Abstract
Background:
This study aims to estimate the risk factors of gastrointestinal (GI) bleeding in patients with acute coronary syndrome (ACS) and to evaluate the optimal duration of dual antiplatelet therapy (DAPT).
Materials and Methods:
We enrolled 1266 patients with ACS in a telephone follow-up program to determine whether any of the patients were hospitalized for GI bleeding. We collected baseline data, laboratory tests, electrocardiograms, and echocardiography covering all ACS patients. Multivariable regression was performed to adjust for confounders and predictors of GI bleeding. At the same time, the optimal duration of DAPT for ACS patients was evaluated.
Results:
A total of 1061 ACS patients were included in the study. After 13–68 months, 48 patients (4.5%) were hospitalized for GI bleeding. The risk of GI bleeding was significantly increased in patients treated with DAPT for more than 18 months (hazard ratio 12.792, 5.607–29.185, P < 0.01). Receiver Operating Characteristic curve showed that the duration of DAPT using a cutoff of 14.5 months resulted in a sensitivity of 66.7% and a specificity of 77%.
Conclusion:
In patients with ACS, DAPT time are the main risk factors of GI bleeding. The optimal duration of DAPT is 14.5 months.
Keywords: Acute coronary syndrome, dual antiplatelet therapy, gastrointestinal bleeding
INTRODUCTION
Acute coronary syndrome (ACS) is the most common acute and severe disease in cardiology, and gastrointestinal (GI) bleeding is a commonly found acute and severe disorder in the digestive department. With a better therapeutic approach to chronic duodenal ulcers and an increasing use of nonsteroidal anti-inflammatory drugs in the elderly, the etiology of GI bleeding has changed to drug-induced in the past 20 years. And the number of deaths due to GI bleeding has not decreased.[1] One of the reasons for this occurrence is that in the treatment of cardiovascular disease, widespread use of antiplatelet drugs leads to an increase in the number of patients with ACS complicated with potential GI bleeding. At the same time, patients with ACS are prone to stress-related mucosal disease or GI bleeding in the state of stress.[2] Because patients with GI bleeding cannot tolerate antiplatelet therapy, GI bleeding is a fatal complication in patients with ACS.[3]
The main aim of this study is to identify the causes of GI bleeding in patients with ACS and to reduce the risk of GI bleeding. Meanwhile, through comprehensive analysis and weighing the advantages and disadvantages, the duration of dual antiplatelet therapy (DAPT) should be determined as far as possible.
METHODS
Study population
The Improving Care for Cardiovascular Disease in China-ACS (CCC-ACS) project is a national registry and quality improvement study with a database that focuses on the quality of treatment and care for ACS. The project was launched in 2014 by a collaborative initiative of the American Heart Association (AHA) and Chinese Society of Cardiology. Because the design and methods of the CCC-ACS project have been published in a previous report,[4] and the study was registered at ClinicalTrials.gov (NCT02306616), we will not go into details here. Our study was a hospital-based, retrospective, case-control study based on the CCC-ACS project, and it included all ACS patients in the First Affiliated Hospital of Henan University of Science and Technology from January 2014 to June 2019. During this period, we collected baseline data, laboratory tests, electrocardiograms, and echocardiography covering all ACS patients.
Inclusion criteria
Patients with acute ST segment elevation myocardial infarction, or non-ST segment elevation myocardial infarction, unstable angina pectoris. The subjects received dual antiplatelet (aspirin and clopidogrel or ticagrelor) and statin therapy.
Exclusion criteria
The patient could not be reached, or the information about the patients were unavailable; Death from non-GI bleeding; Had a history of GI hemorrhage before admission. Taking oral anticoagulants for an extended period of time (more than 6 months) or having abnormal coagulation function. Severe liver and kidney failure.
From June 17, 2021, to July 16, 2021, all subjects were followed up by telephone and were asked if there was GI bleeding after discharge from our Department of Cardiology. If there was GI bleeding, it was clearly asked when GI bleeding occurred and whether the patient was hospitalized.
