kemble G. et al. Live, attenuated influenza a H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med. 2006;3(9):e360. doi: 10.1371/journal.pmed.0030360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fan S, Gao Y, Shinya K, Li CK, Li Y, Shi J, Jiang Y, Suo Y, Tong T, Zhong G. et al. Immunogenicity and protective efficacy of a live attenuated H5N1 vaccine in nonhuman primates. PloS Pathog. 2009;5(5):e1000409. doi: 10.1371/journal.ppat.1000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang W, Yin X, Guan L, Li M, Ma S, Shi J, Deng G, Suzuki Y, Chen H. A live attenuated vaccine prevents replication and transmission of H7N9 highly pathogenic influenza viruses in mammals. Emerg Microbes Infect. 2018;7(1):1–0. doi: 10.1038/s41426-018-0154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang X, Zhao J, Wang C, Duan Y, Zhao Z, Chen R, Zhang L, Xing L, Lai C, Zhang S. et al. Immunization with a live attenuated H7N9 influenza vaccine protects mice against lethal challenge. PloS One. 2015;10(4):e0123659. doi: 10.1371/journal.pone.0123659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rudenko L, Kiseleva I, Krutikova E, Stepanova E, Isakova-Sivak I, Donina S, Rekstin A, Pisareva M, Bazhenova E, Kotomina T. et al. Two live attenuated vaccines against recent low–and highly pathogenic H7N9 influenza viruses are safe and immunogenic in ferrets. Vaccines. 2018;6(4):74. doi: 10.3390/vaccines6040074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kong H, Zhang Q, Gu C, Shi J, Deng G, Ma S, Liu J, Chen P, Guan Y, Jiang Y. et al. A live attenuated vaccine prevents replication and transmission of H7N9 virus in mammals. Sci Rep. 2015;5(1):11233. doi: 10.1038/srep11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen H, Matsuoka Y, Swayne D, Chen Q, Cox NJ, Murphy BR. et al. Generation and characterization of a cold-adapted influenza a H9N2 reassortant as a live pandemic influenza virus vaccine candidate. Vaccine. 2003;21(27–30):4430–6. doi: 10.1016/s0264-410x(03)00430-4. [DOI] [PubMed] [Google Scholar]
- 93.Matsuoka Y, Suguitan A Jr., Orandle M, Paskel M, Boonnak K, Gardner DJ, Feldmann F, Feldmann H, Marino M, Jin H. et al. African green monkeys recapitulate the clinical experience with replication of live attenuated pandemic influenza virus vaccine candidates. J Virol. 2014;88(14):8139–52. doi: 10.1128/JVI.00425-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin J, Zhang J, Dong X, Fang H, Chen J, Su N, Gao Q, Zhang Z, Liu Y, Wang Z. et al. Safety and immunogenicity of an inactivated adjuvanted whole-virion influenza a (H5N1) vaccine: a phase I randomised controlled trial. Lancet. 2006;368(9540):991–7. doi: 10.1016/S0140-6736(06)69294-5. [DOI] [PubMed] [Google Scholar]
- 95.van Boxtel RA, Verdijk P, de Boer OJ, van Riet E, Mensinga TT, Luytjes W. Safety and immunogenicity of influenza whole inactivated virus vaccines: a phase I randomized clinical trial. Hum Vaccin Immunother. 2015;11(4):983–90. doi: 10.1080/21645515.2015.1012004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Duong TN, Thiem VD, Anh DD, Cuong NP, Thang TC, Huong VM, Chien VC, Phuong NTL, Montomoli E, Holt R. et al. A phase 2/3 double blinded, randomized, placebo-controlled study in healthy adult participants in Vietnam to examine the safety and immunogenicity of an inactivated whole virion, alum adjuvanted, A(H5N1) influenza vaccine (IVACFLU-A/H5N1). Vaccine. 2020;38(6):1541–50. doi: 10.1016/j.vaccine.2019.11.059. [DOI] [PubMed] [Google Scholar]
