Abstract
Human cognition fundamentally depends on memory. Alzheimer's disease exhibits a strong correlation with a decline in this factor. Phosphodiesterase-4 B (PDE4B) plays a crucial role in neurodegenerative disorders, and its inhibition is one of the promising approaches for memory enhancement. This study aimed to identify secondary metabolites in white cabbage, coffee, and red onion extracts and identify their molecular interaction with PDE4B by in silico and in vitro experiments. Crushed white cabbage and red onion were macerated separately with ethanol to yield respective extracts, and ground coffee was boiled with water to produce aqueous extract. Thin layer chromatography (TLC)–densitometry was used to examine the phytochemicals present in white cabbage, coffee, and red onion extracts. Molecular docking studies were performed to know the interaction of test compounds with PDE4B. TLC-densitometry analysis showed that chlorogenic acid and quercetin were detected as major compounds in coffee and red onion extracts, respectively. In silico studies revealed that alpha-tocopherol (binding free energy (∆Gbind) = −38.00 kcal/mol) has the strongest interaction with PDE4B whereas chlorogenic acid (∆Gbind = −21.50 kcal/mol) and quercetin (∆Gbind = −17.25 kcal/mol) exhibited moderate interaction. In vitro assay showed that the combination extracts (cabbage, coffee, and red onion) had a stronger activity (half-maximal inhibitory concentration (IC50) = 0.12 ± 0.03 µM) than combination standards (sinigrin, chlorogenic acid, and quercetin) (IC50 = 0.17 ± 0.03 µM) and rolipram (IC50 = 0.15 ± 0.008 µM). Thus, the combination extracts are a promising cognitive enhancer by blocking PDE4B activity.
1. Introduction
Cognitive dysfunction is a serious health issue worldwide; this condition is primarily caused by Alzheimer's disease (AD), which is characterized by the aggregation of amyloid-β plaques and neurofibrillary tangles of hyperphosphorylated tau proteins [1]. In 2020, dementia was estimated to affect 55 million individuals globally [2]. The declining concentrations of cyclic adenosine monophosphate (cAMP), cyclic guanosine monophosphate (cGMP), and brain-derived neurotrophic factor (BDNF) in the hippocampus have been observed to be attributed to AD development [1]. The exploration of nootropics or cognitive enhancers, known as “smart drugs,” is catching intrigue on a daily basis. Healthy participants (students and workers) generally take smart drugs to enhance their memory, attention, learning, executive functions, and vigilance. The benefits and potential risks are associated with the use of the most common synthetic nootropics in healthy adults, including piracetam, benzodiazepine inverse agonists, methylphenidate, donepezil, amphetamine-type stimulants, and unifiram analogues [3–5]. Nootropics that target phosphodiesterases (PDEs) in the brain aid in the normal regulation of neuronal cells [6–8]. To date, the world is paying more attention to cognitive enhancement using natural sources, but the research on the effects of white cabbage, coffee, and red onion as smart drugs is still unavailable [9, 10].
Phosphodiesterase-4 (PDE4), the largest family of PDE, is the most complicated and maybe the most broadly expressed PDE in the body [11], and it particularly hydrolyzes cAMP into its inactive form, 5-AMP [12]. Phosphodiesterase-4B (PDE4B) is strongly expressed in the cornu ammonis 2 and cornu ammonis 3 areas of the hippocampus, the cerebellar granular layer, piriform, and parietal cortex [13]. The continuous rise in the production of PDE4 has been observed in the brains of AD patients, specifically those with hippocampal dysfunction and cognitive impairment [14–16]. PDE4 inhibitors (first generation), such as rolipram ameliorate cognition, mitigate despair-like behaviors, anxiety, and promote neuroplasticity in the hippocampus subsequent to transient global cerebral ischemia [17], and they also exhibited serious side effects (nausea and emesis) at therapeutic doses. In similar manner, second-generation PDE4 inhibitors, including roflumilast and cilomilast, augment memory and cognition, increase neurogenesis, and promote neuroplasticity of the hippocampus [18]. In clinical trials, the maximal dose remained limited owing to adverse effects [19]. For the resolution of this problem, an alternative PDE4B inhibitor, specifically one that originates from natural sources (plants), is required. According to the previous studies, dichloromethane Uvaria alba (U. alba) subextract was used to target PDE4 B2B and acetylcholinesterase (AChE) in in vitro assay due to its neuroprotective properties, especially in AD for the discovery of drugs [20]. The flavonol-enriched n-butanol fraction of U. alba attenuated lipopolysaccharide-induced inflammatory responses through the inhibition of proinflammatory NF-κB pathway and the increased NRF2 levels [21] that are beneficial in neuroprotection. Moreover, in in vitro assay, sappanone A (obtained from heartwood of Caesalpinia sappan L.) effectively suppressed PDE4B1 enzyme activity and decreased TNF-α production in RAW264.7 macrophages leading to dual antioxidant and anti-inflammatory activities [22]. Vascular dementia, which makes up around 20% of dementia cases, is the second most prevalent type of senile dementia after AD. For vascular dementia, no medications are currently licensed. A wide range of risk factors, such as cerebral hemorrhage, cerebral infarct, arterial hypotension, and cerebrovascular disorders, have been linked to vascular dementia, even if the exact cause of the condition is still unknown. Moreover, changes in cyclic nucleotide levels, neuroinflammation, and excitotoxicity could all be part of the underlying mechanism. In a recent study, twenty-five α-mangostin derivatives from Garcinia mangostana, administered at a dose of 10 mg/kg, showed impressive therapeutic effects in a vascular dementia model and spared beagle dogs from vomiting, suggesting that these derivatives have potential as a novel antivascular dementia drug [23]. Therefore, white cabbage, red onion, and coffee extracts were considered for this study to find a natural cognitive enhancer.
White cabbage (Brassica oleracea), family Brassicaceae, is consumed worldwide both as a vegetable and in traditional medicine [24]. This vegetable has been investigated for antipsychotic [25], anticholinesterase [26], anti-inflammatory [27, 28], and antioxidant activities [29]. White cabbage contains abundant amounts of alkenyl glucosinolate, also known as sinigrin. Previously, sinigrin has been shown to increase the levels of cyclic nucleotides (cAMP) by blocking PDE4 in the lungs [30]. On the flip side, coffee (Coffea robusta), family Rubiaceae, is consumed as a beverage, and because of its chlorogenic acid presence, effervescent granules are used as an antidiabetic agent [31, 32] as well as often preferred because of its alertness enhancing, drowsiness, and fatigue-removing properties [33]. Coffee has a wide range of pharmacological applications [31, 32], including anti-Parkinson, anti-Alzheimer, and memory enhancement, and reduces the incidence of dementia [33, 34], antiamyloidogenic [35], anti-inflammatory [36], antioxidant [37, 38], and antiglycation activities [38]. Its phytochemical components, such as caffeine and chlorogenic acid, are linked to these effects [33]. C. robusta is also a rich source of hydroxycinnamic acid derivatives, such as chlorogenic acid [39]. Chlorogenic acid and its products can cross the blood-brain barrier (BBB) and offer neuroprotection. In addition, chlorogenic acid has been studied for its potential to boost memory in those who have memory deficiencies caused by sleep deprivation by inhibiting PDE4 activity [40]. On the other hand, red onion (Allium caepa), family Amaryllidaceae, is an extensively consumed vegetable [41]. A. cepa has shown some reported biological activities, namely, antioxidant [42], anti-inflammatory [43], neuroprotective properties [44, 45], and its flavonoids help to reduce the risk of dementia [46]. A. cepa is a complete source of beneficial bioactive compounds, especially flavonoids [47]; among them, quercetin is a major phytochemical [48] that can penetrate BBB, inhibit PDE4, and promote the activation of kinases (such as protein kinase A). These kinases phosphorylate the transcriptional factor cAMP-responsive element-binding protein (CREB). The CREB induces the expression of genes for neuronal plasticity, offers neuroprotection, and improves memory function [46]. Different components [49] or combinations of herbs function synergistically and are, therefore, an essential part of their therapeutic efficacy; many of the phytomedicines on the drug market are complete extracts of plants [50, 51]. The three aforementioned were selected for this study because their major compounds (chlorogenic acid, quercetin, and sinigrin) have been previously shown to interact with PDE4. Furthermore, because these plants are safe and widely accessible, further research into their effects, either alone or in combination, can be done in vitro and in silico to increase PDE4B inhibition.
There is currently no evidence, either in vitro or in silico, that white cabbage, coffee, and red onion extracts as nootropics alone or in combination inhibit PDE4B and identify the chief bioactive chemicals present in coffee (in aqueous), red onion, and white cabbage (in ethanol) extracts by thin layer chromatography (TLC)–densitometry has been conducted. This investigation aimed to identify the major bioactive compounds in white cabbage, coffee, and red onion and to evaluate their activity against PDE4B via in silico and in vitro studies as well as computational pharmacokinetics and toxicities prediction.
2. Materials and Methods
2.1. Materials
Fresh white cabbage (Magelang District, Middle Java, Indonesia), roasted coffee seeds (Lampung, South Sumatra, Indonesia), fresh red onion (Magelang District, Middle Java, Indonesia), deionized water (PT Bratachem, Yogyakarta, Indonesia), 0.9% saline (PT Braun Pharmaceutical Indonesia), aquabidest, ethanol (Sigma-Aldrich, USA), 1,4-dithiothreitol (Sigma-Aldrich, USA), Whatman no. 1 (filter paper) (GE HealthCare, USA), silica-gel F254 (20 cm × 20 cm) (Merck, Germany), sinigrin (Sigma-Aldrich, USA), chlorogenic acid (Sigma-Aldrich, USA), quercetin (Merck, Germany), ethyl acetate (Merck, Germany), formic acid (Merck, Germany), n-Hexane (Merck), acetone (Merck, Germany), toluene (Merck, Germany) and chloroform (Merck, Germany), sprays (FeCl3 and citro borate) (Merck, Germany), PDE4B1 assay kit (BPS Bioscience, USA), test ligands (Table 1; total = 61 compounds), PDE4B_HUMAN (4KP6) enzyme (UniProt ID: Q07343), known ligands (total = 1863), and Molecular Operating Environment (MOE) software (MOE 2022.10) were used. CAMAG® TLC Scanner 3 (Muttenz, Switzerland) and Spark® 20M Multimode Reader Tecan (Switzerland) were also employed in this research.
Table 1.
List of 67 reported test compounds from white cabbage, coffee, and red onion extracts excluding six repeated components.
| Sr. no. | Test compound | Structure | Biological activity | References |
|---|---|---|---|---|
| 1. White cabbage | ||||
| A | Glucosinolates | |||
|
| ||||
| 1 | Sinigrin (PubChem ID: 6911854) |

|
Wound healing and antiproliferative properties | [52–54] |
| 2 | Progoitrin (PubChem ID: 5281139) |

|
Antithyroid activity | [52, 55] |
| 3 | 4-Methoxyglucobrassicin (PubChem ID: 9576738) |

|
Antioxidative activity | [52, 56] |
| 4 | Glucobrassicin (PubChem ID: 6602378) |

|
Antioxidant activity | [52, 57] |
| 5 | Gluconapin (PubChem ID: 9548620) |

|
Hypotriglyceridemia | [52, 58] |
| 6 | 4-Hydroxyglucobrassicin (PubChem ID: 656561) |

|
Antioxidant activity | [52, 59] |
| 7 | Glucoalyssin (PubChem ID: 656523) |

|
Antimicrobial activity | [60, 61] |
| 8 | Glucobrassicanapin (PubChem ID: 5485207) |

|
Antioxidant activity | [60, 62] |
|
| ||||
| B | Flavonoids | |||
|
| ||||
| 9 | Rutin (PubChem ID: 5280805) |

|
Anticancer, antioxidant, anti-inflammatory, and antiamyloidogenic activities | [63–66] |
| 10 | Quercetin (PubChem ID: 5280343) |

|
Antiamyloidogenic, antioxidant, and anti-inflammatory activities | [63, 66, 67] |
| 11 | Catechin (PubChem ID: 9064) |
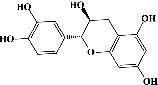
|
Antioxidant, antibacterial, and antimetastatic activities | [63, 68–70] |
| 12 | Kaempferol (PubChem ID: 5280863) |

|
Antimicrobial, antioxidant antitumor, anti-inflammatory and antiproliferative activities | [63, 71–73] |
| 13 | Luteolin (PubChem ID: 5280445) |

|
Antiviral, anti-inflammatory, antioxidant, antiallergic, and neuropharmacological activities | [63, 74–77] |
| 14 | Cyanidin (PubChem ID: 128861) |

|
Antioxidative, anti-inflammatory, and DNA cleavage-protecting activities | [63, 78–81] |
|
| ||||
| C | Phenolic acids | |||
|
| ||||
| 15 | Chlorogenic acid (PubChem ID: 1794427) |

|
Antiulcerogenic, antioxidant, antihepatitis B virus, and DNA-protective activities | [82–87] |
| 16 | p-Coumaric acid (PubChem ID: 637542) |

|
Antioxidant, antiangiogenic, and antibacterial activities | [63, 88–90] |
| 17 | Caffeic acid (PubChem ID: 689043) |

|
Antihepatitis B virus and antioxidant activities | [63, 85, 91, 92] |
| 18 | Gallic acid (PubChem ID: 370) |

|
Antifungal, cytotoxicity, and antioxidant activities and antibacterial activity | [63, 93–95] |
|
| ||||
| D | Other classes of compounds | |||
|
| ||||
| 19 | Alpha-tocopherol (PubChem ID: 14985) |

|
Antioxidant | [96, 97] |
| 20 | Ascorbic acid (PubChem ID: 54670067) |

|
Antioxidative and anti-inflammatory activities | [98, 99] |
| 21 | β-carotene (PubChem ID: 5280489) |

|
Antioxidant, antitumor, and anticancer activities | [100–103] |
| 22 | Violaxanthin (PubChem ID: 448438) |

|
Antiproliferative, antioxidant, and anti-inflammatory | [104–108] |
| 23 | Neoxanthin (PubChem ID: 5282217) |

|
Antiproliferative and apoptosis in PC-3 human prostate cancer cells activities | [104, 109, 110] |
|
| ||||
| 2. Coffee | ||||
| E | Phenolic acids | |||
|
| ||||
| 24 | Chlorogenic acid∗ (PubChem ID: 1794427) | Same as no. 15 | Same as no. 15 | [37] |
| 25 | Cryptochlorogenic acid (PubChem ID: 9798666) |

|
Anti-inflammatory | [111–114] |
| 26 | Neochlorogenic acid (PubChem ID: 5280633) |

|
Anti-inflammatory | [111, 115] |
|
| ||||
| F | Alkaloids | |||
|
| ||||
| 27 | Caffeine (PubChem ID: 2519) |
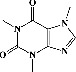
|
Cognitive enhancing, hepatoprotective activity | [37, 116–118] |
| 28 | Trigonelline (PubChem ID: 5570) |

|
Anti-invasive, antitumor, antidiabetic, and antimicrobial activities | [37, 119–122] |
|
| ||||
| 3. Red onion | ||||
| G | Flavonoids | |||
|
| ||||
| 29 | Quercetin∗ (PubChem ID: 5280343) | Same as no. 10 | Same as no. 10 | [123] |
| 30 | Quercetin 3,4′-diglucoside (PubChem ID: 5320835) |
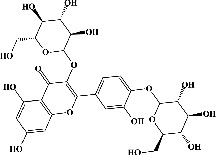
|
Antioxidant and anticarcinogenic activities | [124, 125] |
| 31 | Quercetin 4′-O-glucoside (PubChem ID: 12442954) |

|
Antioxidant activity | [124, 126] |
| 32 | Myricetin (PubChem ID: 5281672) |

|
Anticancer, hypoglycemic, and anti-inflammatory activities | [127–131] |
| 33 | Taxifolin (PubChem ID: 439533) |

|
Anti-inflammatory, digestive enzymes inhibitory, antihyperglycemic, and antiglycation activities | [132–136] |
| 34 | Laricitrin (PubChem ID: 5282154) |

|
Anticancer, antioxidant, and anti-hypertension | [132, 137–139] |
| 35 | Rutin∗ (PubChem ID: 5280805) | Same as no. 9 | Same as no. 9 | [140] |
| 36 | Isorhamnetin (PubChem ID: 5281654) |

|
Antituberculosis, antiplatelet, antitumor, anti-inflammatory, anticancer, and antioxidant activities | [141–147] |
| 37 | Hyperoside (PubChem ID: 5281643) |

|
Antiviral, antifungal, antiplatelet, anticancer, and antioxidant activities | [132, 148–152] |
| 38 | Spiraeoside (PubChem ID: 5320844) |

|
Antitumor, antioxidant, antiviral, antiradical, and enzyme inhibitory activities | [132, 153, 154] |
| 39 | Tectorigenin (PubChem ID: 5281811) |

|
Anti-inflammatory, antioxidant, antifungal activity, and antineuroinflammatory activities | [132, 155–158] |
|
| ||||
| H | Phenolic acids | |||
|
| ||||
| 40 | Ferulic acid (PubChem ID: 445858) |

|
Antibacterial, antioxidant, and antimicrobial activities | [93, 159–161] |
| 41 | Protocatechuic acid (PubChem ID: 72) |

|
Antioxidant, antihyperlipidaemic, and antibacterial activities | [159, 162–165] |
| 42 | p-Coumaric acid∗ (PubChem ID: 5281139) | Same as no. 16 | Same as no. 16 | [124] |
| 43 | Caffeic acid∗ (PubChem ID: 689043) | Same as no. 17 | Same as no. 17 | [124] |
| 44 | Chlorogenic acid∗∗ (PubChem ID: 1794427) | Same as no. 15 | Same as no. 15 | [124] |
|
| ||||
| I | Organosulfurs | |||
|
| ||||
| 45 | Onionin A |

|
Antioxidant, antibacterial, cytotoxic, antitumoral, and antimetastatic activities | [166–169] |
| 46 | Cycloalliin (PubChem ID: 12305351) |

|
Antioxidant activity | [170, 171] |
| 47 | Isoalliin (PubChem ID: 5281112) |
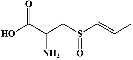
|
Antibacterial activity | [172, 173] |
| 48 | Methiin (PubChem ID: 9578071) |

|
Antidiabetic and antioxidative activities | [172, 174] |
| 49 | Propiin (PubChem ID: 91819955) |

|
— | [172] |
| 50 | Allicin (PubChem ID: 65036) |

|
Antioxidant, antibacterial, antimalarial, and antimicrobial activities | [175–179] |
| 51 | Alliin (PubChem ID: 9576089) |

|
Antioxidant and antimicrobial activities | [172, 180–182] |
| 52 | S-methyl-L-cysteine (PubChem ID: 24417) |
|
Antibacterial and antimicrobial activities | [172, 183–185] |
| 53 | S-ethylcysteine (PubChem ID: 25203803) |

|
Antioxidative and antiglycative activities | [172, 186] |
| 54 | S-propyl-L-cysteine (PubChem ID: 125198) |

|
Antioxidant, antibacterial activities, and anticancer activities | [172, 187, 188] |
| 55 | S-allyl-cysteine (PubChem ID: 9793905) |