Baseline measurements and biochemical analysis
When the subjects were admitted to the Department of Cardiology for the first time because of ACS, the clinical data, such as age, alcohol abuse habits, heart rate, history of myocardial infarction, and history of percutaneous coronary intervention (PCI), were recorded. GI bleeding was defined as hematemesis, bleeding due to the nasogastric tube, positive fecal occult blood test, or anal bleeding. DAPT was defined as aspirin combined with clopidogrel or ticagrelor. Alcohol abuse was defined as drinking at least 3 times a week with a minimum quantity of 50 mL each time. Diabetes was defined as glycosylated hemoglobin > 6% or long-term use of oral drugs or subcutaneous insulin injection to control blood sugar. Quality control of laboratory indicators and definition of medical history standard were performed with reference to the CCC Project.
Statistical analysis
The baseline of clinical data were divided into continuous variables and categorical variables. For continuous variables, we utilized the Kolmogorov–Smirnov method to test the normality. When P > 0.05, it was assumed to be a normal distribution, which was expressed by the mean, standard deviation. Otherwise, log transformation was carried out, and it was represented by the interquartile range, median (Q1, Q3). For Categorical variable data, it was represented as percentages. By univariate Cox proportional hazard regression analysis, the hazard ratio (HR) values of the baseline variables were calculated. The variables with statistical significance will entered into the next step of multivariable analysis. Subsequently, multivariable regression was performed to adjust for confounders for predictors of GI bleeding. Establish a risk scoring system for GI bleeding based on a logistic regression model. All statistical tests and confidence intervals (CIs) were two-sided, and P < 0.01 was used to identify statistically significant results. Statistical analysis of data was performed by SPSS19 software.
RESULTS
Baseline characteristics and follow-up results
We obtained the complete clinical data of 1266 ACS patients. Finally, 1061 subjects were followed up successfully. There were 525 patients (49.4%) with acute ST segment elevation myocardial infarction, 356 patients (33.6%) with acute non-ST segment elevation myocardial infarction, and 180 (17.0%) with unstable angina pectoris. The baseline characteristics of the subjects are shown in Table 1. The average age of the participants was 64 ± 12 years, 78.4% of whom were men. Over time, the cumulative risk of GI bleeding increased [Figure 1]. After 13–68 months, a total of 48 patients (4.5%) were hospitalized for GI bleeding. Moreover, 5 of those 48 patients died during hospitalization.
Table 1.
Baseline characteristics
| Clinical | n=1061 | |
|---|---|---|
| Age (years) | 64±12 | |
| Sex (man), n (%) | 832 (78.4) | |
| Weight (kg) | 67.1±12.9 | |
| Heart rate (bpm) | 76±16 | |
| Systolic blood pressure (mmHg) | 128±25 | |
| Alcohol abuse, n (%) | 317 (29.9) | |
| Diabetes, n (%) | 188 (17.7) | |
| History of heart failure, n (%) | 92 (8.6) | |
| History of myocardial infarction, n (%) | 67 (6.3) | |
| History of PCI, n (%) | 64 (6.0) | |
| Hypertension, n (%) | 487 (45.9) | |
| History of atrial fibrillation, n (%) | 72 (6.8) | |
| NT-proBNP (pg/mL) | 313 (123–756) | |
| CK-MB (IU/L) | 122 (59–227) | |
| Creatinine (μmol/L) | 89 (80–101) | |
| Hb (g/L) | 124 (110–134) | |
| LDL-C (mmol/L) | 2.70 (2.11–3.29) | |
| LVEF (%) | 51 (45–58) | |
| Dual-antiplatelet therapy ≤12 months, n (%) | 777 (73.2) | |
| Dual-antiplatelet therapy 12–18 months, n (%) | 188 (17.7) | |
| Dual-antiplatelet therapy >18 months, n (%) | 96 (9.0) | |
| Dual-antiplatelet and anticoagulant therapy >2 months, n (%) | 2 (0.19) | |
| Aspirin treatment ≤36 months, n (%) | 325 (30.6) | |
| Aspirin treatment >36 months, n (%) | 736 (69.4) |
Continuous values are represented by mean±SD. Nonnormal distribution values are represented by median (25th–75th). PCI=Percutaneous coronary intervention; LVEF=Left ventricular ejection fraction; Hb=Hemoglobin; LDL-C=Low density lipoprotein cholesterol; NT-proBNP=N-terminal pro-brain natriuretic peptide
Figure 1.