- 97.Wang S, Xie Z, Huang L, Zhou X, Luo J, Yang Y, Li C, Duan P, Xu W, Chen D. et al. Safety and immunogenicity of an alum-adjuvanted whole-virion H7N9 influenza vaccine: a randomized, blinded, clinical trial. Clin Microbiol Infect. 2020;27(5):775–81. doi: 10.1016/j.cmi.2020.07.033. [DOI] [PubMed] [Google Scholar]
- 98.Aichinger G, Grohmann-Izay B, van der Velden MV, Fritsch S, Koska M, Portsmouth D, Hart MK, El-Amin W, Kistner O, Barrett PN. et al. Phase I/II randomized double-blind study of the safety and immunogenicity of a nonadjuvanted vero cell culture-derived whole-virus H9N2 influenza vaccine in healthy adults. Clin Vaccine Immunol. 2015;22(1):46–55. doi: 10.1128/CVI.00275-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stephenson I, Nicholson KG, Gluck R, Mischler R, Newman RW, Palache AM, Verlander NQ, Warburton F, Wood JM, Zambon MC. et al. Safety and antigenicity of whole virus and subunit influenza A/Hong Kong/1073/99 (H9N2) vaccine in healthy adults: phase I randomised trial. Lancet. 2003;362(9400):1959–66. doi: 10.1016/S0140-6736(03)15014-3. [DOI] [PubMed] [Google Scholar]
- 100.Beyer WE, Nauta JJ, Palache AM, Giezeman KM, Osterhaus AD. Immunogenicity and safety of inactivated influenza vaccines in primed populations: a systematic literature review and meta-analysis. Vaccine. 2011;29(34):5785–92. doi: 10.1016/j.vaccine.2011.05.040. [DOI] [PubMed] [Google Scholar]
- 101.FDA . Clinical data needed to support the licensure of pandemic influenza vaccines. Center for Biologics Evaluation and Research (CBER). 2007. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-data-needed-support-licensure-pandemic-influenza-vaccines.
- 102.Bresson JL, Perronne C, Launay O, Gerdil C, Saville M, Wood J, Höschler K, Zambon MC. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet. 2006;367(9523):1657–64. doi: 10.1016/S0140-6736(06)68656-X. [DOI] [PubMed] [Google Scholar]
- 103.Levie K, Leroux-Roels I, Hoppenbrouwers K, Kervyn AD, Vandermeulen C, Forgus S, Leroux‐Roels G, Pichon S, Kusters I. An adjuvanted, low-dose, pandemic influenza a (H5N1) vaccine candidate is safe, immunogenic, and induces cross-reactive immune responses in healthy adults. J Infect Dis. 2008;198(5):642–9. doi: 10.1086/590913. [DOI] [PubMed] [Google Scholar]
- 104.Leroux-Roels I, Van der Wielen M, Kafeja F, Vandermeulen C, Lazarus R, Snape MD, John T, Carre C, Nougarede N, Pepin S. et al. Humoral and cellular immune responses to split-virion H5N1 influenza vaccine in young and elderly adults. Vaccine. 2009;27(49):6918–25. doi: 10.1016/j.vaccine.2009.08.110. [DOI] [PubMed] [Google Scholar]
- 105.Lazarus R, Kelly S, Snape MD, Vandermeulen C, Voysey M, Hoppenbrouwers K, Hens A, Van Damme P, Pepin S, Leroux-Roels I. et al. Antibody persistence and booster responses to split-virion H5N1 avian influenza vaccine in young and elderly adults. PLOS ONE. 2016;11(11):e0165384. doi: 10.1371/journal.pone.0165384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chotpitayasunondh T, Thisyakorn U, Pancharoen C, Pepin S, Nougarede N. Safety, humoral and cell mediated immune responses to two formulations of an inactivated, split-virion influenza A/H5N1 vaccine in children. PLOS ONE. 2008;3(12):e4028. doi: 10.1371/journal.pone.0004028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Langley JM, Frenette L, Ferguson L, Riff D, Sheldon E, Risi G, Johnson C, Li P, Kenney R, Innis B. et al. Safety and cross-reactive immunogenicity of candidate AS03-adjuvanted prepandemic H5N1 influenza vaccines: a randomized controlled phase 1/2 trial in adults. J Infect Dis. 2010;201(11):1644–53. doi: 10.1086/652701. [DOI] [PubMed] [Google Scholar]