|
Antioxidant and anti-inflammatory activities | [172, 189, 190] |
| 56 | S-propylmercapto-cysteine (PubChem ID: 192762) |

|
Antioxidant activity | [172, 191] |
| 57 | Trisulfide dipropyl (PubChem ID: 22383) |

|
Antimicrobial activity | [172, 192] |
| 58 | Diallyl disulfide (PubChem ID: 16590) |

|
Antimicrobial and anticarcinogenic activities | [172, 193, 194] |
| 59 | Diisopropyl disulfide (PubChem ID: 77932) |

|
Antioxidative activity | [172, 195] |
| 60 | Methyl propenyl disulfide (PubChem ID: 5366552) |

|
Antioxidant, antibacterial, and antimicrobial activities | [172, 196, 197] |
| 61 | 6-Methyl-4,5-dithia-1-heptene (PubChem ID: 525503) |

|
— | [172] |
|
| ||||
| J | Anthocyanin | |||
|
| ||||
| 62 | Cyanidin 3-glucoside (PubChem ID: 12303220) |

|
— | [198] |
| 63 | Cyanidin 3-laminaribioside (PubChem ID: 44256721) |

|
Antioxidant activity | [141, 199, 200] |
| 64 | Cyanidin 3-malonylglucoside (PubChem ID: 10143380) |

|
Antimicrobial and antiobesity activities | [141, 201–203] |
| 65 | Petunidin 3′-glucoside acetate (PubChem ID: 443651) |

|
Antioxidant activity | [141, 204] |
| 66 | Malvidin 3′-glucoside (PubChem ID: 11249520) |

|
Antioxidant and anti-inflammatory activities | [141, 205, 206] |
| 67 | Peonidin-3′-glucoside (PubChem ID: 443654) |

|
Anti-inflammatory and antioxidant activities | [141, 207, 208] |
∗ = first-time repeated compound; ∗∗ = second-time repeated compound. PubChem IDs were taken from the PubChem website (https://pubchem.ncbi.nlm.nih.gov/) in order to get the 2D structure of compounds for molecular docking.
2.2. Collection and Identification of Plant Material
White cabbage and red onion (fresh whole bulb) were collected from Magelang, Jawa Tengah, and coffee seeds were purchased from Tokopedia (Lampung origin). All these test materials were authenticated at the Department of Pharmaceutical Biology, Faculty of Pharmacy, Universitas Gadjah Mada, Yogyakarta, Indonesia (Identification no. 20.25.1 UN1/FFA.2/BF/PT/2022).
2.3. Preparation of Extracts
White cabbage (1 kg) and red onion bulb (1 kg) were rinsed with water and chopped separately. The chopped materials were later placed in an electrical blender one by one and processed to obtain a finer form for extraction. Roasted coffee (1 kg) was also blended with an electrical blender. The blended samples (white cabbage and red onion) were macerated with pure ethanol 100% (percent) (5 L) for each sample and placed overnight for at least 24 hours. The coffee was macerated by the infusion method, where ground coffee was boiled in distilled water for 15 minutes. Whatman No. 1 filter paper was used to filter all extracts, and the solvents were evaporated using a rotary evaporator at 50–70°C, and the extracts were stored in a desiccator at room temperature until dryness.
2.4. TLC and TLC-Densitometry Analysis
TLC analysis was performed on an aluminum plate precoated with silica-gel F254 as the stationary phase and the mixtures of formic acid, ethyl acetate, and aquabidest (1 : 8 : 1.5; 25) and n-hexane, ethyl acetate, and formic acid (6 : 4 : 0.5; 19) as a mobile phase for coffee and red onion, respectively. To visualize the compounds, the TLC spots were observed under a ultraviolet (UV) light at 254 and 366 nm wavelength, as well as with FeCl3 and citroborate for chlorogenic acid and quercetin, respectively. TLC-densitometry analysis was used to identify chlorogenic acid (1 mg/mL) and quercetin (1 mg/mL) in coffee (10 mg/mL) and red onion (10 mg/mL) extracts solution, respectively. The maximum wavelengths of 330 nm and 380 nm were set for the TLC-densitometry analysis of chlorogenic acid and quercetin, respectively.
2.5. In Silico Molecular Docking Studies
To determine the binding interaction of the test ligand with PDE4B (4KP6), molecular docking was carried out using the Molecular Operating Environment (MOE) software (MOE 2022.10), and the majority of the default tools (pocket atom, placement method (Triangle Matchter, London dG), refinement method (Induced fit and ASE), and energy minimization as 0.1 kcal/mol) were followed. Protein Data Bank (RCSB PDB, https://www.rcsb.org/) was used to acquire protein crystals of the PDE4B_HUMAN enzyme (UniProt ID: Q07343). For the desired expression, only 4KP6 was selected for docking, and the two-dimensional structures of previously investigated compounds were attained using SMILES ID in PubChem (https://www.pubchem.ncbi.nlm.nih.gov/).
2.5.1. Preparation of Known Ligand Dataset
The dataset of all known ligands (inhibitors of PDE4B; total = 1863 known ligands) for PDE4B, which was selected based on the ligands' activity (half-maximal inhibitory concentration (IC50)), was downloaded from the ZINC database (https://zinc.docking.org/). Subsequently, the known ligand dataset was created using MOE's default parameters following minimizing energy by MOPAC in MOE (0.1 kcal/mol).
2.5.2. Preparation of Test Ligands
All test ligands' 3D structures (of total = 61 bioactive compounds from white cabbage, coffee, and red onion; Table 1) were created by MOE by minimizing energy following minimizing energy by MOPAC in MOE (0.1 kcal/mol) and saving them in a (.mdb) file, and their 2D structures were retrieved from their smiles ID in PubChem.
2.5.3. Preparation of Protein-Ligand Complex
A total of forty-two protein (PDE4B) crystal structures (including 4KP6) were retrieved from the PDB. Using quickprep in MOE software with the default parameters, the 3D structure of PDE4B (4KP6) was prepared by eliminating metal atoms and minimizing energy by MOPAC in MOE (0.1 kcal/mol). The crystal protein 4KP6 complex was then saved as an MOE file for further docking validation.
2.5.4. Validation of Docking Protocols
To validate the pose or placement algorithm of the docked ligand, 4KP6 was redocked (by applying Induced fit, London dG, and ASE protocols) with its native ligand. Redocking and scoring function validation were used to validate the docking protocol. The flexible docking on the protein's pocket atoms was done using the induced fit method. The placement method was chosen as Triangle Matcher and London dG, and the scoring function was chosen as ASE. Before molecular docking of the test ligands, pose validation and scoring function of known ligands were analyzed. To evaluate pose validation, the binding affinities for ligand-enzyme complexes were calculated as kcal/mol, and the root mean square deviation (RMSD) was computed. The RMSD value was regarded to be within the threshold limit, <2 Å (angstrom). The association between docking score and IC50 values of recognized ligands was calculated in order to examine the scoring function validation.
2.5.5. Docking of Test Ligands with Protein
All the prepared 61 test ligands (excluding their six repetitions of the same compounds in the table) from white cabbage, coffee, and red onion (Table 1) were docked with the prepared PDE4B (4KP6) using the Induced fit, London dG, and ASE protocols, with 10 poses being set for each component.
2.6. Profiles of ADME
SwissADME software (Molecular Modeling Group, Swiss Institute of Bioinformatics, 2019) was used to computationally predict the ADME parameters of sixty-one secondary metabolites from white cabbage, red onion, and coffee extracts. Pharmacokinetic profiles were assessed using Lipinski's “rule of five,” which examines a drug's biochemical characteristics that may affect how well it absorbs and permeates through cell membranes. A compound must meet at least three of Lipinski's criteria (molecular weight <500 Da, estimated lipophilicity (log P) <5, number of hydrogen-bond donors <5, and number of hydrogen-bond acceptors <10) in order to show drug similarity. Additionally, for in silico toxicity prediction, which considers the possible mutagenicity, tumorigenicity, irritating effects, and reproductive toxicity of the secondary metabolites from white cabbage, red onion, and coffee extracts, the OSIRIS property explorer program (Thomas Sander, Idorsia Pharmaceuticals Ltd., 2017) was utilized [20].
2.7. In Vitro PDE4B1 Inhibitory Assay
PDE4B1 inhibitory assay was performed in accordance with the manufacturer's protocol using certain concentrations of white cabbage extract (100, 50, 25, 12.5, 6.25, 3.125, 1.5625 µg/mL), coffee extract (100, 50, 25, 12.5, 6.25, 3.125, 1.5625 µg/mL), and red onion extract (100, 50, 25, 12.5, 6.25, 3.125, 1.5625 µg/mL) and the combination (white cabbage extract (100, 50, 25, 12.5, 6.25, 3.125, 1.5625 µg/mL), coffee extract (100, 50, 25, 12.5, 6.25, 3.125, 1.5625 µg/mL), and red onion extract (100, 50, 25, 12.5, 6.25, 3.125, 1.5625 µg/mL)) extracts. The same concentrations were applied for sinigrin, chlorogenic acid, and quercetin. In this protocol, the fluorescently labeled cAMP was incubated with a sample containing PDE4B1 for 1 hour, and a binding agent which was added to the reaction mixture to induce changes in fluorescent polarization was then measured using a fluorescence reader equipped for the measurement of fluorescence polarization, which was measured at 485 ± 5 nm (excitation) and 528 ± 10 nm (emission) using a properly equipped microtiter-plate fluorescence reader.
2.8. Statistical Analysis
The data are presented as the mean ± standard errors of means (SEM). To generate sigmoidal % activity versus concentration graphs, IC50 was calculated using nonlinear regression curve fitting. One-way ANOVA was applied for analysis followed by Tukey's multiple comparison test, and a p value of 0.05 was established as the statistically significant value. GraphPad Prism version 5.0 was used to analyze the data.
3. Results
3.1. Identification of Chief Bioactive Compounds by TLC-densitometry Analysis
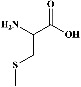
We identified chlorogenic acid in coffee. On a silica-gel F254 plate, the compound was detected at UV 254 and 366 nm (Figures 1(a)(i) and 1(a)(ii), respectively). TLC-densitometry analysis was performed to confirm this finding (Figure 1(b)). Quercetin was detected at UV 254 and 366 nm in red onion (Figures 1(c)(iii) and 1(c)(iv), respectively). This result was confirmed by TLC-densitometry analysis (Figure 1(c)).
Figure 1.
TLC profile of coffee, onion, chlorogenic acid, and quercetin along with their spectrums (b) and (d). TLC plates indicate (a(i)) and (c(iii)) (at 254 nm); (a(ii)) and (c(iv)) (at 366 nm); CE = coffee extract; OE = onion extract; Cl = chlorogenic acid; Qu = quercetin; AUC = area under the curve; Rf = retention factor.
3.2. In Silico Molecular Docking Studies
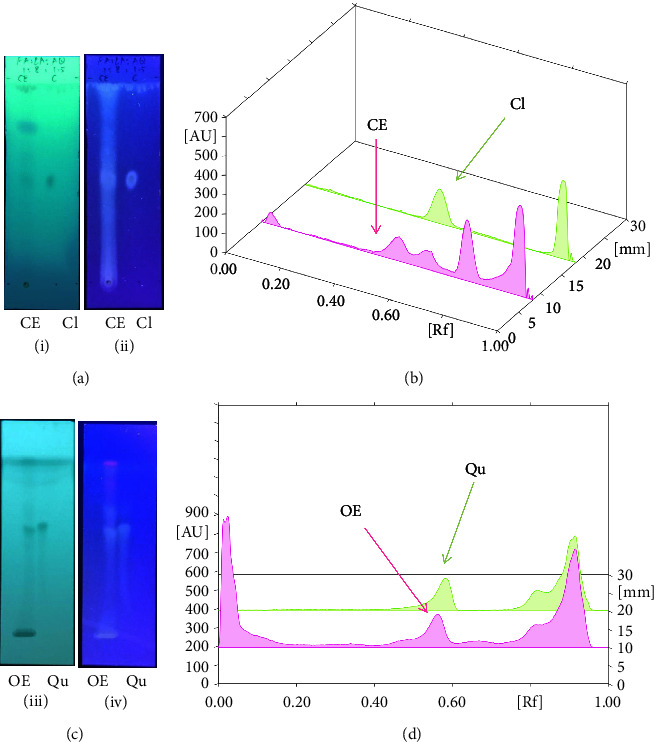
Figure 2(a) exhibits captioned 3D images of the interaction between a redocked native ligand [(2-ethyl-2-{[4-(methylamino)-6-(1H-1,2,4-triazol-1-yl)-1,3,5-triazin-2-yl]amino}butanenitrile) (PubChem CID: 71598545)] and amino acids of PDE4B (PDB ID: 4KP6) with an RMSD value of 0.834 Å and binding energy (−21.494 kcal/mol). Figure 2(b) shows their 2D interaction. In the current docking research, it was discovered that the triazine ring of native ligand had a π-hydrogen bonding with Ile410 and its nitrogen on amine group on the same ring with Asn395 while its triazole ring had π-hydrogen interaction with Phe414 and π–π binding with Phe446; besides this, nitrogen in its triazole ring represented attachment with Met431 (Figures 2(a) and 2(b)). On the other hand, the known ligand (ZINC000043194322) (binding energy −35.123 kcal/mol) was found to interact in 2D with amino acids of PDE4B (PDB ID: 4KP6) in Figure 2(c), whereas Figure 2(d) represents a known ligand's 2D association with amino acids. Additionally, the known ligand's benzene ring displayed a π–π interaction with Phe446, and the pyridine ring had π-hydrogen bonding with Met347; however, the oxygen on the pyrimidine ring served as a sidechain donor at Met431 (Figures 2(c) and 2(d)).
Figure 2.
The 3D caption of native and known ligands with native ligand, and the 2D caption of native ligand and known ligands with amino acid residues. (a) = redocked native ligand 3D interaction with amino acid residues; (b) = native ligand 2D interaction with amino acid residues; (c) = docked known ligand (ZINC000043194322) 3D interaction with amino acid residues; (d) = known ligand (ZINC000043194322) 2D interaction with amino acid residues. Trp = tryptophan; Phe = phenylalanine; Asp = aspartic acid; Gln = glutamine; Met = methionine; Ser = serine; Ile = isoleucine; His = histidine; H = hydrogen; N = nitrogen; F = fluorine; O = oxygen; H2O = water.
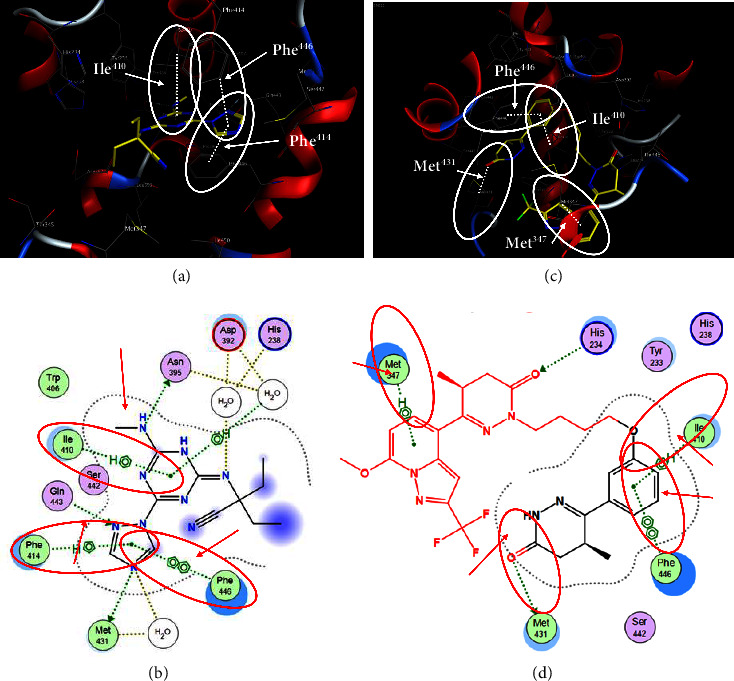
Quercetin (PubChem CID: 5280343), the bioactive component in red onion, and reference drug rolipram (PubChem CID: 5092) have also been studied to find out how well they interact with PDE4B (PDB ID: 4KP6). Figure 3 shows the 3D interaction of rolipram (binding energy −22.801 kcal/mol) with amino acids of PDE4B (PDB ID: 4KP6) in Figure 3(a) and the 2D interaction in Figure 3(b). This study revealed that the nitrogen in the pyrrolidine ring in rolipram contributed sidechain to Asp395 and the benzene ring interacted with Phe446 by π–π binding interaction (Figures 3(a) and 3(b)). Alternatively, Figure 3(c) exhibits 3D interaction of quercetin (binding energy −17.252 kcal/mol) with amino acids of PDE4B (PDB ID: 4KP6) while Figure 3(d) represents 2D interaction of known ligand with amino acids. The quercetin's benzene-1,2-diol ring interacted with Phe446 through π–π binding and π-hydrogen binding with Ile410, while its hydroxyl group served as a donor to Asn395 in these interactions. Moreover, the hydroxyl group on the benzene-1,2-diol ring of quercetin served as a donor to Thr414 (Figures 3(c) and 3(d)).
Figure 3.
The 3D caption of rolipram and quercetin with amino acid residues, and the 2D caption of rolipram and quercetin with amino acid residues. (a) = rolipram 3D interaction with amino acid residues; (b) = rolipram 2D interaction with amino acid residues; (c) = quercetin 3D interaction with amino acid residues; (d) = quercetin 2D interaction with amino acid residues. Phe = phenylalanine; Asp = aspartic acid; Thr = threonine; Ile = isoleucine; His = histidine; N = nitrogen; H = hydrogen; O = oxygen; OH = hydroxyl group.
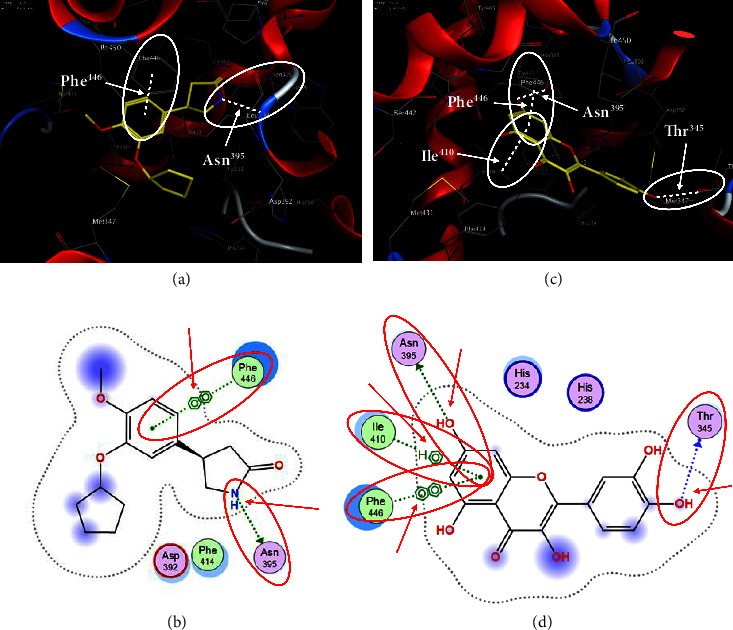
In order to evaluate chlorogenic acid (PubChem CID: 1794427) and alpha-tocopherol's (PubChem CID: 1742129) affinity for the PDE4B (PDB ID: 4KP6), chlorogenic acid, a coffee bioactive component, has also been studied. In Figure 4, Figure 4(a) depicts the 3D interaction of chlorogenic acid (binding energy −21.501 kcal/mol) with amino acids of PDE4B (PDB ID: 4KP6), while Figure 4(b) shows the 2D interaction. This finding exhibited that the benzene ring in benzene-1,2-diol of chlorogenic acid interacted with Phe446 by π–π binding and Ile410 by π-hydrogen binding, and oxygen on carboxylic acid in chlorogenic acid served as side chain acceptor for His234 while hydroxyl group acted as side chain donor interaction of the known ligand with amino acids. Additionally, the alpha-tocopherol's indole ring showed an engagement with Phe446 through π–π binding and Ile410 by π-hydrogen binding while the hydroxyl group on the benzene ring served as a side chain acceptor for Gln443 (Figures 4(c) and 4(d)). The active component of white cabbage, sinigrin, has also been investigated to determine its affinity for PDE4B (PDB ID: 4KP6). Unfortunately, sinigrin from white cabbage extract did not interact with PDE4B in all scoring functions. In terms of binding interactions with functional residues, the docking results of alpha-tocopherol were investigated excellently, and it also had the strongest effects on the PDE4B (PDB ID: 4KP6) target protein out of the 28 phytochemicals, including rolipram, that were chosen for Glu304 (Figures 4(a) and 4(b)). In contrast, Figure 4(c) represents the 3D interaction of alpha-tocopherol (binding energy = −38.007 kcal/mol) with amino acids of PDE4B (PDB ID: 4KP6), whereas Figure 4(d) exhibits 2D.
Figure 4.
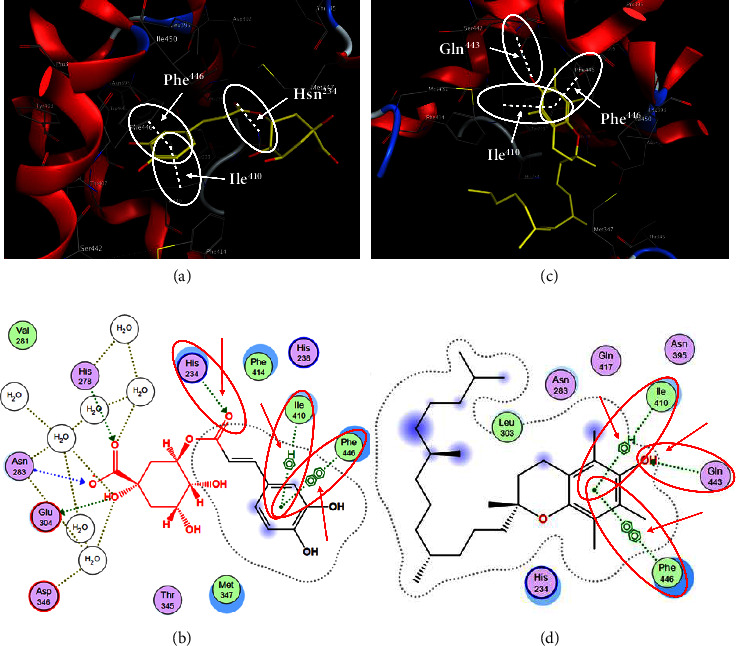
The 3D caption of chlorogenic acid and alpha-tocopherol with native ligand, and the 2D caption of chlorogenic acid and alpha-tocopherol with amino acid residues. (a) = chlorogenic acid 3D interaction with amino acid residues; (b) = chlorogenic acid 2D interaction with amino acid residues; (c) = alpha-tocopherol 3D interaction with amino acid residues; (d) = alpha-tocopherol (PubChem CID 1742129) 2D interaction with amino acid residues. Phe = phenylalanine; Asn = asparagine; Glu = glutamic acid; Asp = aspartic acid; Gln = glutamine; Met = methionine; Thr = threonine; Ile = isoleucine; Val = valine; His = histidine; H = hydrogen; O = oxygen; OH = hydroxyl group; H2O = water.
In the table (Table 2), the compounds' names, their structures (along with rolipram, as a reference), free bind energies (∆Gbind [kcal/mol {kilocalorie/molar}]), and pIC50 values have been enlisted.
Table 2.
The list of 28 compounds, their structures (along with rolipram, as standard), free bind energies (∆Gbind [kcal/mol]), and pIC50 values.
| Sr. no. | Compounds | ∆Gbind (kcal/mol) | pIC50 |
|---|---|---|---|
| 1 |