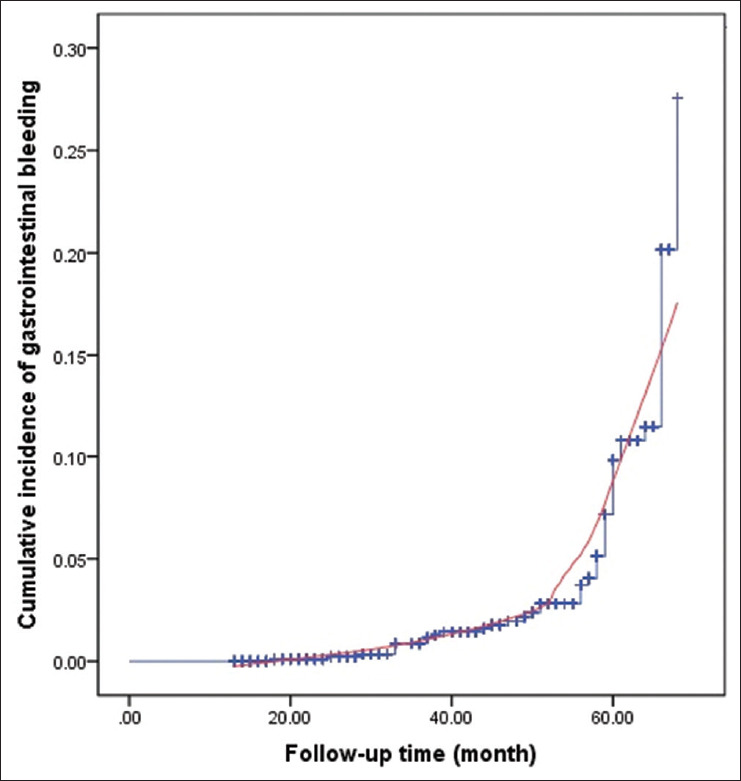
Cumulative risk of gastrointestinal bleeding
Risk factors for gastrointestinal bleeding
To analyze the risk factors for GI bleeding in patients with ACS, we first screened out the possible risk factors through the single factor Cox proportional risk model [Table 2], and then we analyzed these risk factors using the multifactor Cox proportional hazard model.
Table 2.
Univariable proportional hazards regression models for gastrointestinal bleeding
| Factors | HR | P | ||
|---|---|---|---|---|
| Age | 1.020 (0.958–1.239) | 0.593 | ||
| Sex | 1.002 (0.628–2.013) | 0.808 | ||
| Systolic pressure | 0.856 (0.568–1.012) | 0.082 | ||
| Heart rate | 1.058 (0.989–1.815) | 0.905 | ||
| Alcohol abuse | 5.265 (2.912–10.289) | <0.01 | ||
| History of heart failure | 2.169 (1.098–4.864) | 0.843 | ||
| History of PCI | 13.107 (4.587–29.058) | <0.01 | ||
| History of atrial fibrillation | 11.919 (4.685–25.074) | <0.01 | ||
| history of type 2 diabetes | 5.105 (2.254–8.022) | 0.035 | ||
| history of myocardial infarction | 3.233 (1.956–5.052) | 0.051 | ||
| Hypertension | 1.967 (1.005–3.752) | 0.912 | ||
| BMI<18.5 | Reference | |||
| BMI=18.5–23.9 | 0.101 (0.063–0.289) | <0.01 | ||
| BMI>23.9 | 0.052 (0.024–0.282) | <0.01 | ||
| Dual-antiplatelet therapy ≤12 months | Reference | |||
| Dual-antiplatelet therapy 12–18 months | 6.133 (1.189–18.658) | <0.01 | ||
| Dual-antiplatelet therapy >18 months | 17.892 (7.705–29.834) | <0.01 | ||
| LVEF | 0.751 (0.524–0.896) | <0.01 | ||
| FBS | 1.988 (0.822–2.931) | 0.081 | ||
| NT-proBNP | 1.265 (0.709–3.130) | 0.075 | ||
| CK-MB | 1.060 (0.786–2.982) | 0.298 | ||
| Creatinine | 1.130 (1.039–1.919) | 0.089 | ||
| Hb | 0.891 (0.790–0.981) | 0.032 | ||
| LDL-C | 0.934 (0.809–1.070) | 0.677 | ||
| Aspirin treatment >36 months | 3.176 (0.890–7.128) | 0.052 |
HR=Hazard ratio; PCI=Percutaneous coronary intervention; BMI=Body mass index; LVEF=Left ventricular ejection fraction; FBS=Fasting blood glucose; Hb=Hemoglobin; LDL-C=Low density lipoprotein cholesterol; NT-proBNP=N-terminal pro-brain natriuretic peptide
Finally, as shown in Table 3, it was found that alcohol abuse (HR 4.565, 2.087–9.984, P < 0.01), history of PCI (HR 10.107, 3.675–27.800, P < 0.01), and history of atrial fibrillation (HR 9.419, 3.845–23.074, P < 0.01) were the risk factors for GI bleeding in ACS patients. Compared with the patients whose body mass index (BMI) was less than normal, normal BMI (HR 0.139, 0.053–0.360, P < 0.01) and high BMI (HR 0.099, 0.034–0.285, P < 0.01) were less likely to have GI bleeding. There was no significant difference in the risk of GI bleeding between patients treated with DAPT for <12 months and those treated for 12–18 months (HR 4.133, 1.049–16.287, P = 0.043). However, the risk of GI bleeding was significantly increased in patients treated with DAPT for more than 18 months (HR 12.792, 5.607–29.185, P < 0.01). The longer the use of dual antiplatelet drugs, the higher the cumulative risk of GI bleeding [Figure 2].
Table 3.
Multivariable-adjusted proportional hazards regression models for gastrointestinal bleeding
| Adjustment factors | HR | P | ||
|---|---|---|---|---|
| Alcohol abuse | 4.565 (2.087–9.984) | <0.01 | ||
| History of PCI | 10.107 (3.675–27.800) | <0.01 | ||
| History of atrial fibrillation | 9.419 (3.845–23.074) | <0.01 | ||
| BMI <18.5 | Reference | |||
| BMI=18.5–23.9 | 0.139 (0.053–0.360) | <0.01 | ||
| BMI >23.9 | 0.099 (0.034–0.285) | <0.01 | ||
| Dual-antiplatelet therapy ≤12 months | Reference | |||
| Dual-antiplatelet therapy 12–18 months | 4.133 (1.049–16.287) | 0.043 | ||
| Dual-antiplatelet therapy >18 months | 12.792 (5.607–29.185) | <0.01 |
HR=Hazard ratio; PCI=Percutaneous coronary intervention; BMI=Body mass index
Figure 2.

The relation between duration of dual platelet therapy and risk of gastrointestinal bleeding
Optimal duration of dual antiplatelet therapy
An receiver operating characteristic (ROC) curve was drawn to determine the optimal duration of DAPT, which can prevent acute coronary events and minimize the risk of GI bleeding [Figure 3]. The results showed that the duration of DAPT using a cutoff of 14.5 months resulted in a sensitivity of 66.7% and a specificity of 77%. The area under curve for this risk score was 0.735 (CI 0.657–0.813), P < 0.01.
Figure 3.

Receiver operating characteristic curve indicates the optimal duration of dual antiplatelet therapy. ROC = Receiver operating characteristic
Risk score for gastrointestinal bleeding
We created a risk score system for GI bleeding according to a logistic risk regression model to further examine the weight of various risk variables on GI bleeding. In this system, we assigned a score of 1 to alcohol addiction, combined with history of PCI, history of atrial fibrillation, BMI, DAPT time, and sex, for a total score of 14.31 [Table 4]. The higher the score, the higher the danger of GI bleeding. Through this scoring system, it is helpful to quickly and accurately determine the risk of GI bleeding in patients in clinical practice.