- 108.Langley JM, Risi G, Caldwell M, Gilderman L, Berwald B, Fogarty C, Poling T, Riff D, Baron M, Frenette L. et al. Dose-sparing H5N1 A/Indonesia/05/2005 pre-pandemic influenza vaccine in adults and elderly adults: a phase III, placebo-controlled, randomized study. J Infect Dis. 2011;203(12):1729–38. doi: 10.1093/infdis/jir172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Izurieta P, Kim WJ, Wie SH, Lee J, Lee JS, Drame M, Vaughn DW, Schuind A. Immunogenicity and safety of an AS03-adjuvanted H5N1 pandemic influenza vaccine in Korean adults: a phase IV, randomized, open-label, controlled study. Vaccine. 2015;33(24):2800–7. doi: 10.1016/j.vaccine.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 110.Diez-Domingo J, Garces-Sanchez M, Baldo JM, Planelles MV, Ubeda I, JuBert A, Marés J, Moris P, Garcia-Corbeira P, Dramé M. et al. Immunogenicity and safety of H5N1 A/Vietnam/1194/2004 (Clade 1) AS03-adjuvanted prepandemic candidate influenza vaccines in children aged 3 to 9 years: a phase ii, randomized, open, controlled study. Pediatr Infect Dis J. 2010;29(6):e35–46. doi: 10.1097/INF.0b013e3181daf921. [DOI] [PubMed] [Google Scholar]
- 111.Chanthavanich P, Anderson E, Kerdpanich P, Bulitta M, Kanesa-Thasan N, Hohenboken M. Safety, tolerability and immunogenicity of an MF59-adjuvanted, Cell Culture-derived, A/H5N1, subunit influenza virus vaccine: results from a Dose-finding Clinical Trial in healthy pediatric subjects. Pediatr Infect Dis J. 2019;38:757–64. doi: 10.1097/INF.0000000000002345. [DOI] [PubMed] [Google Scholar]
- 112.Peterson J, Van Twuijver E, Versage E, Hohenboken M. Phase 3 randomized, multicenter, placebo-controlled study to evaluate safety, immunogenicity, and lot-to-lot consistency of an adjuvanted cell culture-derived, H5N1 subunit influenza virus vaccine in healthy adult subjects. Vaccines. 2022;10(4):497. doi: 10.3390/vaccines10040497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Frey SS, Versage E, Van Twuijver E, Hohenboken M. Antibody responses against heterologous H5N1 strains for an MF59-adjuvanted cell culture–derived H5N1 (aH5n1c) influenza vaccine in adults and older adults. Hum Vaccin Immunother. 2023;19(1):2193119. doi: 10.1080/21645515.2023.2193119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Frey SE, Shakib S, Chanthavanich P, Richmond P, Smith T, Tantawichien T, Kittel C, Jaehnig P, Mojares Z, Verma B. et al. Safety and Immunogenicity of MF59-Adjuvanted Cell Culture–derived A/H5N1 subunit influenza virus vaccine: Dose-Finding Clinical Trials in adults and the elderly. Open Forum Infect Dis. 2019;6(4):ofz107. doi: 10.1093/ofid/ofz107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vanni T, Thome BC, Sparrow E, Friede M, Fox CB, Beckmann AM, Huynh C, Mondini G, Silveira DH, Viscondi JYK. et al. Dose-sparing effect of two adjuvant formulations with a pandemic influenza A/H7N9 vaccine: a randomized, double-blind, placebo-controlled, phase 1 clinical trial. PLOS ONE. 2022;17(10):e0274943. doi: 10.1371/journal.pone.0274943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ortiz JR, Spearman PW, Goepfert PA, Cross K, Buddy Creech C, Chen WH, Parker S, Overton ET, Dickey M, Logan HL. et al. Safety and immunogenicity of monovalent H7N9 influenza vaccine with AS03 adjuvant given sequentially or simultaneously with a seasonal influenza vaccine: a randomized clinical trial. Vaccine. 2022;40(23):3253–62. doi: 10.1016/j.vaccine.2022.