|
−38.007 | 25.46 |
| 2 |

|
−29.557 | 10.9 |
| 3 |

|
−25.637 | 8.23 |
| 4 |

|
−25.318 | 7.78 |
| 5 |

|
−24.354 | 6.44 |
| 6 |
|
−24.036 | 5.99 |
| 7 |

|
−23.865 | 5.7 |
| 8 |

|
−23.789 | 5.6 |
| 9 |

|
−22.801 | 4.27 |
| 10 |

|
−21.833 | 2.93 |
| 11 |

|
−21.501 | 2.5 |
| 12 |

|
−21.479 | 2.43 |
| 13 |

|
−21.169 | 2 |
| 14 |

|
−20.423 | 0.96 |
| 15 |

|
−19.686 | −0.06 |
| 16 |

|
−18.621 | −1.55 |
| 17 |

|
−17.410 | −3.24 |
| 18 |

|
−17.287 | −3.41 |
| 19 |

|
−17.271 | −3.43 |
| 20 |

|
−17.252 | −3.45 |
| 21 |

|
−16.574 | −4.4 |
| 22 |

|
−16.461 | −4.56 |
| 23 |

|
−15.621 | −7.53 |
| 24 |

|
−14.850 | −6.8 |
| 25 |

|
−14.099 | −7.85 |
| 26 |

|
−13.874 | −8.16 |
| 27 |

|
−11.439 | −11.55 |
| 28 |

|
−10.316 | −13.12 |
∆Gbind = free bind energy; kcal/mol = kilocalorie/molar; IC50 = half-maximal inhibitory concentration; pIC50 = −log(IC50 (M)).
3.3. ADME and Drug-Likeness Prediction
Through the use of in silico absorption, distribution, metabolism, and excretion (ADME) screening, the overall pharmacokinetic behavior of 61 compounds was defined. Using the following descriptors—molecular weight, lipophilicity (given as octanol-water partition coefficient), and the number of hydrogen-bond donors and acceptors—the druggability of the compounds was predicted using Lipinski's rule of five. Partial bioavailability and drug-likeness were demonstrated by all secondary metabolites, which included forty-one highly ranked compounds. Furthermore, passive intestinal absorption and brain permeation of the substances were predicted using the intuitive graphical representation of the functions of lipophilicity and apparent polarity known as the BOILED-Egg (brain or intestinal estimated permeation predictive model) (Figure 5).
Figure 5.
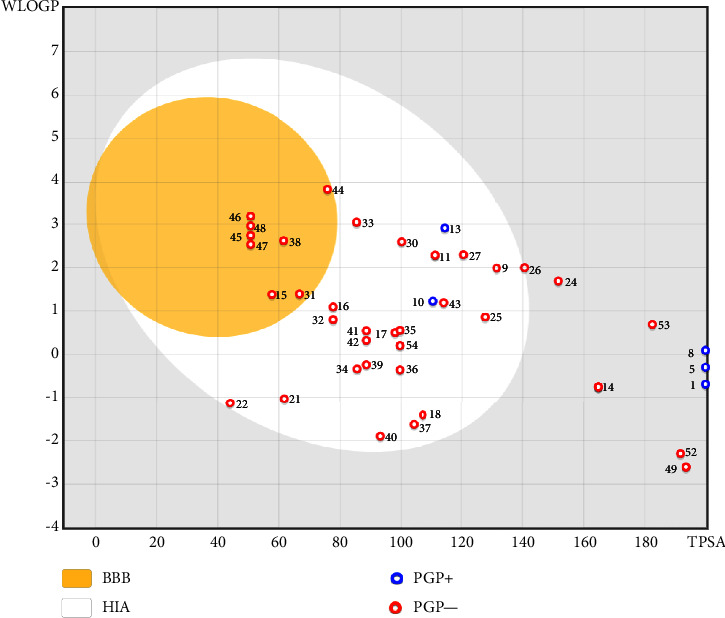
GI tract and brain permeation prediction of the 41 top-ranked secondary metabolites in white cabbage, red onion, and coffee by brain or the intestinal estimated permeation predictive model (BOILED-Egg) method. GI = gastrointestinal, BBB = blood-brain barrier, HIA = human intestinal absorption, PGP+ = P-glycoprotein positive, PGP- = P-glycoprotein negative, TPSA = topological polar surface area, secondary metabolites: 1 = sinigrin, 5 = gluconapin, 8 = glucobrassicanapin, 9 = quercetin, 10 = catechin, 11 = kaempferol, 13 = cyanidin, 14 = chlorogenic Acid, 15 = p-coumaric acid, 16 = caffeic acid, 17 = gallic acid, 18 = ascorbic acid, 21 = caffeine, 22 = trigonelline, 24 = myricetin, 25 = taxifolin, 26 = laricitrin, 27 = isorhamnetin, 30 = tectorigenin, 31 = ferulic acid, 32 = protocatechuic acid, 33 = onionin (A) 34 = cycloalliin, 35 = isoalliin, 36 = methiin, 37 = propiin, 38 = allicin, 39 = S-methyl-L-cysteine, 40 = S-ethylcysteine, 41 = S-propyl-L-cysteine, 42 = S-allylcysteine, 43 = S-propylmercaptocysteine, 44 = dipropyl trisulfide, 45 = diallyl disulfide, 46 = diisopropyl disulfide, 47 = methyl propenyl disulfide, 48 = 6-methyl-4,5-dithia-1-heptene, 49 = cyanidin 3-glucoside, 52 = malvidin 3-glucoside, 53 = peonidin-3-glucoside, and 54 = alliin.
The BBB penetration prediction is very probable for the eight compounds (p-coumaric acid, ferulic acid, allicin, dipropyl trisulfide, diallyl disulfide, diisopropyl disulfide, methyl propenyl disulfide, and 6-methyl-4,5-dithia-1-heptene) present in the yellow region (yolk), but passive absorption through the gastrointestinal (GI) tract is more likely for those in the white region. With the significant binding affinity to the target enzyme PDE4B, the twenty-five compounds (quercetin, catechin, kaempferol, cyanidin, caffeic acid, gallic acid, ascorbic acid, caffeine, trigonelline, taxifolin, laricitrin, isorhamnetin, tectorigenin, protocatechuic acid, onionin A, cycloalliin, isoalliin, methiin, propiin, S-methyl-L-cysteine, S-ethylcysteine, S-propyl-L-cysteine, S-allylcysteine, S-propylmercaptocysteine, and alliin) were expected to have low BBB bridging capacities and good GI absorption. The bioavailability pattern of diallyl disulfide, diisopropyl disulfide, methyl propenyl disulfide, and 6-methyl-4,5-dithia-1-heptene was similar. Sinigrin, gluconapin, and glucobrassicanapin's location outside of the white region of the BOILED-Egg model can be explained by its expected poor absorption in the GI tract, even though it has a good binding affinity to PDE4B. The OSIRIS Property Explorer was also utilized to estimate the reproductive toxicity, mutagenicity, and tumorigenicity, irritating impact toxicities of the sixty-one secondary metabolites derived from white cabbage, red onion, and coffee extracts. The thirty-four compounds (catechin, luteolin, chlorogenic acid, ascorbic acid, cryptochlorogenic acid, neochlorogenic acid, trigonelline, taxifolin, hyperoside, onionin A, cycloalliin, isoalliin, methiin, propiin, allicin, S-methyl-L-cysteine, S-ethylcysteine, S-propyl-L-cysteine, S-allylcysteine, S-propylmercaptocysteine, dipropyl trisulfide, diallyl disulfide, diisopropyl disulfide, methyl propenyl disulfide, 6-methyl-4,5-dithia-1-heptene, cyanidin 3-glucoside, malvidin 3-glucoside, alliin, rutin, violaxanthin, alpha-tocopherol, neoxanthin, quercetin 3,4′-diglucoside, and β-carotene) did not show any predicted toxicity among the top-ranked compounds. However, the rest twenty-three compounds exhibited potential toxicities, including mutagenicity, tumorigenic, irritant, and reproductive effective toxicities. It was predicted that sinigrin, progoitrin, 4-methoxyglucobrassicin, glucobrassicin, 4-hydroxyglucobrassicin, glucoalyssin, chlorogenic acid, p-coumaric acid, and tectorigenin would be harmful to reproduction while gluconapin and glucobrassicanapin were expected to have irritant and reproductive effects. Caffeic acid, caffeine, and ferulic acid would be predicted as highly dangerous due to possessing mutagenicity, tumorigenic, and reproductive effective properties. Moreover, kaempferol, quercetin-4′-o-glucoside, myricetin, laricitrin, isorhamnetin, spiraeoside, protocatechuic acid, and quercetin 3,4′-diglucoside expected to have mutagenicity effects (Table 3). Therefore, to enhance their toxicity profile, structural iteration is strongly advocated.
Table 3.
The computational pharmacokinetic and toxicities studies of 61 reported compounds derived from Swiss ADME and OSIRIS.
| Compound | GI absorptiona | Solubilityb | BBB permeantc | PAINSd | Log Po/we | Mutagenecityf | Tumorigenicg | Irritanth | Reproductive effectivei | Pgp substrate | CYP1A2 inhibitor | CYP2C19 inhibitor | CYP2C9 inhibitor | CYP2D6 inhibitor | CYP3A4 inhibitor | Bioavailability score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Low | Very soluble | No | No | −1.26 | No | No | No | Yes | Yes | No | No | No | No | No | 0.11 |
| 2 | Low | Very soluble | No | No | −1.54 | No | No | No | Yes | Yes | No | No | No | No | No | 0.11 |
| 3 | Low | Soluble | No | No | −0.2 | No | No | No | Yes | Yes | No | No | No | No | No | 0.11 |
| 4 | Low | Soluble | No | No | −0.45 | No | No | No | Yes | No | No | No | No | No | No | 0.11 |
| 5 | Low | Very soluble | No | No | −0.94 | No | No | Yes | Yes | Yes | No | No | No | No | No | 0.11 |
| 6 | Low | Soluble | No | No | −0.71 | No | No | No | Yes | No | No | No | No | No | No | 0.11 |
| 7 | Low | Very soluble | No | No | −1.1 | No | No | No | Yes | Yes | No | No | No | No | No | 0.11 |
| 8 | Low | Very soluble | No | No | −0.52 | No | No | Yes | Yes | Yes | No | No | No | No | No | 0.11 |
| 9 | High | Soluble | No | Yes | 1.23 | Yes | Yes | No | No | No | Yes | No | No | Yes | Yes | 0.55 |
| 10 | High | Soluble | No | Yes | 0.83 | No | No | No | No | Yes | No | No | No | No | No | 0.55 |
| 11 | High | Soluble | No | No | 1.58 | Yes | No | No | No | No | Yes | No | No | Yes | Yes | 0.55 |
| 12 | High | Soluble | No | Yes | 1.73 | No | No | No | No | No | Yes | No | No | Yes | Yes | 0.55 |
| 13 | High | Soluble | No | Yes | 0.32 | — | — | — | — | Yes | Yes | No | No | No | No | 0.55 |
| 14 | Low | Very soluble | No | Yes | −0.39 | No | No | No | No | No | No | No | No | No | No | 0.11 |
| 15 | High | Soluble | Yes | No | 1.26 | No | No | No | Yes | No | No | No | No | No | No | 0.85 |
| 16 | High | Very soluble | No | Yes | 0.93 | Yes | Yes | No | Yes | No | No | No | No | No | No | 0.56 |
| 17 | High | Very soluble | No | Yes | 0.21 | Yes | No | No | Yes | No | No | No | No | No | Yes | 0.56 |
| 18 | High | Highly soluble | No | No | −1.24 | No | No | No | No | No | No | No | No | No | No | 0.56 |
| 19 | Low | Very soluble | No | Yes | −0.37 | No | No | No | No | No | No | No | No | No | No | 0.11 |
| 20 | Low | Very soluble | No | Yes | −0.46 | No | No | No | No | No | No | No | No | No | No | 0.11 |
| 21 | High | Very soluble | No | No | 0 | Yes | Yes | No | Yes | No | No | No | No | No | No | 0.55 |
| 22 | High | Very soluble | No | No | −0.61 | No | No | No | No | No | No | No | No | No | No | 0.55 |
| 23 | Low | Soluble | No | No | −0.19 | Yes | No | No | No | Yes | No | No | No | No | No | 0.17 |
| 24 | Low | Soluble | No | Yes | 0.79 | Yes | No | No | No | No | Yes | No | No | No | Yes | 0.55 |
| 25 | High | Soluble | No | Yes | 0.51 | No | No | No | No | No | No | No | No | No | No | 0.55 |
| 26 | Low | Soluble | No | Yes | 1.3 | Yes | No | No | No | No | Yes | No | No | Yes | Yes | 0.55 |
| 27 | High | Soluble | No | No | 1.65 | Yes | No | No | No | No | Yes | No | No | Yes | Yes | 0.55 |
| 28 | Low | Soluble | No | Yes | −0.38 | No | No | No | No | No | No | No | No | No | No | 0.17 |
| 29 | Low | Soluble | No | No | −0.19 | Yes | No | No | No | Yes | No | No | No | No | No | 0.17 |
| 30 | High | Soluble | No | No | 2.06 | No | No | No | Yes | No | Yes | No | No | Yes | Yes | 0.55 |
| 31 | High | Soluble | Yes | No | 1.36 | Yes | Yes | No | Yes | No | No | No | No | No | No | 0.85 |
| 32 | High | Very soluble | No | Yes | 0.65 | Yes | No | No | No | No | No | No | No | No | Yes | 0.56 |
| 33 | High | Very soluble | No | No | 1.34 | No | No | No | No | No | No | No | No | No | No | 0.55 |
| 34 | High | Highly soluble | No | No | −1.44 | No | No | No | No | No | No | No | No | No | No | 0.55 |
| 35 | High | Highly soluble | No | No | −1.21 | No | No | No | No | No | No | No | No | No | No | 0.55 |
| 36 | High | Highly soluble | No | No | −1.91 | No | No | No | No | No | No | No | No | No | No | 0.55 |
| 37 | High | Highly soluble | No | No | −1.87 | No | No | No | No | No | No | No | No | No | No | 0.55 |
| 37 | High | Very soluble | Yes | No | 1.61 | No | No | No | No | No | No | No | No | No | No | 0.55 |
| 39 | High | Highly soluble | No | No | −1.06 | No | No | No | No | No | No | No | No | No | No | 0.55 |
| 40 | High | Highly soluble | No | No | −1.42 | No | No | No | No | No | No | No | No | No | No | 0.55 |
| 41 | High | Highly soluble | No | No | −0.34 | No | No | No | No | No | No | No | No | No | No | 0.55 |
| 42 | High | Highly soluble | No | No | −0.45 | No | No | No | No | No | No | No | No | No | No | 0.55 |
| 43 | High | Very soluble | No | No | 0.15 | No | No | No | No | No | No | No | No | No | No | 0.55 |
| 44 | High | Soluble | Yes | No | 2.98 | No | No | No | No | No | No | No | No | No | No | 0.55 |
| 45 | High | Very soluble | Yes | No | 2.39 | No | No | No | No | No | No | No | No | No | No | 0.55 |
| 46 | High | Soluble | Yes | No | 2.54 | No | No | No | No | No | No | No | No | No | No | 0.55 |
| 47 | High | Very soluble | Yes | No | 1.85 | No | No | No | No | No | No | No | No | No | No | 0.55 |
| 48 | High | Very soluble | Yes | No | 2.46 | No | No | No | No | No | No | No | No | No | No | 0.55 |
| 49 | Low | Soluble | No | Yes | −1.99 | No | No | No | No | No | No | No | No | No | No | 0.17 |
| 50 | Low | Soluble | No | Yes | −1.31 | — | — | — | — | No | No | No | No | No | No | 0.17 |
| 51 | Low | Soluble | No | Yes | −0.95 | — | — | — | — | No | No | No | No | No | No | 0.17 |
| 52 | Low | Soluble | No | No | −1.9 | No | No | No | No | No | No | No | No | No | No | 0.17 |
| 53 | Low | Soluble | No | No | −0.69 | — | — | — | — | No | No | No | No | No | No | 0.17 |
| 54 | High | Highly soluble | No | No | −1.33 | No | No | No | No | No | No | No | No | No | No | 0.55 |
| 55 | — | — | — | — | — | No | No | No | No | — | — | — | — | — | — | — |
| 56 | — | — | — | — | — | No | No | No | No | — | — | — | — | — | — | — |
| 57 | — | — | — | — | — | No | No | No | No | — | — | — | — | — | — | — |
| 58 | — | — | — | — | — | No | No | No | No | — | — | — | — | — | — | — |
| 59 | — | — | — | — | — | Yes | No | No | No | — | — | — | — | — | — | — |
| 60 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 61 | — | — | — | — | — | No | No | No | No | — | — | — | — | — | — | — |
a = GI (gastrointestinal absorption), b = water solubility, c = BBB (blood-brain barrier) permeant, d = the potential for a particular molecule to act as PAINS and obstruct biological assay is predicted by PAINS (pan-assay interference compounds), e = consensus logP (lipophilicity): the mean of five forecasts made with various methods (value < 5), f = mutagenicity (OSIRIS Property Explorer was used to carry out the evaluation), g = tumorigenic (OSIRIS Property Explorer was used to carry out the evaluation), h = irritant (OSIRIS Property Explorer was used to carry out the evaluation), i = reproductive effective (OSIRIS Property Explorer was used to carry out the evaluation), compounds (1 = sinigrin, 2 = progoitrin, 3 = 4-methoxyglucobrassicin, 4 = glucobrassicin, 5 = gluconapin, 6 = 4-hydroxyglucobrassicin, 7 = glucoalyssin, 8 = glucobrassicanapin, 9 = quercetin, 10 = catechin, 11 = kaempferol, 12 = luteolin, 13 = cyanidin, 14 = chlorogenic acid, 15 = p-coumaric acid, 16 = caffeic acid, 17 = gallic acid, 18 = ascorbic acid, 19 = cryptochlorogenic acid, 20 = neochlorogenic acid, 21 = caffeine, 22 = trigonelline, 23 = quercetin-4′-o-glucoside, 24 = myricetin, 25 = taxifolin, 26 = laricitrin, 27 = isorhamnetin, 28 = hyperoside, 29 = spiraeoside, 30 = tectorigenin, 31 = ferulic acid, 32 = protocatechuic acid, 33 = onionin A, 34 = cycloalliin, 35 = isoalliin, 36 = methiin, 37 = propiin, 38 = allicin, 39 = S-methyl-L-cysteine, 40 = S-ethylcysteine, 41 = S-propyl-L-cysteine, 42 = S-allylcysteine, 43 = S-propylmercaptocysteine, 44 = dipropyl trisulfide, 45 = diallyl disulfide, 46 = diisopropyl disulfide, 47 = methyl propenyl disulfide, 48 = 6-methyl-4,5-dithia-1-heptene, 49 = cyanidin 3-glucoside, 50 = cyanidin 3-malonylglucoside, 51 = petunidin 3-glucoside, 52 = malvidin 3-glucoside, 53 = peonidin-3-glucoside, 54 = alliin, 55 = rutin, 56 = violaxanthin, 57 = alpha-tocopherol, 58 = neoxanthin, 59 = quercetin 3,4′-diglucoside, 60 = cyanidin 3-laminaribioside, and 61 = β-carotene).
3.4. In Vitro PDE4B1 Assay
An in vitro assay was performed to confirm the activities of the tested extracts, combination extract (white cabbage, red onion, and coffee), standards, and combination standard (sinigrin, quercetin, and chlorogenic acid) (Figure 6; Table 4). All samples were assayed at a concentration of 100 µg/ml with serial dilutions, and it was found that the sinigrin standard showed a very weak PDE4B1 inhibitory activity (Figure 6(a); Table 4); alternatively, white cabbage extract (Figure 6(b); Table 4) presented higher PDE4B1 inhibition than sinigrin standard. Similarly, the quercetin standard (Figure 6(c); Table 4) displayed PDE4B1 inhibitory activity which was greater than white cabbage extract but less than red onion extract (Figure 6(d); Table 4). Moreover, red onion extract showed this effect significantly more than the quercetin standard, and it is probably owing to the rest compounds such as polyphenols and organosulfates present in the extract. Likewise, chlorogenic acid (Figure 6(e); Table 4) exhibited PDE4B1 inhibition more than red onion while less than coffee extract (Figure 6(f); Table 4). The coffee extract showed a PDE4B1 inhibitory effect significantly greater than chlorogenic acid, and this maybe due to additional compounds such as caffeine present in the extract. Lastly, it was observed that combination extract (Figure 6(h); Table 4) represented significantly excellent PDE4B1 inhibitory activity with IC50 at a concentration of 0.12 ± 0.03 µM among all under test standard and reference samples, even over combination standard (Figure 6(g); Table 4); however, our results were in accordance with the reference (rolipram). In the present study, rolipram (Figure 6(i); Table 4) was employed as a reference drug against all the test extracts and their standards and exhibited a PDE4B1 inhibitory activity slightly less than combination extracts, and contrarily, near to combination standard. IC50 values (PDE4B1 inhibitory activities) of test compounds, extracts, and rolipram are summarized in Table 4 while Figure 6 represents nonlinear regression curves between PDE4 B1% activity and concentration (Log (µM)) of nine tested samples.