Table 4.
Risk score for gastrointestinal bleeding
| Risk factor | Score | |
|---|---|---|
| Alcohol abuse | 1 | |
| History of PCI | 2.5 | |
| History of atrial fibrillation | 2.8 | |
| BMI <18.5 | 2.2 | |
| DAPT >12–18 months | 2.1 | |
| DAPT >18 months | 3.4 | |
| Female | 0.31 |
DAPT=Dual-antiplatelet therapy; PCI=Percutaneous coronary intervention; BMI=Body mass index
DISCUSSION
There are many contradictions in the treatment of ACS patients with GI bleeding. Antiplatelet therapy is the basis of the treatment of ACS. Especially in patients who have undergone PCI, DAPT including a P2Y12 inhibitor should be used. However, the use of antiplatelet drugs often aggravates GI bleeding.[5,6]
Previous studies have shown that ACS combined with GI bleeding predicts a higher mortality rate.[7,8] A study of 13,819 patients with ACS showed that the incidence of GI bleeding was 1.3%, and the 30-day all-cause mortality associated with GI bleeding was 4.87%.[9] By analyzing the insurance and medical insurance claims data in the United States, it was found that anticoagulation combined with antiplatelet therapy significantly increased the risk of bleeding compared with simple antiplatelet therapy, and the elderly patients over 75 years were more likely to have bleeding.[10]
Thus, we followed up 1061 patients with ACS and tried to determine the risk of GI bleeding through statistical analysis. We found that 4.5% of patients had GI bleeding, and the risk of GI bleeding increased with the extension of follow-up time. In our study, multivariate Cox proportional hazard model analysis showed that patients with alcohol abuse, history of atrial fibrillation, low body weight, and those who had undergone PCI were more likely to have GI bleeding. Previous studies have found that patient with renal dysfunction, old age, and heart failure are the risk factors for GI bleeding.[11,12] Our study did not find a significant correlation between these factors and GI bleeding, which may be because the patients in our study had fewer and single underlying diseases at admission. Other studies have found that patients with atrial fibrillation, especially those with low body weight or who have undergone PCI, are more likely to have GI bleeding,[13,14] which is consistent with our findings. Patients who have had previous PCI may be at a higher risk of GI bleeding because they require longer and more intensive antiplatelet therapy. Moreover, for atrial fibrillation, it has become more common in patients who have undergone PCI, which may be due to higher comorbidity, especially in elderly patients with nonacute indications. At the same time, patients with a history of atrial fibrillation were likely to receive anticoagulant therapy, which increases the risk of bleeding. Whether it is atrial fibrillation, PCI, or weight factors, the root cause of GI bleeding seems to be a lack of balance between antithrombosis therapy and prevention of bleeding.
The greatest risk of prolonged DAPT is that it can easily lead to hemorrhage in the brain, GI tract, and other important organs. A study of 78,133 veterans showed that antithrombotic therapy increased the risk of hospitalization and blood transfusion due to GI bleeding.[15] In particular, third-generation P2Y12 inhibitors, such as ticagrelor and prasugrel, increase the risk of GI bleeding.[16] Our study found that the risk of GI bleeding within 12 months of DAPT was comparable to that with DAPT for 12–18 months. However, when DAPT lasted more than 18 months, the risk of GI bleeding increased significantly. In patients with ACS, the duration of DAPT has been controversial. For high-risk patients with extensive coronary artery disease and complex anatomical structure, different studies have reported different conclusions on whether to adopt a short DAPT strategy or long-term therapy.[17] The European Society of Cardiology and the AHA American College of Cardiology/AHA suggest that DAPT should last at least 12 months in patients with ACS, regardless of whether they are undergoing coronary intervention. In patients with low risk of bleeding, the duration of DAPT should be appropriately extended to 18 months or even longer according to the situation.[18,19] Our study estimated that the optimal duration of DAPT was 14.5 months by drawing an ROC curve. If the duration of DAPT exceeds 14.5 months, the risk of GI bleeding will be greatly increased; it is consistent with the results of other randomized clinical trials in recent years.[20]
Limitations
We should pay attention to a few limitations of this study. First, the number of patients included in our study was not large enough, and some patients (16.2%) were lost to follow-up. Second, there was no comparison of the bleeding risk between clopidogrel and ticagrelor. Finally, the follow-up time was not long enough. The research results need to be validated in large-scale, long-term follow-up randomized controlled trials.