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Madan A, Segall N, Ferguson M, Frenette L, Kroll R, Friel D, Soni J, Li P, Innis BL, Schuind A. Immunogenicity and safety of an AS03-adjuvanted H7N9 pandemic influenza vaccine in a randomized trial in healthy adults. J Infect Dis. 2016;214(11):1717–27. doi: 10.1093/infdis/jiw414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Madan A, Collins H, Sheldon E, Frenette L, Chu L, Friel D, Drame M, Vaughn DW, Innis BL, Schuind A. et al. Evaluation of a primary course of H9N2 vaccine with or without AS03 adjuvant in adults: a phase I/II randomized trial. Vaccine. 2017;35(35):4621–8. doi: 10.1016/j.vaccine.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 119.Atmar RL, Keitel WA, Patel SM, Katz JM, She D, El Sahly H, Pompey J, Cate TR, Couch RB. Safety and immunogenicity of nonadjuvanted and MF59-adjuvanted influenza A/H9N2 vaccine preparations. Clin Infect Dis. 2006;43(9):1135–42. doi: 10.1086/508174. [DOI] [PubMed] [Google Scholar]
- 120.Nicolodi C, Groiss F, Kiselev O, Wolschek M, Seipelt J, Muster T. Safety and immunogenicity of a replication-deficient H5N1 influenza virus vaccine lacking NS1. Vaccine. 2019;37(28):3722–9. doi: 10.1016/j.vaccine.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 121.Rudenko L, Desheva J, Korovkin S, Mironov A, Rekstin A, Grigorieva E, Donina S, Gambaryan A, Katlinsky A. Safety and immunogenicity of live attenuated influenza reassortant H5 vaccine (phase I–II clinical trials). Influenza Other Respir Viruses. 2008;2(6):203–9. doi: 10.1111/j.1750-2659.2008.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Karron RA, Talaat K, Luke C, Callahan K, Thumar B, Dilorenzo S, McAuliffe J, Schappell E, Suguitan A, Mills K. et al. Evaluation of two live attenuated cold-adapted H5N1 influenza virus vaccines in healthy adults. Vaccine. 2009;27(36):4953–60. doi: 10.1016/j.vaccine.2009.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pitisuttithum P, Boonnak K, Chamnanchanunt S, Puthavathana P, Luvira V, Lerdsamran H, Kaewkungwal J, Lawpoolsri S, Thanachartwet V, Silachamroon U. et al. Safety and immunogenicity of a live attenuated influenza H5 candidate vaccine strain A/17/turkey/Turkey/05/133 H5N2 and its priming effects for potential pre-pandemic use: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2017;17(8):833–42. doi: 10.1016/S1473-3099(17)30240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Romanova J, Krenn BM, Wolschek M, Ferko B, Romanovskaja-Romanko E, Morokutti A, Shurygina A-P, Nakowitsch S, Ruthsatz T, Kiefmann B. et al. Preclinical Evaluation of a Replication-Deficient Intranasal ΔNS1 H5N1 Influenza Vaccine. PLOS ONE. 2009;4(6):e5984. doi: 10.1371/journal.pone.0005984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rudenko L, Isakova-Sivak I, Naykhin A, Kiseleva I, Stukova M, Erofeeva M, Korenkov D, Matyushenko V, Sparrow E, Kieny M-P. et al. H7N9 live attenuated influenza vaccine in healthy adults: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect Dis. 2016;16(3):303–10. doi: 10.1016/S1473-3099(15)00378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kiseleva I, Isakova-Sivak I, Stukova M, Erofeeva M, Donina S, Larionova N, Krutikova E, Bazhenova E, Stepanova E, Vasilyev K. et al. A phase 1 randomized placebo-controlled study to assess the safety, immunogenicity and genetic stability of a new potential pandemic H7N9 live attenuated influenza vaccine in healthy adults. Vaccines. 