Figure 6.
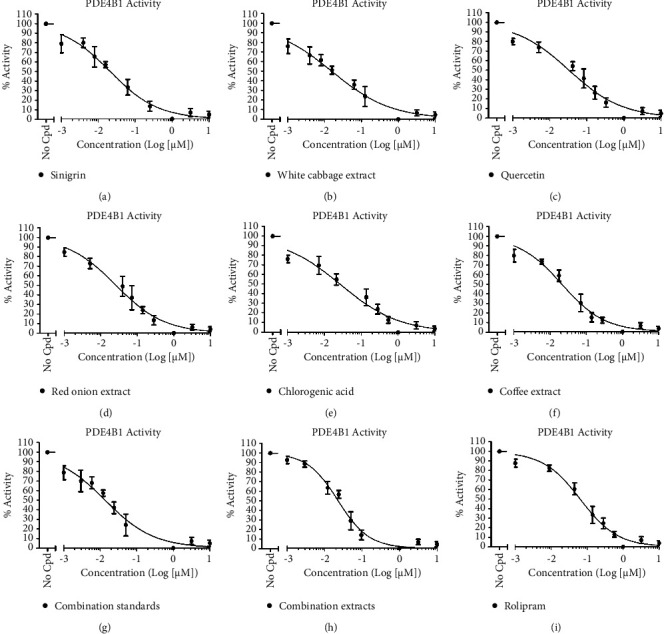
Nonlinear regression curves between the activity (%) of nine tested samples against PDE4B1 and their concentrations (Log (µM)). (a) = sinigrin (IC50 = 0.24 ± 0.01 µM); (b) = white cabbage extract (IC50 = 0.27 ± 0.009 µM); (c) = quercetin (IC50 = 0.25 ± 0.01 µM); (d) = red onion extract (IC50 = 0.22 ± 0.02 µM); (e) = chlorogenic acid (IC50 = 0.20 ± 0.03 µM); (f)=coffee extract (IC50 = 0.18 ± 0.03 µM); (g) = combination (sinigrin, quercetin, and chlorogenic acid) standard (IC50 = 0.17 ± 0.03 µM); (h) = combination (white cabbage, red onion, and coffee) extract (IC50 = 0.12 ± 0.03 µM); (i) = rolipram (a PDE4B inhibitor) (IC50 = 0.15 ± 0.008 µM). PDE4B1 = phosphodiestrase-4B1; No Cpd = no compound.
Table 4.
Summary of PDE4B1 inhibitory activities (IC50 values) of test compounds, extracts, and rolipram.
| Sr. no. | Test sample | IC50 values (µM)∗ |
|---|---|---|
| 1 | Sinigrin | 0.27 ± 0.009 |
| 2 | Chlorogenic acid | 0.20 ± 0.03 |
| 3 | Quercetin | 0.25 ± 0.01 |
| 4 | Combo standard | 0.17 ± 0.03 |
| 5 | Coffee extract | 0.18 ± 0.03 |
| 6 | Red onion | 0.22 ± 0.02 |
| 7 | White cabbage extract | 0.24 ± 0.01 |
| 8 | Combo extract | 0.12 ± 0.03 |
| 9 | Rolipram | 0.15 ± 0.008 |
∗ The values were the average of the three independent experiments, expressed as means standard deviation (SD). IC50 = median inhibitory concentration and µM = micromolar.
4. Discussion
Plants are the hidden source of mysterious therapeutic components [209]. Over the last several decades, the therapeutic value of natural products has long gone unreported in health services [210]. Alternatively, numerous available data indicate that people used to consume herbs to treat a variety of ailments [211]. Contrary to popular belief, plants play a crucial role in the provenance of current allopathic medications [212]. Plant products have been examined based on folklore uses to enhance the quality of life and define the scientific horizon [213]. Cabbage, coffee, and red onion are rich sources of glucosinolates, phenolic acids, and flavonoids, respectively. These phytochemicals participate in controlling multiple diseases, such as neurodegenerative diseases, especially memory dysfunction by targeting PDE4 [30, 39, 40, 46, 47]. The chemical profile of coffee and red onion extracts was analyzed using a TLC-densitometry. Coffee and red onion extracts were dissolved in methanol as stated in the method section and analyzed on a precoated silica-gel TLC plate for the identification of chlorogenic acid and quercetin as major compounds. The current study detected chlorogenic acid and quercetin in coffee and red onion, respectively, and evaluated their in silico and in vitro activities against PDE4B1 as well as pharmacokinetic and toxicity studies. In our previous study, Ahmad et al. 2023 followed the same procedure that identified sinigrin (with an Rf 0.77 at maximum wavelength 399 nm) in white cabbage by using TLC-densitometry analysis [214]; hence, we excluded sinigrin in our current analysis.
Molecular docking was carried out to gain insight into the binding affinity and potential interaction of PDE4B (PDB ID: 4KP6) with the bioactive compounds of white cabbage, coffee, and red onion. The protein targets were validated (redocked) prior to conducting the docking research, and RMSD values were used as a parameter. RMSD is a distinctive characteristic that demonstrates the capacity of both native ligand complexes and proteins to duplicate themselves in the development of an appropriate configuration. The preferred RMSD value is 1 Å, though 2 Å is also appropriate [215]. Sixty-three compounds were tested against PDB4B (PDB ID: 4KP6) by performing their molecular docking with MOE. The authors found that 28 compounds interacted with PDE4B (PDB ID: 4KP6) by binding majorly with amino acid residues (Phe446 and Ile410); however alpha-tocopherol, which is one of the previously reported bioactive components of white cabbage, exhibited the strongest binding affinity for PDE4B (PDB ID: 4KP6) by interacting with Phe446, Ile410, and Gln443 residues while chlorogenic acid with Phe446, Ile410, and His234 and quercetin with Phe446, Ile410, and Asp395 interacted moderately. Unfortunately, sinigrin did not represent any interaction with PDE4B (PDB ID: 4KP6). The present study also exhibits that rolipram showed its affinity by creating an attachment with Phe446 and Asp392 residues. In an earlier investigation, test ligands interacted with PDE4 (1XMU) amino acids (Phe446, Asn395, Gln443, and Ile410) [216]. Similarly, in another study, rolipram generated a contact with Phe446, Asp392, and Ile410 residues of PDE4 [217]. It means that interaction with any one of the amino acids, such as Phe446, Asp392, and Ile410, can result in the inhibition of PDE4B activity. Based on these investigations, our docking results are in accordance with Xia et al. reports [216, 217]. ∆Gbind is a crucial parameter because it shows how strongly compounds attach to the receptor. Even, the stability and strength of the interaction between an enzyme and its ligand are also indicated by a meager ∆Gbind. The pharmacological impacts are influenced by ∆Gbind [215]. The compounds' ∆Gbind was identical to one another, with the standard compound having the lowest free-bond energy. As a result, white cabbage, coffee, and red onion can be able to target PDE4B because their compounds (sinigrin, chlorogenic acid, and quercetin, respectively) interacted with PDE4B during docking, and they may also be an excellent source of a natural cognitive enhancer that can help treat memory impairment. Therefore, it can be suggested that white cabbage, coffee, and red onion extracts should be researched more thoroughly in in vivo investigations.
Drug development has advanced to a new stage that uses computer-based drug design in predicting the ADME characteristics of the medications [218]. A free online tool called SwissADME was used to assess the pharmacokinetics, drug-likeness, and medicinal chemistry friendliness of small compounds. Most of the compounds among sixty-one compounds from white cabbage, red onion, and coffee extracts exhibited to have no predictive toxicities during computational analysis. However, one by third part of the analyzed compounds possess predictive harmful effects which were predicted by SwissADME and OSIRIS. In a previous study, though there was a low GI absorption, quinadoline pharmacophore had an adequate ADME profile with logP and solubility in acceptable limits [219]. The findings from brain and intestinal estimated permeation predictive model (BOILED-Egg) approach for the GI tract and brain permeation for the prediction of the top-ranked secondary metabolites were corroborated with the recent earlier study [20]. The results of this study indicate that additional research on the bioactive compounds of white cabbage, red onion, and coffee extracts against their respective target proteins is necessary to find new therapeutic leads against memory function and AD, as the top-ranked ligands have good pharmacokinetic features.
The idea of virtual screening of secondary metabolites from white cabbage, coffee, and red onion extracts was sparked by the high global prevalence of memory impairments and the absence of effective and safe treatments. According to the previous studies, the computational identification of kobophenol A as a promising lead drug against SARS-CoV-2 infection was confirmed through experimental validation, demonstrating its ability to prevent the binding of S1-RBD from SARS-CoV-2 to the host ACE2 receptor. The data obtained indicated that kobophenol A might be explored further as a pharmacological treatment for SARS-CoV-2 infection that is safe, efficacious, and nontoxic [220]. According to the in silico ADMET prediction, quinadoline B confers high drug-likeness, poor blood-brain barrier penetrability, and high gastrointestinal absorption, in accordance with Lipinski's rule of five. The complexes generated between the top-ranked ligands and their respective protein targets were dynamically stable and had high total free energy of binding, as demonstrated by MD modeling and subsequent total free energy calculation. Consequently, these substances may serve as models or models for the creation of multitargeting ligands directed against SARS-CoV-2 [221]. In another study, MD simulations were performed on the compounds that rated highest in order to assess the stability of the systems over a predetermined period of time and to thoroughly examine the binding mechanism of the most promising derivatives. Ultimately, five compounds with a better affinity for SARS-CoV-2 RdRp were found using the in silico searching procedure, piqueing researchers' interest in pursuing additional research on these compounds as potential antiviral medicines. Notably, the suggested computational methodology offers a convenient computational procedure for hit-to-lead optimization, with implications for anti-SARS-CoV-2 drug development and generally in the drug optimization process [219]. One more study revealed that the complexes formed between the protein targets of the top-ranked ligands were found to be thermodynamically stable by MD modeling. These results, along with the advantageous pharmacokinetic characteristics of the top-ranked ligands, call for additional research on U. alba's secondary metabolites in relation to their individual target proteins in order to identify novel therapeutic approaches against AD and cancer [20]. However, the current work on cabbage, coffee, and red onion extracts could serve as a foundation for the future search and creation of unique cognitive-enhancing drugs.
PDE4B is widely expressed in the different brain regions. PDE4B1, a long isoform of PDE4B [222], is mainly present in hippocampal CA2 and CA3 regions being more specific than the PDE4B gene knockouts, thereby considering it a possible therapeutic target in cognition by promoting neuroplasticity [222, 223] while PDE4B2B, a short isoform, usually expressed in cortex and hippocampus [224] and imparts crucial role in neuroinflammation [225, 226]. Its structural superposition showed a little conformational change, suggesting that the inhibitors might not be PDE4 subfamily-specific [227]. To determine the IC50 values of the tested compounds (sinigrin, quercetin, and chlorogenic acid and their combination), white cabbage, coffee, and red onion and their combination extracts against PDE4B1, an in vitro assay was performed. Rolipram was used as a reference drug for PDE4B1 inhibitory activity. This study revealed different IC50 values between sinigrin and white cabbage extract against PDE4B1 that may be due to the extract containing other phytoconstituents. In one study, sinigrin enhanced intracellular cAMP levels by PDE4 inhibition at its highest concentration 10−5 mol/L [30]. However, literature is still lacking studies on sinigrin and white cabbage extract in connection with PDE4B1. Likewise, quercetin and red onion extract were assayed against PDE4B1 in order to determine IC50 values. Our findings indicated that red onion extract had more PDE4B1 inhibitory activity as compared to quercetin, which is a naturally occurring PDE4-selective inhibitor present in vegetables, tea, and fruits [228]. Although quercetin inhibitory activity for PDE4D is available currently, a study gap for quercetin and red onion extract on PDE4B1 is still present. However, only quercetin 3-O–α-L-arabinopyranosyl-(1⟶2)-O–α-L-rhamnopyranoside, a derivative of quercetin, was found to show inhibitory activity against PDE4B with an inhibition 100% in an in vitro assay [229]. Similarly, chlorogenic acid and coffee extract were assayed against PDE4B1 in order to examine their PDE4B1 inhibitory activities. The results of the current study indicated that coffee extract had more PDE4B1 inhibitory activity as compared to chlorogenic acid. Though chlorogenic acid was evaluated against PDE4 only in previous studies, the data are not available on chlorogenic acid and coffee extract against PDE4B1. The authors employed rolipram as a reference drug against tested compounds (sinigrin, quercetin, and chlorogenic acid and their combination), combination (white cabbage, coffee, and red onion and their combination) extracts for PDE4B1 inhibitory activity. Our investigation exhibited that rolipram inhibited PDE4B1 more than all tested samples except combination extracts. In a previous study, Xia et al. determined the PDE4B1 inhibitory activity of rolipram in an in vitro fluorescence polarization assay where it showed IC50 at a concentration of 0.13 ± 0.02 µM [216]. Additionally, rolipram was also analyzed against PDE4B1 in an in vitro enzymatic assay and showed IC50 at a concentration of 0.15 ± 0.02 µM by Tang et al. [230]. Our results for all test compounds, extracts, and reference rolipram were consistent with the earlier studies against PDE4 B. As per the previous report, a single-dose triple combination has more efficacy, safety, and fewer side effects than high-dose single therapy and/or twice-a-day double therapy [231]. Therefore, based on our in vitro experiment, a single-dose triple combination of extracts treatment can be safer and more effective than mono and double extracts in treating memory function. However, a further, in vivo approach is guaranteed to strengthen this evidence.
To explain how cabbage, coffee, and red onion extracts work, the author made a proposed mechanism of action targeting PDE4B that can help in further in vivo studies and drug development as well (Figure 7). According to the current findings of the molecular analysis, cabbage, coffee, and red onion extracts work to prevent memory loss by acting as a PDE4B inhibitor. The previous study embarks that inhibition of PDE4B enzyme triggers the activation of cAMP/PKA/CREB/BDNF signaling pathway and subsequently raises the expression of BDNF protein in the hippocampus, thereby improving memory function [232]. According to the previously reported study findings [30, 40, 46], chlorogenic acid, quercetin, and sinigrin from coffee, red onion, and white cabbage extracts, respectively, have both in vitro and in vivo PDE4 inhibitory actions. Effective methods for raising intracellular cAMP levels include inhibition of this enzyme which is beneficial for improving memory function [30, 39, 46, 233]. The cabbage, coffee, and red onion extracts activate the adenylate cyclase pathway, as cAMP/PKA/CREB/BDNF pathway, thereby increasing the level of BDNF, a protein found in synapses, BDNF controls the expansion, upkeep, and maturation of brain neurons, expression in the hippocampus and preventing the memory loss. The brain's temporal lobe, cerebral cortex, and hippocampal regions are where this protein is primarily expressed. BDNF is crucial for preserving the form and functionality of neurons. A deficiency of neurotrophic protein can raise the risk of neuronal loss, which can cause damage and death to brain neurons. This loss of neurons impairs brain function physiologically and results in cognitive issues, including memory loss [1]. However, the compounds present in white cabbage, coffee, and red onion extracts have the ability to block PDE4B and reverse memory loss.
Figure 7.
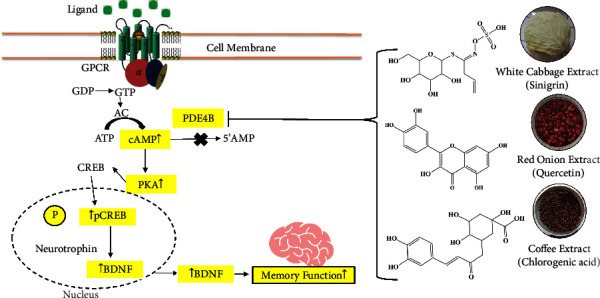
Mechanism of action of cabbage, coffee, and red onion extracts. GPCR = G-protein coupled receptor, GDP = guanosine diphosphate, GTP = guanosine triphosphate, AC = adenylyl cyclase, ATP = adenosine triphosphate, cAMP = cyclic adenosine monophosphate, 5′-AMP = 5-adenosine monophosphate, PKA = protein kinase (A) BDNF = brain-derived neurotrophic factor, CREB = cAMP-response element binding, pCREB = phosphorylated cAMP-response element binding.