CONCLUSION
In patients with ACS, DAPT time, alcohol abuse, low body weight, history of PCI, and history of atrial fibrillation are the main risk factors for GI bleeding. The optimal duration of DAPT is 14.5 months, which can provide patients with higher cardiovascular benefits and lower risk of GI bleeding.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. No animal studies were carried out by the authors for this article. Institutional review board approval was granted for the aggregate data set for research and quality improvement by the Ethics Committee of Beijing Anzhen Hospital, Capital Medical University. The Ethics Committee of the First Affiliated Hospital of Henan University of Science and Technology accepted central ethics approval.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Financial support and sponsorship
This study was supported by the Medical and health scientific research Special project of Luoyang 2020 (grant number 2001029A); Luoyang science and technology Foundation, medical and health project (grant number 2030002A).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
REFERENCES
- 1.Lau LH, Sung JJ. Treatment of upper gastrointestinal bleeding in 2020: New techniques and outcomes. Dig Endosc. 2021;33:83–94. doi: 10.1111/den.13674. [DOI] [PubMed] [Google Scholar]
- 2.Pioppo L, Bhurwal A, Reja D, Tawadros A, Mutneja H, Goel A, et al. Incidence of non-variceal upper gastrointestinal bleeding worsens outcomes with acute coronary syndrome: Result of a national cohort. Dig Dis Sci. 2021;66:999–1008. doi: 10.1007/s10620-020-06266-7. [DOI] [PubMed] [Google Scholar]
- 3.Pemmasani G, Elgendy I, Mamas MA, Leighton JA, Aronow WS, Tremaine WJ. Epidemiology and clinical outcomes of patients with inflammatory bowel disease presenting with acute coronary syndrome. Inflamm Bowel Dis. 2021;27:1017–25. doi: 10.1093/ibd/izaa237. [DOI] [PubMed] [Google Scholar]
- 4.Hao Y, Liu J, Liu J, Smith SC, Jr., Huo Y, Fonarow GC, et al. Rationale and design of the improving care for cardiovascular disease in China (CCC) project: A national effort to prompt quality enhancement for acute coronary syndrome. Am Heart J. 2016;179:107–15. doi: 10.1016/j.ahj.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Khan MY, Siddiqui WJ, Alvarez C, Aggarwal S, Hasni SF, Ahmad A, et al. Reduction in postpercutaneous coronary intervention angina in addition to gastrointestinal events in patients on combined proton pump inhibitors and dual antiplatelet therapy: A systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30:847–53. doi: 10.1097/MEG.0000000000001125. [DOI] [PubMed] [Google Scholar]
- 6.Hallas J, Dall M, Andries A, Andersen BS, Aalykke C, Hansen JM, et al. Use of single and combined antithrombotic therapy and risk of serious upper gastrointestinal bleeding: Population based case-control study. BMJ. 2006;333:726. doi: 10.1136/bmj.38947.697558.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng FH, Wong SY, Lam KF, Chang CM, Lau YK, Chu WM, et al. Gastrointestinal bleeding in patients receiving a combination of aspirin, clopidogrel, and enoxaparin in acute coronary syndrome. Am J Gastroenterol. 2008;103:865–71. doi: 10.1111/j.1572-0241.2007.01715.x. [DOI] [PubMed] [Google Scholar]
- 8.Chin MW, Yong G, Bulsara MK, Rankin J, Forbes GM. Predictive and protective factors associated with upper gastrointestinal bleeding after percutaneous coronary intervention: A case-control study. Am J Gastroenterol. 2007;102:2411–6. doi: 10.1111/j.1572-0241.2007.01460.x. [DOI] [PubMed] [Google Scholar]