2020;8(2):296. doi: 10.3390/vaccines8020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sobhanie M, Matsuoka Y, Jegaskanda S, Fitzgerald T, Mallory R, Chen Z, Luke C, Treanor J, Subbarao K. Evaluation of the safety and immunogenicity of a Candidate pandemic live attenuated influenza vaccine (pLAIV) against influenza A(H7N9). J Infect Dis. 2016;213(6):922–9. doi: 10.1093/infdis/jiv526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Karron RA, Callahan K, Luke C, Thumar B, McAuliffe J, Schappell E, Joseph T, Coelingh K, Jin H, Kemble G, et al. A live attenuated H9N2 influenza vaccine is well tolerated and immunogenic in healthy adults. J Infect Dis. 2009;199(5):711–6. doi: 10.1086/596558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Krammer F, Palese P. Universal influenza virus vaccines that target the conserved hemagglutinin stalk and conserved sites in the head domain. J Infect Dis. 2019;219(Supplement_1):62–7. doi: 10.1093/infdis/jiy711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Krammer F, Palese P, Steel J. Advances in universal influenza virus vaccine design and antibody mediated therapies based on conserved regions of the hemagglutinin. Curr Top Microbiol Immunol. 2015;386:301–21. doi: 10.1007/82_2014_408. [DOI] [PubMed] [Google Scholar]
- 131.Galarza JM, Latham T, Cupo A. Virus-like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol. 2005;18:244–51. doi: 10.1089/vim.2005.18.244. [DOI] [PubMed] [Google Scholar]
- 132.Kapczynski DR, Tumpey TM, Hidajat R, Zsak A, Chrzastek K, Tretyakova I, Pushko P. Vaccination with virus-like particles containing H5 antigens from three H5N1 clades protects chickens from H5N1 and H5N8 influenza viruses. Vaccine. 2016;34(13):1575–81. doi: 10.1016/j.vaccine.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ren Z, Ji X, Meng L, Wei Y, Wang T, Feng N, Zheng X, Wang H, Li N, Gao X. et al. H5N1 influenza virus-like particle vaccine protects mice from heterologous virus challenge better than whole inactivated virus. Virus Res. 2015;200:9–18. doi: 10.1016/j.virusres.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 134.Hu J, Zhang Q, Peng P, Li R, Li J, Wang X, Gu M, Hu Z, Hu S, Liu X. et al. Baculovirus-derived influenza virus-like particle confers complete protection against lethal H7N9 avian influenza virus challenge in chickens and mice. Vet Microbiol. 2022;264:109306. doi: 10.1016/j.vetmic.2021.109306. [DOI] [PubMed] [Google Scholar]
- 135.Pushko P, Tumpey TM, Bu F, Knell J, Robinson R, Smith G. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine. 2005;23(50):5751–9. doi: 10.1016/j.vaccine.2005.07.098. [DOI] [PubMed] [Google Scholar]
- 136.Pushko P, Tumpey TM, Van Hoeven N, Belser JA, Robinson R, Nathan M, Smith G, Wright DC, Bright RA. Evaluation of influenza virus-like particles and Novasome adjuvant as candidate vaccine for avian influenza. Vaccine. 2007;25(21):4283–90. doi: 10.1016/j.vaccine.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 137.Tretyakova I, Pearce MB, Florese R, Tumpey TM, Pushko P. Intranasal vaccination with H5, H7 and H9 hemagglutinins co-localized in a virus-like particle protects ferrets from multiple avian influenza viruses. Virology. 2013;442(1):67–73. doi: 10.1016/j.virol.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 138.Pillet S, Couillard J, Trepanier S, Poulin JF, Yassine-Diab B, Guy B, Ward BJ, Landry N. Immunogenicity and safety of a quadrivalent plant-derived virus like particle influenza vaccine candidate—two randomized phase II clinical trials in 18 to 49 and ≥50 years old adults. PLOS ONE. 2019;14(6):e0216533. doi: 10.1371/journal.pone.0216533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lopez-Macias C, Ferat-Osorio E, Tenorio-Calvo A, Isibasi A, Talavera J, Arteaga-Ruiz O, Arriaga-Pizano L, Hickman SP, Allende M, Lenhard K. et al. Safety and immunogenicity of a virus-like particle pandemic influenza a (H1N1) 2009 vaccine in a blinded, randomized, placebo-controlled trial of adults in Mexico. Vaccine. 2011;29(44):7826–34. doi: 10.1016/j.vaccine.2011.07.