It is unknown if PDE4B negatively influences the pathway involved in CREB-mediated neuronal survival. The discovery of PDE4B-selective inhibitors has opened up new avenues for research into the dual function of PDE4B as a pro-survival and anti-inflammatory target for a variety of neurological conditions and traumas especially in cognition.
5. Conclusion
In summary, quercetin and chlorogenic acid were identified by TLC-densitometry as the major compounds in red onion and coffee, respectively. The molecular docking study showed that among 28 bioactive components, alpha-tocopherol had the best binding interaction with the PDE4B whereas chlorogenic acid and quercetin demonstrated moderate interaction. In this experiment, sinigrin did not show the interaction with PDE4B. The majority of compounds presented no toxicities. The combination extracts showed stronger inhibition against PDE4B1 as compared to the combination standard and reference (rolipram). Further research on an in vivo model is needed to confirm this activity.
Acknowledgments
The authors thank Universitas Gadjah Mada for funding our work with the Hibah RTA grant (Grant ID: 5075/UN1.PII/DITLIT/Dit-Lit/PT.01.00/2023).
Data Availability
The data used to support the findings of this study are included in the article.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors' Contributions
N.A. carried out the experiments, analyzed the data, and wrote the manuscript. Z.I., N.F., and N.A. contributed to the experimental design. K.N.L. drew chemical structures, proofread, and checked for grammar. N.S.O.U. reviewed and edited the manuscript. Z.I., N.F., and A.S. supervised the project. All authors read and approved the final manuscript.
References
- 1.Ahmad N., Lesa K. N., Sudarmanto A., Fakhrudin N., Ikawati Z. The role of phosphodiesterase-1 and its natural product inhibitors in alzheimer’s disease: a review. Frontiers in Pharmacology . 2022;13(12):1070677–1070716. doi: 10.3389/fphar.2022.1070677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarawneh H. Y., Menegola H. K., Peou A., Tarawneh H., Jayakody D. M. P. Central auditory functions of alzheimer’s disease and its preclinical stages: a systematic review and meta-analysis. Cells . 2022;11(6):1007–1030. doi: 10.3390/CELLS11061007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fakhrudin N., Fakhrudin N., Nurrochmad A., Sudarmanto B. S. A., Ikawati Z. In vitro and in silico studies of secang wood (Caesalpinia sappan L.) extracts and Brazilin as natural phosphodiesterase-1 (PDE1) inhibitor for herbal cognitive enhancer development. Research Journal of Pharmacy and Technology . 2020;13(5):2269–2274. doi: 10.5958/0974-360X.2020.00409.6. [DOI] [Google Scholar]
- 4.Schifano F., Catalani V., Sharif S., et al. Benefits and harms of “smart drugs” (nootropics) in healthy individuals. Drugs . 2022;82(6):633–647. doi: 10.1007/S40265-022-01701-7. [DOI] [PubMed] [Google Scholar]
- 5.Malík M., Tlustoš P. Nootropics as cognitive enhancers: types, dosage and side effects of smart drugs. Nutrients . 2022;14(16):3367–3394. doi: 10.3390/NU14163367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Nema M. Y., Gaurav A. Phosphodiesterase as a target for cognition enhancement in schizophrenia. Current Topics in Medicinal Chemistry . 2020;20(26):2404–2421. doi: 10.2174/1568026620666200613202641. [DOI] [PubMed] [Google Scholar]
- 7.Yanai S., Toyohara J., Ishiwata K., Ito H., Endo S. Long-term cilostazol administration ameliorates memory decline in senescence-accelerated mouse prone 8 (SAMP8) through a dual effect on CAMP and blood-brain barrier. Neuropharmacology . 2017;116(4):247–259. doi: 10.1016/j.neuropharm.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Xu Y., Deng C., Zheng Y., Liu N., Fu B. Applying vinpocetine to reverse synaptic ultrastructure by regulating BDNF-related PSD-95 in alleviating schizophrenia-like deficits in rat. Comprehensive Psychiatry . 2019;94(10):152122–152130. doi: 10.1016/J.COMPPSYCH.2019.152122. [DOI] [PubMed] [Google Scholar]
- 9.Van Puyvelde M., Van Cutsem J., Lacroix E., Pattyn N. A state-of-the-art review on the use of modafinil as A performance-enhancing drug in the context of military operationality. Military Medicine . 2022;187(11-12):1286–1298. doi: 10.1093/milmed/usab398. [DOI] [PubMed] [Google Scholar]
- 10.Ienca M., Shaw D. M., Elger B. Cognitive enhancement for the ageing world: opportunities and challenges. Ageing and Society . 2019;39(10):2308–2321. doi: 10.1017/S0144686X18000491. [DOI] [Google Scholar]
- 11.McDonough W., Aragon I. V., Rich J., et al. PAN-selective inhibition of CAMP-phosphodiesterase 4 (PDE4) induces gastroparesis in mice. The FASEB Journal . 2020;34(9):12533–12548. doi: 10.1096/fj.202001016RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H., Gaur U., Xiao J., Xu B., Xu J., Zheng W. Targeting phosphodiesterase 4 as a potential therapeutic strategy for enhancing neuroplasticity following ischemic stroke. International Journal of Biological Sciences . 2018;14(12):1745–1754. doi: 10.7150/ijbs.26230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhat A., Bishir M., Pandi-Perumal S. R., Chang S. L., Chidambaram S. B. Roflumilast, a phosphodiesterase-4 inhibitor, ameliorates sleep deprivation-induced cognitive dysfunction in C57bl/6J mice. ACS Chemical Neuroscience . 2022;13(13):1938–1947. doi: 10.1021/acschemneuro.2c00127. [DOI] [PubMed] [Google Scholar]
- 14.Bhat A., Ray B., Mahalakshmi A. M., et al. Phosphodiesterase-4 enzyme as a therapeutic target in neurological disorders. Pharmacological Research . 2020;160:105078–105095. doi: 10.1016/J.PHRS.2020.105078. [DOI] [PubMed] [Google Scholar]
- 15.Teich A. F., Nicholls R. E., Puzzo D., et al. Synaptic therapy in alzheimer’s disease: a CREB-centric approach. Neurotherapeutics . 2015;12(1):29–41. doi: 10.1007/s13311-014-0327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar A., Sharma V., Singh V. P., et al. Herbs to curb cyclic nucleotide phosphodiesterase and their potential role in alzheimer’s disease. Mechanism of Ageing and Development . 2015;149:75–87. doi: 10.1016/j.mad.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Soares L. M., De Vry J., Steinbusch H. W. M., Milani H., Prickaerts J., Weffort de Oliveira R. M. Rolipram improves cognition, reduces anxiety- and despair-like behaviors and impacts hippocampal neuroplasticity after transient global cerebral ischemia. Neuroscience . 2016;326:69–83. doi: 10.1016/j.neuroscience.2016.03.062. [DOI] [PubMed] [Google Scholar]
- 18.Sugin L. J. S., Murugesan A., Bindu M., Sunil K. N. Roflumilast: a potential drug for the treatment of cognitive impairment? Neuroscience Letters . 2020;736:135281–135328. doi: 10.1016/j.neulet.2020.135281. [DOI] [PubMed] [Google Scholar]
- 19.Gewald R., Grunwald C., Egerland U. Discovery of triazines as potent, selective and orally active PDE4 inhibitors. Bioorganic and Medicinal Chemistry Letters . 2013;23(15):4308–4314. doi: 10.1016/j.bmcl.2013.05.099. [DOI] [PubMed] [Google Scholar]
- 20.Quimque M. T., Notarte K. I., Letada A., et al. Potential cancer- and alzheimer’s disease-targeting phosphodiesterase inhibitors from Uvaria alba: insights from in vitro and consensus virtual screening. ACS Omega . 2021;6(12):8403–8417. doi: 10.1021/acsomega.1c00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Notarte K. I. R., Quimque M. T. J., Macaranas I. T., et al. Attenuation of lipopolysaccharide-induced inflammatory responses through inhibition of the NF-κb pathway and the increased NRF2 level by a flavonol-enriched n-butanol fraction from Uvaria alba. ACS Omega . 2023;8(6):5377–5392. doi: 10.1021/acsomega.2c06451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y. Z., Wang Y. L., Che H. J., et al. Sappanone A: a natural PDE4 inhibitor with dual anti-inflammatory and antioxidant activities from the heartwood of Caesalpinia sappan L. Journal of Ethnopharmacology . 2023;304:116020–116116. doi: 10.1016/j.jep.2022.116020. [DOI] [PubMed] [Google Scholar]
- 23.Liang J., Huang Y. Y., Zhou Q., et al. Discovery and optimization of α-mangostin derivatives as novel PDE4 inhibitors for the treatment of vascular dementia. Journal of Medicinal Chemistry . 2020;63(6):3370–3380. doi: 10.1021/acs.jmedchem.0c00060. [DOI] [PubMed] [Google Scholar]
- 24.Ray L. R., Alam M. S., Junaid M., et al. Brassica oleracea var. Capitata f. Alba: a review on its botany, traditional uses, phytochemistry and pharmacological activities. Mini Reviews in Medicinal Chemistry . 2021;21(16):2399–2417. doi: 10.2174/1389557521666210111150036. [DOI] [PubMed] [Google Scholar]
- 25.Yadav M., Parle M., Dhingra M. S. Protective effect of Brassica oleracea juice against ketamine-induced stereotyped behaviours in mice. Journal of Medicinal Plants . 2017;5:200–204. [Google Scholar]
- 26.Güller U., Güller P., Çiftci M. Radical scavenging and antiacetylcholinesterase activities of ethanolic extracts of carob, clove, and linden. Alternative Therapies in Health and Medicine . 2021;27(5):33–37. [PubMed] [Google Scholar]
- 27.Kim H., Lee Y., Kim S., et al. Anti-inflammatory effects of Brassica oleracea Var. capitata L. (Cabbage) methanol extract in mice with contact dermatitis. Pharmacognosy Magazine . 2018;14(54):174–179. doi: 10.4103/pm.pm_152_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryou S. H., Cho I. J., Choi B. R., Kim M. B., Kwon Y. S., Ku S. K. Brassica oleracea var. Capitata L. Alleviates indomethacin-induced acute gastric injury by enhancing anti-inflammatory and antioxidant activity. Processes . 2021;9(2):372–386. doi: 10.3390/pr9020372. [DOI] [Google Scholar]
- 29.Dal Prá V., Dolwitsch C. B., Lima F. O., et al. Ultrasound-assisted extraction and biological activities of extracts of Brassica oleracea var. capitata. Food Technology and Biotechnology . 2015;53(1):102–109. doi: 10.17113/ftb.53.01.15.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chu S., Liu W., Lu Y., et al. Sinigrin enhanced antiasthmatic effects of beta adrenergic receptors agonists by regulating CAMP-mediated pathways. Frontiers in Pharmacology . 2020;11(5):723–728. doi: 10.3389/fphar.2020.00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoang M. H., Nguyen T. A. T., Nguyen V. N. H., Vo T. N. Cafestol analogues from coffea canephora: in vitro inhibition and molecular docking to α-glucosidase. Natural Product Research . 2022;38(3):379–385. doi: 10.1080/14786419.2022.2123479. [DOI] [PubMed] [Google Scholar]
- 32.Faradilla M., Maysarah H., Sari I., Kwok K. Formulation of effervescent granule from robusta green coffee bean ethanolic extract (coffea canephora) Journal of Pharmacy and BioAllied Sciences . 2020;12(6):S743–S746. doi: 10.4103/jpbs.JPBS_258_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Husni M. A., Nugroho A. K., Sulaiman T. N. S., Fakhrudin N. Microencapsulation of green coffee beans (coffea canephora) extract using whey protein concentrate. Iraqi Journal of Pharmaceutical Sciences . 2022;31(1):20–31. doi: 10.31351/vol31iss1pp20-31. [DOI] [Google Scholar]
- 34.Husni M. A., Nugroho A. K., Fakhrudin N., Sulaiman T. N. S. Microencapsulation of ethyl acetate extract from green coffee beans (coffea canephora) by spray drying method. Indonesian Journal of Pharmacy . 2021;32(2):221–231. doi: 10.22146/ijp.1457. [DOI] [Google Scholar]
- 35.Ciaramelli C., Palmioli A., De Luigi A., et al. NMR-driven identification of anti-amyloidogenic compounds in green and roasted coffee extracts. Food Chemistry . 2018;252:171–180. doi: 10.1016/J.FOODCHEM.2018.01.075. [DOI] [PubMed] [Google Scholar]
- 36.Fatimatuzzahro N., Prasetya R. C., Anggara K. D. N. Robusta coffee (coffea canephora) down regulation TNF-α expression in carotid artery endothelial cell of hyperlipidemia rat model. Trends in Sciences . 2022;19(4):2199–2210. doi: 10.48048/tis.2022.2199. [DOI] [Google Scholar]
- 37.Tarigan E. B., Wardiana E., Hilmi Y. S., Komarudin N. A. The changes in chemical properties of coffee during roasting: a review. IOP Conference Series: Earth and Environmental Science . 2022;974(1):012115–012124. doi: 10.1088/1755-1315/974/1/012115. [DOI] [Google Scholar]
- 38.Herawati D., Giriwono P. E., Dewi F. N. A., Kashiwagi T., Andarwulan N. Three major compounds showing significant antioxidative, α-glucosidase inhibition, and antiglycation activities in robusta coffee brew. International Journal of Food Properties . 2019;22(1):994–1010. doi: 10.1080/10942912.2019.1622562. [DOI] [Google Scholar]
- 39.Cornelis M. C., Bennett D. A., Weintraub S., Schneider J. A., Morris M. C. Caffeine consumption and dementia: are lewy bodies the link? Annals of Neurology . 2022;91(6):834–846. doi: 10.1002/ANA.26349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maher A., El Sayed N., Nafea H., Gad M. Rolipram rescues memory consolidation deficits caused by sleep deprivation: implication of the CAMP/PKA and CAMP/epac pathways. CNS and Neurological Disorders- Drug Targets . 2022;21(7):631–639. doi: 10.2174/1871527320666210816105144. [DOI] [PubMed] [Google Scholar]
- 41.Jaiswal N., Kumar D., Rizvi S. I. Red onion extract (Allium cepa L.) supplementation improves redox balance in oxidatively stressed rats. Food Science and Human Wellness . 2013;2(2):99–104. doi: 10.1016/j.fshw.2013.05.003. [DOI] [Google Scholar]
- 42.Zaki N. L., Abd-Elhak N. A., Abd El-Rahman H. S. M. The utilization of yellow and red onion peels and their extracts as antioxidant and antimicrobial in preservation of beef burger during storage. Advance Journal of Food Science and Technology . 2022;10(1):1–9. doi: 10.12691/ajfst-10-1-1. [DOI] [Google Scholar]
- 43.Sami R., Elhakem A., Alharbi M., et al. In-vitro evaluation of the antioxidant and anti-inflammatory activity of volatile compounds and minerals in five different onion varieties. Separations . 2021;8(5):57–65. doi: 10.3390/SEPARATIONS8050057. [DOI] [Google Scholar]
- 44.Marefati N., Ghorani V., Shakeri F., et al. A review of anti-inflammatory, antioxidant, and immunomodulatory effects of Allium cepa and its main constituents. Pharmaceutical Biology . 2021;59(1):285–300. doi: 10.1080/13880209.2021.1874028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alipour Nosrani E., Tamtaji O. R., Alibolandi Z., et al. Neuroprotective effects of probiotics bacteria on animal model of Parkinson’s disease induced by 6-hydroxydopamine: a behavioral, biochemical, and histological study. Journal of Immunoassay and Immunochemistry . 2021;42(2):106–120. doi: 10.1080/15321819.2020.1833917. [DOI] [PubMed] [Google Scholar]
- 46.Vauzour D. Dietary polyphenols as modulators of brain functions: biological actions and molecular mechanisms underpinning their beneficial effects. Oxidative Medicine and Cellular Longevity . 2012;2012:16. doi: 10.1155/2012/914273.914273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sagar N. A., Pareek S., Benkeblia N., Xiao J. Onion (Allium cepa L.) bioactives: chemistry, pharmacotherapeutic functions, and industrial applications. Food Frontiers . 2022;3(3):380–412. doi: 10.1002/FFT2.135. [DOI] [Google Scholar]
- 48.Saleem U., Ahmad N., Shah M. A., Anwar F., Ahmad B. Anti-urolithiatic activity of salvia hispanica L. Seeds in ethylene glycol induced urolithiasis rat’s model. Anais da Academia Brasileira de Ciências . 2020;92(4):202000677–e20200112. doi: 10.1590/0001-3765202020200067. [DOI] [PubMed] [Google Scholar]
- 49.Corrigan H., Dunne A., Purcell N., et al. Conceptual functional-by-design optimisation of the antioxidant capacity of trans-resveratrol, quercetin, and chlorogenic acid: application in a functional tea. Food Chemistry . 2023;428(4):136764–136775. doi: 10.1016/j.foodchem.2023.136764. [DOI] [PubMed] [Google Scholar]