- 9.Nikolsky E, Stone GW, Kirtane AJ, Dangas GD, Lansky AJ, McLaurin B, et al. Gastrointestinal bleeding in patients with acute coronary syndromes: Incidence, predictors, and clinical implications: Analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial. J Am Coll Cardiol. 2009;54:1293–302. doi: 10.1016/j.jacc.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Abraham NS, Noseworthy PA, Inselman J, Herrin J, Yao X, Sangaralingham LR, et al. Risk of gastrointestinal bleeding increases with combinations of antithrombotic agents and patient age. Clin Gastroenterol Hepatol. 2020;18:337–46. doi: 10.1016/j.cgh.2019.05.017. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye Z, Reintam Blaser A, Lytvyn L, Wang Y, Guyatt GH, Mikita JS, et al. Gastrointestinal bleeding prophylaxis for critically ill patients: A clinical practice guideline. BMJ. 2020;368:l6722. doi: 10.1136/bmj.l6722. [DOI] [PubMed] [Google Scholar]
- 12.Mahady SE, Margolis KL, Chan A, Polekhina G, Woods RL, Wolfe R, et al. Major GI bleeding in older persons using aspirin: Incidence and risk factors in the ASPREE randomised controlled trial. Gut. 2021;70:717–24. doi: 10.1136/gutjnl-2020-321585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SR, Choi EK, Park CS, Han KD, Jung JH, Oh S, et al. Direct oral anticoagulants in patients with nonvalvular atrial fibrillation and low body weight. J Am Coll Cardiol. 2019;73:919–31. doi: 10.1016/j.jacc.2018.11.051. [DOI] [PubMed] [Google Scholar]
- 14.Morita Y, Hamaguchi T, Yamaji Y, Hayashi H, Nakane E, Haruna Y, et al. Temporal trends in prevalence and outcomes of atrial fibrillation in patients undergoing percutaneous coronary intervention. Clin Cardiol. 2020;43:33–42. doi: 10.1002/clc.23285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abraham NS, Hartman C, Richardson P, Castillo D, Street RL, Jr., Naik AD. Risk of lower and upper gastrointestinal bleeding, transfusions, and hospitalizations with complex antithrombotic therapy in elderly patients. Circulation. 2013;128:1869–77. doi: 10.1161/CIRCULATIONAHA.113.004747. [DOI] [PubMed] [Google Scholar]
- 16.Guo CG, Chen L, Chan EW, Cheung KS, Isshiki T, Wong IC, et al. Systematic review with meta-analysis: The risk of gastrointestinal bleeding in patients taking third-generation P2Y (12) inhibitors compared with clopidogrel. Aliment Pharmacol Ther. 2019;49:7–19. doi: 10.1111/apt.15059. [DOI] [PubMed] [Google Scholar]
- 17.Verdoia M, Camaro C, Kedhi E, Marcolongo M, Suryapranata H, De Luca G. Dual antiplatelet therapy duration in acute coronary syndrome patients: The state of the art and open issues. Cardiovasc Ther. 2020;2020:6495036. doi: 10.1155/2020/6495036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2018;39:213–60. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 19.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Jr., Ganiats TG, Holmes DR, Jr., et al. 2014 AHA/ACC Guideline for the management of patients with non-ST-elevation acute coronary syndromes: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64:e139–228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Verdoia M, Khedi E, Ceccon C, Suryapranata H, De Luca G. Duration of dual antiplatelet therapy and outcome in patients with acute coronary syndrome undergoing percutaneous revascularization: A meta-analysis of 11 randomized trials. Int J Cardiol. 2018;264:30–8. doi: 10.1016/j.ijcard.2018.02.095. [DOI] [PubMed] [Google Scholar]