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Landry N, Ward BJ, Trepanier S, Montomoli E, Dargis M, Lapini G, Vézina L-P. Preclinical and clinical development of plant-made virus-like particle vaccine against avian H5N1 influenza. PloS One. 2010;5(12):e15559. doi: 10.1371/journal.pone.0015559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chung KY, Coyle EM, Jani D, King LR, Bhardwaj R, Fries L, Smith G, Glenn G, Golding H, Khurana S. et al. ISCOMATRIX™ adjuvant promotes epitope spreading and antibody affinity maturation of influenza a H7N9 virus like particle vaccine that correlate with virus neutralization in humans. Vaccine. 2015;33(32):3953–62. doi: 10.1016/j.vaccine.2015.06.047. [DOI] [PubMed] [Google Scholar]
- 142.Turner JS, O’Halloran JA, Kalaidina E, Kim W, Schmitz AJ, Zhou JQ, Lei T, Thapa M, Chen RE, Case JB. et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021;596(7870):109–13. doi: 10.1038/s41586-021-03738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Furey C, Ye N, Kercher L, DeBeauchamp J, Crumpton JC, Jeevan T, Patton C, Franks J, MG Alameh, SH Fan, AT Phan. Development of a nucleoside-modified mRNA vaccine against clade 2.3.4.4b H5 highly pathogenic avian influenza virus. bioRxiv. 2023. doi: 10.1101/2023.04.30.538854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bahl K, Senn JJ, Yuzhakov O, Bulychev A, Brito LA, Hassett KJ, Laska ME, Smith M, Almarsson Ö, Thompson J. et al. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses. Mol Ther. 2017;25(6):1316–27. doi: 10.1016/j.ymthe.2017.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xiong F, Zhang C, Shang B, Zheng M, Wang Q, Ding Y, Luo J, Li X. An mRNA-based broad-spectrum vaccine candidate confers cross-protection against heterosubtypic influenza a viruses. Emerg Microbes Infect. 2023;12(2):2256422. doi: 10.1080/22221751.2023.2256422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Feldman RA, Fuhr R, Smolenov I, Mick Ribeiro A, Panther L, Watson M, Senn JJ, Smith M, Almarsson Ӧ, Pujar HS. et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine. 2019;37(25):3326–34. doi: 10.1016/j.vaccine.2019.04.074. [DOI] [PubMed] [Google Scholar]
- 147.Pillet S, Aubin E, Trepanier S, Poulin JF, Yassine-Diab B, Ter Meulen J, Ward BJ, Landry N. Humoral and cell-mediated immune responses to H5N1 plant-made virus-like particle vaccine are differentially impacted by alum and GLA-SE adjuvants in a phase 2 clinical trial. NPJ Vaccines. 2018;3(1):3. doi: 10.1038/s41541-017-0043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Treanor JJ, Chu L, Essink B, Muse D, El Sahly HM, Izikson R, Goldenthal KL, Patriarca P, Dunkle LM. Stable emulsion (SE) alone is an effective adjuvant for a recombinant, baculovirus-expressed H5 influenza vaccine in healthy adults: a phase 2 trial. Vaccine. 2017;35(6):923–8. doi: 10.1016/j.vaccine.2016.12.053. [DOI] [PubMed] [Google Scholar]
- 149.Treanor JJ, Essink B, Hull S, Reed S, Izikson R, Patriarca P, Goldenthal KL, Kohberger R, Dunkle LM. Evaluation of safety and immunogenicity of recombinant influenza hemagglutinin (H5/Indonesia/05/2005) formulated with and without a stable oil-in-water emulsion containing glucopyranosyl-lipid a (SE+GLA) adjuvant. Vaccine. 2013;31(48):5760–5. doi: 10.1016/j.vaccine.2013.08.064. [DOI] [PubMed] [Google Scholar]
- 150.Stadlbauer D, Rajabhathor A, Amanat F, Kaplan D, Masud A, Treanor JJ, Izikson R, Cox MM, Nachbagauer R, Krammer F. et al. Vaccination with a recombinant H7 hemagglutinin-based influenza virus vaccine induces broadly reactive antibodies in humans. mSphere. 2017;2(6). doi: 10.1128/mSphere.00502-17. [DOI] [PMC free article] [PubMed] [Google Scholar]