- 50.Prabhakar P. K., Kumar A., Doble M. Combination therapy: a new strategy to manage diabetes and its complications. Phytomedicine . 2014;21(2):123–130. doi: 10.1016/J.PHYMED.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 51.Caesar L. K., Cech N. B., Kubanek J., Linington R., Luesch H. Synergy and antagonism in natural product extracts: when 1 + 1 does not equal 2. Natural Product Reports . 2019;36(6):869–888. doi: 10.1039/C9NP00011A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kapusta-Duch J., Kusznierewicz B. Young shoots of white and red headed cabbages like novel sources of glucosinolates as well as antioxidative substances. Antioxidants . 2021;10(8):1277–1292. doi: 10.3390/antiox10081277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mazumder A., Dwivedi A., du Preez J. L., du Plessis J. In vitro wound healing and cytotoxic effects of sinigrin–phytosome complex. International Journal of Pharmaceutics . 2016;498(1-2):283–293. doi: 10.1016/j.ijpharm.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 54.Jie M., Cheung W. M., Yu V., Zhou Y., Tong P. H., Ho J. W. S. Anti-proliferative activities of sinigrin on carcinogen-induced hepatotoxicity in rats. PLoS One . 2014;9(10):e110145–e110149. doi: 10.1371/journal.pone.0110145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greer M. A., Deeney J. M. Antithyroid activity elicited by the ingestion of pure progoitrin, A naturally occurring thioglycoside of the turnip family. Journal of Clinical Investigation . 1959;38(9):1465–1474. doi: 10.1172/jci103923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim S. J., Ishii G. Glucosinolate profiles in the seeds, leaves and roots of rocket salad (eruca sativa mill.) and anti-oxidative activities of intact plant powder and purified 4-methoxyglucobrassicin. Soil Science and Plant Nutrition . 2006;52(3):394–400. doi: 10.1111/j.1747-0765.2006.00049.x. [DOI] [Google Scholar]
- 57.Cabello-Hurtado F., Gicquel M., Esnault M. A. Evaluation of the antioxidant potential of cauliflower (Brassica oleracea) from a glucosinolate content perspective. Food Chemistry . 2012;132(2):1003–1009. doi: 10.1016/j.foodchem.2011.11.086. [DOI] [Google Scholar]
- 58.Washida K., Miyata M., Koyama T., Yazawa K., Nomoto K. Suppressive effect of yamato-mana (Brassica rapa L. Oleifera group) constituent 3-butenyl glucosinolate (gluconapin) on postprandial hypertriglyceridemia in mice. Bioscience, Biotechnology, and Biochemistry . 2010;74(6):1286–1289. doi: 10.1271/bbb.100018. [DOI] [PubMed] [Google Scholar]
- 59.Lee S. Y., Kwon H., Kim J. K., Park C. H., Sathasivam R., Park S. U. Comparative analysis of glucosinolate and phenolic compounds in green and red kimchi cabbage (Brassica rapa L. Ssp. pekinensis) hairy roots after exposure to light and dark conditions. Horticulturae . 2023;9(4):466–480. doi: 10.3390/horticulturae9040466. [DOI] [Google Scholar]
- 60.Park M. H., Arasu M. V., Park N. Y., et al. Variation of glucoraphanin and glucobrassicin: anticancer components in Brassica during processing. Food Science and Technology . 2013;33(4):624–631. doi: 10.1590/S0101-20612013000400005. [DOI] [Google Scholar]
- 61.Blažević I., Radonić A., Mastelić J., Zekić M., Skočibušić M., Maravić A. G. Glucosinolates, glycosidically bound volatiles and antimicrobial activity of Aurinia sinuata (Brassicaceae) Food Chemistry . 2010;121(4):1020–1028. doi: 10.1016/j.foodchem.2010.01.041. [DOI] [Google Scholar]
- 62.Lee M. K., Chun J. H., Byeon D. H., et al. Variation of glucosinolates in 62 varieties of Chinese cabbage (Brassica rapa L. Ssp. pekinensis) and their antioxidant activity. LWT- Food Science and Technology . 2014;58(1):93–101. doi: 10.1016/j.lwt.2014.03.001. [DOI] [Google Scholar]
- 63.Oboh G., Ademiluyi A. O., Ogunsuyi O. B., Oyeleye S. I., Dada A. F., Boligon A. A. Cabbage and cucumber extracts exhibited anticholinesterase, antimonoamine oxidase and antioxidant properties. Journal of Food Biochemistry . 2017;41(3):e12358–e12364. doi: 10.1111/jfbc.12358. [DOI] [Google Scholar]
- 64.Caparica R., Júlio A., Araújo M. E. M., et al. Anticancer activity of rutin and its combination with ionic liquids on renal cells. Biomolecules . 2020;10(2):233–247. doi: 10.3390/biom10020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Afanas’eva I. B., Ostrakhovitch E. A., Mikhal’chik E. V., Ibragimova G. A., Korkina L. G. Enhancement of antioxidant and anti-inflammatory activities of bioflavonoid rutin by complexation with transition metals. Biochemical Pharmacology . 2001;61(6):677–684. doi: 10.1016/S0006-2952(01)00526-3. [DOI] [PubMed] [Google Scholar]
- 66.Jiménez-Aliaga K., Bermejo-Bescós P., Benedí J., Martín-Aragón S. Quercetin and rutin exhibit antiamyloidogenic and fibril-disaggregating effects in vitro and potent antioxidant activity in APPswe cells. Life Sciences . 2011;89(25-26):939–945. doi: 10.1016/j.lfs.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 67.Lesjak M., Beara I., Simin N., et al. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. Journal of Functional Foods . 2018;40(1):68–75. doi: 10.1016/j.jff.2017.10.047. [DOI] [Google Scholar]
- 68.You J., Luo Y., Wu J. Conjugation of ovotransferrin with catechin shows improved antioxidant activity. Journal of Agricultural and Food Chemistry . 2014;62(12):2581–2587. doi: 10.1021/jf405635q. [DOI] [PubMed] [Google Scholar]
- 69.Kajiya K., Hojo H., Suzuki M., Nanjo F., Kumazawa S., Nakayama T. Relationship between antibacterial activity of (+)-Catechin derivatives and their interaction with a model membrane. Journal of Agricultural and Food Chemistry . 2004;52(6):1514–1519. doi: 10.1021/jf0350111. [DOI] [PubMed] [Google Scholar]
- 70.Menon L. G., Kuttan R., Kuttan G. Anti-metastatic activity of curcumin and catechin. Cancer Letters . 1999;141(1-2):159–165. doi: 10.1016/S0304-3835(99)00098-1. [DOI] [PubMed] [Google Scholar]
- 71.Tatsimo S. J. N., Tamokou J. d. D., Havyarimana L., et al. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from bryophyllum pinnatum. BMC Research Notes . 2012;5(1):158–163. doi: 10.1186/1756-0500-5-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang J., Fang X., Ge L., et al. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS One . 2018;13(5):01975633–e197612. doi: 10.1371/journal.pone.0197563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liao W., Chen L., Ma X., Jiao R., Li X., Wang Y. Protective effects of kaempferol against reactive oxygen species-induced hemolysis and its antiproliferative activity on human cancer cells. European Journal of Medicinal Chemistry . 2016;114(5):24–32. doi: 10.1016/j.ejmech.2016.02.045. [DOI] [PubMed] [Google Scholar]
- 74.Fan W., Qian S., Qian P., Li X. Antiviral activity of luteolin against Japanese encephalitis virus. Virus Research . 2016;220(7):112–116. doi: 10.1016/j.virusres.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 75.Coleta M., Campos M. G., Cotrim M. D., Lima T. C. M. d., Cunha A. P. d. Assessment of luteolin (3′,4′,5,7-tetrahydroxyflavone) neuropharmacological activity. Behavioural Brain Research . 2008;189(1):75–82. doi: 10.1016/j.bbr.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 76.Seelinger G., Merfort I., Schempp C. M. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Medica . 2008;74(14):1667–1677. doi: 10.1055/s-0028-1088314. [DOI] [PubMed] [Google Scholar]
- 77.Ziyan L., Yongmei Z., Nan Z., Ning T., Baolin L. Evaluation of the anti-inflammatory activity of luteolin in experimental animal models. Planta Medica . 2007;73(3):221–226. doi: 10.1055/s-2007-967122. [DOI] [PubMed] [Google Scholar]
- 78.Tsuda T., Watanabe M., Ohshima K., et al. Antioxidative activity of the anthocyanin pigments cyanidin 3-O-.Beta.-D-Glucoside and cyanidin. Journal of Agricultural and Food Chemistry . 1994;42(11):2407–2410. doi: 10.1021/jf00047a009. [DOI] [Google Scholar]
- 79.Noda Y., Kaneyuki T., Mori A., Packer L. Antioxidant activities of pomegranate fruit extract and its anthocyanidins: delphinidin, cyanidin, and pelargonidin. Journal of Agricultural and Food Chemistry . 2002;50(1):166–171. doi: 10.1021/jf0108765. [DOI] [PubMed] [Google Scholar]
- 80.Wang H., Nair M. G., Strasburg G. M., et al. Antioxidant and antiinflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. Journal of Natural Products . 1999;62(2):294–296. doi: 10.1021/np980501m. [DOI] [PubMed] [Google Scholar]
- 81.Acquaviva R., Russo A., Galvano F., et al. Cyanidin and cyanidin 3-O-β-D-glucoside as DNA cleavage protectors and antioxidants. Cell Biology and Toxicology . 2003;19(4):243–252. doi: 10.1023/B:CBTO.0000003974.27349.4e. [DOI] [PubMed] [Google Scholar]
- 82.Kapusta-Duch J., Leszczyńska T., Filipiak-Florkiewicz A. Comparison of total polyphenol contents and antioxidant activity in cruciferous vegetables grown in diversified ecological conditions. Acta Scientiarum Polonorum Technologia Alimentaria . 2012;11(4):335–346. [Google Scholar]
- 83.Shimoyama A. T., Santin J. R., Machado I. D., et al. Antiulcerogenic activity of chlorogenic acid in different models of gastric ulcer. Naunyn-Schmiedeberg’s Archives of Pharmacology . 2013;386(1):5–14. doi: 10.1007/s00210-012-0807-2. [DOI] [PubMed] [Google Scholar]
- 84.Sato Y., Itagaki S., Kurokawa T., et al. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. International Journal of Pharmaceutics . 2011;403(1-2):136–138. doi: 10.1016/j.ijpharm.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 85.Wang G. F., Shi L. P., Ren Y. D., et al. Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro. Antiviral Research . 2009;83(2):186–190. doi: 10.1016/j.antiviral.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 86.Wu L. Effect of chlorogenic acid on antioxidant activity of flos lonicerae extracts. Journal of Zhejiang University- Science B . 2007;8(9):673–679. doi: 10.1631/jzus.2007.B0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu J. G., Hu Q. P., Liu Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. Journal of Agricultural and Food Chemistry . 2012;60(46):11625–11630. doi: 10.1021/jf303771s. [DOI] [PubMed] [Google Scholar]
- 88.Kiliç I., Yeşiloğlu Y. Spectroscopic studies on the antioxidant activity of P-coumaric acid. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy . 2013;115(11):719–724. doi: 10.1016/j.saa.2013.06.110. [DOI] [PubMed] [Google Scholar]
- 89.Kong C. S., Jeong C. H., Choi J. S., Kim K. J., Jeong J. W. Antiangiogenic effects of P-coumaric acid in human endothelial cells. Phytotherapy Research . 2013;27(3):317–323. doi: 10.1002/PTR.4718. [DOI] [PubMed] [Google Scholar]
- 90.Lou Z., Wang H., Rao S., Sun J., Ma C., Li J. P. p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control . 2012;25(2):550–554. doi: 10.1016/j.foodcont.2011.11.022. [DOI] [Google Scholar]
- 91.Gülçin İ. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid) Toxicology . 2006;217(2-3):213–220. doi: 10.1016/j.tox.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 92.Spagnol C. M., Assis R. P., Brunetti I. L., Isaac V. L. B., Salgado H. R. N., Corrêa M. A. In vitro methods to determine the antioxidant activity of caffeic acid. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy . 2019;219:358–366. doi: 10.1016/j.saa.2019.04.025. [DOI] [PubMed] [Google Scholar]
- 93.Borges A., Ferreira C., Saavedra M. J., Simões M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microbial Drug Resistance . 2013;19(4):256–265. doi: 10.1089/MDR.2012.0244. [DOI] [PubMed] [Google Scholar]
- 94.de Cristo Soares Alves A., Mainardes R. M., Khalil N. M. Nanoencapsulation of gallic acid and evaluation of its cytotoxicity and antioxidant activity. Materials Science and Engineering: C . 2016;60:126–134. doi: 10.1016/j.msec.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 95.Li Z. J., Liu M., Dawuti G., et al. Antifungal activity of gallic acid in vitro and in vivo. Phytotherapy Research . 2017;31(7):1039–1045. doi: 10.1002/PTR.5823. [DOI] [PubMed] [Google Scholar]
- 96.Singh J., Upadhyay A. K., Bahadur A., Singh B., Singh K. P., Rai M. Antioxidant phytochemicals in cabbage (Brassica oleracea L. Var. Capitata) Scientia Horticulturae . 2006;108(3):233–237. doi: 10.1016/j.scienta.2006.01.017. [DOI] [Google Scholar]
- 97.Östman B., Michaëlsson K., Helmersson J., et al. Oxidative stress and bone mineral density in elderly men: antioxidant activity of alpha-tocopherol. Free Radical Biology and Medicine . 2009;47(5):668–673. doi: 10.1016/j.freeradbiomed.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 98.Martinez-Villaluenga C., Peñas E., Sidro B., Ullate M., Frias J., Vidal-Valverde C. White cabbage fermentation improves ascorbigen content, antioxidant and nitric oxide production inhibitory activity in LPS-induced macrophages. LWT-Food Science and Technology . 2012;46(1):77–83. doi: 10.1016/j.lwt.2011.10.023. [DOI] [Google Scholar]
- 99.Gęgotek A., Skrzydlewska E. Antioxidative and anti-inflammatory activity of ascorbic acid. Antioxidants . 2022;11(10):p. 1993. doi: 10.3390/antiox11101993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chandra-Hioe M. V., Rahman H. H., Arcot J. Lutein and β-carotene in selected asian leafy vegetables. Journal of Food Chemistry and Nanotechnology . 2017;03(03):93–97. doi: 10.17756/jfcn.2017-043. [DOI] [Google Scholar]
- 101.Mueller L., Boehm V. Antioxidant activity of β-carotene compounds in different in vitro assays. Molecules . 2011;16(2):1055–1069. doi: 10.3390/molecules16021055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jayappriyan K. R., Rajkumar R., Venkatakrishnan V., Nagaraj S., Rengasamy R. In vitro anticancer activity of natural β-carotene from Dunaliella salina EU5891199 in PC-3 cells. Biomedicine and Preventive Nutrition . 2013;3(2):99–105. doi: 10.1016/j.bionut.2012.08.003. [DOI] [Google Scholar]
- 103.Mathews-Roth M. M. Antitumor Activity of <i>β</i>-Carotene, Canthaxanthin and Phytoene. Oncology . 1982;39(1):33–37. doi: 10.1159/000225601. [DOI] [PubMed] [Google Scholar]
- 104.Kruk J. Occurrence of chlorophyll precursors in leaves of cabbage heads- the case of natural etiolation. Journal of Photochemistry and Photobiology B: Biology . 2005;80(3):187–194. doi: 10.1016/j.jphotobiol.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 105.Soontornchaiboon W., Joo S. S., Kim S. M. Anti-inflammatory effects of violaxanthin isolated from microalga chlorella ellipsoidea in RAW 264.7 macrophages. Biological and Pharmaceutical Bulletin . 2012;35(7):1137–1144. doi: 10.1248/bpb.b12-00187. [DOI] [PubMed] [Google Scholar]
- 106.Wang F., Huang L., Gao B., Zhang C. Optimum production conditions, purification, identification, and antioxidant activity of violaxanthin from microalga eustigmatos Cf. Polyphem (eustigmatophyceae) Marine Drugs . 2018;16(6):190–202. doi: 10.3390/md16060190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Araki M., Kaku N., Harada M., Ando Y., Yamaguchi R., Shindo K. Production of auroxanthins from violaxanthin and 9-cis-violaxanthin by acidic treatment and the antioxidant activities of violaxanthin, 9-cis-violaxanthin, and auroxanthins. Journal of Agricultural and Food Chemistry . 2016;64(49):9352–9355. doi: 10.1021/acs.jafc.6b04506. [DOI] [PubMed] [Google Scholar]
- 108.Pasquet V., Morisset P., Ihammouine S., et al. Antiproliferative activity of violaxanthin isolated from bioguided fractionation of dunaliella tertiolecta extracts. Marine Drugs . 2011;9(5):819–831. doi: 10.3390/md9050819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kotake-Nara E., Sugawara T., Nagao A. Antiproliferative effect of neoxanthin and fucoxanthin on cultured cells. Fisheries Science . 2005;71(2):459–461. doi: 10.1111/j.1444-2906.2005.00986.x. [DOI] [Google Scholar]
- 110.Kotake-Nara E., Asai A., Nagao A. Neoxanthin and fucoxanthin induce apoptosis in PC-3 human prostate cancer cells. Cancer Letters . 2005;220(1):75–84. doi: 10.1016/j.canlet.2004.07.048. [DOI] [PubMed] [Google Scholar]
- 111.Šeremet D., Fabečić P., Vojvodić Cebin A., Mandura Jarić A., Pudić R., Komes D. Antioxidant and sensory assessment of innovative coffee blends of reduced caffeine content. Molecules . 2022;27(2):448–465. doi: 10.3390/MOLECULES27020448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Francisco V., Costa G., Figueirinha A., et al. Anti-inflammatory activity of cymbopogon citratus leaves infusion via proteasome and nuclear factor-κb pathway inhibition: contribution of chlorogenic acid. Journal of Ethnopharmacology . 2013;148(1):126–134. doi: 10.1016/j.jep.2013.03.077. [DOI] [PubMed] [Google Scholar]
- 113.Zhao X. L., Yu L., Zhang S. D., et al. Cryptochlorogenic acid attenuates LPS-induced inflammatory response and oxidative stress via upregulation of the Nrf2/HO-1 signaling pathway in RAW 264.7 macrophages. International Immunopharmacology . 2020;83:106436–106445. doi: 10.1016/j.intimp.2020.106436. [DOI] [PubMed] [Google Scholar]
- 114.Ma X., Okyere S. K., Hu L., et al. Anti-inflammatory activity and mechanism of cryptochlorogenic acid from Ageratina adenophora. Nutrients . 2022;14(3):439–453. doi: 10.3390/nu14030439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Park S. Y., Jin M. L., Yi E. H., Kim Y., Park G. Neochlorogenic acid inhibits against LPS-activated inflammatory responses through up-regulation of Nrf2/HO-1 and involving AMPK pathway. Environmental Toxicology and Pharmacology . 2018;62:1–10. doi: 10.1016/j.etap.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 116.Ahmad N., Lesa K. N., Fakhrudin N., Ikawati Z. Potentiality of coffee (coffea robusta) and its bioactive compounds in memory function: a review. Tropical Journal of Natural Product Research . 2023;7(11):5015–5025. [Google Scholar]
- 117.Carter A. J., O’Connor W. T., Carter M. J., Ungerstedt U. Caffeine enhances acetylcholine release in the Hippocampus in vivo by a selective interaction with adenosine A1 receptors. Journal of Pharmacology and Experimental Therapeutics . 1995;273(2):637–642. [PubMed] [Google Scholar]
- 118.Gajewska M., Paini A., Sala Benito J. V., et al. In vitro-to-in vivo correlation of the skin penetration, liver clearance and hepatotoxicity of caffeine. Food and Chemical Toxicology . 2015;75:39–49. doi: 10.1016/j.fct.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 119.Hirakawa N., Okauchi R., Miura Y., Yagasaki K. Anti-invasive activity of niacin and trigonelline against cancer cells. Bioscience Biotechnology and Biochemistry . 2005;69(3):653–658. doi: 10.1271/bbb.69.653. [DOI] [PubMed] [Google Scholar]
- 120.Jeong Y. Il, Kim D. H., Chung K. D., Kim Y. H., Lee Y. S., Choi K. C. Antitumor activity of trigonelline-incorporated chitosan nanoparticles. Journal of Nanoscience and Nanotechnology . 2014;14(8):5633–5637. doi: 10.1166/JNN.2014.8818. [DOI] [PubMed] [Google Scholar]
- 121.Kar A., Mukherjee S. K., Barik S., Hossain S. T. Antimicrobial activity of trigonelline hydrochloride against Pseudomonas aeruginosa and its quorum-sensing regulated molecular mechanisms on biofilm formation and virulence. ACS Infectious Diseases . 2024;10(2):746–762. doi: 10.1021/acsinfecdis.3c00617. [DOI] [PubMed] [Google Scholar]
- 122.Zhou J., Zhou S., Zeng S. Experimental diabetes treated with trigonelline: effect on β cell and pancreatic oxidative parameters. Fundamental and Clinical Pharmacology . 2013;27(3):279–287. doi: 10.1111/J.1472-8206.2011.01022.X. [DOI] [PubMed] [Google Scholar]
- 123.Kumar M., Barbhai M. D., Hasan M., et al. Onion (Allium cepa L.) peels: a review on bioactive compounds and biomedical activities. Biomedicine and Pharmacotherapy . 2022;146:512–112. doi: 10.1016/J.BIOPHA.2021.112498. [DOI] [PubMed] [Google Scholar]
- 124.Muscolo A., Papalia T., Settineri G., Mallamaci C., Panuccio M. R. Sulfur bentonite-organic-based fertilizers as tool for improving bio-compounds with antioxidant activities in red onion. Journal of the Science of Food and Agriculture . 2020;100(2):785–793. doi: 10.1002/JSFA.10086. [DOI] [PubMed] [Google Scholar]
- 125.Williamson G., Plumb G. W., Uda Y., Price K. R., Rhodes M. J. C. Dietary quercetin glycosides: antioxidant activity and induction of the anticarcinogenic phase II marker enzyme quinone reductase in Hepalclc7 cells. Carcinogenesis . 1996;17(11):2385–2387. doi: 10.1093/carcin/17.11.2385. [DOI] [PubMed] [Google Scholar]
- 126.Zheng Y. Z., Deng G., Liang Q., Chen D. F., Guo R., Lai R. C. Antioxidant activity of quercetin and its glucosides from propolis: a theoretical study. Scientific Reports . 2017;7(1):7543–7611. doi: 10.1038/s41598-017-08024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shabir I., Pandey V. K., Dar A. H., et al. Nutritional profile, phytochemical compounds, biological activities, and utilisation of onion peel for food applications: a review. Sustainability . 2022;14(19):972–11. doi: 10.3390/SU141911958. [DOI] [Google Scholar]
- 128.Ha T. K., Jung I., Kim M. E., Bae S. K., Lee J. S. Anti-cancer activity of myricetin against human papillary thyroid cancer cells involves mitochondrial dysfunction–mediated apoptosis. Biomedicine and Pharmacotherapy . 2017;91:378–384. doi: 10.1016/j.biopha.2017.04.100. [DOI] [PubMed] [Google Scholar]
- 129.Qian J., Zhang J., Chen Y., Dai C., Fan J., Guo H. Hypoglycemic activity and mechanisms of myricetin. Natural Product Research . 2022;36(23):6177–6180. doi: 10.1080/14786419.2022.2058941. [DOI] [PubMed] [Google Scholar]
- 130.Wang S. J., Tong Y., Lu S., et al. Anti-Inflammatory Activity of Myricetin Isolated from Myrica Rubra Sieb. et Zucc. Leaves. Planta Medica . 2010;76(14):1492–1496. doi: 10.1055/S-0030-1249780. [DOI] [PubMed] [Google Scholar]
- 131.Sun F., Zheng X. Y., Ye J., Wu T. T., Wang J. L., Chen W. Potential anticancer activity of myricetin in human T24 bladder cancer cells both in vitro and in vivo. Nutrition and Cancer . 2012;64(4):599–606. doi: 10.1080/01635581.2012.665564. [DOI] [PubMed] [Google Scholar]
- 132.Chernukha I., Kupaeva N., Kotenkova E., Khvostov D. Differences in antioxidant potential of Allium cepa husk of red, yellow, and white varieties. Antioxidants . 2022;11(7):1243–1257. doi: 10.3390/antiox11071243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gupta M. B., Bhalla T. N., Gupta G. P., Mitra C. R., Bhargava K. P. Anti-inflammatory activity of taxifolin. The Japanese Journal of Pharmacology . 1971;21(3):377–382. doi: 10.1016/S0021-5198(19)36228-6. [DOI] [PubMed] [Google Scholar]
- 134.Su H., Ruan Y. T., Li Y., Chen J. G., Yin Z. P., Zhang Q. F. In vitro and in vivo inhibitory activity of taxifolin on three digestive enzymes. International Journal of Biological Macromolecules . 2020;150:31–37. doi: 10.1016/j.ijbiomac.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 135.Yoon K. D., Lee J. Y., Kim T. Y., et al. In vitro and in vivo anti-hyperglycemic activities of taxifolin and its derivatives isolated from pigmented rice (oryzae sativa L. Cv. Superhongmi) Journal of Agricultural and Food Chemistry . 2020;68(3):742–750. doi: 10.1021/acs.jafc.9b04962. [DOI] [PubMed] [Google Scholar]
- 136.Muramatsu D., Uchiyama H., Kida H., Iwai A. Cell cytotoxity and anti-glycation activity of taxifolin-rich extract from Japanese larch, larix kaempferi. Heliyon . 2019;5(7):e02047–e2053. doi: 10.1016/j.heliyon.2019.e02047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chang W. A., Hung J. Y., Jian S. F., et al. Laricitrin ameliorates lung cancer-mediated dendritic cell suppression by inhibiting signal transducer and activator of transcription 3. Oncotarget . 2016;7(51):85220–85234. doi: 10.18632/oncotarget.13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee S., Choi Y. J., Huo C., et al. Laricitrin 3-rutinoside from ginkgo biloba fruits prevents damage in TNF-α-stimulated normal human dermal fibroblasts. Antioxidants . 2023;12(7):1432–1448. doi: 10.3390/antiox12071432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jha T., Reddy M. Laricitrin as a potential phytoconstituent for drug designing of hypertension. Biomedical Sciences and Computational Biology . 2022:125–264. [Google Scholar]
- 140.Guss E. V., Ziyatdinova G. K., Zhupanova A. S., Budnikov H. C. Voltammetric determination of quercetin and rutin on their simultaneous presence on an electrode modified with polythymolphthalein. Journal of Analytical Chemistry . 2020;75(4):526–535. doi: 10.1134/S106193482004005X. [DOI] [Google Scholar]
- 141.Fredotovíc Ž., Šprung M., Soldo B., et al. Chemical composition and biological activity of Allium cepa L. And allium × cornutum (clementi ex visiani 1842) methanolic extracts. Molecules . 2017;22(3):448–460. doi: 10.3390/MOLECULES22030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jnawali H. N., Jeon D., Jeong M. C., et al. Antituberculosis activity of a naturally occurring flavonoid, isorhamnetin. Journal of Natural Products . 2016;79(4):961–969. doi: 10.1021/acs.jnatprod.5b01033. [DOI] [PubMed] [Google Scholar]
- 143.Rodríguez L., Badimon L., Méndez D., et al. Antiplatelet activity of isorhamnetin via mitochondrial regulation. Antioxidants . 2021;10(5):p. 666. doi: 10.3390/antiox10050666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Teng B., Lu Y. H., Wang Z. T., Tao X. Y., Wei D. Z. In vitro anti-tumor activity of isorhamnetin isolated from hippophae rhamnoides L. Against BEL-7402 cells. Pharmacological Research . 2006;54(3):186–194. doi: 10.1016/j.phrs.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 145.Chirumbolo S. Anti-inflammatory action of isorhamnetin. Inflammation . 2014;37(4):1200–1201. doi: 10.1007/s10753-014-9846-9. [DOI] [PubMed] [Google Scholar]
- 146.Zhang B. Y., Wang Y. M., Gong H., et al. Isorhamnetin flavonoid synergistically enhances the anticancer activity and apoptosis induction by cis-platin and carboplatin in non-small cell lung carcinoma (NSCLC) International Journal of Clinical and Experimental Pathology . 2015;8(1):25–37. [PMC free article] [PubMed] [Google Scholar]
- 147.Zuo A., Yanying Y., Li J., et al. Study on the relation of structure and antioxidant activity of isorhamnetin, quercetin, phloretin, silybin and phloretin isonicotinyl hydrazone. Free Radicals and Antioxidants . 2011;1(4):39–47. doi: 10.5530/ax.2011.4.7. [DOI] [Google Scholar]
- 148.Wu L., Yang X., Huang Z., Liu H., Wu G. In vivo and in vitro antiviral activity of hyperoside extracted from abelmoschus manihot (L) medik. Acta Pharmacologica Sinica . 2007;28(3):404–409. doi: 10.1111/j.1745-7254.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 149.Li S., Zhang Z., Cain A., Wang B., Long M., Taylor J. Antifungal activity of camptothecin, trifolin, and hyperoside isolated from camptotheca acuminata. Journal of Agricultural and Food Chemistry . 2005;53(1):32–37. doi: 10.1021/jf0484780. [DOI] [PubMed] [Google Scholar]
- 150.Gao Y., Fang L., Wang X., et al. Antioxidant activity evaluation of dietary flavonoid hyperoside using Saccharomyces cerevisiae as a model. Molecules . 2019;24(4):788–799. doi: 10.3390/molecules24040788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ku S. K., Yoo H., Zhou W., Na M. K., Bae J. S. Antiplatelet activities of hyperoside in vitro and in vivo. Animal Cells and Systems . 2014;18(3):204–209. doi: 10.1080/19768354.2014.925970. [DOI] [Google Scholar]
- 152.Wu P., Sun W., Wu P. Hyperoside exerts potent anticancer activity in skin cancer. Frontiers in Bioscience . 2020;25(3):463–479. doi: 10.2741/4814. [DOI] [PubMed] [Google Scholar]
- 153.Nile A., Nile S. H., Cespedes-Acuña C. L., Oh J. W. Spiraeoside extracted from red onion skin ameliorates apoptosis and exerts potent antitumor, antioxidant and enzyme inhibitory effects. Food and Chemical Toxicology . 2021;154:112327–112335. doi: 10.1016/j.fct.2021.112327. [DOI] [PubMed] [Google Scholar]
- 154.Kostikova V. A., Zarubaev V. V., Esaulkova I. L., et al. The antiviral, antiradical, and phytochemical potential of dry extracts from spiraea hypericifolia, S. Media, and S. Salicifolia (rosaceae) South African Journal of Botany . 2022;147:215–222. doi: 10.1016/j.sajb.2022.01.013. [DOI] [Google Scholar]
- 155.Ha L. M., Que D. T. N., Huyen D. T. T., Long P. Q., Dat N. T. Toxicity, analgesic and anti-inflammatory activities of tectorigenin. Immunopharmacology and Immunotoxicology . 2013;35(3):336–340. doi: 10.3109/08923973.2013.770521. [DOI] [PubMed] [Google Scholar]
- 156.Han T., Cheng G., Liu Y., Yang H., Hu Y., Huang W. In vitro evaluation of tectoridin, tectorigenin and tectorigenin sodium sulfonate on antioxidant properties. Food and Chemical Toxicology . 2012;50(2):409–414. doi: 10.1016/j.fct.2011.10.066. [DOI] [PubMed] [Google Scholar]
- 157.Lim H. S., Kim Y. J., Kim B. Y., Park G., Jeong S. J. The anti-neuroinflammatory activity of tectorigenin pretreatment via downregulated NF-κb and ERK/JNK pathways in BV-2 microglial and microglia inactivation in mice with lipopolysaccharide. Frontiers in Pharmacology . 2018;9:462–513. doi: 10.3389/fphar.2018.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Oh K. B., Kang H., Matsuoka H. Detection of antifungal activity in belamcanda chinensis by a single-cell bioassay method and isolation of its active compound, tectorigenin. Bioscience, Biotechnology, and Biochemistry . 2001;65(4):939–942. doi: 10.1271/bbb.65.939. [DOI] [PubMed] [Google Scholar]
- 159.Teshika J. D., Zakariyyah A. M., Zaynab T., et al. Traditional and modern uses of onion bulb (Allium cepa L.): a systematic review. Critical Reviews in Food Science and Nutrition . 2019;59(1):39–70. doi: 10.1080/10408398.2018.1499074. [DOI] [PubMed] [Google Scholar]
- 160.Kikuzaki H., Hisamoto M., Hirose K., Akiyama K., Taniguchi H. Antioxidant properties of ferulic acid and its related compounds. Journal of Agricultural and Food Chemistry . 2002;50(7):2161–2168. doi: 10.1021/jf011348w. [DOI] [PubMed] [Google Scholar]
- 161.Shi C., Zhang X., Sun Y., et al. Antimicrobial activity of ferulic acid against cronobacter sakazakii and possible mechanism of action. Foodborne pathogens and disease . 2016;13(4):196–204. doi: 10.1089/FPD.2015.1992. [DOI] [PubMed] [Google Scholar]
- 162.Li X., Wang X., Chen D., Chen S. Antioxidant activity and mechanism of protocatechuic acid in vitro. Functional Foods in Health and Disease . 2011;1(7):232–244. doi: 10.31989/ffhd.v1i7.127. [DOI] [Google Scholar]
- 163.Liu J., Meng C., Liu S., Kan J., Jin C. Preparation and characterization of protocatechuic acid grafted chitosan films with antioxidant activity. Food Hydrocolloids . 2017;63:457–466. doi: 10.1016/j.foodhyd.2016.09.035. [DOI] [Google Scholar]
- 164.Harini R., Pugalendi K. V. Antioxidant and antihyperlipidaemic activity of protocatechuic acid on streptozotocindiabetic rats. Redox Report . 2010;15(2):71–80. doi: 10.1179/174329210X12650506623285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu K., Tsao S., Yin M. In vitro antibacterial activity of roselle calyx and protocatechuic acid. Phytotherapy Research . 2005;19(11):942–945. doi: 10.1002/ptr.1760. [DOI] [PubMed] [Google Scholar]
- 166.Pan C., Fujiwara Y., Horlad H., Iriki T., Shiraishi D., Komohara Y. Cyclic sulfur compounds targeting macrophage polarization into M2/protumor phenotype and their anti-tumor effects. Cancer Immunology, Immunotherapy . 2022;71(6):1331–1343. doi: 10.1007/S00262-021-03085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhou Y., Li C., Feng B., Chen B., Jin L., Shen Y. UPLC-ESI-MS/MS based identification and antioxidant, antibacterial, cytotoxic activities of aqueous extracts from storey onion (Allium cepa L. Var. Proliferum regel) Food Research International . 2020;130:977–108. doi: 10.1016/j.foodres.2019.108969. [DOI] [PubMed] [Google Scholar]
- 168.Kim J. H. Anti-bacterial action of onion (Allium cepa L.) extracts against oral pathogenic bacteria. The Journal of Nihon University School of Dentistry . 1997;39(3):136–141. doi: 10.2334/josnusd1959.39.136. [DOI] [PubMed] [Google Scholar]
- 169.Silva R. M. G. d., Alves C. P., Barbosa F. C., et al. Antioxidant, antitumoral, antimetastatic effect and inhibition of collagenase enzyme activity of eleutherine bulbosa (dayak onion) extract: in vitro, in vivo and in silico approaches. Journal of Ethnopharmacology . 2024;318 doi: 10.1016/j.jep.2023.117005.117005 [DOI] [PubMed] [Google Scholar]
- 170.Galavi A., Hosseinzadeh H., Razavi B. M. The effects of Allium cepa L. (onion) and its active constituents on metabolic syndrome: a review. Iranian journal of basic medical sciences . 2021;24(1):3–16. doi: 10.22038/ijbms.2020.46956.10843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kim S., Kim D. B., Lee S., Park J., Shin D., Yoo M. Profiling of organosulphur compounds using HPLC-PDA and GC/MS system and antioxidant activities in hooker chive (allium hookeri) Natural Product Research . 2016;30(24):2798–2804. doi: 10.1080/14786419.2016.1164700. [DOI] [PubMed] [Google Scholar]
- 172.Borah P., Banik B. K. Diverse therapeutic applications of onion. Archives of Pharmacology and Therapeutics . 2019;1(1):14–16. doi: 10.33696/pharmacol.1.003. [DOI] [Google Scholar]
- 173.Adjei-Mensah B., Koranteng A. A. A., Hamidu J. A., Tona K. Antibacterial activities of garlic (allium sativum) in broiler and laying hens production. World’s Poultry Science Journal . 2023;79(1):155–176. doi: 10.1080/00439339.2023.2164236. [DOI] [Google Scholar]
- 174.Ghosh D., Bera T. K., Chatterjee K., Ali K. M., De D. Antidiabetic and antioxidative effects of aqueous extract of seed of Psoralea corylifolia (somraji) and seed of trigonella foenum‐graecum L., (methi) in separate and composite manner in streptozotocin‐induced diabetic male albino rat. Tropical Journal of Pharmaceutical Research . 2009;1(7):1–10. [Google Scholar]
- 175.Stice S. P., Thao K. K., Khang C. H., Baltrus D. A., Dutta B., Kvitko B. H. Thiosulfinate tolerance is a virulence strategy of an atypical bacterial pathogen of onion. Current Biology . 2020;30(16):3130–3140.e6. doi: 10.1016/j.cub.2020.05.092. [DOI] [PubMed] [Google Scholar]
- 176.Prasad K., Laxdal V. A., Yu M., Raney B. L. Antioxidant activity of allicin, an active principle in garlic. Molecular and Cellular Biochemistry . 1995;148(2):183–189. doi: 10.1007/BF00928155. [DOI] [PubMed] [Google Scholar]
- 177.Ankri S., Mirelman D. Antimicrobial properties of allicin from garlic. Microbes and Infection . 1999;1(2):125–129. doi: 10.1016/S1286-4579(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 178.Coppi A., Cabinian M., Mirelman D., Sinnis P. Antimalarial activity of allicin, a biologically active compound from garlic cloves. Antimicrobial Agents and Chemotherapy . 2006;50(5):1731–1737. doi: 10.1128/AAC.50.5.1731-1737.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cutler R. R., Wilson P. Antibacterial activity of a new, stable, aqueous extract of allicin against methicillin-resistant Staphylococcus aureus. British Journal of Biomedical Science . 2004;61(2):71–74. doi: 10.1080/09674845.2004.11732646. [DOI] [PubMed] [Google Scholar]
- 180.Zhang Z., Lei M., Liu R., Gao Y., Xu M., Zhang M. Evaluation of alliin, saccharide contents and antioxidant activities of black garlic during thermal processing. Journal of Food Biochemistry . 2015;39(1):39–47. doi: 10.1111/JFBC.12102. [DOI] [Google Scholar]
- 181.Choi M. K., Chae K. Y., Lee J. Y., Kyung K. H. Antimicrobial activity of chemical substances derived from S-Alk(En)Yl-L-Cysteine sulfoxide (alliin) in garlic, allium sativum L. Food Science and Biotechnology . 2007;16(1):1–7. [Google Scholar]
- 182.Zhang M., Lei N., Zhu T., Zhang Z. Thermal processing effects on the chemical constituent and antioxidant activity of S-Alk(En)Ylcysteine s-oxides (alliin) extract. LWT-Food Science and Technology . 2013;51(1):309–313. doi: 10.1016/j.lwt.2012.09.024. [DOI] [Google Scholar]
- 183.Kyung K. H., Fleming H. P. S-Methyl-L-Cysteine sulf oxide as the precursor of methyl methanethiolsulfinate, the principal antibacterial compound in cabbage. Journal of Food Science . 1994;59(2):350–355. doi: 10.1111/J.1365-2621.1994.TB06964.X. [DOI] [Google Scholar]
- 184.Kyung K. H., Han D. C., Fleming H. P. Antibacterial activity of heated cabbage juice, S-Methyl-L-Cysteine sulfoxide and methyl methanethiosulfonate. Journal of Food Science . 1997;62(2):406–409. doi: 10.1111/J.1365-2621.1997.TB04013.X. [DOI] [Google Scholar]
- 185.Kyung K. H., Lee Y. C. Antimicrobial activities of sulfur compounds derived from S-alk (en) yl-L-cysteine sulfoxides in allium and Brassica. Food Reviews International . 2001;17(2):183–198. doi: 10.1081/FRI-100000268. [DOI] [Google Scholar]
- 186.Huang C. N., Horng J. S., Yin M. C. Antioxidative and antiglycative effects of six organosulfur compounds in low-density lipoprotein and plasma. Journal of Agricultural and Food Chemistry . 2004;52(11):3674–3678. doi: 10.1021/jf0307292. [DOI] [PubMed] [Google Scholar]
- 187.Xiao H., Parkin K. L. Antioxidant functions of selected allium thiosulfinates and S-Alk(En)Yl-l-Cysteine sulfoxides. Journal of Agricultural and Food Chemistry . 2002;50(9):2488–2493. doi: 10.1021/jf011137r. [DOI] [PubMed] [Google Scholar]
- 188.Kulikova V., Morozova E., Rodionov A., et al. Non-stereoselective decomposition of (±)-S-alk(en)yl-l-cysteine sulfoxides to antibacterial thiosulfinates catalyzed by C115H mutant methionine γ-lyase from Citrobacter freundii. Biochimie . 2018;151:42–44. doi: 10.1016/j.biochi.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 189.Ruiz-Sánchez E., Pedraza-Chaverri J., Medina-Campos O. N., Maldonado P. D., Rojas P. S. S-Allyl cysteine, a garlic compound, produces an antidepressant-like effect and exhibits antioxidant properties in mice. Brain Sciences . 2020;10(9):592–609. doi: 10.3390/brainsci10090592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Johnson P., Loganathan C., Iruthayaraj A., Poomani K., Thayumanavan P. S-allyl cysteine as potent anti-gout drug: insight into the xanthine oxidase inhibition and anti-inflammatory activity. Biochimie . 2018;154:1–9. doi: 10.1016/j.biochi.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 191.Dini I., Tenore G. C., Dini A. Chemical composition, nutritional value and antioxidant properties of allium caepa L. Var. Tropeana (red onion) seeds. Food Chemistry . 2008;107(2):613–621. doi: 10.1016/j.foodchem.2007.08.053. [DOI] [Google Scholar]
- 192.Kim J. W., Huh J. E., Kyung S. H., Kyung K. H. Antimicrobial activity of alk(En)Yl sulfides found in essential oils of garlic and onion. Food Science and Biotechnology . 2004;13(2):235–239. [Google Scholar]
- 193.Yi L., Su Q. Molecular mechanisms for the anti-cancer effects of diallyl disulfide. Food and Chemical Toxicology . 2013;57:362–370. doi: 10.1016/j.fct.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 194.Casella S., Leonardi M., Melai B., Fratini F., Pistelli L. The role of diallyl sulfides and dipropyl sulfides in the in vitro antimicrobial activity of the essential oil of garlic, Allium sativum L., and leek, Allium porrum L. Phytotherapy Research . 2013;27(3):380–383. doi: 10.1002/ptr.4725. [DOI] [PubMed] [Google Scholar]
- 195.Higuchi O., Tateshita K., Nishimura H. Antioxidative activity of sulfur-containing compounds in allium species for human low-density lipoprotein (LDL) oxidation in vitro. Journal of Agricultural and Food Chemistry . 2003;51(24):7208–7214. doi: 10.1021/jf034294u. [DOI] [PubMed] [Google Scholar]
- 196.Woo K. S., Yoon H. S., Lee S. K., Chung Y. G. Antimicrobial activity and distilled components of garlic (allium sativum L.) and ginger (zingiber officinale roscoe) Applied Biological Chemistry . 1997;40(6):514–518. [Google Scholar]
- 197.Woo K. S., Yoon H. S., Lee Y. R., et al. Characteristics and antioxidative activity of volatile compounds in heated garlic (allium sativum) Food Science and Biotechnology . 2007;16(5):822–827. [Google Scholar]
- 198.Stoica F., Condurache N. N., Horincar G., et al. Value-added crackers enriched with red onion skin anthocyanins entrapped in different combinations of wall materials. Antioxidants . 2022;11(6):1048–1066. doi: 10.3390/ANTIOX11061048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pérez-Gregorio M. R., Regueiro J., González-Barreiro C., Rial-Otero R., Simal-Gándara J. Changes in antioxidant flavonoids during freeze-drying of red onions and subsequent storage. Food Control . 2011;22(7):1108–1113. doi: 10.1016/j.foodcont.2011.01.006. [DOI] [Google Scholar]
- 200.Metrani R., Singh J., Acharya P., Jayaprakasha G. S., Patil B. Comparative metabolomics profiling of polyphenols, nutrients and antioxidant activities of two red onion (Allium cepa L.) cultivars. Plants . 2020;9(9):1077–1094. doi: 10.3390/plants9091077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhao X., Zhang C., Guigas C., et al. Composition, antimicrobial activity, and antiproliferative capacity of anthocyanin extracts of purple corn (Zea mays L.) from China. European Food Research and Technology . 2009;228(5):759–765. doi: 10.1007/s00217-008-0987-7. [DOI] [Google Scholar]
- 202.Pertuzatti P. B., Barcia M. T., Rebello L. P. G., et al. Antimicrobial activity and differentiation of anthocyanin profiles of rabbiteye and highbush blueberries using HPLC–DAD–ESI-MSn and multivariate analysis. Journal of Functional Foods . 2016;26:506–516. doi: 10.1016/j.jff.2016.07.026. [DOI] [Google Scholar]
- 203.Kaume L., Gilbert W. C., Brownmiller C., Howard L. R., Devareddy L. Cyanidin 3-O-β-d-glucoside-rich blackberries modulate hepatic gene expression, and anti-obesity effects in ovariectomized rats. Journal of Functional Foods . 2012;4(2):480–488. doi: 10.1016/j.jff.2012.02.008. [DOI] [Google Scholar]
- 204.Wang F., Li H., Qin Y., Mao Y., Zhang B., Deng Z. Effects of heat, ultrasound, and microwave processing on the stability and antioxidant activity of delphinidin and petunidin. Journal of Food Biochemistry . 2019;43(5):128188–e12911. doi: 10.1111/jfbc.12818. [DOI] [PubMed] [Google Scholar]
- 205.Sun J., Li X., Luo H., et al. Comparative study on the stability and antioxidant activity of six pyranoanthocyanins based on malvidin-3-glucoside. Journal of Agricultural and Food Chemistry . 2020;68(9):2783–2794. doi: 10.1021/acs.jafc.9b06734. [DOI] [PubMed] [Google Scholar]
- 206.Huang W. Y., Liu Y. M., Wang J., Wang X. N., Li C. Y. Anti-inflammatory effect of the blueberry anthocyanins malvidin-3-glucoside and malvidin-3-galactoside in endothelial cells. Molecules . 2014;19(8):12827–12841. doi: 10.3390/molecules190812827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhang J., Sun L., Dong Y., et al. Chemical compositions and α-glucosidase inhibitory effects of anthocyanidins from blueberry, blackcurrant and blue honeysuckle fruits. Food Chemistry . 2019;299:125102–125112. doi: 10.1016/j.foodchem.2019.125102. [DOI] [PubMed] [Google Scholar]
- 208.Song J., Zhang H., Wang Z., Wang J. The antioxidant activity, α-glucosidase and acetylcholinesterase inhibition activity, and chemical composition of paeonia delavayi petal. Food Quality and Safety . 2022;6:1–12. doi: 10.1093/fqsafe/fyac020. [DOI] [Google Scholar]
- 209.Abdel-Tawab M., De Vita D. Considerations to Be taken when carrying out medicinal plant research—what we learn from an insight into the IC50 values, bioavailability and clinical efficacy of exemplary anti-inflammatory herbal components. Pharmaceuticals . 2021;14(5):437–470. doi: 10.3390/PH14050437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Frontiers in Pharmacology . 2014;4:177–210. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Li F. S., Weng J. K. Demystifying traditional herbal medicine with modern approach. Nature Plants . 2017;3(8):17109–17117. doi: 10.1038/nplants.2017.109. [DOI] [PubMed] [Google Scholar]
- 212.Tulp O. L., Rizvi S. A. A. Case study review: pharmacognosy of complementary and alternative medicines as effective adjuncts in the treatment of mild to moderate hypertension. International Journal of Computational and Applied Mathematics . 2023;16(1):44–49. doi: 10.15406/ijcam.2023.16.00631. [DOI] [Google Scholar]
- 213.Shah Z. A., Abu-Izneid T., Rauf A., et al. Phosphodiesterase 1 inhibition and molecular docking study of phytochemicals isolated from stem heartwood of heterophragma adenophyllum seem. South African Journal of Botany . 2020;135:274–279. doi: 10.1016/j.sajb.2020.08.013. [DOI] [Google Scholar]
- 214.Ahmad N., Lesa K. N., Ujiantari N. S. O., Sudarmanto A., Ikawati Z., Fakhrudin N. Phytochemical identification and in silico study of ethanolic extract of white cabbage as a phosphodiesterase 1B inhibitor. Journal of Herbmed Pharmacology . 2023;12(4):521–535. doi: 10.34172/jhp.2023.45004. [DOI] [Google Scholar]
- 215.Fakhrudin N., Nurrochmad A., Sudarmanto A., Ikawati Z., Caesalpinia sappan L. Wood is a potential source of natural phosphodiesterase-1 inhibitors. Pharmacognosy Journal . 2020;12(6):1206–1217. doi: 10.5530/PJ.2020.12.169. [DOI] [Google Scholar]
- 216.Xia C., He J. P., Feng K. W., et al. Discovery of novel 3-amino-4-alkoxyphenylketones as PDE4 inhibitors with improved oral bioavailability and safety against spatial memory impairments. ACS Chemical Neuroscience . 2022;13(3):390–405. doi: 10.1021/acschemneuro.1c00762. [DOI] [PubMed] [Google Scholar]
- 217.Sánchez-Mendoza E., Campos-Xolalpa N., Pérez-Gutiérrez S., Rodríguez-Ramos F. Mechanism of action via inhibition of phosphodiesterases and molecular docking of antiinflammatory secondary metabolites and NSAIDs. Revista Latinoamericana de Quimica . 2023;50(1-3):52–65. [Google Scholar]
- 218.Kamble A. N. S., Mitkar A. A. Swiss ADME predictions of pharmacokinetics and drug-likeness properties of secondary metabolites present in trigonella foenum-graecum. Journal of Pharmacognosy and Phytochemistry . 2023;12(5):341–349. doi: 10.22271/phyto.2023.v12.i5d.14745. [DOI] [Google Scholar]
- 219.Brogi S., Quimque M. T., Notarte K. I., et al. Virtual combinatorial library screening of quinadoline B derivatives against SARS-CoV-2 RNA-dependent RNA polymerase. Computation . 2022;10(1):7–22. doi: 10.3390/computation10010007. [DOI] [Google Scholar]
- 220.Gangadevi S., Badavath V. N., Thakur A., et al. Kobophenol A inhibits binding of host ACE2 receptor with spike rbd domain of SARS-CoV-2, a lead compound for blocking COVID-19. The Journal of Physical Chemistry Letters . 2021;12(7):1793–1802. doi: 10.1021/acs.jpclett.0c03119. [DOI] [PubMed] [Google Scholar]
- 221.Quimque M. T. J., Notarte K. I. R., Fernandez R. A. T., et al. Virtual screening-driven drug discovery of SARS-CoV2 enzyme inhibitors targeting viral attachment, replication, post-translational modification and host immunity evasion infection mechanisms. Journal of Biomolecular Structure and Dynamics . 2021;39(12):4316–4333. doi: 10.1080/07391102.2020.1776639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Blauvelt A., Langley R. G., Gordon K. B., et al. Next generation PDE4 inhibitors that selectively target PDE4B/D subtypes: a narrative review. Dermatology and therapy . 2023;13(12):3031–3042. doi: 10.1007/s13555-023-01054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lugnier C. The complexity and multiplicity of the specific CAMP phosphodiesterase family: PDE4, open new adapted therapeutic approaches. International Journal of Molecular Sciences . 2022;23(18):10616–10637. doi: 10.3390/ijms231810616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wilson N. M., Gurney M. E., Dietrich W. D., Atkins C. M. Therapeutic benefits of phosphodiesterase 4B inhibition after traumatic brain injury. PLoS One . 2017;12(5):e0178013–e0178030. doi: 10.1371/journal.pone.0178013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Reyes-Irisarri E., Sánchez A. J., García-Merino J. A., Mengod G. Selective induction of CAMP phosphodiesterase PDE4B2 expression in experimental autoimmune encephalomyelitis. Journal of Neuropathology and Experimental Neurology . 2007;66(10):923–931. doi: 10.1097/nen.0b013e3181567c31. [DOI] [PubMed] [Google Scholar]
- 226.Tibbo A. J., Baillie G. S. Phosphodiesterase 4B: master regulator of brain signaling. Cells . 2020;9(5):1254–1270. doi: 10.3390/cells9051254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Feng X., Wang H., Ye M., et al. Identification of a PDE4-specific pocket for the design of selective inhibitors. Biochemistry . 2018;57(30):4518–4525. doi: 10.1021/acs.biochem.8b00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Townsend E. A., Emala C. W. Quercetin acutely relaxes airway smooth muscle and potentiates β-agonist-induced relaxation via dual phosphodiesterase inhibition of PLCβ and PDE4. American Journal of Physiology- Lung Cellular and Molecular Physiology . 2013;305(5):396–403. doi: 10.1152/ajplung.00125.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lourenço E. M. G., Fernandes J. M., Carvalho V. d. F., et al. Identification of a selective PDE4B inhibitor from bryophyllum pinnatum by target fishing study and in vitro evaluation of quercetin 3-O-α-LArabinopyranosyl-(1⟶2)-O-α-LRhamnopyranoside. Frontiers in Pharmacology . 2020;10:1–12. doi: 10.3389/fphar.2019.01582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Tang L., Huang C., Zhong J., et al. Discovery of arylbenzylamines as PDE4 inhibitors with potential neuroprotective effect. European Journal of Medicinal Chemistry . 2019;168:221–231. doi: 10.1016/j.ejmech.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 231.Lipson D. A., Barnacle H., Birk R., et al. FULFIL trial: once-daily triple therapy for patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine . 2017;196(4):438–446. doi: 10.1164/rccm.201703-0449OC. [DOI] [PubMed] [Google Scholar]
- 232.Kim S. K., Ko Y. H., Lee S. Y., Jang C. G. Memory-enhancing effects of 7,3′,4′-trihydroxyisoflavone by regulation of cholinergic function and BDNF signaling pathway in mice. Food and Chemical Toxicology . 2020;137 doi: 10.1016/j.fct.2020.111160.111160 [DOI] [PubMed] [Google Scholar]
- 233.Maher A., El Sayed N., Nafea H., Gad M. Rolipram rescues memory consolidation deficits caused by sleep deprivation: implication of the CAMP/PKA and CAMP/epac pathways. CNS and Neurological Disorders- Drug Targets . 2022;21(7):631–639. doi: 10.2174/1871527320666210816105144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included in the article.


